Antifungal and Antiviral Therapy
SPECIFIC INDICATIONS AND USES FOR ANTIFUNGAL THERAPY
AREAS OF CONTROVERSY IN ANTIFUNGAL THERAPY
Empirical Antifungal Therapy for the Febrile ICU Patient
Antifungal Prophylaxis in the ICU
Systemic Antifungal Agents
This chapter will focus on systemic antifungal therapy as it is relevant to the critical care setting. Topical therapy of candidiasis and therapy of other systemic fungal infections will be omitted or only briefly reviewed. The interested reader is referred to the Infectious Diseases Society of America (IDSA) guidelines on the topic.1
Polyenes
Polyenes act by binding to ergosterol in the fungal cytoplasmic membrane, causing ionic leakage and osmotic instability.2 Additionally, they cause oxidation of the cytoplasmic membrane.2 Polyenes are fungicidal in most settings and the most prominent members of the family are amphotericin B and nystatin. Both drugs have important toxic effects that often limit their use in patients with organ/system failure. Newer formulations of the drugs have been developed to reduce this limitation.
Amphotericin B and Its Lipid Formulations
Amphotericin B is produced by Streptomyces nodosus and is one of the oldest and most widely used antifungal agents. There is much experience with its use in mycoses and hosts, and it is usually the comparison standard for new therapies.3 Because of significant acute and chronic toxicities, there is an extensive anecdotal literature describing ways to ameliorate these toxicities, as well as limitations of all these strategies.4 The advent of lipid-based formulations of amphotericin B has enhanced our ability to limit toxicities and provide adequate courses of therapy.5
Amphotericin B has in vitro and in vivo activity against most isolates of Candida spp., Cryptococcus neoformans, Histoplasma capsulatum, Blastomyces dermatitidis, Mucorales, Coccidioides immitis, Paracoccidioides brasiliensis, Aspergillus spp., Fusarium spp., and Sporothrix schenckii.6 The activity of amphotericin B is so broad that it is almost easier to think of this in terms of the few species that are consistently less susceptible or actually resistant to amphotericin B: Aspergillus terreus,7 Trichosporon spp.,8 and Pseudallescheria boydii (Scedosporium prolificans).9 One species of Candida, C. lusitaniae, readily becomes resistant to the polyenes.10 Resistance among isolates of Candida is otherwise rare.11,12
Amphotericin B is hydrophobic and is combined with deoxycholate to permit intravenous (IV) administration in an aqueous solvent. Once in the bloodstream, it dissociates and binds to plasma proteins and lipoproteins. It is stored in the liver and other organs and slowly eliminated. Drug metabolism is complex and not affected by renal or liver failure.3 Hemodialysis and peritoneal dialysis do not remove the drug. Measuring drug levels is possible13 but not of obvious clinical relevance. Cerebrospinal fluid (CSF) penetration is poor. Although amphotericin B is primarily used in IV infusion, it can also be used topically for localized gastrointestinal (GI) or urinary infections or instilled directly to treat central nervous system (CNS) infections.
The most common toxicity is the systemic reaction associated with IV infusion, thought to be caused by release of inflammatory mediators from monocytes and macrophages and producing fever, hypotension, and on occasion severe dyspnea. Two forms of renal toxicity are seen. First, a very acute form of renal dysfunction appears to be related to amphotericin B–induced renal arteriolar constriction.14 Second, cumulative dose-dependent tubular damage is almost invariably seen if significant doses are given. The tubular injury is characterized by potassium/magnesium wasting and azotemia. It is generally reversible when the drug is stopped, but it is also particularly aggravated if the patient is volume depleted or given other nephrotoxic drugs. The renal injury also reduces erythropoietin production15 and thus causes a mild anemia during chronic therapy (the hematocrit will typically fall to about 30%). Finally, amphotericin B may precipitate cardiac arrhythmias, especially if the patient is already hypokalemic and hypomagnesemic.16–19 The deoxycholate formulation of amphotericin B is given intravenously at 0.5 to 1.0 mg/kg/day, but it can also be administered every other day by doubling the dose. To avoid producing a precipitate, it must be diluted in an electrolyte-free solution at no more than 0.1 mg/mL. It should be administered over no less than 1 hour and most authorities prefer a 2- to 3-hour infusion.20 Because the occasional patient reacts violently to the drug, an initial test dose of 1 mg of the drug may be given prior to infusing the entire first dose. Premedication with acetaminophen, diphenhydramine, and steroids may be used if the patient develops reactions to the infusion. Volume loading appears to reduce nephrotoxicity, and many authorities give (if possible) 500 to 1000 mL normal saline just prior to each dose of amphotericin B. Administration via a central line is advisable because amphotericin B given peripherally often produces phlebitis.
Treatment goals have been traditionally and arbitrarily cumulative (such as a total of 1 or 2 g), but a time-based approached is increasingly used for some diseases (e.g., for candidemia, in which 2 weeks of therapy at 0.6-0.7 mg/kg after the last positive blood culture has been shown to produce a late relapse rate of about 1%).21 Although the importance of therapy in the typical patient is unclear,22,23 candiduria is sometimes treated with amphotericin B bladder washes. A typical dose is 50 mg of amphotericin B diluted in 1000 mL of water and irrigated over 24 hours. A small number of intracranial fungal infections benefit from intrathecal dosing at 0.1 to 0.5 mg three times per week, but expert advice should be sought if this therapy is considered.
There are three lipid-based formulations of the drug: amphotericin B colloidal dispersion (ABCD, marketed as Amphotec and Amphocil), amphotericin B lipid complex (ABLC, marketed as Abelcet), and liposomal amphotericin (L-AmB, marketed as AmBisome). The names of these compounds are often confusing. Only one of the drugs (L-AmB) is a true liposome. However, it is not uncommon for physicians to refer to therapy with these compounds in general as therapy with “liposomal amphotericin B.” The preferred terminology is “lipid-associated formulation of amphotericin B” (LFAB).5 When speaking of specific compounds, we find that use of the names ABCD, ABLC, and AmBisome minimizes confusion.
All three LFABs have comparable efficacy among themselves and when compared to regular amphotericin B, but significantly less nephrotoxicity.5,24 They are also thought to be concentrated and distributed in the reticuloendothelial system, theoretically achieving higher tissue dose delivery and concentration. The lipid carrier does, however, dramatically change the pharmacology and delivery of these compounds, and higher doses than are typical for amphotericin B are both safe and necessary for optimal activity: The licensed dosages are 5 mg/kg/day (ABLC), 3 to 6 mg/kg/day (ABCD), and 3 to 5 mg/kg/day (AmBisome). The optimal dosage of these compounds is unclear and the agents appear generally equipotent. Dosages of approximately 3 mg/kg/day would appear suitable for treatment of most serious Candida infections. Doses of at least 5 mg/kg/day are used for mold infections, with some authors recommending even higher doses.25–27 However, a recent study showed no advantage to using 10 mg/kg/day over 3 mg/kg/day for invasive aspergillosis and other invasive mold infections.28
All three LFABs appear active against the same range of fungal infections that can be treated with amphotericin B. The compounds do differ in their relative toxicities.24 ABCD appears to have significant administration-related toxicity and is not often used. AmBisome and ABLC are both well tolerated, but AmBisome has been associated with somewhat less nephrotoxicity in some patient settings.29 Some patients will tolerate one formulation better than another.30,31
Because of the cost of the LFABs, there has been great interest in the concept of making a pseudo-LFAB by suspending amphotericin B deoxycholate in commercially available lipid emulsions.32–34 This practice does not, however, consistently reduce toxicity.35 This may be due to preparation-dependent precipitation of the amphotericin B noted by some36,37 but not all38 authors. Most authorities have concluded that further work with this approach should be undertaken as part of a controlled clinical trial that addresses these issues.39 A recent meta-analysis on the use of these formulations has been published, but because of heterogeneity, the results are inconclusive.40
The cost of the LFABs is significant, but the counterbalancing reduction in nephrotoxicity is also valuable and should be considered when choosing an LFAB versus a conventional amphotericin B.41 A recent study of the impact of nephrotoxicity of amphotericin B deoxycholate in patients with invasive aspergillosis suggested that this patient population suffered significant morbidity due to the amphotericin B itself.42 Use of amphotericin B deoxycholate produces significant nephrotoxicity in about 30% of patients, increasing hospital length of stay and hospitalization costs by nearly $30,000.43,44 Nevertheless, the context of amphotericin B–induced toxicity should also be considered. A rise of the creatinine to 3 mg/dL in an otherwise well patient who is being treated with amphotericin B for (say) osteoarticular sporotrichosis may be clinically imperceptible owing to the fact that the patient has no other acute medical problems. On the other hand, a rise in creatinine from 1 mg/dL to 2 mg/dL may be disastrous in a surgical patient who is also suffering from nosocomial pneumonia and cardiac insufficiency. No firm guidelines in this area have yet to emerge. We currently believe that an LFAB is appropriate for patients who have failed amphotericin B deoxycholate (FDA [Food and Drug Administration] indication), or who are intolerant of amphotericin B deoxycholate (FDA indication), or who are highly likely to be intolerant (no FDA indication). We define intolerance broadly: a creatinine clearance (CrCl) less than 50% of the normal for the patient’s age or a fall in CrCl with therapy. Predicting intolerance is difficult, but such factors as concomitant use of highly nephrotoxic agents (e.g., an aminoglycoside) or underlying primary/intrinsic renal disease (e.g., diabetes mellitus) associated renal dysfunction should be considered.
Flucytosine
Flucytosine (5-FC) is the fluorine analog of cytosine.45 It was originally synthesized as an antineoplastic agent, but poor antitumor activity and discovery of its antifungal properties led to its further development as an antifungal agent. It acts by deamination to 5-fluorouracil and then conversion to a noncompetitive inhibitor of thymidylate synthase that interferes with fungal DNA and RNA synthesis. It has been demonstrated effective in cryptococcosis, candidiasis, and chromomycosis, being the drug of choice for the latter infection. Drug resistance in vivo develops quickly, so the standard practice is to combine it with another agent.46,47 5-FC is water soluble and has high bioavailability. Protein binding is negligible and approximately 90% is excreted in urine. CSF penetration is good, and both hemodialysis and peritoneal dialysis remove it. It is teratogenic in rats and therefore contraindicated during pregnancy. Adverse effects include rash, diarrhea, and hepatic dysfunction. High blood levels (>100 µg/mL) are associated with profound leukopenia and thrombocytopenia.48 This has prompted the recommendation of monitoring drug levels, renal/liver function, and blood counts in patients receiving 5-FC. Dosage of 150 mg/kg/day divided in four doses has usually been suggested, but recent in vivo49 and human experience50 suggests that dosage of 100 mg/kg/day (again, divided into four doses) may be as effective and better tolerated. Renal failure requires dosage adjustment to half the dose if CrCl is 25 to 50 mL/minute and a quarter dose if it falls below 25 mL/minute. Patients on hemodialysis should be given the latter dose after dialysis. Combination with amphotericin requires constant monitoring of toxicity parameters and dose adjustment. The target blood level is approximately 50 µg/mL. Most experts would discontinue use of this agent if blood counts start dropping, regardless of the blood levels.
The Azole Antifungal Agents
The introduction of this class of drugs was a major advance in antifungal therapy, because they offer both IV and oral formulations for the treatment of systemic mycosis. Their widespread use has also prompted the emergence of resistance. Azoles act by blocking the activity of lanosterol demethylase, a cytochrome enzyme in both fungal and mammalian cells. Fungal cell membrane synthesis of ergosterol is inhibited and other sterol intermediates are substituted in the membrane, resulting in a nonviable cell. This effect is much slower than that of amphotericin, so these drugs are generally regarded as fungistatic. Because these drugs reduce production of the ergosterol to which polyenes must bind to produce their effect, there is a potential for the azoles and polyenes to appear antagonistic. However, these effects are drug-, organism-, and model-dependent and a range of effects may be seen. This area is complex and has recently been reviewed.51,52 At present, use of such combinations should be avoided outside a clinical trial. All of the azoles have the ability to interfere with mammalian sterol synthesis. This was most notable with ketoconazole, which can produce gynecomastia and adrenal insufficiency.53,54 Subsequent azoles have been selected for lack of such effects. All of the azoles can, however, produce hepatic dysfunction. The most common pattern is that of increased transminases. However, any form of dysfunction may be seen. This can be life-threatening if not recognized. The hepatic dysfunction is reversible upon discontinuation of the offending azole.
Another concern with azoles is drug interactions. This becomes particularly important in the critical care setting in which many drugs are being used concomitantly. Table 52.1 summarizes the most important drug interactions that have been reported. The critical interactions generally have to do with drugs cleared by the liver. Some agents (e.g., rifampin and phenobarbital) induce the enzymes that clear the azoles. In other cases, the azole interferes with clearance of another agent (e.g., the azoles predictably increase blood levels of cyclosporine). It is not possible to list all of the known interactions. Consultation with a pharmacy specialist is suggested when using azoles in the setting of polypharmacy, particularly in the critical care setting and when caring for patients with multiple comorbid conditions.
Table 52.1
Selected Azole-Drug Interactions*
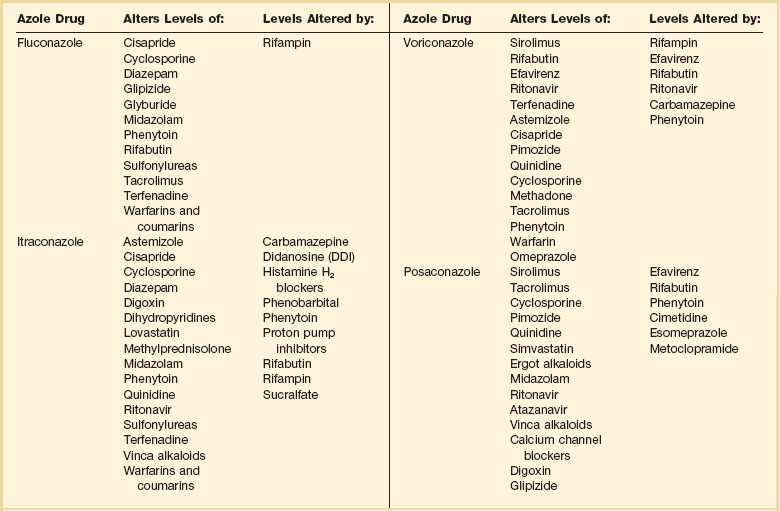
*Generally, azoles tend to increase the levels of other drugs, whereas other drugs tend to decrease the level of the azole. This list is not meant to be comprehensive, and the practitioner should review the full prescribing information. Consultation with a pharmacy specialist is recommended when azoles are going to be used in complex polypharmacy situations.
