Chapter 59 Anterior Cruciate Ligament Reconstruction in Skeletally Immature Patients
Intrasubstance tears of the anterior cruciate ligament (ACL), a common injury in adults, are relatively rare in children and adolescents.1 Presumably, this difference in prevalence of ACL tears is due to anatomical and biomechanical differences that predispose skeletally immature knees to physeal and bone injury rather than ACL tears. Despite being an uncommon occurrence, ACL tears in children and adolescents have recently been reported with increasing frequency.2–20 The increased recognition of this injury may be attributed to an increase in sports participation combined with improved examination and diagnostic methods. When a skeletally immature patient presents with a torn ACL, the physician is confronted with a difficult decision because nonoperative treatment may result in instability with subsequent meniscal tears and early degenerative changes,2,6,9–11 and surgery may cause iatrogenic leg length discrepancy or angular deformities.5–7,12–15
Management of ACL tears in skeletally immature patients remains controversial because of a deficiency in the basic science literature on physeal growth and response to injury. Clinical studies published on the treatment of this condition have contributed to the confusion by having poor methodology with low levels of evidence and combining patients with different levels of maturation and methods of treatment.*
Natural History
The natural history of ACL tears in children and adolescents has not been clearly documented, but it can be extrapolated from the results of studies published on nonoperative treatment. The unique challenge of treating intrasubstance ACL tears in skeletally immature patients combined with the absence of an efficacious surgical procedure resulted in a historical approach of nonoperative treatment, consisting of bracing, quadriceps and hamstring strengthening, counseling, and activity modification. A growing body of evidence from studies of nonoperative treatment proves that the natural history of ACL tears in children and adolescents is generally poor for behavioral or other reasons. ACL deficient patients in this age group are noncompliant with activity modification, and consequently they often experience recurrent instability, meniscal damage, and sports-related disability. Kannus and Jarvinen10 treated 25 patients with grade II partial ACL tears and seven patients with grade III complete ACL tears. Eight years after the initial injury, the results were excellent or good for the patients with Grade II ACL tears. The long-term results of grade III ACL injuries were poor because these patients developed chronic instability and posttraumatic arthritis. Kannus and Jarvinen reported that the results of nonoperative treatment for complete ACL tears in this age group were not acceptable. Angel and Hall3 evaluated 27 children and adolescents who had a torn ACL. At the time of follow-up, the majority had pain and limitations of activity. Eleven of 12 patients in their series of children younger than age 14 were disabled with knee function. Graf et al9 found that seven of eight children treated conservatively sustained new meniscal tears within 15 months. McCarroll et al11 found that 37 of 38 adolescent patients had episodes of instability, and 27 of 38 had symptomatic meniscal tears. Eleven of 18 patients in the series of Mizuta et al20 developed degenerative changes within 51 months, and the researchers stated the results were “poor and unacceptable.” Millet et al21 also found that the incidence of meniscal injuries increased significantly in chronic cases.
Assessing Skeletal Maturity
For large populations, chronological age is an excellent predictor of skeletal maturity; however, patients may show a significant variance from the average. Consequently, it is important to determine the skeletal age with radiographs. The most common method of estimating skeletal age is by comparing an anteroposterior radiograph of the patient’s left hand and wrist with the age-specific radiographs in the Greulich and Pyle atlas.22
Physiological age can be determined with Tanner staging of sexual maturation.23 Patients are preliminarily staged prior to surgery by questioning them about the onset of menarche or growth of axillary hair. After induction of anesthesia and prior to surgery, Tanner staging is determined by examining the patient’s secondary sexual development, including the growth of pubic and axillary hair, breasts, and genitalia. Prepubescent patients are categorized in Tanner stage I and II of development, pubescent patients are in stage III, and postpubescent patients are in Tanner stage V (Table 59-1).
Table 59-1 Tanner Staging Classification of Secondary Sexual Characteristics
| Tanner Stage | Male Sexual Characteristics | Female Sexual Characteristics |
|---|---|---|
| Stage I (prepubescent) | Testes <4 mL or <2.5 cm No pubic hair |
No breast development No pubic hair |
| Stage II | Testes 4 mL or 2.5–3.2 cm Minimal pubic hair at base of penis |
Breast buds Minimal pubic hair on labia |
| Stage III (pubescent) | Testes 12 mL or 3.6 cm Pubic hair over pubis Voice changes Muscle mass increases |
Elevation of breast; areolae enlarge Pubic hair of mons pubis Axillary hair Acne |
| Stage IV | Testes 4.1–4.5 cm Pubic hair as adult Axillary hair Acne |
Areolae enlarge Pubic hair as adult |
| Stage V (postpubescent) | No growth Testes as adult Pubic hair as adult Facial hair as adult Mature physique |
No growth Adult breast contour Pubic hair as adult |
| Other | Peak height velocity: 13.5 years | Peak height velocity: 11.5 years Menarche: 12.7 years |
Normal Growth and Development
The physes of the distal femur and proximal tibia are the most rapidly growing in the body. Anderson et al24 estimated that the distal femoral physis contributes 40% and the proximal tibia physis contributes 27% of the overall lower extremity length. More recently, Pritchett25 reported that the distal femur grows at 1.3 cm per year until the last 2 years of maturity, when the growth rate drops to 0.65 cm per year. The rates of the proximal tibial growth are 0.9 cm per year and 0.5 cm per year in the last 2 years. The peak height velocity for males is 13 to 15 years of age (average 13.5 years), and it rarely occurs before Tanner stage IV. Twenty percent of males do not hit peak height velocity until Tanner stage V. For females, the peak height velocity occurs in Tanner stage III between 11 and 13 years of age (average 11.5 years). Peak height velocity in females precedes menarche by approximately 1 year.
