Chapter 31 Applied Anatomy of the Cervical Spine
Spinal Column
Occipitocervical Junction
Osseous Elements
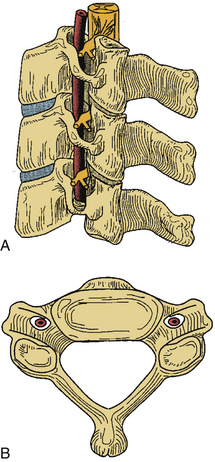
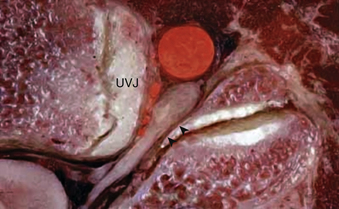
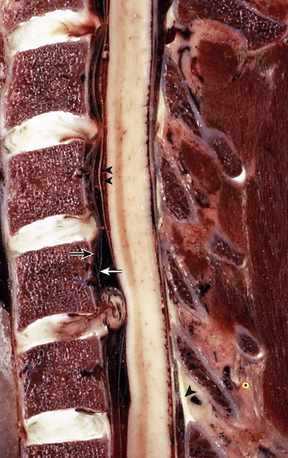
The first and second vertebral segments attach to the occiput to form the craniocervical junction, which is a complex articular system permitting rotational and nutational movement. The first cervical segment, also known as C1 or the atlas, is composed of a ventral and dorsal arch, joined laterally by symmetrical lateral masses (Figs. 31-1 to 31-3). The superior and inferior articulating surfaces of the lateral mass are concave to enable articulation with the occipital condyles, superiorly, and the shoulders of the axis, inferiorly (Figs. 31-4 and 31-5). The ventral arch forms a short bridge between the lateral masses, with a dorsal articular surface forming a synovial joint with the odontoid process of C2 and an anterior tubercle ventrally to which the longus colli muscle attaches. The dorsomedial walls of the C1 lateral masses form a small tubercle that serves as the attachment for the transverse atlantoaxial ligament. The dorsal arch of the atlas typically forms a posterior tubercle in the midline that is a rudimentary equivalent to subaxial spinous processes. Caution should be exercised in dissection of this structure because the dorsal arch may be incomplete. The dorsal arch is round in cross-section in the midline and attenuates laterally to form a flatter surface where it attaches to the dorsal lateral masses. The superior surface of the lateral dorsal arch forms a groove known as the sulcus arteriosus on which the vertebral arteries run bilaterally; it is often thin or even dehiscent and great care must be taken when exposing the inferior surface of the C1 lateral mass. The lateral masses are bounded laterally by short transverse processes that are fenestrated by the transverse foramen, or foramen transversarium. As in the subaxial spine, the vertebral artery traverses the transverse foramen of C1 before turning 90 degrees medially to run along the superior surface of the dorsal arch, as described previously. This genu lies 1.5 to 2 cm lateral to midline1 and dorsal dissection should seldom require exposure of this precarious region.
The second vertebral segment, or axis (see Figs. 31-1 to 31-4), is a distinctive osseous structure. The odontoid process, a peglike rostral projection, forms a synovial joint with the atlas along its ventral border and allows the atlas to rotate on C2, with translational restriction created by a complex of ligamentous structures. The tip of the odontoid process serves as the attachment for the apical ligament, which connects C2 to the basion (the ventral lip of the foramen magnum). The tip is flanked by two bony prominences that serve as the attachments for the paired alar ligaments that span the divide between C2 and the occipital condyles. The transverse atlantoaxial ligament traverses the dorsal border of the odontoid process, which often carries a groove for this strong ligament. The neck of the odontoid process narrows to meet the vertebral body of C2 and is a common site for C2 fracture. The vertebral body culminates rostrally to form two bilaterally symmetrical “shoulders” that flank the odontoid process and articulate with the lateral masses of C1. A relatively long pars interarticularis spans the interval between superior and inferior articulating processes and is laterally bounded by the transverse process of C2. The disproportionate length of the C2 pars interarticularis has important clinical implications. Because the superior and inferior articulating processes of C2 are coronally offset, extension applies significant strain on the C2 pars interarticularis. Under forceful hyperextension, the pars interarticularis may fracture, giving rise to the mechanism and morphology of the so-called hangman’s fracture. As at other levels, this process is fenestrated by the transverse foramen, which lies immediately lateral to the C2 pedicle and serves as the conduit for the vertebral artery. Unlike at other levels, the C2 foramen is angulated 45 degrees laterally so that the vertebral artery is partially roofed by the superior articular process.2 The inferior articular process forms an articulation with the C3 superior articular process and assumes the more typical orientation of subaxial lateral masses.
