Antigens and Antibodies
At the conclusion of this chapter, the reader should be able to:
• Define the terms antigen and antibody.
• Compare the characteristics of major histocompatibility complex (MHC) classes I and II.
• Name and compare the characteristics of each of the five immunoglobulin classes.
• Draw and describe a typical immunoglobulin G (IgG) molecular structure.
• Name the four phases of an antibody response.
• Describe the characteristics of a primary and secondary (anamnestic) response.
• Compare the terms antibody avidity and antibody affinity.
• Describe the method of production of a monoclonal antibody.
• Analyze a case study related to antigens or antibodies.
• Correctly answer case study related multiple choice questions.
• Be prepared to participate in a discussion of critical thinking questions.
• Describe the principle and agglutination reactions in ABO blood grouping.
• Describe the principle, expected results, reference values, and clinical interpretation of the serum protein electrophoresis procedure.
Antigen Characteristics
General Characteristics of Immunogens and Antigens
Histocompatibility Antigens
Nucleated cells such as leukocytes and tissues possess many cell surface–protein antigens that readily provoke an immune response if transferred into a genetically different (allogenic) individual of the same species. Some of these antigens, which constitute the major histocompatibility complex (MHC) (see Color Plate 2), are more potent than others in provoking an immune response. The MHC is referred to as the human leukocyte antigen (HLA) system in humans because its gene products were originally identified on white blood cells (WBCs, leukocytes). These antigens are second only to the ABO antigens in influencing the survival or graft rejection of transplanted organs. HLAs are the subject of numerous scientific investigations because of the strong association between individual HLAs and immunologic disorders (see Chapter 31 for more discussion of the MHC).
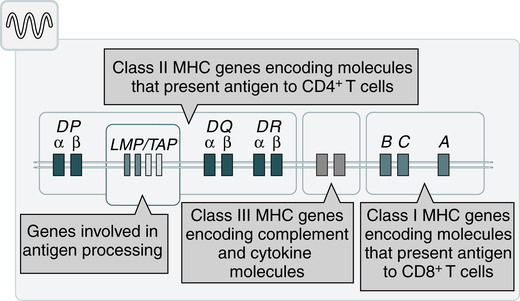
Major Histocompatibility Complex Regions
The MHC is divided into four major regions (Fig. 2-1)—D, B, C, and A. The A, B, and C regions are the classic or class Ia genes that code for class I molecules. The D region codes for class II molecules. Class I includes HLA-A, HLA-B, and HLA-C. The three principal loci (A, B, and C) and their respective antigens are numbered, for example, as 1, 2, 3. The class II gene region antigens are encoded in the HLA-D region and can be subdivided into three families, HLA-DR, HLA-DC (DQ), and HLA-SB (DP).
Classes of HLA Molecules
Structurally, there are two classes of HLA molecules, class I and class II (Table 2-1). Both class I and class II antigens function as targets of T lymphocytes (see Chapter 4 for a further discussion of lymphocytes) that regulate the immune response (Fig. 2-2). Class I molecules regulate interaction between cytolytic T cells and target cells and class II molecules restrict the activity of regulatory T cells. Thus, class II molecules regulate the interaction between helper T cells and antigen-presenting cells (APCs). Cytotoxic T cells directed against class I antigens are inhibited by CD8 cells; cytotoxic T cells directed against class II antigens are inhibited by CD4 cells. Many genes in the class I and class II gene families have no known function.
Table 2-1
Comparison of MHC Class I and Class II
| Class I | Class II | |
| Loci | HLA-A, -B, and -C | HLA-DN, -DO, -DP, -DQ, and -DR |
| Distribution | Most nucleated cells | B lymphocytes, macrophages, other antigen-presenting cells, activated T lymphocytes |
| Function | To present endogenous antigen to cytotoxic T lymphocytes | To present endogenous antigen to helper T lymphocytes |
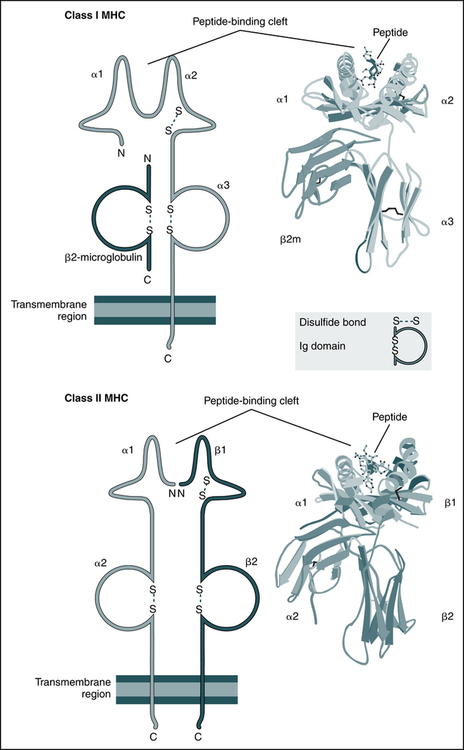
The schematic diagrams (left) and models (right) of the crystal structures of class I and class II MHC molecules illustrate the domains of the molecules and the fundamental similarities between them. Both types of MHC molecules contain peptide-binding clefts and invariant portions that bind CD8 (the α3 domain of class I) or CD4 (the β2 domain of class II). β2m, β2-Microglobulin. (From Abbas AK, Lichtman AH: Basic immunology: functions and disorders of the immune system, updated edition, ed 3, Philadelphia, 2011, Saunders; crystal structures courtesy Dr. P. Bjorkman, California Institute of Technology, Pasadena, Calif.)
