CHAPTER 32 APRAXIA
Apraxia is one of the more frequent consequences of brain damage and can lead to severe disabilities in daily life. The term apraxia covers a wide spectrum of higher order motor disorders caused by acquired brain disease that affects the performance of skilled, learned movements with or without preservation of the ability to perform the same movement outside the clinical setting in the appropriate situation or environment. The disturbance of purposive movements cannot be termed apraxia, however, if it results from a language comprehension disorder or from dementia or if the patient suffers from any elementary motor or sensory deficit (i.e., paresis, dystonia, ataxia) that could fully explain the abnormal motor behavior.1–3
Apraxia is found mostly in patients with stroke, but the disorder can result from a wide variety of other focal lesions (i.e., trauma, tumors) or from diffuse brain damage as observed in Alzheimer’s disease or corticobasal degeneration.2
LIMB APRAXIAS
Hugo Liepmann originally posited that the idea of the action, or movement formula, containing the space-time picture of the movement, was stored in the left parietal lobe and that in order to carry out a skilled movement, the space-time plan must first be retrieved and associated via cortical connections with the innervatory pattern stored in the left sensorimotorium—mainly the premotor cortex—which in turn conveys the information on formula to the left primary motor areas. When the left limb performs the movement, the information must be transmitted from the left to the right sensorimotorium through the corpus callosum to activate, thereafter, the right motor cortex. Liepmann conceived of ideational apraxia as a disruption of the space-time plan or its proper activation, so that it was impossible to construct the idea of the movement; the patient would not know what to do. In contrast, in ideomotor apraxia, the space-time plan was intact but it could no longer guide the innervatory engrams that implemented the movement because it was disconnected from them; the patient knew what to do but not how to do it. Finally, LKA appeared when the disruption of the innervatory engrams interfered with the selection of the muscle synergies necessary to perform the skilled movement.4,5 Liepmann’s initial description and classification of these three types of apraxia have such clarity and influence that they still underlie the most widely used existing schemes of apraxic disturbances.
In 1985, Roy and Square6 advanced a model for the organization of action that was based on the operation of a two-part system involving both conceptual and production components. The conceptual system involves knowledge of objects and tools in terms of the actions and functions they serve and knowledge of actions independent of tools or objects but in which the use of tools and objects may be incorporated. On the other hand, the production system incorporates a sensorimotor component of knowledge, as well as encompassing the perceptual motor processes for organizing and executing action. According to this model, dysfunction of the praxis conceptual system would give rise to conceptual or ideational apraxia, whereas impairment of the praxis production system would induce ideomotor apraxia.6 Thereafter, an influential cognitive neuropsychological model, also mapped onto the model of language processing, was introduced by Rothi and colleagues.7 They proposed to separate input pathways for verbal and visual stimuli to explain the dissociation between the ability to perform an action on command versus on imitation; to separate semantic and nonsemantic pathways to account for dissociations in the ability to represent meaningful versus meaningless actions; and to separate input and output lexicons to allow for differences in the ability to conceptualize actions and to perform them.7
More recently, Buxbaum and associates8 proposed an interplay between a dynamic body-centered representation of actions and stored representation of learned actions in order to explain the different forms of ideomotor apraxia, and Leiguarda and Marsden9 suggested that the most common form of ideomotor apraxia as well as of LKA can be interpreted as caused by disruption of multiple parallel parietofrontal circuits involved in sensorimotor transformations.
Evaluation of Limb Praxis
A systematic evaluation of limb praxis is crucial in order to (1) identify the presence of apraxia, (2) classify correctly the nature of limb praxis deficit according to the errors committed by the patient and the modality through which these errors are elicited, and (3) gain an insight into the underlying mechanism of the patient’s abnormal motor behavior (Table 32-1).
| Intransitive movements | Nonrepresentational (e.g., touch your nose, wiggle your fingers). |
| Representational (e.g., wave goodbye, hitchhike) | |
| Transitive movements | (e.g., use a hammer or use a screwdriver) under verbal, visual, and tactile modalities |
| Imitation of meaningful and meaningless movements, postures, and sequences | |
| Tool* selection tasks | To select the appropriate tool to complete a task, such as a hammer for a partially driven nail |
| Alternative tool selection tasks | To select an alternative tool such as pliers to complete a task such as pounding a nail, when the appropriate tool (i.e., hammer) is not available |
| Mechanical problem-solving task | (e.g., to select the appropriate one of three novel tools for lifting a wooden cylinder out of a socket). |
| Multiple-step tasks | (e.g., to prepare requiring actions such as prepare a letter for mailing) |
| Gesture recognition and discrimination tasks | To assess the capacity to comprehend gestures, either verbally (to name gestures performed by the examiner) or nonverbally (to match a gesture performed by the examiner with cards depicting the tool/object† corresponding to the pantomime); and to assess the ability to discriminate a well from a wrongly performed gesture |
* Tool: implement with which an action is performed (e.g., hammer, screwdriver).
† Object: the recipient of the action (e.g., nail, screw).
From Leiguarda R: Apraxias as traditionally defined. In Freund H, Hallett M, Jeannerod M, et al, eds: Higher-Order Motor Disorders. Oxford, UK: Oxford University Press, 2005, pp 303-338.
Transitive movements should be assessed under different modalities, including verbal, visual (seeing the tool or the object on which the tool works), and tactile (using actual tools and/or objects), as well as on imitation, because impairment can be seen under some performance conditions but not others. Nevertheless, the most sensitive test for apraxia is to ask patients to pantomime to verbal commands, because actions must be performed without guidance through visual or tactile feedback from the object and thus are almost entirely dependent on stored movement representations. In addition to the specific praxis assessment tasks listed in Table 32-1, it is important to carry out a complete cognitive evaluation, because findings may contribute to an understanding of the neural mechanisms of some praxic deficits.
Analysis of a patient’s performance is based on both accuracy and error patterns (Table 32-2). Detailed error analysis is crucial both for unveiling and for properly classifying an apraxic disorder; patients with ideational apraxia have difficult mainly with sequencing actions (e.g., making coffee) and exhibit content errors or semantic parapraxias (e.g., mimicking use of a hammer when requested to use a knife). Patients with ideomotor apraxia show primarily temporal and spatial errors, which are more evident when they perform transitive rather than intransitive movements. Errors in LKA represent slowness, coarseness, and fragmentation of finger and hand movements.2,3

TABLE 32-2 Types of Praxis Errors
Rights were not granted to include this table in electronic media. Please refer to the printed book.
From Rothi LJG, Heilman KM, eds: Apraxia: The Neuropsychology of Action. East Sussex, UK: Psychology Press, 1997.
