Chapter 45 Application of Current Radiation Delivery Systems and Radiobiology
• Radiosurgery involves the delivery of a high dose of ionizing radiation to a target in a single or a few fractions.
• Physical properties of high-energy photons or protons used for radiosurgery determine their biological effects.
• The biological consequences of high-dose radiation used for radiosurgery are poorly understood, and may differ from those of conventionally fractionated radiation therapy.
• A number of delivery systems are available for modern radiosurgery, with either photons or protons, each of which has unique characteristics for the patient and the physician.
• In retrospective and prospective studies, there are no data that demonstrate a consistent clinical advantage for one type of radiosurgery device over another.
Radiation therapy has been used for the treatment of benign and malignant central nervous system (CNS) diseases for over 50 years. Ionizing radiation, either from machines known as linear accelerators (linacs) or from radioactive sources such as cobalt-60, can be used for either conventional fractionated radiotherapy or single-dose, stereotactic radiosurgery (SRS). Radiosurgery, as defined by both the American Society for Radiation Oncology (ASTRO) and the American Association of Neurologic Surgeons (AANS) is “a distinct discipline that utilizes externally generated ionizing radiation in certain cases to inactivate or eradicate a defined target(s) in the head or spine without the need to make an incision.”1 The definition specifies that radiosurgery is usually delivered in one to five sessions, and uses a stereotactic image guidance system. This definition is in contrast to fractionated stereotactic radiotherapy (fSRT), which uses stereotactic localization and immobilization, but delivers a series of fractions of lower daily dose. This chapter explains the basic physics and biology of radiation therapy, and describes the most common delivery systems used for radiosurgery.
Physics of Radiotherapy
Photon Radiation
The most common form of ionizing radiation used in radiotherapy is the photon, which is a particle that has no mass, travels at the speed of light, and carries the energy present in microwaves, visible light, ultraviolet light, and x-rays. The nature of the interaction of a photon with matter depends upon the energy of the photon. In the kiloelectron volt (keV) range, which is used in most diagnostic x-ray units, photons interact with matter via the photoelectric effect.2 A photon interacts with a tightly bound electron, which absorbs most of the energy and is ejected from the atom and is then free to interact with other atoms in the vicinity. This interaction is highly dependent on the atomic number of the material being irradiated. Diagnostic x-ray studies take advantage of this phenomenon, as bone, which is high in calcium, is much more likely to interact with these photons than soft tissue, which is mostly carbon, hydrogen, and oxygen. This differential interaction is the basis of diagnostic x-ray imaging.
At higher energies, which is the energy of most therapeutic radiation, the Compton effect predominates.2 A high-energy photon in the megaelectron volt (MeV) range interacts with a loosely held orbital electron, which results in ejection of the electron, and scattering of the photon (with a change in energy of the scattered photon). This interaction is independent of the atomic number of the material being irradiated, but it is dependent on electron density. Thus, images generated from therapeutic energy x-rays are less useful for imaging than diagnostic x-rays, as there is little contrast between bone and soft tissue. However, this energy range is useful in radiation therapy, as it is highly penetrating, and is able to interact equally with all tissues.
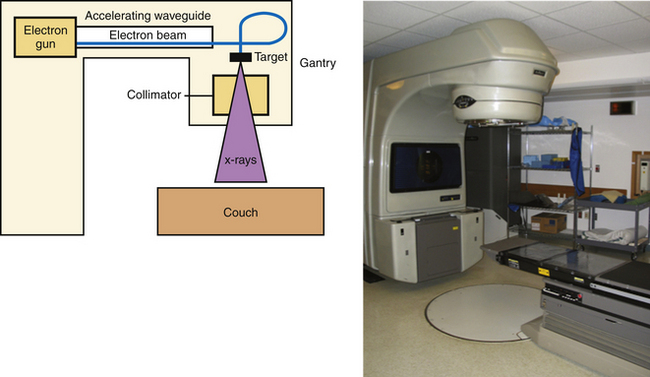
The other main source of high-energy photons is a linac, which uses microwaves to accelerate electrons to a high energy. These electrons are directed to collide with a high-molecular-weight target, and the resulting interactions between nuclei and electrons result in the production of high-energy photons (X-rays) via a process known as bremsstrahlung, or “braking” radiation. The difference between X-rays and γ-rays is simply the site of origin: X-rays are produced by electron interactions but γ-rays are produced by nuclear decay. Unlike the γ-rays produced by 60Co, the energy of the X-rays generated in a linac is based on the characteristics of the machine, and most commercial units offer multiple energy options. These X-rays are produced in the head of the unit (known as the gantry), and can be shaped by a system of controllable leaves, known as a multileaf collimator (MLC), placed between the source of the X-rays and the patient (Fig. 45.1).
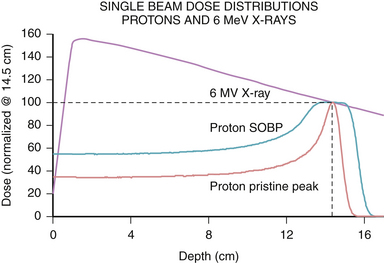
The physical characteristics of photons determine their biological effects. Photons deposit dose in a characteristic pattern, in which there is an initial buildup of energy after entry into the patient, followed by a steady loss of energy as they pass through the tissue according to the law of exponential decay. The energy of the photon is lost in inverse proportion to the square of the distance traveled. Thus, photons spare the skin to some degree, but deposit the majority of their dose upon entrance into tissue, and continue to deposit decreasing amounts of energy as they exit the body (Fig. 45.2). The shape of this depth-dose curve is dependent on the energy of the photon involved. Higher energy photons exhibit greater skin-sparing effect, but have a slower rate of attenuation as they pass through tissue. In general, photons in the 1- to 6-MeV range are used for SRS, as the desired target depth in the cranium is relatively shallow, and higher energies result in unnecessary increased dose delivered beyond the target point.