Autoantigens
The evolution of a recognition system that can recognize and destroy nonself material must also have safeguards to prevent damage to self antigens. The body’s immune system usually exercises tolerance to self antigens but, in some situations, antibodies may be produced in response to normal self antigens. This failure to recognize self antigens can result in autoantibodies directed at hormones, such as thyroglobulin (see Chapter 28).
Blood Group Antigens
Blood group substances are widely distributed throughout the tissues, blood cells, and body fluids. When foreign RBC antigens are introduced to a host, a transfusion reaction or hemolytic disease of the fetus and newborn can result (see Chapter 26). In addition, certain antigens, especially those of the Rh system, are integral structural components of the erythrocyte (RBC) membrane. If these antigens are missing, the erythrocyte membrane is defective and results in hemolytic anemia. When antigens do not form part of the essential membrane structure (e.g., A, B, and H antigens), the absence of antigen has no effect on membrane integrity.
General Characteristics of Antibodies
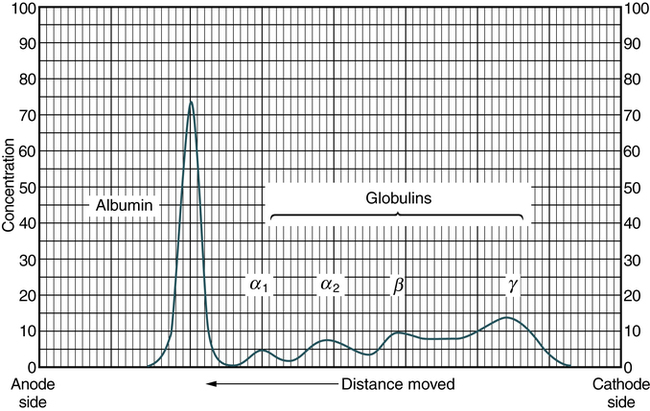
Antibodies are specific proteins referred to as immunoglobulins. Many antibodies can be isolated in the gamma globulin fraction of protein by electrophoresis separation (Fig. 2-3). The term immunoglobulin (Ig) has replaced gamma globulin because not all antibodies have gamma electrophoretic mobility. Antibodies can be found in blood plasma and in many body fluids (e.g., tears, saliva, colostrum).
Immunoglobulin (Ig) Classes
Five distinct classes of immunoglobulin molecules are recognized in most higher mammals—IgM, IgG, IgA, IgD, and IgE. These Ig classes differ from each other in characteristics such as MW and sedimentation coefficients (Table 2-2).
Table 2-2
Characteristics of Immunoglobulin Classes
| IgM | IgG | IgA | IgE | IgD | |
| Molecular weight (daltons, Da) | 900,000 | 160,000 | 360,000 | 200,000 | 160,000 |
| Sedimentation coefficient (Σ) | 19 | 7 | 11 | 8 | 7 |
| Carbohydrate (%) | 12 | 8 | 7 | 12 | 12 |
| Subclasses | — | IgG1-4 | α1, α2 | — | — |
| Serum concentration, adults (mg/mL) | 1.5 | 13.5 | 3.5 | 0.05 | Trace |
| Serum half-life (days)∗ | 5 | 23 | 6 | 2.5 | 3 |
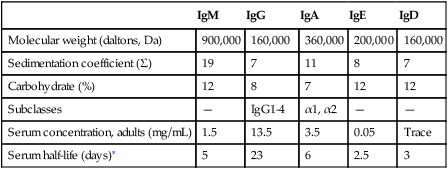
∗Half life (days) = the amount of time to reach ½ activity concentration. Serum values are average concentrations in normal, healthy individuals.
