CHAPTER 178 Arachnoid Cysts
Arachnoid cysts are benign, congenital, non-neoplastic, extra-axial, intra-arachnoid lesions filled with fluid similar to or exactly like cerebrospinal fluid (CSF).1–3 Bright’s4 early observation that these entities are “serous cysts forming in connection with the arachnoid, and apparently lying within its layers” has been confirmed in more recent pathologic evaluation.3
Arachnoid cysts constitute about 1% of all nontraumatic intracranial space-occupying lesions. As neuroimaging has become more widely available, incidentally discovered cysts are frequently found.5–9 Although they may present at any age, 60% to 80% of arachnoid cysts are discovered in children10,11 and appear with a male-to-female ratio of 2:1 to 3:1.
Primary arachnoid cysts are congenital cysts lined by a single layer of flattened, arachnoid cells in a vascular collagenous membrane that lies entirely within the arachnoid layer. The cysts may be loculated, compartmentalized, or freely communicating with the surrounding CSF cisterns. Secondary arachnoid cysts develop as a result of another condition, although many of the cysts attributed to meningitis, trauma, or hemorrhage are probably congenital in origin.12 Secondary arachnoid cysts may show signs of previous inflammatory changes such as gliosis or hemosiderin within the walls.13,14 The cyst fluid may be xanthochromic, proteinaceous, or hemorrhagic.15
Epidemiology
The incidence of arachnoid cysts in the general population is estimated to be around 0.1% from autopsy series.16 The incidence is similar in computed tomography (CT) imaging series (0.2%)6 and greater in magnetic resonance imaging (MRI) series (0.8% to 1.7%).5,17 The greater incidence in autopsy and imaging series than suggested by clinical symptoms indicates that arachnoid cysts are asymptomatic and incidental in most cases. Most arachnoid cysts are found in the supratentorial space (90%). Rengachary and Watanabe18 first reported on the distribution of 208 arachnoid cysts reported between 1831 and 1980, and other authors have found a similar distribution.19–22 Abtin and Walker23 summarized the distribution of arachnoid cysts from several series (Table 178-1). These figures from academic centers represent the distribution of symptomatic arachnoid cysts referred to neurosurgical centers rather than the true distribution.
TABLE 178-1 Distribution of Intracranial Arachnoid Cysts in Pediatric Series*
| LOCATION | INCIDENCE (%) |
|---|---|
| Sylvian fissure/middle fossa | 42 |
| Posterior fossa | 24 |
| Suprasellar | 10 |
| Quadrigeminal cistern | 7.5 |
| Interhemispheric | 7.3 |
| Cerebral convexity | 5.7 |
| Other | 2.5 |
| Supratentorial/infratentorial | 1.1 |
* Data from references 18 to 22.
Pathology
The sine qua non of arachnoid cysts is splitting of the arachnoid layers at the margins of the cyst, and these cysts are therefore best described as intra-arachnoid cysts. They usually arise within and expand the margins of CSF cisterns rich in arachnoid (i.e., the sylvian fissure, suprasellar and quadrigeminal cisterns, cerebellopontine angle, interhemispheric fissure, and posterior fossa midline cisterns).24,25
Pathogenesis
Arachnoid cysts may coexist with other genetic disturbances in collagen formation. Several genetic disorders are now associated with a higher incidence of arachnoid cysts, including Marfan syndrome,26 neurofibromatosis type I,27–29 glutaric aciduria,30–32 acrocallosal syndrome,33 other hereditary disorders,33–41 and autosomal dominant polycystic kidney disease (PKD).42–45 Shievink and coworkers45 found intracranial arachnoid cysts in 8% of patients with PKD who underwent prospective radiologic screening for intracranial aneurysms.
Embryology
The origin of arachnoid cysts has been a controversial subject, and the details remain unclear. The formation of arachnoid cysts is hypothesized to result from abnormal embryologic development of the subarachnoid space. Early in normal embryonic development, a loose layer of connective tissue, called the meninx primitiva, or perimedullary mesh, a precursor to the pia mater and arachnoid, lines the surface of the dura and surrounds the neural tube. At about 15 weeks’ gestation, the rhombic roof ruptures, CSF pulses through this mesh, and the pia mater and arachnoid separate incompletely,46 resulting in the cobweb-like appearance of the arachnoid. The leading hypothesis for arachnoid cyst formation proposes that this separation of the superficial arachnoid and deep pia is aberrant, and enclosed, loculated chambers form and develop into a cystic mass. Alternatively, the meninx primitiva formation is aberrant, which may also lead to cyst formation.47 If this hypothesis is correct, arachnoid cysts should be in close proximity to an arachnoid cistern, and this has been found to be true in most cases.
More likely, a slit-valve or ball-valve communication may exist between the subarachnoid space and the cyst, allowing entry but not egress of CSF.48,49 CT cisternography or cine-phase contrast-enhanced MRI sequences often demonstrate slow filling or emptying of the arachnoid cyst.48 Normal CSF pulsation or Valsalva maneuvers and the local geometry of the split membrane may provide the pressure gradient to drive fluid into the cyst. Slit valves have been directly observed with endoscopy and currently are the most direct explanation for why the cysts expand.12,48,50,51
Clinical Presentation
Arachnoid cysts most commonly present during childhood. About 75% of intracranial arachnoid cysts presented before 3 years of age in one series,22 and the median age at presentation was 2.2 years in another series.19 In the European cooperative study, the average age at presentation was slightly older (6 years).52
Clinical presentation varies primarily by age and location. Some arachnoid cysts remain asymptomatic throughout life.53 The most common presenting symptom is headache, which may be due to local mass effect, high intracranial pressure (ICP), or hydrocephalus, or may be unrelated to the coexisting cyst. Infants may present with macrocephaly, enlarged tense fontanelle, and splayed sutures with irritability, failure to thrive, and developmental delay.
Arachnoid cysts of the middle cranial fossa may be associated with hemorrhage, but this complication has been considered very infrequent. Of just 42 cases reported in the literature, half were associated with a blunt head trauma. In a series reported by Parsch and colleagues,17 2.43% of the personally observed subdural hematomas and hygromas were related to the rupture of a sylvian fissure arachnoid cyst. In a series of 168 temporal arachnoid cysts,54 6.5% were found to have intracystic or subdural hematomas. There has been no longitudinal study to date monitoring patients with asymptomatic arachnoid cysts to determine the rate of hemorrhage.
Supratentorial Arachnoid Cysts
Sylvian fissure cysts compose about one third of all arachnoid cysts in children and about half of those presenting in adulthood. Presentation is more common in children than in adults, and there is a nearly 3:1 male-to-female predominance. The left side is affected more often than the right. The common middle fossa location may be explained by meningeal maldevelopment if the arachnoid covering of the temporal and frontal lobes fails to merge when the sylvian fissure is formed in early fetal life.55
The most common symptom is a unilateral headache in the supraorbital or temporal region that may be exacerbated with physical exertion. The headaches may rarely be associated with other signs and symptoms of increased ICP such as nausea, vomiting, and papilledema.21 The next most frequent associated symptom is focal, complex-partial, or generalized seizures occurring in up to one third of patients.19,22,56 The cause of seizures in patients with arachnoid cysts is unknown and the relationship between seizure focus and cyst location is unclear57,58 (see Koch 1994, Yalcin 2002).
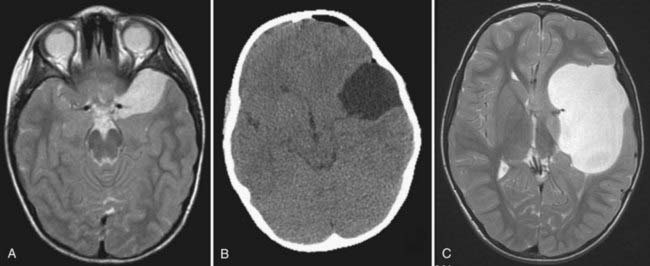
Middle fossa and sylvian fissure cysts were classified by Galassi and associates57 on the basis of their CT appearance and apparent communication with adjacent normal CSF spaces (Fig. 178-1). Type I cysts are small, lenticular, biconvex collections located at the anterior pole of the middle fossa directly posterior to the sphenoid ridge that appear to communicate freely with the adjacent cisterns. There is little associated mass effect, and they usually do not have associated calvarial deformities. They do not require treatment.
