45 Arrhythmias
Although infrequent in the pediatric population, arrhythmias represent potentially significant causes of morbidity and mortality. The diagnosis and management of arrhythmias require an understanding of age-dependent normal variations in heart rate (Table 45-1). This chapter describes the etiology, clinical significance, and treatment options of common arrhythmias found in infants, children, and adolescents, including bradyarrhythmias, tachyarrhythmias, and rhythm disturbances leading to syncope and sudden death.
Table 45-1 Normal Heart Rate for Age
| Age | Heart Rate (beats/min) |
|---|---|
| Newborn | 110-160 |
| 1-6 months | 100-180 |
| 6-12 months | 95-170 |
| 1-3 years | 95-150 |
| 3-5 years | 70-130 |
| 5-8 years | 65-120 |
| 8-12 years | 65-120 |
| 12-16 years | 60-110 |
| >16 years | 60-100 |
Sinus Arrhythmias and Premature Impulses
Bradyarrhythmias
Atrioventricular Conduction Abnormalities
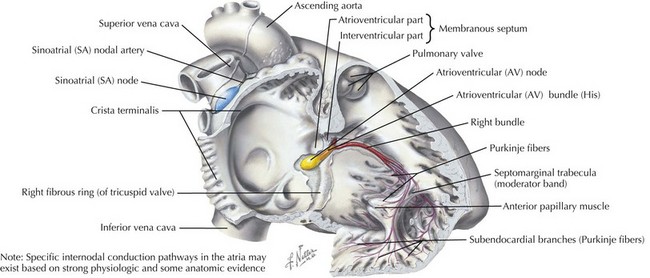
Abnormal AV conduction occurs when transmission of the normal sinus node impulses is delayed or blocked because of an abnormality in the conduction system, specifically the AV node or His-Purkinje system (Figure 45-1).
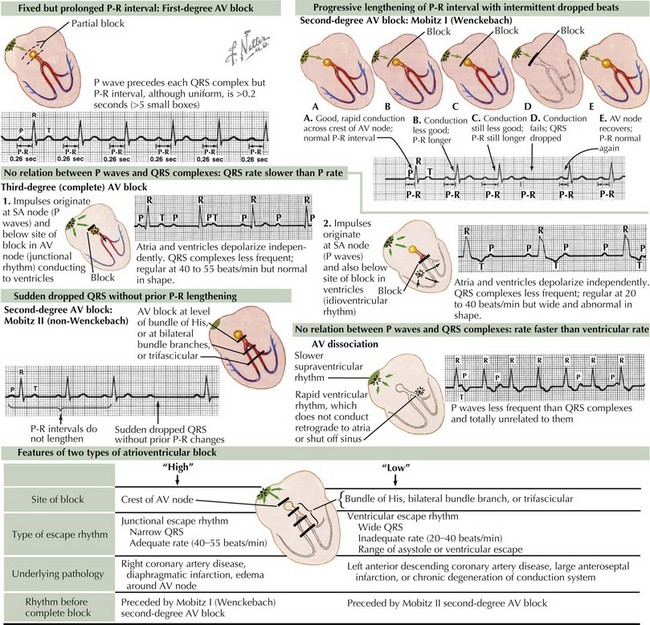
First-Degree Atrioventricular Block
Because of an abnormal delay in conduction through the AV node, first-degree AV block results in prolongation of the PR interval above the upper limits of normal for age and heart rate (Figure 45-2). It is important to note that bradycardia does not occur because of first-degree AV block alone. This type of block can appear in otherwise healthy children as a benign phenomenon, usually related to increased vagal tone. Other causes may include cardiac surgery, rheumatic fever, Lyme disease, digoxin toxicity, and electrolyte imbalance. Isolated first-degree AV block does not require treatment unless there is progression to more advanced AV block.
Second-Degree Atrioventricular Block
Mobitz Type I (Wenckebach phenomenon) occurs at the level of the AV node, yielding progressive lengthening in the PR interval until one QRS complex or ventricular depolarization fails to occur (see Figure 45-2). This may occur in otherwise healthy children with parasympathetic dominance as well as in trained athletes and is frequently observed during monitoring of heart rates during sleep. After confirmation via surface ECG, management should focus on treating any underlying pathologic causes.
Mobitz type II occurs at the level of the bundle of His and is defined as the intermittent loss of AV conduction without preceding lengthening of the PR interval. This form of second-degree AV block is less common and suggests a more serious form of AV conduction disorder in which progression to complete heart block with hemodynamic compromise is more likely. Although similar causes exist between Mobitz type I and type II, consultation with a pediatric cardiologist is indicated (see Figure 45-2).
