CHAPTER 6
Anorectum
INTRODUCTION
Management of diseases of the anorectum has traditionally been relegated to the proctologist, often a surgeon. The ease of sigmoidoscopy and the advent of video technology have provided the tools for endoscopists to assume a more active role in the evaluation and treatment of these disorders. Visual inspection of the perianal area coupled with digital examination should always precede endoscopic examination because subtle clues to underlying pathology may be identified. Retroflexion best permits evaluation of distal lesions, particularly those at or above the dentate line. A careful inspection of the anal canal should also be performed, especially for patients reporting anorectal pain and for patients with pain elicited on digital examination. Evaluation of the anal canal can also be performed with a disposable plastic anoscope.
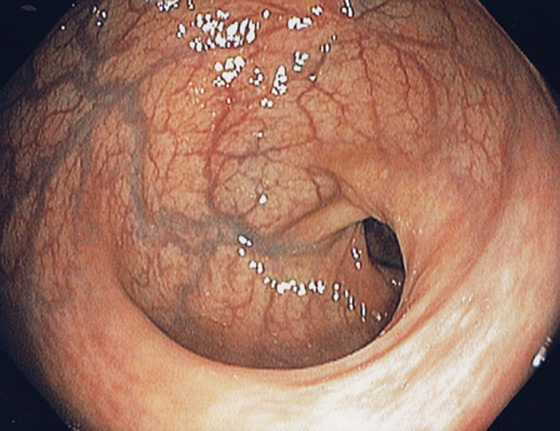
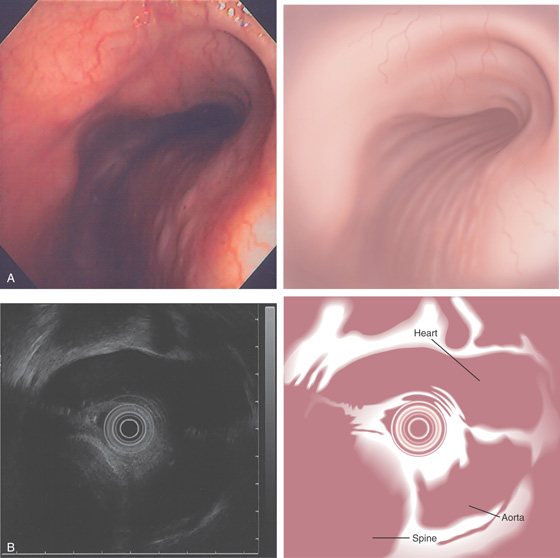
Figure 6.1 RECTUM
The rectum is characterized by prominent vascularity, in contrast with the sigmoid and proximal colon.
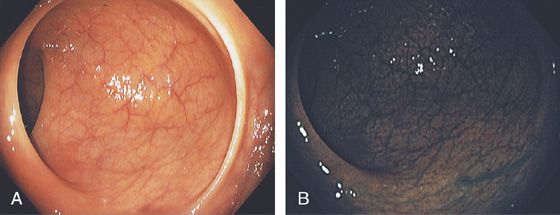
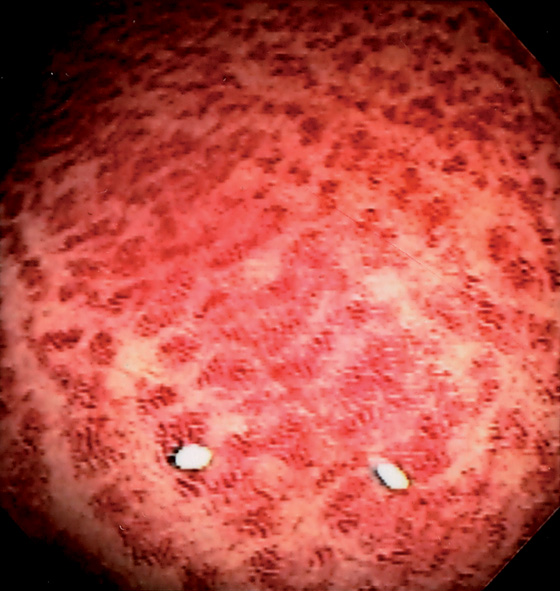
Figure 6.2 VALVES OF HOUSTON
A, The three valves of Houston are semilunar structures, two on the right and one on the left. In this patient in the left lateral decubitus position, the two valves on the right are anterior. B, Narrow band imaging demonstrates the subtle vascular pattern of the rectum.
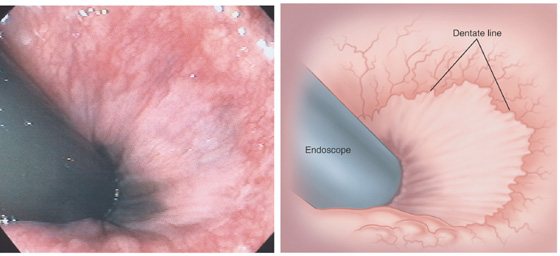
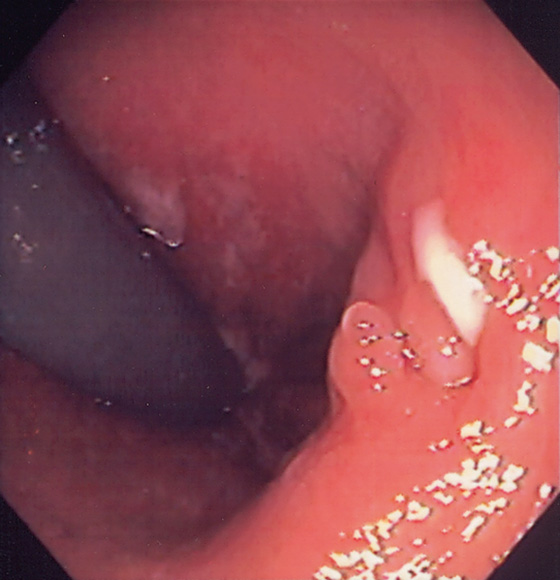
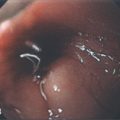
Figure 6.3 ANORECTAL JUNCTION ON RETROFLEXION
Retroflexion at the anorectal junction shows the dentate line demarcating the squamous mucosa from the colonic mucosa. In this case, black pigment of the squamous mucosa is near the endoscope.
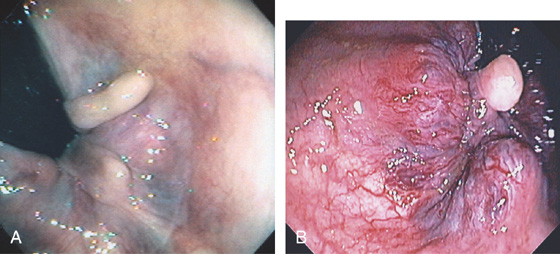
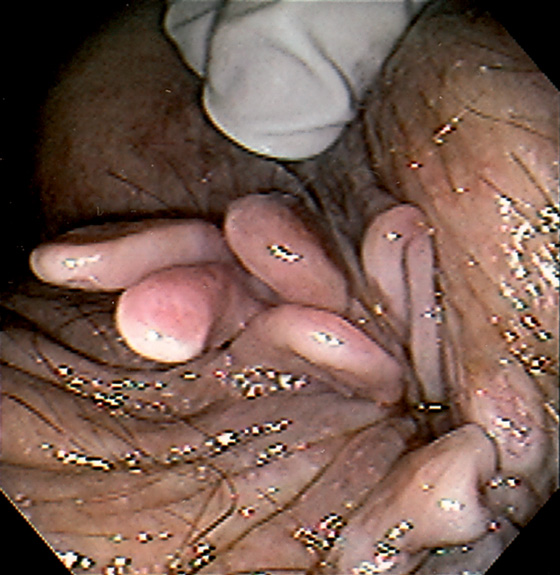
Figure 6.4 HYPERTROPHIED ANAL PAPILLAE
A, Three white polypoid structures are distal to the dentate line. These normal structures are hypertrophied, simulating a polyp on digital rectal examination. B, Solitary skin tag associated with enlarged hemorrhoidal tissue.
