Chapter 111
Aortoiliac
Extra-Anatomic Bypass
Joseph R. Schneider
The term extra-anatomic bypass refers to any bypass graft, autologous or otherwise that is placed in a site different from that of the arterial segment that is being bypassed. The term is imprecise at best since many common procedures, for example femorotibial bypass with in situ great saphenous vein or carotid artery to subclavian artery bypass might be considered “extra-anatomic.” However, the term extra-anatomic bypass is generally used to refer to procedures addressing disease of the aortoiliac and femoral arterial systems and include the basic procedures discussed in this chapter: femorofemoral bypass, axillofemoral bypass, obturator bypass, and thoracofemoral and supraceliac–to–iliofemoral bypass.
General Concepts
The basic situations for which extra-anatomic bypass procedures were developed include patients at unusually high risk for direct aortoiliac replacement (coronary artery atherosclerosis or other severe medical comorbidities), “hostile” abdomen (previous surgery or infection with adhesions, intestinal stomas, active intra-abdominal infection, or otherwise contaminated fields), infected prosthetic intra-abdominal vascular grafts, aortoenteric fistulae, and infected inguinal and infrainguinal arterial bypass grafts or other groin sepsis. These problems were apparent almost as soon as aortic and femoropopliteal vascular reconstructions were first performed in the 1950s and 1960s. Thus all of the basic procedures to be discussed were developed during that era. Although questions remain and data are contradictory regarding several aspects of outcomes with these extra-anatomic bypass procedures, the basic techniques are “mature.”
With respect to aortoiliac atherosclerotic occlusive disease, the rapid advances in endovascular therapy have included techniques that may be effective in patients who would previously have been treated with extra-anatomic bypass. However, younger patients with aortoiliac atherosclerosis, who would have been treated with direct aortofemoral bypass in the past, tend to have more discrete disease1–3 and patients with more discrete disease are precisely the group who tend to enjoy the best results with endovascular therapy. Older patients with more advanced comorbidities tend to have more diffuse atherosclerosis and for that reason tend to have less favorable results with endovascular therapy.4–9 Such older, higher-risk patients are more likely to require open surgical therapy, so extra-anatomic techniques would be expected to represent an increasing fraction of open surgical therapies for aortoiliac atherosclerosis. However, endovascular techniques continue to improve, to be applied to a rapidly increasing fraction of patients treated for iliac arterial occlusive disease including total occlusions,10 and may be applicable to a larger proportion of patients with diffuse aortoiliac disease in the future.11–13 Thus although the procedures themselves may be considered mature, their role and relative importance in the care of arterial problems is likely to continue to evolve.
History
Freeman and Leeds appear to have been the first to describe femorofemoral bypass,14 although a similar (ilioiliac) operation may have been performed earlier as part of a secondary procedure for iliac limb thrombosis after homograft repair for aortic bifurcation thrombosis and reported later by Oudot and Beaconsfield.15 However, Vetto’s 1962 report provided the first comprehensive description of a series of patients undergoing prosthetic femorofemoral bypass using techniques similar to those still in use today.16 Lewis17 described a truly remarkable subclavian artery to distal aortic homograft bypass as part of reconstruction for a patient with both a ruptured infrarenal aortic aneurysm and what seems likely to have been a type B aortic dissection,18 but the first uses of the axillary artery for inflow for axillofemoral extra-anatomic bypass appear to have been virtually simultaneous by Blaisdell and Hall19 and Louw.20 Blaisdell in particular continued to write about axillofemoral reconstruction and associated outcomes.21,22 Shaw and Baue included what are probably the first three cases of obturator bypass in an article describing suggested approaches to a number of infected arterial graft problems,23 although Courbier and Monties suggested the possibility of a transobturator graft route 3 years earlier.24 The first use of the thoracic aorta as the inflow for an extra-anatomic bypass was likely that described by Stevenson et al.25 Axillofemoral bypass and descending thoracic aorta–to–iliofemoral bypass (the latter, for simplicity, hereafter termed thoracofemoral bypass in this chapter) rapidly became important options for aortic prosthetic graft infection. For example, Fry and Lindenauer in 1967 reported the application of a number of these procedures, including at least one each axillofemoral, thoracofemoral, and supraceliac aorta–to–femoral bypass to cases of aortic graft infection.26 Each of these procedures has now been refined, and the experience with each is now sufficient to derive some general conclusions about their applicability and results.
Indications
The indications for extra-anatomic bypass are diverse and vary by the specific procedure contemplated. Femorofemoral and axillofemoral bypass are appropriate for patients with unilateral or bilateral chronic or acute arterial occlusive processes, most commonly atherosclerosis, with disease that is unfavorable for endovascular treatment and who are at high risk for aortofemoral bypass. Outcomes are particularly poor in patients with critical limb ischemia (CLI) who do not undergo arterial revascularization of some sort.27,28 Therefore, even high-risk patients with CLI due to aortoiliac occlusive disease will in most cases be suitable candidates for some sort of reconstruction, and femorofemoral and axillofemoral bypass are certainly among the interventions to be considered in such patients. These procedures may also be appropriate for revascularization in patients with active intra-abdominal infection (including “mycotic” aortic aneurysm or infected aortic prostheses), aortoenteric fistulae, or otherwise “hostile” abdomen that would be unusually difficult to treat with aortofemoral bypass. Obturator bypass is most commonly employed for femoral arterial reconstruction in patients with groin sepsis including primary vascular infection—for example, femoral mycotic aneurysm after puncture for a diagnostic or therapeutic endovascular procedure or recreational drug use, patients requiring removal of an infected arterial prosthesis in the groin, or patients with an otherwise “hostile” groin (e.g., after radiation therapy or previous surgery). Thoracofemoral and supraceliac aorta–to–iliofemoral bypass are most often employed to avoid reoperation in the infrarenal aorta after failure of standard aortofemoral bypass or after removal of infected prosthetic aortic grafts.
Contraindications
Femorofemoral and axillofemoral bypass are contraindicated in patients with extreme “medical” risk for surgery or with unusually short life expectancy. Obturator bypass with vein is a formidable procedure, and likely of greater magnitude than femorofemoral or axillofemoral bypass (or conventional femoropopliteal bypass), but the indications for obturator bypass are most often such that the only alternative may be to ligate the arteries in the groin and accept a substantial risk of major amputation. Consequently, the patient and surgeon may need to proceed with obturator bypass even if the patient is “high risk.” Thoracofemoral and supraceliac to iliofemoral bypass are both very formidable procedures, certainly equally or more “invasive” than conventional aortofemoral bypass, and they are inappropriate for patients at high risk for open abdominal or thoracic surgery. With respect to femorofemoral, axillofemoral, thoracofemoral, and supraceliac aorta–to–iliofemoral bypass, aortofemoral bypass remains the standard against which all other methods of reconstruction for iliac artery occlusive disease must be measured.29–34
Femorofemoral Crossover Bypass
Basic Concepts, Indications, and Patient Selection
Femorofemoral bypass depends on the capacity of one iliac arterial system to supply adequate blood flow to support both legs and is one possible reconstructive alternative for patients with symptoms related to unilateral stenosis or occlusion of a common or external iliac artery. Hemodynamic studies (see later) confirm that one iliac artery can support both legs, at least at rest, in the absence of flow limiting lesions in the planned “donor” iliac arterial system. Even a diseased donor iliac arterial system may be improved with endovascular techniques to allow a less invasive, yet effective femorofemoral bypass when a more invasive procedure would otherwise be required.
One attractive alternative to femorofemoral bypass is direct (ipsilateral) iliofemoral bypass. Kretschmer et al found no difference between femorofemoral bypass and unilateral iliofemoral bypass with respect to patency,35 but virtually all other published studies have found iliofemoral bypass to yield somewhat better patency than femorofemoral bypass, assuming an appropriate common iliac artery for inflow to the graft.36–45 Indeed, in a truly remarkable study, van der Vliet et al compared results of 184 unilateral iliac reconstructions (62% based on iliac artery inflow) and compared them with 350 contemporaneous patients undergoing aorta–to–bilateral iliac or femoral reconstruction over a 10-year time period and found no difference in patency between the groups, implying that iliofemoral bypass actually does yield results comparable to the “benchmark” aortofemoral bypass.46 However, even if the ipsilateral common iliac artery is relatively free of disease and would be an adequate inflow source for iliofemoral bypass, iliofemoral bypass is more invasive than femorofemoral bypass, and femorofemoral bypass may be preferred by some surgeons for that reason. Femorofemoral bypass may also be used as a component of endovascular repair of aortic aneurysm.47–57
Technique and Graft Configuration
Femorofemoral bypass is performed with the patient positioned supine. The operation can be performed with local infiltration anesthesia but is most often performed under spinal or general anesthesia. The abdomen is prepped along with the groin area and anterior thighs to allow access to the abdomen in case of unexpected findings during surgery. Longitudinal incisions are generally used to expose and control the femoral arteries on both sides. Although incisions parallel to the groin creases may be used, such oblique incisions are somewhat less versatile if exposure must be extended much more distal than the femoral artery bifurcation. General considerations described elsewhere in this text, as well as geometric considerations discussed in the following paragraphs, may affect the selection of the site of the arterial anastomoses.58
Femorofemoral bypass is certainly an operation of lesser magnitude than aortofemoral bypass, and the technique is regarded as simple by many surgeons. However, femorofemoral bypass, both as a stand-alone procedure and as part of an axillofemoral, thoracofemoral, or other bypass procedure, presents unique technical (geometric) challenges. The graft is tunneled from one groin incision to the other within the abdominal wall superior to the pubis (Fig. 111-1). The tunnel is created bluntly with fingers, a large clamp, or tubular tunneler. The prefascial subcutaneous plane will be the appropriate location for the graft tunnel in most patients, but a preperitoneal position59,60 may be elected or may be appropriate if unfavorable conditions exist in the abdominal wall such as prior surgery, radiation-damaged skin or other skin changes, an unusually thin subcutaneous fat layer, or even obesity predisposing to unfavorable graft geometry. The latter tunnel position may be associated with injury to bowel or urinary bladder61,62 and must be used with great caution, especially if there has been previous abdominal surgery. However, inflow for “crossover” bypasses may be provided in some cases by a contralateral iliac artery,36,63–68 and these iliac origin crossover grafts are usually most conveniently placed in the preperitoneal position. With the exception of Ng et al,69 most authors have found the patencies of these iliofemoral crossover bypasses to be comparable to or perhaps slightly better than femorofemoral bypasses. The outflow of such crossover grafts may be the popliteal or even tibial arteries70–72 or may be “sequential” grafts with an intermediate anastomosis to the femoral system in the groin, as well as a popliteal or other distal anastomosis to address both “inflow” (aortoiliac) and “outflow” (femoropopliteal) disease, especially in the setting of tissue loss due to multilevel disease.73,74 Even more unusual presentations may be addressed using a transperineal graft route.75–80 Readers are directed to the original publications for a more detailed description of these approaches.
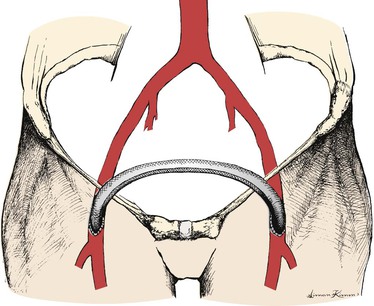
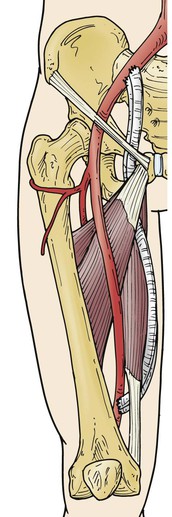
Figure 111-1 Standard “inverted C,” perhaps better termed “inverted U” configuration of femorofemoral bypass graft.
The graft is roughly confined to a plane tipped forward superiorly from the coronal plane to an extent that varies considerably with patient habitus. Anastomoses are made to some component of the femoral arterial system and are “end-to-side” in nearly all cases so that the graft is directed roughly longitudinally at the anastomoses. Whether the graft is configured as an “inverted C”81 (see Fig. 111-1; perhaps better termed “inverted U” and preferred by most surgeons) or a “lazy S,”82 the graft will make two abrupt changes in direction within the tipped-from-coronal plane just described. The likelihood of kinking can be reduced by using a slight excess of graft material, which reduces the tendency of the graft to kink at the “heel” of the anastomoses and by making the tunnel as a continuous curve between the groin incisions and several centimeters superior to the proposed anastomoses to try to increase the radii of the graft curves transitioning from a roughly longitudinal direction to a transverse direction coursing from one groin to the other.
The tendency of the graft to kink within the sagittal plane is a separate concern. The protuberant abdomen presents a problem not encountered in the aortofemoral graft that parallels the distal external iliac artery and emerges from beneath the inguinal ligament. The more obese or more protuberant the abdomen, the more the plane of the graft is tipped forward from coronal within the sagittal plane. This tends to cause the angle between the graft and the native artery to become less acute and, thereby, to cause a standard-length end-to-side anastomosis (roughly 3 times as long as the graft diameter) to kink in the sagittal plane. This can usually be prevented by making a shorter femoral arteriotomy (and anastomosis) or by making the anastomosis to a more distal part of the femoral arterial system, which has the effect of bringing the graft and the femoral artery into a more parallel (and desirable) relationship. Extending the anastomosis(es) at least partially onto the deep femoral artery may also be very helpful in reducing the tendency of the graft to kink in both the sagittal and coronal planes.
Systemic heparin is administered after completion of dissection and tunneling. Endarterectomized superficial femoral artery was used in Freeman and Leeds’ first femorofemoral bypass,14 and autologous vein grafts were common and even preferred conduits in some surgeons’ early experiences with femorofemoral bypass.83–85 A prosthetic graft is now used in nearly all cases, although almost every reported series of femorofemoral grafts has a few constructed of vein or other autologous vessel, generally placed in situations with high concern for infection.14,60,86–91 Another alternative, especially in the face of infection, is the use of femoral popliteal vein. D’Addio et al reported excellent results with femoral popliteal vein conduit in 54 patients, 16 of whom were undergoing femorofemoral bypass as the sole procedure.92 Either anastomosis may be performed first when using prosthetic conduits, but we would perform the donor anastomosis first when using venous conduits so that the presence of one or more competent valves in the conduit would not interfere with flushing just before completion of the second (recipient) anastomosis. Great care must be taken to allow some redundancy in the graft as noted earlier. It is very important to confirm enhancement of flow in the recipient vessels and continued flow in the outflow vessels beyond the donor side anastomosis using a continuous wave Doppler or other suitable test after anastomoses are completed and all clamps are removed.
Results
The perioperative mortality associated with femorofemoral bypass is highly dependent on patient selection but should be well under 5% in elective operations. Patients undergoing femorofemoral bypass are likely to have somewhat less advanced comorbidities and are consequently likely to enjoy longer survival than patients undergoing axillofemoral bypass. For example, we estimated patient survival of 71% for femorofemoral versus 35% for axillofemoral bypass at 3 years in our previous reports.93,94 Furthermore, far more patients appear to undergo femorofemoral than axillofemoral bypass procedures (for example, more than 4 times as many femorofemoral bypasses compared with axillofemoral bypasses were performed in Johnson and Lee’s prospective study95). Consequently, reports including 5-year results are much more common for femorofemoral bypass, allowing more confident assessment of long-term performance. We suggested in the sixth edition of this textbook that primary and secondary patency for femorofemoral bypass at 3 to 5 years should be expected to be about 60% and 70%, respectively,93,96–98 but results appear to have improved somewhat since that time. Ricco and Probst reported the results of a multicenter comparison of femorofemoral–to–inline (aortofemoral or iliofemoral) bypass and have also tabulated results of previously published studies of femorofemoral bypass including at least 40 patients, follow-up of at least 5 years, and with life-table estimates of graft patency at 5 years.45 Table 111-1 is modified from those tabulations to include only reports that also provide the number of grafts at risk at 5 years and with the addition of some previous and subsequent articles that also qualify.* This table demonstrates some variability in the patency estimates among these studies, likely due to diversity with respect to risk factors such as the fraction of patients with critical limb ischemia and failed previous aortofemoral bypass, which as noted elsewhere, can significantly affect femorofemoral graft patency. This variation is much less than is seen with axillofemoral bypass (see later). These tabulated data allow calculation of a weighted-average estimate of 5-year primary patency of 66%; however, a similar calculation confined to reports after the year 2000 yields a weighted-average estimate of 72%.
Table 111-1
Femorofemoral Bypass Patency
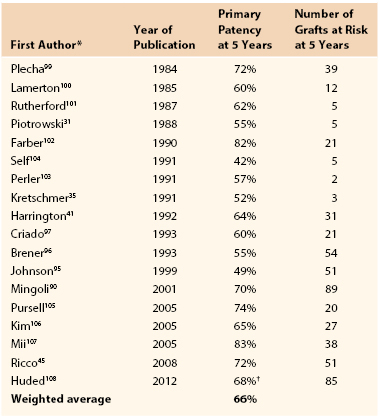
* Previously reported studies of femorofemoral bypass including at least 40 patients, follow-up of at least 5 years, and with life-table estimates of 5 year primary patency that appear to be compliant with current reporting standards.261,262
† Composite of femorofemoral bypass subjects with no adjunctive inflow (n = 74 at 5 years) and those with endovascular adjunctive inflow (n = 11 at 5 years) procedure.
Modified from Ricco J-B, et al: Long-term results of a multicenter randomized study on direct versus crossover bypass for unilateral iliac artery occlusive disease. J Vasc Surg 47:45-54, 2008.
Causes of Failure
The causes of extra-anatomic bypass graft failure are elusive, but are likely to include progression of disease in both inflow and outflow arteries. Although some early authors may have viewed femorofemoral bypass as a durable procedure not vulnerable to progression of disease109,110 and da Gama actually suggested that the donor artery enlarges over time after placement of an extra-anatomic bypass,111 other early adopters viewed femorofemoral bypass to be at risk from progression of disease in the donor iliac system.112,113
Surveillance
The role of noninvasive surveillance of femorofemoral and other extra-anatomic bypass grafts remains unclear, but Stone et al presented a unique exploration of the possible predictive value of duplex scan surveillance and concluded that a duplex scan estimated peak systolic velocity of greater than 300 cm/sec in the inflow artery or a midgraft peak systolic velocity less than 60 cm/sec were predictive of impending graft thrombosis.114 Early experience since adopting this approach in our practice seems to confirm the findings of Stone et al, although it should be noted that these findings have not been confirmed by others.
Effect of Surgical Indications and Patient Characteristics
Pursell et al noted a trend toward better patency in claudicants, consistent with the observation in virtually every other lower extremity arterial intervention.105 In contrast, Criado et al and, in what is among the largest, longest, and most completely followed series on femorofemoral bypasses, Brener et al noted no apparent difference in femorofemoral bypass patency comparing claudicants to patients with CLI.96,97 Our previous investigation actually suggested a trend toward reduced patency in patients with claudication compared with those with CLI, although the trend was not significant and would be difficult to explain except as a result of random error,93 and Huded et al’s recent report including data from our original publication found no difference between patency in claudicants versus subjects with CLI.108 On balance, it appears likely that results in claudicants are as good or better than those in subjects with critical limb ischemia, but not comparable to those after aortofemoral bypass.
Femorofemoral bypass is one of many methods of treating patients with symptomatic occlusion of one limb of a previously placed aortobifemoral graft.65,115,116 Although most authors have reported results in these patients to be inferior to those in patients undergoing primary femorofemoral bypass,87,93,96,101,117–119 others have described more favorable results.120–124 Most patients who suffer thrombosis of an aortobifemoral graft limb will be quite symptomatic and require urgent intervention.115,125–128 Femorofemoral bypass is one option, and the surgeon and patient may need to accept the prospect of a greater risk of subsequent failure in exchange for more expedient reperfusion of the leg. We have also noted a trend toward an inverse relationship between age and patency for femorofemoral bypass.93 However, we observed a similar trend after aortobifemoral bypass and this may represent the effect of more aggressive biology of atherosclerosis in patients who require intervention at a younger age.
