Applied anatomy of the lumbar spine
Human posture
The human spine is a self-supporting construction of skeleton, cartilage, ligaments and muscles. Erect, there are four sagittal curves, which are the result of man’s evolution from quadruped to biped. This began in Africa 3 million years ago with Homo australo-pithecus, which had a pelvis strong enough to support an erect posture. After a further 1.5 million years, the definitive erect posture had been adopted – Homo erectus.1 The four curves resulted: cervical lordosis, thoracic kyphosis, lumbar lordosis and sacrococcygeal kyphosis. This S form seems to be a compromise between the static and the dynamic qualities of the spine2; theoretical considerations suggest that the S form is the shape an elastic bar adopts when it is subjected to axial compression.3
The phylogenetic evolution from the large thoracolumbar kyphotic spine of a quadruped into two kyphotic and lordotic curves is also reflected in the spine’s ontogeny. In intrauterine life and during the first 5 months after birth, the spinal curves are absent and there is only one slight kyphosis of the whole spine. At 13 months the lumbar spine is straight, at 3 years some lumbar lordosis is present, and by 8 years the lumbar spine has attained its normal adult posture (Fig. 31.1).4
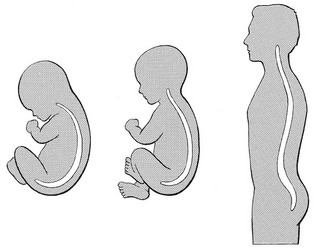
Fig 31.1 Development of the lumbar lordosis.
The development of the erect posture is recent and it seems that, apart from the compensatory lordosis, not much in the way of structural adaptation has taken place. Comparative anatomical evidence suggests that the spine has evolved as a hanging structure between the anterior and the posterior parts of the body. However, during development from quadruped to biped, the function of the spine had to alter completely (Table 31.1); this has serious consequences. In an upright position, the spine is submitted to axial load, which probably leads to the premature disc degeneration from which humans are apt to suffer. In the upright position, the lumbar spine has to resist flexion, whereas the quadruped spine has to resist extension because it is structurally undesirable for a ‘bridge’ to sag in the middle. The change to an upright position, however, has not yet been followed by anatomical adaptation, and the human spine has an anatomy that more readily withstands extension than flexion: the anterior part of the annulus fibrosus is stronger and thicker than the posterior, and the anterior longitudinal ligament is almost twice as thick and broad as the posterior.5
Table 31.1
Comparison of the quadruped and biped lumbar spines
| Quadruped | Biped | |
| Structure | Horizontal | Vertical |
| Load | Horizontal | Axial |
| Curve | Slight kyphosis | Lordosis |
| Strength | Against extension | Against flexion |
| Strong structures | Anterior | (Posterior?) |
Vertebrae
Vertebral bodies
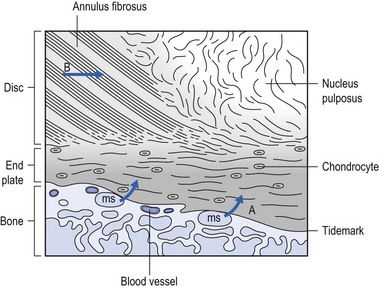
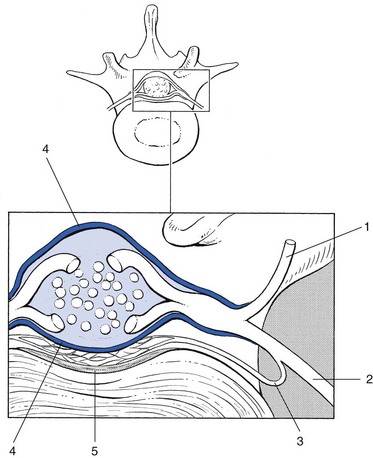
Each vertebral body is more or less a cylinder with a thin cortical shell which surrounds cancellous bone. From L1 to L5, the posterior aspect changes from slightly concave to slightly convex, and the diameter of the cylinder increases gradually because of the increasing loads each body has to carry. At the upper and lower surfaces, two distinct areas can be seen: each is a peripheral ring of compact bone – surrounding and slightly above the level of the flat and rough central zone – which originates from the apophysis and fuses with the vertebral body at the age of about 16. The central zone – the bony endplate – shows many perforations, through which blood vessels can reach the disc. A layer of cartilage covers this central zone, which is limited by the peripheral ring. This is the cartilaginous endplate, forming the transition between the cortical bone and the rest of the intervertebral disc. A sagittal cut through the vertebral body shows the endplates to be slightly concave, which consequently gives the disc a convex form.6
Pedicles
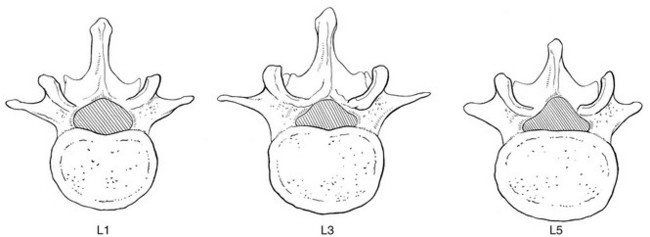
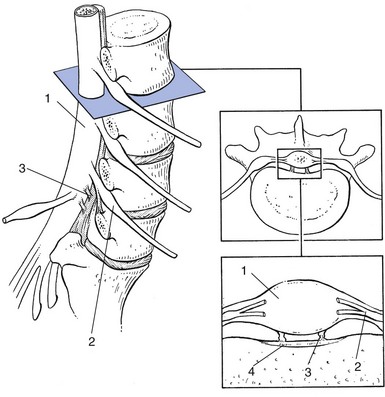
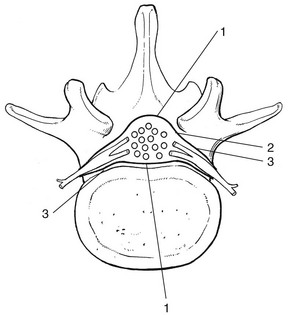
The two pedicles originate posteriorly and attach to the cranial half of the body. Together with the broad and flat lamina, they form the vertebral arch. From L1 to L5, the pedicles become shorter and broader, and are more lateral. This narrows the anteroposterior diameter and widens the transverse diameter of the vertebral canal from above downwards. Together with the increasing convexity of the posterior aspect of the vertebral body, these changes in the position of the pedicles alter the shape of the normal bony spinal canal from an ellipse at L1 to a triangle at L3 and more or less a trefoil at L5 (Fig. 31.2).
Laminae
The part of the lamina between the superior and inferior articular processes is called the ‘pars interlaminaris’. It runs obliquely from the lateral border of the lamina to its upper medial border. This portion of the lamina is subjected to considerable bending forces, as it lies at the junction between the vertically oriented lamina and the horizontally oriented pedicle. This ‘interlaminar part’ will therefore be susceptible to fatigue fractures or stress fractures (spondylolysis) (see Ch. 39).7
Intervertebral discs
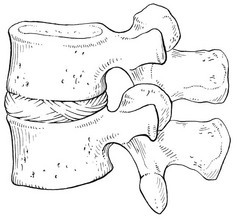
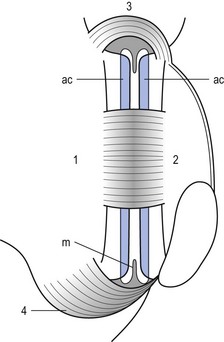
Two adjacent vertebral bodies are linked by an intervertebral disc. Together with the corresponding facet joints, they form the ‘functional unit of Junghans’ (Fig. 31.3).8
Endplates
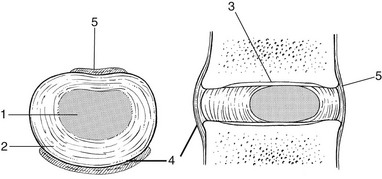
An upper and a lower cartilaginous endplate (each about 0.6–1 mm thick) cover the superior and inferior aspects of the disc. They are plates of cartilage that bind the disc to their respective vertebral bodies. Each endplate covers almost the entire surface of the adjacent vertebral body; only a narrow rim of bone, called the ring apophysis, around the perimeter of the vertebral body is left uncovered by cartilage. That portion of the vertebral body to which the cartilaginous endplate is applied is referred to as the vertebral endplate. The endplate covers the nucleus pulposus in its entirety; peripherally it fails to cover the entire extent of the annulus fibrosus.9 The collagen fibrils of the inner lamellae of the annulus enter the endplate and merge with it, resulting in all aspects of the nucleus being enclosed by a fibrous capsule.10
The endplate permits diffusion and provides the main source of nutrition for the disc.11,12 Up to the age of 8 years, the cartilaginous endplates are penetrated by blood vessels which pass into the peripheral layers of nucleus and annulus. Thereafter, the disc’s nutrition is achieved by diffusion through the endplate. The hyaline endplate is also the last part of the disc to wear through during severe disc degeneration.13
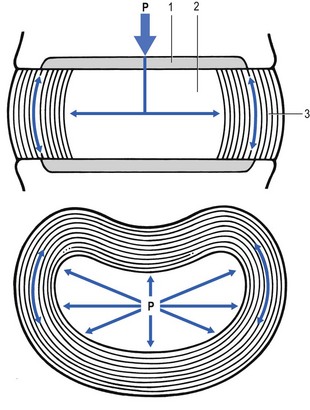
Annulus fibrosus
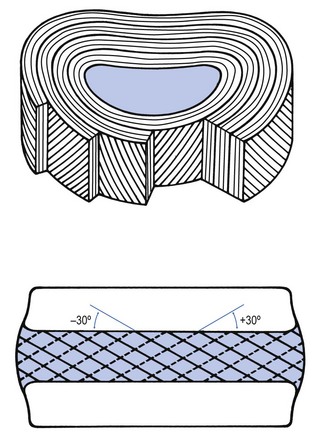
This is made up of 15–25 concentric fibrocartilaginous sheets or ‘lamellae’ (Fig. 31.4), each formed by parallel fibres, running obliquely at a 30° angle between the vertebral bodies.14 Because the fibres of two consecutive layers are oriented in opposite directions, they cross each other at an angle of approximately 120°.15 This arrangement of the annular fibres gives the normal disc great strength against shearing and rotational stresses,16 while angular movements remain perfectly possible.17,18 The outermost fibres are attached directly to bone, around the ring apophysis, and for that reason they are referred to as the ligamentous portion of the annulus fibrosus. The inner third merges with the cartilaginous endplate and is referred to as the capsular portion of the annulus fibrosus (Fig. 31.5).
Nucleus pulposus
This consists of a gelatinous substance, made of a meshwork of collagen fibrils suspended in a mucoprotein base which contains mucopolysaccharides and water.19 With advancing age, the amount of mucopolysaccharides diminishes, as does that of the water they bind. A young nucleus is 85% water, whereas it is only 65% water in the elderly.20 These biological changes are mirrored in the macroscopic aspects of the nucleus. In the second and third decades the nucleus is clear, firm and gelatinous but subsequently it becomes drier and more friable. In the elderly, the nucleus has the texture of thickened cream cheese, and is dry, brownish and friable.
At birth the nucleus pulposus occupies the centre of the intervertebral space. As the anterior part of the vertebral body grows faster than the posterior part, the nucleus comes to lie more posteriorly. Consequently, the anterior part of the annulus will have thicker and stronger fibres,21 which means that the annulus gives better protection against anterior than posterior displacements of the nucleus; this is disadvantageous with respect to the contiguous nerve roots and dura.
