Chapter 78 Arrhythmias Associated with Congenital Heart Disease
Arrhythmia Substrates in Patients with Congenital Heart Disease
Arrhythmia Substrates in Unrepaired Patients with Congenital Heart Disease
Accessory Pathways
Congenital heart disease is relatively common in the general population (~1% of births), and accessory pathways are also somewhat common (1.6 to 3 of 1000 live births). Therefore, one would expect to observe Wolff-Parkinson-White (WPW) syndrome and supraventricular tachycardia (SVT) on the basis of these incidences alone. Indeed, in the Pediatric Radiofrequency Ablation Registry, ablations for accessory pathways have been reported in patients with most types of defects.1 However, it is well known that certain types of congenital cardiac disease are more commonly associated with WPW syndrome. The most prominent of these defects is Ebstein’s anomaly of the tricuspid valve.2,3 In patients with these defects, the prevalence of WPW is approximately 9%. Other defects reported to demonstrate an increased association with WPW include L-transposition of the great vessels (ventricular inversion and congenitally corrected transposition) and hypertrophic cardiomyopathy.4–8 In patients with Ebstein’s anomaly, accessory pathways are often multiple and are generally right sided, although occasionally a posteroseptal pathway location is present. In L-transposition, one sees an increased incidence of Ebstein’s anomaly of the left-sided (systemic) atrioventricular (AV) valve, which is morphologically always a tricuspid valve and is related to a left-sided, morphologic right ventricle. In these patients, it is thought that the increased incidence of WPW is explained by the coexistence of Ebstein’s anomaly.4–6 Finally, WPW has perhaps been overdiagnosed in patients with hypertrophic cardiomyopathy (HCM) because of the pre-existing common QRS abnormality that may resemble pre-excitation. Specifically, some patients actually have a fasciculoventricular pathway of no clinical significance.9 However, SVT mediated by accessory pathways does occur in some patients with HCM; in these patients, SVT may be very poorly tolerated because of a coexisting hemodynamic abnormality.7 Specifically, Danon disease, a form of glyocogen storage disease, is associated with ventricular pre-excitation.10
Other Substrates for Tachycardia
Other arrhythmias are occasionally seen in patients with congenital heart disease who have not had surgical repair. Atrial flutter, when seen, usually is a complication of atrial dilation on hemodynamic grounds. For example, patients with Ebstein’s anomaly or other causes of severe tricuspid regurgitation may have atrial flutter, which, in turn, is most likely caused by right atrial dilation.3 Patients with mitral valve disease, and in particular mitral stenosis, are at risk for atrial fibrillation (AF), depending on their left atrial dilation. Patients with left ventricular failure and those with pulmonary hypertension, suprasystemic right ventricular pressure, or both may also have increased atrial pressures, with consequent atrial arrhythmias, and may also have ventricular ectopy, ventricular tachycardia (VT), or ventricular fibrillation (VF).
Atrioventricular Block
Certain groups of patients are predisposed to the development of complete AV block, independent of attempted surgical repair. First, patients who have L-transposition, also known as ventricular inversion or congenitally corrected transposition of the great vessels, are at risk for the development of complete AV block spontaneously throughout their lives. The yearly incidence has been estimated at approximately 2% per year.11 This is attributed to the abnormalities of the conduction system, in which malalignment exists between the atrial and the ventricular septa, and the normal compact AV node cannot make contact during embryologic development with the distal conducting system. A more anterior AV node forms instead and is thought to be more fragile.
Second, a syndrome of familial AV block associated with various septal defects has recently been described. Heterozygous mutations in NKX2.5, a homeobox transcription factor, lead to the spontaneous development of complete AV block, as well as associated cardiac defects, of which atrial septal defects (ASDs) are the most common.12 Ventricular septal defects (VSDs) may also be seen, particularly those associated with tetralogy of Fallot.
Arrhythmia Substrates Following Surgery for Congenital Heart Disease
Anatomic and Developmental Considerations
During cardiac embryologic development, the right atrium is derived from three sources13: (1) The primitive right atrium forms adjacent to the tricuspid annulus and gives rise to the heavily trabeculated right atrial free wall and right atrial appendage. (2) The sinus venosus is incorporated into the right atrium and provides the origin for the smooth-walled portion of the right atrium (sinus venarum) that exists between the cavae posterior to the primitive right atrial structures. (3) Finally, septation of the primitive common atrium is accomplished by the formation of the atrial septum from the septum primum and the septum secundum. The ostium secundum is a foramen that forms in the septum primum, which is subsequently closed by the septum secundum, which forms a flap over this ostium to create the foramen ovale. In the fetus, the foramen ovale provides a route for right atrial blood to cross to the left atrium. All along the junction between the primitive right atrium and the sinus venosus portion of the right atrium is the crista terminalis (“terminal crest”), which appears as a ridge along the inner surface of the right atrium. The crista terminalis runs superior to inferior along the lateral wall of the right atrium. At its superior edge, near the junction between the superior vena cava (SVC) and the right atrium, is the sinus node pacemaker complex. As it arches inferiorly toward the inferior vena cava (IVC), it gives rise to the eustachian valve ridge (EVR), which appears as more of a flap than a ridge. The EVR is a remnant of the primitive right sinoatrial valve, guarding the ostium between the sinus venosus and the primitive right atrium. The EVR runs anterior to the IVC orifice and posterior to the posterior portion of the tricuspid valve annulus. As such, in the fetal circulation, the EVR acts to direct the IVC flow away from the tricuspid annulus and toward the foramen ovale. As the EVR arches toward the inferior atrial septum, it passes just superior to the ostium of the coronary sinus. It joins with the valve of the coronary sinus to form the tendon of Todaro, which inserts on the atrial septum near the bundle of His. With the coronary sinus ostium and the tricuspid annulus, the tendon of Todaro forms the triangle of Koch, and at the apex of this triangle, the compact AV node is found.
Adult-Type Atrial Flutter
Classic atrial flutter is characterized by atrial rates of up to 300 beats/min, with typical and very characteristic saw-tooth flutter waves visible on the surface electrocardiogram (ECG). This suggests the presence of nearly continuous atrial electrical activity because of the relative lack of a long atrial isoelectric interval in most patients. In typical atrial flutter, the flutter waves are prominent and are negative in leads II, III, and AVF, suggesting inferior to superior atrial activation. Although initially thought to represent re-entry around the caval veins or around the tricuspid valve annulus, the work of multiple investigators has clearly established the actual circuit. Impulses emerge from an isthmus of atrial tissue between the IVC and the tricuspid annulus to spread up the atrial septum, activating the atrium at the site where the bundle of His is recorded, and then down the right atrial free wall to enter the isthmus again.14 This counterclockwise activation has been categorized as typical atrial flutter, whereas atrial flutter that uses the same circuit but in the clockwise order of activation has been categorized as reverse typical atrial flutter.15
In addition, further details have been provided using techniques of entrainment pacing, which depend on the demonstration of equivalence of the postpacing interval (PPI) during entrainment and the tachycardia cycle length (TCL) to establish that any given site is in the circuit (PPI = TCL). These studies have demonstrated the importance of the crista terminalis and the EVR as sites of conduction block during atrial flutter.16–18 Conduction block is suggested by the demonstration that sites along the ridge where double potentials can be recorded are present.17–20 The importance of such areas of conduction block is strengthened by entrainment pacing, demonstrating that the atrial myocardium on one or another side of the line of conduction block is part of the circuit. The critical nature of these lines of block is proved by RF ablation lesions that are designed to bridge from one line of block to another, with resultant abolition of the atrial flutter.21–23 These criteria have been satisfied with both typical and reverse typical atrial flutter, and the features of this arrhythmia circuit now seem well characterized.
As previously described, the wave of activation leaves the region of the tricuspid valve–IVC isthmus to climb the interatrial septum and enter the heavily trabeculated right atrial free wall in the region of the SVC. It then spreads down the right atrial free wall, with the crista terminalis behind and the tricuspid annulus in front, turning counterclockwise around the tricuspid annulus when viewed from below (left anterior oblique view fluoroscopically). As the wave of activation turns posterior, it enters a “funnel,” as described by Nakagawa and colleagues, created because the distance between the crista and the tricuspid annulus becomes progressively shorter.18 The wave is funneled to the isthmus between the IVC and the tricuspid valve annulus, now with the annulus anterior and inferior and the EVR (the extension of the crista terminalis) posterior and superior. It is important to note that at this site, the EVR bisects the isthmus between the IVC and the tricuspid valve and that it is the EVR, not the IVC, that provides the critical site of conduction block. As it enters the interatrial septum, the wave again spreads in a superior fashion along the septum for the next circuit. Atrial flutter can be effectively dealt with using RF ablation either at the septal site of EVR insertion, by lesions that bridge from the tricuspid valve to the EVR, or posterior, from the tricuspid annulus down to the IVC.18,21
Simple Atriotomy-Based Atrial Flutter
When performing surgery to repair a simple secundum ASD, the surgeon typically places a long incision in the right atrial free wall, which is oblique and runs from the right atrial appendage laterally down toward, but not to, the tricuspid annulus or the IVC. Care is taken to avoid the sinus node, and this concern results in the crista terminalis typically not being incised by the surgeon. This incision gives adequate exposure for the repair of ASDs and is also used for the atrial approach to repair VSDs, either alone or as part of tetralogy of Fallot and related defects. The ASD itself is commonly closed by using sutures, but for large defects a patch may be employed. Occasionally, a patent foramen is left open. The occurrence of atrial flutter in patients with repaired tetralogy of Fallot deserves special mention; in some series, it is at least as common as VT, and the presence of right bundle branch block (RBBB) may lead to its being confused with VT.24
This surgical approach clearly creates a long line of permanent conduction block that is entirely in the trabeculated right atrium, anterior to the crista terminalis. This anatomy potentially creates a tunnel of atrial tissue between the crista and the atriotomy and another between the atriotomy and the tricuspid annulus. Such tunnels can easily be imagined as the required protected zones of conduction, mediating IART. Numerous cases of patients who exhibit “incisional” IART in which the atriotomy seems to act as the critical barrier have been reported.25 In such patients, RF application from the atriotomy to the IVC, the tricuspid annulus, or the SVC has been successful in terminating tachycardia and preventing re-induction.
