CHAPTER 280 Anterior Approach for Cervical Spondylotic Myelopathy
The progression of cervical spondylosis can be insidious, and symptoms can range from relatively asymptomatic or minor findings to significant spinal cord compression with associated myelopathic findings. These symptoms result from degenerated cervical intervertebral disks, herniated disks, bulging disks, or disk-osteophyte complexes. The radiographic incidence of cervical spondylosis has been quoted as 20% to 25% in the population 50 years or younger and 70% to 95% in the 65-year-old age group.1,2 Despite radiographic findings suggestive of degenerative cervical disk disease, relatively few patients are symptomatic and most have transient episodes that respond to conservative measures.
Historical Perspective
At the turn of the 20th century, Elliot first reported how arthritis in the cervical spine appeared to be responsible for the development of radicular symptoms caused by compression at the neural foramina.3 Stookey later revealed pathologic syndromes caused by cervical disk herniations that were incorrectly attributed to chondromas.4 Stookey divided the compression of these extradural chondromas into three regions: (1) those compressing half of the ventral aspect of the spinal cord, (2) those compressing both halves of the spinal cord, and (3) those compressing laterally on the nerve roots. Elsberg classified these chondroma-like lesions as ecchondrosis and local hyperplasia of the cartilage.5 Stookey and Elsberg were the first surgeons to address the surgical removal of chronic degenerated cervical disks. The classic paper by Mixter and Barr first presented the correlation between radicular symptoms and lateral herniation of the lumbar disks.6 This observation propelled an intense interest in the role of cervical disk degeneration and herniation in the development of cervical radiculopathy and myelopathy.
The surgical approach to the treatment of cervical disk disease was refined in the 20th century. In 1955, Smith and Robinson described their anterior approach for removing the disk and performing an arthrodesis with a horseshoe-shaped bone graft7 and later expanded the success of this technique.8,9 In 1958, Cloward presented his technique for removing the disk and performing an interbody fusion with a cylindrical bone graft.10 In 1960, Bailey and Badgley introduced interbody fusion through a keystone technique.11 In the late 1960s, Verbiest expanded the anterior approach to incorporate a more anterolateral exposure for resecting the foramen transversarium and controlling the vertebral artery.12 These procedures have enjoyed a tremendous amount of success in the treatment of cervical spondylosis and degenerative disk disease. The advent of new imaging modalities has further improved understanding of the natural history of cervical spondylosis. Moreover, new spinal instrumentation permits safe and extensive decompression with excellent clinical outcomes.
Cervical Spine Anatomy
The articulation between vertebral bodies is formed by the disks. Intervertebral disks consist of a semifluid gelatinous matrix, the nucleus pulposus, surrounded by the annulus fibrosus. The intervertebral disk adheres firmly to the vertebral bodies via the cartilaginous end plate. More posteriorly, the position of the nucleus pulposus is slightly eccentric. In younger age groups, the nucleus pulposus has a high water content. With time, however, it dehydrates, thereby leading to degeneration. The annulus fibrosus consists of well-defined fibrous lamellae that adhere strongly to the vertebral end plate. In addition to containing the nucleus pulposus, the annulus fibrosus withstands considerable shearing and tensile forces from all directions. The annulus fibrosus is innervated by the sinuvertebral nerve, which might play a role in the origin of diskogenic pain.13
Biomechanics
Within the cervical spine, several functional regions are responsible for motion. Approximately 40% of axial rotation occurs at the atlantoaxial joints. The anatomic configuration of the atlantoaxial joint, which is stabilized by the transverse and alar ligaments, permits only rotation. The remaining axial rotation is evenly distributed among the subaxial vertebrae, but the middle and lower (C4-7) segments provide the most motion, partially because of the orientation of their facets. Almost 30% to 50% of flexion occurs at the occipital-C2 region; the remainder is distributed unevenly throughout the spine, with the lower cervical segments being mostly responsible.14
Pathophysiology: the Degenerative Process
Most reports on the natural history of cervical spondylosis and myelopathy were published before the advent of contemporary imaging techniques and do not accurately represent the true natural history of cervical spondylosis.15–21 New imaging techniques, however, are improving our understanding of the natural history and progression of symptomatic cervical spondylosis and its pathophysiology. Gore and coauthors1 reported that the radiographic incidence of cervical spondylosis in asymptomatic patients was about 95% in men and 70% in women in the seventh decade of life.
In 1963, Lees and Turner reported their data on a group of patients suffering from cervical stenosis with and without myelopathy.18 They found that the development of symptoms was typically followed by periods of improvement or stabilization. Of the 44 patients with myelopathy, only 5 showed progressive symptoms at the time of last follow-up. In contrast, in 1967 Symon and Lavender reported that 67% of their patients with conservatively treated cervical myelopathy had a steadily progressive course.22
More recent studies have also described a more worrisome course for cervical myelopathy. Shimomura and associates observed 56 patients after nonsurgical treatment of cervical spondylotic myelopathy as defined by a Japanese Orthopedic Association score of 13 or higher.23 Although most patients remained stable, 11 deteriorated and suffered moderate or severe symptoms. In contrast to partial compression, circumferential compression of the spinal cord was predictive of deterioration.
The onset of symptoms is generally in the sixth decade, and men are affected more than women. C5-6 and C6-7 are the levels most involved because of their relatively extensive range of motion. The onset of symptoms is usually insidious, with long periods of stabilization and intermittent episodes of decline. The outcome of symptomatic cervical spondylosis depends on the severity of the radiculopathy and myelopathic signs and the age of patients when they seek treatment.15,16,19,21
The anatomy of the cervical spine and its relationship to the neural elements are unique in comparison to other regions of the spine. The cross-sectional area of the cervical spinal canal is almost entirely occupied by the spinal cord and exiting nerve roots. In contrast, the lumbar region is mostly occupied by nerve roots. This anatomic relationship, coupled with increasing cervical motion, makes the cervical spine vulnerable to small degenerative changes that might become manifested clinically.24,25
As degeneration ensues, fissures in the annulus make the disk more susceptible to herniation and narrow its height. As the disk narrows, the facet joints override each other, and the neurocentral joints rub against the superior end plates. Reparative efforts between adjacent end plates and the joints cause sclerosis of subchondral bone and the formation of osteophytes. As the extent of contact surfaces and the transfer of force to equilibrate the new biomechanical demands increase, the surfaces expand and form disk-osteophyte complexes that constrict the neural foramen and spinal canal.19,25–27
Unlike the lumbar nerve roots, which exit the foramen after a long oblique course, the cervical nerve roots exit through the neural foramen in a shorter, more direct transverse route. Moreover, the cervical neural foramen is mostly filled by the nerve root. These features make it difficult for the nerve roots to accommodate a decrease in the surface area of their foraminal exit. Consequently, clinical radiculopathy can be associated with an insignificantly small to moderately sized bone spur. Another potential cause of a disk-osteophyte complex is calcified herniated disk material.28 Overt trauma might exacerbate the symptoms of cervical spondylosis; however, its association with the development of cervical spondylosis has not been clearly established.29