Cartilage is devoid of nerves and it has been conventional to draw the same conclusions about the disc. However, over the last few decades, there has been a great deal of research on the possibility that there is some innervation. The presence of free nerve endings has been demonstrated as far as one-third of the way into cadaveric annuli fibrosi,22 and as far as halfway into annuli fibrosi obtained during posterior fusion operations.23 Other research has shown a few nervous elements in the periphery of the annulus fibrosus.24,25
More recent studies have demonstrated mechanoreceptors to be present in the outer two or three lamellae of the human intervertebral disc and the anterior longitudinal ligament.26 Although the presence of substance P – generally accepted as an important nociceptive neurotransmitter – has so far not been demonstrated in human intervertebral discs,27 other neuropeptides have.28,29 The exact relationship between the existence of small nerve endings in the outer layers of the disc and back pain therefore still remains controversial.
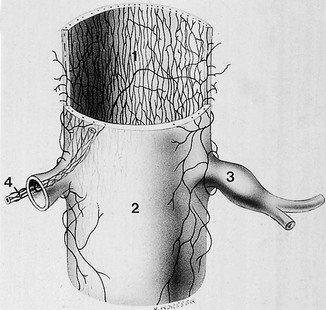
The lack of blood supply to the intervertebral disc has been shown by microangiographic studies.30 There is some vascularization of the vertebral borders of the disc in children but by the age of 8 years all cartilaginous penetration by blood vessels has disappeared. Vascular buds in the bony endplate remain during adulthood as a vascular bed under the cartilaginous endplate, and diffusion from these through the endplates remains the main nutritional pathway for the disc during adult life,31,32 although some nutrition via contact with the intimate anterior and posterior longitudinal ligaments is also possible (Fig. 31.6).33 The disc is thus the largest non-vascular structure in the body, which causes difficulties in healing and regeneration after damage.
Behaviour of the disc
The disc as an osmotic system
The main structural components of the intervertebral disc are collagen, proteoglycans (PGs) and water. The water is not free but is bound by the PGs,34 which, because of their pronounced osmotic properties, maintain the hydration and turgor of the disc. The proportion of the three constituents varies across the disc. Fluid and PG concentrations are highest in the nucleus and lowest in the annulus, whereas the reverse is true for collagen.
Proteoglycans are complex chemical structures, existing as monomer subunits and aggregates. The former are made up of a central protein molecule with an attached long-chain glucosaminoglycan; the latter consist of monomers, attached to a long hyaluronic acid filament (see Ch. 3). The synthesis of PGs is performed by the cartilage cells and is a continuous process, demanding a well-balanced metabolism.35 For its nutrition, the disc, devoid as it is of any penetrating vascular structure, depends entirely on diffusion through the central portion of the endplates and the outer annulus (see Fig. 31.6).11,36 Consequently, the disc is vulnerable and changes in its composition are inevitable as age advances. Although the total collagen content remains fairly constant during adult life,37 the PG concentration falls.38,39 The result is that the osmotic properties and the turgor of the disc will also decline as age advances (see p. 438).40
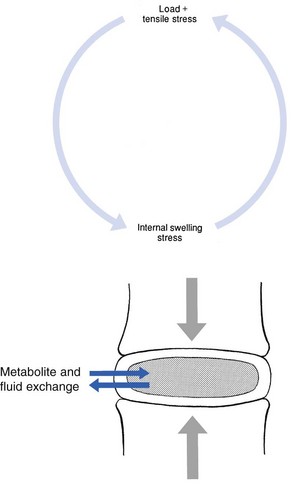
Proteoglycans play a key role in the osmotic system of the intervertebral joint, which incorporates the nucleus, annulus and cartilaginous endplates, and also the cancellous bone of the vertebrae. Two compartments – nucleus and paravertebral tissues – are separated by the semipermeable barrier formed by the cartilaginous endplate and the annulus fibrosus, which permits the transport of small molecules only: water, ions and substances of low molecular weight. Diffusion tests with dye demonstrate that only substances with a molecular weight under 400 can pass the disc tissue barrier.41 The PGs of the inner compartment take up water, until the hydrostatic pressure that results is in balance with the physical tension that arises from the tensile forces of the annulus and the loads applied by muscles, ligaments and gravity (Fig. 31.7).
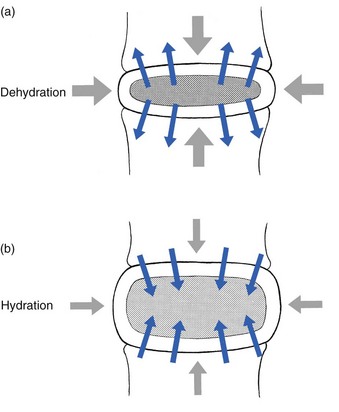
At this point, there is no net fluid loss or gain. If the external stress is increased – say, by an increase in load – the balance will be disturbed and fluid is expressed from the nucleus (Fig. 31.8a). This loss of fluid has two consequences: tensile stress in the collagen network falls and the concentration of PGs in the nucleus and thus the osmotic pressure rise. In other words, loss of fluid increases the internal swelling pressure, until the latter has risen to the physical stress and a new balance is achieved.43 The reverse happens when the external load decreases: the internal osmotic pressure is momentarily higher than the external load and fluid is attracted (Fig. 31.8b). The concentration of PGs and the swelling pressure decrease until external and internal pressures again reach an equilibrium.
Influence of the external load on hydration of the disc
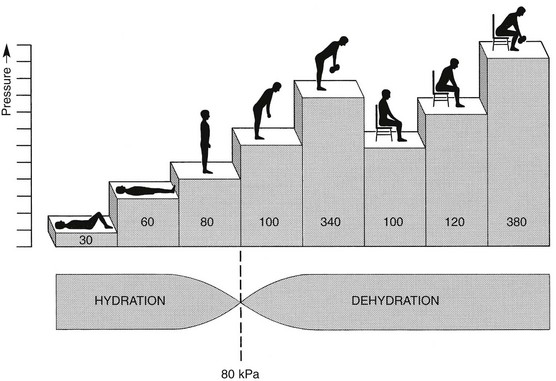
Using diffusion techniques with dye and with radioactive substances, Krämer was able to show that in normal non-degenerated discs there is an extravasation of fluid when a load of more than 80 kPa is applied. Absorption takes place when the load is lower than 80 kPa.45
From 1966 on, Nachemson and co-workers demonstrated the relationship between body posture and intradiscal pressure by intravital recordings.46–51 They demonstrated that the pressure in an L3 disc of a healthy individual, weighing 70 kg, is 30 kPa in a supine lying position. Standing and walking around sets up a pressure between 70 and 85 kPa, whereas sitting raises the pressure to 100 kPa and slightly bending forwards to 120 kPa. Lifting a 20 kg object with a bent back and straight legs increases the intradiscal pressure to a surprising 340 kPa.
These findings suggest the dehydration–hydration point, found by Krämer to be around the standing and walking position (Fig. 31.9). The supine position causes hydration of the disc, whereas sitting, bending or lifting squeezes fluid out of the disc.
Since the transport processes in the disc depend largely on fluid flow, continuous change in intradiscal pressure could be of utmost importance for the nutrition of the disc.53 Load and de-load acts as a pump, and transports water and metabolites from and to the intervertebral disc.54 In order to protect discs against early degeneration, it is therefore important to keep the intradiscal pressure as low as possible during daily activities. This can be achieved by adopting a slight lordosis at the lumbar spine which protects the disc against excessive pressure. Also, regular changes in position continuously alter the intradiscal pressure, so causing a nutritional fluid flow to and from the disc.55 The prophylactic measures derived from these findings will be discussed in the section on back schools (see p. 582).
Biomechanical properties of the disc
The fibroelastic annulus provides the disc with hydraulic properties and provides resistance to tensile forces. Retained by this fibroelastic mesh, the nucleus pulposus acts like a fluid-filled balloon. During load, it distributes the axial pressure equally over the cartilage plates and the annulus fibrosus (Fig. 31.10). The annular fibres are under a constant slight stretch, because of the turgor of the nucleus. McNab56 compares the annulus with a coiled spring that pulls the vertebral bodies together against the turgor of the nucleus. If the load is axial and symmetrical, the nucleus pulposus distributes the force to all sides and therefore perpendicularly on the stretched annular fibres. In this position the disc is very strong and, during high compressive loads, outward herniation of the nucleus is not seen but there is a collapse of the cartilaginous endplates.57,58
Asymmetrical loading, however, simultaneously involves tension, compression and shear stresses at different locations in the disc. Bending results in a tensile stress on the convex side and a compressive stress on the concave side: that under tension stretches, while that under compression bulges.59,60 Tensile stresses on the convex side are increased by the migration of the nucleus. During such asymmetrical loading, the nucleus pulposus is pushed away from the area of compression, following the simple mathematical parallelogram of forces (Fig. 31.11). This means that, in bending forwards, the nucleus will move posteriorly, and therefore greater stress will fall on the posterior annular fibres, which are already subjected to a strong tensile stress.
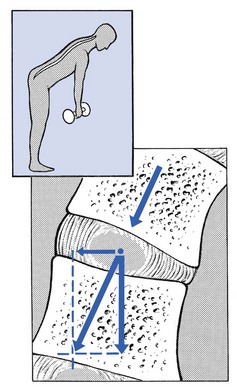
Fig 31.11 Asymmetrical loading.
The posterior migration of the nucleus pulposus in bending has been demonstrated experimentally by putting a metal pin in the nucleus pulposus.61 On continuous forward bending, the nucleus migrated backwards at a speed of 0.6 mm/min during the first 3 minutes and this continued very slowly during the next hour. After bending ceased, the nucleus regained its original position only very slowly. These findings have been confirmed by discography62 and by magnetic resonance imaging (MRI) studies.63–65 Biomechanical studies, conducted in vitro, have also demonstrated that the normal nucleus moves posteriorly in kyphosis and anteriorly in lordosis.66–68
The weak zone of the disc
Several anatomical, biochemical and biomechanical properties make the posterior aspect of the disc the most critical and vulnerable part of the whole intervertebral joint.69,70
• The posterior annular fibres are sparser and thinner than the anterior.
• Because the area available for diffusion is smaller posteriorly than anteriorly, the posterior part of the nuclear–annular boundary receives less nutrition and again the posterior part of the disc is the most strained part.71
• The posterior longitudinal ligament affords only weak reinforcement, whereas the anterior fibres are strengthened by the powerful anterior longitudinal ligament.
• Because of the special mechanical arrangements of the annular fibres, the tangential tensile strain on the posterior annular fibres is 4–5 times the applied external load.72
In order to prevent early degeneration and internal derangement, the erect body has developed only one adequate defence system: namely, a slightly lordotic lumbar posture. Cyriax was the first clinician to point out the importance of the lumbar lordosis. Long before biomechanical experiments, such as those of Nachemson, and purely on clinical findings, he demonstrated the importance of correct posture in the avoidance of backache and sciatica.73 This is the physiological lumbar lordosis, which diminishes the intradiscal pressures and protects the disc against backward displacements of the nucleus pulposus. ‘Keep your back hollow’ is still the best advice against recurrent discogenic backache, even more so in recent decades because of the more sedentary jobs many people have. A sitting position not only increases the intradiscal pressure but also forces the lumbar spine into kyphosis by a backwards inclination of the sitting pelvis.74 Increased sedentary work is probably one of the reasons for the rising rate of lumbar syndromes.
