CHAPTER 71 Arterial Anatomy of the Thorax
THORACIC AORTA
Normal Anatomy
The thoracic aorta can be divided into five segments: the aortic root, the ascending aorta, the proximal and posterior aortic arch (or aortic isthmus), and the descending aorta (Fig. 71-1). The aortic root is the short segment of the aorta that arises from the left ventricle to include the aortic valve and the sinuses of Valsalva. The right and left coronary arteries arise from the sinuses of Valsalva (Fig. 71-2). The sinotubular junction delineates this segment from the ascending aorta, which extends from the sinotubular junction to the first branch of the aortic arch. Although the average diameter of the ascending aorta is 3.5 cm, the diameter at which surgical repair is considered is 5.5 cm.1 The ascending aorta is branchless. It remains enveloped within the uppermost extent of the serous pericardium, and in some cases, in the presence of pericardial fluid, the transverse pericardial recess can be seen surrounding part of the ascending aorta. As the ascending aorta travels cephalad, it curves toward the right just above the right atrium and slightly anterior to the superior vena cava. It conventionally lies to the right of the main pulmonary artery and anterior to the right pulmonary artery, which branches from the main pulmonary artery at a lower level than that of the left pulmonary artery (Figs. 71-3 and 71-4).
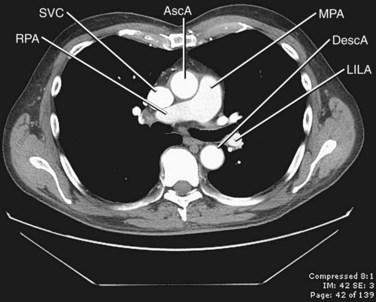
 FIGURE 71-4 Contrast-enhanced CT image through the middle mediastinum inferior to Figure 71-3 shows the origin of the right pulmonary artery (RPA) arising from the main pulmonary artery (MPA). The main pulmonary artery lies to the left of the ascending aorta (AscA), and the right pulmonary artery runs posterior to the superior vena cava (SVC). DescA, descending aorta; LILA, left interlobar artery.
FIGURE 71-4 Contrast-enhanced CT image through the middle mediastinum inferior to Figure 71-3 shows the origin of the right pulmonary artery (RPA) arising from the main pulmonary artery (MPA). The main pulmonary artery lies to the left of the ascending aorta (AscA), and the right pulmonary artery runs posterior to the superior vena cava (SVC). DescA, descending aorta; LILA, left interlobar artery.
The aortic arch is defined from the origin of the right brachiocephalic artery to the insertion of the ductus arteriosus, the ligamentum arteriosum in the adult. The arch courses obliquely through the anterior mediastinum from anterior to posterior and horizontally from right to left. Anterior to the arch lies the prevascular space, which is typically composed of mediastinal fat. The trachea is posterior to the proximal portion of the arch and is located to the right of the distal or posterior arch. The superior vena cava is conventionally to the right of the proximal arch (Fig. 71-5). The proximal aortic arch typically includes three branches in the following order from right to left: the right brachiocephalic, the left common carotid, and the left subclavian artery (Fig. 71-6). The distal segment of the arch, also known as the aortic isthmus, includes the portion extending from the left subclavian artery to the ligamentum arteriosum (Fig. 71-7). The ligamentum arteriosum represents the remnant of fetal circulation that shunts blood from the pulmonary artery to the arch and can often be identified in adults by associated calcification.
The descending aorta is divided into thoracic and abdominal portions. The thoracic segment of the descending aorta extends from the ligamentum arteriosum to the diaphragmatic aortic hiatus, at approximately T10. The average diameter is 2.48 cm, ranging from 1.6 to 3.7 cm. However, surgical intervention is considered when the diameter is greater than 6.5 cm.1 The descending thoracic aorta tapers distally and just above the diaphragm has an average diameter of 2.42 cm, with ranges from 1.4 to 3.3 cm.2 It courses along the left aspect of the spine within the posterior mediastinum. There are several paired branches arising from the descending thoracic aorta, which include the bronchial arteries, the esophageal arteries, and the posterior intercostal arteries. Phrenic branches and pericardial branches are also typically present.
Variant Anatomy
Variant branching patterns of the arch vessels are frequently encountered, particularly within the black population. The term bovine arch is a commonly used misnomer that refers to common origin of the brachiocephalic and left common carotid arteries (Fig. 71-8). Cadaveric studies show an overall incidence of 13%, with an incidence of 25% in blacks and 8% in whites.3 A variant of this, also erroneously referred to as bovine arch, consists of the left common carotid arising as a branch from the brachiocephalic artery, usually between 1 and 2.5 cm from the brachiocephalic origin.4 The incidence is 9% in the overall population, with 10% seen in blacks and 5% seen in whites.4
Anomalies of the aortic arch are rare, with the exception of the left aortic arch with aberrant right subclavian artery, which has an occurrence of 1 : 200.5 The incidence of the remaining anomalies is reported as less than 1% of all congenital cardiac anomalies; 85% to 95% of the arch anomalies are double aortic arch and right aortic arch with aberrant left subclavian artery.6 Arch anomalies are characterized by their sidedness as well as by the branching patterns off of the arch. There are five described anomalies: double aortic arch, right-sided aortic arch with mirror-image branching, right-sided aortic arch with abnormal branching, left-sided arch with abnormal branching, and cervical aortic arch.
