Chapter 64 Anterior Cruciate Ligament Strain Behavior During Rehabilitation Exercises
Description of the Devices, Methods, and Approaches Used to Measure Anterior Cruciate Ligament Biomechanics in Vivo
Henning et al1 were the first researchers to measure the elongation behavior of the ACL in vivo. A hooked pin was attached to the partially disrupted ACL in two patients, and the peak displacement of the pin was measured during different rehabilitation activities. Absolute strain values were not reported. The displacement measurements for various activities were compared with that produced by a 350N, anteriorly directed shear load applied to the tibia during the Lachman test. Although this method has several obvious limitations, it was one of the first studies that measured the ACL in vivo.
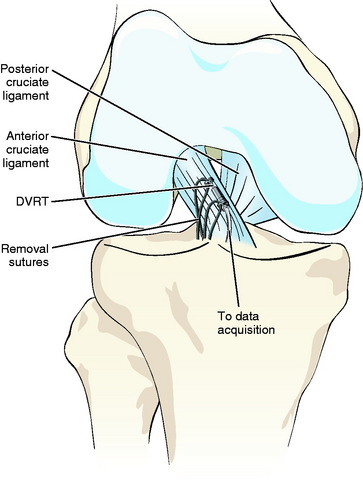
Subsequent to this work, ACL strain measurements have been performed in vivo using both the Hall Effect Strain Transducer (HEST, MicroStrain, Williston, VT) and the Differential Variable Reluctance Transducer (DVRT, MicroStrain, Williston, VT) (Fig. 64-1). Both displacement transducers are small (4–5 mm in length), are highly compliant, have a similar barbed attachment technique, can be sterilized, and can be implanted arthroscopically to the anteromedial aspect of the ACL in vivo.2,3 Although the devices have many similarities, the sensing technology is different, and the DVRT is now more frequently used than the HEST, mainly due to its improved accuracy, better precision, and lower profile.3
The HEST is composed of an inner tube that slides with an outer tube. At the end of each tube are barbs that attach the sensor to the ligament. The inner tube houses a magnet, and the outer tube has a Hall effect magnetic sensor. As the length of the ligament changes, the magnet moves relative to the Hall effect sensor, and this produces the relative change in length between the two barbs. In comparison, the DVRT detects the movement of the two barbs attached to the ligament by measuring the differential change in reluctance produced by the position change of a magnetically permeable core within two small coil windings that are excited with an alternating current (AC) signal.2
The DVRT is currently the displacement transducer of choice.2,3 The monotonic sensing range of a 5-mm DVRT is 1.75 mm, creating a linear sensing range of 35%. The displacement sensitivity is typically 2 V/mm, and the signal:noise ratio is 1000:1. The DVRT has 3.5 μm of nonlinearity, 1 μm of hysteresis, 1 μm nonrepeatability, 0.1 μm/°C temperature error coefficient, and 7 μm root mean square (RMS) error (or 0.1% strain). The DVRT is calibrated with a specially designed micrometer system (AutoCal, MicroStrain, Burlington, VT).2,3
The displacement transducer is implanted into the knee joint through a lateral parapatellar arthroscopic portal (incision) of the joint capsule with the knee at approximately 90 degrees of flexion. The sensing axis of the device is aligned with the anteromedial fibers of the ACL. The two fixation barbs of the device are then pressed into the ligament. Repeated anteroposterior shear loading tests (Lachman) are performed at the beginning and end of a protocol to determine the reference for strain calculation and to serve as a “repeated normal” test to ensure that the transducer measurements are reproducible.2,3
For calculations of ACL strain, it is important to determine a reference length (the length of the transducer when the ACL becomes taut in response to palpation).4 When a posteriorly directed shear load is applied to the tibia with the knee at 30 degrees of flexion, the ACL becomes unstrained and is unloaded in response to palpation. When an anteriorly directed shear load is applied to the tibia, the ACL becomes taut.2–4 This slack–taut transition is identified from the applied anteroposterior loading versus DVRT output plot as the inflection point.4 For the anteromedial portion of the ACL, this slack–taut transition point can estimate the absolute reference within 0.7% strain.4 The wire connections for data acquisition and transducer removal are allowed to course through the lateral portal, and the function of the sensor through the desired range of motion is checked prior to closing the arthroscopic portals and applying sterile dressing such as Tegaderm.3
The DVRT has many advantageous characteristics for measuring ACL strain in vivo. It is relatively small (approximately 5 mm), is lightweight, and can be attached to the ACL arthroscopically. Ligaments have a strain distribution about their length and cross-section, and the DVRT allows accurate, reliable, and repeatable strain measurements of specific regions of a ligament. In addition, the calibration remains stable in environments that range between room temperature and body temperature, making it very practical. Over the years, the DVRT has been shown to be biotolerable and safe, without any adverse long-term reactions.2,3
The limitations of the DVRT must be appreciated. First, although the DVRT is small, the anatomy of the femoral intercondylar notch, combined with the constraints produced by the arthroscopic portals, constrains placement of the sensor to the anteromedial portion of the ACL in humans.2,3 Although the current ACL reconstruction techniques aim to reproduce the function of the anteromedial bundle, recent reports suggest that it may be important to replicate the function of both bundles of the ACL to better restore rotational and anteroposterior limits of motion of the knee.5,6 Second, impingement of the device against the roof of the femoral intercondylar notch does not allow measurement of ACL strain when the knee is in extension or hyperextension. Therefore it is difficult to study activities such as gait and landing from a jump.
