Anterior Cruciate Ligament Reconstruction
Jim Magnusson, Richard Joreitz and Luga Podesta
Anterior cruciate ligament (ACL) injuries can occur at any stage of life from 5 to 85 years old.1–3 However, most often they occur in the relatively young active (athletic) population. The age group more commonly associated with ACL ruptures is between 14 and 29 years old.3–8 The extent of the injury and desired level of activity usually dictate when surgical intervention is required. This chapter describes the current surgical considerations, techniques, and rehabilitative guidelines with supportive rationale. The individual clinician must determine the speed and intensity appropriate for each patient.
Surgical Indications And Considerations
Cause and Epidemiologic Factors
ACL injury has been well documented and classically involves a noncontact mechanism involving rapid deceleration in anticipation of a change of direction (i.e., pivoting motion) or landing motion.9–13 Boden and colleagues14 reported that 72% of ACL tears occurred as a result of noncontact. Most injuries are sustained at foot strike, with the knee close to full extension and with the ground reaction forces lateral to the knee joint causing a “valgus collapse9,15”; sagittal plane motion seems to have less influence on the ACL during injury.9,16,17 The incidence of individuals sustaining a ruptured ACL has been reported at 1 in 3000.12
Patients describe feeling and sometimes hearing a “pop18” and are 1000 times more likely to be participating in a sporting event.4 Swelling is immediate, which implicates a ligamentous injury because of its associated vascularity. Patients exhibiting instability of the knee that affects pivot shift demonstrate a positive Lachman test; positive magnetic resonance imaging (MRI) for ACL rupture should be thoroughly evaluated for surgical considerations. Functionally, these patients have difficulty performing pivoting and deceleration related to activities of daily living (ADLs) or sports. Although individuals who have sustained isolated rupture of the ACL may continue to be functional, their level of function is compromised and may require future surgical intervention because of secondary restraint pathology.19–21 The surgeon should thoroughly evaluate the patient’s desired level of activity to ensure a successful outcome. Multiple studies have made reference to the sequelae of degenerative arthritis and potential for meniscal tears in the ACL-deficient knee.21–25
Both anatomic and physiologic risk factors have been researched. Some of the anatomic risk factors that may predispose an individual to ACL injury include the following: hypermobility (laxity of joints), hormonal influences on hypermobility, a narrow intercondylar notch, ligament width, tibial rotation, pronated feet, and increased width of the pelvis in the female athlete.26 Although some causes exist to suggest certain anatomic features, conclusive evidence has not been established between ligament failure and the anatomic risk factors. Physiologic risk factors include poor core strength, lower extremity (LE) deficits in muscular strength and coordination, and foot wear–ground interface. It may be a combination of the previously listed factors that leads to ACL injury, but women are two to eight times more likely to sustain injury than males.13,27–29 Hormonal influences that affect ligament laxity have been explored, with evidence leaning toward this as a nonfactor. However, menstrual hormones may indirectly contribute to injury by influencing neuromuscular performance and muscle function.29,30 Although there may be some influence on laxity, more compelling arguments point to strength and coordination differences. Many researchers have further studied the relationship of neuromuscular performance as a potential risk factor. They have identified significant differences in neuromuscular control after the onset of maturation. This deficit was observed in females landing after a jump. The neuromuscular deficit allowed migration of the knee into a valgus collapse position, placing the ACL at risk.9,30–32 Hewett, Myer, and Ford30also noted that after maturation (i.e., neuromuscular spurt) males regained their control; however, females did not make similar adaptations. The “drop jump” screening test is a useful examination to help prevent and further understand the mechanisms of an ACL injury.33 Leetun and associates34 looked at lumbopelvic (core) stability as a risk factor for LE injury in female athletes. They concluded that athletes who did not sustain an injury demonstrated better hip abduction and external rotation strength, and that hip external rotation strength was the only useful predictor of injury status.
![]() Overall, the therapist must be aware of the potential risk factors that were present leading up to the ACL injury. In this way, the rehabilitation program can safely return the patient to the sport and prevent future injury.
Overall, the therapist must be aware of the potential risk factors that were present leading up to the ACL injury. In this way, the rehabilitation program can safely return the patient to the sport and prevent future injury.
Treatment Options
The timing of when to perform reconstruction (acute versus chronic) has been a source of debate. It has been accepted that a higher risk for complications exists if surgery is performed (1) before obtaining a homeostatic environment, (2) if range of motion (ROM) is limited (especially extension), and (3) when quadriceps and hamstring contraction is inadequate (i.e., unable to perform a straight leg raise [SLR]).23,35 It is also apparent that with postponing reconstruction in an active population, the risk is higher for meniscal and chondral surface damage.22,36–38
A topic of debate is how soon after injury should reconstructive surgery be performed. When using a bone-patella tendon-bone (BPTB) autograft, evidence exists that surgery should not occur before 3 weeks after injury to decrease the risk of arthrofibrosis.23,39–41
Other authors propose that loss of motion is not dependent upon time when performing surgery after an injury.42–45
Bottoni and associates also showed through a randomized controlled trial that early ACL reconstructions with a hamstring autograft can be performed and will not increase the likelihood of arthrofibrosis because long rehabilitation emphasizes extension and early ROM.45 Sterett and associates did not find an association between incidence of motion loss and timing of surgery but used the minimal criteria of active ROM of 0° to 120°, active quadriceps control, and the ability to perform an SLR without a lag as determinants of successful outcome. In a systematic review, Smith and associates did not find a consensus for the optimal time after injury to perform reconstructive surgery to return to activity faster with limited complications.46,47
Typically, surgeons will require the patient to achieve full extension, be able to do an SLR without a lag, and have minimal to no swelling present before operating.
Researchers have speculated about an age when reconstruction is not recommended; however, to date no literature has noted any detrimental outcomes based on the age of the patient. In fact, studies have shown no significant difference in outcomes in comparing individuals at the age breaks of 35 and 40 years.7,48–50 Reconstruction of the skeletally immature (SI) patient remains controversial, but the current literature appears to be leaning toward performing reconstruction. Younger populations are sustaining ACL tears; although it has been generally advisable to await physeal closure before reconstruction, some surgeons are having successful outcomes.51,52 Appropriateness for reconstruction should be evaluated based on chronologic age, Tanner stage, radiologic findings in the knee, and developmental-psychologic factors.53,54 Drilling across the physis has not been advocated because of the risks of arresting bone growth. However, Shelbourne and colleagues51 presented information on a small group of SI patients (Tanner stage 3 or 4 with clearly open growth plates) who underwent intraarticular patella tendon graft. Surgery emphasized the importance of not overtensioning the graft and meticulous placement of the bone plugs proximal to the physes. The patients had no growth disturbances on follow-up; when confronted with the potential of new meniscal tears, recurrent instability, effusion, and pain, ACL reconstruction in the SI patient appears to be a viable option.26,55
The anticipated functional limitations (modification of activities involving pivoting and deceleration) must be explored and explained to the patient who chooses not to have an ACL-deficient knee reconstructed. Ciccotti and associates56 reported on nonoperative management of patients from 40 to 60 years. They found that 83% of the patients had a satisfactory result with guided rehabilitation. However, they also mentioned that surgery might be an option for individuals wishing to continue sporting and pivoting activities.
Surgical techniques to replace the deficient ACL continue to evolve. Advances in arthroscopic surgery provide surgeons with the ability to perform these reconstructive procedures using a one-incision endoscopic technique. Research continues in the search for the optimal graft, fixation technique, and surgical reconstructive procedure. In 1920, Hey-Groves57 and Campbell58 (in 1939) first described the use of the patella tendon as an ACL graft. Because of these original surgical descriptions, numerous procedures to repair or reconstruct the ACL have been advocated. Attempts at primary repair of the ACL with and without augmentation59–61 were of limited success.37 Extraarticular ACL reconstruction also was suggested as a technique to reconstruct the ACL-deficient knee.62,63 However, long-term results were disappointing.64,65 Intraarticular ACL reconstruction using various tissues, including the patellar tendon, iliotibial band, and combinations of hamstring tendons (semitendinosus, semitendinosus-gracilis), has been extensively described in the literature.20,66–69
The biologic grafts most widely used today are the central third patellar tendon (i.e., BPTB complex) or multistrand hamstring tendon grafts. Although the hamstring graft has some advantages,70,71 both procedures are equally successful (surgeon preference dictates choice if problems such as patella dysfunction are not present).72–75
In general the endoscopic patellar tendon autograft reconstruction remains the most popular.10,76–78
Graft Selection
The selection of the appropriate graft to replace the ACL is crucial to the ultimate success of the reconstruction. Primary concerns in the selection of an autogenous graft to replace the incompetent ACL include the biomechanical properties of the graft (e.g., initial graft strength and stiffness relative to the normal ACL), ease of graft harvest and fixation, potential for donor-site morbidity, and individual patient concerns. Other factors that ultimately influence graft performance include biologic changes in graft materials over time and their ability to withstand the effects of repetitive loading and stress.79 Noyes and colleagues80 studied the biomechanical properties of a number of autograft tissues and showed that an isolated 14-mm-wide BPTB graft has 168% the strength of an intact ACL. A graft 10 mm wide is about 120% as strong. The study also determined that a single-strand semitendinosus graft displayed only 70% of the normal ACL strength. The data show that BPTB grafts have comparable tensile strength but increased stiffness in relation to the normal ACL, whereas single-strand semitendinosus grafts have decreased tensile strength but comparable stiffness. Other researchers have shown that multiple strands of semitendinosus or semitendinosus-gracilis composite grafts are stronger relative to the normal ACL.