Femorofemoral bypass has also been used as part of endovascular aneurysm repair using aortouniiliac systems.47–57 Local femoral wound complications have been a concern in several of these publications. However, patency of the femorofemoral graft in such patients has been excellent and clearly better than in those patients treated for occlusive disease. These results will be described in more detail in chapters dedicated to endovascular aneurysm repair.
Femorofemoral bypass was first used in patients considered unfit for aortofemoral bypass.129 However, the relative ease of the procedure and the substantially lower level of physiologic insult to the patient prompted many to extend the procedure to better risk patients who would be candidates for aortofemoral bypass.96,101,119,130–134 Femorofemoral bypass may also be preferred by some patients and surgeons because the risk of erectile dysfunction in men is likely lower than after aortofemoral bypass.67,87,93,135–137 We are aware of no randomized prospective comparisons of femorofemoral versus aortofemoral bypass, and even contemporaneous case control studies are unusual. We previously examined the results of patients who would have been candidates for aortofemoral bypass, but who instead underwent femorofemoral bypass, and we compared these results with patients undergoing aortofemoral bypass during the same time period in the same institution. The results of femorofemoral bypass, even in these good-risk patients, were clearly inferior to the results in patients undergoing aortofemoral bypass.93 Mingoli et al retrospectively reviewed and compared the performance of femorofemoral and aortofemoral bypass in two institutions between 1973 and 1993 and found no differences.138 However, this latter study must be considered unique since other studies of femorofemoral bypass consistently report inferior patency when compared with contemporaneous studies of aortofemoral bypass. Furthermore, many of the patients undergoing aortofemoral bypass in the past would be more appropriately treated with transluminal balloon angioplasty or iliofemoral bypass today, and it is unlikely that such a study could be repeated in a single institution today. In practice, it is rare that patient characteristics and arterial anatomy leave only two alternatives, femorofemoral or aortofemoral bypass, but when this is the case we continue to recommend the former for poor-risk patients and the latter for good-risk patients.
Effect of Graft Material and External Support
Most femorofemoral grafts have been constructed of prosthetic material. Dacron (polyester) was used almost exclusively until expanded polytetrafluoroethylene (ePTFE) became available. It is difficult to determine whether either of these classes of materials dominate femorofemoral bypass today. We could find no convincing evidence that either ePTFE or externally supported polytetrafluoroethylene (xPTFE) is superior to polyester grafts as measured by patency and hemodynamic performance. We are aware of no published data regarding the PTFE grafts with support within the wall (Gore Intering, W.L. Gore, Flagstaff, Ariz) in this application. At least one study found a trend toward inferior patency for ePTFE femorofemoral grafts.97 However, the remainder of published articles have reported comparable patency results using PTFE and polyester grafts in this application.45,90,95,139,140 Thus it currently appears that surgeons may choose either polyester or PTFE grafts and expect similar outcomes.
External or internal support is certainly a plausible contributor to improving the performance of femorofemoral and other subcutaneous bypass grafts. However, with the exception of Mingoli et al, who found an advantage of external support in a retrospective study including both polyester and PTFE grafts,90 we are aware of no clear evidence that external support provides superior outcomes in this application. Indeed, Kim et al106 reported results with mostly unsupported ePTFE grafts (Y.W. Kim, personal communication) that are indistinguishable from those with xPTFE in a report by Mingoli et al. Pursell et al also reported excellent results using unsupported polyester grafts (R.B. Galland, personal communication).105 Finally, we examined a series of 6-, 7-, 8-, and 10-mm diameter ePTFE grafts and could detect no relationship between diameter and hemodynamic performance or patency,93 findings confirmed by Ricco and Probst.45 Consequently, I have chosen to use 6-mm diameter xPTFE or Intering PTFE grafts for convenience and the possibility of decreased infection risk when performing femorofemoral bypass, but the hemodynamic performance and patency profiles of the available prosthetic grafts are probably indistinguishable in this application.
Hemodynamic Considerations
Many surgeons have been concerned that one iliac artery could not adequately supply blood flow to both legs or that the femorofemoral graft could “steal” from the donor limb and produce new or worsened symptoms of ischemia in the donor limb.85,86,113,141 Ehrenfeld et al showed that the capacity of a healthy iliac artery in an animal model far exceeds the resting flow requirements of both legs.141 Several authors have investigated the hemodynamic performance of the femorofemoral graft in patients and have concluded that there is rarely, if ever any significant deleterious effect on the donor limb and that the recipient limb remains well reperfused as long as there is no hemodynamically significant lesion in the donor iliac arterial system.60,130,142–144 The conclusions of these authors have dominated thought about femorofemoral graft hemodynamics to the present day. However, others have detected a slight fall in the mean resting ankle pressure on the donor side, a finding subsequently duplicated by several authors.60,93,143,145,146 Investigators for Veterans Affairs Cooperative Study 141 noted that the combination of hemodynamic deterioration and clinical symptoms of steal was present in only 3% of patients, although a much higher fraction of patients developed hemodynamic evidence of donor limb steal at rest, and angiographic findings did not predict the occurrence of donor limb steal.147 Harris et al made the remarkable observation that 45% of patients suffered deterioration in donor limb hemodynamics under exercise conditions despite normal resting donor limb hemodynamics after femorofemoral bypass.148 However, on balance, the evidence supports the contention that although some fall in donor limb pressure may occur after femorofemoral bypass, the donor limb is not functionally adversely affected by placement of a femorofemoral bypass as long there is no hemodynamically significant lesion in the donor iliac arterial system.
Significance of Iliac Artery “Inflow” Lesions.
Assessment of the hemodynamic significance of angiographically detected stenoses in the prospective donor iliac arteries has also been a topic of great interest in patients considered for femorofemoral bypass. Angiography alone is unreliable with respect to detection and assessment of hemodynamic significance of aortoiliac arterial disease.82,143,149–155 The increased flow in a donor arterial system after a bypass may unmask previously hemodynamically occult lesions.149,154,156 Sako was probably the first author to report the use of directly measured femoral artery pressures at rest and after injection of papaverine, a potent arterial vasodilator, to assess the capacity of the iliac arterial inflow to support a significant increase in flow.157 The test was subsequently further evaluated by Flanigan et al.158 The test as described by Flanigan is performed by directly measuring both systemic pressure and the arterial pressure in the proposed “donor” femoral artery before and after injection of 30 mg of papaverine into the femoral artery and has been used to predict whether an angiographically detected iliac lesion is of clinical importance when selecting “inflow” versus “outflow” reconstructive procedures or to determine whether an iliac artery will support a femorofemoral bypass. Flanigan et al found that femoral pressures more than 15% less than radial or brachial pressures were associated with an unsatisfactory outcome.
The concept of a physiologic test to predict how a potential donor iliac arterial system would behave under the stress of supporting two legs instead of just one is quite appealing. However, Archie has pointed out that only one study158 has examined the predictive value of the papaverine test.82 Furthermore, the technique has not been standardized. “Papaverine tests” are often performed with vasodilators other than papaverine, typically nitroglycerin or tolazoline. I have not been able to find literature to confirm that tests performed with other vasodilators are valid. I have also observed the test being performed without a separate radial artery or other catheter to measure systemic pressure during the test. This may cause a false positive result since systemic pressure often falls briefly but significantly during the 1 or 2 minutes immediately after injection of papaverine into the femoral artery. Finally, Flanigan stressed the importance of Doppler confirmation of increased flow in the proposed donor femoral arteries after papaverine injection, an often neglected portion of the test.158 Absence of such an increase in flow implies a technical problem with the test or significant occlusive disease limiting femoral outflow on the donor side and is likely to lead to a false negative test.
Archie has reported the largest and most detailed study of papaverine testing and its value in femorofemoral bypass.82 He found the test’s sensitivity and specificity inadequate to be reliable. However, despite the concerns expressed so eloquently by Archie, his work focused on the value of papaverine test data to predict patency and hemodynamic results as assessed in the noninvasive vascular laboratory. The value of papaverine testing as a predictor of relief of symptoms after femorofemoral bypass has not been so carefully examined. Thus we have continued to employ papaverine testing as one decision-making factor in femorofemoral bypass, particularly when balloon angioplasty of the donor iliac system is used, although we have devalued the test’s importance in view of Archie’s work.
Hemodynamic Results.
The ability of femorofemoral bypass to normalize perfusion of the recipient limb is more in question. Recipient limb perfusion is predictably improved by technically successful femorofemoral bypasses with appropriate anatomy.146 However, many surgeons have noted a significant number of clinical failures of femorofemoral bypass as measured by persistent claudication, rest pain, or failure to heal gangrenous lesions.145,159 The Veterans Affairs Cooperative Study 141 study cited earlier included more than 300 patients, clearly the largest study of femorofemoral hemodynamics to date, but these researchers presented no information about exercise testing of these patients.147 We previously examined the postoperative resting hemodynamics of femorofemoral bypass in 91 patients and concluded that recipient limb pressures would not be normal even at rest with completely normal femoral and other infrainguinal outflow vessels and as noted earlier; this finding was independent of the diameter of the graft used.93 Despite less than complete normalization of hemodynamics, femorofemoral bypass will in most cases provide adequate improvement in perfusion to maintain the limb in patients with critical limb ischemia.136,160 Although many surgeons view femorofemoral bypass as a satisfactory treatment for claudication,96,135,161–163 the persistent resting hemodynamic abnormalities in the recipient limb after femorofemoral bypass raise concern that it will perform poorly under exercise conditions.93,119,148 For this reason, we recommend inline revascularization for claudicants, which is usually possible with current interventional techniques.
Angioplasty of “Donor” Iliac Artery Before Femorofemoral Bypass
Successful femorofemoral bypass is highly dependent on a hemodynamically satisfactory donor iliac arterial system. Endovascular intervention for selected iliac artery lesions provides excellent short- and long-term results in terms of hemodynamic improvement and patency, as discussed in Chapter 112. It is not surprising that endovascular procedures to improve suboptimal donor iliac arteries might be considered before or concomitant with femorofemoral bypass. Porter et al164 described two patients who underwent graduated dilation (as previously described by Porter’s coauthor Dotter et al165) of the donor iliac artery before femorofemoral bypass with satisfactory early results in both. This procedure was performed before the development of balloon angioplasty by Grüntzig166,167 and others. Several authors have reported experience with transluminal balloon angioplasty before or concomitant with femorofemoral bypass.93,96,97,108,168–176 With rare exceptions,39,96,108 results of these studies have generally supported the view that donor iliac artery balloon angioplasty with stenting in selected cases is associated with a satisfactory hemodynamic outcome and patency rate. Furthermore, the results of balloon angioplasty have probably improved since the initial studies were published.175 On balance, it appears that as long as the donor iliac lesion would be considered favorable for angioplasty apart from the proposed femorofemoral bypass (ideally a short, minimally calcified lesion of the common iliac artery), then it is reasonable to proceed with angioplasty of the donor iliac artery and femorofemoral bypass.
Effect of Outflow Disease
Vascular surgeons recognized the critical importance of outflow and particularly the deep femoral artery very early in the experience with aortofemoral reconstruction.177–180 This principle is almost certainly applicable to extra-anatomic bypass as well. As with other operations for iliac arterial occlusive disease, many surgeons have suspected that disease of the femoral outflow arteries has a major impact on long-term femorofemoral bypass patency.* However, the patency of the superficial femoral artery has been found by many other authors to have no detectable impact on long-term patency.87,93,96,97,182 Ensuring outflow to at least one healthy artery, either the superficial femoral or more often the deep femoral artery, appears to provide adequate outflow to support patency, just as seems the case with direct aortofemoral bypass.93,183,184 Some have argued that routine “profundaplasty” is indicated in “inflow” operations including femorofemoral, axillofemoral, and aortofemoral bypass,185 but this probably has no impact on hemodynamic performance or patency in the absence of significant deep femoral origin stenosis.34 The ability to pass a 3.5-mm diameter probe into the outflow artery after completing the “toe” portion but before completion of the anastomosis is reassuring with respect to the adequacy of outflow, and we have always confirmed that this maneuver could be accomplished before completing any “inflow” operation. On balance, despite our own previous observations, I believe that the quality of outflow may affect patency, but I also believe that maneuvers to ensure the best possible outflow are likely to minimize this effect.
Complications
Complications of femorofemoral bypass are those common to nearly all arterial operations including those discussed in Section 8 (Complications) of this text. Complications relatively unique to femorofemoral bypass include possible perforation of the bladder or intraperitoneal viscera during creation of the graft tunnel, particularly when a preperitoneal tunnel is used,61,62 although transvesical and transbowel passage of conventional aortofemoral grafts have also been reported.186
Axillofemoral Bypass
Basic Concepts, Indications, and Patient Selection
Axillofemoral bypass depends on the ability of a healthy axillary artery to supply adequate blood to the ipsilateral arm and one or both legs, at least at rest. With the exception of occasional use as treatment of patients with aortic coarctation,187–189 axillofemoral bypass is used almost exclusively as treatment for patients with primary or secondary disease of the infrarenal aorta or iliac arteries. Axillofemoral bypass, just as any other method of intervention for primary or secondary infrarenal aortoiliac disease, must be judged against aortofemoral bypass.30,32–34 Axillofemoral bypass is an essential tool for treatment of many patients with infected aorta or prosthetic arterial grafts or aortoenteric fistulae,190–199 although in situ alternatives, discussed in Section 8 (Complications) and elsewhere in this text, have been proposed even for these patients.199–207 Early experience demonstrated axillofemoral bypass to be an excellent alternative for frail, older adult patients with bilateral iliac artery disease and those with other comorbidities such as multiple prior abdominal operations, abdominal stomas, or prior radiation therapy.86,132,146,208–221 However, the choice between aortofemoral and axillofemoral bypass is often less than clear. The definition of “high risk” is quite subjective, and the threshold for choosing axillofemoral over aortofemoral bypass is likely to vary significantly among surgeons. For example, approximately 25% of our open reconstructions for bilateral iliac artery occlusive disease were axillofemoral bypasses (and we were criticized for this “high ratio” when we presented our work in 1991),94,222 whereas Hepp et al performed axillofemoral bypass about 10 times as often as aortofemoral bypass.223 This has led to markedly different profiles of patient samples in published series of axillofemoral bypass, a topic that will be further in the following section.
Technique and Graft Configuration
Axillofemoral bypass is nearly always performed under general anesthesia.224 We have on rare occasions performed the entire operation with local anesthesia and sedation, but the exploration of the axillary artery and tunneling of the axillofemoral graft segment are difficult to perform under such circumstances. The operation may be expedited by the use of two operating teams, especially for axillobifemoral bypass. Either axillary artery may be an appropriate donor unless there is disease in the subclavian or axillary artery, although the right side is preferred in cases of aortic infection since subsequent remedial operations may involve left flank or thoracic exposures. We have approached this question by measuring blood pressures in both arms and recording continuous-wave Doppler waveforms in the brachial arteries. If a systolic pressure discrepancy of 10 mm Hg or greater exists between the arms, the axillary artery on the side with the higher blood pressure is chosen.
We insist on a triphasic Doppler waveform in the brachial artery in the proposed donor limb. Some authors have recommended routine preoperative arch and subclavian arteriography, citing a substantial frequency of occult disease in the axillosubclavian arterial system of patients considered for axillofemoral bypass.225 If both axillary arteries appear hemodynamically adequate to support axillofemoral bypass, we generally choose the arm ipsilateral to the patient’s more ischemic lower extremity, even when performing axillobifemoral bypass. However, the contralateral axillary artery may be used as the inflow even for axillounifemoral bypass if required by the circumstances. In the case of infection, it may occasionally be necessary to place bilateral axillounifemoral or axillodistal grafts,226 for instance, to avoid infected groin wounds. These choices are often made on the basis of other practical issues—for example, to avoid stomas or other pathology or to avoid placing the graft on the side preferred by the patient for sleeping. We would discourage placement of an axillofemoral graft on the side of a patent arteriovenous hemodialysis fistula, although we are unaware of any objective examination of the results of such procedures. Intraoperative arterial pressure-monitoring catheters should generally be placed in the nondonor arm.
Some surgeons perform the operation with the donor side arm at the patient’s side, but we prefer a supine position with the arm abducted to 90 degrees on the donor side. We also place a rolled towel under the patient on that same side to lift the torso several centimeters from the operating table deck. We believe these maneuvers improve exposure of the most medial portion of the axillary artery and allow visualization of the flank and lateral chest wall while passing the graft tunneler. We have used the 65-cm Gore tubular tunneler (W.L. Gore, Flagstaff, Ariz) and found that it allows easy passage of the axillofemoral graft limb without an intermediate incision in the flank. Femorofemoral limbs are positioned using the same approach described previously for isolated femorofemoral bypass. We have always draped widely to allow thoracotomy, sternotomy, or celiotomy, if necessary, to manage intraoperative bleeding or other complications that would dictate these approaches (Fig. 111-2), although we have never found it necessary to perform any of these maneuvers.
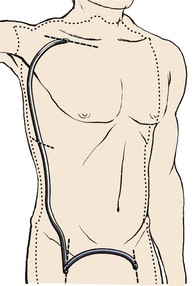
Figure 111-2 Typical area of exposure for an axillobifemoral bypass graft. The right axillary artery is the donor artery in this case. The intermediate incision in the right lower chest or upper flank is generally unnecessary if an appropriate tunneler is used.
Axillary Anastomosis
A transverse infraclavicular incision is carried through the clavipectoral fascia, exposing the pectoralis major muscle. The pectoralis major muscle fibers are pushed superiorly and inferiorly exposing the deep fascia and beneath that, the fat containing the axillary vein, artery, and brachial plexus elements. The axillary artery is exposed from the clavicle medially to the pectoralis minor muscle laterally, often requiring ligation of crossing veins or small arterial branches. The axillosubclavian arteries are considerably more fragile than the femoral arteries and care must be taken not to injure them or the adjacent veins and brachial plexus elements during dissection or during placement of retractors or vascular clamps. Similarly, care must be taken when performing anastomoses to the axillary arteries since sutures are much more likely to pull through these more fragile vessels. Conventional longitudinal or oblique groin incisions are used for femoral artery exposure.
Some early adopters of axillofemoral bypass recommended proximal anastomosis to the third portion of the axillary artery (lateral to the pectoralis minor muscle) since that portion of the artery is so accessible.208,214,218,227 However, Blaisdell21,228 from the beginning of his experience, and others since that time, have stressed medial placement of the axillary anastomosis on the first portion of the axillary artery (medial to the pectoralis minor muscle) to avoid tension (see next section). We have generally placed the graft posterior to the pectoralis minor muscle unless the patient has had prior axillary surgery, but placement of the axillary end of the graft anterior or posterior to the pectoralis minor muscle is probably unimportant with respect to results. It is far more important to place the axillary-graft anastomosis as medially as possible to avoid tension on the axillary anastomosis when the arm is abducted.229 Furthermore, medial placement of the axillary anastomosis eliminates the need to divide the pectoralis minor muscle in most cases. Leaving a slight excess length of graft in the axilla has also been advocated to reduce the likelihood of tension on the anastomosis.230,231 The axillofemoral graft must be tunneled subcutaneously in the midaxillary line to prevent kinking of the graft with torso flexion or kinking over the costal margin, which tends to be more prominent anteriorly than in the midaxillary line. Care must also be taken to avoid injury to the neurovascular structures of the axilla during tunneling.