An in vitro technique measuring both strain and resultant force in the entire ACL was developed by Markolf et al7 and is useful in interpreting the DVRT data. The technique involves mechanically isolating the bone insertion of the ACL and attaching a load cell to the bone–ligament complex. Throughout the procedure the anatomical origin and insertion are maintained in space.7 Loads and torques can be applied to the knee, and forces, stresses, and strains can be directly measured.8–12 Markolf et al13,14 tested the DVRT and the ACL mechanical isolation technique in the same experiment, creating calibration curves to estimate resultant forces in the ACL from strain measurements made in vivo. In so doing, all the data from the prior DVRT measurements can be related to resultant force measurements for common activities when the forces and moments produced across the knee in vivo are replicated in vitro.2,3,15–17
Recently, noninvasive imaging techniques have been introduced for measuring the in vivo kinematics of the tibia relative to the femur, and these data have been used to estimate ACL biomechanics.5,18,19 Sheehan and Rebmann19 used a cine–phase contrast magnetic resonance imaging (MRI) technique to evaluate the orientation of the attachment sites of the ACL during non–weight-bearing flexion, whereas Li et al5,18 used a combined imaging and three-dimensional (3D) computer-modeling technique to evaluate the orientation of the attachment sites of the ACL during weight-bearing flexion of the knee (one-legged lunge). Although these new, MRI-based, noninvasive techniques have apparent limitations, they have opened a new era for measuring the in vivo kinematics of the knee.
For the cine–phase contrast MRI technique, the cine MRI produced the anatomical images during periodic motion, and phase contrast MRI measured the 3D velocities in the imaging plane.19 The ACL strain was calculated by combining the velocity and anatomical data obtained from the cine–phase contrast MR images. The insertions of the ACL were identified, and the lengths of the anterior and posterior regions of the ACL were calculated for a selection of different knee flexion angles. When compared with DVRT measurements, the cine–phase contrast MRI method revealed a similar strain pattern of the anterior region of the ACL during active extension of the knee. However, for the cine–phase contrast MRI method, the strain values were more than three times greater, approaching the failure strains of the ACL, and thus this approach may overestimate the ACL strain values.19
For the technique that combined imaging and 3D computer modeling, MR images were first taken of human subjects to construct a 3D model for each knee.5,18 After modeling, each subject performed a lunge, and two orthogonal fluoroscopic images were taken at four selected flexion angles to re-create the in vivo knee positions. These orthogonal images and the 3D knee model were then manually matched to reproduce the kinematics of the knee. The tibial and femoral insertion sites were identified to investigate the ACL attachment site’s biomechanics. The position of knee at full extension was used as reference. During the one-legged lunge, Li et al18 demonstrated that the anteromedial bundle of the ACL decreased in length by 7% when the knee moved from extension to flexion. These results are in agreement with those measured with the DVRT.
