Antiviral Therapy, Susceptibility Testing, and Prevention
1. Define antiviral resistance and explain what may lead a health care provider to believe that resistance to antiviral therapy is occurring?
2. Define antiviral susceptibility testing and list some of the factors that may vary the end results of testing.
3. Explain the lack of standardization of protocols for antiviral susceptibility testing.
4. Define the criteria that determine whether antiviral susceptibility testing should be performed.
5. Explain the difference between phenotypic and genotypic antiviral susceptibility testing.
6. Name some of the types of phenotypic susceptibility testing and list some of the advantages and disadvantages of this method of susceptibility testing.
7. Describe the methodology of genotypic susceptibility testing and list some of the illnesses for which it is used.
8. List the reasons for drug susceptibility testing for individuals infected with the human immunodeficiency virus (HIV).
9. List the vaccinations used to prevent influenza infection. Also, explain why this vaccine must be reformulated every year and why ongoing surveillance of influenza isolates is crucial to the global vaccination program.
10. Name the two classes of antiviral medications used to treat and prevent influenza. Also, list the four antiviral medications that have been approved by the U.S. Food and Drug Administration (FDA) and briefly explain their mode of action.
Methods of Antiviral Susceptibility Testing
Influenza
For the 2010 influenza season, resistance to the adamantanes remained high; both circulating influenza A viruses (H3N2 and 2009 H1N1) showed high levels of resistance to the these drugs. These viruses are still susceptible to the neuraminidase inhibitors, and this class of antiviral medication is the current therapy of choice for antiviral treatment and for chemoprophylaxis of current circulating influenza A virus strains (Table 67-1).
TABLE 67-1
| Virus | Mode of Action | Target | Examples of Common Drugs |
| CMV | Nucleoside analog | Viral DNA | Ganciclovir |
| HIV* | Nucleoside analog Nucleotide analog Nonnucleoside analog Protease inhibitor Fusion inhibitor |
Viral DNA Viral DNA Reverse transcriptase Viral protease Virus, host cell membrane |
Efavirenz Tenofovir disoproxil fumarate Emtricitabine |
| HSV/VZV | Nucleoside analog Pyrophosphate analog |
Viral DNA DNA polymerase |
Acyclovir Foscarnet |
| Hepatitis B | Nucleoside analog Nucleotide analog |
Reverse transcriptase DNA polymerase |
Lamivudine Adefovir dipivoxil |
| Influenza A | Inhibit penetration and uncoating of virus | Host cell membrane | Amantadine, imantadine |
| Influenza A and B | Prevent release of virus | Neuraminidase inhibitors | Zanamivir, oseltamivir |
| RSV | Inhibit expression of viral mRNA and protein synthesis | Viral mRNA | Ribavirin |
| HCV | Inhibit expression of viral mRNA; increase resistance to virus | Viral mRNA or neighboring host cells | Ribavirin plus interferon-alpha |
| Picornaviruses (enteroviruses and rhinoviruses) | Inhibit attachment and uncoating of virus | Binds to virus | Pleconaril |
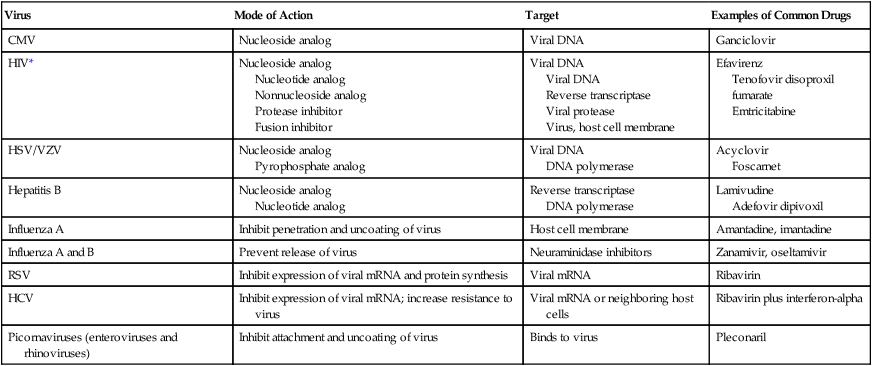
CMV, Cytomegalovirus; HIV, human immunodeficiency virus; HSV, herpes simplex virus; VZV, varicella-zoster virus; RSV, respiratory syncytial virus; HCV, hepatitis C virus.
*More than 20 antiretroviral drugs in six different mechanistic classes are available to design treatment regimens. See the most recent guidelines at http://aidsinfo.nih.gov/guidelines
Prevention of Other Viral Infections
Vaccination
Control of many viral diseases has been accomplished by vaccination. Since Jenner developed the first vaccine against smallpox 200 years ago, attenuated-live or inactivated-dead viral vaccines have been used successively to prevent yellow fever, poliomyelitis, measles, mumps, rubella, hepatitis B, and influenza (Table 67-2). Smallpox was eliminated in 1977 by an effective vaccination program. Additional vaccines continue to appear. New smallpox vaccines with fewer side effects are being developed to prevent outbreaks in the event of bioterrorism. A live-attenuated varicella (chickenpox) vaccine is now recommended for all children, and an inactivated hepatitis A vaccine is available for travelers and others entering areas of higher endemicity. Rotavirus vaccines are approved by the FDA and are now available. Recombinant vaccines are also available for the prevention of HPV infection.
TABLE 67-2
Examples of Vaccines for Preventing Viral Diseases
| Disease | Type of Vaccine |
| Yellow fever | Attenuated-live |
| Poliomyelitis | Attenuated-live and inactivated |
| Measles | Attenuated-live |
| Mumps | Attenuated-live |
| Rubella | Attenuated-live |
| Hepatitis B | Inactivated |
| Influenza | Inactivated |
| Smallpox | Attenuated-live |
| Chickenpox | Attenuated-live |
| Hepatitis A | Inactivated |
| Rabies | Inactivated |
| Rotavirus | Attenuated-live |
Immune Prophylaxis and Therapy
Immune prophylaxis is used to prevent serious viral infection in patients who are immunocompromised or functionally compromised. Instead of actively immunizing an individual with an antiviral vaccine, limited protection can be conferred by intramuscular inoculation of human immunoglobulin. Pooled human immunoglobulin contains antibody against all common viruses. Specific high-titered immunoglobulin can be collected from patients recovering from a specific infection to ensure maximum antibody levels. Immune prophylaxis should be considered an emergency procedure. Table 67-3 lists immune prophylaxis available for viral infections.
TABLE 67-3
Immune Prophylaxis or Therapy for Viral Diseases
| Disease | Circumstances of Use |
| Prophylaxis | |
| Hepatitis A | Traveler to developing country |
| Hepatitis B | Newborns of infected mothers or unimmunized laboratory worker following needlestick |
| Rabies | After bite from potentially rabid animal |
| Measles | Unimmunized close contact with infected individual |
| Varicella | Newborns of infected mothers at time of delivery |
| Respiratory | Infants younger than 2 years of age with underlying lung syncytial disease virus |
| Therapy | |
| Lassa fever | During disease to reduce severity |
Passive immunization occasionally is effective as therapy for viral infection (see Table 67-3). Therapy with immune serum for some hemorrhagic fevers, such as Lassa fever, has also been successful in reducing mortality associated with the disease.