Ligamentous Structures
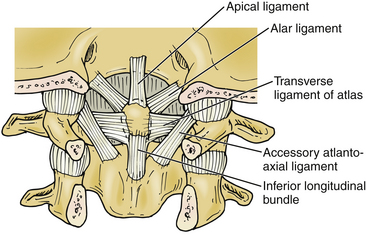
Although the articular surfaces of the occipitocervical junction are oriented to permit constrained flexibility, a complex of intervening ligamentous structures acts in concert to restrict excessive translation and rotation (see Figs. 31-2 to 31-4). Three ligaments span the divide between the odontoid process and the occiput. In the midline, the apical ligament spans the interval between the basion and the tip of the odontoid process. This ligament, also known as the middle odontoid ligament or suspensory ligament, is of unclear biomechanical significance because it has been described as absent in 20% of specimens.3 The alar ligaments are bilaterally symmetrical structures approximately 1 cm in length spanning from the dorsolateral odontoid tip to the medial occipital condyles. Each alar ligament restricts excessive rotation to the contralateral side and excessive lateral bending to the contralateral side.4
The cruciate, or cruciform, ligament is the most important ligamentous structure of the craniovertebral junction. It is composed of four limbs that unite over the dorsal odontoid process. The superior limb, or ascending band, inserts on the occiput, whereas the inferior limb, or descending band, inserts on the dorsal body of C2. The transverse atlantoaxial ligament forms the transverse limbs of this complex and attaches to bony tubercles on the medial borders of the C1 lateral masses. The transverse ligament is a strong, inelastic structure composed of primarily dense collagen with a ventrolateral transition to fibrocartilaginous tissue near its insertion on the C1 lateral masses. As a consequence, this lateral transition portion is the zone most susceptible to traumatic rupture, and the transverse atlantoaxial ligament has been demonstrated to rupture under loads of 400 to 1100 N.5 Rupture of this ligament may be identified on MRI, particularly on gradient-echo sequences,6 and is associated with atlantoaxial instability. On occasion, the insertion of this ligament will fracture off the medial wall of one or both lateral masses while the ligament itself remains intact. CT and MRI characterization of these disruption patterns has important implications for the management of traumatic atlantoaxial instability.
Subaxial Cervical Spine
Osseous Structures
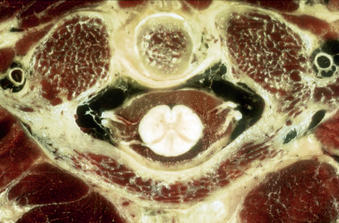
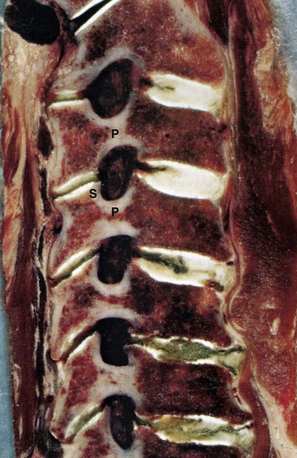
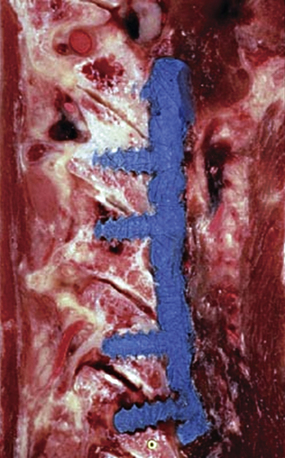
Each vertebral segment can be divided into a ventral portion, the vertebral body, and a dorsal portion, the dorsal or vertebral arch. The cervical vertebral body is roughly cylindrical in geometry, although the anteroposterior diameter is typically smaller than the transverse diameter. Relative to the vertebral arch, the cervical vertebral bodies are smaller than the vertebral bodies of the thoracolumbar spine, likely because they bear significantly less load. The superior and inferior surfaces of the vertebral body serve as the superior and inferior end plates, respectively. The lateral edges of the superior end plate curve sharply upward to form the uncinate processes bilaterally, a unique feature of the cervical spine. These processes articulate with complementary bevels on the lateral surfaces of the adjacent inferior end plate. Although this articulation is referred to as the “uncovertebral joint,” it contains no synovial fluid and as a consequence is not a true joint7 (Figs. 31-6 and 31-7).
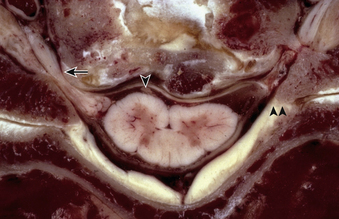
The most distinctive feature of the cervical spine is the fenestration of the transverse processes that flank each vertebral body. The transverse process projects ventrolaterally with a deep groove along its superior surface; this serves to carry the cervical spinal nerves. The transverse process terminates laterally with two prominences—the anterior and posterior tubercles. The anterior tubercle serves as an attachment for the ventral cervical musculature and the posterior tubercle serves as an attachment for the dorsal cervical musculature. The transverse foramen, or foramen transversarium, fenestrates the transverse process and carries the vertebral arteries bilaterally (Figs. 31-8 to 31-10).
