Chapter 73 Arrhythmias During Pregnancy
Cardiac arrhythmias during pregnancy, although not a routine problem, are not uncommon. Fortunately, life-threatening tachyarrhythmias in women of childbearing age are infrequent; sustained symptomatic bradyarrhythmias in this population are also exceedingly rare. Additionally, most inherent rhythm disorders manifest long before the patient reaches the childbearing age, suggesting that a more aggressive therapeutic approach may be warranted if the individual plans for a future pregnancy.1 The need to manage arrhythmias in this patient population, however, is likely to become a larger issue as women delay pregnancy until later in life and those with significant congenital heart abnormalities routinely survive into their reproductive years. Few adult cardiologists have had significant exposure to these patients, either in training or in clinical practice. As such, these patients are often approached with a great deal of trepidation, given the recognized pitfall that any management decision on the mother’s behalf could potentially adversely affect the health of the unborn child. This need not be the case if straightforward principles of evaluation and management are followed. Most arrhythmias during pregnancy can be safely and adequately managed with conservative therapies that have excellent outcomes for both mother and child.
Physiological Changes During Pregnancy
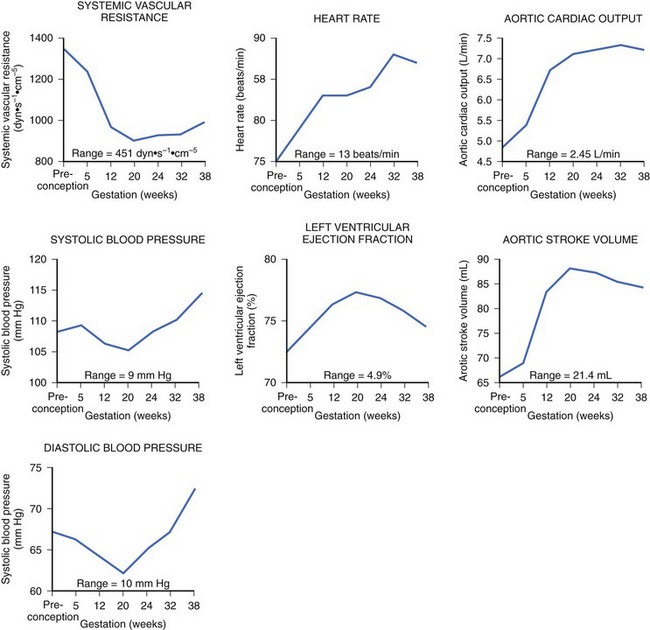
During pregnancy, the mother’s cardiovascular system must accommodate significant hemodynamic changes to meet her own needs and those of the developing fetus. Total body water increases by approximately 5 to 8 liters in a normal pregnancy, and even more so in patients with clinical edema. This process begins during the first trimester and continues until mid-pregnancy, at which time total body water levels stabilize and then gradually diminish until the last week of pregnancy.2 This rise in blood volume results in an approximately 40% increase in cardiac output, significantly increasing the mechanical demands on the maternal heart throughout this period. The increase in cardiac output in early pregnancy is disproportionately greater than the observed increase in heart rate, most likely secondary to the augmentation of stroke volume (Figure 73-1).3 Peripheral vascular resistance is seen to fall throughout pregnancy in proportion to the rise in total body water, resulting in a decrease in blood pressure in early pregnancy; the blood pressure reaches its nadir by mid-pregnancy and returns to baseline levels at term.
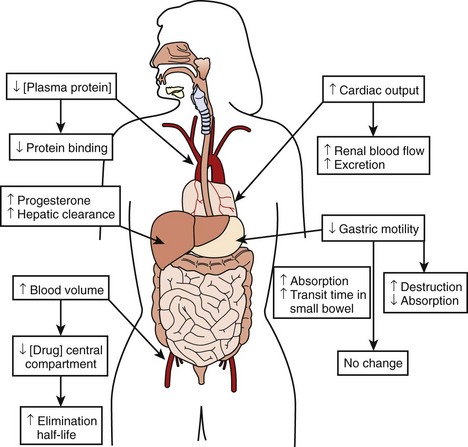
The physiological effects of pregnancy on drug therapy are significant. The observed increase in cardiac output increases renal blood flow by mid-pregnancy by as much as 60% to 80%, resulting in a peak glomerular filtration rate (GFR) that is 50% higher than that before the pregnancy. This GFR level is sustained until the end of pregnancy, resulting in markedly increased metabolism of renally cleared drugs.3 Changes in gastric motility and secretion affect drug absorption both upward and downward.4 Additionally, an increased level of progesterone during pregnancy results in the increased metabolism of hepatically cleared drugs.5 An increased blood volume with the resulting increased volume of distribution may lower the concentration of drugs in the central compartment, increasing their elimination half-life. At the same time, decreases in plasma protein concentration result in less protein binding of susceptible drugs, thus increasing their bioavailability and potential effect.3,6,7 The combined result of these changes makes careful assessment of clinical drug effect essential, as the measured serum drug concentration may be misleading because of altered protein binding. Total measured serum drug concentration may be low because of decreased protein binding, when, in fact, the drug’s active free fraction may have remained unchanged. Thus, although the drug level may appear low, therapeutic efficacy may have been achieved, making increases in drug dosage unnecessary and perhaps dangerous (Figure 73-2).
Principles of Evaluation and Management
There is no place for empirical treatment in pregnant patients.8 This is the cardinal rule that must be followed in the treatment of patients who are pregnant. Careful documentation, correct diagnosis, and correlation of the arrhythmia, whatever it may be, with symptom severity is vitally important. On the one hand, arrhythmias causing hemodynamic compromise to the mother obviously compromise the fetus via reduced placental blood flow and therefore are of primary concern. On the other hand, patients who complain of palpitations, but in whom no arrhythmia can be documented, have a low likelihood of having a life-threatening arrhythmia. In most cases, no further evaluation is warranted.9 In patients with minimal or minor symptoms and a structurally normal heart, the conservative principle “less is better” applies. The need for treatment must be clear. Symptom severity should guide clinical judgment as to whether the benefit of therapy outweighs the risks to both mother and fetus.
History
The recorded history should include detailed questioning of onset, length, and degree of symptoms as well as aggravating and alleviating factors. Symptoms of concern for hemodynamic instability include dizziness, syncope, or near-syncope. Precipitants of arrhythmias such as electrolyte imbalances, renal failure, thyrotoxicosis, and gestational diabetes should be considered.10 Structural heart disease and previously diagnosed tachyarrhythmias are of concern as the women with these conditions are at an increased risk of recurrence or worsening of their arrhythmia throughout the pregnancy. Patients with a history of repaired congenital heart disease will inherently carry the risk of developing scar-related tachyarrhythmias. A new arrhythmia during pregnancy may also be the first manifestation of underlying heart disease.11–13
Physical Examination
From the cardiovascular standpoint, physical examination of the pregnant patient is essentially identical to that of patients who are not pregnant. Normal findings during pregnancy include a widely split first heart sound, expiratory splitting of the second heart sound late in pregnancy, a soft systolic flow murmur believed to represent increased blood flow across the pulmonic valve, and mild peripheral edema.14 Findings of specific interest in a patient presenting with an arrhythmia would include murmurs of mitral stenosis, signs consistent with congestive heart failure, and murmurs consistent with congenital heart disease.
Diagnostic Testing
Electrocardiography
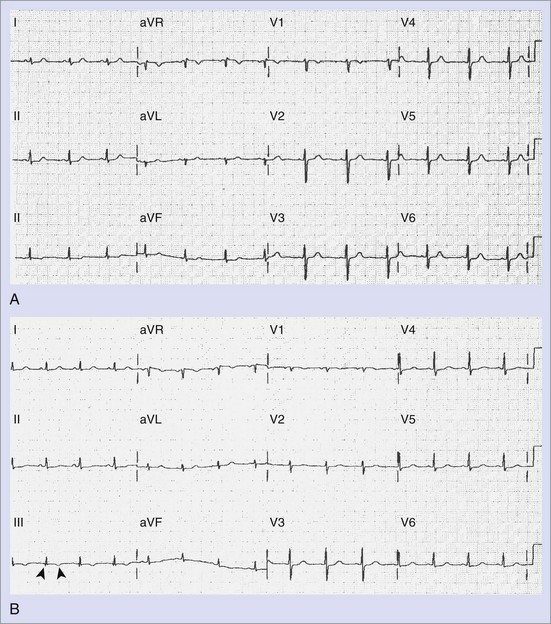
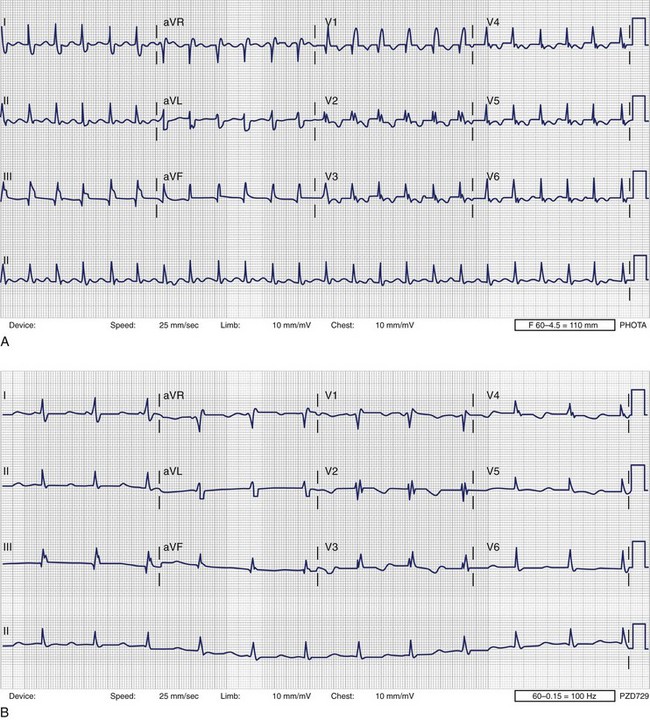
The standard 12-lead electrocardiogram (ECG) is, of course, immensely helpful in making the diagnosis if it can be obtained during an arrhythmia episode. A baseline recording may provide clues to the presence of underlying conduction system disease, chamber hypertrophy, ventricular pre-excitation, QT prolongation, and evidence of arrhythmogenic right ventricular dysplasia. The normal ECG during pregnancy may demonstrate a shift in the QRS axis to the left in the frontal plane with a small Q wave with an inverted T wave in lead III (Figure 73-3, A and B). These are normal changes during pregnancy resulting from a gradual shift in the position of the heart within the thorax.15
Electrophysiology Study
Although currently electrophysiology studies (EPSs) are routinely performed in the diagnosis and treatment of arrhythmias, the use of fluoroscopy for the positioning of catheters during diagnostic and ablative procedures limits this option during pregnancy.8 Radiation exposure of the fetus during the first and second trimesters of pregnancy has been linked to congenital malformations and mental retardation.16 In addition, radiation exposure in utero increases the risk of childhood malignancies, especially leukemia.17–20 If absolutely necessary, an EPS can be performed, with a lead apron draped over the mother’s abdomen; with this approach, good outcomes have been reported in the literature.21 Using intracardiac echocardiography and an electroanatomic mapping system can (and should) also be used for catheter placement and manipulation to minimize fluoroscopic time if ablation appears to be the only effective treatment.22,23 Because of the small, but finite, increased risk of childhood malignancy, routine use of fluoroscopy, even with appropriate shielding, is not recommended.