Proton Radiation
Another form of ionizing radiation used in SRS involves charged particles, most commonly protons. Proton production starts by stripping the electron from molecular hydrogen gas, and the resulting protons are then accelerated to a therapeutic energy level using alternating magnetic fields in a cyclotron or synchrotron. The physical properties of protons result in a different dose distribution from that of photon radiation. Protons have a defined distance of travel, known as the range, which is dependent on their energy. Protons release their energy primarily at the end of their range, which is known as the Bragg peak, after William Henry Bragg, the Nobel Prize–winning physicist. The consequence of the Bragg peak is that protons deposit relatively lower doses of radiation prior to the end of their range compared to photons, and have no exit dose, that is, no dose delivered past their range (see Fig. 45.2). By modulating the energy of protons, a spread-out Bragg peak (SOBP) can be generated to cover wider areas, with the tradeoff of increased entrance dose. Because of their sharp range and lack of exit dose, protons provide a favorable dose profile for use in SRS. The disadvantage of protons is that their availability is limited to a few centers due to the cost and complexity of maintaining such facilities for clinical use.
Biology of Radiation Therapy
The primary target of ionizing radiation is generally thought to be damage to the DNA of target cells. As described previously, ionizing radiation results in the ejection of electrons from the atoms in the tissue being irradiated. Because most of the cell is water, the photon or proton is most likely to interact with a molecule of water, resulting in the production of superoxide, hydroxyl radicals, and other reactive oxygen species, which damage the DNA and result in replicative failure. Thus, radiotherapy is believed to be more effective in the presence of oxygen,3 and it is thought that hypoxic areas of tumors may be less sensitive to the effects of ionizing radiation.4
Although many different types of DNA damage can be produced by ionizing radiation, the most critical form of damage in the use of therapeutic radiation is the double strand break. Double strand breaks are more difficult for cells to repair, and the repair process can generate aberrant chromosomes that result in mitotic catastrophe, or mutations that result in reduced replicative fitness.5 The critical nature of the double strand break is evidenced by the fact that patients with mutations in the ataxia-telangiectasia mutated (ATM) gene, one of the key sensors of DNA double strand breaks,6 and an integral part of double strand break repair, are extremely sensitive to ionizing radiation damage.7
The production of double strand breaks is related to the efficiency of the particle in transferring its energy to the surrounding matter. This concept is quantified as the linear energy transfer (LET) of differing radiation modalities, and results in differences in the relative biological effectiveness (RBE) of different types of radiation beams. Photons produced by 60Co are considered to have a low LET and are defined to have an RBE of 1. However, the LET varies with both energy and with the type of particle used in irradiation. For example, neutrons, which are large, noncharged particles, have a very high LET, and produce more double strand breaks for a given dose of radiation, resulting in a higher RBE.8 Protons at therapeutic energies, although of similar mass to neutrons, do not exhibit a particularly high LET, presumably because their positive charge leads to repulsive forces with atomic nuclei. Protons used in radiotherapy have been calculated to have an RBE of 1.1 compared to photons produced by 60Co.9 This means that for a given absorbed dose, protons will have a 10% greater biological effect. To avoid confusion, proton therapy doses are typically reported as gray (relative biological effectiveness) (Gy[RBE]), taking this correction factor into account.
Although DNA damage is known to be the primary mechanism of action of radiation, the cellular target of radiotherapy is more controversial. For malignant tumors, which are highly proliferative, and often have impaired DNA repair,10 the target is thought to be the cancer cells themselves, as they are unable to repair the DNA damage inflicted by ionizing radiation. Furthermore, their rapid progression through the cell cycle results in more potential checkpoints that can trigger cell death. Thus, cancer cells are more sensitive than normal cells to radiation. This effect is seen clinically, as radiation of malignant tissues often causes clinical or radiographic regression of the lesion.
In contrast, for benign disease, the cells are not as proliferative, and may be in resistant phases of the cell cycle. These observations have led some to speculate that benign tumors are relatively radioresistant.11 However, in clinical practice, radiation appears to induce a quiescent state, which corresponds to radiographic and clinical stability,12,13 suggesting that benign tumors respond to radiotherapy in some way. It is possible that these tumors undergo DNA damage that limits their replication, but the biology of this process is still unclear.
Although tumor cells have been thought to be the primary target of radiotherapy, many have suggested that radiotherapy has an effect on vascular endothelium, which mediates the primary mode of cell death, especially at the higher doses used in SRS.14,15 Irradiation of B16 melanoma cell explants in mice with doses of 15 to 20 Gy result in waves of endothelial cell apoptosis 1 to 6 hours after irradiation.15 The endothelial response was critical to tumor control, as endothelial- specific mutation of Bax, a critical regulator of apoptosis, rendered these explants insensitive to doses of 15 Gy. Thus, endothelial cell death may lead to direct hypoxic necrosis of the tumor, or may secrete signaling molecules that may cause tumor death. However, other studies have shown that at even higher radiation doses (>20 Gy), the mode of death in an irradiated gastrointestinal tract appears to become independent of endothelial cell apoptosis,16 suggesting that the dose response relationship is complex, and may be differentially regulated in different tissues. These differences are the subject of much investigation and may become more important as combination chemotherapy or targeted therapy is considered.
These observations correlate with data from radiosurgical experiments exposing normal rat brain or tumor explants to radiosurgical doses of ionizing radiation. Examination of human acoustic schwannoma xenografts after irradiation with 10 to 40 Gy showed a significant decrease in tumor vascularity, as well as significant intramural vascular hyalinization.17 Furthermore, irradiation of rat brains with 15 to 30 Gy resulted in changes in local blood flow, leukocyte/endothelial interaction, formation of aneurysmal structures, and thrombus formation.18 These data suggest that vascular damage by radiosurgery may be an important component of its clinical effect.