Facet joints
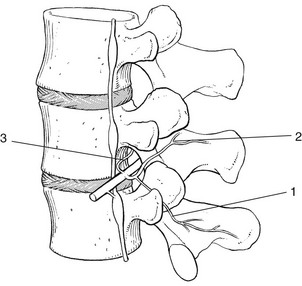
The joints between the lower and upper articular processes are called zygapophyseal joints, apophyseal joints or ‘facet’ joints. They are true synovial joints, comprised of cartilaginous articular surfaces, synovial fluid, synovial tissue and a joint capsule (Fig. 31.12). The superior articular surface is slightly concave and faces medially and posteriorly. The convex inferior articular surface points laterally and slightly anteriorly. In general terms, there is a change from a relatively sagittal orientation at L1–L3, to a more coronal orientation at L5 and S1 (Fig. 31.13).75,76
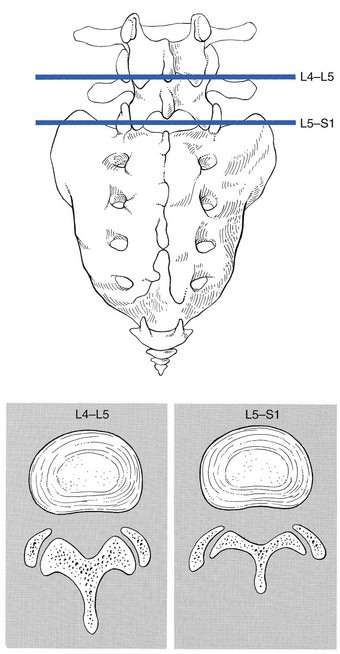
Fig 31.13 Facet joints at L4–L5 and L5–S1.
Unlike the disc, the facet joints normally do not bear weight and during normal loads they are not subjected to compression strain.72 In degenerative fragmentation of the disc, however, intervertebral height diminishes and the articular surfaces are subjected to abnormal loading, setting up spondylarthrosis.77 The main function of the facet joints is to guide lumbar movements and keep the vertebrae in line during flexion–extension and lateral flexion. Because of the more sagittal slope of the articular surfaces, very little rotation takes place at the four upper lumbar levels. More distally, at the lumbosacral level, the joint line has a more coronal plane, which makes rotational movements potentially possible, but these are limited by the iliolumbar ligaments (see p. 424).78 The total range of rotation in the lumbar spine is therefore very limited, although not completely zero.79
The capsule of the joints is well developed and thick and elastic at the dorsal, superior and inferior aspects. At rest, the fibres run slightly diagonally from lateral–caudal to medial–cranial. As the articular excursion is about 0.5 cm at each level, the capsule must have a considerable laxity to follow the points of insertion during flexion. It therefore possesses capsular recesses of varying size, at the superior and inferior poles of the joint, which gives the joint the appearance of a dumb-bell during arthrographic examinations.80 In extension the posterior capsule can become pinched between the apex of the inferior facet and the lamina below. In order to prevent this, some fibres of the multifidus blend with the posterior capsular fibres and keep the capsule taut.81 The ventral aspect of the capsule is an extension of the ligamentum flavum. It is very thin82 and may rupture during intra-articular injections.83
During flexion, the inferior articular process slides upwards on the superior articular process. The lower part of the latter loses contact and becomes exposed. Similarly, the lower part of the inferior articular process becomes exposed ventrally. In order to protect these exposed surfaces, and to maintain a film of synovial fluid over the articular cartilages, the facet joints are endowed with small intra-articular ‘meniscoids’.84–86 These small fibro-adipose crescent wedges have a base attached to the joint capsule and an apex that projects into the capsular pouches.87 Stretching of the capsule during flexion makes them disappear. Some believe that these fibro-adipose enlargements could become pinched between the articular surfaces, constituting a probable source of backache.88–91
The facet joints are innervated by fibres of the medial branch of the dorsal root. The same nerve supplies the inferior aspect of the capsule and the superior aspect of the joint below.92
Ligaments
The broad, thick anterior longitudinal ligament (Fig. 31.14) originates from the anterior and basilar aspect of the occiput and ends at the upper and anterior part of the sacrum. It consists of fibres of different lengths: some extend over 4–5 vertebral bodies; the short fibres attach firmly to the fibres of the outermost annular layers and the periosteum of two adjacent vertebrae.
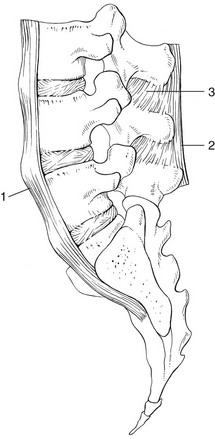
Fig 31.14 Anterior longitudinal (1) and supraspinous and interspinous ligaments (2 and 3, respectively).
The posterior longitudinal ligament (Fig. 31.15) is smaller and thinner than its anterior counterpart: 1.4 cm wide (versus 2 cm in the anterior ligament) and 1.3 mm thick (versus 2 mm). This is another fact in favour of the theory that the lumbar spine was originally designed to be a horizontal hanging structure: to withstand extension strains, the back had to be stronger anteriorly than posteriorly.5 The posterior longitudinal ligament is narrow at the level of the vertebral bodies, and gives lateral expansions to the annulus fibrosus at the level of the disc, which bestow on it a denticulated appearance.93
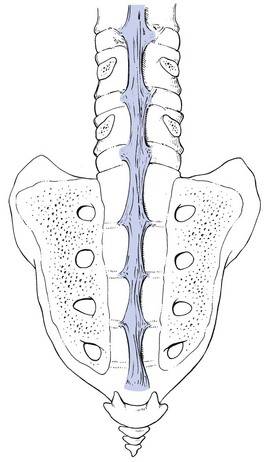
Fig 31.15 Posterior longitudinal ligament.
Although the posterior ligament is rather narrow, it is important in preventing disc protrusion.94 Its resistance is the main factor in restricting posterior prolapse and accounts for the regular occurrence of spontaneous reduction in lumbago. This characteristic is also exploited in manipulative reduction, when a small central disc displacement is moved anteriorly when the ligament is tightened. The fact that the ligament occupies only the midline of the vertebral column is one of the predetermining factors in the progression of sciatica: as a central protrusion enlarges, it tends to move in the direction of least resistance – lateral to the ligament. Once free from ligamentous resistance, it further enlarges and starts to compress the nerve root. This anatomical evolution is mirrored by the change in the clinical picture: a central backache is replaced by a unilateral sciatica.
The ligamentum flavum (Fig. 31.16) connects two consecutive laminae and has a very elastic structure with an elastin content of more than 80%.82 The lateral extensions form the anterior capsule of the facet joints and run further laterally to connect the posterior and inferior borders of the pedicle above with the posterior and superior borders of the pedicle below. These lateral fibres form a portion of the foraminal ring and the lateral recess.95,96
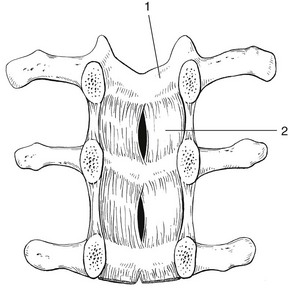
Fig 31.16 Lamina (1) and ligamentum flavum (2).
The interspinous ligament (see Fig. 31.14) lies deeply between two consecutive spinal processes. Unlike the longitudinal ligaments, it is not a continuous fibrous band but consists of loose tissue,97 with the fibres running obliquely from posterosuperior to anteroinferior.98 This particular direction may give the ligament a function over a larger range of intervertebral motion than if the fibres were vertical.99 The ligament is also bifid, which allows the fibres to buckle laterally to both sides when the spinous processes approach each other during extension.97
The supraspinous ligament is broad, thick and cord-like. It joins the tips of two adjacent spinous processes, and merges with the insertions of the lumbodorsal muscles. Some authors consider the supraspinous ligament as not being a true ligament, as it seems to consist largely of tendinous fibres, derived from the back muscles.100 The effect of the supraspinous ligaments on the stability of the lumbar spine should not be underestimated.101 Because the ligament is positioned further away from the axis of rotation and due to its attachments to the thoracolumbar fascia,102 it will have more effect in resisting flexion than all the other dorsal ligaments. Pearcy103 showed that the distance between the tips of the spinous processes increases during full flexion by 360% at L3–L4 and 129% at L5–S1. By contrast, the posterior longitudinal ligament only increases by 55% at L3–L4 and 34% at L5–S1. This demonstrates the limiting effect of the ligament on the increasing posterior disc height during stooping. The importance of a strong supraspinous ligament in the prophylaxis of recurrent disc protrusions will be discussed later.
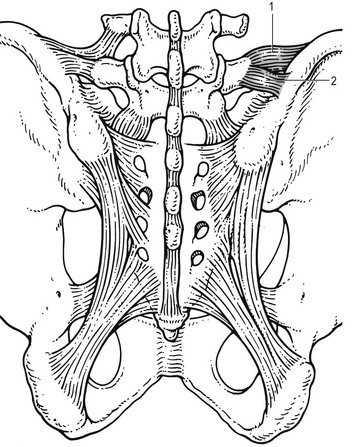
The iliolumbar ligaments (Fig. 31.17) are thought to be related to the upright posture.104 They do not exist at birth but develop gradually from the epimysium of the quadratus lumborum muscle in the first decade of life to attain full differentiation only in the second decade.105 The ligament consists of an anterior and a posterior part.106–108 The anterior band of the iliolumbar ligament is a well-developed, broad band. Its fibres originate from the anterior–inferior part of the L5 transverse process from as far medially as the body of the L5 vertebra to the tip of the transverse process, and expand as a wide fan before inserting on the anterior part of the iliac tuberosity. The posterior band of the iliolumbar ligament originates from the apex of the L5 transverse process and is thinner than the anterior. It inserts on the iliac crest, behind the origin of the quadratus lumborum.108
The iliolumbar ligaments play an important role in the stability of the lumbosacral junction by restricting both side flexion and rotational movement at the L5–S1 joint and forward sliding of L5 on the sacrum.104,109,110 One clinical consequence of this is that posterolateral disc protrusions at the L5–S1 level will not be followed by large lateral flexions of L5 on the sacrum. Marked adaptive deformity will therefore be absent here. Consequently, a large lateral tilt in a patient with acute backache means a displacement at L3–L4 or L4–L5, since these intervertebral joints can open up more easily. Also, stabilization of the lumbosacral junction by the strong iliolumbar ligaments may explain the fact that L5–S1 pars defects are more stable than L4–L5 lesions (see spondylolisthesis, Ch. 58).111,112
Muscles and fasciae
The spine is unstable without the support of the muscles that power the trunk and position the spinal segments.113 Back muscles can be divided in four functional groups: flexors, extensors, lateral flexors and rotators (Fig. 31.18).
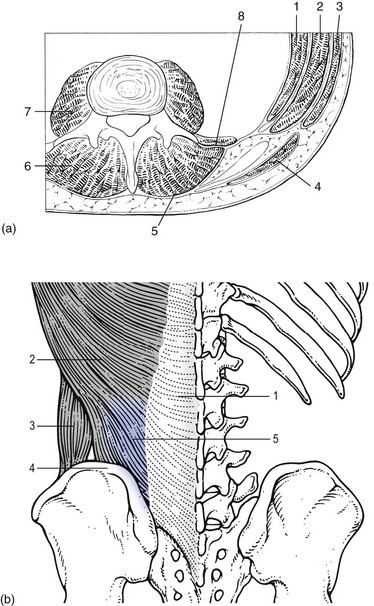
The extensors are arranged in three layers: the most superficial is the strong erector spinae or sacrospinalis muscle. Its origin is in the erector spinae aponeurosis, a broad sheet of tendinous fibres attached to the iliac crest, the median and lateral sacral crests and the spinous processes of the sacrum and lumbar spine.114 The middle layer is the multifidus. The fibres of the multifidus are centred on each of the lumbar spinous processes. From each spinal process, fibres radiate inferiorly to insert on the lamina, one, two or three levels below. The arrangement of the fibres is such that it pulls downwards on each spinal process, thereby causing the vertebra of origin to extend.115 The third layer is made up of small muscles arranged from level to level, which not only have an extension function but are also rotators and lateral flexors.
