Chapter 155 Anterior Approaches for Multilevel Cervical Spondylosis
Pathophysiology of Multilevel Cervical Spondylosis
Cervical radiculopathy and myelopathy are phenomena that are based on a compressive insult to the spinal cord and nerves. These can be either continuous or repetitive in nature. Degenerative phenomena, which ultimately result in the restriction of the spinal canal diameter and exit foramen narrowing, are part of a cascade that includes disc dehydration and loss of height with buckling of ligaments and periosteum and the formation of osteophytes and both bony and ligamentous hypertrophy. In addition, ossification of the posterior longitudinal ligament (OPLL) can occur. Although these processes are mainly responsible for a static compression and reduction of the canal, in cases where joint laxity predominates, varying degrees of listhesis during flexion-extension and rotation movements can lead to transitory compressions, which, in the long run, can lead to repetitive insults of the spinal cord long before becoming overt deformities. In the presence of significant osteophytes, the draping of the cord over these bony prominences with movement may account for a dynamic insult in the absence of instability.1–3
Myelopathy thus seems to develop from direct pressure insult. The hypothesis of hypoperfusion of the cord due to vascular compression, such as compression of the anterior spinal artery, seems appealing; however, no animal studies carried out on the topic seem to have confirmed the involvement of such a mechanism.4
Once myelopathic signs manifest, the history of CSM is characterized by progression.5,6 Only a minority of patients with myelopathic signs deteriorate rapidly. Given that the prognosis and postoperative outcome seem to correlate directly with the preoperative deficits and functional status, however, excessive waiting before proceeding with decompressive surgery does not appear to be justified as long as the general condition of the patient is favorable.7,8
Recently, the concept of origin of degenerative changes in spine has been speculated to be related to muscle weakness related facetal overriding.9,10 Facetal “retrolisthesis” could be a primary event rather than a secondary phenomenon. The pathologic events related to degeneration could be related to facetal overriding, rather than primary disc space reduction or loss of “water-content” of the disc. Facetal distraction, as proposed by Goel et al, has been successfully used as an alternative modality of treatment of single and multiple-level cervical and lumbar degeneration.11,12 The treatment has been identified to result in restoration of intervertebral distances and neural foraminal and spinal canal diameter. The treatment has been shown to result in increase of neural and spinal canal dimensions and restoration of disc space height and disc “water content”. Surgery is done without removal of any part of disc, bone, and ligament and aims at arthrodesis of the spinal segment.
Diagnosis
Clinical Features
The patient with multilevel cervical spondylosis (MCS) presents with a heterogeneous array of findings that show either polyradicular involvement or findings suggestive of cervical myelopathy. Difficulty in walking due to stiffness of the legs is a primary symptom that suggests myelopathy and, when present, can have diagnostic significance. Paresthesia and weakness of the limbs are dominant symptoms, but radiating pain in the hands can be less frequent. Long-standing dull pain at the nape of the neck is common. Apart from a thorough neurologic examination, the patient should also be examined and questioned for subtle signs that might not be identified in simple strength, sensation, and reflex assessments. Initial signs of myelopathy might be difficulty in fine finger movements such as buttoning a shirt or writing. These may not be accompanied by any other deficits and can thus be overlooked if the patient is not specifically asked about them. The “myelopathy hand,” which is characterized by sensory disturbances without any particular radicular distribution, clumsiness, and interosseous wasting, is also a warning sign in the absence of the possibility of peripheral polyneuropathy.13,14
Imaging Features
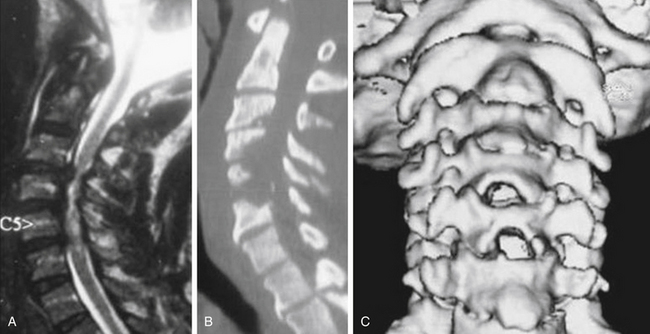
MRI is the best available imaging procedure for assessing the spinal cord and other soft-tissue structures, such as discs, ligaments, and capsules, that can be at the origin of compressive phenomena. It will also show compression of the spinal cord and nerves due to bony structures, but is less accurate in definition. Patients with impending or frank myelopathy due to compressive spondylosis show varying degrees of signal changes in the cord. These are hyperintense on T2-weighted images (T2WI) and hypointense on T1WI. Given that T2WI cord changes are more easily detected and appear earlier in the course of compressive myelopathy, MRI also has a prognostic value in that the finding of hypointensity on T1WI indicates a poorer prognosis when found preoperatively.15,16,
Computed tomography (CT) is the investigative modality of choice for the assessment of the bony spine. Although this is more relevant in trauma patients, where three-dimensional reconstructions on all planes show the exact relationships of the bony structures and potential fractures, it is not indispensable in most degenerative disease states. It can again be used in selected cases for assessment of bony conformation and osteophytes in particular, during preoperative planning, and postoperatively for the assessment of fusion and hardware position. When integrated with myelography, CT represents a good alternative to MRI if the latter is not available or feasible.17
Surgery for Multilevel Cervical Spondylosis
If appropriately selected, surgery for MCS is a very rewarding operation. Although the need for decompression of the cord from the osteophytic compression is necessary, the indication and methodology for fixation is a debated subject. Wide decompression of the cord by removal of the osteophytic bars should be the aim. The need to remove the vertebral bodies or to perform a corpectomy to remove the osteophytic bars is also a debated subject. However, the relative ease of performance of decompression by performing corpectomy has led several authors to recommend such an operative approach.