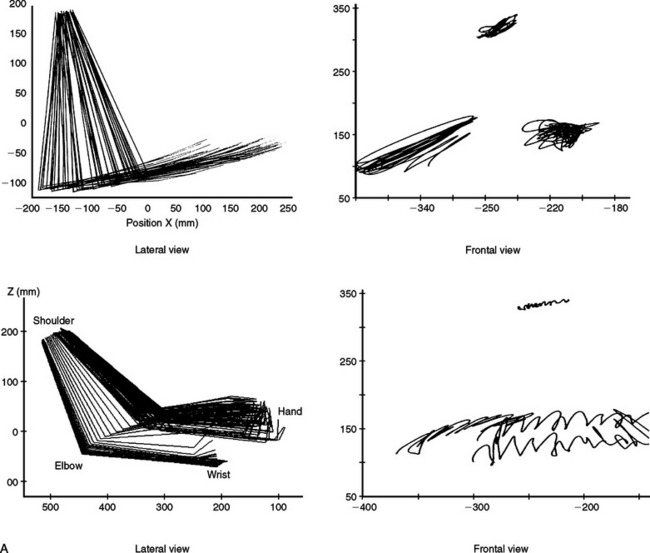
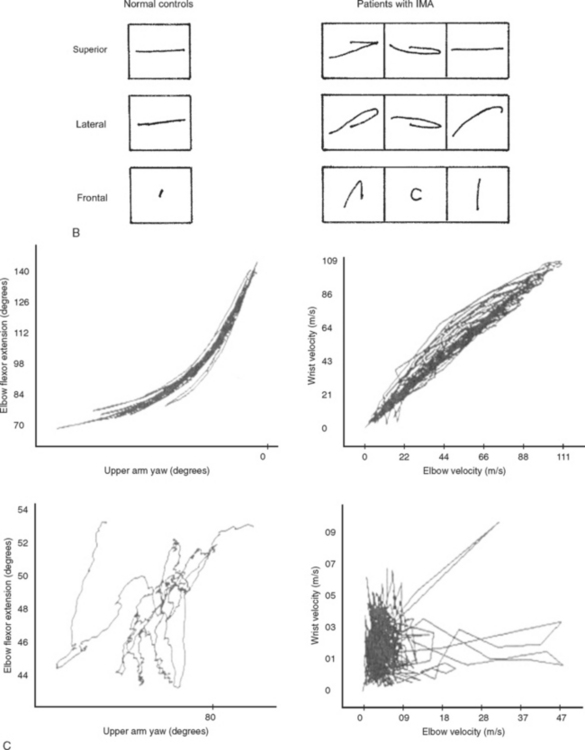
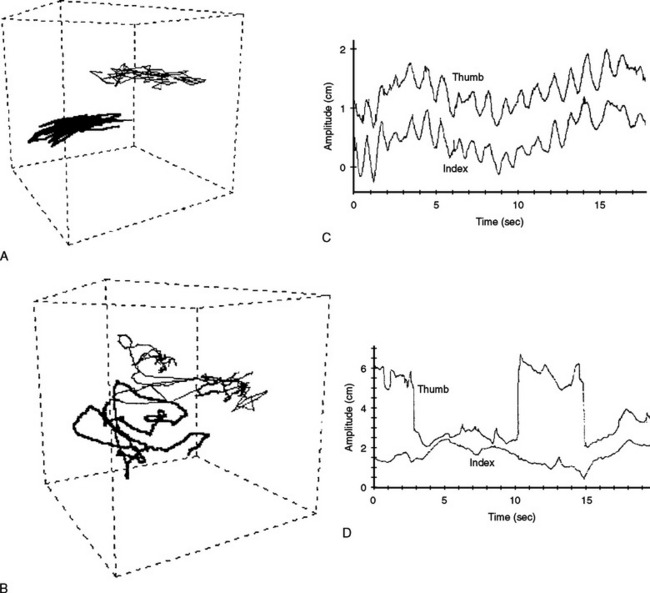
Three-dimensional motion analysis of different types of movements has provided a better and more accurate method of objectively capturing the nature of the praxis errors observed in clinical examination. Patients with ideomotor apraxia caused by focal left hemisphere lesions, by different asymmetrical cortical degenerative syndromes, and by basal ganglion disease have shown several kinematic abnormalities of dissimilar complexity, such as slow and hesitant build up of hand velocity, irregular and nonsinusoidal velocity profiles, abnormal amplitudes, alterations in the plane of motion and in the directions and shapes of wrist trajectories, decoupling of hand speed and trajectory curvature, and loss of interjoint coordination (Fig. 32-1).10,11 The study of manipulating finger movements in patients with LKA also disclosed severe abnormalities that unveiled the nature of the motor deficit. The workspace is highly irregular and of varying amplitude, there is breakdown of the temporal profiles of the scanning movements, and, overall, severe lack of coordination between fingers has been found (Fig. 32-2).12
Lateralization of Praxic Functions
Apraxia, as tested by the imitation of gestures and object use pantomime, has been found in about 50% of patients with left hemisphere damage and in fewer than 10% of those with right hemisphere damage,1 which means that some praxic functions or some specific components of learned skilled movement are bilaterally represented or are preferentially processed in the right hemisphere. Nevertheless, most of the errors exhibited by patients with ideomotor apraxia are seen equally in patients with left or right hemisphere damage when they pantomime nonrepresentative and representative/intransitive gestures, but they are observed predominantly in patients with left hemisphere damage when they pantomime transitive movements, because this action is performed outside the natural context. Moreover, it has been suggested that, whereas either hemisphere would be able to process both intransitive movements and transitive movements with tools/objects, the left hemisphere would be dominant not only for the “abstract” performance (pantomiming to verbal command) of transitive movements but also for learning and reproducing novel movements such as meaningless movements and sequences.3 The left hemisphere also seems to be specialized for the selection of limb movements that are appropriate for the use of an object and for the retrieval of action knowledge in general, including knowledge related to tools.3
Most functional neuroimaging studies in which researchers have evaluated pantomiming tool-use gestures have revealed activation of parietofrontal areas predominantly in the left hemisphere, regardless of which hand was used.13–15 Frydman and colleagues16 specifically studied the lateralization of praxis assessed through pantomiming transitive gestures. They found that transitive gestures involving mainly distal muscles when pantomimed with the right hand activated frontoparietal association areas in the left hemisphere. When the same movement was performed with the left hand, activation also predominated on the left hemisphere, with the exception of the premotor cortex, which showed bilateral activation in most subjects. In turn, transitive gestures involving proximal limb movements performed with either the right or the left hand caused bilateral parietofrontal activation. Thus, transitive gestures, when pantomimed in response to verbal command, are differentially represented interhemispherically and intrahemispherically, depending on whether the movement involves predominantly proximal or distal musculature and whether it is performed with the right or the left hand.16
Types of Limb Apraxia
Ideational or Conceptual Apraxia
Liepmann defined ideational apraxia as an impairment in performing tasks that required a sequence of several acts with tools and objects (e.g., prepare a letter for mailing).5 However, other authors use the term to denote a failure to use single tools appropriately.2 To overcome this confusion, Ochipa and associ ates17 suggested restricting the term ideational apraxia to a failure to conceive a series of acts leading to an action goal, and they introduced the term conceptual apraxia to denote a loss of knowledge of how objects are used. However, a strict difference between ideational and conceptual apraxia is not always feasible, inasmuch as patients with ideational apraxia not only fail on tests of multiple object use but may also perform abnormally when using a single object. Thus, ideational apraxia or conceptual apraxia could be defined as a deficit in the conception of a single movement or of a sequence of them, so that the patient does not know what to do.3
Patients with ideational or conceptual apraxia exhibit primarily content errors or semantic parapraxias (e.g., using a comb as a toothbrush) in the performance of transitive movements (see Table 32-2). They are unable to associate tools with the objects that receive their action; thus, when a partially driven nail is shown, the patient may select a pair of scissors rather than a hammer from an array of tools to complete the action and may also fail to describe the function of a tool or be unable to point out a tool when its function is described by the examiner. In addition, a patient may have difficulties in matching objects for shared purposes: for example, when asked to complete an action and the appropriate tool is not available (e.g., a hammer to drive a nail), the patient may select not the most adequate tool for that action (e.g., a wrench) but rather one that is inadequate (e.g., a screwdriver).18 Patients with ideational apraxia are impaired in the sequencing of tool/object use, exhibiting many types of errors including deletion, addition, omission, misuse, substitution, and perseveration and are disabled in everyday life, because they use tools/objects improperly, select the wrong tools/objects for an intended activity, perform a complex sequential activity (e.g., making espresso) in a wrong order, or cannot complete the task at all.19 Ideational apraxia has been traditionally allocated to the left parieto-occipital and parietotemporal regions, although left frontal and frontotemporal lesions may also cause ideational apraxia or conceptual apraxia.5,18,19 Nevertheless, semantic or conceptual errors are observed particularly in patients with temporal lobe pathology (e.g., semantic dementia).20
