Chapter 19 Anovulation and Ovulatory Dysfunction
INTRODUCTION
Anovulation and ovulatory dysfunction are frequent reasons for gynecologic referral. The most common form of ovulatory dysfunction, polycystic ovary syndrome (PCOS), is estimated to occur in up to 6% to 10% of women.1 Ovulatory dysfunction may result in a spectrum of clinical symptoms ranging from amenorrhea to frequent, irregular, and heavy menses. In addition to menstrual irregularities, many women will experience subfertility or other endocrine symptoms, such as hirsutism. Choice of therapy will depend on numerous factors, including the presenting symptom, age, desire for fertility, and associated conditions.
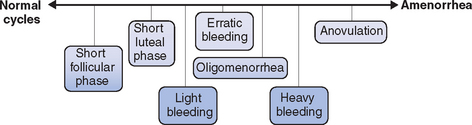
Regardless of the etiology, a spectrum of ovulatory disorders exists that may precede the eventual development of amenorrhea or occur during the recovery from amenorrhea (Fig. 19-1). Underlying abnormalities in ovulatory function may present clinically with either quantitative or qualitative changes in the menstrual cycle. A prolonged follicular phase or intermittent anovulation both result in infrequent (cycles greater than 35 days) and often heavy menstrual flow; a shortened follicular or luteal phase is associated with frequent menstrual flow and often premenstrual spotting.
PATHOPHYSIOLOGY
The Hypothalamus
The pathway ultimately responsible for ovulation is initiated by pulsatile release of gonadotropin-releasing hormone (GnRH) from neurons in the arcuate nucleus of the hypothalamus into the portal circulation. From there, GnRH induces release of the anterior pituitary hormones, luteinizing hormone (LH) and follicle-stimulating hormone (FSH). The amplitude and frequency of GnRH release are critical to normal functioning of the HPO axis.2 Numerous hormones and neurotransmitters are known to modulate GnRH release, including dopamine, norepinephrine, neuropeptide Y, and endorphins. Factors affecting release of these hormones and neurotransmitters or the physical delivery of GnRH to the anterior pituitary may ultimately disrupt ovulation.
Excessive cortisol secretion (due to high corticotropin-releasing hormone activity), high levels of endogenous neuropeptide Y and opioids (specifically endorphins) are all thought to play a role in suppression of GnRH activity associated with psychological stress and eating disorders. Less commonly, injury, infection, or central nervous system (CNS) tumors may interfere with delivery of GnRH to the anterior pituitary via the portal circulation. Hypothalamic dysfunction or failure has been reported following severe deceleration injuries that cause pituitary stalk transection, CNS tumors such as craniopharyngiomas or hamartomas that cause stalk compression, and infiltrative diseases such as tuberculosis or sarcoidosis.
More recently, the role of leptin in maintenance of BMI, regulation of appetite, and control of reproductive function has been investigated. Leptin is a 146-amino acid protein hormone expressed in numerous sites but mainly in adipose tissue. It has been shown to have a direct stimulatory effect on GnRH pulsatility, as well as LH/FSH secretion from the anterior pituitary, and is likely central to control of the HPO axis through a number of complex endocrine and paracrine actions.3 Leptin may act as a sensor of energy deficiency, limiting the high-energy cost of reproduction and growth during periods of starvation. Concentrations decrease rapidly with fasting, resulting in suppression of reproductive, thyroid, and growth hormones. A preliminary study suggests that administration of recombinant human leptin may reverse the neuroendocrine abnormalities associated with hypothalamic amenorrhea, inducing a return of hormone secretion reminiscent of puberty.4
Pituitary Gonadotropins
The secretion of LH and FSH in response to GnRH stimulation varies as puberty progresses. As the HPO axis matures, it is common to see abnormalities in ovulatory function. The average time from onset of menses to establishment of regular ovulatory cycles is 2 to 3 years.5
The Ovary
Depletion of follicles is a common cause of abnormalities in ovulatory function during the perimenopausal transition. Early follicular (day 3) FSH levels rise due to the relative resistance of the remaining follicles to FSH-dependent stimulation and a concomitant decrease in serum inhibin. Accelerated follicular development may occur in response to increased FSH levels, with elevated estradiol secretion early in the follicular phase, ovulation as early as day 8 to 11, and corresponding shortening of the menstrual interval (e.g., from a 28-day to a 23-day pattern).6,7 As follicular number and responsiveness decline, the pattern progresses to oligo-ovulation or anovulation and eventual amenorrhea. This inevitable process of reproductive aging usually begins in the late 30s and may become clinically manifest with changing menstrual patterns throughout the 40s.7