Importantly, the cervical pedicle serves as the dorsomedial wall of the foramen transversarium, exposing the vertebral artery to hazard when pedicle screws breach laterally. The cervical pedicles connect the dorsal arch with the vertebral body and are angled medially between 38 and 48 degrees from the midsagittal plane.8 The pedicles of C3 through C7 range in outer diameter from 6.0 to 6.5 mm9 and in inner diameter from 2.7 to 3.1 mm.8 The pedicle is thinnest along its lateral wall. In aggregate, these features make safe placement of cervical pedicle screws a challenge. The medial wall is close to the thecal sac, the lateral wall is close to the vertebral artery, and the superior wall is close to the superjacent nerve root.
The superior and inferior articular processes of the cervical spine are oriented obliquely on sagittal projection, with complementary surfaces on adjacent segments. Together with the intervening bone, these articular processes combine to form lateral masses at each level that are parallelogram-shaped in sagittal cross-section. Adjacent lateral masses in the subaxial cervical spine are in close apposition so that in aggregate they form a flexible, cylindrical column of bone dorsolateral to the vertebral bodies. This pillar-like architecture affords axial load-bearing capacity to the dorsal vertebral arches. The oblique configuration of articular surfaces imparts a shingle effect to the lateral masses and allows for flexion and extension while restricting translation, affording osseous neuroprotection for the enveloped cervical spinal cord and nerve roots (see Fig. 31-6). The lateral mass serves as a common anchor point for instrumentation, but an awareness of adjacent neurovascular structures is essential for safe placement of lateral mass screws that avoid the vertebral artery and cervical nerve roots. A rostral screw trajectory is protective of the exiting nerve root, whereas a lateral trajectory protects the vertebral artery (Figs. 31-11 to 31-13).
The dorsal arch is completed by bilateral laminae that unite in the midline to form a bifid spinous process rostrally (typically from C3 to C5) and a monofid spinous process caudally (typically from C6 and below). The laminae are narrow, with a thinner superior than inferior edge. The height of the lamina is 10 to 11 mm at C4, whereas the thickness of the lamina is about 2 mm at C5.10 The spinous processes act as insertions for the semispinalis cervicis muscle. In aggregate, the pedicles, lateral masses, laminae, and spinous processes form the dorsal vertebral arch, which circumscribes the spinal cord and affords neuroprotective function (see Fig. 31-8).
Discoligamentous Structures
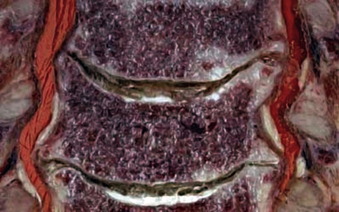
The cervical disc space is created by the interval between adjacent superior and inferior vertebral end plates. The inferior end plate is typically concave, creating a ventral lip on the superjacent vertebral body and taller disc space at midbody. Together, the cervical discs constitute 20% of the total cervical height.11 In a normal cervical spine the disc space height increases with consecutive levels from C3-4 to C7-T1 and the disc space depth increases from approximately 16 mm at C3 to 20 mm at C7.12 These progressive dimensional changes must be factored into instrumentation selection for ventral cervical procedures.
The intervertebral disc is composed of two components: a central nucleus pulposus that is circumscribed in axial cross-section by the anulus fibrosus. The nucleus pulposus is an avascular structure composed of loose fibrous strands suspended in a proteoglycan gel. It has a high water content that can be readily evaluated on MRI and its hydration declines with normal aging, with 88% water content at birth declining to 64% by the seventh decade13 (see Fig. 31-6). The intervertebral disc receives nutrition by diffusion14 and creates an immune-isolated avascular space susceptible to infection. The anulus fibrosus is composed of concentric rings of obliquely oriented fibers spanning the disc space from one vertebral body to the other. These fibers insert on the epiphyseal ring of the end plate, where the anchoring fibers are called Sharpey fibers. These concentric fibrous rings confer multiaxial shear resistance, like the steel belt of an automobile tire (Fig. 31-14).
The ALL is composed of interdigitating collagen fibers running longitudinally from the anterior tubercle of C1 along the ventral vertebral bodies to the sacrum. It is a broad-based, flat structure without clear lateral boundaries that attenuates laterally where it merges with paramedian, prevertebral connective tissue. The fibers of the ALL are lamellated, with the deepest fibers spanning only adjacent levels, its more superficial layers spanning two to three levels, and its most superficial layers spanning many levels. It serves to restrict hyperextension and excessive axial traction and is adherent to the underlying vertebral bodies and intervertebral discs. Along with the anulus fibrosus, the ALL is the predominant restricter to hyperextension. The posterior longitudinal ligament runs along the dorsal vertebral bodies from C2 to the sacrum. Its rostral extension, called the tectorial membrane, inserts at the basion, along the clivus of the occiput. Like the ALL, it is a flat band of fibrous collagen tissue, although it is composed of two layers. The ventral layer is adherent to the dorsal vertebral bodies and intervertebral discs and the dorsal layer is adjacent to the thecal sac. The epidural venous plexus is sandwiched between these two layers. The posterior longitudinal ligament is three to four times thicker in the cervical spine than in the thoracolumbar spine and serves to restrict hyperflexion and axial traction.15 It is believed to provide further resistance to disc herniation and neural compression (Fig. 31-15).