To understand the origin of these variants, it is necessary to understand the embryology of the aortic arch. There are six paired aortic arches in the fetus as well as two dorsal aortae. The first and second aortic arches contribute to the formation of the stapedial artery. The third arches form the carotid system, including the right and left common, external, and internal carotids. The left fourth arch forms the aortic arch; the right forms a portion of the right subclavian artery. The fifth pair of aortic arches regress in their entirety. The sixth arches contribute to the development of the right and left pulmonary arteries, and the left also forms the ductus arteriosus. The left seventh segmental artery forms the left subclavian artery. The right seventh segmental artery contributes to the distal right subclavian artery. The right subclavian artery also receives contributions from the dorsal aorta.7 It is the abnormal regression or persistence of these structures that contributes to the anomalies discussed next (Fig. 71-9).8
In the normal development of the arch, the right dorsal aorta as well as a portion of the right sixth aortic arch regresses. In double aortic arch development, both of the paired dorsal aortae persist, with regression or persistence of the sixth arch, which contributes to the formation of the ductus arteriosus. Because there is complete encasement of the trachea and esophagus, patients often present early in life with nonpositional stridor. Double aortic arch can be seen on plain film radiography as a posterior indentation on the trachea by the right arch on lateral view and often on the posteroanterior view as tracheal indentations by the more superior right arch and inferior left arch. However, CT and MRI are the preferred methods for characterization of the anatomy as well as of any associated cardiac anomalies. Axial images demonstrate the “four artery sign,” representing paired carotid and subclavian arteries, evenly spaced around the trachea, just cephalad to the aortic arch (Fig. 71-10).9
When there is persistence of the right dorsal aorta and the left dorsal aorta regresses, a right-sided arch develops. There are three subtypes of right aortic arch, depending on the branching pattern. Mirror-image branching replicates the order of branching seen with a left-sided aorta, with a left brachiocephalic artery arising first, followed by the right common carotid and then the right subclavian artery (Fig. 71-11). A left-sided ductus arteriosus is typically seen with this entity, passing between the descending aorta and left pulmonary artery. Right aortic arch with mirror-image branching is almost always associated with congenital heart anomalies, most commonly tetralogy of Fallot, reported in 25% of cases.10
With right aortic arch with aberrant left subclavian artery, there is abnormal dorsal aortic regression between the origins of the left common and the left subclavian arteries. The left subclavian arises from a retroesophageal diverticulum, which is the remnant of the regressed dorsal segment.11 The ductus arteriosus arises from this diverticulum and connects to the left pulmonary artery, creating a complete vascular ring (Fig. 71-12). Because this condition forms a complete vascular ring, these patients often present with nonpositional stridor due to airway compression.11
Right aortic arch can also be seen with an isolated left subclavian artery, which has no direct communication to the aorta. Instead of originating from the arch, an isolated left subclavian artery arises from the left pulmonary artery through the ductus arteriosus. The embryologic origin of this anomaly is hypothesized to involve regression of the fourth arch as well as a portion of the sixth arch, with migration of the seventh intersegmental artery to the level of the sixth arch, forming the communication between the ductus and the pulmonary artery (which arises from the left sixth arch) and the left subclavian artery (arising from the seventh intersegmental artery).12 Because of the subclavian’s lack of oxygenated blood supply from the arch, this anomaly can present with extremity ischemia and subclavian steal phenomena. Although isolated subclavian artery has been reported on the right, it occurs much more commonly on the left. Right aortic arch with isolated left subclavian artery is almost always associated with congenital heart disease, most commonly with transposition and tetralogy of Fallot. Luetmer11 described 39 reported cases from 1970 to 1990.
Left-sided aortic arch with aberrant right subclavian artery is the most common aortic arch anomaly, with an occurrence of 1 : 200.5 Similar to right arch with aberrant left subclavian, this vascular anomaly results when there is abnormal regression between the right common carotid and right subclavian. The aberrant right subclavian arises from the diverticular remnant of the right aortic arch, also called Kommerell diverticulum. This aberrant artery most commonly passes posterior to the esophagus, but it can pass between the trachea and esophagus in 18% and anterior to the trachea in 4%.13 Because this condition does not produce a complete vascular ring, patients are usually asymptomatic (Fig. 71-13).
The aortic arch is typically located between the second costosternal junction on the right and the T4 vertebral body.14 An anomalous position of the arch, known as the high-riding cervical arch, is variable in position from just above the expected location of the arch to a supraclavicular location, within the soft tissues of the neck. A cervical arch often passes posterior to the esophagus and can be associated with both left and right arches. Although it is controversial, this anomaly is thought to develop as a result of persistent second or third branchial arches,15 with regression of the normal contributions from the fourth branchial arches. The aortic arch branches can also be anomalous in origin with this entity as well. On plain film radiography, a cervical arch is manifested as a superior mediastinal mass with tracheal deviation. CT shows the arch at or above the thoracic inlet, typically supraclavicular in location (Fig. 71-14). Clinically, patients can present with a pulsatile neck mass but generally are asymptomatic.