The greatest concern, however, is not leg length discrepancy but angular deformity. An over-the-top femoral groove may result in a valgus/flexion deformity of the distal femur by damaging the perichondral ring of LaCroix. Damage to the anterior tibial physis may result in recurvatum. In the worst-case scenario, Wester et al26 estimated that a 14-year-old boy with 2 cm of remaining distal femoral growth could develop a 14-degree valgus deformity with a lateral femoral epiphysiodesis or 11-degree recurvatum with partial tibial physeal arrest.
Basic Science Research on Physeal Injury
Although a deficiency exists in the age-specific basic science on physeal injuries, research has demonstrated the effects of drill hole damage to the physis and the results of placing a soft tissue graft through a transphyseal hole in animal models. Makela et al27 in 1988 drilled 2- and 3.2-mm transphyseal femoral holes in a rabbit model. The 2-mm holes destroyed 3% of the cross-sectional area of the physis, and the 3.2-mm holes destroyed 7% of the cross-sectional area. The destruction of 7% of the cross-sectional area of the growth plate caused permanent growth disturbance.
Other researchers have evaluated the effect of placing a soft tissue graft across the physis. Guzzanti et al28 performed an ACL reconstruction with the semitendinosus tendon using 2-mm transphyseal femoral and tibial holes in immature rabbits. The femoral holes damaged 11% of the transverse diameter and 3% of the cross-sectional area of the physis, and the tibial holes damaged 12% of the transverse diameter and 4% of the cross-sectional area of the physis. Two of the 21 tibiae developed a valgus deformity, and one was shorter. The researchers recommended careful evaluation of the percentage of damage to the area of the physis before performing intraarticular methods of reconstruction of the ACL in adolescents.
Houle et al29 performed a transphyseal ACL reconstruction in a rabbit model using four tunnel diameters ranging from 1.95 to 3.97 mm. They found that the larger drill holes caused more marked deformity and the soft tissue graft did not offer protection to physeal arrest. They recommended that tunnels involve 1% or less of the area of the physis when reconstructing the ACL in children.
Janarv et al30 drilled 1.7-, 2.5-, and 3.4-mm holes in rabbit femurs. The hole in one femur was left empty, and the hole in the contralateral femur was filled with a soft tissue autograft. They found that growth retardation occurred when the drill injury destroyed 7% to 9% of the distal femoral physis, but no retardation was seen in injuries of 4% to 5% of the cross-sectional area of the physis. The soft tissue grafts prevented solid bone bridging, but a bone cylinder formed around the grafts. They also measured the tibial and femoral physis of a 12-year-old girl and estimated that an 8-mm drill hole would destroy 3% to 4% of the physis. In contrast to the results of Houle et al29 and Guzzanti et al,28 Stadelmaier et al31 found that a soft tissue graft placed in transphyseal drill holes of a canine model prevented formation of a bony bridge and subsequent growth disturbance.
The effect of tensioning a graft across open physes has also been evaluated. Edwards et al32 found that tensioning a fascia lata autograft at 80N in a canine model caused valgus femoral and varus tibial deformities without radiographic or histological evidence of physeal bar formation, indicating the physes were responding to the Hueter-Volkmann principle, which states that application of compressive force perpendicular to the physes will inhibit longitudinal growth. This study illustrates the potential risks for ACL reconstruction in this age group, even with physeal-sparing procedures.
Risk Factors for Iatrogenic Growth Disturbance
The potential consequences of growth disturbance after ACL reconstruction in the skeletally immature knee have a major influence on decisions about surgical technique. Although the results of basic science studies on physeal injury in animals may not be entirely applicable to humans, several important risk factors for growth disturbance after physeal injury have been identified. The studies of Guzzanti et al28 and Houle et al29 demonstrated that the proximal tibial physis seems to be more vulnerable than the femoral physis to growth arrest.
In general, the risk of growth disturbance is related to the extent of damage relative to the cross-sectional area of the physis. However, uncertainty still exists, even in animal models, regarding the size and orientation of the drill holes that can be made without causing growth disturbance. The drill hole size threshold for growth disturbance in animal models has been between 1% and 7% of the cross-sectional area of the physis.27,28–30 The holes should be drilled perpendicularly, rather than obliquely, to limit the area of damage to the physis. Although results have been mixed, placing a soft tissue graft across the physis appears to offer protection from bone bridging and growth arrest. Research also demonstrates that the physes are sensitive to compressive forces,32 and consequently ACL grafts, including those used in physeal sparing procedures, should not be overtensioned.