The dorsal elements are spanned by numerous ligamentous structures that permit constrained flexibility in the cervical spine. The ligamentum flavum is a two-layered structure that spans the interlaminar space between adjacent segments, originating on the ventral surface of the superjacent lamina approximately halfway up the lamina and inserting on the superior edge of the subjacent lamina.16 Importantly, it also inserts laterally on the medial edge of the superior articular process. The ligamentum flavum derives its name from its high elastin content, which confers a yellow appearance to it.13 The ligamentum flavum restricts hyperflexion; with aging, the contractile elasticity of the ligamentum flavum diminishes and hyperextension creates redundancy in this structure, which may narrow the anteroposterior diameter of the spinal canal, potentially contributing to spinal cord compression.
Intertransverse ligaments are short bands of fibrous tissue that bridge adjoining transverse processes. These ligaments serve to restrict lateral cervical bending. The interspinous ligaments are paired midline structures that bridge adjoining spinous processes. In the cervical spine the nuchal ligament represents the rostral extension of the supraspinous ligament. It spans the interval from the occipital protuberance rostrally to the spinous process of C7 caudally. It is a thick, elastic fibrous band that serves to resist hyperflexion. These ligaments are predominant elements of the so-called posterior tension band (Fig. 31-16; see also Fig. 31-15).
Neural Elements
Cervical Spinal Cord
The spinal cord is a roughly cylindrical neural continuation of the caudal medulla. In cross-section, the spinal cord is bilaterally symmetrical and can be separated into the central, butterfly-shaped gray matter and the circumferential white matter, which is mostly composed of longitudinally oriented spinal tracts. The perimeter of the spinal cord is demarcated by longitudinally oriented sulci and fissures that serve to divide the spinal cord longitudinally into white matter columns. The ventral median fissure is a true, pia-lined space in which the anterior spinal artery runs. The other circumferential demarcations are less defined. The posterior median sulcus separates the left and right hemicords, the anterolateral sulcus is marked by the emergence of the ventral/motor roots, and the dorsolateral sulcus is marked by the entry of the dorsal/sensory roots. These sulci divide each half of the spinal cord into three principal columns: the anterior column (in the interval between the ventral median fissure and the anterolateral sulcus), the lateral column (between the anterolateral and posterolateral sulci), and the posterior column (between the posterolateral sulcus and the posterior median sulcus). In the cervical spinal cord, the posterior column is further subdivided by the intermediate sulcus into a lateral fasciculus cuneatus and a medial fasciculus gracilis. In the cervical spine, the spinal cord segment is at approximately the same level as the same-numbered spinal column segment (see Fig. 31-16).
Cervical Spinal Nerves
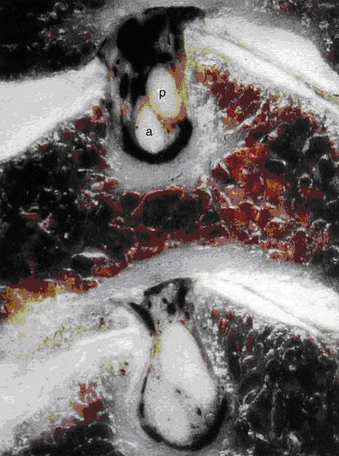
The cervical spinal nerves represent the union of ventral and dorsal roots that arise independently from the cervical spinal cord, as outlined previously. Each root, in turn, represents the union of numerous rootlets that arise from the anterolateral sulcus (for the ventral root) and posterolateral sulcus (for the dorsal root). The anterolateral sulcus is only 1 to 3 mm lateral to midline, and, as a consequence, midline ventral compressive lesions may exert pressure on these exiting ventral rootlets17 (see Fig. 31-16). Moreover, this branching architecture allows for intradural anastomoses between nerve roots, which may cause atypical dermatomal or myotomal distribution of radiculopathy.18–20 Within the dorsal root, before splitting into numerous dorsal rootlets, large fibers for proprioception run medially and ventrally and small pain fibers are located laterally and dorsally. The ventral and dorsal roots both traverse the intervertebral neural foramen above their same-numbered pedicle (i.e., the C5 nerve roots exit in the C4-5 neural foramen, above the C5 pedicle). The C8 pedicle exits the C7-T1 intervertebral foramen. The first cervical root does not have a dorsal root ganglion and consequently does not have a corresponding sensory dermatome.