Femoral Anastomoses
Systemic heparin is given after graft tunneling. The proximal-end-graft to side-axillary artery anastomosis is generally performed first. The order of distal anastomoses for axillobifemoral grafts may vary depending on whether there are one or two surgeons. The distal anastomosis is conventionally performed end-to-side to an appropriate artery in the groin. It is important to ensure adequate outflow. In the case of axillobifemoral configuration, the femorofemoral component may be placed by “piggy-backing” the femorofemoral graft onto the distal anastomotic hood of the axillofemoral graft (Fig. 111-3A). Alternatively, the femorofemoral graft may be placed first as described earlier and the distal anastomosis of the axillofemoral component may be piggy-backed onto the ipsilateral femorofemoral graft anastomotic hood. Either of these two variations would qualify as an inverted U femorofemoral component and would maintain maximum flow throughout the axillofemoral component, a characteristic thought to be desirable with respect to graft patency. Femorofemoral graft components should be placed using the principles described earlier. Blaisdell22,232 and Rutherford et al233 have described alternative configurations that theoretically prevent competitive inflow from the native iliac arterial system on the side of the distal axillofemoral anastomosis and which may thereby decrease the risk of graft thrombosis due to stasis (Fig. 111-3B and 111-3C). In Figure 111-3B, the common femoral artery is divided superior to the distal axillofemoral anastomosis and the femorofemoral graft component is anastomosed end-to-end to the distal common femoral artery. In Figure 111-3C, the common femoral artery is divided as in Figure 111-3B, but the common femoral artery is anastomosed to the side of the axillofemoral graft, which is then continued from the right groin to the left groin for the left femoral anastomosis. This latter configuration has the potential advantage of reducing the total number of anastomoses from four to three. Another alternative approach has been advocated by Wittens et al.234 These authors have examined a variety of grafts with manufactured bifurcations for axillobifemoral reconstructions. They found that a graft with a flow divider similar to that in bifurcated aortobifemoral grafts provided both superior hemodynamic performance and patency, although I could not identify any published confirmatory data since the original publication in 1992 except Dr. Wittens’ later doctoral thesis.234,235 Thus I would consider this approach as potentially useful but unconfirmed at this time. Indeed, this configuration appears similar to that first used by Sauvage and Wood210 but later described as “incorrect” by the same group.81 These grafts would appear to have long ipsilateral and contralateral distal graft limbs beyond the graft bifurcation, contrary to the most typical strategy as mentioned earlier. Furthermore, such a configuration would seem to require either a much longer ipsilateral groin incision or a counter-incision on the ipsilateral flank to avoid kinking of the contralateral distal graft limb. It is very important to confirm enhancement of flow in the recipient vessels using a continuous wave Doppler or other suitable test after anastomoses are completed and all clamps are removed. It is also essential to confirm adequate blood flow in the donor arm beyond the axillary anastomosis by confirming a good radial pulse or satisfactory oxygen saturation as indicated by pulse oximetry in the hand with both the axillofemoral graft and axillary outflow vessels unclamped.
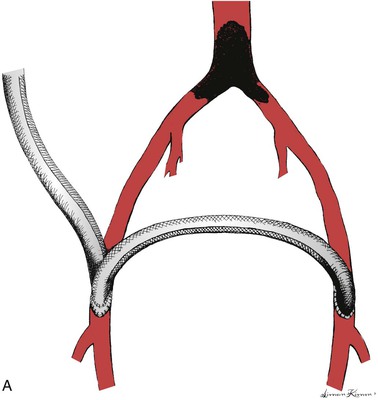
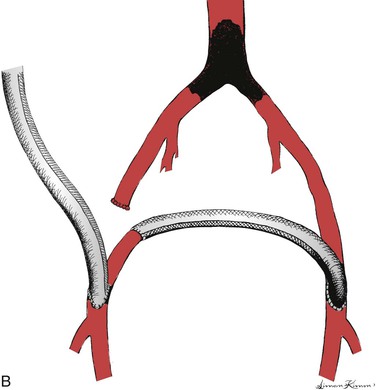
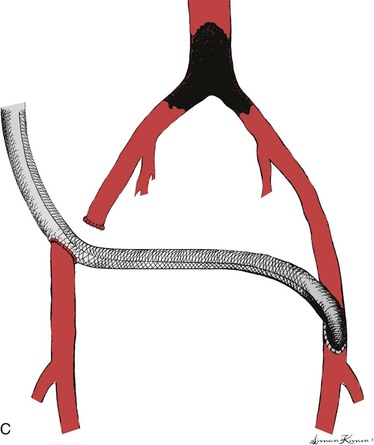
Figure 111-3 Three configurations of axillobifemoral bypass grafts. All three are shown with a right-sided axillofemoral graft component. A, The most common configuration.81 B and C, Modifications described by Blaisdell22,232 and Rutherford et al,233 respectively, designed to prevent competitive inflow from a patent ipsilateral iliac system.22
Results
Effects of Surgical Indications and Patient Characteristics on Results
Early Literature.
Earlier series of axillofemoral bypasses made it clear that axillofemoral bypass provided acceptable patencies in high-risk patients212–214,236 but the emergence and adoption of life-table methods for patency estimates allowed more meaningful comparisons between axillofemoral bypass and aortofemoral bypass and between series of axillofemoral bypass. Examination of reports of axillofemoral bypass yields perhaps the broadest range of long-term patencies of any arterial reconstructive procedure.101,237 A prior review of pertinent articles regarding axillofemoral bypass and confined primarily to patients with chronic lower extremity arterial occlusive disease published between 1960 and 1993 yielded 3-year primary patency estimates as low as 39% and as high as 85%.238 A subsequent 1996 report from Passman et al reported 5-year estimated primary patency of 74%.239
There are many potential explanations for these discrepancies. Patients undergoing axillofemoral bypass as part of the treatment for infection of the aorta or an aortic prosthesis or for aortoenteric fistula tend to suffer high perioperative morbidity and mortality, but with respect to graft patency and limb salvage results have varied. Oblath et al240 and Hennes et al199 reported generally mediocre results, but other investigators report that survivors are likely to enjoy better patency than those with chronic severe arterial occlusive disease.101,198,241,242 Series including substantial numbers of such patients will generally report a more favorable patency experience. On the other hand, it is likely that patients undergoing emergent axillofemoral bypass for acute lower extremity ischemia suffer substantially more early and late complications than do those undergoing surgery in more elective circumstances.243,244 Results after primary operations, including axillofemoral bypass can be expected to be superior to those for secondary operations.101 Patient characteristics and surgical indications influence outcome and must be considered when reading published reports of results of axillofemoral bypass. Axillofemoral bypass was also initially proposed only for high-risk patients,210,211,215,216 but by the mid- to late-1970s, some authors advocated extending these procedures to more patients and in some cases even advocated axillofemoral bypass as the procedure of choice for all but the youngest and healthiest of patients when anatomy allowed.130 Many surgeons were strongly influenced by three specific favorable reports,81,245,246 but the favorable experience reported in these articles may have been due to the characteristics of the patients reported and the approach to patency reporting.
Claudication versus Critical Limb Ischemia.
Claudicants have generally been found to enjoy better patency than patients with CLI for virtually every type of arterial reconstruction for chronic lower extremity arterial disease (with the possible exception of femorofemoral bypass, see earlier). Furthermore, claudicants tend to live longer than patients with CLI and through that mechanism tend to contribute to patency life tables longer than do those with CLI. Thus series with substantial numbers of low-risk claudicants tend to report disproportionately favorable patency experience than do those restricted to high-risk patients with CLI.247 If analysis is confined to high-risk patients, then most patients are likely to suffer from critical limb ischemia, patency is likely to be poorer, and late mortality is likely to be high. These issues and considerations have probably not changed significantly over time. For example, Bliss and Barrett reported estimated survival of only 43% at 28 months after axillofemoral bypass,214 Devolfe et al observed an approximately 35% 3-year survival 10 years after Bliss,219 and we reported 35% 3-year estimated survival 20 years after Bliss.93 However, as longevity of patients with cardiac and other comorbid diseases has improved over the last several years, the survival of patients undergoing axillofemoral bypass may also have improved. Huded et al reported 5-year survival of more than 40% in the most recent substantial study including axillofemoral bypass patients.108
Secondary Patency.
Finally, some of these favorable reports were based on the use of what is currently termed “secondary patency.” Nearly all authors have noted that axillofemoral bypass grafts are more likely than aortofemoral grafts to thrombose, and the favorable secondary patency of the former was at the expense of a significantly more frequent requirement for graft thrombectomy. Some reports have also considered the axillofemoral and femorofemoral components of axillobifemoral grafts as two distinct grafts, thus doubling the total number of “observed” grafts. Using this approach, thrombosis of one component has only half as much impact on the patency calculations as it would if the entire graft is considered as a unit. We consider this approach to be misleading since patients would find little consolation in persistent patency of half of their graft when told they will require amputation because of thrombosis of the other component of their graft.
Systematic Review.
Review of the published literature addressing axillofemoral bypass for chronic lower extremity ischemia since those earlier favorable reports has generally been much less optimistic. Two exceptions have been the favorable experience of Ray et al and El-Massry et al from the same group in Seattle and reports from the group at the Oregon Health Sciences University.81,239,248,249 It is instructive to examine these reports after stratification by operative indication. For example, Corbett et al reported 38% secondary patency at 2 years in a series composed of only patients with CLI,250 whereas Ray et al (roughly contemporary with Corbett et al and including 59% claudicants) reported 79% primary patency at 3 years. Dé and Hepp had to perform at least 1 and as many as 5 explorations for thrombosis in half of 131 axillofemoral bypass grafts.251 KA Harris et al reported only a 53% 3-year patency,252 whereas EJ Harris et al reported 78% 5-year primary patency in roughly the same era.248 Urayama et al noted markedly reduced primary patency for extra-anatomic bypass compared with direct aortofemoral reconstruction, but the former group was older, more likely to have critical limb ischemia, and had markedly lower survival to at least 10 years after the procedure.253
Table 111-2 represents a review including reports that appeared to adhere to current reporting standards.* We selected articles that we thought provided adequate information about indications for axillofemoral bypass, comorbidities, and late mortality; clearly used life-table methods to calculate patency as defined using Society for Vascular Surgery criteria261,262; and when possible, included data only from patients with chronic or acute arterial occlusive disease, excluding data related to arterial or prosthetic graft infection. It was unclear in some of these articles whether patency was calculated by number of graft limbs or number of patients. These studies are arranged in Table 111-2 in order of ascending primary patency. Only two primary reports of axillofemoral bypass that I could identify published in the last 20 years appear to qualify for inclusion in Table 111-2 based on the just-described criteria, although there does seem to be a trend toward lower operative mortality in some more recent reports that are not included in Table 111-2.263–266 With the exception of the report of Donaldson et al,257 the results of the reports included in Table 111-2 are fairly easily separated into series with few claudicants, including our own previous report and series that include significant numbers of claudicants, the former comprising seven of the first eight citations and the latter consisting of Donaldson et al, plus the last five citations in the table. Thus it appears that inclusion of as few as 20% claudicants in such a series has a potentially dramatic impact on the patency experience of axillofemoral bypass. Operative and late mortality, where reported, are also lower in those series with larger numbers of claudicants, thus enhancing the patency predictions by the mechanisms discussed earlier. Finally, a wide range exists in the frequencies of various comorbidities in patients undergoing axillofemoral bypass, reinforcing the contention that the definition of “high-risk” differs among institutions. Jämsén et al and Huded et al have provided an excellent description of their results, but these reports did not clearly distinguish claudicants from those with CLI to an adequate extent to allow inclusion in Table 111-2.108,263 However, review of those authors’ results show them to be generally consistent with those described by others and included in Table 111-2, except for improving mortality as noted earlier.
Table 111-2
Comparison of Indications, Patient Characteristics, and Mortality with Axillofemoral Graft Patency
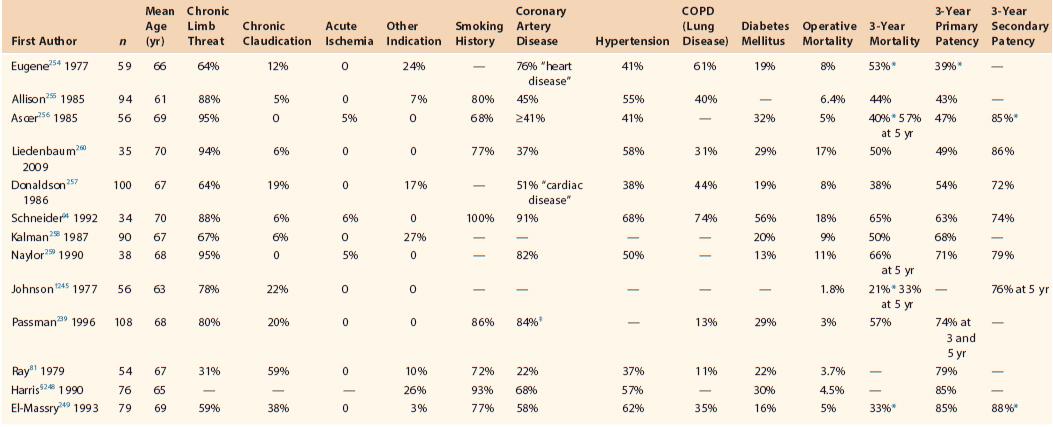
* Not quoted for 3 years but estimated from life table graphs in original article.
† Method of patency calculation in this article appears consistent with SVS definition of secondary patency.261
‡ Listed as “heart disease;” not clearly restricted to coronary artery disease.
§ Included because of unusually high graft patency despite lack of adequate information regarding indications for surgery and late mortality. Subsequent update of this series showed 78% 3-year primary patency.282
Modified from Schneider JR: The role of extraanatomic bypass in the management of bilateral aortoiliac occlusive disease. Semin Vasc Surg 7:35-44, 1994.
We have identified no prospective randomized comparisons of axillofemoral and aortofemoral bypass and we are aware of only five reports using the case-control approach to analyzing outcome in contemporaneous patient cohorts treated with axillofemoral and aortofemoral bypass in a single institution.94,239,245,264,267 Johnson et al compared primary axillofemoral bypass to contemporaneous results with aortofemoral bypass in the same institution and observed 76% (axillofemoral) versus 77% (aortofemoral) estimated secondary graft patency at 5 years.245 Our experience was substantially different yielding 63% (axillofemoral) versus 85% (aortofemoral) estimated primary patency at 3 years for contemporaneous patients in a study with only 6% claudicants in the axillofemoral group.94 Inspection of the life-table patency graph in Mason et al suggests significantly lower patency for axillofemoral bypass as compared with aortobifemoral bypass.267 Passman et al reported 5-year estimated primary patency of 74% (axillofemoral) versus 80% (aortofemoral), a difference that was not statistically significant.239 The discrepancy in results is almost certainly related to significant differences in patient mix, the use of secondary patency in Johnson’s work and primary patency in ours, and the fact that our own review included both primary and secondary axillofemoral and aortobifemoral bypass procedures. The results reported by Passman et al remain impressive even after this type of scrutiny. Despite the enthusiasm for axillofemoral bypass among a few authors, most continue to view this operation as most appropriate for patients at very high-risk for aortofemoral bypass and who cannot be treated with iliac endovascular techniques or as part of the treatment for infection of the native aorta or previously placed aortic prostheses.265 A more recent publication from the same institution as Passman et al268 does not report life-table estimates of patency, but review of the material in that article suggests that patency rates in patients treated more recently are not as favorable as those reported previously and are more consistent with others’ experience. Finally, Onohara et al have performed a multivariate analysis of patients undergoing axillofemoral and aortofemoral bypass hoping to adjust for several possible predictors of patency, and their analysis suggests that axillofemoral bypass patency is comparable to that for aortofemoral bypass after adjustment for other factors.264 Such a conclusion is intriguing but would require larger sample sizes and validation by others before it could be generally accepted.
Limb Salvage.
Limb salvage may be the best criterion by which to assess operations for limb-threatening ischemia, but appropriate life-table estimates of limb salvage are unusual in published reports of axillofemoral bypass. Limb salvage may also include results in claudicants or other patients whose surgical indications did not include CLI. Examination of reports in which life-table methods are clearly used in patients suffering predominantly from CLI or in which separate results are tabulated for patients whose initial presentation was CLI yields 3-year limb salvage estimates ranging from 69% to slightly more than 80%, a range much narrower than that for patency.94,258,259,269 Most important, although axillofemoral bypass does not provide complete hemodynamic normalization, it appears to provide limb salvage in most patients whose initial indication for reconstruction is CLI and is an excellent compromise in that regard when confronted with patients at extreme risk of direct aortic reconstruction.266
Surveillance.
The role of noninvasive surveillance of axillofemoral grafts is questionable. Sanchez et al270 and Calligaro et al271 found duplex scanning to be of some utility but did not distinguish between axillofemoral and other graft positions. Musicant et al were unable to identify a clear duplex scan–derived threshold predictive of impending axillofemoral graft failure.268 Thus it does not appear that routine surveillance of axillofemoral grafts can be justified at this time.
Effect of Graft Material and External Support
The first axillofemoral bypasses were performed when only saphenous vein or other autologous grafts and unsupported prosthetic (polyester or textile PTFE) grafts were available.20,227,272 Autologous grafts may be appropriate in some circumstances,272,273 but prosthetic grafts are used almost exclusively today. Externally supported polyester,274,275 ePTFE,218,276 and xPTFE248 have subsequently become available, and each has been touted as superior to their predecessors. Each of these materials appears mechanically durable and material failures are extremely rare.277–279 Several retrospective studies comparing polyester and PTFE detected no differences in patency.220,232,280,281 Published studies that suggest improved results with a new graft material suffer from the problem of comparison with historical controls. Harris et al248 reported excellent results with xPTFE when compared with historical controls from their own group with unsupported grafts of unspecified material. A subsequent update of this same series produced a downward revision of patency, although the results remain impressive.282 However, the number of claudicants was not specified in that study, and we observed much less favorable results with the identical xPTFE graft placed during roughly the same time period in a high-risk group consisting nearly exclusively of patients with limb-threatening ischemia and severe outflow disease.94 El-Massry et al249 reported excellent results with externally supported polyester grafts in a series with 38% claudicants, but these results were only marginally better than those with unsupported polyester prostheses reported by Ray et al from the same group published 14 years earlier.81 Indeed, Ray et al reported one of the highest axillofemoral graft patencies ever, despite the fact that his report is now more than 30 years old (see Table 111-2). Finally, hemodynamic studies attempting to assess the importance of external compression as a potential cause of graft thrombosis have yielded conflicting results283,284 and the basic assumptions that graft compression is a cause of failure or that external support prevents compressive occlusion of the graft remain unconfirmed.
Johnson and Lee, in the only identified randomized prospective comparison of supported polyester versus xPTFE axillofemoral bypass grafts, demonstrated no detectable difference between externally supported polyester and xPTFE axillofemoral grafts.95 PTFE, collagen-impregnated polyester, and gelatin-coated polyester grafts are all convenient, avoiding the requirement for preclotting and possible bleeding within the subcutaneous tunnel, and any of these can be used with similar outcome expectations. Thus although many have concluded that external support is of value in axillofemoral bypass,136,248,282 a critical review of the precedent literature does not support this conclusion. The concept of external support has “face validity,” and we continue to use externally supported grafts, although this is not based on solid evidence. Development of new prosthetic grafts may alter our preferences in the future.
