http://evolve.elsevier.com/McCuistion/pharmacology
Millions of people in the United States have active epilepsy, a seizure disorder that results from abnormal electric discharges from the cerebral neurons characterized by a loss or disturbance of consciousness and usually involuntary, uncontrolled movements. The electroencephalogram (EEG), computed tomography (CT), and magnetic resonance imaging (MRI) are useful in diagnosing epilepsy. The EEG records abnormal electric discharges of the cerebral cortex. Of all seizure cases, 75% are considered to be primary, or idiopathic (of unknown cause), and the remainder are secondary to brain trauma, brain anoxia (absence of oxygen), infection, or cerebrovascular disorders (e.g., cerebrovascular accident [CVA], stroke). Epilepsy is a chronic, usually lifelong disorder. The majority of persons with seizure disorder had their first seizure before 20 years of age.
Seizures that are not associated with epilepsy could result from fever, hypoglycemic reaction, electrolyte imbalance (hyponatremia), metabolic imbalance (acidosis or alkalosis), and alcohol or drug use. When these conditions are corrected, the seizures cease. Recurrent seizures may result from birth and perinatal injuries, head trauma, congenital malformations, neoplasms (tumors), or idiopathic, or unknown, causes.
International Classification of Seizures
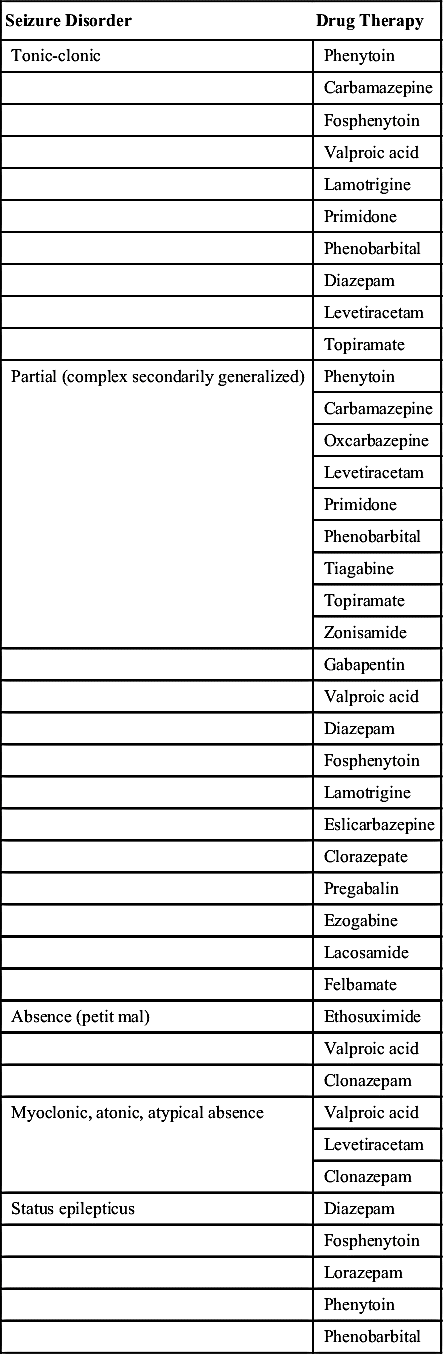
There are various types of seizures, such as tonic-clonic (formerly known as grand mal), absence (formerly known as petit mal), and psychomotor. The International Classification of Seizures (Table 19.1) describes two seizure categories: generalized and partial. A person may also have mixed seizures that comprise more than one type.
