Chapter 171 Anterior Lumbar Interbody Fusion
Indications and Techniques
History
Anterior approaches to the spine have been used to treat lumbar degenerative disc disease for 70 years. The earliest reports of anterior spine surgery arise from the late 19th century. Tuberculosis of the spine was a significant problem, which often led to paraplegia. Vincent of Paris, in 1892, evacuated a tuberculosis abscess using costotransversectomy.1 Three years later, the other pioneer of anterior spine surgery, Victor Menard, authenticated the benefits of the anterior approach by performing a posterolateral costotransversectomy for abscess drainage.2 The first attempted anterior approach to the lumbar spine was in 1906 by Muller, wherein he used a transperitoneal approach to debride tuberculosis of the lumbar spine.3 Postoperatively, his patient did well; however, subsequent patients had significant morbidities and mortalities, which slowed the progression of the approach. Almost 15 years later, in 1921, Royle used a retroperitoneal approach to remove a congenital hemivertebra in the lumbar spine.4 The next year, MacLennon was the first to treat scoliosis in children with an anterior retroperitoneal approach.5
During the 1930s, Chaklin performed an anterior retroperitoneal osteotomy of the lumbar spine, while Burns, whom many credit to be the first to perform an anterior fusion of the lumbar spine, used bone grafting to treat lumbar spondylolisthesis via a transperitoneal approach.6,7 Ito et al. used both an anterior costotransversectomy approach to the thoracic spine to treat Pott’s disease and a retroperitoneal approach to the lumbar spine to perform structural bone grafting.8 In 1944, Iwahara treated lumbar degenerative disease by an interbody fusion done using a retroperitoneal approach.9 Four years later, Lane and Moore used a transperitoneal approach to treat lumbar degenerative disease.10 In 1945, Capener described a transperitoneal approach using a tibial allograft graft; in 1959, he modified this approach by removing an additional rib laterally.11 In 1956 and then in 1960, Hodgson and Stock, who were able to successfully eradicate the disease in hundreds of tuberculosis patients, extended the anterior approach to the thoracic and lumbar spine for debridement of tuberculosis abscesses with subsequent interbody fusion.12 Acceptance of the anterior interbody fusion technique as an efficacious procedure has evolved as one of the predominant techniques to treat discogenic back pain.
Advantages
The anterior approach to the lumbar spine has been shown to have several advantages over posterior approaches. The anterior approach provides direct access to the ventral surface of the vertebral bodies and disc spaces, and it provides a direct and more complete view of the anterior surface of the lumbar spine from L3 to S1.13 In addition, the anterior approach avoids dissection through the soft tissue and musculature encountered in the posterior approach, especially the paraspinal muscles. Avoidance of injury to these tissues may result in significantly decreased postoperative pain and subsequently decreased length of postoperative hospital stay.13,14
Lumbar interbody fusion via the anterior approach has also shown to be advantageous over posterior techniques, such as posterior lumbar interbody fusion (PLIF). One significant advantage is the absence of neural retraction required in the anterior approach, as compared to the degree of retraction required on the neural structures in the PLIF technique.15 One recent study compared the anterior lumbar interbody fusion (ALIF) technique to the transforaminal lumbar interbody fusion technique and shows an increased ability of ALIF to improve foraminal height, local disc angle, and lumbar lordosis.16 Other studies have demonstrated decreased rate of adjacent segment degeneration with ALIF compared to PLIF.17
Indications
The indications for ALIF have increased over the last several years. The most common indication for ALIF is discogenic back pain that occurs between the levels of L3 to S1.13,18 Another common indication for this procedure is spondylolisthesis, namely, the isthmic and degenerative types.19,20 ALIF has also been used as revision surgery after a failed posterior fusion involving segments from L3 to S1.13,21 A secondary indication for anterior fusion is intervertebral foraminal stenosis secondary to loss of disc height,22 in conjunction with the need for interbody fusion.
