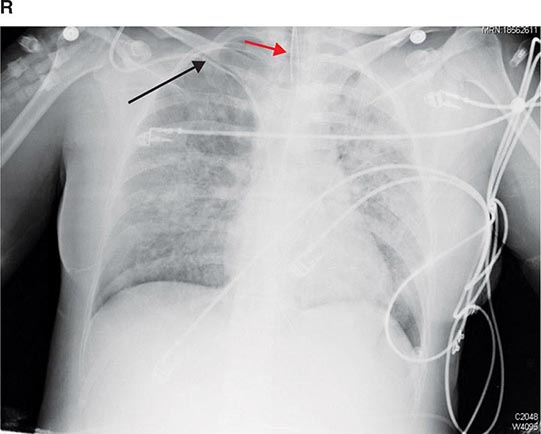
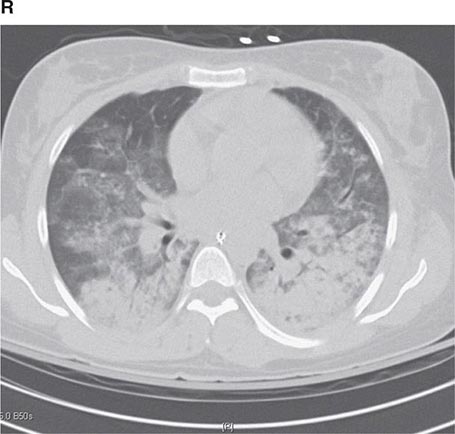
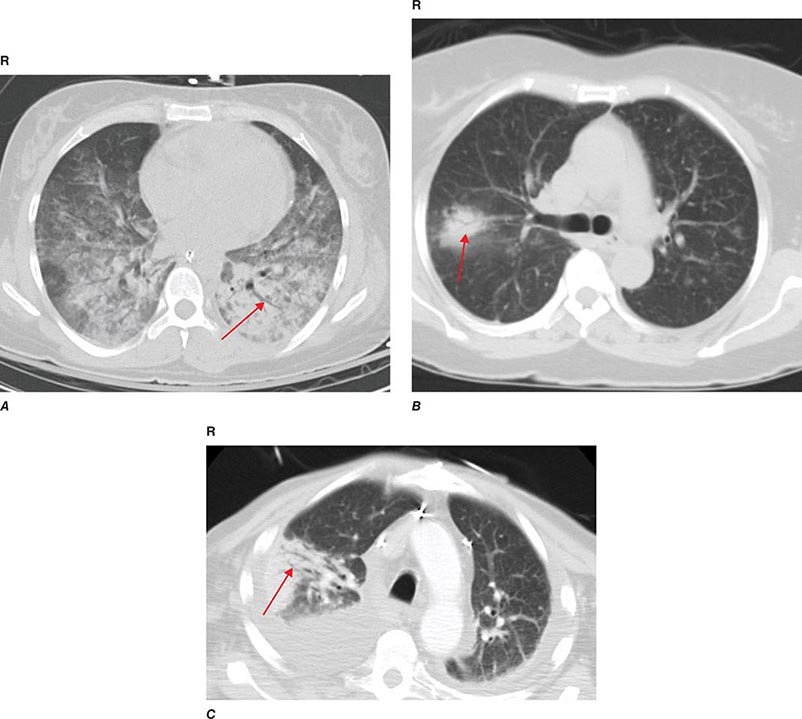
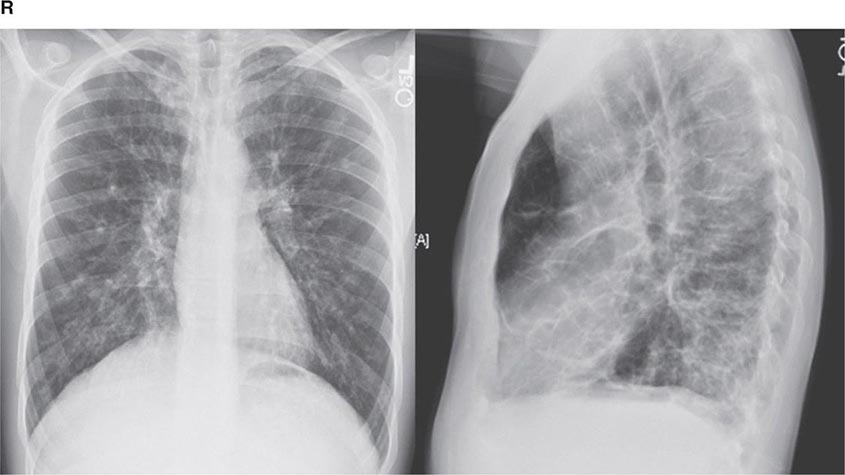
PART 11: Disorders of the Respiratory System
SECTION 1 |
DIAGNOSIS OF RESPIRATORY DISORDERS |
305 |
Approach to the Patient with Disease of the Respiratory System |
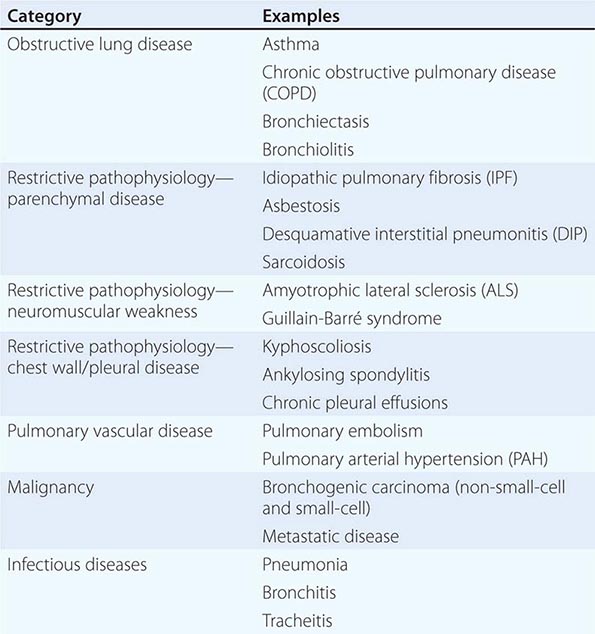
The majority of diseases of the respiratory system fall into one of three major categories: (1) obstructive lung diseases; (2) restrictive disorders; and (3) abnormalities of the vasculature. Obstructive lung diseases are most common and primarily include disorders of the airways, such as asthma, chronic obstructive pulmonary disease (COPD), bronchiectasis, and bronchiolitis. Diseases resulting in restrictive pathophysiology include parenchymal lung diseases, abnormalities of the chest wall and pleura, and neuromuscular disease. Disorders of the pulmonary vasculature include pulmonary embolism, pulmonary hypertension, and pulmonary veno-occlusive disease. Although many specific diseases fall into these major categories, both infective and neoplastic processes can affect the respiratory system and result in myriad pathologic findings, including those listed in the three categories above (Table 305-1).
|
CATEGORIES OF RESPIRATORY DISEASE |
Disorders can also be grouped according to gas exchange abnormalities, including hypoxemic, hypercarbic, or combined impairment. However, many diseases of the lung do not manifest as gas exchange abnormalities.
As with the evaluation of most patients, the approach to a patient with disease of the respiratory system begins with a thorough history and a focused physical examination. Many patients will subsequently undergo pulmonary function testing, chest imaging, blood and sputum analysis, a variety of serologic or microbiologic studies, and diagnostic procedures, such as bronchoscopy. This stepwise approach is discussed in detail below.
HISTORY
Dyspnea and Cough The cardinal symptoms of respiratory disease are dyspnea and cough (Chaps. 47e and 48). Dyspnea has many causes, some of which are not predominantly due to lung pathology. The words a patient uses to describe shortness of breath can suggest certain etiologies for dyspnea. Patients with obstructive lung disease often complain of “chest tightness” or “inability to get a deep breath,” whereas patients with congestive heart failure more commonly report “air hunger” or a sense of suffocation.
The tempo of onset and the duration of a patient’s dyspnea are likewise helpful in determining the etiology. Acute shortness of breath is usually associated with sudden physiologic changes, such as laryngeal edema, bronchospasm, myocardial infarction, pulmonary embolism, or pneumothorax. Patients with COPD and idiopathic pulmonary fibrosis (IPF) experience a gradual progression of dyspnea on exertion, punctuated by acute exacerbations of shortness of breath. In contrast, most asthmatics have normal breathing the majority of the time with recurrent episodes of dyspnea that are usually associated with specific triggers, such as an upper respiratory tract infection or exposure to allergens.
Specific questioning should focus on factors that incite dyspnea as well as on any intervention that helps resolve the patient’s shortness of breath. Asthma is commonly exacerbated by specific triggers, although this can also be true of COPD. Many patients with lung disease report dyspnea on exertion. Determining the degree of activity that results in shortness of breath gives the clinician a gauge of the patient’s degree of disability. Many patients adapt their level of activity to accommodate progressive limitation. For this reason, it is important, particularly in older patients, to delineate the activities in which they engage and how these activities have changed over time. Dyspnea on exertion is often an early symptom of underlying lung or heart disease and warrants a thorough evaluation.
Cough generally indicates disease of the respiratory system. The clinician should inquire about the duration of the cough, whether or not it is associated with sputum production, and any specific triggers that induce it. Acute cough productive of phlegm is often a symptom of infection of the respiratory system, including processes affecting the upper airway (e.g., sinusitis, tracheitis), the lower airways (e.g., bronchitis, bronchiectasis), and the lung parenchyma (e.g., pneumonia). Both the quantity and quality of the sputum, including whether it is blood-streaked or frankly bloody, should be determined. Hemoptysis warrants an evaluation as delineated in Chap. 48.
Chronic cough (defined as that persisting for >8 weeks) is commonly associated with obstructive lung diseases, particularly asthma and chronic bronchitis, as well as “nonrespiratory” diseases, such as gastroesophageal reflux and postnasal drip. Diffuse parenchymal lung diseases, including IPF, frequently present as a persistent, nonproductive cough. As with dyspnea, all causes of cough are not respiratory in origin, and assessment should encompass a broad differential, including cardiac and gastrointestinal diseases as well as psychogenic causes.
Additional Symptoms Patients with respiratory disease may report wheezing, which is suggestive of airways disease, particularly asthma. Hemoptysis can be a symptom of a variety of lung diseases, including infections of the respiratory tract, bronchogenic carcinoma, and pulmonary embolism. In addition, chest pain or discomfort is often thought to be respiratory in origin. As the lung parenchyma is not innervated with pain fibers, pain in the chest from respiratory disorders usually results from either diseases of the parietal pleura (e.g., pneumothorax) or pulmonary vascular diseases (e.g., pulmonary hypertension). As many diseases of the lung can result in strain on the right side of the heart, patients may also present with symptoms of cor pulmonale, including abdominal bloating or distention and pedal edema (Chap. 279).
Additional History A thorough social history is an essential component of the evaluation of patients with respiratory disease. All patients should be asked about current or previous cigarette smoking, as this exposure is associated with many diseases of the respiratory system, most notably COPD and bronchogenic lung cancer but also a variety of diffuse parenchymal lung diseases (e.g., desquamative interstitial pneumonitis and pulmonary Langerhans cell histiocytosis). For most disorders, longer duration and greater intensity of exposure to cigarette smoke increases the risk of disease. There is growing evidence that “second-hand smoke” is also a risk factor for respiratory tract pathology; for this reason, patients should be asked about parents, spouses, or housemates who smoke. Possible inhalational exposures should be explored, including those at the work place (e.g., asbestos, wood smoke) and those associated with leisure (e.g., excrement from pet birds) (Chap. 311). Travel predisposes to certain infections of the respiratory tract, most notably the risk of tuberculosis. Potential exposure to fungi found in specific geographic regions or climates (e.g., Histoplasma capsulatum) should be explored.
Associated symptoms of fever and chills should raise the suspicion of infective etiologies, both pulmonary and systemic. A comprehensive review of systems may suggest rheumatologic or autoimmune disease presenting with respiratory tract manifestations. Questions should focus on joint pain or swelling, rashes, dry eyes, dry mouth, or constitutional symptoms. In addition, carcinomas from a variety of primary sources commonly metastasize to the lung and cause respiratory symptoms. Finally, therapy for other conditions, including both irradiation and medications, can result in diseases of the chest.
Physical Examination The clinician’s suspicion of respiratory disease often begins with a patient’s vital signs. The respiratory rate is often informative, whether elevated (tachypnea) or depressed (hypopnea). In addition, pulse oximetry should be measured, as many patients with respiratory disease have hypoxemia, either at rest or with exertion. The classic structure of the respiratory examination proceeds through inspection, percussion, palpation, and auscultation as described below. Often, however, auscultatory findings will lead the clinician to perform further percussion or palpation in order to clarify these findings.
The first step of the physical examination is inspection. Patients with respiratory disease may be in distress, often using accessory muscles of respiration to breathe. Severe kyphoscoliosis can result in restrictive pathophysiology. Inability to complete a sentence in conversation is generally a sign of severe impairment and should result in an expedited evaluation of the patient.
Percussion of the chest is used to establish diaphragm excursion and lung size. In the setting of decreased breath sounds, percussion is used to distinguish between pleural effusions (dull to percussion) and pneumothorax (hyper-resonant note).
The role of palpation is limited in the respiratory examination. Palpation can demonstrate subcutaneous air in the setting of barotrauma. It can also be used as an adjunctive assessment to determine whether an area of decreased breath sounds is due to consolidation (increased tactile fremitus) or a pleural effusion (decreased tactile fremitus).
The majority of the manifestations of respiratory disease present as abnormalities of auscultation. Wheezes are a manifestation of airway obstruction. While most commonly a sign of asthma, peribronchial edema in the setting of congestive heart failure can also result in diffuse wheezes, as can any other process that causes narrowing of small airways. For this reason, clinicians must take care not to attribute all wheezing to asthma.
Rhonchi are a manifestation of obstruction of medium-sized airways, most often with secretions. In the acute setting, this manifestation may be a sign of viral or bacterial bronchitis. Chronic rhonchi suggest bronchiectasis or COPD. Stridor, a high-pitched, focal inspiratory wheeze, usually heard over the neck, is a manifestation of upper airway obstruction and should prompt expedited evaluation of the patient, as it can precede complete upper airway obstruction and respiratory failure.
Crackles, or rales, are commonly a sign of alveolar disease. A variety of processes that fill the alveoli with fluid may result in crackles. Pneumonia can cause focal crackles. Pulmonary edema is associated with crackles, generally more prominent at the bases. Interestingly, diseases that result in fibrosis of the interstitium (e.g., IPF) also result in crackles often sounding like Velcro being ripped apart. Although some clinicians make a distinction between “wet” and “dry” crackles, this distinction has not been shown to be a reliable way to differentiate among etiologies of respiratory disease.
One way to help distinguish between crackles associated with alveolar fluid and those associated with interstitial fibrosis is to assess for egophony. Egophony is the auscultation of the sound “AH” instead of “EEE” when a patient phonates “EEE.” This change in note is due to abnormal sound transmission through consolidated parenchyma and is present in pneumonia but not in IPF. Similarly, areas of alveolar filling have increased whispered pectoriloquy as well as transmission of larger-airway sounds (i.e., bronchial breath sounds in a lung zone where vesicular breath sounds are expected).
The lack or diminution of breath sounds can also help determine the etiology of respiratory disease. Patients with emphysema often have a quiet chest with diffusely decreased breath sounds. A pneumothorax or pleural effusion may present with an area of absent breath sounds.
Other Systems Pedal edema, if symmetric, may suggest cor pulmonale; if asymmetric, it may be due to deep venous thrombosis and associated pulmonary embolism. Jugular venous distention may also be a sign of volume overload associated with right heart failure. Pulsus paradoxus is an ominous sign in a patient with obstructive lung disease, as it is associated with significant negative intrathoracic (pleural) pressures required for ventilation and impending respiratory failure.
As stated earlier, rheumatologic disease may manifest primarily as lung disease. Owing to this association, particular attention should be paid to joint and skin examination. Clubbing can be found in many lung diseases, including cystic fibrosis, IPF, and lung cancer. Cyanosis is seen in hypoxemic respiratory disorders that result in >5 g of deoxygenated hemoglobin/dL.
DIAGNOSTIC EVALUATION
The sequence of studies is dictated by the clinician’s differential diagnosis, as determined by the history and physical examination. Acute respiratory symptoms are often evaluated with multiple tests performed at the same time in order to diagnose any life-threatening diseases rapidly (e.g., pulmonary embolism or multilobar pneumonia). In contrast, chronic dyspnea and cough can be evaluated in a more protracted, stepwise fashion.
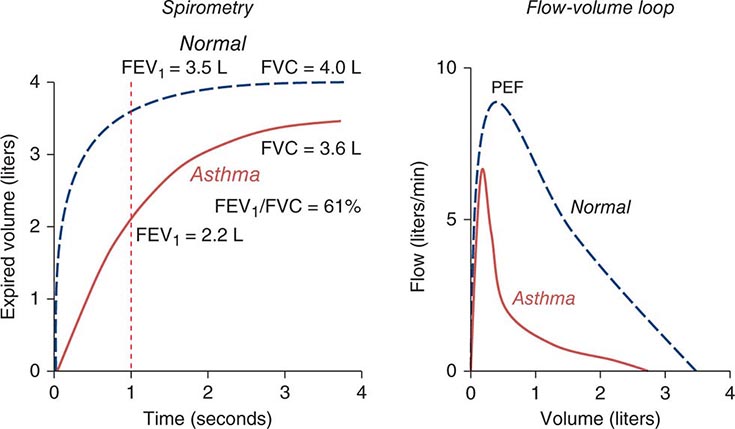
Pulmonary Function Testing (See also Chap. 307) The initial pulmonary function test obtained is spirometry. This study is an effort-dependent test used to assess for obstructive pathophysiology as seen in asthma, COPD, and bronchiectasis. A diminished-forced expiratory volume in 1 sec (FEV1)/forced vital capacity (FVC) (often defined as <70% of the predicted value) is diagnostic of obstruction. In addition to measuring FEV1 and FVC, the clinician should examine the flow-volume loop (which is effort-independent). A plateau of the inspiratory and expiratory curves suggests large-airway obstruction in extrathoracic and intrathoracic locations, respectively.
Spirometry with symmetric decreases in FEV1 and FVC warrants further testing, including measurement of lung volumes and the diffusion capacity of the lung for carbon monoxide (DLCO). A total lung capacity <80% of the predicted value for a patient’s age, race, sex, and height defines restrictive pathophysiology. Restriction can result from parenchymal disease, neuromuscular weakness, or chest wall or pleural diseases. Restriction with impaired gas exchange, as indicated by a decreased DLCO, suggests parenchymal lung disease. Additional testing, such as measurements of maximal expiratory pressure and maximal inspiratory pressure, can help diagnose neuromuscular weakness. Normal spirometry, normal lung volumes, and a low DLCO should prompt further evaluation for pulmonary vascular disease.
Arterial blood gas testing is often helpful in assessing respiratory disease. Hypoxemia, while usually apparent with pulse oximetry, can be further evaluated with the measurement of arterial PO2 and the calculation of an alveolar gas and arterial blood oxygen tension difference ([A–a]DO2). Patients with diseases that cause ventilation-perfusion mismatch or shunt physiology have an increased (A–a) DO2 at rest. Arterial blood gas testing also allows the measurement of arterial PCO2. Hypercarbia can accompany severe airway obstruction (e.g., COPD) or progressive restrictive physiology, as in patients with neuromuscular weakness.
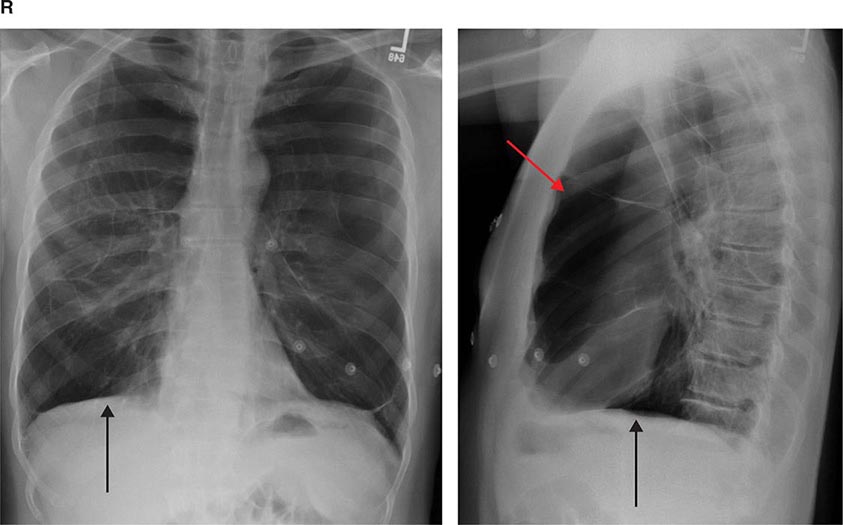
Chest Imaging (see Chap. 308e) Most patients with disease of the respiratory system undergo imaging of the chest as part of the initial evaluation. Clinicians should generally begin with a plain chest radiograph, preferably posterior-anterior and lateral films. Several findings, including opacities of the parenchyma, blunting of the costophrenic angles, mass lesions, and volume loss, can be very helpful in determining an etiology. However, many diseases of the respiratory system, particularly those of the airways and pulmonary vasculature, are associated with a normal chest radiograph.
CT of the chest is often performed subsequently and allows better delineation of parenchymal processes, pleural disease, masses or nodules, and large airways. If the test includes administration of contrast, the pulmonary vasculature can be assessed with particular utility for determination of pulmonary emboli. Intravenous contrast also allows lymph nodes to be delineated in greater detail.
FURTHER STUDIES
Depending on the clinician’s suspicion, a variety of other studies may be done. Concern about large-airway lesions may warrant bronchoscopy. This procedure may also be used to sample the alveolar space with bronchoalveolar lavage or to obtain nonsurgical lung biopsies. Blood testing may include assessment for hypercoagulable states in the setting of pulmonary vascular disease, serologic testing for infectious or rheumatologic disease, or assessment of inflammatory markers or leukocyte counts (e.g., eosinophils). Sputum evaluation for malignant cells or microorganisms may be appropriate. An echocardiogram to assess right- and left-sided heart function is often obtained. Finally, at times, a surgical lung biopsy is needed to diagnose certain diseases of the respiratory system. All of these studies will be guided by the preceding history, physical examination, pulmonary function testing, and chest imaging.
306e |
Disturbances of Respiratory Function |
The primary functions of the respiratory system—to oxygenate blood and eliminate carbon dioxide—require virtual contact between blood and fresh air, which facilitates diffusion of respiratory gases between blood and gas. This process occurs in the lung alveoli, where blood flowing through alveolar wall capillaries is separated from alveolar gas by an extremely thin membrane of flattened endothelial and epithelial cells, across which respiratory gases diffuse and equilibrate. Blood flow through the lung is unidirectional via a continuous vascular path, along which venous blood absorbs oxygen from and loses CO2 to inspired gas. The path for airflow, in contrast, reaches a dead end at the alveolar walls; thus the alveolar space must be ventilated tidally, with inflow of fresh gas and outflow of alveolar gas alternating periodically at the respiratory rate (RR). To provide an enormous alveolar surface area (typically 70 m2) for blood-gas diffusion within the modest volume of a thoracic cavity (typically 7 L), nature has distributed both blood flow and ventilation among millions of tiny alveoli through multigenerational branching of both pulmonary arteries and bronchial airways. As a consequence of variations in tube lengths and calibers along these pathways as well as the effects of gravity, tidal pressure fluctuations, and anatomic constraints from the chest wall, the alveoli vary in their relative ventilations and perfusions. Not surprisingly, for the lung to be most efficient in exchanging gas, the fresh gas ventilation of a given alveolus must be matched to its perfusion.