Significant leg length discrepancy or angular deformity, although rare, has been reported after ACL reconstruction in skeletally immature patients.5,6,14 Kocher et al5 surveyed Herodicus and the ACL study group. One hundred and forty respondents reported 15 cases of growth disturbance. We have also seen two cases of valgus knee deformity after ACL reconstruction in adolescents. One of these patients had a staple placed across the lateral femoral physis.6 The other patient was a 12-year-old boy who was recently seen 6 months after a transphyseal ACL reconstruction with an Achilles tendon allograft. The graft had failed, and the patient had a 3-degree valgus alignment of the normal knee and a 7-degree valgus alignment of the ACL deficient knee, without physeal arrest.
Treatment Options
Case reports and animal studies showing iatrogenic growth disturbance after intraarticular transphyseal replacement have prevented clinicians from routinely applying proven methods of ACL reconstruction for adults to skeletally immature patients. Nonoperative management of ACL tears in children and adolescents is an especially seductive approach. The advantages of delaying surgery include additional psychological maturation of the patient, which facilitates compliance with postoperative rehabilitation, and greater skeletal maturity, which allows for less risky and more familiar traditional procedures. For these reasons, some surgeons still advocate a nonoperative approach despite the poor results.7,12,14
Other surgeons have performed primary repair33,34 or extraarticular replacement in this age group.9,11 Unfortunately, these procedures have been found to be no more successful in children than they are in adults.
Modified physeal-sparing intraarticular replacements have also been advocated to minimize the risk of physeal injury.19 Parker et al35 reconstructed the ACL by passing the hamstring tendons through a groove in the anterior aspect of the tibia and over the top of the lateral femoral condyle. Kocher et al13,18 reported the results of 44 Tanner stage I or II patients who were treated with a combined intraarticular and extraarticular reconstruction of the ACL. The iliotibial band was placed extraarticularly around the outside of the lateral femoral condyle and through the intercondylar notch and then sutured to the periosteum of the proximal tibia. Two patients in their series required reconstruction for graft failure. The mean IKDC subjective score for the remaining 42 patients was 96.7, and the mean growth from surgery to follow-up was 21 cm. The Lachman examination revealed that 23 patients were normal, 18 were nearly normal, and one was abnormal. The results of the pivot-shift test were normal in 31 patients. The researchers concluded that this procedure provided an excellent functional outcome with minimal risk of growth disturbance. Some surgeons have used transphyseal tibial holes and the over-the-top femoral position with autografts16,17 and allografts.12 Recently, Guzzanti et al36 recommended ACL reconstruction with single-stranded semitendinosus and gracilis tendon graft in Tanner stage I patients with a transepiphyseal tibial hole and a femoral over-the-top femoral position. Although these over-the-top procedures have not caused growth disturbances, they do not provide isometry of the graft. Odensten and Gillquist37 demonstrated that the femoral over-the-top position resulted in an average of 10 mm of graft elongation as the knee approached extension. If the over-the-top femoral position is used, the clinician should avoid rasping, which may damage the perichondral ring.
ACL replacement procedures with intraarticular transphyseal placement of the graft remain controversial because of the potential for physeal injuries. Clinical studies documenting the safety of transphyseal replacement have primarily involved postmenarchal adolescent females or postpubescent adolescent males with physes that were near closure.11,12,38–40 Pressman et al15 performed an intraarticular replacement in 18 patients, only seven of whom had open physis and 11 of whom had closed or closing physis. Andrews et al12 and McCarroll et al7 also performed intraarticular replacements, but postoperatively their patients grew only 4.5 cm and 2.3 cm of height, respectively. The average age of the patients at the time of ACL reconstruction was greater than 14 years in other case series.38,40,41 The potential for leg length discrepancy or angular deformity is relatively low in these cohorts. Surgical treatment of patients who are in Tanner stage I or II of maturation presents greater consequences if growth disturbance occurs, potentially resulting in significant limb length discrepancy or angular deformity. Only a few patients who were that immature were included in the previous clinical reports on transphyseal replacement. Consequently, the safety of transphyseal procedures for preadolescent children has not been substantiated in the clinical literature, and basic science studies have also failed to clearly demonstrate the safety of transphyseal drilling or placement of a soft tissue graft across the physis.
Guzzanti et al42 recommended transphyseal reconstruction in Tanner stage II and III patients with semitendinosus gracilis grafts. They emphasized that the holes should not be larger than 6 mm.