Review of Studies That Have Characterized Anterior Cruciate Ligament Strain Behavior During Rehabilitation Exercises
In vivo ACL strain measurements of patient volunteers with normal ACLs have been carried out to describe the strain behavior of the ACL during commonly prescribed rehabilitation exercises and have been used to establish clinical criteria for ACL reconstruction. These studies also serve as a basis for development of rehabilitation programs that do not jeopardize the survival of the ACL graft but still allow exercises for optimal recovery of muscle strength and range of motion following ACL reconstruction. Rank comparison of peak ACL strain values produced during common rehabilitation activities are summarized in Table 64-1.
Table 64-1 Rank Comparison of Average Peak Anterior Cruciate Ligament Strain Values Measured During Various Rehabilitation Activities
| Rehabilitation Activity | Resistance | Peak Strain |
|---|---|---|
| Isometric quadriceps contraction at 15 degrees | 30 Nm of extension torque | 4.4% |
| Squatting | Sport Cord | 4.0% |
| Active flexion–extension | 45N weight boot | 3.8% |
| Lachman test | 150N anterior shear load | 3.7% |
| Squatting | 3.6% | |
| Gastrocnemius contraction at 15 degrees of knee flexion | 15 Nm of ankle torque | 3.5% |
| Active extension of the knee | 12 Nm of extension torque | 3.0% |
| One-legged sit to stand | 2.8% | |
| Active extension | Leg weight only | 2.8% |
| Combined isometric quadriceps and hamstring contraction at 15 degrees | 2.8% | |
| Gastrocnemius contraction at 5 degrees of knee flexion | 15 Nm of ankle torque | 2.8% |
| Stair climbing | 2.7% | |
| Isometric quadriceps contraction at 30 degrees | 30 Nm of extension torque | 2.7% |
| Step-down (during extension phase of the exercise cycle) | 2.6% | |
| Step-up | 2.5% | |
| Lunge (during extension phase of the exercise cycle) | 2.0% | |
| Anterior drawer | 150N anterior shear load | 1.8% |
| Stationary bicycling | 1.7% | |
| Active flexion of the knee | 12 Nm of flexion torque | 1.5% |
| Isometric hamstring contraction at 15 degrees | 10 Nm of flexion torque | 0.6% |
| Combined isometric quadriceps and hamstring contraction at 30 degrees | 0.4% | |
| Passive flexion–extension | 0.1% | |
| Gastrocnemius contraction at 30 and 45 degrees of knee flexion | 15 Nm of ankle torque | 0% |
| Isometric quadriceps contraction at 60 degrees | 30 Nm of extension torque | 0% |
| Isometric quadriceps contraction at 90 degrees | 30 Nm of extension torque | 0% |
| Combined isometric quadriceps and hamstring contraction at 60 degrees | 0% | |
| Combined isometric quadriceps and hamstring contraction at 90 degrees | 0% | |
| Isometric hamstring contraction at 30, 60, and 90 degrees | 0% |
Most of the strain transducer measurements have been performed under local (intraarticular) anesthesia, allowing the patients to have full control of their muscles. Typically the study participants have been candidates for arthroscopic partial meniscectomy or diagnostic arthroscopy without known ligament trauma. Preoperatively the patients have had normal gait, range of motion, and normal ligament function as documented by clinical examination and arthroscopic visualization.2,3
The ACL strain measurements have revealed that during passive flexion–extension motion of the lower leg with the thigh held in a horizontal position, the ACL is unstrained between 110 and 11.5 degrees of flexion and becomes strained as the knee is moved into terminal extension.20 Although the impingement of the strain transducer against the roof of the femoral intercondylar notch has not allowed measurements of the ACL strain during hyperextension of the knee in all patients, these findings indicate that the ACL strain continues to increase with increasing extension of the knee and is greatest when the knee is in hyperextension. To support this, in vitro studies have demonstrated that ACL strain and force increase as the knee is passively moved from a flexed position to an extended (0 degrees) position and then to a hyperextended position.9–11,21,22
The following general conclusions can be made regarding the effect of externally applied loads on ACL strain values: The ACL is a primary restraint to anterior displacement of the tibia relative to the femur when the knee is near extension, and it also restrains internal (but not external) axial rotation of the tibia.2,3,17 Although cadaver studies have revealed that the ACL serves as an important secondary restraint to applied varus-valgus moments, in vivo measurements have revealed that ACL strain values are not increased when varus and valgus moments are applied to the knee at 20 degrees of flexion.3,17 In addition, in vivo measurements have shown different ACL strain values during non–weight-bearing versus weight-bearing conditions. For example, transitioning from non–weight-bearing to weight-bearing conditions increases ACL strain values when varus-valgus moments and external torque are applied to the knee.17 In addition, when anteriorly directed shear loads are applied to the tibia, the strain values are higher during weight-bearing conditions in comparison with non–weight-bearing conditions.17