Because the atrial structures that support typical atrial flutter are also present and because these patients often have other risk factors for the development of flutter (atrial dilation, fibrosis, etc.), they may also have typical atrial flutter postoperatively. Furthermore, as has become apparent in patients with otherwise structurally normal hearts, such IART and flutter circuits can run in either direction (counterclockwise or clockwise). Indeed, several series reported a more common occurrence of typical or reverse typical atrial flutter than true incisional flutter in these patients.26,27 In patients who have undergone patch closure of a secundum ASD, the patch itself has been reported to be a possible site of conduction block, mediating tachycardia, although this is less common.21 The potential variability in circuits and rotation creates the possibility for several distinct P-wave morphologies and AT cycle lengths.
Intra-atrial Re-entry Following Atrial Repair of Transposition
The Senning and Mustard procedures, which are similar operations to address the hemodynamic abnormality in transposition, direct systemic return to the left ventricle and pulmonary artery and the pulmonary venous return to the right ventricle and aorta.28,29Although very successful, these operations are rarely performed in the current era, in part because of the success of the arterial switch procedure and in part because of the high incidence of sinus node dysfunction, atrial arrhythmias, and increased risk of sudden cardiac death (SCD). However, interest has recently increased in the so-called double switch procedure as a strategy for managing patients with L-transposition (congenitally corrected transposition). A Senning atrial baffle is constructed in this procedure.30 Thus, this surgical substrate may not, in fact, disappear. In the Mustard procedure, after a long atriotomy anterior to the crista terminalis and the resection of the atrial septum, a baffle is constructed and sewn into place around each caval vein, through the isthmus between the IVC and the tricuspid annulus, and to the posterior wall of the left atrium so that caval flow is direct to the mitral annulus.29 Pulmonary venous flow travels around the baffle and finds the tricuspid annulus. It is important to note that the baffle, where it is sewn into place along the tricuspid annulus, has the same function as the EVR in fetal life, which is to prevent IVC flow from reaching the tricuspid valve. Furthermore, surgical technique is directed at avoiding injury to the sinus node, so the crista terminalis is not disturbed. Finally, various approaches are used to avoid AV block, and often these lead to the coronary sinus being incorporated into the pulmonary venous atrium rather than the systemic venous atrium.31 These details leave the entire right atriotomy as well as the isthmus of atrial tissue between the EVR and tricuspid annulus in the new pulmonary venous atrium.32,33 The one exception is the situation where the coronary sinus drainage is the systemic venous atrium, in which a catheter can reach the flutter isthmus from the IVC.32
In most respects, the Senning procedure is similar electrophysiologically to the Mustard procedure. The Senning procedure was designed to use mostly atrial tissue versus artificial material to construct the baffle.28 In order to accomplish this, two atrial incisions are made. The first is in the right atrium, longitudinal, parallel, and anterior to the crista terminalis. The second, in the left atrium, is parallel to the first and between the right pulmonary veins and the interatrial septum. A U-shaped incision is made in the atrial septum, just above the coronary sinus, leaving the flutter isthmus intact. This flap of atrial septum is sewn to the back of the left atrium, to the left of the left pulmonary veins. The flap of right atrial free wall is sewn into place near or at the site of the EVR, preventing IVC flow from crossing the tricuspid valve. The left atrial incision is closed by sewing to the other edge of the right atrial incision. As in the Mustard procedure, both the flutter isthmus and the right atriotomy are part of the new pulmonary venous atrium.
Intra-atrial Re-entry Following the Fontan Procedure
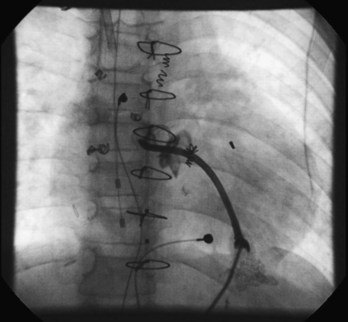
The Fontan procedure has changed many times since its development as a palliative procedure for patients without two functional ventricles, as a way of relieving ventricular volume overload and of normalizing arterial saturations.34 Initially, it was thought that the right atrium could be used as an effective pumping chamber, provided that pulmonary artery pressures were low (atriopulmonary connection). Largely as a result of an extremely high incidence of atrial arrhythmias after such procedures, as well as concerns about hydraulic energy loss in the system and pulmonary venous obstruction, this approach has been abandoned in favor of approaches that bypass the heart entirely (total cavopulmonary connection via the lateral tunnel or via an extracardiac conduit).35–37 Within each of the two categories, many modifications exist. Despite the approach of total cavopulmonary connection, atrial arrhythmias continue to be observed, although some large series now report a lower incidence of arrhythmias with the external conduit Fontan when compared with the lateral tunnel.38 In any case, surgical details are critical in planning mapping and ablation procedures in these patients. In particular, difficulties in access are common and may limit the number of catheters that can be placed in the heart. Novel approaches such as the direct transthoracic approach are reported (Figure 78-1), and one may also consider perforating the baffle or approaching the atrial mass via the SVC and the left pulmonary artery.39–41
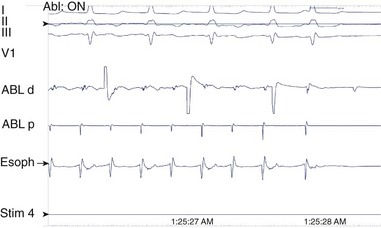
A long atriotomy is placed with the various forms of atriopulmonary connection. In patients who had a conduit from the right atrium to the pulmonary artery and in those in whom the right atrial appendage was connected directly to the pulmonary artery or to the right ventricular outflow tract (RVOT; the Bjork modification), this atriotomy was anterior to the crista terminalis. Often in these patients, patch augmentation of the right atrium was performed, using a piece of pericardium or other material incorporated into the closure. Invariably, closure of a large ASD was also necessary. As in the simpler situation of ASD repair (see earlier), both typical atrial flutter and incisional re-entry around the anterior atriotomy are possible and have been observed. Triedman and colleagues demonstrated slow conduction up the lateral wall in their excellent multiple-site mapping studies using basket catheters42; and this configuration fits the concept of conduction in a long isthmus bounded by the atriotomy and the crista terminalis. Re-entry around the ASD patch is also possible. Finally, patch closure of the tricuspid annulus has been occasionally performed in patients with a single ventricle without tricuspid atresia, potentially creating areas of slow atrial conduction on the other side of the suture line. Entrainment pacing is useful in identifying areas that are in the circuit and might be potential targets for ablation (Figures 78-2 and 78-3).43 Alternatively, the use of dense voltage maps has been reported to be of value in identifying areas of scar and corridors of low-voltage myocardium, which can be targeted for ablation lesions, with some success.44
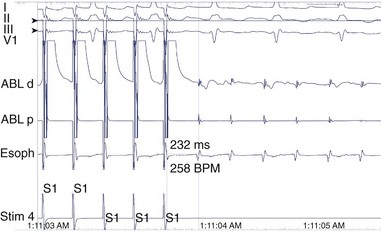
FIGURE 78-2 Surface and intracardiac electrogram tracings during the electrophysiology study in the patient shown in Figure 78-1. Atrial tachycardia is entrained via pacing through the ablation catheter at a candidate site, and the postpacing interval is equivalent to the tachycardia cycle length, demonstrating that this site is in the atrial tachycardia circuit.
Ventricular Tachycardia Following Repair of Tetralogy of Fallot
VT continues to be a difficult problem in the management of patients who have undergone surgical repair of tetralogy of Fallot and other related congenital heart defects. The actual etiology of SCD in patients following tetralogy repair is still somewhat uncertain. However, because of the frequent occurrence of premature ventricular contractions (PVCs), nonsustained and sustained VT in patients who have undergone complete repair of tetralogy of Fallot and related defects such as double-outlet right ventricle, VT has been implicated in the etiology of SCD in this patient group.45–51 It is known that postoperative tetralogy of Fallot is the single most common condition that causes SCD among children between the ages of 1 and 16 years.52
Most of the available information concerning patients with VT and congenital heart disease pertains to tetralogy of Fallot compared with other forms of congenital heart disease. Ventricular arrhythmias do occur but are much rarer in patients with other lesions.53 For the purposes of management, tetralogy of Fallot can be viewed as an archetype for other lesions when patients with other lesions present with ventricular arrhythmias in the setting of ventriculotomy, right ventricular dysfunction, or both.
Patients with tetralogy of Fallot prior to repair have a large VSD with (usually severe) RVOT obstruction, which leads to cyanosis. The placement of a systemic-to-pulmonary artery shunt as a palliative procedure adds the element of potential left ventricular volume overload. Correction of the defect involves patch closure of the VSD with relief of the right ventricular obstruction. In nearly all patients, this requires resection of a significant amount of right ventricular muscle. Early in the experience, this was not done via an atriotomy with retraction of the tricuspid valve but, instead, required a ventriculotomy. Finally, in tetralogy of Fallot, the pulmonary annulus is typically smaller than normal. This has been approached by the placement of a transannular patch, which leads to chronic pulmonic insufficiency. Pulmonic insufficiency may be very severe if it is associated with downstream obstruction related to significant pulmonary arterial stenosis. It has been hypothesized that ventricular arrhythmias are caused by the effects of years of chronic cyanosis, followed by the placement of a ventriculotomy, with elevation of right ventricular pressures arising from inadequate relief of obstruction, and severe pulmonic regurgitation with right ventricular dysfunction and enlargement.45,54–56 Factors such as wall stress and chronic cyanosis, coupled with the passage of time, may lead to myocardial fibrosis and result in the substrate for re-entrant ventricular arrhythmias. This hypothesis is supported by histologic examination of the hearts of patients with tetralogy of Fallot who died suddenly. These studies have shown extensive fibrosis.57 The hypothesis is also supported by the observation of fractionated electrograms and late potentials recorded from the right ventricle at EPS, suggesting the presence of slow conduction.58,59 Despite the presence of a 5% incidence of coronary artery abnormalities in tetralogy of Fallot that put the left anterior descending coronary artery or other large branches at risk at the time of complete repair, such potential damage has not been implicated in the etiology of ventricular arrhythmias or of SCD in most patients.
Careful studies in patients with VT following surgery for tetralogy, as reported by Zeppenfeld et al, have supported the concept that the mechanism of VT is macro–re-entry, which involves a limited number of critical isthmuses in the right ventricle, either at the site of anterior right ventriculotomy or related to the site of a VSD patch.60 Transient entrainment has been documented, with constant fusion at the paced cycle length and progressive fusion at decreasing cycle lengths.61,62 Successful ablation of these isthmuses is effective in preventing the recurrence of VT.
