Antimicrobial Drugs
• Discuss the general goal of antimicrobial drug actions
• Describe the five basic categories of mechanisms used by antimicrobial drugs
• Explain the various spectra of activity for antimicrobial drugs
• Name and describe factors that need to be considered in selecting an antimicrobial drug
• Explain the therapeutic index and discuss possible side effects associated with antimicrobial therapy
• Discuss the development of drug resistance by microorganisms
• Explain the mechanisms leading to drug resistance, including the development of multiple resistances
• Describe how drug resistance can be controlled
• Name and describe the actions of antibacterial, antiviral, antiprotozoan, and antihelminthic agents
Mechanisms of Antimicrobial Action
The general goal of any antimicrobial agent is to disrupt the metabolism or structure of an organism to such a point that it can no longer survive or reproduce. The organisms can thus be affected in two different ways: by microbicidal action or by microbiostatic action. Microbicidal action simply kills the microorganism whereas microbiostatic action reversibly inhibits growth. If the antimicrobial agent is microbiostatic, once it is removed the microorganism will usually recover and grow again normally. Factors that can influence the effectiveness of either type of antimicrobial action include the concentration of the agent as well as the type and nature of the target organisms. An agent that may be microbicidal at a certain concentration may only be microbiostatic at lower levels and one that is lethal to one type of organism may only inhibit another (see Dose Effects in Chapter 21, Pharmacology). The effectiveness of microbiostatic agents may also be dependent on external factors such as the host’s own immune system. Because this agent does not directly destroy the target pathogen, the infection must ultimately be eliminated by the host’s own defensive mechanisms.
1. Cell wall synthesis inhibition
2. Protein synthesis inhibition
Inhibition of Cell Wall Synthesis
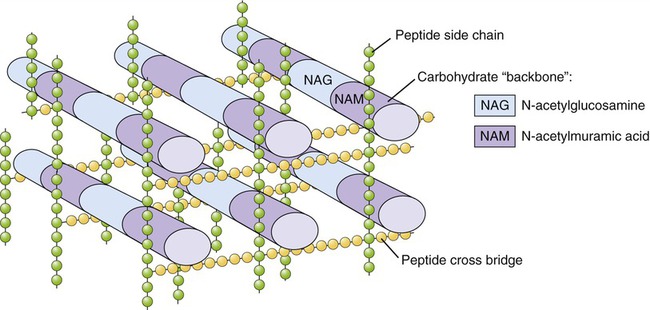
As discussed in Chapter 3 (Cell Structure and Function) and Chapter 6 (Bacteria and Archaea), most bacterial cells contain a rigid cell wall composed of peptidoglycan, which protects the cell from rupturing in hypotonic environments. Young, growing cells must constantly synthesize new peptidoglycan and transport it to the wall (Figure 22.1). Agents such as penicillins and cephalosporins chemically react with the enzymes responsible for completing the peptidoglycan layer, thus preventing the assembly process. This disruption may occur at numerous different sites in the layer and at different points in the process, but in all cases it causes weaknesses at growth points in the layer, which in turn renders the cell vulnerable to lysis. In a hypotonic environment, water then enters the cell and eventually causes it to burst.
Inhibition of Protein Synthesis
Most of the agents that inhibit protein synthesis involve disrupting the process of translation (see Chapter 3, Cell Structure and Function) at the ribosome–mRNA complex (Figure 22.2). The ribosomes of eukaryotic cells are structurally different from those of prokaryotic cells, and because of this human cells are usually not affected by the inhibitory action of these agents because they selectively act against bacteria. The specific chemical reactions that disrupt the translation of the mRNA vary and include the following:
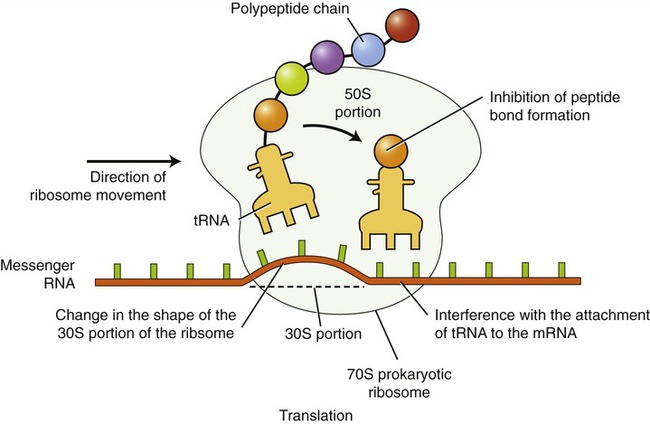
The inhibition of translation can occur at a number of points, including the following: inhibition of peptide bond formation, interference with the attachment of transfer RNA (tRNA) to mRNA, and changing of the shape of the ribosome, thereby preventing translocation along the mRNA strand.
Inhibition of Nucleic Acid Synthesis
The metabolic process involved in the synthesis of DNA and RNA is a long and complex series of enzyme-catalyzed reactions (see Chapter 3, Cell Structure and Function). The complexity of the process lends itself to disruption at many points along the way and inhibition at any point can block subsequent events in the pathway. As with the other mechanisms the specific target within the process is often dependent on the specific antimicrobial agent being used, but the end result is the same no matter where the point of attack is—disruption of the synthesis of nucleic acids. The interference with nucleic acid synthesis by an antimicrobial drug can be due to:
Characteristics of Antimicrobial Agents
• Whether it is microbicidal rather than microbiostatic
• How readily and easily the agent may be delivered to the site of infection
• Whether it is readily soluble in body fluids and will maintain its potency long enough to be effective
• How long the agent remains active in the body
• The degree of antimicrobial resistance to the agent
• Whether it complements or aids in host body defenses
• Does it have a long shelf life?