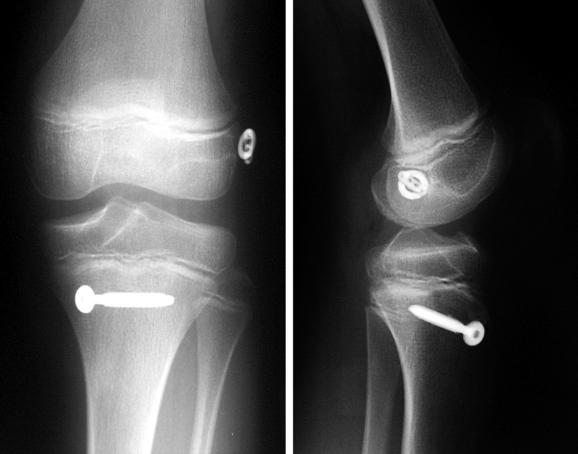
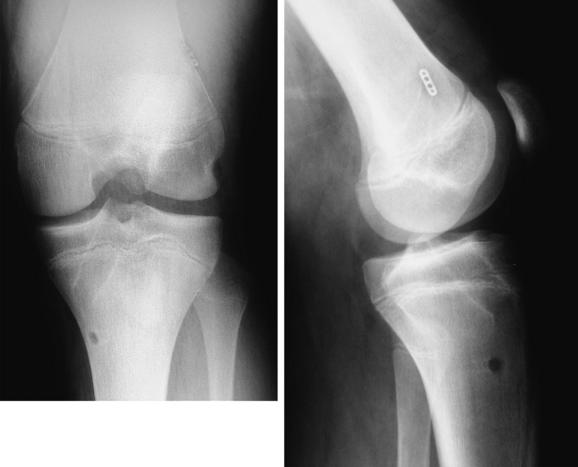
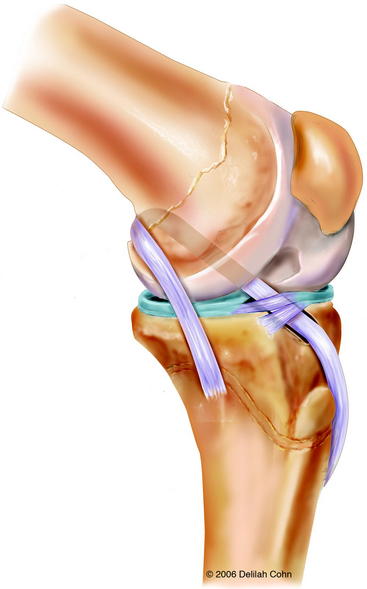
Anderson43 reported the preliminary results of a transepiphyseal replacement that followed the generally accepted principles of ACL reconstruction in adults but theoretically minimized the risk of physeal injury by not transgressing either the tibial or femoral physes (Figs. 59-1 and 59-2). Twelve patients, including three who were in Tanner stage I, four in Tanner stage II, and five in Tanner stage III, were evaluated at a mean of 4.1 years after surgery. The mean growth from the time of surgery to follow-up was 16.5 cm. The difference in lengths of the lower limbs, as measured on long leg radiographs, was not clinically relevant. The mean score on the IKDC Subjective Knee Form was 96.5. Ligament laxity testing with a KT-1000 arthrometer revealed a mean side-to-side difference of 1.5 mm. The rating, according to the criteria of the Objective 2001 IKDC Knee Form,44 was normal for seven patients and nearly normal for five patients.
Treatment and Recommendations
Transepiphyseal ACL reconstruction is recommended for high-risk patients because this procedure adheres to the generally accepted principles of ACL replacement in adults but theoretically minimizes the risk of physeal injury by not transgressing either the tibial or the femoral physis. For surgeons who are worried about the technical difficulty of a transepiphyseal ACL replacement, the physeal-sparing reconstruction described by Kocher et al13 may be an alternative. Although the iliotibial band is a relatively weak graft and is not placed isometrically on either the tibia or femur, the functional results appear to be good.
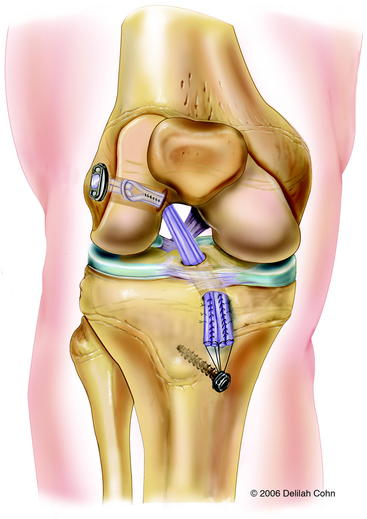
More mature, low-risk, pubescent Tanner stage IV patients are treated with a transphyseal replacement using quadruple hamstring grafts fixed with an Endobutton proximally and screw and post distally (Fig. 59-3). Postpubescent patients in Tanner stage V of development, including males older than 16 years and females older than 14 years, may be treated safely with a standard adult ACL replacement.
