Approach to the urogenital system
Anatomy
The oval-shaped kidneys, measuring 9 to 15 cm in length and 4 to 6 cm in width, are located in retroperitoneum between the 12th thoracic and 4th lumbar vertebrae, just lateral to the psoas muscles. The kidneys are surrounded by the Gerota fascia (echogenic capsule) and a fat layer of variable thickness. Ultrasound examination, in both longitudinal and transverse planes, is performed using convex or microconvex (2.5-5 MHz) transducers and identifies the hilum, containing renal vessels and ureter; the pelvis, receiving two to three major calyces; and the cortex and the medulla, containing renal pyramids. Normal cortical echogenicity should be less than or equal to the echogenicity of liver (Figure 42-1). The right and left kidneys are visualized in the right (midaxillary line) and left (posterior-axillary line) lower intercostal spaces, respectively. The right kidney is lower than the left one because of the right lobe of the liver. In the intensive care unit (ICU), intercostal ultrasound approaches as well as scans along both hypochondriac regions can be used.1–4
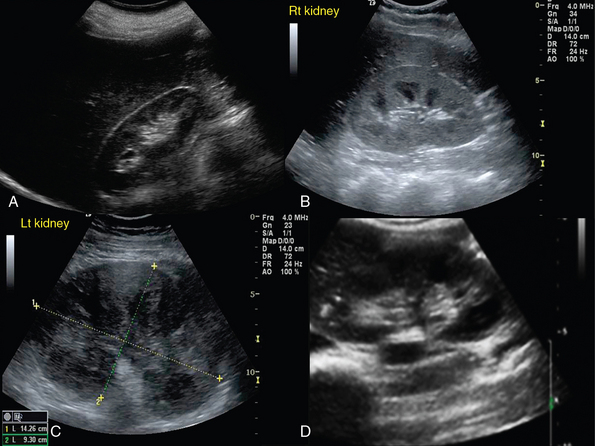
Figure 42-1 A, Normal kidney. B, Enhanced cortical echogenicity in lupus-related glomerulonephritis. C, Kidney enlargement with loss of parenchymal differentiation in a septic patient with acute kidney injury. D, Heterogeneous cortical appearance in pyelonephritis.
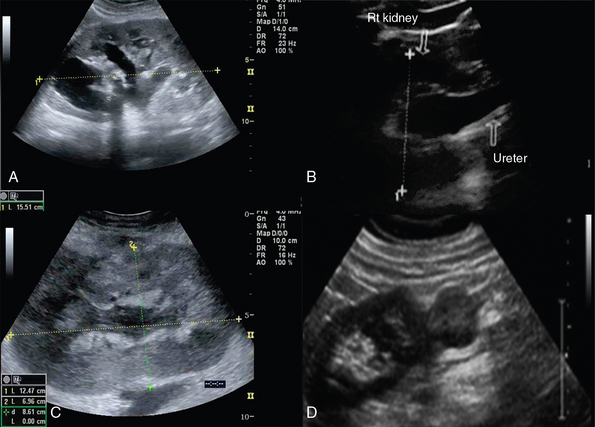
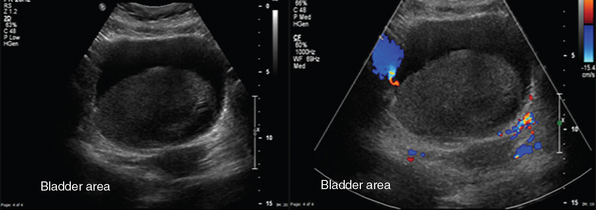
The right and left main renal arteries (RAs) originate laterally from the abdominal aorta, inferior to the superior mesenteric artery (SMA). Segmental (lobar) arteries originate before or immediately after entering the kidney, and their branches (interlobar arteries) travel through the kidney parenchyma. The right and left renal veins (RV) originate off the inferior vena cava (IVC). Ureters travel retroperitoneally, crossing over the iliac vessels and along the pelvic sidewalls, before curving anteriorly and medially at the level of ischial spines, and thus entering the bladder at ureterovesical junction. Ureters are not well visualized by ultrasound but, when distended (hydroureter), appear as hypoechoic, tubular structures (Figure 42-2). The round-shaped bladder is depicted by ultrasound just above the pubis. When not