Chapter 79 Arrhythmias and Adult Congenital Heart Disease
Congenital heart defects complicate approximately 0.5% to 1% of all live births. More than 1 million adults in the United States are estimated to have repaired or unrepaired congenital heart disease.1 Approximately 50% of this group has a congenital heart defect categorized as moderate or severe by the American Heart Association/American College of Cardiology (AHA/ACC) task force on adult congenital heart disease (ACHD); this group of patients is at the greatest risk for cardiac arrhythmias.2 This number can only be expected to increase in the future.3 Advances in surgical technique now allow many patients with previously fatal congenital defects to survive into adulthood. However, the electrophysiological and physiological/hemodynamic consequences of these lesions often manifest as arrhythmias in young adults. In many patients, arrhythmias are a leading cause of functional decline as well as sudden cardiac arrest.4,5 A recent survey showed that hospital admissions for ACHD doubled between 1998 and 2005; the most common reason for hospital admission was a cardiac arrhythmia.6 Knowledge of the anatomy and physiology of congenital heart defects is critical to managing cardiac arrhythmias in this unique ACHD patient population.
Percutaneous catheter mapping and ablation of atrial and ventricular arrhythmias in the ACHD population have undergone significant advances in the past decade. In particular, the advent of electroanatomic three-dimensional mapping and its integration with cardiac imaging such as computed tomography (CT) or magnetic resonance imaging (MRI) have helped the electrophysiologist define the precise electroanatomic substrate for the arrhythmias that develop in this complex population.7 Percutaneous access to the cardiac chambers often presents unique challenges in this patient population, requiring detailed knowledge of the lesion and anatomic repair as well as familiarity with procedures such as trans-septal and trans-baffle puncture. In addition, ACHD patients present significant hemodynamic challenges because they have lower cardiac reserve and greater physiological instability in response to anesthesia or with the occurrence of rapid heart rates.
General Principles
It cannot be overemphasized that a thorough understanding of the individual anatomy and physiology of the patient with congenital heart disease is essential before planning and performing any invasive electrophysiological procedure. Prior operative and catheterization reports should be reviewed in detail. Noninvasive imaging such as echocardiography, MRA, or CTA should be reviewed with a physician experienced in interpreting studies of patients with ACHD.8 Patients must be viewed within the historical context of the type of cardiac repair they have received; different variations of surgical repairs for the same congenital heart defect may yield vastly different electrophysiological outcomes. Venous access may be altered by the surgical procedure or by prior catheterizations, and standard approaches to the chamber of interest may not be possible.9
Antiarrhythmic Drug Therapy
FDA class 1C agents (flecainide, propafenone) have been shown to be proarrhythmic in adults with ischemic heart disease. Nevertheless, they are often efficacious in the treatment for supraventricular and ventricular arrhythmias and are sometimes used in the pediatric patient with congenital heart disease because these agents are well tolerated and pose no risk of organ toxicity.10 These agents should be used with caution in the patient with ACHD; they may have a role in the presence of a backup implantable cardioverter-defibrillator (ICD).
A more recent addition to the antiarrhythmic armamentarium is dofetilide. Although studies have demonstrated safety in the adult population with prior myocardial infarction (MI), studies on its efficacy or toxicity in the ACHD patient population have not been done.11 Dofetilide is a potent potassium channel blocker and must be initiated in the inpatient setting, where monitoring of the QTc interval can occur. Unlike sotalol, dofetilide has no β-blocking properties and therefore is less likely to cause significant bradycardia or AV conduction block. However, concomitant β-blocking agents typically are required to slow the ventricular rate in response to atrial arrhythmias. Verapamil is contraindicated in patients taking dofetilide because of its interaction with hepatic metabolism; diltiazem, diuretics, and digoxin may also increase the risk of proarrhythmia and should be used with caution.
In patients with significant systemic ventricular dysfunction, amiodarone is the agent of choice. Amiodarone therapy often slows conduction velocity in an already diseased atrial substrate and may therefore facilitate 1 : 1 A : V conduction during atrial flutter or intra-atrial re-entrant tachycardia (IART) leading to hemodynamic compromise or silent ventricular dysfunction. This is uncommon because of the associated effect of slowing AV nodal conduction. The known risk of hepatic dysfunction with amiodarone may be worsened in the ACHD population because chronic hepatic venous congestion is common. In addition, patients should be monitored for pulmonary, thyroid, and ocular toxicities. Because of its long-term toxicities, amiodarone is typically reserved for patients with recurrent, poorly tolerated arrhythmias not amenable to catheter ablative therapy or for those with significant coexistent ventricular dysfunction. Typically, the lowest dose of amiodarone effective in suppressing arrhythmias—often 100 mg daily—is sufficient. Amiodarone can be safely initiated in the outpatient setting, typically at 400 mg daily and then titrated downward; however, in patients at risk for significant bradycardia, inpatient monitoring is advised. Dronedarone—a new antiarrhythmic agent with similar structure to amiodarone, but without amiodarone’s iodine moiety or long-term organ toxicity—has recently been approved for use in atrial arrhythmias and may provide another option for the patient with ACHD.12
Invasive Electrophysiology Study
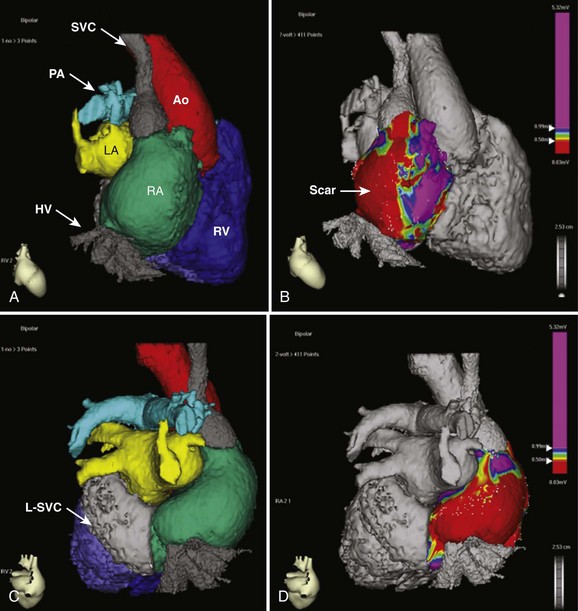
The advent of three-dimensional electroanatomic mapping techniques allows realistic depiction of the patient’s anatomy combined with three-dimensional visualization of the tachycardia circuit. Electroanatomic mapping systems use magnetic fields or impedance measurements to locate a catheter in space. By moving the catheter around the chamber of interest, a shell of the chamber can be created. At each point on the shell, both the local recorded bipolar voltage and the activation time during a tachycardia can be annotated and color coded. The voltage map, in combination with pace mapping, can be quite useful for denoting areas of scar (low voltage) or prosthetic baffle material (no voltage) when developing an ablation strategy.13 Activation mapping denotes the local activation time relative to another fixed reference catheter. In the general adult patient with atrial arrhythmias, the coronary sinus (CS) catheter is used as the reference. In the ACHD population, the CS may not be accessible and any stable catheter position such as the atrial appendage can be used. The use of active fixation leads for this purpose in complex cases has been described.14 The combined voltage and activation maps, coupled with standard entrainment mapping, can be used to tailor an ablation strategy. These advanced technologies have improved the outcomes of catheter ablation but do not substitute a thorough understanding of the anatomy and electrophysiology associated with the arrhythmia occurring in these patients. Integration with CTA or MRA angiographic imaging can be performed to obtain a more realistic depiction of the complex anatomy of the patient with ACHD (Figure 79-1). In our experience, the main usefulness of CTA image integration is to confirm that the entirety of a particular cardiac chamber has been mapped.