CAROTIDS: COMMON, EXTERNAL, AND INTERNAL
The carotid arteries arise from the third aortic arches embryologically. The right common carotid arises from the right brachiocephalic artery, most commonly at the level of the sternoclavicular joint. In approximately 12% of the population, the origin is above the sternoclavicular joint.16 The left common carotid can be divided into a thoracic and a cervical portion; the thoracic portion courses from the arch through the superior mediastinum, to the level of the sternoclavicular joint, where it becomes the cervical portion. The common carotid arteries are invested in a carotid sheath, which contains the internal jugular vein and the vagus nerve as well, with the artery medial to the vein and anteromedial to the nerve. The common carotids course obliquely cephalad, passing posterior to the sternoclavicular joints and deep to the sternocleidomastoid muscle, to the level of the thyroid cartilage, approximately C4, where they bifurcate into the internal and external carotid arteries. The bifurcation is the location of the carotid body (also called the carotid glomus or glomus caroticum), which is a cluster of chemoreceptors that can give rise to glomus tumors. In approximately 80% to 85% of patients, the internal carotid artery is posterior or posterolateral to the external carotid (Fig. 71-15).17
The external carotid artery supplies the face, scalp, and neck. It courses from the bifurcation at the level of the thyroid cartilage to the posterior aspect of the mandibular neck, where it branches into the superficial temporal and internal maxillary arteries. The external carotid has several branches, which typically arise in the following order: superior thyroid, ascending pharyngeal, lingual, facial, occipital, and posterior auricular arteries, followed by the terminal branches, the maxillary artery and superficial temporal artery (Fig. 71-16).
The internal carotid artery is the primary supply of oxygenated blood to the intracranial structures, including the anterior brain and the orbits. The internal carotid remains within the carotid sheath and retains the same relationship to the jugular vein and vagus nerve as the common carotid artery. The older classification delineated four segments: the cervical, petrous, cavernous, and cerebral segments. The newer classification system described by Bouthillier17 divides the internal carotid into the following seven segments: cervical (C1), petrous (C2), lacerum (C3), cavernous (C4), clinoid (C5), ophthalmic (C6), and communicating (C7). The cervical portion extends from the carotid bifurcation to the level of the carotid canal in the petrous bone, anterior to the jugular foramen (Fig. 71-17). The carotid bifurcation and the proximal portion of the cervical segment lie within an external anatomic landmark known as the carotid triangle. The triangle is defined anatomically by the sternocleidomastoid muscle posteriorly, the omohyoid muscle anteriorly, and the stylohyoid and digastric muscles superiorly (Fig. 71-18). The petrous segment of the internal carotid artery travels in the carotid canal of the petrous bone, taking a short vertical course before coursing medially and horizontally through the canal. The lacerum segment is a short segment of the internal carotid artery that begins at just above the foramen lacerum (typically filled with fibrocartilage in living patients) and continues to the petrolingual ligament. On exiting the carotid canal, the cavernous segment begins at the petrolingual ligament, ascends to the posterior clinoid process, then passes alongside the body of the sphenoid bone anteriorly, and finally courses vertically on the medial side of the anterior clinoid process, where it perforates the dura and becomes the clinoid segment at the proximal dural ring. The clinoid segment includes the portion of internal carotid artery between the proximal dural ring and the distal dural ring, where it then becomes the ophthalmic segment, which is intradural. The ophthalmic segment extends from the distal dural ring to the posterior communicating artery, with a course that is parallel and posterolateral to the optic nerve. This segment gives off the ophthalmic and superior hypophyseal arteries. The final segment of the internal carotid artery, called the communicating segment, passes between the optic and oculomotor nerves, giving rise to the posterior communicating artery and the anterior choroidal artery before bifurcating into the terminal branches of the internal carotid artery, the anterior cerebral artery and the middle cerebral artery.
Variant Anatomy
The level of the carotid bifurcation can be variable, most commonly above C4 when it occurs. Huber17a reported the bifurcation at C4-5 in 48% of 658 bifurcations and at C3-4 in 34%. The most stable anatomic landmark for the bifurcation is the superior thyroid cartilage.18 The common carotids are most commonly branchless, although sometimes they may give rise to the superior thyroid artery, the inferior thyroid, or, uncommonly, the vertebral artery.