• Is it affordable/available to all patients who might need it?
Spectrum of Action
Most antimicrobial drugs do not inhibit the growth of all pathogens. Antimicrobial drugs have a spectrum of activity, which is the range of pathogen types against which a given drug is effective (Table 22.1). On the basis of the spectrum of activity, drugs can be classified as antimicrobials with a broad spectrum of activity and those that have a narrow spectrum of activity.
TABLE 22.1
Spectrum of Activity of Some Antimicrobial Drugs
| Drug | Activity against: |
| Penicillin G | Gram-positive bacteria |
| Streptomycin | Gram-negative bacteria, mycobacteria |
| Tetracycline | Gram-negative bacteria, gram-positive bacteria, chlamydias, rickettsias |
| Isoniazid | Mycobacteria |
| Acyclovir | Viruses |
| Ketoconazole | Fungi |
| Mefloquine (malaria) | Protozoans |
| Niclosamide (tapeworms) | Helminths |
| Biltricide (flukes) | Helminths |
• Drugs with a broad spectrum of activity are effective against a large variety of microorganisms. An example would be the sulfonamides, which are effective against both gram-positive and gram-negative bacteria, actinomycetes, chlamydias, and some protozoans. These drugs exert their effects on all cell components of the microbes. An advantage of using broad-spectrum antibiotics is the high possibility of efficacy against an unidentified pathogen. The disadvantage of using such a drug is the likelihood of also destroying the normal flora (see Chapter 9, Infection and Disease). Destruction of the normal flora can lead to disease if one organism of the normal flora is replaced by a pathogen. This then leads to what is referred to as a superinfection.
• Drugs with a narrow spectrum of activity, such as isoniazid and penicillin G, are effective only against a relatively small number of microbes, and generally avoid destruction of the normal flora. They target a specific component that is present only in certain bacteria.
• Drugs with a medium spectrum of activity are effective against some gram-positive and gram-negative bacteria, but not all of them.
Selective Toxicity
An ideal antimicrobial drug will kill harmful microorganisms without significant damage to the host. This principle is referred to as selective toxicity. The differences in structure and/or metabolism between the microbe and the host make selective toxicity possible. With greater differences between the pathogen and the host it is easier to develop an effective antimicrobial drug. Regarding toxicity, it is preferable to have a wide range between the toxic dosage level, which can cause damage to the host, and the therapeutic dosage level, which will eliminate the infectious pathogen over a prescribed period of time (see Chapter 21, Pharmacology).
Delivery to the Site of Infection
To be effective, drugs need to be easily and effectively delivered to the site of infection. Therefore, an important criterion for the effectiveness (efficacy) of a drug is its ability to cross biological barriers (see Chapter 21, Pharmacology). Potential barriers may be physiological, such as the lining of the body compartments and the blood–brain barrier, or those induced by inflammation and disease. Abscess walls, exudates, and necrotic material are classic examples of host reactions to pathogens and can impede the transport of drugs to the invading microorganisms.
Time of Activity
It is desirable for an antimicrobial drug to be active over a long period of time for an optimal effect. As covered in Chapter 21 (Pharmacology), the duration of action time is a major consideration when evaluating the overall effect of a drug on an infection/pathogen. If the destruction of a population of pathogens requires a sustained concentration of agent over a specified period of time, any shortening of this time may significantly decrease the effectiveness of the treatment. By subjecting the organisms to the agent for a time that proves to be nonlethal to many of them, the risk of producing a resistant population is increased.
Not Subject to Antimicrobial Resistance
The ideal antimicrobial drug is an agent that is not subject to the development of antimicrobial resistance. If the nature of the agent and its mechanism is such that populations of pathogens are less likely to develop resistance to the agent, its effectiveness and scope of use would be dramatically increased. Because of microbial genetic mutations as well as the acquisition of resistance genes from other cells, viruses, and the environment; it is difficult to develop an antibiotic against which microbes will not eventually develop some resistance (see Resistance to Antimicrobial Drugs).
Complements/Aids in Host’s Own Defenses
The natural defenses of a host, including barriers at portals of entry, protective cells and environments within the host, and an acquired immune system (both active and passive), do an excellent job in fighting off infections in the healthy host (see Chapter 20, The Immune System). When these layers of defense are compromised, antimicrobial drugs need to be administered in order to aid the immune system. Any time a foreign substance is introduced into a host there is a risk of upsetting this complex balance of defenses and actually inhibiting the body’s natural response to pathogens. For example, if an antibiotic harms an organ or system it may adversely affect the natural defenses of the host, causing such things as excessive damage to the kidneys.
Nonallergenic
Certain antimicrobial agents are capable of inducing a hypersensitivity reaction (Chapter 20, The Immune System) in certain individuals. Patients with allergies to specific agents such as penicillin must be careful to inform medical personnel about their allergy, or the shock that may result from receiving penicillin could be fatal.
