Antipsychotics
Perspective
The first antipsychotic, chlorpromazine, was used for the treatment of psychosis in France in 1951 and in the United States in 1954. Antipsychotic use has expanded significantly since then. The historic term neuroleptic, used with antipsychotic medication, is no longer appropriate because newer agents cause less sedation. The term antipsychotic is now preferred. In 2009, U.S. poison control centers reported more than 4700 exposures to phenothiazines and 43,000 exposures to atypical antipsychotics, resulting in 0 and 8 deaths, respectively.1
Principles of Disease
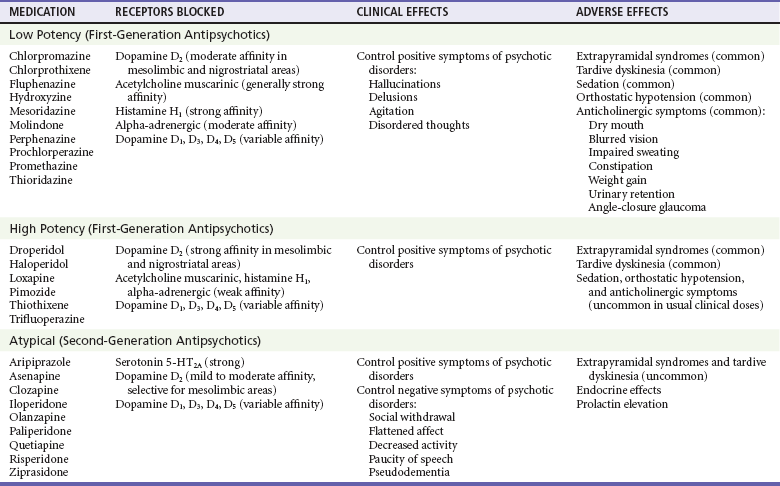
Antipsychotic medications are used to treat agitation and psychosis caused by schizophrenia, mania, acute idiopathic psychosis, substance-induced psychosis, and dementia. The antipsychotic medications are divided into three broad categories on the basis of their receptor profiles, clinical effects, and adverse effects (Table 161-1). All antipsychotic medications effectively treat the positive symptoms of psychotic disorders; they reduce hallucinations, control agitation, and aid in restructuring of disordered thinking. In general, the low-potency first-generation antipsychotics (FGAs) are the most sedating. Movement disorders, including extrapyramidal symptoms (EPS) and tardive dyskinesia (TD), are significant problems with both low-potency and high-potency FGAs. In addition to producing less sedation and fewer movement disorders, the atypical or second-generation antipsychotics (SGAs) assist with the negative symptoms of psychotic disorders, such as flat affect, avolition, social withdrawal, and impoverished thought and speech.2 Although neuroleptic malignant syndrome (NMS) occurs with all agents, it occurs least with SGAs.
Anatomy and Physiology
Antipsychotic drugs block dopamine receptors in several areas of the brain, including the cerebral cortex, basal ganglia, limbic system, hypothalamus, and chemoreceptor trigger zone. All antipsychotic agents reduce the positive symptoms of schizophrenia by blocking the dopamine D2 receptor subtype in the mesolimbic region of the brain. However, blockade of D2 receptors in the nigrostriatal brain region produces undesired EPS. In addition, blockade of D2 receptors in the mesocortical brain region impairs cognition and worsens the negative symptoms of schizophrenia. SGAs block both D2 and serotonin 5-HT2A receptors. Because these agents have lower affinity for dopamine receptor antagonism and more selective binding of D2 receptors in the mesolimbic versus nigrostriatal areas of the brain, SGAs should have a lower rate of EPS and TD. The serotonin receptor antagonism is thought to reduce EPS effects and to improve the negative symptoms of schizophrenia.3,4 However, studies have found no significant difference in reported rates of EPS and TD between perphenazine (a FGA) and several SGAs.5,6 Antipsychotic medications also block other receptor types (see Table 161-1).