For the respiratory system to succeed in oxygenating blood and eliminating CO2, it must be able to ventilate the lung tidally and thus to freshen alveolar gas; it must provide for perfusion of the individual alveolus in a manner proportional to its ventilation; and it must allow adequate diffusion of respiratory gases between alveolar gas and capillary blood. Furthermore, it must accommodate severalfold increases in the demand for oxygen uptake or CO2 elimination imposed by metabolic needs or acid-base derangement. Given these multiple requirements for normal operation, it is not surprising that many diseases disturb respiratory function. This chapter considers in some detail the physiologic determinants of lung ventilation and perfusion, elucidates how the matching distributions of these processes and rapid gas diffusion allow normal gas exchange, and discusses how common diseases derange these normal functions, thereby impairing gas exchange—or at least increasing the work required by the respiratory muscles or heart to maintain adequate respiratory function.
VENTILATION
It is useful to think about the respiratory system as three independently functioning components: the lung, including its airways; the neuromuscular system; and the chest wall, which includes everything that is not lung or active neuromuscular system. Accordingly, the mass of the respiratory muscles is part of the chest wall, while the force these muscles generate is part of the neuromuscular system; the abdomen (especially an obese abdomen) and the heart (especially an enlarged heart) are, for these purposes, part of the chest wall. Each of these three components has mechanical properties that relate to its enclosed volume (or—in the case of the neuromuscular system—the respiratory system volume at which it is operating) and to the rate of change of its volume (i.e., flow).
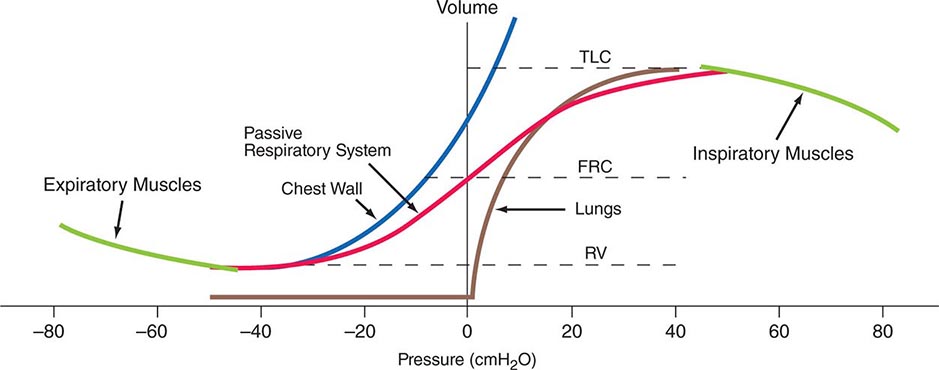
Volume-Related Mechanical Properties—Statics Figure 306e-1 shows the volume-related properties of each component of the respiratory system. Due both to surface tension at the air-liquid interface between alveolar wall lining fluid and alveolar gas and to elastic recoil of the lung tissue itself, the lung requires a positive transmural pressure difference between alveolar gas and its pleural surface to stay inflated; this difference is called the elastic recoil pressure of the lung, and it increases with lung volume. The lung becomes rather stiff at high volumes, so that relatively small volume changes are accompanied by large changes in transpulmonary pressure; in contrast, the lung is compliant at lower volumes, including those at which tidal breathing normally occurs. At zero inflation pressure, even normal lungs retain some air in the alveoli because the small peripheral airways are tethered open by radially outward pull from inflated lung parenchyma attached to adventitia; as the lung deflates during exhalation, those small airways are pulled open progressively less, and eventually they close, trapping some gas in the alveoli. This effect can be exaggerated with age and especially with obstructive airway diseases, resulting in gas trapping at quite large lung volumes.
FIGURE 306e-1 Pressure-volume curves of the isolated lung, isolated chest wall, combined respiratory system, inspiratory muscles, and expiratory muscles. FRC, functional residual capacity; RV, residual volume; TLC, total lung capacity.
The elastic behavior of the passive chest wall (i.e., in the absence of neuromuscular activation) differs markedly from that of the lung. Whereas the lung tends toward full deflation with no distending (transmural) pressure, the chest wall encloses a large volume when pleural pressure equals body surface (atmospheric) pressure. Furthermore, the chest wall is compliant at high enclosed volumes, readily expanding even further in response to increases in transmural pressure. The chest wall also remains compliant at small negative transmural pressures (i.e., when pleural pressure falls slightly below atmospheric pressure), but as the volume enclosed by the chest wall becomes quite small in response to large negative transmural pressures, the passive chest wall becomes stiff due to squeezing together of ribs and intercostal muscles, diaphragm stretch, displacement of abdominal contents, and straining of ligaments and bony articulations. Under normal circumstances, the lung and the passive chest wall enclose essentially the same volume, the only difference being the volumes of the pleural fluid and of the lung parenchyma (both quite small). For this reason and because the lung and chest wall function in mechanical series, the pressure required to displace the passive respiratory system (lungs plus chest wall) at any volume is simply the sum of the elastic recoil pressure of the lungs and the transmural pressure across the chest wall. When plotted against respiratory system volume, this relationship assumes a sigmoid shape, exhibiting stiffness at high lung volumes (imparted by the lung), stiffness at low lung volumes (imparted by the chest wall or sometimes by airway closure), and compliance in the middle range of lung volumes. In addition, a passive resting point of the respiratory system is attained when alveolar gas pressure equals body surface pressure (i.e., when the transrespiratory system pressure is zero). At this volume (called the functional residual capacity [FRC]), the outward recoil of the chest wall is balanced exactly by the inward recoil of the lung. As these recoils are transmitted through the pleural fluid, the lung is pulled both outward and inward simultaneously at FRC, and thus its pressure falls below atmospheric pressure (typically, –5 cmH2O).
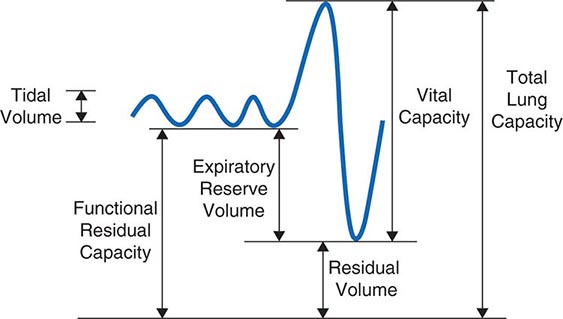
The normal passive respiratory system would equilibrate at the FRC and remain there were it not for the actions of respiratory muscles. The inspiratory muscles act on the chest wall to generate the equivalent of positive pressure across the lungs and passive chest wall, while the expiratory muscles generate the equivalent of negative transrespiratory pressure. The maximal pressures these sets of muscles can generate vary with the lung volume at which they operate. This variation is due to length-tension relationships in striated muscle sarcomeres and to changes in mechanical advantage as the angles of insertion change with lung volume (Fig. 306e-1). Nonetheless, under normal conditions, the respiratory muscles are substantially “overpowered” for their roles and generate more than adequate force to drive the respiratory system to its stiffness extremes, as determined by the lung (total lung capacity [TLC]) or by chest wall or airway closure (residual volume [RV]); the airway closure always prevents the adult lung from emptying completely under normal circumstances. The excursion between full and minimal lung inflation is called vital capacity (VC; Fig. 306e-2) and is readily seen to be the difference between volumes at two unrelated stiffness extremes—one determined by the lung (TLC) and the other by the chest wall or airways (RV). Thus, although VC is easy to measure (see below), it provides little information about the intrinsic properties of the respiratory system. As will become clear, it is much more useful for the clinician to consider TLC and RV individually.
FIGURE 306e-2 Spirogram demonstrating a slow vital capacity maneuver and various lung volumes.
Flow-Related Mechanical Properties—Dynamics The passive chest wall and active neuromuscular system do exhibit mechanical behaviors related to the rate of change of volume, but these behaviors become quantitatively important only at markedly supraphysiologic breathing frequencies (e.g., during high-frequency mechanical ventilation) and thus will not be addressed here. In contrast, the dynamic airflow properties of the lung substantially affect its ability to ventilate and contribute importantly to the work of breathing, and these properties are often deranged by disease. Understanding dynamic airflow properties is therefore worthwhile.
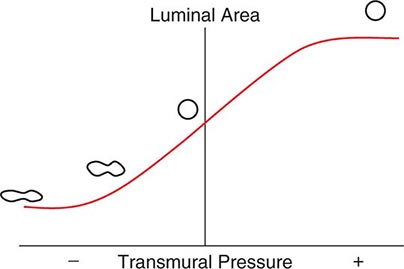
As with the flow of any fluid (gas or liquid) in any tube, maintenance of airflow within the pulmonary airways requires a pressure gradient that falls along the direction of flow, the magnitude of which is determined by the flow rate and the frictional resistance to flow. During quiet tidal breathing, the pressure gradients driving inspiratory or expiratory flow are small owing to the very low frictional resistance of normal pulmonary airways (Raw, normally <2 cmH2O/L per second). However, during rapid exhalation, another phenomenon reduces flow below that which would have been expected if frictional resistance were the only impediment to flow. This phenomenon is called dynamic airflow limitation, and it occurs because the bronchial airways through which air is exhaled are collapsible rather than rigid (Fig. 306e-3). An important anatomic feature of the pulmonary airways is its treelike branching structure. While the individual airways in each successive generation, from most proximal (trachea) to most distal (respiratory bronchioles), are smaller than those of the parent generation, their number increases exponentially such that the summed cross-sectional area of the airways becomes very large toward the lung periphery. Because flow (volume/time) is constant along the airway tree, the velocity of airflow (flow/summed cross-sectional area) is much greater in the central airways than in the peripheral airways. During exhalation, gas leaving the alveoli must therefore gain velocity as it proceeds toward the mouth. The energy required for this “convective” acceleration is drawn from the component of gas energy manifested as its local pressure, which reduces intraluminal gas pressure, airway transmural pressure, airway size (Fig. 306e-3), and flow. This is the Bernoulli effect, the same effect that keeps an airplane airborne, generating a lifting force by decreasing pressure above the curved upper surface of the wing due to acceleration of air flowing over the wing. If an individual tries to exhale more forcefully, the local velocity increases further and reduces airway size further, resulting in no net increase in flow. Under these circumstances, flow has reached its maximum possible value, or its flow limit. Lungs normally exhibit such dynamic airflow limitation. This limitation can be assessed by spirometry, in which an individual inhales fully to TLC and then forcibly exhales to RV. One useful spirometric measure is the volume of air exhaled during the first second of expiration (FEV1), as discussed later. Maximal expiratory flow at any lung volume is determined by gas density, airway cross-section and distensibility, elastic recoil pressure of the lung, and frictional pressure loss to the flow-limiting airway site. Under normal conditions, maximal expiratory flow falls with lung volume (Fig. 306e-4), primarily because of the dependence of lung recoil pressure on lung volume (Fig. 306e-1). In pulmonary fibrosis, lung recoil pressure is increased at any lung volume, and thus the maximal expiratory flow is elevated when considered in relation to lung volume. Conversely, in emphysema, lung recoil pressure is reduced; this reduction is a principal mechanism by which maximal expiratory flows fall. Diseases that narrow the airway lumen at any transmural pressure (e.g., asthma or chronic bronchitis) or that cause excessive airway collapsibility (e.g., tracheomalacia) also reduce maximal expiratory flow.
FIGURE 306e-3 Luminal area versus transmural pressure relationship. Transmural pressure represents the pressure difference across the airway wall from inside to outside.
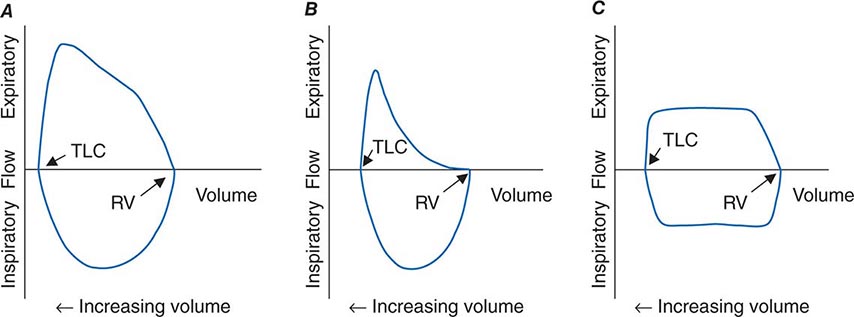
The Bernoulli effect also applies during inspiration, but the more negative pleural pressures during inspiration lower the pressure outside of airways, thereby increasing transmural pressure and promoting airway expansion. Thus inspiratory airflow limitation seldom occurs due to diffuse pulmonary airway disease. Conversely, extrathoracic airway narrowing (e.g., due to a tracheal adenoma or post-tracheostomy stricture) can lead to inspiratory airflow limitation (Fig. 306e-4).
FIGURE 306e-4 Flow-volume loops. A. Normal. B. Airflow obstruction. C. Fixed central airway obstruction. RV, residual volume; TLC, total lung capacity.
The Work of Breathing In health, the elastic (volume change–related) and dynamic (flow-related) loads that must be overcome to ventilate the lungs at rest are small, and the work required of the respiratory muscles is minimal. However, the work of breathing can increase considerably due to a metabolic requirement for substantially increased ventilation, an abnormally increased mechanical load, or both. As discussed below, the rate of ventilation is primarily set by the need to eliminate carbon dioxide, and thus ventilation increases during exercise (sometimes by more than twentyfold) and during metabolic acidosis as a compensatory response. Naturally, the work rate required to overcome the elasticity of the respiratory system increases with both the depth and the frequency of tidal breaths, while the work required to overcome the dynamic load increases with total ventilation. A modest increase of ventilation is most efficiently achieved by increasing tidal volume but not respiratory rate, which is the normal ventilatory response to lower-level exercise. At high levels of exercise, deep breathing persists, but respiratory rate also increases. The pattern chosen by the respiratory controller minimizes the work of breathing.
The work of breathing also increases when disease reduces the compliance of the respiratory system or increases the resistance to airflow. The former occurs commonly in diseases of the lung parenchyma (interstitial processes or fibrosis, alveolar filling diseases such as pulmonary edema or pneumonia, or substantial lung resection), and the latter occurs in obstructive airway diseases such as asthma, chronic bronchitis, emphysema, and cystic fibrosis. Furthermore, severe airflow obstruction can functionally reduce the compliance of the respiratory system by leading to dynamic hyperinflation. In this scenario, expiratory flows slowed by the obstructive airways disease may be insufficient to allow complete exhalation during the expiratory phase of tidal breathing; as a result, the “functional residual capacity” from which the next breath is inhaled is greater than the static FRC. With repetition of incomplete exhalations of each tidal breath, the operating FRC becomes dynamically elevated, sometimes to a level that approaches TLC. At these high lung volumes, the respiratory system is much less compliant than at normal breathing volumes, and thus the elastic work of each tidal breath is also increased. The dynamic pulmonary hyperinflation that accompanies severe airflow obstruction causes patients to sense difficulty in inhaling—even though the root cause of this pathophysiologic abnormality is expiratory airflow obstruction.
Adequacy of Ventilation As noted above, the respiratory control system that sets the rate of ventilation responds to chemical signals, including arterial CO2 and oxygen tensions and blood pH, and to volitional needs, such as the need to inhale deeply before playing a long phrase on the trumpet. Disturbances in ventilation are discussed in Chap. 318. The focus of this chapter is on the relationship between ventilation of the lung and CO2 elimination.
At the end of each tidal exhalation, the conducting airways are filled with alveolar gas that had not reached the mouth when expiratory flow stopped. During the ensuing inhalation, fresh gas immediately enters the airway tree at the mouth, but the gas first entering the alveoli at the start of inhalation is that same alveolar gas in the conducting airways that had just left the alveoli. Accordingly, fresh gas does not enter the alveoli until the volume of the conducting airways has been inspired. This volume is called the anatomic dead space (VD). Quiet breathing with tidal volumes smaller than the anatomic dead space introduces no fresh gas into the alveoli at all; only that part of the inspired tidal volume (VT) that is greater than the VD introduces fresh gas into the alveoli. The dead space can be further increased functionally if some of the inspired tidal volume is delivered to a part of the lung that receives no pulmonary blood flow and thus cannot contribute to gas exchange (e.g., the portion of the lung distal to a large pulmonary embolus). In this situation, exhaled minute ventilation (![]() E = VT × RR) includes a component of dead space ventilation (
E = VT × RR) includes a component of dead space ventilation (![]() D = VD × RR) and a component of fresh gas alveolar ventilation (
D = VD × RR) and a component of fresh gas alveolar ventilation (![]() A = [VT – VD] × RR). CO2 elimination from the alveoli is equal to
A = [VT – VD] × RR). CO2 elimination from the alveoli is equal to ![]() A times the difference in CO2 fraction between inspired air (essentially zero) and alveolar gas (typically ~5.6% after correction for humidification of inspired air, corresponding to 40 mmHg). In the steady state, the alveolar fraction of CO2 is equal to metabolic CO2 production divided by alveolar ventilation. Because, as discussed below, alveolar and arterial CO2 tensions are equal, and because the respiratory controller normally strives to maintain arterial PCO2 (PaCO2) at ~40 mmHg, the adequacy of alveolar ventilation is reflected in PaCO2. If the PaCO2 falls much below 40 mmHg, alveolar hyperventilation is present; if the PaCO2 exceeds 40 mmHg, then alveolar hypoventilation is present. Ventilatory failure is characterized by extreme alveolar hypoventilation.
A times the difference in CO2 fraction between inspired air (essentially zero) and alveolar gas (typically ~5.6% after correction for humidification of inspired air, corresponding to 40 mmHg). In the steady state, the alveolar fraction of CO2 is equal to metabolic CO2 production divided by alveolar ventilation. Because, as discussed below, alveolar and arterial CO2 tensions are equal, and because the respiratory controller normally strives to maintain arterial PCO2 (PaCO2) at ~40 mmHg, the adequacy of alveolar ventilation is reflected in PaCO2. If the PaCO2 falls much below 40 mmHg, alveolar hyperventilation is present; if the PaCO2 exceeds 40 mmHg, then alveolar hypoventilation is present. Ventilatory failure is characterized by extreme alveolar hypoventilation.
As a consequence of oxygen uptake of alveolar gas into capillary blood, alveolar oxygen tension falls below that of inspired gas. The rate of oxygen uptake (determined by the body’s metabolic oxygen consumption) is related to the average rate of metabolic CO2 production, and their ratio—the “respiratory quotient” (R = ![]() CO2/
CO2/![]() O2)—depends largely on the fuel being metabolized. For a typical American diet, R is usually around 0.85, and more oxygen is absorbed than CO2 is excreted. Together, these phenomena allow the estimation of alveolar oxygen tension, according to the following relationship, known as the alveolar gas equation:
O2)—depends largely on the fuel being metabolized. For a typical American diet, R is usually around 0.85, and more oxygen is absorbed than CO2 is excreted. Together, these phenomena allow the estimation of alveolar oxygen tension, according to the following relationship, known as the alveolar gas equation:
PAO2 = FIO2 × (Pbar – PH2O) – PACO2/R
The alveolar gas equation also highlights the influences of inspired oxygen fraction (FIO2), barometric pressure (Pbar), and vapor pressure of water (PH2O = 47 mmHg at 37°C) in addition to alveolar ventilation (which sets PACO2) in determining PAO2. An implication of the alveolar gas equation is that severe arterial hypoxemia rarely occurs as a pure consequence of alveolar hypoventilation at sea level while an individual is breathing air. The potential for alveolar hypoventilation to induce severe hypoxemia with otherwise normal lungs increases as Pbar falls with increasing altitude.
GAS EXCHANGE
Diffusion For oxygen to be delivered to the peripheral tissues, it must pass from alveolar gas into alveolar capillary blood by diffusing through alveolar membrane. The aggregate alveolar membrane is highly optimized for this process, with a very large surface area and minimal thickness. Diffusion through the alveolar membrane is so efficient in the human lung that in most circumstances a red blood cell’s hemoglobin becomes fully oxygen saturated by the time the cell has traveled just one-third the length of the alveolar capillary. Thus the uptake of alveolar oxygen is ordinarily limited by the amount of blood transiting the alveolar capillaries rather than by the rapidity with which oxygen can diffuse across the membrane; consequently, oxygen uptake from the lung is said to be “perfusion limited.” CO2 also equilibrates rapidly across the alveolar membrane. Therefore, the oxygen and CO2 tensions in capillary blood leaving a normal alveolus are essentially equal to those in alveolar gas. Only in rare circumstances (e.g., at high altitude or in high-performance athletes exerting maximal effort) is oxygen uptake from normal lungs diffusion limited. Diffusion limitation can also occur in interstitial lung disease if substantially thickened alveolar walls remain perfused.
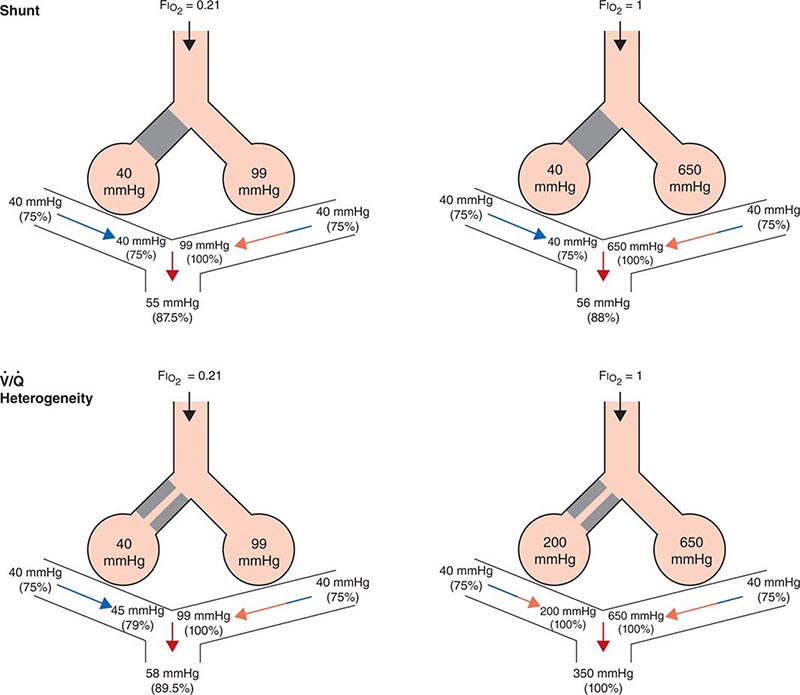
Ventilation/Perfusion Heterogeneity As noted above, for gas exchange to be most efficient, ventilation to each individual alveolus (among the millions of alveoli) should match perfusion to its accompanying capillaries. Because of the differential effects of gravity on lung mechanics and blood flow throughout the lung and because of differences in airway and vascular architecture among various respiratory paths, there is minor ventilation/perfusion heterogeneity even in the normal lung; however, ![]() /
/![]() heterogeneity can be particularly marked in disease. Two extreme examples are (1) ventilation of unperfused lung distal to a pulmonary embolus, in which ventilation of the physiologic dead space is “wasted” in the sense that it does not contribute to gas exchange; and (2) perfusion of nonventilated lung (a “shunt”), which allows venous blood to pass through the lung unaltered. When mixed with fully oxygenated blood leaving other well-ventilated lung units, shunted venous blood disproportionately lowers the mixed arterial PaO2 as a result of the nonlinear oxygen content versus PO2 relationship of hemoglobin (Fig. 306e-5). Furthermore, the resulting arterial hypoxemia is refractory to supplemental inspired oxygen. The reason is that (1) raising the inspired FIO2 has no effect on alveolar gas tensions in nonventilated alveoli and (2) while raising inspired FIO2 does increase PACO2 in ventilated alveoli, the oxygen content of blood exiting ventilated units increases only slightly, as hemoglobin will already have been nearly fully saturated and the solubility of oxygen in plasma is quite small.
heterogeneity can be particularly marked in disease. Two extreme examples are (1) ventilation of unperfused lung distal to a pulmonary embolus, in which ventilation of the physiologic dead space is “wasted” in the sense that it does not contribute to gas exchange; and (2) perfusion of nonventilated lung (a “shunt”), which allows venous blood to pass through the lung unaltered. When mixed with fully oxygenated blood leaving other well-ventilated lung units, shunted venous blood disproportionately lowers the mixed arterial PaO2 as a result of the nonlinear oxygen content versus PO2 relationship of hemoglobin (Fig. 306e-5). Furthermore, the resulting arterial hypoxemia is refractory to supplemental inspired oxygen. The reason is that (1) raising the inspired FIO2 has no effect on alveolar gas tensions in nonventilated alveoli and (2) while raising inspired FIO2 does increase PACO2 in ventilated alveoli, the oxygen content of blood exiting ventilated units increases only slightly, as hemoglobin will already have been nearly fully saturated and the solubility of oxygen in plasma is quite small.