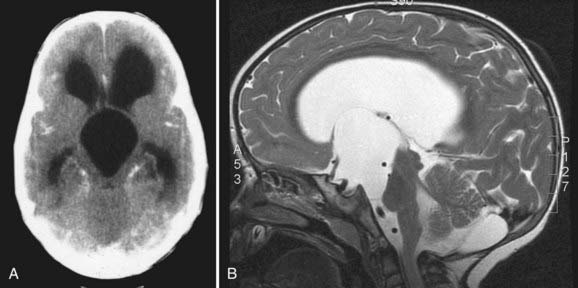
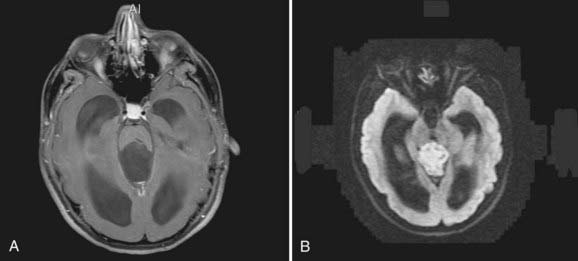
Parasellar intra-arachnoid cysts are subdivided into intrasellar and suprasellar subgroups according to their relationship to the diaphragma sella. Suprasellar cysts are much more common and found almost exclusively in children. Nearly 50% of suprasellar cysts are diagnosed in children younger than 5 years old and 20% in children younger than 1 year old.60 They typically present with hydrocephalus by extending into the third ventricle and obstructing the aqueduct of Sylvius (Fig. 178-2). They may also present with visual abnormalities, including hemianopia or decline in acuity. Gait ataxia and opisthotonos may occur in patients with large cysts that displace the midbrain posteriorly. The “bobble-head doll syndrome” has been described in relation to suprasellar arachnoid cyst.61–68 Ten to 60% of patients with suprasellar arachnoid cysts may present with endocrinopathy. Precocious puberty and growth hormone deficiency are the most common.69–72
Intrasellar arachnoid cysts are uncommon and typically present in the fourth or fifth decade of life.73 The most common presenting symptom is headache, and these cysts rarely present with visual abnormalities or endocrinopathy. Most of these intrasellar cysts are discovered incidentally, and they may be difficult to differentiate from an intrasellar craniopharyngioma or Rathke’s cleft cyst on imaging alone.
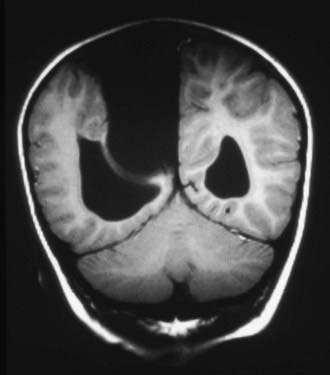
Arachnoid cysts of the cerebral convexities usually present with headaches or seizures, or both. Age, size, and location typically determine their clinical presentation. Small cysts may focally compress the underlying brain and thin the overlying bone, whereas large cysts may lead to asymmetric calvarial expansion, suture diastasis, and hemispheric distortion (Fig. 178-3). These cysts tend to occur more commonly in females than males.
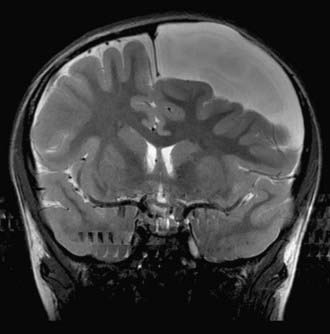
Interhemispheric cysts are often associated with agenesis of the corpus callosum. They most often present with macrocephaly and asymmetric growth of the calvaria. They tend to be associated with increased ICP, developmental delay, hypertonia or hypotonia, hemiparesis, ocular changes, and epilepsy (Fig. 178-4).74,75
Arachnoid cysts in the quadrigeminal cistern abutting the collicular plate may cause hydrocephalus by obstructing the sylvian aqueduct. Progressive macrocephaly in an infant is the most common presentation. Other symptoms and signs associated with quadrigeminal plate cistern arachnoid cysts include Parinaud’s syndrome, nystagmus, hearing deficits, trochlear nerve palsy, and apneic spells.22,76–80
Infratentorial Arachnoid Cysts
Arachnoid cysts of the cerebellopontine angle can present with tinnitus, vertigo, facial weakness, facial sensory loss, hearing loss, or ataxia.81 The presentation may be indistinguishable from Meniere’s disease. Other presentations have included trigeminal neuralgia and hemifacial spasm.82–88 Large midline posterior fossa cysts often present with obstructive hydrocephalus (Fig. 178-5). This entity must be differentiated from mega cisterna magna, Dandy-Walker malformation, epidermoid cyst, and large cystic tumors. Infrequently, posterior fossa cysts may present with cerebellar signs such as ataxia, nystagmus, and cranial nerve dysfunction and progressive quadriparesis.
Imaging
Intracranial arachnoid cysts are often diagnosed in utero by ultrasound. This is a noninvasive, inexpensive test and very effective screening tool for congenital brain malformations. Prenatal ultrasound has detected arachnoid cysts as early as 13 weeks’ gestation,89 but most are diagnosed during the second trimester.90–92 Arachnoid cysts appear sonolucent with enhanced transmission of the ultrasound beam through the collection and are thus hypoechogenic to surrounding brain. Ultrasound may also be useful intraoperatively for placement of cystoperitoneal shunts, guidance of endoscopic fenestration, and detection and monitoring the progression of arachnoid cysts postnatally.
Imaging of congenital arachnoid cysts with CT or MRI reveals sharply circumscribed, smoothly marginated lesions having a homogeneous density very near to CSF. A noncontrast CT is usually sufficient to differentiate an arachnoid cyst from other cystic lesions. Arachnoid cyst walls are so thin that they are not visible on CT, whereas the walls of cystic tumors may be visible. The wall of an arachnoid cyst does not enhance if contrast agent is administered. Hyperdense calcium flecks are often visible in the walls of a parasellar craniopharyngioma but are never present in an arachnoid cyst. The fluid of an arachnoid cyst is identical to CSF on CT imaging unless there has been an intracystic hemorrhage, in which case it will appear hyperdense to CSF. CT ventriculography, cisternography, or cystography may show communication of the cyst with the basal cisterns and subarachnoid space.11,59,93 CT alone is not capable of demonstrating CSF flow dynamics and communication of the arachnoid cyst to the surrounding subarachnoid space. CT with intrathecal or intraventricular injection of metrizamide can simultaneously provide anatomic detail of the subarachnoid and intraventricular space as well as physiologic information. In communicating arachnoid cysts, the cyst fills with metrizamide, but the clearance of contrast from the cyst is delayed with respect to the surrounding subarachnoid space and basal cisterns. In noncommunicating cysts, there is no early entry of contrast into the cyst (2 to 6 hours), but contrast accumulates around the cyst in the cisterns creating a halo demarcating the cyst boundary (about 24 hours). The contrast then clears from the subarachnoid space and slowly accumulates within the arachnoid cyst.
The three-dimensional relationship of arachnoid cysts to the basal cisterns is best visualized with the multiplanar acquisition of MRI. The mass effect on adjacent structures and associated anomalies can also best be visualized, particularly when combined with contrast imaging to highlight the surrounding vascular anatomy. The greater resolution of MRI allows for better detection of smaller cysts and cysts adjacent to bony structures. MRI can distinguish between the CSF-like fluid of most arachnoid cysts and the proteinaceous fluid of other cystic lesions. MRI sequences can also differentiate epidermoids from arachnoid cysts by their hyperintense appearance on proton density–weighted and diffusion-weighted sequences.94 Cysts associated with parasites are also hyperintense on proton density–weighted images.