Determination of Antibiotic Effectiveness: Efficacy
• The disk diffusion or Kirby-Bauer method
• The dilution or minimal inhibitory concentration (MIC) method
Disk Diffusion or Kirby-Bauer Method
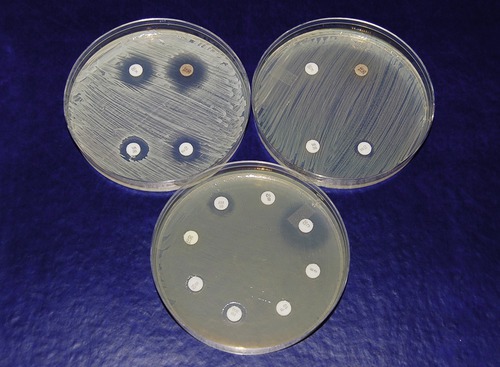
In the disk diffusion or Kirby-Bauer method a culture of the pathogen is uniformly spread over the entire surface of an agar plate, using the spread plate method, or in a modified streak pattern, using a sterile swab to literally paint the surface of the plate. Paper filter disks impregnated with specific concentrations of selected antibiotics are then evenly spaced on the agar surface. The inoculated plate with disks is then incubated. As they are incubated the antibiotic in each disk begins to diffuse into the agar. If the antibiotic affects the growth of the organism that has been spread on the surface this will be evident as a clear area of no growth surrounding the disk (Figure 22.3). This area is called a zone of inhibition because it delineates the area where the diffused antibiotic inhibited growth of the organism. Because larger molecules diffuse into the agar at a slower rate than smaller molecules, the size of the zone of inhibition is not necessarily a measure of effectiveness. Standard zone diameters have been established for each concentration of antibiotic, as well as approximate number of cells plated on the specific medium being used. The diameter of each individual zone can then be measured and evaluated in order to determine whether the organism is sensitive or resistant to the antibiotic.
Dilution/Minimal Inhibitory Concentration Method
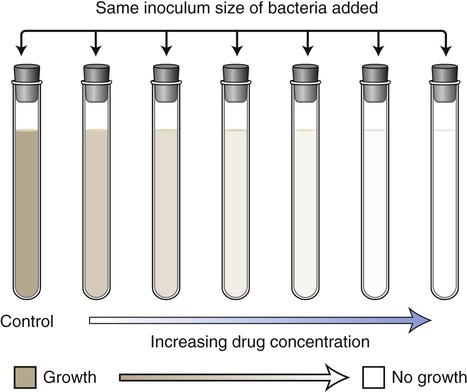
In the dilution/minimal inhibitory concentration method a given quantity of the pathogen culture is added to a series of tubes containing decreasing concentrations of a specific antibiotic. The tubes are then incubated and examined for growth. Turbidity (cloudiness) indicates bacterial growth; lack of turbidity signifies that bacterial growth is either inhibited or the organisms have been killed by the antimicrobial agent (Figure 22.4). The goal is to identify the tube with the lowest concentration of drug that inhibits visible growth. This concentration is referred to as the minimal inhibitory concentration (MIC) for a specific agent acting on a selected microorganism. In the past this test was performed in individual test tubes. However, kits are now available wherein the test is performed in shallow wells on standard testing plates. This test is widely used in hospitals and clinics today; however, there are also shortcomings with this method. As with the Kirby-Bauer method, the basic test procedure will not indicate whether an antibiotic is microbicidal or microbiostatic. A second test using this method can be added to determine whether the organisms are being inhibited or killed. In this test a sample from the tube with the lowest concentration showing no growth is subcultured in order to see whether there are any organisms still surviving in the tube. This lowest concentration containing no living organisms is called the minimal bactericidal concentration (MBC).
Side Effects
All drugs are potential poisons (see Chapter 21, Pharmacology) and the administration of therapeutic drugs can possibly lead to side effects that can be potentially serious. Drugs can adversely affect the liver (hepatotoxic), kidneys (nephrotoxic), gastrointestinal tract, cardiovascular system, red bone marrow (hemotoxic), nervous system (neurotoxic), respiratory system, integument, bones, and teeth. The administration of drugs involves a previous assessment of the risks and benefits. This type of assessment is referred to as the therapeutic index (see Chapter 21).
Another example of an unwanted side effect is when an individual develops a hypersensitivity (allergic) reaction. Allergic reactions have been reported for most antimicrobial drugs, with penicillin accounting for the majority of antimicrobial drug–induced allergies, followed by sulfonamides. Individuals allergic to these drugs become sensitized during the first exposure, not showing any symptoms. After sensitizing the immune system, a second exposure will result in hives, respiratory problems, and occasionally anaphylaxis that may become fatal (see Hypersensitivity Reactions in Chapter 20, The Immune System). For pregnant women the U.S. Food and Drug Administration (FDA) has identified and classified antibiotics that present no evidence of risk to the fetus.
Resistance to Antimicrobial Drugs
One of the major challenges in healthcare today is the problem caused by bacteria that are already resistant to antimicrobial drugs as well as those that are acquiring resistance at an alarming rate. The evolution of bacteria toward antibiotic resistance, including multidrug resistance, represents an aspect of the general evolution of bacteria and most likely cannot be stopped. Bacterial drug resistance goes beyond the traditional evolutionary mechanisms, because bacteria can pass on drug resistance without having to inherit it. This process occurs via plasmids, the nonchromosomal DNA of bacteria (see Genetics in Chapter 6, Bacteria and Archaea). Furthermore, these plasmids can also be transferred to different species of microorganisms.