Transepiphyseal Anterior Cruciate Ligament Reconstruction
Surgical Technique
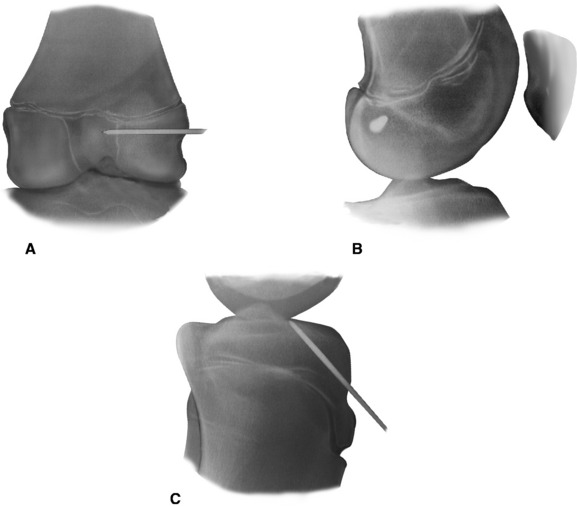
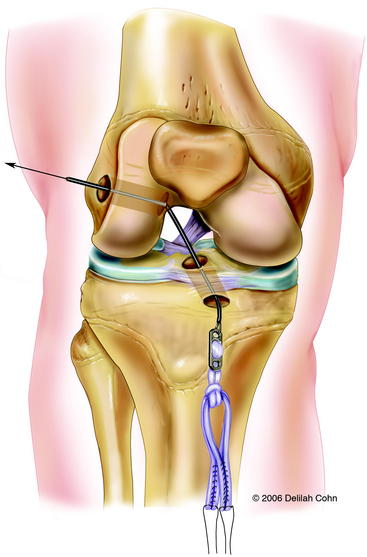
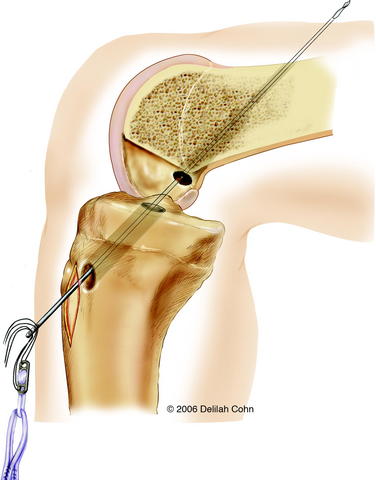
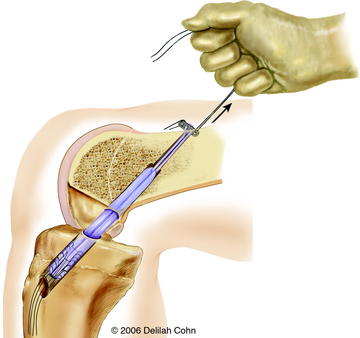
With the C-arm in the lateral position, the limb is adjusted to show a perfect lateral view (Fig. 59-4, B). The point of the guidewire is placed on the skin over the lateral femoral condyle, corresponding with the location of the footprint of the ACL on the femur. This point is approximately one-fourth of the distance from posterior to anterior along the Blumensaat line and one fourth of the distance down from the Blumensaat line (see Fig. 59-4, B). A 2-cm lateral incision is made at this point, the iliotibial tract is incised longitudinally, and the periosteum is stripped from a small area of the lateral femoral condyle. The C-arm is used to visualize the entry point of the guidewire in both the anteroposterior and the lateral planes. With the C-arm in the lateral planes and using a freehand technique, the point of the guidewire is introduced 2 to 3 mm into the femoral epiphysis. The pin is not angulated anteriorly or posteriorly but is kept perpendicular to the femur in the coronal plane. The C-arm is then rotated to the anteroposterior plane to make sure that the guidewire is not angulated superiorly or inferiorly. The guidewire is then driven across the femoral epiphysis, perpendicular to the femur and distal to the physis (see Fig. 59-4, A and B). Entrance of the guidewire into the intercondylar notch is subsequently visualized arthroscopically. The guidewire should enter at the center of the anatomical footprint of the ACL on the femur. This femoral guidewire is left in place, and a second guidewire is then inserted into the anteromedial aspect of the tibia through the epiphysis with the aid of a tibial drill guide. From the direct lateral position, the C-arm is rotated externally approximately 30 degrees to clearly demonstrate the physis extending into the tibial tubercle. The guidewire is then drilled into the tibial epiphysis under real-time fluoroscopic imaging (see Fig. 59-4, C). The handle of the drill guide must be lifted for the pin to clear the anterior part of the tibial physis. The pin should enter the joint at the level of the free edge of the lateral meniscus and in the posterior footprint of the ACL on the tibia. The appropriate position of both guidewires should be confirmed arthroscopically at this point. Tendon sizers are used to measure the diameter of the quadruple tendon graft (which typically ranges from 6 to 8 mm). A tight fit is important; consequently, the smallest appropriate drill is used to ream over both guidewires. The edge of the femoral hole is chamfered intraarticularly, and the width of the lateral femoral condyle is measured. The appropriate Endobutton-CL (2–3 cm) is chosen so that approximately 2 cm of the quadruple hamstring tendon graft will remain within the lateral femoral condyle. The Endobutton-CL is then passed around the middle of the double tendons and is looped inside of itself to secure the tendons proximally (Fig. 59-5). Alternatively, the tendons can be placed through the continuous loop before the tendon ends are sutured together. However, this requires drilling and measuring the length of the femoral hole before graft preparation. Otherwise, it is difficult to determine the appropriate length of the Endobutton-CL necessary to leave 2 cm of the tendon graft within the lateral femoral condyle.