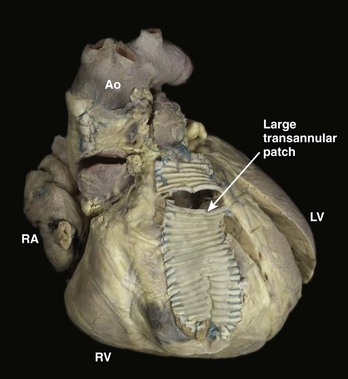
As described above, re-entry around anatomic obstacles such as surgical incisions or patch material is the dominant mechanism of both atrial and ventricular arrhythmias in patients with ACHD. A typical example is seen in repaired tetralogy of Fallot (TOF), in which patch material is often used to close the ventricular septal defect (VSD) and may be needed to expand the right ventricular outflow tract (Figure 79-2). During ablation, linear lesions can be created between low-voltage regions or anatomic barriers to transect a critical isthmus of the tachycardia circuit and interrupt the tachycardia. Creation of long linear lesions in scarred atrial tissue often is difficult; irrigated catheters have improved the outcome and should be considered when performing an ablation in this patient population. Whenever possible, proof of block across a linear lesion should be obtained with pacing maneuvers to minimize the likelihood of slow conduction across the line leading to a recurrent tachycardia. Less frequently, atrial stretch can lead to “focal” atrial tachycardia. This tachycardia can be focally ablated at the site of earliest activation, often identified by low-amplitude fractionated electrograms.
The remainder of this chapter is dedicated to a discussion of the most common groups of ACHD lesions seen in patients with arrhythmias: atrial septal defects (ASDs), Fontan procedures, transposition of the great arteries after an intra-atrial repair, and TOF. These repairs represent the majority of patients seen in the ACHD arrhythmia clinic. Although less common, Ebstein’s anomaly also is discussed given its unique electrophysiological consequences. The common strategies applied to the arrhythmias encountered with these lesions can often be extrapolated to other patient groups not discussed here, including those with scar-related arrhythmias after VSD repair or AV canal repair. The reader is directed to the recently published guidelines for the management of ACHD as well as for device therapy.2,15
Atrial Septal Defects
ASDs are among the most common congenital heart lesions and often are associated with atrial arrhythmias, both preoperatively and after repair.16 The majority of ASDs are of the secundum type (75%). Secundum ASDs occur within the oval fossa, although they may extend outside the fossa when an associated deficiency of the atrial septum exists. The electrocardiogram (ECG) in patients with secundum ASDs typically shows evidence of a vertically oriented P wave, right-axis deviation of the QRS complex, and incomplete right bundle branch block (RBBB). Primum ASDs, which are a type of endocardial cushion defect, occur less commonly (15% to 20%) than secundum ASDs and often are associated with mitral or tricuspid valve regurgitation. The ECG in a patient with a primum ASD typically has left-axis deviation of the QRS complex. Primum ASDs can be associated with AV conduction disturbances and, occasionally, with complete heart block. Sinus venosus ASDs (5% to 10%) occur in either the superior or inferior paraseptal region, at the mouth of the IVC or the SVC. Superior sinus venosus ASDs may be associated with sinus node disease and typically have left-axis deviation of the P wave on the ECG. Least common is the coronary sinus septal defect, a defect between the wall of the coronary sinus and the left atrium. All the above ASDs lead to left atrial enlargement, fibrosis, and changes in atrial refractoriness.17 These changes in response to the chronic volume overload often lead to the development of both AF and atrial flutter.
Of the patients referred for the surgical closure of an ASD, 10% to 20% would have had at least one episode of AF or atrial flutter.15 Risk factors for atrial arrhythmia after surgical ASD closure include older age at the time of repair (>40 years), elevated pulmonary arterial pressure, preoperative atrial arrhythmia, and postoperative junctional rhythm. Given the continued risk of arrhythmia, combining the Maze procedure with septal defect repair should be considered in those patients with a history of AF or atrial flutter. Whether prophylactic Maze surgery has a role in the treatment of those patients at highest risk of AF undergoing ASD repair remains controversial. Incisional atrial flutters after ASD repair typically occur around the posterior right atriotomy incision rather than around the ASD patch itself. Catheter ablation can be extremely effective. The long-term occurrence of atrial arrhythmias after transvenous ASD closure is unknown, but one promise of this technology is a potential reduction in incisional re-entrant tachycardias.
Ebstein’s Anomaly of the Tricuspid Valve
A wide range of severity in hemodynamic abnormalities and arrhythmia occurrence exists in patients with Ebstein’s anomaly of the tricuspid valve. The structural abnormality involves the apical displacement of the septal leaflet of the tricuspid valve, often with displacement of the mural (posterior) leaflet and atrialization of the basal portion of the right ventricle. The valve itself is malformed and regurgitant, and an ASD or patent foramen ovale, often with right-to-left shunting, is present in one third of patients.19 This combination leads to severe right atrial dilation and a susceptibility to atrial arrhythmias. Ebstein’s anomaly can also be seen in congenitally corrected transposition of the great arteries (L-TGA).
Ebstein’s anomaly is commonly associated with Wolff-Parkinson-White (WPW) syndrome, with a reported prevalence of 10% to 40%.20–22 Accessory pathways in patients with Ebstein’s anomaly are typically right sided, and multiple accessory pathways are often present (30% to 50%). In addition to classic AV pathways, variants such as slowly conducting atriofascicular fibers are also more common in patients with Ebstein’s anomaly.23 In this population, the clinician should take care not to overlook subtle pre-excitation, which may manifest as absence of the expected RBBB.24 Because Ebstein’s anomaly may be clinically silent into adulthood, echocardiography should be performed in any adult with right-sided accessory pathways and evidence of right atrial enlargement on the ECG to exclude this abnormality.
As with many other congenital lesions associated with right atrial enlargement, atrial flutter and AF are commonly seen in adults with Ebstein’s anomaly.25 The atrial arrhythmia burden remains high even after surgical repair, with at least one third of patients having AF observed in long-term follow-up.26
Ablation of supraventricular tachycardia (SVT) related to accessory pathways has become standard therapy for these patients. If surgical repair is planned, an electrophysiology study (EPS) should be performed before the operation in any patient with known or suspected pre-excitation. The absence of a RBBB pattern in lead V1 has been shown to be predictive of an occult accessory pathway in this group.27 Catheter ablation should be attempted before surgery, with intraoperative mapping performed if catheter ablation cannot be performed or has been unsuccessful.
