Anterior Cervical Discectomy and Fusion
Derrick G. Sueki, Erica V. Pablo, Rick B. Delamarter and Paul D. Kim
Cervical spondylosis or degeneration presents as different clinical syndromes with the most common being degenerative disc disease, radiculopathy, and myelopathy. Cervical degenerative disc disease may present as axial neck pain, neck stiffness, or as headaches. Cervical radiculopathy classically shows symptoms of arm pain with sensory or motor deficits in the upper extremities (UEs), which is caused by disc herniation or osteophyte formation. Cervical spondylotic myelopathy (Figs. 14-1 and 14-2) may occur with gait abnormalities, hand clumsiness, or upper motor neuron signs. Studies on the natural history of degenerative disc disease demonstrate that the majority of patients suffering from axial neck pain or radiculopathy improve with conservative treatment. Cervical myelopathy, however, tends to progress with time and close clinical follow-up is warranted.
Pathophysiology And Clinical Evaluation
Cervical spondylosis is a progressive degenerative cascade that occurs with aging. Annular tears and biochemical changes in the cervical disc can lead to decreased water content, shrinking or herniation of nuclear pulposus tissue, and disc collapse. This places increased stress on associated facet and uncovertebral joints, causing them to degenerate, eventually leading to axial neck pain and stiffness. In addition, this can lead to the formation of bony spurs and disc herniations that may encroach on the neuroforamina, resulting in radiculopathy.1
The clinical presentation of cervical spondylosis can vary and must be distinguished from referred shoulder or visceral pain. A careful history and physical examination must be done to determine the exact cause of the neck pain. Nonmechanical neck pain is less likely to be related to disc disease, and other sources including tumor and infection must be considered. Radicular symptom neck pain will often be exacerbated by neck extension and rotation to the affected side (Spurling sign). In contrast, muscular neck pain is often exacerbated by neck flexion and rotation away from the more painful side. In cases of lower cervical degenerative disease, the pain often radiates to the shoulder, upper arm, or infrascapular areas, and upper cervical disease may present as temporal pain and retroorbital headaches.2
Cervical radiculopathy typically presents as pain and paresthesia in a single or multiple nerve root distribution. Spurling sign is a reproduction of radicular pain caused by extending the neck and rotating the head to the symptomatic side, which leads to narrowing of the neuroforamina. Axial compression and the Valsalva maneuver may also reproduce symptoms. The “shoulder abduction sign” is the reduction of radicular symptoms caused by placing the hand of the affected arm on top of the head, which decreases tension on the nerve roots.3
Cervical myelopathy typically has gait abnormalities, hyperreflexia, and loss of fine motor skills, which result from mechanical compression of the spinal cord in the cervical region. Motor weakness and muscle wasting may be present, as well as sensory abnormalities. Patients may also complain of neck pain and/or radicular symptoms, so careful evaluation must be done to determine the exact cause of symptoms. Typical examination findings include upper motor neuron signs and hyperreflexia manifested as a positive Hoffman reflex, clonus of deep tendon reflexes, and an upgoing Babinski reflex.
History and physical examination remain the most important processes in the diagnostic workup. Imaging and electromyography or nerve conduction studies can be used to supplement the diagnostic workup. Plain radiographs, including anteroposterior, lateral, oblique, and lateral flexion and extension views, can demonstrate developmental stenosis, disc space narrowing, abnormal alignment, dynamic instability, and osteophyte formation. Radiographic findings may occur with normal age-related degenerative changes, so radiographic findings must be correlated with clinical findings.4 Magnetic resonance imaging (MRI) is commonly used and is the most sensitive modality for demonstrating spinal cord morphology in relation to the surrounding bony and soft-tissue structures (Fig. 14-3). Computed tomography myelography is highly sensitive for detecting foraminal stenosis, but it is invasive and does have a risk of complications.5 Electromyography and nerve conduction studies can help distinguish between nerve root compression and a peripheral neuropathy and are useful in patients with unclear diagnose. In cases of mechanical neck pain without radiculopathy, several studies support the use of provocative discography to confirm discogenic origin of the pain and to clarify which disc levels are appropriate to treat.6,7
Treatment And Surgical Indications
The majority of patients with axial neck pain experience acceptable resolution of symptoms without surgical intervention. Cervical radiculopathy responds well to conservative treatment, but many patients progress to experience recurrent or persistent symptoms.8 Initially, activity modification and a brief soft collar immobilization are often recommended, but prolonged inactivity may lead to deconditioning. Early pharmacologic treatment is initiated with nonsteroidal antiinflammatory drugs or acetaminophen. With severe acute pain, narcotic analgesics may be used. Paraspinal muscle spasm may be relieved with muscle relaxants but is often improved with a soft collar immobilization alone. Some patients also may respond to oral corticosteroids.9 All medications should be prescribed only with careful regard for the potential adverse reactions and interactions with other medications that the patient is taking. Physical therapy is an essential component of conservative treatment and includes modalities, such as traction and heat or cold therapy, as well as an isometric neck and shoulder-stabilizing exercise program. The specifics of a physical therapy program are often left up to the discretion of the particular therapist.
Surgical treatment depends on the clinical entity treated and success of nonoperative treatment. Conservative treatment is the mainstay of initial treatment for cervical radiculopathy and degenerative disc disease with acceptable results.10 Surgical intervention for patients with cervical radiculopathy is indicated when the symptoms are persistent or recurrent or they are severe or debilitating enough to merit surgery.11 A prolonged conservative course is recommended for treatment of axial neck pain. If surgery is being considered for axial neck pain and diagnostic evaluation has failed, a discogram is obtained to identify the exact correct level(s) responsible for discogenic pain. As with any elective surgical procedure, appropriate patient expectations and selection must be considered before any surgical intervention (Box 14-1). In general, workers’ compensation patients and those involved in litigation can be expected to have worse outcomes even after successful fusion surgery.12,13 Cervical myelopathy must be considered separately, however, because clinical progression usually occurs even with conservative treatment. Classically, patients with cervical myelopathy have periods of clinical stability interspersed with “stepwise degeneration” and careful follow-up must be used to monitor disease progression.14
Surgical Procedure
Single-level cervical disc disease is most commonly treated with anterior cervical discectomy and fusion (ACDF). For one or more adjacent levels, some surgeons choose to perform a corpectomy of the intervening vertebral bodies instead of multilevel ACDF. After the discectomy, graft choices include an iliac crest bone graft, structural allograft, or a synthetic/metallic spacer. Currently, most surgeons use anterior cervical plating to prevent graft displacement anteriorly and to provide stability while cervical fusion occurs. In cases of severe stenosis or instability, intraoperative neuromonitoring is often used in an attempt to prevent injury and assess adequacy decompression.
Surgery begins with the induction of general endotracheal anesthesia. The patient is then placed in the supine position on a radiolucent operative table to allow imaging in both the anterior-posterior and lateral planes. A soft bump is placed beneath the scapula, and gentle traction is then applied to the cervical spine. In addition, gentle skin traction pulling toward the foot of the bed is applied with wide tape on the shoulders. Traction helps to radiographically visualize the lower cervical levels during surgery. The anterior neck is then prepped and draped, with care taken not to restrict the surgical field. Palpating the bony landmarks (or alternatively by using a radiopaque skin marker and a lateral radiograph) determines the level of the skin incision. A transverse incision is then made through the skin and subcutaneous fat, and bleeding is controlled using electrocautery. The platysma muscle is carefully cut in line with the incision to avoid cutting the large superficial veins just beneath it. Beneath the platysma muscle, the deep cervical fascia is identified and divided laterally to the anterior border of the sternocleidomastoid muscle, where it is dissected inferiorly and superiorly off of the muscle belly. A finger is then used for blunt dissection between the carotid sheath laterally and the trachea and esophagus medially down to the prevertebral fascia. Retractors are then used to retract the midline structures, allowing direct visualization of prevertebral fascia and underlying longus colli muscles and disc spaces.
Once the appropriate level is confirmed, the longus colli muscles are dissected off of the bone laterally and a self-retaining retractor is placed, exposing the disc space to the uncovertebral joints. The operating microscope, sterilely draped, is then brought into the field (Fig. 14-4). Under direct visualization using the microscope, the disc is incised with a scalpel and the anterior portion is removed using a pituitary forceps and an angled curette. A high-speed drill may be used to complete the discectomy and expose the posterior longitudinal ligament (PLL). After exposure, the PLL is elevated off of the posterior aspect of the vertebral bodies using a small 4-0 forward-angled curette; it is then excised using 1 mm and 2 mm Kerrison rongeurs. The PLL does not need to be routinely removed if no nuclear protrusion or extrusion is found, but this has to be carefully explored. The foramina can be probed with the 90° angled nerve hook to confirm adequate decompression or any remaining loose disc fragments. When the discectomy and foraminotomies are complete, the disc space is measured and an appropriately sized graft is chosen. While increased traction is applied on the halter traction device, the graft is gently impacted into position. When it is adequately positioned, all traction is removed. An appropriate-sized plate is then chosen and applied on the anterior aspect of the cervical spine. Care is taken when drilling screw holes to choose a length that will be contained in the vertebral body and be parallel with the endplate of the disc space. When the plate is in position, a lateral radiograph is obtained and graft and hardware positioning is checked (Figs. 14-5 and 14-6).
After instrumentation is complete, the wound is copiously irrigated and thoroughly checked for hemostasis. Often a drain is used even if the wound appears very dry, because a postoperative hematoma may cause significant morbidity. The platysma muscle and subcutaneous tissue are then closed with interrupted absorbable sutures. A running subcuticular layer of suture may follow this closure, followed by a sterile dressing. The patient is then placed into a rigid cervical orthosis before extubation.
Postoperatively, the head of the patient’s bed is maintained in an elevated position to decrease swelling in the neck. The patient should be able to walk, void, swallow liquids, and tolerate a diet before discharge. Most patients are discharged the day after surgery. Patients commonly complain of a sore throat and pain with swallowing a few days after surgery. If these complaints seem more severe than usual, then a single dose or short course of oral corticosteroids may be given in an attempt to minimize swelling.
Outcomes
Postoperatively, patients with radicular symptoms will often note immediate relief of pain after surgery. Most patients report a change in the quality of their axial neck pain to one more typical of postoperative pain. Generally, patients treated for radicular symptoms achieve excellent clinical results (up to 90% satisfactory results), whereas those treated for axial neck pain achieve good results.15,16 One concern in the postoperative period is overactivity before fusion is achieved. Solid consolidation of fusion often requires 6 to 12 weeks, so excessive motion and loading are discouraged during this period. Often patients are maintained in a rigid cervical collar for 6 to 12 weeks to restrict their activities, but patients frequently recover from surgery much sooner and desire to remove the orthosis and resume normal activities. This relative immobilization can result in significant patient deconditioning, which can be a challenge to the therapist. In the early period of return to activity and therapy, it is important to avoid injury caused by an overly strenuous exercise program or an overzealous patient.
Future Directions
ACDF is a generally well-tolerated and successful procedure; however, recent data has shown that cervical fusion may lead to less satisfying results than previously thought.17 Concerns about adjacent segment disease has led to the development of cervical total disc replacement.18 Recently, results of a multicenter, randomized, prospective Federal Drug Administration clinical trial of cervical disc replacement (Synthes Prodisc-C) versus ACDF has shown clinical equivalence or superiority of cervical disc replacement over fusion.19 Other clinical studies have demonstrated similar consistent evidence with other cervical disc prosthese.20,21 These treatments have the potential of offering shorter recovery times and more rapid return to activity and may help prevent the progression of adjacent-level degeneration. Our view is that cervical disc replacement is superior to fusion.