The extensor muscles are enveloped by the thoracolumbar fascia (Fig. 31.18b), which in turn consists of three layers. The anterior layer is quite thin and covers the anterior surface of the quadratus lumborum. Medially, it is attached to the anterior surfaces of the lumbar transverse processes, and in the intertransverse space it merges with the intertransverse ligaments. The middle layer lies behind the quadratus lumborum muscle. Medially, it also continues into the intertransverse ligament to attach to the lateral border of the lamina. The posterior layer covers the back muscles. It arises from the lumbar spinous processes and from the supraspinous ligaments to envelop the back muscles and blend with the other layers of the thoracolumbar fascia along the lateral border of the iliocostalis lumborum. The union of the fasciae is quite dense and forms a strong raphe (the lateral raphe116) which fuses with the fibres of transversus abdominis, internal oblique and latissimus dorsi muscles. The lateral raphe further inserts at the posterior segment of the iliac crest and the posterior superior iliac spine.117
Spinal canal
The spinal canal is made up of the canals of individual vertebrae so that bony segments alternate with intervertebral and articular segments. The shape of the transverse section changes from round at L1 to triangular at L3 and slightly trefoil at L5 (see Fig. 31.2).118
The posterior wall is formed by the uppermost portions of the laminae and the ligamenta flava. Because the superoinferior dimensions of the laminae tend to decrease at the L4 and L5 levels, the ligamenta flava consequently occupy a greater percentage of the posterior wall at these levels.95 The posterolateral borders of the posterior wall are formed by the anterior capsule of the facet joint and the superior articular process, which is located well anterior of the articulating inferior articular process.
The spinal canal contains the dural tube, the spinal nerves and the epidural tissue.
Dura mater
The dura mater is a thick membranous sac, attached cranially around the greater foramen of the occiput, where its fibres blend with the inner periosteum of the skull, and anchored distally to the dorsal surface of the distal sacrum by the filum terminale. The latter descends to the coccyx, where its fibres merge with the connective tissue of the sacroiliac ligaments.119 The dural sac itself ends blind, usually at S2. There is an inconstant dural attachment, the ‘Hofmann complex’,120 made up of bands of connective tissue and loosely joining the anterior dura to the vertebral column (Fig. 31.19). Ventral meningovertebral ligaments pass from the ventral surface of the dura to the posterior longitudinal ligament. They are variable in structure and may present either as tight bands, bifurcations in a Y shape or paramedian bands.121–123 Others reported on more lateral ligaments, passing from the lateral surface of the dural sac and blending with the periosteum of the pedicles.124–127
Dural mobility
The vertebral canal lengthens considerably during flexion: O’Connell128 showed by radiological measurements that in full flexion the length of the cervical canal increases by 3 cm, compared with its neutral position. The dura mater, a structure situated in the vertebral canal but anchored at the top and at the bottom, will consequently move in the spinal canal. Breig129 suggests that the dura mater unfolds and stretches. Other authors have found a gliding of the dural sac in relation to the spinal canal during flexion and extension.130–133 Using gas myelography, Decker134 showed that the dura moved towards the front of the canal during flexion: like a rubber band, it shifts towards a position of less tension, and is pulled against the anterior wall. Klein135 demonstrated an upward displacement of the dura by more than 5 mm at L3 level during full flexion of the spine.
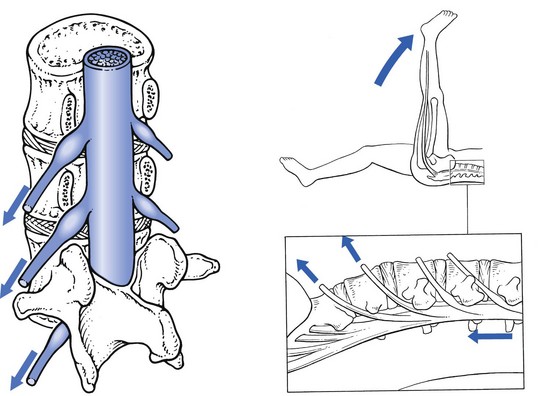
Straight leg raising can put considerable traction on the dural sac. During this manœuvre the L4, L5, S1 and S2 nerve roots are dragged downwards and forwards (Fig. 31.20). At the level of the intervertebral foramen, the degree of downward movement is between 1 and 4 mm.136–138 As the root is connected through its dural investment with the distal part of the dura, the latter will also be involved in the downward movement. Therefore, straight leg raising drags on the dura mater and pulls it caudally, laterally and forwards.135,139
During neck flexion and straight leg raising, the dura thus moves slightly in relation to the anterior wall of the spinal canal, despite some loose attachments between the posterior longitudinal ligament and the dural sac. Anatomical changes at the anterior walls – for instance, a disc protrusion bulging dorsally into the canal – compress the dura. Conversely it can be pulled against this protrusion, whether from below during straight leg raising or from above during neck flexion. The observation that the dura is mobile thus has considerable clinical significance, since an increase of lumbar pain during neck flexion or during straight leg raising implicates the dura mater as the source. In fact, these signs have been accepted for decades as being positive in meningeal irritation (Kernig’s sign and neck retraction), but this mechanism of dural pain was not elucidated until Cyriax’s paper was published in 1945.140 In the differential diagnosis of lumbar pain syndromes, ‘dural signs’ are extremely important in distinguishing a lesion in which the anterior part of the dura mater is involved (disc displacements) from possible lesions at the posterior wall (facet joints and ligaments).
Dural sensitivity
Clinical experiments have shown that the anterior part of the dura is sensitive to both mechanical and chemical stimulation.141,142 Back pain is also well known in the context of neurological diseases in which the dura becomes inflamed143 or compressed.144 Further evidence for dural pain comes from neurosurgical studies that report relief of postlaminectomy pain after resection of the nerves to the dura.145
From the 1950s on, numerous neuroanatomical studies have been conducted that describe the innervation of the dural tube.146 Several authors have shown that the ventral half of the dura mater is supplied by small branches of the sinuvertebral nerve.147,148 Further work has confirmed that the innervation is from the sinuvertebral nerves and is confined to the anterior part of the dura only.149,150
During the last decade, immunohistochemical studies clearly demonstrated a significant number of free nerve endings, containing substance P, calcitonin-generated peptides and other neurotransmitters contributing to nociception.151,152 All these findings have been confirmed and extended recently so that the present concept is of a dense, longitudinally orientated nerve plexus in the ventral spinal dura, extending over up to eight segments, showing a great deal of overlap between adjacent levels and crossing the midline.153,154 The anterior part of the dura mater is thus innervated by a mesh of nerve fibres which belong to different and consecutive sinuvertebral nerves (Fig. 31.21). This probably explains the phenomenon of ‘dural pain’, which is a pattern of large and broad reference of pain covering different dermatomes, commonly found in low back syndromes. The patient then describes lumbar pain, radiating to the abdomen or up to the chest, to the groin or to the front of both legs.155
Nerve roots
Definition
The pairs of spinal roots join at the level of the foramen. Immediately proximal to its junction with the ventral root, the dorsal root forms an enlargement – the dorsal root ganglion – which contains the cell bodies of the sensory fibres in the dorsal root. Distal to the junction at the foramen, the dura mater merges with the epineurium of the spinal nerve. From here the extraspinal part of the spinal nerve begins.125
Boundaries
The entire course of the intraspinal part of the spinal nerve is enveloped by the radicular canal156 or spinal nerve root canal.157 The term ‘lateral recess’ has been applied to the bony boundaries of this radicular canal.158
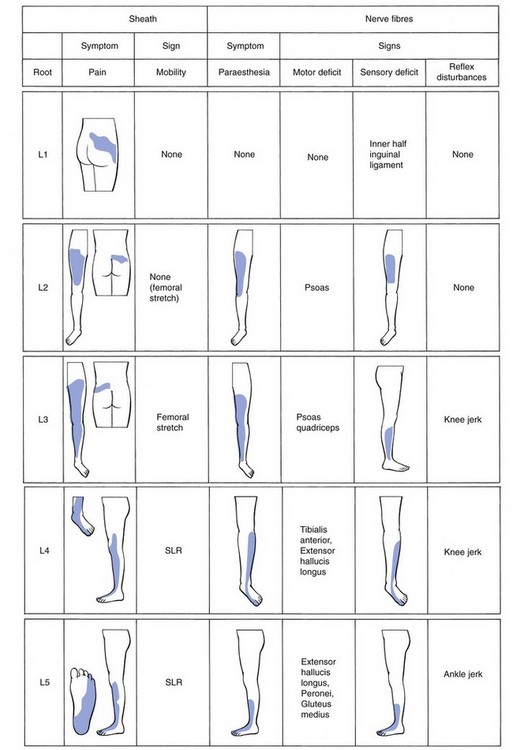
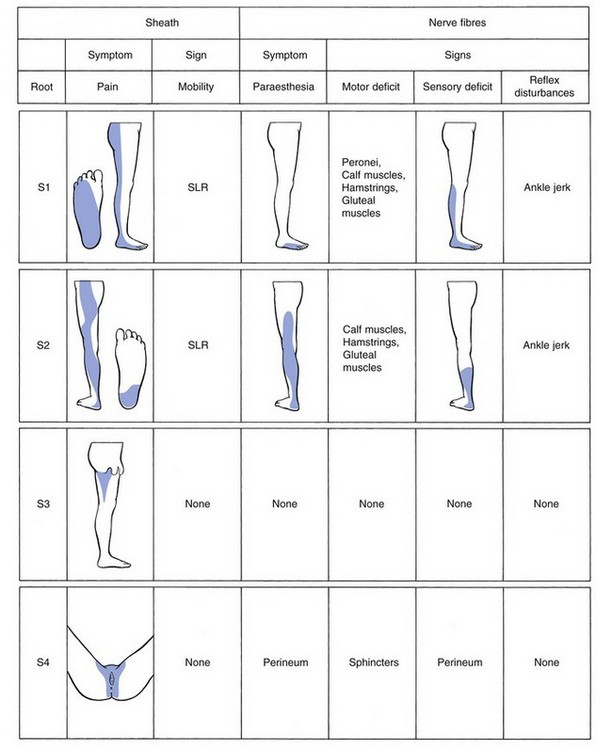
The length of the radicular canal increases from L3 to S1, so making the L5 and S1 roots more liable to compression. The L3 nerve root travels behind the inferior aspect of the vertebral body and the L3 disc. The L4 nerve root crosses the whole vertebral body to leave the spinal canal at the upper aspect of the L4 disc. The L5 nerve root emerges at the inferior aspect of the fourth lumbar disc and crosses the fifth vertebral body to exit at the upper aspect of the L5 disc (Fig. 31.22).159,160 Further clinical applications of this downward direction of the nerve roots are:
• At L4 level, a disc protrusion can pinch the fourth root, the fifth root or, with a larger protrusion, both roots.
• At L5 level, a disc can compress the fifth root, the first sacral root or both.
