Chapter 55 Anterior Lumbar Interbody Fusion
Possibly more than any other spinal surgical procedure, a careful consideration of the indications for ALIF is essential for a good surgical outcome. Chronic low back pain is a frequent indication for ALIF.1–10 ALIFs are not ordinarily used to decompress the neural elements because of less-than-optimal visualization. Therefore, safe ventral decompression is difficult to perform.1,11,12
History
Anterior lumbar interbody fusion has evolved considerably over the past century. Ventral approaches to the lumbar spine were first reported for the treatment of tuberculosis. In 1892, Vincent13 described a costotransversectomy that was used to treat tuberculosis of the ventral lumbar spine. In 1906, Muller14 described a transperitoneal decompressive operation for the lumbar spine. Ito et al.,15 in 1934, described a transperitoneal approach to the lumbar spine that incorporated fusion.
Spondylolisthesis has been treated with ventral lumbar surgery since 1933. At that time, Burns described a transperitoneal approach using a tibial graft for fusion of the L5-S1 interspace.16 Numerous other investigators have followed with modifications of ventral lumbar surgery for spondylolisthesis.17–26 The retroperitoneal approach to the lumbar spine was first reported by Iwahara27 in 1944. In 1948, Lane and Moore28 described a series of patients with isolated lumbar disc disease who were treated with ALIF.
Preoperative Considerations
Proper patient selection is vital to the success of an ALIF procedure12 and is the best predictor of a good surgical outcome. Operative indications for ALIF include degenerative disc disease with intractable back pain.2,6,9,10,29,30 Degenerative spondylolisthesis with back pain often accompanies degenerative disc disease.4,30–36 A failure of prior dorsal surgery may also be an indication for ALIF.1,2,7–9,11,12,31–33,37,38 Most important, the symptoms should have persisted for a prolonged period. In general, symptoms are present for 6 months or more before a patient undergoes ALIF. Likewise, a prolonged trial of conservative therapy should have been attempted and failed before planning an ALIF procedure.6,29,38,39 Contraindications to ALIF are absolute or relative. The only true absolute contraindication is advanced osteoporosis that prevents the vertebral bodies from maintaining a bone graft.1 Relative contraindications occur in varying degrees. Neural compression as a result of either disc disease or stenosis is a relative contraindication. If neural compression is present, it is most safely and thoroughly approached from a dorsal approach. Other relative contraindications to ALIF include prior retroperitoneal surgery, severe peripheral vascular disease,1,40–42 an active disc space infection, a neoplasm,42 an infrarenal aortic aneurysm, an anomalous genitourinary system3 with only a single ureter, and systemic medical illness that precludes the surgery. Also, ALIF may be relatively contraindicated in men who wish to reproduce because a small but present risk of retrograde ejaculation has been described.43–49 As a general rule, an ALIF procedure is associated with less blood loss and is better tolerated than the majority of dorsal lumbar surgical procedures.5,8,50
A trial of nonoperative therapy is mandatory before undergoing ALIF.6,29,38,39,51 Mainstays of nonoperative treatment include optimizing body weight, which usually consists of weight loss in most patients. Cessation of smoking is also important because smoking is known to decrease fusion rates.39,52,53 In addition, a core-strengthening exercise program that includes back and abdominal muscle strengthening and stretching exercises should be instituted. Consultations with a pain management specialist,7 as well as a psychological evaluation,7,10,13,31,33,35,38,51,54–56 may be useful adjuncts in determining the optimal candidates for this procedure. Chronic pain may cause varying degrees of depression, anxiety, and aggression, and these behavioral changes can be reversed if the cause of pain is discovered and corrected.54,57 The efficacy of other conservative therapies such as epidural steroid injections, acupuncture, chiropractic, and biofeedback is more debatable. A preoperative trial of bracing is often recommended, and advocates of preoperative bracing believe that surgical results are superior if there is a good response to preoperative use of an external orthosis.58–60
It is essential for both the surgeon and the patient to have realistic expectations of the surgical outcome, and this is one of the reasons for a prolonged, conservative trial. Surgical success should be evaluated by a return to normal activities of daily living rather than by the hope for a return to high-level athletic activities. Surgical success cannot be fully evaluated until a minimum of 6 to 12 months after the operation.6,9,29,31–33,37,38,54 In general, it is not until the fusion process has been completed that surgical success can be fully evaluated.
Well-known negative prognostic indicators include involvement in workers’ compensation or litigation cases,8,9,31,39,50,51,55 because these patients are known to have suboptimal long-term clinical results. This is particularly worrisome because the operative indications for ALIF are often subjective (low back pain) rather than objective (neurologic deficit).
Before considering an ALIF procedure, a detailed radiographic imaging workup is required. The initial workup includes a plain-film radiographic series with flexion and extension lateral views.50 Flexion and extension radiographs may not reveal segmental instability because many painful motion segments are caused by rotational destabilization.61 These radiographs are used to evaluate spinal stability as well as to confirm the number of true lumbar vertebrae. In preoperative planning, these images are correlated with advanced radiographic imaging studies, such as MRI, CT scans, and myelography with postmyelography CT scanning. It is important to evaluate lumbar interspaces beyond the zone of the previous spinal surgery to identify additional sources of pain.
A myelogram, with a postmyelography CT scan, is considered by some to be the gold standard to rule out neural compression.7,20,29,37,50 A myelogram and CT scan are particularly useful in cases of lateral recess stenosis. In addition, CT scanning is an excellent tool for evaluating bony pathology and recording measurements of the cross-sectional diameters of the vertebral bodies on axial views.
MRI is extremely useful in defining soft tissue pathology. In particular, T2-weighted sagittal images are valuable in determining disc dehydration as evidenced by decreased signal intensity.10,62 In addition, MRI can evaluate motion segments adjacent to a previously operated level, neural compression secondary to a herniated lumbar disc, and central stenosis. Furthermore, axial measurements to be used in preoperative planning can be accurately obtained from MRIs. MRI will also reveal the presence of facet disease or other dorsal disease, which may influence the decision to back up the ALIF with a dorsal fusion. An ideal candidate for ALIF would have an isolated degenerative disc at L5-S1 with at least 50% loss of height.
Discography is a more controversial preoperative radiographic imaging technique. Some advocates of discography promote the use of a provocative discogram to evaluate patients who are considering lumbar interbody fusion.8,12,29,37,39,50,53,56,63 With a provocative discogram, the patient’s typical pain pattern must be reproduced at the involved level in an awake, nonsedated state. In addition, injection at other levels of the lumbar spine must not reproduce the patient’s typical low back pain pattern. Discography should be used to confirm MRI findings. It can be most effective in determining the number of levels to be fused in cases of multilevel disease. In patients who show discrepancies between the results of discography and MRI, consideration should be given to delaying surgical therapy and pursuing other conservative treatments. In other words, diagnostic imaging techniques should be used to decrease, not increase, the percentage of surgical candidates.
The number of levels to be fused is an important yet controversial topic. Many studies have clearly demonstrated that pseudarthrosis rates are increased with each additional level of fusion.9,33,37,39,41,56 Thus, clinical success rates are lessened with each additional fused level.33 Furthermore, there is an increased chance of adjacent motion segment degeneration with longer fusions, which is particularly problematic at the level immediately rostral to a lumbar interbody fusion.29,64 The number of levels fused is most frequently determined by a review of the radiographic imaging studies. Typically, plain flexion and extension radiographs that demonstrate pathologic motion at the proposed fusion site are helpful in demonstrating segmental instability.50,65 In addition, MRI scans, especially T2-weighted sagittal images, which demonstrate loss of signal consistent with disc dehydration, may help to define the involved segments.10,62 Finally, it is the opinion of some authors that a provocative discogram confirming the results of the MRI or the flexion and extension radiographs may be useful in determining the number of levels to be fused.*
Before undergoing ALIF, a detailed general medical evaluation should be completed. Smoking is known to decrease fusion rates in spinal surgical procedures,39,52,53 and as a general guideline, patients are requested to quit smoking a minimum of 8 weeks before surgery and to continue to not smoke until radiographic fusion has been confirmed. Additional factors known to detrimentally affect both clinical and radiographic fusion rates include diabetes mellitus, an immunocompromised state, and chronic corticosteroid use.
ALIF is performed for chronic low back pain. As such, patients who have experienced prolonged courses of narcotic analgesic use have a poorer clinical outcome with respect to overall pain control than patients who have not used narcotics preoperatively.38,54 It is unclear whether the worsened clinical outcome in patients requiring narcotic analgesics before surgery is due to more severe disease, changes in the pain receptor mechanisms, or psychological factors.