Therapy Guidelines For Rehabilitation
Rehabilitation after a surgery is a science and an art. The science of rehabilitation relies on a solid understanding of the body’s normal response to injury and trauma. The art of rehabilitation rests in the clinician’s ability to interpret the individual patient’s unique signs and symptoms. The ability to formulate a plan of care that maximizes an individual’s healing potential relies on the ability to blend the science and the art of rehabilitation. The initial portion of this chapter is designed to provide the clinician with an understanding of the role that tissue healing plays in the development of a rehabilitation program. This will serve as a scientific foundation upon which a clinician can base his or her clinical reasoning process. This tissue-healing model will then be placed in the context of ACDF. The activities and precautions of each phase of the rehabilitation process will be rooted in current understanding of the phases of tissue healing. Specific treatment options are provided throughout the chapter, but these should only serve as a guide to treatment and should not replace sound clinical reasoning or judgment when rehabilitating after ACDF.
The decision to operate on the cervical spine may be driven by localized tissue damage and subsequent focal pain, but the majority of spinal surgeries are initiated because of damage to (or threat of damage to) the neural network of the body. Myelographic computed tomography and MRI studies have all demonstrated that 20% to 30% of people who have disc herniation and stenosis do not have radicular symptoms, and many of these people do not have neck pain.22 It has also been shown that under anesthesia, only nerves that are inflamed will produce radicular symptoms when compressed or placed under traction. Therefore, although the intervertebral disc or stenosis can be the source of neck pain, it is generally injury to the nerve that drives the decision to undergo surgery. Protecting the nervous system from further damage and providing an environment in which the nerve can heal are primary goals of the surgery and rehabilitation thereafter.
Within the spine, injury or damage to the nerve often occurs at the spinal nerve root or the dorsal root ganglia. Anatomically, differences in the nerve root make it more susceptible to injury than at other regions of the peripheral nerve. The nerve root is not as well protected, less able to withstand deformation, and less able to repair itself than the remainder of the peripheral nerve. The other structure within the intervertebral foramen that is susceptible to damage is the dorsal root ganglia. The position of the dorsal root ganglia is not constant and can be found inside the foramen, outside the foramen, or in the spinal canal, which can increase the likelihood that it will be injured. In addition, unlike the spinal nerve root and peripheral nerve, the dorsal root ganglia do not have a blood-nerve barrier, which is necessary to prevent foreign substances from invading the nerve. These anatomic differences predispose the dorsal root ganglia to edema and mechanical compression.22–24
Nerves must also be able to move and glide within the tissue. For this to occur, some slack in the system must exist. The spinal cord changes length by 7 cm from flexion to extension. Studies in the arm show that a 7-mm excursion occurs in the nerves with movement. In addition to compression, increased tension of the nerve can result in nerve damage.
![]() More specifically, tension in nerves causing a 20% to 30% increase in length will cause the nerve to break. Boyd and associates25 demonstrated that as little as 6% strain decreases the amplitude of action potentials by 70%, and 10% to 12% strain causes complete conduction block. They have also shown that nerve stretch of as little as 8% greater than the resting length will cause a 50% decrease in blood flow to the nerve and stretch of 15% will cause 80% to 100% reduction in blood flow. Therefore, exercises that place undue stress and tension on the nerves should be avoided.26
More specifically, tension in nerves causing a 20% to 30% increase in length will cause the nerve to break. Boyd and associates25 demonstrated that as little as 6% strain decreases the amplitude of action potentials by 70%, and 10% to 12% strain causes complete conduction block. They have also shown that nerve stretch of as little as 8% greater than the resting length will cause a 50% decrease in blood flow to the nerve and stretch of 15% will cause 80% to 100% reduction in blood flow. Therefore, exercises that place undue stress and tension on the nerves should be avoided.26
Neurons are incapable of dividing and migrating; therefore regeneration occurs only through existing neurons. If the connective tissue sheathing remains intact, then a potential for nerve regrowth exists. If the sheath is disrupted, then the potential for regrowth diminishes. Initially, like any tissue, an inflammatory process is seen within the nerve. Within hours after injury, the nerves start to grow back from the distal stump at 1 to 2 mm per day. In addition to transmitting nerve impulses, the axon of the nerve functions to transmit nutrients and chemicals down its lumen. These axons are filled with axoplasm, which is necessary for nerve health and survival. Axoplasm is a viscous substance and is thixotropic, which means that it needs constant agitation or it will gel.22–24 ![]() Thus care must be taken to encourage movement and gliding of the nerve, but at the same time, positions that place tension on the nerve should be avoided.
Thus care must be taken to encourage movement and gliding of the nerve, but at the same time, positions that place tension on the nerve should be avoided.
ACDF surgery affects the sternocleidomastoid, platysma, anterior scalene, middle scalene, and the longus colli muscles. It also requires the resection of the anterior longitudinal ligament, PLL, joint capsule, and synovium.27–30 After the trauma incurred during surgery, the body is only capable of repairing small muscle lesions with regeneration of muscle tissue. Large lesions will fill in with dense connective scar tissue. Although dense connective scar tissue can function to reestablish tissue continuity, it lacks the contractile elements of normal muscle tissue and the tensile strength of normal ligament and tendon tissue. Therefore the ability to generate contractile forces or resist tensile loading through the region of repair is compromised.31–34
Bone grafts from the iliac crest or from bone donors are often used within the disc space, between two vertebral bodies, to aid in the mineralization and fixation of the region. The iliac crest is used as the primary source of graft material because of its cancellous bone composition. Cancellous bone has a greater potential for revascularization and osteogenesis than grafts from denser cortical bone sources. Healing after a cortical bone graft can take up to two times longer than its cancellous bone graft counterpart.31,32 As will become apparent later in the chapter, the healing and mineralization of bone at the site of fusion is a major factor driving progression through the rehabilitation process.
Phase I (Inflammation)
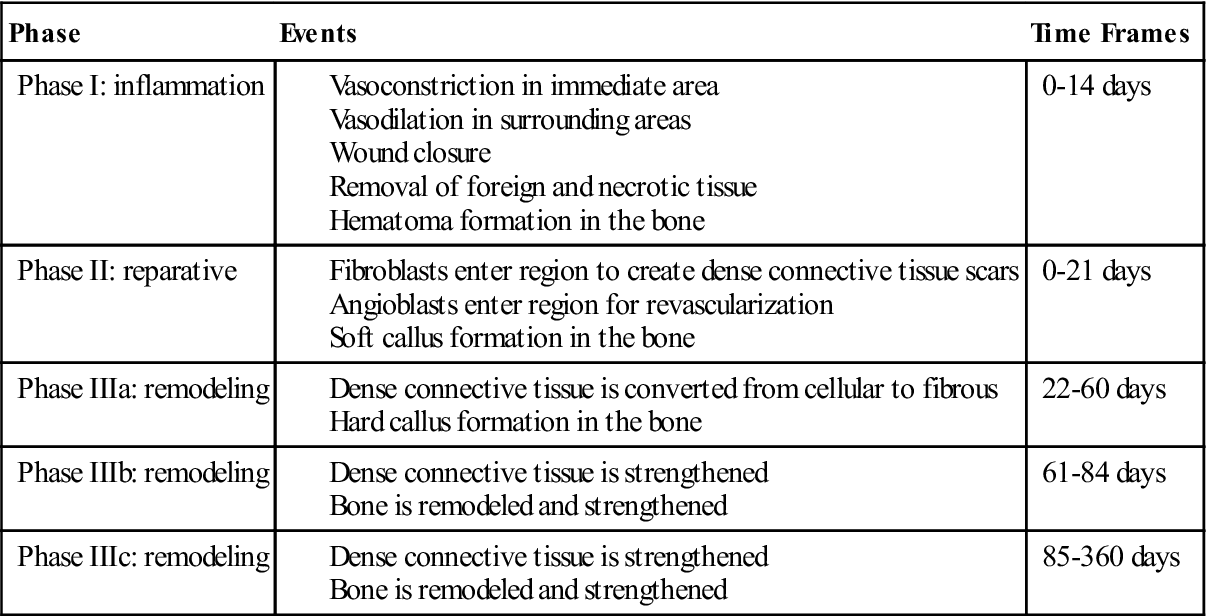
The inflammation phase is the first phase of tissue healing. It begins with injury to the tissue, reaches its peak within the first 72 hours after injury, and is generally completed within 14 days. During these first 14 days, several events occur. Vascular structures in the immediate area constrict to prevent blood loss, and vascular tissues in the surrounding areas dilate to provide conduits through which healing materials can enter the injured site. Cells and chemical mediators are brought into the area to remove all foreign debris and dead or dying tissue and are responsible for the closure of the wound. Both of these actions are important in the prevention of infection.31,32 During the inflammation phase in bone healing, a hematoma is formed at the site of the surgery. This begins immediately after surgery and is usually completed within 7 days. The hematoma will form around the graft and fusion site, and granulation tissue will fill any open space between the graft, the vertebral bodies, and the instrumentation.31–34 Clinically, rehabilitation during the inflammation phase of tissue and bone healing should focus on the prevention of blood loss, reduction of inflammation, and managing the pain that accompanies tissue damage (see Table 14-1).
TABLE 14-1
Soft Tissue and Bone Healing Time Frames
< ?comst?>
| Phase | Events | Time Frames |
| Phase I: inflammation | 0-14 days | |
| Phase II: reparative | 0-21 days | |
| Phase IIIa: remodeling | 22-60 days | |
| Phase IIIb: remodeling | 61-84 days | |
| Phase IIIc: remodeling | 85-360 days |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Phase II (Reparative)
The reparative phase is the second phase of tissue healing. This phase begins almost immediately after injury and is completed in 21 days. The primary function of this phase is the formation of the dense connective tissue needed to repair the wound and reestablish structural continuity of the affected region. The process of repairing the tissue to its original state is a time-consuming process, and little evidence supports the notion that tendons, ligaments, or large muscle injuries heal by regenerating into their original tissue. Thus the reestablishment of structural continuity and integrity of tendons, ligaments, and large muscle lesions is completed through the creation of dense connective scar tissue. Reparation with dense connective tissue patches or scar tissue is a fast process that can allow for quicker recovery of the tissue. Angioblasts and fibroblasts begin to enter the injured region within 5 days of the injury. These cells begin the process of tissue repair and the revascularization of the region. Most of the actual dense connective tissue development is completed by day 21. During bone healing at this time, a synthesis and organization of collagen is seen in the hematoma. Once the hematoma is organized, blood vessels invade the area. This allows osteoblasts to migrate into the region and form woven bone, which is known as a soft callus.31–34 Clinically, the goal of rehabilitation in this phase should be to promote the development of the new dense connective reparative tissue and woven bone (see Table 14-1).
Phase III (Remodeling)
The remodeling phase is the last phase of the tissue healing process. The purpose of this phase is to strengthen the newly formed scar tissue. Two subphase make up tissue remodeling: (1) consolidation and (2) maturation. During the consolidation subphase, tissue is undergoing conversion from a cellular type to one that is fibrous in nature. The actual size of the scar stops growing by 21 days, although the scar will continue to strengthen in response to stress. This subphase lasts from 22 to 60 days. During this phase of bone remodeling, the soft callus phase begins to mineralize and form a hard callus. Variations in mineralization time exist, but generally mineralization is completed by day 64. Mineralization of the callus is used diagnostically as a marker for when it is appropriate to begin rehabilitation. The patient will not be referred for rehabilitation until radiographic evidence indicates that the callus has mineralized.31–34 Clinically, rehabilitation should address protection and prevention of excessive motion through the fusion site.