Contraindications
Several contraindications exist to the ALIF technique. Severe and numerous medical comorbidities pose a relative complication to any major surgical procedure. In addition, severe obesity is a contraindication to the anterior approach, given the hindrance the obesity would have during exposure and retraction of intra-abdominal tissues and structures.
Preoperative Evaluation
The preoperative evaluation of patients who will undergo ALIF first requires careful selection of those patients likely to have a successful outcome after an anterior fusion. For example, in patients with discogenic pain, the following criteria have been shown to be associated with a good outcome after ALIF13: (1) axial back pain aggravated by spinal loading and fusion, (2) radiographic studies consistent with disc degeneration, (3) provocative discography that produces pain only at the affected levels, and (4) dynamic studies demonstrating motion/sagittal deformity on sagittal views.
Careful consideration also needs to be placed on the type of approach used to access the anterior spine. This depends on the patient’s characteristics, as well as the surgeon’s preference. ALIF has three major types of approaches: open, mini-open, and laparoscopic. Each approach has advantages and disadvantages. Open ALIF requires a larger incision and opening into the retroperitoneal space. However, the drawbacks of this approach include increased blood loss during surgery, as well as increased operative time, compared to other approaches.23
Next, the surgeon has to decide whether to approach the anterior spine via a transperitoneal or an extraperitoneal approach. The retroperitoneal approach seems to be the favored approach, given the complications that seem to be associated with the transperitoneal approach. The transperitoneal approach has been shown to have an increased of retrograde ejaculation compared to the retroperitoneal approach by approximately a factor of 10,23,24 secondary to an increased rate of injury to the hypogastric plexus.25 In addition, the number of levels than can be accessed with the transperitoneal approach is typically decreased. Finally, the transperitoneal approach requires direct manipulation of intra-abdominal contents, including visceral structures. This may lead to an increased rate of postoperative ileus, as well as an increased rate of injury to the intraperitoneal structures.
Careful preoperative assessment must also be made in regard to whether ALIF should also be supplemented with additional posterior fusion. The addition of posterior fusion introduces a second step to the surgery. Since it requires dissection of posterior soft tissues, it counteracts the advantage that anterior lumbar approaches have in avoiding dissection of paraspinal musculature. However, biomechanical studies have shown a significant decrease in the relative motion between segments and a decrease in bone stress