The use of oral contraceptives or any drugs containing estrogens is known to increase the incidence of deep venous thromboses during the postoperative period.67 As a result, these drugs should be discontinued for a minimum of 3 months before surgery. If there is any chance that an ongoing spinal infection or neoplastic process is present, it should be thoroughly evaluated before considering ALIF surgery.10 The presence of peripheral vascular disease should also be investigated preoperatively, with a detailed evaluation by physical examination of the aorta and lower extremities.1,41,65 Likewise, if there are genitourinary abnormalities, imaging studies should be performed to confirm the presence of two functioning kidneys and two functioning ureters. Abnormalities of the reproductive system, particularly evidence for ejaculatory dysfunction in a male patient, should be evaluated before surgery. The presence of osteoporosis should be evaluated by bone mineral density testing before any consideration of surgery.42
The preoperative regimen for ALIF may include a bowel preparation the night before surgery. A bowel preparation makes retraction of the intraperitoneal structures easier and increases safety by decreasing the possibility of an iatrogenic bowel injury.6,8,40 In addition, routine preoperative antibiotic coverage directed at Staphylococcus and Streptococcus species should be administered. This is usually accomplished with a first-generation cephalosporin antibiotic.
Intraoperative Considerations
Positioning
Operative positioning depends on the level to be operated on. Most ALIFs are performed at the two most caudal motion segments of the spine, L4-5 and L5-S1.†
General principles of spine surgery should be followed for operative positioning. As a rule, all bony prominences must be padded, with particular attention paid to protection of the ulnar nerve at the elbow38 and the peroneal nerve at the fibular head.53 In addition, antiembolic stockings and sequential compression devices are useful intraoperative adjuncts. Because no neural decompression is performed during an ALIF procedure, there is no need for intraoperative evoked potential monitoring or the use of electromyography. The operating table must allow for both AP and lateral fluoroscopy because this feature is essential for accurate placement of a bone graft or intervertebral cage.
Incision
In any surgical procedure, a properly placed skin incision is essential for achieving optimal exposure. The usual approach for an ALIF procedure is by retroperitoneal exposure from the patient’s left side. The left side is safer than the right because it allows the great vessels to be approached from the side of the aorta. It is easier to retract the infrarenal aorta from left to right than to retract the inferior vena cava from the patient’s right side. A left-sided retroperitoneal approach minimizes the need for any retraction of the inferior vena cava and thus decreases the possibility of injuring5,69 or thrombosing13 this structure. Some surgeons advocate the use of contrast-enhanced CT to identify the arterial and venous anatomy to help with surgical planning.
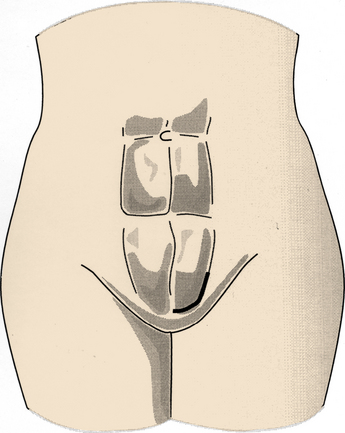
Incisions vary based on the level of surgery. Single-level ALIF at either the L4-5 or L5-S1 interspace may be performed using a right-angled incision. The standard retroperitoneal incision includes a horizontal limb extending from the midline to the linea semilunaris (lateral border of the rectus abdominis muscle). This horizontal limb is located at a point between the umbilicus and the pubic tubercle. The vertical limb of the incision extends up the linea semilunaris for a variable length, depending on the number of levels to be fused. For a single-level ALIF, the vertical limb is approximately the same length as the horizontal limb (Fig. 55-1). When multiple levels are to be fused, the vertical limb extends a longer distance than the horizontal limb. At progressively higher levels, it is necessary to orient the incision in an oblique fashion, extending toward the tip of the left 12th rib. This oblique incision may be necessary to gain access to the retroperitoneal space of the L1-2 and L2-3 interspaces. At more rostral levels, care must be taken to avoid retraction on the spleen, which is susceptible to minor blunt trauma from the retractors.70
Exposure
Anterior lumbar interbody fusion can be approached by a variety of exposures. The most commonly used technique is a left-sided retroperitoneal dissection. Other methods include a transperitoneal open procedure as well as a laparoscopic approach.52,64,71–78 The difficulties with transperitoneal surgery include prolonged postoperative ileus, as well as problems with fluid management and “third spacing” secondary to fluid shifts from an edematous bowel. As such, the open transperitoneal approach is less frequently used in favor of retroperitoneal exposure. Laparoscopic techniques are discussed elsewhere; however, they do not have long-term follow-up data that support their use because they typically have a steep learning curve and prolonged operative time. Although laparoscopic approaches were popularized in the 1990s, they have recently become less often used owing to prolonged operative times.
It is mandatory that a surgeon who is comfortable with the surgical approach provide the exposure. This procedure can be performed by a general surgeon or a vascular surgeon familiar with the retroperitoneal region, as well as management of the potential complications that may occur during the operation. Proper techniques of vessel ligation are vital to successful exposure of the disc spaces.2 With a left retroperitoneal approach, the intraperitoneal contents are dissected away from the retroperitoneal space from the left side toward the midline. The ureter must be identified and protected. During the exposure, it is best to avoid cutting longitudinally oriented structures.10,42 The dissection must be performed ventral to the psoas muscle. In thin patients, it is possible to dissect into the retropsoas space, which can result in excessive postoperative fluid collections, blood loss, and the possibility of injury to the genitofemoral and ilioinguinal nerves.
In patients who have not undergone prior retroperitoneal surgery, gentle blunt dissection is the safest, quickest, and easiest technique. If the peritoneum is violated during the retroperitoneal approach, it should be immediately closed to prevent development of a postoperative hernia.57 Closure is accomplished by isolating the edges of the peritoneum and using an absorbable suture with a continuous stitch. An unrecognized peritoneal violation can result in a postoperative hernia and subsequent bowel obstruction or incarceration. If the bowel proper is violated during retroperitoneal exposure, it should be treated with immediate irrigation, followed by layered closure, with the mucosal and serosal layers repaired separately, and abortion of the ALIF procedure. In addition, broad-spectrum antibiotics with anaerobic coverage should be instituted immediately. In spite of the excellent bony and vascular anatomic landmarks present in the ventral lumbar spine, radiographic confirmation of the correct level is mandatory before performing ALIF. Intraoperative radiographs should be compared with preoperative plain radiographs to confirm the appropriate level.65,67,68 Surgery at the incorrect level has been previously reported.9
Each level of the lumbar spine has distinct anatomic structures that must be addressed during a retroperitoneal approach. In general, lumbar segmental arteries should be preserved unless specific arterial damage has occurred. There is no need routinely to ligate these vessels, which are involved in supplying blood to the vertebral body proper.67
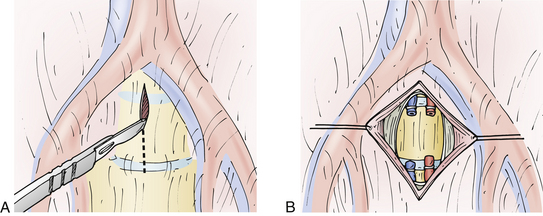
At the L5-S1 interspace, there is no need to mobilize the iliac vessels. This interspace is routinely located between the right and left common iliac vessels and is easily visualized without vessel dissection. Important structures at the L5-S1 level include the autonomic nerves that traverse the prevertebral space at this level. These nerves may be damaged by a monopolar electrocautery, and this can lead to autonomic dysfunction. In men, this is manifested as retrograde ejaculation. To avoid this complication, a monopolar electrocautery should not be used in the prevertebral space. In addition, a vertical opening of the midline prevertebral space should be used with gentle blunt dissection laterally to free all overlying soft tissues and, in the process, to gently dissect the hypogastric plexus away from the ventral disc space at the L5-S1 level10,65 (Fig. 55-2). Rates of postoperative retrograde ejaculation have been reported as ranging from 0.42% to 22.5%, with the transperitoneal approach carrying an approximately 10-fold greater risk.43–49
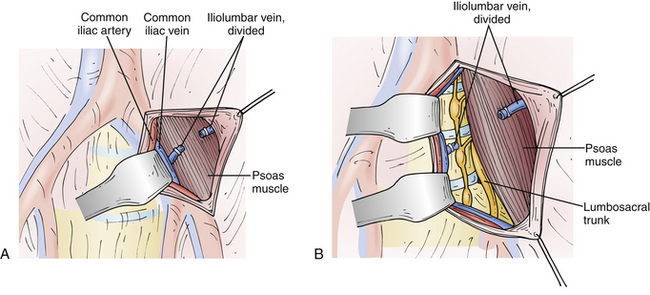
At the L4-5 interspace, the left common iliac vessels routinely traverse the prevertebral space and thus prevent direct access to the space. These vessels must therefore be mobilized from left to right to expose the bony midline. Before mobilizing the iliac vessels, however, vessel loops should be passed proximally and distally to obtain vascular control of both the left common iliac artery and the left common iliac vein. In addition, the left iliolumbar vein routinely enters the left common iliac vein laterally at a variable distance caudal to the inferior vena cava. It is necessary to locate and ligate the left iliolumbar vein before medially mobilizing the left common iliac vein. This ligation will allow for better exposure of the L4-5 interspace because the vessel typically courses over the L5 vertebral body and tethers the iliac vein.79,80 If the left common iliac vein is mobilized without ligation of the iliolumbar vein, extensive hemorrhaging can occur secondary to avulsion of this vessel5,69 (Fig. 55-3A). If an iliac vein is damaged, direct pressure should be applied and proximal and distal vascular control obtained. Fine, nonabsorbable suture is used to close the venous defect primarily with a continuous suturing technique. Common iliac vein injuries have been described in 2% to 4.5% of cases.35,57,81,82 Although they have been reported, injuries of the common iliac artery82 and inferior vena cava5,69 are very rare.