distended, its wall appears thickened. When fully distended, its wall should measure less than 0.4 cm thick. The trigone is a triangular region of the bladder base, formed by two ureteral orifices and the internal urethral orifice, which are identified by depicting ureteral jets, emanating from the ureters. In critical care patients, the balloon of the Foley catheter should be visible in the bladder (catheter is clamped to avoid bladder emptying, Figure 42 E-1). Inability to visualize the balloon should prompt a search for its location because extravesical inflation may result in local trauma and confusing appearances. The normal adult testis measures about 3 to 4 cm (longitudinal section) and is normally hypoechoic. The upper testicular pole is capped with the head of the epidydimis. A thin rim of anechoic fluid may be seen around the testicle.1–4
Disorders
Kidney
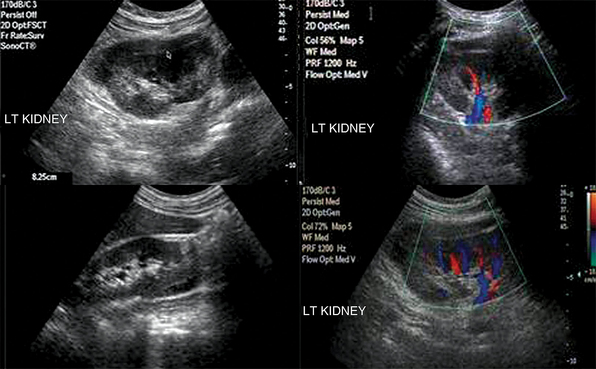
Renal failure resulting from various causes is commonly encountered in the ICU. Ultrasound may depict loss of parenchymal differentiation and rule out obstruction. In acute failure, kidneys may appear normal or increased in size (see Figure 42-1), whereas in chronic failure, kidneys appear small with thin parenchyma and irregular borders. The Doppler-derived resistive index [(RI) = (peak systolic velocity − end diastolic velocity)/(peak systolic velocity)] of renal and interlobar arteries has been used to study many different renal diseases. The normal mean RI value is approximately 0.60, whereas values greater than 0.8 indicate parenchymal disease. These criteria require further validation, and inaccurate readings may occur, especially in ICU patients. Doppler measurements may be obtained when the sample volume is adjusted to 1.5 to 2 mm and the angle to 0 degrees when evaluating interlobar arteries, whereas the angle should be 60 degrees or less in the RA. Despite its limitations, the above method may be promising in the monitoring of critical care patients with failing renal function (Figure 42 E-2).4–8
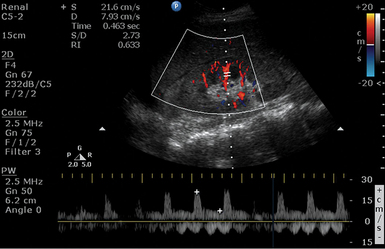
Figure 42 E-2 A septic patient presented with “anuric” acute kidney injury. Doppler-derived resistive index (RI) (0.63 in this image) values ranged from 0.45 to 0.65 in repeated measurements. After supportive therapy, renal function and urine output gradually normalized.