Catheter ablation of the accessory pathways can be highly successful, although it remains a challenging procedure. Overall acute success rates for catheter ablation of accessory pathways are lower (80%), and recurrence rates are higher than in patients who do not have Ebstein’s anomaly. This is most likely caused by the presence of multiple pathways and catheter instability along the tricuspid annulus (which is displaced from the valve leaflets themselves) and because of the presence of tricuspid regurgitation.28 Use of long sheaths and a multipolar halo catheter along the tricuspid annulus may be helpful to guide the ablation and improve catheter stability. In addition, the use of a microcatheter placed within the right coronary artery may help with pathway localization when traditional endocardial catheters such as the multipolar halo catheter are unhelpful.29 As with patients who do not have Ebstein’s anomaly, coronary artery occlusion remains a risk of ablation in this region.30
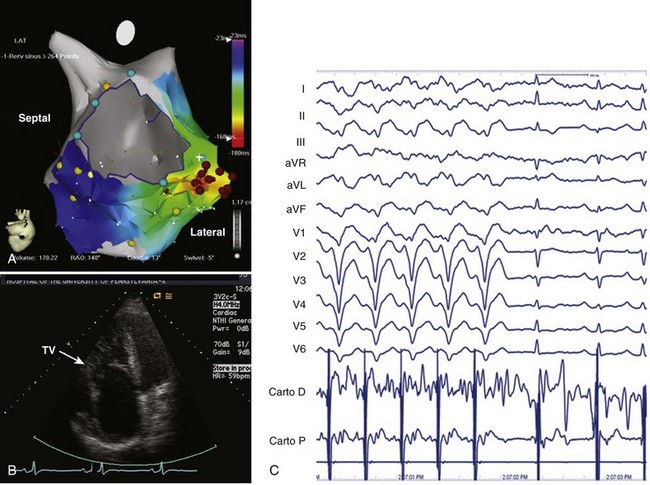
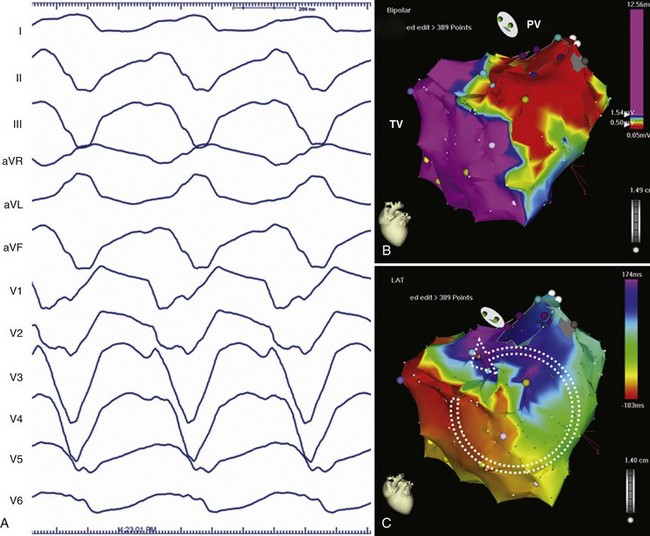
Because the right ventricle is the primary site of abnormality in Ebstein’s anomaly, it is not surprising that ventricular tachycardia (VT) has been described in Ebstein’s anomaly.31,32 VT can arise from within the functional right ventricle itself or from within the atrialized portion of the right ventricle, which retains ventricular electrophysiological properties. As with atrial arrhythmias, the occurrence of VT in a patient with unrepaired Ebstein’s anomaly who has significant tricuspid regurgitation should initiate consideration of surgical repair. Because repair of the tricuspid valve may not always be feasible, we recommend an EPS and attempted ablation of VT before surgery. VT is typically focal and located in the basal atrialized portion of the right ventricle (Figure 79-3). Catheter ablation can be helpful in these patients and, if unsuccessful, can guide surgical cryoablation at the time of valve repair or replacement. This is particularly important if mechanical tricuspid valve replacement is required because future percutaneous access to the right ventricle will be eliminated.
Fontan Operation
Originally performed for the palliation of tricuspid atresia, the Fontan operation is applied to numerous congenital heart lesions when single-ventricle physiology exists. The classic Fontan operation involved the creation of a direct connection from the right atrium to the pulmonary artery, completely bypassing the right ventricle, leading to passive filling of the pulmonary arterial tree and elevated right atrial filling pressures. The occurrence of atrial arrhythmias over time is the major complication of the subsequent right atrial volume overload. In addition to AF and typical atrial flutter, incisional or IART may be seen.18
The Fontan operation has undergone numerous modifications in an effort to reduce perioperative mortality rates and to improve hemodynamic and electrophysiological outcomes. The most recent modification is the extracardiac cavopulmonary Fontan operation, which creates a conduit between the IVC and the SVC, which is then anastomosed to the pulmonary artery, completely bypassing the right atrium and the right ventricle. The lateral tunnel Fontan operation and the extracardiac Fontan operation have largely replaced the atriopulmonary connection in patients with a single ventricle and have resulted in improvements in hemodynamic and arrhythmia outcomes.33
The onset of atrial arrhythmias in patients with atriopulmonary Fontan repairs often prompts evaluation for conversion to a cavopulmonary Fontan repair. The risks of a repeat operation need to be balanced against the benefit of Fontan conversion and individualized for each patient. In experienced centers, conversion to a total cavopulmonary Fontan repair in patients with preserved left ventricular function has been associated with reduced long-term morbidity and mortality rates. Concomitant arrhythmia surgery is recommended with a modified right-sided Maze procedure in patients with IART or atrial flutter.34 Some have recommended the Maze as a prophylactic procedure in those undergoing Fontan conversion regardless of prior arrhythmias. A standard lesion set involves cryoablation lesions applied at the time of Fontan conversion connecting the superior atrial septum to the right atrial appendage, connecting the posterior atrial septum to the incised posterolateral atrial wall transecting the crista terminalis, and a cavotricuspid isthmus ablation.35,36 Lesion sets may need to be modified depending on the underlying anatomic substrate. Despite these remarkable technical advancements, atrial arrhythmias remain a common problem in this population.
Nearly half the adult patients with congenital heart disease who have undergone a Fontan operation will be seen to have atrial arrhythmias in follow-up.37 These arrhythmias include AF, atrial flutter, IART, and SVT. The presence of AV valvular regurgitation is associated with atrial tachyarrhythmias and the development of Fontan obstruction; therefore a hemodynamic evaluation often is warranted in any patient with a new atrial arrhythmia after a Fontan repair. Given the lack of active transport within the right atrium and the presence of prosthetic material in these patients, atrial arrhythmias should be anticoagulated with warfarin.5 Once diagnosed, atrial arrhythmias tend to recur in these patients. Concomitant sinus node dysfunction as well as AV conduction disease can complicate medical therapy for tachyarrhythmias, making a percutaneous ablative approach very appealing in patients who have not undergone surgery.
Intra-atrial Re-entrant Tachycardia
Although typical isthmus-dependent right atrial flutter revolving around the tricuspid valve is commonly seen in patients who have had the Fontan operation, multiple re-entrant and non–re-entrant circuits are often present.38 IART seen in patients who have had the Fontan operation is often caused by a combination of the atrial barriers created at the time of surgery and progressive atrial scarring. Risk factors for IART include older age at the time of surgery, sinus node dysfunction, and abnormal right atrial and ventricular hemodynamics.37 The combination of atrial enlargement, atrial scarring with resultant slow conduction, and the use of antiarrhythmic drug therapy can often lead to slow IARTs with the potential for 1 : 1 AV conduction or increased heart rates that can cause significant hemodynamic deterioration, especially in poorly functioning single or systemic right ventricles.
Antiarrhythmic therapy for IART has been disappointing, even when potent antiarrhythmic agents such as amiodarone are used. Many centers now consider catheter ablation for IART as an early therapy depending on the underlying substrate. In patients who have had the Fontan operation, if percutaneous access to the right atrium can be obtained, catheter ablation for IART can be reasonably successful, with acute efficacy ranging from 60% to 80% but long-term recurrence rates of approximately 40%.39 Patients who have had the Fontan operation with a lateral tunnel may require a surgical approach; however, trans-baffle puncture into the native right atrium is an option for experienced surgeons. Catheter-based treatment of IART requires careful definition of the anatomic circuit. Locating scar material within the atrium usually allows the identification of a critical isthmus between the anatomic barriers and the creation of a linear ablation lesion between these barriers to interrupt the tachycardia circuit.40 The use of three-dimensional electroanatomic mapping along with entrainment is critical for defining the circuit and the anatomic barriers more completely.41–42
Mustard and Senning Repairs for D-Transposition of the Great Arteries
Only 40% to 50% of patients will remain in sinus rhythm 15 to 20 years after a Mustard repair.43,44 Both bradycardias and tachycardias develop over time in this group. Sinus node dysfunction is thought to be related to progressive scarring near the suture lines in the high right atrium–SVC junction or to damage to the sinus node artery during surgery. Patients with postoperative sinus node dysfunction after intra-atrial repairs present most commonly with exercise intolerance, fatigue, presyncope, or syncope. In addition, the long-short cycles created by ectopy in the setting of sinus node dysfunction can precipitate re-entrant atrial arrhythmias such as typical atrial flutter or IART.45,46 AV node conduction abnormalities are relatively rare in this population, which allows the use of single-chamber atrial lead pacemakers if AV nodal function is determined to be normal.