Adapted from Peakman M, Vergani D: Basic and clinical immunology, St Louis, 2009, Elsevier, p 41.
Immunoglobulin M
• Infectious diseases, such as subacute bacterial endocarditis, infectious mononucleosis, leprosy, trypanosomiasis, malaria, and actinomycosis
• Collagen disorders, such as scleroderma
• Hematologic disorders, such as polyclonal gammopathies, monocytic leukemia, and monoclonal gammopathies (e.g., Waldenström’s macroglobulinemia)
Immunoglobulin G
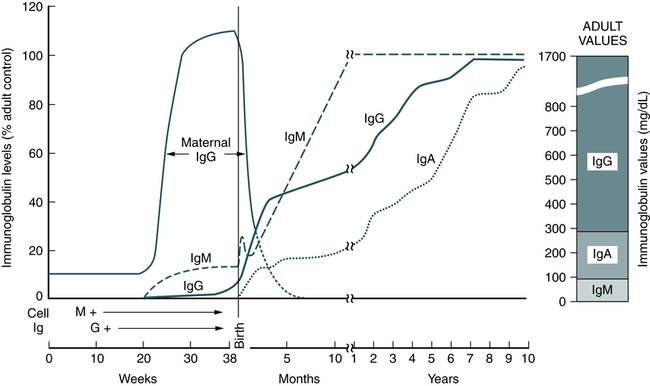
Normal human adult serum values of IgG are 800 to 1800 mg/dL (90 to 210 IU/mL). In infants 3 to 4 months old, the IgG level is approximately 350 to 400 mg/dL (40 to 45 IU/mL), gradually increasing to 700 to 800 mg/dL (80 to 90 IU/mL) by the end of the first year of life (Fig. 2-4). The average adult level is achieved before age 16 years. Other body fluids containing IgG include cord blood (800 to 1800 mg/dL) and CSF (2 to 4 mg/dL).
Immunoglobulin A
• Infectious diseases, such as tuberculosis and actinomycosis
• Collagen disorders, such as rheumatoid arthritis
• Hematologic disorders, such as polyclonal gammopathies, monocytic leukemia, and monoclonal gammopathy (e.g., IgA myeloma)
• Liver disease, such as Laennec’s cirrhosis and chronic active hepatitis
Antibody Structure
Typical Immunoglobulin Molecule
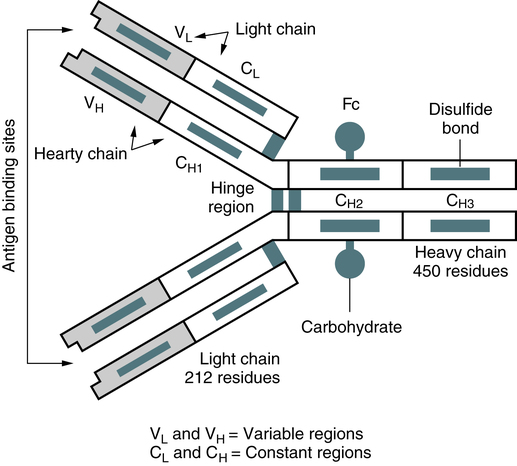
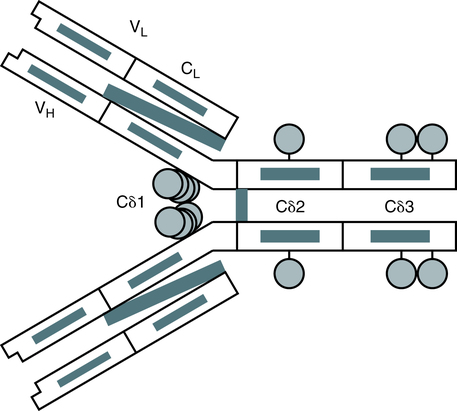
The basic unit of an antibody structure is the homology unit, or domain. A typical molecule has 12 domains, arranged in two heavy (H) and two light (L) chains, linked through cysteine residues by disulfide bonds so that the domains lie in pairs (Fig. 2-5). The antigen-binding portion of the molecule (N-terminal end) shows such heterogeneity that it is known as the variable (V) region; the remainder is composed of relatively constant amino acid sequences, the constant (C) region. Short segments of about 10 amino acid residues within the variable regions of antibodies (or T cell receptor [TCR] proteins) form loop structures called complementary-determining regions (CDRs). Three hypervariable loops, also called CDRs, are present in each antibody H chain and L chain. Most of the variability among different antibodies or TCRs is located within these loops.