Axillouni-versus-Axillobifemoral Configuration, Graft Diameter, and Other Hemodynamic Considerations
The average resting flow in the axillofemoral limb of an axillobifemoral graft is approximately 600 to 900 mL/min (somewhat less in axillounifemoral grafts) when measured intraoperatively with an electromagnetic flowmeter81,246,285 or using duplex scan–derived estimates of volume flow.94 This is consistent with the estimated resting flow of 300 to 400 mL/min in each normal common femoral artery286 and is comparable to the average estimated flow in upper extremity arteriovenous dialysis fistulae. Thus it is not surprising that axillofemoral bypass can provide adequate flow to support the legs. However, despite the observation that one axillary artery may accommodate “the entire cardiac output,”21 axillofemoral bypass may not provide a normal hemodynamic result, probably because of resistance posed by the long graft length. Some clinical evidence suggests that axillofemoral bypass does not result in complete improvement in claudication symptoms.101 We previously noted that axillofemoral bypass resulted in a predicted ankle brachial index of only about 0.7 with normal outflow vessels, much inferior to the results with conventional aortofemoral bypass, and we also noted a trend toward less satisfactory improvement as measured by ankle brachial index in patients with greater estimated graft flow, implying that the axillofemoral graft itself may be flow limiting.94 Given the length and diameter of the grafts used for axillofemoral bypass in particular, it is not surprising that these reconstructions do not provide a hemodynamically normal result.287 Nevertheless, as is the case with femorofemoral bypass, axillofemoral bypass provides adequate enhancement of perfusion to allow limb salvage in most patients.136
Axillofemoral bypass was originally described as a unilateral (unifemoral) procedure. Sauvage and Wood were probably the first to suggest that patency of axillobifemoral grafts would be superior to axillounifemoral grafts because of the increased blood flow in the former.210 The majority opinion for many years was that axillobifemoral grafts would enjoy patency superior to that of axillounifemoral grafts and that axillofemoral bypass should virtually always be performed in a bifemoral configuration.* More recent evidence suggests that this dogma may be flawed. Diameter of graft components is not stated in all published reports. Ray et al suggested an increased rate of thrombosis for 8-mm diameter grafts when flow is less than 240 mL/min.81 Published estimates of flow in axillounifemoral grafts are only slightly more than this 240 mL/min threshold, whereas estimated flows are roughly twice as high in axillobifemoral grafts,94,246 thus providing a plausible theoretical explanation for the alleged superior patency of the axillobifemoral configuration. Dr. Ray’s work also implies that larger diameter grafts would be at risk of thrombosis at even higher minimum flow rates, suggesting that axillofemoral grafts should be no more than 8-mm diameter and that 10-mm and 12-mm diameter grafts would be at very high risk of thrombosis in axillounifemoral configuration.
Despite these observations and the theoretical arguments, other authors have found no difference† or no more than an insignificant trend toward improved patency257 for axillobifemoral grafts as compared with axillounifemoral grafts. In some cases, this may reflect the use of smaller diameter grafts, consistent with the arguments just discussed. For example, Ascer et al, used 6-mm diameter axillofemoral components for axillounifemoral and axillobifemoral grafts and observed no discrepancy in patency between these configurations.256 Ray’s work would suggest a thrombosis threshold of substantially less than 240 mL/min for a 6-mm graft.81 It is also ironic that the highest reported patency for axillofemoral grafts that we have been able to identify came from a series dominated by axillounifemoral grafts and performed by the group that originally championed the axillobifemoral configuration (all uni- and bifemoral grafts were constructed of 8-mm diameter externally supported polyester).249 Thus it appears that axillounifemoral bypass performed with a 6- or 8-mm diameter graft will perform as well as an axillobifemoral bypass performed with an 8-mm axillofemoral component. I prefer an 8-mm xPTFE axillofemoral component and 6-mm xPTFE femorofemoral component for axillobifemoral grafts. I have generally used an 8-mm xPTFE graft for axillounifemoral grafts unless the patient is small, in which case I have chosen a 6-mm xPTFE graft.
Effect of Outflow Disease
Just as with femorofemoral bypass (see earlier), the patency of the superficial femoral artery has been found by some authors to be an important determinant of axillofemoral graft patency21,81,101,246 and by others to have no impact on axillofemoral patency.94,239,256 We and others have found it necessary to perform local procedures to ensure good outflow, usually to the deep femoral artery, in a substantial fraction of cases, certainly more frequently than we have found in aortofemoral bypass.94,181,184,234 We have used the same principles, including passage of a 3.5-mm probe into either the superficial or deep femoral artery as in aortofemoral and femorofemoral bypass to ensure adequate outflow. Thus the inability to demonstrate a difference between patients with patent arteries and those with occluded superficial femoral arteries may reflect the effect of an aggressive approach to ensuring adequate deep femoral arterial outflow.
Axillopopliteal Bypass
Axillofemoral bypass has occasionally been extended to the popliteal artery, primarily for cases in which there is groin sepsis and the superficial femoral artery is an unacceptable distal target vessel or the surgeon believes that foot perfusion will not be adequately enhanced by a bypass to the groin because of infrainguinal occlusive disease. Bastounis et al noted very poor patency among patients undergoing axillopopliteal or axillotibial bypass,292 but Ascer et al, McCarthy et al, and Keller et al all noted acceptable performance of this configuration.293–295 However, these authors all found that patency was inferior to that expected with more conventional reconstructions. This finding is not surprising, given the long graft length and requirement to cross at least one flexion point. Indeed, it is more surprising that any of these compromised grafts remain patent. Nevertheless, this technique may occasionally be the only reasonable approach in patients with groin sepsis or in whose who are unacceptable risks for more conventional reconstruction and whose arterial occlusive anatomy is not amenable to either an “inflow” or “outflow” procedure alone. Readers are directed to the original publications for more information on these rarely required procedures.
Complications
Although axillofemoral bypass is subject to the same complications as other arterial operations discussed in Section 8 (Complications), several complications are unique to axillofemoral bypass, including brachial plexus injuries, “axillary pullout syndrome” (disruption of the axillary artery to graft anastomosis), and thromboembolic risks to the donor arm and recipient legs following thrombosis of the graft.181,229,296–309 Taylor et al have provided an excellent review of the literature describing axillary pullout syndrome and have described a proposed modification of the technique to allow some redundancy of the graft to prevent this complication.231,310 It is likely that axillary pullout most often occurs as a result of tension on the anastomosis with abduction of the arm when the axillary artery to graft anastomosis has been placed too far laterally on the axillary artery or because the reconstruction has failed to provide some graft redundancy, as has been recommended earlier. Mannick et al cautioned against placing grafts with tension on the axillary anastomosis and indicted this as a possible cause of axillary thrombosis.213 We have observed thromboembolic complications to occur in three episodes, all three of which involved extremities in a single patient with a thrombosed axillobifemoral graft. Infection of axillofemoral bypass grafts poses unique problems since these patients have often been treated for failure of prior reconstructions, often have multiple comorbidities making them extremely poor candidates for more reconstructive surgery to deal with this problem, and have extremely limited anatomic options for revascularization.203,311,312 One recent article describes use of the femoral vein and endarterectomized superficial femoral artery to perform in situ replacement of an infected axillofemoral graft,313 extending the use of this autologous conduit even further.202 Graft disruption due to trauma appears to be extremely rare.314,315
Obturator Bypass and Other Extra-Anatomic Alternatives to Direct Femoral Artery Bypass
Indications and Patient Selection
Shaw and Baue first described obturator bypass as a strategy to avoid frankly contaminated fields during reconstruction of patients after removal of infected grafts in the groin.23 This indication has accounted for a large fraction of patients requiring obturator bypass in the past,316–322 but we and others have also used obturator bypass to reconstruct patients after removal of infected ePTFE dialysis access grafts based on the femoral arteries, patients with infected femoral pseudoaneurysms after diagnostic or therapeutic femoral arterial access or recreational drug use or even primary mycotic femoral artery aneurysms,323–326 patients with groin neoplasms requiring en bloc removal of tumor and artery with residual soft tissue defect that would expose an in situ reconstruction,327–329 and patients who have undergone therapeutic radiation in the groin.327,330–332 With respect to the risk of persistent infection and hemorrhage and the concerns about recidivist injection of recreational drugs into bypass grafts placed for infected femoral pseudoaneurysms, the most conservative approach is to simply debride and ligate the arteries in the groin, although this is associated with a significant risk of limb loss.323,333 The question of whether one should routinely reconstruct patients in addition to local debridement and ligation for femoral mycotic aneurysms and other vascular infections in the groin has aroused significant debate324,333–336 and is addressed elsewhere in this text.
The term obturator bypass is imprecise at best. Alternative terms such as transobturator foramen bypass would be preferable, but obturator bypass is already in common usage. The original description employed polyester, but ePTFE, xPTFE, saphenous vein in various configurations, autologous deep vein, and human umbilical vein have all been used.320,337–340 The presence of an infected prosthetic aortofemoral graft limb on the side to be reconstructed is a relative contraindication for obturator bypass, particularly if there is frank pus adjacent to the graft limb. Preoperative imaging studies may indicate involvement of the aortofemoral graft limb and in such cases, other techniques such as axillopopliteal bypass may be the only alternatives.
Technique and Graft Configuration
Guida and Moore have provided an excellent review of the anatomic principles and technique of obturator bypass, although few surgeons would use the third (upper thigh) incision originally recommended by those authors (and by Courbier and Monties in their original cadaver study24) to facilitate tunneling.341 The operation is typically performed with general anesthesia. The patient is placed supine, and the abdomen is prepped. The leg to be reconstructed is prepped circumferentially to allow manipulation during the operation. Infected wounds are excluded from the field as much as is possible. The donor artery is most often exposed using an oblique curvilinear lower quadrant incision and a retroperitoneal approach, although a transperitoneal approach may also be used. The common or external iliac artery may serve as the donor artery. As noted earlier, an aortofemoral graft limb may also serve as the donor vessel if this portion of the graft is uninvolved with the infection.342–344 This alternative requires that the graft be divided and the distal infected portion excluded from the operative field until the bypass has been completed and the wounds closed before exploring the groin wound for removal of infected prosthetic material and native tissue. None of our patients has had a prosthetic inflow source and we have not had occasion to use this approach. The obturator foramen is approached from this same incision by dissection medial to the external iliac vein and posterior to the pubic ramus and blunt dissection of the obturator internus muscle away from the obturator membrane (Fig. 111-4). The obturator artery and nerve perforate this membrane posterolaterally, so it is safest to avoid these structures by passing the graft through the anteromedial aspect of the obturator foramen. The membrane is extremely strong and cannot be perforated bluntly without risk of injuring adjacent structures and must be opened sharply or with electrocauterization.
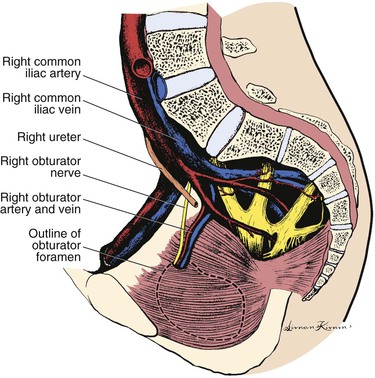
Figure 111-4 Approach to the obturator foramen with adjacent iliac vessels as viewed from the medial side of the pelvis.
Target Artery and Tunneling
The target vessel is often the popliteal or distal superficial femoral artery but may also be the deep femoral artery.345,346 However, approaching the deep femoral artery from the posteromedial side, although technically possible, risks entry into the infected groin and for this reason should be avoided in most cases, unless there is no alternative target artery. In such a case, an incision is made along the mid- to upper medial thigh, taking care not to enter the contaminated area of the groin. The tunnel between the inflow and target arteries is made most often in the potential space between the adductor longus and brevis muscles anteriorly and the adductor magnus muscle posteriorly, which leads directly to the obturator foramen339 (Fig. 111-5), although some authors have recommended that the tunnel be maintained posterior to the adductor magnus to reduce the risk of entry into the contaminated groin.341 Some authors have recommended passing the tunneling device from superior to inferior, but we have found it easier to pass the tunneling device from inferiorly,347,348 especially when the iliac exposure is limited by factors such as obesity, stomas, or the very noncompliant lower abdominal wall often observed in patients after radiation. The superficial femoral artery is easily identified and controlled in the plane of the tunnel anterior to the adductor longus and brevis muscles in the mid-thigh and anterior to the adductor magnus muscle more distally. However, if the popliteal artery is the target, the graft will either require tunneling through the tendinous portion of the adductor magnus (as the native superficial femoral artery does at the adductor hiatus) or be brought around the adductor tendon as a conventional femoropopliteal graft would be. The proximal and distal anastomoses are then completed using standard techniques, usually end-to-side to both donor and target arteries. The surgeon may wish to ligate the external iliac artery downstream from the proximal anastomosis to reduce blood loss during the subsequent femoral artery exploration. Once the reconstruction has been completed, the surgical incisions are closed and excluded before the groin is explored to remove any infected prosthetic or native material and ligate vessels as necessary to prevent hemorrhage. It is critically important to debride sufficient artery to permit oversewing of grossly noninfected segments of the femoral arteries proximally and distally.349
Thigh Perfusion
The thigh may be “isolated” and may be ischemic after obturator bypass with ligation of the femoral arteries.350 Consequently, we have always ligated the superficial femoral artery as high as possible in the thigh and on occasion have anastomosed the superficial femoral to the deep femoral artery after debridement of the common femoral artery, thus allowing blood at systemic pressures to reach the deep femoral artery by retrograde flow when the superficial femoral artery is patent.337,350 This has been done only in cases when we felt the sepsis was well controlled, the arteries were debrided back to grossly healthy tissue, and the infecting organism was an antibiotic-sensitive Staphylococcus aureus. We would recommend against this practice with gram-negative organisms, especially Pseudomonas species.321,351
Results
As with all extra-anatomic bypasses, results vary somewhat from one report to another. van Det and Brands estimated 80% patency (likely, primary patency as defined currently) at 6 years in their personal series of 13 obturator bypasses and compared this with a number of previous reports.332 Sautner et al provided an excellent review of mortality, graft patency, and limb salvage in patients undergoing obturator bypass.352 Estimated patency rates of 73% and 57% at 1 and 5 years, respectively, and limb salvage of 77% at 5 years are somewhat less than would be expected with conventional femoropopliteal bypass, but these figures are better than would be expected with simple excision of infected graft material with local measures including muscle flap treatment353 or nonoperative treatment. However, Nevelsteen et al337 and Kretschmer et al354 presented substantially less optimistic estimates of 5-year patency, and the surgeon can expect to be faced with revision or new reconstruction in a significant number of these patients. Despite its technical demands and the relative difficulty of creating the tunnel, transobturator grafts appear to provide good hemodynamic performance and appear relatively insensitive to hip movement.327
Graft Material
Most early obturator bypasses were performed with prosthetic grafts,* but placing prosthetic grafts in the setting of active infection, even if one can physically exclude the operative field from frank infection, carries a substantial risk of secondary infection of the newly placed prosthesis.334 Indeed, Shaw and Baue placed polyester grafts in their original three patients and two of three of these prosthetic grafts ultimately became infected.23 However, Patel et al have described a series of prosthetic obturator bypass grafts (eight PTFE, four polyester) without an apparent case of infection of the new graft.344 Others, including our own group, have used autologous vein in all cases and have not had problems of persistent infection in any case.316,319,320,338,339 On balance, I believe that obturator bypass should be performed with autologous vein whenever possible since the risk of infectious complications is much less when autologous conduit is used. Second choices for conduit would include femoral-popliteal vein or cephalic or basilic veins from the arm. Benjamin et al have described eight patients with infected pseudoaneurysms, five of whom had femoral pseudoaneurysms due to recreational drug use and in whom autologous deep vein conduit was used to construct obturator bypasses with control of sepsis and limb salvage in all five patients.340 Bell et al used femoral-popliteal veins in 11 cases, 4 of which were placed in a transobturator foramen position to reconstruct infected femoral pseudoaneurysms.358 One may be willing to use prosthetic conduit in cases without infection, for example, in irradiated groins, but the long-term patency advantage of autologous conduit almost certainly parallels that in conventional femoropopliteal bypass, where autologous great saphenous vein is clearly superior to current prosthetic conduits.
Alternatives to Standard Obturator Bypass
It may in some cases be possible to route bypass grafts lateral to the groin wound, anterior to the pelvis, and sufficiently remote from an infected groin to a popliteal, superficial femoral, or deep femoral arterial target, thus obviating the more difficult transobturator tunnel.340,349,358–363 Certain situations may dictate other extra-anatomic bypass routes such as a route through the iliac bone.362,364–366 A number of variations have been described including a subscrotal transperineal crossover graft route (see earlier) that obviates the need to pass through the obturator foramen. Thompson et al have described a remarkable aortobilateral transobturator bypass for combined aortic and femorofemoral graft infection.367 Readers are directed to the original publications for more information about these very unusual situations.
In situ replacement of the femoral artery, even with autologous arterial or venous grafts in the face of active infection, is associated with a substantial risk of graft or anastomotic disruption and likely exsanguination. Obturator bypass is the most conservative and should be considered the favored approach in patients with infection involving the femoral artery who require reconstruction and who are considered candidates for obturator bypass.354 However, in situ replacement with autologous material may be appropriate in highly selected patients with limited local inflammation and limited autologous conduit or whose medical comorbidities appear to place them at very high risk for an operation as large as obturator bypass with autologous vein.368
Complications
The most serious complications of obturator bypass are the complications of persistent or recurrent infection including generalized sepsis and hemorrhage from the groin vessels. As noted earlier, the use of autologous conduit seems to effectively reduce the risk of infection of the obturator bypass graft and the operative sites associated with grafting. The risk of hemorrhage is higher in the face of persistent infection or nutritional or other immunocompromise369 and is likely reduced by generous debridement of infected and necrotic tissue, secure closure in healthy portions of the groin vessels, and coverage with well-vascularized adjacent tissue, such as the sartorius muscle370 or gracilis muscle flap.371 Injury to the obturator nerve and artery is possible, especially if the tunnel is not kept in the anteromedial portion of the obturator fossa. Even more unusual complications of transvaginal352 and transvesical372 passage of the graft have been reported, although as noted earlier, the latter has also been described as a complication of aortofemoral bypass.186
Thoracofemoral and Supraceliac–to–Iliofemoral Bypass
Indications and Patient Selection
These procedures have in most cases been applied to patients with failure or infection of previous infrarenal aortic reconstruction, previous abdominal surgery for other than aortic pathology, radiation, or other reasons that would make a conventional transperitoneal or retroperitoneal approach to infrarenal aortic surgery difficult or impossible and for patients who are not candidates for endovascular intervention. Blaisdell et al’s original patient actually had his thoracofemoral graft placed during the same operation in which the infected infrarenal aortic graft was removed and died with sepsis 1 month after thoracofemoral bypass, although the thoracofemoral graft did not appear to be infected at autopsy.373 Nevertheless, placement of a thoracofemoral graft in the face of simultaneous active infection elsewhere is associated with a significant risk of infection of the graft, and this is probably not a reasonable indication for this procedure.374,375 Repeat aortic replacement with conventional infrarenal aorta–to–graft anastomosis may be possible after a suitable period of delay following removal of infected aortic prostheses,376–378 but I believe most surgeons would view a previously undissected supraceliac or descending thoracic aorta as a preferable inflow source in such patients.
The first known use of thoracofemoral bypass was to treat a patient with prior abdominal surgery and failed prior infrarenal aortic replacement,25 and shortly thereafter, a similar technique was used to treat a patient with an infected aortic prosthesis.373 These remain the primary indications for thoracofemoral bypass today. However, results of thoracofemoral bypass have been so encouraging that some groups have advocated the use of thoracofemoral bypass as primary treatment in selected patients with aortoiliac occlusive disease, at least when the disease extends into the visceral segment of the abdominal aorta.379,380 The procedure is formidable, certainly more so than axillofemoral bypass. Thus the procedure is most applicable to patients who are physiologically appropriate for thoracotomy.