It is, however, important to remember that aberrant courses and anastomoses exist between the lumbar nerve roots,161 which may be present in about 4% of the population.162
The intervertebral foramen163 is the point where the spinal nerve emerges from the canal (Fig. 31.23). It is located in a sagittal plane, so it can be demonstrated perfectly on a plain lateral radiograph. The foramen is limited cranially by the upper pedicle and caudally by the pedicle below. The anterior wall corresponds to the posterior aspect of the vertebral body and the disc. The posterior wall of the intervertebral foramen is formed by the articular facets. The size of the foramen increases from T12–L1 to L4–L5, but the foramen L5–S1 is the smallest of all and is located slightly more anteriorly.
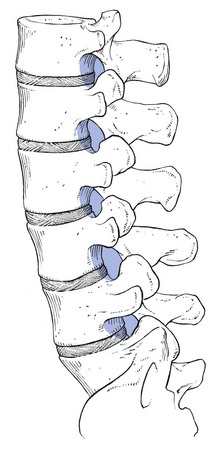
Fig 31.23 Intervertebral foramina.
Anatomy
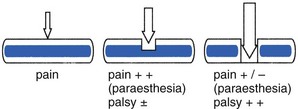
The radicular canal contains the intraspinal extrathecal nerve root. The nerve root consists of a sheath (dural sleeve) and fibres. Each structure has a specific behaviour and function, responsible for typical symptoms and clinical signs (Box 31.1). This has some clinical consequences: slight pressure and inflammation only involve the sleeve and provoke pain and impaired mobility. More substantial compression of the root will also affect the nerve fibres, which leads to paraesthesia and loss of function.
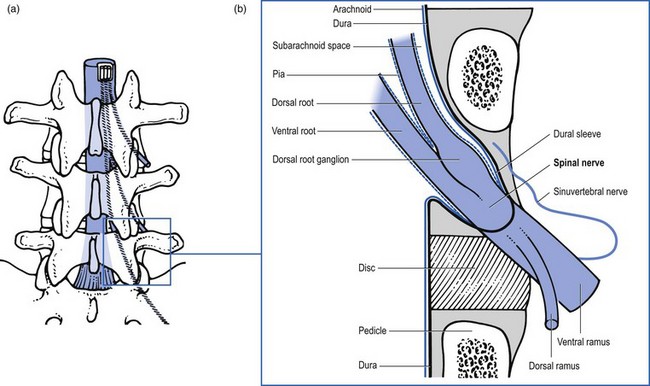
The dural sheath
The dural sheath (Fig. 31.24) starts as a funnel-shaped pouch, enclosing the anterior and posterior roots at their exit from the dural sac. The dural nerve root sleeve proper is formed at the end of this short pouch and continues distally to the foramen, where it merges with the connective tissue sheath of the ganglion and the spinal nerve. The dural investment of the nerve root therefore does not extend beyond the lateral border of the vertebral foramen. In this sleeve, the anterior and posterior roots no longer lie free but are firmly bound to the dural sleeve by the arachnoid membrane. In other words, the subarachnoid space forms a bilaminar tube within the root sleeve as a whole.164
At the foramen, the epidural tissue becomes more condensed and forms a loose ligamentous fixation of the epineural sheath to the bony boundaries of the intervertebral foramen.165,166 A stronger ligament (the so-called ‘lateral root ligament’; Fig. 31.25), connecting the epineural sheath to the pedicle, has also been described.167 It has been suggested that the fixation of the dural sleeve, together with the anterior attachment of the dura to the posterior longitudinal ligament, could be of some importance in the mechanism of sciatica.168 Simple mechanical analysis suggests that pressure applied to the nerve root by a disc protrusion is determined by the extent of the dural ligament fixation rather than by compression of the root against the posterior wall.169
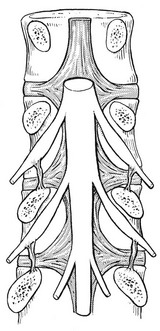
Fig 31.25 The lateral root ligament.
The dural investment of the nerve root is sensitive and mobile, like the dural sac.
Mobility
Although the intervertebral foramen represents a point of relative fixation of the nerve, some caudal migration of the latter remains possible.170 Distal traction on the sciatic nerve and lumbosacral plexus thus pulls the nerve root downwards and drags on the dural sheath and the dura. This occurs during straight leg raising, when the nerve roots of L4, L5, S1 and S2 are moved downwards at the level of the intervertebral foramen.136,171,172 The main range of motion of the S1 root is 4 mm, of L5 3 mm and of L4 1.5 mm. Straight leg raising does not pull directly on the L3 root. This structure can only be moved caudally during knee flexion in the prone position, which stretches the femoral nerve.173 It is not possible to test the mobility of the S3 and S4 roots because they do not reach the lower limb.
Because of the downward and anterior direction of the nerve roots and the relative fixation of the dural investment at the anterior wall, a downward movement of the nerve always involves anterior displacement, which pulls the root against the posterolateral aspect of disc and vertebra. Restriction of nerve root mobility therefore always means anterior compression of the root. Internal rotation of the hip during straight leg raising adds more tension to the lumbosacral plexus and nerve roots.174 To clinicians this is not surprising, because it is common to see patients with considerable limitation of straight leg raising actively rotating their hips laterally when it is performed, thus protecting the inflamed root against further traction.
Cyriax drew attention to two interesting phenomena in relation to the mobility of the nerve root sheath: namely, the existence of a painful arc and the aggravation of the pain during neck flexion.175
• It is a common clinical finding that patients with sciatica show momentary pain during straight leg raising: there is pain only in a certain sector of movement (usually between 45 and 60°). The most acceptable explanation for this curious sign is that a small discal bulge exists over which the root slips and thereafter the rest of the movement is painless. This painful arc during straight leg raising always implies a small disc displacement and is a good indication that reduction by manipulation or traction is possible.
• The dural sheath can also be stretched from above. As we have seen previously (in the section on dural mobility), the dura can slip upwards during neck flexion. If pain brought on by straight leg raising is aggravated by neck flexion, the tissue thus stretched must run in a continuous line from the lumbosacral plexus to the neck. Only the dura mater and its continuations, the dural investments, can possibly be stretched from above and below at the same time.
Sensitivity of the dural investment
Dural root sheaths are innervated by the sinuvertebral nerve,149 and each sheath receives branches from the nerve of the corresponding side and level only. In contrast to the anterior aspect of the dural sac, anastomoses between branches of adjacent sinuvertebral nerves do not exist. Pain originating from the dural sheath is therefore strictly segmental and follows the corresponding dermatomes in the limb.176
Compression of the spinal nerve beyond the intervertebral foramen does not generate pain but only pins and needles, numbness and paresis. This is the case when a disc protrusion has passed very laterally, when the fifth lumbar nerve is compressed between a corporotransverse ligament and the ala of the sacrum,56 or in some spondylolytic compressions of the nerve root. Experience during the performance of a sinuvertebral block also confirms the insensitivity of the nerve root fibres. When the needle, just before touching the posterior aspect of the vertebral body, brushes against the nerve root, no pain but a sharp ‘electric’ shock results. As the dural investment of the root ends at the same level, it must be concluded that the latter is responsible for the radicular pain in sciatica.175
Nerve root
The structure of the nerve root differs from that of the peripheral nerves in three ways: the epineurium is less abundant, the fasciculi do not branch and the perineurium is missing. Thus, compared with a peripheral nerve, the parenchyma of the nerve root is more susceptible to injury, by either mechanical or chemical irritation.177
Irritation of the parenchyma leads to paraesthesia. Unlike ‘radicular’ pain, which is merely a symptom of compression of the dural sheath, pins and needles indicate that the nerve fibres are irritated as well. Paraesthesia is thus a symptom of direct involvement of the nerve root. Further irritation and destruction of the neural fibres leads to interference with conduction, resulting in a motor and/or sensory deficit. The fact that the motor and sensory components of the nerve root remain completely separated during the course of the nerve root along the radicular canal has some clinical consequences: it is possible for a nerve root compression to cause a pure motor paresis or a pure sensory deficit. If pressure is exerted from above, sensory impairment may result, whereas an impingement from below can induce a motor paresis. A larger protrusion, pressing between two roots, can result in a motor palsy of the root above, together with a sensory deficit of the nerve root below (Fig. 31.26).
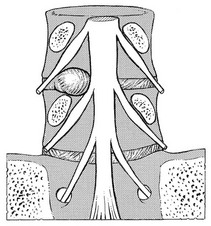
Fig 31.26 Protrusion pressing between two roots.
Controversy still exists over the mechanism of nerve root compression by a protruded disc. Inman and Saunders178 stated that the nerve root is rarely ‘compressed’ between anterior and posterior walls, but is merely brought under tension by the disc herniation. Others have observed that the extrathecal, intraspinal nerve root is relatively fixed to the anterior wall and the intervertebral foramen by the dural ligamentous complex and the foraminal complex (see p. 429).171,179 Therefore, this particular part of the root cannot easily slip away from a disc protrusion and is tethered over it, and a pressure-induced nerve lesion can develop.168
Conclusion
For clinical purposes it is as well to divide the components of the nerve root into an external aspect (the sheath), which is mobile and is responsible for pain, and an internal aspect (the nerve fibres), which serves conduction only. This helps to distinguish the symptoms and signs of each, so permitting proper assessment of the location of a lesion, the magnitude of compression and the degree of functional incapacity (Box 31.2).
In nerve root compressions by a displaced disc, the development of symptoms and signs allows the anatomical changes in the radicular canal to be followed: slight pressure will only involve the sheath of the root (Fig. 31.27), giving rise to pain in the corresponding dermatome and probably impaired mobility, reflected by alterations in straight leg raising. Greater pressure will result in pressure on nerve fibres, reflected in paraesthesia at the distal end of the dermatome. Clinical examination will now reveal not only interference of mobility but also impaired conduction – a sensory deficit and/or loss of motor power. Greater pressure causes root atrophy, which results in loss of sensitivity of the dural sheath and gives a painless straight leg raising test. At the same time the sensory deficit and motor palsy become complete (Fig. 31.28).
Epidural space
The virtual space between the dural sac, the dural sheaths of the nerve roots, and the spinal canal is the epidural space. This space is quite narrow because the dural sac lies very close to the boundaries of the vertebral canal and is filled with a network of loose connective tissue, fat, arteries and a dense network of veins.180
The sinuvertebral nerve is in the anterior half of the epidural space. The venous system is extensive and valveless, with multiple cross-connections. Batson181 has described retrograde venous flow from the lower pelvis to the lumbosacral spine, which probably provides the route for metastases and infections spreading from the pelvic organs to the spine.
Innervation
The spine is innervated by the sinuvertebral nerve and the posterior primary ramus. All the tissues lying posterior to the plane of the intervertebral foramina at each level (i.e. the facet, the vertebral arch, the related tendinous and aponeurotic attachments and the flaval and interspinous ligaments) are innervated from the posterior primary rami. Those anterior to the intervertebral foramina (longitudinal ligaments, anterior dura and dural sleeves) are supplied by branches of the sinuvertebral nerves (Wyke, cited by Cyriax182).