Cardiac Catheterization
Left heart catheterization performed during pregnancy has been reported infrequently.24 Although rarely indicated for the evaluation of an arrhythmia, knowledge of the coronary anatomy may be crucial to the management of certain patients such as an individual who has experienced aborted sudden cardiac death (SCD) during pregnancy. As the maternal age increases, the occurrence of acute ischemic events during pregnancy may rise. The same precautions mentioned above should be employed, with radiation exposure minimized as much as possible.
Frequency of Arrhythmias Complicating Pregnancy
Life-threatening arrhythmias during pregnancy are rare. Arrhythmias, when present, most commonly present as symptomatic palpitations, which, in most cases, represent an increased level of background atrial or ventricular ectopic activity. Although no single mechanism has been definitively cited, increased mechanical stress, intravascular volume shifts, elevated hormonal levels, and emotional changes occurring during pregnancy likely account for this increase. As most of these women will have structurally normal hearts, this ectopy is generally well tolerated, should be approached as benign, and can be anticipated to substantially resolve in the postpartum period.10,25,26
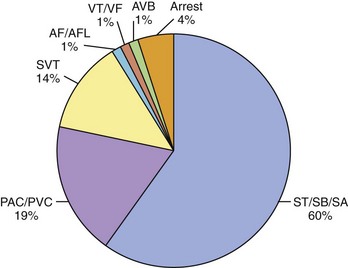
Women with previously diagnosed tachyarrhythmias are at an increased risk of recurrence of their arrhythmia during pregnancy, possibly initiated by increased ectopic activity serving as a trigger for its onset.11,12 Patients with no previous history may present for the first time with an arrhythmia for the same reason. Arrhythmias severe enough to require hospitalization, however, are rare. In a recently published paper, 9 years of hospitalizations at a high-volume obstetric service with the admission diagnosis of “arrhythmia” were reviewed.27 The prevalence of arrhythmia-associated admissions was 166 in 100,000 with an average patient age of 25 years. Sixty percent of arrhythmia admissions comprised a group consisting of patients with sinus tachycardia, sinus bradycardia, and sinus arrhythmia. Premature atrial or ventricular contractions were attributed to 19% of arrhythmia admissions and 14% to supraventricular tachycardia (SVT). Atrial fibrillation (AF) and flutter were counted separately from SVT and comprised only 1% of the arrhythmia admissions. The diagnosis of ventricular tachycardia (VT) or ventricular fibrillation (VF) was made in only 1% of admissions as was atrioventricular (AV) block (Figure 73-4).
Supraventricular Tachycardia
Sinus tachycardia is a normal response to pregnancy. The risk of paroxysmal SVT is generally equally distributed throughout pregnancy, although a rise in the frequency of onset of new or recurrent PSVT in the second and third trimesters has been reported.8,12 Several mechanisms have been postulated to explain the increased incidence, including hemodynamic, autonomic, hormonal, and emotional changes occurring in the pregnant patient. Increased mechanical stress, intravascular volume shifts, elevated estrogen levels, and physiological increases in heart rate have all been implicated.13 AV re-entrant tachycardia—which comprises the pre-excited, Wolff-Parkinson-White (WPW) form of AV reciprocating tachycardia and AV nodal re-entrant tachycardia as well as the non–pre-excited form—is the most common sustained arrhythmia seen in pregnancy and is seen with equal frequency.28,29 Atrial tachycardia, atrial flutter, and AF are encountered most often in patients with structural heart disease, repaired congenital heart disease, and valvular heart disease.
Ventricular Tachycardia
In 1921, Mackenzie reported that nonsustained ventricular arrhythmias were present in approximately 50% of pregnant women in his study.30 The recognized causes of recurrent or paroxysmal VT during pregnancy include arrhythmogenic right ventricular dysplasia, long QT syndrome (LQTS), hypertrophic cardiomyopathy, peripartum cardiomyopathy, and, in rare circumstances, coronary artery disease. Patients without organic heart disease who experience nonsustained VT during pregnancy are at a low risk for subsequent morbidity and mortality.31 Fortunately, very few women during their childbearing years have coronary artery disease, with an even smaller number having re-entrant VT. Multiple cases of sustained VT during pregnancy have been reported. The vast majority of these women have normal hearts and no history of VT before pregnancy. Some of these patients will invariably be found to have catecholamine-sensitive idiopathic VT.32,33 Right ventricular outflow tract (RVOT) tachycardia, for example, is often associated with exercise, typically has a left bundle branch block (LBBB) morphology with an inferior axis of depolarization, and is often responsive to β-blocker therapy (see Chapter 45). Onset of such VT during pregnancy with complete resolution after delivery has been reported in the literature.34 Fascicular VT is also seen in patients with structurally normal hearts. This form of VT generally arises from one of the left ventricular fascicles, most commonly the left posterior fascicle giving a right bundle branch block (RBBB), superiorly directed VT on the 12-lead ECG. This form of VT during pregnancy has also been reported in the literature and is often responsive to calcium channel blockers. Peripartum cardiomyopathy should always be considered with new-onset VT within 6 months of delivery.
Cardiac Arrest
Cardiac arrest during pregnancy is fortunately rare. It is estimated to occur in 1 in 30,000 deliveries as a result of complications occurring during pregnancy, labor, and delivery and in the immediate postpartum period.35 It is important to recognize that in this patient population, the etiology is often different from that for the general population, including amniotic fluid embolism, pulmonary embolism, hemorrhage, and eclampsia.36 Table 73-1 lists the most frequent causes of cardiac arrest in pregnancy. Cardiopulmonary resuscitation (CPR) in the pregnant patient will be reviewed later in this chapter.
Arrhythmias in Patients with Previous Tachyarrhythmia and Structural Heart Disease
In women with pre-existing cardiac rhythm disorders, exacerbation of their arrhythmia during pregnancy is common, increasing the risk of fetal complications.37 In a recently published study, Silversides et al reported on the recurrence rates of arrhythmias during pregnancy in women with known cardiac rhythm disorders.12 Initially, in sinus rhythm, 44% developed tachyarrhythmia recurrences during the pregnancy or in the early postpartum period. Nearly 50% of the women studied with a prior history of SVT had recurrence of their SVT complicating the pregnancy. AF or atrial flutter occurred in 52% of patients with a prior history of AF or atrial flutter. Almost all of the patients (96%) known to have AF or atrial flutter before their pregnancy had underlying structural heart disease.12
Congenital heart disease is a significant risk factor for cardiac arrhythmias and is found increasingly in women of childbearing years. Atrial tachyarrhythmias are frequently encountered, as are ventricular arrhythmias that are associated with such congenital conditions as corrected tetralogy of Fallot, hypertrophic cardiomyopathy, arrhythmogenic right ventricular dysplasia, and congenital LQTS. Silversides et al reported VT complications in 27% of pregnancies in women with a previous history of VT caused by LQTS or structural heart disease.12
Use of Drugs in the Management of Arrhythmias
Once the correct diagnosis has been made and arrhythmia has been documented, an accounting of the severity of the patient’s symptoms must be undertaken. The reasons for instituting treatment must be clear. Severity of symptoms helps determine whether the risks of therapy outweigh the benefits. The only difference in pregnancy is that the physician must consider the risk/benefit ratio for both the mother and the fetus. An arrhythmia that is hemodynamically compromising to the mother is of major concern because of the resulting compromised blood flow to the placenta. Under normal conditions this will be 6 to 7 L/min during the last trimester. The mother accomplishes this by means of a 15% increase in heart rate and 35% to 40% increase in cardiac output.36 Pharmacologic therapy should be reserved for patients with hemodynamically unstable arrhythmias, whereas bothersome symptoms in a patient with a normal heart should be treated conservatively with reassurance, if feasible.
