Chapter 8 Anticancer natural products
Plants have been the basis of sophisticated medical systems for thousands of years, particularly in China and India, and they have an essential role in health care. The World Health Organization has estimated that 80% of the Earth’s inhabitants rely on traditional medicines for primary health care, and plant products are highly important in the remaining 20% of the population (Farnsworth et al 1985). During the period 1959–1980 approximately 25% of US prescription medicines contained plant extracts, plant constituents or agents that were derived from natural sources. Over 119 chemicals from 90 plants species are important drugs in many countries and three-quarters of these were derived from studying the chemistry of traditional medicines (Farnsworth et al 1985).
The National Cancer Institute
The National Cancer Institute (NCI) was established in the USA in 1937 ‘to provide for, foster and aid in coordinating research related to cancer’. This organization has been involved in many of the great discoveries in fundamental research of cancer and particularly in the characterization of cytotoxic natural products. During the 1950s it was realized that there was a need for screening of natural extracts in a discovery programme and the Cancer Chemotherapy National Service Center was set up (Cragg et al 1993). The objectives of this organization were to procure compounds and to screen and evaluate them in a preclinical and, finally, full clinical setting. This service developed into the modern organization known as the Developmental Therapeutics Program (DTP).
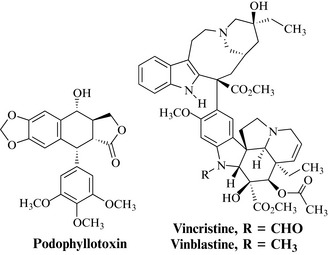
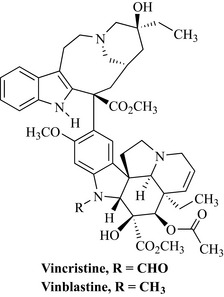
Early successes to exploit plants as a source of anticancer agents included the characterization of podophyllotoxin-type lignans from Podophyllum peltatum, and their semi-synthetic derivatives, and vincristine and vinblastine from Catharanthus roseus (Fig. 8.1). These discoveries drove further interest in plants at the NCI, and collections of plant material were expanded to include 60 countries. This collection strategy was quite extensive and, between 1960 and 1982, 114,000 extracts from 35,000 plants species were screened against a number of tumour types (Cragg et al 1993).
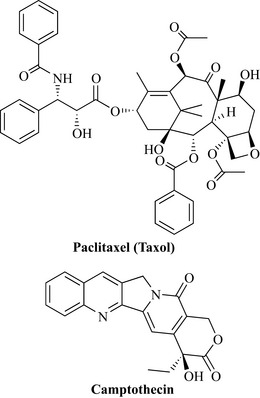
Bioassay-guided fractionation of active extracts led to the characterization of a large number of agents from many different natural product classes. Arguably the most significant discovery from the NCI screening programme was named taxol, from Taxus brevifolia (Taxaceae) (Fig. 8.2). The compound had been previously isolated in 1971 by Wall and co-workers and its activity against a melanoma cell line and in the human xenograft model led to its selection for preclinical development in 1977. Initially, there were problems in acquiring large amounts of the compound, but solutions to these problems and the report of its unique mode of action by promotion of tubulin polymerization and stabilization of microtubules against depolymerization increased interest. Taxol (now known as paclitaxel; see below) has excellent activity against ovarian and breast cancers and was approved in the USA by the FDA in 1993.
Another agent of note, again from the group of Wall, is camptothecin from the Chinese ornamental tree, Camptotheca acuminata (Nyssaceae) (Fig. 8.2). Chinese clinical trials were conducted on this agent, which showed promise against a number of different cancer types, including gastric, liver, head, neck and bladder cancers. However, US clinical trials of the sodium salt were terminated due to a limited number of responses. There has been much work on this class of natural product, with two companies (SmithKline Beecham and Daiichi) having developed and released two products: topotecan and irinotecan.