As a degenerating disk fails biomechanically, load shared by the facets increases. Together with the ligamentum flavum, these structures become hypertrophied, thus further compromising the spinal canal posteriorly and the neural foramen.30 As the facet joints degenerate, this region becomes incompetent to shear stress, thereby leading to spondylolisthesis or retrolisthesis. Posterior disk-osteophyte complexes form from the inferior articular joint and may further constrict the dimensions of the intervertebral foramen and cause clinical symptoms.31 Occasionally, disk-osteophyte complexes become quite large and yet are clinically silent. However, in individuals with congenital cervical stenosis, relatively small osteophytes can compromise a large percentage of the spinal canal and produce significant clinical findings. In patients with spinal canals larger than 13 mm in the AP dimension, symptomatic cervical myelopathy rarely develops.32,33
In addition to anatomic constraints, the area of the cervical canal changes during flexion and extension.30,34 During flexion, the spinal cord lengthens and bows anteriorly such that it abuts the posterior surfaces of the vertebral column.35 In the presence of a posteriorly displaced osteophytic complex, the spinal cord stretches over these bars. As documented in autopsy studies, chronic changes develop.30,35 Local changes in areas of significant compression might affect the physiologic state of local neurons or axons via compressive forces or vascular compromise (venous or arterial).30,32,36
During extension, the posterior elements become a critical factor in the development of cervical stenosis. Extension of the cervical spine allows the ligamentum flavum to buckle inward and shortens the cross-sectional area available for the spinal cord. The spinal cord also shortens during extension, which increases its cross-sectional area and further compromises the cervical canal.32,37 In patients with degenerated disks, loss of height, disk-osteophyte complexes, and hypertrophied joints, as seen in older adults, extension injuries can be neurologically devastating.38
The overriding hypertrophied facets narrow the foramen and impale the exiting nerve roots. The size of the foramen can be further compromised by lateral bending and flexion, which causes radicular symptoms.32
The critical size of the spinal canal and foramen responsible for clinical symptoms is difficult to characterize with current imaging modalities. Individual anatomic and local biomechanical factors are responsible for the development of these symptoms and must be evaluated carefully on an individual basis.30,39 Excessive motion can cause ligamentous laxity and segmental instability and thereby contribute to myelopathic symptoms. The ligaments become incompetent and hypertrophied, and this condition is usually seen adjacent to surgically or degenerated fused levels. Excessive segmental loading results in degenerative subluxation, which further compromises the spinal canal.29
The actual pathophysiologic mechanism of myelopathy is not fully understood. Proposed theories include direct compression of the neural elements, ischemia from compromise of the vasculature, and repetitive microtrauma to the spinal cord with neck movements. The actual mechanism is probably a combination of direct microtrauma and vascular compromise. A study using a canine model of chronic cervical compression showed the characteristic irreversible pathologic findings of large motor neuron loss, necrosis, and cavitation in the region of the anterolateral gray matter at the level of greatest compression.40 There were also potentially reversible changes such as edema and demyelination. The authors postulated that changes in the dimensions of the spinal cord and canal in response to movement pinches the cord when the neck is in certain positions. The pinching then interferes with the microvasculature and causes ischemia in watershed areas.
Clinical Manifestations
Physical findings in myelopathic patients include lower motor neuron disease at the level of the lesion and upper motor neuron disease below the level of the lesion. Hoffman’s sign may be elicited by flicking the middle finger and observing flexion contractions of the thumb and index finger. Reflex abnormalities can include hyporeflexia at the level of the compromised nerve root and hyperreflexia distally. Other findings may include altered suspended sensory disturbances, diffuse spasticity, diffuse hand weakness, proximal lower extremity weakness, clonus, and Babinski’s sign. The differential diagnosis of patients with symptoms of cervical spondylosis who may have myelopathy can include intrinsic medullary tumors, syringomyelia, multiple sclerosis, extramedullary tumors in the cervical or thoracic spine, subacute combined degeneration, hereditary spastic paraplegia, amyotrophic lateral sclerosis, normal-pressure hydrocephalus, and arteriovenous malformations.2,20,29,41–43
Axial neck pain can result from segmental instability caused by disk degeneration and from direct nerve root compression. Myofascial syndromes have also been implicated in the development of axial neck pain.44 Occipital pain has been attributed to arthritic changes or instability at the C1 and C2 junction causing compression of the exiting C2 nerve root.45 Shoulder pain may be related to cervical disk degeneration, brachial neuritis, or nerve root compression at C3, C4, or C5.46–49
The symptoms of cervical radiculopathy follow a specific dermatomal pattern corresponding to the involved nerve root. These features can include loss of motor strength, decreased reflexes, loss of sensation, and well-delineated pain along the dermatome of the nerve root. The most common radiculopathies involve the C5, C6, and C7 nerve roots (also see Chapter 278).24,50
Unusual manifestations of cervical spondylosis can include Brown-Séquard syndrome with ipsilateral hemiparesis, contralateral loss of pain and temperature sensation, and ipsilateral loss of joint perception.51 Although rare, an anterior spinal artery syndrome can result from thrombosis caused by compression of this vessel. This syndrome is associated with complete loss of motor function and sensation below the lesion with preserved joint and vibratory sensation. Vertebrobasilar insufficiency, manifested by nausea, vertigo, dizziness, and visual disturbances, has also resulted from osteophytic overgrowth in the transverse foramen.52,53 Anterior osteophytic spurs can grow quite large and cause dysphagia. This condition, however, is rare because osteophytes grow slowly and the esophagus is flexible and mobile.54,55
Diagnostic Studies
The diagnosis of cervical spondylotic myelopathy and radiculopathy includes the use of radiologic or electrophysiologic studies. Radiographic evaluation of cervical disease begins with comprehensive plain radiographic studies that include AP, lateral, and oblique views. Swimmer’s views are used to assess the cervicothoracic junction. Flexion and extension views can be helpful in patients with a history of trauma, evidence of spondylolisthesis, and previous surgical fusions. Plain radiographs must be evaluated carefully for the presence of congenital stenosis, misalignment, degenerative changes, and instability. A spinal canal less than 12 mm in diameter is considered to be abnormally narrow. One difficulty in relying on plain radiographs is the almost ubiquitous findings of cervical spondylosis in the aging population and the presence of degenerative changes in asymptomatic patients.1,34,56,57 Moreover, plain radiographs are inadequate for the assessment of soft tissue compression, such as ligamentous and disk herniation.
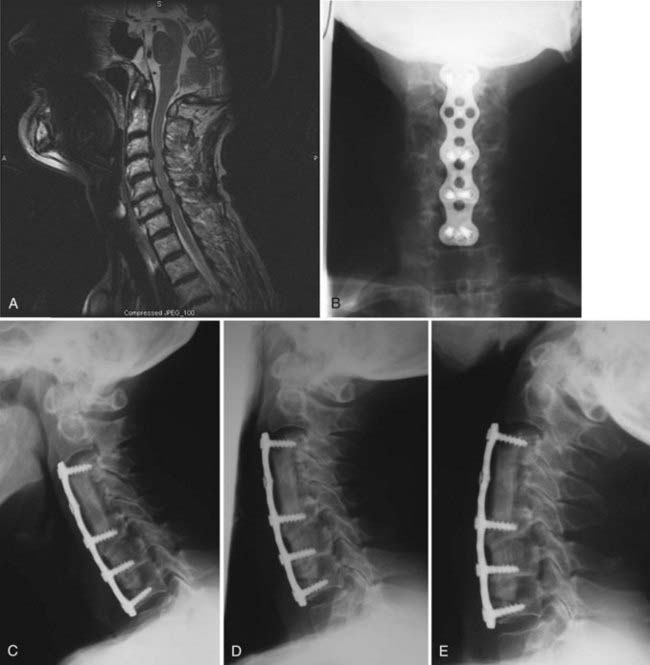
Magnetic resonance imaging (MRI) has become the mainstay for assessing cervical degenerative changes. It is rapid and accurate and does not involve the use of ionizing radiation. It is now the preferred method for screening patients suspected of having radiculopathy or myelopathy (Fig. 280-1).58–60 MRI clearly visualizes the neural elements, as well as ligamentous and soft tissue structures. High-intensity signals within the spinal cord occur in areas of severe compression and suggest intrinsic neural damage, which has implications for postoperative prognosis.36,61–63 MRI can provide excellent visualization of ligamentous disruption caused by trauma.64 This modality images the cervical spine in a multiplanar fashion, thereby facilitating visualization and anatomic definition of the disks and neural foramina and their compressive relationship to the exiting nerve roots. MRI can visualize bone marrow changes that suggest neoplastic, degenerative, inflammatory, or infectious processes. Moreover, MRI is a valuable tool for postoperative evaluation. However, it cannot adequately assess bony or osseous features, which should be imaged with computed tomography (CT).