The graft of choice varies among surgeons. They currently include BPTB autografts and allografts; single-, double-, and quadruple-stranded semitendinosus autografts; and composite grafts using semitendinosus-gracilis autografts. The enthusiasm surrounding the use of allograft replacement of the ACL has recently declined because of the small but tangible risk of infectious disease transmission. The risk of human immunodeficiency virus transmission has been estimated to be 1 in 1.6 million using currently available bone- and tissue-banking techniques.81 Sterilization by means of fresh freezing of allograft tissue may have an advantage over gamma radiation and ethylene oxide. Fielder and associates82 have determined that 3 mrads or more of gamma radiation are required to sterilize HIV. Furthermore, sterilization procedures have been associated with alterations in graft properties and shown to cause a significant average decrease in stiffness (12%) and maximal load (26%),83 and a marked inflammatory response with ethylene oxide use. Further studies must be conducted regarding poststerilization ACL allograft performance. Although the use of allografts as ACL replacements can diminish operative time and prevent graft harvest site morbidity, they are not recommended for routine use in primary ACL deficiency. Currently, either BPTB or multistrand semitendinosus autografts are the most widely used ACL substitutes to reconstruct the ACL-deficient knee.
Graft Fixation
Adequate fixation of the biologic ACL graft is crucial during the early postoperative period after ACL reconstruction. Fixation devices must transfer forces from the fixation device to the graft and provide stability under repetitive loads and sudden traumatic loads. Various techniques are now available for fixation, including interference screws, staples, sutures through buttons, sutures tied over screw posts, and ligament and plate washers. Kurosaka, Yoshiyas, and Andrish84 determined the interference screw to be the strongest method of fixation of BPTB grafts. Interference screw strength depends on compression of the bone plug,79 bone quality,79,84 length of screw thread-bone contact,85 and direction of ligament forces.79 Robertson, Daniel, and Biden86 studied soft tissue fixation to bone and determined the screw with washer and the barbed staple to be the strongest methods of fixation.
Graft Maturation
Graft maturation has an influence on the patient whose goals include a return to sports, most of which require pivoting and cutting. The healing properties of autografts have been discussed in the literature.14,87–90 Although a majority of the studies we have reviewed describe the maturity of the graft at 100% 12 to 16 months postoperatively, return to sports participation in some protocols occurs at 6 months (if functional tests and isokinetics meet criteria).91,92
The graft maturation process begins at implantation and progresses over the next 1 to 2 years. Autografts are strongest at the time of implantation. The implanted graft undergoes a process of functional adaptation (ligamentization), with gradual biologic transformation. The tendon graft undergoes four distinct stages of maturation14,87,89:
1 Necrosis
Within the first 3 weeks after implantation, necrosis occurs in the patella tendon intrinsic graft cells. The graft consists of a collagen network that to this point has relied on a blood supply. As this blood supply is interrupted, the graft undergoes a necrotizing process. Necrosis commences immediately and generally lasts 2 weeks.88–90 Native patella tendon (graft) cells diminish, and replacement cells can be present as early as the first week. Cellular repopulation occurs before revascularization. These cells are thought to arise from both extrinsic sources (i.e., synovial cells, mesenchymal stem cells, bone marrow, blood, ACL stump) and intrinsic sources (i.e., surviving graft cells). Early full ROM is desirable because as new collagen is formed, its formation and strength are dictated by the stresses placed on it.
As the new cells find their way to this frame and add stability to this weak structure, rehabilitation must be careful not to disrupt or stretch them. Necrosis of the graft allows the metamorphosis of the graft from tendon to ligamentous process. Necrosis of the graft is highlighted by the formation of granulation tissue and inflammation. The bone blood supply and synovial fluid nourish the graft by synovial diffusion.93 Revascularization occurs within the first 6 to 8 weeks after implantation. By this time the graft is revascularized via the fat pads, synovium, and endosteum,88–90 and the inflammatory response should be under control. Further inflammatory problems signify a delayed healing process and potential graft problems; the physician and therapist should be alert for them.94,95
Amiel and colleagues93 in 1986 described ligamentization of the rabbit patella tendon ACL graft. However, the graft never obtained all the cellular features of normal ACL tissue. Although the graft takes on many of the physical properties of the normal ACL, the cellular microgeometry of the remodeling graft does not closely resemble that of a normal ACL. The revascularization process progresses from peripheral to central.
Bone plugs incorporate into their respective bone tunnels over a 12-week period but are felt to near completion by approximately the sixth postoperative week. The comparative strength of the healed tendon-to-bone attachment versus the healed bone-plug attachment is unknown. Tendon-bone healing begins as a fibrovascular interface develops between the bone and tendon. Bony ingrowth occurs into these interfaces, which extends into the outer tendon tissue. A gradual reestablishment of collagen fiber continuity between bone and tendon occurs, and the attachment strength increases as collagen fiber continuity increases. These ACL autografts approximate 30% to 50% of the normal ACL strength 1 to 2 years postoperatively.89
Cellular proliferation and collagen formation take place as a continuing process throughout the maturation process. The function of collagen in the ligament is to withstand tension, and certain types of catalysts are present during the healing process. Transforming growth hormone factor b1 has been isolated during the healing of the medial collateral ligament in rats. Administration of this growth hormone during the first 2 weeks after injury was found to increase strength, stiffness, and braking energy of the ligament.90 Other catalysts of collagen formation (platelet-derived growth factor 1 [basic fibroblast growth factor]) have had equally good results in improving the tensile strength of healing ligaments. Since our last edition, more human studies are being presented with varying degrees of success.96 With the relatively recent expansion of platelet rich plasma injections to assist in soft tissue repair, more research is coming out on its use in anterior cruciate ligament reconstruction (ACLR). Recent studies have looked at the use of platelet rich growth factor in assisting the reconstruction.97 Future studies should be performed to validate this intervention.
During the rehabilitation program, pain and edema should dictate the speed at which the patient may progress. In clinics in which it is available, an assessment using the KT-1000 (Medmetric, San Diego) is helpful as well.4,23,98–102
Surgical Procedure
Endoscopic Bone-Patella Tendon-Bone Complex Anterior Cruciate Ligament Reconstruction
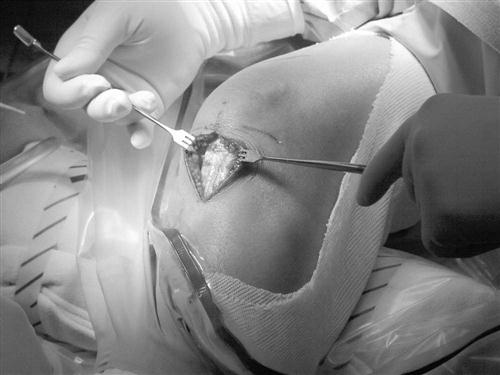
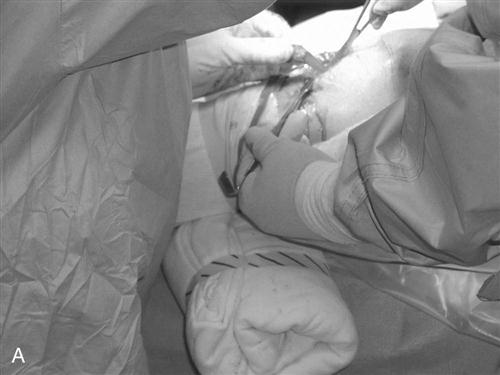
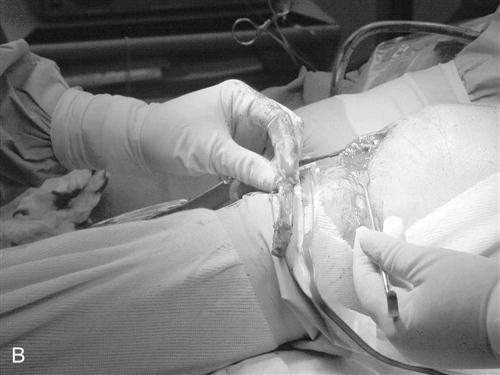
The procedure begins with a complete examination of the knee under anesthesia followed by a thorough diagnostic arthroscopic evaluation. The menisci, joint surfaces, and ligamentous structures are evaluated and additional injuries assessed arthroscopically. The leg is then exsanguinated, and a tourniquet is inflated with 350 mm of pressure. A medial parapatellar incision is made from the inferior pole of the patella to the tibial tuberosity. The skin is dissected down to the peritenon, and skin flaps are made superiorly, inferiorly, medially, and laterally. The peritenon is incised and the patella tendon is exposed. The width of the patellar tendon is noted (Fig. 22-1), and a 10-mm graft is measured from the midpatellar tendon. Two small incisions 10 mm apart are made in the patellar tendon and then extended superiorly and inferiorly with a hemostat. The patellar and tibial bone plugs are measured to provide graft lengths of 20 to 25 mm of patella and 25 to 30 mm of tibial bone. To facilitate bone graft harvest, the corners of the bone plugs are predrilled with a 2-mm drill to decrease stress risers. The perimeters of the bone plugs are then sawed out with a reciprocating saw to a depth of 10 to 11 mm, depending on the size of the patella and tibial tubercle. The graft (Fig. 22-2, A and B) is then taken to the back table, where it is prepared and fashioned to allow passage through the appropriate guides. The surgeon completes the graft by placing one No. 5 Tycron suture in the femoral and three No. 5 Tycron sutures into the tibial bone plugs to facilitate graft passage through the knee. The graft is preserved in a saline-moistened gauze sponge for later use.