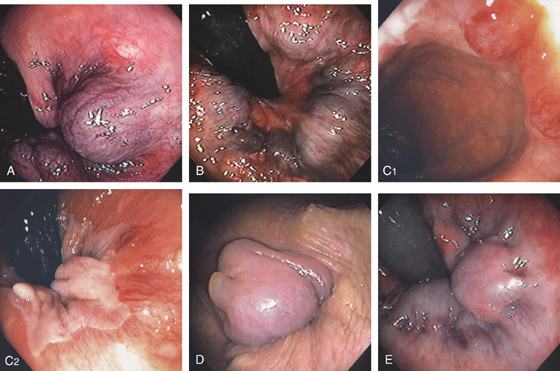
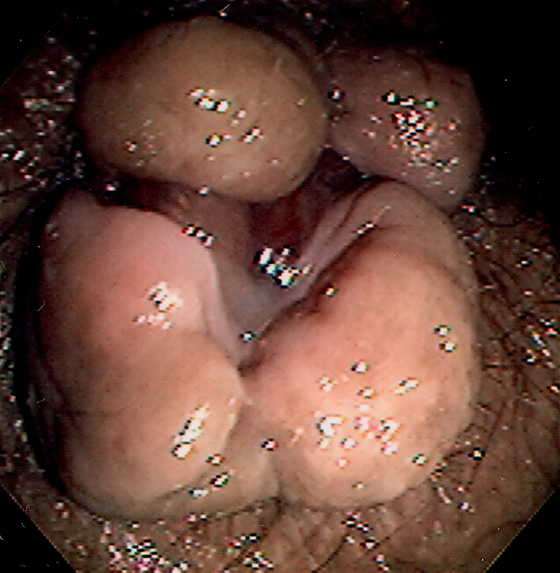
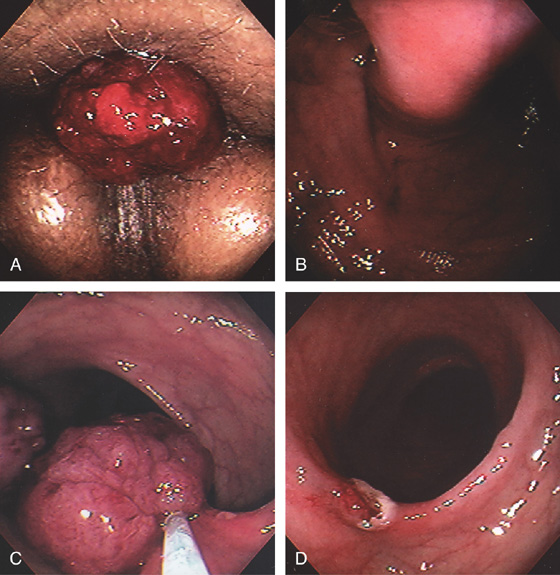
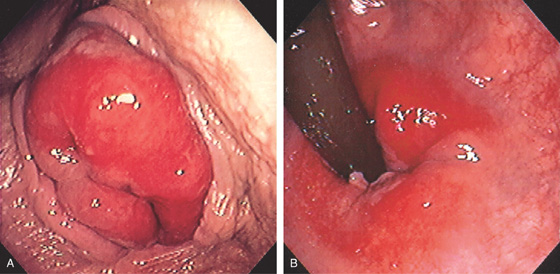
Figure 6.5 HEMORRHOIDAL DISEASE
INTERNAL HEMORRHOIDS
A, Although vascular cushions at the anorectum (hemorrhoids) are normal, this structure is markedly enlarged in this particular patient. The venous enlargement is proximal to the dentate line. B, Multiple internal hemorrhoids are at the dentate line. C1, Two reddish vascular tufts with overlying vasculature at the dentate line as seen on antegrade withdrawal of the colonoscope. C2, Retroflexed view confirms the location of the hemorrhoidal tissue proximal to the dentate line.
EXTERNAL HEMORRHOIDS
D, Thrombosed external hemorrhoid is shown. E, The enlarged vascular cushion is distal to the dentate line, thus representing an external hemorrhoid. The dentate line is well visualized.
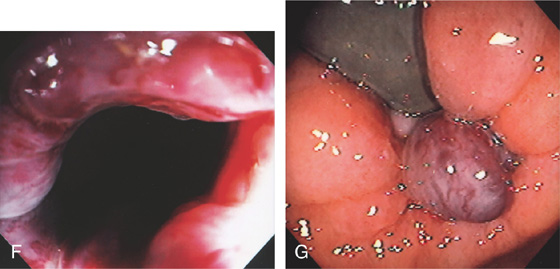
F, Multiple dilated veins on hemorrhoidal tissue are similar to the red signs on esophageal varices. G, External hemorrhoid prolapsed back in the rectum as shown on retroflexion.
Figure 6.6 EXTERNAL HEMORRHOIDS IN PORTAL HYPERTENSION
Prominent external hemorrhoids in a patient with cirrhosis, portal hypertension, and esophageal varices.
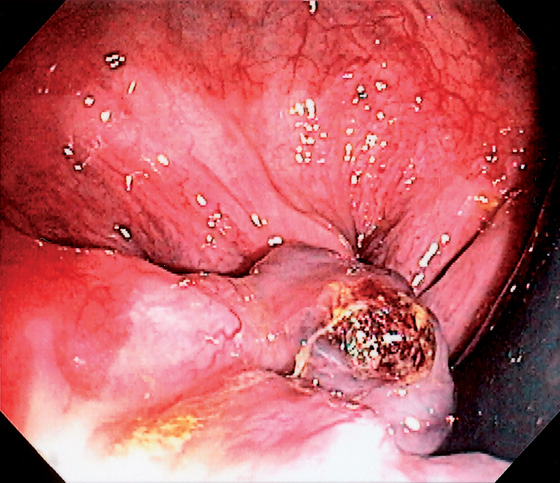
Figure 6.7 EXTERNAL HEMORRHOID WITH BLEEDING STIGMATA
Note the adherent blood clot on the external hemorrhoid as shown on retroflexion.
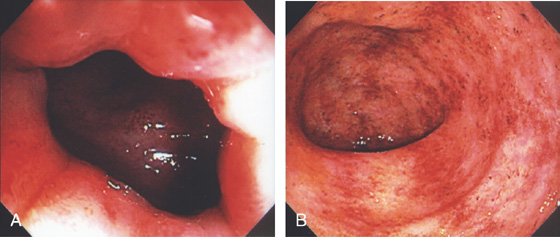
Figure 6.8 ULCERATIVE PROCTITIS
A, The colitis can be seen to begin just inside the anal verge. B, Marked proctitis with all the features of colitis including loss of the mucosal vascular pattern, edema, subepithelial hemorrhage, friability, and mucopus.
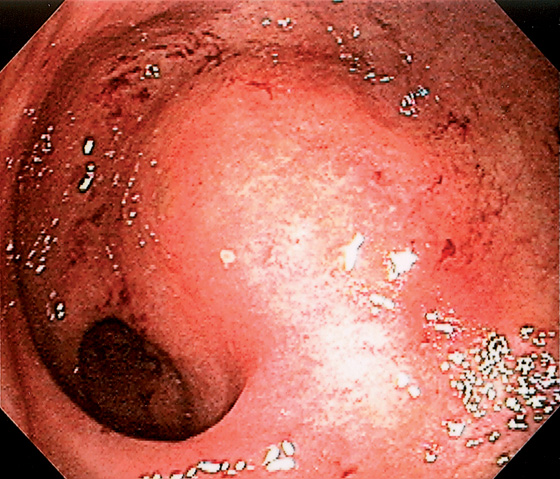
Figure 6.9 NONSPECIFIC HEMORRHAGE
Striking subepithelial hemorrhage in the rectum. Biopsies disclosed no specific cause. Such nonspecific changes can be seen in patients on anticoagulation, or who have thrombocytopenia, or the changes lack specific explanation.