CT provides cross-sectional views of the cervical spine, which allows clear visualization of disk-osteophyte complexes and calcified ridges. Myelography via injection of a water-soluble contrast agent into the subarachnoid space can be used to visualize the spinal cord and segmental nerve roots. Lateral and AP radiographs provide limited definition of compressive lesions. However, the combination of cervical myelography with postmyelography CT provides excellent definition of osteophytic ridges, herniated disks, and their relationship to the nerve roots and spinal cord. In cases of severe compression, CT-myelography allows visualization distal to myelographic blocks.65 CT-myelography is complementary to MRI for patients suffering from an ossified PLL and for those who have previously undergone instrumented fusion. In some cases, CT-myelography has provided enough definition to avoid vertebrectomy, thereby providing another point of fixation for the instrumentation and enhancing stability.
Electrodiagnostic studies can also be used to evaluate patients with radiculopathy or myelopathy. Such tests include electromyography (EMG), nerve conduction velocity (NCV), and somatosensory evoked potentials (SSEPs). These studies are usually performed when there are discrepancies between the clinical and radiographic findings or when other underlying conditions such as amyotrophic lateral sclerosis, multiple sclerosis, or peripheral neuropathy are suspected. EMG and NCV are performed concomitantly and can differentiate among radiculopathy, peripheral nerve pathology, and brachial plexus pathology. All of these electrodiagnostic tools suffer from a lack of specificity and sensitivity in localizing or grading the extent of compression. In selected cases, changes in SSEPs may serve as an objective measure of the progression of cervical spondylosis.66 In their model of chronic spinal cord compression, Al-Mefty and coworkers36 found that changes in SSEPs were present almost immediately before or at the time of initial neurological evaluation.
Preoperative diagnostic images must be evaluated carefully to ensure appropriate surgical planning.
Surgical Decision Making
As with any surgical procedure, the risks and benefits to patients should be evaluated on a case-by-case basis. Factors such as age and medical comorbidity, which clearly influence the surgical outcome, must be considered. Boakye and colleagues pooled the data of more than 46,562 patients from the National Inpatient Sample who underwent anterior spinal fusion for cervical spondylotic myelopathy.67 When compared with patients younger than 45 years, those 65 to 85 years old were 8 times more likely to suffer an adverse event after surgery. When compared with that same group, patients older than 85 years were 60 times more likely to experience an adverse event. Medical comorbid conditions such as hypertension, lung disease, diabetes, and obesity were also found to be predictors of adverse surgical events. Patients with one, two, and three comorbid conditions were 1.4, 2.2, and 2.8 times more likely, respectively, to suffer an adverse event when compared with patients without a significant comorbidity.
Operative Technique for the Anterior Approach
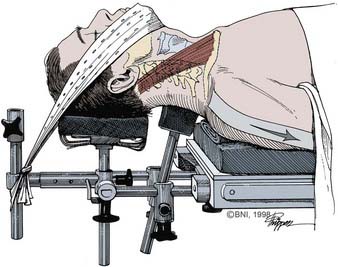
Once the leads are placed, the patient’s neck is extended slightly. A small towel roll may be placed in the interscapular space, or the patient can be placed in a Caspar head holder (Fig. 280-2; Aesculap, San Francisco). Five- to 10-pound traction might be placed with the use of Gardner-Well tongs or an occipitomental traction device.
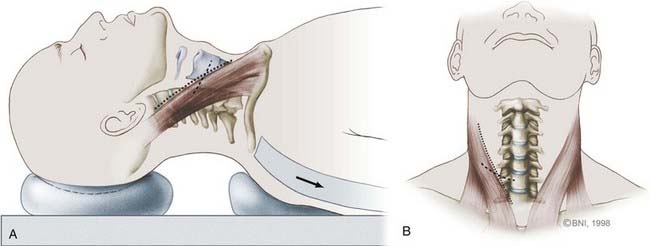
For simple one- or two-level diskectomies, a transverse incision is made along a skin crease. When multilevel diskectomies or corpectomies are being considered, the border of the sternocleidomastoid is incised obliquely (Fig. 280-3). The transverse incision should be 5 or 6 cm long and extend medially near the anatomic midline. Once the incision is delineated, the area is prepared and draped in sterile fashion. If an autograft will be harvested, this area, usually the left iliac crest, is also prepared for surgical access.
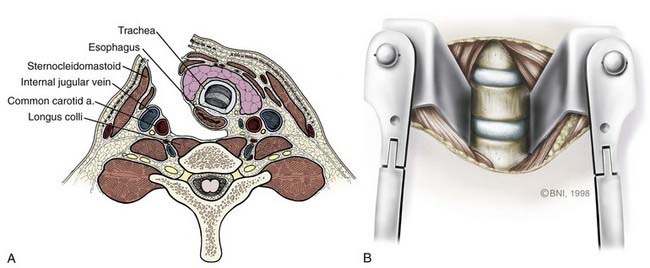
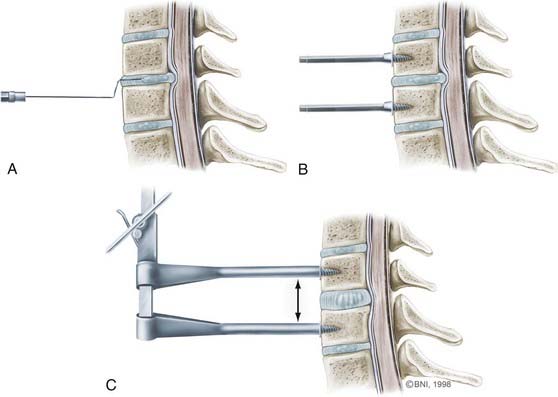
Once the sternocleidomastoid muscle is mobilized, the surgeon can feel the pulsations of the carotid artery with digital palpation. The carotid sheath is retracted laterally with Cloward retractors, and the trachea and esophagus are retracted medially (Fig. 280-4A). Two fascial layers, the pretracheal and prevertebral layers, are identified and easily dissected to expose the spine. The longus colli muscles are identified laterally, and the anterior longitudinal ligaments are seen to overlie the anterior aspect of the cervical spine. To identify the level of interest, lateral radiographs are obtained with a bayonetted spinal needle in the disk space or a Caspar distracting pin in an adjacent vertebral body (see Fig. 280-5A).