The remnant of the ACL is resected, along with any hypertrophic tissue. Arthroscopically, the intercondylar notch is then prepared with the aid of a burr to prevent graft impingement. A site is chosen for placement of the tibial tunnel. Through the midline incision, a small area medial to the tibial tubercle is prepared with subperiosteal elevation. Using a tibial guide and under direct visualization, the surgeon drills a guide pin into the knee from the outside in, exiting within the knee at a site chosen anteromedial to the ACL insertion. The tibial tunnel is reamed to the size of the harvested graft. A curette placed over the guide pin during reaming helps protect the articular cartilage and posterior cruciate ligament from damage. The tibial tunnel must be larger than the femoral tunnel to allow passage of the graft into the knee. The tibial tunnel edges are smoothed with a rasp to prevent graft abrasion after implantation. A fenestrated plug is then placed into the tibial tunnel to prevent fluid extravasation yet allow passage of instruments.
The femoral isometric point is determined on the medial aspect of the lateral femoral condyle, usually 3 to 5 mm anterior to the posterior cortex near the superior intercondylar notch margin (over-the-top position); it is marked with a curette or burr. With the knee flexed past 90°, a fenestrated guide pin is inserted into the knee through the tibial tunnel and drilled through the femoral isometric point and out through the skin with the aid of an over-the-top guide. The femoral tunnel is then reamed to the size of the femoral bone plug to a depth of 30 mm.
The sutures from the femoral bone plug are inserted into the femoral pin and pulled out through the skin. The graft is delivered into the knee, through the tibial tunnel, and into the femoral tunnel under direct visualization. A cannulated interference screw is then inserted into the knee over a nidal guide pin and screwed into the femoral tunnel, compressing the femoral bone plug within the tunnel. Graft isometry is evaluated. The tibial bone plug within the tibial tunnel is secured with interference screw fixation. ROM and stability testing are then performed. The graft is evaluated arthroscopically to assess graft excursion and placement within the intercondylar notch.
The tourniquet is released, hemostasis is obtained, and the knee is irrigated. Loose closure of the patellar tendon is performed with the peritenon approximated to close the anterior defect. The subcutaneous tissue is approximated, and a continuous subcuticular skin closure is performed. The wounds are dressed sterilely. A light compressive wrap and continuous ice water cryotherapy system are applied, and the patient is taken to the recovery room with the knee in a knee immobilizer in full extension.
Physical Therapy Guidelines For Rehabilitation
Rehabilitation following ACL reconstruction has dramatically changed over the past 20 years. While the gold standard of graft choice still remains a BPTB autograft, previous rehabilitation protocols were tailored for this surgery.89,91,94,102–119
Physical therapy therefore must adapt its rehab protocols and tailor them to the individual patient based on graft choice and concomitant injuries and/or surgery. Regardless of the surgical procedure, the rehab protocol must be based on biologic healing. This section will discuss preoperative management following injury, including decision making for conservative management, and postoperative management from the acute inflammatory phase to return to activity.
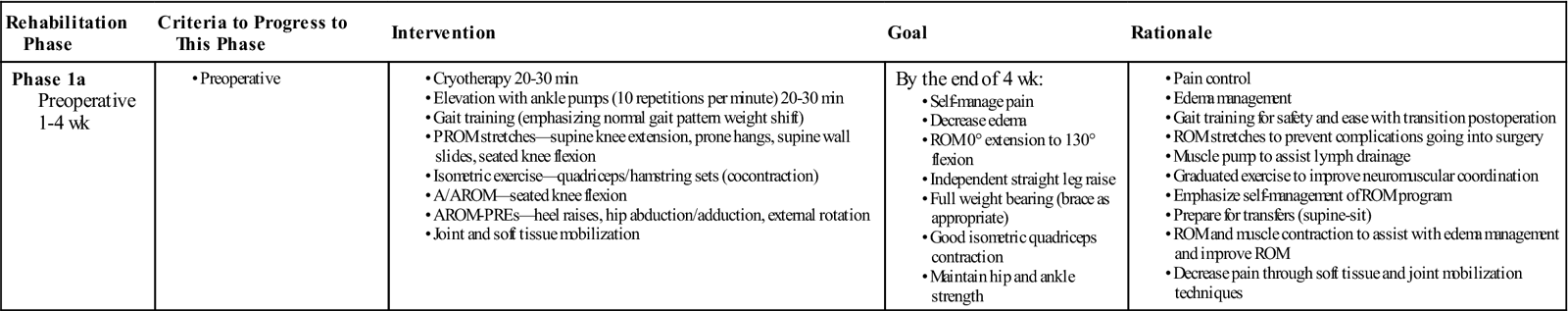
Preoperative Management (Table 22-1)
TABLE 22-1
Preoperative Anterior Cruciate Ligament Reconstruction
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Intervention | Goal | Rationale |
| Phase 1a Preoperative 1-4 wk |
|
By the end of 4 wk: |
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Regardless of when surgery is scheduled, the patient almost always is evaluated and receives treatment to increase ROM, especially extension, increase quadriceps/hamstrings strength, and achieve a normal gait pattern. The evaluation commonly begins with an assessment of gait when the patient enters the premises. The patient will often exhibit a flexed-knee gait or a quadriceps avoidance pattern.120 The patient will commonly have a rehabilitation brace locked in extension and will use two crutches. While the brace is thought to limit ROM and varus-valgus forces to the knee,121 the evidence is inconclusive that braces improve extension, and decrease pain and graft strain following ACL reconstruction.122 Clinically speaking, the brace is used preoperatively and postoperatively to limit external forces that may cause further damage to the knee. For example a patient before having ACL reconstruction may fall and tear their meniscus or have some osteochondral damage. Active and passive knee ROM, patella mobility, presence of edema, hamstring and gastrocnemius flexibility, quadriceps strength, and weight-bearing capacity should be assessed. During the preoperative phase, the patient’s whole kinetic chain should be evaluated. Strength, flexibility, and mobility of the foot, ankle, hip, and core should be assessed. Particular attention should be given to the mechanism of injury to start planning prevention strategies for postoperative rehabilitation. Assessing the entire kinetic chain before the surgery is easier and more comfortable for the patient than after surgery because of the amount of pain and how inflamed the knee will be. Exercises and modalities should be used to decrease inflammation and swelling, restore patellofemoral mobility and increase quadriceps strength as well as global LE strength and flexibility.