A similar process, albeit occurring at an earlier age, may occur in young women who develop premature ovarian failure with loss of ovarian function before age 40.8,9 Some young women with premature ovarian failure experience premature follicular depletion; others likely have a normal complement of follicles that are unresponsive to gonadotropin stimulation, possibly on an autoimmune basis. Young women with premature ovarian failure may also experience sporadic and transient return of ovarian function after diagnosis, ranging from intermittent estrogen-withdrawal bleeding to normal ovulatory cycles and the possibility of conception.10–12
Lastly, the most common cause of ovulatory dysfunction is PCOS, a complex condition of uncertain etiology. PCOS is the most common endocrine disorder in women and is characterized by hyperestrogenic hyperandrogenic chronic anovulation, which may present clinically with various ovulatory and menstrual disorders, infertility, acne, hirsutism, and obesity.13 Numerous metabolic aberrations may also be associated with PCOS, including obesity, insulin resistance, type 2 diabetes, dyslipidemia, and cardiovascular disease. Insulin resistance appears to play a central role in the reproductive dysfunction and long-term health consequences of PCOS.12
When evaluating women with ovulatory dysfunction, it is important to recognize that PCOS is a syndrome with a broad spectrum of clinical phenotypes, and that no existing classification will encompass all possible phenotypes. The Rotterdam PCOS consensus workshop recently published revised criteria for the diagnosis of PCOS.14 After exclusion of other etiologies (congenital adrenal hyperplasia, androgen-secreting tumors, Cushing’s syndrome), two of three of the following criteria must be present to establish a diagnosis of PCOS:
Although frequently associated with PCOS, obesity in itself may contribute to ovulatory dysfunction.15 Numerous studies, including the Nurses’ Health Study, have reported increased rates of ovulatory infertility with increasing BMI.16,17 It is believed that obesity leads to a state of functional hyperandrogenism. Central (abdominal) obesity is associated with insulin resistance and high circulating insulin levels. Because the production of sex hormone-binding globulin (SHBG) in the liver is directly inhibited by insulin, it follows that women with central obesity have lower levels of SHBG and higher levels of free testosterone than age- and weight-matched controls with peripheral obesity.18 This functional hyperandrogenism, in combination with increased peripheral aromatization of estrogen in adipose tissue, leads to alteration in the balance of sex steroids and ultimately contributes to ovulatory dysfunction.
Other Endocrine Disorders
A number of other endocrine disorders may result in ovulatory dysfunction through disruption of the HPO axis. Hyperprolactinemia, for example, leads to suppression of GnRH secretion.19 Similarly, increased thyrotropin-releasing hormone (TRH) activity in women with hypothyroidism ultimately results in reduced GnRH pulsatility through the direct stimulatory effect of TRH on prolactin secretion. Hyperthyroidism may result in a range of menstrual disturbances, from frequent heavy bleeding to amenorrhea.
Congenital Adrenal Hyperplasia
Women with both classic and late onset congenital adrenal hyperplasia (CAH) may present with ovulatory disturbances similar to PCOS.20 Menstrual abnormalities in these women were initially attributed to inadequate glucocorticoid suppression of adrenal androgen production, resulting in suppression of the HPO axis. Particularly in classic CAH, there is evidence of ovarian hyperandrogenism similar to PCOS despite adequate adrenal suppression with dexamethasone.
It has been postulated that perinatal exposure to hyperandrogenism in women with CAH results in hypersecretion of LH in response to GnRH stimulation at puberty. This allows the development of ovarian hyperandrogenism independent of control of adrenal androgen production.21 A similar abnormality has been reported but is less common in late-onset CAH.