With the advent of anterior cervical spinal surgery after its original description by Smith and Robertson and Cloward over 50 years ago, the possibility of treatment solutions for the cervical spine have immensely expanded. It became possible to treat more complex pathologies from the front.18,19 Being able to access the anterior column of the cervical spine enables the surgeon not only to create a biodynamically sound construct in case anterior support is disrupted or lost but also to address a narrowed cervical spinal canal from the aspect where the majority of compressive phenomena originate, which is anteriorly.20 This is particularly true in the majority of cases of CSM, where anterior compression can be due to herniated discs, osteophytes of the posterior vertebral margins, or ossified posterior longitudinal ligaments (OPLLs). The anterior approach has therefore become the favorite route for removal of herniated cervical discs, relegating the posterior approach to the status of exception and feasible only in very selected cases or for simple foraminotomies.21,22
It is beyond the scope of this chapter to make a comparison in terms of efficacy and outcomes between anterior and posterior procedures for MCS. However, it should be mentioned that though no class I clinical trials have been conducted, the data derived from studies published in the literature on the topic seems to favor the anterior approach in the majority of cases.23–26 This seems to be attributable mainly to two facts: (1) As mentioned above, the majority of compression in MCS lies anteriorly in the spinal canal, and (2) the postlaminectomy membrane scars over the decompressed segments, leading to recompression and late deterioration after initial improvement.27 Laminoplasty techniques have been developed and have improved the outcomes achieved by using these procedures.28 Another problem is the kyphosing potential of multilevel laminectomy without fusion; however, this has been clearly established to be significant only in patients younger than 15 years.29,30
Techniques for Anterior Decompression in the Treatment of MCS
Anterior decompressive surgery for MCS can be divided into two groups: radical and conservative.
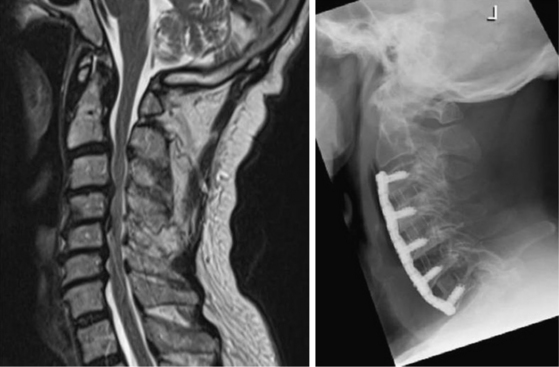
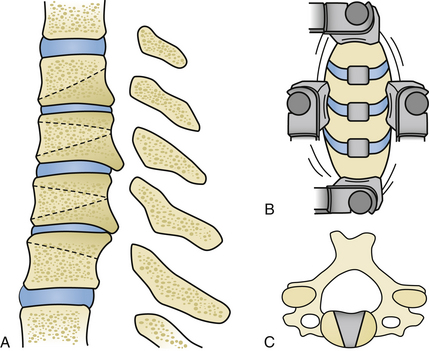
The radical surgical strategy entails maximum decompression with subsequent reconstruction by means of grafts and instrumented fusion. This can be done either by performing multilevel discectomies with extensive osteophytectomies or by performing corpectomies. Both procedures are widely used. The choice of the technique depends essentially on the individual case, the evaluation and experience of the surgeon, and his philosophical understanding. What the advocates of these techniques have in common is the belief that recreation of physiologic alignment and fusion of the operated segments are essential to providing a good long-term outcome (Fig. 155-1 and 155-2).
The following sections describe all these surgical techniques, together with their indications, advantages, and disadvantages. Common to all of them, apart from the oblique partial corpectomy according to George, is the exposure of the anterior cervical spine, which is discussed separately prior to the presentation of the individual techniques.
Exposure of the Anterior Cervical Spine
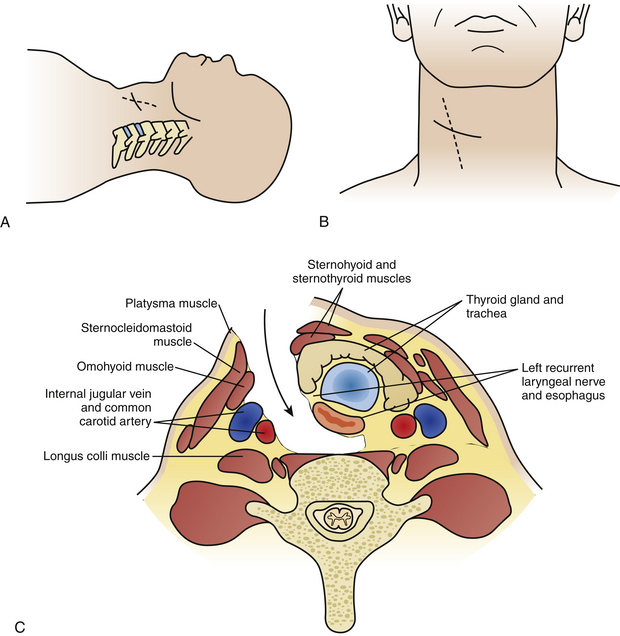
The incision on the chosen side of the neck is usually horizontal, either in a skin crease or along Langer’s lines. This can be as short as an inch, starting from the medial border of the sternocleidomastoid muscle (SCM) and extending medially for one-level procedures, and should be elongated in both directions when more spinal levels are to be exposed. Some surgeons prefer a vertically oblique incision that follows the medial border of the SCM, which has a cosmetically less favorable outcome and appears to be seldom necessary as long as some simple steps in the dissection procedure are observed (Fig. 155-3A, B).
The next step is identification of the medial SCM border and opening of the investing fascia along the whole extent of the medial border with sharp dissection. Blunt finger or pledget dissection should now develop a plane reaching down, palpating the carotid artery, which generally is readily identified and left laterally. Thus the esophagus, larynx, and thyroid are left medially and the anterior aspect of the cervical spine is reached in one point (Fig. 155-3C). The retractor should be inserted underneath the longus colli muscles onto the vertebral body plane to avoid insertion and retraction between the esophagus and the trachea, as this is where the recurrent laryngeal nerve runs and can potentially be injured. A small aspect of the spine is thus exposed, and craniocaudal extension of the exposure is now necessary. This can be done by further blunt dissection, but for a long exposure sharp dissection often becomes necessary to divide the investing fascial layers. Care should be taken not to inadvertently cut the superior thyroid artery, which traverses at the C3 level, and the inferior thyroid artery, which traverses at the C7 level. They may be encountered especially in long exposures, but even then it is not frequently necessary to divide them.31 Should it become necessary, ligation of the vessel is advised prior to its division. The superior belly of the omohyoid will be encountered as a traversing structure during the development of a long craniocaudal plane, and a good dissection of this muscle usually permits its retraction either superiorly or inferiorly without the need to divide it. The prevertebral fascia is now divided and the longus colli muscles are lifted off the vertebral plane bilaterally to allow insertion of the self-retaining retractor blades. Craniocaudal retractor blades can be added for completion of the exposure (Fig. 155-4). Once the desired levels are thus exposed and confirmed by fluoroscopy, the decompressive procedure of choice can follow.