The left common carotid artery most commonly arises from the aortic arch. As previously mentioned, a common origin of the left common carotid with the right brachiocephalic is sometimes referred to as a bovine arch, although this can be considered a misnomer because this is not the usual branching pattern in cows. Less common variations include a single trunk giving rise to both carotids and a common origin with the left subclavian, resulting in symmetric bilateral brachiocephalic trunks, occurring in 1.2% to 1.6% of individuals with anomalous branching.19
Variations of the external carotid include abnormal course and the absence of one or both of the external carotids. An abnormal position has been reported in 5% of the population by Prendes.20 Carotid basilar anastomoses are well known, but rare, vascular anomalies in which there is persistence of the primitive segmental arteries, which connect the carotid system to the vertebrobasilar system. Because the segmental arteries are named according to the cranial nerve with which they are associated, the three most common carotid basilar anastomoses arise from persistence of the trigeminal, otic, and hypoglossal arteries (Fig. 71-19). These anastomoses most commonly occur with the internal carotid but have been reported with the common carotid and external carotid arteries as well. A primitive trigeminal artery is the most common of these, with an incidence between 0.1% and 0.6% by angiography.21 It accounts for 80% to 85% of the persistent carotid basilar anastomoses.22 In primitive trigeminal artery, the persistent trigeminal artery communicates between the basilar and the internal carotid artery and can be further classified by medial or lateral type (supraclinoid internal carotid artery communication or precavernous internal carotid artery communication, respectively) and the presence or origin of the posterior cerebral artery (Saltzman types I and II). With Saltzman type I, the midbasilar and posterior communicating arteries are usually hypoplastic. With type II, the posterior communicating artery supplies the posterior cerebral artery’s territory, and the basilar joins the persistent trigeminal at the level of the superior cerebellar artery (Figs. 71-20 and 71-21).23 The persistent hypoglossal has a reported incidence between 0.03% and 0.26% on cerebral angiography.24 Fetal origin of the posterior cerebral artery arising from the supraclinoid portion of the internal carotid artery with no communication with the basilar artery occurs in 10% of the population on the right and left sides individually and in 8% bilaterally (Fig. 71-22).
BRONCHIAL ARTERIES
The bronchial arteries frequently demonstrate variable origin, branching patterns, and course. The most common configuration includes two bronchial arteries on the left and one on the right. The right bronchial artery most commonly arises from the first aortic intercostal artery. However, most often the left bronchial arteries originate directly from the descending thoracic aorta, between the levels of the T5 and T6 vertebrae. Bronchial arteries that originate outside this area are considered to be anomalous. Hartmann and colleagues25 found that in symptomatic patients presenting with hemoptysis, 36% had at least one bronchial artery of ectopic origin. Of these, 19% had a common bronchial trunk and 81% had isolated right or left bronchial arteries. The most common locations for ectopic origins of the bronchial arteries include the concavity of the aortic arch (74%), the subclavian artery (10.5%), and the descending aorta (8.5%).
PULMONARY ARTERIES
The pulmonary arteries transport deoxygenated blood to the lungs for oxygenation. The transport of deoxygenated rather than of oxygenated blood is a unique feature of the pulmonary arteries; the remainder of the arterial system transports oxygenated blood. The pulmonary trunk arises from the right ventricle, coursing superiorly and posteriorly, passing anterior to the ascending aorta and then to the left of the ascending aorta, where it bifurcates into the right and left main pulmonary arteries at approximately T5-6 (Fig. 71-23). The pulmonary trunk is completely enveloped in pericardium.
The right main pulmonary artery has a horizontal course, posterior to the ascending aorta and superior vena cava, running anterior to the right main bronchus. At the root of the right lung, the right pulmonary artery gives off two branches; the smaller supplies the upper lobe of the lung, and the larger supplies the middle and lower lobes (Fig. 71-24).
The left main pulmonary artery also courses horizontally and lies anterior to the left bronchus. At the root of the left lung, it also gives off two branches, supplying the upper and lower lobes (Fig. 71-25). The remnant of the ductus arteriosus, the ligamentum arteriosum, runs from the proximal aspect of the left pulmonary artery to the concave surface of the aortic arch, just distal to the origin of the left subclavian artery.
The right upper lobe is supplied by three segmental branches, the anterior, posterior, and apical segmental arteries, which arise from the ascending branch of the right main pulmonary artery (Fig. 71-26). The descending branch, the interlobar artery, which supplies the middle and lower lobes on the right, gives rise to the superior basal segmental artery and the middle lobar artery as the first branches at approximately the same level. The superior basal segmental artery originates from the posterior aspect of the interlobar artery; the middle lobar artery originates from the anterior aspect of the interlobar artery and further bifurcates into medial and lateral segmental arteries (Fig. 71-27). More caudally, the interlobar artery gives off the medial basal segmental and the anterior basal segmental artery, followed inferiorly by the posterior basal segmental artery and the lateral basal segmental artery (Fig. 71-28).
The left pulmonary artery divides into the ascending and descending branches, in similar fashion to the right pulmonary arterial system; however, the upper lobe is supplied by the anterior segmental artery and the apicoposterior segmental artery, which arise from the superior lobar pulmonary artery (Fig. 71-29). The descending branch, the interlobar artery, gives off the superior basal segmental artery and the lingular artery first, with the superior basal segmental artery at a slightly higher level than the lingular arteries, followed by the basal segments: anteromedial basal segment, posterior basal segment, and lateral basal segment (Fig. 71-30).