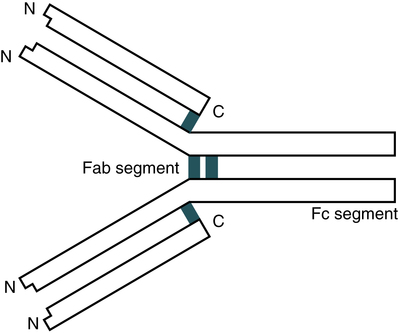
The IgG molecule provides a classic model of antibody structure, appearing Y-shaped under electron microscopy (Fig. 2-6). If the molecule is studies by chemical treatment and the interchain disulfide bonds are broken, the molecule separates into four polypeptide chains. Light chains are small chains (25,000 Da) common to all Ig classes. The L chains are of two subtypes, kappa (κ) and lambda (λ), which have different amino acid sequences and are antigenically different. In humans, about 65% of Ig molecules have κ chains, whereas 35% have λ chains. The larger H chains (50,000 to 77,000 Da) extend the full length of the molecule.
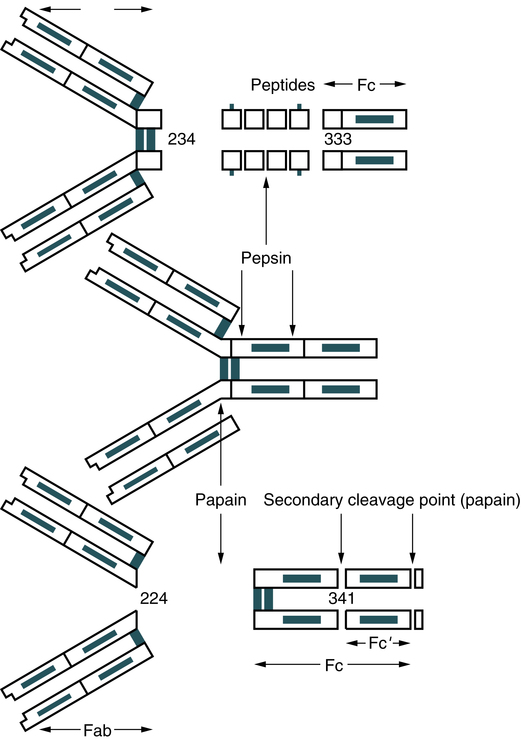
Fab, Fc, and Hinge Molecular Components
A typical monomeric IgG molecule consists of three globular regions (two Fab regions and an Fc portion) linked by a flexible hinge region. If the molecule is digested with a proteolytic enzyme such as papain, it splits into three approximately equal-sized fragments (Fig. 2-7). Two of these fragments retain the ability to bind antigen and are called the antigen-binding fragments (Fab fragments). The third fragment, which is relatively homogeneous and is sometimes crystallizable, is called the Fc portion. If IgG is treated with another proteolytic enzyme, pepsin, the molecule separates somewhat differently. The Fc fragment is split into tiny peptides and thus is completely destroyed. The two Fab fragments remain joined to produce a fragment called F(ab)′2. This fragment possesses two antigen-binding sites. If F(ab)′2 is treated to reduce its disulfide bonds, it breaks into two Fab fragments, each of which has only one antigen-binding site. Further disruption of the interchain disulfide bonds in the Fab fragments shows that each contains a light chain and half of a heavy chain, which is called the Fd fragment.
Structures of Other Immunoglobulins
Immunoglobulin M
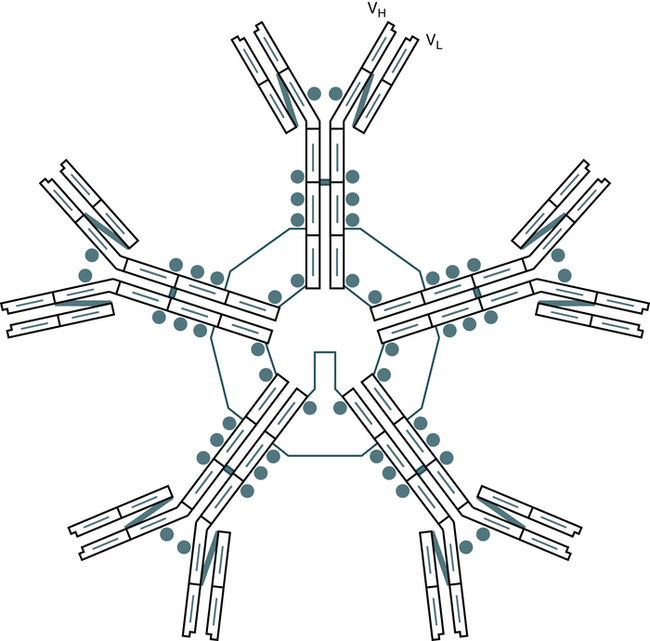
The IgM molecule is structurally composed of five basic subunits. Each subunit consists of two κ or two λ light chains and two mu (µ) heavy chains. The individual monomers of IgM are linked together by disulfide bonds in a circular fashion (Fig. 2-8). A small, cysteine-rich polypeptide, the J chain, must be considered an integral part of the molecule. IgM has carbohydrate residues attached to the CH3 and CH4 domains. The site for complement activation by IgM is located on this CH4 region. IgM is more efficient than IgG in activities such as complement cascade activation and agglutination.