Pathophysiology
EPS can be immediate or delayed after initiation of drug therapy. The acute EPS include dystonia, akathisia, and parkinsonism, which are caused by blockade of nigrostriatal D2 receptors and reduced by blockade of muscarinic receptors. The propensity for antipsychotics to produce EPS is inversely proportional to the agents’ muscarinic receptor antagonism.4 The delayed-onset syndromes, including TD and tardive dystonia, develop after prolonged use of antipsychotic medications and are thought to occur from chronic dopamine receptor blockade in the nigrostriatal area leading to D2 receptor upregulation and hypersensitivity to dopamine.7–9 The pathophysiologic mechanism of NMS, an idiosyncratic reaction to antipsychotic medication, is unknown but thought to be neuroregulatory dysfunction secondary to D2 receptor blockade in the nigrostriatum and hypothalamus, leading to rigidity and hyperthermia, respectively.10 NMS is not associated with ryanodine receptor (RYR) gene mutations, which are associated with malignant hyperthermia.
Antipsychotic medications produce cardiovascular side effects, most commonly orthostatic hypotension with reflexive tachycardia due to alpha-adrenergic blockade. Many agents cause conduction delays, predominantly QT prolongation, resulting in prolonged repolarization and potentially torsades de pointes (TdP).11 The degree of prolongation varies between antipsychotic agents, increasing with dose and with concomitant use of other drugs that prolong the QT interval.12,13 The phenothiazines, particularly thioridazine and mesoridazine, have the greatest risk of cardiac toxicity. QT prolongation has also been reported with the butyrophenones haloperidol and droperidol.12,14 The atypical antipsychotics produce less cardiotoxicity than the traditional agents do, although most can cause repolarization abnormalities in therapeutic doses and overdose.12,15,16 Ultimately, a correlation between QT prolongation and dysrhythmias or TdP has not been established,3 but antipsychotics are associated with an increased risk of sudden death, particularly with heart disease and in elderly patients.17,18 However, psychotic disorders themselves are associated with an increased risk of sudden death.
Clozapine produces agranulocytosis in 1 or 2% of treated patients; however, this has been reduced to 0.4% after strict adherence to labeling requirements.19 Although the mechanism of agranulocytosis is unknown, research supports an immunogenic cause and direct cytotoxic effect on human bone marrow mesenchymal stromal cells.20 Seizure rarely occurs with antipsychotic drugs, but clozapine has the highest seizure incidence. Drug dosing and the patient’s seizure risk profile influence seizure susceptibility.21 FGAs and SGAs have been associated with weight gain, glycemic dysregulation, and dyslipidemia. The cause is not fully understood but partly blamed on the pharmacodynamic profile of these agents.22
Clinical Features
Atypical antipsychotic overdose is similar to that of traditional antipsychotics. Overdoses are characterized by CNS depression and tachycardia.23–30 Miosis may be present, potentially mimicking opioid toxicity.31 Extremity twitching is common. With the exception of clozapine, seizures rarely occur in overdose. Acute EPS have been reported, especially for risperidone overdose.32
Acute Extrapyramidal Syndromes
Acute dystonia is manifested as intermittent, involuntary motor tics or spasms of antagonistic muscle groups that most often involve the facial, neck, back, or limb muscles. This results in trismus, facial grimacing, dysarthria, tongue and lip distortion, torticollis, or oculogyric crisis. Dystonic reactions usually develop within the first several doses of treatment or after a large increase in dosage.33 Laryngeal dystonia is a rare but life-threatening form of dystonia that is manifested as stridor, difficulty in breathing, or choking sensation.5,34 Increased risk of death due to choking has been documented in schizophrenia patients.35