Early reports noted the frequent occurrence of PVCs in patients who had previously undergone repair of tetralogy of Fallot. Gillette and coworkers identified PVCs on routine ECGs in 18% of patients.45 With exercise testing, the incidence may increase—to around 20%, as shown in one study.50 With Holter monitoring, the incidence of ventricular ectopy is reported to be as high as 48%.63 In about half of these patients, ventricular ectopy is complex, defined as multiform beats, couplets, or VT. In the great majority of patients, this ventricular ectopy is entirely asymptomatic.
Many investigators have tried to correlate the incidence of ventricular ectopy with various factors, including age at presentation, age at time of repair, and various hemodynamic features. Four factors seem to be the most important: (1) age at initial repair; (2) time since repair; (3) presence of residual right ventricular obstruction; and (4) presence of significant pulmonic insufficiency. In Chandar and colleagues’ multicenter study, older age at time of repair, especially beyond 10 years of age, was associated with nearly a 100% incidence of ventricular arrhythmias, regardless of the follow-up interval.63 In the same study, time since repair also predicted the occurrence of ventricular ectopy, which occurred in all 4 patients followed up for more than 16 years, despite repair in infancy. Walsh and colleagues, however, showed that in a group of patients who underwent repair at less than 18 months of age, ventricular ectopy was rare on ECG (1%) but more common on Holter monitoring (31%) after an average of 5 years of follow-up.64
Garson and associates, in a study of 488 patients with repaired tetralogy of Fallot, showed that the incidence of ventricular arrhythmias was closely related to right ventricular hemodynamics.65 The incidence of ventricular arrhythmias was significantly higher in those with a right ventricular systolic pressure greater than 60 mm Hg, and in those with a right ventricular end-diastolic pressure greater than 8 mm Hg, suggesting that residual RVOT obstruction and pulmonic insufficiency negatively influence outcome. They also found a relationship to age at surgery, but this was not as important as the follow-up interval. Zahka and coworkers, in a prospective study of 59 patients with tetralogy of Fallot repaired prior to 11 years of age, found that the degree of pulmonary regurgitation was, by far, the most important predictor of the frequency and severity of spontaneously occurring ventricular arrhythmias.54 Although the degree of residual RVOT obstruction was not a predictor in this study, significant residual obstruction was rare in their study group.
Spontaneously occurring sustained VT is, in fact, fairly uncommon among patients with repaired tetralogy, despite the high incidence of ventricular ectopy. The best data in this regard come from the study by Harrison and associates that included patients with repaired tetralogy of Fallot attending an adult congenital heart disease clinic.66 Eighteen of 210 patients (8.6%) had either documented sustained VT, syncope, or near-syncope, with palpitations and inducible sustained monomorphic VT at EPS. VT was closely related to right ventricular hemodynamics, particularly RVOT aneurysms and pulmonic insufficiency. This finding is consistent with the earlier report by Zahka and coworkers, which emphasized the importance of pulmonic insufficiency as a risk factor for ventricular ectopy.54
The occasional but persistent observation of unexpected SCD in this group of patients with repaired congenital heart disease, along with the high incidence of spontaneously occurring ventricular arrhythmias, both simple and complex, has led to the hypothesis that SCD in such patients is caused by VT. During the 1970s and 1980s, it was standard practice to perform EPSs in a large proportion of patients who had undergone repair of tetralogy of Fallot or of related congenital defects. Antiarrhythmic drug therapy was often prescribed on the basis of the results of such studies. This approach has, for the most part, been abandoned because of the lack of strong evidence supporting the proposition that SCD can be prevented with this approach as well as worries about the proarrhythmic effect of the antiarrhythmic medications chosen for treatment. In a large multicenter retrospective review that used a variety of electrophysiological protocols, Chandar and colleagues reported the experience with 359 postoperative patients with tetralogy of Fallot who underwent invasive EPS.63 VT could be induced in 17% of patients but not in any patient who was asymptomatic and had a normal 24-hour ECG. Although late SCD occurred in five patients, none of these patients had inducible VT at EPS.
It is also interesting that the risk of VT can be assessed from QRS duration on the surface ECG. Gatzoulis and associates found that in a group of 48 well-studied postoperative patients with tetralogy of Fallot, those with a QRS duration greater than 180 ms had a greatly increased risk of spontaneous VT, SCD, or both.55 Similarly, Balaji and coworkers showed that a QRS duration greater than 180 ms predicts the finding of inducible sustained monomorphic VT at EPS.67 The cause of this relationship is almost certainly related to the tendency of chronic pulmonic insufficiency to cause right ventricular dilation with more severe right ventricular conduction delay, for which lengthening of the QRS is a marker.
Clear evidence that the risk of SCD may increase at late follow-up exists. In a careful study of 490 survivors of tetralogy of Fallot repair at a single center, Nollert and colleagues constructed actuarial survival curves out to 36 years following surgery.68 In this study, the yearly actuarial mortality rate during the first 25 years was 0.24% per year, but mortality increased dramatically after 25 years to 0.94% per year. Most deaths were from SCD. The mortality risk was also related to date of repair (highest before 1970), the degree of preoperative polycythemia (highest with hematocrit >48), and the use of an RVOT patch (highest with a patch). The last factor is most likely related to the presence of pulmonic insufficiency, as suggested earlier.
The close linkage of pulmonic insufficiency, right ventricular dilation, and VT/SCD suggests that the management of pulmonic insufficiency in patients with tetralogy of Fallot should be more aggressive. However, pulmonary valve placement is problematic in young children with small hearts because of issues related to growth, and valves predictably fail, necessitating reoperation. The earlier the first valve is placed, the more surgeries the patient must undergo over his or her lifetime. However, it is ideal to intervene with pulmonary valve replacement before the development of significant right ventricular dysfunction. While QRS duration is an easily obtained indicator of right ventricular dilation, attention has lately focused on the evaluation of right ventricular volume by cardiac magnetic resonance imaging (MRI). Therrien et al have observed that in adults with tetralogy of Fallot, a right ventricular end-diastolic volume of greater than 170 mL/m2 predicted a failure of the right ventricle to return to normal size following PVR, while all patients with volumes less than 170 mL/m2 had complete normalization.69 Geva has proposed a right ventricular end-diastolic volume of 160 mL/m2 or more as a criterion for PVR, among other criteria and has also recommended an earlier operation for patients with defects originally repaired at age 3 years or later.70 It remains to be seen whether the widespread adoption of these protocols will have an effect on the incidence rates of SCD.71
Surgically Induced Atrioventricular Block
The risk of complete AV block as a result of surgery depends on the type of repair that is attempted. At highest risk are patients who undergo closure of AV septal (canal or endocardial cushion) defects and those having closure of VSDs involving the perimembranous region of the ventricular septum.53,72,73 In such defects, the distal conducting system is in a location that is difficult to avoid during surgical closure of the defect. The repair of subaortic stenosis is often complicated by AV block because of the need, in many cases, to resect muscle from the left side of the ventricular septum. Muscular VSDs and supracristal (doubly committed subarterial) VSDs are distant from the conducting system and, therefore, are not highly associated with AV block. Likewise, the repair of ASDs is only rarely complicated by postoperative AV block, and its occurrence should raise the possibility of an NKX-2.5 mutation.53,74
Postoperative Sinus Node Dysfunction
Any operation that involves surgery on the right atrium may lead to postoperative sinus node dysfunction; this problem is often not evident early after surgery but may take years to manifest. The mechanism of sinus node damage is not well known, but most likely, the damage results from direct injury to the sinus node pacemaker complex, interference with the blood supply to this structure, or both. The repair of simple ASDs is only rarely followed by sinus node dysfunction, whereas the risk is higher with the repair of sinus venosus ASDs. Sinus node dysfunction is most common following the Senning or the Mustard procedure for transposition, the hemi-Fontan procedure, and the Fontan procedure.53
Treatment of Arrhythmias in Patients with Congenital Heart Disease
Antiarrhythmic Drug Therapy
Decisions Regarding Treatment
Sustained tachycardia may be more poorly tolerated in this patient population because of poor systemic ventricular function. In addition, the use of antiarrhythmic agents is problematic in this patient population because of the increased prevalence of sinus node dysfunction after atrial surgery, such as the Mustard, Senning, or Fontan procedures, as well as the potential for proarrhythmia, which is exacerbated by systemic ventricular dysfunction.75 These issues all need to be evaluated in the choice of antiarrhythmic agents in this population.
It is difficult to know which patients require EPS, treatment, or both. When one considers that no prospective studies have demonstrated that SCD can be prevented in any group of postoperative patients by treatment of any type, it does not seem that one should recommend routine EPS and treatment of asymptomatic patients with ventricular ectopy or other arrhythmias. It seems likely in certain subgroups carefully chosen antiarrhythmic therapy may exert a beneficial effect in lowering mortality; it is also quite possible that in some subgroups, the proarrhythmic potential of antiarrhythmic medications more than makes up for any beneficial effect, a result similar to that seen in the Cardiac Arrhythmia Suppression (CAST) trial.76,77 Until such prospective, controlled trials are performed, therapy with drugs such as flecainide, propafenone, and quinidine cannot be recommended for most asymptomatic patients. An argument can be made for selecting certain patients for prophylactic treatment with β-blockers, as Garson and associates have suggested.78 Examples of such patients might include those with significant pulmonic regurgitation, residual right ventricular obstruction and complex ventricular ectopy, or both. One can also argue that these patients should be considered for cardiac surgery to correct hemodynamic abnormalities such as residual obstruction, especially branch pulmonary artery stenosis, and should also be considered for placement of a homograft valve to eliminate pulmonic insufficiency.