![]() Excessive motion at the fusion site can lead to excessive callus formation and delay of the reparative process. The goal of rehabilitation in this phase should be the strengthening of the newly formed connective tissue. Care must be taken during this phase not to exceed the mechanical limits of the newly formed tissue, because overstress of the tissue will result in tissue injury and delay healing.
Excessive motion at the fusion site can lead to excessive callus formation and delay of the reparative process. The goal of rehabilitation in this phase should be the strengthening of the newly formed connective tissue. Care must be taken during this phase not to exceed the mechanical limits of the newly formed tissue, because overstress of the tissue will result in tissue injury and delay healing.
The maturation subphase occurs from day 60 to 360 when the tissues are fully fibrous in nature. For this reason, a progression in the strengthening of the affected tissues may begin. For bone remodeling, the hard callus begins to adapt to the stresses placed upon it. These stressors can be internal and external and include low serum calcium levels, skeletal microdamage, and changes in mechanical stress. The bone-remodeling process generally takes 6 months from initiation to completion, but it can take up to 4 years.31–34 Clinically, rehabilitation programs must provide appropriate levels of stress to the bone to encourage bone strengthening and remodeling without creating or exacerbating tissue injury (see Table 14-1).
Summary
Although guidelines can provide generalized time frames for healing and recovery, it is important to realize that a firm grasp of the factors listed previously will enable the clinician to individualize the rehabilitation program for each patient. No two patients are identical. Therefore no two rehabilitation programs should be identical. Solid clinical reasoning regarding the patient and the nature of the injury and surgery will ultimately drive the rehabilitation process.
Certain key components should be kept in mind during each phase of the rehabilitation process for ACDF.
Phase I:
The initial goal of rehabilitation should be the reduction of inflammation, closure of the wound, and reduction in pain.
Phase II:
The surgical site should be protected until dense connective tissue is formed and the bone shows evidence of mineralization.
Movement of the UEs below shoulder levels to promote nerve mobility and healing should be encouraged.
Phase III:
Gliding of the neural tissue through the surgical site to prevent the formation of adhesions should be promoted.
The clinician should begin placing stress on the soft tissue and bone in graded increments to promote proper soft tissue and bone growth and development.
Description Of Rehabilitation And Rationale For Using Instrumentation
Phase I (Inflammatory Phase)
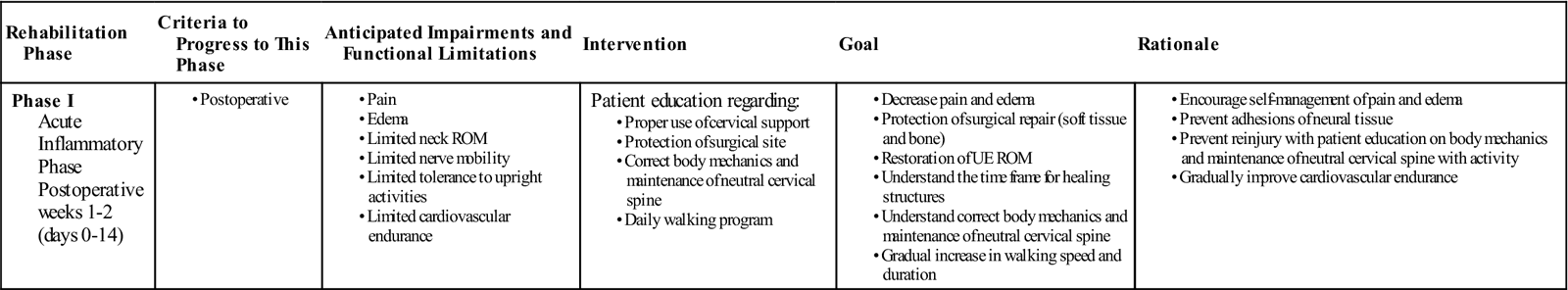
TIME: 1 to 2 weeks after surgery (days 0 to 14)
GOALS: Protect the surgical site, decrease pain and inflammation, maintain UE flexibility, and initiate patient education regarding neutral cervical spine mechanics (Table 14-2)
TABLE 14-2
Anterior Cervical Discectomy and Fusion
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
During the initial phase of rehabilitation, the primary focus of physical therapy is to protect the surgical site and make sure that the patient is educated on the mechanics of maintaining a proper neutral cervical spine (Fig. 14-7). Hospitalization after ACDF in most cases will be for 1 to 2 days. During this time the patient will be given a cervical collar to wear to immobilize the neck and encourage soft tissue and bone healing. Instructions regarding the frequency of collar wear will be determined by the physician and may differ on a case-by-case basis.
Physical evaluation during this time may include wound assessment and the assessment of bed mobility and gait. ![]() Because of the fragility of the wound and fusion sites, assessment of cervical range of motion (ROM) and UE strength are not appropriate in this phase. The primary physical impairments that the patient is likely to experience are pain, limited cardiovascular endurance, and limited tolerance to upright activities. During this time the injured vasculature around the wound begins to close and the noninjured vessels dilate, which may lead to increased warmth and redness around the incision site. This may be accompanied by neck pain and a sore throat. Oral analgesics may be given by the physician to manage the pain and inflammation.
Because of the fragility of the wound and fusion sites, assessment of cervical range of motion (ROM) and UE strength are not appropriate in this phase. The primary physical impairments that the patient is likely to experience are pain, limited cardiovascular endurance, and limited tolerance to upright activities. During this time the injured vasculature around the wound begins to close and the noninjured vessels dilate, which may lead to increased warmth and redness around the incision site. This may be accompanied by neck pain and a sore throat. Oral analgesics may be given by the physician to manage the pain and inflammation.
The inflammatory phase lasts approximately 2 weeks. During this time activities should center on resuming normal daily activities. Ambulation to and from the restroom should begin immediately, with assistance as needed, and progress until the patient is independent. The patient should be encouraged to increase the daily sitting tolerances. Pain and fatigue should guide the progression. Once discharged from the hospital, the patient will be instructed to protect the cervical region. The cervical collar issued earlier should be worn 24 hours a day unless otherwise ordered by the physician.
![]() Before discharge from the hospital, it is important that the therapist educate the patient on proper cervical spine mechanics during activity, as well as the need to restrict large amounts of movement in the neck to prevent soft tissue and bone injury. (Refer to Box 14-2 for specific patient guidelines to follow after discharge.) Because of the many muscular attachments of the shoulder girdle to the cervical spine, the patient should be advised to refrain from heavy lifting and from activities above shoulder level. Before discharge, the need to continue a home walking program and use of the cervical collar should also be addressed.
Before discharge from the hospital, it is important that the therapist educate the patient on proper cervical spine mechanics during activity, as well as the need to restrict large amounts of movement in the neck to prevent soft tissue and bone injury. (Refer to Box 14-2 for specific patient guidelines to follow after discharge.) Because of the many muscular attachments of the shoulder girdle to the cervical spine, the patient should be advised to refrain from heavy lifting and from activities above shoulder level. Before discharge, the need to continue a home walking program and use of the cervical collar should also be addressed.
Phase II (Reparative Phase)
TIME: 3 weeks after surgery (days 0 to 21)
GOALS: Understand neutral spine concepts, increase UE soft tissue mobility and flexibility, improve upright tolerance, improve activities of daily living (ADLs), increase cardiovascular function (Table 14-3)
TABLE 14-3
Anterior Cervical Discectomy and Fusion
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
In many instances, phase II of the rehabilitation process will take place independently in the patient’s home. Home therapy is rarely indicated; therefore education regarding patient progression through the first month after surgery is an important aspect of hospital care. The patient will be progressed to phase III once sufficient radiographic evidence of callus formation and mineralization is seen. During the reparative phase of tissue and bone healing, the body begins to form and lay down scar tissue at the surgical site, enhancing the integrity of the musculature to withstand gradual increases in loads to the tissues. Within the bone fusion site, callus formation is nearing completion. Rehabilitation throughout this phase should be a continuation of phase I, and a broadening of the focus to include the restoration of UE ROM to shoulder level and independence with self-care skills while protecting the surgical site (Fig. 14-8). At this time in the rehabilitation process, the patient may begin active range of motion (AROM) exercises of the shoulders. Nerves and soft tissue require movement to heal properly. Movement also prevents the formation of scar tissue adhesions between the nerve and the healing tissue surrounding the surgery. Therefore movement of the arms below shoulder level should be encouraged. Exercises incorporating flexion and extension of the elbow, wrist, and fingers should also be implemented at this time. ![]() Motion above shoulder level should still be avoided.
Motion above shoulder level should still be avoided.
Throughout all activities and exercises, the patient should be encouraged to maintain a neutral cervical spine. As neck pain and inflammation begin to subside in this phase and the patient continues to progress in activity level, trunk stabilization exercises may be introduced to allow the patient to achieve the overall neutral spine concept. Trunk stabilization exercises will allow loads to be properly distributed along the spine so as not to adversely increase loads to the cervical region during activities. Moreover, improved trunk stability and overall neutral spine will contribute to improving tolerance to upright postures.
Phase IIIa (Remodeling Phase)
TIME: 4 to 8 weeks after surgery (days 22 to 60)
GOALS: Enhance nerve healing and mobility, prevent scar tissue formation, increase UE strength and endurance, improve thoracic spine mobility (Table 14-4)
TABLE 14-4
Anterior Cervical Discectomy and Fusion
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
AROM, Active range of motion; PROM, passive range of motion; UE, upper extremity.
During this period of recovery, the patient (along with the soft tissues and bone of the surgical site) begins to experience numerous changes. Between the end of the fourth week and up to the sixth postoperative week, the physician will reassess the patient. Generally this reassessment will include a new radiographic study.
![]() Protection of the surgical site and proper immobilization should continue until the physician has seen evidence of mineralization and callus formation of the bone graft. Once the surgical site has sufficiently mineralized, the physician may permit additional rehabilitation.
Protection of the surgical site and proper immobilization should continue until the physician has seen evidence of mineralization and callus formation of the bone graft. Once the surgical site has sufficiently mineralized, the physician may permit additional rehabilitation.