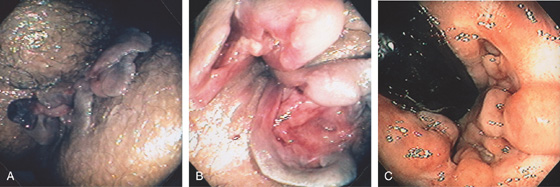
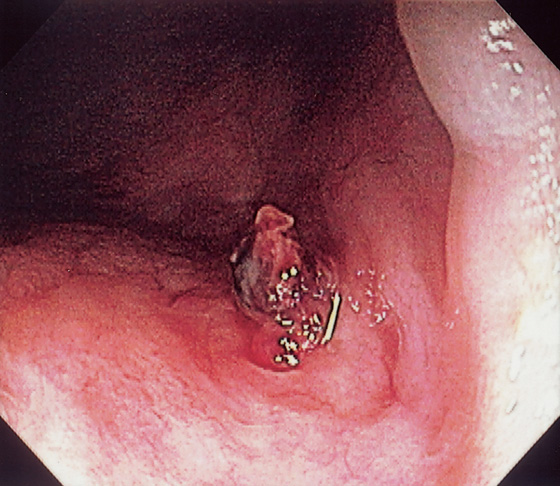
Figure 6.10 PERIANAL CROHN’S DISEASE
A, Multiple large skin tags at the anus simulate thrombosed external hemorrhoids. B, Further inspection reveals fistulae at the base of the tags. Fistulae are also seen in areas of ulcerated mucosae. C, Retroflexion at the anorectal junction demonstrates multiple serpiginous ulcerations, typical of Crohn’s disease.
Figure 6.11 MULTIPLE SKIN TAGS IN CROHN’S DISEASE
Multiple skin tags at the anal verge in a patient with long-standing Crohn’s disease.
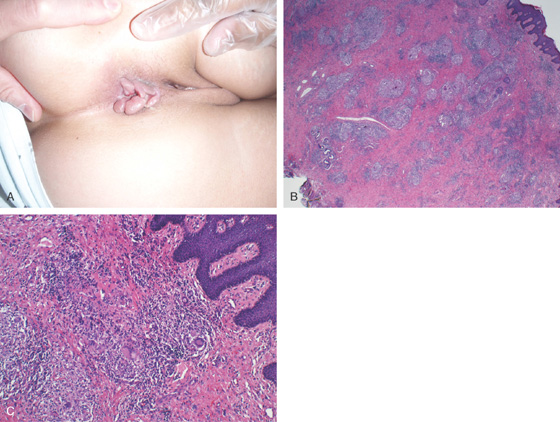
Figure 6.12 MULTIPLE SKIN TAGS IN CROHN’S DISEASE
A, Multiple ulcerated skin tags. B, After surgical resection, granulomas can be seen, as well as multinucleated giant cells (C).
Figure 6.13 SKIN TAGS FROM PRIOR HEMORRHOIDAL DISEASE
A, Multiple skin tags and redundant tissue at the anal verge caused by prior hemorrhoidal disease. B, Solitary large skin tag at the dentate line as shown on antegrade view.

Figure 6.14 BLEEDING SKIN TAG
A, Retroflexed view shows a large skin tag with fresh bleeding. B, After washing, ulceration can be seen at the base of the large skin tag.
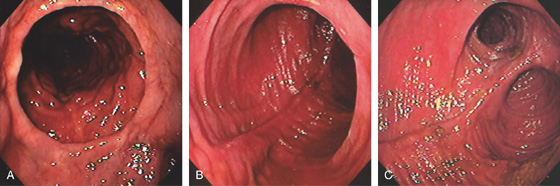
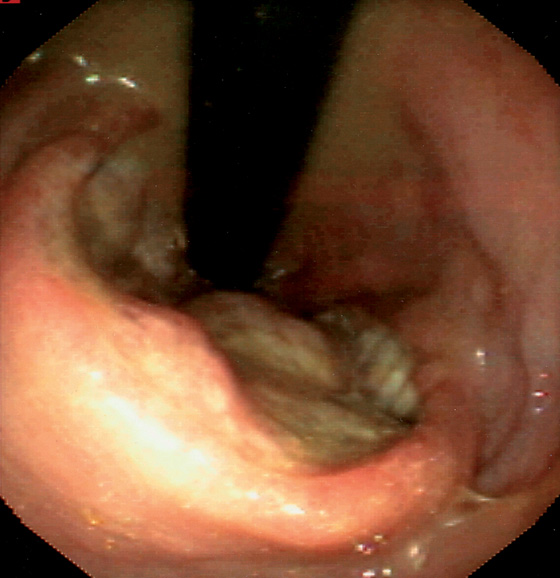
Figure 6.15 J POUCH
A, Distal anastomosis in the foreground. B, Characteristic appearance of the pouch. C, Both limbs are now shown.
Figure 6.16 POUCHITIS
Patchy ulceration and edema in the pouch extending to the limbs (A1, A2). B1, B2, More diffuse disease with edema, ulcerations, and mucopus.
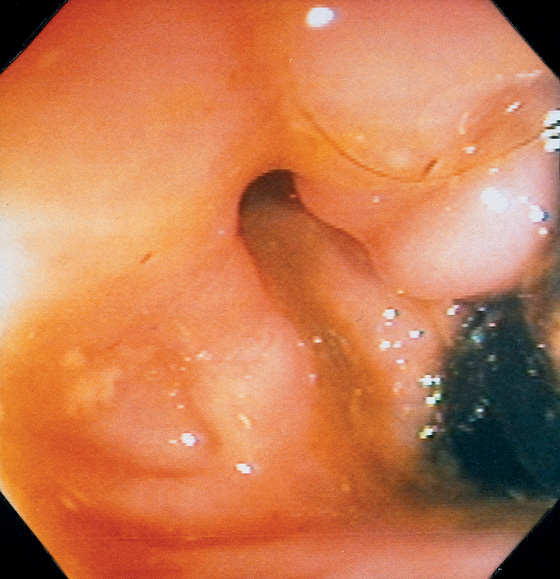
Figure 6.17 RECTAL FISTULA
Retroflexion in the rectum demonstrates an opening near the anorectal junction. This patient had a history of Crohn’s disease. Note the absence of rectal inflammation. The fistula could also be identified in the antegrade view of the anorectum.
Figure 6.18 RECTAL AND PERIANAL FISTULAE
Fistulous opening proximal to the dentate line on retroflexion view (A, B).

Opening is shown antegrade on withdrawal (C). Perianal skin tags with erosions and a fistulous opening are also noted (D).
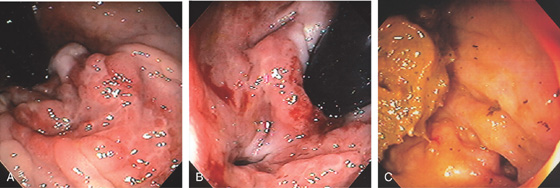
Figure 6.19 RECTAL FISTULA IN CROHN’S DISEASE
A, Ulceration at the anal verge in a patient with Crohn’s disease. B, Fistula site identified. C, After medical therapy, the rectal mucosa is now normal, although the fistula is still widely patent. This patient now reported passage of stool through the vagina.
Figure 6.20 RECTAL FISTULA
Area in the distal rectum as shown on retroflexion with nodularity and where pus was seen to pass spontaneously.
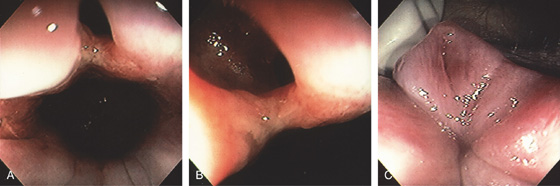
Figure 6.21 FISTULA IN ANO
A, Opening in the anal canal. B, Close-up shows the fistula. C, Redundant tissue at the anal verge with an opening representing the fistulous tract.
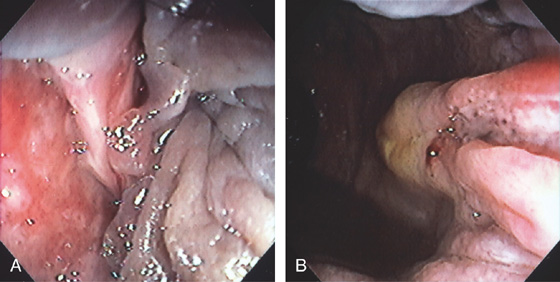
Figure 6.22 FISSURE
Linear tear in the anal canal with associated sentinel pile (A, B).
Figure 6.23 RECTAL VARICES
A, Cluster of varices in the distal rectum. There is bleeding stigmata on one of the variceal trunks. B, A vascular ectasia was identified just proximal to the varices. These can be seen in colonic disease related to portal hypertension.