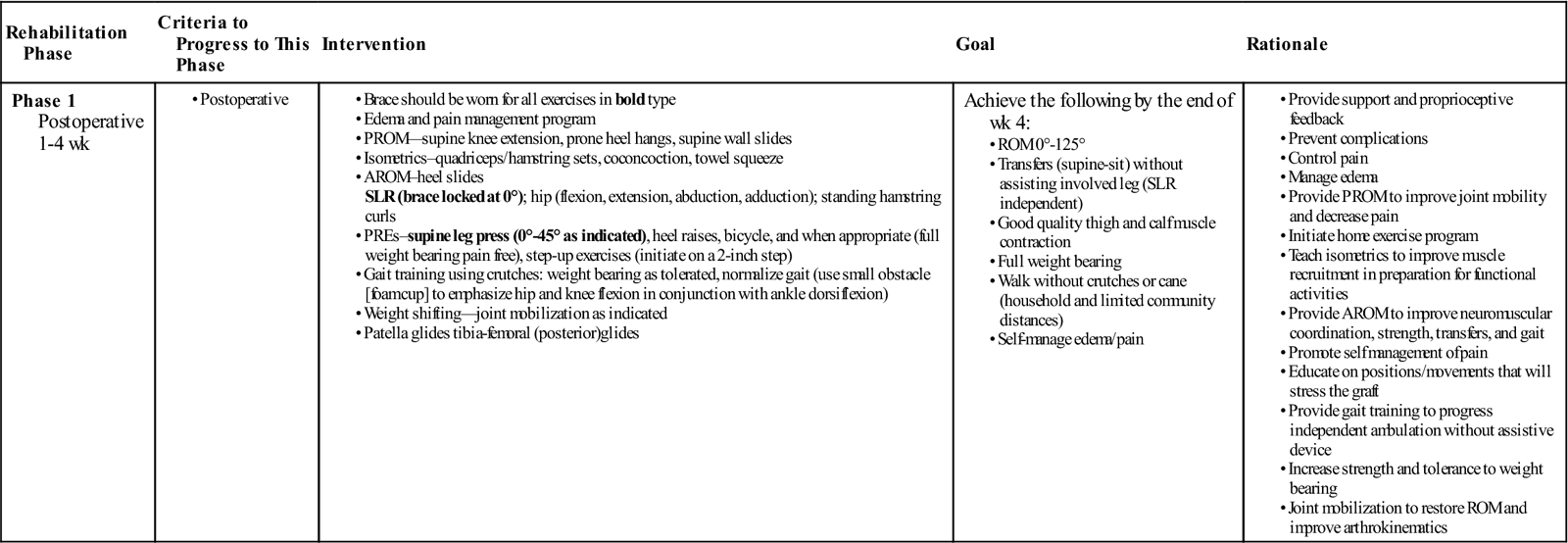
Phase 1: 0 to 4 Weeks (Table 22-2)
TABLE 22-2
Phase 1 Anterior Cruciate Ligament Reconstruction
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Intervention | Goal | Rationale |
| Phase 1 Postoperative 1-4 wk |
Achieve the following by the end of wk 4: |
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Rehabilitation following ACL reconstruction can be broken down into phases. Phase 1 begins immediately after surgery and lasts 4 weeks. In this phase, emphasis is placed on decreasing pain and inflammation, protecting the healing graft, and restoring strength and ROM. While inflammation after surgery is normal, the swelling and subsequent pain must be reduced as soon as possible. Swelling can increase pain and quadriceps muscle inhibition.123,124
Hopkins and associates showed that transcutaneous electric neuromuscular stimulation can be used to control pain and edema.125 Jarit and associates showed that home interferential current therapy can help to reduce pain and swelling, and increase ROM following knee surgery.126 However, the time parameters for transcutaneous electric neuromuscular stimulation125 was 30 min/day and interferential current therapy treatment126 was 3 sessions per day for 28 minutes per session, which may not be feasible for both the patient and treating physical therapist. Cryotherapy, whether in the form of continuous flow cold therapy, crushed ice, or commercial cold gel packs, is effective at reducing secondary hypoxia, pain, and edema.125,127 When available, continuous flow cold therapy should be used over crushed ice.128,129 Elevation with muscle pumping (ankle pumps, quad sets) can help the lymph system remove tissue debris and inflammatory byproducts (free-floating proteins too large to filter through the capillaries).127 Cryotherapy with compression and elevation should occur after each treatment session, as well as up to 5 times daily for 20 minutes when pain, inflammation, and swelling are present. Girth measurements should be taken at the midpatella, as well as proximally and distally, to monitor progress of swelling reduction.
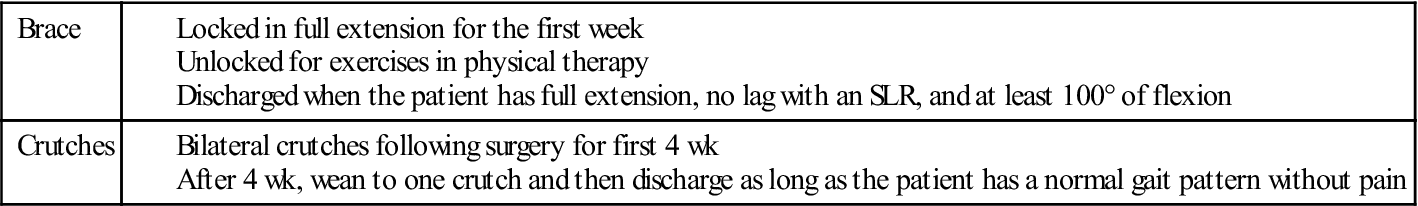
Patients will typically have two crutches and a postoperative brace locked in extension for the first week following surgery. After 1 week, the brace can be unlocked for exercise and gait. If the patient demonstrates a normal pain-free gait pattern, they may wean from two to one crutch, and then discharge them entirely. The brace will typically be discharged once the patient has approximately 100° of flexion, is able to do an SLR without a lag, and has a normal pain-free gait cycle. This process usually occurs 4 to 6 weeks after surgery. Table 22-3 shows commonly used guidelines for using and discharging the brace and crutches.
TABLE 22-3
Guidelines for Using and Discharging Brace and Crutches
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
As previously stated, there should be an emphasis on early ROM following surgery. Full passive knee extension should be achieved within the first week to decrease abnormal joint arthrokinematics and prevent arthrofibrosis.130,131 Bracing in extension22,132 or hyperextension133 can be used as a means to prevent flexion contractures. Patellar mobilizations, especially superiorly and inferiorly, should be applied to regain full mobility. Patellar immobility could result in ROM complications and difficulty recruiting quadriceps contraction.4,92,100,134–145
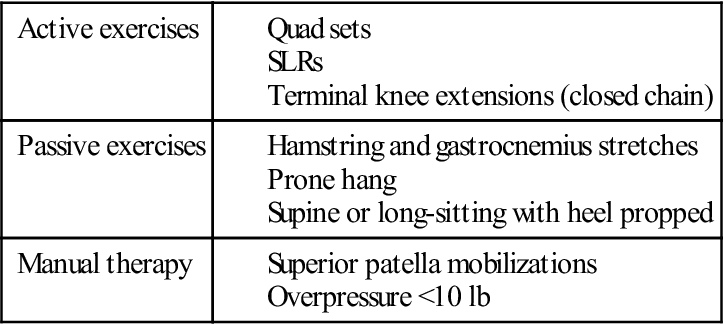
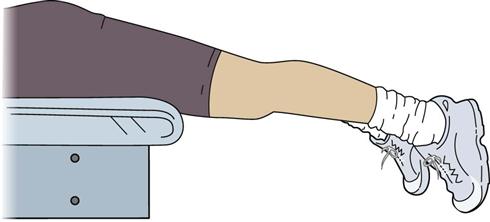
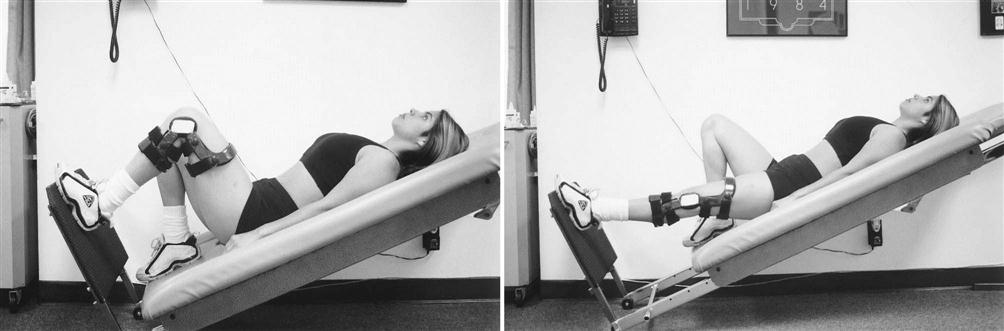
Exercises to achieve full extension include, but are not limited to, hamstring and gastrocnemius stretching, quad sets with the heel propped under a wedge, superior patella mobilizations, a prone or supine (Figs. 22-3 and 22-4) hang, and overpressure of up to 10 lb. Remember that the ACL prevents anterior tibial translation and posterior femoral translation. With joint mobilizations, take caution with overpressure to avoid pushing the joint in the direction that the healing ACL graft limits (Fig. 22-5). The heel should also be propped up when icing and/or resting at home. You must educate the patient to avoid putting a pillow under the knee when resting at home so that the patient does not develop a flexion contracture. Tables 22-4 and 22-5 show examples of commonly used exercises to increase ROM.
TABLE 22-4
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
TABLE 22-5
< ?comst?>
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
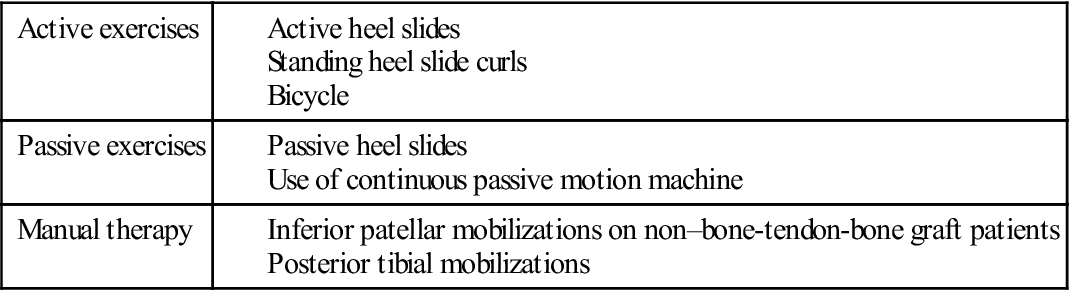
Within the first 2 weeks following isolated ACL reconstruction, the patient should achieve 100° to 120° of flexion. If a concomitant meniscal repair is performed, the patient will be limited to 90° flexion for the first 4 to 6 weeks following surgery. Common causes of decreased flexion include arthrofibrosis, patella immobility (particularly with the inferior glide), posterior capsular hypomobility, decreased quadriceps flexibility, and excessive swelling. Exercises to increase knee flexion include active and active-assisted heel slides, posterior tibial and inferior patella mobilizations (in non–bone-tendon-bone autograft graft patients), and pedaling on a stationary bicycle. It is difficult to stretch the quadriceps when there is decreased knee flexion. However, the physical therapist can stretch the patient in the side-lying position with the knee flexed as much as tolerated and stretching into more hip extension. Despite conflicting evidence of long-term benefits,146–148 the use of a continuous passive motion device may also be used at home for 4 hours per day until the patient reaches 120° flexion.