If the goal of therapy is to prevent the recurrence of clinically documented VT, then proceeding in a fashion similar to that with other forms of VT might be considered. EPS for the induction of VT with subsequent drug testing is reasonable; but it should be kept in mind that some proarrhythmic risk to the use of certain medications, especially quinidine, procainamide, flecainide, and propafenone, may very well be present. Exercise testing may also be used as a means of inducing VT in certain patients. The choice of a pharmacologic agent for long-term treatment is then based both on the suppression of the arrhythmia by the drug and on its proarrhythmic potential. In the former consideration, investigators have reported some success with most class I agents, especially quinidine, procainamide, propafenone, and flecainide, as well as with β-blockers. The latter consideration, proarrhythmia, is difficult to judge. A high incidence of documented proarrhythmia with flecainide and encainide in patients with structural cardiac disease has been reported by Fish and associates in a pediatric series.79 Furthermore, cardiac arrest and deaths occurred predominantly among patients with underlying heart disease. Sotalol is also known to be proarrhythmic, particularly in patients with significant ventricular dysfunction.80 D-sotalol has been found to increase mortality in post-infarction patients with ventricular dysfunction (the SWORD trial).81 These findings suggest that sotalol and class IC agents should be used with extreme caution in patients with repaired tetralogy of Fallot and ventricular dysfunction. This, of course, leaves amiodarone, which has the advantage of little-reported proarrhythmia, even in patients with significant ventricular dysfunction. Concerns have been raised over long-term side effects such as pulmonary fibrosis, ocular abnormalities, thyroid dysfunction, transaminase elevations, and significant bradycardia in children and young adults82–84; the use of this agent in a young person means that the medication is likely to be needed for several decades at least. These concerns naturally have led to the consideration of nonpharmacologic therapy.
Digoxin
Digoxin has a long history of use in children, in particular in patients with atrial flutter and intra-atrial re-entry. Although the drug exerts a direct effect on the cardiac cell membrane, most of its antiarrhythmic properties are a result of its indirect actions mediated through the autonomic nervous system.85 The direct and indirect effects of digoxin on the AV node prolong the refractory period and slow conduction. In general, digoxin is not an effective drug for the acute conversion of atrial flutter. In the collaborative study by Garson and coworkers, digoxin used alone was successful in preventing recurrences of atrial flutter in only 44% of patients.86 Of the conventional agents, the most effective treatment for preventing recurrences (53%) was the combination of digoxin with a type IA agent such as quinidine or procainamide. However, the use of digoxin may be beneficial in the control of ventricular rate during atrial flutter.
Class I Antiarrhythmic Medications
When using the sodium channel blocking agents to manage arrhythmias in patients with congenital heart disease, the likelihood of success must be carefully balanced against the possibility of proarrhythmia. Class IA agents (quinidine, procainamide, and disopyramide) as well as class IC agents (flecainide and propafenone) are useful in treating atrial flutter and ventricular arrhythmias as well as in controlling SVT mediated by concealed or manifest accessory pathways. Because of their vagolytic properties, class IA medications, particularly disopyramide, need to be given in combination with digoxin preparations or other AV node–blocking agents to lessen the likelihood of 1 : 1 AV conduction should atrial flutter occur. Class IB agents (lidocaine, tocainide, mexiletine, phenytoin, and moricizine), however, are not considered particularly useful in atrial arrhythmias, although moricizine has been used effectively in some studies. These agents are primarily used in treating ventricular arrhythmias. They are all effective in suppressing PVCs, and for many years, phenytoin has been a favorite antiarrhythmic medication for suppressing PVCs in patients following tetralogy of Fallot repair.65 Whether suppression of PVCs is an important goal in the population is open to speculation. No clear evidence exists to show that VT and SCD can be prevented by the use of these agents. Furthermore, classes 1A and 1C agents share a propensity to proarrhythmia, particularly in patients with ventricular dysfunction, and so should be used with caution.79
Class II Antiarrhythmic Medications
β-Adrenergic blocking agents such as propranolol and atenolol may be useful for rate control in patients with chronic atrial flutter and have the advantage of not being proarrhythmic. For other forms of SVT, the β-blockers are often effective in preventing recurrences. Patients with concomitant sinus node dysfunction may require permanent pacing if β-adrenergic blocking agents are used. Some evidence exists to show that the use of propranolol is safe and effective in the management of ventricular arrhythmias following repair of tetralogy of Fallot.78
Class III Antiarrhythmic Medications
Class III agents are thought to exert their actions primarily by prolonging action potential duration and refractoriness without significantly affecting conduction. The agents in this category are amiodarone and sotalol. Garson and colleagues had an opportunity to use amiodarone in a group of 39 patients with congenital heart disease, critical tachyarrhythmias, and arrhythmias not responsive to conventional agents.87 Sixteen of the 39 patients had recurrent atrial flutter, and 15 of the 16 had complete elimination of the flutter. Sotalol has been used more recently with good results.88–89 It combines class III action with β-adrenergic blocking activity and so may not be tolerated by patients with poor ventricular dysfunction. Both these agents also may exacerbate sinus node dysfunction.
Class IV Antiarrhythmic Medications
Class IV drugs (e.g., verapamil), given intravenously, have been used almost exclusively as an acute intervention. Conversion of atrial flutter to normal sinus rhythm occurs rarely after the intravenous administration of verapamil. However, what does occur is a lowering of the ventricular rate secondary to delayed conduction through the AV node or conversion from atrial flutter to AF with a reduction in conducted impulses. Verapamil may be an effective agent for a rapidly deteriorating patient with atrial flutter and 1 : 1 AV conduction. However, extreme caution must be exercised when using this medication in younger children, and verapamil should never be given to those younger than 6 months.90 Another option for the rapid control of the ventricular rate is the continuous infusion of diltiazem, another class IV agent.
Results of Antiarrhythmic Drug Therapy
Unfortunately, medical therapy has been disappointing in the management of patients with postoperative arrhythmias. In general, a fairly low success rate has been observed with a variety of antiarrhythmic agents for the prevention of recurrent atrial flutter or IART. Garson and associates showed that even if an antiarrhythmic agent was found to be successful in suppressing episodes of atrial flutter, a significant incidence of SCD was present, which suggests that life-threatening proarrhythmia may be a serious potential problem in postoperative patients.86 Finally, although Garson and colleagues presented retrospective evidence that the suppression of ventricular ectopy was associated with a lower incidence of SCD among patients with tetralogy of Fallot, no prospective trials have been performed to support this concept.65
Ablation of Accessory Pathways in Patients with Unrepaired Congenital Heart Disease
Venous and Arterial Access
Patients who have had multiple procedures or who have had long stays in the intensive care unit with indwelling lines may have limited venous access because of iliofemoral thrombosis. When such thrombosis is bilateral, this problem may prevent a normal approach to the right atrium. In patients who have undergone the bi-directional Glenn procedure, direct access to the right atrium from the SVC is not available because of the direct connection of the SVC to the pulmonary artery. In both situations, approaching the right atrium from the other cava may be considered, for example, from the SVC, when bilateral iliofemoral thrombosis is present. Reports of the use of a transhepatic approach for diagnostic and interventional catheterization suggest that this route might also be efficacious for catheter ablation.91,92 Left-sided accessory pathways may, of course, be ablated by a retrograde approach in larger patients, but this approach is not recommended in children weighing less than 15 to 20 kg or in those with aortic valve disease.93
Situs
The existence of situs abnormalities can render a catheter ablation procedure potentially confusing because of the nonstandard location of veins, arteries, and the heart itself, but these problems are not insurmountable if one possesses knowledge of congenital cardiac pathology. Such abnormalities are, unfortunately, not limited to simple mirror-image arrangements. Standard fluoroscopy planes for normal anatomy may make little sense in the setting of situs abnormalities. When needed, transthoracic or transesophageal echocardiography may be used to confirm catheter tip locations.94–96 Finally, patients with heterotaxy syndromes may have interruption of the IVC with azygous continuation. In these patients, a catheter passed from the femoral vein will traverse the azygous system to join the SVC and enter the atrium from above. In these patients, an ablation catheter may be introduced from the internal jugular or subclavian vein to allow more straightforward catheter manipulation. In this area of difficulty, the use of advanced catheter navigation technologies such as Stereotaxis, which has been reported to aid in the maneuvering of catheters remotely in patients with complex congenital heart disease, can be considered.97
Atrioventricular Conduction Structures
The ability to successfully ablate septal accessory pathways without the complication of AV block in patients with normal intracardiac anatomy depends, in large part, on the electrophysiologist’s detailed knowledge of the location of the compact AV node and the bundle of His in relation to other structures in the heart. In several forms of congenital heart disease, the AV conducting tissue is located differently, and this anatomy must be kept in mind.98 For example, patients with a complete AV canal as well as those with ostium primum ASD have AV conducting tissue displaced posteriorly toward the coronary sinus.99 Ablation of posteroseptal accessory pathways would, therefore, likely carry a higher risk of complete AV block. In tetralogy of Fallot, the conducting system is at risk as well, being at the margin of the VSD.100 Patients with L-transposition have AV conducting tissue located more anteriorly, and it is thought to be more fragile and prone to accidental damage during catheterization.101 The anatomy for other rarer forms of congenital heart disease has also been defined.98 The current availability of transcatheter cryoablation now allows a safer alternative for ablation of septal pathways in patients with abnormal AV conduction systems.102
Defect-Specific Factors
The situation with Ebstein’s anomaly can be quite challenging. The presence of significant tricuspid regurgitation may make stable catheter position difficult on the right AV groove. Similarly, the downward displacement of the tricuspid valve leaflets (principally the septal and posterior leaflets) makes stable positioning on the AV groove difficult. However, the single most difficult factor in such patients is the difficulty in achieving an adequate temperature at the catheter tip despite maximal voltage, most likely because of the capacious right atrium and the atrialized right ventricle. This, combined with the propensity of patients with Ebstein’s anomaly to have multiple accessory pathways and a tendency to AF, often makes such procedures long, grueling, and ultimately unsuccessful. A variety of catheter approaches, both from the IVC as well as from the internal jugular vein or the right subclavian vein should be tried, and the use of long venous sheaths to allow for better catheter stability might be considered.103 For the fluoroscopic identification of the right AV groove and for precise mapping of signals at the AV groove, some investigators have used a 2 Fr custom mapping wire introduced directly into the right coronary artery and advanced around the AV groove.104–106 The use of temperature monitoring, temperature control, or a combination of both is mandatory in patients with Ebstein’s anomaly to allow differentiation between two scenarios: (1) lack of success because of incorrect catheter position and (2) inadequate temperature.107
In patients with L-transposition, the malalignment of the atrial and ventricular septa creates a complex anatomy when dealing with septal accessory pathways. Typically, the ventricular septum is in the sagittal plane, whereas the atrial septum is more normally positioned, but close to the AV groove. The coronary sinus may be difficult to enter. The propensity to AV block in such patients (mentioned earlier) must be kept in mind. The presence of significant left-sided AV valve regurgitation with left-sided Ebstein’s anomaly may dictate an antegrade approach to the ablation of left-sided accessory pathways because positioning under the left-sided tricuspid leaflets might not allow close enough contact with the true AV groove. This approach may be chosen also out of a desire to avoid creating further regurgitation with catheter ablation attempts. Trans-septal puncture can certainly be accomplished, but the angle of attack may not be standard because of the abnormal septal orientation. When in doubt, pulmonary angiography to define the left atrium on levophase, intraoperative echocardiography, or a combination of both may be used.95,96
Saul and associates have documented in patients with more complex anatomy the presence of dual AV conducting systems, which may mediate AV reciprocating tachycardia. These have been patients with AV discordance with or without atrial situs inversus.108 The second, or accessory, AV node and distal conducting system resemble Mahaim-type atriofascicular accessory pathways seen in patients with otherwise normal cardiac anatomy and can be mapped and ablated with similar techniques.