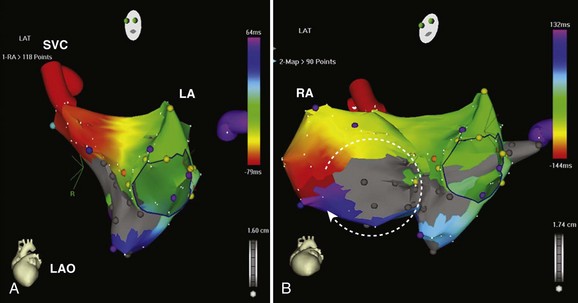
IART is the most common arrhythmia seen after atrial-level repair of D-TGA. Despite the multiple, complex incisions required for the Mustard or the Senning repair, atrial flutter rotating around the tricuspid valve, similar to cavotricuspid isthmus–dependent right atrial flutter, remains the most common form if IART seen.47 IART along the lateral right atrium can also be seen and can be distinguished from peri–tricuspid valve flutter by using entrainment mapping. Given the complicated anatomy and the risks of invasive catheter-based therapy, treatment with antiarrhythmic drugs is often first-line therapy in patients with D-TGA who have atrial arrhythmias after the Mustard or the Senning repair, but this can be limited by concomitant sinus node dysfunction. However, for recurrent or refractory atrial flutters, electrophysiological mapping and ablation can be effective. In cavotricuspid isthmus–dependent atrial flutter, the circuit revolves around the tricuspid valve annulus, which lies anterior to the right atrial baffle suture line and typically is not accessible from the caval venous system. The tricuspid annulus usually requires a retrograde approach via the aortic valve (Figures 79-4 and 79-5). However, a trans-baffle approach can be used via pre-existing baffle leaks or a lateral puncture of the baffle.48 As noted above, access to the coronary sinus during EPSs may be either via the systemic or the pulmonary venous atria and may not be easily entered. IARTs frequently occur in patients with D-TGA and usually involve an activation wavefront passing between the lateral right atrial incision and the tricuspid annulus. AV nodal re-entry tachycardia (AVNRT) has also been described in this population.49,50
Patients with atrial-level repair of transposition remain at risk for sudden cardiac death (SCD) from ventricular arrhythmias as well as from supraventricular arrhythmias with rapid AV conduction. Awareness of the risk for sustained VT in long-term follow-up—previously believed to be a rare occurrence—has increased, with a prevalence reported to be 10%. Risk factors for SCD or VT include systemic ventricular dysfunction or the presence of additional lesions such as VSD or ventricular outflow obstruction.51,52 The presence of atrial arrhythmias or a QRS duration greater than 140 ms has been found to be predictive of sudden death in some studies but not others.51,52 Defibrillator implantation in this population is left to the discretion of the treating physician. Programmed electrical stimulation in these patients has not gained favor. Most devices are being implanted in the highest risk patients as secondary prophylaxis against SCD or a high burden of nonsustained VT (see below).53
Tetralogy of Fallot
TOF is the most common cyanotic congenital heart condition, comprising approximately 10% of the ACHD patient group.54 Adult patients with a history of surgical repair of TOF now have excellent overall long-term survival; however, they remain at risk for atrial and ventricular arrhythmias and SCD (1% to 2.5% per decade).55–56 Fortunately, they are among the most studied group of patients with congenital heart disease. Arrhythmias in patients with TOF primarily appear to arise from the right ventricular substrate as a result of surgical incisions, suture lines, patch insertion, and resection of muscular obstruction at the time of operative repair, often in association with both right and left ventricular dysfunction and residual hemodynamic abnormalities.
The morphologic abnormalities in unrepaired TOF are the presence of some degree of right ventricular infundibular stenosis, a VSD, an overriding aorta, and concomitant right ventricular hypertrophy. Many patients have small pulmonary valves and infundibular stenosis, and some may have peripheral pulmonary artery stenosis.57 The original surgical approach to repair of TOF involved a right ventriculotomy to access the VSD and infundibular stenosis. A trans-annular patch (see Figure 79-2) often is needed to augment the outflow tract, which results in pulmonic regurgitation. This approach has resulted in a significant risk of clinical arrhythmias and SCD. Risk factors have included right ventricular volume and pressure overload. In the current era, surgery at an earlier age with a combined trans-atrial/trans–pulmonary artery approach has led to a reduction, although not elimination, of late ventricular arrhythmias.58
Atrial Arrhythmias
Atrial arrhythmias occur in approximately one third of patients in long-term follow-up after repaired TOF and may be a cause of significant morbidity and even SCD when rapid AV conduction is present.59 Moderately elevated heart rates are poorly tolerated in the presence of residual hemodynamic abnormalities or poor ventricular function. Prolonged QRS duration longer than 160 ms is a risk factor for supraventricular arrhythmias after late reoperation for TOF.60 The QRS width is considered a surrogate marker for right ventricular pressure and volume overload as well as increased right atrial pressure and stretch. Additional risk factors for atrial arrhythmias include older age at repair and moderate or severe tricuspid regurgitation.61
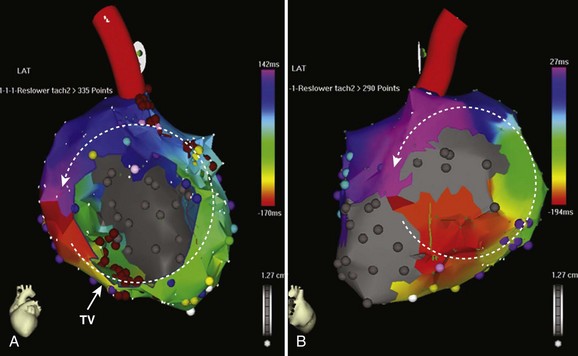
Similar to other congenital lesions with a surgical approach that involves a right atriotomy, the most common atrial arrhythmias include cavotricuspid isthmus–dependent atrial flutter or IART around the lateral right atrial atriotomy scar, occasionally occurring in a figure-of-8 pattern (see Figures 79-4 and 79-5). In contrast to other more complex ACHD lesions, mapping and ablation of these flutters often is more straightforward and can now be considered primary therapy. Ablation of the tricuspid annular circuit and the atriotomy circuit should be performed to minimize recurrences and the need for long-term antiarrhythmic therapy. The tricuspid annular circuit is ablated in the standard fashion, with a linear lesion placed between the IVC and the tricuspid annulus. The posterior atriotomy can be identified by wide split potentials along the posterolateral right atrium. In the case of re-entry around the atriotomy scar, an ablation line can be empirically drawn from the posterior atriotomy scar to the level of the IVC or the tricuspid annulus. Although typically extending the atriotomy incision inferiorly will result in tachycardia termination, the use of noninducibility as the sole endpoint in the early years of IART ablation led to suboptimal long-term efficacy.62 Achieving conduction block across lines of ablation has led to improved arrhythmia control in the long term.63 High output pacing should also be performed along the intended ablation path in the lateral atrium to exclude phrenic nerve capture because the phrenic nerve often is found in this region and can be damaged with ablation.
Ventricular Tachycardia
Surgical repair of TOF relieves RVOT; however, as patients age, progressive pulmonary regurgitation inevitably occurs. The ensuing right ventricular dilation, together with the surgical incisions, scarring, and fibrosis, can lead to the substrate for VT. Approximately 10% to 15% of patients with repaired TOF will have VT during long-term follow-up.61 Sustained VT in a patient with repaired TOF should always prompt a full hemodynamic assessment. Surgical intervention has classically been considered in the case of progressive pulmonic insufficiency or marked right ventricular dilation.5 However, in a retrospective study of patients with TOF undergoing pulmonary valve (PV) replacement, no reduction in VT or SCD was seen compared with a matched group of patients who did not undergo valve replacement.64 However, the group of patients undergoing PV replacement in this retrospective study had larger right ventricle dimensions (RV end diastolic volume index 196 ± 76 vs. 132 ± 38 mL/m2; P < .001) compared with the conservatively managed control group with a similar mortality outcome. Therefore an argument could be made that PV replacement may have been beneficial. We believe that the decision to pursue PV replacement should be based on hemodynmic and right ventricle volume measurements. If surgical valve replacement is to be performed, electroanatomic mapping and ablation of VT before surgery still have a role because the tachycardia circuit and critical isthmuses supporting VT can often be more easily mapped in the electrophysiology laboratory. In the case of unsuccessful catheter ablation, cryoablation can be performed in the operating room on the basis of the mapped tachycardia circuit. The advent of percutaneous PV repair may reduce or eliminate the need for open operative PV replacement. Catheter ablation of VT before percutaneous valve insertion should also be considered because the presence of valve conduit material may limit the efficacy of ablation near the PV.