TABLE 19.1
International Classification of Seizures
| Category | Characteristics |
| Generalized seizure | Seizures involve both cerebral hemispheres of the brain. |
| Tonic-clonic seizure | Also called grand mal seizure, the most common form; in the tonic phase, skeletal muscles contract or tighten in a spasm that lasts 3 to 5 seconds; in the clonic phase, a dysrhythmic muscular contraction occurs with a jerkiness of legs and arms that lasts 2 to 4 minutes. |
| Tonic seizure | Sustained muscle contraction |
| Clonic seizure | Dysrhythmic muscle contraction |
| Absence seizure | Also called petit mal seizure; brief loss of consciousness lasts less than 10 seconds with fewer than three spike waves on the electroencephalogram (EEG) printout. This type usually occurs in children. |
| Myoclonic seizure | Isolated clonic contraction or jerks that last 3 to 10 seconds may be limited to one limb (focal myoclonic) or may involve the entire body (massive myoclonic); may be secondary to a neurologic disorder such as encephalitis or Tay-Sachs disease. |
| Atonic seizure | Head drop, loss of posture, and sudden loss of muscle tone occurs. If lower limbs are involved, the patient could collapse. |
| Infantile spasms | Muscle spasm |
| Partial seizure | Involves one hemisphere of the brain; no loss of consciousness occurs in simple partial seizures, but there is a loss of consciousness in complex partial seizures. |
| Simple seizure | Occurs in motor, sensory, autonomic, and psychic forms; no loss of consciousness occurs. |
| Motor | Formerly called the Jacksonian seizure, this type involves spontaneous movement that spreads; it can develop into a generalized seizure. |
| Sensory | Visual, auditory, or taste hallucinations |
| Autonomic response | Paleness, flushing, sweating, or vomiting |