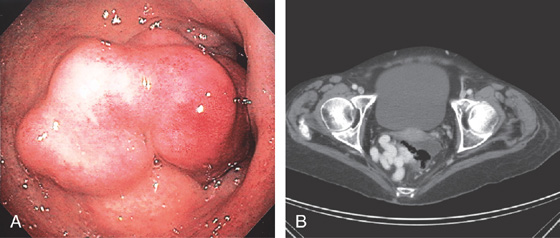
Figure 6.24 RECTAL VARICES
A, Large cluster of veins in the midrectum resembling a mass lesion. B, Contrast examination shows a large variceal trunk in the rectum.
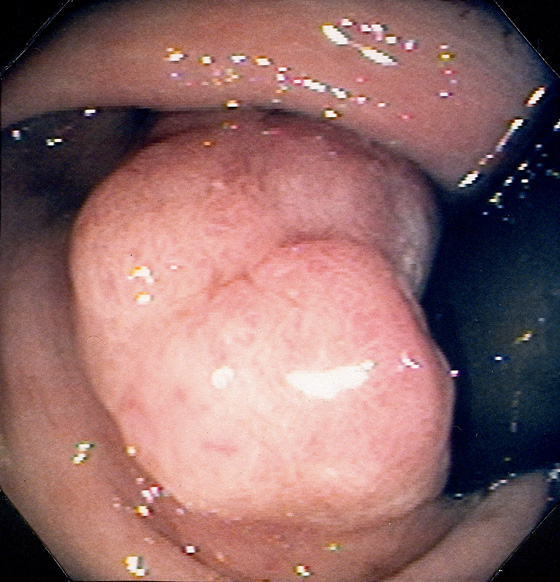
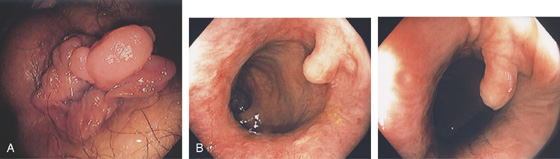
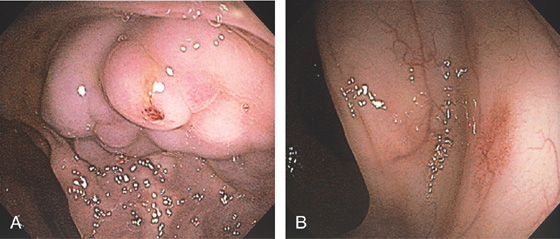
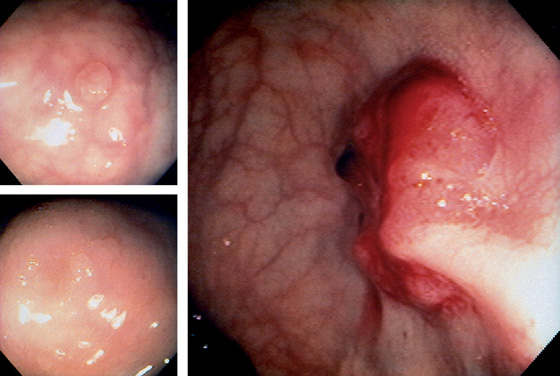
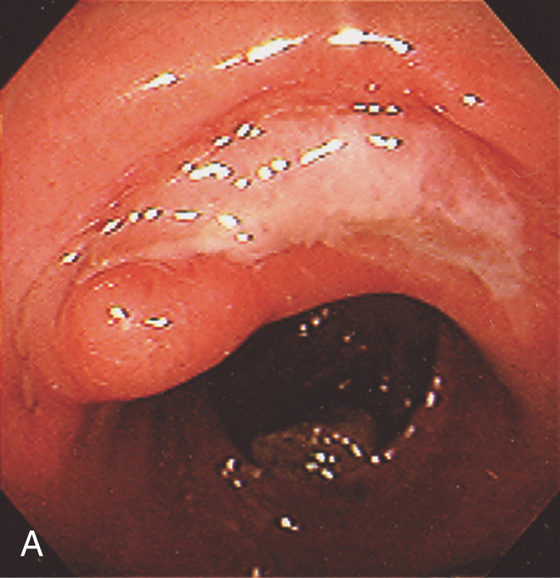
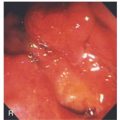
Figure 6.25 ADENOMATOUS POLYP
Large, adenomatous-appearing polyp at the anorectal junction shown on retroflexion. A soft mass lesion was palpated on digital rectal examination. The lesion could not be observed as the endoscope was advanced into the rectum.
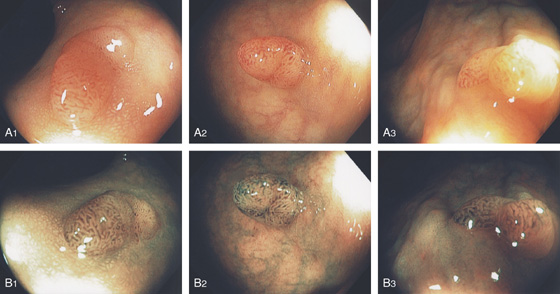
Figure 6.26 ADENOMATOUS POLYP
A1-A3, Typical-appearing small sessile polyp in the distal rectum on standard and narrow band imaging (B1-B3). The narrow band image demonstrates a “brain” appearance suggesting an adenoma.
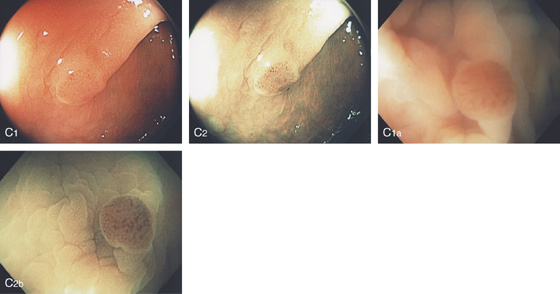
C1, Small polyp with reddish overlying vascular pattern as shown on standard and narrow band imaging (C2). Note the appearance is accentuated by examining the lesion underwater because of magnification (C1a, C2b).
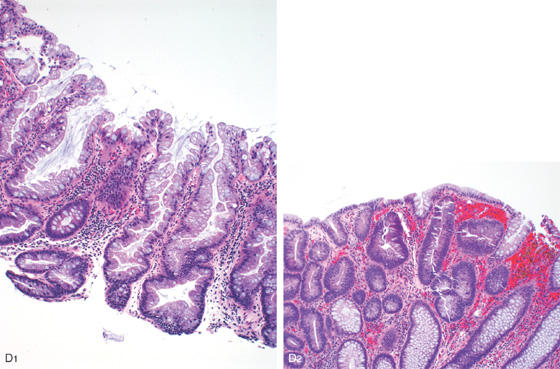
Figure 6.26 ADENOMATOUS POLYP
D1, Neoplastic epithelium with elongated cigar-shaped nuclei, increased nuclear/cytoplasmic ratio, and surrounding lamina propria with focal fresh and remote hemorrhage (D2).
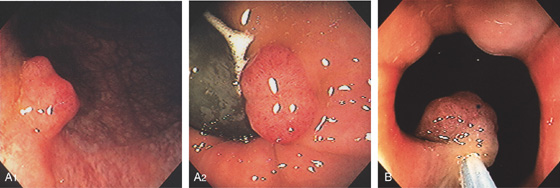
Figure 6.27 ADENOMATOUS POLYP
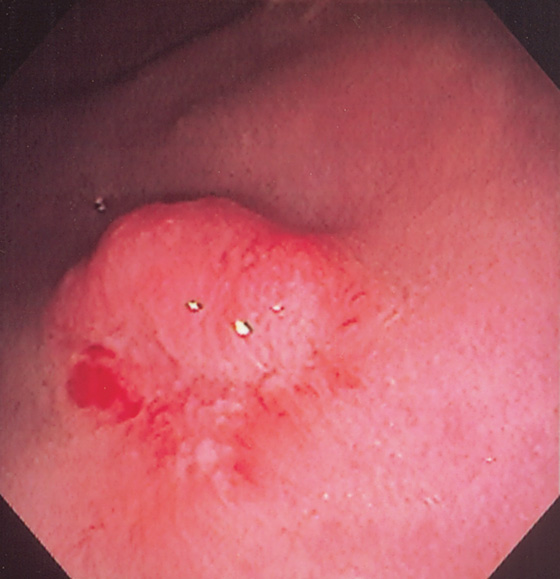
A, Solitary small polyp at the anal verge typical for an adenoma (A1). Polyp as shown on retroflexion (A2). B, The polyp has been snared.