Fluconazole
Fluconazole is one of the newer triazoles that has in vitro and in vivo activity mainly against yeast, such as Candida spp. and Cryptococcus neoformans. It also has efficacy against Coccidioides immitis, Histoplasma capsulatum, and Blastomyces dermatitidis. Unfortunately resistance (mediated by increased production of target enzymes, efflux pumps, and mutations in target enzyme) has become a problem, particularly in Candida albicans (mutation to resistance is seen), C. glabrata (has intrinsically lower susceptibility and may become highly resistant), and C. krusei (intrinsically highly resistant).55,56 Nevertheless, fluconazole is still very active against most strains causing invasive candidiasis, and higher doses can be used for those organisms that are in the “susceptible-dose dependent” range. Fluconazole is water soluble and is available in oral and IV presentations that produce similar blood levels. Bioavailability is excellent and it is well absorbed regardless of the gastric contents. It has a long half-life and can be administered in a single daily dose. It exhibits little binding to serum proteins and is widely distributed in all body fluids. CSF penetration is particularly high, making it particularly useful in the treatment of CNS infections such as cryptococcosis and coccidioidomycosis. Fluconazole is excreted by the kidneys and dosing should be adjusted in proportion to the CrCl. The most common adverse effects are nausea and vomiting. Skin rash is infrequent but can be severe. Hepatitis has also been reported. The usual dose is 400 to 800 mg (or 6-8 mg/kg) per day by mouth or IV, but doses as high as 2 g/day have been tolerated.57
Itraconazole
Itraconazole is a triazole antifungal agent with a wider spectrum than fluconazole. It has in vitro and in vivo activity against Candida spp., Aspergillus spp., Histoplasma spp., Blastomyces dermatitidis, Sporothrix schenckii, Trichophyton spp., Cryptococcus neoformans, Coccidioides immitis, and Paracoccidioides brasiliensis.58,59 The resistance issues observed for fluconazole are also an issue with itraconazole.56 Itraconazole was initially available only in a capsule form that has unpredictable bioavailability. Absorption of the capsule formulation is optimized by ingestion with food.60 Two new formulations have recently been introduced. First, there is a solution in cyclodextrin for oral administration that significantly enhances bioavailability and is now preferred over the capsule for producing maximal blood levels.61 Second, an IV formulation (again, in a cyclodextrin carrier) has recently become available. The IV formulation is valuable in that it quickly and reliably produces significant blood levels.62–64 Itraconazole is highly protein bound and has a prolonged half-life. CSF penetration is negligible, so it is not generally used for CNS infections. Itraconazole is metabolized by the liver and dosing does not need to be modified in renal failure. However, the cyclodextrin carrier used in the IV formulation is cleared by the kidneys and its behavior in patients with renal dysfunction is not known. Thus, this formulation of itraconazole should not be used in patients with a CrCl less than 25 mL/hour.
Adverse effects are mainly GI, with nausea, vomiting, and abdominal pain occurring in up to 10% of patients. Hepatitis is uncommon but liver enzyme monitoring is recommended. Because itraconazole, and its major metabolite hydroxyitraconazole, are inhibitors of CYP3A4, drug interactions are a major issue and the most common ones are summarized in Table 52.1.
Voriconazole
Voriconazole is one of the newest triazoles to arrive into the market. It is licensed for the treatment of invasive candidiasis and invasive aspergillosis, as well as for the treatment of Fusarium and Scedosporium infections. It is considered as the treatment of choice for invasive aspergillosis.65 Voriconazole was not inferior to amphotericin B followed by fluconazole in a large clinical trial, but it is unknown if it has any advantages over fluconazole or the echinocandins for treating infections by fluconazole-resistant C. glabrata.66,67 Although it has activity against the endemic mycoses, data are limited at this point, so it is not recommended for routine use in these infections at this time.68,69
A relevant gap in its activity is the class of Zygomycetes (such as Mucor spp., Absidia spp., and Rhizopus spp.). Although there have been multiple reports of breakthrough Zygomycetes infections in patients receiving voriconazole prophylaxis or treatment, a causal relationship has not been established.70,71 Nevertheless, clinicians should be aware that this drug does not have activity against these organisms, and thus it should not be used for empirical therapy of mold infections if Zygomycetes are in the differential diagnosis.
Voriconazole is available in oral (tablets and suspension) and IV formulations, with 96% bioavailability. Usual dosing is 4 to 6 mg/kg IV every 12 hours or 200 to 300 mg orally every 12 hours. Voriconazole has nonlinear pharmacokinetics; thus, increasing the dose will not necessarily increase blood levels. Routine blood level measurement to document absorption is now recommended, although blood levels associated with specific efficacy and safety margins have not been established. Voriconazole is metabolized by the human hepatic cytochrome P-450 enzymes, CYP2C19, CYP2C9, and CYP3A4, and has extensive drug interactions with many drugs commonly used in the critical care setting. Consultation with a pharmacy specialist is recommended for patients on multiple drugs. Like itraconazole, the IV formulation is prepared in a cyclodextrin-based formulation, and thus it is not recommended for use in patients with CrCl less than 50 mL/minute. In such patients the oral formulation may be safely used. Adverse events include self-limited visual disturbances and hallucinations, infrequent reports of hepatic insufficiency, and as with the other azoles, rare reports of arrhythmias and QT prolongation. Monitoring of liver enzymes is recommended during voriconazole therapy.72–74
Posaconazole
Posaconazole is the newest triazole in the market and although licensed primarily for prophylaxis of fungal infections in high-risk patients,75 it does have promising activity against mold infections, in particular infections by the Zygomycetes and Fusarium.76 It has also shown excellent in vitro and in vivo activity against Candida spp. with demonstrated efficacy in esophageal disease. It is available only in an oral formulation.77,78
Echinocandins
The echinocandin antifungal agents represent an entirely new class of antifungal drugs. There are three echinocandins on the market at this time: caspofungin, micafungin, and anidulafungin. These agents are cyclic lipohexapeptides that act via inhibition of glucan synthesis.79 Preclinical studies have shown efficacy against all species of Candida without any evidence for cross-resistance with polyenes or azoles, Aspergillus spp., and selected other fungi.80,81 Although the target enzyme is present in most fungi, the echinocandins are not active against C. neoformans and molds other than Aspergillus. The echinocandins appear to be rapidly fungicidal for Candida spp., but their activity against Aspergillus may be better described as fungistatic.82 Nonetheless, they are quite active in animal models of both candidiasis and aspergillosis.83,84 These drugs have a long half-life, permitting once-daily dosing, and are excreted in the liver. They do not interfere with the cytochrome system and they are not known to be nephrotoxic, having otherwise remarkable safety profiles. At this time, differences between the three drugs appear to be subtle, requiring further study.
Caspofungin
Caspofungin was the first echinocandin in the market. It has excellent in vitro and in vivo activity against Aspergillus and Candida spp. It has demonstrated efficacy for the treatment of invasive candidiasis, treatment of invasive aspergillosis, and empirical therapy of fungal infections in the setting of febrile neutropenia.85–87 The usual dosing includes loading with 70 mg IV, followed by 50 mg IV every 24 hours. The safety profile is good with mild elevation of liver enzymes. Patients with hepatic insufficiency (Child-Pugh class B or C) require dosage adjustment to 35 mg/kg IV every 24 hours.68,88,89 Drug interactions are infrequent, but it is recommended to monitor cyclosporine levels in patients receiving caspofungin.90 A recent clinical trial showed that higher doses of caspofungin (100 mg/day) are well tolerated but do not necessarily translate into additional clinical benefits.91
Micafungin
Micafungin is approved by the by the FDA for the treatment of esophageal candidiasis, prophylaxis of Candida infections in stem cell transplant patients, and treatment of candidemia. Although micafungin has excellent in vitro and in vivo activity against Aspergillus and Candida spp., clinical data on invasive aspergillosis are limited.92,93 Micafungin has demonstrated efficacy both for the treatment of invasive candidiasis and as a prophylactic agent for fungal infections in allogeneic stem cell transplant recipients.94–96 The prophylactic dose is 50 mg IV every 24 hours, and the therapeutic dose for invasive candidiasis is 100 mg IV every 24 hours. As with the other echinocandins, the most frequent adverse event is mild elevation of liver enzymes. No dosage adjustment is required in the setting of renal insufficiency or moderate hepatic insufficiency. Mild drug interactions have been reported with concomitant use of sirolimus and nifedipine.
Anidulafungin
As with the other echinocandins, anidulafungin has excellent in vitro and in vivo activity against Aspergillus and Candida spp.97 It is currently indicated for the treatment of invasive candidiasis, having demonstrated noninferiority, and perhaps superiority, in a clinical trial versus fluconazole.98 The usual dosing includes a loading dose of 200 mg IV, followed by 100 mg IV every 24 hours. Anidulafungin does not require dosage adjustment for patients with renal or hepatic failure and no significant drug interactions are reported. 97,99,100
Specific Indications and Uses for Antifungal Therapy
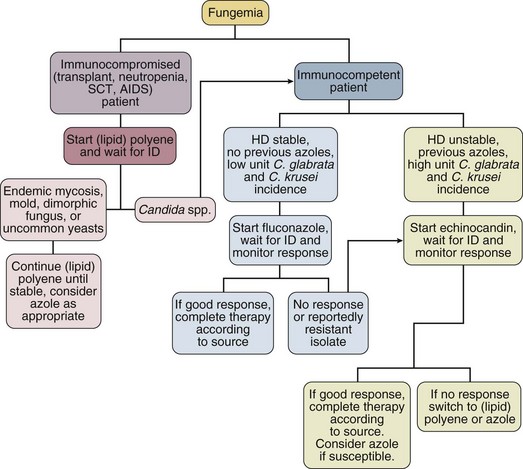
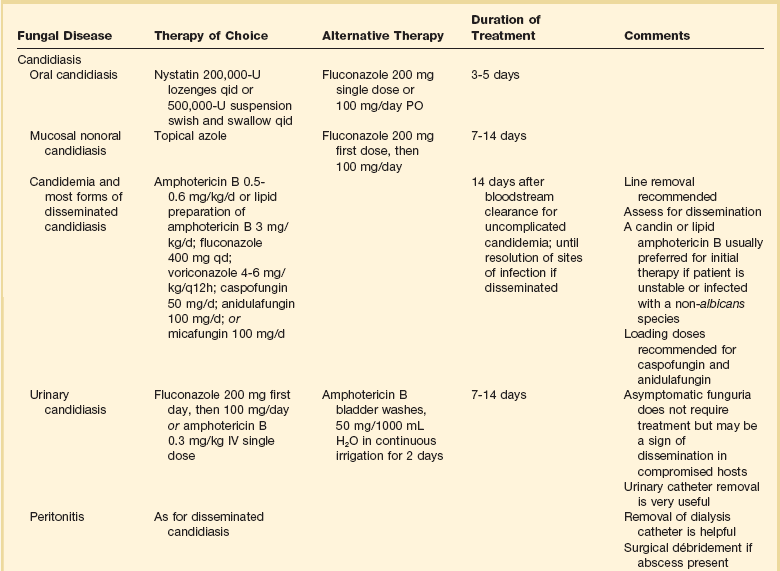
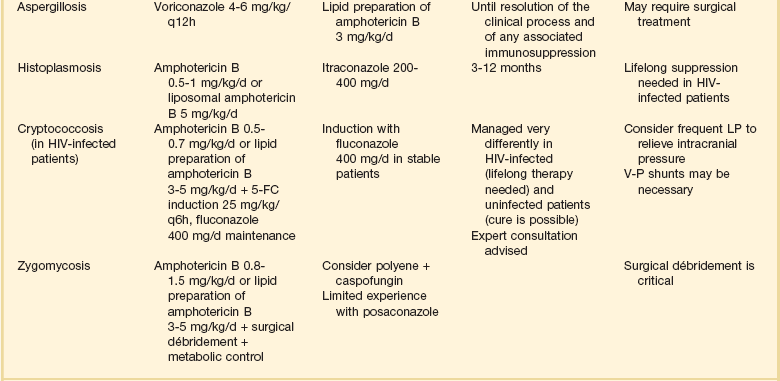
Although the precise diagnosis of a fungal infection may be laborious, time-consuming, and delayed, therapy should never be withheld in a critically ill patient with suspected or confirmed fungal infection. Empirical therapy will often be started with amphotericin B or one of its lipid preparations and then tailored according to the final identification of the organism. This section presents generally accepted treatment recommendations for the most commonly encountered fungal infections in the critical care setting. Infections by less common fungi may be particularly severe and rapidly progressive and require consultation with an infectious diseases specialist for appropriate treatment. Table 52.2 summarizes the most often encountered fungal diseases in the critical care setting with their generally accepted treatment options. Figure 52.1 presents our approach for the critically ill patient with fungemia.
Table 52.2
Candida Infections
Although C. albicans remains the most common pathogen in oropharyngeal and cutaneous candidiasis, non-albicans species of Candida are increasingly frequent causes of invasive candidiasis.101 Guidelines and reviews for therapy of candidiasis in the intensive care setting have recently been published.102–105 These guidelines are extensive and will not be repeated in detail. Rather, the text will focus on several clinical situations in which candidal infections are particularly challenging for the critical care specialist.
Candidemia and Disseminated Candidiasis
The diagnosis of disseminated candidiasis is always a challenge.106 There is no single tool that conclusively makes this diagnosis. Isolation of Candida from the bloodstream is simply the most obvious form of disseminated candidiasis, but clinical experience makes it obvious that disseminated candidiasis can occur in the absence of detectable fungemia.
Candidemia may be treated initially with either fluconazole, voriconazole, amphotericin B (or its lipid preparations), or an echinocandin. In the critically ill and unstable patient, an echinocandin or amphotericin B lipid preparations is preferred because of the broader spectrum of activity and more rapid onset of action.104,107 Owing to their greater safety, echinocandins are increasingly viewed as the initial agents of choice, and in fact, both the anidulafungin study and recent patient level meta-analysis of the candidemia clinical trials suggest that initial therapy with echinocandins may be superior to initial therapy with azoles.98,108
The susceptibility of Candida to the currently available antifungal agents can generally be predicted if the species of the infecting isolate is known.10,12,101,109–117 Bloodstream isolates of C. albicans, C. tropicalis, and C. parapsilosis are generally susceptible to fluconazole and amphotericin B. Isolates of C. glabrata and C. krusei will often (if not always, as is the case with C. krusei) be resistant to fluconazole. Isolates of C. lusitaniae may be resistant to amphotericin B. Antifungal susceptibility testing is becoming increasingly important as a guide in the treatment of these infections.118 In particular, detection of fluconazole resistance is very valuable, as it provides support for continued use of echinocandins or polyenes.
Although all Candida spp. are generally considered to be susceptible to echinocandins, C. parapsilosis has higher minimum inhibitory concentrations (MICs) than the other Candida spp. This, however, has not translated into reduced activity in clinical trials.85,119 Once the organism is speciated, or if the prevalence of more resistant species is low, therapy may be switched to fluconazole. Fluconazole has demonstrated to be as effective in clearing candidemia as amphotericin B in immunocompetent hosts. Duration of treatment is 14 days following the last positive culture,21 and if a central line is present, removal is highly recommended. If evidence of disseminated candidiasis is found, therapy should be prolonged to at least 4 weeks to ensure proper organ clearance.
The Intravenous Catheter in Candidemic Patients
Although long an area of contention, current data strongly suggest that candidemia is often related to (if not primarily propagated by) a central venous catheter. Central venous catheters in particular have been found to be both a risk factor for developing candidemia120–123 and associated with persistent fungemia.121 Removal of the catheter has been associated with shorter duration of subsequent candidemia124 and improved patient outcome.125,126 Unique to the species of Candida, candidemia with C. parapsilosis is almost always due to a catheter.127,128 The situation may be different for neutropenic patients, particularly those who have permanent, lower-risk catheters such as Hickman catheters. Such catheters may of course become infected, but these patients may also have candidemia due to entry of the organisms from the gut into the bloodstream. This concept is supported by demonstrations that Candida can enter the bloodstream from the gut,129 by the relative lack of effect of catheter removal in a large cohort of cancer patients,128 and by the frequent demonstration of gut wall invasion in patients who die with disseminated candidiasis.130,131 Unfortunately, there is no convincing way to tell if a given catheter is involved. Differential quantitative blood cultures through the line and from a peripheral site have been suggested to be one approach to resolving this problem,132 but this technique remains controversial.133 On a practical basis, serious consideration to line removal should be given if fungemia persists for more than a few days. A recent patient level meta-analysis of the major candidemia clinical trials has confirmed a mortality rate reduction and microbiologic cure benefit for this approach.108
Mucosal Infections and Colonization
Although there are many risk factors for development of disseminated candidiasis, colonization at one or more nonsterile sites represents an unusually strong risk factor.134 As discussed earlier, local oral and mucosal candidiasis should thus be considered as a predictor of possible invasive disease in the critically ill or immunocompromised host.135,136 Esophageal candidiasis does require systemic treatment. Fluconazole is generally preferred here, although amphotericin B and the candins can also be used.