| Psychological | Personality changes |
| Complex seizure | Loss of consciousness occurs, and the patient does not recall behavior immediately before, during, and immediately after the seizure. |
| Psychomotor | Complex symptoms include automatisms (repetitive behavior such as chewing or swallowing motions), behavioral changes, and motor seizures. |
| Cognitive | Confusion or memory impairment |
| Affective | Bizarre behavior |
| Compound | May lead to generalized seizures such as tonic or tonic-clonic |
Antiseizure Drugs
Drugs used for epileptic seizures are called antiseizure drugs, anticonvulsants, or antiepileptic drugs (AEDs). Antiseizure drugs stabilize nerve cell membranes and suppress the abnormal electric impulses in the cerebral cortex. These drugs prevent seizures but do not eliminate the cause or provide a cure. Antiseizure drugs are classified as central nervous system (CNS) depressants.
With the use of antiseizure drugs, seizures are controlled in approximately 70% of patients. These drugs are usually taken throughout the person’s lifetime; however, the health care provider might discontinue the medication if no seizures have occurred after 3 to 5 years in some cases.
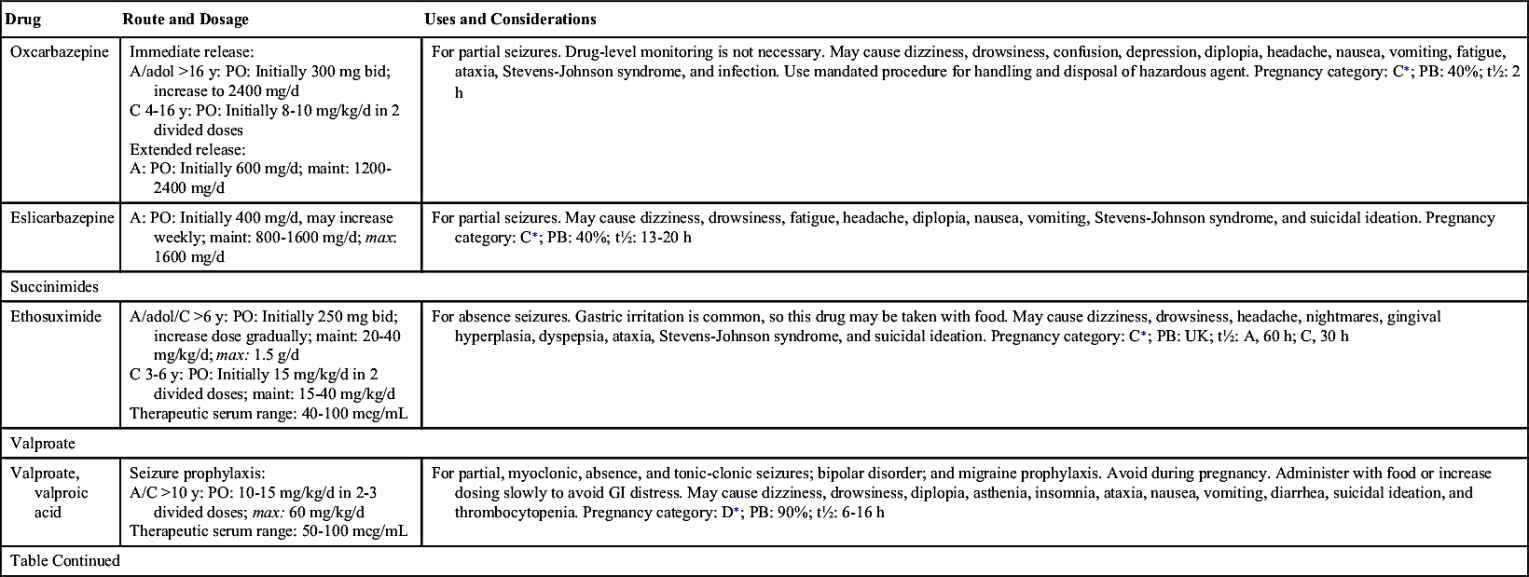
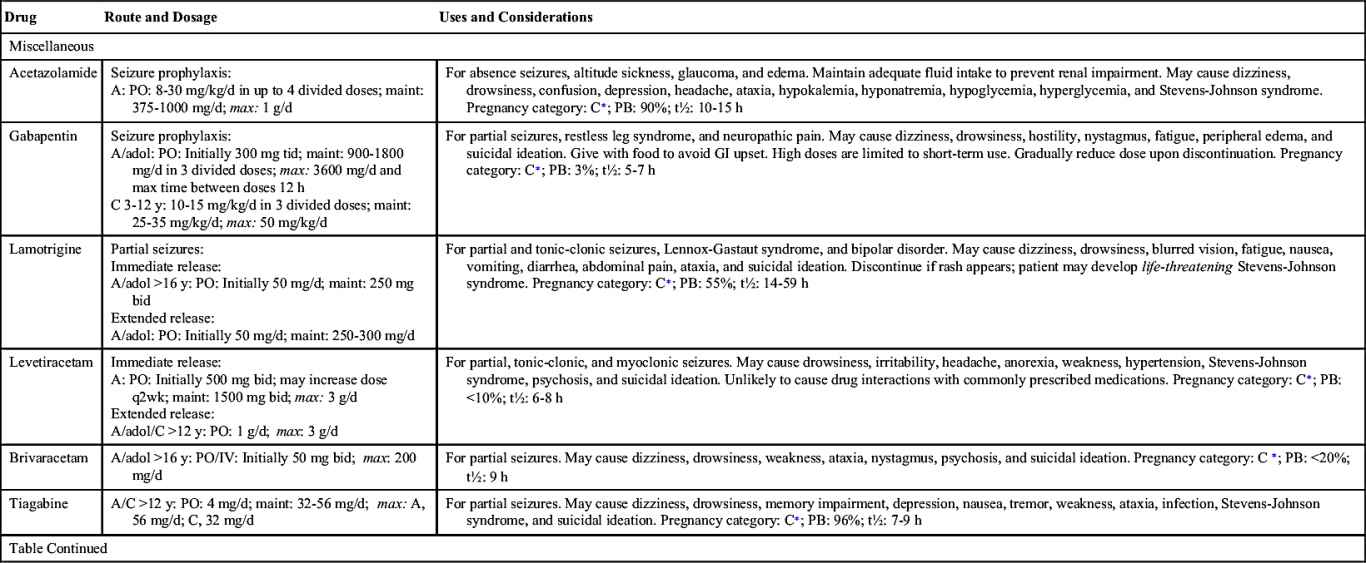
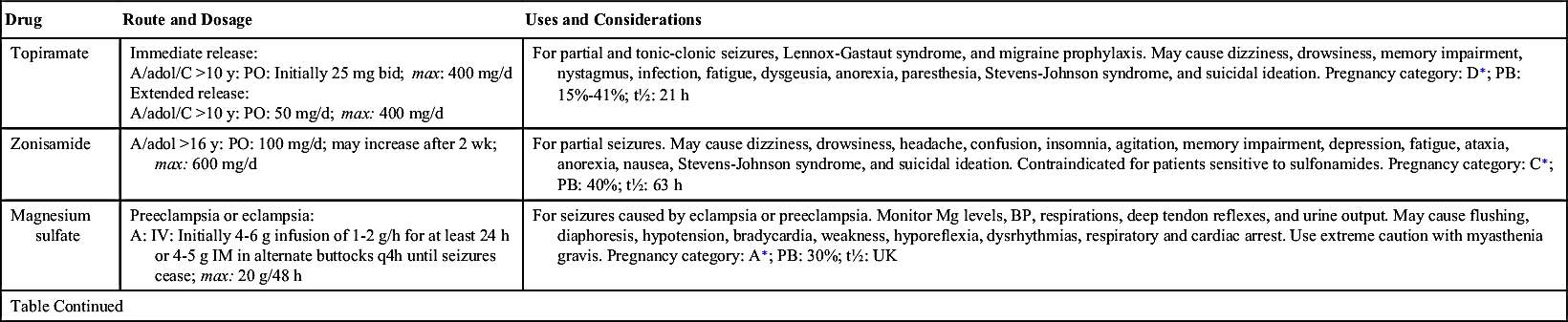
Many types of antiseizure drugs are used to treat seizures, including the hydantoins (phenytoin), long-acting barbiturates (phenobarbital, mephobarbital, primidone), succinimides (ethosuximide), benzodiazepines (diazepam, clonazepam), carbamazepine, and valproate (valproic acid). Antiseizure drugs are not indicated for all types of seizures. For example, phenytoin is effective in treating tonic-clonic and partial seizures but is not effective in treating absence seizures.