Ideomotor Apraxia
Ideomotor apraxia has been defined as “an impairment in the timing, sequencing, and spatial organization of gestural movements.”7 Patients with ideomotor apraxia exhibit mainly temporal and spatial errors. The movements are incorrectly produced, but the goal of the action can usually be recognized. Transitive movements are more affected than intransitive ones when patients pantomime in response to commands, and patients usually do better on imitation than when responses are elicited through verbal commands. Acting with tools/objects is performed better than pantomiming their use, but even so, movements may not be entirely normal. Ideomotor apraxia is commonly associated with damage to the parietal association areas surrounding the intraparietal sulcus, less frequently with lesions of the premotor and prefrontal cortices and supplementary motor area, and usually with disruption of the intrahemispheric white matter bundles that interconnect parietal and frontal areas. Small lesions of the basal ganglia and thalamus may cause ideomotor apraxia, but in the majority of patients, the pathology extends to the internal capsule, as well as to the periventricular and peristriatal white matter.2,9
Limb-Kinetic Apraxia
Many clinicions do not consider LKA a true apraxia but merely the expression of a basic motor (corticospinal) deficit. However, studies performed since 2000 clearly demonstrated—as Kleist and Liepmann originally suggested5,21,22—that LKA is a higher order motor disorder over and above a corticospinal or basal ganglia deficit.9,12 The deficit in LKA is confined mainly to finger and hand movements contralateral to the lesion, regardless of the affected hemisphere, with preservation of power and sensation. Manipulative finger movements are predominantly affected. However, in most cases, all movements, either complex or routine and independently of the modality needed to evoke them, are involved. There is a delay in the initiation of movements, as well as slowing in their execution, but what is especially striking is the temporal disordering of cooperative muscle action and loss of selective muscle activation; the fingers no longer act in concert, and there is lack of interfinger coordination. Simultaneous and sequential actions of individual fingers are distorted, and the resulting movement becomes coarse, fragmented, and mutilated. Fruitless attempts usually precede wrong movements, which in turn are frequently contaminated by extraneous movements. Imitation of finger postures is also abnormal, and some patients use the less affected or normal hand to reproduce the requested posture. The severity of the deficit is consistent, exhibited to the same degree in everyday activities as in the clinical setting; not presenting therefore voluntary-automatic dissociation.5,3,12,23
Performance with the limb-kinetic apraxic hand may superficially resemble tactile apraxia caused by posterior parietal lesions, inasmuch as both are unilateral finger and hand apraxias, with gross disturbances of object exploration and manipulation. However, intransitive and expressive movements are preserved, and imitation of hand and finger movements is normal in tactile apraxia. Tactile apraxia is a unimodal somatosensorimotor transformation disorder characterized by a specific inability to engender adequate finger movements required for the exploration of an object held in the hand. No apraxia is present when the patient sees the object; it appears only when he or she is blindfolded and starts actively touching it. Somatosensory functions, particularly tactile recognition, may be normal or moderately disturbed.3
Callosal Apraxia
Damage to the body of the corpus callosum (with or without associated genu involvement) may induce a unilateral apraxia deficit of the nondominant limb, the characteristics of which may vary according to the type of test given and the lateralization pattern of praxic skills present in each patient, although the most enduring defect is demonstrated when verbal-motor tasks, such as pantomiming in response to command, are used.24–27 Some patients cannot correctly pantomime in response to verbal commands with their left hands but perform normally on imitation and object use,28 whereas others cannot use their left hands on command, by imitation or while holding the object.25,27 Moreover, a few patients cannot pantomime in response to verbal commands or while holding the object, but they perform fairly well on imitation or improve over time on imitation and object use.26
Modality-Specific or Dissociation Apraxias
Modality-specific or dissociation apraxias are praxic deficits exhibited by patients who commit errors only, or predominantly, when the movement is evoked by one but not all modalities.7,29 Thus, some patients may perform abnormally only under verbal commands; this deficit has been attributed to a left hemispheric lesion probably located in the parietal lobe, which disrupts the lexicomotor transformation process, or in the corpus callosum.28,30,31 Investigators have also described patients who performed poorly in response to seeing an object but performed considerably better when given the object tactile input or when asked to gesture to the name of the object.32 As an exception, some patients may be unable to use tool/objects but can correctly pantomime their use on commands.33 Furthermore, investigators have described patients who, unlike those with ideomotor apraxia who improved on imitation, were more impaired when imitating than when pantomiming in response to command (conduction apraxia)34 or could not imitate but performed flawlessly under other modalities; this situation is termed visuoimitative apraxia.35 Deficits may be restricted solely to the imitation of meaningless gestures with preserved imitation of meaningful gestures36,37 (see later discussion).