Postural Rehabilitation
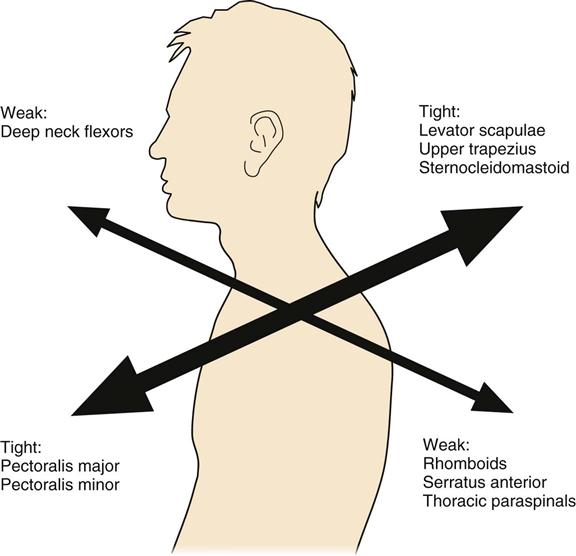
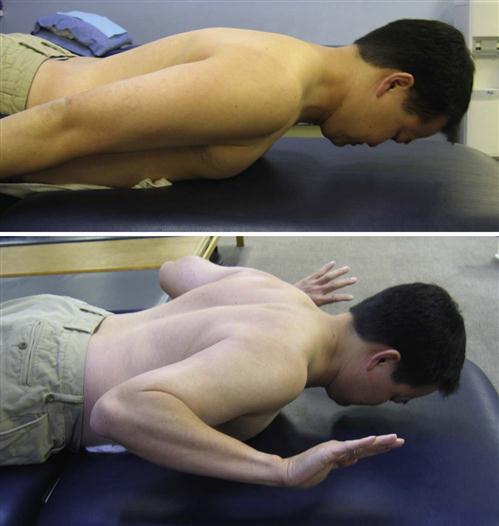
Rehabilitation specialists should expect to see patients in an outpatient setting at approximately 6 weeks after ACDF. Upon initial evaluation, observation of the patient’s posture will give the clinician a significant amount of information concerning weakness, elongation, and strength of specific musculature, as well as the patient’s ability to maintain a neutral cervical spine. According to Janda,35 a common postural alignment seen in people with upper quarter pathology is known as upper crossed syndrome (Fig. 14-9). Regardless of the cause, this alignment will consist of an upper quarter muscle pattern in which certain muscles will be weakened and lengthened and others will be strong and shortened, resulting in an increased thoracic kyphosis, increased midcervical lordosis, and increased upper cervical extension. Protraction of the scapula will often accompany this postural deviation. More specifically, a weakening and lengthening of the rhomboids, middle and lower trapezius, deep neck flexors, supraspinatus, infraspinatus, and the deltoid musculature occurs. This is combined with a tightening and shortening of the pectoralis major and minor, levator scapulae, upper trapezius, scalenes, subscapularis, and sternocleidomastoid muscles. Thus knowledge of how each muscle has been affected after surgery is necessary to guide the rehabilitation program. Postural rehabilitation should be implemented, and interventions should focus on the stretching of shortened musculature, strengthening of the weakened muscles of the trunk and neck, and performing UE movements while maintaining a neutral cervical spine. The clinician should be constantly weighing the intervention required against the limitations imposed by healing tissue. In the case of upper cross patterns, it is appropriate for the patient to stretch the pectoralis and subclavius muscles, but stretching of the sternocleidomastoid or levator scapulae muscle should be postponed due to these muscles’ proximal attachment to the cervical spine. Good evidence of fusion healing should be present before stretching of these cervical muscles commences (Fig. 14-10).
Cervical Stability
ACDF surgery requires the partial resection of the longus colli muscle.27 From a functional recovery perspective, the longus colli has an important role in maintaining cervical stability. Although research is lacking regarding cervical stability, numerous studies have been conducted on the role of lumbar stability to control motion and stabilize spinal segments.36 Richardson and associates37 performed a series of studies on the ability of deep lumbar muscles to stabilize spinal segments in patients with lumbar pain. Their findings suggest that deep muscle activation is a necessary component in the reestablishment of spinal control after a low back injury. Subjects that did not reestablish segmental control continued to experience low back pain. Recently, the same group has turned its attention to the cervical spine.38 They suggest that deep cervical muscles are necessary for normal cervical spine stability. The role may be even greater than that seen in the lumbar region because of the large role cervical spine muscles play in the maintenance and control of a region designed to provide mobility. Thus exercises designed to recruit deep neck flexors will be imperative to provide adequate stability of a highly mobile region. These exercises can include supine chin tucks in a neutral spine using a rolled towel or pillow if necessary, progressing to an inclined position and eventually a sitting position (Fig. 14-11). Jull38 has proposed the use of a blood pressure cuff behind the neck as a means of monitoring the amount of cervical muscle recruitment (Fig. 14-12 and Box 14-3). A recent study by O’Leary and associates39 showed a significant improvement in isometric craniocervical muscle performance with the use of a pressure biofeedback device. In this study, patients were initially instructed to achieve the correct cervicoflexion action without increased activity of superficial musculature. Once achieved, the use of a blood pressure cuff was used to guide the training of the craniocervical muscle contraction at different levels of pressure.39
A progressive resistance exercise (PRE) program for the UEs may be initiated with light weights during this phase. Biceps curls, triceps extensions, wrist and hand exercises, and isometric shoulder exercises are all appropriate at this time.
![]() The strengthening program should still be carried out below 90° of glenohumeral elevation to ensure that the musculature of the neck is not being overstressed. Each patient will need to begin at a different level after taking into account his or her present functional status and familiarity with the exercises. The focus should be on the use of light weights to build endurance of the musculature initially to assist with return-to-work activities and maintenance of prolonged postures.
The strengthening program should still be carried out below 90° of glenohumeral elevation to ensure that the musculature of the neck is not being overstressed. Each patient will need to begin at a different level after taking into account his or her present functional status and familiarity with the exercises. The focus should be on the use of light weights to build endurance of the musculature initially to assist with return-to-work activities and maintenance of prolonged postures.
Joint Mobilization
Decreased flexibility in thoracic spine segments and the soft tissue of the thoracic region may prevent proper body alignment, including full glenohumeral ROM. Thus treatment should include soft tissue mobilization to the mid and lower thoracic spine. ![]() Later mobilization to the midthoracic spine can be included with the authorization of the physician.
Later mobilization to the midthoracic spine can be included with the authorization of the physician.
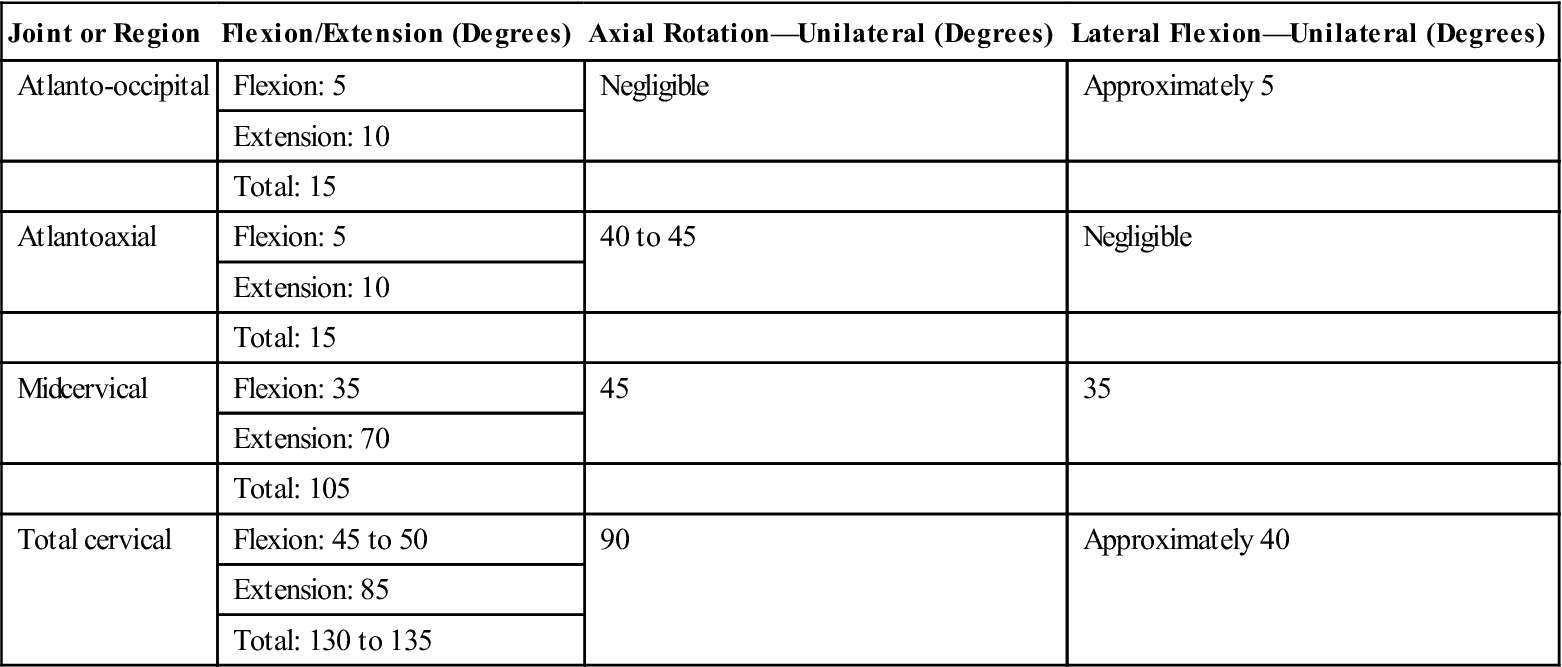
It is appropriate to begin AROM and passive range-of-motion activities at this time (Fig. 14-13). The clinician should keep in mind that the biomechanics of the cervical spine will be altered by cervical fusion surgery. An understanding of how it will be affected is critical in assessing a patient’s progress and ultimate outcome. The cervical vertebrae are the smallest and most mobile of all spinal vertebrae. The cervical region functions to provide mobility for the head on the trunk. It also functions to protect vital structures, such as the spinal cord, as they route distally down the body. In total, the functional units of the cervical region must work together to provide 45° to 50° of flexion and 85° of extension, for a total of 130° to 135° of total sagittal plane motion. In the horizontal plane, the cervical spine must be able to provide 90° of unilateral motion and 180° of total rotational motion. Finally, 40° of unilateral frontal plane motion occurs—or 80° in total (Table 14-5).40,41 Segmentally, two adjacent spinal vertebrae and the intervertebral disc between the two comprise a functional motion segment. Each functional spinal unit provides varying degrees to the total motion seen in the cervical region. The fusing of one or several of the functional motion segments will alter the mechanics of adjacent segments. The body will eventually adjust; the result is transitional degeneration.
TABLE 14-5
Approximate ROM for the Three Planes of Movement for the Joints of the Craniocervical Region
< ?comst?>
| Joint or Region | Flexion/Extension (Degrees) | Axial Rotation—Unilateral (Degrees) | Lateral Flexion—Unilateral (Degrees) |
| Atlanto-occipital | Flexion: 5 | Negligible | Approximately 5 |
| Extension: 10 | |||
| Total: 15 | |||
| Atlantoaxial | Flexion: 5 | 40 to 45 | Negligible |
| Extension: 10 | |||
| Total: 15 | |||
| Midcervical | Flexion: 35 | 45 | 35 |
| Extension: 70 | |||
| Total: 105 | |||
| Total cervical | Flexion: 45 to 50 | 90 | Approximately 40 |
| Extension: 85 | |||
| Total: 130 to 135 |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Adapted from Neumann D: Axial skeleton: osteology and arthrology. In Neumann D, editor, Kinesiology of the musculoskeletal system: Foundations for physical rehabilitation, ed 2, St Louis, 2009, Mosby.
![]() Therefore, initially a fusion to the C5-6 motion segment may result in a loss of 10° to 15° of unilateral rotation.36,42 Therefore the objective goal of rehabilitation should not be to attain 90° of unilateral rotation. Instead normal unilateral rotation after fusion to C5-6 would be 65° to 70° of motion.
Therefore, initially a fusion to the C5-6 motion segment may result in a loss of 10° to 15° of unilateral rotation.36,42 Therefore the objective goal of rehabilitation should not be to attain 90° of unilateral rotation. Instead normal unilateral rotation after fusion to C5-6 would be 65° to 70° of motion.