Immunoglobulin A
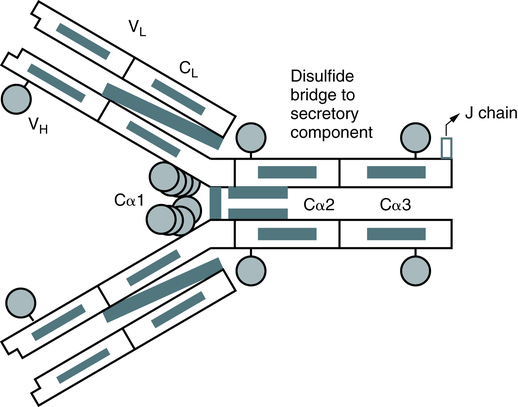
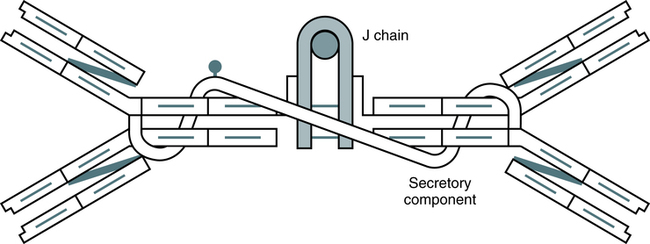
In humans, more than 80% of IgA occurs as a typical four-chain structure consisting of paired κ or λ chains and two heavy chains (Fig. 2-9). The basic four-chain monomer has an MW of 160,000 Da; however, in most mammals, plasma IgA occurs mainly as a dimer. In dimeric IgA, the molecules are joined by a J chain linked to the Fc regions. Secretory IgA exists mainly in the 11S dimeric form and has an MW of 385,000 Da (Fig. 2-10). This form of IgA is present in fluids and is stabilized against proteolysis when combined with another protein, the secretory component. In humans, variations in the heavy chains account for the subclasses IgA1 and IgA2.
Immunoglobulin E
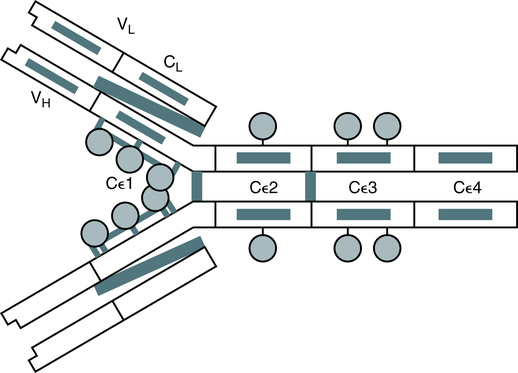
The IgE molecule is composed of paired κ or α light chains and two epsilon (ε) heavy chains (Fig. 2-12). It is unique in that its Fc region binds strongly to a receptor on mast cells and basophils and, together with antigen, mediates the release of histamines and heparin from these cells.
Immunoglobulin Variants
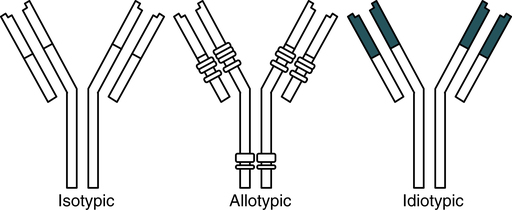
An antigenic determinant is the specific chemical determinant group or molecular configuration against which the immune response is directed. Because they are proteins, immunoglobulins themselves can function as effective antigens when used to immunize mammals of a different species. When the resulting antiimmunoglobulins or antiglobulins are analyzed, three principal categories of antigenic determinants can be recognized—isotype, allotype, and idiotype (Fig. 2-13; Table 2-3).