When compared with the fully relaxed condition, extension torque produced by isometric quadriceps muscle contraction has been shown to strain the ACL near extension of the knee, but not beyond 60 degrees of flexion.15 Isometric hamstring contraction, on the other hand, has not been shown to produce ACL strain at any knee flexion angles.15 When compared with the relaxed condition, combined contraction of quadriceps and hamstring muscles has been shown to produce a significant increase in strain at 15 degrees of knee flexion, but not at 30, 60, or 90 degrees of knee flexion.15 Isometric gastrocnemius muscle contraction has been shown to strain the ACL when the knee is near extension (at 5 and 15 degrees of flexion), and when gastrocnemius muscle contraction was combined with quadriceps or hamstring muscle contraction, the strain was increased in comparison with isolated contractions of these muscles.23
Active extension–flexion motion of the knee (an open kinetic chain exercise) between the limits of 10 and 90 degrees produces peak ACL strains near extension, and these values gradually decrease with increasing knee flexion.15 Beyond 35 degrees of knee flexion, the ACL becomes unstrained.15 Application of weight during this exercise (applied to increase extension torque about the knee) produces significant increases of ACL strain values at 10 and 20 degrees of flexion and shifts the strained–unstrained transition to 45 degrees of knee flexion. A subsequent follow-up study confirmed that the peak ACL strain values increased when knee extension torque increased.16 It was also shown that application of compressive loading, such as that produced by body weight, did not reduce peak ACL strains during extension exercises.16 Application of flexion torque during flexor exercise produced significant decreases of ACL strain values; however, when compressive loading was added, such a decrease was not observed.16
Stair climbing is a closed kinetic chain exercise, and because step-up exercise has been shown to reduce anterior translation of the tibia with respect to the femur, it is commonly considered safe for rehabilitation following ACL reconstruction.24 In vivo measurements during stationary stair-climbing exercises have demonstrated that ACL strain is increased when the knee moves from a flexed to an extended position, and the average strain values were moderate when compared with other commonly prescribed rehabilitation activities tested with the same technique.25 However, the strain values were highly variable, with peak values ranging as much as 7%. These strain magnitudes may produce detrimental effects to the healing graft, and therefore caution should be exercised when making any recommendations for stationary stair climbing following ACL reconstruction.
Most clinicians have considered bicycling to be a relatively safe rehabilitation exercise with many therapeutic qualities, and therefore it is commonly recommended for rehabilitation following ACL injury or reconstruction. In vivo ACL strain measurements during stationary bicycling also support this observation.26 In an in vivo study, stationary biking was performed at six different riding conditions (three power levels and two cadences).26 Power levels 75 Watts (W), 125W, and 175W simulated downhill, level, and uphill riding conditions, respectively. The results revealed that with this selection of power and cadence levels, stationary bicycling produces relatively low peak strain values (mean 1.7%) when compared with other rehabilitation activities commonly prescribed after ACL injury or reconstruction, and thus stationary bicycling can be considered safe for rehabilitation after ACL reconstruction without excessively straining the graft. However, the safety and efficacy of bicycling, or of any rehabilitation exercise for that matter, following ACL reconstruction can only be determined via clinical studies.
Closed kinetic chain squatting exercises are commonly prescribed to improve muscle strength after ACL reconstruction. Because of the compressive joint load and co-contraction of muscles spanning the knee, advocates of closed kinetic chain exercise consider it safer than active flexion–extension exercises. It has been demonstrated that squatting and active flexion–extension exercises produce similar strain patterns (strain is greatest near full extension and gradually decreases toward flexion) and maximum strain values, indicating that compressive joint force does not necessarily protect a healing ACL graft.27 It has to be emphasized, however, that in contrast to active extension of the knee, the increasing resistance during squatting to the limit of 134N did not significantly increase ACL strain values.27
Recently Heijne et al28 measured the strain behavior of the ACL during four different closed kinetic chain exercises: (1) step-up, (2) step-down, (3) lunge, and (4) one-legged sit to stand. They found that the strain produced during these four exercises was not significantly different at all knee positions (knee flexion angles of 30, 50, and 70). The largest strain values were measured when the knee was near extension (at 30 degrees of knee flexion), and the strain values decreased significantly as the knee was flexed.