Fractionation versus Radiosurgery
Conventional radiotherapy is typically fractionated into daily doses, which is thought to result in reduced effects of radiation on normal tissue. Fractionation allows for DNA repair to occur, which is predicted to favor normal cells that retain the full complement of DNA repair proteins.19 Furthermore, fractionation allows for reoxygenation of hypoxic areas, resulting in increased sensitivity of malignant cells that were previously hypoxic.20 Additionally, fractionated therapy allows for reassortment of cells in the cell cycle, as cells are most sensitive to radiation during the G2/M phase of the cell cycle and resistant during the late S phase and G1. Thus, giving radiation in daily fractions allows those cells that are in resistant phases of the cell cycle to move to more sensitive phases of the cell cycle during subsequent fractions.21 The downside of fractionation is that it allows for repopulation of tumor cells during the therapy. Reoxygenation, reassortment, repair, along with repopulation, are known as the “four Rs” of radiobiology, and explain the radiobiological basis for daily fractionated therapy.
The value of fractionation is more apparent for certain tissues, which typically have high rates of proliferation and show relatively less capability to repair sublethal DNA damage. This concept is quantified as the α/β ratio, which is a radiobiological concept, based on a model of radiation response, that attempts to explain the differential sensitivity of tissues to fractionation.22 Tissues with a high α/β ratio respond quickly to radiotherapy, and are sensitive to smaller fraction sizes. Examples of these so-called “early” responding tissues include the gastrointestinal tract, lymphocytes, and skin. Tissues with a low α/β ratio respond more slowly to radiation, are typically less proliferative, and show a high capacity for DNA repair. Examples of late responding tissues include neural tissue and the lung.
The α/β ratio can be used to calculate a biologically equivalent dose (BED), which attempts to equalize total doses that are given in different fraction sizes. The equation is BED = (number of fractions ∗ fractional dose) ∗ (1 + (fractional dose/α/β ratio)).22 Although there is debate over the validity of this model at the high doses used in radiosurgery, it allows one to approximate the effect of doses delivered in fractionated sessions and those given in radiosurgery. For example, if one assumes a tumor to have a high α/β ratio of 10, a dose of 20 Gy in a single fraction is biologically equivalent to a dose of 40 Gy in 8 fractions, or 50 Gy in 25 fractions. However, for normal brain tissue, which has an α/β ratio closer to 3, the schedule 20 Gy × 1 has a BED of 153.3, while 5 Gy × 8 has a BED of 106.7, and 2 Gy × 25 has a BED of 83.3. Thus, the preceding regimens have the same BED for a tissue with a high α/β ratio, but the larger fraction sizes have a much higher BED for tissues with a low α/β ratio. What this means is that although the tumor control would be predicted to be equivalent for 20 Gy in 1 fraction and 50 Gy in 25 fractions, the radiosurgical dose would produce a more profound effect on normal brain tissue. Although the BED is merely an approximation of biological effect and not a real quantity, it can be useful when considering the dose of radiation to be used when the target is close to critical normal structures such as cranial nerves.
Another potential explanation for the excellent clinical effect of radiosurgical doses in malignant disease lies in the possibility that high radiation doses are required to kill radiation-resistant cells. Emerging data suggest that clonogenic cells within tumors may have an intrinsically higher radioresistance due to increased expression of antioxidant genes.23 It may be that very high radiation doses are able to overcome this effect, resulting in death of those clonogenic cells, and leading to tumor control.
The radiobiological concerns outlined here suggest some situations when it may be advantageous to choose fractionated treatment over single-fraction radiosurgery. Given that normal neural tissue, including cranial nerves, are late-responding, with an α/β ratio of approximately 3, they will be more sensitive to large radiation doses compared to small ones. If the target to be treated is in close proximity to one of these structures (e.g., optic nerve sheath meningioma24 or a pituitary tumor very close to the optic chiasm25), it may be more prudent to fractionate treatment to reduce the risk of injury to the cranial nerves. Conversely, for hormone-secreting tumors, there is biological evidence that high-dose radiosurgery is associated with more rapid normalization of hormone levels.26 Thus, the choice between radiosurgery and fractionated treatment must weigh a number of radiobiological and clinical parameters.