Luteal Phase Defect
The role of luteal phase defect in infertility remains controversial, however. Studies designed to address this issue have demonstrated a significant prevalence of luteal phase defect in the fertile population and are hampered by a lack of consensus regarding diagnostic criteria. Performance of repeated endometrial biopsies to assess histology is often impractical and the interpretation of results limited by a high degree of interobserver variability. Similarly there is no agreement on the appropriate normal values for serum progesterone. Finally, lack of a clear treatment effect with progesterone supplementation has led many to question the importance of luteal phase defect as a cause of infertility.24–26
Luteinized Unruptured Follicle
Lastly, luteinized unruptured follicle syndrome has been reported as a transient or ongoing cause of ovulatory disturbance. The syndrome involves absence of sonographic signs of ovulation or lack of an ovulatory stigma at laparoscopy 48 to 72 hours after the LH surge. The phenomena may occur in conjunction with periovulatory nonsteroidal anti-inflammatory ingestion, leading to inhibition of prostaglandin-induced follicular rupture.27,28 Other cases have been reported in conjunction with suboptimal doses or improperly timed human chorionic gonadotropin injections during ovulation induction cycles.29 Aside from these iatrogenic cases, there is no clear etiology, no documented treatment, and considerable controversy over the diagnostic criteria with their inherent variability and observer biases.29,30 A survey of practice patterns in the United States indicated that only 8% of university-based board-certified reproductive endocrinologists would screen for this disorder.31
ESTABLISHING A DIAGNOSIS
Considerable controversy exists over the criteria used to define luteal phase defects.25,32 Histologic dating of a luteal phase endometrial biopsy specimen has been considered the gold standard, requiring a 2- to 3-day difference between histologic and chronologic dating in two separate cycles.22 However, the test is invasive and uncomfortable, and suffers from substantial intraobserver and interobserver variability.33 Serum progesterone measurements are less invasive, although less accepted due to debate over the threshold level used for diagnosis, the number of suboptimal tests needed, and the relatively poor correlation with endometrial response.24,25,34 Progesterone secretion is both pulsatile (in response to episodic LH release) and parabolic (with peak levels in the midluteal phase), so low levels may occur physiologically in the trough prior to each LH pulse and at the beginning and end of each luteal phase.35 The threshold progesterone value used to differentiate ovulatory from anovulatory cycles (6 to 18 nmol/L or 2 to 5 ng/mL) should not be confused with that suggested for the diagnosis of defective luteal phase (21 to 32 nmol/L or 7 to 10 ng/mL).24,34
DIAGNOSIS
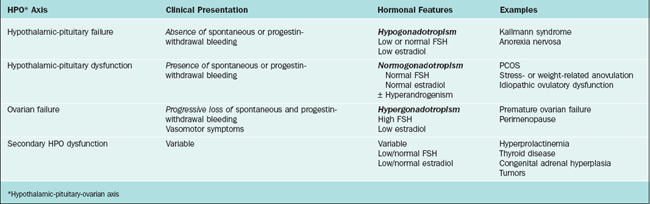
Various classification systems have been developed for ovulatory disorders (Table 19-1). Such systems also provide the basis for a timely and cost-effective evaluation. They revolve around three key principles that can also be used to guide the history, physical examination, investigations, and management (Table 19-2).
Table 19-2 Key Principles for Evaluation of Ovulatory Dysfunction
History
A review of the hypothalamic-pituitary axis may reveal precipitating factors or underlying medical disorders that require specific investigation and treatment. However, many of these symptoms are nonspecific. Hypothalamic factors are common among thin women who present with oligomenorrhea or amenorrhea. Questioning should include sources of excessive life stress, chronic illness, excessive exercise, significant weight change, disordered eating, and an overall preoccupation with thinness.13 Recall that women with hypothalamic causes of amenorrhea rarely report hypoestrogenic symptoms such as vasomotor flushing.
Symptoms of pituitary adenoma or prolactin excess such as severe headaches, visual field change (typically bitemporal hemianopsia), or galactorrhea should be elicited. A medication history may reveal drugs that affect prolactin secretion, including dopamine antagonists (metoclopramide, domperidone) and antipsychotics (risperidone, haloperidol, phenothiazines). Symptoms of thyroid dysfunction (particularly hypothyroidism) are common and notoriously nonspecific, but should be sought in addition to a personal or family history of thyroid disease.