Candiduria
Treatment of asymptomatic candiduria produces only temporary clearing of the urine and is probably not indicated.22,23 However, candiduria should probably be treated in symptomatic patients, immunocompromised patients, low-birth-weight infants, renal transplant patients, and patients who will undergo urologic manipulation or surgery.102 If treatment is indicated, systemic therapy with amphotericin or fluconazole is preferred, and amphotericin bladder washes should be reserved for patients with renal insufficiency and low renal clearance. Removal of the urinary catheter is by itself a useful intervention and should always be considered.
Other Forms of Invasive Candidiasis
There are many other possible forms of invasive candidiasis: meningitis, endocarditis, and osteomyelitis, just to name a few. This area has recently been reviewed,103 and for most forms of this disease there are very few specific studies on therapy. The largest body of data will always be anecdotal reports of use of amphotericin B, and for essentially every form there are at least a few reports of successful therapy with fluconazole. In general, amphotericin B is preferred when the infection is most acute, when data on the nature of the infection are still being generated in the laboratory, or when the patient has previously received azole therapy. Fluconazole provides a good way to step down to an oral agent to complete therapy of infections due to susceptible isolates. Data for the echinocandins are starting to accumulate for these chronic infections.137 Removal of foreign body and standard surgical drainage are often key as well. An excellent example of the need to remove foreign bodies is found in treatment of dialysis catheter-related peritoneal candidiasis in which catheter removal is important. Surgical drainage is of course important in candidal peritonitis related to gut injury and fecal spillage.
Cryptococcosis
Treatment of cryptococcosis has recently been reviewed.138,139 Cryptococcal meningitis in non–human immunodeficiency virus (HIV)-infected adults continues to be seen sporadically. The majority of the published experience as to treatment is with amphotericin B given for 4 to 6 weeks.140,141 Because this therapy is curative in approximately two thirds of patients, this approach is warranted. Expert consultation is also appropriate for this relatively uncommon infection.142,143
On the other hand, cryptococcal meningitis in the HIV-infected patient is a well-established and common problem. Meningeal cryptococcosis in this setting should be treated with a 2-week course of IV amphotericin B or its lipid preparations,139,144,145 followed by life-long suppression with fluconazole.146,147 Current trends favor also using 5-FC unless there is a contraindication.50 Although itraconazole does not penetrate the CSF, anecdotal evidence has shown that it may be useful in treating CNS disease, although it is apparently less potent as a long-term therapy.148
For all forms of cryptococcal meningitis, intracranial hypertension should be aggressively treated with repeated lumbar puctures or a CSF shunt.149,150 The addition of steroids and other immune modulating agents to antifungal therapy has shown promising results in animal models,151 and clinical trials are being conducted. The immune reconstitution syndrome plays a major role in cryptococcal-related morbidity in HIV patients and the current recommendation is to delay antiretroviral therapy for a few weeks after treatment for cryptococcosis is started.139
Aspergillosis
Treatment of aspergillosis has been extensively reviewed.152–154 Invasive aspergillosis should be considered in any severely immunocompromised patient with an unexplained pulmonary or sinonasal process. Biopsy is normally required for definitive diagnosis, although a new generation of galactomannan-based serodiagnostic tests may prove useful as adjuncts to diagnosis.155,156 Although amphotericin B has classically been the initial treatment of choice for invasive aspergillosis, the current treatment of choice is voriconazole65,74 or a lipid preparation of amphotericin B. Aggressive surgical management is necessary and curative in some cases.106,157 Itraconazole has historically been an option for the treatment of aspergillosis in patients who are intolerant or refractory to amphotericin B, but newer and more effective azoles are now available.158 Overall, the outlook for invasive aspergillosis is critically dependent on recovery of immune function. Without this, the prognosis is usually dismal. Of note, recent studies have documented an increase in incidence of invasive aspergillosis in nonhematology, nontransplant patients seen in the intensive care unit (ICU), such as chronic obstructive pulmonary disease and autoimmune diseases.159,160 This trend should be monitored, and the critical care specialist should be suspicious when cultures of Aspergillus from a bronchoalveolar lavage or diagnostic markers such as galactomannan are positive.161
Histoplasmosis
The initial treatment of choice for severe acute histoplasmosis is an amphotericin B preparation.162–164 Noncritical cases may be treated with itraconazole 200 to 400 mg/day. Fluconazole is only moderately effective and should not be used as primary therapy.165 Successful treatment results in a decrease of serum and urine Histoplasma antigen.166 Duration of therapy is a function of disease form and underlying immune status. HIV-infected patients with disseminated disease should be treated as acute histoplasmosis and maintained on life-long suppression with itraconazole. Liposomal amphotericin B has shown excellent efficacy in this setting.167 Non-HIV-infected patients may require from 3 to 12 months of therapy, and expert consultation is generally advised.
Mucormycosis
The treatment of choice for mucormycosis (infections typically caused by Mucor spp., Rhizopus spp., and Absidia spp.) is aggressive surgical débridement and prompt start of high-dose amphotericin B or a lipid preparation.168 Follow-up therapy with itraconazole may be warranted, and expert consultation is advised. Posaconazole has shown efficacy in this setting.78 More recently, and despite the lack of in vitro activity of echinocandins against these agents, investigators have shown in vitro synergy and excellent clinical outcomes with the combination of amphotericin B and caspofungin.169 Correction of metabolic abnormalities (acidosis, iron overload, and hyperglycemia) should also be pursued aggressively.
Other Fungal Infections
Other fungal infections, such as blastomycosis, fusariosis, and infection by Trichosporon spp., Coccidioides immitis, Malassezia furfur, and Penicillium spp. may be occasionally encountered in the critical care setting, particularly when caring for immunocompromised hosts. Although amphotericin B is probably the best empirical drug for any characterized suspected fungal infection, it is not very effective against some of these pathogens. Guidelines for treating some of these infections have been published.1 Expert consultation should be promptly obtained.
Areas of Controversy in Antifungal Therapy
Empirical Antifungal Therapy for the Febrile ICU Patient
Fever in the ICU patient is a complex problem that requires prompt evaluation for many possible sources, including infection, atelectasis, pulmonary embolism, drug fever, thermoregulatory dysfunction, and many more.170 Infection by Candida should be suspected when the patient has risk factors such as immunocompromise, broad-spectrum antibiotic therapy, parenteral nutrition, steroids, surgery (especially if the gut wall is transected), urinary catheters, and burns.171,172
Unfortunately, making a diagnosis of invasive candidiasis is difficult. In the most straightforward scenario, the patient is febrile and has positive blood cultures for Candida. On other occasions, biopsy or aspiration is used to make a clear-cut diagnosis of a localized abscess due to Candida. These situations are, however, the exception. Far more common is the scenario of a persistently febrile ICU patient with a combination of the previously mentioned risk factors. In this setting, we place great importance on the presence of positive cultures from nonsterile sites such as wounds, sputum, or stool. The key concept is that presence of Candida at any of these sites significantly increases the likelihood of developing invasive disease.134,173 Positive cultures from the urine are also considered in this context (even though that is normally a sterile site). Candiduria in the afebrile patient is generally a clinical non-event that does not require therapy,22,23 but candiduria in the febrile ICU patient at the very least represents colonization and increased risk of invasive disease and at the worse represents actual upper urinary tract infection. A similar logic applies to Candida in the sputum. Pneumonia due to Candida occurs but is generally clinically inapparent.174 The presence of Candida in the sputum more often means that the gut, and thus the patient, is colonized. This, in turn, is a risk factor as discussed previously.
Research has shown conclusively that delays to appropriate antifungal therapy in ICU patients with invasive candidiasis are associated with increased mortality rates.175 The latest version of the IDSA guidelines recommend empirical antifungal therapy in critically ill patients who are deemed to be at high risk for the infection based on a variety of recently available scoring systems and risk assessment strategies.105,176–180 On the other hand, there is a negative multicenter study that failed to show a benefit to fluconazole in this setting.181
Antifungal Prophylaxis in the ICU
Prevention is always preferred to therapy, and this is certainly true for invasive candidiasis. Although prevention of mucosal disease can be achieved with almost any regimen, prevention of invasive disease has consistently required systemic therapy. Both fluconazole and amphotericin B are effective in the right setting. The key is to select patient populations with a meaningful chance of contracting invasive candidiasis. The value of prophylaxis has been shown convincingly for bone marrow transplantation patients182,183 and selected liver transplant patients.184–187 Studies of patients receiving standard chemotherapy for leukemia have shown a trend favoring prophylaxis,188,189 but the lower rates of disease in the control group lower the statistical power of the studies. To further confuse matters, even a group that sounds homogeneous (e.g., allogeneic bone marrow transplant recipients) really is not—different forms of chemotherapy and degrees of graft-versus-host disease produce different levels of risk. This point was driven home in an editorial emphasizing the range of variation within the category of “neutropenic patient.”190
In practical terms, three major studies have demonstrated benefits to prophylaxis of invasive candidiasis in the ICU.191–193 No study or meta-analysis has shown benefits in terms of mortality rates, however.194–197 These studies have the limitation of being single center studies and having limited numbers of patients. Most experts would agree with the fact that routine use of antifungal prophylaxis should be reserved for units with a high incidence of invasive candidiasis or for carefully selected patients at the highest risk.103,105
Measurement of Drug Levels
Drug level monitoring is theoretically justified to assure efficacy and avoid toxicity. However, drug level monitoring during antifungal therapy is relatively new and should not be carried out routinely because there is a lack of information on its clinical correlation and meaning. Situations in which drug level monitoring has proved to be useful are itraconazole levels to verify adequate absorption when using the oral forms of this compound, 5-FC levels to watch for possible myelotoxicity, and voriconazole/posaconazole levels to document absorption of the drugs when given orally.198,199
Susceptibility Testing
The Clinical Laboratory Standards Institute has published a guideline for standardized antifungal susceptibility testing that has been widely adopted200 and subsequently revised. This methodology is recommended for testing Candida and Cryptococcus spp. and breakpoints have been developed.201 Antifungal susceptibility testing is now widely available and should be considered when treating serious Candida infections, when treatment failure occurs, or when toxicity or side effects limit the use of a particular drug. It should also be remembered that pharmacology, safety, published experience, and drug interactions must be considered along with susceptibility when selecting a therapy.102,105 Mold susceptibility has also been standardized but is not routinely recommended.
Antiviral Agents
Antiviral chemotherapy has made great advances since the 1980s. Before then a diagnosis of a life-threatening viral infection meant mostly supportive therapy and patience. Although still limited, today we have a host of treatment options for herpetic infections; upper and lower respiratory illness by influenza, cytomegalovirus (CMV), and respiratory syncytial virus (RSV); and some relatively exotic systemic diseases. Therapy for HIV, hepatitis B, and hepatitis C infection has also made gigantic leaps, but it is usually not undertaken in the critical care setting and is thus beyond the scope of this chapter. Table 52.3 summarizes the currently available non-HIV specific antiviral drugs and their general spectrum.
Table 52.3
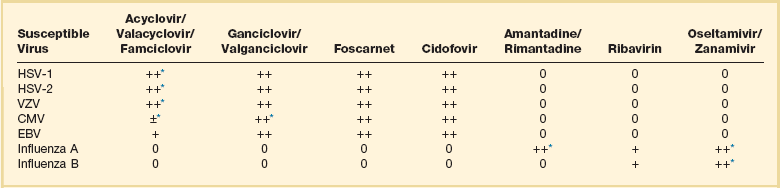
0, no known activity; ±, active under specific circumstances; +, active; ++, very active.
Acyclovir, Famciclovir, and Valacyclovir
Acyclovir is a nucleoside analog of guanine that has in vitro and in vivo activity against several viruses in the herpesvirus family, particularly against herpes simplex virus type 1 (HSV-1), HSV-2, varicella-zoster virus (VZV), and Epstein-Barr virus (EBV). High concentrations also inhibit CMV. It inhibits DNA polymerase causing DNA chain termination.202–204 Acyclovir is the treatment of choice for severe herpetic infections in immunocompetent and immunocompromised hosts. Resistance due to viral thymidine kinase mutations or, less often, DNA polymerase mutations may emerge in patients with severe immunocompromise such as transplant recipients and advanced HIV infection during treatment of HSV and VZV infections.205–207 Acyclovir has also been used for CMV prophylaxis in transplant patients.208,209 Acyclovir is available in oral, IV, and topical forms. Bioavailability of the oral form is poor (15-21%), requiring high dosage/frequent doses. Protein binding is less than 20%. CSF penetration is low, but acyclovir is active for CNS infections. The topical form is virtually unabsorbed. The half-life is short and the drug is cleared by the kidneys, so dosage adjustment in renal failure and hemodyalisis is required. Supplementation is not needed in peritoneal dialysis. Side effects are uncommon.210 The main concern with acyclovir therapy is the crystallization of the drug in the renal tubules, leading to renal failure. Aggressive hydration and monitoring of renal function are recommended during IV acyclovir therapy. Oral acyclovir may cause nausea and vomiting. The usual oral dose is 200 to 800 mg every 4 hours. The IV dosing range for severe infections is 8 to 12 mg/kg IV every 8 hours. Proven encephalitis is treated with 10 to 12 mg/kg every 8 hours for 14 to 21 days. Doses of up to 20 mg/kg may be more effective in premature infants.211
Famciclovir, the prodrug of penciclovir (a guanosine analog),212,213 is a well-absorbed oral agent that has shown excellent activity against first-episode or recurrent genital herpes and HSV/VZV infection in both HIV and immunocompetent hosts.214–216 A dose of 500 mg orally three times a day was shown to be as effective as acyclovir for treatment of herpes zoster.217
Valacyclovir, an analog of acyclovir that has a similar profile of side effects, is now available in oral formulations with the advantage of longer dosing intervals. It appears comparable to acyclovir for treatment of mucocutaneous HSV infections, but more effective in herpes zoster. It is also effective orally for prophylaxis of CMV in renal transplant patients.218 The dosing range is 500 to 1000 mg orally two to three times a day.219–221 Although absorption is excellent, there is little data to support treating invasive disease, such as CNS disease, with this drug.