Complications and Pitfalls with the Exposure of the Anterior Cervical Spine
All complications inherent to the anterior approach are likely to be more pronounced in multilevel surgery because of the extent of the dissection and retraction. Recurrent laryngeal nerve injuries have been reported to make up about 17% of all neurologic injuries in anterior cervical surgery, with the majority being transitory.32 Injury to a recurrent nerve leads to paralysis of a vocal cord, with a hoarse voice and risk of aspiration. This risk is minimized by blunt dissection while avoiding excessive coagulation and overzealous retraction. Langenbeck retractors should be placed in contact with and exerting pressure on the vertebral plane much rather than in the tissues, and orthostatic retractor blades should be inserted under the longus colli muscles.33 When symptoms of hoarseness persist for more than 6 weeks, referral to an otolaryngologist should be made for assessment and evaluation of the vocal cords.34
Transient dysphagia has been reported to be as frequent as 8%,15 but injury to the esophagus is rare. If perforation of the esophagus occurs, however, it can be a life-threatening condition because of the high risk of infection, which can eventually lead to mediastinitis or vertebral osteomyelitis.35
Injury of the carotid artery and the jugular vein are uncommon and can be avoided by minimizing sharp dissection and the use of sharp-edged retractors. The other vessels that can be injured during the anterior approach are the superior and inferior thyroid arteries, which cross at C3 and C7, respectively. Careful extension of the dissection craniocaudally from the middle can quite easily identify the vessels and, if necessary, ligate and divide them. Injury of the vertebral artery is rare but has been reported to be as high as 3%.36
Multilevel Discectomy
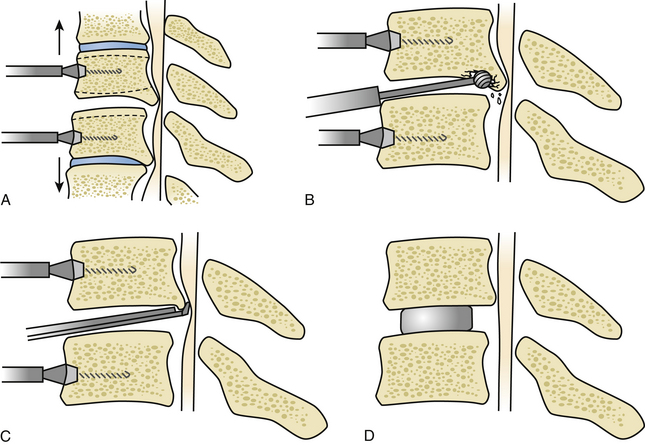
If the main area of compression lies mainly behind the disc spaces as opposed to also behind the vertebral bodies, then multiple levels of discectomy and osteophytectomy at the affected levels are justified (Fig. 155-5). Once the anterior aspect of the spine is prepared with the insertion of the laterolateral self-retaining retractor blades under the bilateral longus colli muscles and the correct level is identified, the disc is incised with a No. 15 scalpel blade, taking care to control the depth of the incision. The disc material is first widely curetted. To expose the posterior limit of the vertebral bodies, vertebral body distractors can be used at each level. However, some surgeons prefer the use of Caspar retractor pins. Retractor pins are inserted into the vertebral bodies adjacent to the disc (Fig. 155-6A). These are usually 12 or 14 mm in length and can be placed slightly convergent toward the depth. This has an effect such that the proximal diverging ends need to be approximated to place the retractor sleeves over them, thus causing the posterior disc space to open up slightly on distraction. A series of curved and straight curettes can now be used to empty the disc and prepare the vertebral end plates by elevating the cartilaginous end plates. Moving toward the posterior end of the disc space, this is often found to be closed by osteophytes, which almost always originate predominantly from the posteroinferior lip of the superior vertebra.
A cutting or diamond burr of appropriate size can now be used to drill down the osteophyte (Fig. 155-6B). The drilling of the osteophytic bars proceeds laterally up to the upward-sloping walls of the uncinate processes and downward until it is possible to insert a small, blunt hook behind the superior and inferior vertebral bodies and the PLL. Enough space should be created to introduce first a 1-mm and then a 2-mm Kerrison Rongeur without too much force (Fig. 155-6C). The posterior lips are thus removed with the rongeurs, and the blunt hook is inserted in a turning motion to penetrate behind the PLL. The PLL is then excised with the rongeurs, and the decompression is carried out laterally into the root exit foramina (Fig. 155-7A-C).
Once all disc spaces have been prepared and decompression is satisfactory, the need for fixation and fusion can be evaluated. If no grafting or fusion is considered, the operation may end at this stage. There are plenty of studies in the literature on single-level anterior cervical discectomy and decompression showing that grafting and fusion are not superior to no grafting at all.37–41
Reports of multilevel discectomies without graft and fusion are unfortunately rare. No comparative studies have been done, but the information that is available seems to suggest that the long-term outcomes associated with uninstrumented discectomy, at least for two spinal levels, are comparable to those of instrumented fusion.42
When it comes to the choice of the ideal graft, an evaluation of the pros and cons based on the objectives and the characteristics of the graft utilized is needed. Autologous bone graft is an ideal material. It has better fusion rates than allograft bone43 or any other artificial material. This needs to be weighed against the problem of donor site pain and morbidity, however, which has been reported to be as high as 8%.44 Cages of material that is not bone, such as PEEK, carbon fiber, or hydroxyapatite, have high stability and a smaller, or almost no, degree of graft deformation.45,46
It is therefore generally difficult to make recommendations, but cages of nonbone material are increasingly popular and widely used because of the combination of good mechanical rigidity and no donor site morbidity, which are opposed only by a slightly smaller fusion rate compared to autologous bone.43
Plate-and-Screw Fixation
The next step is the decision whether to supplement the decompression and fusion procedure with instrumentation. Although initial series reported good outcomes without plating, nowadays the addition of instrumentation is recommended and widely used in anterior cervical multilevel procedures. This is based on the fact that, generally, the higher the number of bone surfaces, the higher the rate of pseudoarthrosis.47 This view continues to be challenged, however, and good results with stand-alone cages, even for multiple cervical levels, have been reported.48
When the decision to use instrumentation is made, there are two fundamental differences in choosing a plate-and-screw fixation device: whether it is constrained or not. Constrained systems consist of the possibility to lock the screw to the plate once positioned, thus forming a rigid entity, whereas this is not the case in unconstrained systems. The downside of a constrained system can be stress-shielding of the graft, a phenomenon that consists of the rigid plate-screw complex’s taking the majority of the axial load, thus not passing on the necessary load to the graft with the potential for pseudoarthrosis to ensue. These factors need to be taken into account; biomechanically, however, constrained systems clearly maintain rigidity more efficiently than nonconstrained systems do.49
At the moment of implantation, when choosing the plate, care should be taken to select one of the appropriate length. The plate should not cover more than two thirds of the anterior aspect of the adjacent vertebrae to avoid the possibility of interference with the free motion of the superior and inferior adjacent moving disc segments. A plate too close in proximity to the next moving segment has been shown to increase the formation of osteophytes, thus leading to accelerated adjacent segment degeneration.