At the L3-4 disc space, the iliac vessels must be mobilized medially. In addition, a minimal amount of mobilization of the distal aorta from left to right is necessary (Fig. 55-3B). There is no need to mobilize the inferior vena cava during a left-sided approach to the lumbar spine at any level. If surgery is necessary at the L1-2 levels, the aorta will need to be mobilized from left to right to gain exposure to the true bony midline. In addition, care must be exerted at the L1-2 level to avoid iatrogenic injury to the left renal artery if overly vigorous medial displacement of the aorta is performed.
Retraction
After exposure of the retroperitoneal space, a table-mounted, self-retaining retraction system is useful. It should have malleable, broad-bladed retractors to disperse pressure evenly. Laparotomy pads are used to cover the retractor blades to protect the sensitive intraperitoneal structures. After the self-retaining retractor system is positioned and the correct level identified, a lateral fluoroscopic image can confirm the appropriate level.10,65,67,68 An AP fluoroscopic image is necessary to mark the true bony midline with an indelible marking pen or methylene blue dye. Maintaining orientation to the bony anatomic midline is critical to the safe performance of an ALIF procedure.
Excessive retraction can lead to injuries to the peritoneum, the intraperitoneal structures, or the major blood vessels. The intraperitoneal contents are best retracted using the self-retaining retractor system; however, the iliac vessels and the aorta should not be retracted by this method. The use of self-retaining retractors on these vessels can be associated with an increased risk of vascular injury and an increased risk of thrombosis of either the artery or the vein.1,67,68 The major vessels are best retracted by hand-held instruments manipulated by a surgical assistant under direct vision. Thus, pressure on the vessels can be frequently released, which helps to prevent development of an intramural thrombus. If one of the iliac vessels or the aorta is injured, the umbilical tapes, which provide distal and proximal vascular control, are used to halt the bleeding. This is followed by primary vessel repair using nonabsorbable sutures. This repair is ordinarily performed by either a general or vascular surgeon. Iliac artery thrombosis can be detected by carefully monitoring peripheral pulses and skin color in the lower extremities. If a thrombus is detected or strongly suspected, an intraoperative angiogram should be obtained immediately. Thrombectomy is the treatment for a positive angiogram.41 In addition, if the ureter is injured during the retraction or exposure, placement of a ureteral stent is ordinarily performed by a general or urologic surgeon.83
Discectomy
After the correct level has been confirmed with lateral fluoroscopy and an AP fluoroscopic image has confirmed the midline, the midline is marked on the vertebral bodies above and below the disc space to help maintain orientation. At this stage in the operation, long-handled instruments are useful. Commercially available curets and rongeurs 13 to 15 inches long facilitate performance of the discectomy. To determine the depth of the discectomy, confirmation should be made based on the preoperative MRI study or CT scan. Particularly at the L5-S1 interspace, it is important to confirm that all tissue overlying the disc space has been gently and bluntly swept laterally before incising the ventral anulus fibrosus. Once again, avoidance of monopolar electrocautery diminishes the incidence of postoperative autonomic dysfunction.2,8,10,33,67,84
A symmetrical window is cut into the ventral anulus fibrosus, taking care to preserve the lateral aspects of the disc bilaterally (Fig. 55-4). It is essential to maintain orientation to the midline and stay within the anulus fibrosus, with care being taken not to violate the anulus laterally or dorsally. The nucleus pulposus is removed, up to the dorsal anulus fibrosus. Calibrated tips on the instruments are useful at this stage. With use of some of the newer cage techniques, a predetermined cylinder of disc material is removed. In a standard lumbar interbody fusion, all articular cartilage is removed from the bony end plates above and below the disc space, with care taken to preserve the subchondral bone of the end plates. As a general rule, disc material is removed 25 to 30 mm in depth and 30 to 35 mm in width, centered at the bony midline. These measurements are quite consistent for the lower lumbar motion segments. Variations exist in the AP dimensions of the vertebral bodies. The oval shape of the lumbar vertebrae accounts for a larger depth in the midline than in the lateral portions of the vertebrae.2 It is important to avoid use of bone wax for bleeding cancellous bone because foreign bodies may decrease the postoperative fusion rates.
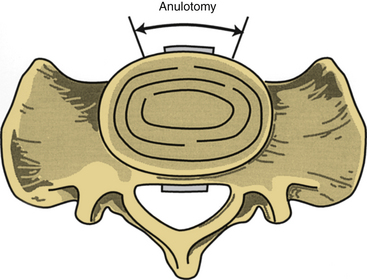
FIGURE 55-4 An anulotomy is performed as depicted, taking great care not to proceed too far laterally.
(Copyright University of New Mexico, Division of Neurosurgery, with permission.)
Excessive depth of the discectomy can be catastrophic. The dorsal anulus fibrosus is not well visualized from an ALIF approach, and if it is violated, epidural bleeding or a dural injury can occur. These are usually not well controlled by a limited exposure and are best avoided. If the dorsal anulus fibrosus is violated, the disc space must be observed for bleeding or a cerebrospinal fluid (CSF) leak. Bleeding is best controlled with copious amounts of irrigation, as well as with the use of thrombin-soaked Gelfoam. Patience is the key because access in this area is extremely poor. It is unrealistic to attempt bipolar cautery on a bleeding epidural vessel unless it is directly visualized. Monopolar electrocautery must be avoided at all times in the epidural space. If a CSF leak occurs, free muscle and fascia grafts, with or without fibrin glue, are used to attempt to control the egress of CSF. Consideration should be given to placement of a lumbar subarachnoid drain to divert CSF during the immediate postoperative period.85 Safe and reliable decompression of the thecal sac or nerve roots is not realistic from an ALIF approach, and if neural decompression is necessary, it should be performed using a dorsal procedure or as part of a combined procedure.
Distraction
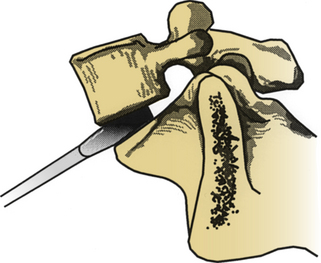
Several commercially available systems have been developed for ALIF. These systems have disc space distractors with calibrated tips to prevent excessively deep disc space distraction (Fig. 55-5). Improved distraction can also be obtained by proper patient positioning. This helps to maintain lumbar lordosis and in the process opens up the ventral aspect of the interspace. Particularly in larger patients, the operating surgeon may choose to wear a headlamp to improve visualization.
Bone Graft Harvest
When harvesting iliac crest autograft, the most medial aspect of the skin incision should be at least 2 cm lateral to the anterior superior iliac spine. The lateral femoral cutaneous nerve is subcutaneous in this location and typically runs within 2 cm of the anterior superior iliac spine. If this nerve is transected, permanent anesthesia of the ventrolateral skin of the thigh can result.7,85 If it is compressed, a temporary sensory deficit, caused by neurapraxic injury, results. When ALIF is performed using structural iliac crest autograft as the sole graft source, the medial and lateral aspects of the crest need to be exposed and an oscillating saw used to harvest tricortical strut grafts. Osteotomes can also be used; however, they have been shown to be associated with an increased incidence of microfractures of the tricortical bone graft.86 If the iliac crest autograft is being used to augment allograft bone or a cage, then a small cortical window can be opened on either the medial or lateral surface of the iliac crest, and curets and gouges can be used to remove cancellous bone from the iliac crest. In extremely thin patients, the medial crest is favored for its cosmetic advantages; in heavier patients, the lateral crest is more easily accessible. Ordinarily, harvesting iliac crest autograft does not result in cosmetic deformity.