If the decision to initiate drug therapy is made, as few drugs at the lowest effective therapeutic doses as possible must be used. Although this is inherently true for the treatment of all patients, it is particularly important in the pregnant patient. This approach exposes the mother and the fetus to the least possible amount of potential toxins. When feasible, drug choice should be limited to those with a history of safe use in pregnancy. The majority of antiarrhythmic drugs are classified as U.S. Food and Drug Administration (FDA) category C—this means that risk cannot be ruled out—as indicated by animal studies that have suggested risk, but no human studies or controlled studies in either humans or animals that suggest risk exists (Table 73-2).38 Keep in mind that these categories have been criticized as being misleading because they suggest the presence of graded risk as one crosses categories and similar risk among drugs in the same category. This is not accurate, as a wide range of severity of adverse effects exist within classes, and often no distinction can be seen between teratogenic and other toxic effects. For example, if given the choice between a category C drug that is new and one that has a long, safe history of use, the drug with the history of safe use is recommended.25 Of course, a chance of fetal harm is always present when any antiarrhythmic drug is taken during pregnancy, but in the right situation, the potential benefits should outweigh the potential risk. It is unlikely that comprehensive clinical trials will ever be performed in this patient population, for obvious ethical reasons. The publication of clinical case reports involving the use of these drugs in pregnancy is therefore vitally important.
Table 73-2 U.S. Food and Drug Administration Use-in-Pregnancy Ratings
| CATEGORY | INTERPRETATION |
|---|---|
| A | Controlled studies show no risk. Adequate, well-controlled studies in pregnant women have failed to demonstrate a risk to the fetus in any trimester of pregnancy. |
| B | No evidence of risk in humans. Adequate, well-controlled studies in pregnant women have not shown increased risk of fetal abnormalities despite adverse findings in animals, or, in the absence of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility. |
| C | Risk cannot be ruled out. Adequate, well-controlled human studies are lacking, and animal studies have shown a risk to the fetus or are lacking as well. A chance of fetal harm exists if the drug is administered during pregnancy, but the potential benefits may outweigh the potential risk. |
| D | Positive evidence of risk. Studies in humans or investigational or postmarketing data have demonstrated fetal risk. Nevertheless, potential benefits from the use of the drug may outweigh the potential risk. For example, the drug may be acceptable if needed in a life-threatening situation or serious disease for which safer drugs cannot be used or are ineffective. |
| X | Contraindicated in pregnancy. Studies in animals or humans or investigational or postmarketing reports have demonstrated positive evidence of fetal abnormalities or risk, which clearly outweighs any possible benefit to the patient. |
From the Physicians’ desk reference: PDR, ed 55, Oradell, NJ, 2001, Medical Economics, p 344.
Effects on the Fetus
The physician must consider the stage of pregnancy before deciding on a drug. The risk of teratogenicity is generally higher during the first trimester; after 8 weeks, organogenesis is complete, and the risk to the fetus is substantially reduced. The occurrence of teratogenic abnormalities depends on the drug the fetus is exposed to, the duration of exposure, and genetic predisposition.9 To further complicate management, the absorption, distribution, and excretion of drugs are dramatically altered during pregnancy, as previously described, making monitoring of drug levels difficult and therapeutic effects variable. Drug effects at the time of labor and delivery are also of concern and need to be taken into account.39 In most cases, the method of drug delivery (oral versus intravenous administration) has not been reported to be a significant factor in terms of toxicity. The method of delivery, however, will likely affect the speed and immediate potency of the drug effect and therefore needs to be factored into the decision process.
Lactation and Breastfeeding
Antiarrhythmic drug use during breastfeeding has similar concerns as during pregnancy. Many antiarrhythmic drugs and AV nodal blockers are excreted in breast milk, and thus continued use during breastfeeding must be considered on a case-by-case basis. Both warfarin and heparin are safe for use in nursing mothers who require long-term anticoagulation. Refer to Tables 73-3, 73-4, and 73-5 for current FDA ratings on drug safety during lactation for the most common drugs used to treat arrhythmias.
Table 73-3 Calcium Channel Blocker and Digoxin Therapy During Pregnancy
| DRUG | FDA CATEGORY | SAFETY DURING LACTATION |
|---|---|---|
| Verapamil | C | S |
| Diltiazem | C | S |
| Diltiazem IV | C | NS |
| Digoxin | C | S |
FDA, U.S. Food and Drug Administration; S, generally regarded as safe; maternal medication usually compatible with breastfeeding; IV, intravenous; NS, generally regarded as not safe and contraindicated or requires cessation of breastfeeding.
Atrioventricular Nodal Blockers: Adenosine
Although only limited data are available, it appears that adenosine has no direct effect on the fetus when fetal monitoring is performed during bolus intravenous (IV) administration.40,41 Adenosine, which is a purine nucleoside present in all human cells, characteristically depresses AV nodal conduction and sinus node automaticity. These electrophysiological effects have proven useful for the termination of SVT involving the AV node as part of the re-entrant circuit.42 Because of its rapid onset and short duration of action, it appears to be a safe drug for use during pregnancy, although it remains an FDA category C drug.43
Calcium Channel Blockers
Verapamil is a calcium channel blocker with a long history of use in the management of SVT. It crosses the placental barrier and has been reported to affect fetal cardiovascular activity.44 It is rapidly absorbed but has high first-pass metabolism, with only a small portion excreted unchanged in the urine. Ninety percent of the drug is plasma protein bound. Congenital defects in association with verapamil have not been reported. Verapamil is an FDA category C drug.
Diltiazem is a relatively newer drug than verapamil, so less information on the clinical history of its use in pregnancy is available. Nevertheless, it has been used in the treatment of premature labor without reported congenital abnormalities and thus should be safe for use in arrhythmias.45 Diltiazem is an FDA category C drug (see Table 73-3).
Digoxin
Digoxin is a cardiac glycoside, which has a long history of use in pregnancy for the management of supraventricular arrhythmias, although it remains classified as an FDA category C drug. Elimination of the drug is predominantly renal. Digoxin crosses the placenta readily, with fetal plasma concentrations similar to maternal values within 30 minutes.46 These agents have also been administered maternally to manage fetal tachyarrhythmias.47,48 Digoxin is not teratogenic.
β-Blockers
β-Blockers have been used extensively in pregnancy. Adverse outcomes with their use, predominantly consisting of fetal bradycardia, hypotonia, neonatal respiratory depression, low birth weight, and hypoglycemia, have been reported.4,9 The incidence of these adverse outcomes is low. No studies or case reports, to date, implicate any β-blocker in fetal malformations.
Atenolol, however, has recently been reclassified as a category D drug, that is, positive evidence of risk exists, so this drug should not be used in pregnant patients. The risk consists primarily of an increased incidence of reduced birth weight compared with placebo or other β-blockers.39 If in utero β-blocker use is necessary, it is important to watch for low birth weight and neonatal hypoglycemia in the infant following delivery. Infant blood glucose should be monitored for 24 to 48 hours after delivery, but again, the incidence of these adverse outcomes is low.4,9
Acebutolol and pindolol have recently been reclassified by the FDA as category B drugs. This classification designates that no evidence of risk in humans exists. Some data suggest that cardioselective agents (acebutolol) should be used preferentially because they may interfere less with β2-mediated peripheral vasodilation and uterine relaxation.49 Propranolol remains a category C drug but has a long history of safe use in pregnancy (see Table 73-4).50
Vaughn-Williams Class Drugs
Class IA Antiarrhythmic Drugs
Quinidine
Approximately 70% to 80% of an oral dose of quinidine is absorbed from the gastrointestinal (GI) tract, with 80% of the drug bound to plasma proteins. Elimination is predominantly hepatic. Quinidine readily crosses the placental barrier, a property that has been successfully exploited historically to terminate fetal arrhythmias.51 Of the class IA agents, quinidine has the longest record of safe use during pregnancy. Although isolated cases of adverse effects such as fetal thrombocytopenia and eighth nerve toxicity have been reported, the drug is considered relatively safe.25 Given its long history of safe use, quinidine is the drug of choice if one elects to use a class IA agent, although it is becoming clinically more difficult to obtain this drug.
Procainamide
Procainamide is 75% to 90% absorbed in the intestines, with 15% of the drug bound to plasma proteins. Elimination is predominantly renal. Although data are limited, procainamide appears to cross the placenta readily and thus has also been used to treat fetal tachyarrhythmias.52 No adverse fetal outcomes with use of procainamide have been reported, making it an acceptable choice in terminating and managing maternal SVTs. Oral procainamide is no longer clinically available. As it can be administered only intravenously, its use is currently limited to the initial period of arrhythmia management in the hospitalized patient.
Disopyramide
Little published experience in the use of disopryamide in pregnancy is available. It has 60% to 83% absorption in the GI tract and variable protein binding.4 Elimination is predominantly renal. Although it is not teratogenic, it can cause uterine contractions, which makes this drug less desirable for use during pregnancy than other IA agents.26
Class IB Antiarrhythmic Drugs: Lidocaine
Lidocaine is the drug of choice in the initial management of sustained VT and cardiac arrest. It is an FDA category B drug in pregnancy and is believed to be safe to use during lactation. Evidence as to whether or not it alters newborn neurobehavioral responses when administered to the mother during delivery is conflicting.53
Class IC Antiarrhythmic Drugs: Flecainide and Propafenone
Flecainide and propafenone both cross the placenta rather easily. Fetal levels of flecainide have been reported to be as high as 86% of maternal levels.54 Although flecainide is classified as an FDA category C drug, many reports have been made of its safe use during pregnancy with no adverse outcome.55 Neither of the class IC antiarrhythmic drugs has been reported to be teratogenic. Given the available clinical experience, flecainide is a reasonable choice in patients with structurally normal hearts who require antiarrhythmic therapy.