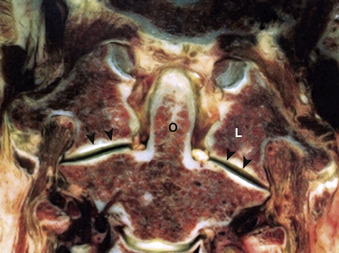
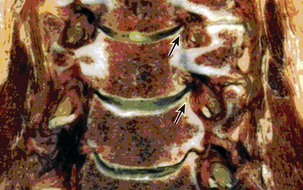
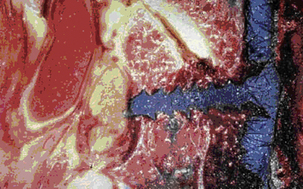
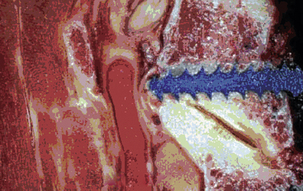
The cervical spinal nerve roots traverse the intervertebral foramen close to numerous adjacent osseous, discoligamentous, and vascular structures. A thorough understanding of these relationships facilitates safe and effective decompression when clinically indicated. The nerve root exits the neural foramen immediately above the like-numbered pedicle, as described previously. Within the intervertebral foramen, the dorsal root is located more rostrally than the ventral root; hence, compressive pathologies may disproportionately affect the ventral or dorsal root, causing dissociation of radicular symptoms. The course of the exiting nerve root is oblique with a ventrolateral trajectory that varies across levels. At its origin, the foramen is bounded ventromedially by the uncovertebral joint; more laterally, the nerve root and dorsal root ganglion abut the vertebral artery. Uncovertebral hypertrophy and lateral disc extrusions may cause compression or distortion of the nerve root, triggering radiculopathy (Fig. 31-17). The vertebral artery, which lies immediately lateral to the uncovertebral joint, may also become distorted by this uncovertebral hypertrophy, causing a secondary compressive effect on the exiting nerve root or, more typically, the dorsal root ganglion21 (see Fig. 31-9). The dorsolateral wall of the foramen is defined by the superior and inferior articular processes of the adjacent vertebral bodies, and facet hypertrophy may similarly precipitate radiculopathy. This topography gives rational basis to the Spurling maneuver on physical examination, in which an irritable nerve root may be triggered by hyperextension and lateral bending toward the affected nerve root. Although the cranial and caudal bounds of the foramen are defined by the superjacent and subjacent pedicles, the cervical nerve roots lie more caudally within this interval, often directly abutting the roof of the subjacent pedicle (Fig. 31-18).
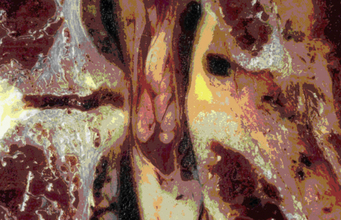
FIGURE 31-17 Disc-osteophyte complex exerting pressure on obliquely oriented ventral and dorsal rootlets.
Vascular Anatomy
Arterial Anatomy
Spinal Cord Perfusion
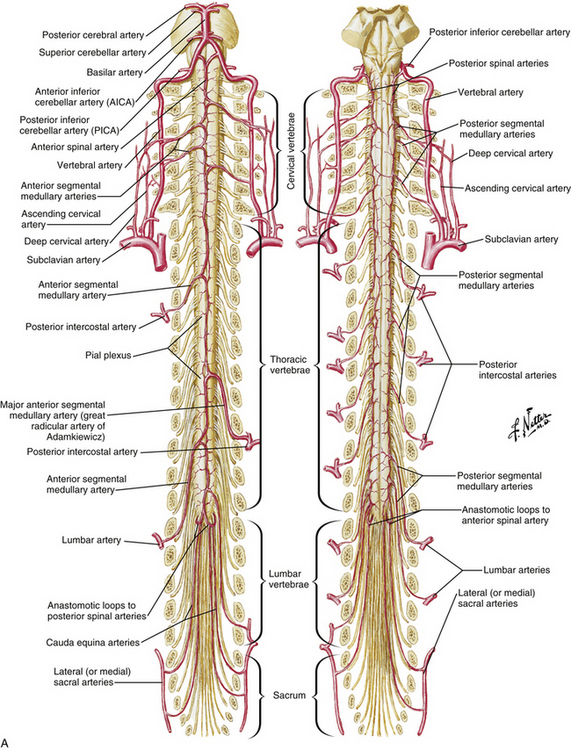
The spinal cord is perfused by three principal arteries: the anterior spinal artery (ASA) and the paired posterior spinal arteries (PSAs; Fig. 31-19). None of these vessels represent single vessels that traverse the length of the spinal cord; rather, each represents a complex of anastomotic, interrupted, longitudinal vessels that together run the length of the cord.22 These interrupted vessels draw supply from numerous segmental vessels along the length of the spine.23,24 Moreover, the intrinsic perfusion of the spinal cord parenchyma is achieved by two independent vascular systems: the centripetal system runs along the perimeter of the spinal cord in a perimedullary plexus that gives rise to arteries that penetrate radially into the spinal cord parenchyma; the centrifugal system originates deep within the anterior median fissure from branches of the ASA.25 In general, the centripetal system perfuses the white matter of the spinal cord and the centrifugal system perfuses the gray matter.