Increasing quadriceps strength is another goal of phase 1. A hallmark in the early rehabilitation process is the ability to perform an SLR without a lag. To achieve full active extension, the patient must also have full passive extension and adequate superior patella mobility. As mentioned previously, there should be minimal pain and swelling to decrease quadriceps inhibition.55,123,124,127
Early initiation of quadriceps strengthening has been shown to increase ROM and quadriceps muscle torque safely.149
Debate exists on the most appropriate type of strengthening. Closed kinetic chain (CKC) exercises are more functional and were previously thought to be safest for the healing graft.92,150–155
CKC exercises can begin with isometrics (Fig. 22-6) and progress to include minisquats and the leg press in the range of 0° to 45° to minimize patellofemoral joint stress (Fig. 22-7).47,156 However, it has been shown that controlled open kinetic chain (OKC) quadriceps strengthening is both safe and advantageous following ACL surgery.157–161 These authors157–161 reported that active OKC knee extension without resistance did not strain the ACL from 90° to 40° of flexion. Mikkelsen and associates159,162 showed that it was safe to add OKC knee extension exercises from the sixth week postsurgery in the range of 90° to 40°, and progressing to 90° to 10° by the twelfth week. Steinkamp and associates156 showed that OKC knee extension can be performed with minimal patellofemoral joint stress from the range of 90° to 45°. Heijne 163 began OKC knee extension exercises at 4 weeks and 12 weeks for both hamstring tendon and patellar tendon grafts. Quadriceps muscle torque was not significantly different for any group. But the early addition of OKC exercises for patients receiving hamstring tendon autografts resulted in significant increased laxity over time. They therefore concluded that further studies are needed to determine the optimal time to add OKC quadriceps strengthening following ACL reconstruction with a hamstring graft. Because of the potential increased strain to the graft with OKC exercises in low levels of flexion (<30°), isometric quadriceps exercises should be done at 0°, 90°, and 60°. Emphasis should be on achieving a superior patellar glide with each quad set, and an inferior patellar glide when they relax. OKC knee extension should be in the range of 90° to 40° in the early weeks following ACL reconstruction. If a hamstring graft is used, the patient should wait until after the fourth week. If a lag is present, an SLR will cause strain to the healing graft because the quadriceps is active with the knee in low levels of flexion. Therefore, the patient should wear their postoperative brace with it locked in extension for the exercise until the lag is gone. When performing SLRs, the patient should actively do a quad set with each repetition to increase neuromuscular control.
Even in the acute phase, the entire kinetic chain can be strengthened. OKC hamstring curls and/or CKC hamstring sets and bridges may be used to strengthen the hamstrings. Caution should be given, if a hamstring autograft was used, when strengthening the hamstrings. If, for example, the patient receives a concomitant meniscal repair, weight-bearing and/or hamstring strengthening exercises may be delayed for 4 to 6 weeks. Four-way SLRs hip external rotation and abduction can be used to strengthen the hip. Weight-bearing heel and toe raises or use of a resistance band or cuff weight can be used to strengthen the ankle. Weight shifting side-to-side and front-to-back should be employed as the precursor to gait training.
Neuromuscular electrical stimulation (NMES) should be used to increase quadriceps strength and improve gait164 following ACL reconstruction. Their protocol used a 2500-Hz alternating current, with intensities that induced at least 50% of maximum voluntary isometric torque measured by a dynamometer positioned at 60° to 85° of flexion. The contraction time was for 10 seconds followed by a 50-second rest, for a duration of 10 to 15 contractions. Their results demonstrated a greater increase in quadriceps strength than voluntary exercise alone.164–166
Fitzgerald and associates167 modified the protocol described by Snyder-Mackler and associates in which the patient receives the NMES with the knee in full extension. The NMES and exercise group demonstrated moderately greater quadriceps strength at 12 weeks and moderately higher levels of self-reported knee function at both 12 and 16 weeks postsurgery compared with the exercise only group. If a dynamometer is available, the physical therapist should apply the parameter described by Snyder-Mackler and associates.164,166 However, Fitzgerald and associates demonstrated positive gains with his modified protocol.167
Some patients are given portable NMES units to use at home in addition to exercises, depending on whether they are unable to receive formal physical therapy twice per week or are having difficulty achieving adequate volitional quadriceps strength. The Empi 300PV (Empi, St. Paul, Minn.) has been shown to produce comparable levels of average peak torque for quadriceps strengthening.44
Because of the limited number of physical therapy visits paid for by insurance, the patient may only be treated once per week for the first 4 to 6 weeks. It is therefore imperative that the patient be educated on the importance of doing his or her home exercise program. It is the job of the treating physical therapist to provide the patient with a detailed yet easily understood list of exercises to perform, teach the patient the proper form for each exercise, and maximize time spent in each physical therapy appointment.
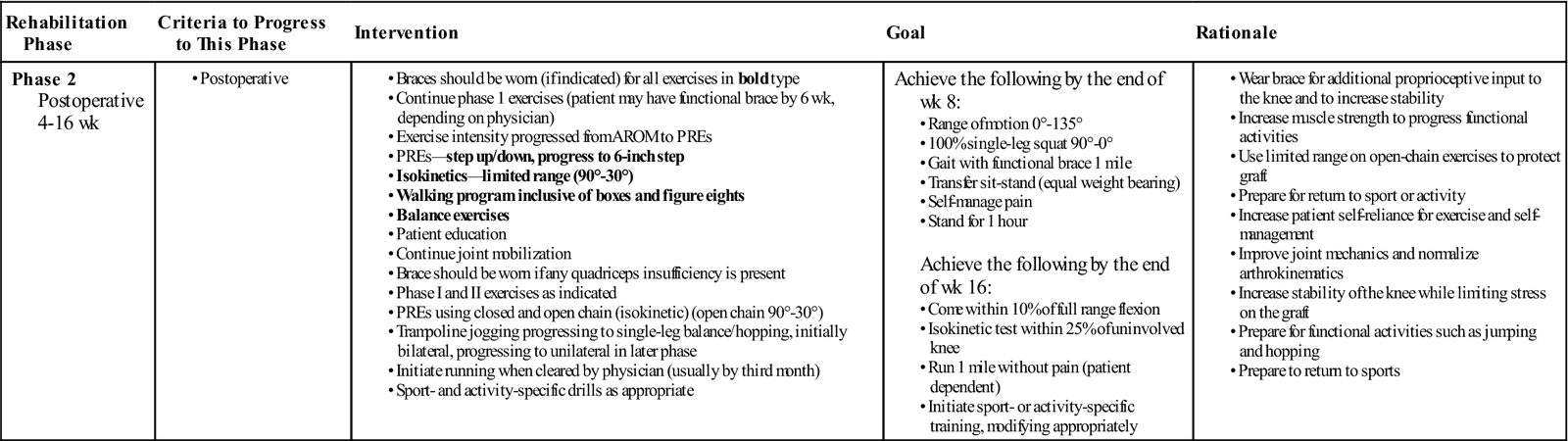
Phase 2: 4 to 16 Weeks (Table 22-6)
TABLE 22-6
Phase 2 Anterior Cruciate Ligament Reconstruction
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to This Phase | Intervention | Goal | Rationale |
| Phase 2 Postoperative 4-16 wk |
|
Achieve the following by the end of wk 8: Achieve the following by the end of wk 16: |
|
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
AROM, Active range of motion; PREs, progressive resistance exercises.
Phase 2 begins when the patient has full passive ROM and normalized pain-free independent gait and lasts until the patient begins to run (if and when appropriate).
In phase 2, the primary focus of rehabilitation is to increase strength and neuromuscular control in preparation for dynamic tasks and be independent with ADLs.
Strength training should follow the principle of progressive overload, which means to gain strength, the muscles must be gradually loaded beyond the point to which they are normally loaded.168 However the clinician must keep in mind that the reconstructed joint (mechanics), patellofemoral control, and joint surfaces may be the limiting factors for progressing exercise intensity.
Ideally, a one repetition maximum (RM) can be found for each exercise, and exercise prescription can be based on that. Initially, strength training in the early phases after surgery will start with 1 to 3 sets of 8 to 12 repetitions with 70% to 80% of 1 RM. Once this initial goal is met, muscle endurance should be progressed, consisting of 3 to 5 sets of 15 to 25 repetitions with 50% to 70% of 1 RM.