Variant Anatomy
Variations of the pulmonary artery include the pulmonary arterial sling and aberrant origin from the ductus arteriosus. Pulmonary arterial sling occurs when the left main pulmonary artery arises from the posterior right main pulmonary artery, courses around the right main stem bronchus, and then passes between the esophagus and the trachea, compressing the trachea (Fig. 71-31). Before the advent of cross-sectional imaging, this anomaly was diagnosed by barium studies, which demonstrated anterior indentation of the esophagus and posterior indentation of the trachea. The ligamentum venosum attaches to the main pulmonary artery, passing to the left of the trachea and attaching to the posterior aortic arch, completing the ring.
1 Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877-S1880.
2 Aronberg DJ, Glazer HS, Madsen K, Sagel SS. Normal thoracic aortic diameters by computed tomography. J Comput Assist Tomogr. 1984;8:247-250.
3 De Garis CF, Black IB, Riemenschneider EA. Patterns of the aortic arch in American white and Negro stocks, with comparative notes on certain other mammals. J Anat. 1933;67:599-618.
4 Layton KF, Kallmes DF, Cloft HJ, et al. Bovine aortic arch variant in humans: clarification of a common misnomer. AJNR Am J Neuroradiol. 2006;27:1541-1542.
5 Donnelly LF, Fleck RJ, Pacharn P, et al. Aberrant subclavian arteries: cross-sectional imaging findings in infants and children referred for evaluation of extrinsic airway compression. AJR Am J Roentgenol. 2002;178:1269-1274.
6 Kocis KC, Midgley FM, Ruckman RN. Aortic arch complex anomalies: 20-year experience with symptoms, diagnosis, associated cardiac defects, and surgical repair. Pediatr Cardiol. 1997;18:127-132.
7 Congdon ED. Transformation of the aortic-arch system during the development of the human embryo. Contrib Embryol. 1992;68:47-110.
8 Lowe GM, Donaldson JS, Backer CL. Vascular rings: 10-year review of imaging. Radiographics. 1991;11:637-646.
9 Kleinman PK, Spevak MR, Nimkin K. Left-sided esophageal indentation in right aortic arch with aberrant left subclavian artery. Radiology. 1990;191:565-567.
10 Nadas AS. Pediatric Cardiology, 2nd ed. Philadelphia: WB Saunders; 1963. p 430
11 Luetmer PH, Miller GM. Right aortic arch with isolation of the left subclavian artery: case report and review of the literature. Mayo Clin Proc. 1990;65:407-413.
12 Kalke BR, Magotra R, Doshi SM. A new surgical approach to the management of symptomatic aberrant right subclavian artery. Ann Thorac Surg. 1987;44:86-89.
13 Gray H, Pick TP, Howden R. Gray’s Anatomy: The Unabridged Running Press Edition of the American Classic. Philadelphia: Running Press; 1991. p 478
14 Harley HR. The development and anomalies of the aortic arch and its branches. With the report of a case of right cervical aortic arch and intrathoracic vascular ring. Br J Surg. 1959;46:561-573.
15 Gray H. Anatomy of the Human Body. Philadelphia: Lea & Febiger; 1918.
16 Trigaux JP, Delchambre F, Van Beers B. Anatomical variations of the carotid bifurcation: implications for digital subtraction angiography and ultrasonography. Br J Radiol. 1990;747:181-185.
17 Bouthillier A, van Loveren H, Keller JT. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996;38:425-433.
17a Huber P. Cerebral Angiography, 4th ed. Stuttgart, Germany: Thieme; 1982.
18 Ribeiro RA, Ribeiro JA, Rodrigues Filho OA, et al. Common carotid artery bifurcation levels related to clinical relevant anatomical landmarks. Int J Morphol. 2006;24:413-416.
19 Uflacker R. Atlas of Vascular Anatomy: An Angiographic Approach. Philadelphia: Lippincott Williams & Wilkins; 2006. p 135
20 Prendes JL, McKinney WM, Buonanno FS, Jones AM. Anatomic variations of the carotid bifurcation affecting Doppler scan interpretation. J Clin Ultrasound. 1980;8:147-150.
21 Hahnel S, Hartmann M, Jansen O, Sartor K. Persistent hypoglossal artery: MRI, MRA and digital subtraction angiometry. Neuroradiology. 2001;43:767-769.
22 Caldemeyer KS, Carrico JB, Mathews VP. The radiology and embryology of anomalous arteries of the head and neck. AJR Am J Roentgenol. 1998;170:197-203.
23 Uchino A, Sawada A, Takase Y, et al. MR angiography of anomalous branches of the internal carotid artery. AJR Am J Roentgenol. 2003;181:1409-1414.
24 De Caro R, Parenti A, Munari PF. The persistent primitive hypoglossal artery: a rare anatomic variation with frequent clinical implications. Ann Anat. 1995;177:193-198.
25 Hartmann IJ, Remy-Jardin M, Menchini L. Ectopic origin of bronchial arteries: assessment with multidetector helical CT angiography. Eur Radiol. 2007;17:1943-1953.