Ganciclovir and Valganciclovir
Ganciclovir is another nucleoside analog of guanine but has a slightly wider antiviral spectrum than acyclovir. It is highly active against CMV, as well as HSV-1, HSV-2, and EBV. Like acyclovir it acts by interference with DNA polymerase, but it is not an obligate chain terminator. Resistance to ganciclovir by CMV and HSV is increasingly reported. HSV isolates that are thymidine kinase–deficient and thus resistant to acyclovir will also be resistant to ganciclovir.207,222 It is mainly indicated in prophylaxis, treatment, and suppression of CMV syndromes in immunocompromised patients.208,223–227 Ganciclovir is available in oral, intraocular, and IV forms, the first two being useful only in chronic suppression of CMV disease. Once it reaches the bloodstream it has body-wide distribution, low protein binding, and low CNS penetration. Excretion is renal and dosage adjustment is required in renal failure. Ganciclovir is removed by hemodialysis, and therefore it should be administered after dialysis. Side effects of IV ganciclovir include severe neutropenia (40%), thrombocytopenia (20%), phlebitis, rash, increased liver enzymes, and azotemia. The nephrotoxicity is potentiated by concomitant use of nephrotoxic agents and acyclovir. Ganciclovir should not be used in pregnant women because it is known to be teratogenic. The usual dose in acute infection is 5 mg/kg every 12 hours. Maintenance therapy at 5 mg/kg IV every day should be continued as long as the patient is immunocompromised. Discontinuation of therapy in acquired immunodeficiency syndrome (AIDS) patients appears feasible following immune reconstitution.208,223,228,229 An intraocular delivery system is also available as an adjunct to the treatment of CMV retinitis.
Valganciclovir is an L-valyl ester of ganciclovir that has significantly increased bioavailability over previous oral formulations of ganciclovir, resulting in levels previously unattainable with the traditional formulations. It allows for prophylaxis and treatment of CMV infections with an oral alternative. The usual dose for prophylaxis is 900 mg orally every 24 hours, and for treatment the recommended induction dose is 900 mg orally every 12 hours, followed by a maintenance dose of 900 mg orally every 24 hours. This drug requires adjustment for patients with renal impairment.230,231
Cidofovir
Cidofovir is a nucleotide analog with activity against most herpesviruses. Its activation is not virus-enzyme dependent, so it has activity against most acyclovir-resistant HSV and ganciclovir-resistant CMV. Resistant strains have also been reported, and synergy with ganciclovir and foscarnet has been reported. Oral bioavailability is very low. It is 6% protein bound and is excreted by the kidneys.232,233 Its side effects include neutropenia and nephrotoxicity, which can manifest as proteinuria, azotemia, and a Fanconi-like renal syndrome. It has been shown to be teratogenic and mutagenic and is contraindicated in pregnancy. It is currently licensed for CMV retinitis in HIV infection but has also shown activity against acyclovir-resistant HSV.234,235 The usual dose is 5 mg/kg every week for 2 weeks, then 5 mg/kg every other week.
Foscarnet
Foscarnet is a pyrophosphate analog that has antiviral activity against the herpesviruses, HIV, and hepatitis B virus. Its mechanism of action is interference with DNA polymerase or reverse transcriptase to block effective viral replication. Viral isolates that are resistant to ganciclovir or acyclovir are often susceptible to foscarnet, but primary resistance to foscarnet has also been described, particularly in HIV-infected patients.205,206,236–238 The main therapeutic indication of foscarnet is ganciclovir-resistant CMV disease. Foscarnet is only available in IV formulations and it has body-wide distribution. Protein binding is 15% and it has good CSF penetration. The drug is excreted almost intact by the kidneys and requires very careful dosage adjustment in renal failure. Foscarnet is removed by hemodialysis but should be avoided in patients with severe renal dysfunction. Its main side effect is nephrotoxicity, which occurs in most patients but is reversible when therapy is stopped.239 Dosage adjustment should be made by following the CrCl closely. Hypocalcemia and hypercalcemia as well as phosphate abnormalities are common, so monitoring of electrolytes is also recommended. The usual dose is 60 mg/kg every 8 hours for CMV infections and 40 mg/kg every 8 hours for treating acyclovir-resistant HSV infections.
Amantadine and Rimantadine
Amantadine is a tricyclic amine inhibitor of influenza A virus. Its mechanism of action involves inhibition of the transmembrane domain of the viral M2 protein, thus preventing viral uncoating during early stages of replication. It has activity against influenza A but no clinical activity against influenza B.240–243 Resistant strains can be developed in vitro and are also seen in household and nursing home contacts exposed to persons treated for acute influenza. It is indicated in influenza A prophylaxis and treatment and also for management of Parkinson’s disease and drug-induced extrapyramidal reactions. It is well absorbed orally and has body-wide distribution. It is 67% bound to plasma proteins and excreted unchanged in the urine by glomerular filtration and tubular secretion. The usual adult dosage for both prophylaxis and treatment is 100 mg twice a day. The dosage must be reduced in elderly patients and patients with renal insufficiency. In individuals who are 65 or older, the dose should be reduced to 100 mg every day. The dose for a CrCl less than 10 mL/minute is 200 mg per week. The most common adverse events are related to the CNS and correlate directly with high levels. Patients may present with confusion, seizures, hallucinations, and coma. Amantadine also causes GI upset.
Rimantadine is another tryciclic amine with similar properties, spectrum, and indications as amantadine.244,245 The main difference lies in its extensive metabolism by the liver and its minimal renal clearance, but it also requires dosage adjustment in advanced liver and kidney insufficiency (CrCl < 10 mL/minute) as well as in the elderly. Its major advantage is less frequent CNS toxicity. Dosage is 100 mg twice a day.
Ribavirin
Ribavirin is a triazole nucleoside analog that has broad-spectrum antiviral activity. It is effective in vitro against RSV, influenza A and B, HSV, HIV, hepatitis C virus, and viruses causing hemorrhagic fevers.215,246–248 Its currently approved clinical indications are treatment of pediatric patients with severe RSV infection, and in combination with injected interferon alfa and ribavirin capsules, it is approved for treatment of chronic hepatitis C.249,250 The IV form has been shown to reduce mortality rate in Korean/Asian hemorrhagic fever with renal syndrome.251 Its mechanism of action is not fully understood and intrinsic resistance has not been reported. It is available in oral, aerosolized, and IV formulations. It has high bioavailability through the oral or aerosolized forms and it is metabolized by the liver. Toxicity in the aerosolized formulation consists primarily of severe bronchospasm and cardiac rhythm abnormalities. The IV formulation also causes hemolytic anemia. Use in ventilated patients requires experienced personnel because environmental leaking and diffusion are common.252 Recommended dosage is 1.1 g/day of ribavirin administered by continuous aerozolization for 12 to 18 hours/day for 3 to 7 days. It should not be combined with other aerosolized medications. Induction of bronchospasm is common, and scheduled use of a bronchodilator may be required during ribarvirin therapy.
Oseltamivir
Oseltamivir is a sialic acid neuraminidase inhibitor that has recently become available for the treatment of both influenza A and B.253–255 Its mechanism of action is inhibition of the viral neuraminidase, causing in turn inhibition of virus release from infected cells and spread in the respiratory tract. Drug resistance has already been reported in vitro in strains with mutations that cause changes in the viral neuraminidase or hemagglutinin. In clinical studies of adults, only 1% to 2% of posttreatment strains showed evidence of mutations with decreased neuraminidase susceptibility. Recent reports have mentioned infrequent resistance in avian influenza and H1N1 strains 256–258 Cross-resistance between oseltamivir and zanamivir, the other member of the class, has also been observed in vitro. It is indicated for the prevention and early treatment of uncomplicated acute influenza A and B.259 Major clinical trials have demonstrated that oseltamivir reduces the duration, severity, and rate of complications requiring antibiotic use.255 Its role in complicated cases such as those seen in the critical care setting is unclear at this time, although the 2009 H1N1 epidemic contributed a wealth of data related to its use in critically ill patients.260,261 Oseltamivir is available in an oral form, which has greater than 75% bioavailability. The drug is converted to oseltamivir carboxylate that is eliminated unchanged in the urine. Less than 20% of the dose is eliminated in feces. Dose adjustment is recommended for renal failure and geriatric patients when CrCl is less than 30 mL/minute.262 The recommended dose in such settings is 75 mg orally every 24 hours for 5 days. Clinically relevant drug interactions have not been identified. Side effects, which include nausea, vomiting, and abdominal pain, are usually mild. The recommended dosage is 75 mg orally twice daily for 5 days and it is recommended that treatment is begun within 2 days of symptom onset. New formulations of IV oseltamivir are being developed.263
Zanamivir
Zanamivir is another sialic acid analog neuraminidase inhibitor that was recently approved for the treatment of both influenza A and B.253,264 Much like oseltamivir, its mechanism of action is by neuraminidase inhibition with subsequent inhibition of viral release and spread. Resistant strains have also been identified in vitro, and a resistant strain was also recovered from an immunocompromised patient with influenza B after 2 weeks of nebulized treatment.265,266 It is indicated in acute cases of influenza with early onset of treatment.254,267–269 Trials on patients with more severe disease are ongoing.270,271 Experience is also building with its use in prophylaxis.240,272,273 Zanamivir is available in a powder form for oral inhalation. A new IV formulation is also under development.274 Approximately 4% to 17% of the dose is absorbed and less than 10% of the drug is protein bound. It is excreted unchanged in the urine and unabsorbed drug is cleared in the feces. No dosage adjustment in renal or hepatic failure is recommended at this time, but experience is limited. Zanamivir does not have significant drug interactions.275,276 Bronchospasm may be precipitated particularly in patients with underlying lung disease.277 The recommended dose is 10 mg (two inhalations) twice a day for 5 days.
Specific Indications and Uses for Antiviral Therapy
Herpes Simplex and Varicella-Zoster
Acyclovir, famciclovir, and valacyclovir are the drugs of choice in the treatment of virtually all severe herpetic infections.203,204, 215, 278–285 The greater oral bioavailability of famciclovir and valacyclovir makes these agents very attractive, especially for treatment of VZV. Although primary HSV-1 and -2 infections can be treated orally, immunocompromised patients or severely ill patients will require IV therapy. A high index of suspicion for dissemination should be maintained in immunocompromised patients—mild, localized, or atypical disease may progress to fulminant disease. Duration of therapy in this setting is usually 7 to 14 days.
Finally, acyclovir-resistant HSV infections, which are an increasing problem in the immunocompromised population,206,207, 286,287 can be treated with foscarnet or cidofovir.
Primary VZV infection (chickenpox) should be treated in all adults, especially in the immunocompromised.278,281,285,288,289 Reactivation (shingles) should be treated because treatment has been shown to decrease duration and intensity of symptoms.290,291 Mild cases may be treated with oral famciclovir or valacyclovir. Severe cases (disseminated, immunocompromised patients with involvement of more than one dermatome, or ophthalmic involvement) should be treated with IV acyclovir. It is important to remember that these patients are contagious and should be suitably isolated using airborne precautions. As with HSV, resistant strains may be treated with foscarnet.292,293
Cytomegalovirus Infection
CMV infections require treatment in immunocompromised patients. Populations at increased risk of the disease are HIV-infected patients and transplant recipients. In HIV patients, both retinal and visceral involvement are initially treated with IV ganciclovir at 5 mg/kg every 12 hours for 2 weeks.208,223,224 Valganciclovir is a suitable oral formulation for the treatment of this infection. Various maintenance options are increasingly available (including ganciclovir ocular implants) but are beyond the scope of this chapter. Resistant cases may be treated with foscarnet 60 mg/kg every 8 hours for 2 weeks 229,236 or cidofovir. Clinical and laboratory monitoring while on either of these drugs is essential to avoid therapy-related complications. Refractory cases may be treated with cidofovir.
In the transplant patient, the combination of ganciclovir and IV immunoglobulin is now the treatment of choice for CMV pneumonia.223,294–296 High doses of acyclovir are useful as prophylaxis of CMV in the transplant patient but not in the HIV setting. Ganciclovir-resistant strains should be treated with foscarnet.
Influenza
Clear benefit in the treatment of influenza is consistently seen only when patients are treated early in the course of the disease.253,297–300 Therapeutic options (which can also be used prophylactically) include amantadine/rimantadine, oseltamivir, and zanamivir.301–306 In patients who present later in the course of the disease, the benefits of therapy are unstudied. Prevention of complications has not been clearly demonstrated. Further trials are required to demonstrate whether one class of drugs is superior to the other and whether they have a role in the critically ill patient. Rapid diagnosis of influenza is now available through different techniques that detect viral components in upper respiratory tract samples. These techniques are useful in detecting and treating infected hospitalized patients early and in regional surveillance of influenza. However, a negative test does not completely rule out influenza, and empirical therapy of ambulatory patients with a clinical syndrome strongly suggesting influenza is probably appropriate.
Avian and pandemic influenza have received much attention from the scientific community and the media in the past 2 years. Considerable efforts are being carried out for surveillance, prompt detection, and containment of these diseases.307–310 Critical care specialists should have increased awareness and involve infection control and public health authorities in patients with particularly severe forms of influenza with the appropriate epidemiologic background. Suspected and confirmed cases should be placed on airborne precautions. As mentioned earlier, although there are scarce reports of neuraminidase inhibitor resistance in some avian influenza strains, these viruses appear to be generally susceptible to oseltamivir and zanamivir.256,257
The 2009 H1N1 epidemic virtually changed how we manage influenza in critically ill patients. During this epidemic oseltamvir and zanamivir were frequently used late in the disease and at high doses, also in the setting of extracorporeal membrane oxygenation (ECMO), with variable results.261, 270, 311–313 At this point the true value of these agents is still unknown, although most experts agree that at the very least they may decrease viral shedding and transmission.
An important consideration is vaccination and postexposure prophylaxis in health care personnel working in acute and chronic care facilities. Appropriate vaccination and influenza control measures in both health care personnel and patients has proved to be effective in decreasing disease and even mortality rates in patients.314–316
Other Viral Infections
Other potentially treatable viral infections that may be encountered in the critical care setting include parvovirus, for which treatment with IV immunoglobulin has shown some beneficial effects;317–319 enterovirus, for which a new drug called pleconaril showed only marginal benefits in the setting of meningitis;320,321 and hemorrhagic fevers and hantavirus, for which ribavirin may be considered despite lack of definitive proof of efficacy.248 Dengue and yellow fever can be particularly severe and are most effectively managed with aggressive supportive care.
References
1. Sobel, JD. Practice guidelines for the treatment of fungal infections. For the Mycoses Study Group. Infectious Diseases Society of America. Clin Infect Dis. 2000; 30(4):652.
2. Ghannoum, MA, Rice, LB. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999; 12(4):501–517.
3. Gallis, HA, Drew, RH, Pickard, WW. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990; 12:308–329.
4. Hoeprich, PD. Clinical use of amphotericin B and derivatives: Lore, mystique, and fact. Clin Infect Dis. 1992; 14(Suppl 1):S114–S119.
5. Ostrosky-Zeichner, L, Marr, KA, Rex, JH, Cohen, SH. Amphotericin B: Time for a new “gold standard”. Clin Infect Dis. 2003; 37(3):415–425.
6. Groll, AH, Piscitelli, SC, Walsh, TJ. Clinical pharmacology of systemic antifungal agents: A comprehensive review of agents in clinical use, current investigational compounds, and putative targets for antifungal drug development. Adv Pharmacol. 1998; 44:343–499.
7. Sutton, DA, Sanche, SE, Revankar, SG, et al. In vitro amphotericin B resistance in clinical isolates of Aspergillus terreus, with a head-to-head comparison to voriconazole. J Clin Microbiol. 1999; 37(7):2343–2345.
8. Walsh, TJ, Melcher, GP, Rinaldi, MG, et al. Trichosporon beigelii, an emerging pathogen resistant to amphotericin B. J Clin Microbiol. 1990; 28:1616–1622.
9. Walsh, M, White, L, Atkinson, K, Enno, A. Fungal Pseudallescheria boydii lung infiltrates unresponsive to amphotericin B in leukaemic patients. Aust N Z J Med. 1992; 22:265–268.