In the MRI era, metrizamide CT has in many cases been replaced by MRI sequences that are sensitive to CSF flow.95,96 Electrocardiogram-gated cine-mode MRI sequences were able to demonstrate communication between intracranial arachnoid cyst and CSF spaces in 95% of patients in a recent study.97 The identification of a jet-like flow void at a potential communication site is the most convincing evidence of communication between the arachnoid cysts and the adjacent subarachnoid space. These results compare favorably with CT cisternography and operative findings.
Magnetic resonance cisternography with T2-weighted imaging96–101 or more recent three-dimensional Fourier transformation constructive interference in steady state or fast imaging employing steady-state acquisition can depict medial arachnoid cyst walls, thin cranial nerves, small vessels, and cortical sulci in detail.100 Magnetic resonance cisternography was originally used to detect small tumors or vascular compression at the cerebellopontine angle102,103 as well as small lesions in the ventricular system.104,105 Fine detail such as the trochlear nerve and Lillequist’s membrane can be resolved using this technique.105,106 The delineation of these structures has been confirmed during neuroendoscopic surgery and has proved useful for planning fenestration into the basal subarachnoid cisterns.
Many cystic lesions appear hyperintense on T2-weighted imaging and hypointense on T1-weighted imaging, and although the appearance of the cystic margin, surrounding edema, and contrast enhancement can provide clues to the diagnosis, the specificity remains low. In vivo proton magnetic resonance spectroscopy allows for the characterization of intracranial cystic lesions based on the presence or combination of specific metabolite resonances.107–110 Arachnoid cysts, because the contents are very similar to CSF, have low concentrations of metabolites and small lactate peaks.
Diffusion-weighted imaging (DWI) evaluates the diffusion properties of water in tissue and has also been used to aid in the differential diagnosis of cystic brain lesions.110,111 Epidermoids, with restricted diffusion of protons and cyst contents dissimilar to CSF, have restricted diffusion, appear dark on apparent diffusion coefficient (ADC) maps, and are hyperintense on DWI. The combination of magnetic resonance spectroscopy and DWI has sensitivity and specificity greater than 95% for distinguishing among arachnoid cysts, epidermoid cysts, and other cystic lesions compared with sensitivity and specificity (about 65%) for conventional MRI (Fig. 178-6).110
Functional imaging modalities, such as fluorodeoxyglucose (FDG) positron emission tomography (PET) and technetium-hexamethylpropyleneamine oxime (Tc-HMPAO) single-photon emission computed tomography (SPECT), have been used in a small number of studies in patients with arachnoid cysts.112–115
Treatment Alternatives
Most cysts remain constant in size, and conservative management has been proposed for most patients. There have been many case reports of arachnoid cysts undergoing spontaneous resolution.53,116–126 Patients who do not demonstrate clear signs of increased ICP or focal neurologic deficits are usually considered for conservative management because surgery has a low but real risk for morbidity. In these patients, close follow-up with CT or MRI is a treatment option.
Conservative Management
Numerous middle fossa arachnoid cysts have been discovered to be associated with hemorrhage.17,44,118,127–152 Of 658 patients with chronic subdural hematoma in one study, 2.4% of the hematomas were thought to be secondary to arachnoid cysts.17 The incidence of middle fossa arachnoid cysts on MRI in patients that presented with hemorrhage (2.4%) is 5 times higher than the incidence of middle fossa arachnoid cysts found incidentally on MRI. This association has led some authors to advocate surgical intervention even in asymptomatic patients.13 In a recent study, Tamburrini and colleagues153 sent a survey to internationally recognized pediatric neurosurgery centers to assess management of children with controversial sylvian arachnoid cysts. Contributors were asked to answer a six-part multiple-choice questionnaire related to a 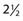 -year-old boy with a Galassi type II left-sided sylvian arachnoid cyst presented in different clinical situations. One sixth of participants were in favor of direct surgical treatment in the case of an incidental diagnosis. These surgeons justified the surgical indication because of the theoretical risk for spontaneous or traumatic intracranial bleeding. More than 60% suggested continued clinical follow-up, with half indicating they would request a baseline MRI and 26% suggesting avoidance of contact sports. These suggestions are almost entirely based on anecdotal case reports, however. The actual rate of cyst-related intracranial hemorrhage after contact sports–related head injury is essentially unknown.153
-year-old boy with a Galassi type II left-sided sylvian arachnoid cyst presented in different clinical situations. One sixth of participants were in favor of direct surgical treatment in the case of an incidental diagnosis. These surgeons justified the surgical indication because of the theoretical risk for spontaneous or traumatic intracranial bleeding. More than 60% suggested continued clinical follow-up, with half indicating they would request a baseline MRI and 26% suggesting avoidance of contact sports. These suggestions are almost entirely based on anecdotal case reports, however. The actual rate of cyst-related intracranial hemorrhage after contact sports–related head injury is essentially unknown.153
Surgery
Headache is a common presenting symptom in children, with sylvian fissure arachnoid cysts reported in up to 70% of symptomatic cases.154,155 A significant portion of these patients, however, complain of a chronic, recurrent type of headache that is not reliably attributed to the presence of a cyst on an imaging study, its effect on surrounding cerebrovascular structures, or its effect on CSF dynamics.
The role of invasive ICP monitoring in patients with sylvian fissure arachnoid cysts is controversial. In one study, the authors considered ICP monitoring an important preoperative tool to rule out the need to operate on children with type I cysts and confirmed almost continuously increased ICP in patients presenting with type III cysts but found ICP monitoring was far less discriminating in children who harbored type II cysts.156 There was an almost homogeneous distribution among patients with normal and pathologic ICP values and type II cysts. Although the causal relationship between clinical symptoms and sylvian arachnoid cysts cannot be identified with absolute values of ICP, the role of abnormally high CSF pressure waves and arterial pulsatility in contributing to symptoms cannot be ruled out. Prolonged ICP monitoring should provide more reliable information about intracranial compliance in patients with arachnoid cyst and headache than ICP evaluations through puncture of the cyst or short-term ICP recordings.
Craniotomy for Cyst Excision and Fenestration
Most authors in the neurosurgical literature advocate microsurgical cyst fenestration with or without marsupialization as the first-line approach to avoid placement of a shunt.20,52,75,157,158 The long-term clinical success rates approach 75% in this type of surgical management. There appears to be no difference with respect to complication rates when compared with shunt placement. Thus, these authors advocate maintaining shunt independence as an important goal while treating the cyst, thus eliminating the inherent risks associated with shunts and leaving implanted foreign material within the intracranial space.
Shunt Placement
Several authors have advocated direct cyst shunting as their initial surgical approach to arachnoid cysts.13,19,22,159 These authors have found lower clinical success rates with fenestration. For example, in one study, two thirds of patients initially treated with craniotomy and cyst fenestration subsequently required cystoperitoneal shunting for either cyst recurrence or no improvement in symptoms.19
Although many authors have reported the benefits of direct shunt placement, other factors must be weighed in the decision to use this protocol. Moreover, patients with arachnoid cysts and associated hydrocephalus may require ventriculoperitoneal shunts in addition to cystoperitoneal shunts. Because ventricular decompression may increase the risk for subdural hematoma, caution should be used when proceeding with this approach. High-pressure valves or flow-control valves may be helpful in an attempt to avoid overdrainage. Quadrigeminal cistern or pineal region arachnoid cysts may be shunted into the cisterna magna without concerns for overdrainage or the requirement of shunt valves (Torkildsen’s shunt variant).160,161
Neuroendoscopic Management
Neuroendoscopy has become increasingly popular as an alternative to shunting in the management of hydrocephalus and arachnoid cysts.49–51,65,95,162–180 The endoscope has been used to improve the placement of the proximal catheter,181 to decrease the number of shunts required as in the treatment of multicompartmental hydrocephalus,182 and to fenestrate cysts in a less invasive manner than craniotomy.50,169,180 The advantage of this approach over microsurgery can be called into question: cyst membranes and adjacent cisternal membranes are often thickened and fibrous. Microsurgery through a small trephine craniotomy offers a better visualization and microinstruments to facilitate a safer and wider fenestration.