Figure 6.28 PROLAPSED ADENOMA
A, Hemorrhagic polyp at the anal verge. B, With passage of the endoscope into the rectum, the polyp was seen to be pedunculated on a long stalk. C, The polyp has been snared. D, The base of the polyp was on the first valve of Houston.
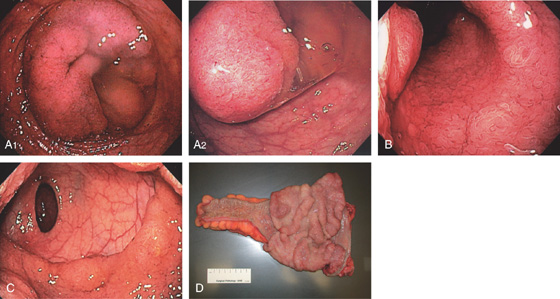
Figure 6.29 VILLOUS ADENOMA
A1, A2, Circumferential lesion of the distal rectum. Note the large amount of mucus. B, The center of the circumferential lesion has a typical villous appearance. C, Note the sharp demarcation at the proximal border. D, Surgical specimen shows a typical-appearing large villous adenoma.
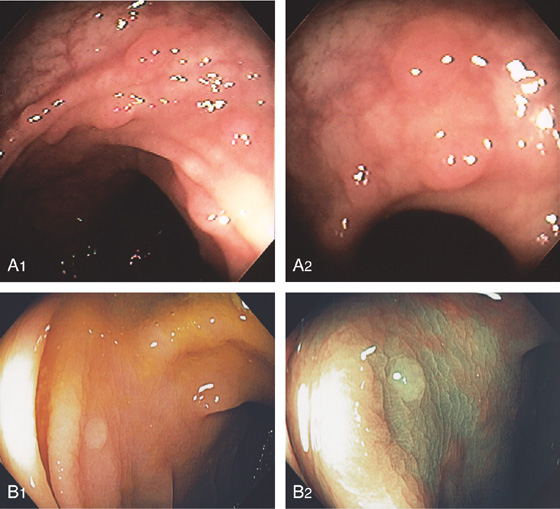
Figure 6.30 HYPERPLASTIC POLYPS
A1, A2, Multiple small polyps in the distal rectum. Close-up shows a smooth surface and slight pink discoloration. B1, Small clear polyp in the rectum. On narrow band imaging, no pattern is appreciated on the mucosal surface (B2).
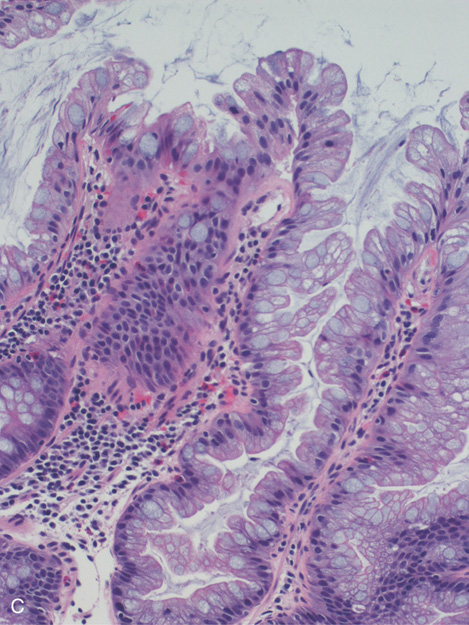
C, Colonic mucosa with cells having increased cytoplasmic mucin content and arranged in a serrated configuration.
Figure 6.31 CARCINOID POLYP
A, Small yellowish-appearing polyp in the distal colon (A1). Close-up shows the submucosal nature of the polyp and typical yellow color (A2). The lesion was firm on biopsy. B1, Yellow smooth sessile polyp in the distal rectum. B2, The polyp has been snared. Note the peculiar overlying vascular pattern as shown on standard and (B3) narrow band imaging.
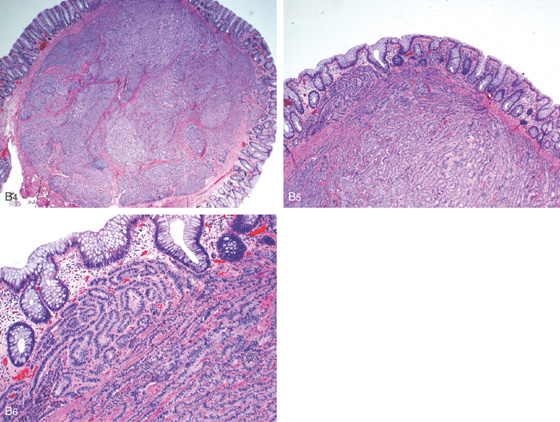
B4-B6, Typical features of a carcinoid lesion demonstrate a predominantly ribbon-like architectural arrangement and neuroendocrine cytologic features including relatively plasmacytoid nuclear placement, “salt and pepper” chromatin, and mild amount of cytoplasm.
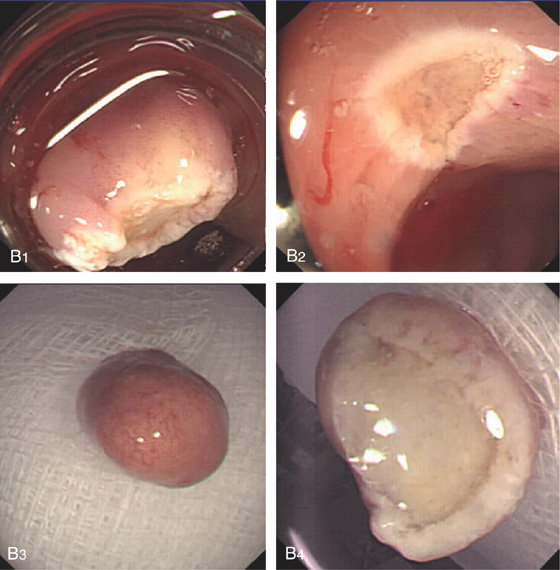
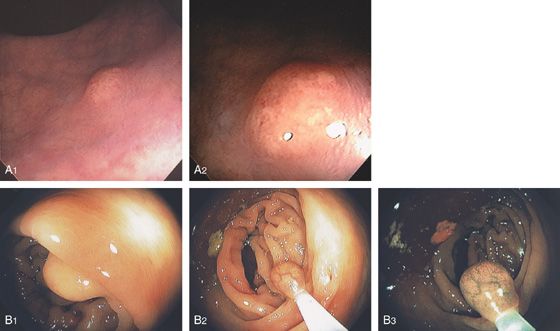
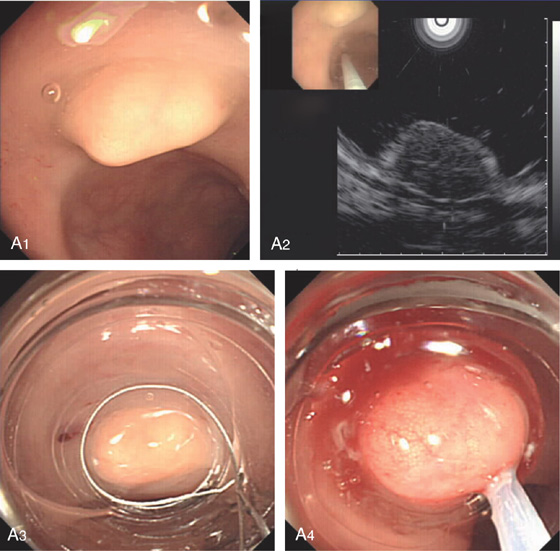
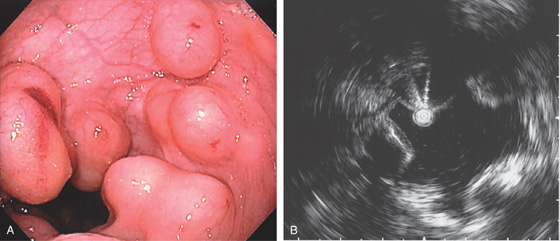
Figure 6.32 CARCINOID LESION
A1, Submucosal lesion. Endoscopic ultrasonography (EUS) shows the submucosal nature of the polyp (A2). A cap device is used for endoscopic mucosal resection (A3, A4). B, The lesion has been snared and completely resected.