Third-Degree Atrioventricular Block or Complete Atrioventricular Block
Complete AV block is defined as complete interruption of atrial impulse propagation, resulting in atrial and ventricular activity that is independent of each other (AV dissociation). Surface ECG morphology will show regular P waves at a rate appropriate for age but with QRS complexes occurring at regular and slower rates than the atrial rate with no consistent relationship between P waves and QRS complexes. Both congenital and acquired forms of complete AV block occur and should be suspected in any patient with symptomatic bradycardia (see Figure 45-2).
Tachyarrhythmias
Supraventricular Tachycardias
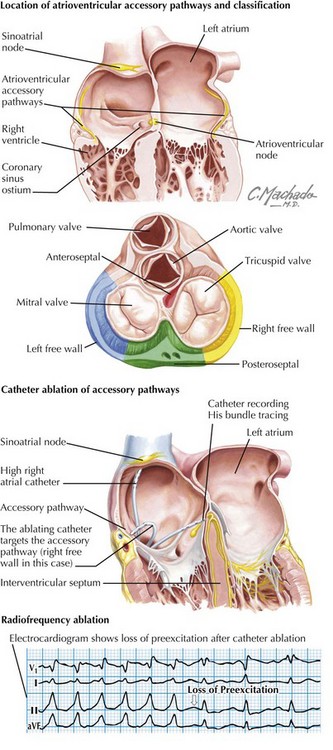
Reentry Tachycardia with an Accessory Pathway
Wolff-Parkinson-White (WPW) syndrome accounts for 10% to 20% of cases of SVT (Figure 45-3). It is diagnosed by the presence of preexcitation (early depolarization via the accessory pathway) on a surface ECG. This is manifested by a short PR, a widened QRS, and a delta wave. WPW may present at any age and can be associated with Ebstein’s anomaly, heterotaxy syndrome, and L-transposition of the great arteries. Sudden death may occur because of rapid ventricular responses with 1 : 1 conduction in the setting of more rapid atrial tachyarrhythmias such as atrial fibrillation. The risk of sudden death in asymptomatic patients incidentally discovered to have WPW is yet unknown, thus making risk stratification a challenge.
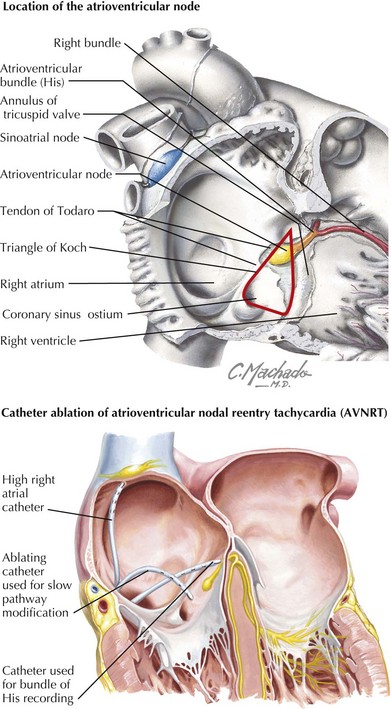
Reentry Tachycardia without an Accessory Pathway
AV nodal reentrant tachycardia (AVNRT) occurs when dual conduction pathways exist within the AV node creating substrate for reentry pathophysiology similar to WPW and other accessory pathway SVT (Figure 45-4). Seen more commonly in older children and adolescents, rates can range from 140 to 200 beats/min.
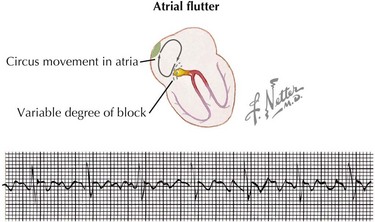
Intraatrial reentrant tachycardia (atrial flutter or fibrillation) is caused by reentrant or “circus” movements within the atria. Atrial flutter usually exhibits regular and regularly irregular intervals with atrial rates greater than 250 beats/min. Variable ventricular responses can be observed because of varying degrees of AV block. Risk factors for atrial flutter include structural heart disease with dilated atria, atrial scarring from prior surgery for congenital heart disease (e.g., Fontan, Mustard, or Senning operations), myocarditis, hyperthyroidism, and digitalis toxicity. ECG reveals rapid and regular atrial sawtoothed flutter waves, which are diagnostic for atrial flutter (Figure 45-5).