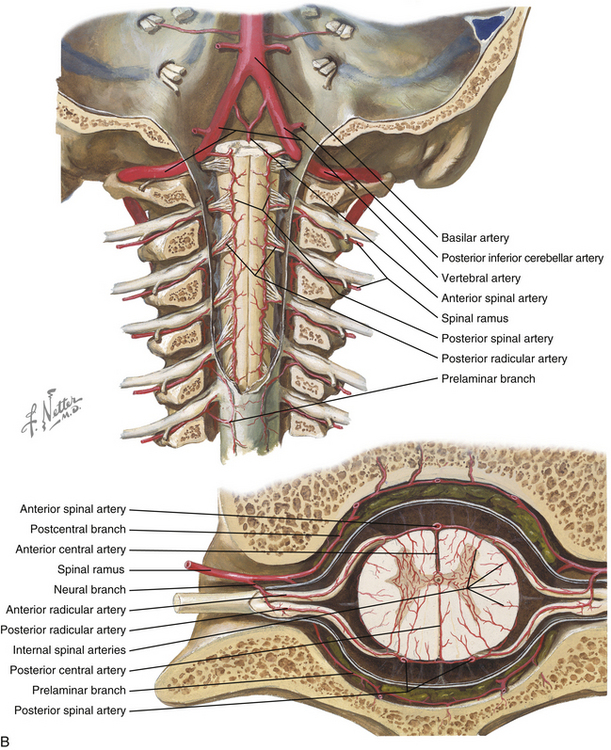
The ASA originates from descending branches of bilateral intracranial vertebral arteries and enters the midline ventral median fissure. It supplies the ventral two thirds of the spinal cord; ASA infarct typically causes dysfunction of the anterior columns, spinothalamic tracts, and corticospinal tracts. The PSAs arise from the intracranial vertebral arteries or posterior inferior cerebellar arteries.11 The paired PSAs run in the dorsolateral sulci and form a peripheral anastomotic plexus perfusing the dorsal one third of the spinal cord.
Vertebral Artery Anatomy
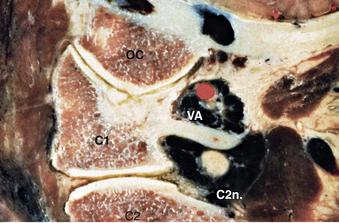
The vertebral arteries are paired vessels that lie close to the cervical spine. The vertebral artery arises from the subclavian artery, from the innominate artery, or directly from the aorta. The course of the vertebral artery is divided into four segments. The V1 segment represents the segment from origin until the artery enters its first foramen transversarium. In 87.5% of cases, this is at the C6 level; in 5.4% it is at C7; in 6.6% it is at C5, and in 0.4% it is at C4.26 The V2 segment is the portion that traverses the foramina transversaria and terminates after the C2 foramen transversarium. This is the most vulnerable segment of the vertebral artery in most common cervical spine surgeries. The V2 segment may have a tortuous course, and awareness of these anomalies must be achieved radiographically before performing cervical surgery (Fig. 31-20). Moreover, the foramen transversarium is closer to the uncovertebral joint at more rostral levels, warranting more cautious uncovertebral drilling. The V3 segment continues until the vertebral artery penetrates the dura. The V4 segment is the intradural portion of the vessel, ending at the vertebrobasilar junction (Fig. 31-21).
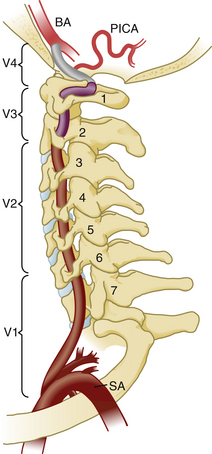
FIGURE 31-21 Vertebral artery anatomy. BA, basilar artery; PICA, posterior inferior cerebellar artery; SA, subclavian artery.
(Used with permission from Dickman CA, Fehlings MG, Gokaslan ZL, editors: Spinal cord and spinal column tumors, New York, 2006, Thieme.)
Numerous muscular and osseous branches arise from the vertebral artery at each level. At each level in the subaxial spine, anterior and posterior central arteries contribute to an epidural plexus that provides perfusion to the vertebral bodies. Anterior and posterior ascending arteries arise from the vertebral arteries at the C2 level and anastomose to perfuse the atlantoaxial complex. Collateral arterial supply is recruited from the thyrocervical and costocervical trunks in the lower cervical spine and the ascending pharyngeal and occipital arteries in the upper cervical spine. The odontoid process is perfused by an arcade of arteries, with the base of the odontoid supplied by the ascending branches of the vertebral artery and the tip supplied by the apical artery of the odontoid process, a branch of the hypoglossal artery.27
Venous Anatomy
The intrinsic spinal cord is drained centrifugally by radially oriented veins that empty into a circumferential venous plexus called the vasa corona. The dorsal half of the spinal cord venous drainage empties into this plexus, eventually converging on the median dorsal longitudinal vein, whereas the ventral half of the spinal cord drainage empties into a comparable plexus, eventually draining into the median ventral longitudinal vein. These two principal longitudinal veins collateralize through the venous vasa corona and empty into the epidural venous plexus by way of medullary veins. The epidural venous plexus is a complex but organized system of valveless vessels that allow bidirectional flow, eventually draining into the vena cava and azygos veins. Just as the Batson venous plexus is implicated in the migration of neoplastic and infectious processes in the caudal spine, the pharyngovertebral veins have been implicated in the migration of parapharyngeal infections to the cervical intradural and epidural compartments.28