Neural Processes Underlying Limb Praxis
Neural Representation of Gestures and the Selection of Actions
Skillful and competent conventional use of objects and tools requires a normal prehension system, intact representations of functional actions for an adequate utilization behavior, and an intact semantic knowledge.3
Visually guided reaching, grasping, and object manipulation are paramount components in any task-related movement. Such object-oriented action implies a cerebral interface set up to align sensory information concerning position and shape of both object and limb, with specific motor commands encoding distance, velocity, direction, and grip.38 Research on primates has identified a series of segregated parietofrontal circuits that work in parallel, each one involved in a specific sensorimotor transformation process. The proposed functions of the main parietofrontal circuits are as follows: (1) visual and somatosensory transformation for reaching; (2) somatosensory transformation for posture, as well as transformation of body part location data into information necessary to control body part movements; (3) visuomotor transformation for grasping and manipulation; (4) coding peripersonal space for limb and neck movements; (5) internal representation of actions; and (6) visual transformation for eye movements.39
Several functional brain imaging studies on reaching, grasping, and object manipulation in humans have demonstrated activation of the parietal (Brodmann areas 7, 39, and 40) and frontal areas (dorsal premotor, ventral premotor, and supplementary motor areas), as well as of the primary sensorimotor cortex, corresponding to those involved in the circuits described in monkeys. In addition, activation has been documented in the caudate and putamen, globus pallidus, thalamus, and cerebellum.40–43 Grasping specifically activates the lateral bank of the anterior intraparietal sulcus, whereas during grasping and manipulation, the ventral premotor cortex is involved.43
Most studies investigating tool and action knowledge have shown activation in posterior left superior and middle temporal gyri. The left posterior temporal areas are usually activated together with neural systems associated with semantic retrieval (left inferior and middle temporal gyri/Brodmann areas 20 and 21); left inferior frontal cortex (Brodmann areas 44, 45, and 47), and left premotor and left frontomarginal gyri (Brodmann areas 10 and 12).44–47 The generation of action verbs related to tool/object use also activates the left angular gyrus, which indicates that the system mediating access to verbs is anatomically close to those the system that supports concepts of movements and space-time relationships.48
Functional brain imaging studies on tool use skills have demonstrated activation, predominantly in the left hemisphere, of an extensively distributed control network made up of the inferior and superior parietal lobules, the posterior superior and middle temporal areas, the premotor (dorsal and ventral) and dorsolateral prefrontal cortices, and the supplementary motor area. The dorsolateral prefrontal cortex and posterior temporal areas are preferentially involved during action planning, whereas parietal, premotor, and supplementary motor areas are engaged during action execution in addition to action planning.15 The only brain region activated during manipulation with the tool, in comparison with the fingers, is the lateral edge of the intraparietal sulcus.49
In conclusion, skillful and competent use of tool/object depends on tool-/object-specific conceptual knowledge, as well as on several sensorimotor transformation processes involved in reaching, grasping, and manipulation; it is therefore subserved by an extensive temporoparietofrontal system that integrates tool/object knowledge with the ideation and generation of actions. A putative temporoparietal route may constitute an intermediate and necessary step for integrating objects’ functional properties into adequate movement patterns such as those required for utilization behavior.3
To date, there have been no studies designed to evaluate the representation of intransitive gestures. Intransitive gestures are usually much less complex than transitive movements, are geared to sociocultural contexts, and are stimulated by environmental cues (e.g., salute) rather than constrained by the shape and function of tools/objects, as in the case of transitive movements. It has therefore been suggested that intransitive movements and postures are subserved by a more widely and differently distributed intrahemispheric network and/or that they are bilaterally represented.3
Neurophysiological, neuroimaging, and clinical studies have delineated at least two well-distributed neural systems essential for the selection of limb movement responses and for the selection of object-oriented responses. The first system consists of the lateral premotor (Brodmann area 6) and parietal cortices, basal ganglia, thalamus, and white matter fascicles participating in the selection of limb movement responses. The other is an adjacent system made up of lateral area 8 and interconnected parietal regions, thalamus, striatum, and white matter fascicles and is concerned with the selection of object-oriented responses.50
Pathophysiology of Limb Apraxia
Ideational or conceptual types of praxic deficits
Competent conventional use of objects and tools depends primarily on an intact semantic knowledge. Two possible models of semantic system functioning have been postulated. According to the model based on a multimodal distributed semantic architecture, objects of all types are represented by visual, tactile, and motor/proprioceptive nodes in proportion to the degree to which these various sensory and motor systems are involved as the representation is acquired and elaborated. In the case of tools and body parts, the dominant “channel” of experience involves sensorimotor (i.e., how the tool is held and used/manipulated) and functional information (i.e., knowing the usage context).51 According to the second model, a verbal, propositional semantic system operates by “reading” the sensorimotor representations or gestural engrams20; thus, skill and appropriate object use require the combination of dorsal stream processing (“how” system) with the product of ventral pathway processing (“what” system), which provides access to semantics.52
Therefore, ideational apraxia or conceptual apraxia may result from disruption of normal integration processes between the system subserving the functional knowledge of action and those involved in object knowledge, or it may result from damage to the putative conceptual system involving in tool-action knowledge.6,9 On the basis of studies of patients with semantic dementia syndrome, however, it has been alternatively proposed that patients with conceptual apraxia are impaired in the use of objects for which they have lost conceptual knowledge (e.g., naming and object descriptions). Their ability to select and use novel tools normally (mechanical problem solving), which unveils the capacity to infer function from structure, is usually preserved.20 The finding that some patients with ideational apraxia or conceptual apraxia may use some objects normally may be ascribed to degraded but partially retained conceptual knowledge about such objects, enhanced by sensorimotor information53 or, more precisely, to reliance on visual/tactile affordance, together with good problem-solving skills, because patients may be able to efficiently manipulate novel tools.20
Finally, the impairment in carrying out sequences of actions requiring the use of various objects (i.e., the original definition of ideational apraxia) may be the consequence of disruption of the subsystem involved in short-term script ordering31 (see later discussion).