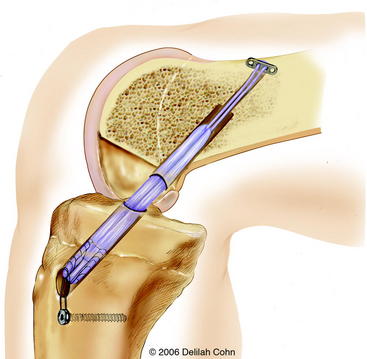
A #5 Fiberwire suture is placed in one end of the Endobutton, and a suture passer is used to pass it from anterior to posterior through the tibia and out the lateral femoral condyle (see Fig. 59-5). The Endobutton and tendons are then pulled up through the tibia and out of the femoral hole with use of the #5 suture. An Endobutton washer (Smith & Nephew, Memphis, TN), 3 to 4 mm larger than the femoral hole, is placed over the Endobutton. Tension is then applied to the tendons distally, pulling the Endobutton and washer to the surface of the lateral femoral condyle (Fig. 59-6). The washer is necessary to anchor the graft proximally because the hole in the lateral femoral condyle is larger than the Endobutton. The graft is placed under tension, and the knee is then extended to determine arthroscopically whether there is impingement of the graft on the intercondylar notch. Although an anterior notchplasty is usually unnecessary when this technique is used, if the anterior outlet of the intercondylar notch touches or indents the graft in terminal extension, a small portion of the anterior outlet may be removed. With the knee in 10 degrees of flexion, the quadruple hamstring graft is secured distally by tying the #5 Fiberwire sutures over a tibial screw and post that is placed medial to the tibial tubercle apophysis and distal to the proximal tibial physis. If the tendon graft extends through the tibial drill hole, it is also secured to the periosteum of the anterior tibia with multiple #1 Ethibond sutures with use of figure-eight stitches (see Fig. 59-6). The subcutaneous tissue and skin are closed in a routine fashion, and a hinged brace is applied.
Physeal-Sparing ACL Reconstruction with the Iliotibial Band
The following is the technique described by Kocher et al.13,18 Although it is not an isometric ACL replacement, the functional results may be good. If this technique is chosen, the defect in the iliotibial band over the vastus lateralis muscle should be closed. Failure to do so may result in a cosmetic problem caused by herniation of the vastus lateralis muscle.
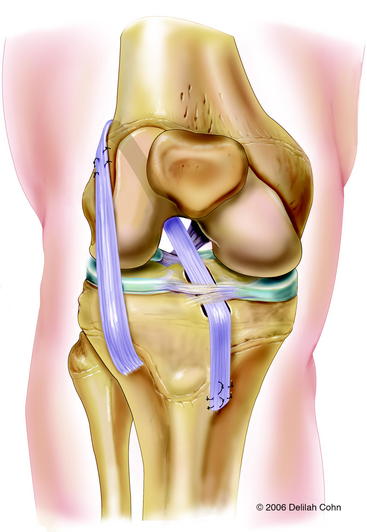
This procedure, a modification of the McIntosh and Darby13 intraarticular and extraarticular ACL reconstruction, is performed with the patient supine and a tourniquet on the proximal thigh. A 6- to 10-cm incision is made from the lateral joint line along the superior border of the iliotibial band. The iliotibial band is exposed, and incisions are made along its superior and inferior margins from Gerdy’s tubercle for a distance of 15 and 20 cm proximal to the joint line, depending on the patient’s size. The iliotibial band is detached proximally, dissected free from the lateral capsule, and tubularized with a whipstitch using a #5 Ethibond suture. Arthroscopy is then performed through anteromedial and anterolateral portals. Remnants of the torn ACL and fat pad are resected, and a small notchplasty is performed. Soft tissue is removed from the over-the-top position of the lateral femoral condyle, but care is taken to avoid injury to the perichondral ring, which is close to the over-the-top position. Another incision is made parallel to the medial border of the patellar tendon, extending from the joint line for a distance of 4 cm distally. Dissection is carried down to the periosteum. The physis is identified with the use of a Keith needle. A curved clamp is placed under the intermeniscus ligament, and a groove is made in the proximal tibial epiphysis with the use of a small curved rasp. Care is taken to avoid damage to the anterior tibial physis. The iliotibial band graft is then pulled into the knee with a full-length clamp or tendon passer that is passed through the anteromedial portal, over the top of the lateral femoral condyle, and out the lateral capsule. The clamp is then passed under the intermeniscal ligament, and the graft is regrasped and pulled into the medial incision. The graft is seated into the groove in the tibial epiphyses, placed under tension, and sutured to the lateral femoral condyle at the insertion of the lateral intermuscular septum with the knee in 90 degrees of flexion and 15 degrees of external rotation (Fig. 59-7). The periosteum is incised distal to the physis, and a trough is made in the metaphysis. The graft is placed under tension and sutured to the periosteum at this location with the knee in 20 degrees of flexion. The defect created by harvesting the iliotibial band is closed over the vastus lateralis muscle. The lateral patella reticulum is left open to avoid excessive pressure on the lateral facet of the patella. The wounds are closed in a routine fashion.