Complications associated with iliac crest autograft harvesting include lateral femoral cutaneous nerve palsies that result from medial placement of the skin incision. A postoperative hematoma may develop in the event of inadequate hemostasis.8 This complication can be avoided by paying attention to hemostasis, and perhaps with the use of a drain. Postoperative wound infections and subsequent pain may also occur,29,37,82 and are avoided by use of generous quantities of irrigation, drainage of the wound, use of prophylactic antibiotics, and consideration of pulse lavage after graft harvest.
Bone Morphogenetic Protein
In 2002, the U.S. Food and Drug Administration (FDA) approved the use of recombinant human bone morphogenetic protein-2 (rhBMP-2) for use in the Medtronic (Minneapolis, MN) LT Cage at the L4-5 and L5-S1 interspaces.87 The study compared the use of rhBMP-2 with iliac crest autograft, citing clinical success with fusion rates of 96.9% versus 92.6% at 12 months and 94.5% versus 88.7% at 24 months. The rhBMP-2 group also did not complain of donor site pain or cosmetic appearance, as opposed to the small percentage (2–10%) of the iliac crest group who did experience these complaints.87
To date, rhBMP-2 is the only osteoinductive growth factor that is FDA approved for use in ALIF. Its presumed mechanism is the stimulation of pluripotent stem cells from bleeding cancellous bone to form bone.87 Other, subsequent studies have used rhBMP-2 in femoral ring allograft, threaded cortical bone dowels, and threaded cylindrical titanium cages, and found similar clinical and radiographic success rates.88,89 In the initial FDA study group’s 6-year follow-up, the clinical and radiographic results remained durable.90
Fusion
Anterior lumbar interbody fusion is performed for low back pain, and long-term bony fusion is necessary for the best postoperative results.9,33,68 Fusion can be judged both clinically, as evidenced by the relief of back pain, and radiographically, as evidenced by radiographic incorporation of the graft. The meticulous performance of ALIF is critical.
After the discectomy is completed, a spacer must be placed in the involved interspace. A variety of materials have been used to function as spacers. Iliac crest autograft can be used as a spacer, in which case two or more tricortical grafts are typically placed side by side. Allograft bone can also be used. The different forms of allografts used in ALIF procedures include femoral ring, tibia, humerus, and iliac crest. Of these, femoral ring is the best choice because it can be sculpted to anatomically fit the defect and then be packed with cancellous autograft bone. The use of iliac crest allograft is a poor choice because this bone does not have the same structural strength as comparable autografts. Threaded dowels of bone derived from femoral allograft have also been used.91 They are placed in predetermined channels, a method similar to the Cloward technique used for the cervical spine. Recently, the use of cages as spacers in ALIF procedures has gained popularity.52,92,93 These cages received FDA approval in 1996 and are available in many forms; threaded titanium cages, polyetheretherketone (PEEK), and carbon fiber–reinforced PEEK cages can be obtained. These cages are routinely packed with iliac crest cancellous autograft bone or rhBMP-2 soaked sponges.
Two principles of bony fusion are applicable to ALIF: (1) the need for compression, and (2) the need for immobilization to increase the chances of acquiring stable bony fusion. Unlike dorsal fusion, with ventral fusion the graft can be maintained in a position of compression.2,6,38,65,67,68 Compression is best achieved by distracting the interspace and placing a slightly oversized spacer in this disc space. Compressive forces felt by a spacer in the intervertebral space are many times greater in magnitude than any compression achieved by an onlay graft dorsally. Immobilization is obtained by firmly fitting a spacer into the intervertebral disc space. In addition, an external orthosis may be used to diminish motion at the lower lumbar spine. There is less need for internal instrumentation with ALIF. Historically, interference plates were placed to prevent cage backout. Newer devices have been used for implantation with ventral plate/screw fixation or with integrated plate/screw fixation in the cage itself.
In sizing the spacer or choosing the number of spacers to place, recent unpublished data recommend 60% vertebral body end-plate coverage.94 Because approximately 80% of the load on the spine and 90% of the articular surface area is supported ventrally,95 this larger footprint is biomechanically more advantageous in stability and allows for optimum fusion across the intervertebral space.
The grafting technique itself involves removal of all articular cartilage overlying the surfaces to be fused. The interspace is distracted, and a slightly oversized spacer is selected. A portion of cancellous bone from the vertebral body immediately above and below the interspace is exposed using a drill, curet, or gouge. The exposed cancellous bone of the vertebral body should be aligned with the exposed cancellous portion of the bone graft or the center of the cage, which allows for optimal fusion as well as providing immediate hemostasis. The ventral-most aspect of the spacer should be slightly countersunk beneath the ventral-most surface of the vertebral bodies above and below. Bone wax should be avoided because it decreases fusion rates; hemostatic agents or grafting agents may be used instead. When placing a spacer, meticulous attention to maintenance of the orientation of the bony midline is important. If two spacers are used, they should be symmetrically placed on each side of the midline, and if a single spacer is used, it should be centered on the midline. After placement of the spacer, AP and lateral fluoroscopic confirmation should be obtained immediately.65,67,68,96 If any modification of the positioning of the spacer is necessary, it should be addressed immediately. A particular concern regarding the placement of two spacers side by side is assurance that when the second spacer is positioned, it does not cause loosening of the first spacer. This must be confirmed after placement of the second spacer, and if the initial spacer has been loosened, modification must be made before wound closure. Strict adherence to symmetrical placement of the spacers with respect to the midline helps avoid this problem.
Plating
Recent biomechanical studies have shown improved stabilization with ventral fixation devices. Stand-alone interbody implants do not confer significant stabilization in extension or rotation compared with flexion and lateral bending.97 The historic buttress/interference plate has evolved into a ventral plate, similar to a cervical plate, to allow for stabilization. Ventral plates can eliminate the need for a separate dorsal approach for instrumentation. The presumed increase in biomechanical stability would protect against graft migration and allow for better bony healing. Biomechanical studies have shown that ventral plating significantly enhances stability in flexion, extension, and lateral bending.98–100 Clinical studies have also supported the effectiveness of ventral plating for ALIF, with excellent fusion rates and improvement in visual analogue pain scores and the Oswestry Disability Index.101
Newer technologies have incorporated the plate and screw into the interbody graft. These devices have also shown improved biomechanical stability over stand-alone ALIF. The long-term clinical outcome of these devices is still under investigation. The benefit of an integrated plate and screws is that it allows for less dissection of soft tissues and mobilization of blood vessels. Some surgeons have reported blood vessel complications such as erosion when the vessels overlie the ventral plate,95 lending further advantage to cages with integrated plate and screws.
Dorsal Supplementation
Biomechanical studies have shown that pedicle fixation offers the strongest construct to resist forces in flexion, extension, axial rotation, and lateral bending.98,100 Novel methods in minimally invasive percutaneous pedicle fixation may be used in patients with facet disease, instability, or dorsal pathology. These patients may have dorsal fixation for enhanced biomechanical stabilization with minimal blood loss and tissue disruption. In experienced hands, surgical times are often short for these percutaneous procedures.
Postoperative Complications
Operative Approach Complications
After ALIF, it is possible to develop a postoperative hernia,30,56,82 which can lead to bowel obstruction1,20,102 and possible bowel infarction. Violation of the peritoneum during ALIF with the retroperitoneal approach, or violation of the transversalis fascia during iliac bone graft harvest, can lead to the development of both internal hernias and subsequent bowel obstruction. When the peritoneum or the transversalis fascia is violated, postoperative hernias are best prevented by immediate operative repair of the defects during the index surgical procedure.57 If these structures are violated and are inadequately repaired or not appreciated, a hernia may result.
An injury to the bowel may occur during retroperitoneal exposure, and this extremely rare complication requires immediate detection and treatment. The treatment is aggressive irrigation followed by direct repair of the bowel, with separate, layered closures of the mucosal and serosal layers. Prophylactic antibiotics are administered and the ALIF procedure is aborted at this point. If not already present during the surgery, a general surgeon should be summoned to perform or assist in the bowel repair. Bowel injuries are best avoided by using a preoperative bowel preparation, including an enema to help decompress the loops of bowel, as well as placement of a nasogastric tube before the retroperitoneal procedure is performed.6,8,41
Major blood vessel injuries are rare during operative exposure. Injuries to the iliac arteries or veins, the inferior vena cava, and the aorta occur in 2% to 4% of ALIF operations.5,35,57,69,81,82 To avoid vessel injuries, a self-retaining retractor should not be used on the vessels during the exposure portion of the operation.1 In addition, before mobilizing the iliac vessels or the aorta, proximal and distal control should be obtained with vessel loops, which are used when a major vessel is injured. Should this type of injury occur, a vascular surgeon should perform or assist in vessel repair. Major vessel injuries are repaired primarily using nonabsorbable sutures. Sometimes smaller vessels are injured. The left iliolumbar vein can be avulsed if it is not ligated before the left common iliac vein is mobilized.5,69 This vessel is safely ligated at any time and should be ligated before common iliac vein mobilization. Likewise, lumbar segmental arteries can be ligated safely at any level; however, these vessels ordinarily are spared during the surgical procedure.