Between 1985 and 1990 the NCI developed a new in vitro screen that uses 60 human cell lines from nine cancer types representing leukaemia, lung, colon, central nervous system (CNS), melanoma, ovarian, renal, prostate and breast cancers. Synthetic or natural products are made up at concentrations ranging from 10–4 M to 10–8 M and then tested against the 60 different tumour cell lines. The data that are acquired from this test system are evaluated to decide if further investigation is warranted (Alley et al 1988, Grever et al 1992).
• GI50: the concentration at which growth is inhibited by 50%
• TGI: the concentration at which cell growth is totally inhibited
• LC50: the concentration at which 50% of the cells are killed.
The importance of these three factors is that a fingerprint of a compound’s anticancer activity is generated which can be correlated, using a mathematical model, with test results for known anticancer drugs (Paull et al 1989). This can even give an idea of whether a compound works by a similar mechanism of action as a known anticancer agent such as vincristine. Promising compounds with interesting selective profiles of activity (e.g. activity against one particular cell line) are then retested to confirm the reproducibility of these results. In 1995 a three cell line prescreen was introduced to increase the throughput of compounds whilst not reducing the number of compounds with activity in the 60 cell line assay. These cell lines were from lung, CNS and breast tumour types.
A new acquisition programme was initiated in 1986 with contracts for the cultivation and extraction of fungi and bacteria and the collection of marine organisms and terrestrial plants (Cragg et al 1997). Marine organisms were collected from many diverse locations, including the Caribbean, Australasia, Central and South Pacific, Indian Ocean and Polynesia. Plant material was collected in over 25 countries in the tropical and subtropical regions by specialist organizations with botanical expertise such as the Missouri Botanical Garden, the University of Chicago and the New York Botanical Garden.
Collaborations with the NCI rely on working closely with qualified organizations (e.g. universities, research institutes, botanical gardens) in the source country that has access to the biological diversity. Botanists and biologists from source countries collaborate in collections, which are invaluable to the NCI, and in many cases training is provided and workshops are organized for local personnel so that there is a transfer of expertise in certain areas. The source countries send biomass back to the National Products Repository in Frederick, Maryland. In the past, invitations have been made to local scientists of source countries to visit the NCI for collaborative research in natural products (Cragg et al 1997). This fosters openness and true collaboration between the NCI and source country.
For biomass collection, dried plant material (usually in the dry weight range of 0.3–1.0 kg) and frozen marine organism samples (1 kg) are shipped to Frederick and stored at –20°C, lessening the possibility of sample degradation. Biomass is extracted with methanol/dichloromethane (1:1) and then water to give organic and aqueous extracts which are given NCI numbers and stored at –20°C until required for screening (Cragg et al 1997). The extracts are then tested in the in vitro human cancer cell line screen and active extracts undergo bioassay-guided fractionation to isolate pure natural products. Structure elucidation is carried out using NMR and mass spectrometry. Compounds demonstrating significant antitumour activity are selected for secondary testing in in vivo systems and those with good activity advance to preclinical and, finally, clinical development. During this process there is the opportunity to license compounds out to companies for development; this is the preferred route due to the large costs of conducting the development process.
Importantly, agreements ensure that any royalties that may arise are shared with the country of origin and, as previously mentioned with the Convention on Biodiversity, it is a legal requirement that the collection of organisms is only possible with the ‘prior informed consent’ of the authorities of the source country (see Chapter 5, p. 55).
A full review of antitumour agents is beyond the scope of this text but we will briefly cover the three main sources of anticancer agents (marine sources, plants and microbes), giving selected examples of agents and their sources. The reader is urged to consult the excellent reviews by Cragg and co-workers on this subject (Cragg & Newman 1999, 2000, Cragg et al 1993, 1997).
Marine anticancer natural products
Marine organisms have no history of medicinal use but, because the oceans cover 70% of the Earth’s surface, there is a vast reserve for the discovery of new natural product drugs. This huge environment is home to a fantastic range of diverse organisms and, of the 28 major animal classes, 26 exist in aquatic areas and eight are exclusively aquatic (Cragg et al 1997). Collection of biomass is usually carried out by diving and in some cases submersibles can give access to organisms that occur in deeper sites, although this is a highly expensive procedure.