The VT circuits in repaired TOF typically involve isthmuses between the right ventriculotomy incision or patch, the VSD patch and the tricuspid and PV annuli (see Figure 79-2). The ECG morphology of VT in these patients is variable but typically has either a left bundle morphology with an inferior axis and late precordial transition if activation occurs in a clockwise direction around the right ventricular free wall or a right bundle branch superior axis pattern in the case of counterclockwise activation of the right ventricular free wall.65 VT can also occur between the VSD patch and the tricuspid annulus and typically results in a left bundle inferior axis morphology with an early precordial transition. Entrainment often indicates a broad wavefront of activation, incorporating much of the free wall of the right ventricle. Ablation typically requires delivery of linear pattern of lesions between the RVOT patch and the tricuspid annulus or the VSD patch. Ablation through areas of late potentials can also be important in slowing and terminating the tachycardia circuit. Because of the heavy fibrosis and scarring associated with the outflow tract repair, ablation with conventional 4-mm or 8-mm tip catheters can be challenging. Irrigated tip catheters should be used when available.66 In the case of a poorly tolerated VT, areas of scar or electrically unexcitable tissue can be identified and the barriers connected with ablation to interrupt the tachycardia circuit. With this approach, successful ablation can be performed even in sinus rhythm (Figure 79-6). Documentation of conduction block across the linear pattern of lesions can be difficult to achieve but is important for reducing recurrences after ablation.67,68 Ablation of VT in repaired TOF has a high acute success rate and will likely be applied on a wider scale to this population in the future.69
In patients with repaired TOF without VT, programmed ventricular stimulation can be used to aid risk stratification of SCD. The largest study to date combined data from 252 patients with repaired TOF and noninvasive risk factors for SCD.70 One third of patients were inducible for monomorphic VT at EPS, and 4.4% were inducible for polymorphic VT. Inducibility for either monomorphic or polymorphic VT had 78% sensitivity and 80% specificity for the combined endpoint of sustained VT or SCD over a 6.5-year follow-up, with a 92% negative predictive value. The presence of inducible polymorphic VT had added value to inducible monomorphic VT, improving sensitivity with only a small decrease in specificity. Whether programmed stimulation should be performed routinely in the otherwise asymptomatic patient with TOF with a wide QRS remains controversial. We reserve invasive electrophysiological evaluation for those patients with evidence of nonsustained VT (NSVT) on a yearly screening Holter monitor, left ventricular dysfunction, presyncope, or syncope. Other noninvasive risk markers of SCD in patients with TOF are discussed in detail in the section on defibrillator therapy.
Device Therapy
When pacemaker or defibrillator implantation is considered in a patient with ACHD, pre-procedural planning is critical. Venous access is often not straightforward, especially with heterotaxy syndromes, and in patients in whom Fontan repairs have been performed. Nonstandard routes of access, such as a trans-hepatic approach or, more commonly, an epicardial surgical approach, may be needed in many patients.71 Intracardiac shunts may necessitate closure before device placement, anticoagulation, or a surgical approach to device placement. Venous or intra-atrial baffle obstruction may require dilation and stent placement before the placement of leads. Concomitant minor vascular anomalies that impact on device placement, such as the presence of a left-sided SVC, may also be present. Left-arm peripheral venography, echocardiography, catheterization, CTA, MRA, or a combination of these may be required before device implantation.
Pacing
Sinus node dysfunction often is seen in patients with ACHD who have had repairs that require surgery near the sinus node such as D-TGA with intra-atrial repairs or in predisposed patients, such as those with heterotaxy syndromes and sinus venosus ASDs. In patients who have undergone a Fontan repair, sinus node dysfunction or AV conduction disturbances, often worsened by the effects of drug therapy, may necessitate pacing. Access to the ventricle is always prohibited, but depending on the type of Fontan repair, limited transvenous access to the atrium may exist as well. Therefore epicardial pacing wires often are placed at the time of surgery if the need for atrial pacing is anticipated. Even in patients with older-generation repairs, transvenous atrial lead placement is not desirable in the presence of any right-to-left atrial shunting because of the risk of systemic embolism.72 In addition, the low-flow state in the Fontan atrium can result in thrombus formation even when no lead is present.
Defibrillator Therapy
Patients with ACHD and sustained VT or abrupt syncope should be considered for implantation of an ICD if they have a reasonable life expectancy (>1 year). This secondary prophylaxis group has been found to have a high rate of appropriate ICD shocks in follow-up.73 Prophylactic defibrillator insertion in the setting of depressed ventricular function is a more controversial issue and has little prospective supportive data in the literature.74,75
The role of programmed electrical stimulation (PES) for risk stratification in the general ACHD population remains unclear. As previously mentioned, patients with repaired TOF comprise the most studied group.70 In one study, a large group of both asymptomatic patients with ACHD and NSVT and symptomatic patients with varied lesions underwent PES, with somewhat conflicting results. Although inducibility was shown to be significantly associated with an increased risk of death in follow-up (hazard ratio, 6.1), a significant false-negative rate was seen in patients with documented VT. In addition, the studies were frequently complicated by both bradyarrhythmias and atrial arrhythmias.76
Endocardial device placement can be surprisingly straightforward in the patient with D-TGA after the Mustard or the Senning repair; however, careful pre-procedure planning is required. An evaluation with CTA, MRA, or cardiac catheterization should be performed to assess potential baffle stenosis, obstruction, or leaks. SVC baffle obstruction often is asymptomatic and may require stenting before device insertion. Baffle leaks can be either left to right or right to left, especially if subsequent baffle obstruction develops. Leaks should always be repaired before device insertion to prevent systemic thromboembolism from debris on device leads; this can typically be performed with percutaneous closure devices. Warfarin can also been used in patients requiring device therapy who have unrepairable baffle leaks, although this remains controversial.72 It should always be noted that the presence of a left-sided SVC may require modification of the implantation approach. During atrial lead insertion, care should be taken to avoid placement of the device into the left atrial appendage, which usually is the region most easily approached from the SVC baffle. Far-field oversensing of ventricular signals and phrenic nerve capture can be problematic in this area. Ventricular leads are placed via the baffle and the mitral valve into the systemic venous, left ventricle (Figure 79-7). When implanting defibrillator leads, a single coil lead is recommended to avoid additional risk of SVC baffle obstruction, which is not uncommon in these patients.
Patients with repaired or nonrepaired TOF are at risk for SCD. Numerous studies have examined the risk factors for future SCD with somewhat mixed results. Generally agreed-on risk factors include a QRS width greater than 180 ms, older age at the time of repair, presence of a trans-annular RVOT patch that permits free pulmonic regurgitation, frequent ventricular ectopy on Holter monitoring, increased right ventricular systolic pressure and volume, and complete heart block.61,77 The presence of left ventricular systolic dysfunction has also recently been shown to be an independent predictor of SCD in patients with repaired TOF.78 Although the majority of ventricular arrhythmias are still thought to arise in the right ventricle, it is possible that they are more poorly tolerated in the setting of bi-ventricular failure.