10. Yoon, SA, Vazquez, JA, Steffan, PE, et al. High-frequency, in vitro reversible switching of Candida lusitaniae clinical isolates from amphotericin B susceptibility to resistance. Antimicrob Agents Chemother. 1999; 43(4):836–845.
11. Law, D, Moore, CB, Denning, DW. Amphotericin B resistance testing of Candida spp. : A comparison of methods. J Antimicrob Chemother. 1997; 40:109–112.
12. Nguyen, MH, Clancy, CJ, Yu, VL, et al. Do in vitro susceptibility data predict the microbiologic response to amphotericin B? Results of a prospective study of patients with Candida fungemia. J Infect Dis. 1998; 177:425–430.
13. Cleary, JD, Chapman, SW, Deng, J, Lobb, CJ. Amphotericin B enzyme-linked immunosorbent assay. Antimicrob Agents Chemother. 1996; 40:637–641.
14. Sabra, R, Branch, RA. Mechanisms of amphotericin B-induced decrease in glomerular filtration rate in rats. Antimicrob Agents Chemother. 1991; 35:2509–2514.
15. Lin, AC, Goldwasser, E, Bernard, EM, Chapman, SW. Amphotericin B blunts erythropoietin response to anemia. J Infect Dis. 1990; 161(2):348–351.
16. Craven, PC, Gremillion, DH. Risk factors of ventricular fibrillation during rapid amphotericin B infusion. Antimicrob Agents Chemother. 1985; 27:868–871.
17. Aguado, JM, Hidalgo, M, Moya, I, et al. Ventricular arrhythmias with conventional and liposomal amphotericin [letter; comment]. Lancet. 1993; 342(8881):1239.
18. Googe, JH, Walterspiel, JN. Arrhythmia caused by amphotericin B in a neonate. Pediatr Infect Dis J. 1988; 7(1):73.
19. Soler, JA, Ibanez, L, Zuazu, J, Julia, A. Bradycardia after rapid intravenous infusin of amphotericin B [letter; comment]. Lancet. 1993; 341(8841):372–373.
20. Oldfield, EC, III., Garst, PD, Hostettler, C, et al. Randomized, double-blind trial of 1- versus 4-hour amphotericin B infusion durations. Antimicrob Agents Chemother. 1990; 34:1402–1406.
21. Rex, JH, Bennett, JE, Sugar, AM, et al. A randomized trial comparing fluconazole with amphotericin B for the treatment of candidemia in patients without neutropenia. N Engl J Med. 1994; 331:1325–1330.
22. Kauffman, CA, Vazquez, JA, Sobel, JD, et al. Prospective multicenter surveillance study of funguria in hospitalized patients. Clin Infect Dis. 2000; 30(1):14–18.
23. Sobel, JD, Kauffman, CA, McKinsey, D, et al. Candiduria: A randomized, double-blind study of treatment with fluconazole and placebo. Clin Infect Dis. 2000; 30(1):19–24.
24. Wong-Beringer, A, Jacobs, RA, Guglielmo, BJ. Lipid formulations of amphotericin B: Clinical efficacy and toxicities. Clin Infect Dis. 1998; 27:603–618.
25. Walsh, TJ, Hiemenz, JW, Seibel, NL, et al. Amphotericin B lipid complex for invasive fungal infections: Analysis of safety and efficacy in 556 cases. Clin Infect Dis. 1998; 26:1383–1396.
26. Walsh, TJ, Hiemenz, JW, Seibel, N, Anaissie, EJ, Amphotericin B lipid complex in the treatment of 225 cases of invasive mycosis 34th Interscience Conference on Antimicrobial Agents and Chemotherapy, Orlando, FL. 1994.
27. Walsh, TJ, Seibel, NL, Arndt, C, et al. Amphotericin B lipid complex in pediatric patients with invasive fungal infections. Pediatr Infect Dis J. 1999; 18(8):702–708.
28. Cornely, O, Maertens, J, Bresnik, M, Herbrecht, R. Liposomal amphotericin B (L-AMB) as initial therapy for invasive filamentous fungal infections (IFFI): A randomized-prospective trial of a high loading regimen vs. standard dosing (AmBiLoad trial). Blood. 2005; 106:900a.
29. Wingard, JR, White, MH, Anaissie, EJ, et al, A Randomized double blind study of Ambisome and Abelcet in febrile neutropenic patients San Diego, CA, March 17-19. Focus on Fungal Infections, Final program presentation summaries abstracts. 1999.
30. Johnson, MD, Drew, RH, Perfect, JR. Chest discomfort associated with liposomal amphotericin B: Report of three cases and review of the literature. Pharmacotherapy. 1998; 18:1053–1061.
31. Kauffman, CA, Wiseman, SW. Anaphylaxis upon switching lipid-containing amphotericin B formulations. Clin Infect Dis. 1998; 26:1237–1238.
32. Ayestaran, A, Lopez, RM, Montoro, JB, et al. Pharmacokinetics of conventional formulation versus fat emulsion formulation of amphotericin B in a group of patients with neutropenia. Antimicrob Agents Chemother. 1996; 40:609–612.
33. Barquist, E, Fein, E, Shadick, D, et al. A randomized prospective trial of amphotericin B lipid emulsion versus dextrose colloidal solution in critically ill patients. J Trauma. 1999; 47(2):336–340.
34. Caillot, D, Chavanet, P, Casanovas, O, et al. Clinical evaluation of a new lipid-based delivery system for intravenous administration of amphotericin B. Eur J Clin Microbiol Infect Dis. 1992; 11:722–725.
35. Torre, D, Banfi, G, Tambini, R, et al. A retrospective study on the efficacy and safety of amphotericin B in a lipid emulsion for the treatment of cryptococcal meningitis in AIDS patients. J Infection. 1998; 37(1):36–38.
36. Trissel, LA. Amphotericin B does not mix with fat emulsion. Am J Health Syst Pharm. 1995; 52:1463–1464.
37. Ericsson, O, Hallmen, A-C, Wikstrom, I. Amphotericin B is incompatible with lipid emulsions. Ann Pharmacother. 1996; 30:298.
38. Owens, D, Fleming, RA, Restino, MS, et al. Stability of amphotericin B 0. 05 and 0. 5 mg/mL in 20% fat emulsion. Am J Health Syst Pharm. 1997; 54:683–686.
39. Cleary, JD. Amphotericin B formulated in a lipid emulsion. Ann Pharmacother. 1996; 30:409–412.
40. Mistro, S, Maciel Ide, M, de Menezes, RG, et al. Does lipid emulsion reduce amphotericin B nephrotoxicity? A systematic review and meta-analysis. Clin Infect Dis. 2012; 54(12):1774–1777.
41. Rex, JH, Walsh, TJ. Editorial response: Estimating the true cost of amphotericin B. Clin Infect Dis. 1999; 29(6):1408–1410.
42. Wingard, JR, Kubilis, P, Lee, L, et al. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin Infect Dis. 1999; 29(6):1402–1407.
43. Bates, DW, Su, L, Yu, DT, et al. Mortality and costs of acute renal failure associated with amphotericin B therapy. Clin Infect Dis. 2001; 32(5):686–693.
44. Bates, DW, Su, L, Yu, DT, et al. Correlates of acute renal failure in patients receiving parenteral amphotericin B. Kidney Int. 2001; 60(4):1452–1459.
45. Francis, P, Walsh, TJ. Evolving role of flucytosine in immunocompromised patients—New insights into safety, pharmacokinetics, and antifungal therapy. Clin Infect Dis. 1992; 15:1003–1018.
46. Whelan, WL. The genetic basis of resistance to 5-fluorocytosine in Candida species and Cryptococcus neoformans. Crit Rev Microbiol. 1987; 15:45–56.
47. King, D, Froggat, W, Smith, D, et al, Molecular epidemiology of an amphotericin B, 5-FC resistant strain of Candida lusitaniae 93rd National Meeting of the American Society for Microbiology, Atlanta, GA. 1993.
48. Vermes, A, van der Sijs, H, Guchelaar, HJ. Flucytosine: Correlation between toxicity and pharmacokinetic parameters. Chemotherapy. 2000; 46(2):86–94.
49. Lewis, RE, Klepser, ME, Pfaller, MA. In vitro pharmacodynamic characteristics of flucytosine determined by time-kill methods. Diagn Microbiol Infect Dis. 2000; 36(2):101–105.
50. Van der Horst, CM, Saag, MS, Cloud, GA, et al. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N Engl J Med. 1997; 337:15–21.
51. Sugar, AS. Use of amphotericin B with azole antifungal drugs: What are we doing? Antimicrob Agents Chemother. 1995; 39:1907–1912.
52. Sugar, AM. Antifungal combination therapy: Where we stand. Drug Resist Updates. 1998; 1:89–92.
53. Thompson, DF, Carter, JR. Drug-induced gynecomastia. Pharmacotherapy. 1993; 13(1):37–45.
54. Sarver, RG, Dalkin, BL, Ahmann, FR. Ketoconazole-induced adrenal crisis in a patient with metastatic prostatic adenocarcinoma: Case report and review of the literature. Urology. 1997; 49(5):781–785.
55. Rex, JH, Rinaldi, MG, Pfaller, MA. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995; 39:1–8.
56. White, TC, Marra, KA, Bowden, RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998; 11:382–402.
57. Anaissie, EJ, Kontonyiannis, DP, Huls, C, et al. Safety, plasma concentrations, and efficacy of high-dose fluconazole in invasive mold infections. J Infect Dis. 1995; 172:599–602.
58. Van Cutsem, J. The in-vitro antifungal spectrum of itraconazole. Mycoses. 1989; 32(Suppl 1):7–13.
59. Van Cutsem, J, Van Gerven, F, Janssen, PA. Activity of orally, topically, and parenterally administered itraconazole in the treatment of superficial and deep mycoses: Animal models. Rev Infect Dis. 1987; 9(Suppl 1):S15–S32.
60. Hardin, TC, Graybill, JR, Fetchick, R, et al. Pharmacokinetics of itraconazole following oral administration to normal volunteers. Antimicrob Agents Chemother. 1988; 32:1310–1313.
61. Stevens, DA. Itraconazole in cyclodextrin solution. Pharmacotherapy. 1999; 19:603–611.
62. Boogaerts, J, Michaux, J-L, Bosly, A, et al, Pharmacokinetics and safety of seven days of intravenous (IV) itraconazole followed by two weeks oral itraconazole solution in patients with haematological malignancy 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, LA. 1996.
63. Vandewoude, K, Vogelaers, D, Decruyenaere, J, et al. Concentrations in plasma and safety of 7 days of intravenous itraconazole followed by 2 weeks of oral itraconazole solution in patients in intensive care units. Antimicrob Agents Chemother. 1997; 41(12):2714–2718.
64. Zhou, HH, Goldman, M, Wu, J, et al. A pharmacokinetic study of intravenous itraconazole followed by oral administration of itraconazole capsules in patients with advanced human immunodeficiency virus infection. J Clin Pharmacol. 1998; 38(7):593–602.
65. Herbrecht, R, Denning, DW, Patterson, TF, et al. Voriconazole versus amphotericin B for primary therapy of invasive aspergillosis. N Engl J Med. 2002; 347(6):408–415.
66. Kullberg, BJ, Sobel, JD, Ruhnke, M, et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: A randomised non-inferiority trial. Lancet. 2005; 366(9495):1435–1442.
67. Ostrosky-Zeichner, L, Oude Lashof, AM, Kullberg, BJ, Rex, JH. Voriconazole salvage treatment of invasive candidiasis. Eur J Clin Microbiol Infect Dis. 2003; 22(11):651–655.
68. Perfect, JR. Use of newer antifungal therapies in clinical practice: What do the data tell us? Oncology (Williston Park). 2004; 18(13 Suppl 7):15–23.
69. Perfect, JR, Marr, KA, Walsh, TJ, et al. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin Infect Dis. 2003; 36(9):1122–1131.
70. Imhof, A, Balajee, SA, Fredricks, DN, et al. Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin Infect Dis. 2004; 39(5):743–746.
71. Marty, FM, Cosimi, LA, Baden, LR. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell transplants. N Engl J Med. 2004; 350(9):950–952.
72. Boucher, HW, Groll, AH, Chiou, CC, Walsh, TJ. Newer systemic antifungal agents: Pharmacokinetics, safety and efficacy. Drugs. 2004; 64(18):1997–2020.
73. Donnelly, JP, De Pauw, BE. Voriconazole—A new therapeutic agent with an extended spectrum of antifungal activity. Clin Microbiol Infect. 2004; 10(Suppl 1):107–117.
74. Herbrecht, R. Voriconazole: Therapeutic review of a new azole antifungal. Expert Rev Anti Infect Ther. 2004; 2(4):485–497.
75. Cornely, OA, Maertens, J, Winston, DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007; 356(4):348–359.
76. Cornely, OA, Vehreschild, JJ, Ruping, MJ. Current experience in treating invasive zygomycosis with posaconazole. Clin Microbiol Infect. 2009; 15(Suppl 5):77–81.
77. Groll, AH, Walsh, TJ. Posaconazole: Clinical pharmacology and potential for management of fungal infections. Expert Rev Anti-Infect Ther. 2005; 3(4):467–487.
78. Torres, HA, Hachem, RY, Chemaly, RF, et al. Posaconazole: A broad-spectrum triazole antifungal. Lancet Infect Dis. 2005; 5(12):775–785.
79. Kurtz, MB, Douglas, CM. Lipopeptide inhibitors of fungal glucan synthase. J Med Vet Mycol. 1997; 35(2):79–86.
80. Espinel-Ingroff, A. Comparison of in vitro activities of the new triazole SCH56592 and the echinocandins MK-0991 (L-743,872) and LY303366 against opportunistic filamentous and dimorphic fungi and yeasts. J Clin Microbiol. 1998; 36(10):2950–2956.
81. Del Poeta, M, Schell, WA, Perfect, JR. In vitro antifungal activity of pneumocandin L-743,872 against a variety of clinically important molds. Antimicrob Agents Chemother. 1997; 41(8):1835–1836.
82. Petraitis, V, Petraitiene, R, Groll, AH, et al. Antifungal efficacy, safety, and single-dose pharmacokinetics of LY303366, a novel echinocandin B, in experimental pulmonary aspergillosis in persistently neutropenic rabbits. Antimicrob Agents Chemother. 1998; 42:2898–2905.
83. Abruzzo, GK, Flattery, AM, Gill, CJ, et al. Evaluation of water-soluble pneumocandin analogs L-733560, L-705589, and L-731373 with mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1995; 39:1077–1081.
84. Abruzzo, GK, Flattery, AM, Gill, CJ, et al. Evaluation of the echincocandin antifungal MK-0991 (L-743,872): Efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob Agents Chemother. 1997; 41:2333–2338.
85. Mora-Duarte, J, Betts, R, Rotstein, C, et al. Comparison of caspofungin and amphotericin B for invasive candidiasis. N Engl J Med. 2002; 347(25):2020–2029.
86. Maertens, J, Raad, I, Petrikkos, G, et al. Efficacy and safety of caspofungin for treatment of invasive aspergillosis in patients refractory to or intolerant of conventional antifungal therapy. Clin Infect Dis. 2004; 39(11):1563–1571.
87. Walsh, TJ, Teppler, H, Donowitz, GR, et al. Caspofungin versus liposomal amphotericin B for empirical antifungal therapy in patients with persistent fever and neutropenia. N Engl J Med. 2004; 351(14):1391–1402.