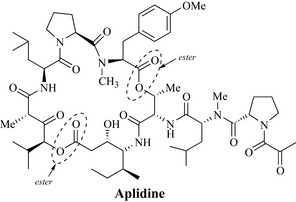
The discipline of marine natural product chemistry is comparatively new compared to phytochemistry, with relatively small numbers of natural products having been reported. At present there is one marine natural product which is used as an anticancer drug clinically (Ecteinascidin 743, Yondelis), and a number are in clinical trials and their potent activities highlight the future importance of this source. A compound in development worth noting is bryostatin-1, which is a novel macrocyclic lactone derived from the marine bryozoan, Bugula neritina. This compound modulates protein kinase C activity and phase I studies have demonstrated activity against several tumour types. Bryostatin-1 has subsequently been investigated extensively in phase II clinical trials as a single agent and data suggest that it may have potential in combination with other cytotoxic agents. Didemnin B (Trididemnum solidum) was the first marine natural product to enter clinical trials and has recently been shown to induce apoptosis (programmed cell death) in a wide range of cell lines. Aplidine (Fig. 8.3) or dehydrodidemnin B, which is structurally related to didemnin B, is a marine depsipeptide obtained from the tunicate Aplidium albicans, originally found in the Mediterranean. Tunicates, which are also known as sea squirts, are organisms that attach themselves to submerged objects and feed by removing microscopic organisms from the water that is drawn through them. Depsipeptides are cyclic peptides that also possess a cyclic ester functional group. Aplidine blocks the cell division cycle in human tumour cell lines and prevents the onset of DNA synthesis. Like didemnin B, it has also been shown to be an inducer of apoptosis in many cell systems. Additionally, using the xenograft assay, grafted into athymic mice, aplidine demonstrated considerable activity against colon, bladder, lung, prostate and stomach tumours, and against melanoma and lymphoma tumours. This agent is currently in clinical trials. Aplidine demonstrates some of the very best features of a natural product, being highly chiral and exhibiting great functionality. Both of these features contribute to the high cytotoxicity of this molecule, which in nature is probably produced by Aplidium species as a chemical defence mechanism.
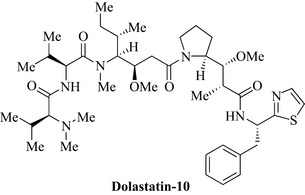
One of the most promising groups of agents comes from the sea hare (Dolabella auricularia), an herbivorous mollusc from the Indian Ocean which produces cytotoxic linear peptides. An interesting member of this class is dolastatin-10 (Fig. 8.4), an inhibitor of microtubule assembly, currently in clinical trials.
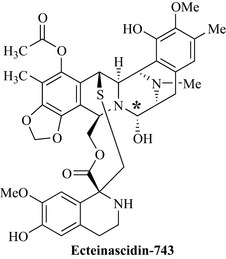
Perhaps the most interesting antitumour marine compounds and the first anti-cancer drug to come from this marvelous source is ecteinascidin-743, now marketed as Yondelis (Trabectidin) (Fig. 8.5), from Ecteinascidia turbinata, which is a tunicate found in the Caribbean and Mediterranean. Ecteinascidin-743 has very potent activity against a broad spectrum of tumour types in animal models; it binds to the minor groove of the DNA double helix and inhibits cell proliferation, leading to apoptosis of cancer cells. This binding to the minor groove of DNA allows an alkylation reaction to occur between a guanine residue of the DNA and an electron-deficient carbon on the molecule (* in Fig 8.5).
Plant antitumour agents
Camptotheca acuminata (Nyssaceae)
In 1957, a small number of ethanolic plant extracts (1000) were screened at the Cancer Chemotherapy National Center (USA) and extracts of Camptotheca acuminata were shown to possess high activity. The late Dr Monroe Wall, who will be remembered as a leading expert in plant antitumour agents and a wonderful enthusiast for natural product research, established a natural product research group at Research Triangle Institute which conducted some research that was funded by the NCI. Dr Wall, who was later joined by Dr Mansukh Wani, became interested in the constituents of Camptotheca, particularly due to the high activity of these extracts. Wood and bark (20 kg) were collected for extraction and the extracts were shown to be active against a mouse leukaemia life prolongation assay in which it was unusual to find activity (Wall & Wani 1995). During the 1960s, the bioassay-guided isolation process was slow and it could take as long as 3 months to get bioassay results from the mouse life-prolongation assay back to the chemists working on the extracts (Wall & Wani 1995).