Akathisia is a subjective feeling of restlessness associated with objective motor restlessness, including repetitive foot shuffling, truncal rocking, or pacing. The subjective distress may precipitate aggressive behavior.5,36 Akathisia usually develops within the first few days of treatment, but 40% of patients given 10 mg of intravenous prochlorperazine developed akathisia within 1 hour.37
A parkinsonian syndrome of bradykinesia, masked facies, shuffling gait, muscle rigidity, and resting tremor frequently develops during the first month of treatment; 90% of cases occur within 3 months of treatment. Perioral tremor (rabbit syndrome), in which the lip and nose movements resemble those of a rabbit, can also develop after prolonged therapy.5
Tardive Dyskinesia
TD is a chronic movement disorder induced by prolonged use of antipsychotic medication. Typical signs of TD include quick, involuntary movements of the face (blinking, grimaces, tongue movements, and chewing), extremities, or trunk. Twenty percent of patients treated with long-term traditional antipsychotics are affected. TD is difficult to treat and is frequently permanent. Anticholinergic agents may worsen TD; reduction of the antipsychotic dose or a change to an alternative agent should be considered.5,38 TD improves in some patients switched to clozapine; amantadine improved symptoms compared with placebo in a small study.39,40
Neuroleptic Malignant Syndrome
NMS is a serious idiosyncratic drug reaction that typically develops during the first month of therapy but has occurred during stable drug regimens. Risk factors include rapid drug loading, high dosage, high-potency antipsychotics, parenteral formulations, dehydration, preceding psychomotor agitation, and previous episodes of NMS.41,42 Other medications may contribute to NMS, including lithium, which inhibits dopamine secretion, as can withdrawal from dopaminergic agents used to treat Parkinson’s disease.5,10,43 The incidence of NMS in patients treated with antipsychotics is approximately 0.02%.41 Atypical antipsychotic agents, including clozapine, risperidone, olanzapine, quetiapine, ziprasidone, amisulpride, and aripiprazole, have been associated with NMS.44
Table 161-2 lists the diagnostic criteria for NMS. Other features of NMS may include sialorrhea, dysarthria, dysphagia, metabolic acidosis, liver function elevations, sodium imbalance, dehydration, elevations in serum catecholamines, coagulopathy, generalized slowing on the electroencephalogram, pulmonary embolism, and renal failure.42 Atypical presentations may lack full diagnostic criteria for NMS.44,45
Table 161-2
Diagnostic Criteria and Clinical Features of Neuroleptic Malignant Syndrome
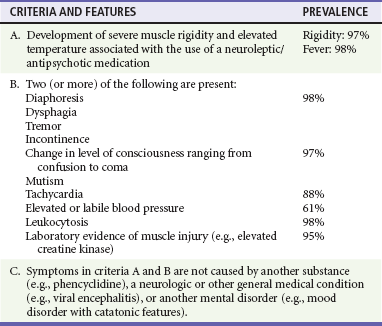
Modified from American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed, text rev. Washington, DC, American Psychiatric Association, 2000. Prevalence data from Caroff SN, Mann SC: Neuroleptic malignant syndrome. Med Clin North Am 77:185, 1993.
Cardiovascular Toxicity and Dysrhythmias
The most common cardiac effect is sinus tachycardia with a normal QRS duration. If QRS prolongation is present, another drug effect should be suspected. QT prolongation occurs during therapeutic dosing of many antipsychotic agents. Therapeutic doses of thioridazine, followed by ziprasidone, prolong the QT interval more than risperidone, olanzapine, quetiapine, or haloperidol does.15,16 QT prolongation associated with TdP is a well-described adverse effect of thioridazine, mesoridazine, droperidol, sertindole, and haloperidol.12 Among FGAs, thioridazine carries the greatest risk of cardiotoxicity. Among SGA overdoses, whereas ziprasidone carries the greatest risk of QTc prolongation,46,47 TdP has been reported with amisulpride overdose.30 QT prolongation should be considered a “class effect” of all antipsychotic medications.