Mobilization techniques are a mainstay of physical therapy. In practice, they are used to increase ROM within targeted regions by moving specific joints or specific muscles. Care must be taken in choosing the appropriate time to begin implementation of soft tissue and particularly passive joint mobilization techniques because of the potential translational effect they may have on the cervical spine at the region of the fusion. Although studies are lacking in the area of mobilization of the cervical spine, several studies have addressed the effects of mobilization in the lumbar region. Researchers43–46 studied the effects of a posterior to anterior force placed on the spinous process of L3. Their study showed a force at L3 could result in movement as far away as T8; in a follow-up study by the same group, the same posterior to anterior force resulted in an anterior rotation of the sacrum. The implications of these findings for the patient after a cervical fusion are that even mobilizations to distant segments may have a translatory effect on the fusion site. ![]() Therefore mobilization of the spine should not be initiated until the fusion site itself has shown radiographic evidence of sufficient mineralization and callus formation. This information and the authorization of mobilization to the cervical spine should come from the surgeon. Moreover, research has revealed transitional degeneration in the segments directly above and below the fusion. Once this is found, proper mobilization and joint forces may be added to begin stimulating proper formation and modeling of bone tissue.
Therefore mobilization of the spine should not be initiated until the fusion site itself has shown radiographic evidence of sufficient mineralization and callus formation. This information and the authorization of mobilization to the cervical spine should come from the surgeon. Moreover, research has revealed transitional degeneration in the segments directly above and below the fusion. Once this is found, proper mobilization and joint forces may be added to begin stimulating proper formation and modeling of bone tissue.
![]() Although it is difficult to imagine an instance when direct mobilization to the fusion site would be warranted, mobilization to adjacent structures and segments is justified to increase spinal ROM and decrease the demands placed upon the fusion site.
Although it is difficult to imagine an instance when direct mobilization to the fusion site would be warranted, mobilization to adjacent structures and segments is justified to increase spinal ROM and decrease the demands placed upon the fusion site.
![]() However, therapists should be wary of applying mobilization techniques close to the fusion site. Research has revealed transitional degeneration in the segments directly above and below the fusion.47 Transitional degeneration is a common long-term complication after a spinal fusion, particularly in multilevel fusions. It consists of segmental articular degeneration and spondylytic changes in the spine. It has been hypothesized that these changes are the result of the increased stress placed on these segments because of the decreased mobility of the fusion spinal segments. Goffin and colleagues48 studied 120 patients after ACDF surgery and at a mean follow-up period of 98 months. They found that 92% of the patients demonstrated segmental degenerative changes. Eventually, when dense connective tissue and bone has been adequately strengthened and stabilized, then distant spinal segments can be mobilized when needed.
However, therapists should be wary of applying mobilization techniques close to the fusion site. Research has revealed transitional degeneration in the segments directly above and below the fusion.47 Transitional degeneration is a common long-term complication after a spinal fusion, particularly in multilevel fusions. It consists of segmental articular degeneration and spondylytic changes in the spine. It has been hypothesized that these changes are the result of the increased stress placed on these segments because of the decreased mobility of the fusion spinal segments. Goffin and colleagues48 studied 120 patients after ACDF surgery and at a mean follow-up period of 98 months. They found that 92% of the patients demonstrated segmental degenerative changes. Eventually, when dense connective tissue and bone has been adequately strengthened and stabilized, then distant spinal segments can be mobilized when needed.
Neural Mobilization and Neural Dynamics
Neural mobility techniques should also be progressed in this phase to prevent neural adhesions to the surrounding tissue. Scar tissue has the ability to restrict joints; scar tissue can adhere to nerves and affect their mobility.
![]() Because the patient is likely to have experienced nerve-related symptoms as a contributing factor for undergoing surgery, an understanding of neurodynamics, or the relationship between the nervous system and associated connective tissues, will ensure proper interventions will be chosen that will not aggravate or overstretch the neural tissue. While the concept of neurodynamics has been around for some time, empirical data supporting its clinical use has been lacking. Neurodynamic techniques are commonly classified into two categories: techniques that glide the nerve and techniques that stretch the nerve. An example of a gliding technique in the upper quarter would be placing tension on the nerve at the wrist with wrist extension while simultaneously reducing tension at the neck by side-bending toward the same side. An example of a tensioning technique would be placing tension on the wrist with wrist extension while simultaneously side-bending the neck toward the opposite side. Research by Coppieters and associates49 has shown that sliding techniques produce a greater amount of nerve excursion through the surrounding tissue than tensioning techniques. Furthermore, an increase in muscle activity also occurred with the addition of dorsiflexion. It has been hypothesized that muscles are recruited to protect the nerve and prevent injury when the nerve is placed in tension. Thus clinicians should ensure that soft tissue surrounding the nerve is free to achieve optimal neural movement.
Because the patient is likely to have experienced nerve-related symptoms as a contributing factor for undergoing surgery, an understanding of neurodynamics, or the relationship between the nervous system and associated connective tissues, will ensure proper interventions will be chosen that will not aggravate or overstretch the neural tissue. While the concept of neurodynamics has been around for some time, empirical data supporting its clinical use has been lacking. Neurodynamic techniques are commonly classified into two categories: techniques that glide the nerve and techniques that stretch the nerve. An example of a gliding technique in the upper quarter would be placing tension on the nerve at the wrist with wrist extension while simultaneously reducing tension at the neck by side-bending toward the same side. An example of a tensioning technique would be placing tension on the wrist with wrist extension while simultaneously side-bending the neck toward the opposite side. Research by Coppieters and associates49 has shown that sliding techniques produce a greater amount of nerve excursion through the surrounding tissue than tensioning techniques. Furthermore, an increase in muscle activity also occurred with the addition of dorsiflexion. It has been hypothesized that muscles are recruited to protect the nerve and prevent injury when the nerve is placed in tension. Thus clinicians should ensure that soft tissue surrounding the nerve is free to achieve optimal neural movement. ![]() Therefore treatment should address the gliding and not stretching of nerves.
Therefore treatment should address the gliding and not stretching of nerves.
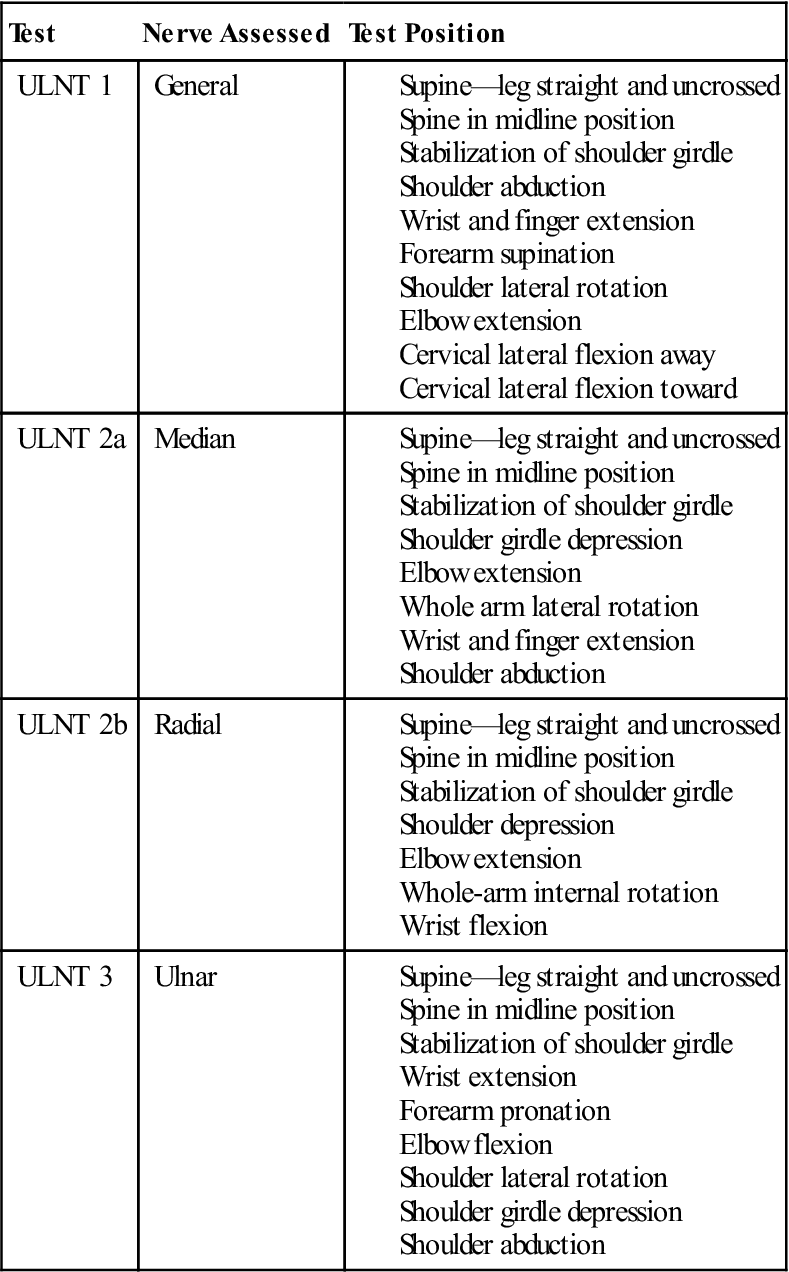
Neurodynamic testing of the upper limb enables the clinician to assess the movement capabilities of neural tissues in relation to the soft tissue structure that surrounds them. Elvy50 developed the upper limb neurodynamic tests (ULNTs) as a method for differentiating potential sources of cervicobrachial symptoms. Upper limb neurodynamic test 1 (ULNT 1) (Fig. 14-14) was designed to assess the movement capabilities of neural tissues associated with the median nerve. Given the mechanical continuity of the nervous system, it has recently been recognized that all upper quarter neural tissues are stressed during ULNT 1; however, components of the test are specifically biased toward the median nerve trunk and C5-C7 nerve roots51 (Table 14-6). Although originally designed to test the median nerve, clinicians are using it as a general clearing test for UE neuromobility because all three major peripheral nerves in the UE are stressed by the ULNT 1 position (Box 14-4).
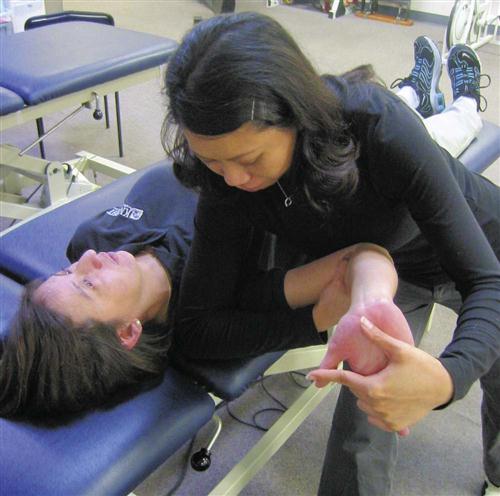
TABLE 14-6
Upper Limb Neurodynamic Testing (ULNT) Positions
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Adapted from Butler D: The sensitive nervous system, Adelaide, Australia, 2000, Noigroup.
Treatments using the ULNTs have generally been a point of confusion for clinicians. A positive test is indicative of restricted mobility in the nerve being tested. Therefore using the test position to stretch the nerve and release any adhesions along the course is a common treatment philosophy. Coppieters and associates52 also found that adding positions of tension to a joint reduced the amount of nerve mobility in the adjacent joints. For example, adding wrist extension to a median nerve mobility test decreases the range of extension available at the elbow. This finding was corroborated in a study by Boyd and associates53 in which straight leg raise ROM was reduced when dorsiflexion was added to the ankle compared with the addition of plantar flexion. Thus when using ULNT test positions to glide the nerve, therapists need to be aware that muscle tissue has the potential to elongate and stretch, whereas neural tissue is not as elastic and responds adversely to stretching as explained above (Fig. 14-15).