The importance of rehabilitation following ACL reconstruction is greatly appreciated; however, there is little consensus regarding how different restrictions and exercises should be administered and how they influence the long-term outcome and healing response of the graft and knee. The previously mentioned studies characterizing the behavior of the ACL during different activities have been used to design accelerated and nonaccelerated rehabilitation programs that gradually increase the strain experienced in the graft. The accelerated program (19 weeks) produces high graft strain early after the reconstruction by allowing immediate full range of motion, weight bearing as tolerated, quadriceps activity with the knee near extension, and return to unrestricted activity within 6 months of reconstruction, whereas in the nonaccelerated program (32 weeks) these same activities are prescribed over a delayed time interval and the graft is therefore not strained as vigorously. The effects of these programs have been subsequently studied via a prospective, randomized, double-blinded clinical trial.29 At 2-year follow-up, both rehabilitation programs produced the same increase of anterior knee laxity and the same effect with regard to clinical assessment, patient satisfaction, functional performance, and the biomarkers of articular cartilage metabolism.29
Review of Studies That Have Measured the Strain of the Bone–Patellar Tendon–Bone Graft
Proper graft placement directly affects knee biomechanics and has been considered one of the most critical surgical variables in determining a successful long-term clinical outcome. In an attempt to place the graft optimally during ACL reconstruction, “isometers” were introduced to help with placement of ACL graft tunnels. In vivo strain measurements of the BPTB graft at the time of reconstruction have, however, shown that local graft elongation did not correlate with the isometric measurements of displacement made prior to preparing the graft tunnels.30 Consequently, isometers are not currently in routine use in ACL reconstructions.
Immediately following fixation of a BPTB graft, cyclical passive extension of the knee between the limits of full extension and 90 degrees of flexion produces a complex seating response of the graft. Unlike the normal ACL, the graft demonstrated a seating behavior by decreasing in length in some patients but increasing in others. Early slippage of the graft bone blocks past the interference screw explains the decrease in length, whereas a creep response of the improperly positioned or overtensioned graft explains the increase in length. Both of these phenomena were found to be associated with increased anteroposterior displacement of the knee during healing.31
The relationship between BPTB graft elongation behavior at the time of surgery and the changes in anteroposterior knee laxity at long-term follow-up have also been evaluated.32 Subjects from the previously mentioned study31 were divided into two groups based on elongation biomechanics of the graft measured after graft fixation. Although both groups had similar anteroposterior knee laxity values at the time of ACL reconstruction, those patients with graft elongation values that were significantly greater than the normal ACL at the time of surgery (outside the 95% confidence interval of the normal ACL) demonstrated a significant increase in anteroposterior knee laxity at the 5-year follow-up, whereas those with elongation values similar to the normal ACL (within the 95% confidence interval of the normal ACL) did not.32 These results suggest that (1) the elongation behavior of the BPTB graft during flexion–extension cycles at the time of surgery may provide important information for long-term success of the knee and (2) anteroposterior laxity measurements at the time of surgery may not adequately predict changes of anteroposterior laxity of the knee during healing and long-term follow-up.