The most common disorder of the HPO axis that will be encountered in anovulatory patients is PCOS. Menstrual disorders, infertility, weight gain, central obesity, hirsutism, and acne are common presenting symptoms. Some affected women will also present with a history of known hypertension, dyslipidemia, or glucose intolerance. Other causes of hyperandrogenism must be excluded, including androgen-secreting tumors, CAH, and Cushing’s syndrome.14
The possibility of premature ovarian failure is high in a young woman who presents with amenorrhea and vasomotor symptoms. However, these patients will frequently progress through a period of shortened menstrual cycles or oligo-ovulation before the onset of amenorrhea.7 Depending on the degree and duration of hypoestrogenism, progestin-withdrawal bleeding may disappear. Symptoms of hypoestrogenism may be absent if the onset was insidious; other women will present with the sudden onset of hot flashes, sleep disturbances, and mood disturbances that may be more marked than those typically seen in the natural menopausal transition. A history of precipitating factors such as chemotherapy, radiation, or extensive ovarian surgery should be sought. A family history of premature ovarian failure is present in 4% to 30%.10 A personal or family history of autoimmune disease such as diabetes, thyroid disease, Addison’s disease, lupus, or vitiligo can also be a valuable clue to potential autoimmune-mediated causes of premature ovarian failure.10
Physical Examination
The physical examination of the anovulatory patient should commence with basic observations including height, weight, calculation of BMI, and assessment of body habitus. Low BMI (>20 kg/m2) may be a clue to hypothalamic dysfunction, whereas elevated BMI (<30 kg/m2) may be suggestive of hypothyroidism (if related to recent increase in weight) or insulin resistance associated with PCOS. Other features of insulin resistance associated with PCOS include central obesity (increased waist/hip ratio) or the presence of acanthosis nigricans, a grey-brown velvety pigmentation frequently seen in the neck, axillae, and groin. Finally, consideration of PCOS should prompt an assessment of clinical hyperandrogenism. The presence of acne or hirsutism and the pattern and severity of each should be documented. An objective tool for assessment of hirsutism is the Ferriman-Gallwey score,36 which is helpful, particularly in the context of research studies in which objective response to antiandrogen treatment is desirable; its utility in routine clinical assessment may be more limited. Male pattern alopecia (temporal hair loss) is also a feature of clinical hyperandrogenism.
Laboratory Evaluation
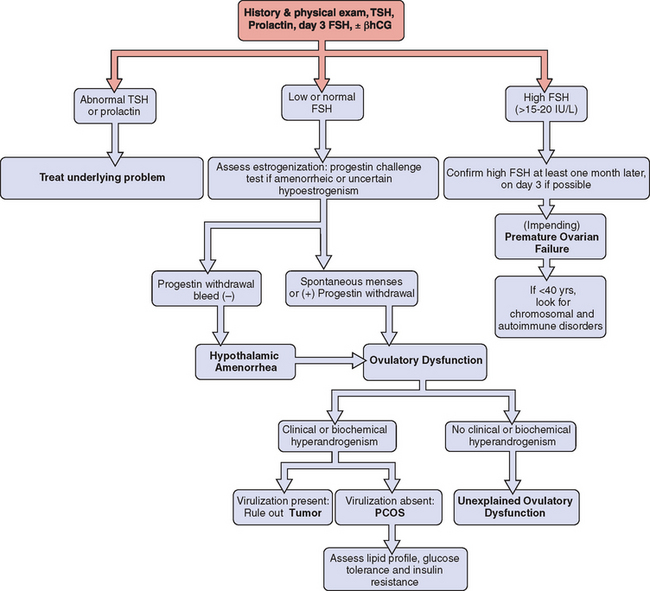
The systematic and cost-effective laboratory evaluation of ovulatory dysfunction is based on the three key principles described in Table 19-2. In the initial investigation, one should always consider the possibility of pregnancy and perform a pregnancy test when clinically indicated. Once pregnancy has been excluded, the investigation should commence with measurement of thyrotropin, prolactin, and FSH (Fig. 19-2).
Thyrotropin and Prolactin
Elevated thyrotropin or prolactin levels will require appropriate investigation and therapy (see Chapter 22), keeping in mind that elevation of both thyrotropin and prolactin is usually the result of hypothyroidism and that hyperprolactinemia will respond to treatment of the underlying thyroid condition.
Gonadotropins
For the purpose of assessing ovarian reserve, measurement of early follicular FSH (day 3) is ideal; however, this may not be practical in the setting of very infrequent menses.6
Elevated FSH concentrations raise the possibility of premature ovarian failure and should be repeated in another cycle for confirmation. Although there is considerable variation among centers, FSH concentrations above 10 to 15 IU/L are suggestive of diminished ovarian reserve, whereas concentrations above 15 to 20 IU/L are highly suggestive of impending or established premature ovarian failure.6 If confirmed, additional testing is prudent in women under age 40 to identify underlying karyotype abnormalities (e.g., Turner’s mosaicism), fragile X premutations, and associated autoimmune disorders.9
Androgens
In the context of clinical hyperandrogenism, it is essential to rule out the possibility of an androgen-secreting tumor. This can frequently be done on the basis of history and physical examination alone. In the absence of rapid onset of hirsutism associated with virilization, an androgen-secreting tumor is extremely unlikely, and serum androgen screening is not required. If uncertain, measurement of testosterone (adrenal or ovarian origin), androstenedione (ovarian origin), and dehydroepiandrostenedione sulfate (adrenal origin) may be helpful. The reliability of different methods of testosterone assessment is highly variable between laboratories. Depending on local assay methodology, free testosterone or free androgen index will likely represent the most accurate assessment of biologically active testosterone.