![]() Soft tissue structures along the course of the nerve can be mobilized to allow for nerve mobility. Movement of the UE can be combined with small movements of the neck to encourage gliding of the nerve rather than stretching. Finally, communication with the patient is essential, because radicular pain or paresthesia is indication that the nerve is being stretched and potentially irritated. The patient and therapist should work in ROMs that do not reproduce the patient’s radicular symptoms.
Soft tissue structures along the course of the nerve can be mobilized to allow for nerve mobility. Movement of the UE can be combined with small movements of the neck to encourage gliding of the nerve rather than stretching. Finally, communication with the patient is essential, because radicular pain or paresthesia is indication that the nerve is being stretched and potentially irritated. The patient and therapist should work in ROMs that do not reproduce the patient’s radicular symptoms.
Cervical Proprioception
As the healing process continues in bone and soft tissue structures, the patient may also have sensorimotor deficits, such as unsteadiness, visual disturbances, and changes in postural stability and cervical joint position sense. Although the exact physiologic mechanism is not clear, these changes are believed to be the result of a traumatic injury to the nerves themselves, chemical mediators within and around the joint that inhibit the proprioceptive nerves, as well as central changes occurring at the spinal cord and cortical regions that alter the body’s response to proprioceptive input. Research has shown that rehabilitation of the cervical spine thus far has traditionally focused on the strength and length of muscles in the region, as well as joint and nerve mobility, which may not be as effective in addressing proprioceptive and sensorimotor disturbances in patients with neck pain.54 According to recent studies by Trevelean,55,56 the abundance of mechanoreceptors in the cervical region plays an important role in providing proprioceptive input to the central nervous system. There are high densities of muscle spindles in the cervical region, especially in the suboccipital muscles, which have up to 200 muscle spindles per gram of muscle. This amount is significant when compared with the 16 muscle spindles per gram of muscle in the first lumbrical.55,56 In addition, the visual and vestibular systems of the cervical spine region have been shown to influence the proprioceptive input provided by the cervical mechanoreceptors. Therefore, the visual, vestibular, and proprioception systems are integrated to provide the sensory input for proper cervical function. In terms of cervical rehabilitation, these findings suggest balance, proprioception, and visual training should be added to a patient’s exercise program to address sensorimotor and proprioceptive deficits.
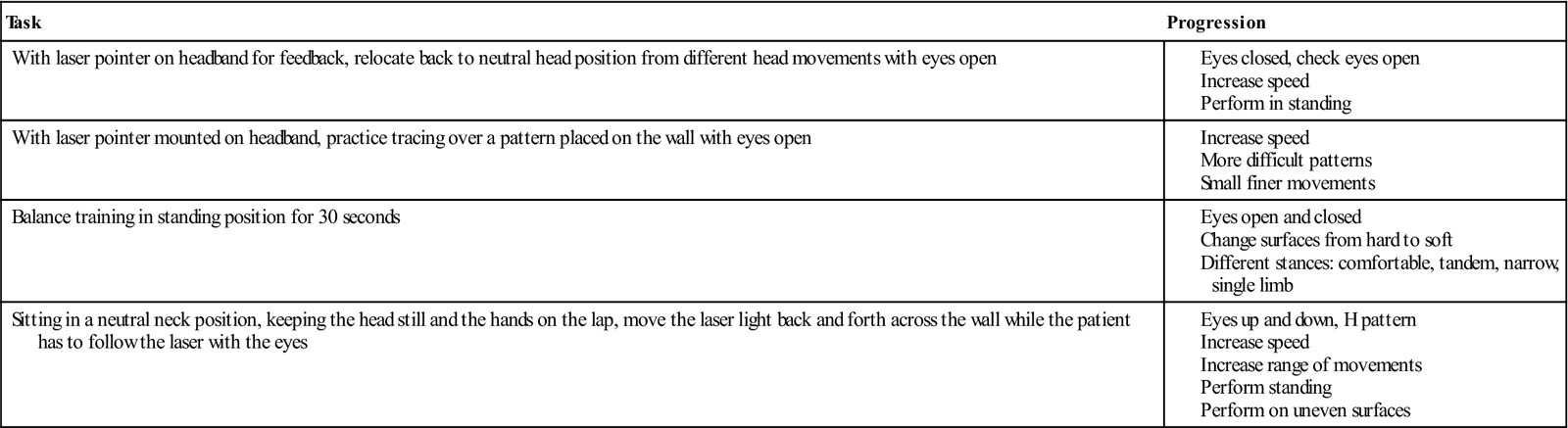
Symptoms of poor head and neck awareness or a “wobbling” head may be due to poor cervical position sense. To assess for disturbed joint position sense, Treleaven advocates the use of a small laser pointer mounted on a lightweight headband. The patient is seated 90 cm away from a wall and the point where the laser shines at initial setup is marked. The patient proceeds to close his or her eyes and then provides a neck motion (for example, right or left rotation). The patient is then instructed to return the head to the initial head position. A greater than 4 to 5 cm error is indicative of cervical proprioception deficits (Fig. 14-16).57,58 This assessment technique can be used as an exercise in which the patient practices relocating the beam of light to the initial position with the eyes open, trying to improve the accuracy of the movements each time. To improve deficits in neck movement control, in which patients complain of fatigue in the neck or difficulty with AROM movements because of increased muscle contraction to protect the cervical spine, the patient may use the laser pointer to trace a pattern on the wall such as a figure eight placed 90 cm in front of the seated patient (Fig. 14-17). The use of joint positioning/neck movement control exercises, coupled with focused treatment on balance training, has proven to be effective in treating patients with acute and chronic neck pain.54 Balance training to improve postural stability included exercises incorporating tandem stance and single-leg stance, with both eyes open and closed, and alterations of stance surfaces. The stance and surface were dependent upon the patient’s capabilities. The visual training exercises consisted of asking the patient to place the head in various positions and then asking the patient to visually track objects or visual targets on the wall. Beams of light or a laser pointer provided a target that the patient could track with his or her eyes (Table 14-7). Since the patient has recently begun AROM exercises, the therapist should use sound clinical judgement as to when these exercises should be incorporated into the patient’s plan of care. In addition, balance exercises may cause increased pain or headaches, and the tasks might need to be altered to more supportive positions. Mild dizziness may occur with balance exercises to allow for vestibular habituation, but the patient’s symptoms should be monitored closely. If the presence of any central nervous system signs appears without explanation or diagnosis, it may be considered a red flag and the therapist should refer the patient to a physician for further tests. Thus far, incorporating proprioceptive, balance, and visual training interventions to a cervical rehabilitation program have achieved positive outcomes.
TABLE 14-7
Exercises and Progressions to Improve Cervical Proprioception
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
To facilitate the return-to-work transition in this phase, cardiovascular endurance should be continued. A daily walking program should be continued and progressed as tolerated.
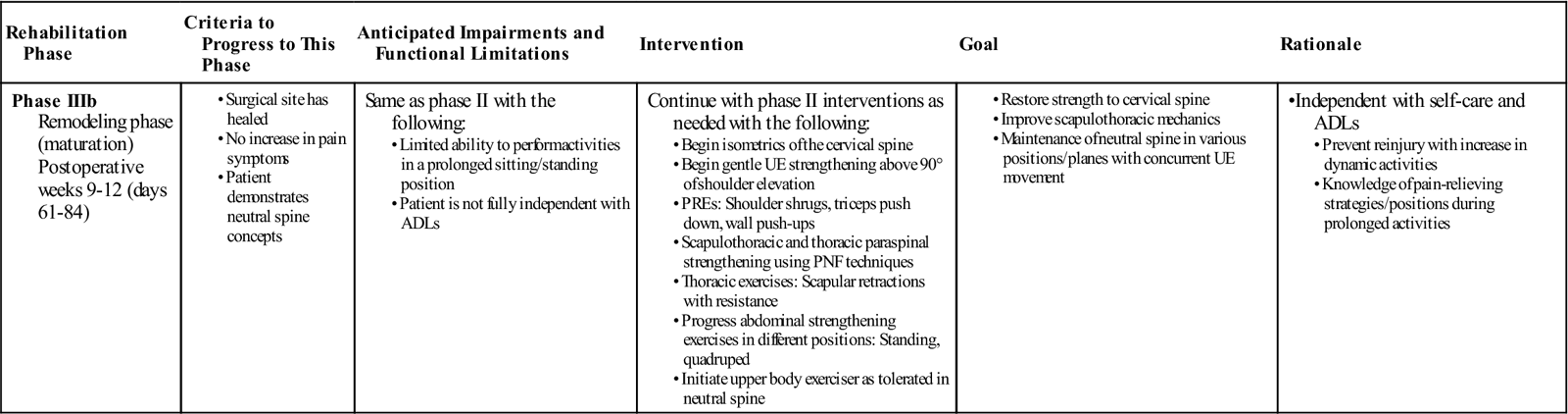
Phase IIIb (Remodeling Phase)
TIME: 9 to 12 weeks after surgery (days 61 to 84)
GOALS: Restoration of strength to the cervical spine, maintenance of neutral spine for prolonged periods of time with concurrent UE movement, improvement in scapulothoracic mechanics (Table 14-8)
TABLE 14-8
Anterior Cervical Discectomy and Fusion
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Progression to this phase of rehabilitation should begin once the patient is able to tolerate the exercises of phase IIIa without an increase in neck or arm symptoms. Interventions from the previous phase have focused on loading the upper and lower body without directly loading the cervical spine. The purpose of this precaution is to prevent overstressing newly healed structures. This particular stage in the rehabilitation process will slowly begin to incorporate direct treatment to the cervical spine structures and therefore should not be started until the patient has adequately demonstrated tolerance to the loads placed on the neck. The patient should also be able to demonstrate proper neutral cervical spine concepts.
The thoracic spine musculature such as the rhomboids and middle and lower trapezius can be further challenged from the previous phase by adding resistance with light weights on a seated or standing rowing machine or through the use of resistance tubing (Figs. 14-18 and 14-19). Proprioceptive neurofacilitation techniques may also be used to strengthen thoracic paraspinals and scapulothoracic musculature. PREs may be increased in weight, sets, and repetitions as tolerated. Exercises above 90° shoulder elevation may be initiated to further strengthen cervical musculature. Isometric cervical spine strengthening may begin in all planes. Attention should be given to complete these exercises in the neutral cervical spine to strengthen the musculature of the cervical spine to allow for proper posture and force production of these muscles. The patient is placed in a comfortable sitting position. For flexion, the patient places both hands on the forehead and brings the forehead into the hands without moving. In extension, the patient places both hands on the back of the head and brings the head backwards into the hands without moving (Fig. 14-20). During the side bend, the patient places one hand on the side of the head and attempts to bring the ear to the shoulder without moving. In rotation, the patient places one hand to the side of the head in front of the ear and looks over the shoulder without allowing movement. Strength and trunk control can be further challenged through the addition of an unstable base of support, such as a half foam roller placed under the feet or the use of a stabilization ball. A clinician should be assisting the patient at all times during these exercises. Synchronized UE movements, such as biceps curls while balancing on an unstable support, will further challenge the trunk and neck complex simultaneously. Placing the patient in positions such as quadruped or prone on the stabilization ball should warrant caution and be delayed if the patient has yet to demonstrate deep neck flexor strength or the ability to maintain a neutral cervical spine in an antigravity position. Proper neck alignment should be maintained during execution of all therapeutic activities.