Exposure and control of the supraceliac aorta was probably first proposed as a method for aortic control during repair of ruptured abdominal aortic aneurysm.381 However, exposure and control of the supraceliac aorta developed into an important technique for treatment of juxtarenal aortic aneurysms,382–384 operations on the visceral segment of the aorta and its branches,287,385,386 “redo” aortic surgery (including “redo” aortofemoral bypass387–391), and as an alternative approach to aortodistal bypass by using the generally less diseased supraceliac aorta in patients with extensive disease of the perirenal and visceral segment of the aorta.392,393
Technique and Graft Configuration
The ascending aorta is favored for inflow for repair of many complex anomalies of the aortic arch and has also been used successfully as an inflow source for lower extremity arterial reconstruction.394,395 However, this is a very invasive procedure requiring median sternotomy, graft positioning, and tunneling, and such a procedure is generally reserved for situations in which access to the descending thoracic or supraceliac aorta would be difficult.
Use of Descending Thoracic Aorta for Inflow
Several authors since Stevenson have described techniques for thoracofemoral bypass based on the descending aorta.374,379,380,393,396–419 The following technique should be considered a composite of those descriptions, but readers are directed to the original publications for variations and alternatives. The operation is performed using general endotracheal anesthesia. Some authors have recommended use of a dual lumen endotracheal tube to allow collapse of the left lung to facilitate surgery374,380,398,413,418 (our preference), but others have found this not to be essential.411,412,414,417 The pelvis is kept as horizontal as possible while the left chest is elevated to approximately 45 degrees off the horizontal. This position is usually facilitated by the use of a “bean bag” to stabilize the patient’s position while simultaneously reducing the risk of any dependent pressure points during surgery.418 The left arm is positioned anteriorly and supported with a Mayo stand or other appropriate device. A left thoracotomy is performed through the seventh, eighth, or ninth intercostal space or with removal of the seventh or eighth rib allowing exposure and control of an adequate portion of the mid- to distal descending thoracic aorta (Fig. 111-6). The inferior pulmonary ligament is generally divided to facilitate this exposure. Distal anastomoses are typically made to the femoral arteries but may be made to the iliac or other arteries as dictated by anatomy and the presence of infection. Standard exposures of these vessels are achieved with groin incisions or retroperitoneal incisions. The graft must be tunneled from the chest into the extraperitoneal abdominal space. Some authors have recommended carrying the intercostal incision across the costal margin414 or even a formal thoracoabdominal incision401 allowing access to the peritoneal, retroperitoneal, or anterior extraperitoneal space. Using this approach, the graft can be tunneled extraperitoneally from the left hemithorax to the left femoral arteries in the anterior axillary line using only a small peripheral incision in the diaphragm and an incision in the left inguinal ligament to gain access to the extraperitoneal space from inferiorly. Tunneling the graft in the retroperitoneum posterior to the left kidney may require a separate incision in the mid- to lower left abdominal wall to gain access to the retroperitoneum380,411 or an extension of the thoracotomy incision across the costal margin to allow access to the retroperitoneum,379,406,414 but this facilitates exposure of the left iliac arteries when one of these is the proposed target. DeLaurentis, Schellack et al, Bowes et al, and Kalman have described techniques whereby the graft may be tunneled blindly from the left groin anterior or posterior to the left kidney up to the left posterior diaphragmatic attachments without complete exposure of the retroperitoneum.374,409,410,417
Figure 111-6 Typical course of the thoracofemoral bypass graft. This illustrates a single graft from the descending aorta to the left common femoral artery with a left-to-right femorofemoral graft. Other approaches, some involving the use of a bifurcated graft, are possible (see text).
Branchereau describes a technique including a flank incision to gain access to the retroperitoneum that he and others have used* and that he believes is “simpler” when compared with the approach of McCarthy et al,406,414 although it does not appear to be “simpler” to me. The graft may be a straight graft from the descending aorta to the left iliofemoral arteries with a crossover graft from the left to the right as necessary or may be a standard bifurcated graft. The former approach is particularly useful when converting a femorofemoral graft or an axillobifemoral graft with a patent femorofemoral component to a thoracobifemoral graft since the crossover component is already in place. The right limb of a standard bifurcated graft is usually too short and must be extended to reach the right groin and also may have a tendency to kink if brought out under the left inguinal ligament and then turned to the right in the subcutaneous suprapubic space as a conventional femorofemoral bypass. Thus it is probably better placed in the preperitoneal space of Retzius,417 although the creation of this preperitoneal tunnel may be difficult and hazardous in patients with previous midline laparotomy for aortofemoral bypass or other surgery. Once the inflow and target arteries have been controlled and the graft has been tunneled, the patient is given heparin and the operation is performed using standard vascular surgical techniques. The aorta is generally clamped completely proximally and distally during performance of the proximal side aorta–to–end graft anastomosis. The graft is clamped and the aortic clamps are removed as early as possible to reperfuse intra-abdominal viscera and, possibly, the spinal cord during performance of distal anastomoses. Both polyester and PTFE grafts have been used with success, and Hughes et al have described a thoracofemoral reconstruction using femoral vein in a patient with an infected axillofemoral bypass originally placed in a patient with an infected infrarenal aortic prosthesis, once again extending the use of these autologous conduits.420 McMillan and McCarthy have described a thoracoscopic approach to the thoracic portion of the operation421 and Fernandez et al have described a robot assisted approach,422 both of which might reduce the morbidity of the operation.
Use of Supraceliac Aorta for Inflow
The supraceliac aorta is generally less likely than the infrarenal aorta to be involved in atherosclerotic disease.390 The supraceliac aorta can be exposed and controlled from an anterior transperitoneal approach,388,390,393 from a thoracoabdominal approach,389,423 or from the left flank retroperitoneal (extrathoracic) approach for repeat or primary surgery of the abdominal aorta and its branches.377,386,389,391,424 Williams et al386 and O’Mara and Williams424 have convincingly demonstrated that a totally retroperitoneal approach without thoracotomy is adequate for nearly all procedures involving the visceral segment and supraceliac portion of the aorta; these authors, along with Sicard and Reilly,425 have provided excellent general descriptions of the approaches to positioning and incisions. Mills et al have also provided a clear description of an extrathoracic retroperitoneal approach (Fig. 111-7) with extension into the 11th intercostal space without entering the pleural cavity and used this approach to perform supraceliac aorta–to–femoral bypass in 11 patients with very good results.391 This approach avoids adhesions that could be problematic from a transperitoneal approach and also allows dissection in a previously undissected area in patients who have had previous exposure and control of the aorta, an especially difficult exposure after previous removal of infected aortic grafts with ligation of the aorta. A further possible advantage of this approach is avoidance of a thoracotomy. Finally, graft tunneling is facilitated compared with thoracofemoral bypass because the entire left common and external iliac arteries are generally easily exposed (and controlled if desired) from this approach. This exposure also provides good access to the celiac, superior and inferior mesenteric, and left renal arteries in most patients. As with thoracofemoral bypass, graft configurations may include aorta–to–left femoral bypass with left-to-right femorofemoral bypass or alternatively, it may be possible to use a conventional bifurcated graft. Tunneling of the right limb of a bifurcated graft may be difficult in the area of a previously placed aorta–to–right femoral graft limb, risking injury to the right ureter, iliac veins, and the colonic cecum in particular, and an anterior preperitoneal tunnel may be used as with thoracofemoral grafts (see earlier). Distal target arteries may be the iliac or femoral arteries, or on even rarer occasions, the target may be some other vessel, such as the popliteal artery426 based on patient characteristics. This approach may be applicable in patients in whom it would be difficult to expose and control the infrarenal aorta.
Figure 111-7 Exposure of the supraceliac aorta from the left flank approach. Sufficient exposure can generally be obtained to allow clamping of the supraceliac aorta to allow anastomosis to a suitable graft to allow bypass to the iliac or femoral arteries distally, just as is possible with thoracic aortic inflow as above. This exposure may also facilitate reconstruction of celiac, superior mesenteric, and left renal branches.
Results
Thoracofemoral bypass is associated with patency approaching that for aortofemoral bypass, an observation that is remarkable given the fact that most of these operations are performed after the failure of one or more prior conventional reconstructions.418 McCarthy et al reported no thromboses before 49 months in their series of 21 grafts.414 Passman et al reported a 5-year primary patency of 79% in 50 patients.380 Carrel et al reported no failures in eight thoracofemoral grafts followed for an average of 2.7 years. Branchereau has reviewed publications before 1993 and concluded that results in series including 10 or more patients suggest a mortality approaching 10%,411 although these publications include patients with infections or other problems clearly associated with increased mortality risk. The mortality risk in uninfected elective patients is generally much lower. Five of the largest known series of these operations380,404,410,412,414 report a combined total of three perioperative deaths out of a total of 112 patients. ICU and total hospital stays have tended to be longer than those for standard aortofemoral bypass, reflecting the fact that this procedure is at least as formidable as aortofemoral bypass.
Results in patients undergoing supraceliac–to–iliofemoral bypass have been quite encouraging. Canepa et al, performed supraceliac–to–distal bypass in seven patients without mortality and with one graft thrombosis and one graft removed for infection.389 Mills et al reported no deaths and 3 graft thromboses, 2 of which were salvaged with thrombectomy, in a series of 11 supraceliac–to–iliofemoral bypass.391 Thus supraceliac–to–iliofemoral bypass is one good reconstructive option for this group of patients.
Acknowledgements
Julie Stielstra, MLS, provided invaluable literature research support.
Selected Key References
Brener BJ, Brief DK, Alpert J, Goldenkranz RJ, Eisenbud DE, Huston J, Parsonnet V, Creighton D, Cross F. Femorofemoral bypass: a 25-year experience. Yao JST, Pearce WH. Long-term results in vascular surgery. Appleton & Lange: East Norwalk; 1993:385–393.
One of the largest, longest, and best-followed series of femorofemoral bypass from a group that has published extensively on this topic..
Criado E. Descending thoracic aorta to femoral artery bypass: surgical technique. Ann Vasc Surg. 1997;11:206–215.
Johnson WC, Lee KK. Comparative evaluation of externally supported Dacron and polytetrafluoroethylene prosthetic bypasses for femorofemoral and axillofemoral arterial reconstructions. Veterans Affairs Cooperative Study #141. J Vasc Surg. 1999;30:1077–1083.
McCarthy WJ, Mesh CL, McMillan WD, Flinn WR, Pearce WH, Yao JS. Descending thoracic aorta-to-femoral artery bypass: ten years’ experience with a durable procedure. J Vasc Surg. 1993;17:336–348.
Mills JL, Fujitani RM, Taylor SM. The retroperitoneal, left flank approach to the supraceliac aorta for difficult and repeat aortic reconstructions. Am J Surg. 1991;162:638–642.
Clearest description of technique and outcomes of this valuable procedure..
Passman MA, Taylor LM, Moneta GL, Edwards JM, Yeager RA, McConnell DB, Porter JM. Comparison of axillofemoral and aortofemoral bypass for aortoiliac occlusive disease. J Vasc Surg. 1996;23:263–271.
Sautner T, Niederle B, Herbst F, Kretschmer G, Polterauer P, Rendl KH, Prenner K. The value of obturator canal bypass. A review. Arch Surg. 1994;129:718–722.
Excellent review of results from the authors’ institution as well as a literature review..
Schneider JR, Besso SR, Walsh DB, Zwolak RM, Cronenwett JL. Femorofemoral versus aortobifemoral bypass: outcome and hemodynamic results. J Vasc Surg. 1994;19:43–57.
Stone PA, Armstrong PA, Bandyk DF, Keeling WB, Flaherty SK, Shames ML, Johnson BL, Back MR. Duplex ultrasound criteria for femorofemoral bypass revision. J Vasc Surg. 2006;44:496–502.
Excellent long-term observation and exploration of possible role of non-invasive surveillance..
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Staple TW. The solitary aortoiliac lesion. Surgery. 1968;64:569–576.
2. Darling RC, et al. Aorto-iliac reconstruction. Surg Clin North Am. 1979;59:565–579.
3. Cronenwett JL, et al. Aortoiliac occlusive disease in women. Surgery. 1980;88:775–784.
4. Johnston KW, et al. 5-year results of a prospective study of percutaneous transluminal angioplasty. Ann Surg. 1987;206:403–413.
5. Johnston KW. Factors that influence the outcome of aortoiliac and femoropopliteal percutaneous transluminal angioplasty. Surg Clin North Am. 1992;72:843–850.
6. Johnston KW. Iliac arteries: reanalysis of results of balloon angioplasty. Radiology. 1993;186:207–212.
7. Kalman PG, et al. Indications and results of balloon angioplasty for arterial occlusive lesions. World J Surg. 1996;20:630–634.
8. Yacyshyn VJ, et al. Predictors of failure of endovascular therapy for peripheral arterial disease. Angiology. 2006;57:403–417.
9. Norgren L, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(Suppl S):S5–67.
10. Colapinto RF, et al. Percutaneous transluminal recanalization of complete iliac artery occlusions. Arch Surg. 1981;116:277–281.
11. Davies AH, et al. Recent changes in the treatment of aortoiliac occlusive disease by the Oxford Regional Vascular Service. Br J Surg. 1990;77:1129–1131.
12. Whiteley MS, et al. Changing patterns in aortoiliac reconstruction: a 7-year audit. Br J Surg. 1996;83:1367–1369.
13. Liapis CD, et al. Advances in the management of iliac artery occlusive disease: a short review. Vasc & Endovasc Surg. 2004;38:541–545.
14. Freeman NE, et al. Operations on large arteries; application of recent advances. Calif Med. 1952;77:229–233.
15. Oudot J, et al. Thrombosis of the aortic bifurcation treated by resection and homograft replacement; report of five cases. Arch Surg. 1953;66:365–369.
16. Vetto RM. The treatment of unilateral iliac artery obstruction with a trans-abdominal, subcutaneous, femoro-femoral graft. Surgery. 1962;52:342–345.
17. Lewis CD. A subclavian artery as the means of blood-supply to the lower half of the body. Br J Surg. 1961;48:574–575.
18. Daily PO, et al. Management of acute aortic dissections. Ann Thorac Surg. 1970;10:237–247.
19. Blaisdell FW, et al. Axillary-femoral artery bypass for lower extremity ischemia. Surgery. 1963;54:563–568.
20. Louw JH. Splenic-to-femoral and axillary-to-femoral bypass grafts in diffuse atherosclerotic occlusive disease. Lancet. 1963;281:1401–1402.
21. Blaisdell FW, et al. Aorto-iliac arterial substitution utilizing subcutaneous grafts. Ann Surg. 1970;172:775–780.
22. Blaisdell FW. Extraanatomical bypass procedures. World J Surg. 1988;12:798–804.
23. Shaw RS, et al. Management of sepsis complicating arterial reconstructive surgery. Surgery. 1963;53:75–86.
24. Courbier R, et al. Les possibilités de pontage ilio-fémoral a travers le trou obturateur [Possibilities of ilio-femoral by-pass through the obturator foramen.]. Mars Chir. 1960;12:244–246.
25. Stevenson JK, et al. A bypass homograft from thoracic aorta to femoral arteries for occlusive vascular disease: case report. Ann Surg. 1961;27:632–637.
26. Fry WJ, et al. Infection complicating the use of plastic arterial implants. Arch Surg. 1967;94:600–609.
27. Lepäntalo M, et al. Outcome of unreconstructed chronic critical leg ischaemia. Eur J Vasc Endovasc Surg. 1996;11:153–157.
28. Second European Consensus Document on chronic critical leg ischemia. [Anonymous] Circulation. 1991;84 [IV1–26] .
29. Hertzer NR, et al. A personal experience with direct reconstruction and extra-anatomic bypass for aortoiliofemoral occlusive disease. J Vasc Surg. 2007;45:527–535.
30. Brewster DC, et al. Optimal methods of aortoiliac reconstruction. Surgery. 1978;84:739–748.
31. Piotrowski JJ, et al. Aortobifemoral bypass: the operation of choice for unilateral iliac occlusion? J Vasc Surg. 1988;8:211–218.
32. Rutherford RB. Aortobifemoral bypass, the gold standard: technical considerations. Semin Vasc Surg. 1994;7:11–16.
33. Brewster DC. Current controversies in the management of aortoiliac occlusive disease. J Vasc Surg. 1997;25:365–379.
34. Rutherford RB. Options in the surgical management of aorto-iliac occlusive disease: a changing perspective. Cardiovasc Surg. 1999;7:5–12.
35. Kretschmer G, et al. Extra-anatomic femoro-femoral crossover bypass (FF) vs. unilateral orthotopic ilio-femoral bypass (IF): an attempt to compare results based on data matching. Eur J Vasc Surg. 1991;5:75–82.
36. Couch NP, et al. The iliac-origin arterial graft: a useful alternative for iliac occlusive disease. Surgery. 1985;97:83–87.
37. Sidawy AN, et al. Retroperitoneal inflow procedures for iliac occlusive vascular disease. Arch Surg. 1985;120:794–796.
38. Kalman PG, et al. Unilateral iliac disease: the role of iliofemoral bypass. J Vasc Surg. 1987;6:139–143.
39. Cham C, et al. Extraperitoneal unilateral iliac artery bypass for chronic lower limb ischaemia. Aust N Z J Surg. 1988;58:859–863.
40. Ricco J-B. Unilateral iliac artery occlusive disease: a randomized multicenter trial examining direct revascularization versus crossover bypass. Association Universitaire de Recherche en Chirurgie. Ann Vasc Surg. 1992;6:209–219.
41. Harrington ME, et al. Iliofemoral versus femorofemoral bypass: the case for an individualized approach. J Vasc Surg. 1992;16:841–854.
42. Oliveira M, et al. Iliofemoral bypass: a 10-year review. Cardiovasc Surg. 1993;1:103–106.
43. Harrington ME, et al. Options in the management of unilateral iliac occlusive disease. Semin Vasc Surg. 1994;7:45–53.
44. Nazzal MM, et al. A comparative evaluation of femorofemoral crossover bypass and iliofemoral bypass for unilateral iliac artery occlusive disease. Angiology. 1998;49:259–265.
45. Ricco J-B, et al. Long-term results of a multicenter randomized study on direct versus crossover bypass for unilateral iliac artery occlusive disease. J Vasc Surg. 2008;47:45–54.
46. van der Vliet JA, et al. Unilateral vascular reconstruction for iliac obstructive disease. J Vasc Surg. 1994;19:610–614.
47. May J, et al. Treatment of complex abdominal aortic aneurysms by a combination of endoluminal and extraluminal aortofemoral grafts. J Vasc Surg. 1994;19:924–933.
48. Yusuf SW, et al. Endoluminal transfemoral abdominal aortic aneurysm repair with aorto-uni-iliac graft and femorofemoral bypass. Br J Surg. 1995;82:916.
49. Yusuf SW, et al. Early results of endovascular aortic aneurysm surgery with aortouniiliac graft, contralateral iliac occlusion, and femorofemoral bypass. J Vasc Surg. 1997;25:165–172.
50. Thompson MM, et al. Aortomonoiliac endovascular grafting: difficult solutions to difficult aneurysms. [[See comment.]] J Endovasc Surg. 1997;4:174–181.
51. Walker SR, et al. Early complications of femorofemoral crossover bypass grafts after aorta uni-iliac endovascular repair of abdominal aortic aneurysms. J Vasc Surg. 1998;28:647–650.
52. Chuter TA, et al. Aortomonoiliac endovascular grafting combined with femorofemoral bypass: an acceptable compromise or a preferred solution? Semin Vasc Surg. 1999;12:176–181.
53. Ohki T, et al. Endovascular graft repair of ruptured aortoiliac aneurysms. J Am Coll Surg. 1999;189:102–113.
54. Rehring TF, et al. Utility and reliability of endovascular aortouniiliac with femorofemoral crossover graft for aortoiliac aneurysmal disease. J Vasc Surg. 2000;31:1135–1141.