Along with the choice of the appropriate plate length, it should also be considered that, especially in longer constructs, the plate should be bent to accommodate the cervical lordosis (Fig. 155-1). Failure to do so can result not only in the plate’s sitting proud at either the cranial or caudal end but also in excessive stress on the screws, thus resulting in increased risk of pullout and dislocation.
As far as the screws are concerned, the appropriate length should be selected. Biomechanical studies have shown that a bicortical fixation, that is, a screw that engages the posterior vertebral body wall, appears superior in terms of stability.50 The absence of a clear clinical advantage, however, together with the increased risk of posterior wall engagement and the newer generations of vertebral body screws and their improved purchase strength, make bicortical purchase a less attractive option, and its use is generally less diffuse.
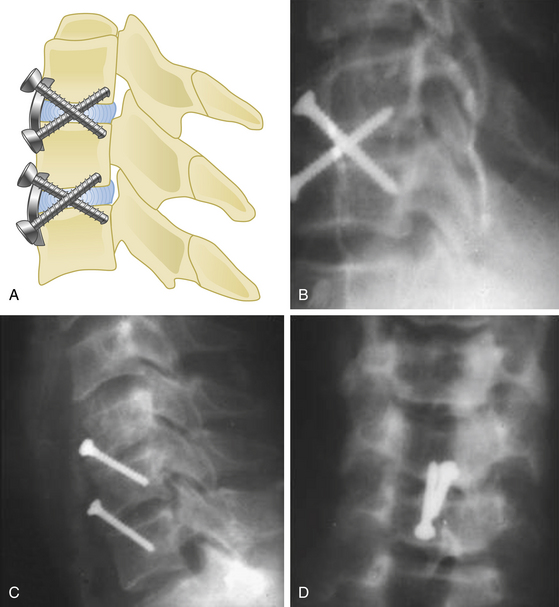
A screw with tricortical purchase fixation was described by Goel in 1997.51 In instances in which strong screw purchase is a concern, by using this method, the screw engages into three cortices (as shown in Fig. 155-8) traversing a disc space. Therefore, there is no need to engage the posterior vertebral body wall, which makes this procedure a safer and stronger option than the classical bicortical type of fixation. Moreover, the screw traverses the relatively firm areas of the vertebral body located in the inferior end of the anterior surface and the region around the disc space. The tricortical screw may not be used where the fusion of the region is not intended. The tricortical screws can be used with plates and even in isolation. The transdiscal, and thus transarticular nature of the screw traverse provides stability. One or more screws can be used for each level (Fig. 155-8A-C).
With regard to multilevel discectomies for decompression of the cervical spine, the insertion of disc replacements at multiple levels seems to be an interesting emerging option. The idea of multilevel disc replacement is appealing, and good results have been reported. It will be interesting to see the long-term data regarding this procedure in terms of delayed fusion and adjacent segment degeneration.52
Complications and Pitfalls in Multilevel Cervical Discectomy
Apart from the approach-related problems and the risk of neurologic injury described above, the main concern with the multilevel cervical discectomy technique is sufficient decompression. It is quite often the case that the major sites of compression lie behind the disc space on multiple levels, and discectomy therefore seems appealing. It has to be considered, however, that obtaining good decompression through the disc space can be technically more demanding than via a trench corpectomy because of the narrower working space. The other problem is pseudoarthrosis, which is not a problem with single-level procedures, but the nonunion rate appears to go up as the number of treated levels increases.53,54 This view has been challenged, however; some surgeons perform multilevel anterior cervical discectomies with spacers without plate fixation and have reported good results.55,56
A point in favor of multilevel discectomies is that the risk of graft dislocation is smaller than in a multilevel corpectomy procedure where a single long graft is inserted, which has a higher potential of mobilization.57
Corpectomy
In performing corpectomy, wide decompression of the cord by removal of the osteophytic bars should be the aim. The need for removal of the vertebral bodies to remove the osteophytic bars is controversial. However, the relative ease of performance of decompression by performing corpectomy has led several authors to recommend such an operative approach. Considering the fact that the entire body of a cervical vertebra is rarely removed, it is more appropriate to talk about a trench corpectomy to illustrate the concept, especially when multiple levels are involved (Fig. 155-9A, B).