To build muscular power, exercise prescription should change to 1 to 3 sets of 3 to 6 repetitions (with >80% of 1 RM) but also performed at a fast velocity (usually performed during phase 3).169 Exercises should be both concentric and eccentric, and isolate specific muscles as well as combine the entire kinetic chain. However, it is still important to remember to protect the healing graft by keeping exercises in the protected ranges. Approximately 6 to 8 weeks after surgery, the mechanical strength of the healing graft is at its weakest.170 However, during that time, bony plugs from BPTB grafts heal within the tibial and femoral tunnels.171 It is not until 8 to 12 weeks after surgery that the soft tissue to bone healing within the tunnels occurs with hamstring grafts.172
Recently, Gerber and associates173 demonstrated increased quadriceps and gluteus maximus strength after a 12-week eccentrically focused resistance training program at 1 year following ACL reconstruction compared with standard rehabilitation. Lately, neuromuscular training protocols are being integrated into the rehabilitation process. Neuromuscular training is such that enhances unconscious motor responses by stimulating both afferent signals and central mechanisms responsible for dynamic joint control. The goal is to induce compensatory changes in muscle activation patterns.174
Previous studies show positive results following ACL injury.93,175–177 These programs consist of balance exercises, dynamic joint stability exercises, plyometric exercises, agility drills, and sport-specific exercises. Risberg and associates178 showed that a 6-month neuromuscular training program versus a traditional strength training program resulted in significantly improved Cincinnati Knee Scores and visual analog scales for pain and function. Risberg and associates179 also showed that at a 2-year follow-up, there were no significant differences between a neuromuscular exercise training protocol and a traditional strength training protocol for the Cincinnati knee score but there was significantly improved knee function and reduced pain. It is the opinion of the authors of this chapter that rehabilitation programs after ACL reconstruction should include traditional strength training, both concentrically and eccentrically focused, as well as neuromuscular training.
Balance exercises can begin as soon as the patient is comfortable with weight bearing. They can begin with forward and lateral weight-shifts and transition to tandem standing. Single-leg stance exercises can be performed in a variety of ways: on the floor, on unstable surfaces such as foam, a trampoline, a tilt-board, and rocker-board, and with the eyes open or closed.180,181
Dynamic single-leg stance exercises can include doing another task while balancing, such as throwing and catching a ball or reaching in multiple directions like a “star.182”
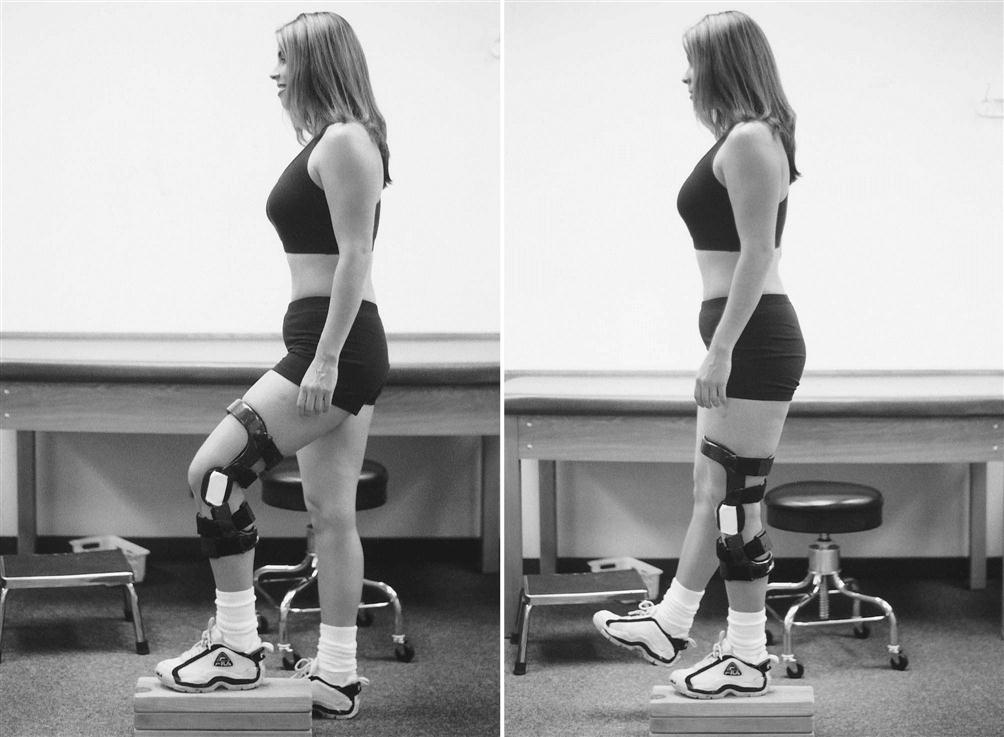
Isolated quadriceps strengthening should progress from simple quad sets and SLRs to knee extensions in the protected ranges. Quadriceps strengthening that incorporates the entire kinetic chain should include double- and single-leg squats, forward and lateral step-ups, wall squats and lunges in multiple planes (Figs. 22-8 through 22-12). Global hip strengthening should progress from SLRs on the table to use of a machine, or sidestepping and diagonal stepping with a resistance band. Hamstring strengthening should progress from prone or standing curls with cuff weights to use of a machine, Romanian deadlifts, and curls with a Physioball while doing a bridge. Core strengthening should include double- and single-leg bridges, prone and side planks, and the chop and lift183 in a lunge position. By the ninth week postoperation, patients should have close to full ROM, be able to perform a unilateral squat with 100% body weight (0° to 90°), walk up to 1 mile, tolerate standing for at least 1 hour, and demonstrate independence in self-management of exercises. The hallmark of this phase is progression from being a functionally independent person with ADLs to initiating exercises/activities in preparation of returning to the previous level of physical activity (Return to running, hiking, and sport-specific activities which will be progressed in phase 3).
We suggest a simple progression for the return to running based on activity or sport need and allowing a rest day between runs (as shown in Box 22-1). For a more complete return to a running program refer to Chapter 34.
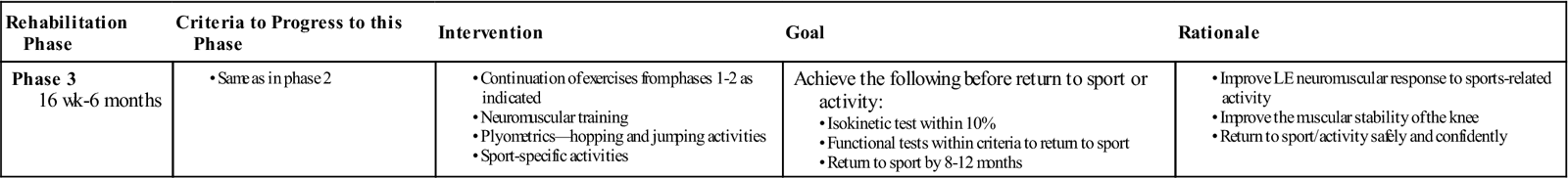
Phase 3: 16 Weeks to 6 Months (Table 22-7)
TABLE 22-7
Phase 3 Anterior Cruciate Ligament Reconstruction
< ?comst?>
| Rehabilitation Phase | Criteria to Progress to this Phase | Intervention | Goal | Rationale |
| Phase 3 16 wk-6 months |
Achieve the following before return to sport or activity: |
< ?comen?>< ?comst1?>
< ?comst1?>
< ?comen1?>
Phase 3 begins when the patient is cleared to run, but there is no consensus about when to start following ACL reconstruction. During phase 3, the patient should progress all exercises to increase LE flexibility and muscular strength, power, and endurance. Commonly, the surgeon will wait between 3 to 6 months following surgery to clear the patient for running based on graft healing and concomitant surgeries.142,170,172,184
Few studies are available that give criteria to begin running following ACL reconstruction. Myer and associates,185 however, used the criteria of: (1) minimum Internation Knee Documentation Committee subjective knee form score of 70, (2) either no postsurgical history of giving way or a negative pivot shift, and (3) a minimum baseline strength knee extension peak torque/body mass of at least 40% (male) and 30% (female) at 300°/sec, and 60% (male) and 50% (female) at 180°/sec. A major obstacle to using their guideline can be if the treating physical therapist does not have access to a dynamometer. The authors of this chapter recommend that the patient use a leg press and knee extension machine (12 weeks after surgery) to find the 1 RM for the involved and uninvolved leg and use 70% for the minimum strength criteria to begin running. It is also recommended that the patient have no episodes of giving way before initiating running and have the ability to walk as fast as possible on a treadmill for 15 minutes without an increase in pain or signs/symptoms of inflammation. A trampoline can be used initially to increase tolerance to absorption of ground reaction forces before running. Patients should simulate bouncing on a trampoline but without letting their feet leave the surface because they are not yet cleared for plyometric activities, much less activities on an unstable surface. Running should begin at a comfortable pace for short durations (1 to 2 minutes), followed by walking for 30 seconds to 1 minute. The running duration should increase as tolerated. The physical therapist should look for any evidence of pain, such as audible differences from foot to foot or different stride lengths. The patient should not progress in time or speed of running if there are any complaints of pain, instability, or signs/symptoms of inflammation.