Agranulocytosis
Clozapine produces agranulocytosis; 75% of occurrences develop within the first 18 weeks after initiation of therapy, peaking at 3 months.19 The concomitant use of other bone marrow–suppressing agents (e.g., carbamazepine) should be avoided. Clozapine administration must be halted if the total white blood cell count falls below 3000 cells/mL or if the absolute neutrophil count is less than 1500 cells/mL. Agranulocytosis has not been reported after an acute overdose. Olanzapine, whose chemical structure is similar to that of clozapine, has also been associated with neutropenia and agranulocytosis, but all patients recovered after discontinuation of olanzapine.48
Seizures
Antipsychotic medications can lower the seizure threshold and induce epileptiform electroencephalographic abnormalities in many asymptomatic patients. However, antipsychotic-induced seizures are rare except for clozapine, which causes a dose-related increase in risk of seizures (approximately 5% at high doses). Antipsychotics can be prescribed for patients with known seizure disorder.20,21,49
Differential Considerations
Differential considerations include a broad list of agents and clinical conditions that produce altered mental status, orthostatic hypotension, anticholinergic syndrome, seizure, QT prolongation, or TdP. Although the signs of NMS are similar, serotonin syndrome is more likely to have clonus, heatstroke is evident from the circumstances, and the causes of these conditions are quite different (Table 161-3). Malignant hyperthermia should be considered in patients receiving inhalational anesthetics or succinylcholine.
Table 161-3
Differential Diagnosis of Neuroleptic Malignant Syndrome
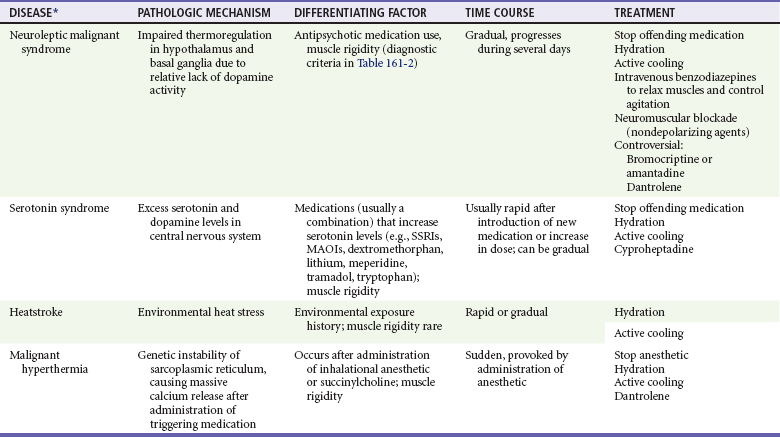
MAOIs, monoamine oxidase inhibitors; SSRIs, selective serotonin reuptake inhibitors.
*Other clinical entities to consider in the diagnosis of these conditions include Addison’s syndrome, central nervous system infection, delirium tremens, hypocalcemia, hypoglycemia, hyponatremia, intracranial hemorrhage, lethal catatonia, poisoning (e.g., amphetamines, anticholinergics, cocaine, nicotine, salicylates, sympathomimetics, strychnine, theophylline), sedative-hypnotic drug withdrawal, sepsis, status epilepticus (including nonconvulsive status), tetanus, thalamic infarct, thyroid storm, and psychotic agitation.
Diagnostic Strategies
Patients who have NMS, parkinsonism with marked muscle rigidity, or prolonged seizures are at risk for rhabdomyolysis and should have serum creatine kinase, renal function, and urine myoglobin measured. Patients taking clozapine or olanzapine who present with infection or fever should be checked for leukopenia. Olanzapine and clozapine have been associated with new-onset diabetes with ketoacidosis.3
Management
Acute Extrapyramidal Syndromes
Dystonia will usually respond within 30 minutes to diphenhydramine 25 to 50 mg intravenously, intramuscularly, or orally or benztropine 1 to 2 mg intramuscularly or orally. Benzodiazepines may also be effective. If relief is not achieved, an alternative diagnosis should be sought. Akathisia is treated similarly or with a lipophilic beta-adrenergic blocker (e.g., propranolol). Treatment of parkinsonism syndrome may include anticholinergic agents. Patients with EPS who respond to diphenhydramine or benztropine should continue therapy for at least 48 hours to prevent recurrence.5,50 In addition, patients should be referred to their treating physician, who may reduce the antipsychotic dose or change to an alternative agent as necessary. Benztropine, diphenhydramine, and the older antipsychotic medications cause anticholinergic effects, so combination therapy may worsen dry mouth, blurred vision, and urinary retention.