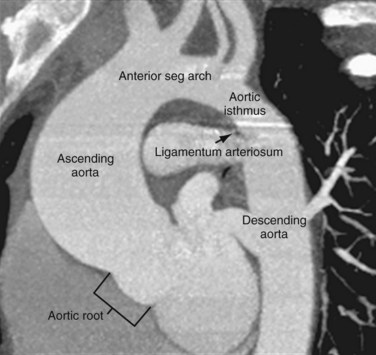
 FIGURE 71-1
FIGURE 71-1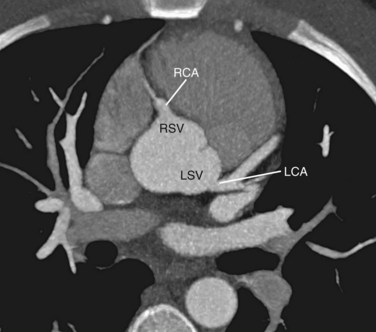
 FIGURE 71-2
FIGURE 71-2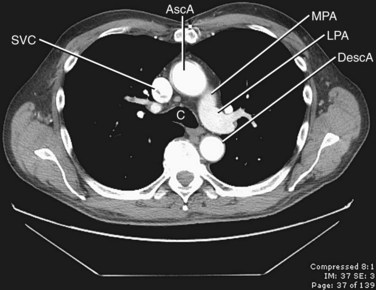
 FIGURE 71-3
FIGURE 71-3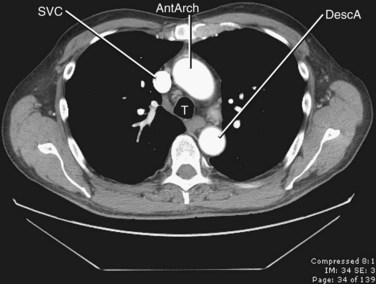
 FIGURE 71-5
FIGURE 71-5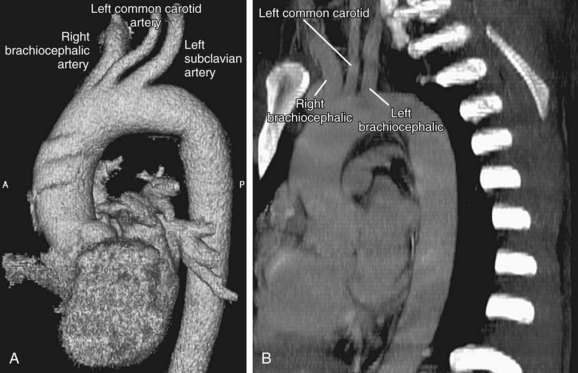
 FIGURE 71-6
FIGURE 71-6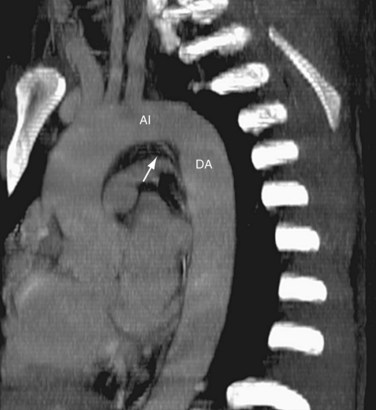
 FIGURE 71-7
FIGURE 71-7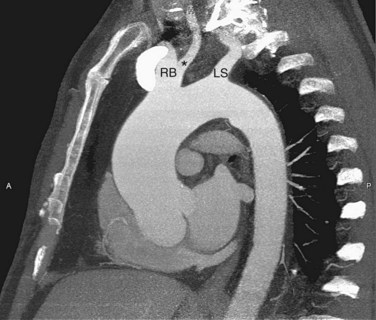
 FIGURE 71-8
FIGURE 71-8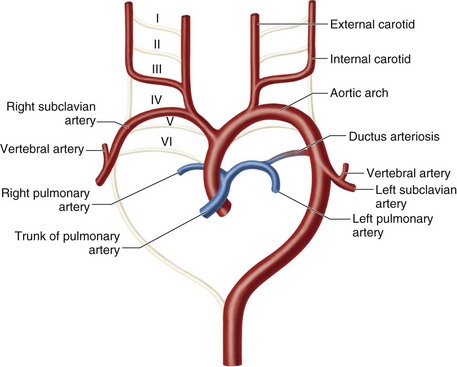
 FIGURE 71-9
FIGURE 71-9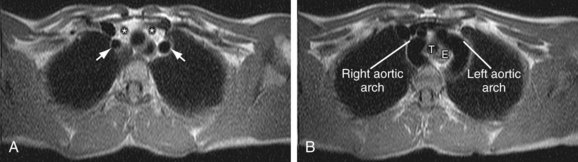
 FIGURE 71-10
FIGURE 71-10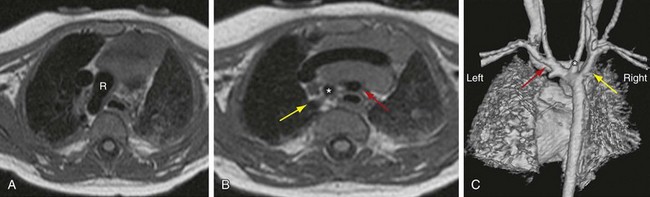
 FIGURE 71-11
