CHAPTER 39 Aqueous tube shunts
Introduction
Glaucoma drainage implants in use today were originally developed by Molteno over 40 years ago, as a single-plate paralimbal implant1. However, the paralimbal position resulted in significant complications; the drainage implants were redesigned so that the plate was made larger, was moved away from the limbus, and was connected to the anterior chamber by a long silicone tube. The physiological principles leading to this design were based on the concepts that a larger surface area would produce a larger drainage area and that the posteriorly positioned plate would result in fewer complications than the original paralimbal plate. Further modifications of Molteno’s implant saw the addition of a second plate, and use of a dual chamber, which restricts drainage initially to a small area anteriorly on the plate surface with later aqueous spread to the remaining plate surface.
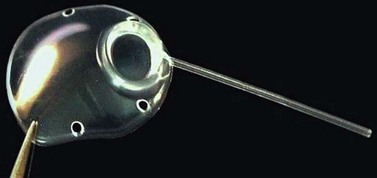
Currently used implants are all long-tube implants, and the most common are single plate large area implants, which are either valved or unvalved. The valved implants include the Ahmed (New World Medical, Rancho Cucamonga, CA) and Krupin (Hood Laboratories, Pembroke, MA) implants. The non-valved implants are the Baerveldt (Advanced Medical Optics, Inc., Santa Ana, CA) and Molteno (IOP, Costa Mesa, CA) implants. Ahmed and Molteno implants are also available as double-plate devices; however, the trend is not to use these double-plate devices. Molteno has recently introduced a larger, thinner and more curved single plate, the Molteno3, which is available in two sizes, 175 mm2 and 230 mm2 (Fig. 39.1). The purpose of glaucoma implants is to create a large drainage bleb, whose permeability controls pressure. Knowledge about drainage bleb physiology should allow modifications that render greater efficiency.
Fundamental principles and physiology of implant blebs
The implant drains aqueous to the plate surface, creating a bleb by elevating the overlying tissue. Drainage efficiency depends on bleb permeability, which in turn depends on aqueous composition, bleb surface area, and tissue reaction. The concept that the bigger the bleb the better the control is only partly true. The original studies supporting ‘bigger is better’ compared single plate Molteno implants with double-plate models over only 12 months2. Subsequently pressure lowering effects of a double-plate Molteno implant were shown to be no more effective than a single plate3. Later studies comparing Baerveldt 350 mm2 with 500 mm2 implants showed the 350 mm implant was more effective in pressure reduction4. Thus, although larger plate size has a minimal effect to reduce pressure further, this effect does not increase exponentially; bleb efficiency does not improve exponentially above a certain size, which may be somewhere between 175 and 240 mm2. Double plate implants become unnecessary, leaving an upper quadrant available for possible future drainage surgery, and eliminating the more arduous and invasive form of implant surgery.
Glaucomatous aqueous contains pro-inflammatory cytokines5,6. Allowing this aqueous to reach the plate surface immediately after implant insertion, as with valved implants, produces a more intense hypertensive phase, and a less efficient bleb7,8. Preventing ‘glaucomatous aqueous’ from reaching the plate surface until IOP has been normalized results in a more efficient bleb7,8. A study has also shown that the lining of the bleb during the ‘hypertensive phase’ produces high levels of pro-inflammatory cytokines, resulting in greater bleb fibrosis9. During the hypertensive phase, the IOP can be decreased by tapping the bleb with a 30-gauge needle, thereby preventing further cytokine production10. Molteno et al.11 described the histopathology of capsules around plates in primary and secondary glaucomas. Without aqueous reaching the plate surface, the plates were encapsulated by a thin avascular collagen layer. The delayed release of aqueous from eyes with a pressure of 25–35 mmHg resulted in capsules with fewer fibrovascular than fibrodegenerative components. Immediate aqueous flow, as occurs in valved or non-ligatured implants, produced thick capsules composed of an outer fibrovascular layer and an inner fibrodegenerative layer of approximately equal thickness. Molteno concluded that without aqueous flow the capsule consists of a thin collagenous avascular layer. With immediate flow of aqueous to the plate surface, an inflammatory reaction occurred, resulting in a capsule containing collagenous and vascular components. After a period of time, a fibrodegenerative process develops in the deeper layers of the capsule, maintained by activation, migration, apoptosis, and production of death messengers by mesodermal cells. This fibrodegenerative process may depend on sufficient IOP for aqueous to displace interstitial fluid from the deeper layers of the capsule. The final thickness of the capsule depends on the timing of these opposing processes, which can be influenced by surgical technique and the use of anti-inflammatory medications.