Retrograde ejaculation is a serious complication that may occur in male patients after ALIF.8,33,41,84 It occurs when the autonomic nerves are injured, usually at the L5-S1 level. The hypogastric plexus is a continuation of the preaortic sympathetic chain that extends down from the thoracic region ventral to the aorta and lumbar vertebrae in the retroperitoneal space.10,42,84 As stated, the use of monopolar electrocautery over the L5-S1 interspace has been associated with an increased incidence of retrograde ejaculation and should be avoided.2,8,10,33,67,84 The rates of retrograde ejaculation have been reported as ranging from 0.42% to 22.5%, with a transperitoneal approach carrying a 10-fold greater risk.43–49 The mechanism of retrograde ejaculation involves the presence of dry ejaculate secondary to relaxation of the internal bladder sphincter, with retrograde flow of ejaculate into the bladder.42,84 There is no surgical management for this complication, and it becomes prograde in 25% to 33% of patients by the end of the second year.45,102
Thrombosis of either venous or arterial structures may occur after ALIF. Venous thrombosis has been reported in 1% to 11% of all ALIF procedures, but arterial thrombosis is extremely rare.* To avoid thromboses, retraction should not be prolonged, and self-retaining retractors should not be used on major vessels. If thrombosis is suspected, an immediate angiogram or venogram should be obtained. The treatment is open surgical thrombectomy.41 Likewise, an embolus in the arterial vascular tree in the lower extremities can occur rarely and is particularly likely if the patient is elderly. To avoid arterial embolization, retraction should not be prolonged and care should be taken not to use self-retaining retractors on blood vessels. An arterial embolus is detected in the perioperative period by loss of distal pulses and a cool extremity and is best treated by immediate vascular surgical intervention.
General systemic medical problems may arise during the postoperative period. Urinary retention occurs in 5% to 27% of cases and is usually temporary.2,4,18,29,33,39,68 It may be related to the use of perioperative narcotic analgesics. If urinary retention occurs, it is important to rule out an injury to the ureter or cauda equina syndrome. If urinary tract dysfunction occurs in the late postoperative period, obstruction of the distal ureter from retroperitoneal scarring must be ruled out.81 Ileus is also common during the postoperative period and usually resolves less than 1 week after the operation. Prolonged postoperative ileus has been reported in 1% to 8% of all ALIF procedures† and also may be related to the use of perioperative narcotic analgesics. An internal hernia that causes bowel obstruction secondary to violation of the peritoneum or transversalis fascia must be ruled out in cases of prolonged postoperative ileus. Serious general medical problems that have been reported include both fatal66,105 and nonfatal4,9 cardiac arrest, fatal4,38 and nonfatal4,5,8,9,53 pulmonary embolus, and aortic aneurysm rupture.82
Neurologic Complications
Specific nerve root syndromes are manifested as radiculopathies and should be worked up immediately with neuroimaging studies. Based on the results of these studies, either expectant observation or dorsal exploration should be undertaken. Injuries to the genitofemoral or ilioinguinal nerves may occur after an ALIF procedure.6,29,41 These injuries are characterized by postoperative numbness in the groin and/or the medial thigh region. They are most common in patients who undergo ALIF procedures at the upper lumbar levels. Palsies in these nerves frequently resolve spontaneously and are usually treated by observation alone.
Compression of the thecal sac during the postoperative period secondary to hematoma, infection, or retropulsed disc material is best treated with dorsal exploration.37,106 A dural tear is ordinarily detectable during surgery. However, it usually cannot be adequately treated through an ALIF approach. Placement of a lumbar subarachnoid drain to divert the CSF during the first week after surgery is the usual treatment for an intraoperative dural tear resulting from an ALIF procedure.85 Injury to the sympathetic nerves on the side ipsilateral to the retroperitoneal approach may occur. Usually, partial sympathectomy occurs and is manifested by vasodilation of vessels in the ipsilateral foot.29,39,107 The usual patient complaint is a cold sensation in the contralateral foot.41,42 Sympathectomy symptoms usually resolve spontaneously in the first 3 to 6 months after surgery. Discitis68,108 and osteomyelitis63,109 each occur in approximately 1% of all ALIF procedures. Their treatment involves reexploration for a confirmed abscess, using either a ventral or a dorsal approach, and wound culture and drainage of the abscess. If infection is present without an abscess, treatment with prolonged antibiotics and immobilization can be used. Surgical exploration may also be necessary in some cases.
Bone Graft Harvest Complications
Postoperative infections of the iliac crest donor site wound occur in 1% to 9% of all ALIF procedures29,37,82 and are usually detected secondary to pain, which is followed by eventual purulent discharge from the wound. Iliac wound infections are best prevented by avoiding the use of foreign material in the wound (e.g., bone wax) and using perioperative prophylactic antibiotics, copious irrigation, and meticulous intraoperative hemostasis. The use of large-bore drains in iliac wounds may prevent accumulation of a hematoma and decrease wound infection rates; however, they lead to an egress of organisms through and around the drain. When iliac crest wound infection occurs, treatment requires surgical reexploration of the wound, cultures, and drainage.
Postoperative iliac crest wound hematomas usually are manifested as pain in the iliac crest region, which may radiate toward the groin.8 These hematomas are best avoided by meticulous hemostasis and generous intraoperative wound irrigation. The use of a postoperative large-bore drain may diminish the incidence of postoperative hematomas. Most of these hematomas resolve spontaneously and can be managed by observation. Based on the surgeon’s preference, it may be necessary to drain them if they become large or painful.
Fusion Complications
Problems related to the bony fusion portion of the operation are the most common complications of ALIF. Persistent low back pain is the most frequent complaint and is usually the result of pseudarthrosis, or nonunion, of the fusion. This complication is reported at extremely variable rates, ranging from 3% to 58%.* It is not possible to diagnose lumbar pseudarthrosis secondary to an ALIF procedure until 6 to 12 months after surgery.6,9,29,31–33,37,38,67 The workup includes plain radiographs to detect evidence of a persistent lucent line between the graft and the vertebral body. Tomograms and CT scans with sagittal reformations also may be useful for detecting this lucent line.112 Flexion and extension lateral radiographs can detect persistent motion consistent with pseudarthrosis.39 MRI is difficult to interpret after ALIF and is not particularly useful in revealing postoperative pseudarthrosis.
The treatment of symptomatic pseudarthrosis secondary to ALIF is prolonged immobilization of the patient, which can be accomplished with a thoracolumbosacral orthosis or with a body cast. Electrical stimulation or a bone growth stimulator may also be helpful. In addition, reoperation using a ventral or dorsal approach may be necessary. Rarely, a ventrodorsal combined procedure is necessary for treatment of pseudarthrosis. Several authors have stated that there is no correlation between radiographic pseudarthrosis rates and clinical outcome results.4,8,35,55,105,111 No treatment is required for asymptomatic pseudarthrosis and the patient should be observed with serial radiographs and clinical evaluations.
Graft collapse occurs in 1% to 2% of all ALIF procedures,1,39,96,103 and ordinarily results from excessive removal of subchondral bone from the vertebral body bony end plate.37 A kyphotic deformity may develop. The treatment is surgical reexploration through a ventral approach. Graft resorption may occur and is particularly likely in patients who are smokers,39,53,113 have diabetes, or are immunosuppressed. Graft dislodgement occurs in 1% of all ALIF procedures, and the treatment is reoperation using a ventral approach.9,39,41 Graft displacement may be minimized using a ventral buttress/interference plate, ventral/ventrolateral plate fixation, or dorsal pedicle fixation to enhance stability.
After a successful ALIF procedure, the motion segments immediately adjacent to the fusion are exposed to increased stress. Months to years after a successful procedure, adjacent-level disc herniation or degeneration may occur and is particularly likely to take place at the motion segment rostral to the ALIF.29,64 Detection of adjacent-level disc problems is best accomplished with diligent long-term follow-up.
Multilevel Anterior Lumbar Interbody Fusion
Complication rates increase when multiple motion segments are fused. Several investigators have described a direct correlation between the number of levels fused and postoperative pseudarthrosis rates. With each additional level fused, the overall pseudarthrosis rate increases.9,29,33,37,39,41,56 Likewise, clinical success rates decrease for each additional level fused.33 In addition, because of the longer lever arms that result from multilevel ALIF, adjacent-level disc problems increase after multilevel procedures. Complications with the retroperitoneal approach also increase because of the larger surgical exposure. Furthermore, systemic medical problems secondary to the longer duration of surgery with increased blood loss are more common in multilevel procedures. Finally, iliac donor site problems are increased when additional bone harvesting is necessary to fuse multiple levels.