Development/Acquisition of Drug Resistance
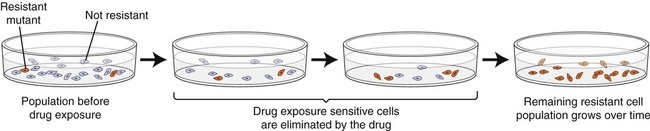
Any large population of microorganisms, especially bacteria, contains a few cells that are already resistant due to prior transfer of plasmids or by mutation. When the drug is not present in the environment the resistant forms will remain small in numbers, but when the drug is administered, the nonresistant organisms will die and the resistant ones will flourish and multiply (Figure 22.5). This natural selection of drug-resistant forms is common and takes place in a variety of natural habitats, laboratories, and medical environments, as well as in individuals during drug therapy.
Mechanisms of Resistance
Once a mutation occurs in bacteria that results in a resistance gene or genes, there are a number of natural mechanisms that can allow the resistance genes to be acquired by nonresistant organisms. The three mechanisms are transduction, transformation, and conjugation (see Chapter 3, Cell Structure and Function). Some cells contain resistance genes on a plasmid called a resistance or R plasmid and may contain as many as six or seven genes, each of which may confer resistance against a number of different antibiotics. Even plasmids can be transferred from one strain or species to another, widening the distribution of the resistance genes even further. These resistance genes confer a variety of different mechanisms of resistance to the microorganisms that possess them.
• Change in membrane permeability: Changes in the proteins of the plasma membrane interfere with the transport mechanisms and this may prevent some antibiotics from crossing the membrane to enter the cell.
• Increased drug elimination: Resistance to tetracycline can arise through plasmid-encoded proteins that pump the drug out of the cell. Aminoglycoside resistance develops through changes in drug permeability caused by point mutation, affecting proteins in the membrane transport system. Many bacteria have multidrug-resistant pumps capable of actively transporting chemicals, including drugs, out of the cell. These protein pumps are encoded in plasmids or chromosomes; they are not selective and are capable of removing a variety of antimicrobial drugs, detergents, and other substances toxic to the microbe.
• Change in the target (receptor) of the agent: The majority of antimicrobial drugs act on a specific target (receptor) such as a protein, RNA, DNA, or a structure within the plasma membranes. Microbes can avoid attack by antimicrobials by altering the drug target. In other words a change in the DNA can occur and a protein important to the functioning of the target site is altered and antibiotics can no longer bind to and disrupt this target protein. For example, bacteria can become resistant to aminoglycosides by changing ribosomal binding sites. Alterations of binding sites in the cell wall of a bacterium can result in resistance to penicillin by Streptococcus pneumoniae and methicillin by Staphylococcus aureus. Some enterococci have developed resistance to vancomycin by a similar mechanism.
• Change in a metabolic pathway: Microbes can develop an alternative metabolic pathway or enzyme that allows the bypassing of a metabolic reaction that could be inhibited by an antibiotic.
• Change in a previously inhibited enzyme: Modifying an enzyme that was previously being inhibited may prevent a reaction or series of reactions in a bacterial cell. With modification of the enzyme this inhibition by the antibiotic may be prevented and the reactions may occur unhampered.
• Development of defensive enzymes: Bacterial enzymes may be developed that are capable of destroying or inactivating antibiotics before they can act on the bacterial cell. A prominent example of this mechanism is the production of β-lactamase, that is, penicillinase, by some bacteria, which breaks the β-lactam ring in penicillin and destroys its effectiveness. Another β-lactamase, cephaloporinase, disrupts the structure of cephalosporin molecules.
Preventing Drug Resistance
• Healthcare professionals and providers should wash their hands thoroughly between patient visits.
• Physicians should not grant patients’ demands for antibiotics when not warranted for the particular infection. Consumers should not demand antibiotics from a physician because the infection may be other than bacterial (e.g., common viral infections).
• Antibiotics when prescribed should target a narrow range of microbes whenever possible.
• Antibiotics may be used in combination in order to kill pathogens that are resistant to one antimicrobial but will be killed by the other (see synergism, discussed previously).
• Hospital and nursing home patients with multidrug-resistant infections should be isolated.
• Physicians should be familiar with local data on antibiotic resistance.
• Patients, when given antibiotics, should take them exactly as prescribed and complete the full course of the treatment to avoid the development of resistant strains.
• Antimicrobials should not be hoarded for later use. Leftover medication should be discarded after completion of the prescribed course of treatment.
• Individuals should not take antimicrobials that have been prescribed to someone else. This may lead to a delay in the correct treatment and also allow bacteria to multiply.
• Antimicrobial soaps and lotions should be used only when protecting a sick individual with a weakened immune system. This will:
• Prevent disruption of the normal flora
• Avoid selecting for resistance
• Prevent the inadvertent selection of naturally resistant bacterial types, which may become emergent pathogens. The human immune system is generally effective at controlling the majority of microbes dominating the environment. The overuse of antimicrobial soaps/lotions may change the composition of microbes in the environment, allowing resistant strains to become dominant.
Specific Antimicrobial Drugs
A great number of antimicrobial drugs are marketed in the United States. Drug companies market the drugs through physicians, and lately also directly to the consumer via television and Internet advertisements. Although a wide variety of commercial names are used, most of them are variants of a small number of drug families. However, different drug companies assign different trade names to the same generic drug (see Chapter 21, Pharmacology). Antimicrobials can be classified as antibacterial, antiviral, antifungal, antiprotozoan, and antihelminthic agents.
Antibacterial Agents
• A natural antibiotic is a chemical substance that is produced by a microorganism and has the capacity to either destroy bacteria or inhibit their growth.
• A semisynthetic antibiotic drug is an antibiotic that has been chemically modified for greater efficacy.