Neuroleptic Malignant Syndrome
Bromocriptine and amantadine have been suggested for treatment of NMS but do not consistently show a benefit.43,51 Bromocriptine is administered orally or by nasogastric tube, beginning with 5 mg every 8 hours and titrated to a maximum of 20 mg per dose. The dose of amantadine for NMS is 200 mg orally every 12 hours. Response to therapy requires at least 24 hours. Dantrolene, which inhibits the release of calcium from the sarcoplasmic reticulum, has no proven benefit.51 Because the muscle rigidity of NMS is thought to be from dopamine blockade in the CNS rather than a muscle abnormality, dantrolene offers no advantage over benzodiazepines and neuromuscular blockade.
References
1. Bronstein, AC, et al. 2009 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS). Clin Toxicol (Phila). 2010;48:979.
2. Oliveira, IR, Juruena, MF. Treatment of psychosis: 30 years of progress. J Clin Pharm Ther. 2006;31:523.
3. Reilly, TH, Kirk, MA. Atypical antipsychotics and newer antidepressants. Emerg Med Clin North Am. 2007;25:477.
4. Horacek, J, et al. Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia. CNS Drugs. 2006;20:389.
5. Caroff, SN, et al. Movement disorders induced by antipsychotic drugs: Implications of the CATIE schizophrenia trial. Neurol Clin. 2011;29:127.
6. Miller, DD, et al. Extrapyramidal side-effects of antipsychotics in a randomized trial. Br J Psychiatry. 2008;193:279.
7. Casey, D. Tardive dyskinesia: Pathophysiology and animal model. J Clin Psychiatry. 2000;61:5.
8. Farver, D. Neuroleptic malignant syndrome induced by atypical antipsychotics. Expert Opin Drug Saf. 2003;2:21.
9. Sachdev, P. Early extrapyramidal side-effects as risk factors for later tardive dyskinesia: A prospective study. Aust N Z J Psychiatry. 2004;38:445.
10. Bhanushali, M, Tuite, P. The evaluation and management of patients with neuroleptic malignant syndrome. Neurol Clin North Am. 2004;22:389.
11. Pacher, P, Kecskemeti, V. Cardiovascular side effects of new antidepressants and antipsychotics: New drugs, old concerns? Curr Pharm Des. 2004;10:2463.
12. Haddad, PM, Anderson, IM. Antipsychotic-related QTc prolongation, torsades de pointes and sudden death. Drugs. 2002;62:1649.
13. Reilly, JG, et al. QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet. 2000;355:1824.
14. Salih, ISM, et al. Comparison of the effects of thioridazine and mesoridazine on the QT interval in healthy adults after single oral doses. Clin Pharm Ther. 2007;82:548.
15. FDA Briefing Document for Zeldox Capsules (Ziprasidone). New York: Pfizer, 2000.
16. Harrigan, EP, et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24:62.
17. Wang, PS, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335.
18. Ray, WA, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225.
19. Honigfeld, G, et al. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59:3.
20. Oja, S, Korhonen, M, Andersson, LC. Clozapine is cytotoxic to primary cultures of human bone marrow mesenchymal stromal cells. J Clin Psychopharmacol. 2010;30:461.
21. Pisani, F, et al. Effects of psychotropic drugs on seizure threshold. Drug Saf. 2002;25:91.
22. Monteleone, P, Martiadis, V, Maj, M. Management of schizophrenia with obesity, metabolic, and endocrinological disorders. Psychiatr Clin North Am. 2009;32:775.
23. Ngo, A, Ciranni, M, Olson, K. Acute quetiapine overdose in adults: A 5-year retrospective case series. Ann Emerg Med. 2008;52:541.
24. Kramer, I, et al. Minimal dose for severe poisoning and influencing factors in acute human clozapine intoxication: A 13-year retrospective study. Clin Neuropharmacol. 2010;33:230.