Once the correct level is identified, the longus colli muscle is dissected laterally off the anterior vertebral body with bipolar cauterization and periosteal elevators. The muscle is mobilized from its medial insertion in a rostrocaudal direction to provide about 20 mm of exposure of the anterior aspect of the vertebral body, disk, or both. Aggressive dissection of the muscle can disrupt the sympathetic fibers that course along its medial edge or inadvertently injure the vertebral artery.68,69
Once the muscle is mobilized, self-retaining retractors are placed with the teeth of the retractor underneath the muscle (Fig. 280-4B). Placing the retractors incorrectly can cause excessive retraction on the esophagus and carotid artery. A second set of retractors can be placed in a rostrocaudal direction to gain full exposure of the area of interest. Alternatively, Caspar distracting pins can be placed at the midlevel of the vertebral body to obtain adequate exposure and provide distraction to facilitate identification of the intervertebral space (see Fig. 280-5B and C).
Diskectomy and Corpectomy
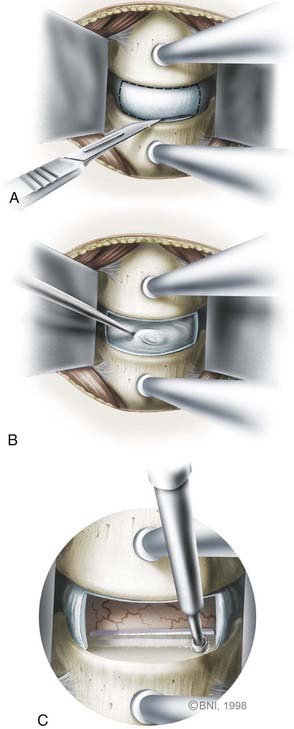
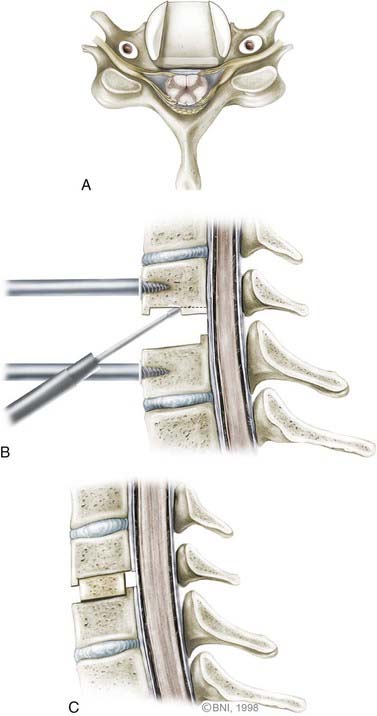
Once the anterior aspect of the spine is exposed, the microscope is brought into the surgical field for the diskectomy. The diskectomy begins by removing the anterior aspect of the annulus fibrosis circumferentially with a sharp knife (Fig. 280-6). The superficial disk is resected with curets and pituitary rongeurs. When significant osteophytic ridges are present, a high-speed, small-diameter bur is used to approach the PLL cautiously. A mental picture of the midline is necessary to avoid wandering too far laterally. The Luschka joints are excellent anatomic landmarks that help the surgeon avoid inadvertent injury to the vertebral artery, which lies immediately lateral to the joint.
Central disk-osteophyte complexes can be removed safely by using thin-plated Kerrison punches and minimizing their protrusion into the spinal canal. Alternatively, osteophytes can be thinned, elevated, and removed with upgoing curets. In patients with radiculopathy, decompression of the foramen must be ample, which might require drilling and resecting the Luschka joints to visualize the exiting nerve root clearly (Fig. 280-7). Nerve root decompression can also be confirmed by palpating the pedicle of the lower cervical vertebrae with a blunt hook. Bleeding from epidural veins is controlled with low-powered bipolar coagulation devices or with other materials such as Surgifoam (Baxter, Deerfield, IL) or Avitene (MecChem, Woburn, MA).
When a corpectomy is performed, diskectomies are performed above and below the corpectomy sites to obtain a visual gauge of where the spinal canal lies (Fig. 280-8). The longus colli muscle is dissected laterally until the body begins to curve laterally and posterolaterally. The vertebrectomy can begin by using a narrow Leksell rongeur spanning from one disk to another. The rest of the corpectomy can be performed by using a high-speed drill and drilling deep into the posterior cortex. At this time the cortex is elevated with upgoing curets, and the PLL is identified posteriorly. Large emissary and epidural veins are carefully controlled with bipolar coagulation. At levels of severe compression, osteophytic ridges that might be adherent to the underlying dura are dissected carefully.
Fusion
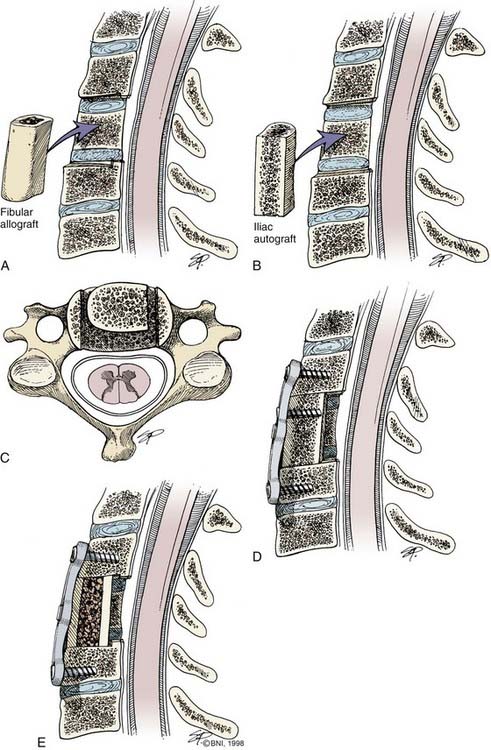
Once the diskectomy or corpectomy is performed, the end plates are prepared to enhance bony fusion. After diskectomy, three types of fusion techniques, which have been modified over the last decades, can be used. The Cloward technique uses a cylindric bone dowel from the iliac crest or a specially prepared iliac allograft.10 The surgeon prepares the diskectomy site by drilling a circular hole 10 to 14 mm deep and 12 to 16 mm in diameter. The bone graft mostly sits on soft cancellous bone, which predisposes it to a slight degree of collapse in comparison to the other techniques. Despite this minor disadvantage, the Cloward technique effectively deals with cervical disk disease.
Another technique, popularized by Simmons and Bhalla,70 uses a keystone-shaped graft. The graft is seated in a triangularly shaped notch located at each end plate and oriented posteriorly. With the use of intraoperative traction, the graft is fitted and locked into place to prevent it from migrating posteriorly or anteriorly. Similar to Cloward’s technique, a substantial portion of the graft sits on soft cancellous bone, which predisposes the graft to settling and potential kyphosis.
Traditionally, the use of allograft or autograft from either the iliac crest or fibula has depended on the surgeon’s preferences and experience. More recently, multiple studies have highlighted the morbidity associated with harvesting autografts.71–75 The proportion of patients with chronic pain after iliac crest harvest is reported to be as high as 26%, with pain medication being used by a significant number.75 Although some surgeons believe that iliac crest donor site morbidity is overestimated, it cannot be ignored as a drawback to the use of autograft.71 Consequently, the use of allograft or synthetic materials in anterior cervical procedures appears to be increasing. Available graft materials include cadaveric allograft, titanium, polyetheretherketone (PEEK), hydroxyapatite, and tantalum, as well as other experimental materials.76–79 Fusion rates with these materials are comparable to those of autograft but without the morbidity associated with harvest of the iliac crest.76,77,80 Synthetic materials are usually packed with bone shavings collected as the end plates are drilled. Allograft is associated with fusion rates similar to those of autograft and is more cost-effective than many synthetic alternatives.