Ideomotor types of praxic deficits
There are two major subtypes of ideomotor apraxia. The largest subtype results from disruption at the movement execution stage of gesture performance (anterior or dynamic ideomotor apraxia) and has been attributed to dysfunction of parietofrontal circuits involved in sensorimotor transformation. The second subtype, posterior or representational ideomotor apraxia, has been suggested to be caused by the inability to store or access representational memories of complex body posture and movements or by a deficit in the selection of actions.2
Dysfunction of frontoparietal circuits involved in sensorimotor transformation
A subgroup of patients with ideomotor apraxia usually commit spatial and temporal errors when performing both transitive and intransitive symbolic or communicative movements under all modalities of elicitation (i.e., verbal command, imitation, seeing and handling the object), although performance usually improves on imitation and with object use. These patients also exhibit errors when imitating meaningless postures and novel motor sequences. It was originally suggested that the crucial underlying neural mechanism in this group of patients with ideomotor apraxia was a disruption of multiple parietofrontal circuits and their subcortical connections, which subserve the computations necessary to translate an action goal into movements by integrating sensory input with central representation of actions that is based on prior experience.9,54 Whereas damage to specific circuits causes unimodal deficit, such as tactile apraxia, involvement of several circuits by a larger lesion or disruption of their integration in supramodal reference frames causes ideomotor apraxia. Thus, damage to circuits devoted to sensorimotor transformation for grasping, reaching, and posture; for transformation of body part location into information required to control body part movements; and for coding extrapersonal space would produce incorrect finger and hand postures and abnormal orientation of the tool/object, inappropriate arm configuration and faulty movement orientation (with regard to both the body and the target of the movement in extrapersonal space), and movement trajectory abnormalities. Patients select the correct movements but have difficulties in translating the selected response into action because of an “execution” disturbance; the online guidance of movements may be defective, and patients may complain of disability in everyday activities.3
Disruption of action selection
Another subgroup of patients with ideomotor apraxia exhibits spatial and temporal errors predominantly when pantomiming in response to verbal command with either hand (i.e., outside the appropriate context). They improve on imitation and when handling the object. These patients do not complain of difficulties in everyday activities; there is an automatic voluntary dissociation. Their online guidance of movements is normal, and they have no pointing and/or grasping deficits; thus, pragmatic representations for object-oriented actions are not directly affected, inasmuch as this is a higher level deficit involving a premovement neural process. The deficits arise when the subject has to shift from a strategy in which object-oriented actions are processed automatically to a more cognitive mode, because of inability to select the appropriate motor schemas from stored motor representations and organize them into purposive action. They may also have deficits in mentally evoking (imaging) the action and may be unable to discriminate correct from incorrect gestures.3
In the study conducted by Rushworth and associates,50 all patients with deficits in the selection of learned actions and apraxia had lesions in the left hemisphere, predominantly in the parietal lobe, but in many, lesions also involved the lateral premotor cortex, as well as interconnecting white matter fascicles and basal ganglia and the thalamus.
Therefore, patients in whom performance is impaired predominantly when pantomiming in response to verbal commands may be those with lesions involving systems subserving movement selection; circuits devoted to sensorimotor transformation are preserved. As a matter of fact, it has been possible to distinguish in monkeys an impairment in movement selection from an impairment in kinematics.3
Finally, some patients with the ideomotor type of apraxia have deficits in forming correct hand configuration appropriate for object use only; this means they show inadequate hand grasp when the object has to be manipulated with the intention to use it, but neither during visually guided (“on-line”) reaching and grasping movements nor when grasping novel objects. These patients can correctly name and recognize fingers and objects and can also define their functions verbally, but they are unable to discriminate between normal and abnormal hand postures, and they exhibit deficits in the perception of selfgenerated movements and in mentally simulating hand gestures. These types of deficits have been associated with left inferior parietal cortex lesions; damage to these regions may degrade the storing of or interrupt the access to representations of learned complex body postures and/or movements associated with familiar objects.55
Limb-kinetic type of praxic deficit
Proper grasping and manipulation require the integrity of the corresponding sensorimotor transformation circuit, the capacity to generate independent finger movements, and the capacity to perform and to exert a delicate somatosensory control process.38 On the basis of the anatomical connections and functional properties of F5 and anterior intraparietal areas, a sensorimotor circuit for grasping has been proposed in which parietal neurons represent the entire hand action and frontal neurons encode particular segments of the action. In turn, direct corticomotoneural projection systems underpin the ability to perform relatively independent finger movements. However, movements of individual digits require activation of a complex set of muscles; this muscular activity must not only generate the digit movement required but also stabilize the bony chain and prevent unwanted digit movements. Both cortical inhibition and corticospinal inhibition seem to be essential for the selection and control of hand muscle activity. When the object is finally grasped, a delicate somatosensory control of finger movement is necessary for precise manipulation to be performed.
Leiguarda and Marsden9 proposed that the most typical examples of LKA, such as those seen in corticobasal degeneration, are caused by disruption of the frontoparietal circuits devoted to grasping and manipulation, combined with impaired generation and control of independent finger movements caused by disruption of intracortical inhibitory circuits, as well as dysfunction of somatosensory control of manipulation. However, because patients with corticobasal degeneration and LKA have neither clinical signs of corticospinal deficit nor involvement of fast-conducting corticomotoneural projections, as evaluated with transcranial magnetic stimulation, and a defect in somaesthesis may not be present, this distinctive apraxic disorder may basically result from dysfunction of the nonprimary cortical motor areas, as previously suggested.9 In support, transcranial magnetic stimulation of Brodmann area 44 produces slowing and clumsiness of fine finger movements without paresis.56 All pathologically confirmed cases of LKA suffered a degenerative process such as corticobasal degeneration and Pick’s disease, involving frontal and parietal cortices or, predominantly, the premotor cortex.9
Imitation of Actions
Imitation is an important component of nonverbal communication. Testing the ability to imitate is an essential aspect of apraxia assessment, particularly in patients with aphasia. Defective performance in gesture imitation has been found in patients with lesions in several cortical regions but essentially with parietofrontal damage. These patients tend to exhibit more errors when imitating transitive than intransitive and meaningless movements.54 Moreover, patients with left parietal lobe damage seem to have more difficulties when imitating meaningful transitive gestures on their own bodies than when imitating movements with reference to external objects.57 Imitation of meaningless hand and finger postures discloses differential susceptibility to right- and left-brain damage. Patients with left-brain damage have more difficulties imitating hand than finger postures, whereas patients with right-brain damage commit more errors with finger postures.58,59 Thus, imitation seems to be body part specific; the gesture’s visual appearance is mentally transformed into categories of body part relationships.