Indications for surgery
Implant insertion remains the procedure of choice in refractory glaucomas, including aphakic and pseudophakic glaucoma, failed glaucoma filtering surgery, neovascular glaucoma, glaucoma associated with corneal transplants, uveitic glaucoma, and congenital glaucomas that are due to iridocorneal dysgenesis. Many of these glaucomas may be treated with anti-metabolites and standard filtration, but due to increasing complications seen with the use of anti-metabolites the indications for glaucoma implant has risen. Scarring of the paralimbal conjunctiva remains a prime indication for a glaucoma implant, providing that the scarring is not too extensive. The findings of the ‘Tube Versus Trabeculectomy’ study may result in the more frequent use of drainage implants as a primary surgical procedure for medically unconcontrolled glaucoma. Primary outcomes for both tubes and trabeculectomies were good with the mean IOP similar for both. The percentage of overall success was higher in the tube group, with the trabeculectomy group requiring less postoperative medications, a finding that was found in the 3- as well as the 1-year follow-up of the study12.
Preoperative assessment
The anatomy of the eye and orbit may determine the implant type, and where it can be placed. A very small orbit may preclude the insertion of any implant. The optimal positioning of different implants has been evaluated using cadaver eyes13, although this study did not include the newer larger single plate Molteno3 implant. The Molteno implant could be placed most posteriorly from the limbus without impinging on the optic nerve, and the Ahmed implant was least amenable to posterior placement. Postmortem findings in an eye with an Ahmed implant led the authors to suggest more anterior placement of the device if used in the supero-nasal quadrant, to avoid possible optic nerve damage14. The size of the orbit needs to be evaluated, as postoperative ocular mobility problems within a small orbit may occur due to bleb size. Implants such as the Baerveldt implant, which gives a lower bleb profile, may be more appropriate in such a case.
The pressure lowering effects of five implants (Molteno single and double plate, Ahmed, Baerveldt, and Krupin implants) was determined to range between 51 and 62%, with no significant differences in either percentage change in IOP or the overall surgical success based on the size of the end plate15. As discussed, larger plate sizes have not been shown to produce significantly lower IOP and a plate size somewhere between 175 mm2 and 250 mm2 may give the maximum pressure lowering potential. This allows the use of single plate implants, which are far easier to insert than double plates, as well as requiring only a single quadrant.of the eye. Achieving immediate postoperative pressure lowering without overt hypotony may seem possible only with a valved implant, but this can be achieved also by occluding the silicone tube in non-valved implants and creating slits in the tubes to control pressure prior to removing the stent16.
The preoperative assessment needs to consider the fibrotic potential of the eye, which is likely to be greater in eyes with previous failed filtration procedures, young patients, and black patients. The use of systemic anti-fibrosis medications, as suggested by Molteno17, or surgical modification such as supra-Tenons implant insertion may be an option in these eyes18.
The presence of a cataract, or corneal opacity, may necessitate a combined cataract extraction and/or corneal transplantation19–21. If there is vitreous in the anterior chamber, more likely in aphakia, a vitrectomy (anterior or posterior) may be required to prevent blockage of the tube by vitreous. Placing the tube in the posterior chamber necessitates a total vitrectomy.