MANAGEMENT
After ruling out underlying medical disorders that require specific therapy, the next priority is to identify predisposing or contributing factors and provide appropriate counseling or therapy. Examples include excessive stress, anxiety or depressive disorders, excessive weight gain, overt or insidious eating disorders, strenuous exercise without appropriate nutritional intake, or a preoccupation with thinness. Remember that the menstrual cycle is often a barometer of underlying health. The etiology of hypothalamic amenorrhea can often be thought of as “energy drain,” whether it be emotional, physical, or caloric. There is a metabolic cost associated with a menstrual cycle, a luteal phase thermal shift, and subsequent menstrual blood loss. Ovulatory dysfunction is unlikely to resolve until the HPO axis senses sufficient “energy” in reserve to cover this metabolic expenditure and sustain an ensuing pregnancy. The process of identifying and discussing factors that contribute to HPO dysfunction is key to initiating the necessary lifestyle changes and/or therapy.13,37
Fertility
For overweight or underweight women, achievement of a healthy body weight and healthy eating habits may be sufficient to correct the underlying ovulatory disorder. Increased activity and modest weight reduction (at least 5% of body weight) among obese oligo-ovulatory women (BMI <30 kg/m2) with PCOS leads to improvement in ovulatory function and an increase in spontaneous and treatment-associated pregnancy rates.38,39 Similarly, ovulatory function frequently improves among women with a low BMI in response to stepwise reductions in exercise frequency or intensity, increased BMI, increased caloric and protein intake, and/or modification of restricted eating patterns.13
After normalization of body weight and other lifestyle changes as appropriate, ovulation can be induced with clomiphene citrate.40 Induction of ovulation with clomiphene citrate is effective in 75% to 80% of patients, although only 40% to 50% will conceive.41 Predictors of clomiphene citrate resistance include high basal FSH concentrations (impending ovarian failure), absence of progestin-induced withdrawal bleeding (hypogonadotropic amenorrhea), and PCOS associated with high BMI (<30 kg/m2) or severe hyperandrogenism.42,43 More recently, aromatase inhibitors such as letrozole have been used for ovulation induction.44,45 Their role in the treatment of PCOS, and specifically clomiphene-resistant PCOS, remains unclear.46
Various approaches have been examined for the treatment of clomiphene-resistant anovulation. Extending the duration of clomiphene citrate administration to 7 to 10 days (i.e., 100 mg from days 3 to 12) is useful in some women, with a total dose that does not exceed the amount used in a conventional protocol of 200 mg for 5 days.42 In clomiphene-resistant women with documented insulin resistance and/or impaired glucose tolerance, addition of metformin may improve the response to clomiphene citrate.47
Appreciating that insulin resistance is felt to be central to the pathophysiology of PCOS, particularly in obese women, there has been great interest in the use of insulin-sensitizing agents as an adjunct to ovulation induction therapy. A number of randomized, controlled trials support improved ovulation rates with metformin, either alone or in conjunction with clomiphene citrate.47,48 A variable improvement in cycle parameters and pregnancy rates has been reported with metformin use during in vitro fertilization treatment cycles.47 Limited information exists regarding the outcome of pregnancies conceived on metformin or those pregnancies in which metformin was continued during pregnancy. Similarly, there is little information about the optimal duration of therapy prior to conception. It has been our practice to discontinue metformin when conception occurs, or after a 3-month trial if there has been no appreciable weight loss or improvement in clinical or endocrine parameters.
Women who do not ovulate or conceive after making appropriate lifestyle changes and receiving therapy with oral agents become candidates for injectable exogenous gonadotropins. Women with hypothalamic amenorrhea or WHO group I ovulatory disorders lack endogenous gonadotropin activity. As such, exogenous gonadotropins can be expected to restore normal ovulation and pregnancy rates.49 A less predictable response is observed in the heterogeneous group of women with WHO group II ovulatory disorders. Predictors of lower ovulation and pregnancy rates include increasing age, obesity, hyperandrogenism, and failure to conceive despite successful ovulation with clomiphene citrate.49,50
Due to the cost associated with exogenous gonadotropin therapy and the increased risk of multiple pregnancy, some women with clomiphene citrate-resistant PCOS will elect to undergo laparoscopic ovarian drilling in an attempt to restore spontaneous ovulatory function. This technique has been shown to reduce circulating androgen levels and restore ovulation in up to 75% of women treated.51 Concern regarding the longevity of its effect and ultimately compromised fertility due to postoperative adhesions has limited the broad application of this technique. However, it is a reasonable alternative in selected patients.