Sinuvertebral nerve
The sinuvertebral nerve was first described by Luschka in 1850.183 It emerges from the anterior aspect of the spinal nerve, distal to the nerve ganglion, and receives some sympathetic branches from the ramus communicans22 (Fig 31.29). In the fetus the nerve is composed of several filaments which may become bound together during later life, to form the adult sinuvertebral nerve.146 The composite nerve is between 0.5 and 1 mm thick,184 passes through the intervertebral foramen and points upwards around the base of the pedicle, to pass along the cranial side of the corresponding disc to reach the medial aspect of the posterior longitudinal ligament. Here it divides into ascending, descending and transverse branches, which anastomose with the sinuvertebral nerves of the contralateral side and with those from adjacent levels. Therefore, instead of a recognizable nerve trunk, the sinuvertebral nerve is represented by a network of overlapping fine filaments from different levels and from both sides (Fig. 31.30).185
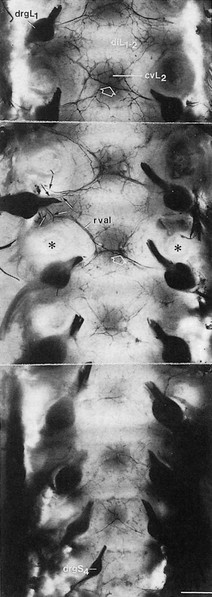
Fig 31.30 Dural nerve branches. *Cut pedicle of a vertebral arch; cv, vertebral body; di, intervertebral disc; drg, spinal ganglion; rv, ventral ramus of spinal nerve. Reproduced with permission from Groen.154
Branches of the sinuvertebral nerve supply the vertebral body, the outermost layers of the annulus fibrosus, the posterior longitudinal ligament, the anterior aspect of the dural sac and the dural investments around the nerve roots.186 Branches of the sinuvertebral nerve also surround the blood vessels of the vertebral canal. The posterior aspect of the dura is devoid of nerve endings. There is still disagreement as to whether the ligamentum flavum and the lamina are innervated by the sinuvertebral nerve.
Posterior primary ramus
Distally from the intervertebral foramen, the spinal nerve divides into a large anterior branch and a smaller posterior ramus (Fig. 31.31). The latter divides almost immediately into a medial and a lateral branch,187 although a smaller intermediate branch has also been identified.92
The medial branch descends posteriorly to the transverse process, where it lies in a groove formed by the junction of the superior articular and transverse processes. A strong fibrous band transforms this osseous groove into an osteofibrous tunnel. At this level a branch innervates the inferior part of the articular capsule of the facet joint. The nerve continues its course caudally on the lamina, to supply the dorsal muscles and the superior part of the articular capsule of the facet joint of the level below.188
Each medial branch thus supplies the facet joints above and below its course. Consequently, each facet joint is innervated by two consecutive medial branches.189,190
The lateral branch of the posterior ramus emerges between the deep layer of the lumbodorsal fascia and the lateral edge of the lamina. It supplies the muscles and the fascia. The lateral branches of the ramus posterior have cutaneous nerves and reach distally as far as the greater trochanter.148
References
1. Washburn, SL, The evolution of man. Sci Am 1978; 239:146. ![]()
2. Lippert, H. Probleme der Statik und Dynamik von Wirbelsäule und Rückenmark. In: Trostdorf E, Stender H, eds. Wirbelsäule und Nervensystem. Stuttgart: Thieme, 1970.
3. Snijders, CJ, On the form of the human spine and some aspects of its mechanical behaviour. Acta Orthop Belg 1969; 35:584. ![]()
4. Kapandji, IA, L’Anatomie fonctionelle du rachis lombosacrée. Acta Orthop Belg 1969; 34:543. ![]()
5. Thaczuk, H, Tensile properties of human longitudinal ligaments. Acta Orthop Scand. 1968;115(suppl). ![]()
6. Hall, LT, Esses, SI, Noble, PC, Kamaric, E, Morphology of the lumbar vertebral endplates. Spine 1998; 23:1517–1523. ![]()
7. Troup, JDG, The etiology of spondylosis. Orthop Clin North Am 1977; 8:57–64. ![]()
8. Farfan, HF. Mechanical Disorders of the Low Back. Philadelphia: Lea & Febiger; 1973.
9. Roberts, S, Menage, J, Urban, PG, Biochemical and structural properties of the cartilage end-plate and its relation to the intervertebral discs. Spine 1989; 14:166–174. ![]()
10. Taylor, JR. The development and adult structure of lumbar intervertebral discs. J Man Med. 1990; 5:43–47.
11. Nachemson, A, Lewin, T, Maroudas, A, Freeman, MAR, In vitro diffusion of dye through the endplates and the annulus fibrosus of human lumbar intervertebral discs. Acta Orthop Scand 1970; 41:589–607. ![]()
12. Roberts, S, Menage, J, Urban, JPG, Biomechanical and structural properties of the cartilage end-plate and its relation to the intervertebral disc. Spine 1989; 14:166–174. ![]()
13. Vernon-Roberts, B. Ageing Lumbar Spine. Lecture to the Society of Back Pain Research; 1975.
14. Joplin, RJ. Intervertebral disc. Surg Gynecol Obstet. 1935; 61:591.
15. Markolf, KL, Morris, JM, The structural components of the intervertebral disc. J Bone Joint Surg 1974; 56A:675–687. ![]()
16. Krismer, M, Haid, C, Rabl, W, The contribution of anulus fibers to torque resistance. Spine 1996; 21:2551–2557. ![]()
17. Galante, J, Tensile properties of the lumbar annulus fibrosus. Acta Orthop Scand. 1967;100(suppl). ![]()
18. Marchand, F, Ahmed, AM, Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine 1990; 15:402–410. ![]()
19. Eyre, DR. Biochemistry of the intervertebral disc. In: Hall DA, Jackson DS, eds. International Reviews of Connective Tissue Research. New York: Academic Press, 1979.
20. Naylor, A. Intervertebral disc prolapse and degeneration. The biomechanical and biophysical approach. Spine. 1976; 1:108.
21. Inoue, H, Takeda, T, Three-dimensional observation of the collagen frame-work of the lumbar intervertebral disc. Acta Orthop Scand 1975; 46:946–956. ![]()
22. Bogduk, N, Tynan, W, Wilson, AS. The innervation of the human lumbar intervertebral discs. J Anat. 1981; 132:39–56.
23. Yoshizawa, H, O’Brien, JP, Smith, WT, Trumper, M, The neuropathology of intervertebral discs removed for low-back pain. J Pathol 1980; 132:95–104. ![]()
24. Konttinen, Y, Grönblad, M, Antti-Poika, I, et al, Neuro-immunohistochemical analysis of peridiscal nociceptive neural elements. Spine 1990; 15:383–386. ![]()
25. Palmgren, T, Grönblad, M, Virri, J, et al, An immunohistochemical study of nerve structures in the anulus fibrosus of human normal lumbar intervertebral discs. Spine (Phila Pa 1976). 1999;24(20):2075–2079. ![]()
26. Roberts, S, Eisenstein, SM, Menage, J, et al, Mechanoreceptors in intervertebral discs; morphology, distribution and neuropeptides. Spine 1995; 20:2645–2651. ![]()
27. Kordala, O, Grönblad, M, Liesi, P, Karahurju, E, Immunohistochemical demonstration of nociceptors in the ligamentous structures of the lumbar spine. Spine 1985; 10:156–157. ![]()
28. Ashton, IK, Roberts, S, Jaffray, DC, et al, Neuropeptides in the human intervertebral disc. J Orthop Res 1994; 12:186–192. ![]()
29. Ozawa, T, Ohtori, S, Inoue, G, et al, The degenerated lumbar intervertebral disc is innervated primarily by peptide-containing sensory nerve fibers in humans. Spine (Phila Pa 1976). 2006;31(21):2418–2422. ![]()
30. Hassler, O, Human intervertebral disc. Acta Orthop Scand 1970; 40:765. ![]()
31. Ogata, K, Whiteside, LA, Nutritional pathways of the intervertebral disc. An experimental study using hydrogen washout technique. Spine 1981; 6:211–216. ![]()
32. Urban, JPG, Holm, S, Maroudas, S, Diffusion of small solutes into the intervertebral disc: an in vivo study. Biorheology 1978; 15:203–221. ![]()
33. Maroudas, A, Nachemson, A, Stockwell, R, Urban, J, In vitro studies of nutrition of intervertebral disc. Society for Back Pain Research, 1973.
34. Mankin, HJ, Thrasher, AZ, Water content and binding in normal and osteoarthrotic human cartilage. J Bone Joint Surg 1975; 57A:76–80. ![]()
35. Jayson, MIV, Barks, JS, Structural changes in the intervertebral disc. Ann Rheum Dis 1973; 32:10–15. ![]()
36. Urban, JP, Smith, S, Fairbank, JC, Nutrition of the intervertebral disc. Spine (Phila Pa 1976). 2004;29(23):2700–2709. ![]()
37. Brickley-Parson, D, Glimcher, M, Is the chemistry of collagen in the intervertebral disc an expression of Wolff’s law: a study of the human lumbar spine. Spine 1984; 9:148–163. ![]()
38. Adam, P, Muir, H, Qualitative changes with age of proteoglycans of human lumbar discs. Ann Rheum Dis 1976; 35:289–295. ![]()
39. Johnstone, B, Bayliss, MT, The large proteoglycans of the human intervertebral disc. Spine 1995; 20:674–684. ![]()
40. Lyons, G, Eisenstein, SM, Sweet, MBE, Biochemical changes in intervertebral disc degeneration. Biochem Biophys Acta 1981; 673:443–453. ![]()
41. Krämer, J. Biomechanische Veränderungen im lumbalen Bewegungssegment. In: Die Wirbelsäule in Forschung und Praxis. Stuttgart: Hippokrates; 1973.
42. Krämer, J. Bandscheibenbedingte Erkrankungen. Stuttgart: Thieme; 1978.
43. Adams, M, Hutton, WC, The effect of posture on the fluid content of the lumbar intervertebral disc. Spine 1983; 8:665–671. ![]()
44. Urban, JPG, McMullin, JF, Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition and degeneration. Spine 1988; 13:179–187. ![]()
45. Krämer, J. Biochemie der Zwischenwirbelscheiben. Wirbelsäule Forsch Prax. 1974; 59:10.
46. Nachemson, A, Lumbar intradiscal pressure. Acta Orthop Scand. 1960;43(suppl). ![]()
47. Nachemson, A, The influence of spinal movements on the lumbar intradiscal pressure and on the tensile stresses in the anulus fibrosus. Acta Orthop Scand 1963; 33:183. ![]()
48. Nachemson, A, Morris, J, In vivo measurements of intradiscal pressure; discometry, a method for the determination of pressure in the lower lumbar discs. J Bone Joint Surg 1964; 46A:1077. ![]()
49. Nachemson, A, The load on lumbar discs in different positions of the body. Clin Orthop 1966; 45:107. ![]()
50. Nachemson, A, Elfström, G. Intravital Dynamic Pressure Measurements in Lumbar Discs. A Study of Common Movements, Maneuvers and Exercises. Stockholm: Almquist & Wiksell; 1970.
51. Nachemson, A. The lumbar spine, an orthopaedic challenge. Spine. 1976; 1:59.
52. Nachemson, A, Towards a better understanding of low-back pain: a review of the mechanics of the lumbar spine. Rheumatol Rehabil 1975; 14:129. ![]()
53. Holm, S, Nachemson, A, Variations in the nutrition of the canine intervertebral disc induced by motion. Spine 1983; 8:866–874. ![]()
54. Holm, S, Urban, JPG. The intervertebral disc: factors contributing to its nutrition and matrix turnover. In: Helminen HJ, ed. Joint Loading. Bristol: Wright, 1987.