Review of Studies Investigating how Functional Knee Bracing Affects Anterior Cruciate Ligament Strain Behavior
Functional knee braces are designed to protect an injured ACL or ACL graft and to prevent further intraarticular damage by reducing anterior translation of the tibia with respect to the femur. Although these braces are commonly prescribed, their effectiveness is controversial and not well documented on human subjects. Several investigations of functional knee braces have been performed using arthrometers or roentgen stereophotogrammetric analysis to measure the displacement of the tibia relative to the femur.33–35 Common limitations of these studies are that the combined effects of compressive load and muscle loading during weight-bearing activities were not included. In vivo strain measurements of the normal ACL have also been performed during various loading conditions to evaluate the efficacy of functional knee braces.36–38 The results reveal that when the subject is non–weight bearing and weight bearing, bracing the knee reduces ACL strains produced in response to anteriorly directed shear loads.36–38 Additionally, in response to applied internal–external torques with the knee non–weight bearing, the ACL of the braced knee was significantly less strained when 2 to 8 N/m of internal torque was applied compared with the unbraced knee.38 At 9 N/m of internal torque, the difference was not statistically significant; however, there was a strong trend that bracing the non–weight-bearing knee reduces ACL strain values.38 In contrast, when the subject was weight bearing, the brace was no longer able to reduce the strain produced during application of internal torque about the tibia.38
Summary
The effect of externally applied loads on ACL strain values is as follows:
The strain behavior of the ACL during rehabilitation exercises is as follows:
1 Henning CE, Lynch MA, Glick KR. An in vivo strain gauge study of elongation of the anterior cruciate ligament. Am J Sports Med. 1985;13:22-26.
2 Beynnon BD, Fleming BC. Anterior cruciate ligament strain in-vivo: a review of previous work. J Biomech. 1998;31:519-525.
3 Fleming BC, Beynnon BD. In vivo measurement of ligament/tendon strains and forces: a review. Ann Biomed Eng. 2004;32:318-328.
4 Fleming BC, Beynnon BD, Tohyama H, et al. Determination of a zero strain reference for the anteromedial band of the anterior cruciate ligament. J Orthop Res. 1994;12:789-795.
5 Li G, DeFrate LE, Rubash HE, et al. In vivo kinematics of the ACL during weight-bearing knee flexion. J Orthop Res. 2005;23:340-344.
6 Woo SL-Y, Kanamori A, Zeminski J, et al. The effectiveness of reconstruction of the anterior cruciate ligament with hamstring and patellar tendon. A cadaveric study comparing anterior tibial and rotational loads. J Bone Joint Surg. 2002;84A:907-914.
7 Markolf KL, Gorek JF, Kabo JM, et al. Direct measurement of resultant forces in the anterior cruciate ligament. An in vitro study performed with a new experimental technique. J Bone Joint Surg. 1990;72A:557-567.
8 Markolf KL, Burchfield DM, Shapiro MM, et al. Combined knee loading states that generate high anterior cruciate ligament forces. J Orthop Res. 1995;13:930-935.
9 Markolf KL, Burchfield DM, Shapiro MM, et al. Biomechanical consequences of replacement of the anterior cruciate ligament with patellar ligament allograft. Part I: insertion of the graft and anterior-posterior testing. J Bone Joint Surg. 1996;78A:1720-1727.
10 Markolf KL, Burchfield DM, Shapiro MM, et al. Biomechanical consequences of replacement of the anterior cruciate ligament with patellar ligament allograft. Part II: forces in the graft compared with forces in the intact ligament. J Bone Joint Surg. 1996;78A:1728-1734.
11 Markolf KL, Wascher DC, Finerman GA. Direct in vitro measurement of forces in the cruciate ligaments. Part I: the effect of multiplane loading in the intact knee. J Bone Joint Surg. 1993;75A:377-386.
12 Markolf KL, Wascher DC, Finerman GA. Direct in vitro measurement of forces in the cruciate ligaments. Part II: the effect of section of the posterolateral structures. J Bone Joint Surg. 1993;75A:387-394.
13 Markolf KL, Willems MJ, Jackson SR, et al. In situ calibration of miniature sensors implanted into the anterior cruciate ligament part I: strain measurements. J Orthop Res. 1998;16:455-463.
14 Markolf KL, Willems MJ, Jackson SR, et al. In situ calibration of miniature sensors implanted into the anterior cruciate ligament part II: force probe measurements. J Orthop Res. 1998;16:464-471.
15 Beynnon BD, Fleming BC, Johnson RJ, et al. Anterior cruciate ligament strain behavior during rehabilitation exercises in vivo. Am J Sports Med. 1995;23:24-34.
16 Fleming BC, Ohlén G, Renström PA, et al. The effects of compressive load and knee joint torque on peak anterior cruciate ligament strains. Am J Sports Med. 2003;31:701-707.
17 Fleming BC, Renstrom PA, Beynnon BD, et al. The effect of weightbearing and external loading on anterior cruciate ligament strain. J Biomech. 2001;34:163-170.