Contraception
If contraception is needed, combination oral contraceptives (OCs) are generally the first line of therapy because of their combined contraceptive and noncontraceptive benefits. In addition to contraception, they provide menstrual regulation, protection of the endometrium from the effects of prolonged unopposed estrogen stimulation, and relief of acne and hirsutism in hyperandrogenic women. The antiandrogenic effects arise predominantly from hypothalamic-pituitary suppression that leads to diminished androgen production from the ovarian stroma. In addition, the estrogen component rapidly produces an increase in hepatic SHBG production, resulting in a decrease in free testosterone levels.52 Where available, cyproterone acetate, the progestational component of some OCs, has intrinsic antiandrogenic activity and is an effective choice for the treatment of menstrual irregularities associated with hyperandrogenism.
Progestin-only contraceptives (tablets, depot injections, and implants) provide contraception, cycle control, and endometrial protection and may be appropriate alternatives for some women unable to tolerate estrogen-containing OCs or in whom such OCs are contraindicated. Relief of androgenic conditions such as acne and hirsutism is more variable. Progestin-only therapy produces suppression of the HPO axis with a resulting reduction in ovarian androgen production, although possibly to a lesser degree than combination OCs. Although testosterone clearance from the circulation is increased, there is no effect on SHBG production.53 In addition, the synthetic progestins possess some intrinsic androgenic activity that, for some women, will result in a paradoxical exacerbation of their androgenic symptoms.52
Cycle Control and Endometrial Protection
If contraception is not required and fertility is not an issue, the need for menstrual cycle control and endometrial protection should be assessed. There are no clear guidelines for the frequency or pattern of menstrual bleeding that ensures endometrial protection in this young population of women. However, chronic anovulation is associated with an increased risk of endometrial hyperplasia and neoplasia,54,55 particularly in obese women (weight <90 kg).56 Clinical experience suggests that progestin-withdrawal bleeds should be induced every 4 to 12 weeks in the face of chronic anovulation or if menses occur less frequently than every 8 weeks.57 Endometrial biopsy should be performed prior to therapy in women with prolonged anovulation, in those with irregular or heavy bleeding, and in women with other risk factors for endometrial hyperplasia (weight <90 kg, infertility with nulliparity, or PCOS).51,56,58–60
Ovarian Failure
Fertility is often still a concern for young women with hypergonadotropism (see Chapter 20). However, women with rising FSH levels, diminished ovarian reserve, or premature ovarian failure are rarely suitable candidates for ovulation induction therapy.60 Depending on age and other factors, there is a small chance of spontaneous conception during occasional ovulatory cycles. Approximately 5% to 10% of young women with premature ovarian failure conceive spontaneously if they enter into a remission after the diagnosis. Beyond this, oocyte donation offers the only realistic possibility of achieving a pregnancy.10,11
Women with rising FSH levels have a presumptive diagnosis of impending ovarian failure, but may still have a relatively normal menstrual pattern and few other symptoms. Although they generally require no specific therapy, they may benefit from counseling about cardiovascular and bone health, including a healthy diet, regular weight-bearing and muscle strengthening exercise, smoking cessation, modest alcohol intake, and appropriate intake of calcium and vitamin D.
Long-term Health Issues
1 Lobo RA, Carmina E. The importance of diagnosing the polycystic ovary syndrome. Ann Intern Med. 2000;132:989-993.
2 Knobil E. The neuroendocrine control of the menstrual cycle. Recent Prog Horm Res. 1980;36:53-88.
3 Moschos S, Chan JL, Mantzoros CS. Leptin and reproduction: A review. Fertil Steril. 2002;77:433-444.
4 Welt CK, Chan JL, Bullen J, et al. Recombinant human leptin in women with hypothalamic amenorrhea. NEJM. 2004;351:987-997.
5 Altchek A. Dysfunctional uterine bleeding in adolescence. Clin Obstet Gynecol. 1977;20:633-650.
6 Scott RTJr, Hofmann GE. Prognostic assessment of ovarian reserve. Fertil Steril. 1995;63:1-11.
7 Sherman BM, Korenman SG. Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest. 1975;55:699-706.
8 Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604-606.