Transphyseal Anterior Cruciate Ligament Reconstruction
Surgical Technique
The knee is flexed to at least 90 degrees prior to insertion of the femoral guidewire. An over-the-top femoral guide is used that leaves 2 mm of bone between the drill hole and the posterior cortex of the lateral femoral condyle. The 2.7-mm passing pin is advanced through the offset guide and through the lateral femoral condyle until it penetrates the lateral femoral cortex. The pin may be palpated under the skin distal to the tourniquet. An acorn reamer that matches the diameter of the graft is used to create the femoral tunnel. The femoral hole is drilled to a depth of 30 to 35 mm at the 10:30 position in the left knee and the 1:30 position in the right knee. The depth of the femoral hole should be 10 mm greater than the desired graft insertion into the lateral femoral condyle so as to allow for rotation of the Endobutton. The Endobutton 4.5-mm reamer is then drilled over the guidewire and out the lateral femoral cortex. The femoral hole is chamfered to minimize graft fraying. The Endobutton depth gauge is used to measure the length of the femoral tunnel from the anterolateral femoral cortex to the opening in the intercondylar notch. The Endobutton-CL that leaves 20 to 25 mm of graft within the femoral tunnel is chosen. A #5 Ethibond suture is passed through one of the outside holes of the Endobutton. This suture is used to pass the Endobutton through the tibia and femur. A #2 Ethibond suture is passed through the other outside hole of the Endobutton, and it is used to rotate the Endobutton after it exits the anterolateral femoral cortex (Fig. 59-8). The hamstring grafts are then passed through the Endobutton-CL, creating a quadruple graft. Both strands of the #5 and #2 sutures in the Endobutton are passed through the eyelet of the passing 2.7-mm pin. The passing pin is inserted up through the tibial and femoral holes, piercing the quadriceps and skin proximal to the knee (see Fig. 59-8). The pin is then pulled out of the femur proximally to pass the sutures. The #5 suture is pulled first, advancing the Endobutton and graft into the femoral hole (Fig. 59-9). The #2 suture is pulled next, rotating the Endobutton external to the femur. The graft is then pulled distally, locking the Endobutton on the outside of the femoral cortex. Secure fixation should be felt. Then the #2 and #5 sutures in the Endobutton are removed. The knee is cycled through a range of motion several times to pretension the graft. The graft is placed under tension, and the knee is then extended to determine arthro- scopically whether there is impingement of the graft on the intercondylar notch. If the anterior outlet of the intercondylar notch touches or indents the graft in terminal extension, a small portion of the anterior outlet may be removed. With the knee in 20 degrees of flexion, the quadruple hamstring graft is secured distally by tying the #5 Fiberwire sutures over a tibial Bioscrew and post that is placed medial to the tibial tubercle apophysis and distal to the proximal tibial physis (Fig. 59-10). If the tendon graft extends through the tibial drill hole, it is also secured to the periosteum of the anterior tibia with multiple #1 Ethibond sutures with use of figure-eight stitches. The subcutaneous tissue and skin are closed in a routine fashion, and a hinged brace is applied.
1 Rang M. Children’s fractures, ed 2, Philadelphia: Lippincott; 1983:290-296.
2 Aichroth PM, Patel DV, Zorilla P. The natural history and treatment of rupture of the anterior cruciate ligament in children and adolescents. A prospective review. J Bone Joint Surg. 2002;84B:618-619.
3 Angel KR, Hall DJ. Anterior cruciate ligament injury in children and adolescents. Arthroscopy. 1989;5:197-200.
4 Brief LP. Anterior cruciate ligament reconstruction without drill holes. Arthroscopy. 1991;7:350-357.
5 Kocher MS, Saxon HS, Hovis WD, et al. Management and complications of anterior cruciate ligament injuries in skeletally immature patients: survey of the Herodicus Society and the ACL Study Group. J Pediatr Orthop. 2002;22:452-457.
6 Lipscomb AB, Anderson AF. Tears of the anterior cruciate ligament in adolescents. J Bone Joint Surg. 1986;68A:19-28.
7 McCarroll JR, Shelbourne KD, Porter DA, et al. Patellar tendon graft reconstruction for midsubstance anterior cruciate ligament rupture in junior high school athletes. An algorithm for management. Am J Sports Med. 1994;22:478-484.
8 Stanitski CL, Harvell JC, Fu F. Observations on acute knee hemarthrosis in children and adolescents. J Pediatr Orthop. 1993;13:506-510.
9 Graf BK, Lange RH, Rujisaki CK, et al. Anterior cruciate ligament tears in skeletally immature patients; meniscal pathology at presentation and after attempted conservative treatment. Arthoscopy. 1992;8:229-233.
10 Kannus P, Jarvinen M. Knee ligament injuries in adolescents. Eight year follow-up of conservative management. J Bone Joint Surg. 1988;70B:772-776.
11 McCarroll JR, Rettig AC, Shelbourne KD. Anterior cruciate ligament injuries in the young athlete with open physes. Am J Sports Med. 1988;16:44-47.
12 Andrews M, Noyes FR, Barber-Westin SD. Anterior cruciate ligament allograft reconstruction in the skeletally immature athlete. Am J Sports Med. 1994;22:48-54.
13 Kocher MS, Sumeet G, Micheli L. Physeal sparing reconstruction of the anterior cruciate ligament in skeletally immature prepubescent children and adolescents. J Bone Joint Surg. 2006;88:283-293.
14 Koman JD, Sanders JO. Valgus deformity after reconstruction of the anterior cruciate ligament in a skeletally immature patient. A case report. J Bone Joint Surg. 1999;81:711-715.
15 Pressman AE, Letts RM, Jarvis JG. Anterior cruciate ligament tears in children: an analysis of operative versus nonoperative treatment. J Pediatr Orthop. 1997;17:505-511.
16 Bisson LJ, Wickiewicz T, Levinson M, et al. ACL reconstruction in children with open physes. Orthopedics. 1998;21:659-663.