FIGURE 306e-5 Influence of air versus oxygen breathing on mixed arterial oxygenation in shunt and ventilation/perfusion heterogeneity. Partial pressure of oxygen (mmHg) and oxygen saturations are shown for mixed venous blood, for end capillary blood (normal versus affected alveoli), and for mixed arterial blood. FIO2, fraction of inspired oxygen; ![]() /
/![]() , ventilation/perfusion.
, ventilation/perfusion.
A more common occurrence than the two extreme examples given above is a widening of the distribution of ventilation/perfusion ratios; such ![]() /
/![]() heterogeneity is a common consequence of lung disease. In this circumstance, perfusion of relatively underventilated alveoli results in the incomplete oxygenation of exiting blood. When mixed with well-oxygenated blood leaving higher
heterogeneity is a common consequence of lung disease. In this circumstance, perfusion of relatively underventilated alveoli results in the incomplete oxygenation of exiting blood. When mixed with well-oxygenated blood leaving higher ![]() /
/![]() regions, this partially reoxygenated blood disproportionately lowers arterial PaO2, although to a lesser extent than does a similar perfusion fraction of blood leaving regions of pure shunt. In addition, in contrast to shunt regions, inhalation of supplemental oxygen does raise the PAO2, even in relatively underventilated low
regions, this partially reoxygenated blood disproportionately lowers arterial PaO2, although to a lesser extent than does a similar perfusion fraction of blood leaving regions of pure shunt. In addition, in contrast to shunt regions, inhalation of supplemental oxygen does raise the PAO2, even in relatively underventilated low ![]() /
/![]() regions, and so the arterial hypoxemia induced by
regions, and so the arterial hypoxemia induced by ![]() /
/![]() heterogeneity is typically responsive to oxygen therapy (Fig. 306e-5).
heterogeneity is typically responsive to oxygen therapy (Fig. 306e-5).
In sum, arterial hypoxemia can be caused by substantial reduction of inspired oxygen tension; by severe alveolar hypoventilation; by perfusion of relatively underventilated (low ![]() /
/![]() ) or completely unventilated (shunt) lung regions; and, in unusual circumstances, by limitation of gas diffusion.
) or completely unventilated (shunt) lung regions; and, in unusual circumstances, by limitation of gas diffusion.
PATHOPHYSIOLOGY
Although many diseases injure the respiratory system, this system responds to injury in relatively few ways. For this reason, the pattern of physiologic abnormalities may or may not provide sufficient information by which to discriminate among conditions.
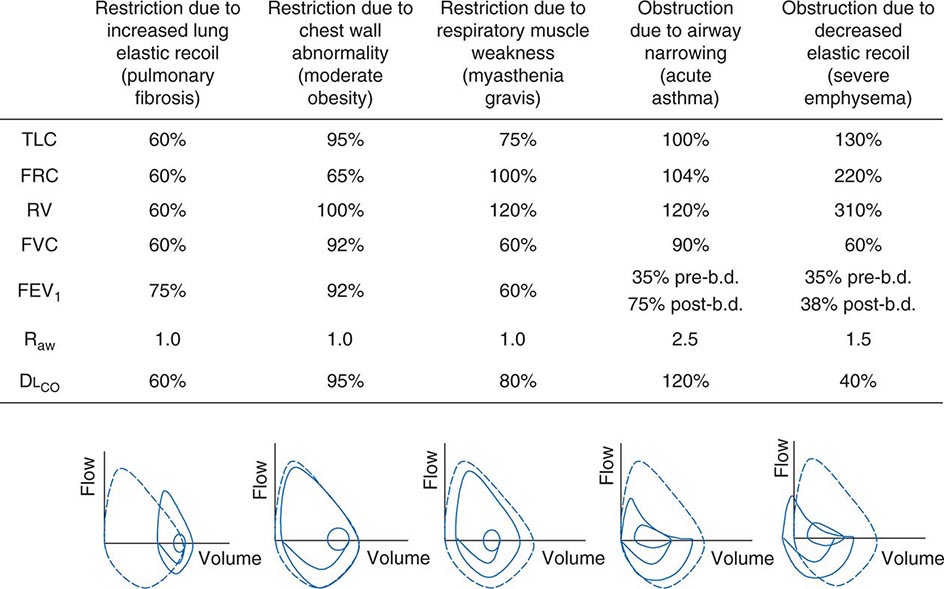
Figure 306e-6 lists abnormalities in pulmonary function testing that are typically found in a number of common respiratory disorders and highlights the simultaneous occurrence of multiple physiologic abnormalities. The coexistence of some of these respiratory disorders results in more complex superposition of these abnormalities. Methods to measure respiratory system function clinically are described later in this chapter.
FIGURE 306e-6 Common abnormalities of pulmonary function (see text). Pulmonary function values are expressed as a percentage of normal predicted values, except for Raw, which is expressed as cmH2O/L per sec (normal, <2 cmH2O/L per second). The figures at the bottom of each column show the typical configuration of flow-volume loops in each condition, including the flow-volume relationship during tidal breathing. b.d., bronchodilator; DLCO, diffusion capacity of lung for carbon monoxide; FEV1, forced expiratory volume in 1 sec; FRC, functional residual capacity; FVC, forced vital capacity; Raw, airways resistance; RV, residual volume; TLC, total lung capacity.
Ventilatory Restriction Due to Increased Elastic Recoil—Example: Idiopathic Pulmonary Fibrosis Idiopathic pulmonary fibrosis raises lung recoil at all lung volumes, thereby lowering TLC, FRC, and RV as well as forced vital capacity (FVC). Maximal expiratory flows are also reduced from normal values but are elevated when considered in relation to lung volumes. Increased flow occurs both because the increased lung recoil drives greater maximal flow at any lung volume and because airway diameters are relatively increased due to greater radially outward traction exerted on bronchi by the stiff lung parenchyma. For the same reason, airway resistance is also normal. Destruction of the pulmonary capillaries by the fibrotic process results in a marked reduction in diffusing capacity (see below). Oxygenation is often severely reduced by persistent perfusion of alveolar units that are relatively underventilated due to fibrosis of nearby (and mechanically linked) lung. The flow-volume loop (see below) looks like a miniature version of a normal loop but is shifted toward lower absolute lung volumes and displays maximal expiratory flows that are increased for any given volume over the normal tracing.
Ventilatory Restriction Due to Chest Wall Abnormality—Example: Moderate Obesity As the size of the average American continues to increase, this pattern may become the most common of pulmonary function abnormalities. In moderate obesity, the outward recoil of the chest wall is blunted by the weight of chest wall fat and the space occupied by intraabdominal fat. In this situation, preserved inward recoil of the lung overbalances the reduced outward recoil of the chest wall, and FRC falls. Because respiratory muscle strength and lung recoil remain normal, TLC is typically unchanged (although it may fall in massive obesity) and RV is normal (but may be reduced in massive obesity). Mild hypoxemia may be present due to perfusion of alveolar units that are poorly ventilated because of airway closure in dependent portions of the lung during breathing near the reduced FRC. Flows remain normal, as does the diffusion capacity of the lung for carbon monoxide (DLCO), unless obstructive sleep apnea (which often accompanies obesity) and associated chronic intermittent hypoxemia have induced pulmonary arterial hypertension, in which case DLCO may be low.
Ventilatory Restriction Due to Reduced Muscle Strength—Example: Myasthenia Gravis In this circumstance, FRC remains normal, as both lung recoil and passive chest wall recoil are normal. However, TLC is low and RV is elevated because respiratory muscle strength is insufficient to push the passive respiratory system fully toward either volume extreme. Caught between the low TLC and the elevated RV, FVC and FEV1 are reduced as “innocent bystanders.” As airway size and lung vasculature are unaffected, both Raw and DLCO are normal. Oxygenation is normal unless weakness becomes so severe that the patient has insufficient strength to reopen collapsed alveoli during sighs, with resulting atelectasis.
Airflow Obstruction Due to Decreased Airway Diameter—Example: Acute Asthma During an episode of acute asthma, luminal narrowing due to smooth muscle constriction as well as inflammation and thickening within the small- and medium-sized bronchi raise frictional resistance and reduce airflow. “Scooping” of the flow-volume loop is caused by reduction of airflow, especially at lower lung volumes. Often, airflow obstruction can be reversed by inhalation of β2-adrenergic agonists acutely or by treatment with inhaled steroids chronically. TLC usually remains normal (although elevated TLC is sometimes seen in long-standing asthma), but FRC may be dynamically elevated. RV is often increased due to exaggerated airway closure at low lung volumes, and this elevation of RV reduces FVC. Because central airways are narrowed, Raw is usually elevated. Mild arterial hypoxemia is often present due to perfusion of relatively underventilated alveoli distal to obstructed airways (and is responsive to oxygen supplementation), but DLCO is normal or mildly elevated.
Airflow Obstruction Due to Decreased Elastic Recoil—Example: Severe Emphysema Loss of lung elastic recoil in severe emphysema results in pulmonary hyperinflation, of which elevated TLC is the hallmark. FRC is more severely elevated due both to loss of lung elastic recoil and to dynamic hyperinflation—the same phenomenon as autoPEEP, which is the positive end-expiratory alveolar pressure that occurs when a new breath is initiated before the lung volume is allowed to return to FRC. Residual volume is very severely elevated because of airway closure and because exhalation toward RV may take so long that RV cannot be reached before the patient must inhale again. Both FVC and FEV1 are markedly decreased, the former because of the severe elevation of RV and the latter because loss of lung elastic recoil reduces the pressure driving maximal expiratory flow and also reduces tethering open of small intrapulmonary airways. The flow-volume loop demonstrates marked scooping, with an initial transient spike of flow attributable largely to expulsion of air from collapsing central airways at the onset of forced exhalation. Otherwise, the central airways remain relatively unaffected, so Raw is normal in “pure” emphysema. Loss of alveolar surface and capillaries in the alveolar walls reduces DLCO; however, because poorly ventilated emphysematous acini are also poorly perfused (due to loss of their capillaries), arterial hypoxemia usually is not seen at rest until emphysema becomes very severe. However, during exercise, PaO2 may fall precipitously if extensive destruction of the pulmonary vasculature prevents a sufficient increase in cardiac output and mixed venous oxygen content falls substantially. Under these circumstances, any venous admixture through low ![]() /
/![]() units has a particularly marked effect in lowering mixed arterial oxygen tension.
units has a particularly marked effect in lowering mixed arterial oxygen tension.
FUNCTIONAL MEASUREMENTS
Measurement of Ventilatory Function • LUNG VOLUMES Figure 306e-2 demonstrates a spirometry tracing in which the volume of air entering or exiting the lung is plotted over time. In a slow vital capacity maneuver, the subject inhales from FRC, fully inflating the lungs to TLC, and then exhales slowly to RV; VC, the difference between TLC and RV, represents the maximal excursion of the respiratory system. Spirometry discloses relative volume changes during these maneuvers but cannot reveal the absolute volumes at which they occur. To determine absolute lung volumes, two approaches are commonly used: inert gas dilution and body plethysmography. In the former, a known amount of a nonabsorbable inert gas (usually helium or neon) is inhaled in a single large breath or is rebreathed from a closed circuit; the inert gas is diluted by the gas resident in the lung at the time of inhalation, and its final concentration reveals the volume of resident gas contributing to the dilution. A drawback of this method is that regions of the lung that ventilate poorly (e.g., due to airflow obstruction) may not receive much inspired inert gas and so do not contribute to its dilution. Therefore, inert gas dilution (especially in the single-breath method) often underestimates true lung volumes.
In the second approach, FRC is determined by measuring the compressibility of gas within the chest, which is proportional to the volume of gas being compressed. The patient sits in a body plethysmograph (a chamber usually made of transparent plastic to minimize claustrophobia) and, at the end of a normal tidal breath (i.e., when lung volume is at FRC), is instructed to pant against a closed shutter, thus periodically compressing air within the lung slightly. Pressure fluctuations at the mouth and volume fluctuations within the body box (equal but opposite to those in the chest) are determined, and from these measurements the thoracic gas volume is calculated by means of Boyle’s law. Once FRC is obtained, TLC and RV are calculated by adding the value for inspiratory capacity and subtracting the value for expiratory reserve volume, respectively (both values having been obtained during spirometry) (Fig. 306e-2). The most important determinants of healthy individuals’ lung volumes are height, age, and sex, but there is considerable additional normal variation beyond that accounted for by these parameters. In addition, race influences lung volumes; on average, TLC values are ~12% lower in African Americans and 6% lower in Asian Americans than in Caucasian Americans. In practice, a mean “normal” value is predicted by multivariate regression equations using height, age, and sex, and the patient’s value is divided by the predicted value (often with “race correction” applied) to determine “percent predicted.” For most measures of lung function, 85–115% of the predicted value can be normal; however, in health, the various lung volumes tend to scale together. For example, if one is “normal big” with a TLC 110% of the predicted value, then all other lung volumes and spirometry values will also approximate 110% of their respective predicted values. This pattern is particularly helpful in evaluating airflow, as discussed below.
AIR FLOW As noted above, spirometry plays a key role in lung volume determination. Even more often, spirometry is used to measure airflow, which reflects the dynamic properties of the lung. During an FVC maneuver, the patient inhales to TLC and then exhales rapidly and forcefully to RV; this method ensures that flow limitation has been achieved, so that the precise effort made has little influence on actual flow. The total amount of air exhaled is the FVC, and the amount of air exhaled in the first second is the FEV1; the FEV1 is a flow rate, revealing volume change per time. Like lung volumes, an individual’s maximal expiratory flows should be compared with predicted values based on height, age, and sex. While the FEV1/FVC ratio is typically reduced in airflow obstruction, this condition can also reduce FVC by raising RV, sometimes rendering the FEV1/FVC ratio “artifactually normal” with the erroneous implication that airflow obstruction is absent. To circumvent this problem, it is useful to compare FEV1 as a fraction of its predicted value with TLC as a fraction of its predicted value. In health, the results are usually similar. In contrast, even an FEV1 value that is 95% of its predicted value may actually be relatively low if TLC is 110% of its respective predictied value. In this case, airflow obstruction may be present, despite the “normal” value for FEV1.
The relationships among volume, flow, and time during spirometry are best displayed in two plots—the spirogram (volume vs. time) and the flow-volume loop (flow vs. volume) (Fig. 306e-4). In conditions that cause airflow obstruction, the site of obstruction is sometimes correlated with the shape of the flow-volume loop. In diseases that cause lower airway obstruction, such as asthma and emphysema, flows decrease more rapidly with declining lung volumes, leading to a characteristic scooping of the flow-volume loop. In contrast, fixed upper-airway obstruction typically leads to inspiratory and/or expiratory flow plateaus (Fig. 306e-4).
AIRWAYS RESISTANCE The total resistance of the pulmonary and upper airways is measured in the same body plethysmograph used to measure FRC. The patient is asked once again to pant, but this time against a closed and then opened shutter. Panting against the closed shutter reveals the thoracic gas volume as described above. When the shutter is opened, flow is directed to and from the body box, so that volume fluctuations in the box reveal the extent of thoracic gas compression, which in turn reveals the pressure fluctuations driving flow. Simultaneous measurement of flow allows the calculation of lung resistance (as flow divided by pressure). In health, Raw is very low (<2 cmH2O/L per second), and half of the detected resistance resides within the upper airway. In the lung, most resistance originates in the central airways. For this reason, airways resistance measurement tends to be insensitive to peripheral airflow obstruction.
RESPIRATORY MUSCLE STRENGTH To measure respiratory muscle strength, the patient is instructed to exhale or inhale with maximal effort against a closed shutter while pressure is monitored at the mouth. Pressures greater than ±60 cmH2O at FRC are considered adequate and make it unlikely that respiratory muscle weakness accounts for any other resting ventilatory dysfunction that is identified.
Measurement of Gas Exchange • DIFFUSING CAPACITY (DLCO) This test uses a small (and safe) amount of carbon monoxide (CO) to measure gas exchange across the alveolar membrane during a 10-sec breath hold. CO in exhaled breath is analyzed to determine the quantity of CO crossing the alveolar membrane and combining with hemoglobin in red blood cells. This “single-breath diffusing capacity” (DLCO) value increases with the surface area available for diffusion and the amount of hemoglobin within the capillaries, and it varies inversely with alveolar membrane thickness. Thus, DLCO decreases in diseases that thicken or destroy alveolar membranes (e.g., pulmonary fibrosis, emphysema), curtail the pulmonary vasculature (e.g., pulmonary hypertension), or reduce alveolar capillary hemoglobin (e.g., anemia). Single-breath diffusing capacity may be elevated in acute congestive heart failure, asthma, polycythemia, and pulmonary hemorrhage.
ARTERIAL BLOOD GASES The effectiveness of gas exchange can be assessed by measuring the partial pressures of oxygen and CO2 in a sample of blood obtained by arterial puncture. The oxygen content of blood (CaO2) depends upon arterial saturation (%O2Sat), which is set by PaO2, pH, and PaCO2 according to the oxyhemoglobin dissociation curve. CaO2 can also be measured by oximetry (see below):
CaO2 (mL/dL) = 1.39 (mL/dL) × [hemoglobin](g) × % O2 Sat
+ 0.003 (mL/dL/mmHg) × PaO2 (mmHg)
If hemoglobin saturation alone needs to be determined, this task can be accomplished noninvasively with pulse oxymetry.
ACKNOWLEDGMENT
The authors wish to acknowledge the contributions of Drs. Steven E. Weinberger and Irene M. Rosen to this chapter in previous editions as well as the helpful contributions of Drs. Mary Strek and Jeffrey Jacobson.
307 |
Diagnostic Procedures in Respiratory Disease |
The diagnostic modalities available for assessing the patient with suspected or known respiratory system disease include imaging studies and techniques for acquiring biologic specimens, some of which involve direct visualization of part of the respiratory system. Methods to characterize the functional changes developing as a result of disease, including pulmonary function tests and measurements of gas exchange, are discussed in Chap. 306e.
IMAGING STUDIES
ROUTINE RADIOGRAPHY
Routine chest radiography, including both posteroanterior (PA) and lateral views, is an integral part of the diagnostic evaluation of diseases involving the pulmonary parenchyma, the pleura, and, to a lesser extent, the airways and the mediastinum (see Chaps. 305 and 308e). Lateral decubitus views are useful for determining whether pleural abnormalities represent freely flowing fluid, whereas apical lordotic views can visualize disease at the lung apices better than the standard PA view. Portable equipment is often used for acutely ill patients who cannot be transported to a radiology suite but are more difficult to interpret owing to several limitations: (1) the single anteroposterior (AP) projection obtained; (2) variability in over- and underexposure of film; (3) a shorter focal spot-film distance leading to lack of edge sharpness and loss of fine detail; and (4) magnification of the cardiac silhouette and other anterior structures by the AP projection. Common radiographic patterns and their clinical correlates are reviewed in Chap. 308e.
Advances in computer technology have allowed the development of digital or computed radiography, which has several benefits: (1) immediate availability of the images; (2) significant postprocessing analysis of images to improve diagnostic information; and (3) ability to store images electronically and to transfer them within or between health care systems.
ULTRASOUND
Diagnostic ultrasound (US) produces images using echoes or reflection of the US beam from interfaces between tissues with differing acoustic properties. US is nonionizing and safe to perform on pregnant patients and children. It can detect and localize pleural abnormalities and is a quick and effective way of guiding percutaneous needle biopsy of peripheral lung, pleural, or chest wall lesions. US is also helpful in identifying septations within loculated collections and can facilitate placement of a needle for sampling of pleural liquid (i.e., for thoracentesis), improving the yield and safety of the procedure. Bedside availability makes it valuable in the intensive care setting. Real-time imaging can be used to assess the movement of the diaphragm. Because US energy is rapidly dissipated in air, it is not useful for evaluation of the pulmonary parenchyma and cannot be used if there is any aerated lung between the US probe and the abnormality of interest.
Endobronchial US, in which the US probe is passed through a bronchoscope, is a valuable adjunct to bronchoscopy, allowing identification and localization of pathology adjacent to airway walls or within the mediastinum.
NUCLEAR MEDICINE TECHNIQUES
Nuclear imaging depends on the selective uptake of various compounds by organs of the body. In thoracic imaging, these compounds are concentrated by one of three mechanisms: blood pool or compartmentalization (e.g., within the heart), physiologic incorporation (e.g., bone or thyroid) and capillary blockage (e.g., lung scan). Radioactive isotopes can be administered by either the IV or inhaled routes or both. When injected intravenously, albumin macroaggregates labeled with technetium-99m (99mTc) become lodged in pulmonary capillaries; the distribution of the trapped radioisotope follows the distribution of blood flow. When inhaled, radiolabeled xenon gas can be used to demonstrate the distribution of ventilation. Using these techniques, ventilation-perfusion lung scanning was a commonly used technique for the evaluation of pulmonary embolism. Pulmonary thromboembolism produces one or more regions of ventilation-perfusion mismatch (i.e., regions in which there is a defect in perfusion that follows the distribution of a vessel and that is not accompanied by a corresponding defect in ventilation [Chap. 300]). However, with advances in computed tomography (CT) scanning, scintigraphic imaging has been largely replaced by CT angiography in patients with suspected pulmonary embolism.
Another common use of ventilation-perfusion scans is in patients with impaired lung function, who are being considered for lung resection. Many patients with bronchogenic carcinoma have coexisting chronic obstructive pulmonary disease (COPD), and the question arises as to whether or not a patient can tolerate lung resection. The distribution of the isotope(s) can be used to assess the regional distribution of blood flow and ventilation, allowing the physician to estimate the level of postoperative lung function.