Bone grafts can be either fibular strut grafts or iliac crest grafts and can be autologous or heterologous in nature. Although the autologous iliac crest graft harvested during the procedure and cut to the appropriate size is probably the most desirable option in terms of fusion rate among all the above options, the associated morbidity due to donor site pain has to be taken into account and included in decision-making and discussion with the patient. Investigators of one patient series reported an incidence of up to 3% in donor site pain and an overall complication rate of around 8% linked to graft harvesting.44
Once the desired implant is thus positioned and a good fit and contact with the adjacent end plates are achieved, the decision needs to be made whether a plate should be integrated for stabilization of the construct if cages or grafts are used that do not have integrated extensions for fixation as mentioned above. A cervical plate and screws seem to be indicated in multilevel decompressions. Although plating appears to make no difference in single-level corpectomy with a well-positioned graft and postoperative movement restriction in a cervical collar, the rate of pseudoarthrosis increases significantly with the number of levels operated on. Furthermore, considering the risk of graft dislodgement increasing with the number of levels, a plate is indicated to prevent it. It may also help to prevent collapse and telescoping (phenomenon of settling of the graft into the vertebral body). Furthermore a plate can help in preventing kyphosis of the segment with restoration of a certain degree of lordosis (Figure 155-2).58–61
As far as the clinical results are concerned, there are a good number of studies in the literature reporting outcomes in patients treated with corpectomy for MCS. The studies are very heterogeneous in terms of patient characteristics, techniques, and tools used to assess outcome measurement. Corpectomy over one to multiple levels is common to all of them, with different implants and plates used only in some of them. Outcome measurements vary from simple description of myelopathic signs and symptoms to application of scales such as Nurick’s, Harsh’s, or that of the Japanese Association of Orthopaedic Surgeons.7,62 Overall, however, results seem to be favorable, with improvement of symptoms ranging from 60% to 100% in the more recent series.63–72
Complications and Pitfalls in Anterior Cervical Corpectomy
As logic dictates, the longer the graft, the higher the chances of dislodgement. An early study examined the phenomenon, and it was found that the dislodgement rate would increase to 50% for three- and four-level corpectomies. In addition to that, biomechanical studies have shown that in multilevel corpectomies with just anterior plating, the plate provides constraint only in flexion, whereas in extension the load is on the graft, which can lead to end plate rupture with impaction of the graft into the vertebral bodies and telescoping and/or graft dislodgement. It was therefore recommended to integrate corpectomies of more than two levels with posterior instrumented fusion. This increased stability and eliminated the cases of graft dislodgement.73–76 Neurologic injury, including damage to the nerve roots or the spinal cord, is rare and has been estimated to be around 1%. This figure is significantly smaller if only injuries of the spinal cord are examined.77
Multivel Discectomy Versus Corpectomy
As can be seen from what has been described so far, the choice of either technique is highly dependent on the specific case and the surgeon’s evaluation and preferences. As far as evidence in the literature goes, a recent systematic review classified the available evidence as class III, stating that both procedures offer equivalent treatment strategies and outcomes. If plating is not performed, corpectomy offers higher fusion rates, but this is counterbalanced by higher rates of graft mobilization. If plating is considered, then both techniques offer equivalent fusion rates.78
Limited Oblique Corpectomy According to Goel
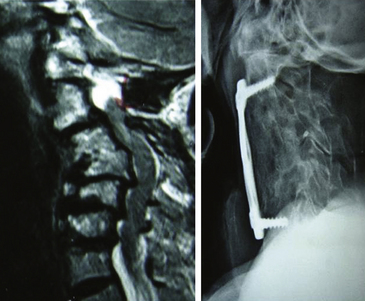
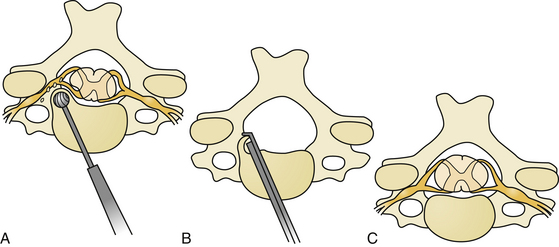
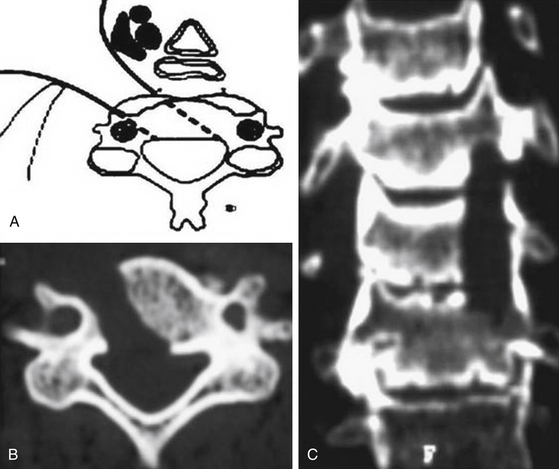
The oblique corpectomy according to Goel was first described in 2005.79 Initially developed for the treatment of OPLL, it continues to be used successfully for MCS caused by anterior compression. It is indicated in patients with a stable cervical spine and can be used for as many cervical levels as needed. The essential aspect of this technique is that the corpectomy is done adjacent to the disc space to expose the part of the osteophytes that lie hidden behind the vertebral bodies. Wide exposure of the region can be obtained by performing oblique corpectomy from both above and below, and the bone cuts can be connected. Such an oblique nature of corpectomy from an anterior approach is suitable to resect the OPLL. The oblique nature of bone cut leaves the anterior and lateral aspects of the vertebral bodies intact and does not destabilize the spine. Under these circumstances, there is no need for bone graft or instrumented fixation and fusion (Fig. 155-10 and 155-11).
Oblique Partial Corpectomy According to George
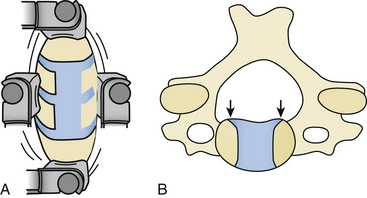
In 1993, George et al described an alternative technique to perform anterior decompression of multilevel cervical cord compression. According to these authors, this technique has the advantage of not causing spinal instability in almost all patients (when spinal instability was excluded preoperatively), as it resects less than 50% of the vertebral body essentially anterolaterally, hence partially oblique, leaving the anterior aspect of the vertebral body and the anterior longitudinal ligament intact (Fig. 155-12).80 Technically, this procedure differs from all other procedures addressing the cervical spine anteriorly in that the approach is more anterolateral than the classical one. The side of approach in this case is based on whether root symptoms are present and can be used only if there are unilateral root symptoms, as the technique does not permit decompression of the contralateral root exit foramina. The stability of the cervical spine was also assessed with flexion-extension x-rays, where the absence of movement of more than 2 mm between two adjacent vertebral bodies qualified as stable. With this assessment, 98% of the patients had a stable cervical spine.
Acknowledgment
Figure 155-1 is courtesy of Mr. Bahram Fakouri, Consultant Spinal Surgeon, Guy’s and St. Thomas Hospital, London, UK; and Figure 155-2 is courtesy of Dr. Ram Chaddha, Spine Surgeon, Lilavati Hospital and Research Centre, Mumbai, India.