Surgical technique
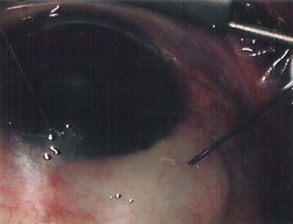
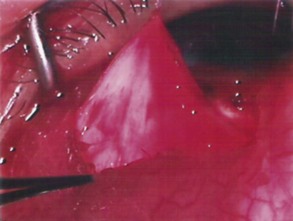
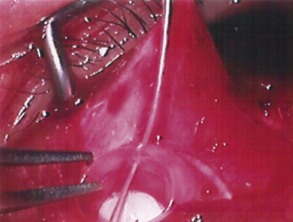
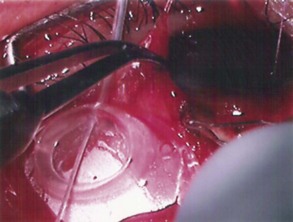
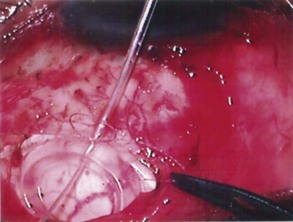
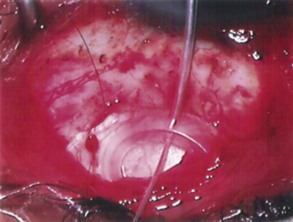
The conjunctival flap may be limbal or fornix based, with most surgeons preferring fornix-based flaps. The conjunctiva is elevated off the underlying sclera with an injection of balanced salt solution (Fig. 39.2). The initial limbal incision is made with a Bard-Parker blade, and continued with spring scissors, for approximately 12 mm. Vertical relieving incisions are then made approximately 5 mm in length parallel to the superior rectus and/or lateral rectus muscles depending on quadrant use. The two edges of the cut limbal conjunctiva are marked with identifying sutures allowing for accurate replacement of the conjunctiva on completion of the surgery (Fig. 39.3). At this stage, if total subconjunctival placement of the implant is the intention, then further posterior dissection of the conjunctiva and Tenons’ capsule off the sclera is continued using a spring scissors, until a pocket approximately 12 mm deep has been created for placement of the implant. The implant may also be placed into a supra-Tenons pocket18. After the initial limbal incision and relieving incisions have been made, a 7-0 Vicryl suture is placed into Tenon’s capsule below the conjunctiva, and Tenon’s capsule is pulled forward, facilitating the dissection of the overlying conjunctiva off Tenon’s capsule below it (see Fig. 39.3). A plane of dissection is then found between Tenon’s and conjunctiva, and dissection in this plane is carried out creating a pocket between the conjunctiva and the underlying Tenon’s into which the implant will be placed (Fig. 39.4). The thickness of the conjunctiva can be titrated by dissecting either closer or further away from the overlying Tenon’s. It is of utmost importance to allow an adequate amount of Tenon’s to remain with the conjunctiva, to prevent possible conjunctival erosion over the ridge of the plate, prior to allowing aqueous onto the plate surface. Tenon’s capsule anterior to the front edge of the plate is dissected off the underlying sclera for approximately 7 mm to allow fixation of the plate to the sclera. This anterior Tenon’s is removed with cautery to prevent bleeding (Fig. 39.5). The Molteno3 implant is the ideal implant for use in supra-Tenon’s placement due to its size and design.
If a Baerveldt or Molteno implant is being used then, prior to insertion of the plate, a 3-0 Supramid suture is placed into the lumen of the silicone tube (Fig. 39.6). The suture is inserted halfway down the tube, leaving the external end long. This suture will act as a stent to prevent postoperative hypotony, as it will be further occluded by cinching it to the tube with a 7-0 Vicryl suture (see Fig. 39.6). The suture will be removed approximately 3 weeks postoperatively, as the free end will be available at the limbus under the conjunctiva.
The plate is fixed to the underlying sclera with an 8-0 nylon suture, the most suitable needle being an 8-0 Ethilon (Ethicon Inc) TG175-8 spatula needle. The curve allows accurate placement of the anterior edge of the implant as the needle emerges very close to the initial entry point. The anterior edge of the plate is sutured 7–10 mm from the limbus (Fig. 39.7). These distances from the limbus allow for adequate coverage of the blebs by the lid, as well as allowing easy access to the bleb should this become necessary, to observe and to tap during the hypertensive phase.
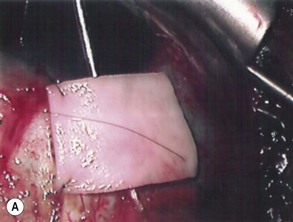
The preparation of the tube takes place after the plate has been secured in position. Preserved donor sclera, cornea, or pericardium is brought onto the operative field to be measured and cut to size so that the tube is covered from the limbus to its insertion into the plate. The prepared graft is sutured at three corners to the sclera with a 10-0 nylon suture, leaving the third suture loose at one side allowing the graft to be retracted to one side, giving access to the underlying tube (Fig. 39.8A&B). The final suturing of the graft, by placing the fourth suture at the remaining corner, takes place after the tube has been introduced into the anterior chamber.