Pharmacophysiology: Action of Antiseizure Drugs
Antiseizure drugs work in one of three ways: (1) by suppressing sodium influx through the drug binding to the sodium channel when it is inactivated, which prolongs the channel inactivation and thereby prevents neuron firing; (2) by suppressing the calcium influx, which prevents the electric current generated by the calcium ions to the T-type calcium channel; or (3) by increasing the action of gamma-aminobutyric acid (GABA), which inhibits neurotransmitters throughout the brain. The drugs that suppress sodium influx are phenytoin, fosphenytoin, carbamazepine, oxcarbazepine, valproic acid, topiramate, zonisamide, and lamotrigine. Valproic acid and ethosuximide are examples of drugs that suppress calcium influx. Examples of drug groups that enhance the action of GABA are barbiturates, benzodiazepines, and tiagabine. Gabapentin promotes GABA release.
Hydantoins
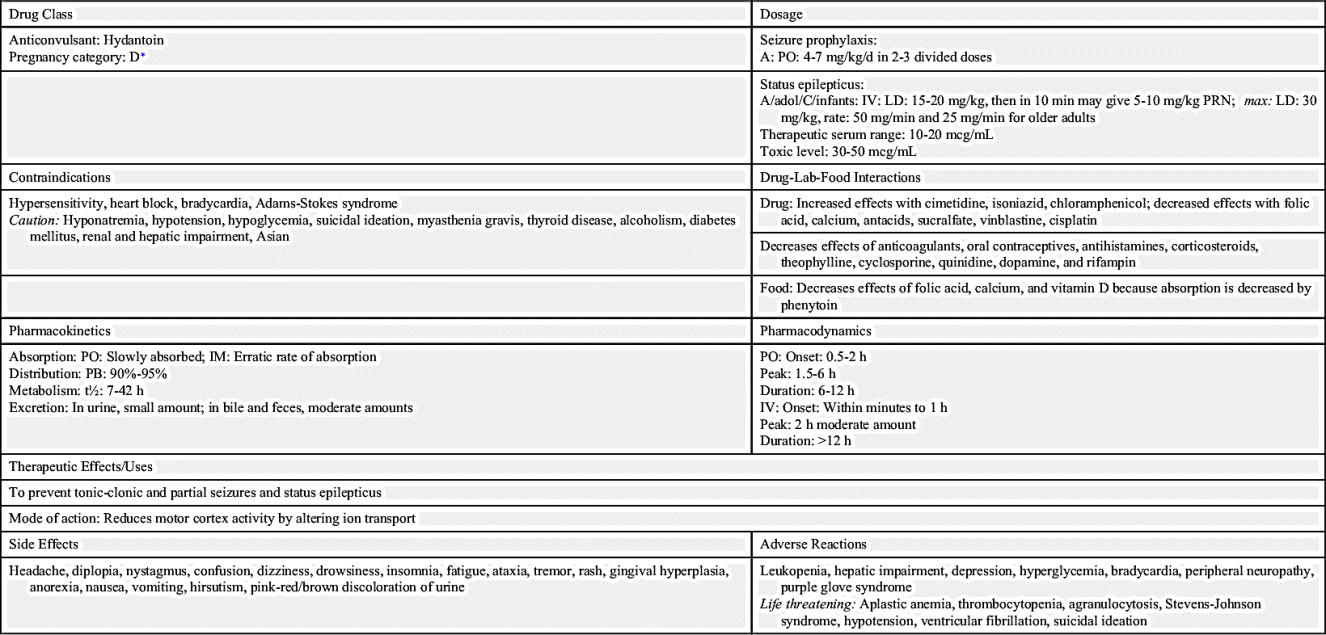
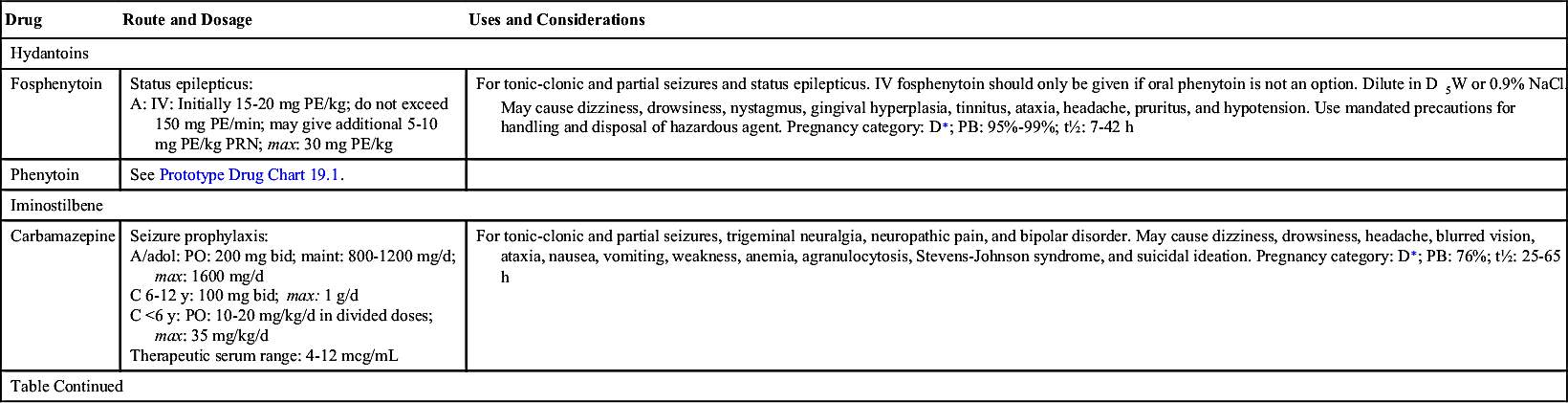
The first antiseizure drug used to treat seizures was phenytoin, a hydantoin discovered in 1938 and still commonly used for controlling seizures. Hydantoins inhibit sodium influx, stabilize cell membranes, reduce repetitive neuronal firing, and limit seizures. By increasing the electrical stimulation threshold in cardiac tissue, it also acts as an antidysrhythmic. It has a slight effect on general sedation, and it is nonaddicting. However, this drug should not be used during pregnancy because it can have a teratogenic effect on the fetus.
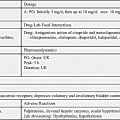
Drug dosage for phenytoin and other antiseizure drugs is age related. Newborns, persons with liver disease, and older adults require a lower dosage because of a decrease in metabolism that results in more available drug. Conversely, individuals with an increased metabolic rate, such as children, may require an increased dosage. The drug dosage is adjusted according to the therapeutic plasma or serum level. Phenytoin has a narrow therapeutic range of 10 to 20 mcg/mL, which is generally considered equivalent to 1 to 2 mcg/mL unbound or free phenytoin. The benefits of an antiseizure drug become apparent when the serum drug level is within the therapeutic range. Typically, if the drug level is below the