History of Radiosurgery
The development of stereotactic radiosurgery (SRS) can be linked to developments in stereotactic localization for neurosurgical procedures by Spiegel and Wycis in 1947.27 The concept behind stereotaxy was to attach a fixed external coordinate system to the skull so that every point within the skull could be defined according to the axes present on the external device. The early system of Spiegel and Wycis used axes correlating to the ventricles as seen on pneumoencephalogram, but as imaging techniques advanced, new systems that relied on computed tomography (CT) or magnetic resonance imaging (MRI) were subsequently developed.28,29 Lars Leksell, a Swedish neurosurgeon at the Karolinska Institute, adapted a similar stereotactic system, and combined this with a variety of types of radiation, ultimately relying on radioactive 60Co to produce multiple small photon beams converging on an intracranial target.30 This system evolved to become a hospital-based therapy known as the Gamma Knife, the first commercially available radiosurgery system. With further research, conventional linacs were adapted to produce a similar effect through the use of multiple convergent noncoplanar arcs, enabling a wider range of radiotherapy centers to perform SRS.31–33
Several investigators, including Dr. Leksell, with his collaborator, radiobiologist Dr. Borje Larsson, began experimenting with protons produced by the cyclotron in Uppsala using a crossfire technique to irradiate the pituitary.34 At the same time, in 1953, Dr. John Lawrence at the University of California at Berkeley cyclotron began to treat the pituitary using the Bragg peak of a proton beam.35 Concurrently, Dr. Raymond Kjellberg, a neurosurgeon at the Massachusetts General Hospital, began to experiment with the Harvard Cyclotron in Cambridge, Massachusetts, and began treating patients in 1961. The initial treatments were limited to the pituitary, as the sella turcica was easily visualized on plain film radiography,36 but he subsequently began to treat arteriovenous malformations based on angiographic images.37
Contemporary Radiation Delivery Systems
Gamma Knife Radiosurgery
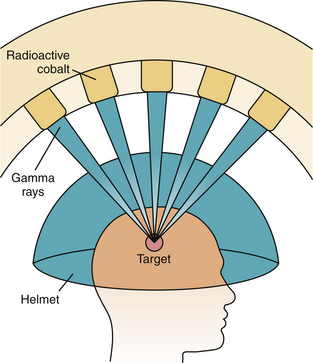
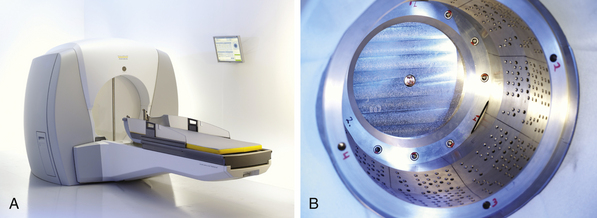
The Gamma Knife was pioneered by Dr. Leksell in the late 1960s, and became the first available commercial radiosurgery unit. The unit, as currently constructed, contains 201 fixed sources of 60Co distributed in a hemisphere, each of which is a thin rod with the long axis oriented along the radius of the sphere, converging on a single point, called the treatment isocenter (Fig. 45.3). These sources are surrounded by a primary collimator, which directs the beams. A series of secondary collimators, or “helmets,” are available with either 4-, 8-, 14-, or 18-mm diameter holes, which can be chosen based on the desired size of the beams to be produced (Fig. 45.4). This secondary collimation determines the width of the radiation beams at the isocenter. By occluding certain collimator holes, the dose distribution can be altered to produce the desired shape and to protect normal structures. Newer models of the Gamma Knife allow for intensity modulation, which can produce more conformal dose distributions.
For radiosurgery, the Leksell frame is attached to the patient, and then a planning CT scan is performed and is uploaded to the treatment planning software. Once a plan is developed, the frame is attached to the treatment couch. The patient is then inserted into the unit, and the frame is positioned relative to the collimator helmet so that the treatment isocenter is aligned with the planned target isocenter. Because there are no moving parts during treatment, there is a high degree of setup accuracy. The dose to be delivered, along with the activity of the sources, determines the length of the treatment time. If multiple isocenters are used, the patient is repositioned relative to the secondary collimator, and the rest of the isocenters are treated. The half-life of 60Co is 5.3 years, and the sources need to be replaced periodically so that treatment times do not become impractical for patients. Dose rates fall 1% per month with 60Co treatment devices.
Linac-Based Radiosurgery
As described earlier, the linac uses microwave energy to accelerate electrons into a therapeutic energy range, and those electrons are used to generate high-energy photons. For radiosurgery, the linac has a secondary collimator attached to the treatment head with a cone-shaped aperture of a specific size, which can be varied based on the size and shape of the lesion to be treated. To produce a dose distribution similar to that of the Gamma Knife, an approach using converging noncoplanar arcs of radiation was adopted38 (Fig. 45.5).
A stereotactic frame, most commonly the Brown-Roberts-Wells (BRW) frame, is placed on the patient, with four pins securing the frame to the skull. The frame has three orthogonal axes—coronal, lateral, and axial—which intersect at the center of the circular frame. The CT localizer frame has nine fiducial rods, which appear in each axial CT slice, and provide a precise correlation between the axes on the frame and any anatomical point on the CT scan. The frame attaches to the CT simulation couch in a similar manner to the linac treatment couch, so that patient setup is identical in the simulator and the treatment unit. The target is outlined on the CT scan, along with any critical normal structures. MRI scans are often fused with the planning CT scan to facilitate target and normal tissue identification.39
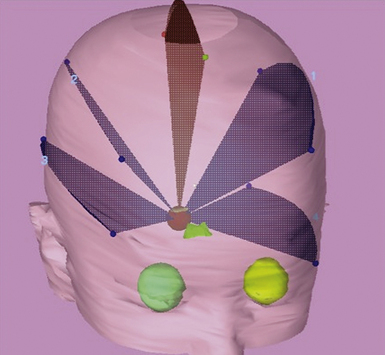
Treatment planning software is used to define 3 to 6 arcs converging on the treatment isocenter, which will result in a high dose at the convergence point, with rapid falloff in any given direction away from treatment isocenter (Fig. 45.6). Because arcs are used, the high-dose volume is usually more ellipsoid than a similar sized volume treated by Gamma Knife. As the number of arcs and the total degrees subtended by each arc increase, the dose to normal tissue decreases, as it is spread out over a larger volume.
When the plan is formed, the patient, with the frame still attached to the skull, is brought to the treatment room, and the frame is attached to the treatment couch. The isocenter of the treatment machine is aligned to the origin of the axes of the stereotactic frame, with an accuracy of less than 1 mm. The treatment isocenter is then aligned with the machine’s isocenter. The gantry rotates during the treatment to produce an arc. After the arc is delivered, the couch can be moved and a new arc created. The end result is that multiple arcs converge on a single point. For irregular volumes, multiple isocenters can be used, similar to those used by the Gamma Knife. When all of the planned arcs are treated, the frame is removed, and the patient can usually return home.