FIGURE 71-11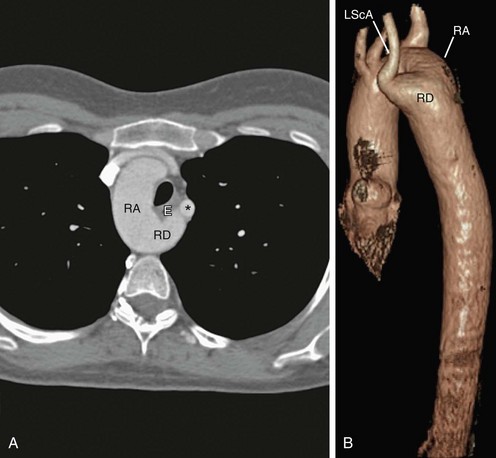
 FIGURE 71-12
FIGURE 71-12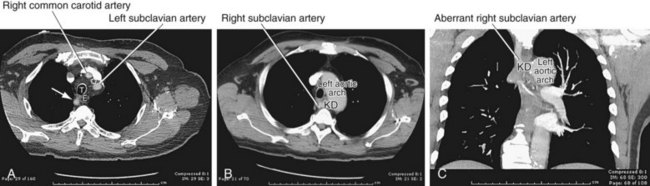
 FIGURE 71-13
FIGURE 71-13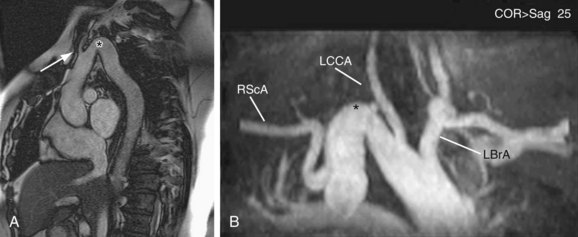
 FIGURE 71-14
FIGURE 71-14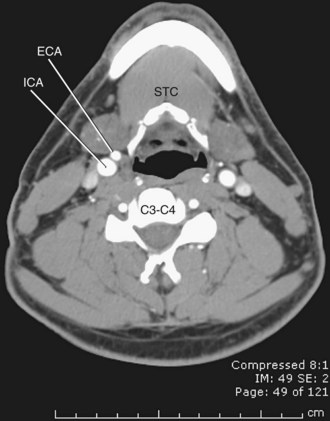
 FIGURE 71-15
FIGURE 71-15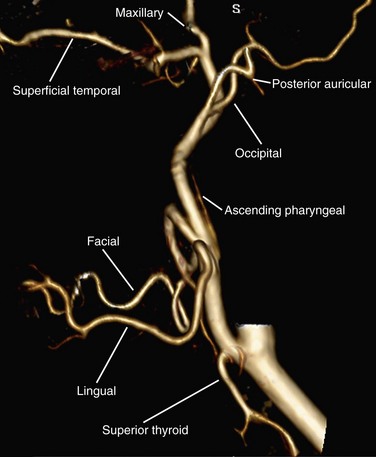
 FIGURE 71-16
FIGURE 71-16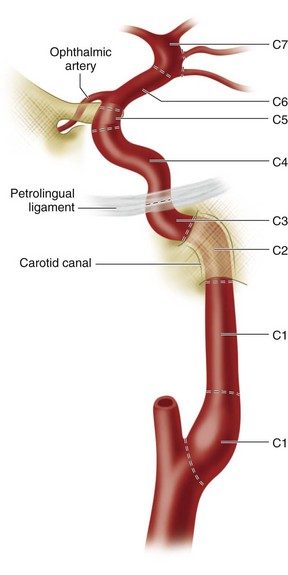
 FIGURE 71-17
FIGURE 71-17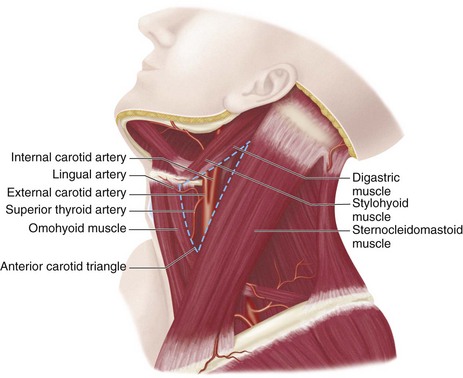
 FIGURE 71-18
FIGURE 71-18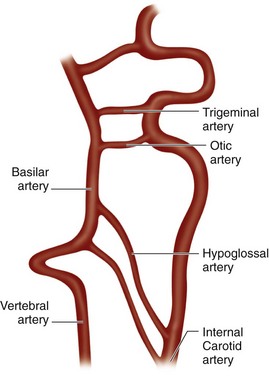
 FIGURE 71-19
FIGURE 71-19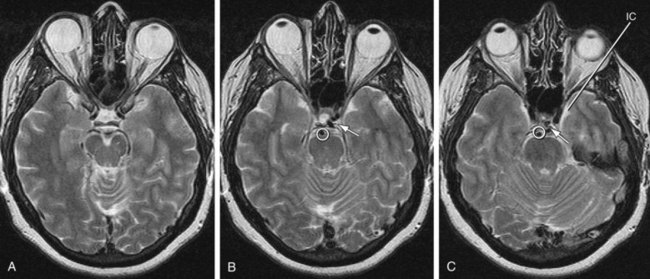
 FIGURE 71-20
FIGURE 71-20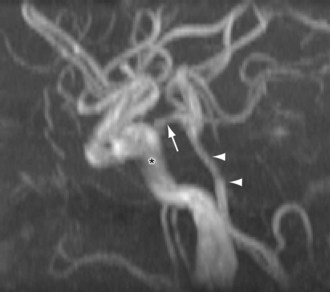
 FIGURE 71-21