Radiofrequency Ablation of Atrial Arrhythmias in Postoperative Patients with Congenital Heart Disease
With the advent of catheter procedures using RF energy and, more recently, cryothermal energy, to eliminate the substrate for conditions such as WPW syndrome and AV node re-entry, ablation of atrial flutter and IART became possible.109–112 Using the surgical experience as a guide, as well as techniques for demonstrating concealed entrainment, Feld and coworkers were able to demonstrate initial success with type 1 (“typical”) atrial flutter by placing RF lesions at the isthmus between the IVC and the tricuspid valve annulus.22 RF lesions placed in these regions were often successful in terminating atrial flutter, and long-term success has been accomplished. Subsequently, other centers have also demonstrated that high success rates are possible in adults with type 1 flutter, with an acceptable incidence of recurrence.18,113
These concepts have further been extended to patients with atypical atrial flutter following extensive atrial surgery.25,106,114–116 In these patients, the substrate for atrial flutter consists of natural anatomic obstacles to impulse propagation such as the IVC, coronary sinus, tricuspid annulus, and so on as well as surgically created obstacles such as atriotomy sites, intra-atrial baffles, and conduits. Transient entrainment may be used to demonstrate the re-entrant mechanism, and sites of slow conduction are sought where concealed entrainment (entrainment without visible fusion on the surface ECG) can be demonstrated. Lesions at these sites are placed in an attempt to bridge the zone of slow conduction and terminate tachycardia. Encouraging results have been reported, but the incidence of recurrence after an initially successful procedure is significant, particularly in more complex heart defects.
Despite having had an atriotomy, many of these patients are found, by careful mapping, to have atrial flutter, which involves the typical isthmus between the tricuspid annulus and the IVC. This is, in fact, quite common in patients who have had simple atrial surgery, as described earlier. In these patients, when typical or reverse typical atrial flutter is documented and clearly involves this isthmus, the ablation procedure may proceed by standard methods for the ablation of typical atrial flutter, and documentation of bi-directional block in the isthmus is a goal of ablation. When such an approach is taken, the results of ablation are very good.26
In patients who have had the Mustard or the Senning procedure for transposition who also have atrial flutter, most of the critical structures that support atrial re-entry are in the new pulmonary venous atrium. The presence of a suture line at the site of the EVR in such patients might cause fibrosis and conduction delay, perhaps dramatically increasing the likelihood that atrial re-entry will occur. In any case, typical and reverse typical atrial flutters are also quite common in this patient population. The approach for ablation is not straightforward when the target is in the pulmonary venous atrium and, often, the arrhythmia must be approached either via a leak or a separation in the baffle or by a retrograde transaortic approach.32 In addition, studies have shown that after successful RF ablation at the flutter isthmus, patients have exhibited a sudden shift from one tachycardia involving the flutter isthmus to a second tachycardia not involving this isthmus but instead involving the atriotomy. Such a phenomenon may be an indication of true “figure of eight” re-entry, with the ablation of one, but not both, limbs of the figure of eight.
Catheter ablation in the atriopulmonary connection type of Fontan has been quite disappointing in contrast to the experience in patients who have had ASD repair and the Mustard and Senning procedures.25,32,114,117,118 Multiple tachycardia circuits and a high incidence of recurrence after initial success have been observed. It may be that with high atrial pressures, the resulting thickening of the atrial wall from atrial hypertrophy prevents the development of full transmural lesions. Alternatively, sluggish blood flow may not allow adequate tip cooling, limiting energy delivery and resulting in ineffective lesions, but this problem is obviated by the use of irrigated tip RF ablation.119
More recent innovations involving total cavopulmonary connection by the lateral tunnel technique are clearly associated with better hemodynamics and lower atrial pressures. Unfortunately, IART is still frequently observed in these patients. In order to exclude the atrium, the SVC is connected directly to the pulmonary artery, and a tunnel is created to direct IVC flow to the underside of the pulmonary artery. The baffle that accomplishes this is similar to that used in the Mustard or the Senning procedure, with a line of sutures going through the region of the EVR and with the baffle directing IVC flow away from the tricuspid annulus. The long atriotomy used to construct the lateral tunnel is closed, and this suture line is in the new pulmonary venous atrium. This anatomy creates the potential for re-entry in the usual flutter circuit, as well as incisional IART involving the right atriotomy, which has been elegantly demonstrated in an animal model by Rodefeld and colleagues.120 Experience with RF ablation in this particular anatomic substrate is not sufficient to comment on its effectiveness, but similar results to those reported for the Senning and Mustard procedures could be expected.
It is encouraging that some authors have reported a lower incidence of atrial arrhythmias in patients who have undergone the Fontan procedure by means of an external conduit.121 In this approach, the SVC is connected to the pulmonary artery directly, and the IVC is connected to the pulmonary artery through the use of aortic allograft material or polytetrafluoroethylene; ideally, this involves no atriotomy, except for cannulation. However, intracardiac repair is often needed, typically via an atriotomy; so generally, no access to the atrium via the IVC or the SVC is available. In this situation, access may be gained by creating a fenestration in the external conduit or by direct transthoracic entry into the atrium.122,123
Radiofrequency Ablation of Ventricular Arrhythmias in Postoperative Patients with Congenital Heart Disease
If VT is easily inducible and well tolerated hemodynamically, RF ablation may be considered. Because most evidence supports the concept of macro–re-entry as the mechanism of such well-tolerated VT, the use of entrainment pacing and mapping techniques is indicated. Investigators have reported successful procedures using RF energy.124–129 Successful sites have included the area between the pulmonic annulus and the outflow tract patch, the isthmus of ventricular tissue between an outflow tract patch and the tricuspid annulus, and the region of the VSD patch.124,126,129
Although well-tolerated VT can be mapped in the electrophysiology laboratory, many patients have ventricular dysfunction, rapid VT rates, or both and will not tolerate this. Several investigators have reported intraoperative mapping and ablation.124,130–133 In particular, Downer and colleagues have used intraoperative mapping of the RVOT in the beating heart, employing an endocardial electrode balloon and a simultaneous epicardial electrode shock array.132 Ablation was carried out with good success in three patients by applying cryotherapy lesions during normothermic cardiopulmonary bypass with the heart beating or during anoxic arrest.
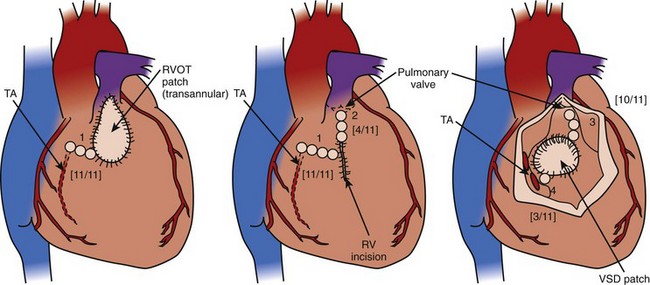
One exciting development is the advent of substrate mapping of the important isthmuses in congenital heart disease and VT by using an electroanatomic map obtained during sinus rhythm, as reported by Zeppenfeld et al.60 A high-density voltage map of the right ventricle is obtained, and areas of likely scar and patch are confirmed by lack of capture using high-output unipolar pacing. This group reported that the substrate-supported VT in patients with tetralogy of Fallot is limited to four isthmuses: (1) the tricuspid annulus and scar or patch in the anterior RVOT, (2) the pulmonary annulus and right ventricular free wall scar or patch, (3) the pulmonary annulus and septal scar or patch, and (4) the septal scar or patch and the tricuspid annulus (Figure 78-4). These observations reveal the possibility of effective ablation of VT by attacking these isthmuses with RF energy, at the time of surgical revision, or both.
Device Therapy
Pacemaker Therapy
Patients with the sick sinus syndrome who require an antiarrhythmic agent other than digoxin to prevent recurrences of arrhythmia are at risk for very slow heart rates. The Joint American College of Cardiology/American Heart Association/Heart Rhythm Society Task Force recommended that these patients have pacemakers implanted.134 Furthermore, many of these patients will have symptoms caused by slow heart rates, such as exercise intolerance or syncope, and should also receive pacemakers. The loss of AV synchrony may not be well tolerated in patients with borderline ventricular function, and pacing is sometimes recommended for patients with few symptoms as a means of optimizing hemodynamic function.
One type of implantable pacemaker is the anti-tachycardia device, which can be used to achieve paced conversion of atrial flutter to sinus rhythm or an atrial paced rhythm. The techniques for conversion are programmable and may include underdrive pacing to overdrive pacing, and programmed extrastimuli to scanning methods.135 Anti-tachycardia pacing is generally chosen if an arrhythmia is refractory to medication, the patient is intolerant of medication, or the attacks are frequent and of long duration. An additional advantage is that many patients have tachycardia-bradycardia syndrome, and after overdrive pacing of atrial flutter, the pacemaker is on standby to begin pacing if the patient’s spontaneous rate is not adequate. Gillette and coworkers reported their extensive experience with anti-tachycardia pacing in patients with congenital heart disease and atrial flutter, using the Intertach II device (Inter Medics, Mumbai, India).135,136 Although many patients with this type of pacemaker still required visits to the hospital for cardioversion, these visits were clearly much less frequent. Of some concern is the observation by Rhodes and associates of a patient whose anti-tachycardia pacemaker converted an episode of atrial flutter to AF with a rapid ventricular response, which resulted in SCD.137 Such pacemakers must be used with concomitant administration of effective AV node blocking agents such as propranolol, diltiazem, or verapamil. Another approach is to program the device to be manually activated so that the patient can be under medical observation when attempts at pace conversion occur.