One of the more recent advances in SRS is the “frameless” radiosurgery system, which does not require an invasive head frame to be fixed to the patient’s cranium. Because conventional SRS required frame placement at the beginning of planning, the patient would often wear the head frame for the entire 5 to 8 hours required for planning and treatment. A number of centers have switched to noninvasive systems that are much more comfortable for the patient, including a thermoplastic mask combined with a dental mold with infrared fiducial markers,40 or use of orthogonal diagnostic energy x-rays to verify patient setup and positioning. The other advantage of the frameless approach is that the planning and treatment can be done on separate days, which facilitates patient and physician scheduling. These approaches have been adopted at a number of centers, and in some preliminary studies appear to be of similar accuracy and clinical efficacy as conventional radiosurgery.41 However, there are some concerns that it may not be as accurate as conventional frames, and a planning margin of 1 to 2 mm is sometimes used to ensure target coverage.42 Thus, this approach has not been universally adopted, and is still under investigation.
Other recent developments in linac-based radiosurgery include the development of special radiosurgical linacs, of which the Novalis Tx is the most common. These linear accelerators can use cone-shaped collimators like conventional linac-based radiosurgery, but also have a multileaf collimator with small leaf size, allowing for very fine shaping of the radiation beam. These MLCs can be used for intensity-modulated radiation therapy (IMRT), in which the leaves attenuate the radiation beam to varying amounts in different areas of the radiation portal. The radiation is then delivered using a set of static (i.e., not arc) fields, with each field having a different intensity pattern. This technique allows for a much more conformal dose distribution, and can be especially useful in the treatment of irregular lesions. Furthermore, using IMRT, these specialized units can use a single isocenter to treat multiple lesions, which reduces treatment time for the patient. Initial planning studies suggest that the use of IMRT for radiosurgery rather than convergent arcs may allow for increased sparing of normal tissue receiving 50% of the prescription dose, and allows for more precise shaping around critical structures.43 The downside of the IMRT approach is that there is more low-dose radiation given to normal brain, and the long-term consequences of this low-dose irradiation are still unknown.
Newer technologies for linac-based radiosurgery are on the horizon, including tomotherapy and volumetric arc therapy (VMAT), which deliver radiation in continuous arcs, similar to a CT scan. In these types of radiation therapies, the radiation is delivered in continuous arcs on a slice-by-slice basis as the patient is moved through the unit. Planning studies suggest that both tomotherapy and VMAT offer improved conformal dose delivery, but may result in larger areas of low-dose radiation to normal tissue,44 although this area is still under investigation.45
Robotic Radiosurgery
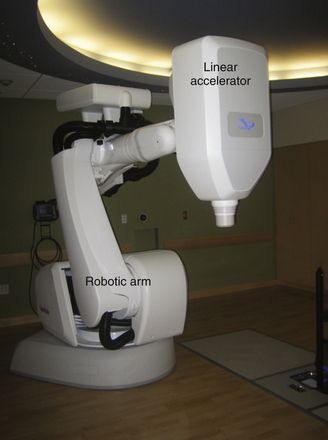
The last major development in photon radiosurgery is the development of the Cyberknife, in which a miniaturized linac is mounted on a robotic arm with 6 degrees of rotational freedom (Fig. 45.7). The linac produces 6-MeV x-rays, and is equipped with either swappable collimators of specific sizes or with a variable collimator that is adjustable to different sizes during the course of treatment. The linac and robotic arm combination can deliver multiple small beamlets of radiation from many different angles to produce a conformal dose plan. The Cyberknife is combined with an in-room diagnostic x-ray unit that takes images of the patient during the treatment, and can be used to modify the treatment plan in real time if there is any change in patient positioning. Thus, there is no need for an invasive frame, and immobilization with a thermoplastic mask is sufficient to achieve the required accuracy.
Proton Beam Radiosurgery
All of the methods outlined previously use photon radiation, but recently there has been a growing interest in using proton radiotherapy. As discussed earlier, protons provide a dosimetric advantage relative to photons owing to the concentration of energy at the Bragg peak and the lack of exit dose. The result is that the integral dose to the normal brain is lower than that for photon radiosurgery. The early radiosurgical approach adopted by Dr. Leksell in the 1950s used a cross-fire technique, in which opposed beams were directed at the target, but the Bragg peak fell outside the patient.34 This approach was used because it was felt that the uncertainty of the dose at the Bragg peak could result in failure to provide adequate dose to the target. However, the team at the Harvard Cyclotron Laboratory, led by Dr. Kjellberg, began to perform Bragg peak radiosurgery beginning in 1961.46 Over the past two decades, there has been a growth in the number of dedicated hospital-based proton therapy sites, beginning with Loma Linda University in 1990, followed by the Massachusetts General Hospital (MGH) in 2001. More recently, a number of centers are opening across the country as interest has increased in proton therapy.
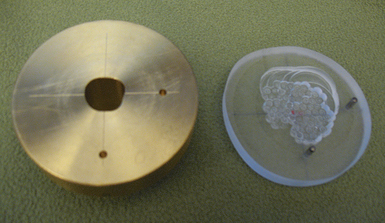
Creation of a proton radiosurgery plan typically uses 1 to 4 conformal portals. Because protons do not have an exit dose, fields can enter the body through a wider range of angles compared to photon arcs (e.g., from the vertex), because the protons will not exit through the rest of the body. The proton range is very sensitive to the tissue density through which the beam passes, and thus attempts are made to avoid sinuses or other air cavities. The energy of the proton beam is modulated to create an SOBP, which can be used to cover targets that are larger than the width of the single Bragg peak. For each field, a custom brass aperture is created to define the field edges, and a Lucite compensator is created to define the distal edge of the Bragg peak (Fig. 45.8).