55. Moore WS, et al. Aorto-uni-iliac endograft for complex aortoiliac aneurysms compared with tube/bifurcation endografts: results of the EVT/Guidant trials. J Vasc Surg. 2001;33:S11–S20.
56. Hinchliffe RJ, et al. Durability of femorofemoral bypass grafting after aortouniiliac endovascular aneurysm repair. J Vasc Surg. 2003;38:498–503.
58. Schneider JR. Femorofemoral Bypass. [Chapter 99] Bell RH Jr. Northwestern handbook of surgical procedures. Landes: Georgetown, Texas; 2005:297–299.
59. Tyson RR, et al. Retropubic femorofemoral bypass: a new route through the space of Retzius. Surgery. 1972;72:401–403.
60. Mosley JG, et al. Long term results of 66 femoral-to-femoral by-pass grafts: a 9-year follow-up. Br J Surg. 1983;70:631–634.
61. Hubert J, et al. An unusual urological complication due to vascular surgery: transvesical polytetrafluoroethylene iliofemoral graft. J Urol. 1996;156:2004–2005.
62. Häcker A, et al. Ein Corpus alienum in der Harnblase nach gefäβchirurgischem Eingriff. [Foreign body in the urinary bladder after vascular surgery.]. Aktuelle Urol. 2005;36:249–251.
63. Wertheimer P, et al. Les dérivations artérielles contro-latérales. A propos de 6 observations. [Contralateral arterial by-passes. Apropos of 6 cases.]. Lyon Chir. 1963;59:350–357.
64. Paineau J, et al. Pontages ilio ou fémoro-fémoraux extra-anatomiques. [Extra-anatomic ilio- or femoro-femoral bypass.]. J Chir (Paris). 1985;122:3–7.
65. Brouwer MH, et al. Long-term results of 44 cross-over bypasses. J Cardiovasc Surg (Torino). 1988;29:290–295.
66. Hanafy M, et al. Comparison of iliofemoral and femorofemoral crossover bypass in the treatment of unilateral iliac arterial occlusive disease. [[See comment.]] Br J Surg. 1991;78:1001–1002.
67. Defraigne JO, et al. Crossover iliofemoral bypass grafting for treatment of unilateral iliac atherosclerotic disease. J Vasc Surg. 1999;30:693–700.
68. do Carmo G, et al. A new approach for the surgical management of unilateral iliac artery occlusive disease: the iliofemoral crossover transposition. J Vasc Surg. 2002;36:404–407.
69. Ng RL, et al. Iliofemoral versus femorofemoral bypass: a 6-year audit. [[See comment.]] Br J Surg. 1992;79:1011–1013.
70. McCaughan JJ Jr, et al. Cross-over graft for unilateral occlusive disease of the ilio-femoral arteries. Ann Surg. 1960;151:26–28.
71. Goetz RH, et al. Crossover femoropopliteal shunt. Surgery. 1968;64:681–684.
72. Schuler JJ, et al. Crossover femorofemoropopliteal sequential bypass for combined ipsilateral iliac and femoropopliteal occlusive disease. Arch Surg. 1984;119:456–461.
73. Dalman RL, et al. Simultaneous operative repair of multilevel lower extremity occlusive disease. J Vasc Surg. 1991;13:211–221.
74. Ellenby MI, et al. A nine-year experience with crossover femoro-femoro-popliteal sequential bypass. Am J Surg. 1991;161:672–676.
75. Hardy JD, et al. Arterial injury and massive blood loss: a case report of management of pelvic gunshot injury with femoro-subscrotal-femoral bypass and 116 units of blood. Ann Surg. 1975;181:245–246.
76. Kieffer E, et al. Pontage veineux fémoro-fémoral par le perinée (6 observations). [Femoro-femoral venous bypass via the perineum (6 cases).]. Chirurgie. 1976;102:420–428.
77. Laurian C, et al. Pontages périnéaux. Technique. Indications à propos de douze cas. [Subscrotal bypass. Technique and indications. A report on twelve cases (author’s transl).]. Ann Chir. 1981;35:455–459.
78. Lawrence PF, et al. Femorofemoral bypass with an infrascrotal perineal approach for the patient with an infected groin wound. J Vasc Surg. 1985;2:485–487.
79. Baird RN. Subscrotal bypass for the infected groin. Greenhalgh RM. Vascular and endovascular surgical techniques. WB Saunders: London; 1994:257–259.
80. Branchereau A, et al. Femorofemoral bypass through the perineum for infection complicating arterial revascularization of the lower limb. Ann Vasc Surg. 1988;2:43–49.
81. Ray LI, et al. Axillofemoral bypass: a critical reappraisal of its role in the management of aortoiliac occlusive disease. Am J Surg. 1979;138:117–128.
82. Archie JP. The value of donor iliac artery pressure gradients in predicting the outcome of femorofemoral bypass. J Vasc Surg. 1996;23:383–393.
83. Ehrenfeld WK, et al. Venous crossover bypass grafts for arterial insufficiency. Ann Surg. 1968;167:287–291.
84. Jepson RP, et al. Femoro-femoral cross-over grafts. Aust N Z J Surg. 1970;39:345–348.
85. Trimble IR, et al. Criteria for femoro-femoral bypass from clinical and hemodynamic studies. Ann Surg. 1972;175:985–993.
86. Warren WD, et al. Rerouting arterial flow to relieve ischemia: femoro-femoral, axillary femoral and carotid-carotid artery bypasses. Ann Surg. 1966;163:131–136.
87. Devolfe C, et al. Ilio-femoral and femoro-femoral crossover grafting. Analysis of an 11-year experience. J Cardiovasc Surg (Torino). 1983;24:634–640.
88. Hakaim AG, et al. Autogenous vein grafts for femorofemoral revascularization in contaminated or infected fields. J Vasc Surg. 1994;19:912–915.
89. Jicha DL, et al. Durability of cross-femoral grafts after aortic graft infection: the fate of autogenous conduits. J Vasc Surg. 1995;22:393–405.
90. Mingoli A, et al. Femorofemoral bypass grafts: factors influencing long-term patency rate and outcome. Surgery. 2001;129:451–458.
91. Pai M, et al. Femoro-femoral arterial bypass is an effective and durable treatment for symptomatic unilateral iliac artery occlusion. Ann R Coll Surg Engl. 2003;85:88–90.
92. D’Addio V, et al. Femorofemoral bypass with femoral popliteal vein. J Vasc Surg. 2005;42:35–39.
93. Schneider JR, et al. Femorofemoral versus aortobifemoral bypass: outcome and hemodynamic results. J Vasc Surg. 1994;19:43–57.
94. Schneider JR, et al. Axillofemoral bypass: outcome and hemodynamic results in high-risk patients. J Vasc Surg. 1992;15:952–963.
95. Johnson WC, et al. Comparative evaluation of externally supported Dacron and polytetrafluoroethylene prosthetic bypasses for femorofemoral and axillofemoral arterial reconstructions. Veterans Affairs Cooperative Study #141. J Vasc Surg. 1999;30:1077–1083.
96. Brener BJ, et al. Femorofemoral bypass: a twenty-five year experience. Yao JST, et al. Long-term results in vascular surgery. Appleton & Lange: East Norwalk; 1993:385–393.
97. Criado E, et al. Femorofemoral bypass graft: analysis of patency and factors influencing long-term outcome. J Vasc Surg. 1993;18:495–505.
98. Criado E, et al. Femorofemoral bypass: appropriate application based on factors affecting outcome. Semin Vasc Surg. 1997;10:34–41.
99. Plecha FR, et al. Femorofemoral bypass grafts: ten-year experience. J Vasc Surg. 1984;1:555–561.
100. Lamerton AJ, et al. The femorofemoral graft. Hemodynamic improvement and patency rate. Arch Surg. 1985;120:1274–1278.
101. Rutherford RB, et al. Extra-anatomic bypass: a closer view. J Vasc Surg. 1987;6:437–446.
102. Farber MA, et al. Femorofemoral bypass: a profile of graft failure. South Med J. 1990;83:1437–1443.
103. Perler BA, et al. Femoro-femoral or ilio-femoral bypass for unilateral inflow reconstruction? Am J Surg. 1991;161:426–430.
104. Self SB, et al. Utility of femorofemoral bypass. Comparison of results with indications for operation. Am Surg. 1991;57:602–606.
105. Pursell R, et al. Critical appraisal of femorofemoral crossover grafts. Br J Surg. 2005;92:565–569.
106. Kim YW, et al. Factors affecting the long-term patency of crossover femorofemoral bypass graft. Eur J Vasc Endovasc Surg. 2005;30:376–380.
107. Mii S, et al. Role of femorofemoral crossover bypass grafting for unilateral iliac atherosclerotic disease: a comparative evaluation with anatomic bypass. Surg Today. 2005;35:453–458.
108. Huded CP, et al. The impact of adjunctive iliac stenting on femoral-femoral bypass in contemporary practice. J Vasc Surg. 2012;55:739–745.
109. Vetto RM. The femorofemoral shunt. An appraisal. Am J Surg. 1966;112:162–165.
110. Papadopoulos CD. Cross-over femorofemoral bypass. Am J Surg. 1966;111:216–219.
111. da Gama AD. The fate of the donor artery in extraanatomic revascularization. J Vasc Surg. 1988;8:106–111.
112. Wylie EJ. The femorofemoral shunt. An appraisal. [Comment on Vetto, R.M] Am J Surg. 1966;112:165.
113. Foley WJ, et al. Crossover femoro-femoral bypass grafts. Arch Surg. 1969;99:83–87.
114. Stone PA, et al. Duplex ultrasound criteria for femorofemoral bypass revision. J Vasc Surg. 2006;44:496–502.
115. Lyons JH Jr, et al. Surgical management of late closure of aortofemoral reconstruction grafts. N Engl J Med. 1968;278:1035–1037.
116. Bernhard VM, et al. The reoperation of choice for aortofemoral graft occlusion. Surgery. 1977;82:867–874.
117. Laurendeau F, et al. Extra-anatomic bypass grafting in the lower extremity. Can J Surg. 1983;26:335–338.
118. Littooy FN, et al. Effect of prior arterial reconstruction on the outcome of femorofemoral bypass. Am Surg. 1987;53:446–450.
119. Kalman PG, et al. The current role for femorofemoral bypass. J Vasc Surg. 1987;6:71–76.
120. Gazzola LM, et al. Femorofemoral bypass. Surgery. 1974;76:841–844.
121. Sethi GK, et al. Femorofemoral bypass graft: choice or compromise? Am Surg. 1975;41:61–66.
122. Crawford FA, et al. Femorofemoral grafts for unilateral occlusion of aortic bifurcation grafts. Surgery. 1975;77:150–153.
123. François F, et al. Femorofemoral crossover bypass for noninfective complications of aortoiliac surgery. Ann Vasc Surg. 1991;5:46–49.
124. Nolan KD, et al. Femorofemoral bypass for aortofemoral graft limb occlusion: a ten-year experience. J Vasc Surg. 1994;19:851–857.
125. Cohn LH, et al. Extra-abdominal management of late aortofemoral graft thrombosis. Surgery. 1970;67:775–779.
126. Najafi H, et al. Late thrombosis affecting one limb of aortic bifurcation graft. Arch Surg. 1975;110:409–412.
127. Stanton PE Jr, et al. Correction of late aortic-bifemoral graft failures. Am Surg. 1977;43:497–502.
128. Benhamou AC, et al. “Redo” surgery for late aorto-femoral graft occlusive failures. J Cardiovasc Surg (Torino). 1984;25:118–125.
129. Ayvazian VH, et al. Limb salvage by extended femorofemoral bypass. Surg Gynecol Obstet. 1972;135:737–741.
130. Parsonnet V, et al. Femorofemoral and axillofemoral grafts–compromise or preference. Surgery. 1970;67:26–33.
131. Mannick JA. Are there practical alternatives to aortoiliac reconstruction? Am J Surg. 1971;122:344–346.
132. Sheiner NM, et al. Axillofemoral and femorofemoral bypass: an alternative to conventional aortoiliac revascularization. Can J Surg. 1973;16:338–342.
133. Descotes J, et al. Les pontages contro-latéraux: procédé de sauvetage ou méthode de choix dans le traitement des oblitérations des artères iliaques? (A propos de 28 observations). [Contralateral shunts: salvage procedure or method of choice in the treatment of occlusion of the iliac arteries? (Apropos of 28 cases).]. J Chir (Paris). 1974;107:537–554.
134. Dick LS, et al. A 12-year experience with femorofemoral crossover grafts. Arch Surg. 1980;115:1359–1365.
135. Davis RC, et al. Broadened indications for femorofemoral grafts. Surgery. 1972;72:990–994.
136. Biancari F, et al. Extra-anatomic bypass surgery for critical leg ischemia. A review. J Cardiovasc Surg (Torino). 1998;39:295–301.
137. Schneider JR. Femorofemoral bypass. Perspectives in vascular surgery. 1999;11:113–123.
138. Mingoli A, et al. Comparison of femorofemoral and aortofemoral bypass for aortoiliac occlusive disease. J Cardiovasc Surg (Torino). 2001;42:381–387.
139. Eiberg JP, et al. Fluoropolymer-coated Dacron versus PTFE grafts for femorofemoral crossover bypass: randomised trial. Eur J Vasc Endovasc Surg. 2006;32:431–438.
140. Lau H, et al. Is the preferential use of ePTFE grafts in femorofemoral bypass justified? Ann Vasc Surg. 2001;15:383–387.
141. Ehrenfeld WK, et al. Vascular “steal” phenomenon. An experimental study. Am J Surg. 1968;116:192–197.
142. Sumner DS, et al. The hemodynamics of the femorofemoral shunt. Surg Gynecol Obstet. 1972;134:629–636.
143. Nicholson ML, et al. Intra-operative inflow resistance measurement: a predictor of steal syndromes following femoro-femoral bypass grafting. Br J Surg. 1988;75:1064–1066.
144. Subram AN, et al. Femorofemoral bypass: prognostic factors. Tex Heart Inst J. 1983;10:257–261.
145. Flanigan DP, et al. Hemodynamic and angiographic guidelines in selection of patients for femorofemoral bypass. Arch Surg. 1978;113:1257–1262.
146. Livesay JJ, et al. Late results of extra-anatomic bypass. Arch Surg. 1979;114:1260–1267.
147. Donor limb vascular events following femoro-femoral bypass surgery. A Veterans Affairs Cooperative Study. [Anonymous] Arch Surg. 1991;126:681–686.
148. Harris JP, et al. Assessment of donor limb hemodynamics in femorofemoral bypass for claudication. Surgery. 1981;90:764–773.
149. Moore WS, et al. Unrecognized aortoiliac stenosis. A physiologic approach to the diagnosis. Arch Surg. 1971;103:633–638.
150. Sethi GK, et al. Multiple-plane angiography for more precise evaluation of aortoiliac disease. Surgery. 1975;78:154–159.
151. Castaneda-Zuniga W, et al. Hemodynamic assessment of obstructive aortoiliac disease. AJR Am J Roentgenol. 1976;127:559–561.
152. Udoff EJ, et al. Hemodynamic significance of iliac artery stenosis: pressure measurements during angiography. Radiology. 1979;132:289–293.
153. Brewster DC, et al. Femoral artery pressure measurement during aortography. Circulation. 1979;60:120–124.
154. Johnston KW, et al. Difficulty in assessing the severity of aorto-iliac disease by clinical and arteriographic methods. Angiology. 1981;32:609–614.
155. Lorentsen E, et al. Evaluation of the functional importance of atherosclerotic obliterations in the aorto-iliac artery by pressure-flow measurements. Acta Med Scand. 1972;191:399–403.
156. Gupta SK, et al. Significance and management of inflow gradients unexpectedly generated after femorofemoral, femoropopliteal, and femoroinfrapopliteal bypass grafting. J Vasc Surg. 1990;12:278–283.
157. Sako Y. Papavarine test in peripheral arterial disease. Surg Forum. 1966;17:141–143.
158. Flanigan DP, et al. Aortofemoral or femoropopliteal revascularization? A prospective evaluation of the papaverine test. J Vasc Surg. 1984;1:215–223.
159. Brief DK, et al. Crossover femorofemoral grafts followed up five years or more. An analysis. Arch Surg. 1975;110:1294–1299.
160. Deruyter L, et al. Femorofemoral bypass grafting in high-risk patients. Acta Chir Belg. 1986;86:271–276.
161. Buchbinder D, et al. Efficacy of femorofemoral bypass for intermittent claudication. Clinical and hemodynamic assessment. Am J Surg. 1986;152:215–219.
162. Capoccia L, et al. Is femorofemoral crossover bypass an option in claudication? Ann Vasc Surg. 2010;24:828–832.
163. Berce M, et al. Femorofemoral crossover grafts for claudication: a safe and reliable procedure. Eur J Vasc Endovasc Surg. 1996;12:437–441.
164. Porter JM, et al. Combined arterial dilatation and femorofemoral bypass for limb salvage. Surg Gynecol Obstet. 1973;137:409–412.
165. Dotter CT, et al. Transluminal treatment of arteriosclerotic obstruction. Description of a new technic and a preliminary report of its application. Circulation. 1964;30:654–670.
166. Grüntzig A, et al. Perkutane Rekanalisation chronischer arterieller Verschlüsse mit einem neuen Dilatationskatheter. Modifikation der Dotter-Technik [Percutaneous recanalization after chronic arterial occlusion with a new dilator-catheter (modification of the Dotter technique) (author’s transl).]. Dtsch Med Wochenschr. 1974;99:2502–2510.
167. Grüntzig A, et al. Technique of percutaneous transluminal angioplasty with the Grüntzig balloon catheter. AJR Am J Roentgenol. 1979;132:547–552.
169. Brewster DC, et al. Long-term results of combined iliac balloon angioplasty and distal surgical revascularization. Ann Surg. 1989;210:324–331.
170. Walker PJ, et al. Combined percutaneous transluminal angioplasty and extraanatomic bypass for symptomatic unilateral iliac artery occlusion with contralateral iliac artery stenosis. Ann Vasc Surg. 1991;5:209–217.
171. Shah RM, et al. Donor iliac angioplasty and crossover femorofemoral bypass. Am J Surg. 1992;164:295–298.
172. Katz SG, et al. Iliac angioplasty as a prelude to distal arterial bypass. J Am Coll Surg. 1994;179:577–582.
173. Perler BA, et al. Does donor iliac artery percutaneous transluminal angioplasty or stent placement influence the results of femorofemoral bypass? Analysis of 70 consecutive cases with long-term follow-up. J Vasc Surg. 1996;24:363–370.
174. Lopez-Galarza LA, et al. Combined percutaneous transluminal angioplasty, iliac stent deployment, and femorofemoral bypass for bilateral aortoiliac occlusive disease. J Am Coll Surg. 1997;184:249–258.
175. AbuRahma AF, et al. Selecting patients for combined femorofemoral bypass grafting and iliac balloon angioplasty and stenting for bilateral iliac disease. J Vasc Surg. 2001;33:S93–S99.
176. Back MR, et al. Duplex selection facilitates single point-of-service endovascular and surgical management of aortoiliac occlusive disease. Ann Vasc Surg. 2002;16:566–574.
177. Leeds FH, et al. Importance of profunda femoris artery in the revascularization of the ischemic limb. Arch Surg. 1961;82:25–31.
178. Morris GC Jr, et al. Surgical importance of profunda femoris artery. Analysis of 102 cases with combined aortoiliac and femoropopliteal occlusive disease treated by revascularization of deep femoral artery. Arch Surg. 1961;82:32–37.
179. Healey SJ, et al. Reconstructive operations for aortoiliac obliterative disease. Results and reflections from an eleven-year experience. N Engl J Med. 1964;271:1386–1391.