Despite these studies, no discrete algorithm exists for primary defibrillator implantation in the patient with repaired TOF. The authors of this chapter typically perform yearly Holter monitoring to screen for ventricular arrhythmias. As stated above, invasive electrophysiological evaluation is done in those patients with NSVT, left ventricular dysfunction, presyncope, or syncope. Asymptomatic patients should have defibrillators implanted if they have inducible VT or are deemed to be at particularly high risk, on the basis of the above criteria.79 Among patients with ACHD, patients with TOF who have defibrillators represent the largest group, with approximately half the devices implanted for primary indications and half for secondary indications.80 Patients with TOF have a reported rate of appropriate ICD therapy it (approximately 10% per year in one study), which would seem to justify it, but these patients have also been reported to have inappropriate shocks and device-related complications.81,82
Cardiac Resynchronization Therapy
Cardiac resynchronization therapy (CRT) has been described in adults with bundle branch block, left ventricular dysfunction, and congestive heart failure. The electrical dyssynchrony of bundle branch block can worsen ventricular function in patients with congestive heart failure. Placement of pacing leads in the right and left ventricles (via the coronary sinus) can improve symptoms of heart failure by “resynchronizing” the ventricles by atrial synchronous biventricular pacing. Biventricular pacemakers have been proven to reduce symptoms, improve left ventricular function, and perhaps even reduce mortality in adults with heart failure and bundle branch block.83–87
CRT has been described in patients with ACHD who have heart failure and bundle branch block, but its role in the population as a whole is unclear. The contribution of conduction system disease to ventricular dyssynchrony in patients with congenital heart disease is complex, especially in those with single-ventricle physiology, and the presence of bundle branch block may be seen in the absence of electrical or mechanical dyssynchrony. The benefit of bi-ventricular pacing in patients with ACHD who have congestive heart failure and bundle branch block is based on several small case series.88 Of particular interest is the patient with a systemic right ventricle. In a patient with transposition who has undergone the Mustard or the Senning repair and has RBBB with systemic right ventricular dysfunction, the coronary sinus travels its usual course along the left AV groove. Therefore a systemic right ventricular lead must be placed either epicardially during a repeat operative procedure or endocardially through the atrial baffle, which we do not favor because of the risk of systemic emboli. The risk of a repeat operation is not small and should be weighed against the potential benefit of resynchronization therapy. Such a procedure may be useful in selected patients. CRT is also being investigated for the prevention of systemic right ventricular failure.89 Although dramatic improvement in cardiac function has been reported, the role of this therapy as primary prevention remains unproven.
One special population worthy of comment is the patient with poor ventricular function who has undergone Fontan repair. The modified Fontan procedure remains a palliative procedure in patients with single-ventricle physiology. Patients who have had Fontan repair remain at risk for progressive ventricular dysfunction, congestive heart failure, and SCD. Multiple-site ventricular pacing via epicardial leads placed surgically on the system ventricle has been shown to improve the immediate postoperative course after the Fontan procedure.90 Some evidence in small series has shown some benefit of the Fontan repair for patients with severe ventricular dysfunction or those awaiting cardiac transplantation.91 However, the potential benefit should be weighed against the risk of repeat sternotomy for epicardial lead placement because the subxyphoid approach rarely gives adequate visualization to allow multiple-site lead placement.
1 Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39(12):1890.
2 Warnes CA, Liberthson R, Danielson GK, et al. Task force 1: The changing profile of congenital heart disease in adult life. J Am Coll Cardiol. 2001;37(5):1170.
3 Congenital heart defects. http://www.nhlbi.nih.gov/health/dci/Diseases/chd/chd_what.html, 2009. Available at Accessed July 2, 2009
4 Walsh EP, Cecchin F. Arrhythmias in adult patients with congenital heart disease. Circulation. 2007;115(4):534.
5 Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;52(23):e1.
6 Opotowsky AR, Siddiqi OK, Webb GD. Trends in hospitalizations for adults with congenital heart disease in the U.S. J Am Coll Cardiol. 2009;54(5):460.
7 Ai T, Ikeguchi S, Watanuki M, et al. Successful radiofrequency current catheter ablation of accessory atrioventricular pathway in Ebstein’s anomaly using electroanatomic mapping. Pacing Clin Electrophysiol. 2002;25(3):374.
8 Kanter RJ. Pearls for ablation in congenital heart disease. J Cardiovasc Electrophysiol. 2010;21(2):223.
9 Khairy P, Triedman JK, Juraszek A, Cecchin F. Inability to cannulate the coronary sinus in patients with supraventricular arrhythmias: Congenital and acquired coronary sinus atresia. J Interv Card Electrophysiol. 2005;12(2):123.
10 Fish FA, Gillette PC, Benson DWJr. Proarrhythmia, cardiac arrest and death in young patients receiving encainide and flecainide. The Pediatric Electrophysiology Group. J Am Coll Cardiol. 1991;18(2):356.
11 Kober L, Thomsen PE, et al. Effect of dofetilide in patients with recent myocardial infarction and left-ventricular dysfunction: A randomised trial. Lancet. 2000;356(9247):2052.
12 Hohnloser SH, Crijns HJ, van Eickels M, et al. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med. 2009;360(7):668.
13 Soejima K, Stevenson WG, Maisel WH, et al. Electrically unexcitable scar mapping based on pacing threshold for identification of the reentry circuit isthmus: Feasibility for guiding ventricular tachycardia ablation. Circulation. 2002;106(13):1678.
14 Lukac P, Pedersen AK, Mortensen PT, et al. Ablation of atrial tachycardia after surgery for congenital and acquired heart disease using an electroanatomic mapping system: Which circuits to expect in which substrate? Heart Rhythm. 2005;2(1):64.
15 Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline update for implantation of cardiac pacemakers and antiarrhythmia devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51(21):e1.
16 Gatzoulis MA, Freeman MA, Siu SC, et al. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med. 1999;340(11):839.
17 Roberts-Thomson KC, John B, Worthley SG, et al. Left atrial remodeling in patients with atrial septal defects. Heart Rhythm. 2009;6(7):1000.
18 Mantovan R, Gatzoulis MA, Pedrocco A, et al. Supraventricular arrhythmia before and after surgical closure of atrial septal defects: Spectrum, prognosis and management. Europace. 2003;5(2):133.
19 Boston US, Dearani JA, O’Leary PW, et al. Tricuspid valve repair for Ebstein’s anomaly in young children: A 30-year experience. Ann Thorac Surg. 2006;81(2):690. discussion 5–6
20 Watson H. Natural history of Ebstein’s anomaly of tricuspid valve in childhood and adolescence. An international co-operative study of 505 cases. Br Heart J. 1974;36(5):417.
21 Kastor JA, Goldreyer BN, Josephson ME, et al. Electrophysiologic characteristics of Ebstein’s anomaly of the tricuspid valve. Circulation. 1975;52(6):987.
22 Delhaas T, Sarvaas GJ, Rijlaarsdam ME, et al. A multicenter, long-term study on arrhythmias in children with Ebstein anomaly. Pediatr Cardiol. 2010;31(2):229.
23 Smith WM, Gallagher JJ, Kerr CR, et al. The electrophysiologic basis and management of symptomatic recurrent tachycardia in patients with Ebstein’s anomaly of the tricuspid valve. Am J Cardiol. 1982;49(5):1223.
24 Vora AM, Lokhandwala YY. Preexcited tachycardia in a patient with Ebstein’s anomaly: Is the preexcitation manifest during sinus rhythm? J Cardiovasc Electrophysiol. 1998;9(9):1012.
25 Celermajer DS, Bull C, Till JA, et al. Ebstein’s anomaly: Presentation and outcome from fetus to adult. J Am Coll Cardiol. 1994;23(1):170.
26 Brown ML, Dearani JA, Danielson GK, et al. Functional status after operation for Ebstein anomaly: The Mayo Clinic experience. J Am Coll Cardiol. 2008;52(6):460.