In the treatment room, the setup and positioning are verified with diagnostic x-rays that confirm the position of the fiducial markers in relation to the bony anatomy of the skull. Some patients are treated using a rotational gantry similar to that of a linac. At the MGH, where one of the treatment rooms has a fixed beamline, some patients are treated in a device known as STAR (stereotactic alignment for radiosurgery), which has greater ability to move and rotate the patient around a fixed beamline (Fig. 45.9). The fixed beam allows for treatment with a slightly higher degree of accuracy as compared to a rotational gantry because the weight of the gantry can result in some positional uncertainty.
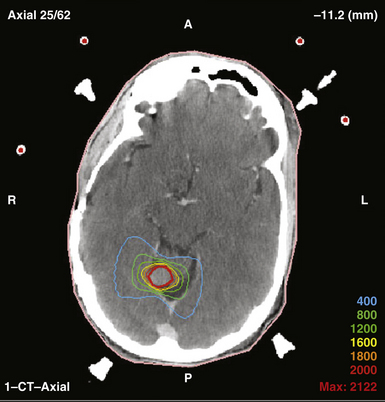
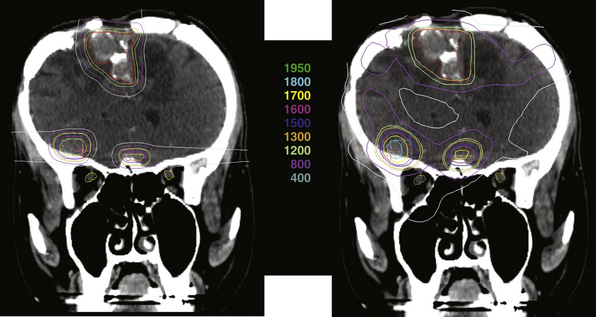
In general, proton radiosurgery results in a very conformal plan, with much less dose inhomogeneity compared to photon-based radiosurgery (Fig. 45.10). These homogeneous plans are desirable for heterogeneous lesions that contain normal tissues such that excessive dose is not wanted. This can be more advantageous than photon plans that produce unacceptable hot spots either within or outside the intended target. The second major advantage of proton radiosurgery is the lack of exit dose, which means that the treatment results in less radiation to the normal brain. Although the risks of low-dose irradiation of normal brain cannot yet be quantified, avoiding this possible complication is desirable.
Evaluation and Comparison of Radiosurgery Techniques
The evaluation of radiosurgical plans relies on a few parameters, which have been defined by ASTRO.47 Key components to evaluate include the ratio between the maximum dose (MD) and the peripheral dose (PD), which gives a sense of the dose homogeneity within the treatment volume. The second key parameter is the ratio between the prescription isodose volume (PIV), which is the total volume that receives at least the prescription dose, and the target volume (TV), which is the volume of the target defined on planning CT scan. This PIV/TV ratio is a measure of target coverage and dose conformality.
Analysis of the Radiation Therapy Oncology Group (RTOG) 90-05 study, a multicenter phase II dose escalation study for SRS of brain metastases, revealed some interesting data regarding both Gamma Knife and linac-based radiosurgery. For Gamma Knife, 94% of the plans had an MD/PD ratio greater than 2, versus only 7% of the linac-based plans.48 These data suggest that the Gamma Knife plans are less homogeneous and more prone to hot spots at the center of the treatment volume. However, it was noted that increasing the number of isocenters used for a given Gamma Knife treatment plan resulted in MD/PD ratios that were more similar to those with linac-based radiosurgery. Current RTOG protocols use a guideline of an MD/PD ratio of less than 2 to qualify as treatment “per protocol.”49
In contrast, the results for the PIV/TV ratio were more favorable for Gamma Knife radiosurgery compared to linac-based radiosurgery. Seventy-seven percent of Gamma Knife plans had a PIV/TV ratio between 1 and 2, with no plans less than 1 or greater than 4. Conversely, only 51% of linac plans had a ratio between 1 and 2, and 10% of plans had a ratio less than 1 and 7% had a ratio greater than 4.48,50 These data suggest that the conformality of plans with Gamma Knife were superior to those produced with linac-based radiosurgery. The RTOG currently recommends a PIV/TV ratio between 1 and 2 as a standard for SRS.49
Although there are differences between plan characteristics, it is important to recognize that there do not appear to be clinical differences in efficacy or toxicity between radiosurgical modalities. Initially, during the development of linac-based radiosurgery, there was some concern that it would prove to be inferior to the Gamma Knife. Multivariate analysis of the RTOG 90-05 study showed that treatment with a linac was associated with an increased risk of local failure, with a hazard ratio of 2.85. The explanation put forward in the paper was that the higher MD/PD ratio and better PIV/TV ratio seen with the Gamma Knife plans resulted in better tumor control compared to linac-based plans. However, proponents of linac-based SRS suggested that this difference could also be due to the fact that Gamma Knife radiosurgery accounted for only 30% of the patients, and was limited to two clinical centers with significant radiosurgical experience, which is difficult to compare to the 70% of treatments performed in 15 institutions with varying degrees of experience with SRS. This hypothesis gained support with data from the RTOG 95-08 study, which randomized patients with one to three brain metastases to receive whole-brain radiotherapy with or without an SRS boost. In this study, there were very few major deviations from the MD/PD and PIV/TV ratios specified by the RTOG with either linac-based radiosurgery or with Gamma Knife, and there was no survival difference based on the type of radiosurgical unit used.51 Thus, the data suggest that when appropriate radiosurgical guidelines are followed, these two modes of SRS are functionally equivalent.