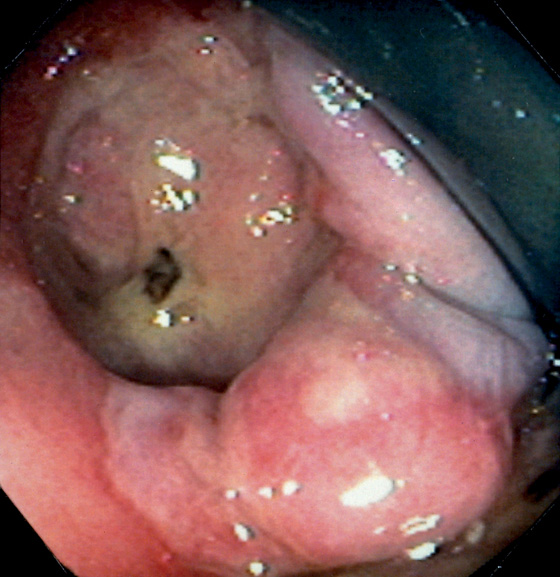
Figure 6.33 MULTIPLE RECTAL NEOPLASMS
Hypervascular sessile lesion in the distal rectum with a fresh blood clot represents recent bleeding (right). The lesion was an adenocarcinoma and was associated with other colonic polyps. In the more proximal rectum, a 3-mm polyp compatible with either an adenoma or a hyperplastic polyp was present (top left). This was a tubulovillous adenoma. In the distal rectum, two clear hyperplastic polyps were found (bottom left).
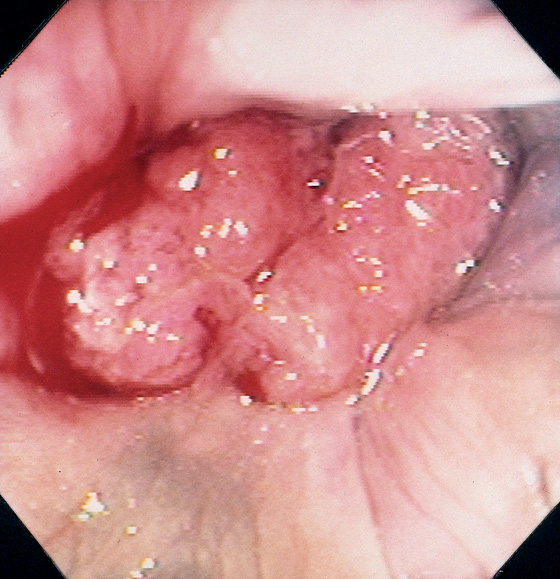
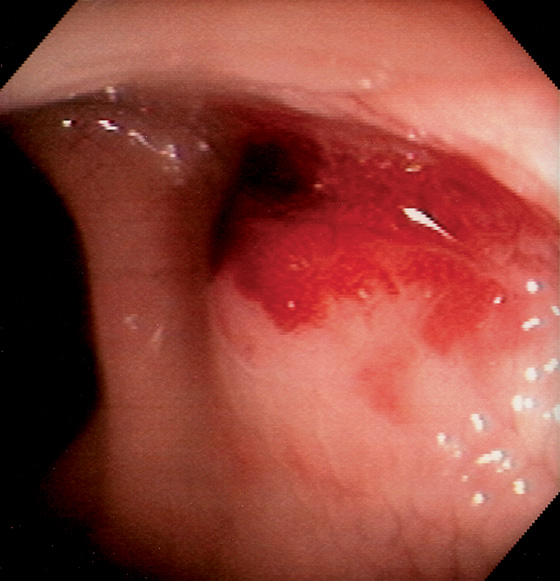
Figure 6.34 ADENOCARCINOMA
This polypoid mass can be seen at the anorectal junction, with active bleeding. Retroflexion in the rectum demonstrated that the lesion arose from the colonic mucosa at the anorectal junction, thus identifying this lesion as a primary colonic carcinoma with prolapse into the anal canal rather than a primary anal carcinoma.
Figure 6.35 ADENOCARCINOMA
Small, friable nodular lesion of the distal rectum. Note the irregularity of the distal margin. This lesion was metastatic to the liver at diagnosis.
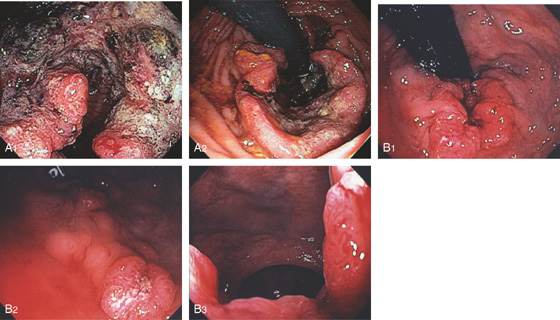
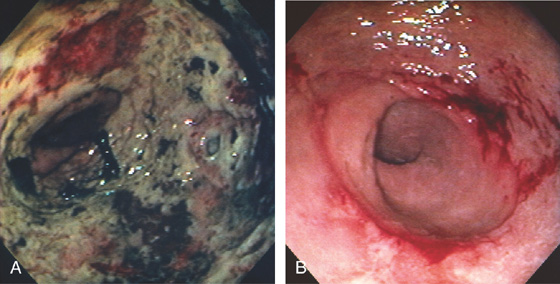
Figure 6.36 ADENOCARCINOMA
A1, Hemicircumferential necrotic tumor just inside the anal verge. A2, Retroflexion shows the nature of the tumor in relation to the anal verge. B1, Circumferential ulcerated mass at the anal verge. B2, Just proximal to the tumor, there is an additional sessile ulcerated lesion, likely adenocarcinoma as well. B3, Tumor on antegrade view in the anal verge.
Figure 6.37 ADENOCARCINOMA
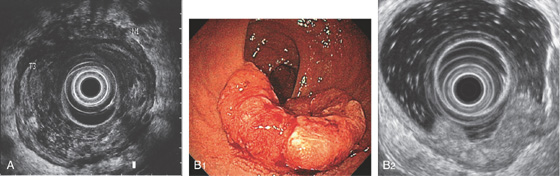
A, Endoscopic ultrasonography stages the lesion to involve the serosa and a lymph node is present (stage T3N1). B1, Donut-shaped lesion of the distal rectum. B2, The tumor involves the muscularis to the adventitia compatible with a stage 3 tumor.
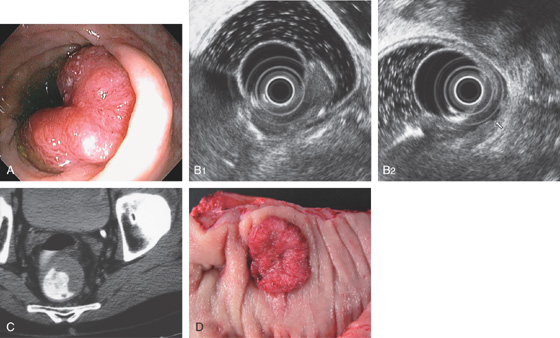
Figure 6.38 ADENOCARCINOMA
A, Hemicircumferential mass lesion in the midrectum. B1, B2, The lesion was limited to the mucosa without evidence of nodal disease. C, Lesion easily seen on CT scan using rectal contrast. D, Solitary midrectal cancer.
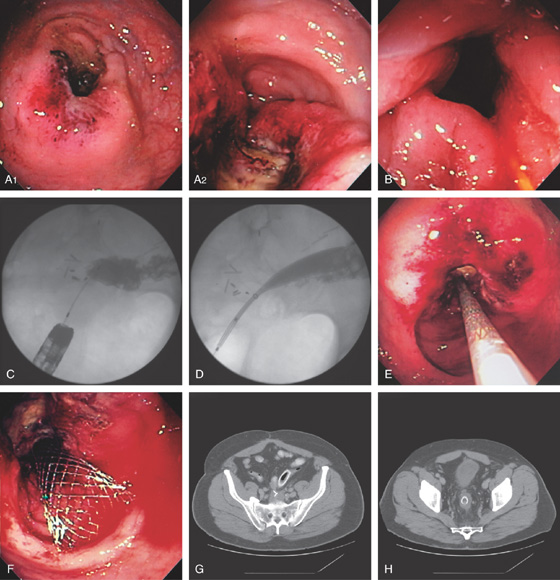
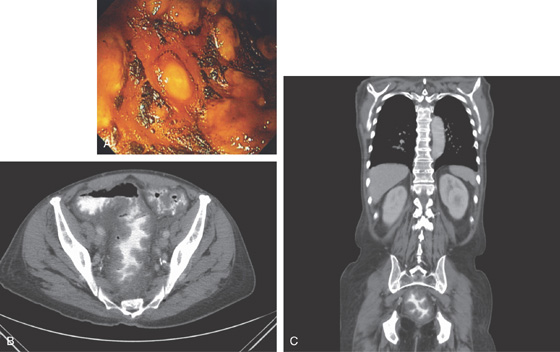
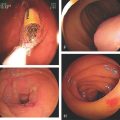
Figure 6.39 ENTERAL STENTING OF OBSTRUCTING RECTAL CANCER
A1, A2, Circumferential ulcerated tumor of the midrectum. B, A guidewire is passed more proximally under fluoroscopic guidance and contrast injected. The stent is deployed (C-F). Stent as seen on follow-up CT scan (G, H).