• Synthetic drugs are agents produced in the laboratory rather than by a microorganism, but can act identically to antibiotics. Sulfonamides, trimethoprim, and quinolones are man-made drugs and therefore are not considered to be antibiotics, but synthetic antibacterial agents.
Synthetic Drugs
Sulfonamides, or sulfa drugs were the first antimicrobial agents that used the metabolic disruption mechanism successfully against bacteria. Since its discovery by Domagk (see “Why You Need to Know”) many sulfonamides have been developed including sulfadoxine, sulfadimidine (short acting), sulfamethoxazole (intermediate acting), sulfametopyrazine (long acting), sulfasalazine (poor absorption by the gastrointestinal tract), and sulfamethoxazole in combination with trimethoprim. This group of antibacterial agents is manufactured synthetically The mechanism of action is to compete with a metabolic intermediate in the folic acid synthesis pathway in bacteria. Folic acid is essential to the synthesis of purines and pyrimidines, which are the bases used in the construction of nucleic acids and other cellular components in both bacteria and mammals (see Chapter 2 [Chemistry of Life] and Chapter 3 [Cell Structure and Function]). Sulfonamides are selectively toxic to bacteria, which must synthesize folic acid because they are unable to obtain it from the environment. In contrast, humans acquire folic acid from dietary sources; therefore the disruption of the folic acid synthesis pathway will not affect the host. The effectiveness of sulfonamides has been seeing some decline as more bacteria have been developing resistance. Mild to moderate side effects include nausea, vomiting, headache, and mental depression. Its use is also limited because of some allergenic reactions, which are experienced by about 5% of patients. These side effects may include rashes, fever, or hives.
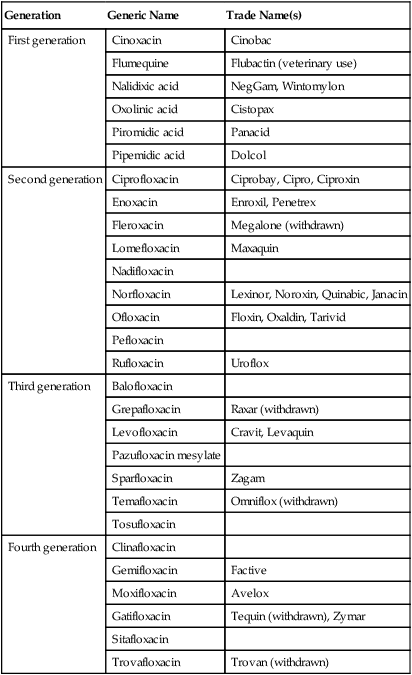
Quinolones, also referred to as fluoroquinolones, are broad-spectrum synthetic drugs used to treat a wide variety of bacterial infections. Nalidixic acid was the first quinolone developed but a family of these drugs is being developed, such as ciprofloxacin and doxycycline, which have gained fame as the drugs of choice for treating anthrax. Quinolones are effective against both gram-negative and gram-positive bacteria and are used in treating urinary tract infections, sexually transmitted diseases, gastrointestinal infections, respiratory tract infections, skin infections, and osteomyelitis. The mechanism used by these agents is the disruption of nucleic acid synthesis through binding to the DNA gyrase complex. This enzyme complex is responsible for the unwinding of DNA for replication, repair, transcription, and other DNA cell processes. Quinolones are divided into generations based on their spectrum of activity. In general, the earlier generations have a narrower spectrum of activity than the later generations (Table 22.2). Some side effects may be experienced including gastrointestinal problems and, in some rare cases, seizures or other neural disturbances.
TABLE 22.2
Generations of Quinolones Based on Their Spectrum of Activity
| Generation | Generic Name | Trade Name(s) |
| First generation | Cinoxacin | Cinobac |
| Flumequine | Flubactin (veterinary use) | |
| Nalidixic acid | NegGam, Wintomylon | |
| Oxolinic acid | Cistopax | |
| Piromidic acid | Panacid | |
| Pipemidic acid | Dolcol | |
| Second generation | Ciprofloxacin | Ciprobay, Cipro, Ciproxin |
| Enoxacin | Enroxil, Penetrex | |
| Fleroxacin | Megalone (withdrawn) | |
| Lomefloxacin | Maxaquin | |
| Nadifloxacin | ||
| Norfloxacin | Lexinor, Noroxin, Quinabic, Janacin | |
| Ofloxacin | Floxin, Oxaldin, Tarivid | |
| Pefloxacin | ||
| Rufloxacin | Uroflox | |
| Third generation | Balofloxacin | |
| Grepafloxacin | Raxar (withdrawn) | |
| Levofloxacin | Cravit, Levaquin | |
| Pazufloxacin mesylate | ||
| Sparfloxacin | Zagam | |
| Temafloxacin | Omniflox (withdrawn) | |
| Tosufloxacin | ||
| Fourth generation | Clinafloxacin | |
| Gemifloxacin | Factive | |
| Moxifloxacin | Avelox | |
| Gatifloxacin | Tequin (withdrawn), Zymar | |
| Sitafloxacin | ||
| Trovafloxacin | Trovan (withdrawn) |
Antibiotic (Nonsynthetic) and Semisynthetic Antibacterials
Cephalosporins are a class of β-lactam antibiotics that are structurally similar to penicillins because of the presence of similar β-lactam rings. They also resemble the penicillins because they also inhibit the successful assembly of peptidoglycan in the cell wall. They tend to be more stable than penicillins and have a broader spectrum of activity. Furthermore, cephalosporins are often given to treat infections in patients with penicillin allergies. Another advantage over penicillin is that they resist hydrolysis by penicillinase, an enzyme secreted by a number of bacteria. Four generations of cephalosporins exist and each generation is more effective against gram-negative bacteria (Table 22.3). In general, each generation has a broader spectrum of activity than the previous one, and typically have improved dosing schedules and fewer side effects.