Minimally Invasive Anterior Lumbar Interbody Fusion
Following the lead developed by gynecologic and general surgeons, clinicians have developed laparoscopic approaches to the lumbar spine for ALIF procedures, and they are particularly useful at the L5-S1 level.71,72,74–77,114 In this region, the iliac vessels are located lateral to the L5-S1 space, providing direct access for an ALIF procedure to be safely performed laparoscopically. Once again, it is essential to avoid using a monopolar electrocautery over the L5-S1 disc because this may result in higher rates of retrograde ejaculation in male patients.2,8,10,33,42,67,84 The laparoscopic approach provides good visualization but makes hemostasis difficult. Likewise, maintaining orientation to the true bony midline is more difficult laparoscopically. AP and lateral fluoroscopy are essential to the laparoscopic approach. Because of the need to mobilize the large blood vessels (iliac artery and vein, inferior vena cava, aorta) at levels rostral to the L5-S1 interspace, laparoscopic methods are more dangerous at these levels.78 There are no long-term data available on the success rates of laparoscopic ALIF. Some surgeons have abandoned laparoscopic ALIF because of the steep learning curve, lack of three-dimensional visualization, and associated complications.
Summary
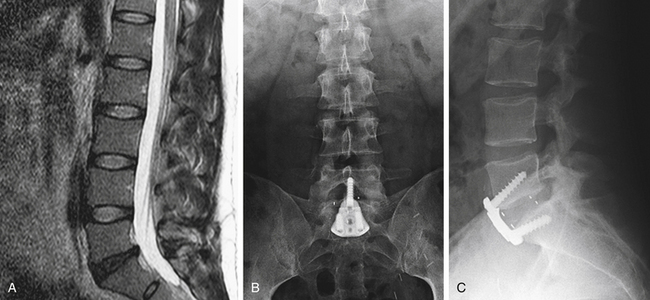
In properly selected patients, the results of ALIF in treating patients with chronic low back pain secondary to degenerative disc disease have been encouraging (Fig. 55-6). ALIF offers the advantages of immediate immobilization of the motion segment, a larger fusion area than is achievable with dorsal or dorsolateral techniques, a fusion construct under compression, improved sagittal plane alignment that can restore lumbar lordosis, restoration of intervertebral disc height, and an increase in the height of the neural foramina. In addition, the spinal canal is avoided, as well as many of the difficulties attributed to scarring that commonly occur with dorsal approaches.11,29 ALIF is generally well tolerated and is associated with less operative blood loss compared with equivalent dorsal procedures.8,37,50
The end result of an ALIF procedure should be a biomechanically strong construct. Long-term follow-up is necessary until both clinical and radiographic fusion are demonstrated. Complication rates are reasonable and many problems can be treated during the index surgery. Complications are (1) related to the operative approach, (2) neurologic, (3) related to the iliac donor site, and (4) directly related to the fusion proper. Fusion complications are the most common. It is the adequacy of the long-term bony arthrodesis that provides long-term pain relief in patients undergoing ALIF procedures, and the long-term results are promising. Continued modifications, including the use of laparoscopic approaches, minimally invasive approaches, rhBMP-2, and integrated fixation cages, must undergo long-term scrutiny to determine their efficacy.1,4,6,8,12,29,35,115
Burkus J.K., Gornet M.F., Schuler T.C., et al. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg [Am]. 2009;91:1181-1189.
Crock H.V. Lumbar vertebral interbody fusion. In: Lin P., Gill K., editors. Lumbar interbody fusion. Rockville, MD: Aspen; 1989:115-125.
Faciszewski T., Winter R.B., Lonstein J.E., et al. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults: a review of 1223 procedures. Spine (Phila Pa 1976). 1995;20:1592-1599.
Long D.M., Filtzer D.L., BenDebba M., et al. Clinical features of the failed-back syndrome. J Neurosurg. 1988;69:61-71.
Sasso R.C., Kenneth Burkus J. LeHuec JC: Retrograde ejaculation after anterior lumbar interbody fusion: transperitoneal versus retroperitoneal exposure. Spine (Phila Pa 1976). 2003;28:1023-1026.
Watkins R. Anterior approaches to the lumbar spine. In: Torrens M.J., Dickson R.A., editors. Operative spinal surgery. New York: Churchill Livingstone; 1991:161-171.
1. Collis J.S., Rojas C., Janack M. Anterior total disc replacement: a modified anterior lumbar interbody fusion. In: Lin P., Gill K., editors. Lumbar interbody fusion. Rockville, MD: Aspen; 1989:149-152.
2. Crock H.V. Anterior lumbar interbody fusion: indications for its use and notes on surgical technique. Clinical Orthop Relat Res. 1982;165:157-163.
3. Dennis S., Watkins R., Landaker S., et al. Comparison of disc space heights after anterior lumbar interbody fusion. Spine (Phila Pa 1976). 1989;14:876-878.
4. Freebody D., Bendall R., Taylor R.D. Anterior transperitoneal lumbar fusion. J Bone Joint Surg [Br]. 1971;53:617-627.
5. Kozak J.A., Heilman A.E., O’Brien J.P. Anterior lumbar fusion options: technique and graft materials. Clin Orthop Relat Res. 1994;300:45-51.
6. Leong J.C.Y. Anterior interbody fusion. In: Lin P., Gill K., editors. Lumbar interbody fusion. Rockville, MD: Aspen; 1989:133-148.
7. Long D.M., Filtzer D.L., BenDebba M., et al. Clinical features of the failed-back syndrome. J Neurosurg. 1988;69:61-71.
8. Sacks S. Anterior interbody fusion of the lumbar spine. J Bone Joint Surg [Br]. 1965;47:211-223.
9. Stauffer R.N., Coventry M.B. Anterior interbody lumbar spine fusion: analysis of Mayo Clinic series. J Bone Joint Surg [Am]. 1972;54:756-768.
10. Watkins R.G. Assessment of results and complications of anterior lumbar fusion. In: Lin P., Gill K., editors. Lumbar interbody fusion. Rockville, MD: Aspen; 1989:153-169.
11. Hammerberg K.W. Anterior lumbar interbody fusion. In: Margulies J.Y., Floman Y., Farcy J.-P.C., et al, editors. Lumbosacral and spinopelvic fixation. New York: Lippincott-Raven; 1996:495-505.
12. O’Brien J.P., Dawson M.H., Heard C.W., et al. Simultaneous combined anterior and posterior fusion: a surgical solution for failed spinal surgery with a brief review of the first 150 patients. Clin Orthop Relat Res. 1986;203:191-195.
13. Vincent E. Contribution a la chirurgie rachidienne, du drainage vertebrale dans la mal de Pott. Rev Chir. 1892;12:273.
14. Muller W. Transperitoneale Freilegung der Wirbelsaule bei tuberkuloser Spondylitis. Dtsch Z Chir. 1906;85:128.
15. Ito H., Tsuchiya J., Asami G. A new radical operation for Pott’s disease. J Bone Joint Surg [Br]. 1934;16:499-515.
16. Burns B. An operation for spondylolisthesis. Lancet. 1933;1:1233.
17. Frieberg S. Studies on spondylolisthesis. Acta Chir Scand. 1939;82(Suppl 55):7-134.
18. Gjessing M.H. Osteoplastic anterior fusion of the lower lumbar spine in spondylolisthesis, localized spondylosis, and tuberculous spondylitis. Acta Orthop Scand. 1951;20:200-213.
19. Henschen C. Operation der Spondylolisthesis durch transabdominelle lumbosacrale Verschraubung und zusätzliche transplantative Spanversteifung. Helv Med Acta. 1942;10:25-28.
20. Ingerbrigtsen R. Indications for anterior transperitoneal fusion in the treatment of spondylolisthesis. Acta Chir Scand. 1953;105:172-181.
21. Jenkins J.A. Spondylolisthesis. Br J Surg. 1936;24:80-85.
22. Mercer W. Spondylolisthesis with a description of a new method of operative treatment and notes of ten cases. Edinb Med J. 1936;43:545-572.
23. Merle d’Aubigne R., Cauchoix J., Faulong M. Anterior transperitoneal arthrodesis in the therapy of spondylolisthesis [in French]. Rev Orthop Chir Appar Mot. 1950;36:490-494.
24. Ramser R. Transabdominelle Operation der nichtraumatischen Spondylolisthesis. Helv Med Acta. 1942;10:365-375.
25. Speed K. Spondylolisthesis: treatment by anterior bone graft. Arch Surg. 1938;37:175-189.
26. Zaaijer J.H. Transabdominal treatment of spondylolisthesis [in Dutch]. Ned Tijdschr Geneeskd. 1952;96:518-520.
27. Iwahara T. A new method of vertebral body fusion. Surgery (Japan). 1944;8:271.
28. Lane J.D., Moore E.S. Transperitoneal approach to the intervertebral disc in the lumbar area. Ann Surg. 1948;127:537-551.