Phase 4: 6 to 9 Months
The last phase of rehabilitation focuses on the actual return to the activity or sport. The correct timing of when to release an athlete back to sport participation has been controversial. Although criteria for return to sport may be fulfilled before the desired time frame, the clinician must discuss and weigh short-term and long-term risks and rewards with the athlete should he or she desire to participate. A recent study found that only 63% of National Football League players who underwent ACLR returned to play. The 63% who returned to play took an average 10.8 months to do so.78
Phase 4 is the final phase of rehabilitation, focusing on preparation for return to sport. In this phase, rehabilitation will focus on sport-specific exercises such as agility, plyometrics, and sprinting. The rehabilitation protocol should be individualized to meet the demands of not only the patient’s sport, but also their position. A receiver in football has different physical demands than a lineman; just as a shortstop has different demands versus a centerfielder in softball. It is difficult to decide when to initiate each of these exercises because there is little evidence available. While there are some guidelines available,138,186,187 they may not apply to your patient based on machines needed, graft type, and/or concomitant surgery. The further direction of initiating functional exercises is the opinion of the treating clinician. Once the patient can tolerate running 1 to 2 miles, they can begin low level agility drills such as forward/backward shuttle runs, lateral shuffling, and carioca. Speed should start at 50% of the patient’s self-perceived effort and increase as tolerated. The physical therapist should look for any compensation when the patient decelerates and pushes off the involved LE. Plyometric training should begin next in all planes of motion. Jumping (two feet) should begin forward, at short distances. Emphasis must be placed on avoiding a valgus collapse when loading before the jump, as well as landing. When the patient demonstrates adequate dynamic control of forward jumps, they should progress in distance jumped, jumping in the frontal and transverse planes, jumping onto boxes, and consecutive jumping. Hopping (one foot) should follow the same progression after the patient demonstrates dynamic control with jumping. The last functional exercises that should be added are high-level agility drills such as cutting, pivoting, and cut-and-spinning. Again, the physical therapist should look for any compensation when decelerating.
Returning to sport should be a collaborative decision made between the surgeon and the physical therapist. It is the responsibility of the physical therapist to prepare the patient for all tasks they could encounter in their particular sport. The patient must demonstrate each exercise at 100% effort with no episodes of giving way, increased pain, or signs/symptoms of inflammation. The patient should return to practice (if applicable) and perform at 100% effort without episodes of giving way, increased pain, or signs/symptoms of inflammation before participating in games. Although the time frame varies with the demands of the activity, Malone and Garrett94 note that it is possible to return to the sport at 6 months if the patient has successfully completed “controlled physiologic rehabilitation.” Thus initiating the training at the 4-month point allows 2 months of functional training and progression. Isokinetic testing is another piece of the puzzle used to determine whether the patient is ready to return to sport.188 Shelbourne and associates136 described criteria for return as follows:
The factors that most rehabilitation programs use to evaluate readiness for return to sport are KT-1000 stability, isokinetic equivalence, and functional tests.4,91,92,135,189–191 ![]() The most useful of these, without denying the importance of others, is functional testing. By using a complement of functional and isokinetic tests, the therapist and physician can determine when return to sport is appropriate. An average timeframe for functional exercises is running at 4.3 months, jumping at 6.5 months, return to light sports at 5.0 months, return to moderate sports at 5.8 months, and return to strenuous sports at 8.1 months.142
The most useful of these, without denying the importance of others, is functional testing. By using a complement of functional and isokinetic tests, the therapist and physician can determine when return to sport is appropriate. An average timeframe for functional exercises is running at 4.3 months, jumping at 6.5 months, return to light sports at 5.0 months, return to moderate sports at 5.8 months, and return to strenuous sports at 8.1 months.142
Currently, there is not a specific, validated return to sport criteria following ACL reconstruction. There are, however, a variety of tests that have been described in the literature. Neeter and associates184 showed a high ability to determine deficits in leg power 6 months after both ACL injury and ACL reconstruction using a test battery of OKC knee extension, OKC knee flexion, and CKC single-leg press. Reid and associates160 found the use of the single hop for distance, a 6-m timed hop, a triple hop, and crossover hops for distance to be reliable and valid following ACL reconstruction with intraclass correlation coefficients for limb symmetry index values ranging from 0.82 to 0.96. The tuck jump may be used to identify LE valgus and side-to-side differences.192
Intrarater within-session reliability was 0.84 (range, 0.72 to 0.97) when scoring the tuck jumps.186 Ground reaction forces can be assessed via hopping on force plates. A decrease in quad strength could result in decreased knee flexion angles when landing, which would increase the force at landing.193 Maximum vertical ground reaction force shows high within-session reliability on both the dominant (r = 0.823) and nondominant (r = 0.877) sides.194
It is recommended that athletes have a side-to-side discrepancy of less than 10%.194
Differences in drop landing195 and drop vertical jump196 should be addressed to within 10%. Because unsuccessful ACL reconstruction can range from 3% to 52%,197 ACL injury prevention techniques should be incorporated throughout the rehabilitation process.
In an effort to avoid excessive genu valgum, the hip abductors should be strengthened. The responsibility of the hip abductors is to prevent and control the excessive Trendelenburg position and subsequent dynamic valgus position at the knee. Studies have found this to be predictive of ACL injuries.15,198
It has been shown that athletes are more injury prone if side-to-side strength and flexibility differences are present.199 In conclusion, rehabilitation following ACL reconstruction must prepare the patient to return to his or her prior level of function. This long process begins immediately following injury with accurate diagnosis and determination if the patient will be a potential “coper.”180,200 Following surgery, protecting the healing graft, immediately decreasing swelling, increasing ROM, and strengthening in protected ranges is warranted. Global kinetic chain strengthening and neuromuscular training, as well as injury prevention tactics, should be incorporated to best prepare the patient to safely return to the prior level of function.
Troubleshooting
Carson and associates201 reviewed 90 failed ACL reconstruction surgeries. Based on their findings, a majority of the failures were the result of surgical technical errors. The most common complications from ACL reconstruction are joint stiffness, flexion contractures, patellar irritability (as high as 34%), and quadriceps weakness.95,202–204 Less frequently, complications include reflex sympathetic dystrophy (less than 1%), neurovascular injury (less than 1%), deep venous thrombosis, infection and possible fluid extravasation, and compartment syndromes (especially with endoscopic techniques).
The incidence of stiffness is reduced after ACL reconstruction by using proper surgical technique combined with an aggressive rehabilitation program. Improper graft placement with the tibial tunnel too far anterior or inadequate notchplasty can cause graft impingement, blocking terminal knee extension. Intraoperative inspection of the graft throughout a full ROM should always be conducted to ensure that the graft is not impinging within the intercondylar notch.205
Arthrofibrosis
One of the most devastating complications after ACL reconstruction is the development of arthrofibrosis. The knee synovium and fat pad become inflamed, leading to a thickened joint capsule. This in turn begins to obliterate the medial and lateral gutters and suprapatellar pouch. The patellar tendon can shorten, produce patella baja, and eventually cause articular damage. Paulos and colleagues206 have defined three stages in the arthrofibrotic knee:
The physical therapist should manage arthrofibrosis early to attempt restoration of full mobility.
A knee with a significant flexion contracture can cause greater impairment than an ACL-deficient knee. Antiinflammatory agents, aggressive physical therapy, and patellar mobilization are the initial treatments for all stages of arthrofibrosis. Arthroscopic débridement, open débridement, and dynamic splinting are usually required in the later stages.207
Another potential complication in obtaining and maintaining full-extension ROM is the presence of a cyclops lesion.208,209 This lesion is usually the result of the proliferation of fibrous tissue surrounding the graft and has been shown to be a cause of failure to regain or to lose full extension in the early postoperative period. Some patients who have achieved full extension will develop a gradual loss of full extension and joint line pain with terminal extension. MRI can verify the presence of this nodule that ultimately must be surgically removed. The patient responds quite well once the lesion is débrided. The therapist must be alert for the patient with delayed onset of ROM loss, especially in extension. They should undergo careful evaluation, including radiographs and MRI. If a cyclops lesion is confirmed, then arthroscopic resection should be performed.208
Anterior Knee Pain
Patellofemoral (PF) pain commonly occurs after ACL reconstruction, although it occurs more frequently after BPTB autograft reconstructions than with hamstring autograft reconstructions. Bach and colleagues42 reported an 18% incidence of mild PF symptoms in a 2- to 4-year follow-up study, whereas Kartus and associates202 reported a 33.6% incidence. Emphasis should be placed on quadriceps strengthening in protected ranges of 0° to 45° for CKC and 90° to 45° for OKC, and avoidance of pain.
Patellar fractures have been reported in the literature as a late complication of BPTB graft harvest. These are believed to be stress fractures that develop because of the decreased vascularity of the patella. Patellar fracture also can occur intraoperatively during graft harvest and has been reported after surgery. Brownstein and Bronner210 reported the incidence of patellar fractures at 0.5% and noted that it usually occurs as a result of a fall. They put the patella at highest risk for fracture during rehabilitation at 10 to 14 weeks postoperation. Pain over the tibial tubercle is less frequently encountered but may occur in those with prominent tibial tubercles. If the patient has limited joint motion before surgery, especially in extension, then a continuous passive motion device should be used immediately after surgery.189
During the first postoperative phase, ROM complications, if present, usually occur in extension. If mobilization and home exercises are not effective, then the patient should try adding weight to the ankle during the prone hanging exercises. Duration and intensity are determined individually, but the authors of this chapter generally start with 3 to 5 lb for a 5-minute increment and have the patient follow through at home three to five times a day. In addition, the patient can add weights to the knee while performing supine knee extension (towel propped under the heel) and progress in a similar manner.