TABLE 22.3
Generations of Cephalosporins Based on Their Spectrum of Activity
| Generation | Generic Name | Trade Name(s) |
| First generation | Cephacetrile | Cephacetrile |
| Cefadroxil | Cefadroxil; Duricef | |
| Cephaloglycin | Kafocin | |
| Cefalonium | Cephalonium | |
| Cephaloridine | Cephaloradine | |
| Cephalothin | Cephalothin; Keflin | |
| Cephapirin | Cephapirin; Cefadyl | |
| Cefatrizine | Axelorax | |
| Cefazolin | Cephazolin; Ancef, Kefzol | |
| Cephradine | Cephradine; Velosef | |
| Cefroxadine | ||
| Ceftezole | ||
| Second generation | Cefaclor | Ceclor, Distaclor, Keflor, Raniclor |
| Cefonicid | Monocid | |
| Cefprozil | Cefprozil; Cefzil | |
| Cefuroxime | Zinnat, Zinacef, Ceftin, Biofuroksym | |
| Cefmetazole | ||
| Cefotetan | ||
| Cefoxitin | ||
| Third generation | Cefcapene | |
| Cefdaloxime | ||
| Cefdinir | Omnicef | |
| Cefditoren | ||
| Cefetamet | ||
| Cefixime | Suprax | |
| Cefmenoxime | ||
| Cefodizime | ||
| Cefoperazone | Cefobid | |
| Cefotaxime | Claforan | |
| Cefpimizole | ||
| Cefpodoxime | Vantin | |
| Cefteram | ||
| Ceftibuten | Cedax | |
| Ceftiofur | ||
| Ceftiolene | ||
| Ceftizoxime | Cefizax | |
| Ceftriaxone | Rocephin | |
| Fourth generation | Cefclidine | |
| Cefepime | Maxipime | |
| Cefluprenam | ||
| Cefoselis | ||
| Cefozopran | ||
| Cefpirome | ||
| Cefquinome |
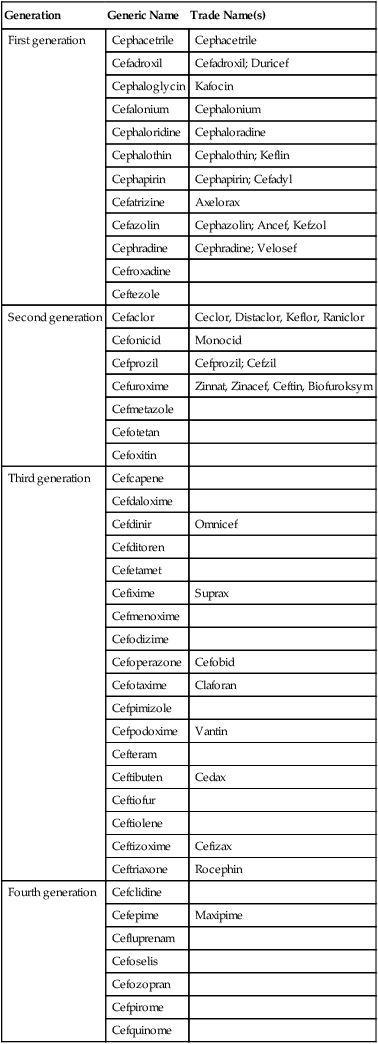
• First-generation drugs have proven to be more effective against gram-positive than gram-negative bacteria.
• Second-generation drugs are effective against gram-positive as well as many gram-negative organisms.
• The third-generation drugs are particularly effective against gram-negative bacteria and are capable of crossing the blood–brain barrier to treat infections in the central nervous system. Furthermore, this generation shows activity against enteric bacteria that produce β-lactamases.
• Fourth-generation cephalosporins have the widest range of activity, are also effective with both gram-positive and gram-negative bacteria, and are rapidly microbicidal.