29. Chow S.P., Leong J.C., Ma A., et al. Anterior spinal fusion or deranged lumbar intervertebral disc. Spine (Phila Pa 1976). 1980;5:452-458.
30. Sacks S. Anterior interbody fusion of the lumbar spine: indications and results in 200 cases. Clin Orthop Relat Res. 1966;44:163-170.
31. Flynn J.C., Hoque M.A. Anterior fusion of the lumbar spine: end-result study with long-term follow-up. J Bone Joint Surg [Am]. 1979;61:1143-1150.
32. Gill K., O’Brien J.P. Observations of resorption of the posterior lateral bone graft in combined anterior and posterior lumbar fusion. Spine (Phila Pa 1976). 1993;18:1885-1889.
33. Goldner J.L., Urbaniak J.R., McCollum D.E. Anterior disc excision and interbody spinal fusion for chronic low back pain. Orthop Clin North Am. 1971;2:543-568.
34. Iwahara T., Ikeda K., Hirabayashi K. Results of anterior spine fusion by extraperitoneal approach for spondylolysis and spondylolisthesis. Nippon Seikeigeka Gakkai Zasshi. 1963;36:1049-1067.
35. Raugstad T.S., Harbo K., Oogberg A., et al. Anterior interbody fusion of the lumbar spine. Acta Orthop Scand. 1982;53:561-565.
36. Sijbrandij S. The value of anterior interbody vertebral fusion in the treatment of lumbrosacral insufficiency, with special reference to spondylolisthesis. Arch Chir Neerl. 1962;14:37-62.
37. Kozak J.A., O’Brien J.P. Simultaneous combined anterior and posterior fusion: an independent analysis of a treatment for the disabled low-back pain patient. Spine (Phila Pa 1976). 1990;15:322-328.
38. Sorensen K.H. Anterior interbody lumbar spine fusion for incapacitating disc degeneration and spondylolisthesis. Acta Orthop Scand. 1978;49:269-277.
39. Loguidice V.A., Johnson R.G., Guyer R.D., et al. Anterior lumbar interbody fusion. Spine (Phila Pa 1976). 1988;13:366-369.
40. Dodge L.D., Bohlman H.H., Rhodes R.S. Concurrent lumbar spinal stenosis and peripheral vascular disease: a report of nine patients. Clin Orthop Relat Res. 1988;230:141-148.
41. Gill K. Technique and complications of anterior lumbar interbody fusion. In: Lin P., Gill K., editors. Lumbar interbody fusion. Rockville, MD: Aspen; 1989:95-106.
42. Watkins R. Anterior approaches to the lumbar spine. In: Torrens M.J., Dickson R.A., editors. Operative spinal surgery. New York: Churchill Livingstone; 1991:161-171.
43. Christensen F.B., Bunger C.E. Retrograde ejaculation after retroperitoneal lower lumbar interbody fusion. Int Orthop. 1997;21:176-180.
44. Faciszewski T., Winter R.B., Lonstein J.E., et al. The surgical and medical perioperative complications of anterior spinal fusion surgery in the thoracic and lumbar spine in adults: a review of 1223 procedures. Spine (Phila Pa 1976). 1995;20:1592-1599.
45. Flynn J.C., Price C.T. Sexual complications of anterior fusion of the lumbar spine. Spine (Phila Pa 1976). 1984;9:489-492.
46. Peng C.W., Bendo J.A., Goldstein J.A., et al. Perioperative outcomes of anterior lumbar surgery in obese versus non-obese patients. Spine J. 2009;9:715-720.
47. Rajaraman V., Vingan R., Roth P., et al. Visceral and vascular complications resulting from anterior lumbar interbody fusion. J Neurosurg. 1999;91:60-64.
48. Sasso R.C., Kenneth Burkus J. LeHuec JC: Retrograde ejaculation after anterior lumbar interbody fusion: transperitoneal versus retroperitoneal exposure. Spine (Phila Pa 1976). 2003;28:1023-1026.
49. Tiusanen H., Seitsalo S., Osterman K., et al. Retrograde ejaculation after anterior interbody lumbar fusion. Eur Spine J. 1995;4:339-342.
50. Harmon P.H. A simplified surgical technique for anterior lumbar discectomy and fusion; avoidance of complications; anatomy of the retroperitoneal veins. Clin Orthop Relat Res. 1964;37:130-144.
51. An H.S., Booth R.E., Rothman R.H. Complications in lumbar disc disease and spinal stenosis surgery. In: Balderston R., An H.S., editors. Spinal surgery. Philadelphia: WB Saunders; 1991:61-78.
52. Brantigan J.W., Steffee A.D. A carbon fiber implant to aid interbody lumbar fusion: two-year clinical results in the first 26 patients. Spine (Phila Pa 1976). 1993;18:2106-2107.
53. Knox B.D., Chapman T.M. Anterior lumbar interbody fusion for discogram concordant pain. J Spinal Disord. 1993;6:242-244.
54. Crock H.V. Observations on the management of failed spinal operations. J Bone Joint Surg [Br]. 1976;58:193-199.
55. Greenough C.G., Taylor L.J., Fraser R.D. Anterior lumbar fusion: a comparison of noncompensation patients with compensation patients. Clin Orthop Relat Res. 1994;300:30-37.
56. Inoue S., Watanabe T., Hirose A., et al. Anterior discectomy and interbody fusion for lumbar disc herniation: a review of 350 cases. Clin Orthop Relat Res. 1984;183:22-31.
57. Southwick W.O., Robinson R.A. Surgical approaches to the vertebral bodies in the cervical and lumbar regions. J Bone Joint Surg [Am]. 1957;39:631-644.
58. Morris J. Low back bracing. Clin Orthop Relat Res. 1974;103:120-132.
59. Saal J.A., Saal S.S. Physical rehabilitation of lower back pain. In: Frymoyer J.W., editor. The adult spine: principles and practice. ed 2. New York: Lippincott-Raven; 1997:1805-1819.
60. Willner S. Effect of a rigid brace on back pain. Acta Orthop Scand. 1985;56:40-42.
61. Fantini G.A., Pappou I.P., Girardi F.P., et al. Major vascular injury during anterior lumbar spinal surgery: incidence, risk factors, and management. Spine (Phila Pa 1976). 2007;32:2751-2758.
62. Schneiderman G., Flannigan B., Kingston S., et al. Magnetic resonance imaging in the diagnosis of disc degeneration: correlation with discography. Spine (Phila Pa 1976). 1987;12:276-281.
63. Fujimaki A., Crock H.V., Bedbrook G.M. The results of 150 anterior lumbar interbody fusion operations performed by two surgeons in Australia. Clin Orthop Relat Res. 1982;165:164-167.
64. Calandruccio R.A., Benton B.F. Anterior lumbar fusion. Clin Orthop Relat Res. 1964;35:63-68.
65. Watkins R. Anterior lumbar interbody fusion: surgical complications. Clin Orthop Relat Res. 1992;284:47-53.
66. Colhoun E., McCall I.W., Williams L., et al. Provocation discography as a guide to planning operations on the spine. J Bone Joint Surg [Br]. 1988;70:267-271.
67. Crock H.V. Lumbar vertebral interbody fusion. In: Lin P., Gill K., editors. Lumbar interbody fusion. Rockville, MD: Aspen; 1989:115-125.
68. Hodgson A.R., Wong S.K. A description of a technic and evaluation of results in anterior spinal fusion for deranged intervertebral disk and spondylolisthesis. Clin Orthop Relat Res. 1968;56:133-162.
69. Baker J.K., Reardon P.R., Reardon M.J., et al. Vascular injury in anterior lumbar surgery. Spine (Phila Pa 1976). 1993;18:2227-2230.
70. Hodge W.A., DeWald R.L. Splenic injury complicating the anterior thoracoabdominal surgical approach for scoliosis: a report of two cases. J Bone Joint Surg [Am]. 1983;65:396-397.
71. Mathews H.H., Evans M.T., Kyles M.K. Laparoscopic discectomy with fusion. In: Proceedings of the 9th annual meeting of the North American Spine Society, October 19–22, 1994, Minneapolis. Burr Ridge, IL: North American Spine Society; 1994:63.
72. McAfee P.C. Laparoscopic fusion and BAK stabilization of the lumbar spine. In: Regan J.J., McAfee P.C., Mack M.J., editors. Atlas of endoscopic spine surgery. St. Louis: Quality Medical Publishing; 1995:306-320.
73. Obenchain T.G. Laparoscopic lumbar discectomy: case report. J Laparoendosc Surg. 1991;1:145-149.
74. Obenchain T.G. Outpatient lumbar discectomy: description of technique and review of first twenty-one cases. Surg Technol Int. 1994;2:415-418.