level, resulting in higher rates of fusion.26 In cases of isthmic spondylolisthesis, the addition of posterior fusion should strongly be considered, given the inherent instability and motion with isthmic spondylolisthesis. In addition, standalone anterior interbody fusion has been associated with increased incidence of sacral fractures over time.19 Finally, in cases requiring multilevel fusion, posterior fixation and fusion should be considered.15
Preoperative imaging should carefully be assessed prior to surgery. Plain radiographs are used to estimate the size and angle of the implant to be used in an effort to achieve optimal postoperative disc height and lordosis. The degree of sagittal imbalance should also be assessed on preoperative imaging, which should be restored.21 One study demonstrated that correction of sagittal imbalance, local lumbar lordosis, disc height, and neural foramen led to better overall long-term outcomes.16
Operative Technique
The positioning of the patient for an ALIF is usually supine on the operating table. In some cases, the lateral position can be used. A break in the operating room table or an inflatable bladder needs to be placed under the lumbar area of the patient at the level of the affected disc to control the amount of lordosis at the level to be operated upon.13,21
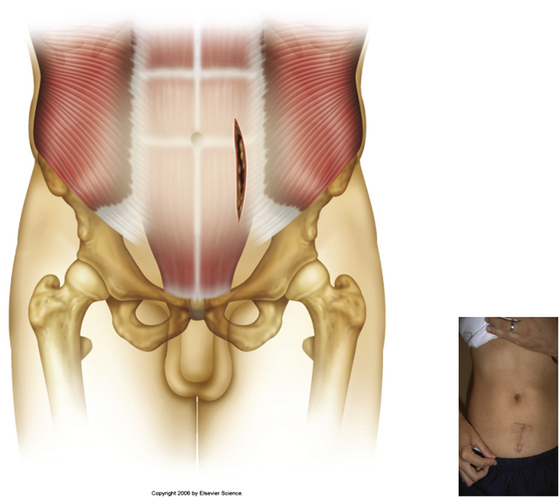
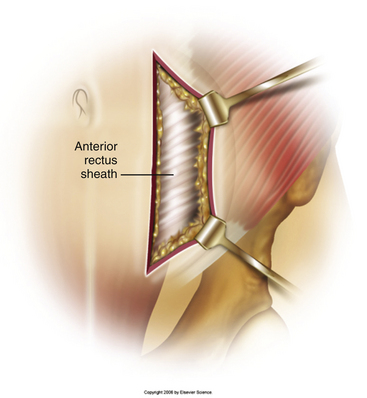
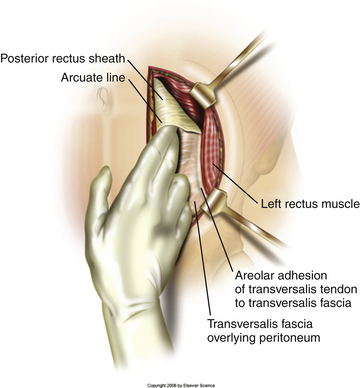
Typically, a vertical skin incision is made on the abdomen to the left of the midline (Fig. 171-1). However, horizontal skin incisions have been used. The incision is made over the appropriate disc space to be fused. Blunt dissection is then carried out through the subcutaneous tissues until the anterior rectus sheath is exposed (Fig. 171-2). A vertical incision is then made through the anterior rectus sheath, exposing the rectus muscle. The rectus muscle is then mobilized and retracted medially to expose the posterior rectus sheath (Fig. 171-3). The exposure of the posterior rectus sheath must include visualization of the arcuate line.
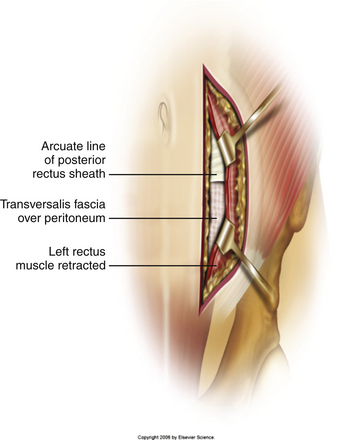
FIGURE 171-3 Retraction of rectus muscle exposing the posterior rectus sheath, including the arcuate line.
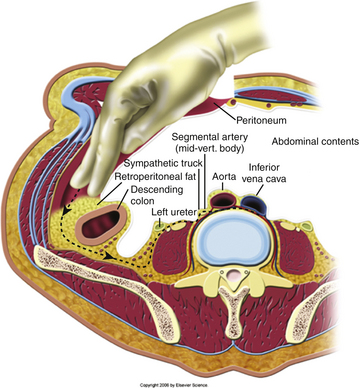
Blunt dissection is then carried out underneath the arcuate ligament between the posterior rectus sheath and the peritoneum (Fig. 171-4). Using blunt dissection, this space is developed until the retroperitoneal space is accessed. Once the accession to the retroperitoneal space has been verified, the peritoneal contents are then swept medially to provide direct visualization of the retroperitoneal space (Fig. 171-5). The ureter sits on the underside of the peritoneum and is retracted away with the peritoneum. At this point, the vascular structures and ureter is carefully identified to minimize injury to these structures. Deep abdominal retractors can then be placed.