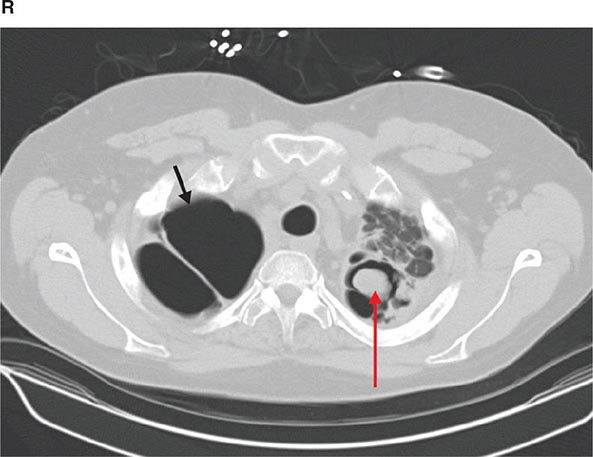
COMPUTED TOMOGRAPHY
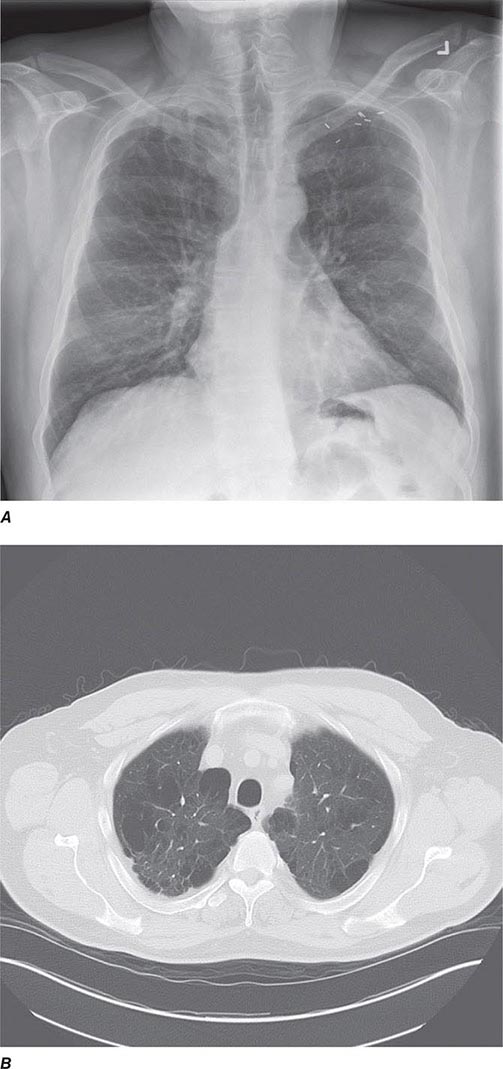
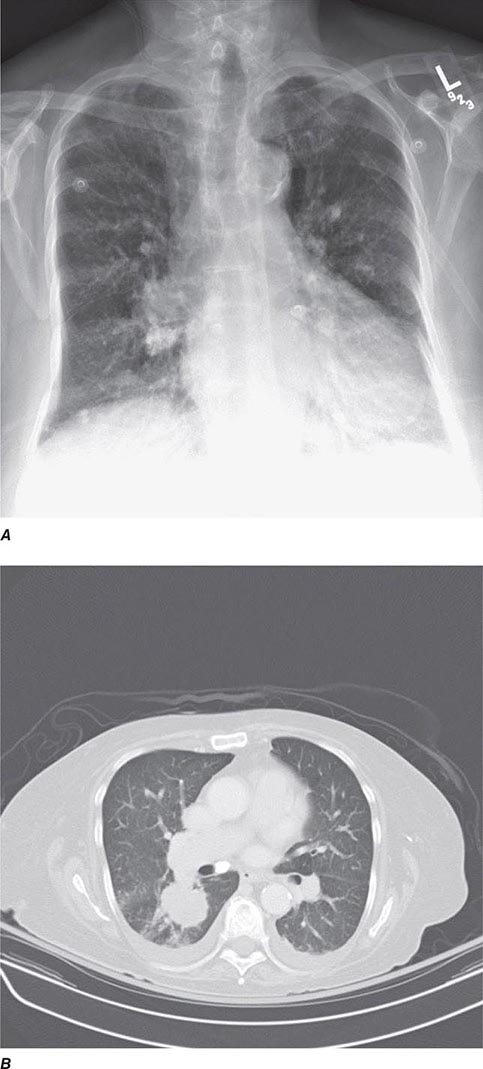
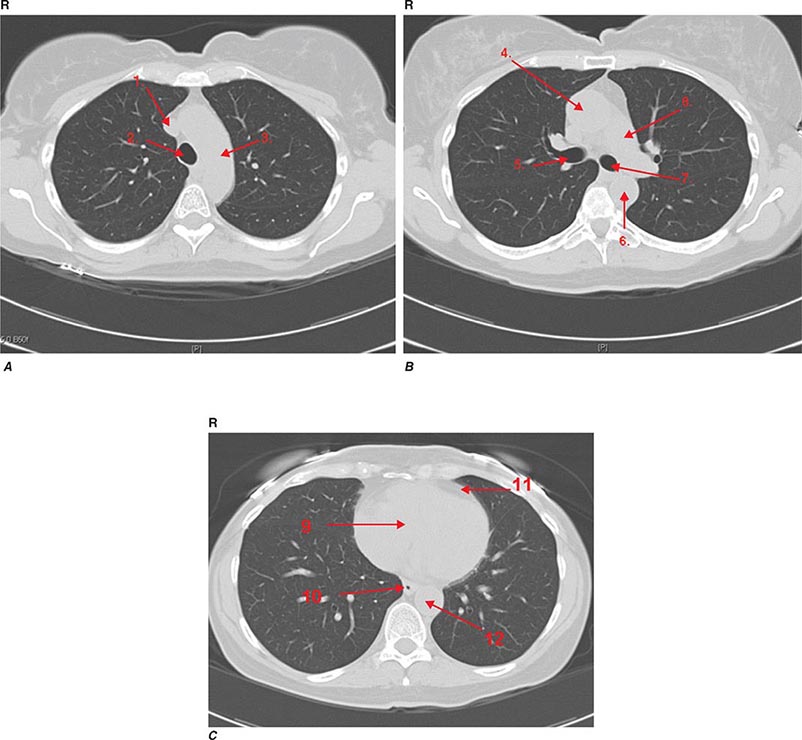
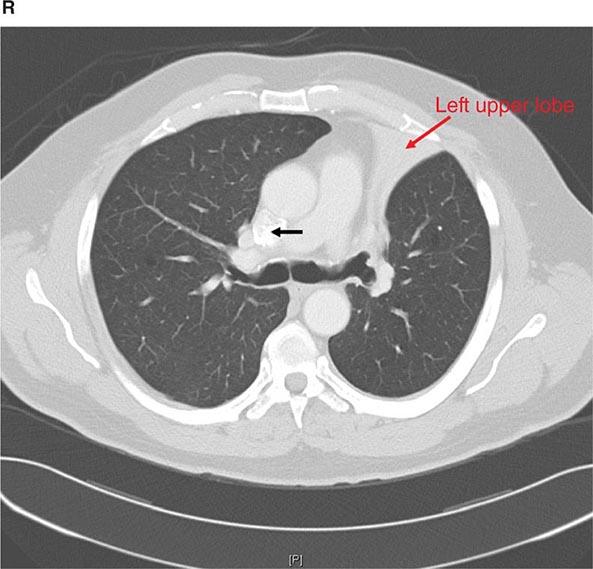
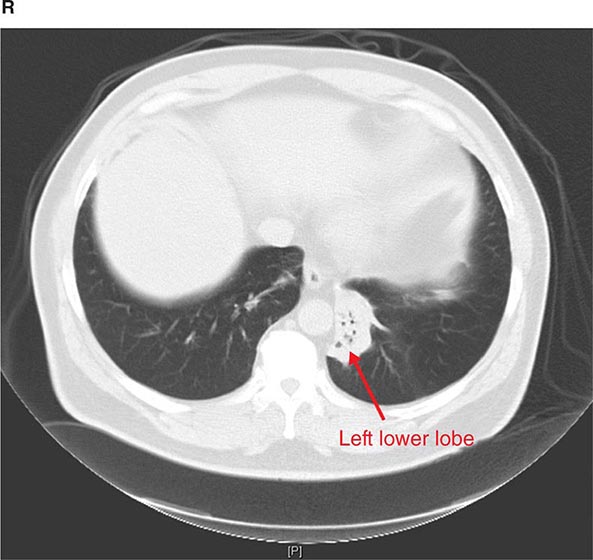
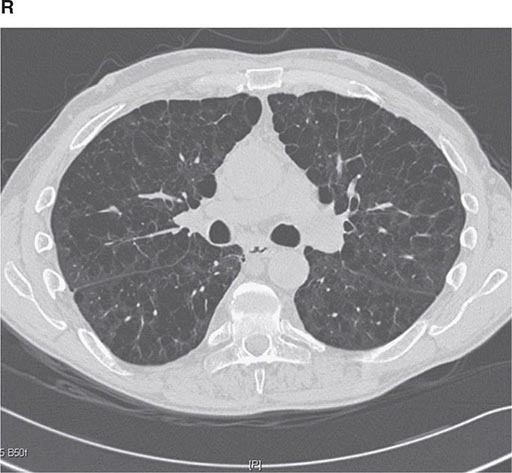
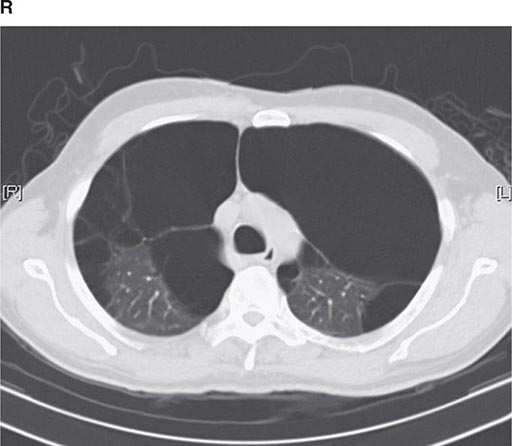
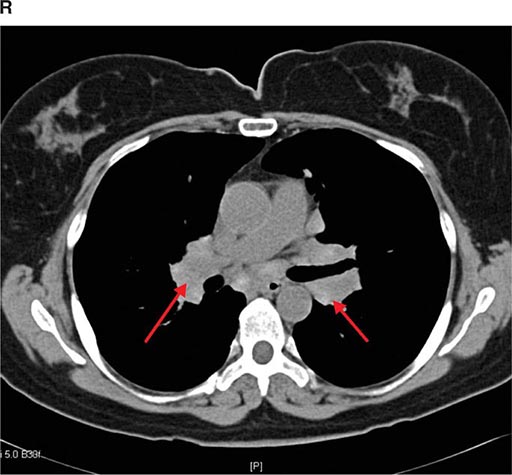
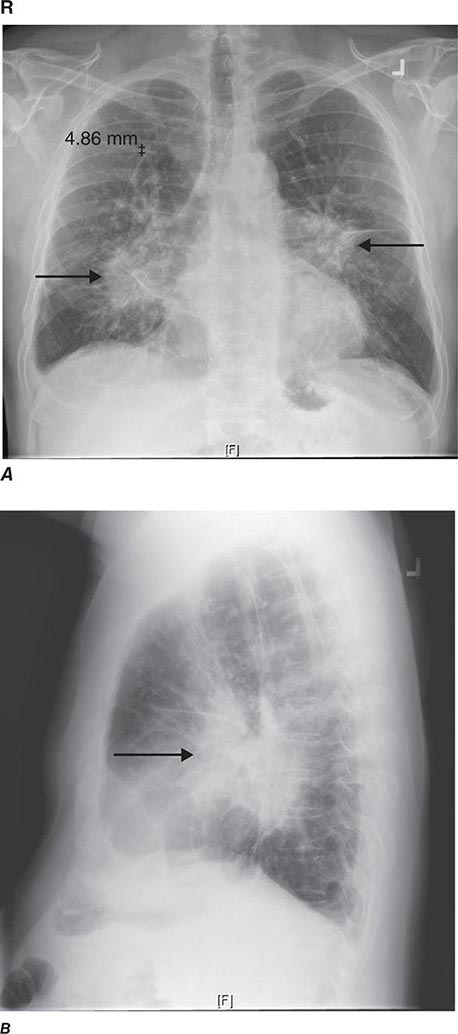
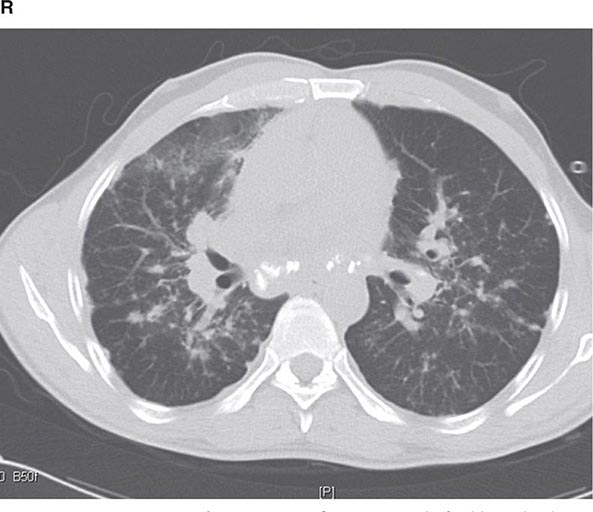
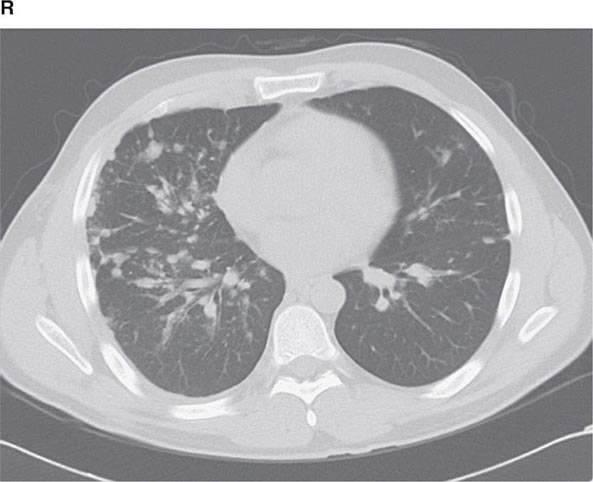
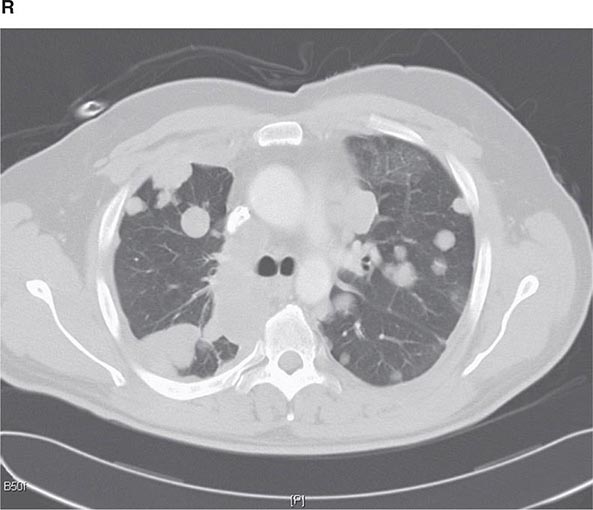
CT offers several advantages over routine chest radiography (Figs. 307-1A, B and 307-2A, B; see also Figs. 315-3, 315-4, and 322-4). First, the use of cross-sectional images allows distinction between densities that would be superimposed on plain radiographs. Second, CT is far better than routine radiographic studies at characterizing tissue density and providing accurate size assessment of lesions.
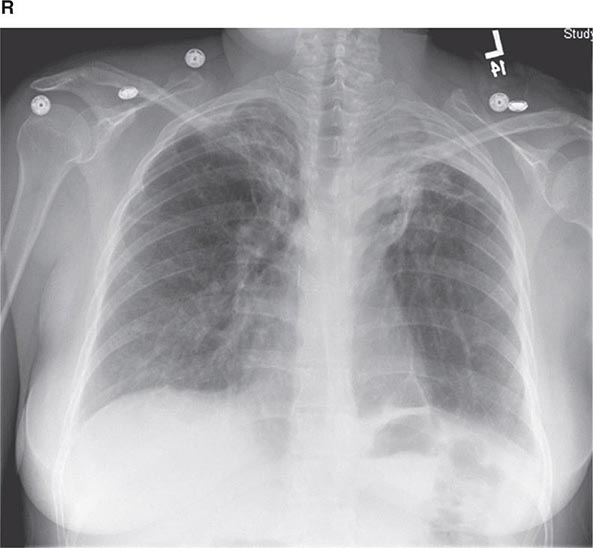
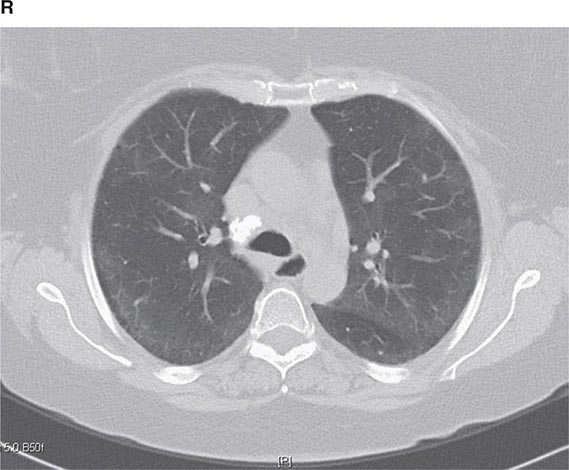
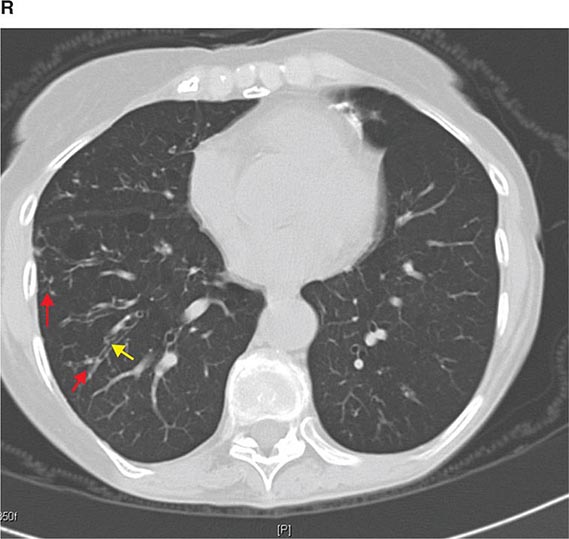
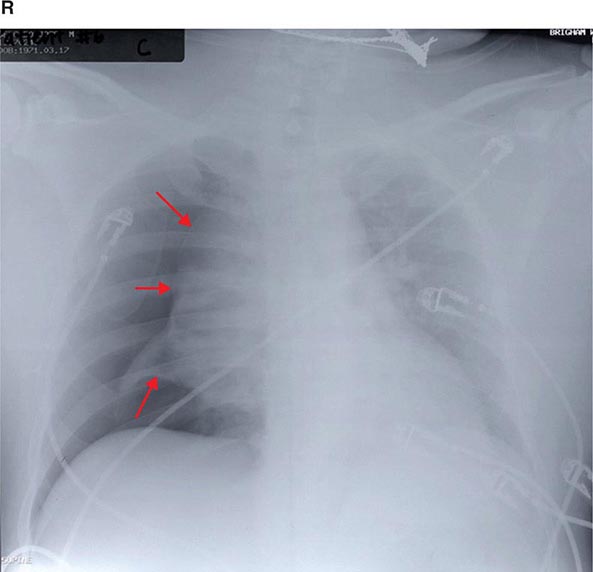
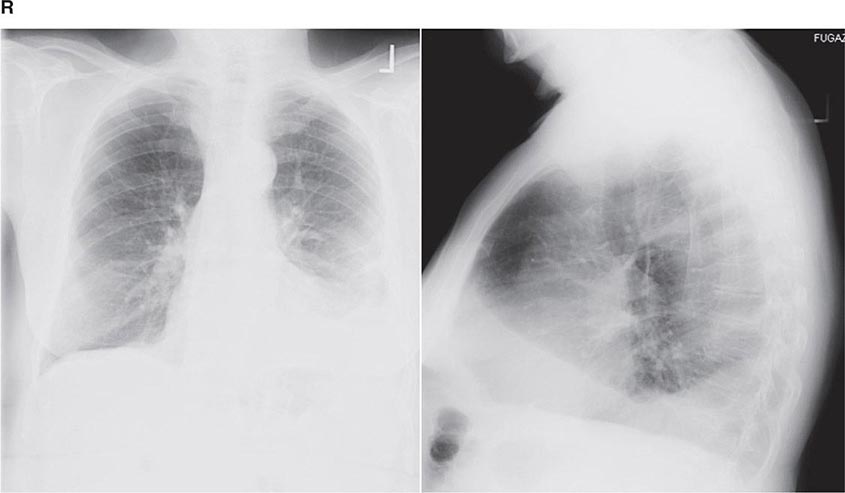
FIGURE 307-1 Chest x-ray (A) and computed tomography (CT) scan (B) from a patient with emphysema. The extent and distribution of emphysema are not well appreciated on plain film but clearly evident on the CT scan obtained.
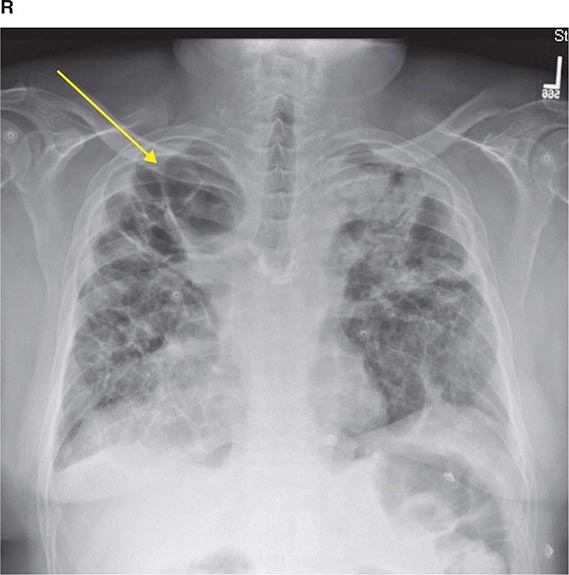
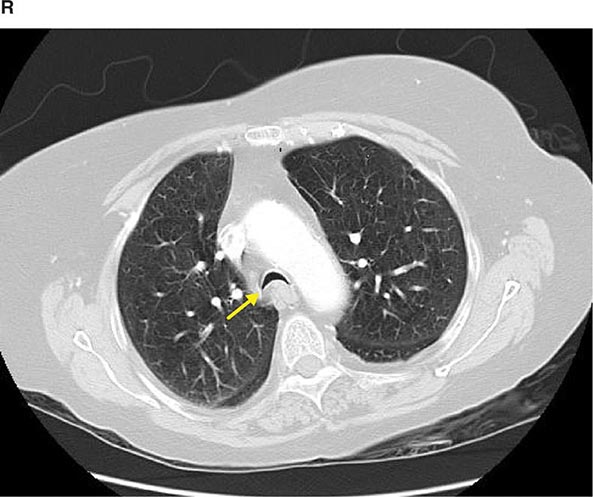
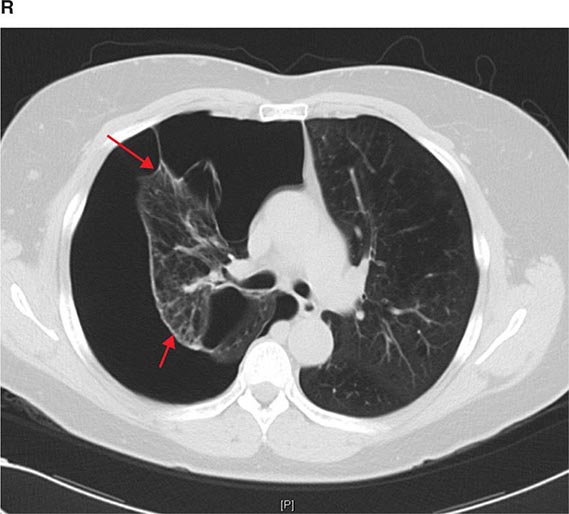
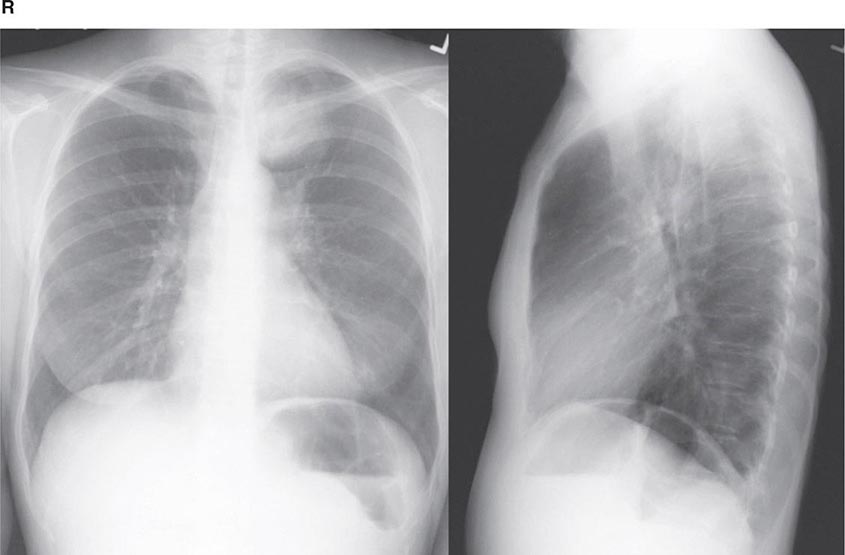
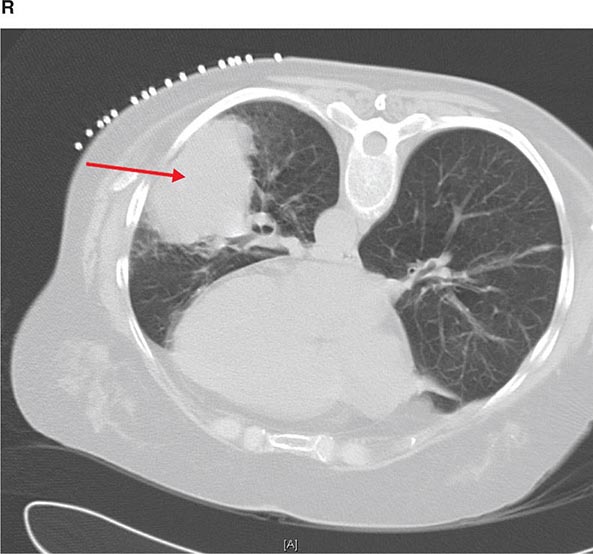
FIGURE 307-2 Chest x-ray (A) and computed tomography (CT) scan (B) demonstrating a right lower-lobe mass. The mass is not well appreciated on the plain film because of the hilar structures and known calcified adenopathy. CT is superior to plain radiography for the detection of abnormal mediastinal densities and the distinction of masses from adjacent vascular structures.
