Chapter 260 Arboviral Encephalitis outside North America
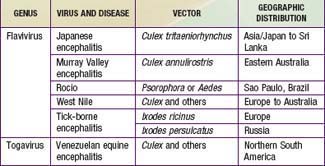
The principal causes of arboviral encephalitis outside North America are Venezuelan equine encephalitis (VEE) virus, Japanese encephalitis (JE) virus, tick-borne encephalitis (TBE), and West Nile (WN) virus (Table 260-1).
260.1 Venezuelan Equine Encephalitis
Treatment
There is no specific treatment for VEE. The treatment is intensive supportive care (Chapter 62), including control of seizures (Chapter 586).
Gubler DJ. The continuing spread of West Nile virus in the western hemisphere. Clin Infect Dis. 2007;45:1039-1046.
Kramer LD, Styer LM, Ebel GD. A global perspective on the epidemiology of West Nile virus. Annu Rev Entomol. 2008;53:61-81.
Siegel-Itzkovich J. Twelve die of West Nile virus in Israel. Br Med J. 2000;321:724.
Tsai TF, Popovici F, Cerescu C, et al. West Nile encephalitis epidemic in southeastern Romania. Lancet. 1998;352:767-771.
260.2 Japanese Encephalitis
Epidemics of encephalitis were reported in Japan from the late 1800s.
Treatment
There is no specific treatment for JE. The treatment is intensive supportive care (Chapter 62), including control of seizures (Chapter 586).
Bista MB, Banerjee MK, Shin SH, et al. Efficacy of a single dose of SA 14–14–2 live-attenuated Japanese encephalitis vaccine: a case-control study. Lancet. 2001;358:791-795.
Gatchalian S, Yao Y, Zhou B, et al. Comparison of the immunogenicity and safety of measles vaccine administered alone or with live-attenuated Japanese encephalitis SA 14–14–2 vaccine in Philippine infants. Vaccine. 2008;26:2234-2241.
Innis BL, Nisalak A, Nimmannitya S, et al. An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg. 1989;40:418-427.
Jones T. IC-51, an injectable vaccine for the prevention of Japanese encephalitis virus infection. Curr Opin Mol Ther. 2009;11:90-96.
Schuller E, Jilma B, Voicu V, et al. Long-term immunogenicity of the new Vero cell-derived, inactivated Japanese encephalitis virus vaccine IC-51: six and 12 month results of a multicenter follow-up phase 3 study. Vaccine. 2008;26:4382-4386.
Tandan JB, Ohrr H, Sohn YM, et al. Single dose of SA 14–14–2 vaccine provides long-term protection against Japanese encephalitis: a case-control study in Nepalese children 5 years after immunization. Vaccine. 2007;25:5041-5045.
Tauber E, Kollaritshe H, von Sonnennburg F, et al. Randomized, double blind, placebo-controlled phase 3 trial of the safety and tolerability of IC51, an inactivated Japanese encephalitis vaccine. J Infect Dis. 2008;198:493-499.
260.3 Tick-Borne Encephalitis
Treatment
There is no specific treatment for TBE. The treatment is intensive supportive care (Chapter 62), including control of seizures (Chapter 586).
Kluger G, Schottler A, Waldvogel K, et al. Tickborne encephalitis despite specific immunoglobulin prophylaxis. Lancet. 1995;346:1502.
McNeil JG, Lednar WM, Stansfield SK, et al. Central European tick-borne encephalitis: assessment of risk for persons in the armed forces and vacationers. J Infect Dis. 1985;152:650-651.