Table 2-3
| Variant | Distribution | Location | Examples |
| Isotype | All variants in normal persons | CH | IgM, IgE |
| CH | IgA1, IgA2 | ||
| CL | Kappa subtype | ||
| CL | Lambda subtype | ||
| Allotype | Genetically controlled alternate forms; not present in all individuals | Mainly CH/CL Sometimes VH/V2 |
Gm groups in humans |
| Idiotype | Individually specific to each immunoglobulin molecule | Variable regions | Probably one or more hypervariable regions forming the antigen-combining site |
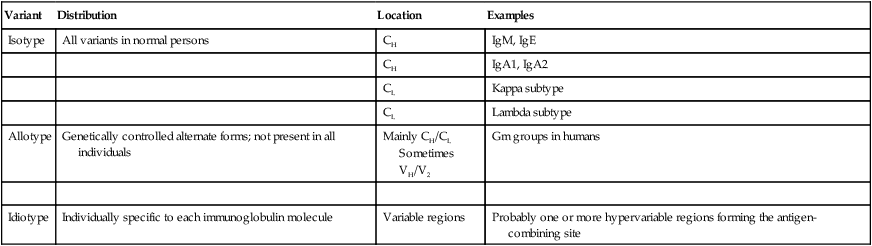
C, Constant regent; Gm, marker on IgG; H, heavy chain; L, light chain; V, variable region.
Antibody Synthesis
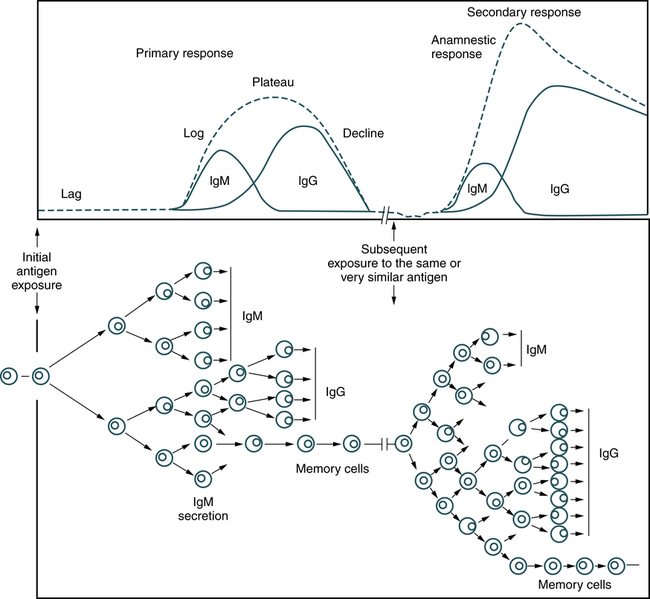
Production of antibodies is induced when the host’s lymphocytes come into contact with a foreign antigenic substance that binds to its receptor. This triggers activation and proliferation, or clonal selection. Clonal expansion of lymphocytes in response to infection is necessary for an effective immune response (Fig. 2-14). However, it requires 3 to 5 days for a sufficient number of clones to be produced and to differentiate into antibody-producing cells. This allows time for most pathogens to damage host tissues and cells.
Secondary (Anamnestic) Response
Subsequent exposure to the same antigenic stimulus produces an antibody response that exhibits the same four phases as the primary response (see Fig. 2-14). Repeated exposure to an antigen can occur many years after the initial exposure, but clones of memory cells will be stimulated to proliferate, with subsequent production of antibody by the individual. An anamnestic response differs from a primary response as follows:
1. Time. A secondary response has a shorter lag phase, longer plateau, and more gradual decline.
2. Type of antibody. IgM-type antibodies are the principal class formed in the primary response. Although some IgM antibody is formed in a secondary response, the IgG class is the predominant type formed.
3. Antibody titer. In a secondary response, antibody levels attain a higher titer. The plateau levels in a secondary response are typically 10-fold or greater than the plateau levels in the primary response.
An example of an anamnestic response can be observed in hemolytic disease, when an Rh-negative mother is pregnant with an Rh-positive baby (see Chapter 26). During the mother’s first exposure, the Rh-positive RBCs of the fetus leak into the maternal circulation and elicit a primary response. Subsequent pregnancies with an Rh-positive fetus will elicit a secondary (anamnestic) response.
Vaccination is the application of primary and second responses. Humans can become immune to microbial antigens through artificial and natural exposure. A vaccine is designed to provide artificially acquired active immunity to a specific disease (e.g., hepatitis B). Booster vaccine (repeated antigen exposure) allows for an anamnestic response, with an increase in antibody titer and clones of memory cells (see Chapter 16).