Class III Antiarrhythmic Drugs
Sotalol
Sotalol has relatively simple pharmacokinetics. It is nearly completely absorbed after oral administration. Approximately 80% to 90% is excreted in urine unchanged, so its use should be avoided in patients with renal insufficiency. It is known to cross the placenta but is the only class I or class III agent to be classified by the FDA as category B.56 Its use in pregnant patients with no adverse outcome has been reported; however, its quality of prolonging the Q-T interval is well described and must be closely watched for to avoid the risk of torsades de pointes.
Amiodarone
Amiodarone is a highly lipophilic compound that accumulates in multiple body tissues with well-described potential toxic side effects. It accumulates in placental tissue as well, although it achieves fetal concentrations of only 9% to 14% of the maternal serum concentration.57 It is clearly an effective antiarrhythmic drug in the general population, but its safety profile in pregnancy is poor. Amiodarone appears to have multiple serious adverse effects on the fetus, the most dangerous being neonatal hypothyroidism.39 (See Table 73-6 for full list of adverse effects.) Whereas amiodarone-induced hypothyroidism is reversible in adults, the newborn infant may suffer irreversible brain damage and seriously compromised respiration caused by a large goiter. Congenital abnormalities in neonates who were exposed to amiodarone have been reported.58,59 This being said, Strasburger et al reported on the safe and effective use of transplacentally administered (oral) amiodarone for the treatment of drug-refractory fetal tachycardia in 26 fetuses under 36 weeks of gestation.60 Low rates of associated fetal mortality and excellent efficacy in the treatment of SVT, junctional tachycardia, and VT in the hydropic fetus were also reported.60 These data suggest that amiodarone may have a role in treating the patient with drug-refractory, symptomatic, and potentially lethal tachyarrhythmias. Nevertheless, this drug carries an FDA category D rating and should be avoided in pregnancy.
Table 73-6 Reported Adverse Fetal Outcome with Amiodarone Use
Dofetilide
Dofetilide is a relatively new class III agent. It is currently classified as a category C drug by the FDA in human pregnancy, although it has been shown to be teratogenic and toxic in animal fetuses at exposure levels slightly above those expected in humans. As this is a new drug with little clinical experience of its use during pregnancy, the authors do not recommend its use as a first-line agent for the treatment of arrhythmias during pregnancy.61
Ibutilide
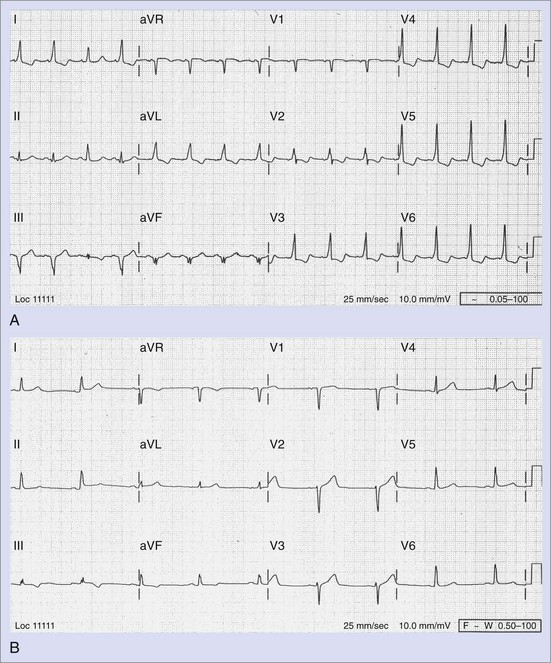
Ibutilide, a class III antiarrhythmic agent, was approved by the FDA in 1995 for intravenous termination of AF and atrial flutter.62 It is particularly useful for the termination of AF and atrial flutter in WPW syndrome, as it prolongs accessory pathway refractoriness as well as that of the atrial myocardium and the ventricular myocardium.63 Published case reports of its safe and successful use in termination of both AF and atrial flutter during pregnancy are now available.64,65 It undergoes extensive first-pass metabolism in the liver with a half-life of 2 to 12 hours and is clinically effective only in the intravenous form. It can cause significant QT prolongation, thus necessitating cardiac monitoring for at least 4 hours after administration (Figure 73-5). Pretreatment with magnesium sulfate is recommended to reduce the risk of torsades de pointes. The safety of ibutilide in early pregnancy has not been established; however, its use as a one-time agent for the termination of SVT suggests that it has a low risk of teratogenicity and may be a reasonable alternative to external cardioversion, particularly when conscious sedation is deemed undesirable. Ibutilide is an FDA category C drug.
Dronedarone
Dronedarone is a new class III antiarrhythmic developed for the treatment of AF. It is a derivative of amiodarone made by the removal of iodine and the addition of a methane-sulfonyl group.66 The later change was made to make the drug less lipophilic, shortening its half-life to approximately 24 hours, thus reducing its accumulation in tissue. These changes to amiodarone were made with the intention of producing a drug with electropharmacologic properties similar to those of amiodarone but having a reduced risk of amiodarone-associated thyroid-related disease and pulmonary disease. It has shown some promise in the treatment of paroxysmal and persistent AF and atrial flutter.67 Currently no data on its use in pregnancy are available, and thus its use cannot be recommended.
Other Cardiac Medications with Possible Antiarrhythmic Effects
Angiotensin-Converting Enzyme Inhibitors
Angiotensin-converting enzyme (ACE) inhibitors have been shown to have positive effects on atrial and ventricular remodeling and may possibly be beneficial in the suppression of AF.68 Nevertheless, they are associated with an increased risk of major congenital malformations when taken during the first trimester. ACE inhibitors should be stopped as soon as possible on becoming pregnant and, ideally, in anticipation of pregnancy. They are an FDA category C drug in the first trimester and a category D drug in the second and third trimesters when exposure of the fetus to this class of drug is known to cause fetal abnormalities, especially those related to the kidneys and associated structures.69
Fish Oil
Conflicting evidence in the efficacy of omega-3 fatty acids in reducing SCD is currently unresolved.70,71 Supplements made from fish livers, such as cod liver oil, contain the retinol form of vitamin A and should be avoided completely during pregnancy. Fish oils not derived from fish livers contain high amounts of docosahexaenoic acid (DHA), which is felt to be beneficial to speed of information processing and attention control in infants.72 At present, no recommendations for the daily intake of DHA by pregnant patients and no indication for its use in arrhythmia suppression exist.
Management of Specific Arrhythmias
Bradyarrhythmias
Congenital complete heart block is usually diagnosed during childhood; however, some cases are discovered incidentally during pregnancy, particularly in the asymptomatic patient. In general, in the asymptomatic patient, no acute intervention is required during pregnancy. No clear guidelines for the treatment of symptomatic patients in the first and second trimesters exist, but permanent pacemaker implantation, using echocardiographic guidance for lead placement, is probably indicated.73 In symptomatic women near term, temporary pacing with induction of labor as soon as possible is the procedure of choice.74 Patients with known congenital third-degree heart block who are considering pregnancy should be referred to a cardiologist for further evaluation. Indications for permanent pacing in these individuals to improve long-term survival have changed on the basis of specific criteria.
Palpitations
Symptomatic palpitations during pregnancy are common and usually represent an increase in “background” atrial, ventricular, and junctional ectopy, likely resulting from increased mechanical stress on the heart, intravascular volume shifts, elevated hormonal levels, and emotional changes occurring during pregnancy. Once documented as such, in a patient with a structurally normal heart, reassurance is the best treatment as these arrhythmias are generally benign, and the ectopy is likely to substantially resolve in the postpartum period.10 If symptoms are severe enough to warrant therapy, a β-blocker is the initial drug of choice.