17 Lo IK, Kirkley A, Fowler PH, et al. The outcome of operatively treated anterior cruciate ligament disruptions in the skeletally immature child. Arthroscopy. 1997;13:627-634.
18 Micheli LJ, Rask B, Gerberg L. Anterior cruciate ligament reconstruction in patients who are prepubescent. Clin Orthop. 1999;364:40-47.
19 Nakhostine M, Bollen SR, Cross MJ. Reconstruction of mid-substance anterior cruciate rupture in adolescents with open physes. J Pediatr Orthop. 1995;15:286-287.
20 Mizuta H, Kubota K, Shiraishi M, et al. The conservative treatment of complete tears of the anterior cruciate ligament in skeletally immature patients. J Bone Joint Surg. 1995;77B:890-894.
21 Millett PJ, Willis AA, Warren RF. Associated injuries in pediatric and adolescent anterior cruciate ligament tears: does a delay in treatment increase the risks of meniscal tear? Arthroscopy. 2002;18:955-999.
22 Greulich WW, Pyle S. Radiographic atlas of skeletal development of the hand and wrist, ed 2. Stanford, CA: Stanford University Press, 1959.
23 Tanner JM, Whitehouse RH. Clinical longitudinal standards for height, weight, height velocity, weight velocity and stages of puberty. Arch Dis Child. 1976;51:170-179.
24 Anderson M, Green WT, Messnet MB. Growth and predictions of growth in the lower extremities. Am J Orthop. 1963;45:1-14.
25 Pritchett JW. Longitudinal growth and growth-plate activity in the lower extremity. Clin Orthop. 1992;275:274-279.
26 Wester W, Canale ST, Dutkowsky JP, et al. Prediction of angular deformity and leg length discrepancy after anterior cruciate ligament reconstruction in skeletally immature patients. J Pediatr Orthop. 1994;14:516-521.
27 Makela EA, Vainionpaa S, Vihtonen K, et al. The effect of trauma to the lower femoral epiphyseal plate. An experimental study in rabbits. J Bone Joint Surg. 1988;70B:187-191.
28 Guzzanti V, Falciglia F, Gigante A, et al. The effect of intraarticular ACL reconstruction on the growth plates of rabbits. J Bone Joint Surg. 1994;75B:960-963.
29 Houle JB, Letts M, Yang J. Effects of a tensioned tendon graft in a bone tunnel across the rabbit physis. Clin Orthop. 2001;Oct:275-281.
30 Janarv PM, Wikstrom B, Hirsch G. The influence of transphyseal drilling and tendon grafting on bone growth: an experimental study in the rabbit. J Pediatr Orthop. 1998;18:149-154.
31 Stadelmaier D, Arnoczky S, Dodds J, et al. The effects of drilling and soft tissue grafting across open growth plates. Am J Sports Med. 1995;23:431-435.
32 Edwards TB, Greene CC, Baratta RV, et al. The effect of placing a tensioned graft across open growth plates. A gross and histologic analysis. J Bone Joint Surg. 2001;83A:725-734.
33 Clanton TO, DeLee JC, Sanders B, et al. Knee ligament injuries in children. J Bone Joint Surg. 1979;61A:1195-1201.
34 Engebretsen L, Svenningsen S, Benum P. Poor results of anterior cruciate ligament repair in adolescence. Acta Orthop Scand. 1988;59:684-686.
35 Parker AW, Drez DJr, Cooper JL. Anterior cruciate ligament injuries in patients with open physes. Am J Sports Med. 1994;22:44-47.
36 Guzzanti V, Falciglia F, Stanitski CL. Physeal-sparing intraarticular anterior cruciate ligament reconstruction in pre-adolescents. Am J Sports Med. 2003;31:949-953.
37 Odensten M, Gillquist J. Functional anatomy of the anterior cruciate ligament and a rationale for reconstruction. J Bone Joint Surg. 1985;67A:257-262.
38 Aronowitz ER, Ganley TJ, Goode JR, et al. Anterior cruciate ligament reconstruction in adolescents with open physes. Am J Sports Med. 2000;28:168-175.
39 Edwards PH, Grana WA. Anterior cruciate ligament reconstruction in the immature athlete: long-term results of intraarticular reconstruction. Am J Knee Surg. 2001;14:232-237.
40 Matava MJ, Siegel MG. Arthroscopic reconstruction of the ACL with semi-tendinosus-gracilis autograft in skeletally immature adolescent patients. Am J Knee Surg. 1997;10:60-69.
41 Shelbourne D, Gray T, Wiley B. Results of transphyseal anterior cruciate ligament reconstruction using patella tendon autograft in Tanner Stage III or IV adolescents with clearly open growth plates. Am J Sports Med. 2004;32:1218-1222.
42 Guzzanti V, Falciglia F, Stanitski CL. Preoperative evaluation and anterior cruciate ligament reconstruction technique for skeletally immature patients in Tanner Stages II and III. Am J Sports Med. 2003;31:941-948.
43 Anderson AF. Transepiphyseal replacement of the anterior cruciate ligament in skeletally immature patients. A preliminary report. J Bone Joint Surg. 2003;85A:1255-1263.
44 Irrgang JJ, Anderson AF, Boland AL, et al. Development and validation of the international knee documentation committee subjective knee form. Am J Sports Med. 2001;29:600-613.