Although the Intertach II device is no longer on the market, a new generation of devices is now available, and these devices provide dual-chamber pacing and anti-tachycardia pacing (AT500, Medtronic, Minneapolis, MN). Some experience is available in patients with congenital heart disease.138 In addition, the latest generation of implantable cardioverter-defibrillators (ICDs) has the capability to perform atrial anti-tachycardia pacing as well as tiered therapy in the ventricle. Finally, ICDs that provide cardioversion shocks to the atrium are in development and may eventually be useful in the population of patients with repaired congenital heart disease.
Implantable Cardioverter-Defibrillators in Tetralogy of Fallot
With the rapid changes recently in lead and generator technology, ICDs have become a more viable option for the treatment of patients with VT following repair of congenital heart disease. Patients with repaired congenital heart disease made up 18% of patients with ICDs in the multiple-center review by Silka and colleagues of 125 pediatric patients.139 Most reports to date have dealt mainly with devices attached to epicardial patches, but recent reports have included patients with transvenous systems.140 SCD continues to be a serious concern in postoperative patients, particularly in patients who have had repair of tetralogy of Fallot who are at least 25 years beyond surgical repair, and in patients who have had the Mustard and the Senning procedures.68,86 The role of ICDs in the management of patients with congenital heart disease is undergoing rapid evolution, as implantable units become smaller and more multifunctional. Now that transvenous lead technology is well developed, implantation of ICDs in patients with prior sternotomies has become less problematic.141 However, congenital heart disease poses many challenges to the placement of such leads. Clearly, patients with persistent intracardiac shunting are not candidates for transvenous defibrillator leads because of the risk of thromboembolism. The use of active-fixation leads may well be necessary because of distortions of ventricular anatomy or the need to place such leads in a smooth-walled, morphologic left ventricle. In the past, the frequent occurrence of atrial arrhythmias in this patient population limited the usefulness of ICDs because of the tendency for atrial tachyarrhythmias with brisk AV conduction to be detected within the rate criteria for ICD therapy, which leads to inappropriate shocks. The availability of dual-chamber ICDs.142,143 with atrial sensing via an atrial lead makes implantation in patients with congenital heart disease more feasible.142,143 The indications for such implants should be similar to those for the population with adult noncongenital disease, namely, aborted SCD. Large multiple-center studies may eventually identify subpopulations of postoperative patients with congenital heart disease who are at high enough risk of SCD to justify prophylactic ICD implantation. Most data will be available in adults with congenital heart disease, and recent studies have shown ICDs to be effective, but with a high incidence of complications.144
Conclusion
1 Kugler JD, Danford DA, Deal BJ, et al. Radiofrequency catheter ablation for tachyarrhythmias in children and adolescents. N Engl J Med. 1994;330:1481.
2 Lev M, Givson S, Miller RA. Ebstein’s disease with Wolff-Parkinson-White syndrome. Am Heart J. 1955;49:724.
3 Scheibler GL, Adams P, Anderson RC, et al. Clinical study of twenty three cases of Ebstein’s anomaly of the tricuspid valve. Circulation. 1959;19:165.
4 Benson DJr, Gallagher JJ, Oldham HN, et al. Corrected transposition with severe intracardiac deformities with Wolff-Parkinson-White syndrome in a child: Electrophysiologic investigation and surgical correction. Circulation. 1980;61:1256.
5 Bokeriia LA, Revishvili A, Makhmudov MM. Syndrome of ventricular preexcitation and corrected transposition of the great vessels with insufficiency of the arterial atrioventricular valve of the Ebstein anomaly type. Kardiologiia. 1984;24:94.
6 Keller N, Soorensen MR. Corrected transposition of the great arteries with a left-sided Ebstein-like anomaly and WPW syndrome: A case diagnosed by two-dimensional echocardiography. Ugeskrift for Laeger. 1981;143:1971.
7 Perosio AM, Suarez LD, Bunster AM, et al. Pre-excitation syndrome and hypertrophic cardiomyopathy. J Electrocardiol. 1983;16:29.
8 MacRae CA, Ghaisas N, Kass S, et al. Familial hypertrophic cardiomyopathy with Wolff-Parkinson-White syndrome maps to a locus on chromosome 7q3. J Clin Invest. 1995;96:1216.
9 Ghosh S, Avari JN, Rhee EK, et al. Hypertrophic cardiomyopathy with preexcitation: Insights from noninvasive electrocardiographic imaging (ECGI) and catheter mapping. J Cardiovasc Electrophysiol. 2008;19:1215.
10 Arad M, Maron BJ, Gorham JM, et al. Glycogen storage diseases presenting as hypertrophic cardiomyopathy. N Engl J Med. 2005;352:362.
11 Huhta JC, Maloney JD, Ritter DG, et al. Complete atrioventricular block in patients with atrioventricular discordance. Circulation. 1983;67:1374.
12 Benson DW, Silberbach GM, Kavanaugh-McHugh A, et al. Mutations in the cardiac transcription factor NKX2.5 affect diverse cardiac developmental pathways. J Clin Invest. 1999;104:1567.
13 Moore KL. The developing human. Clinically oriented embryology. Philadelphia: Saunders; 1974.
14 Cosio FC. Endocardial mapping of atrial flutter. In: Touboul P, Waldo AL, editors. Atrial arrhythmias. St Louis: Mosby–Year Book, 1990.
15 Saoudi N, Cosio F, Waldo A, et al. A new classification of atrial tachycardias based on electrophysiologic mechanisms. Eur Heart J. 2000;21(6):508.
16 Olgin JE, Kalman JM, Fitzpatrick AP, Lesh MD. Role of right atrial endocardial structures as barriers to conduction during human type I atrial flutter: Activation and entrainment mapping guided by intracardiac echocardiography. Circulation. 1995;92:1839.
17 Kalman JM, Olgin JE, Saxon LA, et al. Activation and entrainment mapping defines the tricuspid annulus as the anterior barrier in typical atrial flutter. Circulation. 1996;93:398.
18 Nakagawa H, Lazzara R, Khastgir T, et al. The role of the tricuspid annulus and the eustachian valve/ridge on atrial flutter: Relevance to catheter ablation of the septal isthmus and a new technique for rapid identification of ablation success. Circulation. 1996;93:407.
19 Feld GK, Shahandeh-Rad F. Mechanism of double potentials recorded during sustained atrial flutter in the canine right atrial crush-injury model. Circulation. 1992;86:628.
20 Olshansky B, Okumura K, Henthorn RW, Waldo AL. Characterization of double potentials in human atrial flutter: Studies during transient entrainment. J Am Coll Cardiol. 1990;15:833.
21 Lesh MD, Van Hare GF, Epstein LM, et al. Radiofrequency catheter ablation of atrial arrhythmias: Results and mechanisms. Circulation. 1994;89:1074.
22 Feld GK, Fleck P, Chen PS, et al. Radiofrequency catheter ablation for the treatment of human type 1 atrial flutter: Identification of a critical zone in the reentrant circuit by endocardial mapping techniques. Circulation. 1992;86:1233.
23 Klein G, Guiraudon G, Sharma A, Milstein S. Demonstration of macroreentry and feasibility of operative therapy in the common type of atrial flutter. Am J Cardiol. 1986;57:587.
24 Harrison DA, Siu SC, Hussain F, et al. Sustained atrial arrhythmias in adults late after repair of tetralogy of fallot. Am J Cardiol. 2001;87(5):584.
25 Kalman JM, Van Hare GF, Olgin JE, et al. Ablation of “incisional” reentrant atrial tachycardia complicating surgery for congenital heart disease: Use of entrainment to define a critical isthmus of slow conduction. Circulation. 1996;93:502.
26 Chan DP, Van Hare GF, Mackall JA, et al. Importance of atrial flutter isthmus in postoperative intra-atrial reentrant tachycardia. Circulation. 2000;102:1283.
27 Collins KK, Love BA, Walsh EP, et al. Location of acutely successful radiofrequency catheter ablation of intraatrial reentrant tachycardia in patients with congenital heart disease. Am J Cardiol. 2000;86:969.
28 Senning A. Surgical correction of transposition of the great vessels. Surgery. 1959;45:966.
29 Mustard WT, Keith JD, Trusler GA, et al. The surgical management of transposition of the great vessels. J Thorac Cardiovasc Surg. 1964;48:953.
30 Karl TR, Weintraub RG, Brizard CP, et al. Senning plus arterial switch operation for discordant (congenitally corrected) transposition. Ann Thorac Surg. 1997;64:495.
31 Ebert PA, Gay WA, Engle MA. Correction of transposition of the great arteries: Relationship of the coronary sinus and postoperative arrhythmias. Ann Surg. 1974;180:433.
32 Van Hare GF, Lesh MD, Ross BA, et al. Mapping and radiofrequency ablation of intraatrial reentrant tachycardia after the Senning or Mustard procedure for transposition of the great arteries. Am J Cardiol. 1996;77:985.
33 Kanter RJ, Papagiannis J, Carboni MP, et al. Radiofrequency catheter ablation of supraventricular tachycardia substrates after Mustard and Senning operations for D-transposition of the great arteries. J Am Coll Cardiol. 2000;35:428.
34 Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240.
35 McElhinney DB, Petrossian E, Reddy VM, Hanley FL. Extracardiac conduit Fontan procedure without cardiopulmonary bypass. Ann Thorac Surg. 1998;66:1826.
36 van Son JA, Reddy M, Hanley FL. Extracardiac modification of the Fontan operation without use of prosthetic material. J Thorac Cardiovasc Surg. 1995;110:1766.
37 Jonas RA, Castaneda AR. Modified Fontan procedure: Atrial baffle and systemic venous to pulmonary artery anastomotic techniques. J Cardiac Surg. 1988;3:91.
38 Robbers-Visser D, Miedema M, Nijveld A, et al. Results of staged total cavopulmonary connection for functionally univentricular hearts: Comparison of intra-atrial lateral tunnel and extracardiac conduit. Eur J Cardiothorac Surg. 2010;37(4):934.
39 Nehgme RA, Carboni MP, Care J, Murphy JD. Transthoracic percutaneous access for electroanatomic mapping and catheter ablation of atrial tachycardia in patients with a lateral tunnel Fontan. Heart Rhythm. 2006;3(1):37.