180. Royster TS, et al. Combined aortoiliac and femoropopliteal occlusive disease. Surg Gynecol Obstet. 1976;143:949–952.
181. Haid SP, et al. Innovative applications of extra-anatomic reconstruction. Surg Clin North Am. 1974;54:123–135.
182. Todd GJ, et al. Femorofemoral bypass for unilateral iliac artery occlusion in the presence of bilateral superficial femoral artery occlusions. J Cardiovasc Surg (Torino). 1985;26:12–14.
183. McDonald PT, et al. Femorofemoral grafts: the role of concomitant extended profundaplasty. Am J Surg. 1978;136:622–628.
184. Prendiville EJ, et al. The profunda femoris: a durable outflow vessel in aortofemoral surgery. J Vasc Surg. 1992;16:23–29.
185. Whitemore KE, et al. Special considerations in the revascularization for aortoiliac occlusive disease: anatomic and extraanatomic bypass. Am Surg. 1980;46:279–288.
186. Farkas P, et al. Transvesical placement of one limb of an aortobifemoral bypass graft as a complication of aortic bypass surgery. Urol Int. 2001;66:227–228.
187. Connery CP, et al. Treatment of aortic coarctation by axillofemoral bypass grafting in the high-risk patient. Ann Thorac Surg. 1991;52:1281–1284.
188. Harada T, et al. Axillofemoral bypass for recurrent atypical coarctation of the thoracic aorta. Woman in childbearing age. Jpn J Thorac Cardiovasc Surg. 1999;47:460–464.
189. Attenhofer Jost CH, et al. Spectrum of reoperations after repair of aortic coarctation: importance of an individualized approach because of coexistent cardiovascular disease. Mayo Clin Proc. 2002;77:646–653.
190. Ehrenfeld WK, et al. Subcutaneous arterial bypass grafts in the management of fistulae between the bowel and plastic arterial prostheses. Ann Surg. 1968;168:29–35.
191. Kleinman LH, et al. A diagnostic and therapeutic approach to aortoenteric fistulas: clinical experience with twenty patients. Surgery. 1979;86:868–880.
192. Raithel D. Late results after extra-anatomic bypass routes. J Cardiovasc Surg (Torino). 1979;20:487–492.
193. Reilly LM, et al. Late results following surgical management of vascular graft infection. J Vasc Surg. 1984;1:36–44.
194. Lorentzen JE, et al. Vascular graft infection: an analysis of sixty-two graft infections in 2411 consecutively implanted synthetic vascular grafts. Surgery. 1985;98:81–86.
195. O’Hara PJ, et al. Surgical management of infected abdominal aortic grafts: review of a 25-year experience. J Vasc Surg. 1986;3:725–731.
196. Schmitt DD, et al. Graft excision and extra-anatomic revascularization: the treatment of choice for the septic aortic prosthesis. J Cardiovasc Surg (Torino). 1990;31:327–332.
197. Ricotta JJ, et al. Total excision and extra-anatomic bypass for aortic graft infection. Am J Surg. 1991;162:145–149.
198. Seeger JM, et al. Long-term outcome after treatment of aortic graft infection with staged extra-anatomic bypass grafting and aortic graft removal. J Vasc Surg. 2000;32:451–461.
199. Hennes N, et al. Gefäβprostheseninfekte—eine retrospektive Analyse von 99 Fällen [Infection of a vascular prosthesis–a retrospective analysis of 99 cases]. Chirurg. 1996;67:37–43.
200. Bandyk DF, et al. In situ replacement of vascular prostheses infected by bacterial biofilms. J Vasc Surg. 1991;13:575–583.
201. Kieffer E, et al. In situ allograft replacement of infected infrarenal aortic prosthetic grafts: results in forty-three patients. J Vasc Surg. 1993;17:349–356.
202. Clagett GP, et al. Autogenous aortoiliac/femoral reconstruction from superficial femoral-popliteal veins: feasibility and durability. J Vasc Surg. 1997;25:255–270.
203. Bandyk DF, et al. Use of rifampin-soaked gelatin-sealed polyester grafts for in situ treatment of primary aortic and vascular prosthetic infections. J Surg Res. 2001;95:44–49.
204. Vogt PR, et al. Technical details with the use of cryopreserved arterial allografts for aortic infection: influence on early and midterm mortality. J Vasc Surg. 2002;35:80–86.
205. Oderich GS, et al. Evolution from axillofemoral to in situ prosthetic reconstruction for the treatment of aortic graft infections at a single center. J Vasc Surg. 2006;43:1166–1174.
206. Oderich GS, et al. In situ rifampin-soaked grafts with omental coverage and antibiotic suppression are durable with low reinfection rates in patients with aortic graft enteric erosion or fistula. J Vasc Surg. 2011;53:99–106.
207. Berger P, et al. Aortic graft infections: is there still a role for axillobifemoral reconstruction? Semin Vasc Surg. 2011;24:205–210.
208. Gorman JF, et al. Axillary-femoral artery bypass. Arch Surg. 1965;91:509–512.
209. DeAvila R, et al. Axillary-femoral bypass. Arch Surg. 1966;92:118–119.
210. Sauvage LR, et al. Unilateral axillary bilateral femoral bifurcation graft: a procedure for the poor risk patient with aortoiliac disease. Surgery. 1966;60:573–577.
211. Pierangeli A, et al. The axillo-femoral by-pass as a treatment of the obstruction of the aorto-iliac junction in the poor risk patients. J Cardiovasc Surg (Torino). 1967;8:353–360.
212. Mannick JA, et al. Axillofemoral bypass graft. A safe alternative to aortoiliac reconstruction. N Engl J Med. 1968;278:461–466.
213. Mannick JA, et al. The late results of axillofemoral grafts. Surgery. 1970;68:1038–1043.
214. Bliss BP, et al. Axillo-femoral and axillo-profunda bypass grafts. Their use for limb salvage in the bad-risk patient with occlusion or infection of the abdominal aorta and iliac arteries. Ann R Coll Surg Engl. 1972;50:268–273.
215. Pollock AV. Axillary-femoral by-pass grafts in the treatment of aorto-iliac occlusive disease. Br J Surg. 1972;59:704–707.
216. Lamis PA, et al. Axillofemoral and femorofemoral bypass grafts: safe surgical alternatives in aortoiliac occlusive disease. South Med J. 1973;66:1263–1266.
217. Richardson JV, et al. Extra-anatomic bypass grafting in aortoiliac occlusive disease: a seven-year experience. South Med J. 1977;70:1287–1291.
218. Campbell CD, et al. Extra-anatomic bypass with expanded polytetrafluoroethylene. Surg Gynecol Obstet. 1979;148:525–530.
220. Burrell MJ, et al. Axillofemoral bypass: a ten-year review. Ann Surg. 1982;195:796–799.
221. Glock Y, et al. Les pontages extra-anatomiques destinés aux membres inférieurs. A propos d’une série de 51 cas consécutifs. [Extra-anatomical shunts in the lower limbs. Apropos of a series of 51 consecutive cases.]. J Chir (Paris). 1984;121:593–598.
222. Johnson WC. Axillofemoral bypass: outcome and hemodynamic results in high-risk patients. [Comment on Schneider JR, et al] J Vasc Surg. 1992;15:962.
223. Hepp W, et al. Late results following extra-anatomic bypass procedures for chronic aortoiliac occlusive disease. J Cardiovasc Surg (Torino). 1988;29:181–185.
224. Schneider JR. Axillofemoral bypass. [Chapter 98] Bell RH Jr. Northwestern handbook of surgical procedures. Landes: Georgetown, Texas; 2005:292–296.
225. Calligaro KD, et al. Unsuspected inflow disease in candidates for axillofemoral bypass operations: a prospective study. J Vasc Surg. 1990;11:832–837.
226. Urdaneta LF, et al. Use of bilateral axillofemoral bypass prosthesis for the management of infected aortic bifurcation grafts: report of a case with extended follow-up. Surgery. 1969;65:753–756.
227. Louw JH. The treatment of combined aortoiliac and femoropopliteal occlusive disease by splenofemoral and axillofemoral bypass grafts. Surgery. 1964;55:387–395.
228. Blaisdell FW. Late axillary artery thrombosis in patients with occluded axillary-femoral bypass grafts. J Vasc Surg. 1985;2:925–926.
229. Sullivan LP, et al. Disruption of the proximal anastomosis of axillobifemoral grafts: two case reports. J Vasc Surg. 1989;10:190–192.
230. Bunt TJ, et al. Optimal proximal anastomosis/tunnel for axillofemoral grafts. J Vasc Surg. 1986;3:673–676.
231. Taylor LM Jr, et al. Acute disruption of polytetrafluoroethylene grafts adjacent to axillary anastomoses: a complication of axillofemoral grafting. J Vasc Surg. 1994;20:520–528.
232. Ward RE, et al. New concepts in the use of axillofemoral bypass grafts. Arch Surg. 1983;118:573–576.
233. Rutherford RB, et al. A modified technique for performing axillobifemoral bypass grafting. J Vasc Surg. 1989;10:468–469.
234. Wittens CHA, et al. Winner of the ESVS Prize 1991. European Prospective Randomised Multi-Centre Axillo-Bifemoral Trial. Eur J Vasc Surg. 1992;6:115–123.
235. Wittens CHA. Haemodynamics in axillobifemoral bypass graft. Department of General Surgery, University Hospital: Rotterdam; 1992.
236. Moore WS, et al. Late results of axillary-femoral bypass grafting. Am J Surg. 1971;122:148–154.
237. Pietri P, et al. Long term results of extra anatomical bypasses. Int Angiol. 1987;6:429–433.
238. Schneider JR, et al. The role of extraanatomic bypass in the management of bilateral aortoiliac occlusive disease. Semin Vasc Surg. 1994;7:35–44.
239. Passman MA, et al. Comparison of axillofemoral and aortofemoral bypass for aortoiliac occlusive disease. J Vasc Surg. 1996;23:263–271.
240. Oblath RW, et al. Extra-anatomic bypass of the abdominal aorta: management of postoperative thrombosis. Ann Surg. 1978;187:647–652.
241. Bacourt F, et al. Axillobifemoral bypass and aortic exclusion for vascular septic lesions: a multicenter retrospective study of 98 cases. French University Association for Research in Surgery. Ann Vasc Surg. 1992;6:119–126.
242. Sandmann W, et al. Die Wundinfektion nach Arterienoperationen im Becken-Bein-Bereich [Wound infection following arterial surgery in the pelvis-leg region]. Chirurg. 1976;47:130–139.
243. Savrin RA, et al. Axillofemoral bypass. Expectations and results. Arch Surg. 1986;121:1016–1020.
244. Agee JM, et al. The risk of axillofemoral bypass grafting for acute vascular occlusion. J Vasc Surg. 1991;14:190–194.
245. Johnson WC, et al. Is axillo-bilateral femoral graft an effective substitute for aortic-bilateral iliac/femoral graft? An analysis of ten years experience. Ann Surg. 1977;186:123–129.
246. LoGerfo FW, et al. A comparison of the late patency rates of axillobilateral femoral and axillounilateral femoral grafts. Surgery. 1977;81:33–40.
247. Schneider JR. Comparison of axillofemoral and aortofemoral bypass for aortoiliac occlusive disease. [Comment on Passman et al] J Vasc Surg. 1996;23:270.
248. Harris EJ Jr, et al. Clinical results of axillobifemoral bypass using externally supported polytetrafluoroethylene. J Vasc Surg. 1990;12:416–421.
249. El-Massry S, et al. Axillofemoral bypass with externally supported, knitted Dacron grafts: a follow-up through twelve years. J Vasc Surg. 1993;17:107–115.
250. Corbett CR, et al. Axillofemoral bypass in poor risk patients with critical ischaemia. Ann R Coll Surg Engl. 1984;66:170–172.
251. Dé P, et al. Present role of extraanatomic bypass graft procedures for aortoiliac occlusive disease. Int Angiol. 1991;10:224–228.
252. Harris KA, et al. Extra-anatomic bypass grafting: a rational approach. Can J Surg. 1989;32:113–116.
253. Urayama H, et al. Long-term results of endarterectomy, anatomic bypass and extraanatomic bypass for aortoiliac occlusive disease. Surg Today. 1998;28:151–155.
254. Eugene J, et al. Fifteen year experience with subcutaneous bypass grafts for lower extremity ischemia. Ann Surg. 1977;186:177–183.
255. Allison HF, et al. Axillofemoral bypass. A 2-decade experience reviewed. South African Medical Journal. 1985;68:559–562.
256. Ascer E, et al. Comparison of axillounifemoral and axillobifemoral bypass operations. Surgery. 1985;97:169–175.
257. Donaldson MC, et al. Axillofemoral bypass: a tool with a limited role. J Vasc Surg. 1986;3:757–763.
258. Kalman PG, et al. Current indications for axillounifemoral and axillobifemoral bypass grafts. J Vasc Surg. 1987;5:828–832.
259. Naylor AR, et al. Axillofemoral bypass as a limb salvage procedure in high risk patients with aortoiliac disease. Br J Surg. 1990;77:659–661.
260. Liedenbaum MH, et al. The outcome of the axillofemoral bypass: a retrospective analysis of 45 patients. World J Surg. 2009;33:2490–2496.
261. Rutherford RB, et al. Suggested standards for reports dealing with lower extremity ischemia. [[Erratum appears in J Vasc Surg 4(4):350, 1986.]] J Vasc Surg. 1986;4:80–94.
262. Rutherford RB, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. [[Erratum appears in J Vasc Surg 33(4):805, 2001.]] J Vasc Surg. 1997;26:517–538.
263. Jämsén T, et al. Axillofemoral bypass operations in Kuopio University Hospital 1985-1996. Ann Chir Gynaecol. 1999;88:269–275.
264. Onohara T, et al. Multivariate analysis of long-term results after an axillobifemoral and aortobifemoral bypass in patients with aortoiliac occlusive disease. J Cardiovasc Surg (Torino). 2000;41:905–910.
265. Angle N, et al. The evolution of the axillofemoral bypass over two decades. Ann Vasc Surg. 2002;16:742–745.
266. Tueche SG. Extra-anatomic bypass shunting in aorto-iliac occlusive disease. Clinical results and risk factors in a Belgian population. Ann Med Interne (Paris). 2003;154:489–492.
267. Mason RA, et al. Alternative procedures to aortobifemoral bypass grafting. J Cardiovasc Surg (Torino). 1989;30:192–197.
268. Musicant SE, et al. Postoperative duplex scan surveillance of axillofemoral bypass grafts. J Vasc Surg. 2003;37:54–61.
269. Foster MC, et al. A review of 155 extra-anatomic bypass grafts. Ann R Coll Surg Engl. 1986;68:216–218.
270. Sanchez LA, et al. Is surveillance to detect failing polytetrafluoroethylene bypasses worthwhile? Twelve-year experience with ninety-one grafts. J Vasc Surg. 1993;18:981–990.
271. Calligaro KD, et al. Duplex ultrasonography to diagnose failing arterial prosthetic grafts. Surgery. 1996;120:455–459.
272. Stipa S. Axillofemoral bypass graft with saphenous, cephalic, and basilic veins. Surg Gynecol Obstet. 1971;133:297–300.
274. Kenney DA, et al. Comparison of noncrimped, externally supported (EXS) and crimped, nonsupported Dacron prostheses for axillofemoral and above-knee femoropopliteal bypass. Surgery. 1982;92:931–946.
275. Schultz GA, et al. A five- to seven-year experience with externally-supported Dacron prostheses in axillofemoral and femoropopliteal bypass. Ann Vasc Surg. 1986;1:214–224.
276. Pradhan DJ, et al. Extra-anatomic bypass: review of 60 cases using PTFE grafts. Am Surg. 1982;48:258–260.
277. Orringer MB, et al. An unusual complication of axillofemoral artery bypass. Surgery. 1972;72:769–771.
278. Onoe M, et al. Disruption of the expanded polytetrafluoroethylene (EPTFE) graft of axillofemoral by-pass. J Cardiovasc Surg (Torino). 1994;35:165–168.
279. Shibutani S, et al. Nonanastomotic pseudoaneurysm with complete disruption of an expanded polytetrafluoroethylene axillofemoral bypass graft. Ann Vasc Surg. 2012;26:422.e9–422.e12.
280. Courbier R, et al. Axillo-femoral bypass material of choice. Greenhalgh RM. Extra-anatomic secondary arterial reconstruction. Pittman: Bath; 1982:122–130.
281. Christenson JT, et al. The late results after axillo-femoral bypass grafts in patients with leg ischaemia. J Cardiovasc Surg (Torino). 1986;27:131–135.
282. Taylor LM. Clinical results of axillofemoral bypass using externally supported polytetrafluoroethylene (reply). J Vasc Surg. 1991;13:564–565.
283. Jarowenko MV, et al. Effect of external pressure on axillofemoral bypass grafts. Ann Surg. 1981;193:274–276.
284. Cavallaro A, et al. The effect of body weight compression on axillo-femoral by-pass patency. J Cardiovasc Surg (Torino). 1988;29:476–479.
285. Graham JC, et al. Axillofemoral and femorofemoral grafts: a 6-year experience with emphasis on the relationship of peroperative flow measurement to graft survival. Br J Surg. 1983;70:326–331.
286. Field JP, et al. Duplex arterial flow measurements in normal lower extremities. J Vasc Technol. 1989;13:13–19.
287. Stoney RJ, et al. Revascularization methods in chronic visceral ischemia caused by atherosclerosis. Ann Surg. 1977;186:468–476.
288. Cina C, et al. Indications and role of axillofemoral bypass in high-risk patients. Ann Vasc Surg. 1988;2:237–241.
289. Illuminati G, et al. Results of axillofemoral by-passes for aorto-iliac occlusive disease. Langenbecks Arch Chir. 1996;381:212–217.
290. Schroe H, et al. Extra-anatomical grafting for aorto-occlusive disease: the outcome in 133 procedures. Acta Chir Belg. 1990;90:240–243.
291. Mohan CR, et al. A comparative evaluation of externally supported polytetrafluoroethylene axillobifemoral and axillounifemoral bypass grafts. J Vasc Surg. 1995;21:801–809.
292. Bastounis E, et al. Axillodistal bypass on critical limb ischaemia. Eur J Vasc Surg. 1993;7:263–270.
293. McCarthy WJ, et al. Axillary-popliteal artery bypass provides successful limb salvage after removal of infected aortofemoral grafts. Arch Surg. 1992;127:974–978.
294. Ascer E, et al. Axillopopliteal bypass grafting: indications, late results, and determinants of long-term patency. J Vasc Surg. 1989;10:285–291.
295. Keller MP, et al. Axillopopliteal bypass for limb salvage. J Vasc Surg. 1992;15:817–822.
296. Kempczinski R, et al. Upper extremity complications of axillofemoral grafts. Am J Surg. 1978;136:209–211.
297. Alexander RH, et al. Axillofemoral bypass grafts using polytetrafluoroethylene. South Med J. 1980;73:1325–1329.
298. Bandyk DF, et al. Upper-extremity emboli secondary to axillofemoral graft thrombosis. Arch Surg. 1981;116:393–395.
299. Hartman AR, et al. Late axillary artery thrombosis in patients with occluded axillary-femoral bypass grafts. J Vasc Surg. 1985;2:285–287.
300. White GH, et al. Exertional disruption of axillofemoral graft anastomosis. ‘The axillary pullout syndrome’. Arch Surg. 1990;125:625–627.
301. Khalil IM, et al. Late upper extremity embolic complications of occluded axillofemoral grafts. Ann Vasc Surg. 1991;5:375–380.