HEALTHCARE APPLICATION
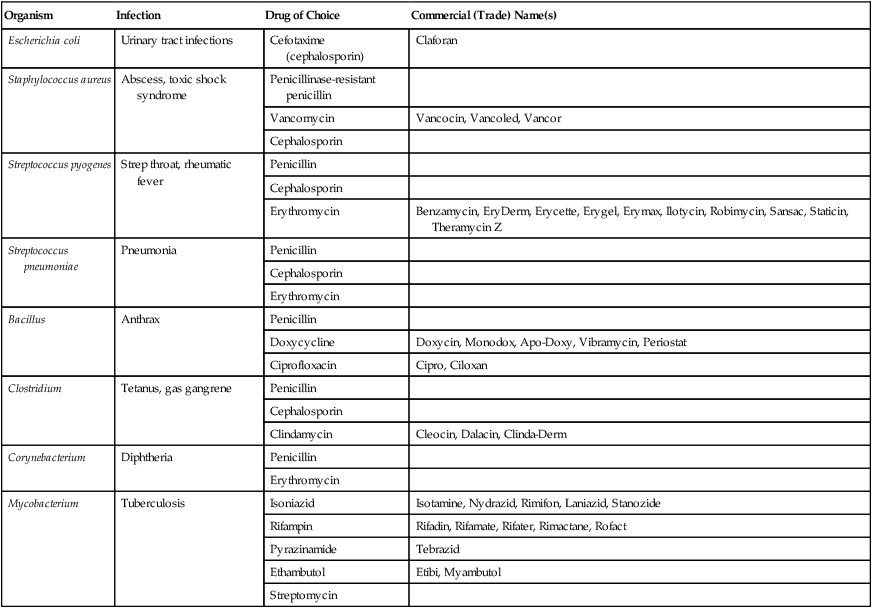
Antibacterial Agents
| Organism | Infection | Drug of Choice | Commercial (Trade) Name(s) |
| Escherichia coli | Urinary tract infections | Cefotaxime (cephalosporin) | Claforan |
| Staphylococcus aureus | Abscess, toxic shock syndrome | Penicillinase-resistant penicillin | |
| Vancomycin | Vancocin, Vancoled, Vancor | ||
| Cephalosporin | |||
| Streptococcus pyogenes | Strep throat, rheumatic fever | Penicillin | |
| Cephalosporin | |||
| Erythromycin | Benzamycin, EryDerm, Erycette, Erygel, Erymax, Ilotycin, Robimycin, Sansac, Staticin, Theramycin Z | ||
| Streptococcus pneumoniae | Pneumonia | Penicillin | |
| Cephalosporin | |||
| Erythromycin | |||
| Bacillus | Anthrax | Penicillin | |
| Doxycycline | Doxycin, Monodox, Apo-Doxy, Vibramycin, Periostat | ||
| Ciprofloxacin | Cipro, Ciloxan | ||
| Clostridium | Tetanus, gas gangrene | Penicillin | |
| Cephalosporin | |||
| Clindamycin | Cleocin, Dalacin, Clinda-Derm | ||
| Corynebacterium | Diphtheria | Penicillin | |
| Erythromycin | |||
| Mycobacterium | Tuberculosis | Isoniazid | Isotamine, Nydrazid, Rimifon, Laniazid, Stanozide |
| Rifampin | Rifadin, Rifamate, Rifater, Rimactane, Rofact | ||
| Pyrazinamide | Tebrazid | ||
| Ethambutol | Etibi, Myambutol | ||
| Streptomycin |
Antifungal Agents
Echinocandins
Echinocandins are made of a ring of six amino acids, linked to a lipophilic side chain.
HEALTHCARE APPLICATION
Antifungal Drugs
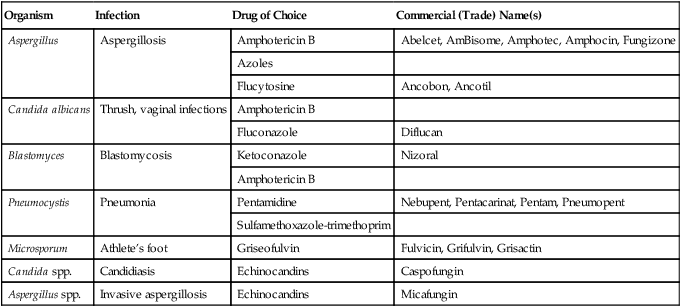
| Organism | Infection | Drug of Choice | Commercial (Trade) Name(s) |
| Aspergillus | Aspergillosis | Amphotericin B | Abelcet, AmBisome, Amphotec, Amphocin, Fungizone |
| Azoles | |||
| Flucytosine | Ancobon, Ancotil | ||
| Candida albicans | Thrush, vaginal infections | Amphotericin B | |
| Fluconazole | Diflucan | ||
| Blastomyces | Blastomycosis | Ketoconazole | Nizoral |
| Amphotericin B | |||
| Pneumocystis | Pneumonia | Pentamidine | Nebupent, Pentacarinat, Pentam, Pneumopent |
| Sulfamethoxazole-trimethoprim | |||
| Microsporum | Athlete’s foot | Griseofulvin | Fulvicin, Grifulvin, Grisactin |
| Candida spp. | Candidiasis | Echinocandins | Caspofungin |
| Aspergillus spp. | Invasive aspergillosis | Echinocandins | Micafungin |
Antiprotozoan Agents
Quinine
HEALTHCARE APPLICATION
Antiprotozoan Drugs
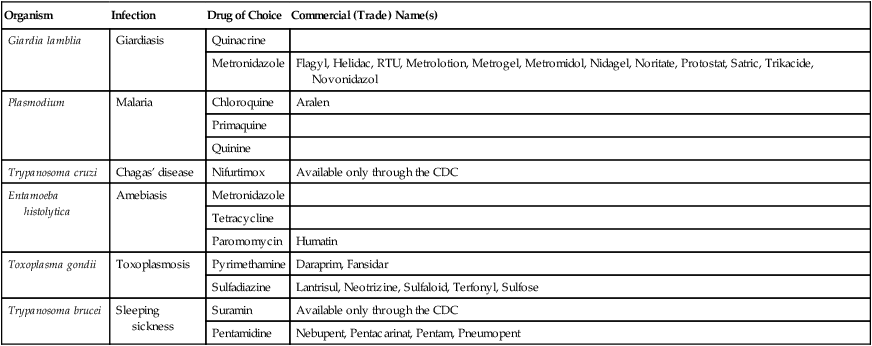
| Organism | Infection | Drug of Choice | Commercial (Trade) Name(s) |
| Giardia lamblia | Giardiasis | Quinacrine | |
| Metronidazole | Flagyl, Helidac, RTU, Metrolotion, Metrogel, Metromidol, Nidagel, Noritate, Protostat, Satric, Trikacide, Novonidazol | ||
| Plasmodium | Malaria | Chloroquine | Aralen |
| Primaquine | |||
| Quinine | |||
| Trypanosoma cruzi | Chagas’ disease | Nifurtimox | Available only through the CDC |
| Entamoeba histolytica | Amebiasis | Metronidazole | |
| Tetracycline | |||
| Paromomycin | Humatin | ||
| Toxoplasma gondii | Toxoplasmosis | Pyrimethamine | Daraprim, Fansidar |
| Sulfadiazine | Lantrisul, Neotrizine, Sulfaloid, Terfonyl, Sulfose | ||
| Trypanosoma brucei | Sleeping sickness | Suramin | Available only through the CDC |
| Pentamidine | Nebupent, Pentacarinat, Pentam, Pneumopent |
Antihelminthic Agents
Ivermectin
HEALTHCARE APPLICATION
Antihelminthic Drugs
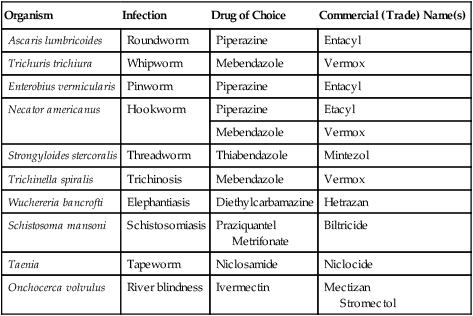
| Organism | Infection | Drug of Choice | Commercial (Trade) Name(s) |
| Ascaris lumbricoides | Roundworm | Piperazine | Entacyl |
| Trichuris trichiura | Whipworm | Mebendazole | Vermox |
| Enterobius vermicularis | Pinworm | Piperazine | Entacyl |
| Necator americanus | Hookworm | Piperazine | Etacyl |
| Mebendazole | Vermox | ||
| Strongyloides stercoralis | Threadworm | Thiabendazole | Mintezol |
| Trichinella spiralis | Trichinosis | Mebendazole | Vermox |
| Wuchereria bancrofti | Elephantiasis | Diethylcarbamazine | Hetrazan |
| Schistosoma mansoni | Schistosomiasis | Praziquantel Metrifonate |
Biltricide |
| Taenia | Tapeworm | Niclosamide | Niclocide |