40 El-Said HG, Ing FF, Grifka RG, et al. 18-year experience with transseptal procedures through baffles, conduits, and other intra-atrial patches. Catheter Cardiovasc Interv. 2000;50(4):434. discussion 440
41 Mehta C, Jones T, De Giovanni JV. Percutaneous transcatheter communication between the pulmonary artery and atrium following an extra-cardiac Fontan: An alternative approach to fenestration avoiding conduit perforation. Catheter Cardiovasc Interv. 2008;71(7):936.
42 Triedman JK, Jenkins KJ, Saul JP, et al. Right atrial mapping in humans using a multielectrode basket catheter [abstract]. PACE. 1995;18:800.
43 Triedman JK, Alexander ME, Berul CI, et al. Electroanatomic mapping of entrained and exit zones in patients with repaired congenital heart disease and intra-atrial reentrant tachycardia. Circulation. 2001;103(16):2060.
44 Nakagawa H, Shah N, Matsudaira K, et al. Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: Isolated channels between scars allow “focal” ablation. Circulation. 2001;103(5):699.
45 Gillette PC, Yeoman MA, Mullins CE, McNamara DG. Sudden death after repair of tetralogy of Fallot: Electrocardiographic and electrophysiologic abnormalities. Circulation. 1977;56:566.
46 James FW, Kaplan S, Chou TC. Unexpected cardiac arrest in patients after surgical correction of tetralogy of Fallot. Circulation. 1975;52:691-695.
47 Quattlebaum TG, Varghese J, Neill CA, Donahoo JS. Sudden death among postoperative patients with tetralogy of Fallot: A follow-up study of 243 patients for an average of twelve years. Circulation. 1976;54:289.
48 Marin-Garcia J, Moller JH. Sudden death after operative repair of tetralogy of Fallot. Br Heart J. 1977;39:1380.
49 Deanfield JE, McKenna WJ, Hallidie-Smith KA. Detection of late arrhythmia and conduction disturbance after correction of tetralogy of Fallot. Br Heart J. 1980;44:248.
50 Garson AJr, Gillette PC, Gutgesell HP, McNamara DG. Stress-induced ventricular arrhythmia after repair of tetralogy of Fallot. Am J Cardiol. 1980;46:1006.
51 Shen WK, Holmes DRJr, Porter CJ, et al. Sudden death after repair of double-outlet right ventricle. Circulation. 1990;81:128.
52 Garson AJr, McNamara DG. Sudden death in a pediatric cardiology population, 1958 to 1983: Relation to prior arrhythmias. J Am Coll Cardiol. 1985;5:134B.
53 Vetter VL, Horowitz LN. Electrophysiologic residua and sequelae of surgery for congenital heart defects. Am J Cardiol. 1982;50:588.
54 Zahka KG, Horneffer PJ, Rowe SA, et al. Long-term valvular function after total repair of tetralogy of Fallot: Relation to ventricular arrhythmias. Circulation. 1988;78:III14.
55 Gatzoulis MA, Till JA, Somerville J, Redington AN. Mechanoelectrical interaction in tetralogy of Fallot: QRS prolongation relates to right ventricular size and predicts malignant ventricular arrhythmias and sudden death. Circulation. 1995;92:231.
56 Gatzoulis MA, Till JA, Redington AN. Depolarization-repolarization inhomogeneity after repair of tetralogy of Fallot: The substrate for malignant ventricular tachycardia? Circulation. 1997;95:401.
57 Deanfield JE, Ho SY, Anderson RH, et al. Late sudden death after repair of tetralogy of Fallot: A clinicopathologic study. Circulation. 1983;67:626.
58 Deanfield J, McKenna W, Rowland E. Local abnormalities of right ventricular depolarization after repair of tetralogy of Fallot: A basis for ventricular arrhythmia. Am J Cardiol. 1985;55:522.
59 Zimmermann M, Friedli B, Adamec R, Oberhansli I. Ventricular late potentials and induced ventricular arrhythmias after surgical repair of tetralogy of Fallot. Am J Cardiol. 1991;67:873.
60 Zeppenfeld K, Schalij MJ, Bartelings MM, et al. Catheter ablation of ventricular tachycardia after repair of congenital heart disease: Electroanatomic identification of the critical right ventricular isthmus. Circulation. 2007;116:2241.
61 Kremers MS, Wells PJ, Black WH, Solodyna MA. Entrainment of ventricular tachycardia in postoperative tetralogy of Fallot. Pacing Clin Electrophysiol. 1988;11:1310.
62 Aizawa Y, Kitazawa H, Washizuka T, et al. Conductive properties of the reentrant pathway of ventricular tachycardia during entrainment from outside and within the zone of slow conduction. Pacing Clin Electrophysiol. 1995;18:663.
63 Chandar JS, Wolff GS, Garson AJr, et al. Ventricular arrhythmias in postoperative tetralogy of Fallot. Am J Cardiol. 1990;65:655.
64 Walsh EP, Rockenmacher S, Keane JF, et al. Late results in patients with tetralogy of Fallot repaired during infancy. Circulation. 1988;77:1062.
65 Garson AJr, Randall DC, Gillette PC, et al. Prevention of sudden death after repair of tetralogy of Fallot: Treatment of ventricular arrhythmias. J Am Coll Cardiol. 1985;6:221.
66 Harrison DA, Harris L, Siu SC, et al. Sustained ventricular tachycardia in adult patients late after repair of tetralogy of Fallot. J Am Coll Cardiol. 1997;30:1368.
67 Balaji S, Lau YR, Case CL, Gillette PC. QRS prolongation is associated with inducible ventricular tachycardia after repair of tetralogy of Fallot. Am J Cardiol. 1997;80:160.
68 Nollert G, Fischlein T, Bouterwek S, et al. Long-term survival in patients with repair of tetralogy of Fallot: 36-year follow-up of 490 survivors of the first year after surgical repair. J Am Coll Cardiol. 1997;30:1374.
69 Therrien J, Provost Y, Merchant N, et al. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol. 2005;95(6):779.
70 Geva T. Indications and timing of pulmonary valve replacement after tetralogy of Fallot repair. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 11, 2006.
71 Harrild DM, Berul CI, Cecchin F, et al. Pulmonary valve replacement in tetralogy of Fallot: Impact on survival and ventricular tachycardia. Circulation. 2009;119(3):445.
72 Goldman BS, Williams WG, Hill T, et al. Permanent cardiac pacing after open heart surgery: Congenital heart disease. Pacing Clin Electrophysiol. 1985;8:732.
73 Bonatti V, Agnetti A, Squarcia U. Early and late postoperative complete heart block in pediatric patients submitted to open-heart surgery for congenital heart disease. Pediatr Med Chir. 1998;20:181.
74 McElhinney DB, Geiger E, Blinder J, et al. NKX2.5 mutations in patients with congenital heart disease. J Am Coll Cardiol. 2003;42:1650.
75 Garson AJr. Medicolegal problems in the management of cardiac arrhythmias in children. Pediatrics. 1987;79:84.
76 The Cardiac Arrhythmia Suppression Trial (CAST) investigators. Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406.
77 The Cardiac Arrhythmia Suppression Trial II investigators. Effect of the antiarrhythmic agent moricizine on survival after myocardial infarction. N Engl J Med. 1992;327:227.
78 Garson AJr, Gillette PC, McNamara DG. Propranolol: The preferred palliation for tetralogy of Fallot. Am J Cardiol. 1981;47:1098.
79 Fish FA, Gillette PC, Benson DWJr. Proarrhythmia, cardiac arrest and death in young patients receiving encainide and flecainide. The Pediatric Electrophysiology Group. J Am Coll Cardiol. 1991;18:356.
80 Hohnloser SH. Proarrhythmia with class III antiarrhythmic drugs: Types, risks, and management. Am J Cardiol. 1997;80:82G.
81 Waldo AL, Camm AJ, deRuyter H, et al. Effect of D-sotalol on mortality in patients with left ventricular dysfunction after recent and remote myocardial infarction. The SWORD Investigators. Survival with oral D-sotalol. Lancet. 1996;348:7.
82 Daniels CJ, Schutte DA, Hammond S, Franklin WH. Acute pulmonary toxicity in an infant from intravenous amiodarone. Am J Cardiol. 1997;80:1113.
83 Celiker A, Kocak G, Lenk MK, et al. Short- and intermediate-term efficacy of amiodarone in infants and children with cardiac arrhythmia. Turk J Pediatr. 1997;39:219.
84 Villain E. Amiodarone as treatment for atrial tachycardias after surgery. Pacing Clin Electrophysiol. 1997;20:2130.
85 Simpson RJJr, Foster JR, Woelfel AK, Gettes LS. Management of atrial fibrillation and flutter: A reappraisal of digitalis therapy. Postgrad Med. 1986;79:241.
86 Garson AJr, Bink BM, Hesslein PS, et al. Atrial flutter in the young: A collaborative study of 380 cases. J Am Coll Cardiol. 1985;6:871.
87 Garson AJr, Gillette PC, McVey P, et al. Amiodarone treatment of critical arrhythmias in children and young adults. J Am Coll Cardiol. 1984;4:749.
88 Tipple M, Sandor G. Efficacy and safety of oral sotalol in early infancy. Pacing Clin Electrophysiol. 1991;14:2062.
89 Maragnes P, Tipple M, Fournier A. Effectiveness of oral sotalol for treatment of pediatric arrhythmias. Am J Cardiol. 1992;69:751.
90 Abinader E, Borochowitz Z, Berger A. A hemodynamic complication of verapamil therapy in a neonate. Helv Paediatr Acta. 1981;36:451.
91 Shim D, Lloyd TR, Cho KJ, et al. Transhepatic cardiac catheterization in children: Evaluation of efficacy and safety. Circulation. 1995;92:1526.
92 Sommer RJ, Golinko RJ, Mitty HA. Initial experience with percutaneous transhepatic cardiac catheterization in infants and children. Am J Cardiol. 1995;75:1289.
93 Lesh MD, Van Hare GF, Scheinman MM, et al. Comparison of the retrograde and transseptal methods for ablation of left free wall accessory pathways. J Am Coll Cardiol. 1993;22:542.
94 Lai WW, al-Khatib Y, Klitzner TS, et al. Biplanar transesophageal echocardiographic direction of radiofrequency catheter ablation in children and adolescents with the Wolff-Parkinson-White syndrome. Am J Cardiol. 1993;71:872.