desired range, the patient is not receiving the required drug dosage to control seizure activity. If the drug level is above the desired range, drug toxicity may result. Monitoring the therapeutic serum drug range is of utmost importance to ensure drug effectiveness. Prototype Drug Chart 19.1 lists the pharmacologic data associated with phenytoin.
Pharmacokinetics
Phenytoin is slowly absorbed from the small intestine. It is a highly protein-bound (90% to 95%) drug, therefore a decrease in serum protein or albumin can increase the free phenytoin serum level. With a small to average drug dose, the half-life of phenytoin is approximately 24 hours, but the range can be from 7 to 42 hours. Phenytoin is metabolized to inactive metabolites, and this portion is excreted in the urine.
Pharmacodynamics
The pharmacodynamics of orally administered phenytoin include onset of action within 30 minutes to 2 hours, peak serum concentration in 1.5 to 6 hours, steady state of serum concentration in 7 to 10 days, and a duration of action dependent on the half-life of up to 45 hours. Oral phenytoin is most commonly ordered as a sustained-release (SR) capsule. The peak SR concentration time is 4 to 12 hours.
Intravenous (IV) infusion of phenytoin should be administered by direct injection into a large vein via a central line or peripherally inserted central catheter (PICC). The drug may be diluted in saline solution; however, dextrose solution should be avoided because of drug precipitation. The manufacturer recommends use of an in-line filter when the drug is administered as an infusion. IV phenytoin, 50 mg or a fraction thereof, should be administered over 1 minute for adults and at a rate of 25 mg/min for older adults. Infusion rates of more than 50 mg/min may cause severe hypotension or cardiac dysrhythmias, especially for older and debilitated patients. Local irritation at the injection site may be noted, and sloughing—formation of dead tissue that separates from living tissue—may occur. The IV line should always be flushed with saline before and after each dose to reduce venous irritation. Intramuscular (IM) injection of phenytoin irritates tissues and may cause damage. For this reason, and because of its erratic absorption rate, phenytoin is not given by the IM route.