At this stage of rehabilitation, the patient may find it difficult to perform activities that require prolonged sitting or standing postures. It is important to assist the patient in recognizing methods or activities that have the ability to relieve some of the pain or soreness. It is also important that he or she be assisted in the development of strategies to increase muscle endurance so that the patient may gradually build a tolerance to these positions. Strategies may include limiting the time spent in any one position, the use of cryotherapy to the neck, or active cervical ROM exercises to relieve stiffness and soreness. Cardiovascular endurance and strength should continue during this phase, and the use of an upper body exerciser may be initiated for short amounts of time.
Phase IIIc (Remodeling Phase)
TIME: 13 to 52 weeks after surgery (days 85 to 360)
GOALS: Return to presurgical strength and endurance, return to prior level of functioning, prepare for discharge from physical therapy (Table 14-9; see also Suggested Home Maintenance Box)
TABLE 14-9
Anterior Cervical Discectomy and Fusion
< ?comst?>
< ?comen?>< ?comst1?>

< ?comst1?>
< ?comen1?>
The remaining phase of the rehabilitation process centers on regaining presurgical strength and endurance. By the end of this phase, the patient should be able to function independently at home and in the workplace. As the patient progresses through the rehabilitation process, functional retraining of work- or sport-specific activities should be assessed. Activities that require increased loads on the cervical spine should be evaluated; pending physician approval, rehabilitation geared toward functional training can be initiated. Return to activities or sports that require contact between players or heavy lifting will require the physician’s approval. At this time the physical therapist may implement a gym- or home-based exercise program to assist in maintenance of proper strength and muscle function. Discharge of the patient should occur once the patient, physical therapist, and physician have all determined that the patient has reached his or her functional goals and is able to continue the rehabilitation process safely and independently.
Clinical Pearl: Cervical Classification
In the 1980s, Sahrmann and associates began to lay the foundation for the development of a clinical reasoning method that focused on subgrouping patients of a larger entity into smaller homogeneous groups to identify specific interventions that will address each subgroup’s specific signs and symptoms.59 Regions such as the neck and low back are large heterogeneous groups and proving efficacy of treatment with such a diversity of patients and pathology is difficult. If the characteristics of this subgroup can be identified and paired with the most effective interventions, then prognosis and the quality of patient care will improve. In recent years, the development of classification-based research has taken on renewed interest. Childs and associates proposed such a classification-based system for the treatment of the cervical region.60 While recovery from cervical fusion surgery does not completely fit within the scope of this classification system, we can apply the principles of their findings toward the treatment of patients following cervical fusion. The study suggests five pairings of outcome goals with specific interventions. The outcome goals are mobility, centralization, conditioning and increased exercise tolerance, pain control, and reduced headaches. Of these five outcomes, pain control and conditioning and increased exercise tolerance most closely reflect the goals following a cervical spine fusion. Pain control is a primary goal in the first and second phases of cervical rehabilitation and the interventions linked to this outcome are AROM exercises within pain tolerance, ROM exercises for adjacent regions, physical modalities as needed, and activity modification. The outcome goal of increased exercise tolerance and conditioning takes place in the second and third phases of cervical rehabilitation. Strengthening/endurance exercises of the upper quarter, as well as aerobic exercises, are the interventions proposed to achieve this clinical outcome.
Troubleshooting
Red Flags
The majority of postoperative complications and red flags will occur within the first several weeks after surgery. Clinicians should be aware of these complications and should educate the patient to notify his or her physician immediately if any of the following complications should occur. Although the majority of the red flags will occur before a patient’s release to outpatient rehabilitation, the outpatient clinician should be cognizant of any drastic changes that would warrant communication with the physician for further assessment and testing.
Infection
The risk of infection after cervical spine surgery is difficult to determine. Postoperative infection rates of up to 6% have been reported. Several factors affect a patient’s risk for acquiring an infection, including a patient’s age, duration of surgical procedure, and the patient’s preoperative physical condition.61 Obesity is also a risk factor for infection because adipose tissue is poorly vascularized. Uncontrolled diabetes also increases the risk of infection. Signs and symptoms of infections include erythema, edema, purulent wound drainage, tenderness, fever, and increased pain.
Dysphagia
The incidence of dysphagia in patients after ACDF has been reported as high as 28%.62,63 Additional studies have noted that 51% of patients will have swallowing difficulty at 1 month after surgery, 31% at 2 months, and 15% at 6 months.64 Therefore speech and swallowing problems after ACDF are not uncommon, although dysphagia is one of the primary symptoms accompanying plate and screw loosening. Persistent symptoms should be cause for further examination by the physician.
Esophageal Injury
Esophageal injury is rare but can occur up to 1 year after surgery.65 The mechanism behind the injury has been attributed to laceration or pressure necrosis of the esophagus by graft displacement. Signs and symptoms of an esophageal injury include increased neck and throat pain, odynophagia, erythema, swelling, tenderness, crepitus, subcutaneous emphysema, unexplained tachycardia, sepsis, and difficulty swallowing.
Neural Injury
The recurrent laryngeal nerve is susceptible to nerve injury after ACDF surgery. Incidence of injury has been reported to be between 0.07% and 11%.66 Injury to the recurrent laryngeal nerve may be the result of endotracheal tubing that may compress and damage the nerve. Symptoms of nerve injury include vocal cord paralysis. Spinal cord injury secondary to ACDF is 0.4%.67 Most often the source of the spinal cord injury is posterior displacement of the bone graft. Nerve root injury is also low at 0.6%. The most affected nerve root is C5. Most incidence of injury resolved in 6 weeks.
Vascular Complications
The exact prevalence of vascular complications after ACDF is unknown. Most studies report values in the neighborhood of 0.6% or less for vertebral artery injury after ACDF surgery.68,69 Signs and symptoms of vertebral artery injury include dizziness, dysphagia, dysarthria, diplopia, and drop attacks.
Cervical Spine Bracing
Skin breakdown and soft tissue injuries are common with the long-term use of cervical bracing. In addition, muscle atrophy, dysphagia, and gastrointestinal dysfunction have also been reported. Signs and symptoms of pressure sores or swallowing dysfunction must be monitored.61
Graft Failure
Graft failure after ACDF may be caused by the following: graft displacement, nonunion, instrumentation failure, host factors including osteoporosis or extreme kyphosis, and technical factors such as a short or long graft. Graft failures are highest with multilevel fusions at 60%, and graft dislodgement has been reported in 5% to 50% of multilevel surgeries without instrumentation. Nonunion rates are higher in iliac crest allografts (60%) versus autografts (17%), although they are the same rate at 5% for single level fusions.70 Clinical symptoms for patients who are symptomatic from nonunion include increasing neck pain and worsening axial pain 6 months after surgery. Patients may have difficulty swallowing and breathing after an anterior graft displacement. Patients who experience a worsening of pain and symptoms should be referred to the physician for additional evaluation and testing procedures.
Chronic Pain
Changes in the peripheral and central nervous system occur almost immediately after an injury. Some of these changes are reversible, and other changes are nonreversible. Many of these changes have been proposed as the pathomechanisms behind the chronicity of pain. It is beyond the scope of this chapter to describe all the neural changes that occur with injury; however, from a clinician’s viewpoint it is important to realize that not all patients will have full resolution of symptoms after surgery. Surgery may have addressed the structures that were originally the source of the patient’s symptoms, but the adaptations that have occurred in the central and peripheral nervous system may not be reversible. Rehabilitation after ACDF has 80% to 90% satisfactory results. There remain an elusive 10% to 20% of patients who continue to experience pain despite the fact that the offending structures have been addressed through removal or fixation. As a clinician, it is important to realize that not all pain is a reflection of actual tissue damage. Some pain is the result of tissue changes, and this will affect the ability to rehabilitate patients.26,71–73
Summary
Rehabilitation of a patient after ACDF surgery is unique in terms of the close relationship the neck has with the shoulder region and its neural network. Unlike other regions of the body, such as the shoulder and the wrist, complete immobilization of the cervical spine is difficult, which can affect the healing potential of the fusion site. Therefore educating the patient on the need to adhere to surgical protection guidelines immediately after surgery is important. Protection of the surgical site is the key aspect of early rehabilitation, and stressing of the fusion site should not begin before mineralization of the callus. Moreover, the shoulder girdle and UE, unlike the hip and lower extremity (LE), rely on coordinated muscle actions to maintain function and stability. Many of these muscles have their proximal attachments at the cervical spine. ![]() Therefore protection of the fusion must also address limiting UE activity until the surgical site is fully healed. Finally, because radicular pain and UE paresthesia are often the symptoms driving the decision for ACDF, the prevention of neural adhesions and promotion of nerve healing should be addressed appropriately.
Therefore protection of the fusion must also address limiting UE activity until the surgical site is fully healed. Finally, because radicular pain and UE paresthesia are often the symptoms driving the decision for ACDF, the prevention of neural adhesions and promotion of nerve healing should be addressed appropriately.
Clinical Case Review
1Angel is a 45-year-old woman who has arrived at the outpatient clinic for an initial evaluation status after ACDF. The surgery was 4 weeks ago. Her chief complaint is stiffness and soreness in her neck with difficulty sleeping at night. She also notes continued difficulty swallowing and complains of a dry mouth. She asks the therapist’s opinion regarding whether she should see her doctor. What should the therapist tell her?
Angel is likely experiencing dysphagia, which is a common short-term side effect of the surgery. The therapist should ask the patient for further information on the duration and intensity of the symptoms. Mild symptoms of dysphagia may be expected, although increased symptoms related to cardiovascular signs such as difficulty breathing, shortness of breath, or symptoms of sleep apnea would warrant a physician consultation.
2Ned is a 41-year-old man who was involved in a motor vehicle accident 2 years ago. His primary pain symptoms because of the accident included paresthesia and a burning sensation throughout his right UE. He underwent ACDF surgery 4 weeks ago and continues to have neural paresthesia in his right arm. What interventions should be administered?
Possible interventions include gentle UE AROM exercises to the elbow in flexion and extension (as well as to the wrists and fingers) below 90° of shoulder elevation to allow the nerves to glide. The therapist should encourage the patient to move the arms below shoulder level and advise the patient not to lift and only move his arms minimally above 90°.
3Sam is a 54-year-old man who underwent ACDF 12 weeks ago. He started outpatient physical therapy services 6 weeks ago and has made significant improvements in his upper quarter ROM and strength. Recently, Sam has begun a gym exercise program as he prepares for discharge from physical therapy. However, after approximately 2 weeks at the gym, Sam reports feeling soreness in his neck and shoulders after performing the following exercises: seated scapular rows, latissimus pull-downs, biceps curls, inclined bench press, and an introductory spinning or cycling class for aerobic conditioning. Which of the previously mentioned exercises may be causing Sam’s symptoms?
The introductory spinning or cycling class may be the cause of Sam’s pain symptoms because of the cervical positioning this type of bike provides. This style of bike usually places the cervical spine in hyperextension and the thoracolumbar spine in flexion, causing Sam to feel soreness after maintaining this extreme position for the duration of the class. Use of a stationary upright bike may place the cervical spine in a more comfortable position and relieve Sam’s symptoms. In addition, it would be prudent for the therapist to advise Sam to postpone latissimus pull-downs at this time. They can be started later in the rehabilitation process; however, at this time it is not wise to complete resisted activities above shoulder level. Finally, the therapist should assess the amount of weight the patient is using.