95 Drant SE, Klitzner TS, Shannon KM, et al. Guidance of radiofrequency catheter ablation by transesophageal echocardiography in children with palliated single ventricle. Am J Cardiol. 1995;76:1311.
96 Tucker KJ, Curtis AB, Murphy J, et al. Transesophageal echocardiographic guidance of transseptal left heart catheterization during radiofrequency ablation of left-sided accessory pathways in humans. Pacing Clin Electrophysiol. 1996;19:272.
97 Wu J, Pflaumer A, Deisenhofer I, et al. Mapping of intraatrial reentrant tachycardias by remote magnetic navigation in patients with D-transposition of the great arteries after Mustard or Senning procedure. J Cardiovasc Electrophysiol. 2008;19:1153.
98 Davies MJ, Anderson RH. Conduction system in congenital heart disease. In: Davies MJ, Anderson RH, editors. The conduction system of the heart. London: Butterworths, 1983.
99 Thiene G, Wenink AC, Frescura C, et al. Surgical anatomy and pathology of the conduction tissues in atrioventricular defects. J Thorac Cardiovasc Surg. 1981;82:928.
100 Dickinson DF, Wilkinson JL, Smith A, et al. Variations in the morphology of the ventricular septal defect and disposition of the atrioventricular conduction tissues in tetralogy of Fallot. Thorac Cardiovasc Surg. 1982;30:243.
101 Anderson RH, Becker AE, Arnold R, Wilkinson JL. The conducting tissues in congenitally corrected transposition. Circulation. 1974;50:911.
102 Collins KK, Rhee EK, Kirsh JA, et al. Cryoablation of accessory pathways in the coronary sinus in young patients: A multicenter study from the Pediatric and Congenital Electrophysiology Society’s Working Group on Cryoablation. J Cardiovasc Electrophysiol. 2007;18:592.
103 Saul JP, Hulse JE, De W, et al. Catheter ablation of accessory atrioventricular pathways in young patients: Use of long vascular sheaths, the transseptal approach and a retrograde left posterior parallel approach. J Am Coll Cardiol. 1993;21:571.
104 Swartz JF, Cohen AI, Fletcher RD, et al. Right coronary epicardial mapping improves accessory pathway catheter ablation success [abstract]. Circulation. 1989;80(Suppl II):II-430.
105 Lesh MD, Van Hare GF, Chien WW, Scheinman MM. Mapping in the right coronary artery as an aid to radiofrequency ablation of right-sided accessory pathways [abstract]. PACE. 1991;14:671.
106 Van Hare GF, Lesh MD, Stanger P. Radiofrequency catheter ablation of supraventricular arrhythmias in patients with congenital heart disease: Results and technical considerations. J Am Coll Cardiol. 1993;22:883.
107 Calkins H, Prystowsky E, Carlson M, et al. Temperature monitoring during radiofrequency catheter ablation procedures using closed loop control. Atakr Multicenter Investigators Group. Circulation. 1994;90:1279.
108 Saul JP, Walsh EP, Triedman JK. Mechanisms and therapy of complex arrhythmias in pediatric patients. J Cardiovasc Electrophysiol. 1995;6:1129.
109 Van Hare GF, Lesh MD, Scheinman MM, Langberg JJ. Percutaneous radiofrequency catheter ablation for supraventricular arrhythmias in children. J Am Coll Cardiol. 1991;17:1613.
110 Dick MD, O’Connor BK, Serwer GA, et al. Use of radiofrequency current to ablate accessory connections in children. Circulation. 1991;84:2318.
111 Saul JP, Walsh EP, Langberg JJ, et al. Radiofrequency ablation of accessory atrioventricular pathways: Early results in children with refractory SVT [abstract]. Circulation. 1990;82(Supplt III):III-222.
112 Walsh EP, Saul JP. Transcatheter ablation for pediatric tachyarrhythmias using radiofrequency electrical energy. Pediatr Ann. 1991;20:386. 388
113 Okumura K, Henthorn RW, Epstein AE, et al. Further observations on transient entrainment: Importance of pacing site and properties of the components of the reentry circuit. Circulation. 1985;72:1293.
114 Treidman JK, Saul JP, Weindling SN, Walsh EP. Radiofrequency ablation of intra-atrial reentrant tachycardia after surgical palliation of congenital heart disease. Circulation. 1995;91:707.
115 Triedman JK, Jenkins KJ, Colan SD, et al. Intra-atrial reentrant tachycardia after palliation of congenital heart disease: Characterization of multiple macroreentrant circuits using fluoroscopically based three-dimensional endocardial mapping. J Cardiovasc Electrophysiol. 1997;8:259.
116 Baker BM, Lindsay BD, Bromberg BI, et al. Catheter ablation of clinical intraatrial reentrant tachycardias resulting from previous atrial surgery: Localizing and transecting the critical isthmus. J Am Coll Cardiol. 1996;28:411.
117 Balaji S, Johnson TB, Sade RM, et al. Management of atrial flutter after the Fontan procedure. J Am Coll Cardiol. 1994;23:1209.
118 Case CL, Gillette PC, Douglas DE, Liebermann RA. Radiofrequency catheter ablation of atrial flutter in a patient with postoperative congenital heart disease. Am Heart J. 1993;126:715.
119 Triedman JK, DeLucca JM, Alexander ME, et al. Prospective trial of electroanatomically guided, irrigated catheter ablation of atrial tachycardia in patients with congenital heart disease. Heart Rhythm. 2005;2:700.
120 Rodefeld MD, Bromberg BI, Schuessler RB, et al. Atrial flutter after lateral tunnel construction in the modified Fontan operation: A canine model. J Thorac Cardiovasc Surg. 1996;111:514.
121 Nurnberg JH, Ovroutski S, Alexi-Meskishvili V, et al. New onset arrhythmias after the extracardiac conduit Fontan operation compared with the intraatrial lateral tunnel procedure: Early and midterm results. Ann Thorac Surg. 2004;78:1979.
122 Michel-Behnke I, Luedemann M, Bauer J, et al. Fenestration in extracardiac conduits in children after modified Fontan operation by implantation of stent grafts. Pediatr Cardiol. 2005;26:93.
123 Nehgme RA, Carboni MP, Care J, Murphy JD. Transthoracic percutaneous access for electroanatomic mapping and catheter ablation of atrial tachycardia in patients with a lateral tunnel Fontan. Heart Rhythm. 2006;3(1):37.
124 Ressia L, Graffigna A, Salerno-Uriarte JA, Vigano M. The complex origin of ventricular tachycardia after the total correction of tetralogy of Fallot. Giornale Italiano di Cardiologia. 1993;23:905.
125 Burton ME, Leon AR. Radiofrequency catheter ablation of right ventricular outflow tract tachycardia late after complete repair of tetralogy of Fallot using the pace mapping technique. Pacing Clin Electrophysiol. 1993;16:2319.
126 Biblo LA, Carlson MD. Transcatheter radiofrequency ablation of ventricular tachycardia following surgical correction of tetralogy of Fallot. Pacing Clin Electrophysiol. 1994;17:1556.
127 Goldner BG, Cooper R, Blau W, Cohen TJ. Radiofrequency catheter ablation as a primary therapy for treatment of ventricular tachycardia in a patient after repair of tetralogy of Fallot. Pacing Clin Electrophysiol. 1994;17:1441.
128 Gonska BD, Cao K, Raab J, et al. Radiofrequency catheter ablation of right ventricular tachycardia late after repair of congenital heart defects. Circulation. 1996;94:1902.
129 Horton RP, Canby RC, Kessler DJ, et al. Ablation of ventricular tachycardia associated with tetralogy of Fallot: Demonstration of bidirectional block. J Cardiovasc Electrophysiol. 1997;8:432.
130 Frank G, Schmid C, Baumgart D, et al. Surgical therapy of life-threatening tachycardic cardiac arrhythmias in children. Monatsschrift Kinderheilkunde. 1989;137:269.
131 Lawrie GM, Pacifico A, Kaushik R. Results of direct surgical ablation of ventricular tachycardia not due to ischemic heart disease. Ann Surg. 1989;209:716.
132 Downar E, Harris L, Kimber S, et al. Ventricular tachycardia after surgical repair of tetralogy of Fallot: Results of intraoperative mapping studies. J Am Coll Cardiol. 1992;20:648.
133 Misaki T, Tsubota M, Tanaka M, et al. Surgical treatment of ventricular tachycardia after radical correction of tetralogy of Fallot. Nippon Kyobu Geka Gakkai Zasshi J Jap Assoc Thorac Surg. 1990;38:130.
134 Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51(21):e1.
135 Gillette PC, Zeigler VL, Case CL, et al. Atrial antitachycardia pacing in children and young adults. Am Heart J. 1991;122:844.
136 Gillette PC. Antitachycardia pacing. Pacing Clin Electrophysiol. 1997;20:2121.
137 Rhodes LA, Walsh EP, Gamble WJ, et al. Benefits and potential risks of atrial antitachycardia pacing after repair of congenital heart disease. Pacing Clin Electrophysiol. 1995;18:1005.
138 Stephenson EA, Casavant D, Tuzi J, et al. Efficacy of atrial antitachycardia pacing using the Medtronic AT500 pacemaker in patients with congenital heart disease. Am J Cardiol. 2003;92(7):871.
139 Silka MJ, Kron J, Dunnigan A, Dick MD. Sudden cardiac death and the use of implantable cardioverter-defibrillators in pediatric patients. The Pediatric Electrophysiology Society. Circulation. 1993;87:800.
140 Wilson WR, Greer GE, Grubb BP. Implantable cardioverter-defibrillators in children: A single-institutional experience. Ann Thorac Surg. 1998;65:775.
141 Silka MJ. Implantable cardioverter-defibrillators in children. A perspective on current and future uses. J Electrocardiol. 1996;29(Suppl):223.
142 Li HG, Thakur RK, Yee R, Klein GJ. Ventriculoatrial conduction in patients with implantable cardioverter defibrillators: Implications for tachycardia discrimination by dual chamber sensing. Pacing Clin Electrophysiol. 1994;17:2304.
143 Lavergne T, Daubert JC, Chauvin M, et al. Preliminary clinical experience with the first dual chamber pacemaker defibrillator. Pacing Clin Electrophysiol. 1997;20:182.
144 Khairy P, Harris L, Landzberg MJ, et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008;117:363.