Pyelonephritis, although difficult to diagnose in the ICU because of blurred clinical picture, may be detected by ultrasound (see Figure 42-1). Ultrasonographic findings are a normal or enlarged kidney, focal or diffuse swelling with decreased echogenicity, loss of corticomedullary differentiation, areas of necrosis (hypoechoic), and microabscess formation (hypoechoic areas with smooth or irregular margins and high-level internal echoes). Renal abscess appears usually as a complex, lobulated cystic mass, whereas hyperechoic foci within indicate presence of gas. Depiction of gas bubbles extended to parenchymal areas, sinus, and perinephric space characterize a severe form of pyelonephritis called emphysematous pyelonephritis, which is usually observed in diabetic patients. Another form of severe infection is pyonephrosis, which may appear as calyceal dilatation (hydronephrosis) with the presence of echogenic material within, consistent with pus or debris formation.4 Glomerulonephritis is a major cause of end-stage kidney disease. In acute and subacute cases, kidneys may appear enlarged because of parenchymal swelling with increased echogenicity and prominent hypoechoic medullary pyramids (see Figure 42-1). In chronic glomerulonephritis, kidneys are small and atrophic with increased echogenicity.4
Renal trauma is usually observed after abdominal injury (see Figure 42-2). Although ultrasound can detect hemoperitoneum, its role in evaluating renal trauma is limited compared with computed tomography (CT). Ultrasound may detect presence of parenchymal (mixed echogenicity) or subcapsular hematomas (perirenal fluid collection), segmental corticomedullary dedifferentiation, linear defect consistent with laceration, or a shattered kidney (multiple areas of disorganized tissue with blood and urine collections) (Figure 42 E-3). Trauma may lead to persistent urine leakage and development of urinomas that manifest as delayed complications (e.g., hydronephrosis, paralytic ileus, etc.). Urinomas have a variety of appearances and may be misdiagnosed as ordinary ascites, abdominal or pelvic abscesses or hematomas, cystic masses, or pancreatic pseudocysts. Urinomas may result from blunt or penetrating trauma, iatrogenic injury, or transmitted back pressure caused by downstream obstruction resulting from a ureteral stone, surgical ligature, or abdominal and pelvic masses. Ureteral urine leaks may be iatrogenic (e.g., surgery or endourologic procedures).9
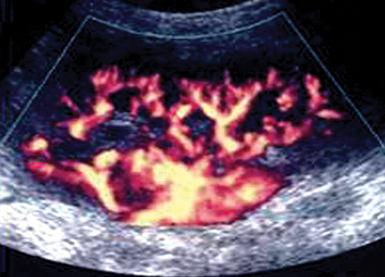
Renal vascular disorders are evaluated by duplex ultrasound techniques. Atherosclerosis is the most common disorder, causing either RA stenosis or occlusion. A renal-aortic Doppler velocity ratio (RAR = peak systolic RA velocity/peak systolic aortic velocity) less than 2.5 indicates the absence of a flow-reducing RA stenosis (reduced RA diameter < 60%), whereas values greater than 3.5 usually correspond to hemodynamically significant stenosis. In cases of aortic disorders (e.g., aneurysm), often observed in the ICU, using RAR is not reliable; instead, peak systolic velocities in either RA greater than 200 cm/sec, along with confirmed poststenotic Doppler signals, could be used to detect flow-reducing stenosis (Figure 42 E-4).4,10 Atherosclerosis may cause bilateral stenosis, while it affects the proximal RA segments. A less common disorder causing RA stenosis is fibromuscular dysplasia, which affects the middistal RA segments, while a “string of beads” RA appearance (narrowing and then widening of arterial segments) can be observed. Acute arterial occlusion may occur because of abdominal trauma. Doppler findings are absence of renal arterial flow and either absence of endonephric flow or detection of low-velocity Doppler signals resulting from collateral channels. Finally, the risk of renal arterial embolism, which may cause partial or total arterial occlusion, increases in patients with atrial fibrillation, mitral stenosis, or previous RA stenosis. RV thrombosis can occur in one or both veins (e.g., nephrotic syndrome) because of extrinsic compression, hypercoaguable states, trauma, or surgery (e.g., posttransplantation). Compression of the left distal RV that may lead to thrombosis between the aorta, and SMA rarely occurs (nutcracker syndrome). Ultrasonographic findings of acute thrombosis include kidney enlargement, loss of parenchymal differentiation, diffuse “snow appearance” (central heterogeneous mass with numerous anechoic spaces), thrombus in the RV or in IVC. In chronic thrombosis, kidneys may appear normal or atrophic and calcified.4,11
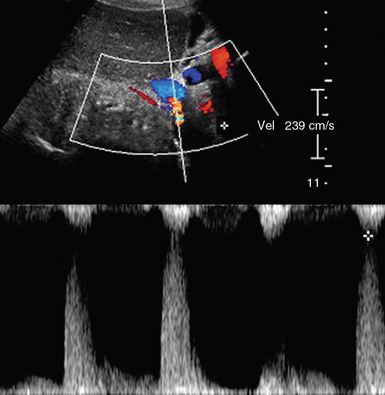
Figure 42 E-4 Color Doppler depicting renal artery (RA) stenosis (peak systolic velocity > 200 cm/sec) in a patient with aneurysmal abdominal aorta.