9 Anasti JN. Premature ovarian failure: An update. Fertil Steril. 1998;70:1-15.
10 van Kasteren YM, Schoemaker J. Premature ovarian failure: A systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5:483-492.
11 Kalantaridou SN, Nelson LM. Premature ovarian failure is not premature menopause. Ann NY Acad Sci. 2000;900:393-402.
12 Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774-800.
13 Perkins RB, Hall JE, Martin KA. Aetiology, previous menstrual function and patterns of neuro-endocrine disturbance as prognostic indicators in hypothalamic amenorrhoea. Hum Reprod. 2001;16:2198-2205.
14 Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19-25.
15 Pasquali R, Pelusi C, Genghini S, et al. Obesity and reproductive disorders in women. Hum Reprod Update. 2003;9:359-372.
16 Rich-Edwards J, Goldman M, Willet W, et al. Adolescent body mass index and infertility caused by ovulatory dysfunction. Am J Obstet Gynecol. 1994;71:171-177.
17 Lake J, Power C, Cole T. Women’s reproductive health—the role of body mass index in early and adult life. Int J Obesity. 1997;21:432-438.
18 VonShoultz B, Calstrom K. On the regulation of sex-hormone binding globulin. A challenge of old dogma and outlines of an alternative mechanism. J Steroid Biochem. 1989;32:327-334.
19 Monroe SE, Levine L, Chang RJ, et al. Prolactin-secreting pituitary adenomas. V. Increased gonadotroph responsivity in hyperprolactinemic women with pituitary adenomas. J Clin Endocrinol Metab. 1981;52:1171-1178.
20 Speiser PW, White PC. Congenital adrenal hyperplasia. NEJM. 2003;349:776-788.
21 Barnes RB, Rosenfield RL, Ehrmann DA, et al. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: Evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79:1328-1333.
22 Noyes RW, Hertig AT, Rock JR. Dating the endometrial biopsy. Fertil Steril. 1950;1:3-25.
23 Tavaniotou A, Albano C, Smitz J, Devroey P. Impact of ovarian stimulation on corpus luteum function and embryonic implantation. J Reprod Immunol. 2000;55:123-130.
24 Li TC, Cooke ID. Evaluation of the luteal phase. Hum Reprod. 1991;6:484-499.
25 Jordan J, Craig K, Clifton DK, Soules MR. Luteal phase defect: The sensitivity and specificity of diagnostic methods in common clinical use. Fertil Steril. 1994;62:54-62.
26 Davis OK, Berkeley AS, Naus GJ, et al. The incidence of luteal phase defect in normal, fertile women, determined by serial endometrial biopsies. Fertil Steril. 1989;51:582-586.
27 Stone S, Khamashta MA, Nelson-Piercy C. Nonsteroidal anti-inflammatory drugs and reversible female infertility: Is there a link? Drug Safety. 2002;25:545-551.
28 Murdoch WJ, Cavender JL. Effect of indomethacin on the vascular architecture of preovulatory ovine follicles: Possible implication in the luteinized unruptured follicle syndrome. Fertil Steril. 1989;51:153-155.
29 Evers JL. The luteinized unruptured follicle syndrome. Baillieres Clin Obstet Gynaecol. 1993;7:363-387.
30 Scheenjes E, te Velde ER, Kremer J. Inspection of the ovaries and steroids in serum and peritoneal fluid at various time intervals after ovulation in fertile women: Implications for the luteinized unruptured follicle syndrome. Fertil Steril. 1990;54:38-41.
31 Glatstein IZ, Harlow BL, Hornstein MD. Practice patterns among reproductive endocrinologists: Further aspects of the infertility evaluation. Fertil Steril. 1998;70:263-269.
32 McNeely MJ, Soules MR. The diagnosis of luteal phase deficiency: A critical review. Fertil Steril. 1988;50:1-15.
33 Duggan MA, Brashert P, Ostor A, et al. The accuracy and interobserver reproducibility of endometrial dating. Pathology. 2001;33:292-297.
34 Daya S, Ward S, Burrows E. Progesterone profiles in luteal phase defect cycles and outcome of progesterone treatment in patients with recurrent spontaneous abortion. Am J Obstet Gynecol. 1988;158:225-232.
35 Filicori M, Butler JP, Crowley WFJr. Neuroendocrine regulation of the corpus luteum in the human. Evidence for pulsatile progesterone secretion. J Clin Invest. 1984;73:1638-1647.