18 Li G, DeFrate LE, Sun H, et al. In vivo elongation of the anterior cruciate ligament during knee flexion. Am J Sports Med. 2004;32:1415-1420.
19 Sheehan FT, Rebmann A. Non-invasive, in vivo measures of anterior cruciate ligament strains. Trans Orthop Res Soc. 2003;28:264.
20 Beynnon BD, Howe JG, Pope MH, et al. The measurement of anterior cruciate ligament strain in vivo. Int Orthop. 1992;16:1-12.
21 Arms SW, Pope MH, Johnson RJ, et al. The biomechanics of anterior cruciate ligament rehabilitation and reconstruction. Am J Sports Med. 1984;12:8-18.
22 Markolf KL, O’Neill G, Jackson SR. Effect of applied quadriceps and hamstring muscle loads on forces in the anterior and posterior cruciate ligaments. Am J Sports Med. 2004;32:1144-1149.
23 Fleming BC, Renström PA, Ohlén G, et al. The gastrocnemius muscle is an antagonist of the anterior cruciate ligament. J Orthop Res. 2001;19:1178-1184.
24 Jonsson H, Karrholm J. Three-dimensional knee joint movements during a step-up: evaluation after anterior cruciate ligament rupture. J Orthop Res. 1994;12:769-779.
25 Fleming BC, Beynnon BD, Renström PA, et al. The strain behavior of the anterior cruciate ligament during stair climbing: an in vivo study. Am J Sports Med. 1999;15:185-191.
26 Fleming BC, Beynnon BD, Renström PA, et al. The strain behavior of the anterior cruciate ligament during bicycling. An in vivo study. Am J Sports Med. 1998;26:109-118.
27 Beynnon BD, Johnson RJ, Fleming BC, et al. The strain behavior of the anterior cruciate ligament during squatting and active flexion-extension. Am J Sports Med. 1997;25:823-829.
28 Heijne A, Fleming BC, Renström PA, et al. Strain on the anterior cruciate ligament during closed kinetic chain exercises. Med Sci Sports Exerc. 2004;36:935-941.
29 Beynnon BD, Uh BS, Johnson RJ, et al. Rehabilitation after anterior cruciate ligament reconstruction. A prospective, randomized, double-blind comparison of programs administered over 2 different time intervals. Am J Sports Med. 2005;33:347-359.
30 Fleming BC, Beynnon BD, Nichols CE, et al. An in vivo comparison between intraoperative isometric measurement and local elongation of the graft after reconstruction of the anterior cruciate ligament. J Bone Joint Surg. 1994;76:511-519.
31 Beynnon BD, Johnson RJ, Fleming BC, et al. The measurement of elongation of anterior cruciate-ligament grafts in vivo. J Bone Joint Surg. 1994;76A:520-532.
32 Beynnon BD, Uh BS, Johnson RJ, et al. The elongation behavior of the anterior cruciate ligament graft in vivo. A long-term follow-up study. Am J Sports Med. 2001;29:161-166.
33 Jonsson H, Karrholm J. Brace effects on the unstable knee in 21 cases. A roentgen stereophotogrammetric comparison of three designs. Acta Orthop Scand. 1990;61:313-318.
34 Wojtys EM, Kothari SU, Huston LJ. Anterior cruciate ligament functional brace use in sports. Am J Sports Med. 1996;24:539-546.
35 Wojtys EM, Loubert PV, Samson SY, et al. Use of a knee-brace for control of tibial translation and rotation. A comparison, in cadavera, of available models. J Bone Joint Surg. 1990;72A:1323-1329.
36 Beynnon BD, Johnson RJ, Fleming BC, et al. The effect of functional knee bracing on the anterior cruciate ligament in the weightbearing and nonweightbearing knee. Am J Sports Med. 1997;25:353-359.
37 Beynnon BD, Pope MH, Wertheimer CM, et al. The effect of functional knee-braces on strain on the anterior cruciate ligament in vivo. J Bone Joint Surg. 1992;74A:1298-1312.
38 Fleming BC, Renstrom PA, Beynnon BD, et al. The influence of functional knee bracing on the anterior cruciate ligament strain biomechanics in weightbearing and nonweightbearing knees. Am J Sports Med. 2000;28:815-824.