Intraspinal Arachnoid Cysts
Surgical decompression of intraspinal arachnoid cysts may result in significant neurological improvement.183 If the cyst lies dorsally within the spinal canal, it is usually excised through a laminectomy. If the cyst lies ventrolaterally, a posterior approach with section of the dentate ligaments to provide access to the cyst may be required.
Arai H, Sato K, Wachi A, et al. Arachnoid cysts of the middle cranial fossa: experience with 77 patients who were treated with cystoperitoneal shunting. Neurosurgery. 1996;39:1108-1112.
Awaji M, Okamoto K, Nishiyama K. Magnetic resonance cisternography for preoperative evaluation of arachnoid cysts. Neuroradiology. 2007;49:721-726.
Caldarelli M. Congenital supratentorial arachnoidal cysts in children. Childs Nerv Syst. 1997;13:358.
Cincu R, Agrawal A, Eiras J. Intracranial arachnoid cysts: current concepts and treatment alternatives. Clin Neurol Neurosurg. 2007;109:837-843.
Ciricillo SF, Cogen PH, Harsh GR, et al. Intracranial arachnoid cysts in children. A comparison of the effects of fenestration and shunting. J Neurosurg. 1991;74:230-235.
Decq P, Brugieres P, Le Guerinel C, et al. Percutaneous endoscopic treatment of suprasellar arachnoid cysts: ventriculocystostomy or ventriculocystocisternostomy? Technical note. J Neurosurg. 1996;84:696-701.
Di Rocco F, Yoshino M, Oi S. Neuroendoscopic transventricular ventriculocystostomy in treatment for intracranial cysts. J Neurosurg. 2005;103:54-60.
Ersahin Y, Kesikci H, Ruksen M, et al. Endoscopic treatment of suprasellar arachnoid cysts. Childs Nerv Syst. 2008;24:1013-1020.
Fewel ME, Levy ML, McComb JG. Surgical treatment of 95 children with 102 intracranial arachnoid cysts. Pediatr Neurosurg. 1996;25:165-173.
Galassi E, Tognetti F, Gaist G, et al. CT scan and metrizamide CT cisternography in arachnoid cysts of the middle cranial fossa: classification and pathophysiological aspects. Surg Neurol. 1982;17:363-369.
Harsh GR4th, Edwards MS, Wilson CB. Intracranial arachnoid cysts in children. J Neurosurg. 1986;64:835-842.
Jallo GI, Woo HH, Meshki C, et al. Arachnoid cysts of the cerebellopontine angle: diagnosis and surgery. Neurosurgery. 1997;40:31-37.
Oberbauer RW, Haase J, Pucher R. Arachnoid cysts in children: a European co-operative study. Childs Nerv Syst. 1992;8:281-286.
Pascual-Castroviejo I, Roche MC, Martinez Bermejo A, et al. Primary intracranial arachnoidal cysts. A study of 67 childhood cases. Childs Nerv Syst. 1991;7:257-263.
Pierre-Kahn A, Capelle L, Brauner R, et al. Presentation and management of suprasellar arachnoid cysts. Review of 20 cases. J Neurosurg. 1990;73:355-359.
Raffel C, McComb JG. To shunt or to fenestrate: which is the best surgical treatment for arachnoid cysts in pediatric patients? Neurosurgery. 1988;23:338-342.
Rengachary SS, Watanabe I, Brackett CE. Pathogenesis of intracranial arachnoid cysts. Surg Neurol. 1978;9:139-144.
Schroeder HW, Gaab MR. Endoscopic observation of a slit-valve mechanism in a suprasellar prepontine arachnoid cyst: case report. Neurosurgery. 1997;40:198-200.
Tamburrini G, Del Fabbro M, Di Rocco C. Sylvian fissure arachnoid cysts: a survey on their diagnostic workout and practical management. Childs Nerv Syst. 2008;24:593-604.
Wester K. Peculiarities of intracranial arachnoid cysts: location, sidedness, and sex distribution in 126 consecutive patients. Neurosurgery. 1999;45:775-779.
Yildiz H, Erdogan C, Yalcin R, et al. Evaluation of communication between intracranial arachnoid cysts and cisterns with phase-contrast cine MR imaging. AJNR Am J Neuroradiol. 2005;26:145-151.
1 Krawchenko J, Collins GH. Pathology of an arachnoid cyst. Case report. J Neurosurg. 1979;50:224-228.
2 Rengachary SS, Watanabe I, Brackett CE. Pathogenesis of intracranial arachnoid cysts. Surg Neurol. 1978;9:139-144.
3 Starkman SP, Brown TC, Linell EA. Cerebral arachnoid cysts. J Neuropathol Exp Neurol. 1958;17:484-500.
4 Bright R. Reports of Medical Cases, Selected with a View of Illustrating the Symptoms and Cure of Diseases by a Reference to Morbid Anatomy. Vol II: Disease of the Brain and Nervous System. London: Longman, Rees, Orme, Brown, Green, and Highley; 1831.
5 Weber F, Knopf H. Incidental findings in magnetic resonance imaging of the brains of healthy young men. J Neurol Sci. 2006;240:81-84.
6 Eskandary H, Sabba M, Khajehpour F, et al. Incidental findings in brain computed tomography scans of 3000 head trauma patients. Surg Neurol. 2005;63:550-553.
7 Robinson RG. Congenital cysts of the brain: arachnoid malformations. Prog Neurol Surg. 1971;4:133-174.
8 Cincu R, Agrawal A, Eiras J. Intracranial arachnoid cysts: current concepts and treatment alternatives. Clin Neurol Neurosurg. 2007;109:837-843.
9 Clemenceau S, Carpentier A. [Intracranial arachnoid cysts. A review]. Rev Neurol (Paris). 1999;155:604-608.
10 Galassi E, Piazza G, Gaist G, et al. Arachnoid cysts of the middle cranial fossa: a clinical and radiological study of 25 cases treated surgically. Surg Neurol. 1980;14:211-219.
11 Hayashi T, Anegawa S, Honda E, et al. Clinical analysis of arachnoid cysts in the middle fossa. Neurochirurgia (Stuttg). 1979;22:201-210.
12 Choi JU, Kim DS. Pathogenesis of arachnoid cyst: congenital or traumatic? Pediatr Neurosurg. 1998;29:260-266.
13 Harsh GR4th, Edwards MS, Wilson CB. Intracranial arachnoid cysts in children. J Neurosurg. 1986;64:835-842.
14 Naidich TP, McLone DG, Radkowski MA. Intracranial arachnoid cysts. Pediatr Neurosci. 1985;12:112-122.
15 Cagnoni G, Fonda C, Pancani S, et al. [Intracranial arachnoid cyst in pediatric age]. Pediatr Med Chir. 1996;18:85-90.
16 Shaw CM, Alford ECJr. Congenital arachnoid cysts and their differential diagnosis. In: Vinken PJ, Bruyn GW, editors. Handbook of Clinical Neurology, Vol 31. Amsterdam: North Holland Publishing Company; 1977:75-136.
17 Parsch CS, Krauss J, Hofmann E, et al. Arachnoid cysts associated with subdural hematomas and hygromas: analysis of 16 cases, long-term follow-up, and review of the literature. Neurosurgery. 1997;40:483-490.
18 Rengachary SS, Watanabe I. Ultrastructure and pathogenesis of intracranial arachnoid cysts. J Neuropathol Exp Neurol. 1981;40:61-83.
19 Ciricillo SF, Cogen PH, Harsh GR, et al. Intracranial arachnoid cysts in children. A comparison of the effects of fenestration and shunting. J Neurosurg. 1991;74:230-235.
20 Fewel ME, Levy ML, McComb JG. Surgical treatment of 95 children with 102 intracranial arachnoid cysts. Pediatr Neurosurg. 1996;25:165-173.