Before insertion into the eye, the tube is shortened. The intraocular portion of the tube should be approximately 2–3 mm. The length may be adjusted according to the condition being managed: in neovascular glaucoma a longer intraocular length may be required to prevent occlusion of the tube by fibrous tissue. Measurement of the tube is done over the external corneal surface, and therefore no traction must be placed on the tube as this will result in leaving the tube shorter than the desired measurement. The tube is cut with scissors, the blades facing up so that the end of the tube has an upward facing bevel, preventing the tip from iris occlusion should it be close to the iris (Fig. 39.9A&B). This bevel-up position also facilitates easier insertion of the tube. The ideal position for the tube is midway between the iris and corneal endothelium. Prior to inserting the tube, a paracentesis opening is made on the temporal side of the limbus, allowing for manipulation of the tube should the need occur following its insertion. The eye is entered into at the limbus with a 22- or 23-gauge needle (Fig. 39.10). The presence of peripheral anterior synechiae, as often in neovascular glaucoma, may necessitate needle entry beyond the limbus, to ensure passage of the tube into the anterior chamber and not into the iris or posterior chamber. The direction of the needle entry is midway between the iris and cornea. The cut end of the tube is grasped in a tying forceps and inserted through the needle entry wound into the anterior chamber. Prior to insertion, in non-valved implants, the tube is totally occluded by cinching the intra-tubal 3-0 Supramid suture with a 7-0 Vicryl suture. The anterior chamber remains well formed as no aqueous is passing to the plate . A venting slit (or slits) is made in the external portion of the tube with a micro-sharp blade (Fig. 39.11). The length of the slit(s) is 2–3 mm. A bubble of aqueous will be seen emerging from the slit indicating aqueous drainage from the anterior chamber (Fig. 39.12). These slits can also be made by passing the needle of a 10-0 suture through the tube16. The tube is then fixed to the sclera with a single 10-0 nylon suture (Fig. 39.13).
The pre-placed graft is secured by tying the final two 10-0 sutures. The Supramid suture emerging from the posterior opening of the tube is now brought forward over the surface of the graft allowing it to emerge at the limbus and finally be covered by the conjunctiva (Fig. 39.14). The conjunctiva is brought forward, and the previously placed locating sutures allow the accurate placement of the conjunctiva to its original position at the limbus The conjunctiva is sutured to the limbus with two 7-0 Vicryl sutures, the medial and lateral relieving incisions being sutured with a running 7-0 Vicryl suture. The 3-0 Supramid suture, which protrudes from below the conjunctiva at the limbus, is trimmed so that the cut end is just at the limbus, allowing for easy access when its removal is required (Fig. 39.15A&B).
Postoperative complications
Hypertensive phase
This occurs usually 4–6 weeks postoperatively. However, the hypertensive phase does not always occur and has been found to be more common in valved implants. This is probably because cytokines, found in glaucomatous aqueous, are able to reach the plate surface immediately after insertion of the device, thereby producing an inflammatory reaction, which intensifies in 4–6 weeks. With high pressure within the bleb, more cytokines are produced, resulting in more inflammation and aggravation of the hypertensive phase. Untreated the hypertensive phase will settle with time, but result in a more fibrous final bleb. Lowering the pressure during the hypertensive phase therefore is mandatory, and this can be achieved with the use of anti-hypertensive medications, or with aqueous removal from the bleb with a 30-gauge needle. This can be done in the office with the use of topical anesthetic and antiseptic. As much as a half cc can be removed without loss of the anterior chamber, and can be repeated usually weekly until pressure settles. Should the capsule appear to be very thick, a burr can be made on the aspirating needle, which can be turned to face the bleb wall, and used to tear open the inner lining of the bleb; this usually results in a second small bleb beneath the overlying conjunctiva (Fig. 39.16).
Tube
Tube–corneal touch may occur in a small localized area, which may produce a localized corneal opacity without any further serious corneal damage, and can be left alone. More extensive contact between tube and cornea may result in corneal endothelial decompensation and will require repositioning of the tube. This is more likely to occur in aphakics, pseudophakics, and eyes that have undergone previous filtering procedures. Multiple intraocular procedures are associated with endothelial cell loss and render the corneas more likely to decompensate, particularly if tubes touch the endothelium, or are close enough to touch the endothelium with eye-rubbing. Corneal transplants are necessary in corneal decompensation associated with glaucoma implants. The tube can be left in the anterior chamber if positioned away from the cornea, or repositioned before the graft. Tubes that appear to be close to the endothelium can be deflected away with a suture placed over the tube within the anterior chamber22. Tubes that appear likely to cause endothelial damage can be removed, cut, and repositioned, or can be removed and placed into the posterior chamber in pseudophakic eyes or into the vitreous cavity in phakic or aphakic eyes, in association with a total vitrectomy.