302. Anderson LS, et al. New mechanism for disruption of axillofemoral bypass. J Vasc Surg. 1992;15:255–256.
303. Brophy CM, et al. Disruption of proximal axillobifemoral bypass graft anastomosis. J Vasc Surg. 1992;15:218–220.
304. McLafferty RB, et al. Upper extremity thromboembolism caused by occlusion of axillofemoral grafts. [[Erratum appears in Am J Surg 170(4):406, 1995.]] Am J Surg. 1995;169:492–495.
305. Mawatari K, et al. The potential risk for upper extremity thromboembolism in patients with occluded axillofemoral bypass grafts: two case reports. Vasc Surg. 2001;35:67–71.
306. Arvanitis DP, et al. Early disruption of proximal anastomosis of a PTFE axillofemoral bypass graft. Vasa. 2002;31:136–137.
307. Siani A, et al. A singular case of iatrogenic axillofemoral bypass disruption: a dramatic event treated by a lucky combined approach. Ann Vasc Surg. 2009;23:413.e415–413.e417.
308. Huang C, et al. Axillary anastomotic disruption after axillofemoral bypass. J Emerg Med. 2007;32:305–307.
309. Daar AS, et al. Graft avulsion: an unreported complication of axillofemoral bypass grafts. Br J Surg. 1978;65:442.
310. Yeager RA, et al. Axillary artery anastomosis to avoid axillofemoral bypass disruption. Semin Vasc Surg. 2000;13:74–76.
311. Marston WA, et al. Management of failed and infected axillofemoral grafts. J Vasc Surg. 1994;20:357–366.
312. de Virgilio C, et al. Infected lower extremity extra-anatomic bypass grafts: management of a serious complication in high-risk patients. Ann Vasc Surg. 1995;9:459–466.
313. Wojciechowski J, et al. Superficial femoral vein and superficial femoral artery as replacement for infected axillofemoral graft. Ann Vasc Surg. 2006;20:544–546.
314. Grochow RM, et al. Chronic traumatic pseudoaneurysm of polytetrafluoroethylene axillofemoral bypass graft in a quadriplegic patient. Ann Vasc Surg. 2008;22:688–691.
315. Kitowski NJ, et al. Traumatic fracture of polytetrafluoroethylene axillofemoral bypass graft. Vasc & Endovasc Surg. 2010;44:131–133.
316. Mahoney WD, et al. Use of obturator foramen in iliofemoral artery grafting: case reports. Ann Surg. 1966;163:215–220.
317. DePalma RG, et al. Arterial bypass via the obturator foramen. An alternative in complicated vascular problems. Am J Surg. 1968;115:323–328.
318. Spiro M, et al. The obturator canal as a route for iliofemoral by-pass. Br J Surg. 1970;57:168–172.
319. Kunlin J. Le pontage ou bypass en chirurgie artérielle restauratrice des membres. Aktuelle Probleme in der Angiologie. 1968;105–113.
320. Tilson MD, et al. Obturator canal bypass grafts for septic lesions of the femoral artery. Arch Surg. 1979;114:1031–1033.
321. Bonelli U, et al. Incidenza e trattamento della infezione protesica vascolare a livello inguinale. Eperienza personale. [Incidence and treatment of vascular prosthesis infection in the groin. Personal experience.]. Minerva Chir. 1994;49:807–811.
322. Geroulakos G, et al. Obturator foramen bypass–the alternative route for sepsis in the femoral triangle. Acta Chir Scand. 1988;154:111–112.
323. Fromm SH, et al. Obturator bypass for mycotic aneurysm in the drug addict. Arch Surg. 1970;100:82–83.
324. Patel KR, et al. Routine revascularization with resection of infection femoral pseudoaneurysms from substance abuse. J Vasc Surg. 1988;8:321–328.
325. Matoussevitch V, et al. Primary extraanatomical revascularization for groin infections in drug addicts. Vasa. 2007;36:210–214.
326. Klicks RJ, et al. False aneurysm formation of the right common femoral artery: a rare complication of a Salmonella infection. Eur J Vasc Surg. 1993;7:747–749.
327. Mentha C, et al. Les pontages artériels ilio-fémoraux par le trou obturateur [Ilio-femoral arterial bridging by the obturator foramen]. J Chir (Paris). 1965;90:131–140.
328. Donahoe PK, et al. Obturator bypass graft in radical excision of inguinal neoplasm. Ann Surg. 1967;166:147–149.
330. Hegarty JC, et al. Revascularization of the lower extremity through the obturator canal. Arch Surg. 1969;98:35–38.
331. Gruß JD, et al. Der Obturator-Bypass. Indikation—Technik—Ergebnisse [The obturator bypass. Indication-technic-results]. Thoraxchir Vask Chir. 1971;19:483–488.
332. van Det RJ, et al. The obturator foramen bypass: an alternative procedure in iliofemoral artery revascularization. Surgery. 1981;89:543–547.
333. Reddy DJ, et al. Infected femoral artery false aneurysms in drug addicts: evolution of selective vascular reconstruction. J Vasc Surg. 1986;3:718–724.
334. Reddy DJ. Treatment of drug-related infected false aneurysm of the femoral artery—is routine revascularization justified? J Vasc Surg. 1988;8:344–345.
335. Padberg F Jr, et al. Femoral pseudoaneurysm from drugs of abuse: ligation or reconstruction? J Vasc Surg. 1992;15:642–648.
336. Arora S, et al. Common femoral artery ligation and local debridement: a safe treatment for infected femoral artery pseudoaneurysms. J Vasc Surg. 2001;33:990–993.
337. Nevelsteen A, et al. Obturator bypass: a sixteen year experience with 55 cases. Ann Vasc Surg. 1987;1:558–563.
338. Panetta T, et al. Obturator bypass with nonreversed translocated saphenous vein. Ann Vasc Surg. 1989;3:56–62.
339. Reddy DJ, et al. Obturator bypass: technical considerations. Semin Vasc Surg. 2000;13:49–52.
340. Benjamin ME, et al. Arterial reconstruction with deep leg veins for the treatment of mycotic aneurysms. J Vasc Surg. 1999;30:1004–1015.
341. Guida PM, et al. Obturator bypass technique. Surg Gynecol Obstet. 1969;128:1307–1316.
342. Diethrich EB, et al. Treatment of infected aortofemoral arterial prosthesis. Surgery. 1970;68:1044–1052.
343. Rawson HD. Arterial grafting through the obturator foramen. Aust N Z J Surg. 1986;56:127–130.
344. Patel A, et al. Obturator bypass: a classic approach for the treatment of contemporary groin infection. Am Surg. 2002;68:653–659.
345. Millis JM, et al. Transobturator aorto-profunda femoral artery bypass using the direct medial thigh approach. Ann Vasc Surg. 1993;7:384–390.
346. Plate G, et al. Obturator bypass to the distal profunda femoris artery using a medial approach–long-term results. Eur J Vasc Endovasc Surg. 1998;16:164–168.
347. Kerdiles Y, et al. Le pontage par le trou obturateur dans la chirurgie artérielle restauratrice des membres inférieurs [Bypass graft through the foramen obturatum in the restorative arterial surgery of the lower limbs]. Ann Chir Thorac Cardiovasc. 1974;13:335–341.
348. Pearce WH, et al. Modified technique of obturator bypass in failed or infected grafts. Ann Surg. 1983;197:344–347.
349. Berguer R, et al. Infected groin aneurysms from heroin addiction. Bergan JJ, et al. Aneurysms: diagnosis and treatment. Grune & Stratton: New York; 1981:643–655.
350. Rudich M, et al. Obturator foramen bypass in the management of infected vascular prostheses. Am J Surg. 1979;137:657–660.
351. Calligaro KD, et al. Are gram-negative bacteria a contraindication to selective preservation of infected prosthetic arterial grafts? J Vasc Surg. 1992;16:337–346.
352. Sautner T, et al. The value of obturator canal bypass. A review. Arch Surg. 1994;129:718–722.
353. Taylor SM, et al. Outcomes in the management of vascular prosthetic graft infections confined to the groin: a reappraisal. Ann Vasc Surg. 1996;10:117–122.
354. Kretschmer G, et al. Groin infections following vascular surgery: obturator bypass (BYP) versus “biologic coverage” (TRP)–a comparative analysis. Eur J Vasc Surg. 1989;3:25–29.
355. Macpherson AI. Iliofemoral arterial by-pass through the obturator foramen. Br J Surg. 1967;54:946–947.
356. Mercier C, et al. Faux anévrysme suppuré du triangle de Scarpa. Pontage ilio-femoral par le trou obturateur. Mars Chir. 1967;19:249–252.
357. Brücke VP, et al. Zur Indikation des Obturator-Bypass [On indications for the obturator bypass]. Zentralbl Chir. 1968;93:489–493.
358. Bell CL, et al. Arterial reconstruction of infected femoral artery pseudoaneurysms using superficial femoral-popliteal vein. J Am Coll Surg. 2005;200:831–836.
359. Smith RF, et al. Surgical treatment of mycotic aneurysms. Arch Surg. 1962;85:663–674.
360. Leather RP, et al. A lateral route for extra-anatomical bypass of the femoral artery. Surgery. 1977;81:307–309.
361. Trout HH 3rd, et al. Lateral iliopopliteal arterial bypass as an alternative to obturator bypass. Am Surg. 1982;48:63–64.
362. Katsamouris AN, et al. Experience with new techniques for extraanatomic arterial reconstruction of the lower limb. Ann Vasc Surg. 2000;14:444–449.
363. Sugawara Y, et al. Retro-sartorius bypass in the treatment of graft infection after peripheral vascular surgery. J Vasc Surg. 2003;37:892–894.
364. Brzezinski W, et al. Transiliac bypass for infected femoral end of an aortofemoral graft. Can J Surg. 1989;32:121–123.
365. Favre J-P, et al. Trans-osseous ilio-femoral by-pass. A new extra-anatomical by-pass. J Cardiovasc Surg (Torino). 1993;34:455–459.
366. Besirli K, et al. An extraanatomic bypass method alternative to the obturator bypass. Vasa. 2005;34:278–280.
367. Thompson JK, et al. Bilateral obturator bypass for combined aortic and femorofemoral graft infection. J Vasc Surg. 2006;44:888.
368. Schneider JR, et al. Superficial femoral vein graft interposition In situ repair for femoral mycotic aneurysm. Ann Vasc Surg. 2009;23:147–149.
369. Ali AT, et al. Graft-associated hemorrhage from femoropopliteal vein grafts. J Vasc Surg. 2005;42:667–672.
370. Meyer JP, et al. The use of sartorius muscle rotation-transfer in the management of wound complications after infrainguinal vein bypass: a report of eight cases and description of the technique. J Vasc Surg. 1989;9:731–735.
371. Hasen KV, et al. Extended approach to the vascular pedicle of the gracilis muscle flap: anatomical and clinical study. Plast Reconstr Surg. 2003;111:2203–2208.
372. Sheiner NM, et al. An unusual complication of obturator foramen arterial bypass. J Cardiovasc Surg (Torino). 1969;10:324–328.
373. Blaisdell FW, et al. Extraperitoneal thoracic aorta to femoral bypass graft as replacement for an infected aortic bifurcation prosthesis. Am J Surg. 1961;102:583–585.
374. DeLaurentis DA. The descending thoracic aorta in reoperative aortic surgery. Bergan JJ, et al. Reoperative arterial surgery. Grune & Stratton: Orlando; 1986:195–203.
375. Sapienza P, et al. Descending thoracic aorta-to-femoral artery bypass grafts. Am J Surg. 1997;174:662–666.
376. Fulenwider JT, et al. Reoperative abdominal arterial surgery–a ten-year experience. Surgery. 1983;93:20–27.
377. Reilly LM, et al. Delayed aortic prosthetic reconstruction after removal of an infected graft. Am J Surg. 1984;148:234–239.
378. DiMuzio PJ, et al. Redo aortic grafting after treatment of aortic graft infection. J Vasc Surg. 1996;24:328–337.
379. Nunn DB, et al. Bypass grafting from the thoracic aorta to femoral arteries for high aortoiliac occlusive disease. Surgery. 1972;72:749–755.
380. Passman MA, et al. Descending thoracic aorta to iliofemoral artery bypass grafting: a role for primary revascularization for aortoiliac occlusive disease? J Vasc Surg. 1999;29:249–258.
381. Crawford ES, et al. Aneurysm of the abdominal aorta. Surg Clin North Am. 1966;46:963–978.
382. Qvarfordt PG, et al. Management of pararenal aneurysms of the abdominal aorta. J Vasc Surg. 1986;3:84–93.
383. Nypaver TJ, et al. Supraceliac aortic cross-clamping: determinants of outcome in elective abdominal aortic reconstruction. J Vasc Surg. 1993;17:868–876.
384. Schneider JR, et al. Supraceliac versus infrarenal aortic cross-clamp for repair of non-ruptured infrarenal and juxtarenal abdominal aortic aneurysm. Cardiovasc Surg. 1997;5:279–285.
386. Williams GM, et al. The extended retroperitoneal approach for treatment of extensive atherosclerosis of the aorta and renal vessels. Surgery. 1980;88:846–855.
387. Crawford ES, et al. “Redo” surgery after operations for aneurysm and occlusion of the abdominal aorta. Surgery. 1977;81:41–52.
388. May J, et al. Transabdominal exposure of the thoracic aorta. Surg Gynecol Obstet. 1980;151:803–805.
389. Canepa CS, et al. Supraceliac aortofemoral bypass. Surgery. 1987;101:323–328.
390. May J, et al. Use of the supraceliac aorta for repeat aortic surgery. Bergan JJ, et al. Reoperative arterial surgery. Grune and Stratton: New York; 1986:175–193.
391. Mills JL, et al. The retroperitoneal, left flank approach to the supraceliac aorta for difficult and repeat aortic reconstructions. Am J Surg. 1991;162:638–642.
392. Barral X, et al. Supraceliac aorta-to-lower extremity arterial bypass. Ann Vasc Surg. 1986;1:30–35.
393. Bacourt F. Revascularization des membres et des artères viscérales à partir de l’aorte thoracique par vois abdominale. Nouvelle technique. J Chir (Paris). 1982;119:749–752.
394. Frantz SL, et al. Ascending aorta-bilateral femoral artery bypass for the totally occluded infrarenal abdominal aorta. Surgery. 1974;75:471–475.
395. Baird RJ, et al. Ascending aorta to bifemoral bypass–a ventral aorta. J Vasc Surg. 1986;3:405–410.
396. Veith FJ, et al. Management of aortoiliac reconstruction complicated by sepsis and hemorrhage. N Engl J Med. 1964;270:1389–1391.
397. Frøysaker T, et al. Bypass procedures in the treatment of obstructions of the abdominal aorta. J Cardiovasc Surg (Torino). 1973;14:317–321.
398. Cevese PG, et al. Thoracic aorta-to-femoral artery by-pass. For the treatment of complete obstruction of the abdominal aorta at the level of the renal arteries. J Cardiovasc Surg (Torino). 1975;16:432–438.
399. Buxton B, et al. Descending thoracic aortofemoral bypass for distal aortic reconstruction after removal of an infected Dacron prosthesis. Med J Aust. 1976;2:133–136.
400. Lakner G, et al. High aortoiliac occlusion: treatment with thoracic aorta to femoral arterial bypass. J Cardiovasc Surg (Torino). 1983;24:532–534.
401. Ochsner JL. Use of thoracic aorta in revascularization of the lower extremity. Bergan JJ, et al. Evaluation and treatment of upper and lower extremity circulatory disorders. Grune & Stratton: Orlando; 1984:361–368.
402. Feldhaus RJ, et al. Thoracic aorta-femoral artery bypass: indications, technique, and late results. Ann Thorac Surg. 1985;40:588–592.
403. Haas KL, et al. Use of thoracic aortobifemoral artery bypass grafting as an alternative procedure for occlusive aortoiliac disease. Am Surg. 1985;51:573–576.
404. Rosenfeld JC, et al. Distal thoracic aorta to femoral artery bypass: a surgical alternative. J Vasc Surg. 1985;2:747–750.
405. Enon B, et al. Revascularisation des membres inférieurs à partir de l’aorte thoracique descendante. [Revascularization of the lower limbs starting from the descending thoracic aorta.]. J Chir (Paris). 1985;122:539–543.
406. McCarthy WJ, et al. Descending thoracic aorta-to-femoral artery bypass. Arch Surg. 1986;121:681–688.
407. Schultz RD, et al. Thoracic aorta as source of inflow in reoperation for occluded aortoiliac reconstruction. Surgery. 1986;100:635–645.
408. Amjad Hussain S. Descending thoracic aorta to bifemoral by-pass graft without laparotomy. Int Surg. 1988;73:260–263.
409. Schellack J, et al. Descending thoracic aortofemoral-femoral bypass: a remedial alternative for the failed aortobifemoral bypass. J Cardiovasc Surg (Torino). 1988;29:201–204.
410. Bowes DE, et al. Long term follow-up of descending thoracic aorto-iliac/femoral bypass. J Cardiovasc Surg (Torino). 1990;31:430–437.
411. Branchereau A, et al. Descending thoracic aorta as an inflow source for late occlusive failures following aortoiliac reconstruction. Ann Vasc Surg. 1991;5:8–15.
412. Kalman PG, et al. Descending thoracic aortofemoral bypass as an alternative for aortoiliac revascularization. J Cardiovasc Surg (Torino). 1991;32:443–446.
413. O’Brien DP, et al. Descending thoracic aorto-bifemoral bypass graft: a safe alternative in the high risk patients. Ir Med J. 1991;84:58–59.
414. McCarthy WJ, et al. Descending thoracic aorta-to-femoral artery bypass: ten years’ experience with a durable procedure. J Vasc Surg. 1993;17:336–348.
415. Carrel T, et al. Le pontage extra-anatomique thoraco-bifémoral: une excellente alternative à la reconstruction in situ lors de revascularisation iterative des membres inférieurs. [Extra-anatomic thoraco-bifemoral bypass: an excellent alternative to in situ reconstruction for repeat revascularization of the lower limbs.]. Schweizerische Medizinische Wochenschrift Journal Suisse de Medecine. 1994;124:961–965.
416. Criado E. Descending thoracic aorta to femoral artery bypass: surgical technique. Ann Vasc Surg. 1997;11:206–215.
417. Kalman PG. Thoracofemoral bypass: proximal exposure and tunneling. Semin Vasc Surg. 2000;13:65–69.
418. Passman MA. Descending thoracic aorta to femoral artery bypass. Perspect Vasc Surg. 1999;11:69–81.
419. Kalman PG. Thoracofemoral bypass: a useful addition to a vascular surgeon’s armamentarium. Perspect Vasc Surg Endovasc Ther. 2004;16:59–64.
420. Hughes R, et al. Thoracofemoral bypass using spliced femoral vein with removal of an infected axillobifemoral bypass graft. Eur J Vasc Endovasc Surg. 2005;29:429–432.
421. McMillan WD, et al. Minimally invasive thoracoscopic thoraco-femoral bypass: a case report. Cardiovasc Surg. 1999;7:251–254.
422. Fernandez JD, et al. Robot-assisted minimally invasive procedure for descending aorta–bifemoral bypass: a case report. Vasc & Endovasc Surg. 2009;43:93–95.
423. Elkins R, et al. Surgical exposure of the upper abdominal aorta and its branches. Surgery. 1971;70:622–627.
424. O’Mara CS, et al. Expanded retroperitoneal approach for abdominal aortic aneurysm repair. Bergan JJ, et al. Aneurysms, diagnosis and treatment. Grune & Stratton: New York; 1982:327–343.
425. Sicard GA, et al. Left retroperitoneal approach to the aorta and its branches: Part I. Ann Vasc Surg. 1994;8:212–219.
426. Wolf YG, et al. Thoracic aorta transobturator bipopliteal bypass as eventual durable reconstruction after removal of an infected aortofemoral graft. J Vasc Surg. 1997;26:693–696.