Difference in action imitation between meaningful and meaningless action/postures can be predicted on the basis of a cognitive imitation model, which postulates disparate processing routes to the motor system. Imitation of meaningless actions/postures would be processed through a nonsemantic route from visual analysis, including mental transformation of another person’s body part and temporary holding in working/short-term memory of the observed movement/posture, to the motor system for actual execution. Imitation of meaningful actions/postures, in turn, can be achieved by either a nonsemantic or a semantic route through a long-term/semantic memory station. This model has received support from functional neuroimaging studies that showed involvement of the dorsal pathway when a meaningless action/posture is imitated and of the dorsal together with the ventral pathway when a meaningful action/posture is perceived with the aim to be imitated.45,46
Representation of sequential movements and actions
Functional brain imaging has shown that different neural systems are actively engaged in planning and executing sequential movements, depending on whether the sequence has been relearned or is a new one and contingent on the complexity of the movement sequence. The supplementary motor area, the primary sensorimotor cortex, the midposterior putamen, and the cerebellum are involved primarily in the execution of automatic, overlearned sequential movements, whereas the prefrontal, premotor, and parietal association cortices and the anterior part of the caudate/putamen are specifically recruited—in addition to such areas engaged in the execution of simple movement sequences—when a complex or newly learned sequence, which requires attention, integration of multimodal information, and working memory processing for its appropriate selection and monitoring, has to be performed.3
Patients with ideomotor apraxia may exhibit several types of errors such as omissions, deletions, additions, transpositions, and perseverations when performing sequencing limb movements and have been found to be particularly impaired in planning and implementing sequences of various hand movements. Abnormalities in movement sequencing have been reported most commonly in patients with left parietal lobe lesions but also with left frontal and basal ganglion involvement.60–63
Thus, different neural systems would be engaged, depending on the characteristics of movement sequences needed to be executed during praxis evaluation. Most of the sequences used to test praxis are new (e.g., sequencing of movements in the movement imitation test) or part of an otherwise well-learned sequence that has to be represented explicitly. In any case, the system comprising the prefrontal, premotor, and parietal cortices and the caudate would be specifically engaged. When the sequence is well known, automated, or overlearned, the supplementary motor area–putamen would be preferentially recruited. Interestingly, activation shifts back to caudate-anterior putamen when attention was paid to the overlearned action. In addition, it might be possible that within this system, there are many different subsystems subserving functionally separate cognitive computations involved in motor sequencing (i.e., working memory, attention, selection of limb movements), which, in turn, may be selectively damaged by the pathological process and so produce different types of sequencing impairment in apraxic patients.9
The sequential organization of actions, rather than movements, has been studied with the use of script event ordering to address the cognitive activity that occurs during action planning at a covert level.31 A script consists of a goal-oriented sequence of events that typically occur in a specific and systematic order. Functional imaging studies have shown that short-term scripts as those used in testing ideational apraxia (e.g., peeling, opening, and eating an orange) cause activation in the left hemisphere of the dorsolateral prefrontal cortex, supramarginal gyrus, inferior temporal gyrus, and middle occipital gyrus. Patients with ideational apraxia caused by damage of the left parietotemporal region or damage of the frontal lobe fail on naturalistic, multiple object tests requiring a sequential structuring of common everyday actions (short-term script ordering, such as making coffee).5,18–20
Recognition of actions and perception of selfgenerated movements
A subset of neurons in area F5 have been found to discharge during the time a monkey observes meaningful hand movements made by the experimenter, particularly when interacting with objects; these were called mirror neurons and were considered to belong to an observation/execution matching system involved in understanding the meaning of motor events, as well as in action imitation.39 Neurons with properties similar to those of mirror neurons in area F5 are also found in the superior temporal sulcus in monkeys. Two other types of neurons that may contribute to the recognition and imitation of postures and actions have also been found in the superior temporal sulcus. One type encodes the visual appearance of particular parts of the body (i.e., fingers, hands, arms), which combine in such a way that the collection of components can specify a particular meaningful posture or action. The second type encodes specific body movements, such as walking and turning. Cells responding to hand-object interaction are also present in the rostral part of the inferior parietal lobe, which sends its cortical output to the F5 area; in turn, the inferior parietal lobe receives projection from the superior temporal sulcus region, and the latter is interconnected with the frontal lobe, thus completing a cortical circuit involved in the perception of hand-object interaction. The crucial cognitive role of the superior temporal sulcus—inferior parietal lobe—F5 network would be the internal representation of actions that, when evoked by an action made by other people, would be involved in two related functions: namely, action recognition and action imitation. Findings of functional neuroimaging studies in humans parallel neurophysiological findings in monkeys.64
Action recognition deficits have been observed in patients with parietal, temporal, frontal, and basal ganglion lesions predominantly in the left hemisphere.65,66 However, Halsband and colleagues57 compared gesture comprehension and imitation in patients with parietal and frontal lesions and found that when lesions affected the left parietal cortex, sparing temporal lobe structures, gesture comprehension was slightly disturbed, although action imitation was severely impaired. The lack of consistent gesture comprehension deficits in these patients could result from preservation of the left temporal cortex, which seems to be crucial for the knowledge of actions.57
Apraxic patients with left parietal damage may also have difficulties when they are required to discriminate from their own hand an external hand that performs the same movement. The impairment in correctly attributing the ownership of the movement may result from the inability to evaluate and compare internal and external feedback about movements.67
Treatment of Limb Apraxia
In one study, investigators used a cross-over design to compare the efficacy of top-down and bottom-up training for the same activities in the same patients. Their top-down approach, “exploration training,” was aimed at teaching patients to infer possible functions of tools and objects from their structural properties. Patients were told to compare tools with similar or different function with regard to their structural properties (e.g., contrasting the teeth of a cutting knife and of a saw with the plain edge of a knife used for spreading) or to make drawings of them that emphasized these structural details. In contrast, “direct training” was intended to establish a routine through performing the task and may hence be classified as being bottom-up. Direct training led to a significant reduction of errors and of the need for assistance, whereas exploration training had no significant practical effects.68
DISTRIBUTION OF THE APRAXIAS IN OTHER BODY PARTS
Although face apraxia has been generally equated with oral nonverbal apraxia—that is, the inability to perform skilled movements of the lips, checks, and tongue1—early reports of patients with facial apraxia described eye and/or eyebrow movement deficits.69 Therefore, face apraxia should refer to a disturbance of upper and lower face movements not explained by elementary motor or sensory deficits. Patients exhibit spatial and temporal errors of similar quality to those observed in limb apraxia when performing representational and nonrepresentational movements such as sticking out the tongue, blowing out a match, smiling, blowing a kiss, showing the teeth, blinking the left or right eye, looking down, or sucking on a straw. Face apraxia often co-exists with Broca’s aphasia and thus is more frequently observed with left hemisphere lesions, particularly those involving the frontal and central operculum, insula, centrum semiovale, and basal ganglia; however, it can also be seen with lesions confined to left posterior cortical regions, as well as with right hemisphere damage.3,69
Trunk movement impairments, labeled trunk apraxia, were originally reported as part of a syndrome associated with bilateral frontal lobe lesions encompassing stance and gait apraxia. However, in some patients, trunk apraxia is overwhelming; they experience difficulties in dancing or turning around and may even be unable to adapt their body in order to use furniture; they have difficulty sitting down in a chair, showing hesitation, sitting in the wrong position (e.g., on the edge of the chair) and in incorrect directions (e.g., facing the back of the chair). When lying in bed, their bodies are not aligned parallel along the major axis of the bed, or they place the pillow in an unusual position. Patients may have minimal or no difficulty in standing or getting up, in contrast to features of some basal ganglion disorders such as parkinsonism.3
It is still controversial whether trunk apraxia results from only left hemisphere damage or whether bilateral hemispheric lesions are necessary. It is often observed in cortical degenera tive syndromes such as progressive apraxia and corticobasal degeneration, in which parietofrontal involvement is prominent, but it has also been found in patients with left hemisphere damage, particularly in those with cortical and subcortical vascular lesions confined to the territory of the middle cerebral artery. Trunk apraxia in these patients can be found without association with limb apraxia.70
The precise nature and localization of gait apraxia still defy exact identification. Gerstmann and Schilder71 described apraxia of gait as a genuine disturbance of walking caused by frontal lesions; more recently, however, it has been considered not as a disorder but a spectrum of higher order walking syndromes.72 Nevertheless, apraxia of gait may be defined as the loss of ability to use the lower limbs properly in the act of walking, a loss that cannot be accounted for by demonstrable sensory impairment or motor weakness.73 Such patients’ gait is characterized by slowness of initiation; loss of balance; “magnetic attraction of the foot to the ground”; counterproductive parasitic movements; difficulty in stopping and turning; and inability to pedal, to kick, or to trace a circle with the foot, as well as increased tone and brisk reflexes in the lower limbs with grasping foot responses. The disorder is caused by bilateral damage mainly to the medial frontal lobes or by white matter lesions that interrupt the connections between premotor cortex, supplementary motor area, and cerebellum and basal ganglia.74
Jeannerod M, Leiguarda R, editors. Higher-Order Motor Disorders. Oxford, UK: Oxford University Press; 2005:303-338.