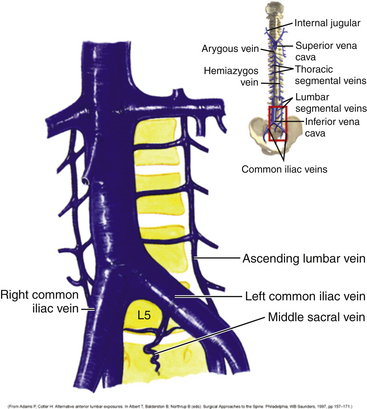
Vascular dissection and retraction then must be carried out to access the ventral surface of the lumbar spine. Knowledge of the vascular anatomy in this area is critical, and there can be a significant amount of variability in the relationship between the bifurcations of the aorta and vena cava and the disc space (Fig. 171-6). If accessing the L3-4 or the L4-5 disc space, then the aorta and vena cava have to be retracted away to visualize the anterior surface of the vertebral bodies. However, if the L5-S1 disc space is to be accessed, the space below the bifurcations of the aorta and vena cava can be accessed. In this case, the middle sacral artery and vein may have to be ligated. Segmental vessels may have to be ligated to maximize the operative field, as well as to prevent bleeding during the case. The iliolumbar vein is also identified and ligated to prevent unnecessary bleeding during the case (Fig. 171-7). The major vessels are retracted away carefully using blunt dissection to expose the anterior spine (Fig. 171-8).
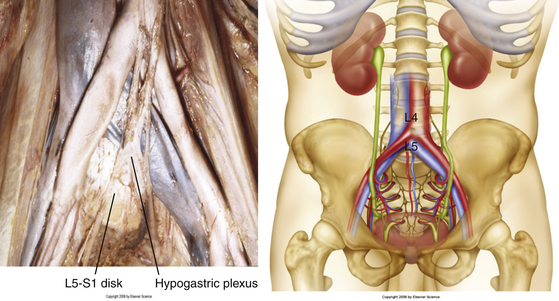
FIGURE 171-6 Vascular anatomy in relation to vertebral levels, including the middle sacral artery and vein.
A variety of interbody implants are available. Each consists of an interbody space and an osteoconductive/osteoinductive substance to fill the spacer. Common substances for spacers include iliac crest autograft, femoral ring allograft, titanium mesh cages, and cylindrical threaded bone dowels and titanium cages. Iliac crest autograft can be harvested at the time of surgery and does not require filling of osteoconductive/osteoinductive material. Nonautograft spacers, however, require filling of osteoconductive/osteoinductive substances. Cylindrical threaded bone dowels and titanium cages are advantageous in that they lower the risk of graft migration or backout, given the threaded surface is in contact with the end plates. Common osteoinductive/osteoconductive filling substances include vertebral autograft, which can be acquired during surgery; cancellous autograft chips; demineralized bone matrix; and bone morphogenetic protein.
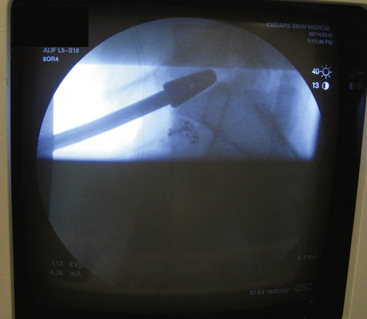
Once the interbody graft has been implanted, intraoperative radiographs are taken to ensure proper placement of the graft in lateral and anteroposterior dimensions (Fig. 171-9). In addition, satisfactory disc height restoration and lordosis are confirmed during intraoperative imaging.
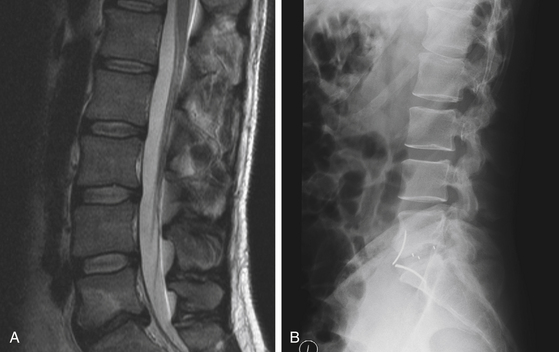
The wound is then irrigated copiously, and hemostasis is meticulously achieved. Retraction on the peritoneum is then released, and the lining of the peritoneum is carefully inspected. Any tears found in the peritoneum should be primarily repaired. The rectus sheath is then closed with interrupted sutures to prevent the risk of ventral hernias. Following this, the skin is closed with staples or subcuticular sutures. Postoperative images are obtained at the discretion of the treating surgeon (Fig. 171-10).