88. Morrison, VA. Echinocandin antifungals: Review and update. Expert Rev Anti-Infect Ther. 2006; 4(2):325–342.
89. Zaas, AK, Alexander, BD. Echinocandins: Role in antifungal therapy, 2005. Expert Opin Pharmacother. 2005; 6(10):1657–1668.
90. Marr, KA, Hachem, R, Papanicolaou, G, et al. Retrospective study of the hepatic safety profile of patients concomitantly treated with caspofungin and cyclosporin A. Transplant Infect Dis. 2004; 6(3):110–116.
91. Betts, RF, Nucci, M, Talwar, D, et al. A multicenter, double-blind trial of a high-dose caspofungin treatment regimen versus a standard caspofungin treatment regimen for adult patients with invasive candidiasis. Clin Infect Dis. 2009; 48(12):1676–1684.
92. Jarvis, B, Figgitt, DP, Scott, LJ. Micafungin. Drugs. 2004; 64(9):969–982.
93. Zaas, AK, Steinbach, WJ. Micafungin: The US perspective. Expert Rev Anti-Infect Ther. 2005; 3(2):183–190.
94. Pappas, PG, Rotstein, CM, Betts, RF, et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin Infect Dis. 2007; 45(7):883–893.
95. Kuse, ER, Chetchotisakd, P, da Cunha, CA, et al. Micafungin versus liposomal amphotericin B for candidaemia and invasive candidosis: A phase III randomised double-blind trial. Lancet. 2007; 369(9572):1519–1527.
96. van Burik, JA, Ratanatharathorn, V, Stepan, DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004; 39(10):1407–1416.
97. Vazquez, JA. Anidulafungin: A new echinocandin with a novel profile. Clin Ther. 2005; 27(6):657–673.
98. Reboli, AC, Rotstein, C, Pappas, PG, et al. Anidulafungin versus fluconazole for invasive candidiasis. N Engl J Med. 2007; 356(24):2472–2482.
99. Krause, DS, Reinhardt, J, Vazquez, JA, et al. Phase 2, randomized, dose-ranging study evaluating the safety and efficacy of anidulafungin in invasive candidiasis and candidemia. Antimicrob Agents Chemother. 2004; 48(6):2021–2024.
100. Pfaller, MA. Anidulafungin: An echinocandin antifungal. Expert Opin Investig Drugs. 2004; 13(9):1183–1197.
101. Pfaller, MA, Jones, RN, Messer, SA, et al. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: Frequency of occurrence and antifungal susceptibility in the SCOPE program. Diagn Microbiol Infect Dis. 1998; 30:121–129.
102. Rex, JH, Walsh, T, Sobel, JD, et al. IDSA treatment guidelines for candidiasis. Clin Infect Dis. 2000; 30:662–678.
103. Pappas, PG, Rex, JH, Sobel, JD, et al. Guidelines for treatment of candidiasis. Clin Infect Dis. 2004; 38(2):161–189.
104. Ostrosky-Zeichner, L, Pappas, PG. Invasive candidiasis in the intensive care unit. Crit Care Med. 2006; 34(3):857–863.
105. Pappas, PG, Kauffman, CA, Andes, D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009; 48(5):503–535.
106. Rex, JH, Walsh, TJ, Anaissie, EA. Fungal infections in iatrogenically compromised hosts. Adv Intern Med. 1998; 43:321–371.
107. Edwards, JE, Jr., Bodey, GP, Bowden, RA, et al. International conference for the development of a consensus on the management and prevention of severe candidal infections. Clin Infect Dis. 1997; 25:43–59.
108. Andes, DR, Safdar, N, Baddley, JW, et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: A patient-level quantitative review of randomized trials. Clin Infect Dis. 2012; 54(8):1110–1122.
109. Rex, JH, Cooper, CR, Jr., Merz, WG, et al. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob Agents Chemother. 1995; 39:906–909.
110. Rex, JH, Pfaller, MA, Barry, AL, et al. Antifungal susceptibility testing of isolates from a randomized, multicenter trial of fluconazole vs. amphotericin B as treatment of non-neutropenic patients with candidemia. NIAID Mycoses Study Group and the Candidemia Study Group. Antimicrob Agents Chemother. 1995; 39:40–44.
111. Pfaller, MA, Bale, MJ, Buschelman, B, Rhomberg, P. Antifungal activity of a new triazole, D0870, compared with four other antifungal agents tested against clinical isolates of Candida and Torulopsis glabrata. Diagn Microbiol Infect Dis. 1994; 19:75–80.
112. Martinez-Suarez, JV, Rodriguez-Tudela, JL. Patterns of in vitro activity of itraconazole and imidazole antifungal agents against Candida albicans with decreased susceptibility to fluconazole from Spain. Antimicrob Agents Chemother. 1995; 39:1512–1516.
113. Wanger, A, Mills, K, Nelson, PW, Rex, JH. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: Enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob Agents Chemother. 1995; 39:2520–2522.
114. Rex, JH, Lozano-Chiu, M, Paetznick, V, et al, Susceptibility testing of current Candida bloodstream isolates from Mycoses Study Group (MSG) collaborative study #34: Isolates of C. krusei are often resistant to both fluconazole and amphotericin B 36th Annual Meeting of the Infectious Diseases Society of America. Denver, CO. 1998.
115. Fisher, MA, Shen, S-H, Haddad, J, Tarry, WF. Comparison of in vivo activity of fluconazole with that of amphotericin B against Candida tropicalis, Candida glabrata, and Candida krusei. Antimicrob Agents Chemother. 1989; 33:1443–1446.
116. Karyotakis, NC, Anaissie, EJ, Hachem, R, et al. Comparison of the efficacy of polyenes and triazoles against hematogenous Candida krusei infection in neutropenic mice. J Infect Dis. 1993; 168:1311–1313.
117. Pfaller, MA, Messer, SA, Hollis, RJ. Strain delineation and antifungal susceptibilities of epidemiologically related and unrelated isolates of Candida lusitaniae. Diagn Microbiol Infect Dis. 1994; 20:127–133.
118. Ghannoum, MA, Rex, JH, Galgiani, JN. Susceptibility testing of fungi: Current status of the correlation of in vitro data with clinical outcome. J Clin Microbiol. 1996; 34:489–495.
119. Ostrosky-Zeichner, L, Rex, JH, Pappas, PG, et al. Antifungal susceptibility survey of 2,000 bloodstream Candida isolates in the United States. Antimicrob Agents Chemother. 2003; 47(10):3149–3154.
120. Lowder, JN, Lazarus, HM, Herzig, RH. Bacteremias and fungemias in oncologic patients with central venous catheters: Changing spectrum of infection. Arch Intern Med. 1982; 142:1456–1459.
121. Fraser, VJ, Jones, M, Dunkel, J, et al. Candidemia in a tertiary care hospital: Epidemiology, risk factors, and predictors of mortality. Clin Infect Dis. 1992; 15:414–421.
122. Wey, SB, Mori, M, Pfaller, MA, et al. Risk factors for hospital-acquired candidemia: A matched case-control study. Arch Intern Med. 1989; 149:2349–2353.
123. Karabinis, A, Hill, C, Leclerq, B, et al. Risk factors for candidemia in cancer patients: A case-control study. J Clin Microbiol. 1988; 26:429–432.
124. Rex, JH, Bennett, JE, Sugar, AM, et al. Intravascular catheter exchanges and the duration of candidemia. Clin Infect Dis. 1995; 21:994–996.
125. Lecciones, JA, Lee, JW, Navarro, EE, et al. Vascular catheter-associated fungemia in patients with cancer: Analysis of 155 episodes. Clin Infect Dis. 1992; 14:875–883.
126. Nguyen, MH, Peacock, JE, Jr., Tanner, DC, et al. Therapeutic approaches in patients with candidemia: Evaluation in a multicenter, prospective, observational study. Arch Intern Med. 1995; 155:2429–2435.
127. Abi-Said, D, Anaissie, E, Uzun, Ö, et al. The epidemiology of hematogenous candidiasis caused by different Candida species. Clin Infect Dis. 1997; 24:1122–1128.
128. Anaissie, EJ, Rex, JH, Uzun, Ö, Vartivarian, S. Predictors of adverse outcome in cancer patients with candidemia. Am J Med. 1998; 104:238–245.
129. Krause, W, Matheis, H, Wulf, K. Fungaemia and funguria after oral administration of Candida albicans. Lancet. 1969; 598–599.
130. Bodey, G, Bueltmann, B, Duguid, W, et al. Fungal infections in cancer patients: An international autopsy survey. Eur J Clin Microbiol Infect Dis. 1992; 11:99–109.
131. Hughes, WT. Systemic candidiasis: A study of 109 fatal cases. Pediatr Infect Dis J. 1982; 1:11–18.
132. Telenti, A, Steckelberg, JM, Stockman, L, et al. Quantitative blood cultures in candidemia. Mayo Clin Proc. 1991; 66:1120–1123.
133. Paya, CV, Guerra, L, Marsh, HM, et al. Limited usefulness of quantitative culture of blood drawn through the device for diagnosis of intravascular-device-related bacteremia. J Clin Microbiol. 1989; 27:1431–1433.
134. Pittet, D, Monod, M, Suter, PM, et al. Candida colonization and subsequent infections in critically ill surgical patients. Ann Surg. 1994; 220:751–758.
135. Cohen, DM, Rex, JH. Antifungal therapy. In: Schlossberg DM, ed. Current Therapy of Infectious Disease. St. Louis: Mosby-Year Book; 1996:609–612.
136. Martins, MD, Rex, JH. Antifungal therapy. In: Parillo JE, ed. Current Therapy in Critical Care Medicine. 3rd ed. St. Louis: Mosby-Year Book; 1997:295–300.
137. Cornely, OA, Lasso, M, Betts, R, et al. Caspofungin for the treatment of less common forms of invasive candidiasis. J Antimicrob Chemother. 2007; 60(2):363–369.
138. Saag, MS, Graybill, RJ, Larsen, RA, et al. Practice guidelines for the management of cryptococcal disease. Clin Infect Dis. 2000; 30:710–718.
139. Perfect, JR, Dismukes, WE, Dromer, F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010; 50(3):291–322.
140. Dismukes, WE, Cloud, G, Gallis, HA, et al. Treatment of cryptococcal meningitis with combination amphotericin B and flucytosine for four as compared with six weeks. N Engl J Med. 1987; 317:334–341.
141. Bennett, JE, Dismukes, WE, Duma, RJ, et al. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptococcal meningitis. N Engl J Med. 1979; 301:126–131.
142. Baddley, JW, Perfect, JR, Oster, RA, et al. Pulmonary cryptococcosis in patients without HIV infection: Factors associated with disseminated disease. Eur J Clin Microbiol Infect Dis. 2008; 27(10):937–943.
143. Pappas, PG, Perfect, JR, Cloud, GA, et al. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin Infect Dis. 2001; 33(5):690–699.
144. Leenders, AC, Reiss, P, Portegies, P, et al. Liposomal amphotericin B (AmBisome) compared with amphotericin B both followed by oral fluconazole in the treatment of AIDS-associated cryptococcal meningitis. AIDS. 1997; 11(12):1463–1471.
145. Baddour, LM, Perfect, JR, Ostrosky-Zeichner, L. Successful use of amphotericin B lipid complex in the treatment of cryptococcosis. Clin Infect Dis. 2005; 40(Suppl 6):S409–S413.
146. Van der horst, C, Saag, M, Cloud, G, et al, Part 1. Randomized double blind comparison of amphotericin B plus flucytosine to amphotericin B alone (Step 1) followed by a comparison of fluconazole to itraconazole (Step 2) in the treatment of acute cryptococcal meningitis in patients with AIDS 35th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA. 1995.
147. Saag, MS, Powderly, WG, Cloud, GA, et al. Comparison of amphotericin B with fluconazole in the treatment of acute AIDS-associated cryptococcal meningitis. N Engl J Med. 1992; 326:83–89.
148. Saag, MS, Cloud, GC, Graybill, JR, et al, Comparison of fluconazole versus itraconzole as maintenance therapy of AIDS-associated cryptococcal meningitis 35th Interscience Conference on Antimicrobial Agents and Chemotherapy, San Francisco, CA. 1995.
149. Denning, DW, Armstrong, RW, Lewis, BH, Stevens, DA. Elevated cerebrospinal fluid pressures in patients with cryptococcal meningitis and acquired immunodeficiency syndrome. Am J Med. 1991; 91:267–272.
150. Rex, JH, Larsen, RA, Dismukes, WE, et al. Catastrophic visual loss due to Cryptococcus neoformans meningitis. Medicine (Baltimore). 1993; 72:207–224.
151. Ostrosky-Zeichner, L, Soto-Hernandez, JL, Angeles-Morales, V, et al. Effects of pentoxifylline or dexmethasone in combination with amphotericin B in experimental murine cerebral cryptococcosis: Evidence of neuroexcitatory pathogenic mechanisms. Antimicrob Agents Chemother. 1996; 40:1194–1197.
152. Stevens, DA, Kan, VL, Judson, MA, et al. Practice guidelines for diseases caused by Aspergillus. Clin Infect Dis. 2000; 30:696–709.
153. Perfect, JR. Management of invasive mycoses in hematology patients: Current approaches. Oncology (Williston Park). 2004; 18(13 Suppl 7):5–14.
154. Walsh, TJ, Anaissie, EJ, Denning, DW, et al. Treatment of aspergillosis: Clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008; 46(3):327–360.
155. Verweij, PE, Denning, DW. Diagnostic and therapeutic strategies for invasive aspergillosis. Semin Respir Crit Care Med. 1997; 18:203–215.
156. Denning, DW. Early diagnosis of invasive aspergillosis. Lancet. 2000; 355(9202):423–424.
157. Denning, DW, Stevens, DA. Antifungal and surgical treatment of invasive aspergillosis: Review of 2,121 published cases. Rev Infect Dis. 1990; 12:1147–1201.
158. Denning, DW, Lee, JY, Hostetler, JS, et al. NIAID Mycoses Study Group multicenter trial of oral itraconazole therapy for invasive aspergillosis. Am J Med. 1994; 97:135–144.
159. Meersseman, W, Van Wijngaerden, E. Invasive aspergillosis in the ICU: An emerging disease. Intensive Care Med. 2007; 33(10):1679–1681.
160. Vandewoude, KH, Blot, SI, Benoit, D, et al. Invasive aspergillosis in critically ill patients: Attributable mortality and excesses in length of ICU stay and ventilator dependence. J Hosp Infect. 2004; 56(4):269–276.
161. Pfeiffer, CD, Fine, JP, Safdar, N. Diagnosis of invasive aspergillosis using a galactomannan assay: A meta-analysis. Clin Infect Dis. 2006; 42(10):1417–1427.
162. Wheat, J, Sarosi, G, McKinsey, D, et al. Practice guidlines for the management of patients with histoplasmosis. Clin Infect Dis. 2000; 30:688–695.
163. Wheat, J, Sarosi, G, McKinsey, D, et al. Practice guidelines for the management of patients with histoplasmosis. Infectious Diseases Society of America. Clin Infect Dis. 2000; 30(4):688–695.
164. Wheat, LJ, Freifeld, AG, Kleiman, MB, et al. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007; 45(7):807–825.
165. McKinsey, DS, Kauffman, CA, Pappas, PG, et al. Fluconazole therapy for histoplasmosis. Clin Infect Dis. 1996; 23:996–1001.