21 Hanieh A, Simpson DA, North JB. Arachnoid cysts: a critical review of 41 cases. Childs Nerv Syst. 1988;4:92-96.
22 Pascual-Castroviejo I, Roche MC, Martinez Bermejo A, et al. Primary intracranial arachnoidal cysts. A study of 67 childhood cases. Childs Nerv Syst. 1991;7:257-263.
23 Abtin K, Walker ML. Congenital arachnoid cysts and the Dandy-Walker complex. In: Albright L, Pollack I, Adelson D, editors. Principle and Practice of Pediatric Neurosurgery. New York: Thieme Medical; 1999:125-141.
24 Gandy SE, Heier LA. Clinical and magnetic resonance features of primary intracranial arachnoid cysts. Ann Neurol. 1987;21:342-348.
25 Heier LA, Zimmerman RD, Amster JL, et al. Magnetic resonance imaging of arachnoid cysts. Clin Imaging. 1989;13:281-291.
26 Ikeda M, Tsuchiya K, Kurosawa T, et al. [Marfan’s syndrome associated with a frontal arachnoid cyst]. Rinsho Shinkeigaku. 1988;28:1076-1078.
27 Martinez-Lage JF, Poza M, Rodriguez Costa T. Bilateral temporal arachnoid cysts in neurofibromatosis. J Child Neurol. 1993;8:383-385.
28 Shehu BB, Hassan I. Cervicothoracic arachnoid cyst in a patient with neurofibromatosis: case report. East Afr Med J. 2006;83:515-517.
29 Yoshioka H, Iino S, Ishimura K, et al. An arachnoid cyst in an 8-year-old boy with neurofibromatosis. Brain Dev. 1984;6:551-553.
30 Hald JK, Nakstad PH, Skjeldal OH, et al. Bilateral arachnoid cysts of the temporal fossa in four children with glutaric aciduria type I. AJNR Am J Neuroradiol. 1991;12:407-409.
31 Martinez-Lage JF, Casas C, Fernandez MA, et al. Macrocephaly, dystonia, and bilateral temporal arachnoid cysts: glutaric aciduria type 1. Childs Nerv Syst. 1994;10:198-203.
32 Jamjoom ZA, Okamoto E, Jamjoom AH, et al. Bilateral arachnoid cysts of the sylvian region in female siblings with glutaric aciduria type I. Report of two cases. J Neurosurg. 1995;82:1078-1081.
33 Koenig R, Bach A, Woelki U, et al. Spectrum of the acrocallosal syndrome. Am J Med Genet. 2002;108:7-11.
34 Arriola G, de Castro P, Verdu A. Familial arachnoid cysts. Pediatr Neurol. 2005;33:146-148.
35 Guzel A, Tatli M, Bilguvar K, et al. Apparently novel genetic syndrome of pachygyria, mental retardation, seizure, and arachnoid cysts. Am J Med Genet A. 2007;143:672-677.
36 Handa J, Okamoto K, Sato M. Arachnoid cyst of the middle cranial fossa: report of bilateral cysts in siblings. Surg Neurol. 1981;16:127-130.
37 Jadeja KJ, Grewal RP. Familial arachnoid cysts associated with oculopharyngeal muscular dystrophy. J Clin Neurosci. 2003;10:125-127.
38 Orlacchio A, Gaudiello F, Totaro A, et al. A new SPG4 mutation in a variant form of spastic paraplegia with congenital arachnoid cysts. Neurology. 2004;62:1875-1878.
39 Pomeranz S, Constantini S, Lubetzki-Korn I, et al. Familial intracranial arachnoid cysts. Childs Nerv Syst. 1991;7:100-102.
40 Sinha S, Brown JI. Familial posterior fossa arachnoid cyst. Childs Nerv Syst. 2004;20:100-103.
41 Su PH, Chen JY, Chen SJ, et al. Clinical manifestations of chromosome 21 interstitial deletion: report of four cases. Acta Paediatr Taiwan. 2006;47:303-308.
42 Alehan FK, Gurakan B, Agildere M. Familial arachnoid cysts in association with autosomal dominant polycystic kidney disease. Pediatrics. 2002;110:e13.
43 Howe G, Liddell J, Hunn A. Adult polycystic kidney disease and arachnoid cyst formation: case report. J Clin Neurosci. 1995;2:269-270.
44 Leung GK, Fan YW. Chronic subdural haematoma and arachnoid cyst in autosomal dominant polycystic kidney disease (ADPKD). J Clin Neurosci. 2005;12:817-819.
45 Schievink WI, Huston J3rd, Torres VE, et al. Intracranial cysts in autosomal dominant polycystic kidney disease. J Neurosurg. 1995;83:1004-1007.
46 Pilu G, De Palma L, Romero R, et al. The fetal subarachnoid cisterns: an ultrasound study with report of a case of congenital communicating hydrocephalus. J Ultrasound Med. 1986;5:365-372.
47 McLone DG. The subarachnoid space: a review. Childs Brain. 1980;6:113-130.
48 Santamarta D, Aguas J, Ferrer E. The natural history of arachnoid cysts: endoscopic and cine-mode MRI evidence of a slit-valve mechanism. Minim Invasive Neurosurg. 1995;38:133-137.
49 Santamarta D, Morales F, Sierra JM, et al. Arachnoid cysts: entrapped collections of cerebrospinal fluid variably communicating with the subarachnoid space. Minim Invasive Neurosurg. 2001;44:128-134.
50 Caemaert J, Abdullah J, Calliauw L, et al. Endoscopic treatment of suprasellar arachnoid cysts. Acta Neurochir (Wien). 1992;119:68-73.
51 Schroeder HW, Gaab MR. Endoscopic observation of a slit-valve mechanism in a suprasellar prepontine arachnoid cyst: case report. Neurosurgery. 1997;40:198-200.
52 Oberbauer RW, Haase J, Pucher R. Arachnoid cysts in children: a European co-operative study. Childs Nerv Syst. 1992;8:281-286.
53 Sommer IE, Smit LM. Congenital supratentorial arachnoidal and giant cysts in children: a clinical study with arguments for a conservative approach. Childs Nerv Syst. 1997;13:8-12.
54 Wester K, Helland CA. How often do chronic extra-cerebral haematomas occur in patients with intracranial arachnoid cysts? J Neurol Neurosurg Psychiatry. 2008;79:72-75.
55 Robertson SJ, Wolpert SM, Runge VM. MR imaging of middle cranial fossa arachnoid cysts: temporal lobe agenesis syndrome revisited. AJNR Am J Neuroradiol. 1989;10:1007-1010.
56 Arai H, Sato K, Wachi A, et al. Arachnoid cysts of the middle cranial fossa: experience with 77 patients who were treated with cystoperitoneal shunting. Neurosurgery. 1996;39:1108-1112.
57 Koch CA, Voth D, Kraemer G, et al. Arachnoid cysts: does surgery improve epileptic seizures and headaches. Neurosurg Rev. 1995;18:173-181.
58 Yalcin AD, Oncel C, Kaymaz A, et al. Evidence against association between arachnoid cysts and epilepsy. Epilepsy Res. 2002;49:255-260.
59 Galassi E, Tognetti F, Gaist G, et al. CT scan and metrizamide CT cisternography in arachnoid cysts of the middle cranial fossa: classification and pathophysiological aspects. Surg Neurol. 1982;17:363-369.
60 Hoffman HJ, Hendrick EB, Humphreys RP, et al. Investigation and management of suprasellar arachnoid cysts. J Neurosurg. 1982;57:597-602.
61 Bhattacharyya KB, Senapati A, Basu S, et al. Bobble-head doll syndrome: some atypical features with a new lesion and review of the literature. Acta Neurol Scand. 2003;108:216-220.
62 Desai KI, Nadkarni TD, Muzumdar D, et al. Suprasellar arachnoid cyst presenting with bobble-head doll movements: a report of 3 cases. Neurol India. 2003;51:407-409.
63 Fioravanti A, Godano U, Consales A, et al. Bobble-head doll syndrome due to a suprasellar arachnoid cyst: endoscopic treatment in two cases. Childs Nerv Syst. 2004;20:770-773.