Figure 6.40 PROSTATE BIOPSY SITE
Area of fresh hemorrhage from recent transrectal prostate biopsy.
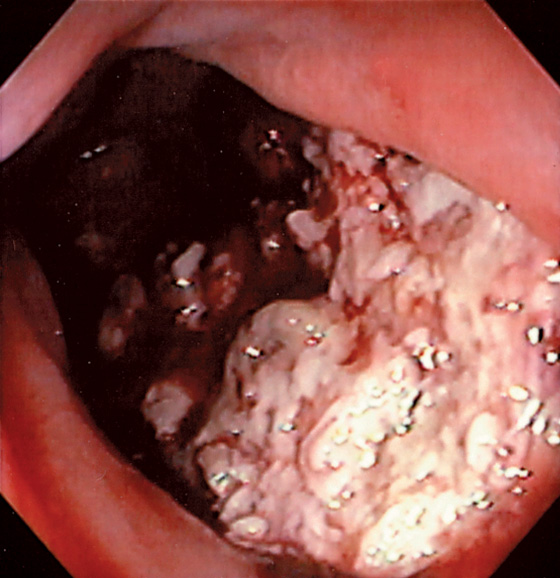
Figure 6.41 PROSTATE CANCER
Diffuse ulceration anteriorly extending from the anal verge proximally.
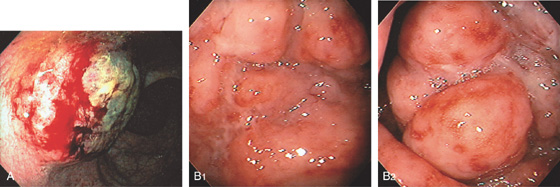
Figure 6.42 NON-HODGKIN’S LYMPHOMA
A, Round, elevated, ulcerated mass lesion in the posterior rectum. B1, Edema and shallow ulceration of the midrectum with areas of nodularity (B2).
Figure 6.43 CLOACOGENIC CARCINOMA
Large ulcerated mass at the anal verge resembling an adenocarcinoma.
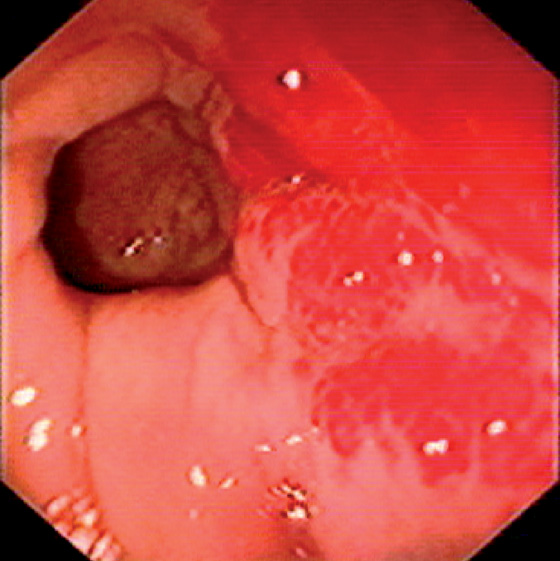
Figure 6.44 KAPOSI’S SARCOMA
Hemorrhagic subepithelial lesions.
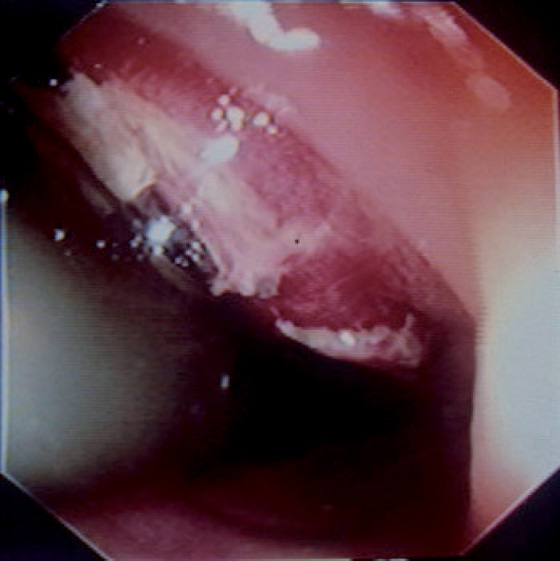
Figure 6.45 GASTROINTESTINAL STROMAL TUMOR
Hemorrhagic raised lesion in the distal rectum shown on retroflexion.
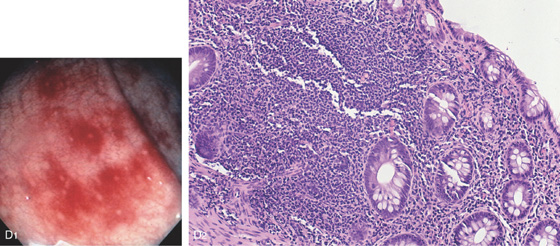
Figure 6.46 SOLITARY RECTAL ULCER
A, Large, well-circumscribed ulceration with a clean base is associated with edema of the proximal margin. This elderly patient had no evidence of infection, ischemia, or anorectal trauma.
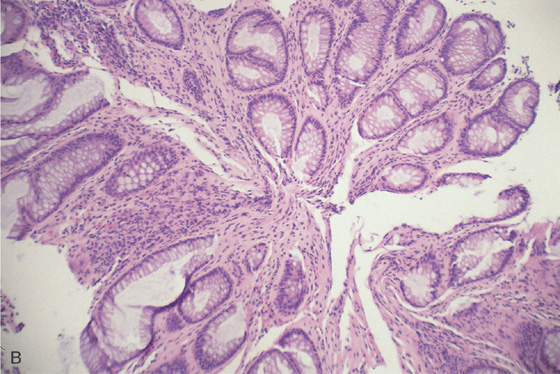
B, Typical histopathologic findings for the solitary rectal syndrome are present, including superficial ulceration, hyperplastic mucosal changes, and obliteration of the lamina propria by fibroblasts and smooth muscle cells.
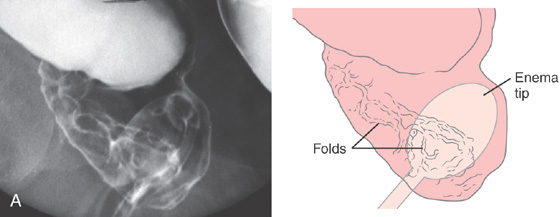
Figure 6.47 SOLITARY RECTAL ULCER
A, Linear folds in distal rectum. The surrounding mucosa is normal.
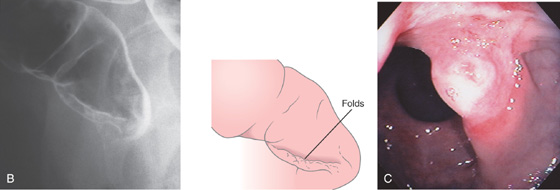
B, Lateral view. These findings are consistent with hemorrhoids. C, Solitary ulceration in the distal rectum.
Figure 6.48 STERCORAL ULCER
Large, well-circumscribed, deep ulcer in the distal rectum is shown by retroflexion. This patient is an elderly woman with chronic constipation and fecal impaction.