Supraventricular Tachycardia
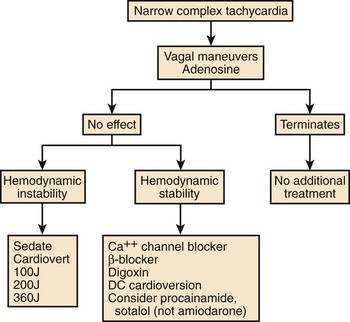
AV re-entrant tachycardia (AVRT) with or without pre-excitation on the 12-lead ECG and AV nodal re-entrant tachycardia (AVNRT) are the most common sustained arrhythmias seen during pregnancy. When they are present even before pregnancy, they often recur with increased frequency in the second and third trimesters and may be particularly resistant to treatment. Pre-existing focal AT may also increase in severity during this time.11 Atrial flutter and AF are seen less commonly and are most often encountered in patients with underlying structural heart disease. Figure 73-6 outlines a possible treatment algorithm for the acute management of SVT. The mainstay of initial therapy is adenosine for rapid termination. Alternatively, β-blockers or calcium channel blockers can be used for acute termination of SVT and are recommended in the most recent Advanced Cardiac Life Support (ACLS) guidelines as second-line therapy.75 Procainamide, sotalol, and, to a lesser extent, digoxin are other alternatives. One deviation from the guidelines that should be noted is the use of amiodarone in the SVT algorithm. In pregnant patients, even short-term infusion of amiodarone should be avoided, if possible. Transesophageal overdrive pacing to acutely terminate re-entrant SVT is an older technique, which is infrequently used currently but has proven efficacy. Its desirability for use in pregnancy would be its relative noninvasiveness. Recent case reports of its use during pregnancy and with neonatal SVT are available.76,77 Electrical cardioversion may be necessary for refractory SVT and is always indicated for hemodynamic instability.78,79
Chronic management of these arrhythmias is similar to that in the patient who is not pregnant. If overt pre-excitation is not present on the surface ECG, any of the AV nodal blockers can be used. Verapamil and digoxin should be avoided in the presence of pre-excitation because of their potential to enhance conduction over the accessory pathway. The concern here is with regard to the development of AF and subsequent rapid conduction to the ventricles through the accessory pathway inducing VF. The use of membrane-sensitive antiarrhythmic drugs should be reserved for patients with clearly defined, symptomatic arrhythmias. Flecainide, quinidine, procainamide, and sotalol are included in this group, with sotalol being the drug of choice in the presence of underlying structural heart disease. In the presence of pre-excited AVRT (WPW syndrome), β-blockers are the best choice for initial therapy, with the addition of flecainide or procainamide, if necessary, to impair conduction over the accessory pathway (Figure 73-7).80,81
Atrial Fibrillation and Atrial Flutter
AF and atrial flutter are very common arrhythmias in the general population but infrequent in women of childbearing age, except in the presence of underlying heart disease. Ventricular rate control is generally the immediate clinical goal, with verapamil, diltiazem, β-blockers, and digoxin all being acceptable agents in the attempt at achieving it. The risk factors for stroke in AF are at least the same as those in patients who are not pregnant, if not slightly higher because of the hypercoagulable state of pregnancy. If spontaneous conversion to sinus rhythm does not occur, early cardioversion (within 48 hours of the onset) should be performed to avoid the need for anticoagulation. During and immediately after cardioversion, the fetus should be monitored for signs of fetal distress. Cardioversion with up to 300 J has been demonstrated to be safe during pregnancy; however, direct-current cardioversion leading to sustained uterine contraction has been reported in some cases.82,83 IV ibutilide has also shown promise in the rapid termination of both AF and atrial flutter and may serve as an alternative to direct external current cardioversion in select cases.64,65 A further alternative is to use one-time high-dose oral flecainide (300 mg) or propafenone (600 mg) to promote conversion to sinus rhythm.84 If the arrhythmia is recurrent or does not easily convert to sinus rhythm, it is almost always because of underlying heart disease. In the case of refractory AF, the therapeutic goal should be adequate rate control to prevent the hemodynamic compromise of the placental blood supply (ideally <90 beats/min at rest and 140 beats/min with exercise).8 For the maintenance of sinus rhythm, the same drugs that are acceptable for the treatment of SVT are used for treating AF and atrial flutter. Quinidine, procainamide, flecainide, and sotalol have all been successfully used in this population, and all appear to be relatively safe. See below for recommendations on anticoagulation in the pregnant patient.
Ventricular Tachycardia
As discussed earlier, nonsustained VT during pregnancy is common and generally benign in the setting of a structurally normal heart. Patients without organic heart disease who experience brief nonsustained VT during pregnancy are at low risk for subsequent morbidity and mortality.31 Withholding drug therapy in this subset of patients, particularly if they are only mildly symptomatic, is reasonable. Idiopathic VT arising from a structurally normal heart is often catecholamine sensitive. In the case of RVOT tachycardia, β-blocker therapy, specifically cardioselective β-blockers, is often effective.32 Fascicular VT is often responsive to calcium channel blockers.
The risk of sustained VT during pregnancy is low but can occur because of genetic defects (LQTS, hypertrophic cardiomyopathy, right ventricular dysplasia, anomalous coronary artery), peripartum cardiomyopathy, and, less commonly, coronary artery disease. In the presence of hemodynamically unstable VT, immediate direct-current cardioversion is appropriate. If the VT is hemodynamically stable, IV antiarrhythmic drugs can be used. Lidocaine remains the drug of choice in pregnant patients if emergent antiarrhythmic drug therapy is needed, despite recent revisions in the ACLS guidelines. Few fetal side effects have been reported with the use of lidocaine, even in early pregnancy and despite significant placental drug transfer.85,86
Unfortunately, chronic antiarrhythmic therapy may be unavoidable in the face of sustained or recurrent VT. Most drugs currently used in the suppression of VT are FDA category C category for use in pregnancy. Quinidine, procainamide, and flecainide have all been used in pregnancy for this purpose, with good effect and no adverse fetal outcome.55,87 Class I antiarrhythmics should be avoided in abnormal hearts because of their proven proarrhythmic effects in this substrate, as in the patient who is not pregnant. Sotalol, however, is an FDA category B drug in pregnancy and remains a good choice in this subgroup of patients so long as renal function is not impaired. The preference of the authors of this chapter for medical therapy after starting a β-blocker would be flecainide in patients with normal hearts and sotalol in patients with structural heart disease. Amiodarone should be avoided in all but the most refractory cases because of its significant adverse fetal effects.
Congenital Long QT Syndrome
A retrospective study of patients with diagnosed LQTS found that the pregnant state was not significantly associated with an increased risk of cardiac events, although the risk did increase in the postpartum period, which suggests that management of these patients should include β-blocker therapy throughout pregnancy and the postpartum period.88 Syncope is a particularly alarming presentation in these patients and should be interpreted as heralding possible SCD. Patients deemed at high risk may require hospitalization until after delivery, at which time definitive therapy with an implanted cardioverter defibrillator (ICD) should be performed.89,90 Telemetric monitoring during labor and delivery is essential, as prolongation of the QTc interval during labor has been described, suggesting the physical and emotional stress during labor might precipitate cardiac arrest in women with LQTS.91 More recently, Seth et al reported a 2.7-fold increased risk of a cardiac event among women with LQTS during the immediate 9-month postpartum period. Additionally, women with the LQT2 genotype were found to be at a considerably higher risk for cardiac events during this period than those with LQT1 or LQT3 genotypes. β-Blocker therapy was associated with a reduction in cardiac events during the high-risk postpartum period.92 Of note, successful pregnancy after left stellate ganglionectomy has been reported in a patient with LQTS.93
Arrhythmogenic Right Ventricular Dysplasia
Case reports of the management of patients with arrhythmogenic right ventricular dysplasia (ARVD) in pregnancy are scarce. No arrhythmias were reported during pregnancy in those reviewed by the authors of this chapter.94,95 Sotalol is often used in the management of these patients and could be considered during pregnancy in a patient who demonstrates frequent ventricular ectopy or nonsustained VT. Theoretically, the management of these patients should be the same as that of those who are not pregnant, with the caveat that ablation of the VT substrate should be considered following delivery, possibly in conjunction with ICD implantation.
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) during pregnancy is fairly well described in the literature.96–98 The physiological changes that are most devastating for patients with HCM, particularly those with an obstructive component, are peripheral vasodilatation and a reduction in preload. Pregnancy characteristically causes an increase in intravascular volume and a decrease in systemic vascular resistance. Thus, a vast majority of patients with this abnormality do quite well during pregnancy, although maternal and fetal death from ventricular arrhythmias associated with HCM have been reported.97 Aortocaval compression during late-term pregnancy or large blood loss during delivery can compromise cardiac preload, thereby precipitating hemodynamic collapse and impeding successful resuscitation.
Implantable Cardioverter-Defibrillator
Implantable cardioverter-defibrillators (ICDs) are now the mainstay of the treatment of life-threatening ventricular arrhythmias. Guidelines for their use in primary and secondary prevention of SCD in the ischemic and nonischemic patient based on multiple large, randomized, prospective clinical trials have been published.99,100 Having an ICD is not a contraindication to becoming pregnant and may, in fact, simplify patient management by decreasing the reliance on suppressive drug therapy until proven absolutely necessary (i.e., the patient experiences an appropriate shock).101 In rare circumstances, it may be desirable to implant an ICD during the pregnancy, in which case lead placement should be accomplished with echocardiographic guidance to prevent radiation exposure to the fetus, or it should be performed under traditional fluoroscopic guidance with appropriate shielding of the fetus, if device implantation is attempted during later-term pregnancy when lesser risk to the fetus from such exposure is present. Pregnancy does not increase the risk of major ICD-related complications or result in a higher number of ICD discharges.101 Wearable external defibrillator harnesses are now commercially available. They do not yet have FDA approval for use during pregnancy but would seem to be a logical alternative as a bridge to permanent ICD implantation for the duration of the pregnancy. Unpublished documentation of the successful use of LifeVest (ZOLL LifeCor; Pittsburgh, PA) for termination of VT caused by LQTS in a pregnant patient (ZOLL Corporation, personal communication) exists.