36 Ferriman D, Gallwey J. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440-1447.
37 Speroff L, Glass RH, Kase NG. Clinical Gynecologic Endocrinology and Infertility. Baltimore: Williams and Wilkins, 1999;421-485.
38 Clark AM, Thornley B, Tomlinson L, et al. Weight loss in obese infertile women results in improvement in reproductive outcome for all forms of fertility treatment. Hum Reprod. 1998;13:1502-1505.
39 Kiddy DS, Hamilton-Fairley D, Bush A, et al. Improvement in endocrine and ovarian function during dietary treatment of obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 1992;36:105-111.
40 American Society for Reproductive Medicine Committee Opinion. Use of clomiphene citrate in women. Fertil Steril. 2003;80:1302-1308.
41 Garcia J, Jones GS, Wentz AC. The use of clomiphene citrate. Fertil Steril. 1977;28:707-717.
42 Fluker MR, Wang IY, Rowe TC. An extended 10–day course of clomiphene citrate (CC) in women with CC-resistant ovulatory disorders. Fertil Steril. 1996;66:761-764.
43 Eijkemans MJ, Habbema JD, Fauser BC. Characteristics of the best prognostic evidence: An example on prediction of outcome after clomiphene citrate induction of ovulation in normogonadotropic oligoamenorrheic infertility. Semin Reprod Med. 2003;21:39-47.
44 Mitwally MF, Casper RF. Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril. 2001;75:305-309.
45 de Ziegler D. The dawning of the non-cancer uses of aromatase inhibitors in gynaecology. Hum Reprod. 2003;18:1598-1602.
46 Al-Omari WR, Sulaiman WR, Al-Hadithi N. Comparison of two aromatase inhibitors in women with clomiphene-resistant polycystic ovary syndrome. Int J Gynaecol Obstet. 2004;85:289-291.
47 Costello MF, Eden JA. A systematic review of the reproductive system effects of metformin in patients with polycystic ovary syndrome. Fertil Steril. 2003;79:1-13.
48 Lord JM, Flight IH, Norman RJ. Insulin-sensitising drugs (metformin, troglitazone, rosiglitazone, pioglitazone, D-chiro-inositol) for polycystic ovary syndrome. Cochrane Database Syst Rev. 2003. CD003053.
49 Fluker MR, Urman B, Mackinnon M, et al. Exogenous gonadotropin therapy in World Health Organization groups I and II ovulatory disorders. Obstet Gynecol. 1994;83:189-196.
50 Mulders AG, Laven JS, Eijkemans MJ, et al. Patient predictors for outcome of gonadotrophin ovulation induction in women with normogonadotrophic anovulatory infertility: A meta-analysis. Hum Reprod Update. 2003;9:429-449.
51 Farquhar CM, Lethaby A, Sowter M, et al. An evaluation of risk factors for endometrial hyperplasia in premenopausal women with abnormal menstrual bleeding. Am J Obstet Gynecol. 1999;181:525-529.
52 van der Vange N, Blankenstein MA, Kloosterboer HJ, et al. Effects of seven low-dose combined oral contraceptives on sex hormone binding globulin, corticosteroid binding globulin, total and free testosterone. Contraception. 1990;41:345-352.
53 Gordon GG, Southren AL, Tochimoto S, et al. Effect of medroxyprogesterone acetate (Provera) on the metabolism and biological activity of testosterone. J Clin Endocrinol Metab. 1970;30:449-456.
54 Coulam CB, Annegers JF, Kranz JS. Chronic anovulation syndrome and associated neoplasia. Obstet Gynecol. 1983;61:403-407.
55 Cheung AP. Ultrasound and menstrual history in predicting endometrial hyperplasia in polycystic ovary syndrome. Obstet Gynecol. 2001;98:325-331.
56 Ballard-Barbash R, Swanson CA. Body weight: Estimation of risk for breast and endometrial cancers. Am J Clin Nutr. 1996;63:437S-441S.
57 Balen A. Polycystic ovary syndrome and cancer. Hum Reprod Update. 2001;7:522-525.
58 Gibson M. Reproductive health and polycystic ovary syndrome. Am J Med. 1995;98:67S-75S.
59 Munro MG. Abnormal uterine bleeding in the reproductive years. Part I—pathogenesis and clinical investigation. J Am Assoc Gynecol Laparosc. 1999;6:393-416.
60 Scott RT, Opsahl MS, Leonardi MR, et al. Life table analysis of pregnancy rates in a general infertility population relative to ovarian reserve and patient age. Hum Reprod. 1995;10:1706-1710.