Recombinant human bone morphogenetic protein-2 (rhBMP-2) has been used in conjunction with synthetic materials such as PEEK to increase the rate of fusion with good results. Currently, the only U.S. Food and Drug Administration–approved use for rhBMP-2 is anterior lumbar fusion, but its use in the cervical spine is being investigated. Caution is necessary because a dose-dependent increase in the rate of postoperative hematoma, respiratory distress, and dysphagia appears to be associated with the use of rhBMP-2 in the cervical spine.81,82 It is thought that rhBMP-2 diffuses into the surrounding soft tissues and causes excess inflammation. More studies are needed to clarify the appropriate use of rhBMP-2 in the cervical spine.
Careful preparation of the end plate ensures successful incorporation of the graft and prevents it from being dislodged.83,84 Meticulous attention is needed to measure the height of the graft accurately and to modify it to preserve normal cervical lordosis. In both diskectomy and corpectomy, the grafts are placed while the end plates are distracted. After the graft is placed, the distraction is removed slowly to provide compression along the graft site to enhance fusion according to Wolfe’s law. Lateral radiographs are obtained to assess for evidence of overdistraction, which could cause postoperative pain, and to confirm good bone-to-graft surface contact.
Plating
A variety of plates are currently available for the cervical spine. The intention of this chapter is not to promote or discuss any particular plating system in detail. Rather, the basic principles of anterior instrumentation and plating will be discussed. Some of the advantages of cervical plating include increased segmental stiffness, prevention of graft-related complications, and restoration of cervical lordosis. Improvement in fusion success rates with anterior cervical plating for a one-level fusion has been questioned.85,86 However, more compelling data support the use of anterior plating for multilevel fusions.87,88
Once the interbody graft is placed and plating is considered, the surgeon has to remove any anteriorly located osteophytes to allow flush contact between the plate and vertebral body. Such removal can be performed with a high-speed drill or with Leksell or pituitary rongeurs. Careful attention is needed to avoid removing the anterior cortex of the vertebral body because it provides a significant amount of resistance to screw pullout. The interbody graft is not countersunk; it is placed flushed with the anterior vertebral body margin to maximize the contact surface with the fusion construct. When a corpectomy autograft is used, the graft itself can be secured to the plate with a bicortical screw. This maneuver cannot be performed with fibular allograft, which is too brittle to accept any screw without affecting its structural integrity.89,90 Depending on the plating system used, the surgeon has the option of using fixed or variably angled screws, and most of the new systems have locking mechanisms to resist screw pullout.
Real-time fluoroscopy helps in monitoring screw placement. The screw should be placed in dense bone tissue in the subchondral region of the bone without violating the end plate. Violation of the end plate by the screw can result in physiomechanical alteration of a normal adjacent segment. The ideal torque is two-finger tightness to avoid stripping the screw. If stripping occurs, the construct can be secured with a larger diameter rescue screw or by moving the entire plate and redirecting new screw trajectories. In addition, methyl methacrylate can be infused into the initial hole to bolster purchase of the screw. The cervical plates can also be contoured to maximize surface contact. However, manipulation can fatigue the plate and should be minimized. When multilevel diskectomies or corpectomies are being performed, multiple fixation points can provide a more biomechanically sound construct. Multiple fixation points are particularly important at the caudal levels, where most of the stress is placed on the construct.89,90
Complications
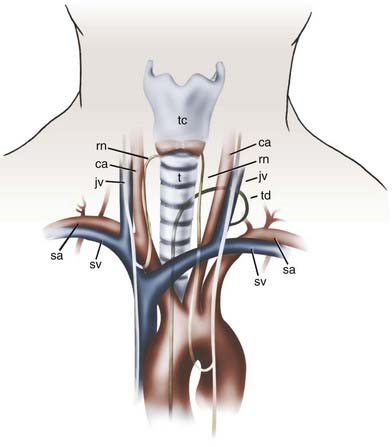
Various complications have been reported after anterior spinal surgery, the most common of which are transient vocal cord paralysis, breathing difficulties, dysphagia, and odynophagia.89,90 Permanent vocal cord paralysis is rare, and its incidence has been estimated to be 0.5% to 1%.91,92 When hoarseness is present postoperatively, it usually resolves within weeks to months. Breathing difficulties may result from swelling of soft tissue after prolonged and excessive retraction or from postoperative hematomas. Large hematomas that compromise the airway should be addressed surgically. Several vital structures are also at risk for injury during anterior spinal approaches, including the carotid artery, jugular veins, trachea, and esophagus (Fig. 280-9).
Careful dissection and meticulous attention to retractor placement should prevent injuries to these structures. The retractors need to be placed under direct vision beneath the longus colli muscle bilaterally to avoid inadvertent injury to the esophagus. Although rare, esophageal injuries carry significant morbidity with the possible development of mediastinitis and lethal abscesses. After surgery is completed, the length of the esophagus needs to be inspected thoroughly to check for rents. Methylene blue dye can be instilled into the pharynx while any suspicious areas are carefully visualized for extravasation of dye. If a rent is identified, it needs to be addressed and repaired primarily. Treatment of an infected esophageal repair requires surgical revision, drainage, nasogastric aspiration, intravenous or local antibiotics (or both), and in some cases esophageal diversion.93,94
Severe neurological injuries are very rare after anterior cervical procedures.95 Such injuries might result from surgical trauma, overdistraction with spinal cord impingement, or retropulsion of interbody grafts. If postoperative neurological deterioration is encountered, an epidural hematoma should be suspected and addressed in emergency fashion. Vertebral artery injuries are also rare and result from loss of midline orientation, screw placement, or aggressive resection of laterally placed disk osteophytes.96 Cerebrospinal fluid fistulas are uncommon. They usually occur in patients with an ossified PLL and extensive dural adhesions. Most defects are small and can be handled by the local application of a Gelfoam pledget and fibrin glue and elevation of the head of the bed after surgery. In such cases, direct closure is technically difficult. If there are any concerns, lumbar drainage can be implemented with good results. Other rare complications can include Horner’s syndrome (anhydrosis, miosis, and ptosis) from interruption of the sympathetic chain located along the anterior surface of the longus colli, pneumothorax when addressing pathology located at the cervicothoracic junction, and thoracic duct disruption when the lower cervical region is approached through the left side.
Complications from iliac crest harvest can include localized pain, meralgia paresthetica from disruption of the lateral femoral cutaneous nerve, wound infections, and hip fractures. Use of an oscillating saw rather than an osteotome might reduce the incidence of hip fractures.97 Graft displacement and angulation have been reported in 2% to 8% of cases.70,98 A well-fitted graft under compression may reduce the incidence of this problem. Depending on the symptoms, these extruded grafts might require surgical intervention.
A risk for pseudarthrosis has been reported in most series addressing anterior cervical diskectomy and fusion.70,85,86,88,98–106 The rate of pseudarthrosis with a one-, two-, and three-level fusion has been reported to be as high as 20%, 50%, and 56%, respectively.107–114 Recent studies of cervical plates have reported significant improvements in fusion rates for two- and three-level fusions.87,115 The development of pseudarthrosis does not necessarily result in poor surgical outcomes. However, after patients with symptomatic pseudarthrosis undergo posterior fusion at the involved levels, their symptoms could resolve. Other potential complications with instrumentation include esophageal erosion and hardware failure. Use of a nonconstraining screw-plate interface might allow graft settling and prevent the plate and screw from breaking.