Side Effects and Adverse Reactions
The adverse effects of hydantoins include psychiatric effects such as depression, suicidal ideation, Stevens-Johnson syndrome, ventricular fibrillation, and blood dyscrasias, such as thrombocytopenia (low platelet count), leukopenia (low white blood cell count), and purple glove syndrome (swollen, discolored, and painful extremities that may require amputation). Patients on hydantoins for long periods might have elevated blood glucose (hyperglycemia) that results from the drug inhibiting the release of insulin. Less severe side effects include nausea, vomiting, gingival hyperplasia (overgrowth of gums or reddened gums that bleed easily), constipation, drowsiness, headaches, slurred speech, confusion, alopecia, hirsutism, and nystagmus (constant, involuntary, cyclical movement of the eyeball).
Drug-Drug Interactions
Drug interaction is common with hydantoins because they are highly protein bound. Hydantoins compete with other drugs (e.g., anticoagulants, aspirin) for plasma protein-binding sites. The hydantoins displace anticoagulants and aspirin, causing more free-drug availability and increasing their activity. Barbiturates, rifampin, and chronic ingestion of ethanol increase hydantoin metabolism. Drugs like sulfonamides and cimetidine can increase the action of hydantoins by inhibiting liver metabolism, which is necessary for drug excretion. Antacids, calcium preparations, sucralfate, and antineoplastic drugs also decrease the absorption of hydantoins. Antipsychotics and certain herbs can lower the seizure threshold, the level at which seizure may be induced, and they increase seizure activity (Complementary and Alternative Therapies 19.1). The patient should be closely monitored for seizure occurrence.
Barbiturates
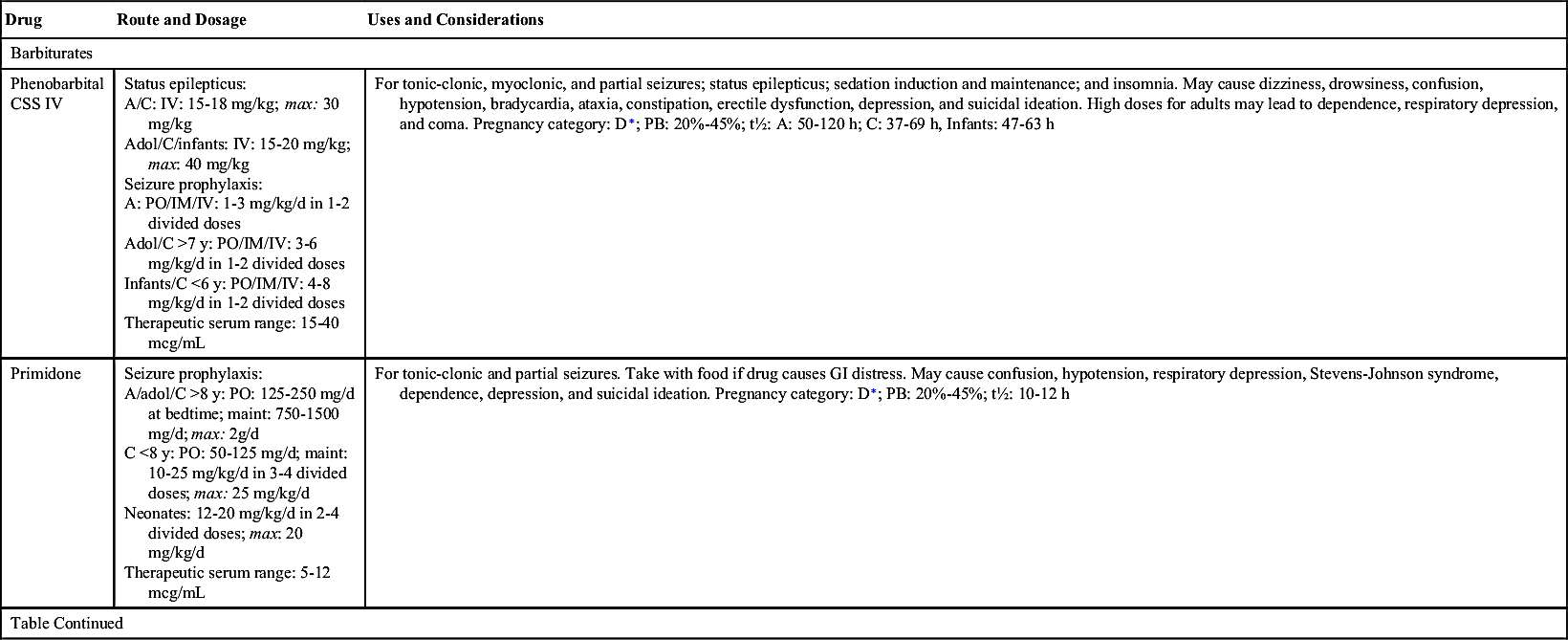
Phenobarbital, a long-acting barbiturate, is prescribed to treat tonic-clonic, partial, and myoclonic seizures and status epilepticus, a rapid succession of epileptic seizures. Barbiturates reduce seizures by enhancing the activity of GABA, an inhibitory neurotransmitter. Possible teratogenic effects and other side effects related to phenytoin are less pronounced with phenobarbital. The therapeutic serum range of phenobarbital is 20 to 40 mcg/mL. Risks associated with the use of phenobarbital include sedation and tolerance to the drug. Discontinuance of phenobarbital should be gradual to avoid recurrence of seizures.