Cardiopulmonary Resuscitation in the Pregnant Patient
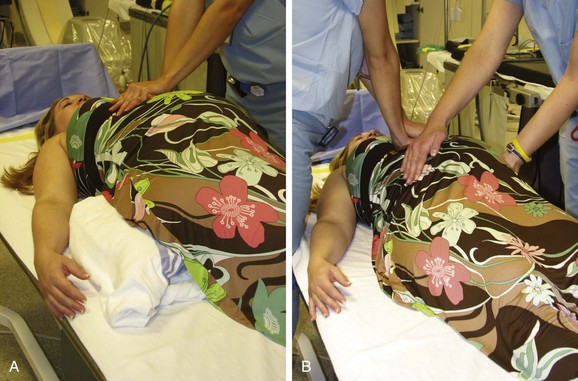
Cardiac arrest in the pregnant patient is complicated by several physiological factors, particularly in late-term pregnancy. Until the fetus becomes viable, at approximately 25 weeks, CPR should be performed as in the patient who is not pregnant. After 25 weeks, the presence of the increasing mass in the abdomen results in aortocaval obstruction and reduced venous return and forward blood flow with chest compression. This can occur as early as 20 weeks into the pregnancy. At term, the vena cava is completely occluded in 90% of supine pregnant patients.102 Thus, one of the most important steps that must be taken before initiating CPR in a noticeably pregnant patient is to displace the uterus to decrease aortocaval compression. One method is to place a wedge or rolled towel under the patient`s right hip to tip the uterus toward her left hip creating a tilt of at least 15 degrees. As anything more may decrease the effectiveness of chest compressions, the degree of pelvic tilt should be no more than 30 degrees. An alternative is manual displacement of the uterus during two-person CPR, with one rescuer on the patient’s left side using both arms to pull the uterus toward the left while the second performs chest compressions (Figure 73-8).103,104
Resting oxygen demand increases 20% during pregnancy to meet the requirements of the mother and the growing fetus. In addition, mothers experience a 20% decrease in lung functional residual capacity because of upward pressure on the diaphragm and lungs by the gravid uterus. The diaphragm is displaced upward by 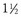 to 2 inches, and the anteroposterior chest diameter will increase slightly to maximize lung capacity, which leads to decreased chest compliance. As a result, pregnant patients become hypoxic much more quickly. These changes make it difficult to provide adequate oxygenation and circulation with standard CPR. Consequently, chest compressions require increased force and are generally performed higher on the chest than usual to accommodate for the shift in the abdominal contents toward the head. Early intubation should be strongly considered to maximize oxygenation and minimize the risk for aspiration of gastric contents during CPR, which is significantly increased in these patients.105
to 2 inches, and the anteroposterior chest diameter will increase slightly to maximize lung capacity, which leads to decreased chest compliance. As a result, pregnant patients become hypoxic much more quickly. These changes make it difficult to provide adequate oxygenation and circulation with standard CPR. Consequently, chest compressions require increased force and are generally performed higher on the chest than usual to accommodate for the shift in the abdominal contents toward the head. Early intubation should be strongly considered to maximize oxygenation and minimize the risk for aspiration of gastric contents during CPR, which is significantly increased in these patients.105
The most recent ACLS guidelines list amiodarone as the drug of choice in cases of refractory VF. In pregnancy, patients should receive lidocaine or procainamide first if defibrillation is unsuccessful. However, if the fetus is not yet viable and the death of the mother is imminent because of refractory VF, amiodarone would certainly be worth a try. After 25 weeks, cesarean section to save the fetus should be seriously considered within 5 minutes of arrest. It may facilitate the successful resuscitation of the mother as well.8 Neonatal and obstetric personnel should be involved early in the resuscitation effort.
The literature documents success with no adverse effect to the mother or fetus with energies as high as 300 J if defibrillation is performed.81 The risk of inducing a fetal arrhythmia is small, primarily because only a fraction of the total energy reaches the fetal heart.106 Some case reports, however, have described sustained uterine contraction causing fetal distress and necessitating emergency cesarean section when direct-current cardioversion is used.82
Anticoagulation Therapy in Pregnancy
Anticoagulation should be instituted in the presence of AF in patients with significant risk factors for embolic stroke (diabetes, hypertension, congestive heart failure, previous stroke, rheumatic heart disease) and should be maintained throughout pregnancy. Warfarin, the standard means of long-term anticoagulation in the general population, is, unfortunately, an FDA category X drug and cannot be used in pregnancy. The drug passes the placental barrier and may lead to spontaneous abortion, fetal hemorrhage, mental retardation, and birth malformations, particularly when used during the first trimester.107 High-dose subcutaneous heparin has been used as a substitute, particularly in the first trimester of pregnancy, as its large molecular weight prevents placental transfer. It should be discontinued at the onset of labor or 24 hours before the induction of labor. (See Table 73-7 for the advantages and disadvantages of heparin and warfarin.) Enoxaparin is a low-molecular-weight heparin (LMWH) and is classified as an FDA category B drug in pregnancy. A recent shift has occurred in practice, as many physicians are now using enoxaparin almost exclusively during pregnancy with no reported adverse effects to the pregnancy or the fetus. This shift in practice can be attributed to several factors, including the more favorable pharmacokinetic profile of the drug, its lower risk of heparin-induced thrombocytopenia, and ease of administration. To date, no evidence of teratogenicity exists.108,109 A recent consensus report suggests that enough data are available to support the safe and effective use of LMWH in the patient with a nonmechanical heart valve for the prevention and treatment of thromboembolism and the prevention of adverse obstetric complications during a high-risk pregnancy.110 Protocols for the use of this drug during pregnancy have been published.106
| AGENT | ADVANTAGES | DISADVANTAGES |
|---|---|---|
| Heparin | Does not cross the placenta | Must be administered parenterally |
| Easily and rapidly reversed | Maternal osteopenia | |
| Short half-life | Maternal thrombocytopenia | |
| Safe with breastfeeding | Maternal mechanical valve thrombosis | |
| Risk of systemic infection with continuous intravenous infusion | ||
| Warfarin | Highest efficacy for preventing thomboembolic events | Crosses the placenta |
| Safe with breastfeeding | Cannot be easily or rapidly reversed | |
| Teratogenic during the first trimester | ||
| Increased risk of hemorrhage during labor and delivery | ||
| Enoxaparin | Lower incidence of osteopenia or osteoporosis with long-term use | Maternal valve thrombosis |
| Lower incidence of heparin-induced thrombocytopenia | ||
| Favorable pharmacokinetics |
The prevention of maternal mechanical valve thromboses presents a difficult dilemma. Unfortunately, enoxaparin is not fully effective in preventing mechanical heart valve thrombosis, thus committing the mother to continuous IV heparin infusion or warfarin throughout the pregnancy.110 The use of warfarin during pregnancy has been reported and has been limited to those patients with mechanical heart valves from weeks 13 through the middle of the third trimester.108 Limited data are available to support this practice (see Table 73-7).
Labor and Delivery
During labor, cardiac output and blood pressure increase and continue to increase in the immediate period following delivery. Within the first hour following delivery, cardiac output begins to fall, returning to baseline levels by 2 weeks after delivery. SVTs occurring in the peripartum period can be managed as previously described. If the arrhythmia proves to be difficult to manage or fetal compromise is a concern, cesarean section may be advantageous. Adenosine remains a safe, rapid means of terminating re-entrant SVTs by using the AV node. Patients with underlying heart disease should have continuous cardiac monitoring during labor and delivery, even if no previous arrhythmia has been documented, as the stress of labor may provoke arrhythmia onset.111
Complete heart block occurring during labor and delivery is uncommon but has been described in the literature.111 Treatment of sudden complete heart block during labor and delivery is limited to those patients who become hemodynamically unstable. The best approach would be the placement of a temporary transvenous pacing wire under echocardiographic guidance.74
Key References
American Heart Association and the International Liaison Committee on Resuscitation. Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Part 8: Advanced challenges in resuscitation. Section 3: Special challenges in ECC. 3F: Cardiac arrest associated with pregnancy. Circulation. 2000;102(Suppl 8):I247.
Barnes EJ, Eben F, Patterson D. Direct current cardioversion during pregnancy should be performed with facilities available for fetal monitoring and emergency caesarean section. Br J Obstet Gynecol. 2002;109:1406.
Brent RL. The effect of embryonic and fetal exposure to x-ray, microwaves, and ultrasound: Counseling the pregnant and nonpregnant patient about these risks. Semin Oncol. 1989;16:347.
Burkart TA, Kron J, Miles WM, et al. Successful termination of atrial flutter by ibutilide during pregnancy. PACE. 2007;30:283.
Chandra NC, Gates EA, Thamer M. Conservative treatment of paroxysmal ventricular tachycardia during pregnancy. Clin Cardiol. 1991;14:347.
Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol. 1997;70:130.
Ginsberg JS, Greer I, Hirsh J. Use of antithrombotic agents during pregnancy. Chest. 2001;119:122S.
Hunter S, Robson S. Adaptation of the cardiovascular system to pregnancy. In: Oakley C, editor. Heart disease in pregnancy. London: BMJ Publishing Group, 1997.
Klein LL, Galan HL. Cardiac disease in pregnancy. Obstet Gynecol Clin. 2004;31:2.
Lee JM, Nguyen C, Joglar JA, et al. Frequency and outcome of arrhythmias complicating admission during pregnancy: Experience from a high-volume and ethnically diverse obstetric service. Clin Cardiol. 2008;31(11):538.
Natale A, Davidson T, Geiger MJ, Newby K. Implantable cardioverter-defibrillators and pregnancy. A safe combination? Circulation. 1997;96:2808.
Qasqas SA, McPherson C, Frishman WH, Elkayam U. Cardiovascular pharmacotherapeutic considerations during pregnancy and lactation. Cardiol Rev. 2004;12(4):201.
Silversides CK, Harris L, Haberer K, et al. Recurrence rates of arrhythmias during pregnancy in women with previous tachyarrhythmia and impact on fetal and neonatal outcomes. Am J Cardiol. 2006;97:1206.
Szumowski L, Walczak F, Dangel J, et al. Ablation of atypical, fast atrio-ventricular nodal tachycardia in a pregnant woman—a case report. Kardiol Pol. 2006;64(2):221.
Wladimiroff JW, Stewart PA. Treatment of fetal cardiac arrhythmias. Br J Hosp Med. 1985;34:134.