Functions of Antibodies
The principal function of an antibody is to bind antigen, but antibodies may also exhibit secondary effector functions and behave as antigens. The significant secondary effector functions of antibodies are complement fixation and placental transfer (Table 2-4). The activation of complement is one of most important effector mechanisms of IgG1 and IgG3 molecules (see Chapter 5). IgG2 seems to be less effective in activating complement; IgG4, IgA, IgD, and IgE are ineffective in terms of complement activation. IgG-4 related disease is a newly recognized inflammatory condition characterized by often but not always elevated serum IgG4 concentrations.
Table 2-4
Comparison of Properties of Immunoglobulins
| IgM | IgG | IgA | IgD | IgE | |
| Complement fixation | 3+ | 0-2+ | No | No | No |
| Placental transfer | No | Yes | No | No | No |

Antigen-Antibody Interaction: Specificity and Cross-Reactivity
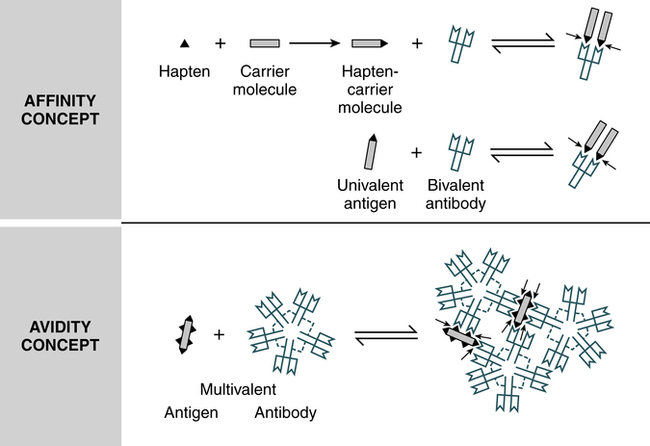
Antibody Avidity
Each four-polypeptide–chain antibody unit has two antigen-binding sites, which allows them to be potentially multivalent in their reaction with an antigen. The functional combining strength of an antibody with its antigen is called avidity, in contrast to affinity, the binding strength between an antigenic determinant (epitope) and an antibody-combining site (Fig. 2-15). When a multivalent antigen combines with more than one of an antibody’s combining sites, the strength of the bonding is significantly increased. For the antigen and antibody to dissociate, all the antigen-antibody bonds must be broken simultaneously.
Molecular Basis of Antigen-Antibody Reactions
Goodness of Fit
The strongest bonding develops when antigens and antibodies are close to each other and when the shapes of the antigenic determinants and the antigen-binding site conform to each other. This complementary matching of determinants and binding sites is referred to as goodness of fit (Fig. 2-16).
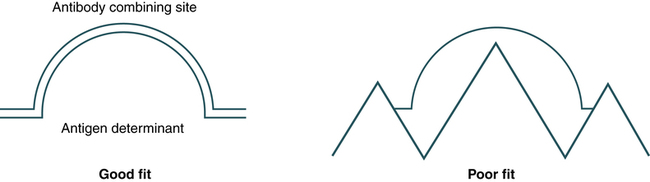
Detection of Antigen-Antibody Reactions
• Precipitation reactions combine soluble antigen with soluble antibody to produce insoluble complexes that are visible.
• Hemolysis testing involves the reaction of antigen and antibody with a cellular indicator (e.g., lysed RBCs).
• The enzyme-linked immunosorbent assay (ELISA) measures immune complexes formed in an in vitro system.
The principles of immunologic methods are discussed in Part II of this text. Detection and quantitation of immunoglobulins is important in the laboratory investigation of infectious diseases and immunologic disorders (Table 2-5).
Table 2-5
Role of Specific Immunoglobulins in Diagnostic Tests
| IgG | IgM | IgA | |
| Agglutination | 1+ | 3+ | Negative |
| Complement fixation | 1+ | 3+ | 1+ |
| Time of appearance after exposure to antigen (days) | 3-7 | 2.5 | 3-7 |
| Time to reach peak titer (days) | 7-21 | 5-14 | 7-21 |

Influence of Antibody Types on Agglutination
Immunoglobulins are relatively positively charged and, after sensitization or coating of particles, they reduce the zeta potential, which is the difference in electrostatic potential between the net charge at the cell membrane and the charge at the surface of shear (see Fig. 10-4). Antibodies can bridge charged particles by extending beyond the effective range of the zeta potential, which results in the erythrocytes closely approaching each other, binding, and agglutinating.