64 Goikhman I, Zelnik N, Peled N, et al. Bobble-head doll syndrome: a surgically treatable condition manifested as a rare movement disorder. Mov Disord. 1998;13:192-194.
65 Hagebeuk EE, Kloet A, Grotenhuis JA, et al. Bobble-head doll syndrome successfully treated with an endoscopic ventriculocystocisternostomy. Case report and review of the literature. J Neurosurg. 2005;103:253-259.
66 Mussell HG, Dure LS, Percy AK, et al. Bobble-head doll syndrome: report of a case and review of the literature. Mov Disord. 1997;12:810-814.
67 Turgut M, Ozcan OE. Suprasellar arachnoid cyst as a cause of precocious puberty and bobble-head doll phenomenon. Eur J Pediatr. 1992;151:76.
68 Wiese JA, Gentry LR, Menezes AH. Bobble-head doll syndrome: review of the pathophysiology and CSF dynamics. Pediatr Neurol. 1985;1:361-366.
69 Pierre-Kahn A, Capelle L, Brauner R, et al. Presentation and management of suprasellar arachnoid cysts. Review of 20 cases. J Neurosurg. 1990;73:355-359.
70 Adan L, Bussieres L, Dinand V, et al. Growth, puberty and hypothalamic-pituitary function in children with suprasellar arachnoid cyst. Eur J Pediatr. 2000;159:348-355.
71 Mohn A, Schoof E, Fahlbusch R, et al. The endocrine spectrum of arachnoid cysts in childhood. Pediatr Neurosurg. 1999;31:316-321.
72 Starzyk J, Kwiatkowski S, Urbanowicz W, et al. Suprasellar arachnoidal cyst as a cause of precocious puberty—report of three patients and literature overview. J Pediatr Endocrinol Metab. 2003;16:447-455.
73 Baskin DS, Wilson CB. Transsphenoidal treatment of non-neoplastic intrasellar cysts. A report of 38 cases. J Neurosurg. 1984;60:8-13.
74 Caldarelli M. Congenital supratentorial arachnoidal cysts in children. Childs Nerv Syst. 1997;13:358.
75 Caldarelli M, Di Rocco C. Surgical options in the treatment of interhemispheric arachnoid cysts. Surg Neurol. 1996;46:212-221.
76 Hayashi T, Kuratomi A, Kuramoto S. Arachnoid cyst of the quadrigeminal cistern. Surg Neurol. 1980;14:267-273.
77 Starshak RJ, Meyer GA, Choi SK, et al. Arachnoid cyst of the collicular cistern. Childs Nerv Syst. 1986;2:144-148.
78 Ohtsuka K, Hashimoto M, Nakamura Y. Bilateral trochlear nerve palsy with arachnoid cyst of the quadrigeminal cistern. Am J Ophthalmol. 1998;125:268-270.
79 Pagni CA, Canavero S, Vinci V. Left trochlear nerve palsy, unique symptom of an arachnoid cyst of the quadrigeminal plate. Case report. Acta Neurochir (Wien). 1990;105:147-149.
80 Topsakal C, Kaplan M, Erol F, et al. Unusual arachnoid cyst of the quadrigeminal cistern in an adult presenting with apneic spells and normal pressure hydrocephalus—case report. Neurol Med Chir (Tokyo). 2002;42:44-50.
81 Jallo GI, Woo HH, Meshki C, et al. Arachnoid cysts of the cerebellopontine angle: diagnosis and surgery. Neurosurgery. 1997;40:31-37.
82 Babu R, Murali R. Arachnoid cyst of the cerebellopontine angle manifesting as contralateral trigeminal neuralgia: case report. Neurosurgery. 1991;28:886-887.
83 Altinors N, Kars Z, Cepoglu C. Rare causes of hemifacial spasm. Report of two cases. Clin Neurol Neurosurg. 1991;93:155-158.
84 Higashi S, Yamashita J, Yamamoto Y, et al. Hemifacial spasm associated with a cerebellopontine angle arachnoid cyst in a young adult. Surg Neurol. 1992;37:289-292.
85 Takano S, Maruno T, Shirai S, et al. Facial spasm and paroxysmal tinnitus associated with an arachnoid cyst of the cerebellopontine angle—case report. Neurol Med Chir (Tokyo). 1998;38:100-103.
86 Felicio AC, Godeiro Cde OJr, Borges V, et al. Bilateral hemifacial spasm and trigeminal neuralgia: a unique form of painful tic convulsif. Mov Disord. 2007;22:285-286.
87 Achilli V, Danesi G, Caverni L, et al. Petrous apex arachnoid cyst: a case report and review of the literature. Acta Otorhinolaryngol Ital. 2005;25:296-300.
88 Ogutcen-Toller M, Uzun E, Incesu L. Clinical and magnetic resonance imaging evaluation of facial pain. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:652-658.
89 Bretelle F, Senat MV, Bernard JP, et al. First-trimester diagnosis of fetal arachnoid cyst: prenatal implication. Ultrasound Obstet Gynecol. 2002;20:400-402.
90 Blaicher W, Prayer D, Kuhle S, et al. Combined prenatal ultrasound and magnetic resonance imaging in two fetuses with suspected arachnoid cysts. Ultrasound Obstet Gynecol. 2001;18:166-168.
91 Golash A, Mitchell G, Mallucci C, et al. Prenatal diagnosis of suprasellar arachnoid cyst and postnatal endoscopic treatment. Childs Nerv Syst. 2001;17:739-742.
92 Fujimura J, Shima Y, Arai H, et al. Management of a suprasellar arachnoid cyst identified using prenatal sonography. J Clin Ultrasound. 2006;34:92-94.
93 Handa J, Nakano Y, Aii H. CT cisternography with intracranial arachnoidal cysts. Surg Neurol. 1977;8:451-454.
94 Dutt SN, Mirza S, Chavda SV, et al. Radiologic differentiation of intracranial epidermoids from arachnoid cysts. Otol Neurotol. 2002;23:84-92.
95 Decq P, Brugieres P, Le Guerinel C, et al. Percutaneous endoscopic treatment of suprasellar arachnoid cysts: ventriculocystostomy or ventriculocystocisternostomy? Technical note. J Neurosurg. 1996;84:696-701.
96 Yildiz H, Erdogan C, Yalcin R, et al. Evaluation of communication between intracranial arachnoid cysts and cisterns with phase-contrast cine MR imaging. AJNR Am J Neuroradiol. 2005;26:145-151.
97 Hoffmann KT, Hosten N, Meyer BU, et al. CSF flow studies of intracranial cysts and cyst-like lesions achieved using reversed fast imaging with steady-state precession MR sequences. AJNR Am J Neuroradiol. 2000;21:493-502.
98 Eguchi T, Taoka T, Nikaido Y, et al. Cine-magnetic resonance imaging evaluation of communication between middle cranial fossa arachnoid cysts and cisterns. Neurol Med Chir (Tokyo). 1996;36:353-357.
99 Tali ET, Ercan N, Kaymaz M, et al. Intrathecal gadolinium (gadopentetate dimeglumine)-enhanced MR cisternography used to determine potential communication between the cerebrospinal fluid pathways and intracranial arachnoid cysts. Neuroradiology. 2004;46:744-754.
100 Awaji M, Okamoto K, Nishiyama K. Magnetic resonance cisternography for preoperative evaluation of arachnoid cysts. Neuroradiology. 2007;49:721-726.
101 Munoz A, Hinojosa J, Esparza J. Cisternography and ventriculography gadopentate dimeglumine-enhanced MR imaging in pediatric patients: preliminary report. AJNR Am J Neuroradiol. 2007;28:889-894.
102 Girard N, Poncet M, Caces F, et al. Three-dimensional MRI of hemifacial spasm with surgical correlation. Neuroradiology. 1997;39:46-51.