FIGURE 71-21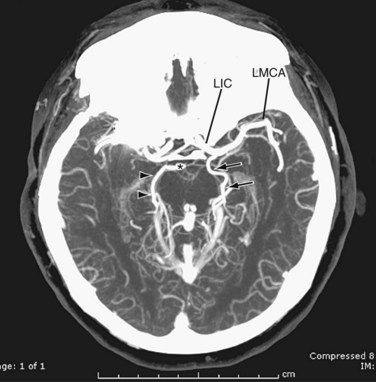
 FIGURE 71-22
FIGURE 71-22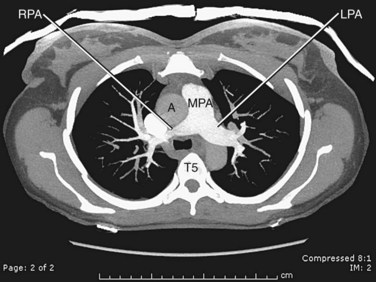
 FIGURE 71-23
FIGURE 71-23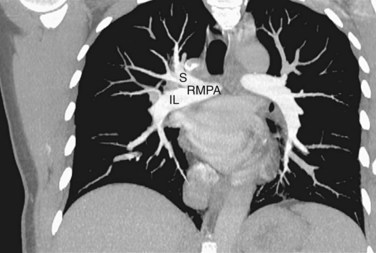
 FIGURE 71-24
FIGURE 71-24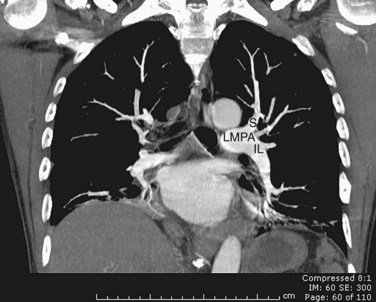
 FIGURE 71-25
FIGURE 71-25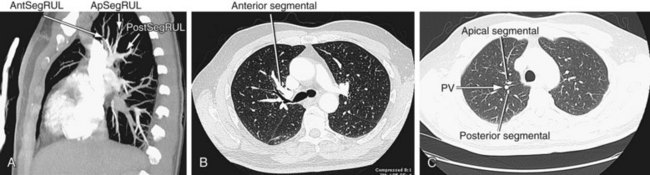
 FIGURE 71-26
FIGURE 71-26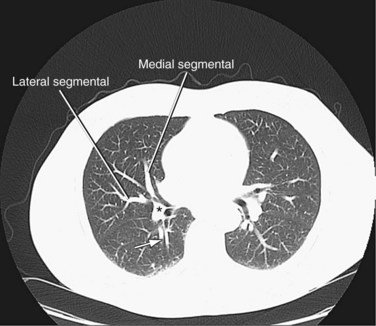
 FIGURE 71-27
FIGURE 71-27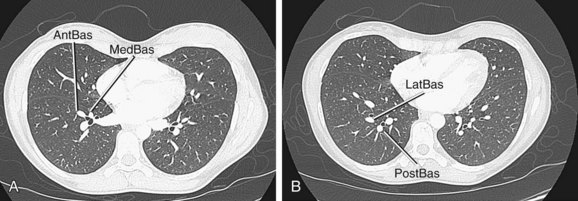
 FIGURE 71-28
FIGURE 71-28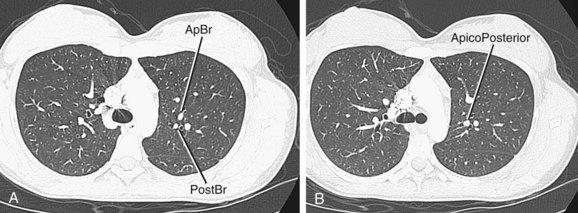
 FIGURE 71-29
FIGURE 71-29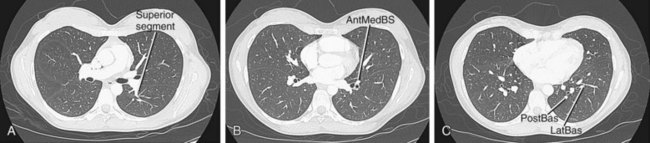
 FIGURE 71-30
FIGURE 71-30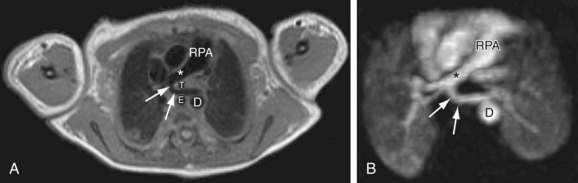
 FIGURE 71-31
FIGURE 71-31