1 Michaelsson M, Engle MA. Congenital complete heart block: An international study of the natural history. Cardiovasc Clin. 1972;4:85.
2 Krauer B, Krauer F. Drug kinetics in pregnancy. Clin Pharmacokinet. 1977;2:167.
3 Dean M, Stock B, Patterson RJ, et al. Serum protein binding of drugs during and after pregnancy in humans. Clin Pharmacol Ther. 1980;28:253.
4 Mitani GM, Steinberg I, Lien EJ, et al. The pharmacokinetics of antiarrhythmic agents in pregnancy and lactation. Clin Pharmacokinet. 1987;12:253.
5 Cox JL, Gardner MJ. Treatment of cardiac arrhythmias during pregnancy. Prog Cardiovasc Dis. 1993;36:137.
6 Chen SS, Perucca E, Lee JN, et al. Serum protein binding and free concentration of phenytoin and phenobarbitone in pregnancy. Br J Clin Pharmacol. 1982;13:547.
7 Herngren L, Ehrnebo M, Boreus LO. Drug binding to plasma proteins during human pregnancy and in the perinatal period. Studies on cloxacillin and alprenolol. Dev Pharmacol Ther. 1983;6:110.
8 Anderson MH. Rhythm disorders. In: Oakley C, editor. Heart disease in pregnancy. London: BMJ Publishing Group, 1997.
9 Rotmensch HH, Elkayam U, Frishman W. Antiarrhythmic drug therapy during pregnancy. Ann Intern Med. 1983;98:487.
10 Shotan A, Ostrzega E, Mehra A, et al. Incidence of arrhythmias in normal pregnancy and relation to palpitations, dizziness, and syncope. Am J Cardiol. 1997;79:1061.
11 Tawam M, Levine J, Mendelson M, et al. Effect of pregnancy on paroxysmal supraventricular tachycardia. Am J Cardiol. 1993;72:838.
12 Silversides CK, Harris L, Haberer K, et al. Recurrence rates of arrhythmias during pregnancy in women with previous tachyarrhythmia and impact on fetal and neonatal outcomes. Am J Cardiol. 2006;97:1206.
13 Lee SH, Chen SA, Wu TJ, et al. Effects of pregnancy on first onset and symptoms of paroxysmal supraventricular tachycardia. Am J Cardiol. 1995;76:675.
14 Hunter S, Robson S. Adaptation of the cardiovascular system to pregnancy. In: Oakley C, editor. Heart disease in pregnancy. London: BMJ Publishing Group, 1997.
15 Nihoyannopoulos P. Cardiovascular examination in pregnancy and the approach to diagnosis of cardiac disorder. In: Oakley C, editor. Heart disease in pregnancy. London: BMJ Publishing Group, 1997.
16 Miller RW. Intrauterine radiation exposures and mental retardation. Health Phys. 1988;55:295.
17 Stewart A, Webb J, Giles D. Malignant disease in childhood and diagnostic irradiation in utero. Lancet. 1956;2:447.
18 Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol. 1997;70:130.
19 Brent RL. The effect of embryonic and fetal exposure to x-ray, microwaves, and ultrasound: Counseling the pregnant and nonpregnant patient about these risks. Semin Oncol. 1989;16:347.
20 Brent R, Meistrich M, Paul M. Ionizing and nonionizing radiations. In: Paul M, editor. Occupational and environmental reproductive hazards: A guide for clinicians. Baltimore: Williams & Wilkins, 1993.
21 Dominguez A, Iturralde P, Hermosillo AG, et al. Successful radiofrequency ablation of an accessory pathway during pregnancy. Pacing Clin Electrophysiol. 1999;22:131.
22 Lee MS, Evans SJ, Blumberg S, et al. Echocardiographically guided electrophysiologic testing in pregnancy. J Am Soc Echocardiogr. 1994;7:182.
23 Marchlinski F, Callans D, Gottlieb C, et al. Magnetic electroanatomical mapping for ablation of focal atrial tachycardias. Pacing Clin Electrophysiol. 1998;21:1621.
24 Shalev Y, Ben-Hur H, Hagay Z, et al. Successful delivery following myocardial ischemia during the second trimester of pregnancy. Clin Cardiol. 1993;16:754.
25 Joglar JA, Page RL. Treatment of cardiac arrhythmias during pregnancy: Safety considerations. Drug Saf. 1999;20:85.
26 Gowda RM, Khan IA, Mehta NJ, et al. Cardiac arrhythmias in pregnancy: Clinical and therapeutic considerations. Int J Cardiol. 2003;88:129.
27 Lee JM, Nguyen C, Joglar JA, et al. Frequency and outcome of arrhythmias complicating admission during pregnancy: Experience from a high-volume and ethnically diverse obstetric service. Clin Cardiol. 2008;31(11):538.
28 Wilbur SL, Marchlinski FE. Adenosine as an antiarrhythmic agent. Am J Cardiol. 1997;79:30.
29 Widerhorn J, Widerhorn AL, Rahimtoola SH, et al. WPW syndrome during pregnancy: Increased incidence of supraventricular arrhythmias. Am Heart J. 1992;123:796.
30 Mackenzie J. Heart disease and pregnancy. London: Henry Frowde & Hodder & Stoughton; 1921.
31 Chandra NC, Gates EA, Thamer M. Conservative treatment of paroxysmal ventricular tachycardia during pregnancy. Clin Cardiol. 1991;14:347.
32 Brodsky M, Doria R, Allen B, et al. New-onset ventricular tachycardia during pregnancy. Am Heart J. 1992;123:933.
33 Wolbrette D, Patel H. Arrhythmias and women. Curr Opin Cardiol. 1999;14:36.
34 Marchlinski FE, Deely MP, Zado ES. Sex-specific triggers for right ventricular outflow tract tachycardia. Am Heart J. 2000;139:1009.
35 Kloeck W, Cummins RO, Chamberlain D, et al. Special resuscitation situations: An advisory statement from the International Liaison Committee on Resuscitation. Circulation. 1997;95:2196.
36 Whitty J. Maternal cardiac arrest in pregnancy. Clin Obstet Gynecol. 2002;245(2):574.
37 Siu SC, Colman JM, Sorensen S, et al. Adverse neonatal and cardiac outcomes are more common in pregnant women with cardiac disease. Circulation. 2002;105:2179.
38 Physicians’ Desk Reference: PDR, 54th ed. Medical Economics, Oradell, NJ, 2000.
39 Qasqas SA, McPherson C, Frishman WH, Elkayam U. Cardiovascular pharmacotherapeutic considerations during pregnancy and lactation. Cardiol Rev. 2004;12(4):201.
40 Afridi I, Moise KJJr, Rokey R. Termination of supraventricular tachycardia with intravenous adenosine in a pregnant woman with Wolff-Parkinson-White syndrome. Obstet Gynecol. 1992;80:481.
41 Mason BA, Ricci-Goodman J, Koos BJ. Adenosine in the treatment of maternal paroxysmal supraventricular tachycardia. Obstet Gynecol. 1992;80:478.
42 DiMarco JP, Sellers TD, Lerman BB, et al. Diagnostic and therapeutic use of adenosine in patients with supraventricular tachyarrhythmias. J Am Coll Cardiol. 1985;6:417.
43 Elkayam U, Goodwin M. Safety and efficacy of intravenous adenosine therapy for supraventricular tachycardia during pregnancy—results of a national survey. J Am Coll Cardiol. 1994;23(Suppl A):91A.
44 Klein V, Repke JT. Supraventricular tachycardia in pregnancy: Cardioversion with verapamil. Obstet Gynecol. 1984;63(Suppl 3):16S.
45 El-Sayed YY, Holbrook RHJr, Gibson R, et al. Diltiazem for maintenance tocolysis of preterm labor: Comparison to nifedipine in a randomized trial. J Matern Fetal Med. 1998;7:217.
46 Saarikoski S. Placental transfer and fetal uptake of 3H-digoxin in humans. Br J Obstet Gynaecol. 1976;83:879.
47 Heaton FC, Vaughan R. Intrauterine supraventricular tachycardia: Cardioversion with maternal digoxin. Obstet Gynecol. 1982;60:749.
48 Wladimiroff JW, Stewart PA. Treatment of fetal cardiac arrhythmias. Br J Hosp Med. 1985;34:134.
49 Page RL. Treatment of arrhythmias during pregnancy. Am Heart J. 1995;130:871.
50 Pruyn SC, Phelan JP, Buchanan GC. Long-term propranolol therapy in pregnancy: Maternal and fetal outcome. Am J Obstet Gynecol. 1979;135:485.
51 Hill LM, Malkasian GDJr. The use of quinidine sulfate throughout pregnancy. Obstet Gynecol. 1979;54:366.
52 Dumesic DA, Silverman NH, Tobias S, et al. Transplacental cardioversion of fetal supraventricular tachycardia with procainamide. N Engl J Med. 1982;307:1128.
53 Kileff MB, James FMIII, Dewan D, et al. Neonatal neurobehavioral responses after epidural anesthesia for cesarean section with lidocaine and bupivacaine. Anesthesiology. 1983;57:A403.
54 Kofinas AD, Simon NV, Sagel H, et al. Treatment of fetal supraventricular tachycardia with flecainide acetate after digoxin failure. Am J Obstet Gynecol. 1991;165:630.
55 Ahmed K, Issawi I, Peddireddy R. Use of flecainide for refractory atrial tachycardia of pregnancy. Am J Crit Care. 1996;5:306.