Succinimides
The succinimide drug group is used to treat absence seizures. Succinimides act by decreasing calcium influx through the T-type calcium channels. The therapeutic serum range of ethosuximide is 40 to 100 mcg/mL. Adverse effects include blood dyscrasias, renal and liver impairment, and systemic lupus erythematosus.
Benzodiazepines
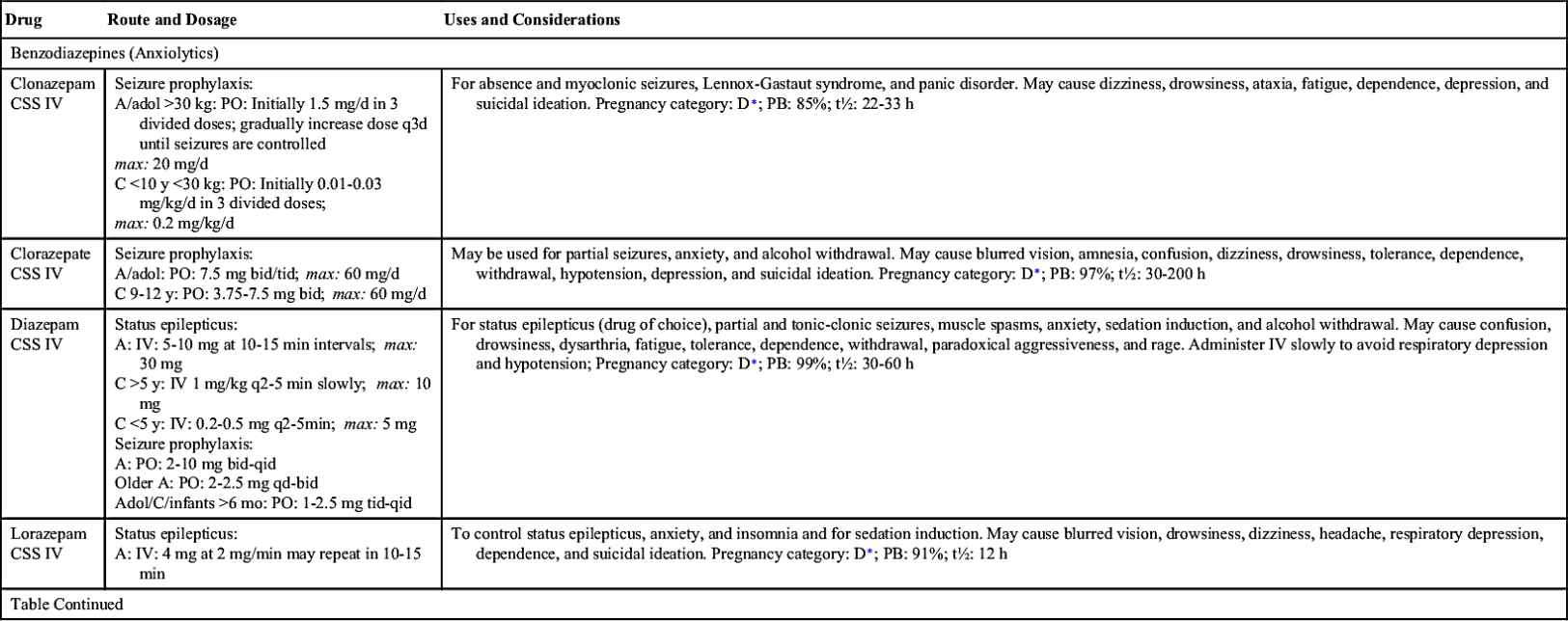
The benzodiazepines that have antiseizure effects are clonazepam, clorazepate dipotassium, lorazepam, and diazepam. Clonazepam is effective in controlling absence and myoclonic seizures, but tolerance may occur 6 months after drug therapy starts; consequently, clonazepam dosage must be adjusted. Clorazepate dipotassium is administered for treating partial seizures.
Diazepam is administered by IV to treat status epilepticus. The drug has a short-term effect; thus other antiseizure drugs, such as phenytoin or phenobarbital, must be given during or immediately after administration of diazepam.