Casotto A., Buoncristiani P. Posterior approach in cervical spondylotic myeloradiculopathy. Acta Neurochir (Wien). 1981;57:275-285.
Cattell H.S., Clark G.L.Jr. Cervical kyphosis and instability following multiple laminectomies in children. J Bone Joint Surg Am. 1967;49:713-720.
Clarke E., Robinson P.K. Cervical myelopathy: a complication of cervical spondylosis. Brain. 1956;79:483-510.
Cloward R.B. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602-617.
Cusick J.F. Pathophysiology and treatment of cervical spondylotic myelopathy. Clin Neurosurg. 1991;37:661-681.
Goel A., Shah A. Facetal distraction as treatment for single- and multilevel cervical spondylotic radiculopathy and myelopathy: a preliminary report. J Neurosurg Spine. 2011;14:689-696. (6)
35. Goel A, Shah A, Jadhav M, Nama S. Distraction of facets with intraarticular spacers as treatment for lumbar canal stenosis: report on a preliminary experience with 21 cases. J Neurosurg Spine. Sep 16, 2011: [Epub ahead of print].
Henderson C.M., Hennessy R.G., Shuey H.M.Jr., Shackelford E.G. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery. 1983;13:504-512.
Hukuda S., Mochizuki T., Ogata M., et al. Operations for cervical spondylotic myelopathy: a comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. 1985;67:609-615.
Kawaguchi Y., Kanamori M., Ishihara H., et al. Minimum 10-year followup after en bloc laminoplasty. Clin Orthop Rel Res. 2003;411:129-139.
Lees F., Turner J.W. Natural history and prognosis of cervical spondylosis. Br Med J. 1963;2:1607-1610.
Mehalic T.F., Pezzuti R.T., Applebaum B.I. Magnetic resonance imaging and cervical spondylotic myelopathy. Neurosurgery. 1990;26:217-227.
Morio Y., Teshima R., Nagashima H., et al. Correlation between operative outcomes of cervical compression myelopathy and MRI of the spinal cord. Spine (Phila Pa 1976). 2001;26:1238-1245.
Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:101-108.
Ono K., Ebara S., Fuji T., et al. Myelopathy hand: new clinical signs of cervical cord damage. J Bone Joint Surg Br. 1987;69:215-219.
Phillips D.G. Surgical treatment of myelopathy with cervical spondylosis. J Neurol Neurosurg Psychiatry. 1973;36:879-884.
Rao R. Neck pain, cervical radiculopathy and myelopathy: pathophysiology, natural history, and clinical evaluation. Instr Course Lect. 2003;52:479-488.
Robinson R.A., Smith G.W. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull Johns Hopkins Hosp. 1955;96:223-224.
Shafaie F.F., Wippold F.J.2nd, Gado M., et al. Comparison of computed tomography myelography and magnetic resonance imaging in the evaluation of cervical spondylotic myelopathy and radiculopathy. Spine (Phila Pa 1976). 1999;24:1781-1785.
Spillane J.D., Lloyd G.H. The diagnosis of lesions of the spinal cord in association with osteoarthritic disease of the cervical spine. Brain. 1952;75:177-186.
Voskuhl R.R., Hinton R.C. Sensory impairment in the hands secondary to spondylotic compression of the cervical spinal cord. Arch Neurol. 1990;47:309-311.
Wada E., Suzuki S., Kanazawa A., et al. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: a long-term follow-up study over 10 years. Spine (Phila Pa 1976). 2001;26:1443-1448.
White A.A.3rd, Panjabi M.M. Biomechanical considerations in the surgical management of cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1988;13:856-860.
Yonenobu K., Fuji T., Ono K., et al. Choice of surgical treatment for multisegmental cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1985;10:710-716.
Yonenobu K., Okada K., Fuji T., et al. Causes of neurologic deterioration following surgical treatment of cervical myelopathy. Spine (Phila Pa). 1986;11:818-823.
Yonenobu K., Yamamoto T., Ono K. Laminoplasty for myelopathy. In: Clark C.R., editor. The Cervical Spine. 3rd ed. Philadelphia, PA: Lippincott-Raven; 1998:849-856.
1. White A.A.3rd, Panjabi M.M. Biomechanical considerations in the surgical management of cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1988;13:856-860.
2. Rao R. Neck pain, cervical radiculopathy and myelopathy: pathophysiology, natural history, and clinical evaluation. Instr Course Lect. 2003;52:479-488.
3. Clarke E., Robinson P.K. Cervical myelopathy: a complication of cervical spondylosis. Brain. 1956;79:483-510.
4. Cusick J.F. Pathophysiology and treatment of cervical spondylotic myelopathy. Clin Neurosurg. 1991;37:661-681.
5. Spillane J.D., Lloyd G.H. The diagnosis of lesions of the spinal cord in association with osteoarthritic disease of the cervical spine. Brain. 1952;75:177-186.
6. Lees F., Turner J.W. Natural history and prognosis of cervical spondylosis. Br Med J. 1963;2:1607-1610.
7. Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:101-108.
8. Phillips D.G. Surgical treatment of myelopathy with cervical spondylosis. J Neurol Neurosurg Psychiatry. 1973;36:879-884.
9. Goel A. Facet distraction spacers for treatment of degenerative disease of the spine: Rationale and an alternative hypothesis of spinal degeneration. J Craniovertebr Junction Spine. 2010;1(2):65-66.
10. Goel A. Facet distraction-arthrodesis technique: Can it revolutionize spinal stabilization methods? J Craniovertebr Junction Spine. 2011;2(1):1-2.
11. Goel A., Shah A. Facetal distraction as treatment for single- and multilevel cervical spondylotic radiculopathy and myelopathy: a preliminary report. J Neurosurg Spine. 2011;14(6):689-696.
12. Goel A., Shah A., Jadhav M., Nama S. Distraction of facets with intraarticular spacers as treatment for lumbar canal stenosis: report on a preliminary experience with 21 cases. J Neurosurg Spine. Sep 16, 2011. [Epub ahead of print]
13. Ono K., Ebara S., Fuji T., et al. Myelopathy hand: new clinical signs of cervical cord damage. J Bone Joint Surg Br. 1987;69:215-219.
14. Voskuhl R.R., Hinton R.C. Sensory impairment in the hands secondary to spondylotic compression of the cervical spinal cord. Arch Neurol. 1990;47:309-311.