Johnson-Frey S, Newman-Norlund R, Grafton S. A distributed left hemisphere networks active during planning of everyday tool-use skills. Cereb Cortex. 2005;15:681-695.
Leiguarda R. Apraxias as traditionally defined. In: Freund H, Hallett M, Jeannerod M, et al, editors. Higher-Order Motor Disorders. Oxford, UK: Oxford University Press; 2005:303-338.
Leiguarda R, Merello M, Nouzeilles MI, et al. Limb-kinetic apraxia in corticobasal degeneration: clinical and kinematic findings. Mov Disord. 2003;18:49-59.
Nutt J. Higher-order disorders of gait. In: Freund H, Hallett M, Jeannerod M, et al, editors. Higher-Order Motor Disorders. Oxford, UK: Oxford University Press; 2005:237-248.
Rossetti I, Rode G, Goldenberg G. Perspectives on higher-order motor deficit rehabilitation. In: Freund H, Hallett M, Jeannerod M, et al, editors. Higher-Order Motor Disorders. Oxford, UK: Oxford University Press; 2005:475-498.
1 De Renzi E. Apraxia. Boller F, Grafman J, editors. Handbook of Neuropsychology. vol 2. Amsterdam: Elsevier Science; 1989:245-263.
2 Rothi LJG, Heilman KM, editors. Apraxia: the Neuropsychology of Action. East Sussex, UK: Psychology Press, 1997.
3 Leiguarda R. Apraxias as traditionally defined. In: Freund H, Hallett M, Jeannerod M, et al, editors. Higher-Order Motor Disorders. Oxford, UK: Oxford University Press; 2005:303-338.
4 Liepmann H. Die linke hemisphare und das handeln [The left hemisphere and action]. Munch Med Wochenschr. 1907;49:2322-2326. 2375–2378. [Translations from Liepmann’s essays on apraxia. In Research Bulletin 506, Department of Psychology, University of Western Ontario, 1980.]
5 Liepmann H. Apraxie. Ergenbnisse der Gesamten Medizin. 1920;1:516-543.
6 Roy EA, Square PA. Common considerations in the study of limb, verbal, and oral apraxia. In: Roy EA, editor. Neuropsychological Studies of Apraxia and Related Disorders. Amsterdam: North-Holland; 1985:111-161.
7 Rothi LJG, Ochipa C, Heilman KM. A cognitive neuropsychological model of limb praxis. Cogn Neuropsychol. 1991;8:443-458.
8 Buxbaum LJ, Giovannetti, Libon D. The role of the dynamic body schema in praxis: evidence from primary progressive apraxia. Brain Cogn. 2000;44:166-191.
9 Leiguarda R, Marsden CD. Limb apraxias: higher-order disorders of sensorimotor integration. Brain. 2000;123:860-879.
10 Poizner H, Mack L, Verfaellie M, et al. Three-dimensional computer graphic analysis of apraxia. Brain. 1990;113:85-101.
11 Leiguarda R, Merello M, Balej J, et al. Disruption of spatial organization and interjoint coordination in Parkinson’s disease, progressive supranuclear palsy, and multiple system atrophy. Mov Disord. 2000;15:627-640.
12 Leiguarda R, Merello M, Nouzeilles MI, et al. Limb-kinetic apraxia in corticobasal degeneration: clinical and kinematic findings. Mov Disorder. 2003;18:49-59.
13 Moll J, de Oliveira-Souza R, Passman LJ, et al. Functional MRI correlates of real and imagined tool-use pantomimes. Neurology. 2000;54:1331-1336.
14 Choi SH, Na DL, Kang E, et al. Functional magnetic resonance imaging during pantomiming tool-use gestures. Exp Brain Res. 2001;139:311-317.
15 Johnson-Frey S, Newman-Norlund R, Grafton S. A distributed left hemisphere networks active during planning of everyday tool-use skills. Cerebral Cortex. 2005;15:681-695.
16 Fridman E, Carpintiero S, Amengual A, et al: Hemispheric lateralization of pantomiming tool-use gestures: a fMRI study. Manuscript in preparation.
17 Ochipa C, Rothi LJG, Heilman KM. Conceptual apraxia in Alzheimer’s disease. Brain. 1992;115:1061-1071.
18 Heilman KM, Maher LH, Greenwald L, et al. Conceptual apraxia from lateralized lesions. Neurology. 1997;49:457-464.
19 De Renzi E, Lucchelli F. Ideational apraxia. Brain. 1988;113:1173-1188.
20 Hodges J, Bozeat S, Lambon Ralph M, et al. The role of conceptual knowledge in object use evidence from semantic dementia. Brain. 2000;123:1913-1925.
21 Kleist K. Kortikale (innervatorische) Apraxie. Jahrb Psychiat Neurol. 1907;28:46-112.
22 Kleist K. Gehirnpathologische und lokalisatorische Ergebnisse: das Stirnhirn im engeren Sinne und seine Störungen. Z ges Neurol Psychiatry. 1931;131:442-448.
23 Faglioni P, Basso A. Historical perspectives on neuroanatomical correlates of limb apraxia. In: Roy EA, editor. Neuropsychological Studies of Apraxia and Related Disorders. Amsterdam: North-Holland; 1985:3-44.
24 Liepmann H, Maas O. Eie Fall von linksseitiger Agraphie und Apraxie bei rechtsseitiger Lähmung. Monatsschrift fur Psychiatrie und Neurologie. 1907;10:214-227.
25 Watson RT, Heilman KM. Callosal apraxia. Brain. 1983;106:391-403.
26 Graff-Radford NR, Welsh K, Godersky J. Callosal apraxia. Neurology. 1987;37:100-105.
27 Leiguarda R, Starkstein S, Berthier M. Anterior callosal haemorrhage: a partial interhemispheric disconnection syndrome. Brain. 1989;112:1019-1037.
28 Geschwind N, Kaplan E. A human cerebral disconnection syndrome. Neurology. 1962;12:675-685.
29 De Renzi E, Faglioni P, Sorgato P. Modality-specific and supramodal mechanisms of apraxia. Brain. 1982;105:301-312.
30 Heilman KM. Ideational apraxia: a re-definition. Brain. 1973;96:861-864.
31 Ruby P, Sirigu A, Decety J. Distinct areas in the parietal cortex involved in long-term and short-term action planning: a PET investigation. Cortex. 2002;38:321-339.