Surgically Relevant Adjacent Structures
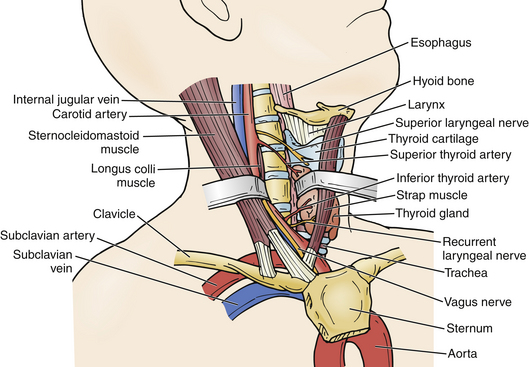
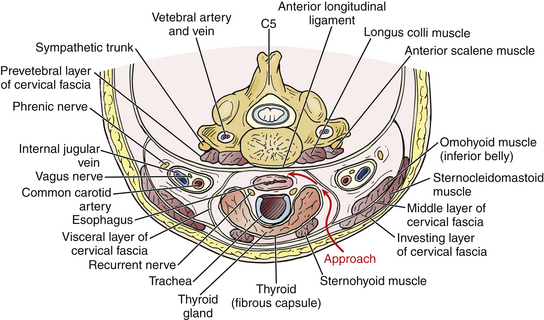
Several structures are readily visible or palpable along the ventral neck and serve as landmarks for underlying structures. The hyoid bone is the most rostral palpable landmark in the midline and roughly correlates with the C3 vertebral body. The thyroid cartilage, immediately inferior to this, corresponds to the C4 vertebral body. The cricoid cartilage is a general landmark for the C6 vertebral body. On occasion, the anterior tubercle of the C6 transverse process, also known as the Chassaignac tubercle, may be palpable. Palpation of these structures facilitates optimal placement of ventral neck incisions (Fig. 31-22).
The platysma muscle lies immediately deep to the skin and subcutaneous tissue. It can be divided to expose the medial border of the sternocleidomastoid and strap muscles. As its name implies, the sternocleidomastoid runs from the mastoid rostrally to the sternum and clavicle caudally. Deep to this muscle is the carotid sheath, containing the carotid artery, internal jugular vein, and vagus nerve. In the midline, the trachea and esophagus are critical adjacent structures that lie immediately ventral to the ventral cervical spine. A dissection plane may be developed medial to the sternocleidomastoid and carotid sheath, lateral to the trachea and esophagus, dividing the pretracheal fascia and bluntly extending the plane down to the prevertebral fascia. This plane may be traversed by the omohyoid muscle, which runs from the scapula to the hyoid bone obliquely approximately over the C5 level. It may be retracted rostrally or caudally to facilitate exposure; however, if needed, it may be divided without significant clinical consequence (Fig. 31-23; see also Fig. 31-22).
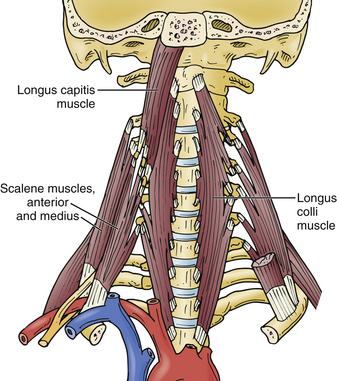
The longus colli muscles flank the ventral cervical vertebral bodies, originating on the anterior tubercle of C1 and extending down to the T3 level, where they insert on the ventral T3 vertebral body. The sympathetic chain runs on the ventral surface of the longus colli muscles and injury must be avoided to prevent an iatrogenic Horner syndrome.29 Although the longus colli may be safely elevated to facilitate placement of ventral cervical retractor blades, awareness of vertebral artery tortuosity is necessary to avoid iatrogenic arterial injury (Fig. 31-24).
Hassler O. Blood supply to human spinal cord: a microangiographic study. Arch Neurol. 1966;15:302-307.
Lu J., Ebraheim N.A., Nadim Y., et al. Anterior approach to the cervical spine: surgical anatomy. Orthopedics. 2000;23:841-845.
Maiman D.J., Pintar F.A. Anatomy and clinical biomechanics of the thoracic spine. Clin Neurosurg. 1992;38:296-324.
McCormick P.C., Stein B.M. Functional anatomy of the spinal cord and related structures. Neurosurg Clin N Am. 1990;1:469-489.
Pait T.G., Killefer J.A., Arnautovic K.I. Surgical anatomy of the anterior cervical spine: the disc space, vertebral artery, and associated bony structures. Neurosurgery. 1996;39:769-776.
Rhoton A., de Oliveira E. Anatomical basis of surgical approaches to the region of the foramen magnum. In: Dickman C.A., Spetzler R.E., Sonntag V.K.H., editors. Surgery of the craniovertebral junction. New York: Thieme; 1998:13-57.
1. Gonzalez L.F., Dickman C.A. Anatomy of the spine and spinal cord. In: Dickman C.A., Fehlings M.G., Gokaslan Z.L., editors. Spinal cord and spinal column tumors. New York: Thieme; 2006:1-23.
2. Abou Madawi A., Solanki G., Casey A.T., et al. Variation of the groove in the axis vertebra for the vertebral artery: implications for instrumentation. J Bone Joint Surg [Br]. 1997;79:820-823.