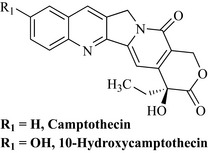
When compared with cellular assays today, in which it may only take a few days to decide if extracts and fractions are active, it is worth noting how diligent, persistent and thorough these researchers were. Extraction of the plant material was carried out first using a non-polar solvent (hot heptane) to remove very non-polar natural products. The plant material was then extracted with ethanol and this extract was treated with chloroform and aqueous ethanol to give two phases, the lipophilic chloroform phase being highly active. Additional extraction of the aqueous phase with chloroform gave a further active chloroform extract. High activity was associated with all of the chloroform extracts of the ethanolic portion, indicating that the active natural product had a degree of lipophilicity. Column chromatography was initially attempted on these active extracts using alumina as the sorbent, but what was not known at the time was that the active component binds very tightly to alumina and could not be eluted from the column. Extensive partitioning of the extract was tried in separating funnels using the quaternary system (four solvents) chloroform/carbon tetrachloride/methanol/water and each of the fractions were assayed in the mouse life-prolongation assay and in vitro cellular cytotoxicity assay. This partitioning system gave excellent separation and active fractions were combined to yield a yellow precipitate, which was subjected to chromatography on silica to which the active agent did not irreversibly bind. Further crystallization of active fractions led to the isolation of the active agent, camptothecin (Fig. 8.6). The structure elucidation of this natural product was carried out by conversion to its chloroacetate, which was then further converted to the iodoacetate salt which gave crystals of sufficient quality for X-ray structural analysis.
Camptothecin was shown to be extremely active in the life-prolongation assay of mice treated with leukaemia cells and in solid tumour inhibition. These activities encouraged the NCI to initiate clinical trials with the water-soluble sodium salt. Trials were conducted in a small number of patients (18) and there were five partial responses against gastrointestinal tumours of short duration (Gottlieb & Luce 1972). There were, however, toxic side effects. A phase II study was conducted but unfortunately there were only two responses to the treatment (Moertel et al 1972). The sodium salt was also studied in a much larger clinical trial in China using 1000 patients with better results against head, neck, gastric, intestinal and bladder carcinomas. These results were more promising than those of the US trial possibly due to the fact that the US patients had already been treated with other cytotoxic drugs and their tumours may have become multidrug-resistant. It is also now known that the lactone (which is absent in the lactone ring-opened sodium salt) of the compound is important for activity.
Wall and Wani continued to isolate and evaluate Camptotheca metabolites and found that one such product in particular, 10-hydroxycamptothecin (Fig. 8.6), was more active than camptothecin, and this may have stimulated a number of companies to prepare further water-soluble analogues of 10-hydroxycamptothecin.
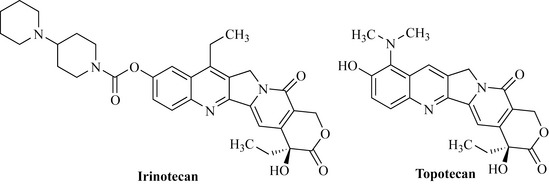
Interest in this natural product and its analogues was low until 1985 when it was discovered that camptothecin works by inhibition of topoisomerase I, which is an enzyme involved in many important cellular processes by interacting with DNA. This finding fuelled further interest in this class of compound as the mechanism by which it functioned as an inhibitor of tumour growth was understood. Much work was conducted by pharmaceutical companies on this template and two products became available as a result of this research, irinotecan and topotecan (Fig. 8.7). The structural importance of the parent natural product, 10-hydroxycamptothecin, as a template can be seen in both of these products.
Topotecan (Fig. 8.7) was approved for use in the USA in 1996. It is active against ovarian cancer.