Plate
Cataracts and retinal detachments are potential complications of tube shunt surgery23,24.
Glaucoma implants may be used to treat glaucoma occurring after retinal detachment surgery, and generally require modification of routine implantation. The two situations that require special attention are the presence of a scleral buckle and silicone oil. Removal of a buckle, where possible, will allow an implant to be inserted more easily. Placing an implant over a buckle increases bulk, which may confound conjunctival coverage. Placement of an implant over a buckle requires a low profile implant such as the Baerveldt. The Ahmed is more bulky and would be less desireable. The author has placed a Molteno3 implant in a supra-Tenon’s pocket above a pre-existing buckle. This method of placing an implant has proven to be relatively easy, as the conjunctival pocket does not require exposure of the underlying sclera or buckle, and subsequent conjunctival closure is easy. The implant extends posteriorly beyond the buckle, which lies undisturbed below. The ability to avoid contact with the underlying buckle avoids many of the complications that have been reported with implants placed directly in contact with buckles25,26. The presence of silicone oil is a major impediment to the use of glaucoma implants, although placement of implants inferiorly has been reported with good results27. Removal of the oil followed by implant insertion has also been reported with a success rate of 57%28. Silicone oil does not filter well through implants and its presence subconjunctivally is not ideal.
Assessment of surgery with glaucoma implants
The surgical results obtained by the various implants have been compared, mainly in non-comparative retrospective trials. Ahmed implants and Baerveldt implants, when compared in separate studies with similar patient groups, demonstrated similar success rates after 12 months’ follow-up29.While the Ahmed implant showed less postoperative hypotony, there may be a higher incidence of the hypertensive phase7,8. Long-term follow-up with glaucoma implants has demonstrated a decrease in success rate from between 73 and 83%, to an average of 63%23.
Hong et al. reported from a literature review the results obtained from the use of the Molteno, Baerveldt, Ahmed, and Krupin implants. The findings revealed that the overall surgical success rate averaged between 72% and 79% among the five devices with no statistical difference at the last follow-up time. The double plate Molteno had a higher success rate, but this was with a shorter follow-up period than for the other devices. All the devices reduced the intraocular pressure by greater than 50% when compared with the preoperative pressure. Success rate has been reported to decrease by 10–15% every year in the first 3 years in a life time analysis comparing the Ahmed and double-plate Molteno implants30.
1 Molteno ACB, Luntz MH. The use of plastics in glaucoma surgery. Proceedings of the first South African International Ophthalmological Symposium. London: Butterworths; 1969.
2 Heuer DK, Lloyd MA, Abrams DA, et al. Which is better? One or two? A randomized clinical trial of single plate versus double plate Molteno implantation in aphelia and pseudophakia. Ophthalmology. 1992;99:1512-1519.
3 Molteno ACB, Bevin TH, Herbison P, et al. Otago glaucoma surgery outcome study. Long term follow-up of primary glaucoma with additional risk factors drained by Molteno implants. Ophthalmology. 2001;12:2193-2200.
4 Britt MT, Labree ID, Lloyd MA, et al. Randomized trial of the 350 mm2 versus the 500 mm2 Baerveldt implant: longer term results. Is bigger better? Ophthalmology. 1999;106:2312-2318.
5 Jampel HD, Roche N, Stoke WJ, et al. Transforming growth factor beta in human aqueous humor. Curr Eye Res. 1990;9:963-969.
6 Tripathi RC, Borisuth NSC, Li J, et al. Growth factors in the aqueous humor and their clinical significance. J Glaucoma. 1994;3:248-258.
7 Nour-Mahdavi K, Caprioli J. Evaluation of the hypertensive phase after insertion of the Ahmed glaucoma valve. Am J Ophthalmol. 2003;136:1001-1008.
8 Tsai JC, Johnson CC, Dietrich MS. The Ahmed shunt versus the Baerveldt for refractory glaucoma A single surgeon comparison of outcome. Ophthalmology. 2003;110:1814-1821.