Ventricular Tachycardia
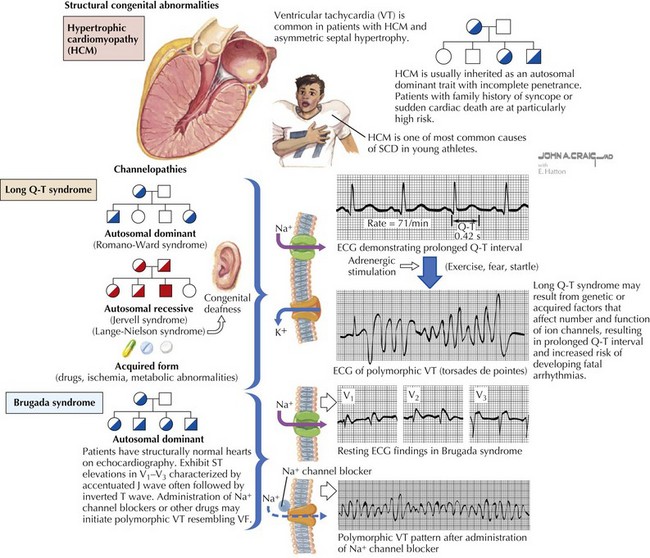
Ventricular tachycardia (VT) is defined as three or more PVCs in series at a heart rate greater than 120 beats/min. These may be self-limited or may present with sudden death (Figure 45-6). Etiologies of VT can be divided into acute and chronic causes. Acute causes include electrolyte imbalance, infections (myocarditis, pericarditis, rheumatic fever), use of toxins or drugs (cocaine, antiarrhythmics, general anesthetics, sympathomimetics, psychotropics), trauma, and myocardial ischemia (infarction, anomalous coronary arteries, Kawasaki disease). Chronic causes of VT include postoperative congenital heart disease (e.g., tetralogy of Fallot), cardiomyopathies (especially hypertrophic cardiomyopathy), tumors or infiltrative processes, primary channelopathies (long QT syndrome, Brugada syndrome), and idiopathic forms. The clinical diagnosis of VT must be distinguished from other arrhythmias that may present with a wide QRS complex tachycardia such as SVT with aberrant conduction. Any wide QRS complex tachycardia, however, should first be considered VT until proven otherwise. Hemodynamically unstable patients with mental status changes or evidence of low cardiac output must be treated promptly with DC synchronized cardioversion. For hemodynamically stable infants and older pediatric patients, intravenous amiodarone and lidocaine are the initial drugs of choice. Ventricular arrhythmias caused by acute etiologies such as electrolyte imbalance, hypoxia, or drug toxicity resolve after the offending abnormality has been corrected. For patients with chronic VT, medical therapies should be aimed at preventing recurrence.
Long Qt Syndromes And Sudden Death
The long QT syndromes are composed of a group of genetic abnormalities created by ion channel mutations that result in abnormal ventricular repolarization. To date, mutations in 12 genes have been identified involving potassium, sodium, and calcium channels. These alterations in conduction properties are associated with malignant ventricular arrhythmias, exercise- and stress-related syncope, and sudden cardiac death (see Figure 45-6). Approximately half of all cases are familial; the remainder are from sporadic mutations. Initial reports consisted of familial studies by Jervell and Lange-Nielson, who described QT prolongation with associated deafness (Jervell-Lange-Nielson syndrome). Romano-Ward also described familial cases of prolonged QT with autosomal dominant transmission and without associated deafness or anomalies (Romano-Ward syndrome).
Ackerman MJ. Molecular basis of congenital and acquired long QT syndromes. J Electrocardiol. 2004;37(suppl):1-6.
Delacretaz E. Supraventricular tachycardia. N Engl J Med. 2006;354:1039-1050.
Epstein AE, Dimarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117(21):e350-e408.
Kaltman JR, Madan N, Vetter VL, Rhodes LA. Arrhythmias and sudden cardiac death. In: Bell LM, Vetter VL, editors. Pediatric Cardiology: The Requisites in Pediatrics. Philadelphia: Elsevier Mosby; 2006:171-194.
Kaltman H, Shah M. Evaluation of the child with an arrhythmia. Pediatr Clin North Am. 2004;51:1537-1551.
Maron BJ. Sudden death in young athletes. N Engl J Med. 2003;349:404-410.
Walsh EP. Clinical Approach to diagnosis and acute management of tachycardias in children. In: Walsh EP, Saul JP, Triedman JK, editors. Cardiac Arrhythmias in Children and Young Adults with Congenital Heart Disease. Philadelphia: Lippincott Williams & Wilkins; 2001:95-113.
Walsh EP, Cecchin F. Recent advances in pacemaker and implantable defibrillator therapy for young patients. Curr Opin Cardiol. 2004;19(2):91-96.