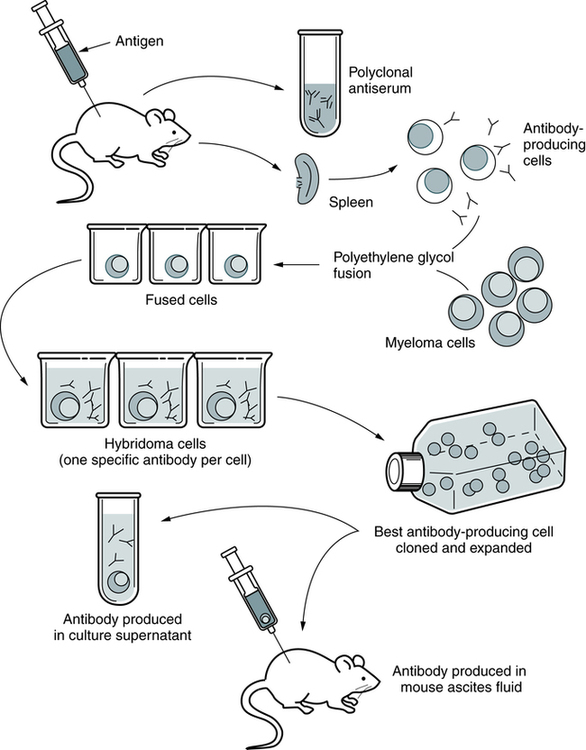
Monoclonal Antibodies
Monoclonal Antibody Production
Modern methods for producing MAbs are refinements of the original technique. Basically, the hybridoma technique enables scientists to inoculate crude antigen mixtures into mice and then select clones producing specific antibodies against a single cell surface antigen (Fig. 2-17). The process of producing MAbs takes 3 to 6 months.
 ABO Blood Grouping (Forward Antigen Typing)
ABO Blood Grouping (Forward Antigen Typing)
Principle
| Anti-A | Anti-B | Blood Group |
| Positive | Negative | A |
| Negative | Positive | B |
| Positive | Positive | AB |
| Negative | Negative | O |
Refer to ![]() for the procedural protocol, sources of error, and clinical notes.
for the procedural protocol, sources of error, and clinical notes.
Chapter Highlights
• Foreign substances can be immunogenic if their membrane or molecular components contain structures (antigenic determinants or epitopes) recognized as foreign by the immune system. The normal immune system responds to foreignness by producing antibodies.
• Cellular antigens of importance to immunologists include MHC groups and HLAs, autoantigens, and blood group antigens. Some of these antigens (e.g., MHC) are more potent than others in provoking an immune response.
• Antigens are usually large organic molecules that are proteins or polysaccharides. Although large foreign molecules are better antigens, haptens can bind to larger carrier molecules and behave like antigens.
• Antibodies that are specific proteins are known as immunoglobulins. Many antibodies can be isolated in the gamma globulin fraction of protein by electrophoretic separation. The primary function of an antibody in body defenses is to combine with antigen.
• Five distinct classes of immunoglobulin molecules are recognized—IgM, IgG, IgA, IgD, and IgE. Antibodies exhibit diversity among the different classes, suggesting different functions in addition to their primary function of antigen binding.
• A typical monomeric IgG molecule consists of three globular regions (two Fab regions and Fc portion) linked by a flexible hinge region.
• An antigenic determinant is the specific chemical determinant group or molecular configuration against which the immune response is directed. Because they are proteins, immunoglobulins can function as effective antigens when used to immunize mammals of a different species. When the resulting antiimmunoglobulins or antiglobulins are analyzed, three principal categories of antigenic determinants can be recognized—isotype, allotype, and idiotype.
• Production of antibodies is induced when the host’s immune system comes into contact with a foreign antigenic substance and reacts to this antigenic stimulation. When an antigen is encountered initially, the cells of the immune system recognize the antigen as nonself and elicit an immune response or become tolerant of it. An immune reaction can be cell-mediated immunity (dependent on T cells and macrophages) or may involve the production of antibodies directed against the antigen.
• After a foreign antigen challenge, an IgM antibody response proceeds in four phases—lag, log, plateau, and decline. Subsequent exposure to the same antigenic stimulus produces an anamnestic (secondary) response, which exhibits the same four phases but differs from a primary response in time, type of antibody produced, and antibody titer.
• Specificity is the ability of a particular antibody to combine with one antigen instead of another.
• Affinity is the bonding strength between an antigenic determinant and antibody-combining site, whereas avidity is the strength with which a multivalent antibody binds a multivalent antigen.
• Agglutination and other tests (e.g., precipitation reactions, hemolysis testing, ELISA) are widely used in immunology to detect and measure the consequences of antigen-antibody interaction.
• Monoclonal antibodies (MAbs) are purified antibodies cloned from a single cell. MAbs bound to cell surface antigens now provide a method for classifying and identifying specific cellular membrane characteristics and leukocyte antigens.