27 Iturralde P, Nava S, Salica G, et al. Electrocardiographic characteristics of patients with Ebstein’s anomaly before and after ablation of an accessory atrioventricular pathway. J Cardiovasc Electrophysiol. 2006;17(12):1332.
28 Chetaille P, Walsh EP, Triedman JK. Outcomes of radiofrequency catheter ablation of atrioventricular reciprocating tachycardia in patients with congenital heart disease. Heart Rhythm. 2004;1(2):168.
29 Shah MJ, Jones TK, Cecchin F. Improved localization of right-sided accessory pathways with microcatheter-assisted right coronary artery mapping in children. J Cardiovasc Electrophysiol. 2004;15(11):1238.
30 Bertram H, Bokenkamp R, Peuster M, et al. Coronary artery stenosis after radiofrequency catheter ablation of accessory atrioventricular pathways in children with Ebstein’s malformation. Circulation. 2001;103(4):538.
31 Lo HM, Lin FY, Jong YS, et al. Ebstein’s anomaly with ventricular tachycardia: Evidence for the arrhythmogenic role of the atrialized ventricle. Am Heart J. 1989;117(4):959.
32 Obioha-Ngwu O, Milliez P, Richardson A, et al. Ventricular tachycardia in Ebstein’s anomaly. Circulation. 2001;104(18):E92.
33 Stamm C, Friehs I, Mayer JEJr, et al. Long-term results of the lateral tunnel Fontan operation. J Thorac Cardiovasc Surg. 2001;121(1):28.
34 Mavroudis C, Backer CL, Deal BJ, et al. Evolving anatomic and electrophysiologic considerations associated with Fontan conversion. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2007:136.
35 Mavroudis C, Backer CL, Deal BJ, et al. Total cavopulmonary conversion and maze procedure for patients with failure of the Fontan operation. J Thorac Cardiovasc Surg. 2001;122(5):863.
36 Deal BJ, Mavroudis C, Backer CL, et al. Comparison of anatomic isthmus block with the modified right atrial maze procedure for late atrial tachycardia in Fontan patients. Circulation. 2002;106(5):575.
37 Ghai A, Harris L, Harrison DA, et al. Outcomes of late atrial tachyarrhythmias in adults after the Fontan operation. J Am Coll Cardiol. 2001;37(2):585.
38 Triedman JK. Atypical atrial tachycardias in patients with congenital heart disease. Heart Rhythm. 2008;5(2):315.
39 Triedman JK, Alexander ME, Love BA, et al. Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 2002;39(11):1827.
40 Kalman JM, VanHare GF, Olgin JE, et al. Ablation of “incisional” reentrant atrial tachycardia complicating surgery for congenital heart disease. Use of entrainment to define a critical isthmus of conduction. Circulation. 1996;93(3):502.
41 Triedman JK, Alexander ME, Berul CI, et al. Electroanatomic mapping of entrained and exit zones in patients with repaired congenital heart disease and intra-atrial reentrant tachycardia. Circulation. 2001;103(16):2060.
42 Delacretaz E, Ganz LI, Soejima K, et al. Multi- atrial maco-re-entry circuits in adults with repaired congenital heart disease: Entrainment mapping combined with three-dimensional electroanatomic mapping. J Am Coll Cardiol. 2001;37(6):1665.
43 Deanfield J, Camm J, Macartney F, et al. Arrhythmia and late mortality after Mustard and Senning operation for transposition of the great arteries. An eight-year prospective study. J Thorac Cardiovasc Surg. 1988;96(4):569.
44 Puley G, Siu S, Connelly M, et al. Arrhythmia and survival in patients >18 years of age after the mustard procedure for complete transposition of the great arteries. Am J Cardiol. 1999;83(7):1080.
45 Vetter VL, Tanner CS, Horowitz LN. Inducible atrial flutter after the Mustard repair of complete transposition of the great arteries. Am J Cardiol. 1988;61(6):428.
46 Anand N, McCrindle BW, Chiu CC, et al. Chronotropic incompetence in young patients with late postoperative atrial flutter: A case-control study. Eur Heart J. 2006;27(17):2069.
47 Khairy P, Van Hare GF. Catheter ablation in transposition of the great arteries with Mustard or Senning baffles. Heart Rhythm. 2009;6(2):283.
48 El Yaman MM, Asirvatham SJ, Kapa S, et al. Methods to access the surgically excluded cavotricuspid isthmus for complete ablation of typical atrial flutter in patients with congenital heart defects. Heart Rhythm. 2009;6(7):949.
49 Kanter RJ, Papagiannis J, Carboni MP, et al. Radiofrequency catheter ablation of supraventricular tachycardia substrates after mustard and senning operations for d-transposition of the great arteries. J Am Coll Cardiol. 2000;35(2):428.
50 Greene AE, Skinner JR, Dubin AM, et al. The electrophysiology of atrioventricular nodal reentry tachycardia following the Mustard or Senning procedure and its radiofrequency ablation. Cardiol Young. 2005;15(6):611.
51 Kammeraad JA, van Deurzen CH, Sreeram N, et al. Predictors of sudden cardiac death after Mustard or Senning repair for transposition of the great arteries. J Am Coll Cardiol. 2004;44(5):1095.
52 Schwerzmann M, Salehian O, Harris L, et al. Ventricular arrhythmias and sudden death in adults after a Mustard operation for transposition of the great arteries. Eur Heart J. 2009;30(15):1873.
53 Khairy P, Harris L, Landzberg MJ, et al. Sudden death and defibrillators in transposition of the great arteries with intra-atrial baffles: A multicenter study. Circ Arrhythm Electrophysiol. 2008;1(4):250.
54 Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. Second of two parts. N Engl J Med. 2000;342(5):334.
55 Murphy JG, Gersh BJ, Mair DD, et al. Long-term outcome in patients undergoing surgical repair of tetralogy of Fallot. N Engl J Med. 1993;329(9):593.
56 Silka MJ, Hardy BG, Menashe VD, Morris CD. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol. 1998;32(1):245.
57 Gatzoulis MA. Tetralogy of Fallot. In: Gatzoulis MA, Webb G, Daubeney PEF, editors. Diagnosis and management of adult congenital heart disease. Philadelphia: Churchill Livingston, 2003.
58 Karl TR, Sano S, Pornviliwan S, Mee RB. Tetralogy of Fallot: Favorable outcome of nonneonatal transatrial, transpulmonary repair. Ann Thorac Surg. 1992;54(5):903.
59 Roos-Hesselink J, Perlroth MG, McGhie J, Spitaels S. Atrial arrhythmias in adults after repair of tetralogy of Fallot. Correlations with clinical, exercise, and echocardiographic findings. Circulation. 1995;91(8):2214.
60 Karamlou T, Silber I, Lao R, et al. Outcomes after late reoperation in patients with repaired tetralogy of Fallot: The impact of arrhythmia and arrhythmia surgery. Ann Thorac Surg. 2006;81(5):1786. discussion 93
61 Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: A multicentre study. Lancet. 2000;356(9234):975.
62 Triedman JK, Bergau DM, Saul JP, et al. Efficacy of radiofrequency ablation for control of intraatrial reentrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 1997;30(4):1032.
63 Nakagawa H, Shah N, Matsudaira K, et al. Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: Isolated channels between scars allow “focal” ablation. Circulation. 2001;103(5):699.
64 Harrild DM, Berul CI, Cecchin F, et al. Pulmonary valve replacement in tetralogy of Fallot: Impact on survival and ventricular tachycardia. Circulation. 2009;119(3):445.
65 Horton RP, Canby RC, Kessler DJ, et al. Ablation of ventricular tachycardia associated with tetralogy of Fallot: Demonstration of bidirectional block. J Cardiovasc Electrophysiol. 1997;8(4):432.