75. Obenchain T.G. Laparoscopic lumbar retroperitoneal and transperitoneal discectomy. In: Regan J.J., McAfee P.C., Mack M.J., editors. Atlas of endoscopic spine surgery. St. Louis: Quality Medical Publishing; 1995:243-254.
76. Sacks B.L. Laparoscopic lumbrosacral discectomy and interbody fusion technique: early clinical experience. In: Minneapolis. Burr Ridge, IL: North American Spine Society; 1994:67. Proceedings of the 9th annual meeting of the North American Spine Society, October 19–22, 1994
77. Sacks B.L., Scwaitzberg S.D. Lumbosacral (L5-S1) discectomy and interbody fusion technique. In: Regan J.J., McAfee P.C., Mack M.J., editors. Atlas of endoscopic spine surgery. St. Louis: Quality Medical Publishing; 1995:275-292.
78. Zuckerman J.F., Hsu K., Implacito D. Instrumented transperitoneal laparoscopic fusion. In: Margulies J.Y., Floman Y., Farcy J.-P.C., et al, editors. Lumbosacral and spinopelvic fixation. New York: Lippincott-Raven; 1996:579-585.
79. Gumbs A.A., Bloom N.D., Bitan F.D., et al. Open anterior approaches for lumbar spine procedures. Am J Surg. 2007;194:98-102.
80. Gumbs A.A., Hanan S., Yue J.J., et al. Revision open anterior approaches for spine procedures. Spine J. 2007;7:280-285.
81. Kostuik J.P. Anterior fixation for burst fractures of the thoracic and lumbar spine with or without neurological involvement. Spine (Phila Pa 1976). 1988;13:286-293.
82. Raney F.L., Adams J.E. Anterior lumbar disc excision and interbody fusion used as salvage procedure. J Bone Joint Surg [Am]. 1963;45:667-668.
83. McMaster W.C., Silber I. An urological complication of Dwyer instrumentation. J Bone Joint Surg [Am]. 1975;57:710-711.
84. Johnson R.M., McGuire E.J. Urogenital complications of anterior approaches to the lumbar spine. Clin Orthop Relat Res. 1981;154:114-118.
85. Marshall L.F. Cerebrospinal fluid leaks: etiology and repair. In: Rothman R.S., Simeone F.A., editors. The spine. ed 3. Philadelphia: WB Saunders; 1992:1892-1898.
86. Jones A.A., Dougherty P.J., Sharkey N.A., et al. Iliac crest bone graft: osteotome versus saw. Spine (Phila Pa 1976). 1993;18:2048-2052.
87. Burkus J.K., Gornet M.F., Dickman C.A., et al. Anterior lumbar interbody fusion using rhBMP-2 with tapered interbody cages. J Spinal Disord Tech. 2002;15:337-349.
88. Burkus J.K. Bone morphogenetic proteins in anterior lumbar interbody fusion: old techniques and new technologies. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:254-260.
89. Slosar P.J., Josey R., Reynolds J. Accelerating lumbar fusions by combining rhBMP-2 with allograft bone: a prospective analysis of interbody fusion rates and clinical outcomes. Spine J. 2007;7:301-307.
90. Burkus J.K., Gornet M.F., Schuler T.C., et al. Six-year outcomes of anterior lumbar interbody arthrodesis with use of interbody fusion cages and recombinant human bone morphogenetic protein-2. J Bone Joint Surg [Am]. 2009;91:1181-1189.
91. Lekovic G.P., Han P.P., Kenny K.J., et al. Bone dowels in anterior lumbar interbody fusion. J Spinal Disord Tech. 2007;20:374-379.
92. Enker P., Steffee A.D. Interbody fusion and instrumentation. Clin Orthop Relat Res. 1994;300:90-101.
93. Leong J.C., Chow S.P., Yau A.C. Titanium-mesh block replacement of the intervertebral disk. Clin Orthop Relat Res. 1994;300:52-63.
94. Heary R.F., Kheterpal A., Mammis A., et al. Stackable carbon fiber cages for thoracolumbar interbody fusion after corpectomy: long-term outcome analysis. Neurosurgery. 2011;68:810-818. discussion 818-819
95. Mummaneni P.V., Haid R.W., Rodts G.E. Lumbar interbody fusion: state-of-the-art technical advances. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:24-30.
96. Whitecloud T.S.3rd, Butler J.C. Anterior lumbar fusion utilizing transvertebral fibular graft. Spine (Phila Pa 1976). 1988;13:370-374.
97. Oxland T.R., Lund T. Biomechanics of stand-alone cages and cages in combination with posterior fixation: a literature review. Eur Spine J. 2000;9(Suppl 1):S95-S101.
98. Johnson W.M., Nichols T.A., Jethwani D., et al. In vitro biomechanical comparison of an anterior and anterolateral lumbar plate with posterior fixation following single-level anterior lumbar interbody fusion. J Neurosurg Spine. 2007;7:332-335.
99. Schleicher P., Gerlach R., Schar B., et al. Biomechanical comparison of two different concepts for stand alone anterior lumbar interbody fusion. Eur Spine J. 2008;17:1757-1765.
100. Tzermiadianos M.N., Mekhail A., Voronov L.I., et al. Enhancing the stability of anterior lumbar interbody fusion: a biomechanical comparison of anterior plate versus posterior transpedicular instrumentation. Spine (Phila Pa 1976). 2008;33:E38-E43.
101. Aryan H.E., Lu D.C., Acosta F.L.Jr., et al. Stand-alone anterior lumbar discectomy and fusion with plate: initial experience. Surg Neurol. 2007;68:7-13. discussion 13
102. Humphries A.W., Hawk W.A., Berndt A.L. Anterior fusion of lumbar vertebrae: a surgical technique. Surg Clin North Am. 1961;41:1685-1700.
103. Hirabayashi K. Anterior lumbar spinal body fusion with the addition of A-O screwing and wiring. In: Lin P., Gill K., editors. Lumbar interbody fusion. Rockville, MD: Aspen; 1989:127-131.
104. Storen H. Remarks in discussion of spondylolisthesis. Nord Med. 1950;44:1802.
105. Taylor T.K.F. Anterior interbody fusion in the management of disorders of the lumbar spine. J Bone Joint Surg [Br]. 1970;52:784.
106. Sicard A. [Sacrolumbar arthrodesis]. Mem Acad Chir (Paris). 1953;79:763-764.
107. Harmon P.H. Anterior disc excision and fusion of the lumbar vertebral bodies: a review of diagnostic level testing, with operative results in more than seven hundred cases. J Int Coll Surg. 1963;40:572-586.
108. Stein S. Laparoscopic lumbar discectomy. In: Minneapolis. Burr Ridge, IL: North American Spine Society; 1994:61. Proceedings of the 9th annual meeting of the North American Spine Society, October 19–22, 1994
109. Stray K. Remarks in discussion of spondylitis. Nord Med. 1950;44:1802.
110. Kostuik J.P. Anterior spinal cord decompression for lesions of the thoracic and lumbar spine, techniques, new methods of internal fixation results. Spine (Phila Pa 1976). 1983;8:512-531.
111. Nisbet N.W., James A. Results of intervertebral bony fusions. J Bone Joint Surg [Br]. 1956;38:952-953.
112. Rothman S.L., Glenn W.V.Jr. CT evaluation of interbody fusion. Clin Orthop Relat Res. 1985;193:47-56.
113. Brown C.W., Orme T.J., Richardson H.D. The rate of pseudarthrosis (surgical nonunion) in patients who are smokers and patients who are nonsmokers: a comparison study. Spine (Phila Pa 1976). 1986;11:942-943.
114. Novotney S.R., Guyer R.D., Regan J.J. Laparoscopic assisted anterior lumbar interbody fusion. In: Minneapolis. Burr Ridge, IL: North American Spine Society; 1994:61-62. Proceedings of the 9th annual meeting of the North American Spine Society, October 19–22, 1994
115. Harmon P.H. Anterior excision and vertebral body fusion operation for intervertebral disk syndromes of the lower lumbar spine: three-to five-year results in 244 cases. Clin Orthop Relat Res. 1963;26:107-127.
* See references 1, 5, 9, 20, 29, 31, 33, 37–39, 41, 50, 63, 68, 82, 96, 103, 105, and 110–112.
* See references 1, 4, 6, 8, 25, 29, 39, 53, 56, 68.
* See references 1, 5, 8, 9, 29, 33, 56, 64, 68, 103.
* See references 4, 8, 9, 29, 33, 37, 41, 56, 57, 68, 104.
* See references 1, 5, 9, 20, 29, 31, 33, 37–39, 41, 50, 63, 68, 82, 96, 103, 105, 110–112.