Al-Mefty O, Harkey HL, Marawi I, et al. Experimental chronic compressive cervical myelopathy. J Neurosurg. 1993;79:550-561.
Bakay L, Leslie EV. Surgical treatment of vertebral artery insufficiency caused by cervical spondylosis. J Neurosurg. 1965;23:596-602.
Baskin JJ, Vishteh AG, Sonntag VKH. Techniques of anterior cervical plating. Oper Tech Neurosurg. 1998;1:90-102.
Bertalanffy H, Eggert HR. Complications of anterior cervical discectomy without fusion in 450 consecutive patients. Acta Neurochir (Wien). 1989;99:41-50.
Boakye M, Patil CG, Santarelli J, et al. Cervical spondylotic myelopathy: complications and outcomes after spinal fusion. Neurosurgery. 2008;62:455-461.
Bulger RF, Rejowski JE, Beatty RA. Vocal cord paralysis associated with anterior cervical fusion: considerations for prevention and treatment. J Neurosurg. 1985;62:657-661.
Cloward RB. The anterior approach for removal of ruptured cervical disks. Neurosurgery. 1958;15:602-617.
Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11:521-524.
Henderson CM, Hennessy RG, Shuey HMJr, et al. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: A review of 846 consecutively operated cases. Neurosurgery. 1983;13:504-512.
Lees F, Turner JW. Natural history and prognosis of cervical spondylosis. Br Med J. 1963;2:1607-1610.
Montgomery DM, Brower RS. Cervical spondylotic myelopathy. Clinical syndrome and natural history. Orthop Clin North Am. 1992;23:487-493.
Panjabi M, White AIII. Biomechanics of nonacute cervical spinal cord trauma. Spine. 1988;13:838-842.
Robinson RA, Smith GW. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull Johns Hopkins. 1955;96:223-224.
Rowland LP. Surgical treatment of cervical spondylotic myelopathy: time for a controlled trial. Neurology. 1992;42:5-13.
Shields LB, Raque GH, Glassman SD, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31:542-547.
Shimomura T, Sumi M, Nishida K, et al. Prognostic factors for deterioration of patients with cervical spondylotic myelopathy after nonsurgical treatment. Spine. 2007;32:2474-2479.
Wang JC, McDonough PW, Endow KK, et al. Increased fusion rates with cervical plating for two-level anterior cervical discectomy and fusion. Spine. 2000;25:41-45.
White AAIII, Panjabi MM. Biomechanical considerations in the surgical management of cervical spondylotic myelopathy. Spine. 1988;13:856-860.
1 Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11:521-524.
2 Montgomery DM, Brower RS. Cervical spondylotic myelopathy. Clinical syndrome and natural history. Orthop Clin North Am. 1992;23:487-493.
3 Elliott G. A contribution to spinal osteoarthritis involving the cervical region. J Bone Joint Surg Am. 1926;8:42-52.
4 Stookey B. Compression of the spinal cord due to ventral extradural chordomas: diagnosis and surgical treatment. Arch Neurol Psychiatry. 1928;20:275-291.
5 Elsberg CA. Extradural spinal tumors—primary, secondary, metastatic. Surg Gynecol Obstet. 1928;46:10-20.
6 Mixter WJ, Barr JS. Rupture of the intervertebral disc with involvement of the spinal canal. N Engl J Med. 1934;211:210-214.
7 Robinson RA, Smith GW. Anterolateral cervical disc removal and interbody fusion for cervical disc syndrome. Bull Johns Hopkins. 1955;96:223-224.
8 Robinson R, Walker AE, Ferlic DC, et al. The results of an anterior interbody fusion of the cervical spine. J Bone Joint Surg Am. 1962;44:1587.
9 Smith GW, Robinson RA. The treatment of certain spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg Am. 1958;40:607-624.
10 Cloward RB. The anterior approach for removal of ruptured cervical disks. Neurosurgery. 1958;15:602-617.
11 Bailey RW, Badgley CE. Stabilization of the cervical spine by anterior fusion. J Bone Joint Surg Am. 1960;42:565-594.
12 Verbiest H. A lateral approach to the cervical spine: technique and indications. J Neurosurg. 1968;28:191-203.
13 Bogduk N, Windsor M, Inglis A. The innervation of the cervical intervertebral discs. Spine. 1988;13:2-8.
14 Panjabi M, White AIII. Biomechanics of nonacute cervical spinal cord trauma. Spine. 1988;13:838-842.
15 Campbell AM, Phillips DG. Cervical disk lesions with neurological disorder. Differential diagnosis, treatment, and prognosis. Br Med J. 1960;2:481-485.
16 Clarke E, Robinson PK. Cervical myelopathy: a complication of cervical spondylosis. Brain. 1956;79:483-510.
17 Gregorius FK, Estrin T, Crandall PH. Cervical spondylotic radiculopathy and myelopathy. A long-term follow-up study. Arch Neurol. 1976;33:618-625.
18 Lees F, Turner JW. Natural history and prognosis of cervical spondylosis. Br Med J. 1963;2:1607-1610.
19 Nurick S. The pathogenesis of the spinal cord disorder associated with cervical spondylosis. Brain. 1972;95:87-100.
20 Rowland LP. Surgical treatment of cervical spondylotic myelopathy: time for a controlled trial. Neurology. 1992;42:5-13.
21 Phillips DG. Upper limb involvement in cervical spondylosis. J Neurol Neurosurg Psychiatry. 1975;38:386-390.
22 Symon L, Lavender P. The surgical treatment of cervical spondylotic myelopathy. Neurology. 1967;17:117-127.
23 Shimomura T, Sumi M, Nishida K, et al. Prognostic factors for deterioration of patients with cervical spondylotic myelopathy after nonsurgical treatment. Spine. 2007;32:2474-2479.
24 Friedenberg ZB, Miller WT. Degenerative disc disease of the cervical spine. J Bone Joint Surg Am. 1963;45:1171-1178.
25 Vernon-Roberts B, Pirie CJ. Degenerative changes in the intervertebral discs of the lumbar spine and their sequelae. Rheumatol Rehabil. 1977;16:13-21.
26 Payne EE, Spillane JD. The cervical spine; an anatomico-pathological study of 70 specimens (using a special technique) with particular reference to the problem of cervical spondylosis. Brain. 1957;80:571-596.
27 Raynor RB, Koplik B. Cervical cord trauma. The relationship between clinical syndromes and force of injury. Spine. 1985;10:193-197.
28 Wilkinson M. The morbid anatomy of cervical spondylosis and myelopathy. Brain. 1960;83:589-617.
29 Brain WR, Northfield D, Wilkinson M. The neurological manifestations of cervical spondylosis. Brain. 1952;75:187-225.
30 White AAIII, Panjabi MM. Biomechanical considerations in the surgical management of cervical spondylotic myelopathy. Spine. 1988;13:856-860.
31 Kelsey JL, White AAIII, Pastides H, et al. The impact of musculoskeletal disorders on the population of the United States. J Bone Joint Surg Am. 1979;61:959-964.