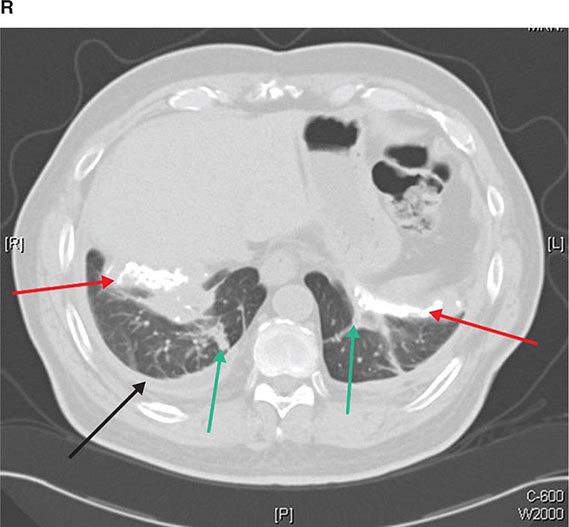
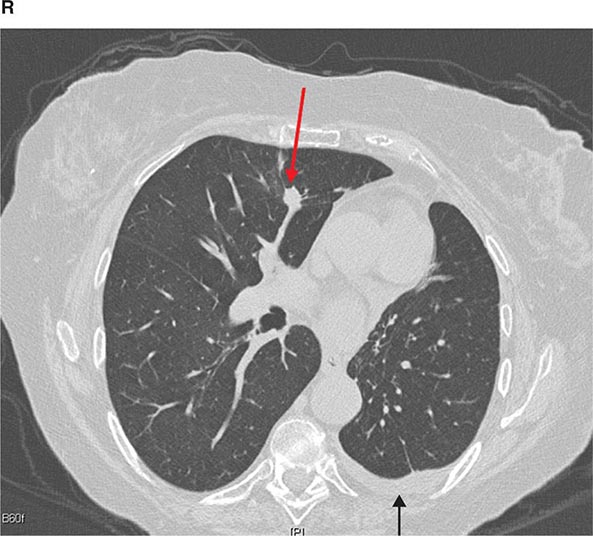
CT is particularly valuable in assessing hilar and mediastinal disease (often poorly characterized by plain radiography), in identifying and characterizing disease adjacent to the chest wall or spine (including pleural disease), and in identifying areas of fat density or calcification in pulmonary nodules (Fig. 307-2). Its utility in the assessment of mediastinal disease has made CT an important tool in the staging of lung cancer (Chap. 107). With the additional use of contrast material, CT also makes it possible to distinguish vascular from nonvascular structures, which is particularly important in distinguishing lymph nodes and masses from vascular structures primarily in the mediastinum, and vascular disorders such as pulmonary embolism.
In high-resolution CT (HRCT), the thickness of individual cross-sectional images is ~1–2 mm, rather than the usual 7–10 mm in conventional CT. The visible detail on HRCT scans allows better recognition of subtle parenchymal and airway disease, thickened interlobular septa, ground-glass opacification, small nodules, and the abnormally thickened or dilated airways seen in bronchiectasis. Using HRCT, characteristic patterns are recognized for many interstitial lung diseases such as lymphangitic carcinoma, idiopathic pulmonary fibrosis, sarcoidosis, and eosinophilic granuloma. However, there is debate about the settings in which the presence of a characteristic pattern on HRCT eliminates the need for obtaining lung tissue to make a diagnosis.
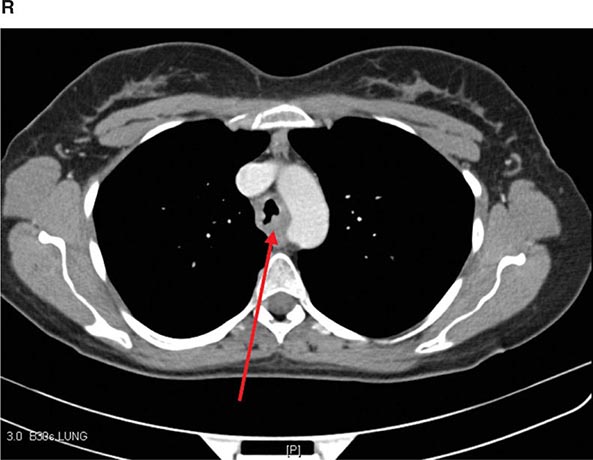
Helical CT and Multidetector CT Helical scanning is currently the standard method for thoracic CT. Helical CT technology results in faster scans with improved contrast enhancement and thinner collimation. Images are obtained during a single breath-holding maneuver that allows less motion artifact and collection of continuous data over a larger volume of lung than is possible with conventional CT. Data from the imaging procedure can be reconstructed in coronal or sagittal planes (Fig. 307-3A), as well as the traditional cross-sectional (axial) view.
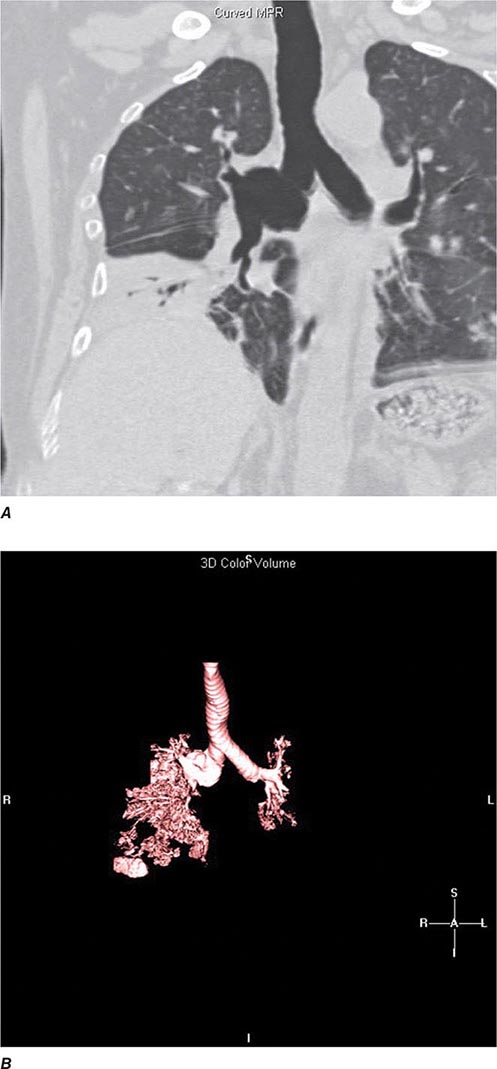
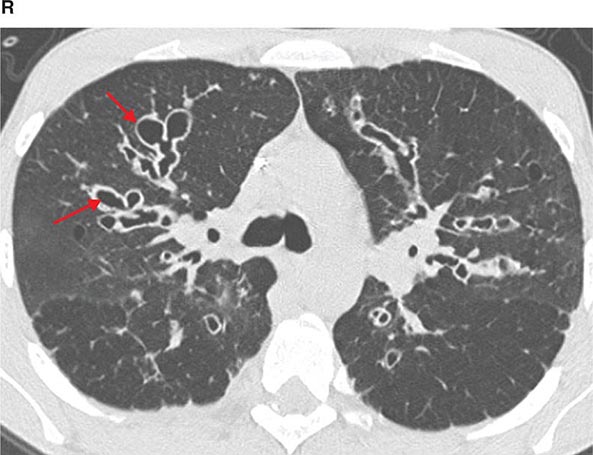
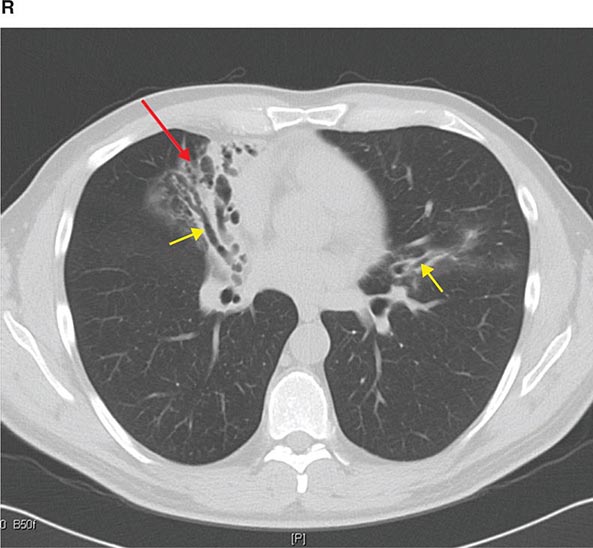
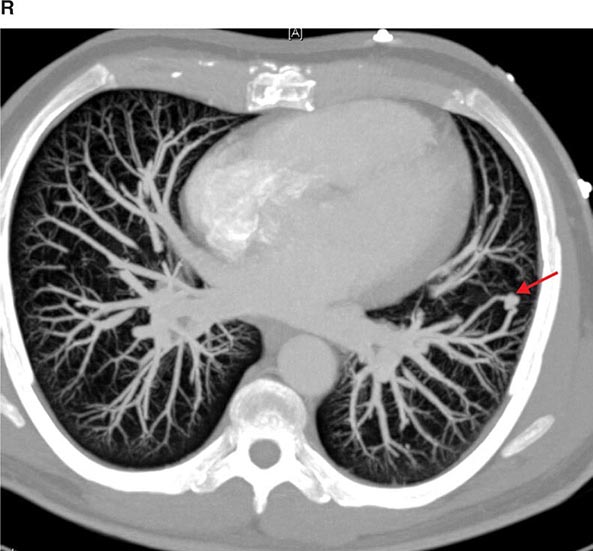
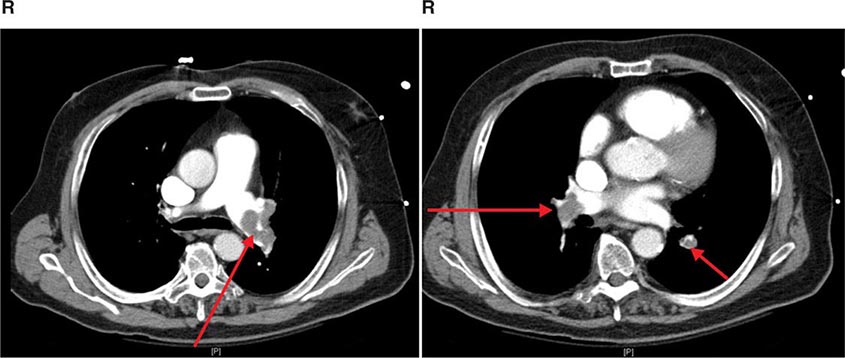
FIGURE 307-3 Spiral computed tomography (CT) with reconstruction of images in planes other than axial view. Spiral CT in a lung transplant patient with a dehiscence and subsequent aneurysm of the anastomosis. CT images were reconstructed in the sagittal view (A) and using digital subtraction to view images of the airways only (B), which demonstrate the exact location and extent of the abnormality.
Further refinements in detector technology have allowed production of scanners with additional detectors along the scanning axis (z-axis). These multidetector CT (MDCT) scanners can obtain multiple slices in a single rotation that are thinner and can be acquired in a shorter period of time. This results in enhanced resolution and increased image reconstruction ability. As the technology has progressed, higher numbers (currently up to 64) of detectors are used to produce clearer final images. MDCT allows for even shorter breath holds, which are beneficial for all patients but especially children, the elderly, and the critically ill. However, it should be noted that despite the advantages of MDCT, there is an increase in radiation dose compared to single-detector CT to consider.
In MDCT, the additional detectors along the z-axis result in improved use of the contrast bolus. This and the faster scanning times and increased resolution have all led to improved imaging of the pulmonary vasculature and the ability to detect segmental and subsegmental emboli. CT pulmonary angiography (CTPA) also allows simultaneous detection of parenchymal abnormalities that may be contributing to a patient’s clinical presentation. Secondary to these advantages and increasing availability, CTPA has rapidly become the test of choice for many clinicians in the evaluation of pulmonary embolism; compared with pulmonary angiography, it is considered equal in terms of accuracy and with less associated risks.
VIRTUAL BRONCHOSCOPY
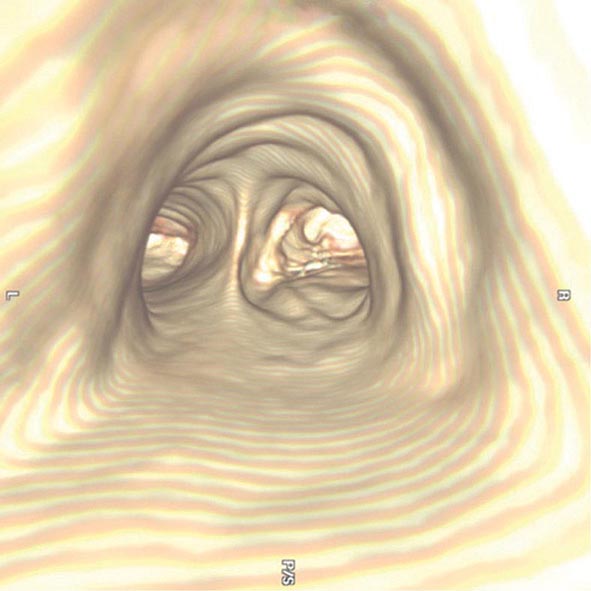
The three-dimensional (3D) image of the thorax obtained by MDCT can be digitally stored, reanalyzed, and displayed as 3D reconstructions of the airways down to the sixth to seventh generation. Using these reconstructions, a “virtual” bronchoscopy can be performed (Fig. 307-4). Virtual bronchoscopy has been proposed as an adjunct to conventional bronchoscopy in several clinical situations: It can allow accurate assessment of the extent and length of an airway stenosis, including the airway distal to the narrowing; it can provide useful information about the relationship of the airway abnormality to adjacent mediastinal structures; and it allows preprocedure planning for therapeutic bronchoscopy to help ensure the appropriate equipment is available for the procedure.
FIGURE 307-4 Virtual bronchoscopic image of the trachea. The view projected is one that would be obtained from the trachea looking down to the carina. The left and right main stem airways are seen bifurcating from the carina.
Virtual bronchoscopy can be used to help target the area of peripheral lung for endobronchial lung volume reduction surgery that is being used in the management of pulmonary emphysema. The extent of emphysema in each segmental region together with other anatomic details may help in choosing the most appropriate subsegments. However, software packages for the generation of virtual bronchoscopic images are relatively early in development, and their utilization and potential impact on patient care are still unknown. Electromagnetic navigational bronchoscopy systems (EMN or ENB) using virtual bronchoscopy have been developed to allow accurate navigation to peripheral pulmonary target lesions, using technology similar to a car global positioning system (GPS) unit.
POSITRON EMISSION TOMOGRAPHIC SCANNING
Positron emission tomographic (PET) scanning is commonly used to identify malignant lesions in the lung, based on their increased uptake and metabolism of glucose. The technique involves injection of a radiolabeled glucose analogue, [18F]-fluoro-2-deoxyglucose (FDG), which is taken up by metabolically active malignant cells. However, FDG is trapped within the cells following phosphorylation, and the unstable [18F] decays by emission of positrons, which can be detected by a specialized PET camera or by a gamma camera that has been adapted for imaging of positron-emitting nuclides. This technique has been used in the evaluation of solitary pulmonary nodules and in staging lung cancer. Detection or exclusion of mediastinal lymph node involvement and identification of extrathoracic disease can be achieved. The limited anatomical definition of radionuclide imaging has been improved by the development of hybrid imaging that allows the superimposition of PET and CT images, a technique known as functional–anatomical mapping. Hybrid PET/CT scans provide images that help pinpoint the abnormal metabolic activity to anatomical structures seen on CT and provide more accurate diagnoses than the two scans performed separately. FDG-PET can differentiate benign from malignant lesions as small as 1 cm. However, false-negative findings can occur in lesions with low metabolic activity such as carcinoid tumors and bronchioloalveolar cell carcinomas, or in lesions <1 cm in which the required threshold of metabolically active malignant cells is not present for PET diagnosis. False-positive results can be seen due to FDG uptake in inflammatory conditions such as pneumonia and granulomatous diseases.
MAGNETIC RESONANCE IMAGING
The role of magnetic resonance imaging (MRI) in the evaluation of respiratory system disease is less well-defined than that of CT. Magnetic resonance (MR) provides poorer spatial resolution and less detail of the pulmonary parenchyma and, for these reasons, is currently not considered a substitute for CT in imaging the thorax. However, the use of hyperpolarized gas in conjunction with MR has led to the investigational use of MR for imaging the lungs, particularly in obstructive lung disease. In addition, imaging performed during an inhalation and exhalation can provide dynamic information on lung function. Of note, MR examinations are difficult to obtain among several subgroups of patients. Patients who cannot lie still or who cannot lie on their backs may have MRIs that are of poor quality; some tests require patients to hold their breaths for 15–25 seconds at a time in order to get good MRIs. MRI is generally avoided in unstable and/or ventilated patients and those with severe trauma because of the hazards of the MR environment and the difficulties in monitoring patients within the MR room. The presence of metallic foreign bodies, pacemakers, and intracranial aneurysm clips also preclude use of MRI.
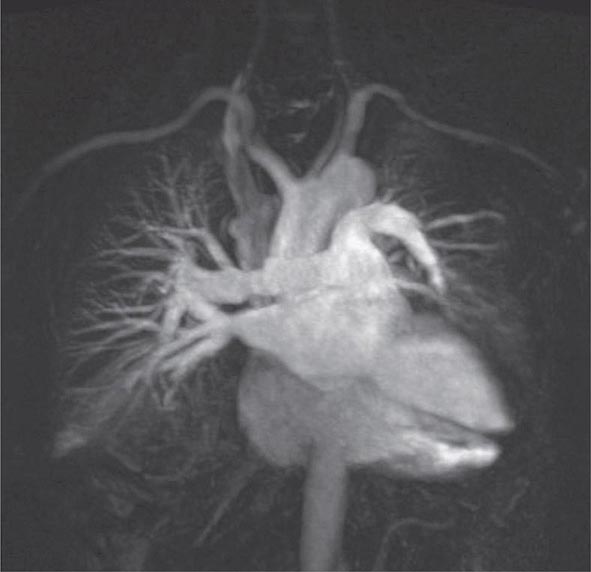
An advantage of MR is the use of nonionizing electromagnetic radiation. Additionally, MR is well suited to distinguish vascular from nonvascular structures without the need for contrast. Blood vessels appear as hollow tubular structures because flowing blood does not produce a signal on MRI. Therefore, MR can be useful in demonstrating pulmonary emboli, defining aortic lesions such as aneurysms or dissection, or other vascular abnormalities (Fig. 307-5) if radiation and IV contrast medium cannot be used. Gadolinium can be used as an intravascular contrast agent for MR angiography (MRA); however, synchronization of data acquisition with the peak arterial bolus is one of the major challenges of MRA. The flow of contrast medium from the peripheral injection site to the vessel of interest is affected by a number of factors including heart rate, stroke volume, and the presence of proximal stenotic lesions.
FIGURE 307-5 Magnetic resonance angiography image of the vasculature of a patient after lung transplant. The image demonstrates the detailed view of the vasculature that can be obtained using digital subtraction techniques. Images from a patient after lung transplant show the venous and arterial anastomosis on the right; a slight narrowing is seen at the site of the anastomosis, which is considered within normal limits and not suggestive of obstruction.
PULMONARY ANGIOGRAPHY
The pulmonary arterial system can be visualized by pulmonary angiography, in which radiopaque contrast medium is injected through a catheter placed in the pulmonary artery. When performed in cases of pulmonary embolism, pulmonary angiography demonstrates the consequences of an intravascular thrombus—either a defect in the lumen of a vessel (a filling defect) or an abrupt termination (cutoff) of the vessel. Other, less common indications for pulmonary angiography include visualization of a suspected pulmonary arteriovenous malformation and assessment of pulmonary arterial invasion by a neoplasm. The risks associated with modern arteriography are small, generally of greatest concern in patients with severe pulmonary hypertension or chronic kidney disease. With advances in CT scanning, MDCT angiography (MDCTA) is replacing conventional angiography for the diagnosis of pulmonary embolism.
MEDICAL TECHNIQUES FOR OBTAINING BIOLOGIC SPECIMENS
COLLECTION OF SPUTUM
Sputum can be collected either by spontaneous expectoration or induced (after inhalation of an irritating aerosol such as hypertonic saline). Sputum induction is used either because sputum is not spontaneously being produced or because of an expected higher yield of certain types of findings. Because sputum consists mainly of secretions from the tracheobronchial tree rather than the upper airway, the finding of alveolar macrophages and other inflammatory cells is consistent with a lower respiratory tract origin of the sample, whereas the presence of squamous epithelial cells in a “sputum” sample indicates contamination by secretions from the upper airways.
In addition to processing for routine bacterial pathogens by Gram’s method and culture, sputum can be processed for a variety of other pathogens, including staining and culture for mycobacteria or fungi, culture for viruses, and staining for Pneumocystis jiroveci. In the specific case of sputum obtained for evaluation of P. jiroveci pneumonia, for example, sputum should be collected by induction rather than spontaneous expectoration, and an immunofluorescent stain should be used to detect the organisms. Traditional stains and cultures are now also being supplemented in some cases by immunologic techniques and by molecular biologic methods, including the use of polymerase chain reaction amplification and DNA probes. Cytologic staining of sputum for malignant cells, using the traditional Papanicolaou method, allows noninvasive evaluation for suspected lung cancer.