| Onchocerca volvulus | River blindness | Ivermectin | Mectizan Stromectol |
Summary
• The goal of antimicrobial agents is to disrupt the metabolism or structure of a pathogen so that it can no longer survive or reproduce.
• Antimicrobial drugs may inhibit cell wall synthesis, protein synthesis, or nucleic acid synthesis, or disrupt the cell membrane and also the metabolism of the microorganism.
• Antimicrobial drugs have a spectrum of activity indicating the range of pathogens against which a given drug is effective. On the basis of their action, antimicrobials are classified as having a broad, medium, or narrow spectrum of activity.
• Several factors need to be considered before selecting an antimicrobial drug. These include selective toxicity, microbicidal or microbiostatic action, delivery to the site of action, time of activity, antimicrobial resistance, host defense, allergies, shelf life, availability, and affordability.
• A number of tests are currently available to determine the most effective antimicrobial against a specific organism including the Kirby-Bauer method, the minimal inhibitory concentration method, and the serum killing power method.
• The administration of drugs can lead to side effects, and an assessment of risks versus benefits needs to be made.
• A challenge to healthcare is the resistance of microbes to antimicrobial drugs.
• The mechanisms of resistance vary and include change in membrane permeability, increased drug elimination, change in the receptor for the agent, change in the metabolic pathways, change in enzymes, and the development of defensive enzymes.
• Antimicrobial resistance is a global problem, and unfortunately multiresistant strains have developed worldwide. The primary sites for multiresistant strain development seem to be hospitals, nursing homes, and other healthcare facilities.
• The spread and also the prevention of the development of resistant microbial organisms involve the action of physicians as well as individuals treated with antimicrobial drugs.
• Specific antimicrobial drugs are subdivided into antibiotics, semisynthetic drugs, and synthetic drugs. Furthermore, antimicrobials are classified as antibacterial, antiviral, antifungal, antiprotozoan, and antihelminthic agents.
Review Questions
1. All of the following are general metabolic or structural targets for antimicrobial drugs except:
2. Which of the following is not a common characteristic used in the selection of an antimicrobial drug?
3. The term “bacteriostatic” means that bacteria:
4. When two antibiotics are given together to increase the therapeutic effect the phenomenon is referred to as:
5. All of the following are mechanisms of resistance used by microbes except:
6. Which of the following is a synthetic antimicrobial drug?
7. Cephalosporins have __________ generations of developed agents.
8. Which of the following antimicrobials is effective against mycobacteria?
9. Which of the following is an antiviral agent?
10. Which of the following drugs is effective against Candida albicans?
11. A drug that kills pathogenic bacteria is referred to as __________.
12. Resistance to only one type of drug may allow resistance to a similar drug. This process is called __________.
13. The range of pathogen type against which a given drug is effective is referred to as the drug’s __________.
14. The effectiveness of a drug against a microbe is also referred to as the drug’s __________.
15. The zone of inhibition is the result of the __________ method.
16. Describe the five basic mechanisms of action that are targeted by antimicrobial drugs.
17. Name five common characteristics that are considered when selecting an antimicrobial drug.
18. Discuss the three types of activity (spectrum of activity) of antimicrobial drugs.
19. Describe how antibiotic effectiveness (efficacy) can be determined.
20. Discuss the possible side effects that can occur during or after treatment with antimicrobial drugs.