103 Yamakami I, Kobayashi E, Hirai S, et al. Preoperative assessment of trigeminal neuralgia and hemifacial spasm using constructive interference in steady state-three-dimensional Fourier transformation magnetic resonance imaging. Neurol Med Chir (Tokyo). 2000;40:545-555.
104 Kurihara N, Takahashi S, Tamura H, et al. Investigation of hydrocephalus with three-dimensional constructive interference in steady state MRI. Neuroradiology. 2000;42:634-638.
105 Laitt RD, Mallucci CL, Jaspan T, et al. Constructive interference in steady-state 3D Fourier-transform MRI in the management of hydrocephalus and third ventriculostomy. Neuroradiology. 1999;41:117-123.
106 Fushimi Y, Miki Y, Ueba T, et al. Liliequist membrane: three-dimensional constructive interference in steady state MR imaging. Radiology. 2003;229:360-365.
107 Chang KH, Song IC, Kim SH, et al. In vivo single-voxel proton MR spectroscopy in intracranial cystic masses. AJNR Am J Neuroradiol. 1998;19:401-405.
108 Poptani H, Gupta RK, Jain VK, et al. Cystic intracranial mass lesions: possible role of in vivo MR spectroscopy in its differential diagnosis. Magn Reson Imaging. 1995;13:1019-1029.
109 Shukla-Dave A, Gupta RK, Roy R, et al. Prospective evaluation of in vivo proton MR spectroscopy in differentiation of similar appearing intracranial cystic lesions. Magn Reson Imaging. 2001;19:103-110.
110 Lai PH, Hsu SS, Ding SW, et al. Proton magnetic resonance spectroscopy and diffusion-weighted imaging in intracranial cystic mass lesions. Surg Neurol. 2007;68(Suppl 1):S25-S36.
111 Nguyen JB, Ahktar N, Delgado PN, et al. Magnetic resonance imaging and proton magnetic resonance spectroscopy of intracranial epidermoid tumors. Crit Rev Comput Tomogr. 2004;45:389-427.
112 Martinez-Lage JF, Valenti JA, Piqueras C, et al. Functional assessment of intracranial arachnoid cysts with TC99 m-HMPAO SPECT: a preliminary report. Childs Nerv Syst. 2006;22:1091-1097.
113 De Volder AG, Michel C, Thauvoy C, et al. Brain glucose utilisation in acquired childhood aphasia associated with a sylvian arachnoid cyst: recovery after shunting as demonstrated by PET. J Neurol Neurosurg Psychiatry. 1994;57:296-300.
114 Pena A, Owler BK, Fryer TD, et al. A case study of hemispatial neglect using finite element analysis and positron emission tomography. J Neuroimaging. 2002;12:360-367.
115 Zaatreh MM, Bates ER, Hooper SR, et al. Morphometric and neuropsychologic studies in children with arachnoid cysts. Pediatr Neurol. 2002;26:134-138.
116 Beltramello A, Mazza C. Spontaneous disappearance of a large middle fossa arachnoid cyst. Surg Neurol. 1985;24:181-183.
117 Weber R, Voit T, Lumenta C, et al. Spontaneous regression of a temporal arachnoid cyst. Childs Nerv Syst. 1991;7:414-415.
118 Inoue T, Matsushima T, Tashima S, et al. Spontaneous disappearance of a middle fossa arachnoid cyst associated with subdural hematoma. Surg Neurol. 1987;28:447-450.
119 Takagi K, Sasaki T, Basugi N. [Spontaneous disappearance of cerebellopontine angle arachnoid cyst: report of a case]. No Shinkei Geka. 1987;15:295-299.
120 Yamanouchi Y, Someda K, Oka N. Spontaneous disappearance of middle fossa arachnoid cyst after head injury. Childs Nerv Syst. 1986;2:40-43.
121 Struck AF, Murphy MJ, Iskandar BJ. Spontaneous development of a de novo suprasellar arachnoid cyst. Case report. J Neurosurg. 2006;104:426-428.
122 Dodd RL, Barnes PD, Huhn SL. Spontaneous resolution of a prepontine arachnoid cyst. Case report and review of the literature. Pediatr Neurosurg. 2002;37:152-157.
123 Pandey P, Tripathi M, Chandra PS, et al. Spontaneous decompression of a posterior fossa arachnoid cyst: a case report. Pediatr Neurosurg. 2001;35:162-163.
124 Bristol RE, Albuquerque FC, McDougall C, et al. Arachnoid cysts: spontaneous resolution distinct from traumatic rupture. Case report. Neurosurg Focus. 2007;22:E2.
125 Seizeur R, Forlodou P, Coustans M, et al. Spontaneous resolution of arachnoid cysts: review and features of an unusual case. Acta Neurochir (Wien). 2007;149:75-78.
126 Muthukumar M. Arachnoid cyst resolution. J Neurosurg. 1996;85:983-984.
127 Aoki N, Sakai T. Intraoperative subdural hematoma in a patient with arachnoid cyst in the middle cranial fossa. Childs Nerv Syst. 1990;6:44-46.
128 Auer LM, Gallhofer B, Ladurner G, et al. Diagnosis and treatment of middle fossa arachnoid cysts and subdural hematomas. J Neurosurg. 1981;54:366-369.
129 Eustace S, Toland J, Stack J. CT and MRI of arachnoid cyst with complicating intracystic and subdural haemorrhage. J Comput Assist Tomogr. 1992;16:995-997.
130 Page A, Paxton RM, Mohan D. A reappraisal of the relationship between arachnoid cysts of the middle fossa and chronic subdural haematoma. J Neurol Neurosurg Psychiatry. 1987;50:1001-1007.
131 Page AC, Mohan D, Paxton RM. Arachnoid cysts of the middle fossa predispose to subdural haematoma formation: fact or fiction? Acta Neurochir Suppl (Wien). 1988;42:210-215.
132 Rogers MA, Klug GL, Siu KH. Middle fossa arachnoid cysts in association with subdural haematomas. A review and recommendations for management. Br J Neurosurg. 1990;4:497-502.
133 Servadei F, Vergoni G, Frattarelli M, et al. Arachnoid cyst of middle cranial fossa and ipsilateral subdural haematoma: diagnostic and therapeutic implications in three cases. Br J Neurosurg. 1993;7:249-253.
134 Hopkin J, Mamourian A, Lollis S, et al. The next extreme sport? Subdural haematoma in a patient with arachnoid cyst after head shaking competition. Br J Neurosurg. 2006;20:111-113.
135 Tsuzuki N, Katoh H, Ohtani N. Chronic subdural hematoma complicating arachnoid cyst secondary to soccer-related head injury: case report. Neurosurgery. 2003;53:242-243.
136 Galassi E, Tognetti F, Pozzati E, et al. Extradural hematoma complicating middle fossa arachnoid cyst. Childs Nerv Syst. 1986;2:306-308.
137 Ziaka DS, Kouyialis AT, Boviatsis EJ, et al. Asymptomatic massive subdural hematoma in a patient with bitemporal agenesis and bilateral temporal arachnoid cysts. South Med J. 2008;101:324-326.
138 Chan JY, Huang CT, Liu YK, et al. Chronic subdural hematoma associated with arachnoid cyst in young adults: a case report. Kaohsiung J Med Sci. 2008;24:41-44.
139 Balak N, Silav G, Kilic Y, et al. Successful surgical treatment of a hemophiliac infant with nontraumatic acute subdural hematoma. Surg Neurol. 2007;68:537-540.
140 Demetriades AK, McEvoy AW, Kitchen ND. Subdural haematoma associated with an arachnoid cyst after repetitive minor heading injury in ball games. Br J Sports Med. 2004;38:E8.
141 Boviatsis EJ, Maratheftis NL, Kouyialis AT, et al. Atypical presentation of an extradural hematoma on the grounds of a temporal arachnoid cyst. Clin Neurol Neurosurg. 2003;105:225-228.
142 Mori K, Yamamoto T, Horinaka N, et al. Arachnoid cyst is a risk factor for chronic subdural hematoma in juveniles: twelve cases of chronic subdural hematoma associated with arachnoid cyst. J Neurotrauma. 2002;19:1017-1027.