55. Stokes, IA, Iatridis, JC, Mechanical conditions that accelerate intervertebral disc degeneration: overload versus immobilization. Spine. 2004;29(23):2724–2732. ![]()
56. McNab, I. Backache. Baltimore: Williams & Wilkins; 1983.
57. Hirsch, C, The reaction of intervertebral discs to compression forces. J Bone Joint Surg 1955; 37A:1188–1191. ![]()
58. Adams, MA, Freeman, BJ, Morrison, HP, et al, Mechanical initiation of intervertebral disc degeneration. Spine. 2000;25(13):1625–1636. ![]()
59. White, A, Panjabi, M. Clinical Biomechanics of the Spine. Philadelphia: Lippincott; 1978.
60. Ebara, S, Iatridis, JC, Setton, LA, et al, Tensile properties of nondegenerate human lumbar anulus fibrosus. Spine (Phila Pa 1976). 1996;21(4):452–461. ![]()
61. Vogel, G. Experimental Untersuchungen zur Mobilität des Nucleus Pulposus in Lumbalen Bandscheiben. Düsseldorf: Medical Dissertation; 1977.
62. Shah, JS, Hampson, WA, Jayson, MIV, The distribution of surface strain in the cadaveric lumbar spine. J Bone Joint Surg 1978; 608:246–251. ![]()
63. Beattie, PF, Brooks, WM, Rothstein, JM, et al, Effect of lordosis on the position of the nucleus pulposus in supine subjects. Spine 1994; 19:2096–2102. ![]()
64. Fennell, AJ, Jones, AP, Hukins, DW, Migration of the nucleus pulposus within the intervertebral disc during flexion and extension of the spine. Spine (Phila Pa 1976). 1996;21(23):2753–2757. ![]()
65. Alexander, LA, Hancock, E, Agouris, I, et al, The response of the nucleus pulposus of the lumbar intervertebral discs to functionally loaded positions. Spine (Phila Pa 1976). 2007;32(14):1508–1512. ![]()
66. Krag, MH, Seroussi, RE, Wilder, DG, Pope, MH, Internal displacement distribution from in vitro loading of human thoracic and lumbar spinal motion segments: experimental results and theoretical predictions. Spine 1987; 12:1001–1007. ![]()
67. Schnebel, BE, Simmons, JW, Chowning, J, Davidson, R, A digitizing technique for the study of movements of intradiscal dye in response to flexion and extension of the lumbar spine. Spine 1988; 13:309–312. ![]()
68. Serrousi, RE, Krag, MH, Muller, DL, Pope, MH, Internal deformations of intact and denucleated human lumbar discs subjected to compression, flexion and extension loads. J Orthop Res 1989; 7:122–131. ![]()
69. Vernon-Roberts, B, Fazzalari, NL, Manthey, BA, Pathogenesis of tears of the anulus investigated by multiple-level transaxial analysis of the T12–L1 disc. Spine (Phila Pa 1976). 1997;22(22):2641–2646. ![]()
70. Kim, Y, Prediction of peripheral tears in the anulus of the intervertebral disc. Spine (Phila Pa 1976). 2000;25(14):1771–1774. ![]()
71. Belytschko, T, Kulak, RF, Schultz, A, Galante, J, Finite element stress analysis of an intervertebral disc. J Biomech 1974; 7:277–285. ![]()
72. Nachemson, A, Some mechanical properties of the lumbar intervertebral disc. Bull Hosp Joint Dis 1962; 23:130. ![]()
73. Cyriax, JH. Disc Lesions. London: Cassell; 1953.
74. Cyriax, JH. The Slipped Disc. London: Scribner; 1980.
75. Schneck, CD, The anatomy of lumbar spondylosis. Clin Orthop Rel Res 1985; 193:20–37. ![]()
76. Ebraheim, NA, Lu, J, Hao, Y, et al, Anatomic considerations of the lumbar isthmus. Spine 1997; 22:941–945. ![]()
77. Groher, W. Diagnostik und konservative Behandlung der Lumbosakralarthrose. Orthop Prax. 1977; 8:564.
78. Chow, D, Luk, K, Leong, J, Woo, C, Torsional stability of the lumbosacral junction. Significance of the iliolumbar ligament. Spine. 1989;14(6):611–615. ![]()
79. McFadden, KD, Taylor, JR, Axial rotation in the lumbar spine, and gaping of the zygapophyseal joints. Spine 1990; 15:295–299. ![]()
80. Kaschner, A, Verhalten der Wirbelgelenkkapsel bei Stellungsänderungen im lumbalen Bewegunssegment, 1976.
81. Lewin, T, Osteoarthritis in lumbar synovial joints. Acta Orthop Scand. 1966;(suppl.):73.
82. Yong-Hing, K, Reilly, J, Kirkaldy-Willis, WH. The ligamentum flavum. Spine. 1976; 1:226–234.
83. Moran, R, O’Connell, D, Walsh, MG, The diagnostic value of facet joint injections. Spine 1988; 13:1407–1410. ![]()
84. Töndury, G. Beitrag zur Kenntnis der kleinen Wirbelgelenken. Z Anat EntwGesch. 1940; 110:568.
85. Emminger, E. Die Gelenkdisci an der Wirbelsäule (eine mögliche Erklärung wirbelsäulenabhängiger Schmerz-zustände). Hefte Unfallheilk. 1955; 48:142.
86. Dörr, WR, Über die Anatomie des Wirbelgelenke. Arch Orthop Unfallchir 1985; 50:222. ![]()
87. Bogduk, N, Engel, R, The menisci of the lumbar zygapophyseal joints. A review of their anatomy and clinical significance. Spine 1984; 9:454–460. ![]()
88. Kos, J, Wolf, J. Les Menisques intervertebraux et leur rôle dans les blocages vertebraux. Ann Med Phys. 1972; 15:203–218.
89. Giles, LGF, Taylor, JR, Inter-articular synovial protrusions. Bull Hosp Joint Dis 1982; 42:248–255. ![]()
90. Bogduk, N, Jull, G. The theoretical pathology of acute locked back: a basis for manipulative therapy. Man Med. 1985; 1:78–82.
91. Berven, S, Tay, BB, Colman, W, Hu, SS, The lumbar zygapophyseal (facet) joints: a role in the pathogenesis of spinal pain syndromes and degenerative spondylolisthesis. Semin Neurol. 2002;22(2):187–196. ![]()
92. Bogduk, N, Wilson, AS, Tynan, W, The human lumbar dorsal rami. J Anat 1982; 134:389–397. ![]()
93. Grenier, N, Gressel, JF, Vital, JM, et al, Normal and disrupted lumbar longitudinal ligaments: correlative MR and anatomic study. Radiology 1989; 171:197–205. ![]()
94. Hukins, DWL, Disc structure and function. The Biology of the Intervertebral Disc. Gosh, P, eds. The Biology of the Intervertebral Disc; vol 1. CRC Press, Boca Raton, 1988:1–37.
95. Ramsey, RH, The anatomy of the ligamenta flava. Clin Orthop 1966; 44:129–140. ![]()
96. Olszewski, AD, Yaszemski, MJ, White, AA, The anatomy of the human lumbar ligamentum flavum; new observations and their surgical importance. Spine 1996; 21:2307–2312. ![]()
97. Yahia, LH, Garron, S, Strykowski, H, Rivard, CH, Ultrastructure of the human interspinous ligament and ligamentum flavum. Spine 1990; 15:262–268. ![]()
98. Heylings, DJA, Supraspinous and interspinous ligaments of the human lumbar spine. J Anat 1978; 125:127. ![]()
99. Dickley, JP, Bednar, DA, Dumas, GA, New insight into the mechanics of the lumbar interspinous ligament. Spine 1996; 21:2720–2727. ![]()
100. Hukins, DWL, Kirby, MC, Sikoryn, TA, et al, Comparison of structure, mechanical properties and functions of lumbar spinal ligaments. Spine 1990; 15:787–795. ![]()
101. Adams, MA, Hutton, WC, Stott, JRR, The resistance to flexion of the lumbar intervertebral joint. Spine 1980; 5:245. ![]()
102. Tesh, KM, Shaw Dunn, J, Evans, JH, The abdominal muscles and vertebral stability. Spine 1987; 12:501–508. ![]()
103. Pearcy, MJ, Tibrewal, SB, Lumbar intervertebral disc and ligament deformations measured in vivo. Clin Orthop Rel Res 1984; 191:281–286. ![]()
104. Leong, JCY, Luk, KDK, Chow, DHK, Woo, CW, The biomechanical functions of the iliolumbar ligament in maintaining stability of the lumbosacral junction. Spine 1987; 12:669–674. ![]()
105. Luk, KDK, Leong, JCY, The iliolumbar ligament: a study of its anatomy, development and clinical significance. J Bone Joint Surg 1986; 68B:197–200. ![]()
106. Hanson, P, Solesson, B, The anatomy of the iliolumbar ligament. Arch Phys Med Rehab 1994; 75:1245–1246. ![]()
107. Fujiwara, A, Tamai, K, Yoshida, H, et al, Anatomy of the iliolumbar ligament. Clin Orthop Relat Res 2000; 380:167–172. ![]()
108. Basadonna, PT, Gasparini, D, Rucco, V, Iliolumbar ligament insertions. In vivo anatomic study. Spine (Phila Pa 1976). 1996;21(20):2313–2316. ![]()
109. Mitchell, GA. The lumbosacral junction. J Bone Joint Surg. 1934; 16:233–254.
110. Yamamoto, I, Panjabi, MM, Oxland, TR, Crisco, JJ, The role of the iliolumbar ligament in the lumbosacral junction. Spine 1990; 15:1138–1141. ![]()
111. Aihara, T, Takahashi, K, Yamagata, M, et al, Biomechanical functions of the iliolumbar ligament in L5 spondylolysis. J Orthop Sci. 2000;5(3):238–242. ![]()
112. Aihara, T, Takahashi, K, Yamagata, M, et al, Does the iliolumbar ligament prevent anterior displacement of the fifth lumbar vertebra with defects of the pars? J Bone Joint Surg Br. 2000;82(6):846–850. ![]()
113. Hasue, M, Fujiwara, M, Kikuchi, S, A new method of quantitative measurement of abdominal and back muscle strength. Spine 1980; 5 153–148. ![]()
114. Bogduk, N, A reappraisal of the anatomy of the human erector spinae. J Anat 1980; 131:525–540. ![]()
115. Bogduk, N. Clinical anatomy of the lumbar spine, 4th ed. Edinburgh: Churchill Livingstone; 2005.
116. Bogduk, N, Macintosh, JE, The applied anatomy of the thoracolumbar fascia. Spine 1984; 9:164–170. ![]()
117. Vleeming, A, Pool-Goudzwaard, A, Stoeckaert, R, et al, The posterior layer of the thoracolumbar fascia. Spine 1995; 20:753–758. ![]()
118. de Berail, A. Exploration radiologique du rachis lombaire normal et son application au syndrôme du canal lombaire étroit. Thesis of Medicine. 1976; 176.
119. Duby, P. Contribution à l’étude anatomique du cul-de-sac dural. Implications en médecine ostéopsyhique. Ann Méd Ostéop. 1985; 1(1):9–14.