Iminostilbenes
Carbamazepine, an iminostilbene, is used to control tonic-clonic and partial seizures. Carbamazepine is also used for psychiatric disorders (e.g., bipolar disorder), trigeminal neuralgia (as an analgesic), and alcohol withdrawal. The therapeutic serum range of carbamazepine is 4 to 12 mcg/mL.
A potentially toxic interaction can occur when grapefruit juice is taken with carbamazepine, and drug concentrations must be carefully monitored.
Valproate
Valproic acid is prescribed for tonic-clonic, absence, and mixed types of seizures, although the safety and efficacy of this drug has not been established for children younger than 2 years of age. Care should be taken when giving this drug to very young children and to patients with liver disorders because hepatotoxicity is one of the possible adverse reactions. Liver enzymes should be monitored. The therapeutic serum range for a patient with seizures is 50 to 100 mcg/mL.
Table 19.2 lists the various antiseizure drugs and their dosages, uses, and considerations, including common side effects and a few serious adverse effects. Table 19.3 lists selected antiseizure drugs frequently prescribed to treat seizure disorders.
Antiseizure drug dosages usually start low and gradually increase over a period of weeks until the serum drug level is within therapeutic range or the seizures cease. Serum antiseizure drug levels should be closely monitored to prevent toxicity.
Antiseizure Drugs and Pregnancy
During pregnancy, seizure episodes increase 25% in women with epilepsy. Hypoxia that may occur during seizures places both the pregnant woman and her fetus at risk.
Many antiseizure drugs have teratogenic properties that increase the risk for fetal malformations. Phenytoin and carbamazepine have been linked to fetal anomalies such as cardiac defects and cleft lip and palate. It has been reported that valproic acid is known to cause major congenital malformations in infants in 4% to 8% of pregnant women who take the drug. As expected, the highest incidence of birth defects occurs when the woman takes combinations of antiseizure drugs.
Antiseizure drugs tend to act as inhibitors of vitamin K, contributing to hemorrhage in infants shortly after birth. Frequently, pregnant women taking antiseizure drugs are given an oral vitamin K supplement during the last week or 10 days of the pregnancy, or vitamin K is administered to the infant soon after birth.
Antiseizure drugs also increase the loss of folate (folic acid) in pregnant women, thus pregnant individuals should take daily folate supplements.
Antiseizure Drugs and Febrile Seizures
Seizures associated with fever usually occur in children between 3 months and 5 years of age. Epilepsy develops in approximately 2.5% of children who have had one or more febrile seizures. Prophylactic antiseizure drug treatment such as phenobarbital or diazepam may be indicated for high-risk patients. Valproic acid should not be given to children younger than 2 years of age because of its possible hepatotoxic effect.
 Cultural Considerations
Cultural Considerations• Communicate respect for cultural beliefs concerning refusal or reluctance to take antiseizure medications daily for life; use an interpreter and the extended family as needed to help the patient understand the importance of keeping to a prescribed drug regimen.
• Use a written drug schedule in the patient’s preferred language to support adherence to the prescribed drug regimen.
• Encourage patient compliance with follow-up by a community nurse.
• Show patient videos or pictures in their own cultural group when language barriers exist to help strengthen compliance with health interventions.
Evaluation
• Evaluate effectiveness of drugs in controlling seizures.
• Monitor serum phenytoin levels to determine whether they are within the desired range. High serum levels of phenytoin are frequently indicators of phenytoin toxicity.
• Monitor patients for hydantoin overdose. Initial symptoms are nystagmus and ataxia (impaired coordination). Later symptoms are hypotension, unresponsive pupils, and coma. Respiratory and circulatory support, as well as hemodialysis, are usually used in the treatment of phenytoin overdose.
Antiseizure Drugs and Status Epilepticus
Status epilepticus, a continuous seizure state, is considered a medical emergency. If treatment is not begun immediately, death could result. The choices of pharmacologic agents are diazepam administered by IV or lorazepam followed by IV administration of phenytoin. For continued seizures, midazolam or propofol and then high-dose barbiturates are used. These drugs should be administered slowly to avoid respiratory depression.
The pharmacologic behavior of specific anticonvulsants, including a few common side effects and severe adverse effects, is summarized in Table 19.2.
Critical Thinking Case Study
SS, a 26-year-old woman, takes phenytoin 100 mg three times daily to control tonic-clonic seizures. She and her husband are contemplating starting a family.
1. What action should the nurse take in regard to the patient’s family planning?
SS complains of frequent upset stomach and bleeding gums when brushing her teeth.
2. To decrease GI distress, what can be suggested?
3. To alleviate bleeding gums, what patient teaching for SS may be included?
4. The nurse checks SS’s serum phenytoin level. What are the indications of an abnormal serum level? What appropriate actions should be taken?