66 Calkins H, Epstein A, Packer D, et al. Catheter ablation of ventricular tachycardia in patients with structural heart disease using cooled radiofrequency energy: Results of a prospective multicenter study. Cooled RF Multi Center Investigators Group. J Am Coll Cardiol. 2000;35(7):1905.
67 Zeppenfeld K, Schalij MJ, Bartelings MM, et al. Catheter ablation of ventricular tachycardia after repair of congenital heart disease: Electroanatomic identification of the critical right ventricular isthmus. Circulation. 2007;116(20):2241.
68 Bala R, Dhruvakumar S, Latif SA, Marchlinski FE. New endpoint for ablation of ventricular tachycardia: Change in QRS morphology with pacing at protected isthmus as index of isthmus block. J Cardiovasc Electrophysiol. 2009;21(3):320.
69 Kriebel T, Saul JP, Schneider H, et al. Noncontact mapping and radiofrequency catheter ablation of fast and hemodynamically unstable ventricular tachycardia after surgical repair of tetralogy of Fallot. J Am Coll Cardiol. 2007;50(22):2162.
70 Khairy P, Landzberg MJ, Gatzoulis MA, et al. Value of programmed ventricular stimulation after tetralogy of fallot repair: A multicenter study. Circulation. 2004;109(16):1994.
71 Fishberger SB, Camunas J, Rodriguez-Fernandez H, Sommer RJ. Permanent pacemaker lead implantation via the transhepatic route. Pacing Clin Electrophysiol. 1996;19(7):1124.
72 Khairy P, Landzberg MJ, Gatzoulis MA, et al. Transvenous pacing leads and systemic thromboemboli in patients with intracardiac shunts: A multicenter study. Circulation. 2006;113(20):2391.
73 Berul CI, Van Hare GF, Kertesz NJ, et al. Results of a multicenter retrospective implantable cardioverter-defibrillator registry of pediatric and congenital heart disease patients. J Am Coll Cardiol. 2008;51(17):1685.
74 Silka MJ, Bar-Cohen Y. Should patients with congenital heart disease and a systemic ventricular ejection fraction less than 30% undergo prophylactic implantation of an ICD? Circ Arrhythm Electrophysiol. 2008;1(4):298.
75 Triedman JK. Should patients with congenital heart disease and a systemic ventricular ejection fraction less than 30% undergo prophylactic implantation of an ICD? Implantable cardioverter defibrillator implantation guidelines based solely on left ventricular ejection fraction do not apply to adults with congenital heart disease. Circ Arrhythm Electrophysiol. 2008;1(4):307.
76 Alexander ME, Walsh EP, Saul JP, et al. Value of programmed ventricular stimulation in patients with congenital heart disease. J Cardiovasc Electrophysiol. 1999;10(8):1033.
77 Garson AJr, Nihill MR, McNamara DG, Cooley DA. Status of the adult and adolescent after repair of tetralogy of Fallot. Circulation. 1979;59(6):1232.
78 Ghai A, Silversides C, Harris L, Webb GD, et al. Left ventricular dysfunction is a risk factor for sudden cardiac death in adults late after repair of tetralogy of Fallot. J Am Coll Cardiol. 2002;40(9):1675.
79 Silversides CK, Kiess M, Beauchesne L, et al. Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: Outflow tract obstruction, coarctation of the aorta, tetralogy of Fallot, Ebstein anomaly and Marfan’s syndrome. Can J Cardiol. 2010;26(3):e80.
80 Dore A, Santagata P, Dubuc M, Mercier LA. Implantable cardioverter defibrillators in adults with congenital heart disease: A single center experience. Pacing Clin Electrophysiol. 2004;27(1):47.
81 Yap SC, Roos-Hesselink JW, Hoendermis ES, et al. Outcome of implantable cardioverter defibrillators in adults with congenital heart disease: A multi-centre study. Eur Heart J. 2007;28(15):1854.
82 Khairy P, Harris L, Landzberg MJ, et al. Implantable cardioverter-defibrillators in tetralogy of Fallot. Circulation. 2008;117(3):363.
83 Cazeau S, Leclercq C, Lavergne T, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344(12):873.
84 Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346(24):1845.
85 Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350(21):2140.
86 Saxon LA, De Marco T, Schafer J, et al. Effects of long-term biventricular stimulation for resynchronization on echocardiographic measures of remodeling. Circulation. 2002;105(11):1304.
87 Cleland JG, Daubert JC, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352(15):1539.
88 Cecchin F, Frangini PA, Brown DW, et al. Cardiac resynchronization therapy (and multisite pacing) in pediatrics and congenital heart disease: Five years experience in a single institution. J Cardiovasc Electrophysiol. 2009;20(1):58.
89 Janousek J, Tomek V, Chaloupecky VA, et al. Cardiac resynchronization therapy: A novel adjunct to the treatment and prevention of systemic right ventricular failure. J Am Coll Cardiol. 2004;44(9):1927.
90 Bacha EA, Zimmerman FJ, Mor-Avi V, et al. Ventricular resynchronization by multisite pacing improves myocardial performance in the postoperative single-ventricle patient. Ann Thorac Surg. 2004;78(5):1678.
91 Sojak V, Mazic U, Cesen M, et al. Cardiac resynchronization therapy for the failing Fontan patient. Ann Thorac Surg. 2008;85(6):2136.
Key References
Berul CI, Van Hare GF, Kertesz NJ, et al. Results of a multicenter retrospective implantable cardioverter-defibrillator registry of pediatric and congenital heart disease patients. J Am Coll Cardiol. 2008;51(17):1685.
Cecchin F, Frangini PA, Brown DW, et al. Cardiac resynchronization therapy (and multisite pacing) in pediatrics and congenital heart disease: Five years experience in a single institution. J Cardiovasc Electrophysiol. 2009;20(1):58.
Chetaille P, Walsh EP, Triedman JK. Outcomes of radiofrequency catheter ablation of atrioventricular reciprocating tachycardia in patients with congenital heart disease. Heart Rhythm. 2004;1(2):168.
Fish FA, Gillette PC, Benson DWJr. Proarrhythmia, cardiac arrest and death in young patients receiving encainide and flecainide. The Pediatric Electrophysiology Group. J Am Coll Cardiol. 1991;18(2):356.
Gatzoulis MA, Balaji S, Webber SA, et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: A multicentre study. Lancet. 2000;356(9234):975.
Gatzoulis MA, Freeman MA, Siu SC, et al. Atrial arrhythmia after surgical closure of atrial septal defects in adults. N Engl J Med. 1999;340(11):839.
Ghai A, Harris L, Harrison DA, et al. Outcomes of late atrial tachyarrhythmias in adults after the Fontan operation. J Am Coll Cardiol. 2001;37(2):585.
Kammeraad JA, van Deurzen CH, Sreeram N, et al. Predictors of sudden cardiac death after Mustard or Senning repair for transposition of the great arteries. J Am Coll Cardiol. 2004;44(5):1095.
Khairy P, Harris L, Landzberg MJ, et al. Sudden death and defibrillators in transposition of the great arteries with intra-atrial baffles: A multicenter study. Circ Arrhythm Electrophysiol. 2008;1(4):250.
Khairy P, Van Hare GF. Catheter ablation in transposition of the great arteries with Mustard or Senning baffles. Heart Rhythm. 2009;6(2):283.
Silka MJ, Bar-Cohen Y. Should patients with congenital heart disease and a systemic ventricular ejection fraction less than 30% undergo prophylactic implantation of an ICD? Circ Arrhythm Electrophysiol. 2008;1(4):298.
Silka MJ, Hardy BG, Menashe VD, Morris CD. A population-based prospective evaluation of risk of sudden cardiac death after operation for common congenital heart defects. J Am Coll Cardiol. 1998;32(1):245.
Triedman JK, Alexander ME, Love BA, et al. Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra-atrial re-entrant tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 2002;39(11):1827.
Warnes CA, Williams RG, Bashore TM, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;52(23):e1.