Complications
Several potential complications exist with ALIF surgery. Many of these complications can be avoided by proper visualization and identification of retroperitoneal structures to minimize subsequent accidental injury to these structures. For example, proper identification of the ureter with subsequent medial retraction with the peritoneum can significantly decrease the risk of injury to this structure.
One possibly devastating risk with anterior lumbar surgery is vascular injury. For this reason, many spine surgeons work with vascular surgeons during the exposure. A recent study demonstrated a 6.1% rate of vascular injury among 212 anterior lumbar interbody and fusion procedures performed.27 Older studies have shown risk of vascular injury to be as high as 17%.25
Injury to the sympathetic hypogastric plexus may result in retrograde ejaculation. A transperitoneal approach to the lumbar spine seems to be associated with a higher rate of injury to the hypogastric plexus as compared to a retroperitoneal approach. The rate of retrograde ejaculation after anterior lumbar surgery has been quoted to be as high as 2.3%.24 The descending sympathetic chain may also be injured with the anterior approach, which may result in a unilateral warm leg syndrome due to the loss of sympathetic activity in the affected extremity.
Conclusion
The ALIF is a highly efficacious technique for fusing the lower lumbar spine in select patients with discogenic back pain.
Adams P., Cotler H. Alternative anterior lumbar exposures. In: Albert T., Balderston R., Northrup B. Surgical Approaches to the Spine. Philadelphia: WB Saunders; 1997:145-155.
Baker J., Reardon P., Reardon M., et al. Vascular injury in anterior lumbar surgery. Spine. 1993;18(15):2227-2230.
Dickerman R., East J., Winters K. Anterior and posterior lumbar interbody fusion with percutaneous pedicle screws: comparison to muscle damage and minimally invasive techniques. Spine. 2009;34(25):E923-E925.
Garg J., Woo K., Hirsch J. Vascular complications of exposure for anterior lumbar interbody fusion. J Vasc Surg. 2010;51(4):946-950.
Hseih P., Koski T., O’Shaughnessy B., et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine. 2007;7:379-386.
Lee D., Lee S., Maeng D., et al. Anterior lumbar interbody fusion with pedicle screw fixation for elderly isthmic spondylolisthesis. J Korean Neurosurg Soc. 2006;40:175-179.
Min J., Jang J., Lee S. Comparison of anterior and posterior approach instrumented lumbar interbody fusion for spondylolisthesis. J Neurosurg Spine. 2007;7:21-26.
Saraph V., Lerch C., Walochnik N., et al. Comparison of conventional versus minimally invasive extraperitoneal approach for anterior lumbar interbody fusion. Eur Spine J. 2004;13:425-431.
Sasso R.C., Burkus J.K., LeHuec J.C. Retrograde ejaculation after lumbar interbody fusion: transperitoneal versus retroperitoneal experience. Spine. 2003;28(10):1023-1026.
1. Vincent E. Contribution a la chirurgie rachidienne du drainage vertebral dans le mal de Pott. Revue de Chirurgie. 1892;12:273-294.
2. Menard V. Traitement de la paraplegie du mal de Pott par le drainage lateral. Revue de Orthopaedie. 1895;6:134-138.
3. Muller W. Transperitoneale freilegung der wirbelsaule bei tuberkuloser spondylitis. Deutsche Zeitschrift für Chirurgie. 1906;85:128-135.
4. Royle N.D. Operative removal of an accessory vertebra. Med J Australia. 1928;1:467-478.
5. MacLennon A. Scoliosis. Brit Med J. 1922;2:864-866.
6. Chaklin V. Anterior fusion of the lumbar spine. Paper presented at: Proceedings of the Scientific Research Institutes of Sverdlosk. 1933.