15. Morio Y., Teshima R., Nagashima H., et al. Correlation between operative outcomes of cervical compression myelopathy and MRI of the spinal cord. Spine (Phila Pa 1976). 2001;26:1238-1245.
16. Mehalic T.F., Pezzuti R.T., Applebaum B.I. Magnetic resonance imaging and cervical spondylotic myelopathy. Neurosurgery. 1990;26:217-227.
17. Shafaie F.F., Wippold F.J.2nd, Gado M., et al. Comparison of computed tomography myelography and magnetic resonance imaging in the evaluation of cervical spondylotic myelopathy and radiculopathy. Spine (Phila Pa 1976). 1999;24:1781-1785.
18. Robinson R.A., Smith G.W. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull Johns Hopkins Hosp. 1955;96:223-224.
19. Cloward R.B. The anterior approach for removal of ruptured cervical disks. J Neurosurg. 1958;15:602-617.
20. Cusick J.F. Pathophysiology and treatment of cervical spondylotic myelopathy. Clin Neurosurg. 1991;37:661-681.
21. Casotto A., Buoncristiani P. Posterior approach in cervical spondylotic myeloradiculopathy. Acta Neurochir (Wien). 1981;57:275-285.
22. Henderson C.M., Hennessy R.G., Shuey H.M.Jr., Shackelford E.G. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery. 1983;13:504-512.
23. Hukuda S., Mochizuki T., Ogata M., et al. Operations for cervical spondylotic myelopathy: a comparison of the results of anterior and posterior procedures. J Bone Joint Surg Br. 1985;67:609-615.
24. Yonenobu K., Fuji T., Ono K., et al. Choice of surgical treatment for multisegmental cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1985;10:710-716.
25. Yonenobu K., Okada K., Fuji T., et al. Causes of neurologic deterioration following surgical treatment of cervical myelopathy. Spine (Phila Pa 1976). 1986;11:818-823.
26. Wada E., Suzuki S., Kanazawa A., et al. Subtotal corpectomy versus laminoplasty for multilevel cervical spondylotic myelopathy: a long-term follow-up study over 10 years. Spine (Phila Pa 1976). 2001;26:1443-1448.
27. Yonenobu K., Yamamoto T., Ono K. Laminoplasty for myelopathy. In: Clark C.R., editor. The Cervical Spine. 3rd ed. Philadelphia, PA: Lippincott-Raven; 1998:849-864.
28. Kawaguchi Y., Kanamori M., Ishihara H., et al. Minimum 10-year followup after en bloc laminoplasty. Clin Orthop Rel Res. 2003;411:129-139.
29. Cattell H.S., Clark G.L.Jr. Cervical kyphosis and instability following multiple laminectomies in children. J Bone Joint Surg Am. 1967;49:713-720.
30. Mayfield F.H. Complications of laminectomy. Clin Neurosurg. 1976;23:435-439.
31. Whitecloud T.S. Complications in anterior cervical fusion. Instr Course Lect. 1978;27:223-227.
32. Flynn T.B. Neurologic complications of anterior cervical interbody fusion. Spine (Phila Pa 1976). 1982;7:536-539.
33. Tew J.M.Jr., Mayfield F.H. Complications of surgery of the anterior cervical spine. Clin Neurol. 1976;23:424-434.
34. Rontal E., Rontal M. Vocal cord injection techniques. Otolaryngol Clin North Am. 1991;24:1141-1149.
35. van Berge Henegouwen D.P., Roukema J.A., de Nie J.C., van de Werken C. Esophageal perforation during surgery on the cervical spine. Neurosurgery. 1991;29:766-768.
36. Smith M.D., Emery S.E., Dudley A., et al. Vertebral artery injury during anterior decompression of the cervical spine: a retrospective review of 10 patients. J Bone Joint Surg Br. 1993;75:410-415.
37. Wilson D.H., Campbell D.D. Anterior cervical discectomy without bone graft: report of 71 cases. J Neurosurg. 1977;47:551-555.
38. Murphy M.G., Gado M. Anterior cervical discectomy without interbody graft fusion. J Neurosurg. 1972;37:71-74.
39. Cuatico W. Anterior cervical discectomy without interbody fusion: an analysis of 81 cases. Acta Neurochir (Wien). 1981;57:269-274.
40. Rosenørm J., Hansen E.B., Rosenørm M.A. Anterior cervical discectomy with and without fusion: a prospective study. J Neurosurg. 1983;59:252-255.
41. Savolainen S., Rinne J., Hernesniemi J. A prospective randomized study of anterior single-level cervical disc operations with long-term follow-up: surgical fusion is unnecessary. Neurosurgery. 1998;43:51-55.
42. Maurice-Williams R.S., Elsmore A. Extended anterior cervical decompression without fusion: a long-term follow-up study. Br J Neurosurg. 1999;13:474-479.
43. Zdeblick T.A., Ducker T.B. The use of freeze-dried allograft bone for anterior cervical fusions. Spine (Phila Pa 1976). 1991;16:726-729.
44. Schnee C.L., Freese A., Weil R.J., Marcotte P.J. Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine (Phila Pa 1976). 1997;22:2222-2227.
45. Stephen D.M., Sherry D.C., Orr D.R., et al. Prospective comparison of cervical lordotic interbody fusion cages versus plated ACF for single level fusion. In Proceedings of the 27the annual meeting of the Cervical Spine Research Society. Rosemont, IL: Cervical Spine Research Society; 1991. 263-265
46. Cook S.D., Dalton J.E., Tan E.H., et al. In vivo evaluation of anterior cervical fusions with hydroxylapatite graft material. Spine (Phila Pa 1976). 1994;19:1856-1866.
47. Emery S.E., Fisher J.R., Bohlman H.H. Three-level anterior cervical discectomy and fusion: radiographic and clinical results. Spine (Phila Pa 1976). 1997;22:2622-2625.
48. Swank M.L., Lowery G.L., Bhat A.L., McDonough R.F. Anterior cervical allograft arthrodesis and instrumentation: multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6:138-143.
49. Spivak J.M., Chen D., Kummer F.J. The effect of locking screws on the stability of anterior cervical plating. Spine (Phila Pa 1976). 1999;24:334-338.