4Sherry is a 50-year-old woman who had ACDF surgery 6 weeks ago and has just recently removed her cervical collar and returned to work as an accountant. She has a forward head posture, rounded and slumped shoulders, and bilateral scapular winging. She also continues to have numbness and tingling in her right UE, and her pain level reaches a 6 out of 10 (10 being the worst) by lunchtime. She is worried that the fusion has been unsuccessful. What is the therapist’s response?
In this case patient education on the need for proper posture throughout the spine appears warranted. The explanation should address proper ergonomic positioning in the workplace to provide the neck and spine an optimal position for work-related activities. In addition, an explanation concerning the effects and stress her posture imposes on nerves and soft tissues will put her at ease. Tight musculature of the pectoralis major and other anterior tissues may be pinching on the brachial plexus, or the nerve roots may be affected secondary to the forward posturing. Interventions to improve her posture should be initiated to relieve undue stresses, and light strengthening of weakened muscles from upper crossed syndrome can be addressed as tolerated. The patient should also be advised to take regular breaks while at work to allow her to change positions and prevent prolonged static postures. The therapist should encourage the patient to lie down or sit in a reclined position during these breaks to allow the postural stabilizers of the neck to rest.
5Angela underwent ACDF surgery to C5-C6 8 weeks ago. She started outpatient physical therapy last week. Her chief complaint is decreased neck mobility. She also reports that she is experiencing a nagging pain in her right anterior superior iliac crest. This is where the graft for the cervical fusion was harvested. Angela wants to know if the hip pain is normal and whether it will resolve. How should the therapist respond?
At approximately 8 weeks after surgery, bone is undergoing a transformation from a soft callus to a hard callus. Although mineralization of bone may have been completed in the cervical region based on radiographs, the affected hip rarely undergoes a series of radiographs before outpatient physical therapy is initiated. Generally, no contraindications exist to physical therapy for hip pain. The harvest site may be tender for several months after the graft removal because of the trauma of surgery and bone-remodeling process, but the pain should gradually abate. Occasionally the lateral femoral cutaneous nerve may be affected by the graft harvest. If this is the case, then the patient will experience numbness or paresthesia down the lateral aspect of the thigh. Recovery of the nerve will depend on whether the nerve was cut during the surgery or simply compressed by inflammation. If it was excised, then recovery potential is poor. If it is simply compressed, then function of the nerve should return once the source of the compression is removed.
6Stacey is a 52-year-old woman who underwent ACDF 8 weeks ago. She has been attending physical therapy for the last 2 weeks but continues to complain that her neck motion is limited. Although she is not currently working, she is worried that she will not be able to resume her position as a bus driver because she is unable to turn her head to look for oncoming traffic. How should the therapist address this problem?
At 8 weeks after surgery, the patient can begin active cervical ROM exercises in all planes. Stacey should be instructed in how to perform these motions beginning in neutral cervical spine position. She should also be told to monitor her symptoms and only move her head until she feels the muscles stretching. Pain should be avoided when completing cervical ROM exercises. This may be added to the patient’s home exercise program, but she should be advised that if she experiences increased pain during or after the exercises, then she should stop them until she has an opportunity to talk with her physical therapist.
7Daniel is a 40-year-old man who underwent C5-6 ACDF surgery 10 weeks ago. He has made improvements with his active cervical ROM but is still unable to side bend much or rotate his head in either direction without moving his trunk. He lacks full shoulder elevation in the sagittal and coronal plane. He also notices difficulty and discomfort while driving slightly longer distances. What form of intervention should the therapist follow?
Daniel’s loss of ROM is normal after ACDF. His cervical rotation ROM will likely continue to improve given that C1-2 is a major source (accounting for up to 50%) of rotation. The therapist should keep in mind that AROM expectations for the cervical spine are less than full (65° to 70° of unilateral rotation versus 90°). The fact that he also has limits in shoulder elevation may indicate a thoracic spine mobility issue. Soft tissue mobilization techniques to the upper and midthoracic segments may relieve the tension on the shoulder and neck, allowing for increases in AROM. Improved posture should also follow, allowing a better distribution and absorption of forces while driving.
8Joe is a 49-year-old man who has been referred to the clinic by his physician 5 months after undergoing ACDF to C4-5 and C5-6. His primary impairments include numbness and tingling in his left UE, decreased cervical ROM, and poor cardiovascular function. After 1 month of physical therapy, Joe has increased his neck ROM and aerobic capacity but still complains of numbness and weakness in his left arm. He asks if the numbness will resolve. What should the therapist tell him?
Several categories of nerve injury are based on the amount of tissue damage occurring at the nerve. A neuropraxia is a local conduction block of the nerve. It usually occurs with compression injuries in which the nerve lumen is compressed and neural and chemical transition down the nerve axon is impaired. The axon and surrounding neurium tissue remains intact. Axonotmesis refers to a condition in which a loss of axon continuity occurs. The neurium tissue remains intact, but because of the loss of axon continuity, degeneration of distal nerve occurs. This condition can be the result of traction to the nerve or severe compression. Neurotmesis is the loss of axon continuity in which the neurium tissue is damaged. Similar degeneration of distal nerve occurs, as seen in axonotmesis; however, because no neurium tissue exists, the nerve has very little chance to heal. This type of injury generally occurs with injuries in which the nerve is severed. Recovery will depend on whether the nerve axon can regrow back to its distal muscular attachment before scar tissue infiltrates the region and blocks axon growth. When the axon and neurium are damaged, little potential exists for full recovery of the nerve. Therefore the therapist should advise the patient that after 6 months, resolution of numbness and weakness is unlikely to occur. Strength can continue to increase, but this is generally the result of muscle hypertrophy and not from innervation of muscle tissue.
9Robert is a 44-year-old man who underwent ACDF surgery 9 weeks ago. He reports that he has made significant gains in terms of overall neck pain and improvements in his original neural symptoms of burning pain in the right hand and forearm. However, since he has removed his collar over 3 weeks ago, he has complained of a “heavy” head and notes he feels “tiredness” in the back of his neck. He reports that he did not have these types of symptoms when he was wearing his collar and although he has been doing his deep neck flexor exercises, he is unsure if he is doing them correctly as he continues to have fatigue in the neck and “jerky” head movements. How should the therapist address his concerns?
The deep neck flexors have shown to be an important factor in maintaining spinal segment support. Fatigue of these muscles might cause an increase in activation of superficial neck muscles, which has the potential to overload painful cervical structures and affect cervical movement control. Furthermore, this can lead to a lack of confidence in the patient and result in increased muscle contraction to try and protect the movements of the cervical spine, which can lead to diminished active ranges of cervical motions. In this case, the therapist may want to reassess the patient’s ability to perform his deep neck flexor exercises and assess the patient’s cervical control by using the laser beam exercise described by Treleaven. In this case, the patient sits in a chair 90 cm away from the wall with a laser pointer mounted onto a lightweight headband. He tries to trace a pattern on the wall with the light beam. The therapist can make a subjective analysis of the accuracy and quality of movement, and if deficits exist, the therapist can use this technique as an intervention to improve joint position sense and cervical movement control.

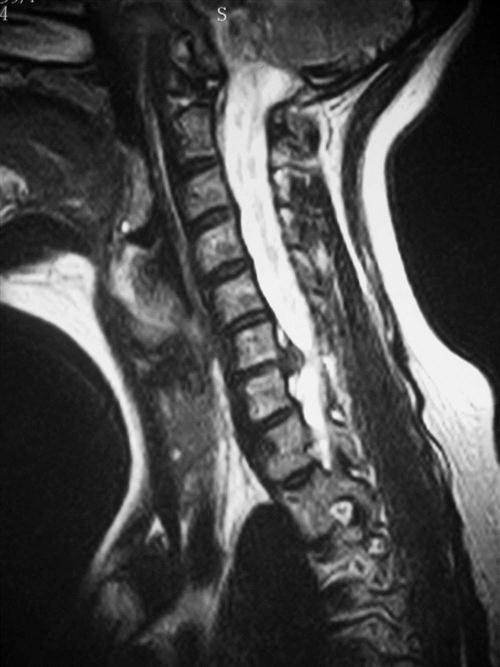
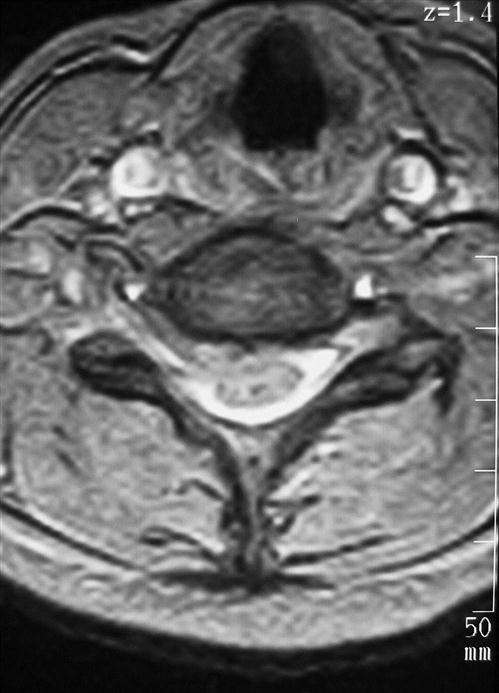
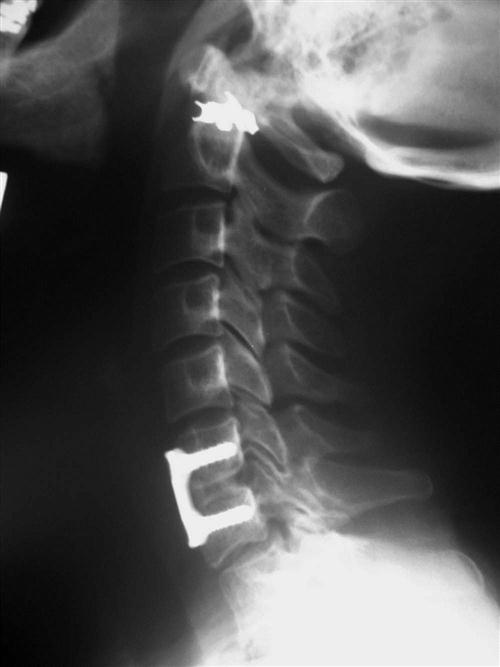
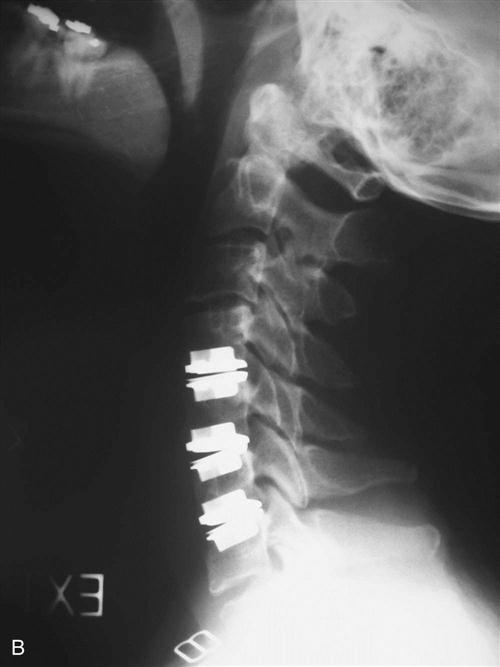
 gallon of milk.
gallon of milk.