3. Tubbs R.S., Grabb P., Spooner A., et al. The apical ligament: anatomy and functional significance. J Neurosurg. 2000;92(Suppl 2):197-200.
4. Willauschus W.G., Kladny B., Beyer W.F., et al. Lesions of the alar ligaments: in vivo and in vitro studies with magnetic resonance imaging. Spine (Phila Pa 1976). 1995;20:2493-2498.
5. Dickman C.A., Mamourian A., Sonntag V.K., et al. Magnetic resonance imaging of the transverse atlantal ligament for the evaluation of atlantoaxial instability. J Neurosurg. 1991;75:221-227.
6. Rhoton A., de Oliveira E. Anatomical basis of surgical approaches to the region of the foramen magnum. In: Dickman C.A., Spetzler R.E., Sonntag V.K.H., editors. Surgery of the craniovertebral junction. New York: Thieme; 1998:13-57.
7. Pait T.G., Killefer J.A., Arnautovic K.I. Surgical anatomy of the anterior cervical spine: the disc space, vertebral artery, and associated bony structures. Neurosurgery. 1996;39:769-776.
8. Rezcallah A.T., Xu R., Ebraheim N.A., et al. Axial computed tomography of the pedicle in the lower cervical spine. Am J Orthop (Belle Mead NJ). 2001;30:59-61.
9. Xu R., Kang A., Ebraheim N.A., et al. Anatomic relation between the cervical pedicle and the adjacent neural structures. Spine (Phila Pa 1976). 1999;24:451-454.
10. Ebraheim N.A., Xu R., Knight T., et al. Morphometric evaluation of lower cervical pedicle and its projection. Spine (Phila Pa 1976). 1997;22:1-6.
11. Parke W., Bono C., Garfin S., et al. Applied anatomy of the spine. In Herkowitz HN, Garfin SR, Eismont FJ, et al. Rothman-Simeone: the spine. ed 5, Philadelphia: Saunders Elsevier, 2006;vol 1. 16–54
12. Lu J., Ebraheim N.A., Yang H., et al. Anatomic bases for anterior spinal surgery: surgical anatomy of the cervical vertebral body and disc space. Surg Radiol Anat. 1999;21:235-239.
13. Maiman D.J., Pintar F.A. Anatomy and clinical biomechanics of the thoracic spine. Clin Neurosurg. 1992;38:296-324.
14. Moore K., Dalley A.I. Clinically oriented anatomy, ed 4. Philadelphia: Lippincott Williams & Wilkins; 1999.
15. Bland J.H., Boushey D.R. Anatomy and physiology of the cervical spine. Semin Arthritis Rheum. 1990;20:1-20.
16. Olszewski A.D., Yaszemski M.J., White A.A.3rd. The anatomy of the human lumbar ligamentum flavum: new observations and their surgical importance. Spine (Phila Pa 1976). 1996;21:2307-2312.
17. Kubo Y., Waga S., Kojima T., et al. Microsurgical anatomy of the lower cervical spine and cord. Neurosurgery. 34, 1994. 890–895 discussion 901-902
18. Shinomiya K., Okawa A., Nakao K., et al. Morphology of C5 ventral nerve rootlets as part of dissociated motor loss of deltoid muscle. Spine (Phila Pa 1976). 1994;19:2501-2504.
19. Pallie W. The intersegmental anastomoses of posterior spinal rootlets and their significance. J Neurosurg. 1959;16:188-196.
20. Marzo J.M., Simmons E.H., Kallen F. Intradural connections between adjacent cervical spinal roots. Spine (Phila Pa 1976). 1987;12:964-968.
21. Alleyne C.H.Jr., Cawley C.M., Barrow D.L., et al. Microsurgical anatomy of the dorsal cervical nerve roots and the cervical dorsal root ganglion/ventral root complexes. Surg Neurol. 1998;50:213-218.
22. Muraszko K.M., Oldfield E.H. Vascular malformations of the spinal cord and dura. Neurosurg Clin N Am. 1990;1:631-652.
23. McCormick P.C., Stein B.M. Functional anatomy of the spinal cord and related structures. Neurosurg Clin N Am. 1990;1:469-489.
24. Gillilan L.A. The arterial blood supply of the human spinal cord. J Comp Neurol. 1958;110:75-103.
25. Hassler O. Blood supply to human spinal cord: a microangiographic study. Arch Neurol. 1966;15:302-307.
26. Daseler E.H., Anson B.J. Surgical anatomy of the subclavian artery and its branches. Surg Gynecol Obstet. 1959;108:149-174.
27. Parke W.W. The vascular relations of the upper cervical vertebrae. Orthop Clin North Am. 1978;9:879-889.
28. Parke W.W., Rothman R.H., Brown M.D. The pharyngovertebral veins: an anatomical rationale for Grisel’s syndrome. J Bone Joint Surg [Am]. 1984;66:568-574.
29. Lu J., Ebraheim N.A., Nadim Y., et al. Anterior approach to the cervical spine: surgical anatomy. Orthopedics. 2000;23:841-845.