56 MacNeil DJ. The side effect profile of class III antiarrhythmic drugs: Focus on d,l-sotalol. Am J Cardiol. 1997;80:90G.
57 Chow T, Galvin J, McGovern B. Antiarrhythmic drug therapy in pregnancy and lactation. Am J Cardiol. 1998;82:58I.
58 Magee LA, Downar E, Sermer M, et al. Pregnancy outcome after gestational exposure to amiodarone in Canada. Am J Obstet Gynecol. 1995;172:1307.
59 Ovadia M, Brito M, Hoyer GL, et al. Human experience with amiodarone in the embryonic period. Am J Cardiol. 1994;73:316.
60 Strasburger JF, Cuneo BF, Michon MM, et al. Amiodarone therapy for drug-refractory fetal tachycardia. Circulation. 2004;109:375.
61 Webster WS, Brown-Woodman PDC, Snow MD, et al. Teratogenic potential of almokalant, dofetilide, and d-sotalol: Drugs with potassium channel blocking activity. Teratology. 1996;53:168.
62 Murray KT. Ibutilide. Circulation. 1998;97:493.
63 Naccarelli GV, Lee KS, Gibson JK, Vanderlugt JT. Electrophysiology and pharmacology of ibutilide. Am J Cardiol. 1996;78:12.
64 Kockova R, Kocka V, Kiernan T, et al. Ibutilide-induced cardioversion of atrial fibrillation during pregnancy. J Cardiovasc Electrophysiol. 2007;18:545.
65 Burkart TA, Kron J, Miles WM, et al. Successful termination of atrial flutter by ibutilide during pregnancy. PACE. 2007;30:283.
66 Zareba KM. Dronedarone: A new antiarrhythmic agent. Drugs Today. 2006;42:75.
67 Hohnloser SH, Crijns H, van Eickels M, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360:668.
68 Serra JL. Review: Atrial fibrillation and the rennin angiotensin system. Ther Adv Cardiovasc Dis. 2008;2(3):215.
69 Klein LL, Galan HL. Cardiac disease in pregnancy. Obstet Gynecol Clin. 2004;31:2.
70 Albert CM, Hennekens CH, O’Donnell CJ. Fish consumption and the risk of sudden cardiac death. JAMA. 1998;279:23.
71 Brouwer IA, Zock PL, Camm AJ, et al. Effect of fish oil on ventricular tachyarrhythmias and death in patients with implantable cardioverter defibrillators. JAMA. 2006;295:2613.
72 Helland IB, Smith L, Saarem K, et al. Maternal supplementation with very-long-chain n-3 fatty acids during pregnancy and lactation augments children’s IQ at 4 years of age. Pediatrics. 2003;111:e39.
73 Pedrinazzi C, Gazzaniga P, Durin O, et al. Implantation of a permanent pacemaker in a pregnant woman under the guidance of electrophysiologic signals and transthoracic echocardiography. J Cadiovasc Med. 2008;9(11):1169.
74 Dalvi BV, Chaudhuri A, Kulkarni HL, et al. Therapeutic guidelines for congenital complete heart block presenting in pregnancy. Obstet Gynecol. 1992;79:802.
75 The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Part 6: Advanced cardiovascular life support: 7D: The tachycardia algorithms. Circulation. 2000;102(Suppl 8):I158.
76 Szumowski L, Walczak F, Dangel J, et al. Ablation of atypical, fast atrio-ventricular nodal tachycardia in a pregnant woman—a case report. Kardiol Pol. 2006;64(2):221.
77 Gimovsky ML, Nazir M, Hashemi E, Polcaro J. Fetal/neonatal supraventricular tachycardia. J Perinatol. 2004;24(3):191.
78 Rotmensch H, Elkayam U. Management of cardiac arrhythmias during pregnancy. Drugs. 1987;33:623.
79 Ogburn P, Schmidt G, Linman J, et al. Paroxysmal tachycardia and cardioversion during pregnancy. J Reprod Med. 1982;27:359.
80 Goldberger J, Helmy I, Katzung B, et al. Use-dependent properties of flecainide acetate in accessory atrioventricular pathways. Am J Cardiol. 1994;73:43.
81 Manolis AS, Estes NAIII. Reversal of electrophysiologic effects of flecainide on the accessory pathway by isoproterenol in the Wolff-Parkinson-White syndrome. Am J Cardiol. 1989;64:194.
82 Curry JJ, Quintana FJ. Myocardial infarction with ventricular fibrillation during pregnancy treated by direct current defibrillation with fetal survival. Chest. 1970;58:82.
83 Barnes EJ, Eben F, Patterson D. Direct current cardioversion during pregnancy should be performed with facilities available for fetal monitoring and emergency caesarean section. Br J Obstet Gynaecol. 2002;109:1406.
84 Reiffel JA. Drug choices in the treatment of atrial fibrillation. Am J Cardiol. 2000;85(10 Suppl 1):12.
85 Briggs GG, Freeman RK, Yaffe SJ. Drugs in pregnancy and lactation, 5th ed. Baltimore: Williams & Wilkins; 1998.
86 American Heart Association and the International Liaison Committee on Resuscitation. Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. Part 8: Advanced challenges in resuscitation. Section 3: Special challenges in ECC. 3F: Cardiac arrest associated with pregnancy. Circulation. 2000;102(Suppl 8):I247.
87 Fagih B, Sami M. Safety of antiarrhythmics during pregnancy: Case report and review of the literature. Can J Cardiol. 1999;15:113.
88 Rashba EJ, Zareba W, Moss AJ, et al. Influence of pregnancy on the risk for cardiac events in patients with hereditary long QT syndrome, for the LQTS Investigators. Circulation. 1998;97:451.
89 McCurdy CMJr, Rutherford SE, Coddington CC. Syncope and sudden arrhythmic death complicating pregnancy. A case report of Romano-Ward syndrome. J Reprod Med. 1993;38:233.
90 Ganta R, Roberts C, Elwood RJ, et al. Epidural anesthesia for cesarean section in a patient with Romano-Ward syndrome. Anesth Analg. 1995;81:425.
91 Minakami H, Nakayam T, Ohno T, et al. Effect of vaginal delivery on the QTc interval in a patient with the long QT (Romano-Ward) syndrome. J Obstet Gynaecol Res. 1999;25:251.
92 Seth R, Moss AJ, McNitt S, et al. Long QT syndrome and pregnancy. J Am Coll Cardiol. 2007;49:1092.
93 Bruner JP, Barry MJ, Elliott JP. Pregnancy in a patient with idiopathic long QT syndrome. Am J Obstet Gynecol. 1984;149:690.
94 Koenig C, Katz M, Gertsch M, et al. Pregnancy and delivery in a patient with Uhl anomaly. Obstet Gynecol. 1991;78:932.
95 Villanova C, Muriago M, Nava F. Arrhythmogenic right ventricular dysplasia: Pregnancy under flecainide treatment. G Ital Gardiol. 1998;28:691.
96 Kolibash AJ, Ruiz DE, Lewis RP. Idiopathic hypertrophic subaortic stenosis in pregnancy. Ann Intern Med. 1975;82:791.
97 Shah DM, Sunderji SG. Hypertrophic cardiomyopathy and pregnancy: Report of a maternal mortality and review of literature. Obstet Gynecol Surv. 1985;40:444.
98 Maron BJ, Bonow RO, Cannon ROIII, et al. Hypertrophic cardiomyopathy. Interrelations of clinical manifestations, pathophysiology, and therapy. N Engl J Med. 1987;316(13):178-179.
99 Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure, for the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. N Engl J Med. 2005;352(20):2146.
100 The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337:1576.
101 Natale A, Davidson T, Geiger MJ, Newby K. Implantable cardioverter-defibrillators and pregnancy. A safe combination? Circulation. 1997;96:2808.
102 Kerr MG, Scott DB, Samuel E. Studies of the inferior vena cava in late pregnancy. BMJ. 1964;1:532.
103 Lee RV, Rodgers BD, White LM, et al. Cardiopulmonary resuscitation of pregnant women. Am J Med. 1986;81:311.
104 Goodwin AP, Pearce AJ. The human wedge. A manoeuvre to relieve aortocaval compression during resuscitation in late pregnancy. Anaesthesia. 1992;47:433.
105 2005 American Heart Association guidelines of cardiopulmonary resuscitation and emergency cardiovascular care. Part 10.8: Cardiac arrest associated with pregnancy. Circulation. 2005;112(Suppl 1):IV-150.
106 DeSilva RA, Graboys TB, Podrid PJ, et al. Cardioversion and defibrillation. Am Heart J. 1980;100:881.
107 Shaul WL, Hall JG. Multiple congenital anomalies associated with oral anticoagulants. Am J Obstet Gynecol. 1977;127:191.
108 Ginsberg JS, Greer I, Hirsh J. Use of antithrombotic agents during pregnancy. Chest. 2001;119:122S.
109 Huxtable LM, Tafreshi MJ, Ondreyco SM. A protocol for the use of enoxaparin during pregnancy: Results from 85 pregnancies including 13 multiple gestation pregnancies. Clin Appl Thromb Hemost. 2005;11:171.
110 Anticoagulation in Prosthetic Valves and Pregnancy Consensus Report (APPCR) panel and scientific roundtable. Anticoagulation and enoxaparin use in patients with prosthetic heart valves and/or pregnancy, Topol EJ, Chairman. Clin Cardiol Consensus Rep. 1, 2002.
111 Spritzer RC, Seldon M, Mattes LM, et al. Serious arrhythmias during labor and delivery in women with heart disease. JAMA. 1970;211:1005.