Taxus brevifolia (Taxaceae)
In the early 1960s a number of plants were collected from the USA and assessed for antitumour activity under an NCI-supported programme. One of these specimens was Taxus brevifolia. This plant, commonly known as the Pacific yew, is a member of the Taxaceae family and is related to the English yew (Taxus baccata), which is common in churchyards in the UK. The Pacific yew is a slow-growing tree common to the western coast of the USA. Active extracts were tested at the Research Triangle Institute in 1964 (Wall & Wani 1995).
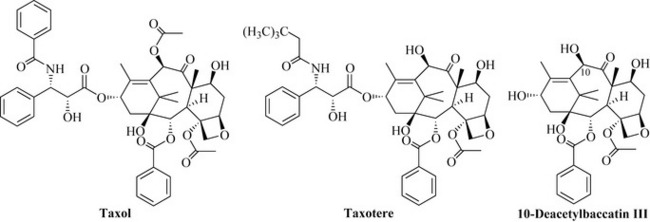
The extraction of the plant material was conducted using a partitioning protocol similar to that employed in the isolation of camptothecin. The isolation of the active component employed a highly extensive partitioning procedure utilizing many steps between aqueous and organic solvents. The biological activity of the fractions was monitored at all stages and 0.5 g of the active compound was isolated. This was an exceptionally low yield (0.004%) from 12 kg of plant material and the name taxol was assigned to the active natural product. Taxol was shown to be active against solid tumours and highly active against leukaemia models in the mouse life-prolongation assay. The compound was also active against a melanoma cell line. As with camptothecin, structure elucidation was not a trivial process (it can still be difficult today!) and there were only a handful of techniques that could be used to determine the structure, including UV, IR, elemental analysis (determination of C, H and N composition) and mass spectrometry; there were not many NMR techniques available in the early 1960s, which made structure elucidation an extremely difficult task. The structure elucidation of this compound is an example of a masterful piece of natural product research and the reader is urged to consult the superb review by Wall & Wani (1995) and the book by Goodman & Walsh (2001).
1H NMR spectra suggested that taxol had a number of ester groups attached to it and was possibly a diterpene natural product of the taxane group. Elemental analysis and mass spectrometry gave a molecular formula of C47H51NO14 for taxol, but it was not possible to obtain crystals of the compound of sufficient quality for X-ray structural analysis. At this stage chemical degradation techniques were used to help elucidate the structure. Base-catalysed methanolysis yielded compounds that could be crystallized and submitted for X-ray structural analysis for structure elucidation. Elegant work, which included the use of further chemical degradation plus 1H NMR and high-resolution mass spectrometry, was conducted to piece these fragments together to give the final structure of taxol (Fig. 8.8) (Wall & Wani 1995).
The fact that taxol worked by a new mechanism and that it was a highly unusual and novel structure encouraged further research into this agent, resulting in clinical trials and the development of analogues such as taxotere (Fig. 8.8).
The supply issues concerning taxol were overcome with its semi-synthesis by the conversion of metabolites present in larger amounts (e.g. 10-deacetylbaccatin III; Fig. 8.8) in the needles of the related English yew (Taxus baccata). As the needles are a renewable resource, there is no need to destroy trees by the removal of bark. Taxol is now commercially produced by plant cell culture by large-scale fermentation of the plant cells.
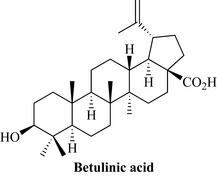
Betula alba (Betulaceae)
Betulinic acid (Fig. 8.9) is a pentacyclic triterpene that occurs in several plants. It can be chemically derived from betulin, a natural product found in abundance in the outer bark of white birch trees (Betula alba).
Podophyllum peltatum (Berberidaceae)
Ethanolic extracts of the rhizomes are known as podophyllin, which is included in many pharmacopoeias for the topical treatment of warts and condylomata acuminata, which are benign tumours. Podophyllin resin is highly irritant and unpleasant and cannot be used systemically. The main natural product is the podophyllotoxin lignan class (see Chapter 6) of which podophyllotoxin was the first to be isolated in 1880 and the structure proposed in 1932. There are many components in podophyllin; the most important from the antitumour perspective are the lignans. Podofilox is a purified form of podophyllin which acts as a cell poison against cells undergoing mitosis, and, although this extract is not a systemic chemotherapeutic agent, it is used topically in creams as a treatment for genital warts.