PERCUTANEOUS NEEDLE ASPIRATION (TRANSTHORACIC)
A needle can be inserted through the chest wall into a pulmonary lesion to obtain an aspirate or tissue core for cytologic/histologic or microbiologic analysis. Aspiration can be performed to obtain a diagnosis or to decompress and/or drain a fluid collection. The procedure is usually carried out under CT or ultrasound guidance to assist positioning of the needle and assure localization in the lesion. The low potential risk of this procedure (intrapulmonary bleeding or creation of a pneumothorax with collapse of the underlying lung) in experienced hands is usually acceptable compared with the information obtained. However, a limitation of the technique is sampling error due to the small size of the tissue sample. Thus, findings other than a specific cytologic or microbiologic diagnosis are of limited clinical value.
THORACENTESIS
Sampling of pleural liquid by thoracentesis is commonly performed for diagnostic purposes or, in the case of a large effusion, for palliation of dyspnea. Diagnostic sampling, either by blind needle aspiration or after localization by US, allows the collection of liquid for microbiologic and cytologic studies. Analysis of the fluid obtained for its cellular composition and chemical constituents allows classification of the effusion and can help with diagnosis and treatment (Chap. 316).
BRONCHOSCOPY
Bronchoscopy is the process of direct visualization of the tracheobronchial tree. Although bronchoscopy is now performed almost exclusively with flexible fiberoptic instruments, rigid bronchoscopy, generally performed in an operating room on a patient under general anesthesia, still has a role in selected circumstances, primarily because of a larger suction channel and the fact that the patient can be ventilated through the bronchoscope channel. These situations include the retrieval of a foreign body and the suctioning of a massive hemorrhage, for which the small suction channel of the bronchoscope may be insufficient.
FLEXIBLE FIBEROPTIC BRONCHOSCOPY
This outpatient procedure is usually performed in an awake but sedated patient (conscious sedation). The bronchoscope is passed through either the mouth or the nose, between the vocal cords, and into the trachea. The ability to flex the scope makes it possible to visualize virtually all airways to the level of subsegmental bronchi. The bronchoscopist is able to identify endobronchial pathology, including tumors, granulomas, bronchitis, foreign bodies, and sites of bleeding. Samples from airway lesions can be taken by several methods, including washing, brushing, and biopsy. Washing involves instillation of sterile saline through a channel of the bronchoscope and onto the surface of a lesion. A portion of the liquid is collected by suctioning through the bronchoscope, and the recovered material can be analyzed for cells (cytology) or organisms (by standard stains and cultures). Brushing or biopsy of the surface of the lesion, using a small brush or biopsy forceps at the end of a long cable inserted through a channel of the bronchoscope, allows recovery of cellular material or tissue for analysis by standard cytologic and histopathologic methods.
The bronchoscope can be used to sample material not only from the regions that can be directly visualized (i.e., the airways) but also from the more distal pulmonary parenchyma. With the bronchoscope wedged into a subsegmental airway, aliquots of sterile saline can be instilled through the scope, allowing sampling of cells and organisms from alveolar spaces. This procedure, called bronchoalveolar lavage, has been particularly useful for the recovery of organisms such as P. jiroveci.
Brushing and biopsy of the distal lung parenchyma can also be performed with the same instruments that are used for endobronchial sampling. These instruments can be passed through the scope into small airways. When biopsies are performed, the forceps penetrate the airway wall, allowing biopsy of peribronchial alveolar tissue. This procedure, called transbronchial biopsy, is used when there is either relatively diffuse disease or a localized lesion of adequate size. With the aid of fluoroscopic imaging, the bronchoscopist is able to determine not only whether and when the instrument is in the area of abnormality, but also the proximity of the instrument to the pleural surface. If the forceps are too close to the pleural surface, there is a risk of violating the visceral pleura and creating a pneumothorax; the other potential complication of transbronchial biopsy is pulmonary hemorrhage. The incidence of these complications is less than several percent.
TRANSBRONCHIAL NEEDLE ASPIRATION (TBNA)
Another procedure involves use of a hollow-bore needle passed through the bronchoscope for sampling of tissue adjacent to the trachea or a large bronchus. The needle is passed through the airway wall (transbronchial), and cellular material can be aspirated from mass lesions or enlarged lymph nodes, generally in a search for malignant cells. Mediastinoscopy has been considered the gold standard for mediastinal staging; however, transbronchial needle aspiration (TBNA) allows sampling from the lungs and surrounding lymph nodes without the need for surgery or general anesthesia.
ENDOBRONCHIAL ULTRASOUND (EBUS)–TRANSBRONCHIAL NEEDLE ASPIRATION (TBNA)
Further advances in needle aspiration techniques have been accomplished with the development of endobronchial ultrasound (EBUS). The technology uses an ultrasonic bronchoscope fitted with a probe that allows for needle aspiration of mediastinal and hilar lymph nodes guided by real-time US images. EBUS allows sampling of mediastinal lymph nodes and masses under direct vision to better identify and localize peribronchial and mediastinal pathology and offers access to more difficult-to-reach areas and smaller lymph nodes in the staging of malignancies. EBUS-TBNA has the potential to access the same paratracheal and subcarinal lymph node stations as mediastinoscopy, but also extends out to the hilar lymph nodes (levels 10 and 11). The usefulness of EBUS for clinical indications other than lung cancer is improving and has been recommended in the evaluation of mediastinal masses of unknown origin early in the diagnostic process.
EMERGING BRONCHOSCOPIC TECHNIQUES
Emerging techniques that can be performed using bronchoscopy include video/autofluorescence bronchoscopy (AFB), narrow band imaging (NBI), optical coherence tomography (OCT), and endomicroscopy using confocal fluorescent laser microscopy (CFM). AFB uses bronchoscopy with an additional light source to screen high-risk individuals and identify premalignant lesions (airway dysplasia) and carcinoma in situ. NBI capitalizes on the increased absorption of blue and green wavelengths of light by hemoglobin to enhance the visibility of vessels of the mucosa and differentiate between inflammatory versus malignant mucosal lesions. CFM uses a blue laser to induce fluorescence, and its high degree of resolution provides a real-time view of living tissue at an almost histologic resolution. OCT uses near-infrared light source and has spatial resolution advantages over CT and MRI. It can penetrate the airway wall up to three times deeper than CFM and is less susceptible to motion artifacts from cardiac pulsation and respiratory movements. However, careful assessment is required before these methods find a place in the evaluation strategy of early lung cancer and other lung diseases.
THERAPEUTIC BRONCHOSCOPY
The bronchoscope may provide the opportunity for treatment as well as diagnosis. A central role of the interventional pulmonology (IP) physician is the performance of therapeutic bronchoscopy. For example, an aspirated foreign body may be retrieved with an instrument passed through the bronchoscope (either flexible or rigid), and bleeding may be controlled with a balloon catheter similarly introduced. Newer interventional techniques performed through a bronchoscope include methods for achieving and maintaining patency of airways that are partially or completely occluded, especially by tumors. These techniques include laser therapy, cryotherapy, argon plasma coagulation, electrocautery, balloon bronchoplasty and dilation, and stent placement. Many IP physicians are also trained in performing percutaneous tracheotomy.
MEDICAL THORACOSCOPY
Medical thoracoscopy (or pleuroscopy) focuses on the diagnosis of pleural-based problems. The procedure is performed with a conventional rigid or a semi-rigid pleuroscope (similar in design to a bronchoscope and enabling the operator to inspect the pleural surface, sample and/or drain pleural fluid, or perform targeted biopsies of the parietal pleura). Medical thoracoscopy can be performed in the endoscopy suite or operating room with the patient under conscious sedation and local anesthesia. In contrast, video-assisted thoracoscopic surgery (VATS) requires general anesthesia and is only performed in the operating room. A common diagnostic indication for medical thoracoscopy is the evaluation of a pleural effusion or biopsy of presumed parietal pleural carcinomatosis. It can also be used to place a chest tube under visual guidance, or perform chemical or talc pleurodesis, a therapeutic intervention to prevent a recurrent pleural effusion (usually malignant) or recurrent pneumothorax.
The increasing availability of advanced bronchoscopic and pleuroscopic techniques has motivated the development of IP programs. IP can be defined as “the art and science of medicine as related to the performance of diagnostic and invasive therapeutic procedures, that which require additional training and expertise beyond that which required in a standard pulmonary medicine training program.” IP physicians provide alternatives to surgery for patients with a wide variety of thoracic disorders and problems.
SURGICAL TECHNIQUES FOR OBTAINING BIOLOGIC SPECIMENS
Evaluation and diagnosis of disorders of the chest commonly involve collaboration between pulmonologists and thoracic surgeons. Although procedures such as mediastinoscopy, VATS, and thoracotomy are performed by thoracic surgeons, there is overlap in many minimally invasive techniques that can be performed by a pulmonologist, an interventional pulmonologist, or a thoracic surgeon.
MEDIASTINOSCOPY AND MEDIASTINOTOMY
Proper staging of lung cancer is of paramount concern when determining a treatment regimen. Although CT and PET scanning are useful for determining the size and nature of mediastinal lymph nodes as part of the staging of lung cancer, tissue biopsy and histopathologic examination are often critical for the diagnosis of mediastinal masses or enlarged mediastinal lymph nodes. The two major surgical procedures used to obtain specimens from masses or nodes in the mediastinum are mediastinoscopy (via a suprasternal approach) and mediastinotomy (via a parasternal approach). Both procedures are performed under general anesthesia by a qualified surgeon. In the case of suprasternal mediastinoscopy, a rigid mediastinoscope is inserted at the suprasternal notch and passed into the mediastinum along a pathway just anterior to the trachea. Tissue can be obtained with biopsy forceps passed through the scope, sampling masses or nodes that are in a paratracheal or pretracheal position (levels 2R, 2L, 3, 4R, 4L). Aortopulmonary lymph nodes (levels 5, 6) are not accessible by this route and thus are commonly sampled by parasternal mediastinotomy (the Chamberlain procedure). This approach involves a parasternal incision and dissection directly down to a mass or node that requires biopsy.
As an alternative to surgery, a bronchoscope can be used to perform TBNA to obtain tissue from the mediastinum, and, when combined with EBUS, can allow access to the same lymph node stations associated with mediastinoscopy, but also extend access out to the hilar lymph nodes (levels 10, 11). Finally, endoscopic ultrasound (EUS)–fine-needle aspiration (FNA) is a second procedure that complements EBUS-FNA in the staging of lung cancer. EUS-FNA is performed via the esophagus and is ideally suited for sampling lymph nodes in the posterior mediastinum (levels 7, 8, 9). Because US imaging cannot penetrate air-filled spaces, the area directly anterior to the trachea cannot accurately be assessed and is a “blind spot” for EUS-FNA. However, EBUS-FNA can visualize the anterior lymph nodes and can complement EUS-FNA. The combination of EUS-FNA and EBUS-FNA is a technique that is becoming an alternative to surgery for staging the mediastinum in thoracic malignancies.
VIDEO-ASSISTED THORACOSCOPIC SURGERY
VATS has become a standard technique for the diagnosis and management of pleural as well as parenchymal lung disease. This procedure is performed in the operating room using single-lung ventilation with double-lumen endotracheal intubation and involves the passage of a rigid scope with a distal lens through a trocar inserted into the pleura. A high-quality image is shown on a monitor screen, allowing the operator to manipulate instruments passed into the pleural space through separate small intercostal incisions. With these instruments the operator can biopsy lesions of the pleura under direct visualization. In addition, this procedure is now used commonly to biopsy peripheral lung tissue or to remove peripheral nodules for both diagnostic and therapeutic purposes. This much less invasive procedure has largely supplanted the traditional “open lung biopsy” performed via thoracotomy. The decision to use a VATS technique versus performing an open thoracotomy is made by the thoracic surgeon and is based on whether a patient can tolerate the single-lung ventilation that is required to allow adequate visualization of the lung. With further advances in instrumentation and experience, VATS can be used to perform procedures previously requiring thoracotomy, including stapled lung biopsy, resection of pulmonary nodules, lobectomy, pneumonectomy, pericardial window, or other standard thoracic surgical procedures, but allows them to be performed in a minimally invasive manner.
THORACOTOMY
Although frequently replaced by VATS, thoracotomy remains an option for the diagnostic sampling of lung tissue. It provides the largest amount of material, and it can be used to biopsy and/or excise lesions that are too deep or too close to vital structures for removal by VATS. The choice between VATS and thoracotomy needs to be made on a case-by-case basis.
308e |
Atlas of Chest Imaging |
This atlas of chest imaging is a collection of interesting chest radiographs and computed tomograms (CTs) of the chest. The readings of the films are meant to be illustrative of specific, major findings. The associated text is not intended as a comprehensive assessment of the images.
EXAMPLES OF NORMAL IMAGING
FIGURE 308e-1 Normal chest radiograph—review of anatomy. 1. Trachea. 2. Carina. 3. Right atrium. 4. Right hemidiaphragm. 5. Aortic knob. 6. Left hilum. 7. Left ventricle. 8. Left hemidiaphragm (with stomach bubble). 9. Retrosternal clear space. 10. Right ventricle. 11. Left hemidiaphragm (with stomach bubble). 12. Left upper lobe bronchus.
FIGURE 308e-2 Normal chest tomogram—note anatomy. 1. Superior vena cava. 2. Trachea. 3. Aortic arch. 4. Ascending aorta. 5. Right mainstem bronchus. 6. Descending aorta. 7. Left mainstem bronchus. 8. Main pulmonary artery. 9. Heart. 10. Esophagus. 11. Pericardium. 12. Descending aorta.
VOLUME LOSS
FIGURE 308e-3 CT scan demonstrating left upper lobe collapse. The patient was found to have an endobronchial lesion (not visible on the CT scan) resulting in this finding. The superior vena cava (black arrow) is partially opacified by intravenous contrast.
FIGURE 308e-4 CT scan revealing chronic left lower lobe collapse. Note dramatic volume loss with minimal aeration. There is subtle mediastinal shift to the left.
FIGURE 308e-5 Left upper lobe scarring with hilar retraction with less prominent scarring in right upper lobe as well. Findings consistent with previous tuberculosis infection in an immigrant from Ecuador.
FIGURE 308e-6 Apical scarring, traction bronchiectasis (red arrow), and decreased lung volume consistent with previous tuberculosis infection. Findings most significant in left lung.
FIGURE 308e-7 Chest radiograph demonstrating right upper lobe collapse (yellow arrow). Note the volume loss as demonstrated by the elevated right hemidiaphragm as well as mediastinal shift to the right. Also apparent on the film are an endotracheal tube (red arrow) and a central venous catheter (black arrow).
FIGURE 308e-8 Opacity in the right upper lobe. Note the volume loss as indicated by the elevation of the right hemidiaphragm, elevation of minor fissure (yellow arrow), and deviation of the trachea to the right (blue arrow).
FIGURE 308e-9 CT scan of the same right upper lobe opacity. Note the air bronchograms and areas of consolidation.
LOSS OF PARENCHYMA
FIGURE 308e-10 Emphysema with increased lucency, flattened diaphragms (black arrows), increased anteroposterior (AP) diameter, and increased retrosternal clear space (red arrow).
FIGURE 308e-11 CT scan of diffuse, bilateral emphysema.
FIGURE 308e-12 CT scan of bullous emphysema.
FIGURE 308e-13 Lymphangioleiomyomatosis—note multiple thin-walled parenchymal cysts.
FIGURE 308e-14 Two cavities on posteroanterior (PA) and lateral chest radiograph. Cavities and air-fluid levels identified by red arrows. The smaller cavity is in the right lower lobe (located below the major fissure, identified with the yellow arrow), and the larger cavity is located in the right middle lobe, which is located between the minor (red arrow) and major fissures. There is an associated opacity surrounding the cavity in the right lower lobe.
FIGURE 308e-15 CT scan of parenchymal cavity.
FIGURE 308e-16 Thick-walled cavitary lung lesions. The mass in the right lung has thick walls and advanced cavitation, whereas the smaller nodule on the left has early cavitary changes (arrow). This patient was diagnosed with Nocardia infection.
INTERSTITIAL PROCESSES
FIGURE 308e-17 Mild congestive heart failure. Note the Kerley B lines (black arrow) and perivascular cuffing (yellow arrow) as well as the pulmonary vascular congestion (red arrow).
FIGURE 308e-18 Pulmonary edema. Note indistinct vasculature, perihilar opacities, and peripheral interstitial reticular opacities. Although this is an anteroposterior film making cardiac size more difficult to assess, the cardiac silhouette still appears enlarged.
FIGURE 308e-19 Chest radiograph demonstrates reticular nodular opacities bilaterally with small lung volumes consistent with usual interstitial pneumonitis (UIP) on pathology. Clinically, UIP is used interchangeably with idiopathic pulmonary fibrosis (IPF).
FIGURE 308e-20 CT scan of usual interstitial pneumonitis (UIP), also known as idiopathic pulmonary fibrosis (IPF). Classic findings include traction bronchiectasis (black arrow) and honeycombing (red arrows). Note subpleural, basilar predominance of the honeycombing.
FIGURE 308e-21 A. PA chest film—note presence of paratracheal (blue arrow), aortopulmonary window (yellow arrow), and hilar lymphadenopathy (purple arrows). B. Lateral film—note hilar lymphadenopathy (purple arrow).
FIGURE 308e-22 Sarcoid—CT scan of stage I demonstrating bulky hilar and mediastinal lymphadenopathy (red arrows).
FIGURE 308e-23 Sarcoid—chest radiograph of stage II. A. PA film with hilar lymphadenopathy (black arrows) and parenchymal changes. B. Lateral film with hilar adenopathy (black arrow) and parenchymal changes.
FIGURE 308e-24 Sarcoid—CT scan of stage II (calcified lymphadenopathy, parenchymal infiltrates).
FIGURE 308e-25 Sarcoid—CT scan of stage II (nodular opacities tracking along bronchovascular bundles).
FIGURE 308e-26 Sarcoid—stage IV with fibrotic lung disease and cavitary areas (yellow arrow).
ALVEOLAR PROCESSES
FIGURE 308e-27 Right middle lobe opacity illustrates major (black arrow) and minor fissures (red arrows) as well as the “silhouette sign” on the right heart border. The silhouette sign is the loss of clear demarcation between normal lung and soft tissue (e.g., heart, diaphragm). This occurs when the lung parenchyma is no longer filled with air and the contrast between air and soft tissue is lost.
FIGURE 308e-28 Right lower lobe pneumonia—subtle opacity on PA film (red arrow), while the lateral film illustrates the “spine sign” (black arrow) where the lower spine does not become more lucent.
FIGURE 308e-29 CT scan of diffuse, bilateral “ground-glass” opacities. This finding is consistent with fluid density in the alveolar space.
FIGURE 308e-30 Chest radiograph reveals diffuse, bilateral alveolar opacities without pleural effusions, consistent with acute respiratory distress syndrome (ARDS). Note that the patient has an endotracheal tube (red arrow) and a central venous catheter (black arrow).
FIGURE 308e-31 CT scan of ARDS demonstrates “ground-glass” opacities with more consolidated areas in the dependent lung zones.
FIGURE 308e-32 Three examples of air bronchograms (red arrows) on chest CT.
BRONCHIECTASIS AND AIRWAY ABNORMALITIES
FIGURE 308e-33 Cystic fibrosis with bronchiectasis, apical disease.
FIGURE 308e-34 CT scan of diffuse, cystic bronchiectasis (red arrows) in a patient with cystic fibrosis.
FIGURE 308e-35 CT scan of focal right middle lobe and lingular bronchiectasis (yellow arrows). Note that there is near total collapse of the right middle lobe (red arrow).
FIGURE 308e-36 “Tree in bud” opacities (red arrows) and bronchiectasis (yellow arrow) consistent with atypical mycobacterial infection. “Tree in bud” refers to small nodules clustered around the centrilobular arteries as well as increased prominence of the centrilobular branching. These findings are consistent with bronchiolitis.
PLEURAL ABNORMALITIES
FIGURE 308e-37 CT scan demonstrating tracheomalacia (yellow arrow). Tracheomalacia is dynamic collapse of the trachea, most prominent during exhalation, due to loss of cartilaginous support.
FIGURE 308e-38 Large right pneumothorax with near complete collapse of right lung. Pleural reflection highlighted with red arrows.
FIGURE 308e-39 Basilar pneumothorax with visible pleural reflection (red arrows). Also note, patient has subcutaneous emphysema (yellow arrow).
FIGURE 308e-40 CT scan of large right-sided pneumothorax. Note significant collapse of right lung with adhesion to anterior chest wall. Pleural reflection highlighted with red arrows. The patient has severe underlying emphysema.
FIGURE 308e-41 Small right pleural effusion (red arrows highlight blunted right costophrenic angles) with associated pleural thickening. Note fluid in the major fissure (black arrow) visible on the lateral film as well as the meniscus of the right pleural effusion.
FIGURE 308e-42 Left pleural effusion with clear meniscus seen on both PA and lateral chest radiographs.
FIGURE 308e-43 Asbestosis. Note calcified pleural plaques (red arrows), pleural thickening (black arrow), and subpleural atelectasis (green arrows).
NODULES AND MASSES
FIGURE 308e-44 Left upper lobe mass, which biopsy revealed to be squamous cell carcinoma.
FIGURE 308e-45 Solitary pulmonary nodule on the right (red arrow) with a spiculated pattern concerning for lung cancer. Note also that the patient is status post left upper lobectomy with resultant volume loss and associated effusion (black arrow).
FIGURE 308e-46 Metastatic sarcoma. Note the multiple, well-circumscribed nodules of different size.
FIGURE 308e-47 Left lower lobe lung mass (red arrow) abutting pleura. Biopsy demonstrated small-cell lung cancer.
FIGURE 308e-48 CT scan of soft tissue mass encircling the trachea (red arrow) and invading tracheal lumen. Biopsy demonstrated adenoid cystic carcinoma (cylindroma).
PULMONARY VASCULAR ABNORMALITIES
FIGURE 308e-49 Mycetoma. Fungal ball (red arrow) growing in preexisting cavity on the left. Right upper lobe has a large bulla (black arrow).
FIGURE 308e-50 Pulmonary arteriovenous malformation (AVM) demonstrated on reformatted CT angiogram (red arrow).
FIGURE 308e-51 Large bilateral pulmonary emboli (intravascular filling defects in contrast scan identified by red arrows).
FIGURE 308e-52 Chest radiograph of a patient with severe pulmonary hypertension. Note the enlarged pulmonary arteries (red arrows) visible on both PA and lateral films.
FIGURE 308e-53 CT scan of the same patient as in Fig. 308e-52. Note the markedly enlarged pulmonary arteries (red arrow).
SECTION 2 |
DISEASES OF THE RESPIRATORY SYSTEM |
309 |
Asthma |
Asthma is a syndrome characterized by airflow obstruction that varies markedly, both spontaneously and with treatment. Asthmatics harbor a special type of inflammation in the airways that makes them more responsive than nonasthmatics to a wide range of triggers, leading to excessive narrowing with consequent reduced airflow and symptomatic wheezing and dyspnea. Narrowing of the airways is usually reversible, but in some patients with chronic asthma there may be an element of irreversible airflow obstruction. The increasing global prevalence of asthma, the large burden it now imposes on patients, and the high health care costs have led to extensive research into its mechanisms and treatment.
PREVALENCE
Asthma is one of the most common chronic diseases globally and currently affects approximately 300 million people worldwide. The prevalence of asthma has risen in affluent countries over the last 30 years but now appears to have stabilized, with approximately 10–12% of adults and 15% of children affected by the disease. In developing countries where the prevalence of asthma had been much lower, there is a rising prevalence, which is associated with increased urbanization. The prevalence of atopy and other allergic diseases has also increased over the same time, suggesting that the reasons for the increase are likely to be systemic rather than confined to the lungs. Most patients with asthma in affluent countries are atopic, with allergic sensitization to the house dust mite Dermatophagoides pteronyssinus and other environmental allergens, such as animal fur and pollens.