32 Pilgrim E, Humphreys GW. Impairment of action to visual objects in a case of ideomotor apraxia. Cogn Neuropsychol. 1991;8:459-473.
33 Motomura N, Yamadori A. A case of ideational apraxia with impairment of object use and preservation of object pantomime. Cortex. 1994;30:167-170.
34 Ochipa C, Rothi LJ, Heilman KM. Conduction apraxia. J Neurol Neurosurg Psychiatry. 1994;57:1241-1244.
35 Merians AS, Clark M, Poizner H, et al. Visual-imitative dissociation apraxia. Neuropsychologia. 1997;35:1483-1490.
36 Mehler MF. Visuoimitative apraxia [Abstract]. Neurology. 1987;34(Suppl 1):129.
37 Goldenberg G, Hagmann S. The meaning of meaningless gestures: a study of visuoimitative apraxia. Neuropsychologia. 1997;35:333-341.
38 Jeannerod M, Arbid MA, Rizzolatti G, et al. Grasping objects: the cortical mechanisms of visuomotor transformation. Trends Neurosci. 1995;18:314-320.
39 Rizzolatti G, Luppino G, Matelli M. The organization of the cortical motor system: new concepts. Electroencephalogr Clin Neurophysiol. 1998;106:283-296.
40 Grafton ST, Arbid MA, Fadiga L, et al. Localization of grasp representation in humans by PET: 2. Observation compared with imagination. Exp Brain Res. 1996;112:103-111.
41 Rizzolatti G, Fadiga L, Matelli M, et al. Localization of grasp representations in humans by positron emission tomography. 1. Observation versus execution. Exp Brain Res. 1996;111:246-252.
42 Faillenot I, Toni I, Decety J, et al. Visual pathways for object-oriented action and object recognition: functional anatomy with PET. Cereb Cortex. 1997;7:77-85.
43 Binkofski F, Phil M, Posse S, et al. Human anterior intraparietal area subserves prehension: a combined lesion and functional MRI activation study. Neurology. 1998;50:1253-1259.
44 Martin A, Haxby JV, Lalonde FM, et al. Discrete cortical regions associated with knowledge of color and knowledge of action. Science. 1995;270:102-105.
45 Decety J, Grezes J, Costes N, et al. Brain activity during observation of action: influence of action content and subject’s strategy. Brain. 1997;120:1763-1777.
46 Grèzes J, Costes N, Decety J. The effects of learning and intention on the neural network involved in the perception of meaningless actions. Brain. 1999;122:1875-1887.
47 Phillips JA, Noppeney U, Humphreys GW, et al. Can segregation within the semantic system account for category-specific deficits? Brain. 2002;125:2067-2080.
48 Grèzes J, Decety J. Functional anatomy of execution, mental simulation, observation, and verb generation of actions: a meta-analysis. Hum Brain Mapp. 2001;12:1-19.
49 Inoue K, Kawashima R, Sugiura M, et al. Activation in the ipsilateral posterior parietal cortex during tool use: a PET study. Neuroimage. 2001;14:1469-1475.
50 Rushworth MFS, Nixon PD, Wade DT, et al. The left hemisphere and the selection of learned actions. Neuropsychologia. 1998;36:11-24.
51 McCarthey RA, Warrington EK. Evidence for modality specific meaning systems in the brain. Nature. 1988;334:428-430.
52 Milner AD, Goodale MA. The Visual Brain in Action. Oxford, UK: Oxford University Press, 1995.
53 Buxbaum LJ, Schwartz MF, Carew TG. The role of semantic memory in object use. Cogn Neuropsychol. 1997;14:219-254.
54 Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123:2306-2313.
55 Sirigu A, Cohen L, Duhamel JR, et al. A selective impairment of hand posture for objects utilization in apraxia. Cortex. 1995;31:41-55.
56 Vozumi T, Tamagawa A, Hashimoto T, et al. Motor hand representation in cortical area 44. Neurology. 2004;62:757-761.
57 Halsband U, Schmitt J, Weyers M, et al. Recognition and imitation of pantomimed motor acts after unilateral parietal and premotor lesions: a perspective on apraxia. Neuropsychologia. 2001;39:200-216.
58 Goldenberg G. Matching and imitation of hand and finger postures in patients with damage in the left or right hemispheres. Neuropsychologia. 1999;37:559-566.
59 Goldenberg G, Straus S. Hemisphere asymmetries for imitation of novel gestures. Neurology. 2002;59:893-897.
60 De Renzi E, Faglioni P, Lodesani M, et al. Performance of left brain-damaged patients on imitation of single movements and motor sequences: frontal and parietal-injured patients compared. Cortex. 1983;19:333-343.
61 Harrington DL, Haaland KY. Motor sequencing with left hemisphere damage: are some cognitive deficits specific to limb apraxia? Brain. 1992;115:857-874.
62 Benecke R, Rothwell JC, Dick JPR, et al. Disturbance of sequential movements in patients with Parkinson is disease. Brain. 1987;110:361-379.
63 Luria AR. Higher Cortical Function in Man, 2nd ed. New York: Basic Books, 1980.
64 Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169-192.
65 Ferro J, Martins I, Mariano G, et al. CT scan correlates of gesture recognition. J Neurol Neurosurg Psychiatry. 1983;46:943-952.
66 Varney N, Damasio H. Locus of lesion in impaired pantomime recognition. Cortex. 1987;23:699-703.
67 Sirigu A, Daprati E, Pradat-Diehl P, et al. Perception of selfgenerated movement following left parietal lesion. Brain. 1999;122:1867-1874.
68 Rossetti I, Rode G, Goldenberg G. Perspectives on higher-order motor deficit rehabilitation. In: Freund H, Hallett M, Jeannerod M, et al, editors. Higher-Order Motor Disorders. Oxford, UK: Oxford University Press; 2005:475-498.
69 Bizzozero I, Costato D, Della Sala S, et al. Upper and lower face apraxia: role of the right hemisphere. Brain. 2000;123:2213-2230.
70 Spinazzola L, Cubelli R, Della Sala S. Impairment of trunk movements following left or right hemisphere lesions: dissociation between apraxic errors and postural instability. Brain. 2003;126:2656-2666.
71 Gerstmann J, Schilder P. Über eine besondere Gangstörung bei Stirnhirner krankung. Wien Med Wochenschr. 1926;76:97-102.
72 Nutt J. Higher-order disorders of gait. In: Freund H, Hallett M, Jeannerod M, et al, editors. Higher-Order Motor Disorders. Oxford, UK: Oxford University Press; 2005:237-248.
73 Meyer JS, Barron DW. Apraxia of gait: a clinico-physiological study. Brain. 1960;83:261-284.
74 Della Sala S, Francescani A, Spinnler H. Gait apraxia after supplementary motor area lesions. J Neurol Neurosurg Psychiatry. 2002;72:77-85.