143 Ulmer S, Engellandt K, Stiller U, et al. Chronic subdural hemorrhage into a giant arachnoidal cyst (Galassi classification type III). J Comput Assist Tomogr. 2002;26:647-653.
144 Kawanishi A, Nakayama M, Kadota K. Heading injury precipitating subdural hematoma associated with arachnoid cysts—two case reports. Neurol Med Chir (Tokyo). 1999;39:231-233.
145 Martinez-Lage JF. Neurosurgical treatment for hydrocephalus, subdural hematomas, and arachnoid cysts in glutaric aciduria type 1. Neuropediatrics. 1996;27:335-336.
146 Attane F, Frerebeau P, Tannier C, et al. [Arachnoid cysts and subdural hematomas]. Rev Neurol (Paris). 1996;152:695-699.
147 Munoz A, Benito-Leon J, Carrasco A, et al. [Subdural hematoma associated with arachnoid cyst]. Neurologia. 1996;11:263-267.
148 Oka Y, Kumon Y, Ohta S, et al. Chronic subdural hematoma associated with middle fossa arachnoid cysts—three case reports. Neurol Med Chir (Tokyo). 1994;34:95-99.
149 Kadiogly HH, Ozturk M, Aydin IH. Extradural hematoma complicating arachnoid cyst—case report. Zentralbl Neurochir. 1994;55:172-174.
150 van Burken MM, Sarioglu AC, O’Donnell HD. Supratentorial arachnoidal cyst with intracystic and subdural haematoma. Neurochirurgia (Stuttg). 1992;35:199-203.
151 Molloy CJ, Jones NR, North JB. Arachnoid cyst presenting as an extradural haematoma. Br J Neurosurg. 1991;5:635-637.
152 Kulali A, von Wild K. Post-traumatic subdural hygroma as a complication of arachnoid cysts of the middle fossa. Neurosurg Rev. 1989;12(suppl 1):508-513.
153 Tamburrini G, Del Fabbro M, Di Rocco C. Sylvian fissure arachnoid cysts: a survey on their diagnostic workout and practical management. Childs Nerv Syst. 2008;24:593-604.
154 Gosalakkal JA. Intracranial arachnoid cysts in children: a review of pathogenesis, clinical features, and management. Pediatr Neurol. 2002;26:93-98.
155 Wester K. Peculiarities of intracranial arachnoid cysts: location, sidedness, and sex distribution in 126 consecutive patients. Neurosurgery. 1999;45:775-779.
156 Di Rocco C, Tamburrini G, Caldarelli M, et al. Prolonged ICP monitoring in sylvian arachnoid cysts. Surg Neurol. 2003;60:211-218.
157 Galassi E, Gaist G, Giuliani G, et al. Arachnoid cysts of the middle cranial fossa: experience with 77 cases treated surgically. Acta Neurochir Suppl (Wien). 1988;42:201-204.
158 Raffel C, McComb JG. To shunt or to fenestrate: which is the best surgical treatment for arachnoid cysts in pediatric patients? Neurosurgery. 1988;23:338-342.
159 Serlo W, von Wendt L, Heikkinen E, et al. Shunting procedures in the management of intracranial cerebrospinal fluid cysts in infancy and childhood. Acta Neurochir (Wien). 1985;76:111-116.
160 Ohnishi YI, Fujimoto Y, Taniguchi M, et al. Neuroendoscopically assisted cyst-cisternal shunting for a quadrigeminal arachnoid cyst causing typical trigeminal neuralgia. Minim Invasive Neurosurg. 2007;50:124-127.
161 Yamane K, Yoshimoto H, Harada K, et al. [Case of spontaneous ventriculocisternostomy: with special reference to a CT finding]. No Shinkei Geka. 1983;11:491-497.
162 Huang Q, Wang D, Guo Y, et al. The diagnosis and neuroendoscopic treatment of noncommunicating intracranial arachnoid cysts. Surg Neurol. 2007;68:149-154.
163 Elhammady MS, Bhatia S, Ragheb J. Endoscopic fenestration of middle fossa arachnoid cysts: a technical description and case series. Pediatr Neurosurg. 2007;43:209-215.
164 Di Rocco F, Yoshino M, Oi S. Neuroendoscopic transventricular ventriculocystostomy in treatment for intracranial cysts. J Neurosurg. 2005;103:54-60.
165 Nakamura Y, Mizukawa K, Yamamoto K, et al. Endoscopic treatment for a huge neonatal prepontine-suprasellar arachnoid cyst: a case report. Pediatr Neurosurg. 2001;35:220-224.
166 Sandberg DI, Souweidane MM. Endoscopic-guided proximal catheter placement in treatment of posterior fossa cysts. Pediatr Neurosurg. 1999;30:180-185.
167 Hopf NJ, Perneczky A. Endoscopic neurosurgery and endoscope-assisted microneurosurgery for the treatment of intracranial cysts. Neurosurgery. 1998;43:1330-1336.
168 Ersahin Y, Kesikci H, Ruksen M, et al. Endoscopic treatment of suprasellar arachnoid cysts. Childs Nerv Syst. 2008;24:1013-1020.
169 Ruge JR, Johnson RF, Bauer J. Burr hole neuroendoscopic fenestration of quadrigeminal cistern arachnoid cyst: technical case report. Neurosurgery. 1996;38:830-837.
170 Cavallo LM, Prevedello D, Esposito F, et al. The role of the endoscope in the transsphenoidal management of cystic lesions of the sellar region. Neurosurg Rev. 2008;31:55-64.
171 Strojnik T. Different approaches to surgical treatment of arachnoid cysts. Wien Klin Wochenschr. 2006;118(suppl 2):85-88.
172 Greenfield JP, Souweidane MM. Endoscopic management of intracranial cysts. Neurosurg Focus. 2005;19(6):E7.
173 Charalampaki P, Filippi R, Welschehold S, et al. Endoscopic and endoscope-assisted neurosurgical treatment of suprasellar arachnoidal cysts (Mickey Mouse cysts). Minim Invasive Neurosurg. 2005;48:283-288.
174 Hayashi N, Hamada H, Umemura K, et al. [Selection of surgical approach for quadrigeminal cistern arachnoid cyst]. No Shinkei Geka. 2005;33:457-465.
175 Nomura S, Akimura T, Imoto H, et al. Endoscopic fenestration of posterior fossa arachnoid cyst for the treatment of presyrinx myelopathy—case report. Neurol Med Chir (Tokyo). 2002;42:452-454.
176 Gangemi M, Maiuri F, Colella G, et al. Endoscopic surgery for large posterior fossa arachnoid cysts. Minim Invasive Neurosurg. 2001;44:21-24.
177 Brunori A, Delitala A, Chiappetta F. Endoscopy for cysts. J Neurosurg. 1999;91:1067-1068.
178 Paladino J, Rotim K, Heinrich Z. Neuroendoscopic fenestration of arachnoid cysts. Minim Invasive Neurosurg. 1998;41:137-140.
179 Furuta S, Hatakeyama T, Nishizaki O, et al. Usefulness of neuroendoscopy in treating supracollicular arachnoid cysts—case report. Neurol Med Chir (Tokyo). 1998;38:107-109.
180 Dhooge C, Govaert P, Martens F, et al. Transventricular endoscopic investigation and treatment of suprasellar arachnoid cysts. Neuropediatrics. 1992;23:245-247.
181 Walker ML, Carey L, Brockmeyer DL. The NeuroNavigational 1.2-mm Neuroview Neuroendoscope. Neurosurgery. 1995;36:617-618.
182 Walker ML, Petronio J, Carey C. Ventriculoscopy. In: Cheek W, editor. Pediatric Neurosurgery: Surgery of the Developing Nervous System. Philadelphia: WB Saunders; 1994:572-581.
183 Osenbach RK, Godersky JC, Traynelis VC, et al. Intradural extramedullary cysts of the spinal canal: clinical presentation, radiographic diagnosis, and surgical management. Neurosurgery. 1992;30:35-42.