32 Parke WW. Correlative anatomy of cervical spondylotic myelopathy. Spine. 1988;13:831-837.
33 Zeidman SM, Ducker TB. Cervical disk disease: part I. Treatment options and outcomes. Neurosurg Q. 1992;2:116-143.
34 Alexander J. Natural history and nonoperative management of cervical spondylosis. In: Menezes AH, Sonntag VKH, editors. Principles of Spinal Surgery. New York: McGraw-Hill; 1996:547-557.
35 Breig A, Turnbull I, Hassler O. Effects of mechanical stresses on the spinal cord in cervical spondylosis. A study on fresh cadaver material. J Neurosurg. 1966;25:45-56.
36 Al-Mefty O, Harkey HL, Marawi I, et al. Experimental chronic compressive cervical myelopathy. J Neurosurg. 1993;79:550-561.
37 Epstein JA, Carras R, Lavine LS, et al. The importance of removing osteophytes as part of the surgical treatment of myeloradiculopathy in cervical spondylosis. J Neurosurg. 1969;30:219-226.
38 Symonds C. The interrelation of trauma and cervical spondylosis in compression of the cervical cord. Lancet. 1953;1:451-454.
39 Mann KS, Khosla VK, Gulati DR. Cervical spondylotic myelopathy treated by single-stage multilevel anterior decompression. A prospective study. J Neurosurg. 1984;60:81-87.
40 Harkey HL, Al-Mefty O, Marawi I, et al. Experimental chronic compressive cervical myelopathy: effects of decompression. J Neurosurg. 1995;83:336-341.
41 Clark CR. Cervical spondylotic myelopathy: history and physical findings. Spine. 1988;13:847-849.
42 Clifton AG, Stevens JM, Whitear P, et al. Identifiable causes for poor outcome in surgery for cervical spondylosis. Post-operative computed myelography and MR imaging. Neuroradiology. 1990;32:450-455.
43 Li TM, Day SJ, Alberman E, et al. Differential diagnosis of motoneurone disease from other neurological conditions. Lancet. 1986;2:731-733.
44 Travell JG, Simons DG. Myofascial Pain Syndromes and Dysfunction. The Trigger Point Manual. Baltimore: Williams & Wilkins; 1983.
45 Ehni G, Benner B. Occipital neuralgia and the C1-2 arthrosis syndrome. J Neurosurg. 1984;61:961-965.
46 Ahlgren BD, Garfin SR. Cervical radiculopathy. Orthop Clin North Am. 1996;27:253-263.
47 McCarty EC, Tsairis P, Warren RF. Brachial neuritis. Clin Orthop Relat Res. 1999;368:37-43.
48 Ruggieri PM. Cervical radiculopathy. Neuroimaging Clin N Am. 1995;5:349-366.
49 Poletti CE. Third cervical nerve root and ganglion compression: clinical syndrome, surgical anatomy, and pathological findings. Neurosurgery. 1996;39:941-948.
50 Henderson CM, Hennessy RG, Shuey HMJr, et al. Posterior-lateral foraminotomy as an exclusive operative technique for cervical radiculopathy: a review of 846 consecutively operated cases. Neurosurgery. 1983;13:504-512.
51 Jabbari B, Pierce JF, Boston S, et al. Brown-Séquard syndrome and cervical spondylosis. J Neurosurg. 1977;47:556-560.
52 Bakay L, Leslie EV. Surgical treatment of vertebral artery insufficiency caused by cervical spondylosis. J Neurosurg. 1965;23:596-602.
53 Smith DR, Vanderark GD, Kempe LG. Cervical spondylosis causing vertebrobasilar insufficiency: a surgical treatment. J Neurol Neurosurg Psychiatry. 1971;34:388-392.
54 Gamache FWJr, Voorhies RM. Hypertrophic cervical osteophytes causing dysphagia. A review. J Neurosurg. 1980;53:338-344.
55 Ladenheim SE, Marlowe FI. Dysphagia secondary to cervical osteophytes. Am J Otolaryngol. 1999;20:184-189.
56 Irvine DH, Foster JB, Newell DJ, et al. Prevalence of cervical spondylosis in a general practice. Lancet. 1965;1:1089-1092.
57 Wilkinson HA, LeMay ML, Ferris EJ. Clinical-radiographic correlations in cervical spondylosis. J Neurosurg. 1969;30:213-218.
58 Brown BM, Schwartz RH, Frank E, et al. Preoperative evaluation of cervical radiculopathy and myelopathy by surface-coil MR imaging. AJR Am J Roentgenol. 1988;151:1205-1212.
59 Larsson EM, Holtas S, Cronqvist S, et al. Comparison of myelography, CT myelography and magnetic resonance imaging in cervical spondylosis and disk herniation. Pre- and postoperative findings. Acta Radiol. 1989;30:233-239.
60 Vanderburgh DF, Kelly WM. Radiographic assessment of discogenic disease of the spine. Neurosurg Clin N Am. 1993;4:13-33.
61 Al-Mefty O, Harkey LH, Middleton TH, et al. Myelopathic cervical spondylotic lesions demonstrated by magnetic resonance imaging. J Neurosurg. 1988;68:217-222.
62 Wada E, Ohmura M, Yonenobu K. Intramedullary changes of the spinal cord in cervical spondylotic myelopathy. Spine. 1995;20:2226-2232.
63 Takahashi M, Yamashita Y, Sakamoto Y, et al. Chronic cervical cord compression: clinical significance of increased signal intensity on MR images. Radiology. 1989;173:219-224.
64 Vaccaro AR, Madigan L, Schweitzer ME, et al. Magnetic resonance imaging analysis of soft tissue disruption after flexion-distraction injuries of the subaxial cervical spine. Spine. 2001;26:1866-1872.
65 Litt AW. Imaging and diagnosis of degenerative disease of the cervical spine. In: Cooper PR, editor. Degenerative Diseases of the Cervical Spine. Park Ridge, IL: American Association of Neurological Surgeons; 1992:73-90.
66 Restuccia D, Di Lazzaro V, Lo Monaco M, et al. Somatosensory evoked potentials in the diagnosis of cervical spondylotic myelopathy. Electromyogr Clin Neurophysiol. 1992;32:389-395.
67 Boakye M, Patil CG, Santarelli J, et al. Cervical spondylotic myelopathy: complications and outcomes after spinal fusion. Neurosurgery. 2008;62:455-461.
68 Ebraheim NA, Lu J, Yang H, et al. Vulnerability of the sympathetic trunk during the anterior approach to the lower cervical spine. Spine. 2000;25:1603-1606.
69 Lu J, Ebraheim NA, Nadim Y, et al. Anterior approach to the cervical spine: surgical anatomy. Orthopedics. 2000;23:841-845.
70 Simmons EH, Bhalla SK. Anterior cervical discectomy and fusion. A clinical and biomechanical study with eight-year follow-up. J Bone Joint Surg Br. 1969;51:225-237.
71 Delawi D, Dhert WJ, Castelein RM, et al. The incidence of donor site pain after bone graft harvesting from the posterior iliac crest may be overestimated: a study on spine fracture patients. Spine. 2007;32:1865-1868.
72 Sasso RC, LeHuec JC, Shaffrey C. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18(suppl:):S77-S81.
73 Sawin PD, Traynelis VC, Menezes AH. A comparative analysis of fusion rates and donor-site morbidity for autogeneic rib and iliac crest bone grafts in posterior cervical fusions. J Neurosurg. 1998;88:255-265.