Renal transplant duplex ultrasound evaluation may depict spectral flow patterns and tissue changes characteristic of normal transplants and those undergoing rejection. Ultrasound can detect RA stenosis-occlusion, RV thrombosis, and the presence of arteriovenous fistulas within the transplant. Renal transplants are often placed subcutaneously in the pelvis, whereas transplant RA and RV are usually anastomosed to the external or internal iliac artery and vein (Figure 42 E-5). All anastomotic sites should be checked by duplex ultrasound; however, this is not always technically feasible. Doppler spectral patterns from all inflow, outflow, and organ vessels must be integrated before drawing any conclusions. Apart from B-mode transplant evaluation, the demonstration of a RI greater than 0.8 or an end-diastolic to systolic velocity ratio greater than 0.2 by Doppler ultrasound, in the absence of RV thrombosis and ureteral obstruction, may be indicative of rejection.12–14
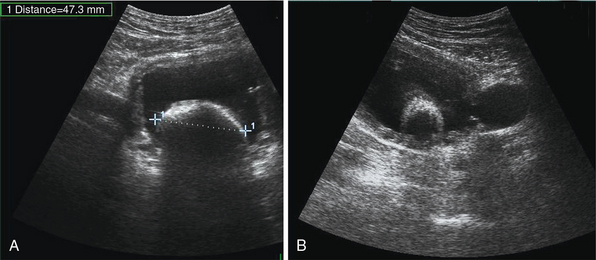
Hydronephrosis is the dilatation of renal pelvis and calyces and may be due to various causes, such as obstruction (blood clot, sloughed papillae, iliac vessels crossing, strictures, etc.), papillary necrosis, reflux nephropathy, chronic infection, urogenital tumors, benign prostatic hyperplasia, ureter narrowing (infection, injury, surgery), neurogenic bladder, pregnancy, retroperitoneal fibrosis and so forth (see Figure 42-2). Ultrasound can detect kidney stones, especially if stones are greater than 0.5 mm because posterior acoustic shadowing is usually present. If the latter is not present, depiction of the “twinkle artifact,” appearing as a quick fluctuating mixture of Doppler signals behind a strongly reflecting interface (stones), might help. A common pitfall is depicting vascular calcifications as renal calculi.4,5
Renal cysts are the most common renal lesions. They are usually benign and may be single, multiple, unilateral, or bilateral. If multiple cysts are found, polycystic kidney disease must be considered. The latter usually affects both kidneys (unilateral in 17% of cases) and may affect other organs, such as the liver, pancreas, heart, and brain. Ultrasonographically, simple cysts are round, anechoic structures composed of a sharply defined smooth wall with posterior acoustic enhancement. Parapelvic cysts may cause hydronephrosis because of pressure; however, in hydronephrosis calyces are usually interconnected with the collecting system. Complex cysts usually have