25. Forrester, MB. Pattern of ziprasidone exposures reported to Texas poison centers, 2001-2005. Hum Exp Toxicol. 2008;27:355.
26. Isbister, GK, Duffull, SB. Quetiapine overdose: Predicting intubation, duration of ventilation, cardiac monitoring and the effect of activated charcoal. Int Clin Psychopharmacol. 2009;24:174.
27. Young, MC, et al. Risk assessment of isolated aripiprazole exposures and toxicities: A retrospective study. Clin Toxicol (Phila). 2009;47:580.
28. Ciranni, MA, Kearney, TE, Olson, KR. Comparing acute toxicity of first- and second- generation antipsychotic drugs: A 10-year, retrospective cohort study. J Clin Psychiatry. 2009;70:122.
29. Melhem, S, et al. Prolonged toxicity in a 2-year-old after accidental ingestion of aripiprazole. Pediatr Emerg Care. 2009;25:105.
30. Isbister, GK, et al. Amusulpride overdose is frequently associated with QT prolongation and torsades de pointes. J Clin Psychopharmacol. 2010;30:391.
31. O’Malley, G. Olanzapine overdose mimicking opioid intoxication. Ann Emerg Med. 1999;34:279.
32. Page, CB, Calver, LA, Isbister, GK. Risperidone overdose causes extrapyramidal effects but not cardiac toxicity. J Clin Psychopharmacol. 2010;30:387.
33. Sachdev, P. Neuroleptic-induced movement disorders: An overview. Psychiatr Clin North Am. 2005;28:255.
34. Christodoulou, C, Kalaitzi, C. Antipsychotic drug–induced acute laryngeal dystonia: Two case reports and a mini review. J Psychopharmacol. 2005;19:307.
35. Ruschena, D, et al. Choking deaths: The role of antipsychotic medication. Br J Psychiatry. 2003;183:446.
36. Kane, JM, et al. Akathisia: An updated review focusing on second-generation antipsychotics. J Clin Psychiatry. 2009;70:627.
37. Drotts, D, Vinson, D. Prochlorperazine induces akathisia in emergency patients. Ann Emerg Med. 1999;34:469.
38. Hubert, F, Friedman, J. Classification and treatment of tardive syndromes. Neurologist. 2003;9:16.
39. Tarsy, D, Baldessarini, RJ, Tarazi, FI. Effects of newer antipsychotics on extrapyramidal function. CNS Drugs. 2002;16:23.
40. Pappa, S, et al. Effects of amantadine on tardive dyskinesia: A randomized, double-blind, placebo-controlled study. Clin Neuropharmacol. 2010;33:271.
41. Strawn, J, Keck, P, Caroff, S. Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164:6.
42. Caroff, S, Mann, S. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77:185.
43. Rosebush, P, Stewart, T. A prospective analysis of 24 episodes of neuroleptic malignant syndrome. Am J Psychiatry. 1989;146:717.
44. Trollor, JN, Chen, X, Sachdev, PS. Neuroleptic malignant syndrome associated with atypical antipsychotic drugs. CNS Drugs. 2009;23:477.
45. Margetic, B, Margetic, B. Neuroleptic malignant syndrome and its controversies. Pharmacoepidemiol Drug Saf. 2010;19:429.
46. Buton, S, et al. Ziprasidone overdose. Am J Psychiatry. 2000;157:835.
47. Bryant, S. A case series of ziprasidone overdoses. Vet Hum Toxicol. 2003;45:81.
48. Tolosa-Vilella, C, et al. Olanzapine-induced agranulocytosis: A case report and review of the literature. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:411.
49. Alper, K, et al. Seizure incidence in psychopharmacological clinical trials: An analysis of Food and Drug Administration summary basis of approval reports. Biol Psychiatry. 2006;62:345.
50. Miller, C, Fleischhacker, W. Managing antipsychotic-induced acute and chronic akathisia. Drug Saf. 2000;22:73.
51. Reulbach, U, et al. Managing an effective treatment for neuroleptic malignant syndrome. Crit Care. 2007;11:R4.