166. Buckley, HR, Richardson, MD, Evans, EG, Wheat, LJ. Immunodiagnosis of invasive fungal infection. J Med Vet Mycol. 1992; 30(Suppl 1):249–260.
167. Johnson, PC, Wheat, LJ, Cloud, GA, et al. Safety and efficacy of liposomal amphotericin B compared with conventional amphotericin B for induction therapy of histoplasmosis in patients with AIDS. Ann Intern Med. 2002; 137(2):105–109.
168. Roden, MM, Zaoutis, TE, Buchanan, WL, et al. Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Clin Infect Dis. 2005; 41(5):634–653.
169. Reed, C, Bryant, R, Ibrahim, AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008; 47(3):364–371.
170. O’Grady, NP, Barie, PS, Bartlett, JG, et al. Practice guidelines for evaluating new fever in critically ill adult patients. Task Force of the Society of Critical Care Medicine and the Infectious Diseases Society of America. Clin Infect Dis. 1998; 26(5):1042–1059.
171. Blumberg, HM, Jarvis, WR, Wenzel, RP, The NEMIS Study, Risk factors for Candida bloodstream infection in surgical intensive care units: NEMIS prospective multicenter study 36th Annual Meeting of the Infectious Diseases Society of America. Denver, CO. 1998.
172. Ostrosky-Zeichner, L. New approaches to the risk of Candida in the intensive care unit. Curr Opin Infect Dis. 2003; 16(6):533–537.
173. Pelz, R, Lipsett, PA, Swoboda, S, et al, Do surveillance cultures predict fungal infeciton in critically ill patients? 36th Annual Meeting of the Infectious Diseases Society of America, Denver, CO. 1998.
174. Rodriguez, LJ, Anaissie, EJ, Rex, JH. Pneumonia due to Candida species. In: Sarosi GA, Davies SF, eds. Fungal Disease of the Lung. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2000:115–122.
175. Garey, KW, Rege, M, Pai, MP, et al. Time to initiation of fluconazole therapy impacts mortality in patients with candidemia: A multi-institutional study. Clin Infect Dis. 2006; 43(1):25–31.
176. Leon, C, Alvarez-Lerma, F, Ruiz-Santana, S, et al. Fungal colonization and/or infection in non-neutropenic critically ill patients: Results of the EPCAN observational study. Eur J Clin Microbiol Infect Dis. 2009; 28(3):233–242.
177. Leon, C, Ruiz-Santana, S, Saavedra, P, et al. A bedside scoring system (“Candida score”) for early antifungal treatment in nonneutropenic critically ill patients with Candida colonization. Crit Care Med. 2006; 34(3):730–737.
178. Ostrosky-Zeichner, L, Pappas, PG, Shoham, S, et al. Improvement of a clinical prediction rule for clinical trials on prophylaxis for invasive candidiasis in the intensive care unit. Mycoses. 2009; 13:R156.
179. Ostrosky-Zeichner, L, Sable, C, Sobel, J, et al. Multicenter retrospective development and validation of a clinical prediction rule for nosocomial invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis. 2007; 26(4):271–276.
180. Hermsen, ED, Zapapas, MK, Maiefski, M, et al. Validation and comparison of clinical prediction rules for invasive candidiasis in intensive care unit patients: A matched case-control study. Crit Care. 2011; 15(4):R198.
181. Schuster, MG, Edwards, JE, Jr., Sobel, JD, et al. Empirical fluconazole versus placebo for intensive care unit patients: A randomized trial. Ann Intern Med. 2008; 149(2):83–90.
182. Goodman, JL, Winston, DJ, Greenfield, RA, et al. A controlled trial of fluconazole to prevent fungal infections in patients undergoing bone marrow transplantation. N Engl J Med. 1992; 326:845–851.
183. Slavin, MA, Osborne, B, Adams, R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after bone marrow transplantation—A prospective, randomized, double-blind study. J Infect Dis. 1995; 171:1545–1552.
184. Kung, N, Fisher, N, Gunson, B, et al. Fluconazole prophylaxis for high-risk liver transplant recipients. Lancet. 1995; 349:1234–1235.
185. Tollemar, J, Hockerstedt, K, Ericzon, BG, et al. Liposomal amphotericin B prevents invasive fungal infections in liver transplant recipients. A randomized, placebo-controlled study. Transplantation. 1995; 59:45–50.
186. Linden, P, Kramer, DJ, Mazariegos, G, et al, Low-dose amphotericin B for the prophylaxis of serious Candida infections in high-risk liver recipients 36th Interscience Conference on Antimicrobial Agents and Chemotherapy, New Orleans, LA. 1996.
187. Winston, DJ, Pakrasi, A, Busuttil, RW. Prophylactic fluconazole in liver transplant recipients: A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999; 131:729–737.
188. Winston, DJ, Chandrasekar, PH, Lazarus, HM, et al. Fluconazole prophylaxis of fungal infections in patients with acute leukemia: Results of a randomized placebo-controlled, double-blind, multicenter trial. Ann Intern Med. 1993; 118:495–503.
189. Schaffner, A, Schaffner, M. Effect of prophylactic fluconazole on the frequency of fungal infections, amphotericin B use, and health care costs in patients undergoing intensive chemotherapy for hematologic neoplasias. J Infect Dis. 1995; 172:1035–1041.
190. Walsh, TJ, Hiemenz, J, Pizzo, PA. Editorial response: Evolving risk factors for invasive fungal infections—All neutropenic patients are not the same. Clin Infect Dis. 1994; 18:793–798.
191. Eggimann, P, Francioli, P, Bille, J, et al. Fluconazole prophylaxis prevents intraabdominal candidiasis in high-risk surgical patients. Crit Care Med. 1999; 27:1066–1072.
192. Garbino, J, Lew, DP, Romand, JA, et al. Prevention of severe Candida infections in nonneutropenic, high-risk, critically ill patients: A randomized, double-blind, placebo-controlled trial in patients treated by selective digestive decontamination. Intensive Care Med. 2002; 28(12):1708–1717.
193. Pelz, RK, Hendrix, CW, Swoboda, SM, et al. Double-blind placebo-controlled trial of fluconazole to prevent candidal infections in critically ill surgical patients. Ann Surg. 2001; 233(4):542–548.
194. Playford, EG, Webster, AC, Sorrell, TC, Craig, JC. Antifungal agents for preventing fungal infections in non-neutropenic critically ill and surgical patients: Systematic review and meta-analysis of randomized clinical trials. J Antimicrob Chemother. 2006; 57(4):628–638.
195. Shorr, AF, Chung, K, Jackson, WL, et al. Fluconazole prophylaxis in critically ill surgical patients: A meta-analysis. Crit Care Med. 2005; 33(9):1928–1935.
196. Ostrosky-Zeichner, L. Prophylaxis and treatment of invasive candidiasis in the intensive care setting. Eur J Clin Microbiol Infect Dis. 2004; 23(10):739–744.
197. Ostrosky-Zeichner, L. Prophylaxis for invasive candidiasis in the intensive care unit: Is it time? Crit Care Med. 2005; 33(9):2121–2122.
198. Andes, D, Lepak, A. Antifungal therapeutic drug monitoring progress: Getting it right the first time. Clin Infect Dis. 2012; 55(3):391–393.
199. Howard, SJ, Felton, TW, Gomez-Lopez, A, Hope, WW. Posaconazole: The case for therapeutic drug monitoring. Ther Drug Monit. 2012; 34(1):72–76.
200. Clinical Laboratory Standards Insitute. Approved standard CLSI document M27-MA3. Wayne, PA: Clinical Laboratory Standards Insitute; 2008.
201. Rex, JH, Pfaller, MA, Walsh, TJ, et al. Antifungal susceptibility testing: Practical aspects and current challenges. Clin Microbiol Rev. 2001; 14(4):643–658.
202. Laskin, OL. Acyclovir. Pharmacology and clinical experience. Arch Intern Med. 1984; 144(6):1241–1246.
203. Whitley, RJ, Gnann, JW, Jr. Acyclovir: A decade later [published errata appear in N Engl J Med 1993;328(9):671 and 1997;337(23):1703]. N Engl J Med. 1992; 327(11):782–789.
204. Whitley, RJ, Middlebrooks, M, Gnann, JW, Jr. Acyclovir: The past ten years. Adv Exp Med Biol. 1990; 278:243–253.
205. Safrin, S. Treatment of acyclovir-resistant herpes simplex virus infections in patients with AIDS. J Acquir Immune Defic Syndr. 1992; 5(Suppl 1):S29–S32.
206. Jacobson, MA, Berger, TG, Fikrig, S, et al. Acyclovir-resistant varicella zoster virus infection after chronic oral acyclovir therapy in patients with the acquired immunodeficiency syndrome (AIDS). Ann Intern Med. 1990; 112(3):187–191.
207. Erlich, KS, Mills, J, Chatis, P, et al. Acyclovir-resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1989; 320(5):293–296.
208. Nichols, WG, Boeckh, M. Recent advances in the therapy and prevention of CMV infections. J Clin Virol. 2000; 16(1):25–40.
209. Meyers, JD, Reed, EC, Shepp, DH, et al. Acyclovir for prevention of cytomegalovirus infection and disease after allogeneic marrow transplantation. N Engl J Med. 1988; 318(2):70–75.
210. Laskin, OL, Longstreth, JA, Saral, R, et al. Pharmacokinetics and tolerance of acyclovir, a new anti-herpesvirus agent, in humans. Antimicrob Agents Chemother. 1982; 21(3):393–398.
211. Scott, LL. Perinatal herpes: Current status and obstetric management strategies. Pediatr Infect Dis J. 1995; 14(10):827–832.
212. Sacks, SL, Wilson, B. Famciclovir/penciclovir. Adv Exp Med Biol. 1999; 458:135–147.
213. Jarvest, RL, Sutton, D, Vere Hodge, RA. Famciclovir: Discovery and development of a novel antiherpesvirus agent. Pharm Biotechnol. 1998; 11:313–343.
214. Luber, AD, Flaherty, JF, Jr. Famciclovir for treatment of herpesvirus infections. Ann Pharmacother. 1996; 30(9):978–985.
215. Keating, MR. Antiviral agents for non-human immunodeficiency virus infections. Mayo Clin Proc. 1999; 74(12):1266–1283.
216. Faro, S. A review of famciclovir in the management of genital herpes. Infect Dis Obstet Gynecol. 1998; 6(1):38–43.
217. Tyring, SK. Efficacy of famciclovir in the treatment of herpes zoster. Semin Dermatol. 1996; 15(2 Suppl 1):27–31.
218. Lowance, D, Neumayer, HH, Legendre, CM, et al. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group [see comments]. N Engl J Med. 1999; 340(19):1462–1470.
219. Perry, CM, Faulds, D. Valaciclovir: A review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy in herpesvirus infections. Drugs. 1996; 52(5):754–772.
220. Stein, GE. Pharmacology of new antiherpes agents: Famciclovir and valacyclovir. J Am Pharm Assoc (Wash). 1997; NS37(2):157–163.
221. Bell, AR. Valaciclovir update. Adv Exp Med Biol. 1999; 458:149–157.
222. Erice, A, Chou, S, Biron, KK, et al. Progressive disease due to ganciclovir-resistant cytomegalovirus in immunocompromised patients. N Engl J Med. 1989; 320(5):289–293.
223. Cheung, TW, Teich, SA. Cytomegalovirus infection in patients with HIV infection. Mt Sinai J Med. 1999; 66(2):113–124.
224. Danner, SA. Management of cytomegalovirus disease. AIDS. 1995; 9(Suppl 2):S3–S8.
225. Emanuel, D, Cunningham, I, Jules-Elysee, K, et al. Cytomegalovirus pneumonia after bone marrow transplantation successfully treated with the combination of ganciclovir and high-dose intravenous immune globulin. Ann Intern Med. 1988; 109(10):777–782.
226. Goodrich, JM, Bowden, RA, Fisher, L, et al. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med. 1993; 118(3):173–178.
227. Hardy, D, Spector, S, Polsky, B, et al. Combination of ganciclovir and granulocyte-macrophage colony-stimulating factor in the treatment of cytomegalovirus retinitis in AIDS patients. The ACTG 073 Team. Eur J Clin Microbiol Infect Dis. 1994; 13(Suppl 2):S34–S40.
228. Holland, GN. New strategies for the management of AIDS-related CMV retinitis in the era of potent antiretroviral therapy. Ocul Immunol Inflamm. 1999; 7(3-4):179–188.
229. Whitley, RJ, Jacobson, MA, Friedberg, DN, et al. Guidelines for the treatment of cytomegalovirus diseases in patients with AIDS in the era of potent antiretroviral therapy: Recommendations of an international panel. International AIDS Society-USA. Arch Intern Med. 1998; 158(9):957–969.
230. Cvetkovic, RS, Wellington, K. Valganciclovir: A review of its use in the management of CMV infection and disease in immunocompromised patients. Drugs. 2005; 65(6):859–878.
231. Razonable, RR, Paya, CV. Valganciclovir for the prevention and treatment of cytomegalovirus disease in immunocompromised hosts. Expert Rev Anti Infect Ther. 2004; 2(1):27–41.
232. Lalezari, JP, Stagg, RJ, Jaffe, HS, et al. A preclinical and clinical overview of the nucleotide-based antiviral agent cidofovir (HPMPC). Adv Exp Med Biol. 1996; 394:105–115.
233. Cundy, KC, Petty, BG, Flaherty, J, et al. Clinical pharmacokinetics of cidofovir in human immunodeficiency virus-infected patients. Antimicrob Agents Chemother. 1995; 39(6):1247–1252.
234. Safrin, S, Cherrington, J, Jaffe, HS. Clinical uses of cidofovir. Rev Med Virol. 1997; 7(3):145–156.
235. Safrin, S, Cherrington, J, Jaffe, HS. Cidofovir. Review of current and potential clinical uses. Adv Exp Med Biol. 1999; 458:111–120.
236. Jacobson, MA, Drew, WL, Feinberg, J, et al. Foscarnet therapy for ganciclovir-resistant cytomegalovirus retinitis in patients with AIDS. J Infect Dis. 1991; 163(6):1348–1351.
237. Pivetti-Pezzi, P, Accorinti, M, Ciapparoni, V, Vullo, V. Treatment of clinically resistant cytomegalovirus retinitis in AIDS patients: Combination of intravenous ganciclovir and intravitreal foscarnet. Eur J Ophthalmol. 1995; 5(4):199–203.
238. Safrin, S, Kemmerly, S, Plotkin, B, et al. Foscarnet-resistant herpes simplex virus infection in patients with AIDS. J Infect Dis. 1994; 169(1):193–196.
239. Jacobson, MA. Review of the toxicities of foscarnet. J Acquir Immune Defic Syndr. 1992; 5(Suppl 1):S11–S17.
240. Mossad, SB. Underused options for preventing and treating influenza. Cleve Clin J Med. 1999; 66(1):19–23.
241. Monto, AS. Viral respiratory infections in the community: Epidemiology, agents, and interventions. Am J Med. 1995; 99(6B):24S–27S.
242. Monto, AS. Using antiviral agents to control outbreaks of influenza A infection. Geriatrics. 1994; 49(12):30–34.
243. Long, JK, Mossad, SB, Goldman, MP. Antiviral agents for treating influenza. Cleve Clin J Med. 2000; 67(2):92–95.
244. Tominack, RL, Hayden, FG. Rimantadine hydrochloride and amantadine hydrochloride use in influenza A virus infections. Infect Dis Clin North Am. 1987; 1(2):459–478.