thick walls, septations, lobulations, calcifications, internal echoes, fluid-filled or echogenic material and are classified as infectious, hemorrhagic, proteinaceous, calcified, neoplasmatic, and hydatid. Renal masses detected by ultrasound, whether cystic or solid, should be further evaluated by other imaging modalities (e.g., contrast-enhanced CT) to make an accurate diagnosis and facilitate surgical treatment (see Figure 42-2). Renal masses are of variable size and may be found at the renal cortex or projecting inside the medulla. Solid tumors are homogeneous or heterogeneous lesions of mixed echogenicity with distinct or irregular borders (Figure 42 E-6). Cystic and hemorrhagic components or evidence of angiogenesis may be found within the tumor.4,15
Bladder
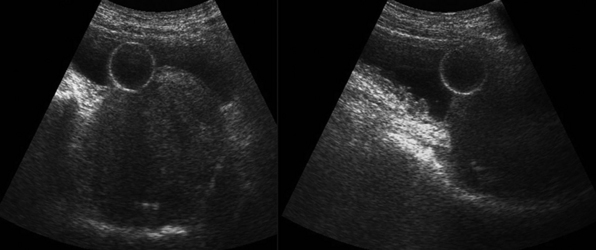
A typical transverse section of the ultrasound examination depicts the bladder behind both rectal muscles and above and behind the rectum. Bladder should be fully filled to achieve a better wall delineation. In males, the prostate gland is visualized under the bladder neck, whereas in females who have not undergone hysterectomy, the uterus may be seen in the midline, posteriorly and superiorly to the bladder. Bladder wall thickening (>0.4 cm) may be focal or diffuse. Focal thickening may be due to cystitis, endometriosis and Crohn disease (possible fistula formation), benign and malignant tumors, and benign prostate hyperplasia (Figures 42 E-7 and 42 E-8). Diffuse thickening may be due to infection, inflammation, prolonged catheterization, drugs, and neurogenic bladder (Figure 42-3). Depending upon the underlying cause additional imaging findings may be present (e.g., echogenic mobile blood clots in case of hemorrhagic cystitis). Ultrasound sensitivity is low for detecting bladder injury; however; indirect findings (e.g., free fluid) may be present. Doppler techniques may help differentiate between tumors and clots (e.g., clots do not exhibit flow); however absence of flow in a bladder mass does not exclude malignancy. Doppler ultrasound is used to enhance ureteral jets (normally apparent within 5-8 minutes) because any asymmetry may indicate obstruction.4,16
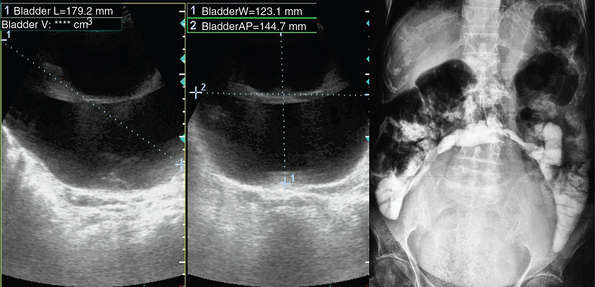
Figure 42-3 Maximally distended (1660 mL) neurogenic bladder (diagnosis of exclusion: co-evaluation with clinical data).
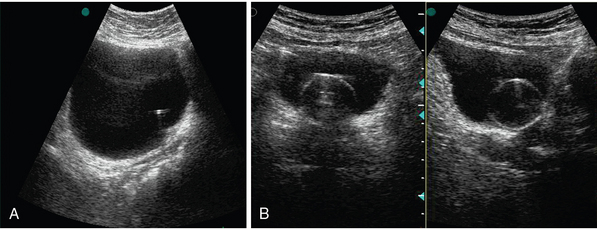
Figure 42 E-8 A, Echogenic stent inserted for subpelvic ureteral stenosis. B, Indwelling catheter inserted for urinary retention.