Fractionated Stereotactic Radiotherapy
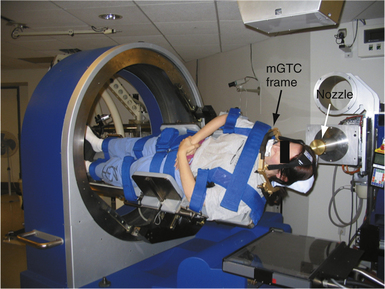
Although the radiosurgical literature centers on single fraction radiosurgery for intracranial targets, there has been a growing interest in fractionating therapy based on the radiobiological advantages outlined here. Thus, several groups became interested in applying the immobilization and localization devices used in radiosurgical procedures with fractionated radiation to reduce the risk of late complications. The biggest challenge in fSRT is the reproducibility of positioning the patient daily such that the targeting accuracy is maintained. Most of the immobilization methods described here, especially the fixed head frame, were not suitable for multiday usage, although some groups have tried this approach.52 One of the most common solutions to this problem was to replace the pins in the head frame with a bite block/dental mold, and an occipital plate to hold the head in position, which became known as the Gill-Thomas-Cosman (GTC) frame.53 This approach is well tolerated for both children and adults with intracranial tumors.54 These approaches have been adopted with all of the technologies described earlier, including IMRT, Cyberknife, and proton radiotherapy. However, because of technical challenges, reports of fractionated therapy with Gamma Knife are much rarer.
Andrews D.W., Scott C.B., Sperduto P.W., et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665-1672.
Khan F.M. The Physics of Radiation Therapy, 3rd ed., Philadelphia: Lippincott Williams & Wilkins, 2003.
Lutz W., Winston K.R., Maleki N. A system for stereotactic radiosurgery with a linear accelerator. Int J Radiat Oncol Biol Phys. 1988;14:373-381.
Shaw E., Scott C., Souhami L., et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291-298.
Thames H.D., Bentzen S.M., Turesson I., et al. Fractionation parameters for human tissues and tumors. Int J Radiat Biol. 1989;56:701-710.
Please go to expertconsult.com to view the complete list of references.
1. Barnett G.H., Linskey M.E., Adler J.R., et al. Stereotactic radiosurgery—an organized neurosurgery-sanctioned definition. J Neurosurg. 2007;106:1-5.
2. Khan F.M. The Physics of Radiation Therapy, 3rd ed., Philadelphia: Lippincott Williams & Wilkins, 2003.
3. Cohen L. Cell population kinetics in radiation therapy: relationship of cellular surviving fraction to RBE and OER. Radiology. 1975;114:213-217.
4. Suit H.D. Application of radiobiologic principles to radiation therapy. Cancer. 1968;22:809-815.
5. Khanna K.K., Jackson S.P. DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet. 2001;27:247-254.
6. Savitsky K., Bar-Shira A., Gilad S., et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science. 1995;268:1749-1753.
7. Taylor A.M., Metcalfe J.A., Thick J., Mak Y.F. Leukemia and lymphoma in ataxia-telangiectasia. Blood. 1996;87:423-438.
8. Hall E.J. The particles compared. Int J Radiat Oncol Biol Phys. 1982;8:2137-2140.
9. Tepper J., Verhey L., Goitein M., Suit H.D. In vivo determinations of RBE in a high energy modulated proton beam using normal tissue reactions and fractionated dose schedules. Int J Radiat Oncol Biol Phys. 1977;2:1115-1122.
10. Bartkova J., Horejsí Z., Koed K., et al. DNA damage response as a candidate anti-cancer barrier in early human tumorigenesis. Nature. 2005;434:864-870.
11. Yeung A.H., Sughrue M.E., Kane A.J., et al. Radiobiology of vestibular schwannomas: mechanisms of radioresistance and potential targets for therapeutic sensitization. Neurosurg Focus. 2009;27:E2.
12. Halasz L.M., Bussiere M.R., Dennis E.R. Proton stereotactic radiosurgery for the treatment of benign meningiomas. Int J Radiat Oncol Biol Phys. 2010 Oct 7. (Epub ahead of print)
13. Lobato-Polo J., Kondziolka D., Zorro O., et al. Gamma knife radiosurgery in younger patients with vestibular schwannomas. Neurosurgery. 2009;65:294-300.
14. Paris F., Fuks Z., Kang A., et al. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293-297.
15. Garcia-Barros M., Paris F., Cordon-Cardo C., et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155-1159.
16. Ch’ang H.J., Maj J.G., Paris F., et al. ATM regulates target switching to escalating doses of radiation in the intestines. Nat Med. 2005;11:484-490.
17. Linskey M.E., Martinez A.J., Kondziolka D., et al. The radiobiology of human acoustic schwannoma xenografts after stereotactic radiosurgery evaluated in the subrenal capsule of athymic mice. J Neurosurg. 1993;78:645-653.
18. Acker J.C., Marks L.B., Spencer D.P., et al. Serial in vivo observations of cerebral vasculature after treatment with a large single fraction of radiation. Radiat Res. 1998;149:350-359.
19. Thames H.D., Bentzen S.M., Turesson I., et al. Fractionation parameters for human tissues and tumors. Int J Radiat Biol. 1989;56:701-710.
20. Urano M., Nishimura Y., Yaes R. The relative significance of repopulation and hypoxic clonogens in the fractionated radiotherapy of a mouse tumor. Radiat Res. 1995;142:204-211.
21. Denekamp J. Cell kinetics and radiation biology. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;49:357-380.
22. Dale R.G., Jones B. The assessment of RBE effects using the concept of biologically effective dose. Int J Radiat Oncol Biol Phys. 1999;43:639-645.
23. Diehn M., Cho R.W., Lobo N.A., et al. Association of reactive oxygen species levels and radioresistance in cancer stem cells. Nature. 2009;458:780-783.
24. Arvold N.D., Lessell S., Bussiere M., et al. Visual outcome and tumor control after conformal radiotherapy for patients with optic nerve sheath meningioma. Int J Radiat Oncol Biol Phys. 2009;75:1166-1172.