120. Hofmann, M. Die Befestigung der Dura mater im Wirbelkanal. Arch Anat Physio (Anat Abt). 1899; 403.
121. Parkin, IG, Harrison, GR, The topographical anatomy of the lumbar epidural space. J Anat 1985; 141:211–217. ![]()
122. Scapinelli, R, Anatomical and radiological studies on the lumbosacral meningovertebral ligaments of humans. J Spinal Disord 1990; 3:6–15. ![]()
123. Posner, I, White, AA, Edwards, WT, Hayes, WC, A biomechanical analysis of the clinical stability of the lumbar and lumbosacral spine. Spine 1982; 7:374–389. ![]()
124. Tencer, AF, Allen, BL, Ferguson, RL, A biomechanical study of thoraco-lumbar spine fractures with bone in the canal. Part III: Mechanical properties of the dura and its tethering ligaments. Spine. 1985;10(8):741–747. ![]()
125. Hollinstead, WH. Anatomy for Surgeons, Vol 3: The Back and Limbs. New York: Harper & Row; 1969.
126. Wiltse, LL, Fonseca, AS, Amster, J, et al, Relationship of the dura, Hofmann’s ligaments, Batson’s plexus and a fibrovascular membrane lying on the posterior surface of the vertebral bodies and attaching to the deep layer of the posterior longitudinal ligament. Spine 1993; 18:1030–1043. ![]()
127. Nowicki, BH, Haughton, VM, Neural foraminal ligaments of the lumbar spine: appearance at CT and NMR imaging. Radiology 1992; 183:257–264. ![]()
128. O’Connell, JEA. The clinical signs of meningeal irritation. Brain. 1946; 69:9–21.
129. Breig, A. Adverse Mechanical Tension in the Central Nervous System. Stockholm: Amqvist & Wiksell; 1978.
130. Reid, JD, Effects of flexion–extension movements of the head and the spine upon the spinal core and nerve roots. J Neurosurg Psychiatry 1960; 23:214–221. ![]()
131. Adams, C, Logue, V, Studies in cervical spondylotic myelopathy. I. Movement of the cervical roots, dura and cord and their relation to the course of extrathecal roots. Brain 1971; 94:557–568. ![]()
132. Bourret, P, Louis, R. Anatomie du système nerveux central, 2nd ed. Paris: Expansion Scientifique Française; 1974.
133. Louis, R. Dynamique vertébro-radiculaire et vertébro-médullaire. Anat Clin. 1981; 3:1–11.
134. Decker, R. La Mobilité de la moelle épinière à l’intérieur du canal vertébral. Ann Radiol (Paris). 1961; 83:883–888.
135. Klein, P, Burnotte, J. Contribution à l’étude biomécanique de la moelle épinière et ses enveloppes. Ann Méd Ostéopath. 1(3), 1985.
136. Goddard, MD, Reid, JD, Movements induced by straight-leg raising in the lumbo-sacral roots, nerves and plexus, and in the intrapelvic section of the sciatic nerve. J Neurol Neurosurg Psychiatry 1965; 28:12–18. ![]()
137. Ko, HY, Park, BK, Park, JH, et al, Intrathecal movement and tension of the lumbosacral roots induced by straight-leg raising. Am J Phys Med Rehabil. 2006;85(3):222–227. ![]()
138. Smith, SA, Massie, JB, Chesnut, R, Garfin, SR, Straight leg raising. Anatomical effects on the spinal nerve root without and with fusion. Spine (Phila Pa 1976). 1993;18(8):992–999. ![]()
139. Martins, AN, Dynamics of the cerebrospinal fluid and the spinal dura mater. J Neurol Neurosurg Psychiatry 1972; 35:468–473. ![]()
140. Cyriax, JH. Lumbago: the mechanism of dural pain. Lancet. 1945; ii:427.
141. El Mahdi, MA, Latif, FYA, Janko, M, The spinal nerve root innervation, and a new concept of the clinicopathological interrelations in back pain and sciatica. Neurochirurgia 1981; 24:137–141. ![]()
142. Smyth, MJ, Wright, V, Sciatica and the intervertebral disc. An experimental study. J Bone Joint Surg 1959; 40A:1401–1418. ![]()
143. Walton JN, ed. Brain’s Diseases of the Nervous System. 8th ed. Oxford University Press, Oxford, 1977:407–408.
144. Guyer, RD, Collier, RR, Ohnmeiss, DD, et al, Extraosseous spinal lesions mimicking disc disease. Spine 1988; 13:228–231. ![]()
145. Cuatico, W, Parker, JC, Further investigations on spinal meningeal nerves and their role in pain production. Acta Neurochir 1989; 101:126–128. ![]()
146. Pedersen, HE, Conrad, FJ, Blunck, MD, Gartner, E, The anatomy of lumbosacral posterior rami and meningeal branches of spinal nerves (sinu-vertebral nerves) with an experimental study of their functions. J Bone Joint Surg. 1956;38A(2):377. ![]()
147. Stilwell, DL, The nerve supply of the vertebral column and its associated structures in the monkey. Anat Red. 1956;125(2):139–162. ![]()
148. Jackson, HC, Winkelmann, RK, Bickel, WH, Nerve endings in the human lumbar spinal column and related structures. J Bone Joint Surg 1966; 48A:1272–1281. ![]()
149. Edgar, MA, Nundy, S. Innervation of the spinal dura mater. J Neurol Neurosurg Psychiatry. 1966; 29:530–534.
150. Edgar, MA, Ghadially, JA, Innervation of the lumbar spine. Clin Orthop Rel Res 1976; 115:35–41. ![]()
151. Ahmed, M, Bjurholm, A, Kreicbergs, A, Schultzberg, M, Neuropeptide Y, tyrosine hydroxylase and vasoactive intestinal polypeptide – immunoreactive nerve fibers in the vertebral bodies, discs, dura mater and spinal ligaments of the rat lumbar spine. Spine 1993; 18:268–273. ![]()
152. Kallakari, S, Cavanaugh, JM, Blagoev, DC, An immunohistochemical study of innervation of lumbar spinal dura and longitudinal ligaments. Spine 1998; 23:403–411. ![]()
153. Groen, GJ, Baljet, B, Drukker, J, The innervation of the spinal dura mater: anatomy and clinical implications. Acta Neurochir (Wien) 1988; 92:39–46. ![]()
154. Groen, GJ, Baljet, B, Drukker, J, Nerves and nerve plexuses of the human vertebral column. Am J Anat 1990; 188:289–296. ![]()
155. Pertuiset, B. Compression extra-durale et extra-médullaire. Rev Prat. 1966; XVI(19):2605–2612.
156. Buchheit, F, Maitrot, D, Middleton, L, Gusmao, S. Narrow radicular canal. In: Wackenheim A, Babin E, eds. The Narrow Lumbar Canal. Berlin: Springer, 1980.
157. Bose, K, Balasubramaniam, P, Nerve root canals of the lumbar spine. Spine 1984; 9:16. ![]()
158. Ciric, I, Mikhael, MA, Tarkington, JA, Vick, NA, The lateral recess syndrome. J Neurosurg 1980; 53:433. ![]()
159. Weinstein, PR, The application of anatomy and pathophysiology in the management of lumbar spine disease. Clin Neurosurg 1980; 27:517. ![]()
160. Crock, HV, Normal and pathological anatomy of the lumbar spinal nerve root canals. J Bone Joint Surg 1981; 63B:487. ![]()
161. Neirdre, A, MacNab, I, Anomalies of the lumbosacral nerve roots. Spine 1983; 8:294–299. ![]()
162. Kadish, LJ, Simmons, EH, Anomalies of the lumbosacral nerve roots. J Bone Joint Surg 1984; 66B:411–416. ![]()
163. Dorwart, RH, Vogler, JB, Helms, CA, Spinal stenosis. Radiol Clin North Am 1983; 21:201. ![]()
164. Brieg, A, Biomechanics of the lumbosacral nerve roots. Acta Radiol (Diagn) (Stockh) 1963; 1:1141. ![]()
165. Akdemir, G, Thoracic and lumbar intraforaminal ligaments. J Neurosurg Spine. 2010;13(3):351–355. ![]()
166. Kraan, GA, Delwel, EJ, Hoogland, PV, et al, Extraforaminal ligament attachments of human lumbar nerves. Spine (Phila Pa 1976). 2005;30(6):601–605. ![]()
167. Forestier, J. Le Trou de conjugaison vertébral et l’espace epidural. Etude Anatomique et Clinique. 1922.
168. Spencer, DL, Irwin, GS, Miller, JA, Anatomy and significance of fixation of the lumbosacral nerve roots in sciatica. Spine. 1983;8(6):672–679. ![]()
169. Grimes, PF, Massie, JB, Garfin, SR, Anatomic and biomechanical analysis of the lower lumbar foraminal ligaments. Spine (Phila Pa 1976). 2000;25(16):2009–2014. ![]()
170. Sunderland, S, Meningeal neural relations in the intervertebral foramen. J Neurosurg 1974; 40:756–763. ![]()
171. Falconer, MA, McGeorge, M, Begg, CA, Observations on the cause and the mechanism of symptom production in sciatica and low back pain. J Neurol Neurosurg Psychiatry 1948; 11:13–26. ![]()
172. Charnley, J, Orthopaedic signs in the diagnosis of disc protrusion. Lancet 1951; i:186–192. ![]()
173. Estridge, MN, Rouhe, SA, Johnson, NG, The femoral stretching test. J Neurosurg 1982; 57:813–817. ![]()
174. Breig, A, Troup, J, Biomechanical considerations in the straight-leg-raising test. Cadaveric and clinical studies of the effect of medial hip rotation. Spine 1979; 4:242–250. ![]()
175. Cyriax, JH. The Slipped Disc, 2nd ed. Epping: Gower Press; 1975.
176. Lindahl, O, Hyperalgesia of the lumbar nerve roots in sciatica. Acta Orthop Scand 1966; 37:367. ![]()
177. Murphy, RW, Nerve roots and spinal nerves in degenerative disc disease. Clin Orthop 1977; 129:46. ![]()
178. Inman, VT, Saunders, JB. The clinico-anatomical aspects of the lumbo-sacral region. Radiology. 1942; 38:669.
179. O’Connell, JEA. Sciatica and the mechanism of the production on the clinical syndrome in protrusions of the lumbar intervertebral discs. Br J Surg. 1943; 30:315.
180. Parkin, IG, Harrison, GR, The topographical anatomy of the lumbar epidural space. J Anat 1995; 141:211–217. ![]()
181. Batson, OV, The function of the vertebral veins and their role in the spread of metastasis. Ann Surg 1940; 112:138. ![]()
182. Cyriax, JH. Textbook of Orthopaedic Medicine, vol I, Diagnosis of Soft Tissue Lesions, 8th ed. London: Baillière Tindall; 1982.
183. Luschka, H. Die Nerven des menschlichen Wirbelkanales. H. Lapschen Buchhandlung. 1850; 4850(8):1.
184. Wiberg, G, Back pain in relation to the nerve supply of the intervertebral disc. Acta Orthop Scand 1948–50; 214:18–19. ![]()
185. Lazorthes, G, Poulhes, J, Espagno, J. Étude sur les nerfs sinu-vertébraux lombaires. Le Nerf de Roofe, existe-t-il? Compt Rend Assoc Anat. 34, 1948.
186. Gilchrist, RV, Isaac, Z, Bhat, AL, Innervation of the anterior spinal canal: an update. Pain Physician. 2002;5(2):167–171. ![]()
187. Bradley, KC, The anatomy of backache. Aust NZ J Surg 1974; 44:227–232. ![]()
188. Sunderland, S. Nerves and Nerve Injuries, 2nd ed. Edinburgh: Churchill Livingstone; 1978.
189. Bogduk, N. The innervation of the lumbar spine. Spine. 1985; 8:286–293.
190. Bogduk, N, Wilson, AS, Tynan, W, The human lumbar dorsal rami. J Anat 1982; 134:383–397. ![]()