In the ICU, a catheterized bladder should be checked for any signs of trapped urine or diverticulum. Often, a sedated patient may develop anuria, which prompts a search for “hemodynamic” causes, whereas scanning the bladder first to exclude distention is a more rational approach. Bladder outlet obstruction may be due to the presence of calculi, pyuria, or blood clots. Calculi can be detected as echogenic foci movable with posterior acoustic shadowing (Figure 42 E-9). Bladder disorders such as diverticulum and ureteroceles may cause diagnostic confusion. Ultrasound findings consistent with diverticulum include an anechoic out-pouching originating from the bladder with a narrow or wide neck, which should be differentiated from ureteroceles, which appear as well-defined, thin-walled echogenic masses (see Figure 42 E-9). Ureteroceles are almost always present inferolaterally at the ureterovesical junction, and if ectopic, a duplicated collecting system is present (80%).17
Scrotum
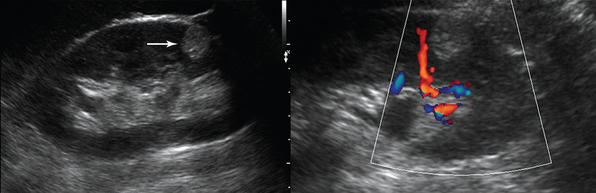
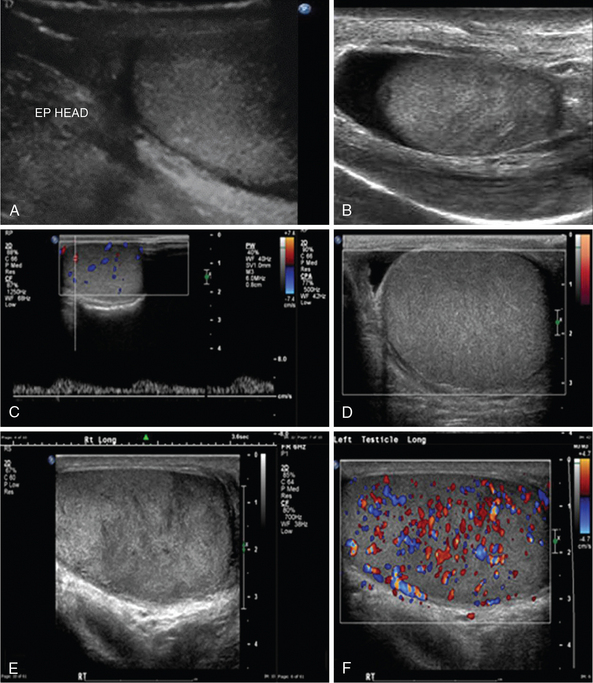
Ultrasound examination of scrotum is performed by high-frequency linear transducers (7-15 MHz). Each testis is examined separately and compared with the other. Epidydimitis starts by affecting epidydimis’ tail and could develop to orchitis in 40% of cases (Figure 42 E-10). Ultrasound findings include edematous enlargement of the gland (the head could measure >17 mm) and thickening of scrotal layers along with increased vascularity. In diffuse orchitis, the testis is enlarged, with a heterogeneous appearance. Seminomas can have the same appearance, as well as testicular torsion. Testicular torsion (twisting of testicular spermatic cord), which most commonly affects patients between 12 to 20 years but can occur at any age, is an emergency condition because it results in ischemia and necrosis (testicular tissue is not viable after more than 6 hours of ischemia). In the acute phase, ultrasound findings may be normal, whereas as it progresses, evidence of testicular and scrotal enlargement along with absent testicular blood flow may be found. Confirmation of both venous and arterial flow on pulsed wave Doppler is critical when ruling out testicular torsion. Scrotal trauma, resulting usually from blunt injury, has poor prognosis if surgical repair is postponed. Ultrasound may reveal irregular testicular shape, heterogenic testicular lesions, intraparenchymal hematoma, and distorted testicular vascularity with associated hematocele (see Figure 42 E-10). The testicles contours should be scanned closely for any irregularity because this could indicate rupture.1–4
Ultrasound-guided interventions
Ultrasound-guidance facilitates interventions, such as emergency percutaneous nephrostomy (PCN) and suprapubic bladder catheterization or placement of internal-external nephroureteral stents. Guided PCN is used to treat urinary obstructions in septic and unstable patients who cannot be transferred to the operating room and carries a lower mortality rate (0.2%) compared with surgery. The kidney is punctured via a posterolateral approach to avoid the colon and the pleura, while the needle is targeting the dilating calyces for the subsequent placement of the PCN tube. Persistent postprocedural hematuria may necessitate a change in tube size or obtaining a new access in a different calyx or even ruling out arteriovenous fistula formation. Guided suprapubic bladder catheterization provides the operator with the potential to choose a more cranial penetration point that may decrease the risk of sepsis in prevesical space.1–4
Pearls and highlights
• Ultrasound-detected renal masses should be evaluated by additional imaging modalities.