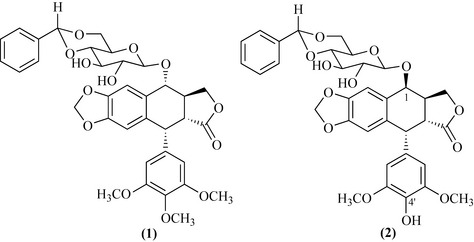
By a serendipitous event, a crude fraction of podophyllin was treated with benzaldehyde, resulting in a mixture of products that were mainly benzyl derivatives of lignan glycosides (Stähelin & von Wartburg 1991). The crude reaction mixture was highly cytotoxic and active in the mouse life-prolongation assay against a leukaemia cell line. Some of the components of this mixture were isolated but were not as active as the whole mixture. Condensation of benzaldehyde with the reaction mixture generated a series of acetals that were not hydrolysed by glucosidase enzymes and were less water-soluble. Additionally, the crude mixture worked by a different mechanism from the purified products by stopping the tumour cells from undergoing mitosis. This preparation was marketed for cancer treatment under the name of Proresid. Work on Proresid with bioassay-guided isolation against a mouse life-prolongation assay with a leukaemia cell line indicated that there was a highly active agent present. As with paclitaxel and camptothecin, in the 1960s it was very difficult to isolate and identify minor components and the process took several years to complete. The most abundant component of Proresid is podophyllotoxin benzylidene glucoside (1) (Fig. 8.10); a minor component is 4′-demethylepipodophyllotoxin benzylidene glucoside (2), which was found to be the most active agent (Fig. 8.10).
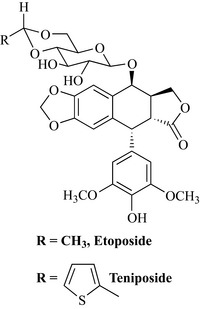
There was much synthetic work conducted to produce analogues that retain the same structural features of compound (2), which is epimeric at position 1 and lacking a methyl at position 4′ with respect to compound (1). The two most important analogues synthesized so far are etoposide and teniposide (Fig. 8.11) which have much more potency than the parent compound.
Catharanthus roseus (Apocynaceae)
The discovery of the vinca alkaloids from the Madagascan periwinkle is a classic example of drug discovery and, although extracts of the species had a reputation as being useful in the treatment of diabetes, a screening programme at the pharmaceutical company Eli Lilly revealed that extracts inhibited the growth of certain types of cancer cells. Bioassay-guided isolation of extracts of the plant led to the characterization of the active alkaloidal compounds vincristine and vinblastine (Fig. 8.12).
Microbial antitumour agents
The anthracyclines
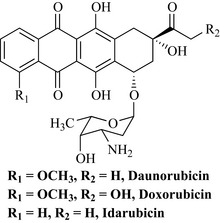
This group is a large complex family of antibiotics and many were investigated before a useful antitumour agent was isolated. They are structurally and biosynthetically related to the tetracyclines (Chapter 6), being derived from the polyketide pathway. One of the first agents to be described in this class was daunorubicin from Streptomyces peucetius and Streptomyces caeruleorubidis (Fig. 8.13).
The bleomycins
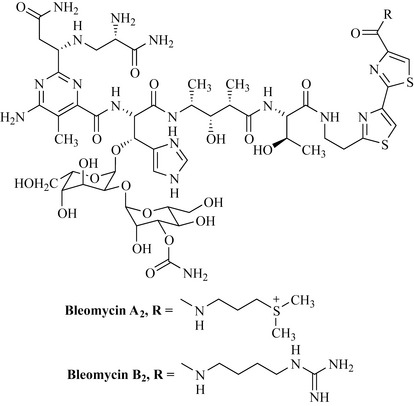
This group consists of a closely related mixture of glycopeptide antibiotics from the filamentous bacterium Streptomyces verticillus. They were discovered in 1966 by screening of culture filtrates against tumours. The bleomycin mixture is partly resolved (purified) prior to formulation for clinical use under the name blenoxane, which consists of a mixture of bleomycin A2 (55–70%) and bleomycin B2 (30%) (Fig. 8.14). These natural products occur as blue copper chelates.