Asthma can present at any age, with a peak age of 3 years. In childhood, twice as many males as females are asthmatic, but by adulthood the sex ratio has equalized. Long-term studies that have followed children until they reach the age of 40 years suggest that many with asthma become asymptomatic during adolescence but that asthma returns in some during adult life, particularly in those with persistent symptoms and severe asthma. Adults with asthma, including those with onset during adulthood, rarely become permanently asymptomatic. The severity of asthma does not vary significantly within a given patient; those with mild asthma rarely progress to more severe disease, whereas those with severe asthma usually have severe disease at the onset.
Deaths from asthma are uncommon, and in many affluent countries have been steadily declining over the last decade. A rise in asthma mortality seen in several countries during the 1960s was associated with increased use of short-acting inhaled β2-adrenergic agonists (as rescue therapy), but there is now compelling evidence that the more widespread use of inhaled corticosteroids (ICS) in patients with persistent asthma is responsible for the decrease in mortality in recent years. Major risk factors for asthma deaths are poorly controlled disease with frequent use of bronchodilator inhalers, lack of or poor compliance with ICS therapy, and previous admissions to hospital with near-fatal asthma.
It has proved difficult to agree on a definition of asthma, but there is good agreement on the description of the clinical syndrome and disease pathology. Until the etiologic mechanisms of the disease are better understood, it will be difficult to provide an accurate definition.
RISK FACTORS AND TRIGGERS
Asthma is a heterogeneous disease with interplay between genetic and environmental factors. Several risk factors that predispose to asthma have been identified (Table 309-1). These should be distinguished from triggers, which are environmental factors that worsen asthma in a patient with established disease.
|
RISK FACTORS AND TRIGGERS INVOLVED IN ASTHMA |
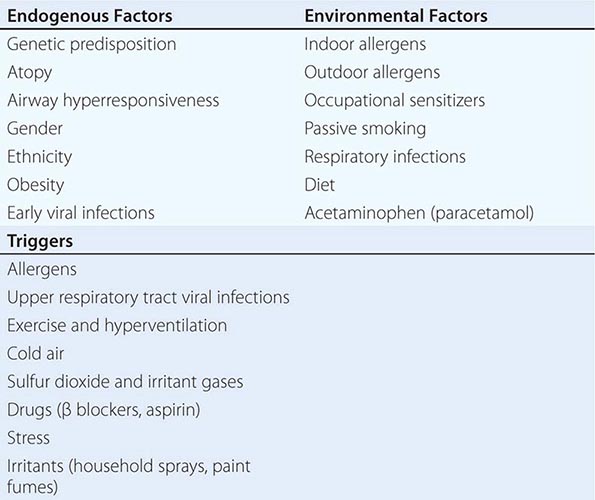
Atopy Atopy is the major risk factor for asthma, and nonatopic individuals have a very low risk of developing asthma. Patients with asthma commonly suffer from other atopic diseases, particularly allergic rhinitis, which may be found in over 80% of asthmatic patients, and atopic dermatitis (eczema). Atopy may be found in 40–50% of the population in affluent countries, with only a proportion of atopic individuals becoming asthmatic. This observation suggests that some other environmental or genetic factor(s) predispose to the development of asthma in atopic individuals. The allergens that lead to sensitization are usually proteins that have protease activity, and the most common allergens are derived from house dust mites, cat and dog fur, cockroaches (in inner cities), grass and tree pollens, and rodents (in laboratory workers). Atopy is due to the genetically determined production of specific IgE antibody, with many patients showing a family history of allergic diseases.
![]() Genetic Predisposition The familial association of asthma and a high degree of concordance for asthma in identical twins indicate a genetic predisposition to the disease; however, whether or not the genes predisposing to asthma are similar or in addition to those predisposing to atopy is not yet clear. It now seems likely that different genes may also contribute to asthma specifically, and there is increasing evidence that the severity of asthma is also genetically determined. Genetic screens with classical linkage analysis and single-nucleotide polymorphisms of various candidate genes indicate that asthma is polygenic, with each gene identified having a small effect that is often not replicated in different populations. This observation suggests that the interaction of many genes is important, and these may differ in different populations. The most consistent findings have been associations with polymorphisms of genes on chromosome 5q, including the T helper 2 (TH2) cells interleukin (IL)-4, IL-5, IL-9, and IL-13, which are associated with atopy. There is increasing evidence for a complex interaction between genetic polymorphisms and environmental factors that will require very large population studies to unravel. Novel genes that have been associated with asthma, including ADAM-33, and DPP-10, have also been identified by positional cloning, but their function in disease pathogenesis is not yet clear. Recent genome-wide association studies have identified further novel genes, such as ORMDL3, although their functional role is not yet clear. Genetic polymorphisms may also be important in determining the response to asthma therapy. For example, the Arg-Gly-16 variant in the β2-receptor has been associated with reduced response to β2-agonists, and repeats of an Sp1 recognition sequence in the promoter region of 5-lipoxygenase may affect the response to antileukotrienes. However, these effects are small and inconsistent and do not yet have any implications for asthma therapy.
Genetic Predisposition The familial association of asthma and a high degree of concordance for asthma in identical twins indicate a genetic predisposition to the disease; however, whether or not the genes predisposing to asthma are similar or in addition to those predisposing to atopy is not yet clear. It now seems likely that different genes may also contribute to asthma specifically, and there is increasing evidence that the severity of asthma is also genetically determined. Genetic screens with classical linkage analysis and single-nucleotide polymorphisms of various candidate genes indicate that asthma is polygenic, with each gene identified having a small effect that is often not replicated in different populations. This observation suggests that the interaction of many genes is important, and these may differ in different populations. The most consistent findings have been associations with polymorphisms of genes on chromosome 5q, including the T helper 2 (TH2) cells interleukin (IL)-4, IL-5, IL-9, and IL-13, which are associated with atopy. There is increasing evidence for a complex interaction between genetic polymorphisms and environmental factors that will require very large population studies to unravel. Novel genes that have been associated with asthma, including ADAM-33, and DPP-10, have also been identified by positional cloning, but their function in disease pathogenesis is not yet clear. Recent genome-wide association studies have identified further novel genes, such as ORMDL3, although their functional role is not yet clear. Genetic polymorphisms may also be important in determining the response to asthma therapy. For example, the Arg-Gly-16 variant in the β2-receptor has been associated with reduced response to β2-agonists, and repeats of an Sp1 recognition sequence in the promoter region of 5-lipoxygenase may affect the response to antileukotrienes. However, these effects are small and inconsistent and do not yet have any implications for asthma therapy.
It is likely that environmental factors in early life determine which atopic individuals become asthmatic. The increasing prevalence of asthma, particularly in developing countries, over the last few decades also indicates the importance of environmental mechanisms interacting with a genetic predisposition.
Infections Although viral infections (especially rhinovirus) are common triggers of asthma exacerbations, it is uncertain whether they play a role in etiology. There is some association between respiratory syncytial virus infection in infancy and the development of asthma, but the specific pathogenesis is difficult to elucidate because this infection is very common in children. Atypical bacteria, such as Mycoplasma and Chlamydophila, have been implicated in the mechanism of severe asthma, but thus far, the evidence is not very convincing of a true association.
The observation that allergic sensitization and asthma were less common in children with older siblings first suggested that lower levels of infection may be a factor in affluent societies that increase the risks of asthma. This “hygiene hypothesis” proposes that lack of infections in early childhood preserves the TH2 cell bias at birth, whereas exposure to infections and endotoxin results in a shift toward a predominant protective TH1 immune response. Children brought up on farms who are exposed to a high level of endotoxin are less likely to develop allergic sensitization than children raised on dairy farms. Intestinal parasite infection, such as hookworm, may also be associated with a reduced risk of asthma. Although there is considerable epidemiologic support for the hygiene hypothesis, it cannot account for the parallel increase in TH1-driven diseases such as diabetes mellitus over the same period.
Diet The role of dietary factors is controversial. Observational studies have shown that diets low in antioxidants such as vitamin C and vitamin A, magnesium, selenium, and omega-3 polyunsaturated fats (fish oil) or high in sodium and omega-6 polyunsaturated fats are associated with an increased risk of asthma. Vitamin D deficiency may also predispose to the development of asthma. However, interventional studies with supplementary diets have not supported an important role for these dietary factors. Obesity is also an independent risk factor for asthma, particularly in women, but the mechanisms are thus far unknown.
Air Pollution Air pollutants, such as sulfur dioxide, ozone, and diesel particulates, may trigger asthma symptoms, but the role of different air pollutants in the etiology of the disease is much less certain. Most evidence argues against an important role for air pollution because asthma is no more prevalent in cities with a high ambient level of traffic pollution than in rural areas with low levels of pollution. Asthma had a much lower prevalence in East Germany compared to West Germany despite a much higher level of air pollution, but since reunification, these differences have decreased as eastern Germany has become more affluent. Indoor air pollution may be more important with exposure to nitrogen oxides from cooking stoves and exposure to passive cigarette smoke. There is some evidence that maternal smoking is a risk factor for asthma, but it is difficult to dissociate this association from an increased risk of respiratory infections.
Allergens Inhaled allergens are common triggers of asthma symptoms and have also been implicated in allergic sensitization. Exposure to house dust mites in early childhood is a risk factor for allergic sensitization and asthma, but rigorous allergen avoidance has not shown any evidence for a reduced risk of developing asthma. The increase in house dust mites in centrally heated poorly ventilated homes with fitted carpets has been implicated in the increasing prevalence of asthma in affluent countries. Domestic pets, particularly cats, have also been associated with allergic sensitization, but early exposure to cats in the home may be protective through the induction of tolerance.
Occupational Exposure Occupational asthma is relatively common and may affect up to 10% of young adults. Over 300 sensitizing agents have been identified. Chemicals such as toluene diisocyanate and trimellitic anhydride, may lead to sensitization independent of atopy. Individuals may also be exposed to allergens in the workplace such as small animal allergens in laboratory workers and fungal amylase in wheat flour in bakers. Occupational asthma may be suspected when symptoms improve during weekends and holidays.
Obesity Asthma occurs more frequently in obese people (body mass index >30 kg/m2) and is often more difficult to control. Although mechanical factors may contribute, it may also be linked to the proinflammatory adipokines and reduced anti-inflammatory adipokines that are released from fat stores.
Other Factors Several other factors have been implicated in the etiology of asthma, including lower maternal age, duration of breast-feeding, prematurity and low birthweight, and inactivity, but are unlikely to contribute to the recent global increase in asthma prevalence. There is also an association with acetaminophen (paracetamol) consumption in childhood, which may be linked to increased oxidative stress.
Intrinsic Asthma A minority of asthmatic patients (approximately 10%) have negative skin tests to common inhalant allergens and normal serum concentrations of IgE. These patients, with nonatopic or intrinsic asthma, usually show later onset of disease (adult-onset asthma), commonly have concomitant nasal polyps, and may be aspirin-sensitive. They usually have more severe, persistent asthma. Little is understood about mechanism, but the immunopathology in bronchial biopsies and sputum appears to be identical to that found in atopic asthma. There is recent evidence for increased local production of IgE in the airways, suggesting that there may be common IgE-mediated mechanisms; staphylococcal enterotoxins, which serve as “superantigens,” have been implicated.
Asthma Triggers Several stimuli trigger airway narrowing, wheezing, and dyspnea in asthmatic patients. Although a previous view held that these stimuli should be avoided, the triggering of asthma by these stimuli is now seen as evidence for poor control and an indicator of the need to increase controller (preventive) therapy.
ALLERGENS Inhaled allergens activate mast cells with bound IgE directly leading to the immediate release of bronchoconstrictor mediators, resulting in the early response that is reversed by bronchodilators. Often, experimental allergen challenge is followed by a late response when there is airway edema and an acute inflammatory response with increased eosinophils and neutrophils that are not very reversible with bronchodilators. The most common allergens to trigger asthma are Dermatophagoides species, and environmental exposure leads to low-grade chronic symptoms that are perennial. Other perennial allergens are derived from cats and other domestic pets, as well as cockroaches. Other allergens, including grass pollen, ragweed, tree pollen, and fungal spores, are seasonal. Pollens usually cause allergic rhinitis rather than asthma, but in thunderstorms, the pollen grains are disrupted and the particles that may be released can trigger severe asthma exacerbations (thunderstorm asthma).
VIRUS INFECTIONS Upper respiratory tract virus infections such as rhinovirus, respiratory syncytial virus, and coronavirus are the most common triggers of acute severe exacerbations and may invade epithelial cells of the lower as well as the upper airways. The mechanism whereby these viruses cause exacerbations is poorly understood, but there is an increase in airway inflammation with increased numbers of eosinophils and neutrophils. There is evidence for reduced production of type I interferons by epithelial cells from asthmatic patients, resulting in increased susceptibility to these viral infections and a greater inflammatory response.
PHARMACOLOGIC AGENTS Several drugs may trigger asthma. Beta-adrenergic blockers commonly acutely worsen asthma, and their use may be fatal. The mechanisms are not clear, but are likely mediated through increased cholinergic bronchoconstriction. All β blockers need to be avoided, and even selective β2 blockers or topical application (e.g., timolol eye drops) may be dangerous. Angiotensin-converting enzyme inhibitors are theoretically detrimental as they inhibit breakdown of kinins, which are bronchoconstrictors; however, they rarely worsen asthma, and the characteristic cough is no more frequent in asthmatics than in nonasthmatics. Aspirin may worsen asthma in some patients (aspirin-sensitive asthma is discussed below under “Special Considerations”).
EXERCISE Exercise is a common trigger of asthma, particularly in children. The mechanism is linked to hyperventilation, which results in increased osmolality in airway lining fluid and triggers mast cell mediator release, resulting in bronchoconstriction. Exercise-induced asthma (EIA) typically begins after exercise has ended and resolves spontaneously within about 30 min. EIA is worse in cold, dry climates than in hot, humid conditions. It is, therefore, more common in sports activities such as cross-country running in cold weather, overland skiing, and ice hockey than in swimming. It may be prevented by prior administration of β2-agonists and antileukotrienes, but is best prevented by regular treatment with ICSs, which reduce the population of surface mast cells required for this response.
PHYSICAL FACTORS Cold air and hyperventilation may trigger asthma through the same mechanisms as exercise. Laughter may also be a trigger. Many patients report worsening of asthma in hot weather and when the weather changes. Some asthmatics become worse when exposed to strong smells or perfumes, but the mechanism of this response is uncertain.
FOOD AND DIET There is little evidence that allergic reactions to food lead to increased asthma symptoms, despite the belief of many patients that their symptoms are triggered by particular food constituents. Exclusion diets are usually unsuccessful at reducing the frequency of episodes. Some foods such as shellfish and nuts may induce anaphylactic reactions that may include wheezing. Patients with aspirin-induced asthma may benefit from a salicylate-free diet, but these are difficult to maintain. Certain food additives may trigger asthma. Metabisulfite, which is used as a food preservative, may trigger asthma through the release of sulfur dioxide gas in the stomach. Tartrazine, a yellow food-coloring agent, was believed to be a trigger for asthma, but there is little convincing evidence for this.
AIR POLLUTION Increased ambient levels of sulfur dioxide, ozone, and nitrogen oxides are associated with increased asthma symptoms.
OCCUPATIONAL FACTORS Several substances found in the workplace may act as sensitizing agents, as discussed above, but may also act as triggers of asthma symptoms. Occupational asthma is characteristically associated with symptoms at work with relief on weekends and holidays. If removed from exposure within the first 6 months of symptoms, there is usually complete recovery. More persistent symptoms lead to irreversible airway changes, and thus, early detection and avoidance are important.
HORMONES Some women show premenstrual worsening of asthma, which can occasionally be very severe. The mechanisms are not completely understood, but are related to a fall in progesterone and in severe cases may be improved by treatment with high doses of progesterone or gonadotropin-releasing factors. Thyrotoxicosis and hypothyroidism can both worsen asthma, although the mechanisms are uncertain.
GASTROESOPHAGEAL REFLUX Gastroesophageal reflux is common in asthmatic patients because it is increased by bronchodilators. Although acid reflux might trigger reflex bronchoconstriction, it rarely causes asthma symptoms, and antireflux therapy usually fails to reduce asthma symptoms in most patients.
STRESS Many asthmatics report worsening of symptoms with stress. Psychological factors can induce bronchoconstriction through cholinergic reflex pathways. Paradoxically, very severe stress such as bereavement usually does not worsen, and may even improve, asthma symptoms.
PATHOPHYSIOLOGY
Asthma is associated with a specific chronic inflammation of the mucosa of the lower airways. One of the main aims of treatment is to reduce this inflammation.
Pathology The pathology of asthma has been revealed through examining the lungs of patients who have died of asthma and from bronchial biopsies. The airway mucosa is infiltrated with activated eosinophils and T lymphocytes, and there is activation of mucosal mast cells. The degree of inflammation is poorly related to disease severity and may even be found in atopic patients without asthma symptoms. This inflammation is usually reduced by treatment with ICS. There are also structural changes in the airways (described as remodeling). A characteristic finding is thickening of the basement membrane due to subepithelial collagen deposition. This feature is also found in patients with eosinophilic bronchitis presenting as cough who do not have asthma and is, therefore, likely to be a marker of eosinophilic inflammation in the airway as eosinophils release fibrogenic mediators. The epithelium is often shed or friable, with reduced attachments to the airway wall and increased numbers of epithelial cells in the lumen. The airway wall itself may be thickened and edematous, particularly in fatal asthma. Another common finding in fatal asthma is occlusion of the airway lumen by a mucous plug, which is comprised of mucous glycoproteins secreted from goblet cells and plasma proteins from leaky bronchial vessels (Fig. 309-1). There is also vasodilation and increased numbers of blood vessels (angiogenesis). Direct observation by bronchoscopy indicates that the airways may be narrowed, erythematous, and edematous. The pathology of asthma is remarkably uniform in different phenotypes of asthma, including atopic (extrinsic), nonatopic (intrinsic), occupational, aspirin-sensitive, and pediatric asthma. These pathologic changes are found in all airways, but do not extend to the lung parenchyma; peripheral airway inflammation is found particularly in patients with severe asthma. The involvement of airways may be patchy, and this is consistent with bronchographic findings of uneven narrowing of the airways.
FIGURE 309-1 Histopathology of a small airway in fatal asthma. The lumen is occluded with a mucous plug, there is goblet cell metaplasia, and the airway wall is thickened, with an increase in basement membrane thickness and airway smooth muscle. (Courtesy of Dr. J. Hogg, University of British Colombia.)
Airway Inflammation There is inflammation in the respiratory mucosa from the trachea to terminal bronchioles, but with a predominance in the bronchi (cartilaginous airways); however, it is still uncertain as to how inflammatory cells interact and how inflammation translates into the symptoms of asthma (Fig. 309-2). There is good evidence that the specific pattern of airway inflammation in asthma is associated with airway hyperresponsiveness (AHR), the physiologic abnormality of asthma, which is correlated with variable airflow obstruction. The pattern of inflammation in asthma is characteristic of allergic diseases, with similar inflammatory cells seen in the nasal mucosa in rhinitis. However, an indistinguishable pattern of inflammation is found in intrinsic asthma, and this may reflect local rather than systemic IgE production. Although most attention has focused on the acute inflammatory changes seen in asthma, this is a chronic condition, with inflammation persisting over many years in most patients. The mechanisms involved in persistence of inflammation in asthma are still poorly understood. Superimposed on this chronic inflammatory state are acute inflammatory episodes, which correspond to exacerbations of asthma. Although the common pattern of inflammation in asthma is characterized by eosinophil infiltration, some patients with severe asthma show a neutrophilic pattern of inflammation that is less sensitive to corticosteroids. However, many inflammatory cells are involved in asthma with no key cell that is predominant (Fig. 309-3).
FIGURE 309-2 Inflammation in the airways of asthmatic patients leads to airway hyperresponsiveness and symptoms. SO2, sulfur dioxide.
FIGURE 309-3 The pathophysiology of asthma is complex with participation of several interacting inflammatory cells, which result in acute and chronic inflammatory effects on the airway.
MAST CELLS Mast cells are important in initiating the acute bronchoconstrictor responses to allergens and several other indirectly acting stimuli, such as exercise and hyperventilation (via osmolality changes), as well as fog. Activated mucosal mast cells are found at the airway surface in asthma patients and also in the airway smooth-muscle layer, whereas this is not seen in normal subjects or patients with eosinophilic bronchitis. Mast cells are activated by allergens through an IgE-dependent mechanism, and binding of specific IgE to mast cells renders them more sensitive to activation by physical stimuli such as osmolality. The importance of IgE in the pathophysiology of asthma has been highlighted by clinical studies with humanized anti-IgE antibodies, which inhibit IgE-mediated effects, reduce asthma symptoms, and reduce exacerbations. There are, however, uncertainties about the role of mast cells in more chronic allergic inflammatory events. Mast cells release several bronchoconstrictor mediators, including histamine, prostaglandin D2, and cysteinyl-leukotrienes, but also several cytokines, chemokines, growth factors, and neurotrophins.
MACROPHAGES AND DENDRITIC CELLS Macrophages, which are derived from blood monocytes, may traffic into the airways in asthma and may be activated by allergens via low-affinity IgE receptors (FcεRII). Macrophages have the capacity to initiate a type of inflammatory response via the release of a certain pattern of cytokines, but these cells also release anti-inflammatory mediators (e.g., IL-10), and thus, their roles in asthma are uncertain. Dendritic cells are specialized macrophage-like cells in the airway epithelium, which are the major antigen-presenting cells. Dendritic cells take up allergens, process them to peptides, and migrate to local lymph nodes where they present the allergenic peptides to uncommitted T lymphocytes to program the production of allergen-specific T cells. Immature dendritic cells in the respiratory tract promote TH2 cell differentiation and require cytokines, such as IL-12 and tumor necrosis factor α (TNF-α), to promote the normally preponderant TH1 response. The cytokine thymic stromal lymphopoietin (TSLP) released from epithelial cells in asthmatic patients instructs dendritic cells to release chemokines that attract TH2 cells into the airways.
EOSINOPHILS Eosinophil infiltration is a characteristic feature of asthmatic airways. Allergen inhalation results in a marked increase in activated eosinophils in the airways at the time of the late reaction. Eosinophils are linked to the development of AHR through the release of basic proteins and oxygen-derived free radicals. Eosinophil recruitment involves adhesion of eosinophils to vascular endothelial cells in the airway circulation due to interaction between adhesion molecules, migration into the submucosa under the direction of chemokines, and their subsequent activation and prolonged survival. Blocking antibodies to IL-5 causes a profound and prolonged reduction in circulating and sputum eosinophils, but is not associated with reduced AHR or asthma symptoms, although in selected patients with steroid-resistant airway eosinophils, there is a reduction in exacerbations. Eosinophils may be important in release of growth factors involved in airway remodeling and in exacerbations but probably not in AHR.
NEUTROPHILS Increased numbers of activated neutrophils are found in sputum and airways of some patients with severe asthma and during exacerbations, although there is a proportion of patients even with mild or moderate asthma who have a predominance of neutrophils. The roles of neutrophils in asthma that are resistant to the anti-inflammatory effects of corticosteroids are currently unknown.
T LYMPHOCYTES T lymphocytes play a very important role in coordinating the inflammatory response in asthma through the release of specific patterns of cytokines, resulting in the recruitment and survival of eosinophils and in the maintenance of a mast cell population in the airways. The naïve immune system and the immune system of asthmatics are skewed to express the TH2 phenotype, whereas in normal airways, TH1 cells predominate. TH2 cells, through the release of IL-5, are associated with eosinophilic inflammation and, through the release of IL-4 and IL-13, are associated with increased IgE formation. Recently, bronchial biopsies have demonstrated a preponderance of natural killer CD4+ T lymphocytes that express high levels of IL-4. Regulatory T cells play an important role in determining the expression of other T cells, and there is evidence for a reduction in a certain subset of regulatory T cells (CD4+CD25+) in asthma that is associated with increased TH2 cells. Recently, innate T cells (ILC2) without T cell receptors have been identified that release TH2 cytokines and are regulated by epithelial cytokines, such as IL-25 and IL-33.