• Doppler-derived RI greater than 0.8 of the interlobar arteries indicates parenchymal disease.
• Loss of parenchymal differentiation and changes in normal kidney size and cortical echogenicity are nonspecific ultrasound findings.
• Ultrasound is used to monitor renal transplants and guide emergency interventions.
• Sudden development of anuria or hematuria indicates bladder ultrasound evaluation.
• Prompt ultrasound detection of testicular trauma and ischemia facilitates early surgical repair.
References
1. Ma OJ, Matteer JR, eds. Emergency ultrasound. New York: McGraw-Hill, 2003.
2. Simon BC, Snoey ER, eds. Ultrasound in emergency and ambulatory medicine. St Louis: Mosby, 1997.
3. Shah, SP, Price, DD, Manual of ultrasound for resource-limited settings. ed 1. Partners in Health, Boston, 2011:219–240.
4. Resnick, MJ, Rifkin, MD. Ultrasonography of the urinary tract. Baltimore: Williams & Wilkins; 1991.
5. Charasse, C, Camus, C, Darnault, P, et al, Acute nondilated anuric obstructive nephropathy on echography: difficult diagnosis in the intensive care unit. Intensive Care Me. 1991; 17:387–391.
6. Tublin, ME, Bude, RO, Platt, JF, The resistive index in renal Doppler sonography: where do we stand. Am J Roentgeno. 2003; 180:885–892.
7. Umgelter, A, Reindl, W, Franzen, M, et al. Renal resistive index and renal function before and after paracentesis in patients with hepatorenal syndrome and tense ascites. Intensive Care Med. 2009; 35:152–156.
8. Keogan, M, Kliewer, M, Hertzberg, B, et al, Renal resistive indexes: variability in Doppler US measurement in a healthy population. Radiology. 1996;199:165–169.
9. Kawashima, A, Sandler, CM, Corl, FM, et al, Education exhibit: imaging of renal traumaa comprehensive review. Radiographic. 2001; 21:557–574.
10. Norris, CS, Pfeiffer, JS, Rittgers, SE, et al. Noninvasive evaluation of renal artery stenosis and renovascular resistance. J Vasc Surg. 1984; 1:192–201.
11. Cai, S, Ouyang, YS, Li, JC, et al. Evaluation of acute renal artery thrombosis or embolism with color Doppler sonography. Clin Imaging. 2008; 32:367–371.
12. Berland, L, Lawson, R, Adams, M, et al. Evaluation of renal transplants with pulsed Doppler duplex ultrasonography. J Ultrasound Med. 1982; 1:215–222.
13. Perrella, RR, Duerinckx, AJ, Tessler, FN, et al, Evaluation of renal transplant dysfunction by duplex Doppler sonography: a prospective study and review of the literature. Am J Kidney Di. 1990; 15:544–550.
14. Rigsby, CM, Burns, PN, Weltin, GW, et al, Doppler signal quantitation in renal allografts: comparison in normal and rejecting transplants with pathologic correlation. Radiolog. 1987; 162:39–42.
15. Israel, GM, Bosniak, MA, How I do it: evaluating renal masses. Radiolog. 2005; 236:441–450.
16. Burge, HJ, Middleton, WD, McClennan, B, Hildebolt, CF, Ureteral jets in healthy subjects and in patients with unilateral ureteral calculi: comparison with color Doppler US. Radiolog. 1991; 180:437–442.
17. Titton, RL, Gervais, DA, Hahn, PF, et al, Urine leaks and urinomas: diagnosis and imaging-guided intervention. Radiographic. 2003; 1133:1147.