The actinomycins
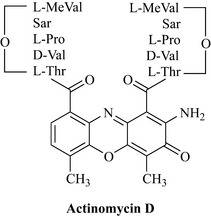
Actinomycin D (dactinomycin) is an antibiotic from Streptomyces parvullus first isolated in 1940. It is an antimicrobial compound which is toxic and has limited use as an antitumour agent. Structurally, it consists of a planar phenoxazinone dicarboxylic acid attached to two identical pentapeptides (Fig. 8.15). A number of analogues of different peptide composition are known.
Alley M.C., Scudiero D.A., Monks A., et al. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res.. 1988;48:589-601.
Cragg G.M., Boyd M.R., Cardellina J.H.II, et al. Role of plants in the National Cancer Institute Drug Discovery and Development Program. In: Kinghorn A.D., Balandrin M.F., editors. Human medicinal agents from plants. Washington, DC: American Chemical Society, 1993.
Cragg G.M., Newman D.J. Cancer Invest.. 1999;17:153-163.
Cragg G.M., Newman D.J. Antineoplastic agents from natural sources: achievements and future directions. Expert Opin. Investig. Drugs. 2000;9:2783-2797.
Cragg G.M., Newman D.J., Weiss R.B. Coral reefs, forests and thermal vents: the world wide exploration of nature for novel antitumour agents. Semin. Oncol.. 1997;24:156-163.
Farnsworth N.R., Akerele O., Bingel A.S., Soejarto D.D., Guo Z. Medicinal plants in therapy. Bull. World Health Organ.. 1985;63:965-981.
Goodman J., Walsh V. The story of taxol. Cambridge: Cambridge University Press; 2001.
Gottlieb J.A., Luce J.K. Cancer Chemother. Rep.. 1972;56:103-105.
Grever M.R., Schepartz S.A., Chabner B.A. The National Cancer Institute: cancer drug discovery and development program. Semin. Oncol.. 1992;19:622-638.
Moertel C.G., Schutt A.J., Reitemeier R.J., et al. Phase II study of camptothecin (NSC-100880) in the treatment of advanced gastrointestinal cancer. Cancer Chemother. Rep.. 1972;56:95-101.
Paull K.D., Shoemaker R.H., Hodes L., et al. Display and analysis of patterns of differential activity of drugs against human tumor cell lines: development of mean graph and COMPARE algorithm. J. Natl. Cancer Inst.. 1989;81:1088-1092.
Stähelin H.F., von Wartburg A. The chemical and biological route from podophyllotoxin glucoside to etoposide. Ninth Cain Memorial Award Lecture. Cancer Res.. 1991;51:5-15.
Wall M.E., Wani M.C. Camptothecin and taxol: discovery to clinic. Thirteenth Bruce F Cain Memorial Award Lecture. Cancer Res.. 1995;55:753-760.
Cragg G.M., Grothaus P.G., Newman D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev.. 2009;109:3012-3043.
Delgado J.N., Remers W.A. Wilson and Gisvold’s textbook of organic medicinal and pharmaceutical chemistry, tenth ed. Philadelphia: Lippincott-Raven; 1998.
Pan L., Chai H., Kinghorn A.D. The continuing search for antitumor agents from higher plants. Phytochem. Lett.. 2010;3:1-8.
Pratt W.B., Ruddon R.W. The anticancer drugs, second ed. Oxford: Oxford University Press; 1994.
Newman D.J., Cragg G.M. Microbial antitumor drugs: natural products of microbial origin as anticancer agents. Curr. Opin. Investig. Drugs. 2009;10:1280-1296.
Newman D.J., Cragg G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod.. 2007;70:461-477.
Rang H.P., Dale M.M., Ritter J.M. Pharmacology, fourth ed. London: Churchill Livingstone; 1999.