9 Freedman J, Goddard D. Elevated levels of transforming growth factor b and prostaglandin E2 in aqueous humor from patients undergoing filtration surgery for glaucoma. Can J Ophthalmol. 2008;43:370.
10 Freedman J, Melamed S, Trope GE. Management of glaucoma implant complications. Glaucoma Surgery. London: Taylor & Francis; 2005.
11 Molteno ACB, Fucik M, Dempster AG, et al. Otago glaucoma surgery outcome study; factors controlling capsule fibrosis around Molteno implants with histological correlation. Ophthalmology. 2003;110:2198-2206.
12 Gedde SJ, Schiffman JC, Feuer WJ, et al. Three year follow-up of the Tube versus Trabeculectomy Study. Am J Ophthalmol. 2009;148:670-684.
13 Kahook MY, Noecker RJ, Pantcheva MB, et al. Location of glaucoma draining devices to the optic nerve. Br J Ophthalmol. 2006;90:1010-1013.
14 Ayyala RS, Layden WE, Slonim CB, et al. Anatomic and histopathologic findings following a failed Ahmed glaucoma device. Ophthal Surg Lasers. 2001;32:248-249.
15 Hong CH, Arosemina A, Zurakowski D, et al. Glaucoma drainage devices: A systematic literature review and current controversies. J Survophthal. 2004;50:48-60.
16 Sherwood MB, Smith MF. Prevention of early hypotony associated with Molteno implants by a new occluding stent technique. Ophthalmology. 1993;100:85-90.
17 Molteno ACB, Straughn JL, Ancker E. Control of bleb fibrosis after glaucoma surgery by anti-inflammatory agents. S Afr Med J. 1976;50:881-885.
18 Freedman J, Chamnongvongse P. Supra-Tenons’ capsule placement of a single-plate Molteno implant. Br J Ophthalmol. 2008;92:669-672.
19 Johnson RH, Nguyen R, Jongsareejit A, et al. Clinical study of combined penetrating keratoplasty, pars plana vitrectomy with temporary keratoprosthesis, and pars plana seton implant. Retina. 1999;19:116-121.
20 Sidoti PA, Mosny AY, Ritterband DC, et al. Pars plana tube insertion of glaucoma drainage implants and penetrating keratoplasty in patients with coexisting glaucoma and corneal disease. Ophthalmology. 2001;108:1050-1058.
21 Hoffman KB, Feldman RM, Budenz DL, et al. Combined cataract extraction and Baerveldt glaucoma drainage implant: indications and outcomes. Ophthalmology. 2002;1089:1916-1920.
22 Freedman J. Management of the Molteno silicone tube in corneal transplant surgery. Ophthalmic Surg Lasers. 1988;29:432-434.
23 Waterhouse WJ, Lloyd MAE, Dugel PU, et al. Rhegmatogenous retinal detachment after Molteno glaucoma implant surgery. Ophthalmology. 1994;101:665-671.
24 Benz MS, Scott IU, Flynn HWJr, et al. Retinal detachment in patients with preexisting glaucoma drainage device: anatomic, visual acuity, and intraocular pressure outcomes. Retina. 2002;22(3):283-287.
25 Sidoti PA, Minckler DS, Baerveldt G, et al. Aqueous tube shunt to a pre-existing episcleral encircling element in the treatment of complicated glaucomas. Ophthalmology. 1994;101:1043.
26 Smith MF, Doyle JW, Famous MM. Modified aqueous drainage implants in the treatment of complicated glaucomas in eyes with pre-existing episcleral bands. Ophthalmology. 1998;105:2237-2242.
27 Al Jazzaf AM, Netland PA, Charles S. Incidence and management of elevated intraocular pressure after silicone oil injection. J Glaucoma. 2005;14:40-46.
28 Nguyen QH, Lloyd, Heuer DK, et al. Incidence and management of glaucoma after intravitreal silicone oil injection for complicated retinal detachments. Ophthalmology. 1992;99:1520-1526.
29 Tsai JC, Johnson CC, Dietrich MS. The Ahmed shunt versus the Baerveldt shunt for refractory glaucoma – a single surgeon comparison of outcome. Ophthalmology. 2003;110:1814-1821.
30 Ayala RS, Zurakowski D, Monshizadeh R, et al. Comparison of double-plate Molteno and Ahmed glaucoma valve in patients with advanced uncontrolled glaucoma. Ophthalmic Surg Lasers. 2002;33:94-101.