245. Wintermeyer, SM, Nahata, MC. Rimantadine: A clinical perspective. Ann Pharmacother. 1995; 29(3):299–310.
246. Crumpacker, C, Heagy, W, Bubley, G, et al. Ribavirin treatment of the acquired immunodeficiency syndrome (AIDS) and the acquired-immunodeficiency-syndrome-related complex (ARC): A phase 1 study shows transient clinical improvement associated with suppression of the human immunodeficiency virus and enhanced lymphocyte proliferation. Ann Intern Med. 1987; 107(5):664–674.
247. Gilbert, BE, Knight, V. Biochemistry and clinical applications of ribavirin. Antimicrob Agents Chemother. 1986; 30(2):201–205.
248. McCormick, JB, King, IJ, Webb, PA, et al. Lassa fever: Effective therapy with ribavirin. N Engl J Med. 1986; 314(1):20–26.
249. Hall, CB, McBride, JT, Walsh, EE, et al. Aerosolized ribavirin treatment of infants with respiratory syncytial viral infection: A randomized double-blind study. N Engl J Med. 1983; 308(24):1443–1447.
250. Levin, MJ. Treatment and prevention options for respiratory syncytial virus infections. J Pediatr. 1994; 124(5 Pt 2):S22–S27.
251. Huggins, JW, Hsiang, CM, Cosgriff, TM, et al. Prospective, double-blind, concurrent, placebo-controlled clinical trial of intravenous ribavirin therapy of hemorrhagic fever with renal syndrome. J Infect Dis. 1991; 164(6):1119–1127.
252. Bradley, JS, Connor, JD, Compogiannis, LS, Eiger, LL. Exposure of health care workers to ribavirin during therapy for respiratory syncytial virus infections. Antimicrob Agents Chemother. 1990; 34(4):668–670.
253. Neuraminidase inhibitors for treatment of influenza A and B infections [published erratum appears in MMWR Morb Mortal Wkly Rep 1999;48(49):1139]. MMWR Morb Mortal Wkly Rep. 1999; 48(RR-14):1–9.
254. Gubareva, LV, Kaiser, L, Hayden, FG. Influenza virus neuraminidase inhibitors. Lancet. 2000; 355(9206):827–835.
255. Treanor, JJ, Hayden, FG, Vrooman, PS, et al. Efficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza: A randomized controlled trial. U. S. Oral Neuraminidase Study Group [see comments]. JAMA. 2000; 283(8):1016–1024.
256. Jefferson, T, Demicheli, V, Di Pietrantonj, C, et al. Neuraminidase inhibitors for preventing and treating influenza in healthy adults. Cochrane Database Syst Rev. (3):2006.
257. Hayden, F, Klimov, A, Tashiro, M, et al. Neuraminidase inhibitor susceptibility network position statement: Antiviral resistance in influenza A/H5N1 viruses. Antivir Ther. 2005; 10(8):873–877.
258. Renaud, C, Kuypers, J, Englund, JA. Emerging oseltamivir resistance in seasonal and pandemic influenza A/H1N1. J Clin Virol. 2011; 52(2):70–78.
259. Hayden, FG, Atmar, RL, Schilling, M, et al. Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza. N Engl J Med. 1999; 341(18):1336–1343.
260. Coffin, SE, Leckerman, K, Keren, R, et al. Oseltamivir shortens hospital stays of critically ill children hospitalized with seasonal influenza: A retrospective cohort study. Pediatr Infect Dis J. 2011; 30(11):962–966.
261. Rodriguez, A, Diaz, E, Martin-Loeches, I, et al. Impact of early oseltamivir treatment on outcome in critically ill patients with 2009 pandemic influenza A. J Antimicrob Chemother. 2011; 66(5):1140–1149.
262. He, G, Massarella, J, Ward, P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin Pharmacokinet. 1999; 37(6):471–484.
263. Ceyhan, M, Karadag Oncel, E, Badur, S, et al. Effectiveness of a new bioequivalent formulation of oseltamivir (Enfluvir(R)) on 2010-2011 seasonal influenza viruses: An open phase IV study. Int J Infect Dis. 2012; 16(4):e273–278.
264. Dunn, CJ, Goa, KL. Zanamivir: A review of its use in influenza. Drugs. 1999; 58(4):761–784.
265. Barnett, JM, Cadman, A, Gor, D, et al. Zanamivir susceptibility monitoring and characterization of influenza virus clinical isolates obtained during phase II clinical efficacy studies. Antimicrob Agents Chemother. 2000; 44(1):78–87.
266. Gubareva, LV, Matrosovich, MN, Brenner, MK, et al. Evidence for zanamivir resistance in an immunocompromised child infected with influenza B virus. J Infect Dis. 1998; 178(5):1257–1262.
267. Hayden, FG, Osterhaus, AD, Treanor, JJ, et al. Efficacy and safety of the neuraminidase inhibitor zanamivir in the treatment of influenza virus infections. GG167 Influenza Study Group. N Engl J Med. 1997; 337(13):874–880.
268. Matsumoto, K, Ogawa, N, Nerome, K, et al. Safety and efficacy of the neuraminidase inhibitor zanamivir in treating influenza virus infection in adults: Results from Japan. GG167 Group. Antivir Ther. 1999; 4(2):61–68.
269. Monto, AS, Fleming, DM, Henry, D, et al. Efficacy and safety of the neuraminidase inhibitor zanamivirin the treatment of influenza A and B virus infections. J Infect Dis. 1999; 180(2):254–261.
270. Wijaya, L, Chua, YY, Cui, L, et al. Intravenous zanamivir in critically ill patients due to pandemic 2009 (H1N1) influenza A virus. Singapore Med J. 2011; 52(7):481–485.
271. Petersen, E, Keld, DB, Ellermann-Eriksen, S, et al. Failure of combination oral oseltamivir and inhaled zanamivir antiviral treatment in ventilator- and ECMO-treated critically ill patients with pandemic influenza A (H1N1). Scand J Infect Dis. 2011; 43(6-7):495–503.
272. Calfee, DP, Peng, AW, Cass, LM, et al. Safety and efficacy of intravenous zanamivir in preventing experimental human influenza A virus infection. Antimicrob Agents Chemother. 1999; 43(7):1616–1620.
273. Monto, AS, Robinson, DP, Herlocher, ML, et al. Zanamivir in the prevention of influenza among healthy adults: A randomized controlled trial [see comments]. JAMA. 1999; 282(1):31–35.
274. Dulek, DE, Williams, JV, Creech, CB, et al. Use of intravenous zanamivir after development of oseltamivir resistance in a critically ill immunosuppressed child infected with 2009 pandemic influenza A (H1N1) virus. Clin Infect Dis. 2010; 50(11):1493–1496.
275. Cass, LM, Efthymiopoulos, C, Bye, A. Pharmacokinetics of zanamivir after intravenous, oral, inhaled or intranasal administration to healthy volunteers. Clin Pharmacokinet. 1999; 36(Suppl 1):1–11.
276. Freund, B, Gravenstein, S, Elliott, M, Miller, I. Zanamivir: A review of clinical safety. Drug Safety. 1999; 21(4):267–281.
277. Williamson, JC, Pegram, PS. Respiratory distress associated with zanamivir [letter]. N Engl J Med. 2000; 342(9):661–662.
278. Balfour, HH, Jr., Rotbart, HA, Feldman, S, et al. Acyclovir treatment of varicella in otherwise healthy adolescents. The Collaborative Acyclovir Varicella Study Group [see comments]. J Pediatr. 1992; 120(4 Pt 1):627–633.
279. Baker, DA. The use of antiviral medications in the treatment of herpes simplex virus infections of women. Int J Fertil Womens Med. 1999; 44(5):227–233.
280. Kaplowitz, LG, Baker, D, Gelb, L, et al. Prolonged continuous acyclovir treatment of normal adults with frequently recurring genital herpes simplex virus infection. The Acyclovir Study Group. JAMA. 1991; 265(6):747–751.
281. Meyers, JD, Wade, JC, Shepp, DH, Newton, B. Acyclovir treatment of varicella-zoster virus infection in the compromised host. Transplantation. 1984; 37(6):571–574.
282. Saral, R, Burns, WH, Laskin, OL, et al. Acyclovir prophylaxis of herpes-simplex-virus infections. N Engl J Med. 1981; 305(2):63–67.
283. Wade, JC, Newton, B, McLaren, C, et al. Intravenous acyclovir to treat mucocutaneous herpes simplex virus infection after marrow transplantation: A double-blind trial. Ann Intern Med. 1982; 96(3):265–269.
284. Wade, JC, Newton, B, Flournoy, N, Meyers, JD. Oral acyclovir for prevention of herpes simplex virus reactivation after marrow transplantation. Ann Intern Med. 1984; 100(6):823–828.
285. Whitley, RJ, Gnann, JW. Herpes zoster: Focus on treatment in older adults. Antiviral Res. 1999; 44(3):145–154.
286. Burns, WH, Saral, R, Santos, GW, et al. Isolation and characterisation of resistant herpes simplex virus after acyclovir therapy. Lancet. 1982; 1(8269):421–423.
287. Crumpacker, CS, Schnipper, LE, Marlowe, SI, et al. Resistance to antiviral drugs of herpes simplex virus isolated from a patient treated with acyclovir. N Engl J Med. 1982; 306(6):343–346.
288. Whitley, RJ. Approaches to the treatment of varicella-zoster virus infections. Contrib Microbiol. 1999; 3:158–172.
289. Whitley, RJ, Gnann, JW, Jr. Therapeutic approaches to the management of herpes zoster. Adv Exp Med Biol. 1999; 458:159–165.
290. Wood, MJ, Johnson, RW, McKendrick, MW, et al. A randomized trial of acyclovir for 7 days or 21 days with and without prednisolone for treatment of acute herpes zoster [see comments]. N Engl J Med. 1994; 330(13):896–900.
291. Whitley, RJ, Weiss, H, Gnann, JW, Jr., et al. Acyclovir with and without prednisone for the treatment of herpes zoster: A randomized, placebo-controlled trial. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group [see comments]. Ann Intern Med. 1996; 125(5):376–383.
292. Reusser, P. Herpesvirus resistance to antiviral drugs: A review of the mechanisms, clinical importance and therapeutic options. J Hosp Infect. 1996; 33(4):235–248.
293. Breton, G, Fillet, AM, Katlama, C, et al. Acyclovir-resistant herpes zoster in human immunodeficiency virus-infected patients: Results of foscarnet therapy. Clin Infect Dis. 1998; 27(6):1525–1527.
294. Hadley, S, Samore, MH, Lewis, WD, et al. Major infectious complications after orthotopic liver transplantation and comparison of outcomes in patients receiving cyclosporine or FK506 as primary immunosuppression. Transplantation. 1995; 59:851–859.
295. Offidani, M, Corvatta, L, Olivieri, A, et al. Infectious complications after autologous peripheral blood progenitor cell transplantation followed by G-CSF. Bone Marrow Transplant. 1999; 24(10):1079–1087.
296. Reed, EC, Bowden, RA, Dandliker, PS, et al. Treatment of cytomegalovirus pneumonia with ganciclovir and intravenous cytomegalovirus immunoglobulin in patients with bone marrow transplants. Ann Intern Med. 1988; 109(10):783–788.
297. Arden, NH. Control of influenza in the long-term-care facility: A review of established approaches and newer options. Infect Control Hosp Epidemiol. 2000; 21(1):59–64.
298. Bergen, GA, Gompf, SG, Sakalosky, PA, Sinnott, JT. Influenza: More than mom and chicken soup. J Fla Med Assoc. 1996; 83(1):19–22.
299. Couch, RB. Measures for control of influenza. Pharmacoeconomics. 1999; 16(Suppl 1):41–45.
300. Demicheli, V, Jefferson, T, Rivetti, D, Deeks, J. Prevention and early treatment of influenza in healthy adults. Vaccine. 2000; 18(11-12):957–1030.
301. Two neuraminidase inhibitors for treatment of influenza. Med Lett Drugs Ther. 1999; 41(1063):91–93.
302. Hayden, FG. Update on influenza and rhinovirus infections. Adv Exp Med Biol. 1999; 458:55–67.
303. Hayden, FG. Antivirals for pandemic influenza. J Infect Dis. 1997; 176(Suppl 1):S56–S61.
304. Wenzel, RP. Expanding the treatment options for influenza [editorial; comment]. JAMA. 2000; 283(8):1057–1059.
305. Shigeta, S. Recent progress in anti-influenza chemotherapy. Drugs R D. 1999; 2(3):153–164.
306. Peters, S. Flu prevention and management. Strategies for the elderly. Adv Nurse Pract. 1997; 5(10):57–59.
307. Chang, SC, Cheng, YY, Shih, SR. Avian influenza virus: The threat of a pandemic. Chang Gung Med J. 2006; 29(2):130–134.
308. de Jong, MD, Hien, TT. Avian influenza A (H5N1). J Clin Virol. 2006; 35(1):2–13.
309. Fauci, AS. Pandemic influenza threat and preparedness. Emerg Infect Dis. 2006; 12(1):73–77.
310. Gruber, PC, Gomersall, CD, Joynt, GM. Avian influenza (H5N1): Implications for intensive care. Intensive Care Med. 2006; 32(6):823–829.
311. Fraaij, PL, van der Vries, E, Beersma, MF, et al. Evaluation of the antiviral response to zanamivir administered intravenously for treatment of critically ill patients with pandemic influenza A (H1N1) infection. J Infect Dis. 2011; 204(5):777–782.
312. Seibert, CW, Rahmat, S, Krammer, F, et al. Efficient transmission of pandemic H1N1 influenza viruses with high-level oseltamivir resistance. J Virol. 2012; 86(9):5386–5389.
313. Lemaitre, F, Luyt, CE, Roullet-Renoleau, F, et al. Impact of extracorporeal membrane oxygenation and continuous venovenous hemodiafiltration on the pharmacokinetics of oseltamivir carboxylate in critically ill patients with pandemic (H1N1) influenza. Ther Drug Monit. 2012; 34(2):171–175.
314. Carman, WF, Elder, AG, Wallace, LA, et al. Effects of influenza vaccination of health-care workers on mortality of elderly people in long-term care: A randomised controlled trial [see comments]. Lancet. 2000; 355(9198):93–97.
315. Nichol, KL. Complications of influenza and benefits of vaccination. Vaccine. 1999; 17(Suppl 1):S47–S52.
316. Hoffmann, CJ, Perl, TM. The next battleground for patient safety: Influenza immunization of healthcare workers. Infect Control Hosp Epidemiol. 2005; 26(11):850–851.
317. Marchand, S, Tchernia, G, Hiesse, C, et al. Human parvovirus B19 infection in organ transplant recipients. Clin Transplant. 1999; 13(1 Pt 1):17–24.
318. van Elsacker-Niele, AM, Kroes, AC. Human parvovirus B19: Relevance in internal medicine. Neth J Med. 1999; 54(6):221–230.
319. Young, NS. Parvovirus infection and its treatment. Clin Exp Immunol. 1996; 104(Suppl 1):26–30.
320. Rogers, JM, Diana, GD, McKinlay, MA. Pleconaril: A broad spectrum antipicornaviral agent. Adv Exp Med Biol. 1999; 458:69–76.
321. Pevear, DC, Tull, TM, Seipel, ME, Groarke, JM. Activity of pleconaril against enteroviruses. Antimicrob Agents Chemother. 1999; 43(9):2109–2115.