Treatment for Complications and Troubleshooting
Phases 1 and 2
Strength complications are addressed using NMES over the affected muscles in conjunction with exercise. The physical therapist also can initiate biofeedback on the vasti to assist with balanced muscle contraction. Anterior knee pain related to PF dysfunction can be treated with modalities (i.e., cryotherapy and ultrasound), soft tissue mobilization, patella taping (after the incision has healed), emphasis on proper LE alignment during the offending activity, and hip strengthening; assessment of foot biomechanics (the need for orthotics) can also be useful in addressing PF issues.
Persistent swelling may indicate hemarthrosis, synovitis, reinjury, or infection.201 If by 4 to 6 weeks the patient has not gained full extension, then the cause may be patellar entrapment. Use of patellar mobilization to a greater extent and with more vigor and serial casting may be considered. Arthroscopy is usually considered if full extension is not obtained by the eighth week.63 If reflex sympathetic dystrophy occurs, then it is usually seen by the fifth week after surgery.
Motion limitations are of primary concern and require aggressive management, as mentioned earlier. Some patients may need to be manipulated or evaluated for surgery during phase 2. As the patient progresses with strengthening exercises, the physical therapist should pay careful attention to any residual pain or edema. The PF mechanism must be continually evaluated as the resistance of the exercises is progressed.
Phase 2
Complications 9 weeks or more after surgery are usually the result of edema or pain after activity. This can result from the addition of new stressors (e.g., exercises) on the knee joint and soft tissue. If not already initiated, then pool exercises may be a helpful adjunct in continuing to develop strength, maintain ROM, and improve mechanics of the LE in a less than full weight-bearing posture. Exercises in the form of deep-water running and activity-specific drills are a good adjunct to land-based rehabilitation.
Summary
In conclusion, rehabilitation following ACL reconstruction must prepare the patient to return to his or her prior level of function. This long process begins immediately following injury with accurate diagnosis and determination if the patient will be a potential “coper.”9,211 Following surgery, protecting the healing graft, immediately decreasing swelling, increasing ROM, and strengthening in protected ranges is warranted. Global kinetic chain strengthening and neuromuscular training, as well as injury prevention tactics, should be incorporated to best prepare the patient to safely return to the prior level of function.
Clinical Case Review
1 Michelle is 3 weeks post-ACLR and wants to know when she can discontinue her postoperation knee brace.
The brace should be discharged when the patient has full extension, no lag with an SLR, and at least 100° of flexion. This typically occurs 2 to 3 weeks after surgery. Most rehabilitation protocols require crutch use with weight bearing as tolerated for 4 weeks following surgery. After 4 weeks, the patient may be weaned from two crutches to one, and then ultimately discharged. A requirement for discharging crutches is that the patient must have a normal pain-free gait pattern. In both situations, consultation and approval from the surgeon should take place.
2 When strengthening the quadriceps, what range is safe for exercises? Why is this important?
For OKC exercises, the quadriceps should be strengthened in the range of 90° to 45° to guard against straining the healing ACL graft and to protect against patellofemoral pain. OKC exercises should begin 4 weeks after surgery. Before that, the patient can perform isometric quadriceps exercises at 90° and 60°. When performing CKC exercises, the patient should stay in the range of 0° to 45° to guard against straining the healing ACL graft and to protect against patellofemoral pain. CKC exercises may be incorporated into the treatment regime when the patient can be full weight bearing without pain.
3 Charlie is 10 weeks post-ACLR and demonstrates good ROM and strength. He is eager to begin running again. In terms of graft healing, when is it generally safe to begin running following ACL reconstruction?
The mechanical strength of the healing graft is at its weakest from 4 to 8 weeks. Bone-to-bone healing, as with the use of a BPTB autograft, occurs approximately at 10 weeks. Soft tissue to bone healing, as with the use of a hamstring or quadriceps autograft, occurs approximately at 12 weeks. Therefore, running should never be initiated before 3 months postsurgery because of the lack of healing. Additionally, the patient must demonstrate adequate strength and neuromuscular control of the LE with particular emphasis on the quadriceps. This typically occurs at 4 to 6 months postsurgery. A collaborative decision with the surgeon as to when the patient should begin running is necessary.
4 George is 4 months post-ACLR and wants to return to playing soccer. How do you best prepare the patient to return to sport following ACL reconstruction?
Given that there are no complications along the way, sport-specific exercises can be introduced into the program (at low intensity) beginning at 3 to 4 months. The best way to prepare the patient is to gradually expose the patient to everything he or she will encounter. Therefore, the patient must be able to tolerate running, going up/down steps, and figure eights, again starting at a low intensity level (walk figure eights before jog, jog before run, etc.). Plyometrics are introduced (usually around 5 months) as the patient begins to show that he or she are able to tolerate and have eccentric as well as concentric control of the LE before agility, plyometric, and cutting exercises are progressed. The patient should eventually perform all of the exercises in all three planes of motion. For example, jumping should be forward/backward, to each side, and clockwise/counterclockwise. Also, the patient should be able to perform any and all exercises at 100% effort in physical therapy before returning to practice. To return to competition, the patient must be able to participate in full practice and perform at 100% effort without any complaints of pain, giving way episodes, or signs/symptoms of inflammation.
5 Tracy is 50 years old and had ACLR 1 week ago. She arrives at her first outpatient visit with moderate edema about the knee and ankle. She states that she has been compliant with weight bearing and uses her crutches and brace as instructed. What further questions can help the therapist provide a successful edema management program?
The most effective way to manage swelling (at home) is with elevation, compression, and ice. Upon further questioning she has not been elevating her leg (above heart level) regularly or performing ROM or isometric exercises. A program was initiated consisting of elevation an ice (20 minutes, four times a day) with ankle pumps (10 repetitions every minute).
6 Randy is 45 years old and tore his ACL while horseback riding. He had ACLR 3 weeks ago. Knee flexion ROM is progressing nicely. Knee extension is limited, with a PROM of −10°. Inflammation is decreasing. He has pain while performing quadriceps sets and SLRs. What treatment techniques can be used to gain knee extension?
Tibiofemoral anterior-posterior mobilization movements into resistance at end range (using grades III and IV) can be performed with the knee in extension. Care must be taken not to elicit any increased tension on the graft. Full extension will decrease stress on the patellofemoral and tibiofemoral articulations.
7 Steve is a 45-year-old fireman. He had an ACLR 9 weeks ago on his left knee. Because of mishaps and personal reasons, physical therapy was not initiated until 5 weeks ago. Presently (9 weeks after surgery) PROM for knee flexion is 110°. The end range is beginning to feel leathery. Soft tissue restrictions appear to be present in the quadriceps. Swelling and complaints of pain are usually minimal. Strength is gradually progressing. What treatment techniques could be used to promote increased knee flexion?
Tibiofemoral posterior glides can also be performed with the knee in flexion (into resistance, grade IV), followed by PROM into flexion with overpressure. Patellofemoral glides can be performed. While PF glides superiorly will assist in gaining extension, PF glides inferiorly will assist with knee flexion and should be mobilized accordingly. In the prone (better for rectus femoris [RF]/Psoas) and supine positions, gentle contraction and relaxation stretches can be performed with the quadriceps. Also, soft tissue mobilization to the distal quadriceps and lateral retinaculum can be performed to address PF restrictions.
8 Nancy is making excellent progress in therapy. However, at 10 weeks after surgery the therapist notices a palpable “clunk” with her AROM, and extension has become quite painful. What could be the origin of her problem?
Limitations with extension usually are the result of posterior capsule or notch problems. The presence of crepitation or a palpable clunk in addition to pain with extension could signify an ACL “nodule.” Nancy was referred back to her surgeon and an MRI confirmed the presence of a cyclops lesion (nodule). Surgery was scheduled to remove the fibroproliferative tissue.







 mile; then run
mile; then run  mile (50% effort) for four repetitions, three times a week.
mile (50% effort) for four repetitions, three times a week.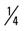 mile; then run
mile; then run 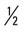 mile (50% effort) for two repetitions, three times a week.
mile (50% effort) for two repetitions, three times a week.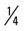 mile, run 1 mile (50% effort); then walk
mile, run 1 mile (50% effort); then walk 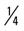 mile for one repetition, three times a week.
mile for one repetition, three times a week.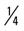 mile, run
mile, run 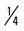 mile (50% effort), walk
mile (50% effort), walk 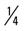 mile, run
mile, run 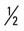 mile (75% effort); then walk
mile (75% effort); then walk 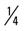 mile for two repetitions, three times a week.
mile for two repetitions, three times a week.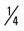 mile, run 1 mile (75% effort); then walk
mile, run 1 mile (75% effort); then walk 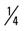 mile for two repetitions, three times a week.
mile for two repetitions, three times a week.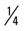 mile, run
mile, run 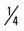 mile (75% effort), walk
mile (75% effort), walk 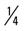 mile, run
mile, run 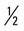 mile (100% effort); then walk
mile (100% effort); then walk 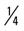 mile for 2 repetitions, 3 times a week.
mile for 2 repetitions, 3 times a week. mile, run 1 mile (100% effort); then walk
mile, run 1 mile (100% effort); then walk  mile for 2 repetitions, three times a week.
mile for 2 repetitions, three times a week.