7. Burns B.H. An operation for spondylolisthesis. Lancet. 1933;1:1233-1235.
8. Ito H., Tsuchiya J., Asami G. A new radical operation for Potts disease: report of 10 cases. J Bone Joint Surg. 1934;16:499-515.
9. Iwahara T. A new method of vertebral body fusion, anterior retroperitoneal surgery. Surgery (Japan). 1944;8:271-279.
10. Lane J.D., Moore E.S. Transperitoneal approach to the intervertebral disc in the lumbar area. Ann Surg. 1948;127:537-544.
11. Capener N. Spondylolisthesis. Betsin J Surg. 1945;19:374.
12. Hodgson A.R., Stock F.E. Anterior spine fusion. Brit J Surg. 1956;44:256-267.
13. Wang M., Laryussen C. Anterior lumbar interbody fusion. In: Kim D.H., Henn J.S., Vacarro A.R., Dickman C.A. Surgical Anatomy and Techniques to the Spine. Philadelphia, PA: Saunders Elsevier, 2006. PP 263–271
14. Hseih P., Koski T., O’Shaughnessy B., et al. Anterior lumbar interbody fusion in comparison with transforaminal lumbar interbody fusion: implications for the restoration of foraminal height, local disc angle, lumbar lordosis, and sagittal balance. J Neurosurg Spine. 2007;7:379-386.
15. Lee D., Lee S., Maeng D. Two-level anterior lumbar interbody fusion with percutaneous pedicle screw fixation: a minimum 3-year follow-up study. Neurol Med Chir. 2010;50:645-650.
16. Min J., Jang J., Lee S. Comparison of anterior and posterior approach instrumented lumbar interbody fusion for spondylolisthesis. J Neurosurg Spine. 2007;7:21-26.
17. Lee D., Lee S., Maeng D., et al. Anterior lumbar interbody fusion with pedicle screw fixation for elderly isthmic spondylolisthesis. J Korean Neurosurg Soc. 2006;40:175-179.
18. Ardern D., Cain C., Hall D. Clinical and radiological outcomes of stand-alone anterior lumbar interbody fusion used to treat discogenic pain: two year results of the Synfix-LR device. J Bone Joint Surg Br. 2010. 92-B:219-a
19. Lastfogel J., Altstadt T., Rodgers R., et al. Sacral fractures following stand-alone L5-S1 anterior lumbar interbody fusion for isthmic spondylolisthesis. J Neurosurg Spine. 2010;13:288-293.
20. Saraph V., Lerch C., Walochnik N., et al. Comparison of conventional versus minimally invasive extraperitoneal approach for anterior lumbar interbody fusion. Eur Spine J. 2004;13:425-431.
21. Kim Y. Finite element analysis of anterior lumbar interbody fusion: threaded cylindrical cage and pedicle screw fixation. Spine. 2007;32:2558-2568.
22. Cho C., Ryu K., Park C.K. Anterior lumbar interbody fusion with stand-alone interbody cage in treatment of lumbar intervertebral foraminal stenosis: comparative study of two different types of cages. J Korean Neurosurg Soc. 2010;47(5):352-357.
23. Sasso R., Burkus K., LeHuec J. Retrograde ejaculation after anterior lumbar interbody fusion: transperitoneal versus retroperitoneal exposure. Spine. 2003;28(10):1023-1026.
24. Adams P., Cotler H. Alternative anterior lumbar exposures. In: Albert T., Balderston R., Northrup B. Surgical Approaches to the Spine. Philadelphia: WB Saunders; 1997:145-155.
25. Baker J., Reardon P., Reardon M., et al. Vascular injury in anterior lumbar surgery. Spine. 1993;18(15):2227-2230.
26. Garg J., Woo K., Hirsch J. Vascular complications of exposure for anterior lumbar interbody fusion. J Vasc Surg. 2010;51(4):946-950.
27. Dickerman R., East J., Winters K. Anterior and posterior lumbar interbody fusion with percutaneous pedicle screws: comparison to muscle damage and minimally invasive techniques. Spine. 2009;34(25):E923-E925.