STRUCTURAL CELLS Structural cells of the airways, including epithelial cells, fibroblasts, and airway smooth-muscle cells, are also important sources of inflammatory mediators, such as cytokines and lipid mediators, in asthma. Indeed, because structural cells far outnumber inflammatory cells, they may become the major sources of mediators driving chronic inflammation in asthmatic airways. In addition, epithelial cells may have key roles in translating inhaled environmental signals into an airway inflammatory response and are probably major target cells for ICS.
Inflammatory Mediators Multiple inflammatory mediators have been implicated in asthma, and they may have a variety of effects on the airways that account for the pathologic features of asthma (Fig. 309-4). Mediators such as histamine, prostaglandin D2, and cysteinyl-leukotrienes contract airway smooth muscle, increase microvascular leakage, increase airway mucus secretion, and attract other inflammatory cells. Because each mediator has many effects, the role of individual mediators in the pathophysiology of asthma is not yet clear. Although the multiplicity of mediators makes it unlikely that preventing the synthesis or action of a single mediator will have a major impact in clinical asthma, recent clinical studies with antileukotrienes suggest that cysteinyl-leukotrienes have clinically important effects.
FIGURE 309-4 Many cells and mediators are involved in asthma and lead to several effects on the airways. AHR, airway hyperresponsiveness; PAF, platelet-activating factor.
CYTOKINES Multiple cytokines regulate the chronic inflammation of asthma. The TH2 cytokines IL-4, IL-5, and IL-13 mediate allergic inflammation, whereas proinflammatory cytokines, such as TNF-α and IL-1β, amplify the inflammatory response and play a role in more severe disease. TSLP is an upstream cytokine released from epithelial cells of asthmatics that orchestrates the release of chemokines that selectively attract TH2 cells. Some cytokines such as IL-10 and IL-12 are anti-inflammatory and may be deficient in asthma.
CHEMOKINES Chemokines are involved in attracting inflammatory cells from the bronchial circulation into the airways. Eotaxin (CCL11) is selectively attractant to eosinophils via CCR3 and is expressed by epithelial cells of asthmatics, whereas CCL17 (TARC) and CCL22 (MDC) from epithelial cells attract TH2 cells via CCR4 (Fig. 309-5).
FIGURE 309-5 T lymphocytes in asthma. Allergen interacts with dendritic cells and releases thymus stimulated lymphopoeitin (TSLP), which stimulates activated dendritic cells to release the chemokines CCL17 and CCL22, which attract T helper 2 (TH2) lymphocytes. Allergens and viral infection may release interleukin (IL)-25 and -33, which recruit and activate type 2 innate lymphoid cells (ILC2). Both TH2 and ILC2 cells release IL-5 and epithelial cells release CCL11 (eotaxin), which together lead to recruitment of eosinophils into the airways.
OXIDATIVE STRESS Activated inflammatory cells such as macrophages and eosinophils produce reactive oxygen species. Evidence for increased oxidative stress in asthma is provided by the increased concentrations of 8-isoprostane (a product of oxidized arachidonic acid) in exhaled breath condensates and increased ethane (a product of lipid peroxidation) in the expired air of asthmatic patients. Increased oxidative stress is related to disease severity, may amplify the inflammatory response, and may reduce responsiveness to corticosteroids.
NITRIC OXIDE Nitric oxide (NO) is produced by NO synthases in several cells in the airway, particularly airway epithelial cells and macrophages. The level of NO in the expired air of patients with asthma is higher than normal and is related to the eosinophilic inflammation. Increased NO may contribute to the bronchial vasodilation observed in asthma. Fractional exhaled NO (FENO) is increasingly used in the diagnosis and monitoring of asthmatic inflammation, although it is not yet used routinely in clinical practice.
TRANSCRIPTION FACTORS Proinflammatory transcription factors, such as nuclear factor-κB (NF-κB) and activator protein-1, are activated in asthmatic airways and orchestrate the expression of multiple inflammatory genes. More specific transcription factors that are involved include nuclear factor of activated T cells and GATA-3, which regulate the expression of TH2 cytokines in T cells.
Effects of Inflammation The chronic inflammatory response has several effects on the target cells of the airways, resulting in the characteristic pathophysiologic and remodeling changes associated with asthma. Asthma may be regarded as a disease with continuous inflammation and repair proceeding simultaneously, although the relationship between chronic inflammatory processes and asthma symptoms is often obscure.
AIRWAY EPITHELIUM Airway epithelial shedding may be important in contributing to AHR and may explain how several mechanisms, such as ozone exposure, virus infections, chemical sensitizers, and allergens (usually proteases), can lead to its development, as all of these stimuli may lead to epithelial disruption. Epithelial damage may contribute to AHR in a number of ways, including loss of its barrier function to allow penetration of allergens; loss of enzymes (such as neutral endopeptidase) that degrade certain peptide inflammatory mediators; loss of a relaxant factor (so called epithelial-derived relaxant factor); and exposure of sensory nerves, which may lead to reflex neural effects on the airway.
FIBROSIS In all asthmatic patients, the basement membrane is apparently thickened due to subepithelial fibrosis with deposition of types III and V collagen below the true basement membrane and is associated with eosinophil infiltration, presumably through the release of profibrotic mediators such as transforming growth factor-β. Mechanical manipulations can alter the phenotype of airway epithelial cells in a profibrotic fashion. In more severe patients, there is also fibrosis within the airway wall, which may contribute to irreversible narrowing of the airways.
AIRWAY SMOOTH MUSCLE In vitro airway smooth muscle from asthmatic patients usually shows no increased responsiveness to constrictors. Reduced responsiveness to β-agonists has also been reported in postmortem or surgically removed bronchi from asthmatics, although the number of β-receptors is not reduced, suggesting that β-receptors have been uncoupled. These abnormalities of airway smooth muscle may be secondary to the chronic inflammatory process. Inflammatory mediators may modulate the ion channels that serve to regulate the resting membrane potential of airway smooth-muscle cells, thus altering the level of excitability of these cells. In asthmatic airways there is also a characteristic hypertrophy and hyperplasia of airway smooth muscle, which is presumably the result of stimulation of airway smooth-muscle cells by various growth factors such as platelet-derived growth factor (PDGF) or endothelin-1 released from inflammatory or epithelial cells.
VASCULAR RESPONSES There is increased airway mucosal blood flow in asthma, which may contribute to airway narrowing. There is an increase in the number of blood vessels in asthmatic airways as a result of angiogenesis in response to growth factors, particularly vascular endothelial growth factor. Microvascular leakage from postcapillary venules in response to inflammatory mediators is observed in asthma, resulting in airway edema and plasma exudation into the airway lumen.
MUCUS HYPERSECRETION Increased mucus secretion contributes to the viscid mucous plugs that occlude asthmatic airways, particularly in fatal asthma. There is hyperplasia of submucosal glands that are confined to large airways and of increased numbers of epithelial goblet cells. IL-13 induces mucus hypersecretion in experimental models of asthma.
NEURAL REGULATION Various defects in autonomic neural control may contribute to AHR in asthma, but these are likely to be secondary to the disease, rather than primary defects. Cholinergic pathways, through the release of acetylcholine acting on muscarinic receptors, cause bronchoconstriction and may be activated reflexly in asthma. Inflammatory mediators may activate sensory nerves, resulting in reflex cholinergic bronchoconstriction or release of inflammatory neuropeptides. Inflammatory products may also sensitize sensory nerve endings in the airway epithelium such that the nerves become hyperalgesic. Neurotrophins, which may be released from various cell types in airways, including epithelial cells and mast cells, may cause proliferation and sensitization of airway sensory nerves. Airway nerves may also release neurotransmitters, such as substance P, which have inflammatory effects.
Airway Remodeling Several changes in the structure of the airway are characteristically found in asthma, and these may lead to irreversible narrowing of the airways. Population studies have shown a greater decline in lung function over time than in normal subjects; however, most patients with asthma preserve normal or near-normal lung function throughout life if appropriately treated.
The accelerated decline in lung function occurs in a smaller proportion of asthmatics, and these are usually patients with more severe disease. There is some evidence that the early use of ICS may reduce the decline in lung function. The characteristic structural changes are increased airway smooth muscle, fibrosis, angiogenesis, and mucus hyperplasia.
Physiology Limitation of airflow is due mainly to bronchoconstriction, but airway edema, vascular congestion, and luminal occlusion with exudate may contribute. This results in a reduction in forced expiratory volume in 1 second (FEV1), FEV1/forced vital capacity (FVC) ratio, and peak expiratory flow (PEF), as well as an increase in airway resistance. Early closure of peripheral airway results in lung hyperinflation (air trapping) and increased residual volume, particularly during acute exacerbations and in severe persistent asthma. In more severe asthma, reduced ventilation and increased pulmonary blood flow result in mismatching of ventilation and perfusion and in bronchial hyperemia. Ventilatory failure is very uncommon, even in patients with severe asthma, and arterial PCO2 tends to be low due to increased ventilation.
Airway Hyperresponsiveness AHR is the characteristic physiologic abnormality of asthma and describes the excessive bronchoconstrictor response to multiple inhaled triggers that would have no effect on normal airways. The increase in AHR is linked to the frequency of asthma symptoms, and, thus, an important aim of therapy is to reduce AHR. Increased bronchoconstrictor responsiveness is seen with direct bronchoconstrictors such as histamine and methacholine, which contract airway smooth muscle, but is characteristically also seen with many indirect stimuli, which release bronchoconstrictors from mast cells or activate sensory nerves. Most of the triggers for asthma symptoms appear to act indirectly, including allergens, exercise, hyperventilation, fog (via mast cell activation), irritant dusts, and sulfur dioxide (via a cholinergic reflex).
CLINICAL FEATURES AND DIAGNOSIS
The characteristic symptoms of asthma are wheezing, dyspnea, and coughing, which are variable, both spontaneously and with therapy. Symptoms may be worse at night, and patients typically awake in the early morning hours. Patients may report difficulty in filling their lungs with air. There is increased mucus production in some patients, with typically tenacious mucus that is difficult to expectorate. There may be increased ventilation and use of accessory muscles of ventilation. Prodromal symptoms may precede an attack, with itching under the chin, discomfort between the scapulae, or inexplicable fear (impending doom).
Typical physical signs are inspiratory, and to a greater extent expiratory, rhonchi throughout the chest, and there may be hyperinflation. Some patients, particularly children, may present with a predominant nonproductive cough (cough-variant asthma). There may be no abnormal physical findings when asthma is under control.
DIAGNOSIS
The diagnosis of asthma is usually apparent from the symptoms of variable and intermittent airways obstruction, but must be confirmed by objective measurements of lung function.
Lung Function Tests Simple spirometry confirms airflow limitation with a reduced FEV1, FEV1/FVC ratio, and PEF (Fig. 309-6). Reversibility is demonstrated by a >12% and 200-mL increase in FEV1 15 min after an inhaled short-acting β2-agonist or in some patients by a 2- to 4-week trial of oral corticosteroids (OCS) (prednisone or prednisolone 30–40 mg daily). Measurements of PEF twice daily may confirm the diurnal variations in airflow obstruction. Flow-volume loops show reduced peak flow and reduced maximum expiratory flow. Further lung function tests are rarely necessary, but whole-body plethysmography shows increased airway resistance and may show increased total lung capacity and residual volume. Gas diffusion is usually normal, but there may be a small increase in gas transfer in some patients.
FIGURE 309-6 Spirometry and flow-volume loop in asthmatic compared to normal subject. There is a reduction in forced expiratory volume in 1 second (FEV1) but less reduction in forced vital capacity (FVC), giving a reduced FEV1/FVC ratio (<70%). The flow-volume loop shows reduced peak expiratory flow and a typical scalloped appearance indicating widespread airflow obstruction.
Airway Responsiveness The increased AHR is normally measured by methacholine or histamine challenge with calculation of the provocative concentration that reduces FEV1 by 20% (PC20). This is rarely useful in clinical practice, but can be used in the differential diagnosis of chronic cough and when the diagnosis is in doubt in the setting of normal pulmonary function tests. Occasionally exercise testing is done to demonstrate the postexercise bronchoconstriction if there is a predominant history of EIA. Allergen challenge is rarely necessary and should only be undertaken by a specialist if specific occupational agents are to be identified.
Hematologic Tests Blood tests are not usually helpful. Total serum IgE and specific IgE to inhaled allergens (radioallergosorbent test [RAST]) may be measured in some patients.
Imaging Chest roentgenography is usually normal but in more severe patients may show hyperinflated lungs. In exacerbations, there may be evidence of a pneumothorax. Lung shadowing usually indicates pneumonia or eosinophilic infiltrates in patients with bronchopulmonary aspergillosis. High-resolution computed tomography (CT) may show areas of bronchiectasis in patients with severe asthma, and there may be thickening of the bronchial walls, but these changes are not diagnostic of asthma.
Skin Tests Skin prick tests to common inhalant allergens (house dust mite, cat fur, grass pollen) are positive in allergic asthma and negative in intrinsic asthma, but are not helpful in diagnosis. Positive skin responses may be useful in persuading patients to undertake allergen avoidance measures.
Exhaled Nitric Oxide FENO is now being used as a noninvasive test to measure airway inflammation. The typically elevated levels in asthma are reduced by ICS, so this may be a test of compliance with therapy. It may also be useful in demonstrating insufficient anti-inflammatory therapy and may be useful in down-titrating ICS. However, studies in unselected patients have not convincingly demonstrated improved clinical outcomes, and it may be necessary to select patients who are poorly controlled.
Differential Diagnosis It is usually not difficult to differentiate asthma from other conditions that cause wheezing and dyspnea. Upper airway obstruction by a tumor or laryngeal edema can mimic severe asthma, but patients typically present with stridor localized to large airways. The diagnosis is confirmed by a flow-volume loop that shows a reduction in inspiratory as well as expiratory flow, and bronchoscopy to demonstrate the site of upper airway narrowing. Persistent wheezing in a specific area of the chest may indicate endobronchial obstruction with a foreign body. Left ventricular failure may mimic the wheezing of asthma, but basilar crackles are present in contrast to asthma. Vocal chord dysfunction may mimic asthma and is thought to be an hysterical conversion syndrome.
Eosinophilic pneumonias and systemic vasculitis, including Churg-Strauss syndrome and polyarteritis nodosa, may be associated with wheezing. Chronic obstructive pulmonary disease (COPD) is usually easy to differentiate from asthma as symptoms show less variability, never completely remit, and show much less (or no) reversibility to bronchodilators. Approximately 10% of COPD patients have features of asthma, with increased sputum eosinophils and a response to OCSs; these patients probably have both diseases concomitantly.
ACUTE SEVERE ASTHMA
Exacerbations of asthma are feared by patients and may be life threatening. One of the main aims of controller therapy is to prevent exacerbations; in this respect, ICS and combination inhalers are very effective.
Clinical Features Patients are aware of increasing chest tightness, wheezing, and dyspnea that are often not or poorly relieved by their usual reliever inhaler. In severe exacerbations, patients may be so breathless that they are unable to complete sentences and may become cyanotic. Examination usually shows increased ventilation, hyperinflation, and tachycardia. Pulsus paradoxus may be present, but this is rarely a useful clinical sign. There is a marked fall in spirometric values and PEF. Arterial blood gases on air show hypoxemia, and PCO2 is usually low due to hyperventilation. A normal or rising PCO2 is an indication of impending respiratory failure and requires immediate monitoring and therapy. A chest roentgenogram is not usually informative but may show pneumonia or pneumothorax.
SPECIAL CONSIDERATIONS
Refractory Asthma Although most patients with asthma are easily controlled with appropriate medication, a small proportion of patients (approximately 5–10% of asthmatics) are difficult to control despite maximal inhaled therapy. Some of these patients will require maintenance treatment with OCS. In managing these patients, it is important to investigate and correct any mechanisms that may be aggravating asthma. There are two major patterns of difficult asthma: some patients have persistent symptoms and poor lung function, despite appropriate therapy, whereas others may have normal or near-normal lung function but intermittent, severe (sometimes life-threatening) exacerbations.
MECHANISMS The most common reason for poor control of asthma is noncompliance with medication, particularly ICS. Compliance with ICS may be low because patients do not feel any immediate clinical benefit or may be concerned about side effects. Compliance with ICS is difficult to monitor because there are no useful plasma measurements that can be made, but measuring the fractional excretion of induced NO (FENO) may identify the problem. Compliance may be improved by giving the ICS as a combination with a LABA that gives symptom relief. Compliance with OCS may be measured by suppression of plasma cortisol and the expected concentration of prednisone/prednisolone in the plasma. There are several factors that may make asthma more difficult to control, including exposure to high, ambient levels of allergens or unidentified occupational agents. Severe rhinosinusitis may make asthma more difficult to control; upper airway disease should be vigorously treated. Drugs such as beta-adrenergic blockers, aspirin, and other cyclooxygenase (COX) inhibitors may worsen asthma. Some women develop severe premenstrual worsening of asthma, which is unresponsive to corticosteroids and requires treatment with progesterone or gonadotropin-releasing factors. Few systemic diseases make asthma more difficult to control, but hyper- and hypothyroidism may increase asthma symptoms and should be investigated if suspected.
Bronchial biopsy studies in refractory asthma may show the typical eosinophilic pattern of inflammation, whereas others have a predominantly neutrophilic pattern. There may be an increase in TH1 cells, TH17 cells, and CD8 lymphocytes compared to mild asthma and increased expression of TNF-α. Structural changes in the airway, including fibrosis, angiogenesis, and airway smooth-muscle thickening, are more commonly seen in these patients.
Corticosteroid-Resistant Asthma A few patients with asthma show a poor response to corticosteroid therapy and may have various molecular abnormalities that impair the anti-inflammatory action of corticosteroids. Complete resistance to corticosteroids is extremely uncommon and affects less than 1 in 1000 patients. It is defined by a failure to respond to a high dose of oral prednisone/prednisolone (40 mg once daily over 2 weeks), ideally with a 2-week run-in with matched placebo. More common is reduced responsiveness to corticosteroids where control of asthma requires OCS (corticosteroid-dependent asthma). In patients with poor responsiveness to corticosteroids, there is a reduction in the response of circulating monocytes and lymphocytes to the anti-inflammatory effects of corticosteroids in vitro and reduced skin blanching in response to topical corticosteroids. There are several mechanisms that have been described, including an increase in the alternatively spliced form of the glucocorticoid receptor (GR)-β, an abnormal pattern of histone acetylation in response to corticosteroids, a defect in IL-10 production, and a reduction in HDAC2 activity (as in COPD). These observations suggest that there are likely to be heterogeneous mechanisms for corticosteroid resistance; whether these mechanisms are genetically determined has yet to be decided.
Brittle Asthma Some patients show chaotic variations in lung function despite taking appropriate therapy. Some show a persistent pattern of variability and may require oral corticosteroids or, at times, continuous infusion of β2-agonists (type 1 brittle asthma), whereas others have generally normal or near-normal lung function but precipitous, unpredictable falls in lung function that may result in death (type 2 brittle asthma). These latter patients are difficult to manage because they do not respond well to corticosteroids, and the worsening of asthma does not reverse well with inhaled bronchodilators. The most effective therapy is subcutaneous epinephrine, which suggests that the worsening is likely to be a localized airway anaphylactic reaction with edema. In some of these patients, there may be allergy to specific foods. These patients should be taught to self-administer epinephrine and should carry a medical warning accordingly.
Aspirin-Sensitive Asthma A small proportion (1–5%) of asthmatics become worse with aspirin and other COX inhibitors, although this is much more commonly seen in severe cases and in patients with frequent hospital admission. Aspirin-sensitive asthma is a well-defined phenotype of asthma that is usually preceded by perennial rhinitis and nasal polyps in nonatopic patients with a late onset of the disease. Aspirin, even in small doses, characteristically provokes rhinorrhea, conjunctival injection, facial flushing, and wheezing. There is a genetic predisposition to increased production of cysteinyl-leukotrienes with functional polymorphism of cys-leukotriene C4 synthase. Asthma is triggered by COX inhibitors but is persistent even in their absence. All nonselective COX inhibitors should be avoided, but selective COX2 inhibitors are safe to use when an anti-inflammatory analgesic is needed. Aspirin-sensitive asthma responds to usual therapy with ICS. Although antileukotrienes should be effective in these patients, they are no more effective than in allergic asthma. Occasionally, aspirin desensitization is necessary, but this should only be undertaken in specialized centers.
Asthma in the Elderly Asthma may start at any age, including in elderly patients. The principles of management are the same as in other asthmatics, but side effects of therapy may be a problem, including muscle tremor with β2-agonists and more systemic side effects with ICS. Comorbidities are more frequent in this age group, and interactions with drugs such as β2-blockers, COX inhibitors, and agents that may affect theophylline metabolism need to be considered. COPD is more likely in elderly patients and may coexist with asthma. A trial of OCS may be very useful in documenting the steroid responsiveness of asthma.
Pregnancy Approximately one-third of asthmatic patients who are pregnant improve during the course of a pregnancy, one-third deteriorate, and one-third are unchanged. It is important to maintain good control of asthma because poor control may have adverse effects on fetal development. Compliance may be a problem because there is often concern about the effects of antiasthma medications on fetal development. The drugs that have been used for many years in asthma therapy have now been shown to be safe and without teratogenic potential. These drugs include SABA, ICS, and theophylline; there is less safety information about newer classes of drugs such as LABA, antileukotrienes, and anti-IgE. If an OCS is needed, it is better to use prednisone rather than prednisolone because it cannot be converted to the active prednisolone by the fetal liver, thus protecting the fetus from systemic effects of the corticosteroid. There is no contraindication to breast-feeding when patients are using these drugs.
Cigarette Smoking Approximately 20% of asthmatics smoke, which may adversely affect asthma in several ways. Smoking asthmatics have more severe disease, more frequent hospital admissions, a faster decline in lung function, and a higher risk of death from asthma than nonsmoking asthmatics. There is evidence that smoking interferes with the anti-inflammatory actions of corticosteroids by reducing HDAC2, necessitating higher doses for asthma control. Smoking cessation improves lung function and reduces the steroid resistance, and thus, vigorous smoking cessation strategies should be used. Some patients report a temporary worsening of asthma when they first stop smoking, possibly due to the loss of the bronchodilating effect of NO in cigarette smoke.
Surgery If asthma is well controlled, there is no contraindication to general anesthesia and intubation. Patients who are treated with OCS will have adrenal suppression and should be treated with an increased dose of OCS immediately prior to surgery. Patients with FEV1 <80% of their normal levels should also be given a boost of OCS prior to surgery. High-maintenance doses of corticosteroids may be a contraindication to surgery because of increased risks of infection and delayed wound healing.
Bronchopulmonary Aspergillosis Bronchopulmonary aspergillosis (BPA) is uncommon and results from an allergic pulmonary reaction to inhaled spores of Aspergillus fumigatus and, occasionally, other Aspergillus species. A skin prick test to A. fumigatus is always positive, whereas serum Aspergillus precipitins are low or undetectable. Characteristically, there are fleeting eosinophilic infiltrates in the lungs, particularly in the upper lobes. Airways become blocked with mucoid plugs rich in eosinophils, and patients may cough up brown plugs and have hemoptysis. BPA may result in bronchiectasis, particularly affecting central airways, if not suppressed by corticosteroids. Asthma is controlled in the usual way by ICS, but it is necessary to give a course of OCS if any sign of worsening or pulmonary shadowing is found. Treatment with the oral antifungal itraconazole is beneficial in preventing exacerbations.