50. Chen I.H. Biomechanical evaluation of subcortical versus bicortical screw purchase in anterior cervical plating. Acta Neurochir (Wien). 1996;138:167-173.
51. Goel A. Tricortical cervical inter-body screw fixation. J Postgrad Med. 1997;43:4-7.
52. Pimenta L., McAfee P.C., Cappuccino A., et al. Superiority of multilevel cervical arthroplasty outcomes versus single-level outcomes: 229 consecutive PCM prostheses. Spine (Phila Pa 1976). 2007;32:1337-1344.
53. Emery S.A., Fisher J.R., Bohlman H.H. Three-level anterior cervical discectomy and fusion: Radiographic and clinical results. Spine. 1997;22:2622-2625.
54. Swank M.L., Lowery G.L., Bhat A.L., et al. Anterior cervical allograft arthrodesis and instrumentation: Multilevel interbody grafting or strut graft reconstruction. Eur Spine J. 1997;6:138-143.
55. Leach JCD, Pereira EAC, Chandran H, Cadoux-Hudson TAD. Multi-level cervical disc disease managed by ACDF with stand-alone intervertebral cages: safe and effective. Paper AB3. Presented at Spine Week 2008. Geneva, Switzerland.
56. Demircan M.N., Kutlay A.M., Colak A., et al. Multilevel cervical fusion without plates, screws or autogenous iliac crest bone graft. J Clin Neurosci. 2007;14:723-728.
57. Hilibrand A.S., Fry M.A., Emery S.E., et al. Improved rate of arthrodesis with strut grafting after multilevel anterior cervical decompression. Spine (Phila Pa 1976). 2002;27:146-151.
58. Wang J.C., McDonough P.W., Endow K., Delamarter R.B. Increased fusion rates with cervical plating for two-level anterior cervical discectomy and fusion. Spine (Phila Pa 1976). 2000;25:41-45.
59. Grubb M.R., Currier B.L., Shih J.S., et al. Biomechanical evaluation of anterior cervical spine stabilization. Spine (Phila Pa 1976). 1998;23:886-892.
60. Zoëga B., Kärrholm J., Lind B. One-level cervical spine fusion: a randomized study, with or without plate fixation, using radiostereometry in 27 patients. Acta Orthop Scand. 1998;69:363-368.
61. Katsuura A., Hukuda S., Imanaka T., et al. Anterior cervical plate used in degenerative disease can maintain cervical lordosis. J Spinal Disord. 1996;9:470-476.
62. Harsh G.R.4th, Sypert G.W., Weinstein P.R., et al. Cervical spine stenosis secondary to ossification of the posterior longitudinal ligament. J Neurosurg. 1987;67:349-357.
63. Okada K., Shirasaki N., Hayashi H., et al. Treatment of cervical spondylotic myelopathy by enlargement of the spinal canal anteriorly, followed by arthrodesis. J Bone Joint Surg Am. 1991;73:352-364.
64. Kojima T., Waga S., Kubo Y., et al. Anterior cervical vertebrectomy and interbody fusion for multi-level spondylosis and ossification of the posterior longitudinal ligament. Neurosurgery. 1989;24:864-872.
65. Zdeblick T.H., Bohlman H.H. Cervical kyphosis and myelopathy: treatment by anterior corpectomy and strut-grafting. J Bone Joint Surg Am. 1989;71:170-182.
66. Seifert V., Stolke D. Multisegmental cervical spondylosis: treatment by spondylectomy, microsurgical decompression, and osteosynthesis. Neurosurgery. 1991;29:498-503.
67. Boni M., Cherubino P., Denaro V., Benazzo F. Multiple subtotal somatectomy: technique and evaluation of a series of 39 cases. Spine (Phila Pa 1976). 1984;9:358-362.
68. Saunders R.L., Bernini P.M., Shirreffs T.G.Jr., Reeves A.G. Central corpectomy for cervical spondylotic myelopathy: a consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74:163-170.
69. Hanai K., Fujiyoshi F., Kamei K. Subtotal vertebrectomy and spinal fusion for cervical spondylotic myelopathy. Spine (Phila Pa 1976). 1986;11:310-315.
70. MacDonald R.L., Fehlings M.G., Tator C.H., et al. Multilevel anterior cervical corpectomy and fibular allograft fusion for cervical myelopathy. J Neurosurg. 1997;86:990-997.
71. Fessler R.G., Steck J.C., Giovanini M.A. Anterior cervical corpectomy for cervical spondylotic myelopathy. Neurosurgery. 1998;43:257-267.
72. Saunders R.I., Pikus H.J., Ball P. Four-level cervical corpectomy. Spine (Phila Pa 1976). 1998;23:2455-2461.
73. Isomi T., Panjabi M.M., Wang J.L., et al. Stabilizing potential of anterior cervical plates in multilevel corpectomies. Spine (Phila Pa 1976). 1999;24:2219-2223.
74. Panjabi M.M., Isomi T., Wang J.L. Loosening at the screw-vertebra junction in multilevel anterior cervical plate constructs. Spine (Phila Pa 1976). 1999;24:2383-2388.
75. Vaccaro A.R., Falatyn S.P., Scuderi G.J., et al. Early failure of long segment anterior cervical plate fixation. J Spinal Disord. 1998;11:410-415.
76. Connolly P.J., Esses S.I., Kostuik J.P. Anterior cervical fusion: outcome analysis of patients fused with and without anterior cervical plates. J Spinal Disord. 1996;9:202-206.
77. Graham J.J. Complications of cervical spine surgery: a five-year report on a survey of the membership of the Cervical Spine Research Society by the Morbidity and Mortality Committee. Spine (Phila Pa 1976). 1989;14:1046-1050.
78. Mummaneni M.V., Kaiser P.G., Matz M.G., et al. Joint Section on Disorders of the Spine and Peripheral Nerves of the American Association of Neurological Surgeons and Congress of Neurological Surgeons. Cervical surgical techniques for the treatment of cervical spondylotic myelopathy. J Neurosurg Spine. 2009;11:130-141.
79. Goel A., Pareikh S. Limited oblique corpectomy for treatment of ossified posterior longitudinal ligament. Neurol India. 2005;53:280-282.
80. George B., Zerah M., Lot G., Hurth M. Oblique transcorporeal approach to anteriorly located lesions in the cervical spinal canal. Acta Neurochir (Wien). 1993;121:187-190.