25. Tishler R.B., Loeffler J.S., Lunsford L.D., et al. Tolerance of cranial nerves of the cavernous sinus to radiosurgery. Int J Radiat Oncol Biol Phys. 1993;27:215-221.
26. Mitsumori M., Shrieve D.C., Alexander E.3rd, et al. Initial clinical results of LINAC-based stereotactic radiosurgery and stereotactic radiotherapy for pituitary adenomas. Int J Radiat Oncol Biol Phys. 1998;42:573-580.
27. al-Rodhan N.R., Kelly P.J. Pioneers of stereotactic neurosurgery. Stereotact Funct Neurosurg. 1992;58:60-66.
28. Heilbrun M.P. Computed tomography-guided stereotactic systems. Clin Neurosurg. 1983;31:564-581.
29. Apuzzo M.L., Sabshin J.K. Computed tomographic guidance stereotaxis in the management of intracranial mass lesions. Neurosurgery. 1983;12:277-285.
30. Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102:316-319.
31. Lutz W., Winston K.R., Maleki N. A system for stereotactic radiosurgery with a linear accelerator. Int J Radiat Oncol Biol Phys. 1988;14:373-381.
32. Loeffler J.S., Alexander E.3rd, Siddon R.L., et al. Stereotactic radiosurgery for intracranial arteriovenous malformations using a standard linear accelerator. Int J Radiat Oncol Biol Phys. 1989;17:673-677.
33. Friedman W.A., Bova F.J. The University of Florida radiosurgery system. Surg Neurol. 1989;32:334-342.
34. Larsson B., Leksell L., Rexed B., et al. The high-energy proton beam as a neurosurgical tool. Nature. 1958;182:1222-1223.
35. Lawrence J.H. Proton irradiation of the pituitary. Cancer. 1957;10:795-798.
36. Kjellberg R.N., Shintani A., Frantz A.G., Kliman B. Proton-beam therapy in acromegaly. N Engl J Med. 1968;278:689-695.
37. Kjellberg R.N., Hanamura T., Davis K.R., et al. Bragg-peak proton-beam therapy for arteriovenous malformations of the brain. N Engl J Med. 1983;309:269-274.
38. Kooy H.M., Nedzi L.A., Loeffler J.S., et al. Treatment planning for stereotactic radiosurgery of intra-cranial lesions. Int J Radiat Oncol Biol Phys. 1991;21:683-693.
39. Kooy H.M., van Herk M., Barnes P.D., et al. Image fusion for stereotactic radiotherapy and radiosurgery treatment planning. Int J Radiat Oncol Biol Phys. 1994;28:1229-1234.
40. Bova F.J., Buatti J.M., Friedman W.A., et al. The University of Florida frameless high-precision stereotactic radiotherapy system. Int J Radiat Oncol Biol Phys. 1997;38:875-882.
41. Nath S.K., Lawson J.D., Simpson D.R., et al. Single-isocenter frameless intensity-modulated stereotactic radiosurgery for simultaneous treatment of multiple brain metastases: clinical experience. Int J Radiat Oncol Biol Phys. 2010;78:91-97.
42. Solberg T.D., Medin P.M., Mullins J., Li S. Quality assurance of immobilization and target localization systems for frameless stereotactic cranial and extracranial hypofractionated radiotherapy. Int J Radiat Oncol Biol Phys. 2008;71:S131-S135.
43. Benedict S.H., Cardinale R.M., Wu Q., et al. Intensity-modulated stereotactic radiosurgery using dynamic micro-multileaf collimation. Int J Radiat Oncol Biol Phys. 2001;50:751-758.
44. Soisson E.T., Mehta M.P., Tome W.A. A comparison of helical tomotherapy to circular collimator-based linear-accelerator radiosurgery for the treatment of brain metastases. Am J Clin Oncol. 2011;34(4):388-394.
45. Lagerwaard F.J., Meijer O.W., van der Hoorn E.A., et al. Volumetric modulated arc radiotherapy for vestibular schwannomas. Int J Radiat Oncol Biol Phys. 2009;74:610-615.
46. Kjellberg R.N., Koehler A.M., Preston W.M., Sweet W.H. Stereotaxic instrument for use with the Bragg peak of a proton beam. Confin Neurol. 1962;22:183-189.
47. Shaw E., Kline R., Gillin M., et al. Radiation Therapy Oncology Group: radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys. 1993;27:1231-1239.
48. Shaw E., Scott C., Souhami L., et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47:291-298.
49. Drzymala R.E., Wasserman T.H., Won M., et al. A phase I-B trial of the radiosensitizer: etanidazole (SR-2508) with radiosurgery for the treatment of recurrent previously irradiated primary brain tumors or brain metastases (RTOG Study 95-02). Radiother Oncol. 2008;87:89-92.
50. Shaw E., Scott C., Souhami L., et al. Radiosurgery for the treatment of previously irradiated recurrent primary brain tumors and brain metastases: initial report of radiation therapy oncology group protocol (90-05). Int J Radiat Oncol Biol Phys. 1996;34:647-654.
51. Andrews D.W., Scott C.B., Sperduto P.W., et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases: phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363:1665-1672.
52. Schwade J.G., Houdek P.V., Landy H.J., et al. Small-field stereotactic external-beam radiation therapy of intracranial lesions: fractionated treatment with a fixed-halo immobilization device. Radiology. 1990;176:563-565.
53. Gill S.S., Thomas D.G., Warrington A.P., Brada M. Relocatable frame for stereotactic external beam radiotherapy. Int J Radiat Oncol Biol Phys. 1991;20:599-603.
54. Dunbar S.F., Tarbell N.J., Kooy H.M., et al. Stereotactic radiotherapy for pediatric and adult brain tumors: preliminary report. Int J Radiat Oncol Biol Phys. 1994;30:531-539.