74 Schnee CL, Freese A, Weil RJ, et al. Analysis of harvest morbidity and radiographic outcome using autograft for anterior cervical fusion. Spine. 1997;22:2222-2227.
75 Silber JS, Anderson DG, Daffner SD, et al. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine. 2003;28:134-139.
76 Bruneau M, Nisolle JF, Gilliard C, et al. Anterior cervical interbody fusion with hydroxyapatite graft and plate system. Neurosurg Focus. 2001;10(4):E8.
77 Fernandez-Fairen M, Sala P, Dufoo MJr, et al. Anterior cervical fusion with tantalum implant: a prospective randomized controlled study. Spine. 2008;33:465-472.
78 Hwang SL, Lee KS, Su YF, et al. Anterior corpectomy with iliac bone fusion or discectomy with interbody titanium cage fusion for multilevel cervical degenerated disc disease. J Spinal Disord Tech. 2007;20:565-570.
79 Tumialan LM, Pan J, Rodts GE, et al. The safety and efficacy of anterior cervical discectomy and fusion with polyetheretherketone spacer and recombinant human bone morphogenetic protein-2: a review of 200 patients. J Neurosurg Spine. 2008;8:529-535.
80 Samartzis D, Shen FH, Matthews DK, et al. Comparison of allograft to autograft in multilevel anterior cervical discectomy and fusion with rigid plate fixation. Spine J. 2003;3:451-459.
81 Shields LB, Raque GH, Glassman SD, et al. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine. 2006;31:542-547.
82 Vaidya R, Carp J, Sethi A, et al. Complications of anterior cervical discectomy and fusion using recombinant human bone morphogenetic protein-2. Eur Spine J. 2007;16:1257-1265.
83 Saunders RL, Bernini PM, Shirreffs TGJr, et al. Central corpectomy for cervical spondylotic myelopathy: a consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74:163-170.
84 Saunders RL. Corpectomy for cervical spondylotic myelopathy. In: Menezes AH, Sonntag VKH, editors. Principles of Spinal Surgery. New York: McGraw-Hill; 1996:559-569.
85 Connolly PJ, Esses SI, Kostuik JP. Anterior cervical fusion: outcome analysis of patients fused with and without anterior cervical plates. J Spinal Disord. 1996;9:202-206.
86 Wang JC, McDonough PW, Endow K, et al. The effect of cervical plating on single-level anterior cervical discectomy and fusion. J Spinal Disord. 1999;12:467-471.
87 Kaiser MG, Haid RWJr, Subach BR, et al. Anterior cervical plating enhances arthrodesis after discectomy and fusion with cortical allograft. Neurosurgery. 2002;50:229-236.
88 Wang JC, McDonough PW, Endow KK, et al. Increased fusion rates with cervical plating for two-level anterior cervical discectomy and fusion. Spine. 2000;25:41-45.
89 Vishteh AG, Baskin JJ, Sonntag VKH. Techniques of cervical discectomy with and without fusion. Oper Tech Neurosurg. 1998;1:85-89.
90 Baskin JJ, Vishteh AG, Sonntag VKH. Techniques of anterior cervical plating. Oper Tech Neurosurg. 1998;1:90-102.
91 Bertalanffy H, Eggert HR. Complications of anterior cervical discectomy without fusion in 450 consecutive patients. Acta Neurochir (Wien). 1989;99:41-50.
92 Bulger RF, Rejowski JE, Beatty RA. Vocal cord paralysis associated with anterior cervical fusion: considerations for prevention and treatment. J Neurosurg. 1985;62:657-661.
93 Gaudinez RF, English GM, Gebhard JS, et al. Esophageal perforations after anterior cervical surgery. J Spinal Disord. 2000;13:77-84.
94 Smith MD, Bolesta MJ. Esophageal perforation after anterior cervical plate fixation: a report of two cases. J Spinal Disord. 1992;5:357-362.
95 Flynn TB. Neurologic complications of anterior cervical interbody fusion. Spine. 1982;7:536-539.
96 Golfinos JG, Dickman CA, Zabramski JM, et al. Repair of vertebral artery injury during anterior cervical decompression. Spine. 1994;19:2552-2556.
97 Porchet F, Jaques B. Unusual complications at iliac crest bone graft donor site: experience with two cases. Neurosurgery. 1996;39:856-859.
98 Gore DR, Sepic SB. Anterior cervical fusion for degenerated or protruded discs. A review of one hundred forty-six patients. Spine. 1984;9:667-671.
99 Aronson N, Filtzer DL, Bagan M. Anterior cervical fusion by the Smith-Robinson approach. J Neurosurg. 1968;29:396-404.
100 Brodke DS, Zdeblick TA. Modified Smith-Robinson procedure for anterior cervical discectomy and fusion. Spine. 1992;17:S427-S430.
101 Clements DH, O’Leary PF. Anterior cervical discectomy and fusion. Spine. 1990;15:1023-1025.
102 Cloward RB. Complications of anterior cervical disc operation and their treatment. Surgery. 1971;69:175-182.
103 Herkowitz HN, Kurz LT, Overholt DP. Surgical management of cervical soft disc herniation. A comparison between the anterior and posterior approach. Spine. 1990;15:1026-1030.
104 Kozak JA, Hanson GW, Rose JR, et al. Anterior discectomy, microscopic decompression, and fusion: A treatment for cervical spondylotic radiculopathy. J Spinal Disord. 1989;2:43-46.
105 Riley LHJr, Robinson RA, Johnson KA, et al. The results of anterior interbody fusion of the cervical spine. Review of ninety-three consecutive cases. J Neurosurg. 1969;30:127-133.
106 Schneeberger AG, Boos N, Schwarzenbach O, et al. Anterior cervical interbody fusion with plate fixation for chronic spondylotic radiculopathy: a 2- to 8-year follow-up. J Spinal Disord. 1999;12:215-220.
107 Brown MD, Malinin TI, Davis PB. A roentgenographic evaluation of frozen allografts versus autografts in anterior cervical spine fusions. Clin Orthop Relat Res. 1976;119:231-236.
108 Cloward RB. Gas-sterilized cadaver bone grafts for spinal fusion operations. A simplified bone bank. Spine. 1980;5:4-10.
109 Emery SE, Fisher JR, Bohlman HH. Three-level anterior cervical discectomy and fusion: radiographic and clinical results. Spine. 1997;22:2622-2624.
110 Grossman W, Peppelman WC, Baum JA, et al. The use of freeze-dried fibular allograft in anterior cervical fusion. Spine. 1992;17:565-569.
111 Malinin TI, Rosomoff HL, Sutton CH. Human cadaver femoral head homografts for anterior cervical spine fusions. Surg Neurol. 1977;7:249-251.
112 Rish BL, McFadden JT, Penix JO. Anterior cervical fusion using homologous bone grafts: a comparative study. Surg Neurol. 1976;5:119-121.
113 Young WF, Rosenwasser RH. An early comparative analysis of the use of fibular allograft versus autologous iliac crest graft for interbody fusion after anterior cervical discectomy. Spine. 1993;18:1123-1124.
114 Zdeblick TA, Ducker TB. The use of freeze-dried allograft bone for anterior cervical fusions. Spine. 1991;16:726-729.
115 Bolesta MJ, Rechtine GR, Chrin AM. Three- and four-level anterior cervical discectomy and fusion with plate fixation: a prospective study. Spine. 2000;25:2040-2044.