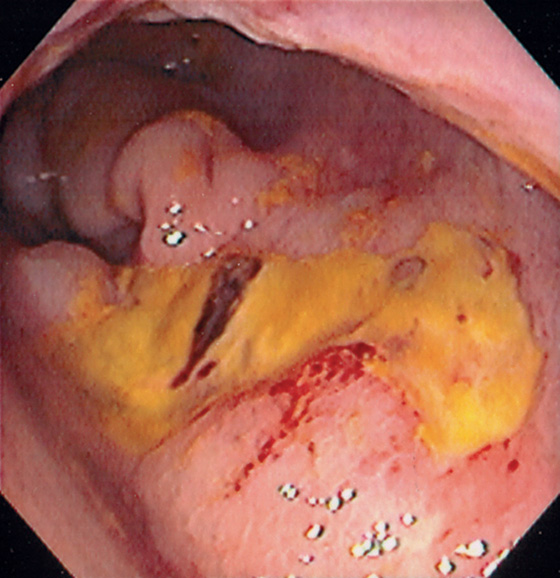
Figure 6.49 RADIATION-INDUCED RECTAL ULCER
Large ulcer on the anterior wall of the rectum in a patient with a prior history of radiation therapy for prostate cancer.
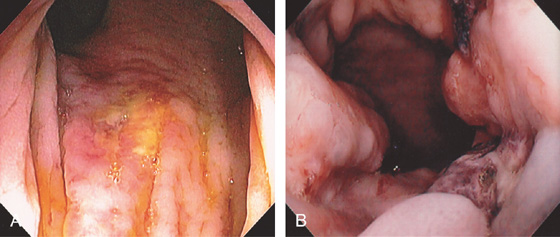
Figure 6.50 RECTAL ULCER SECONDARY TO RECTAL TUBE
A, Shallow ulcer covered with exudate just inside the anal verge. B, Hemicircumferential ulceration with fresh clot.
Figure 6.51 RECTAL ULCER WITH VISIBLE VESSEL
Small ulcer just proximal to the dentate line with a visible vessel.
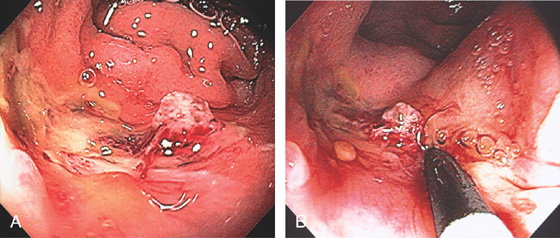
Figure 6.52 RECTAL ULCER WITH RECENT BLEEDING
A, Ulcer in the distal rectum with visible vessel. Note the proximity to the dentate line. B, Large-volume epinephrine injection was performed with the endoscope in the anal canal.
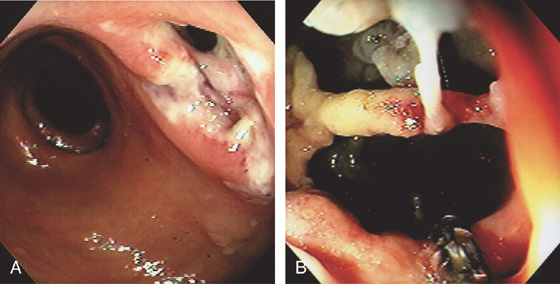
Figure 6.53 IDIOPATHIC ULCER IN ACQUIRED IMMUNODEFICIENCY SYNDROME
A, Ulceration in the distal rectum with an apparent opening. B, The opening was entered demonstrating a complex cystic cavity. Biopsy forceps are visible.
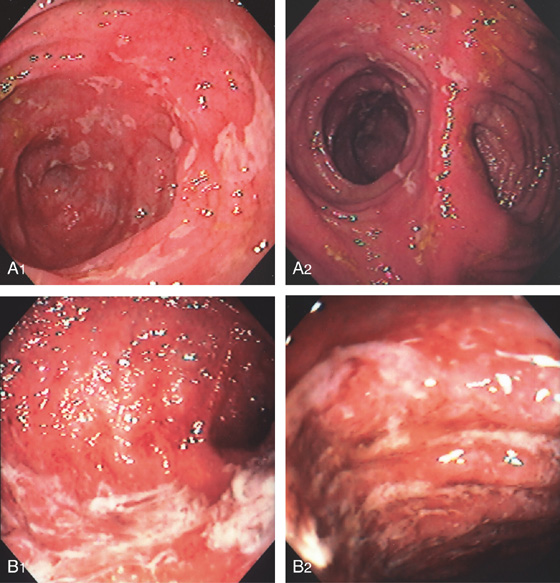
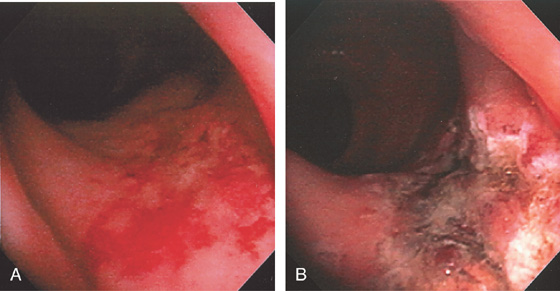
Figure 6.54 ISCHEMIC PROCTITIS
A, Circumferential ulceration extends from the anal verge to the proximal rectum, with sharp demarcation of the proximal border. Biopsy specimens demonstrated necrosis involving soft tissue, nerve, and fat, suggestive of an abscess cavity. B, Repeat examination 3 weeks later demonstrated almost complete reepithelialization, with areas of normal vascular pattern present.
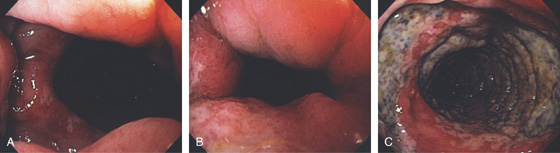
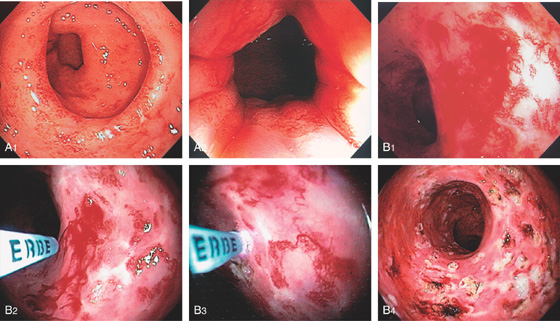
Figure 6.55 ISCHEMIC PROCTITIS
A, As viewed from the anal canal, the ulceration spares the anus and the disease begins just proximal to the anal verge. B, Circumferential ulceration in the distal rectum that extends proximally. C, Note the disease becomes more severe proximally.
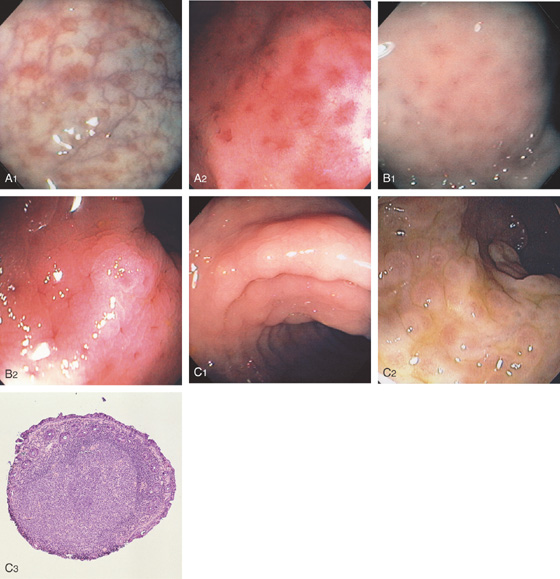
Figure 6.56 LYMPHOID TISSUE
Figure 6.56 LYMPHOID TISSUE
D1, Patchy subepithelial hemorrhage shown with central spared areas. The uninvolved rectum is normal. D2, Lymphoid follicular aggregate is observed. Some subepithelial hemorrhage is apparent at this magnification. The mucosal architecture is normal. The lymphoid tissue is apparent because of the surrounding mucosal hemorrhage, which does not involve the aggregate.
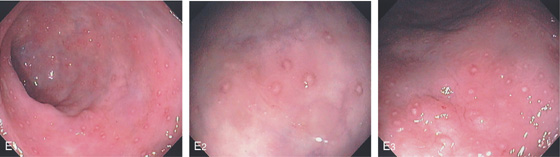
E1, E2, Multiple aphthous ulcer–appearing lesions in the rectum. E3, With the lumen slightly collapsed, these lesions are shown to be on a mound of tissue representing a lymphoid follicle.
Figure 6.57 CLOSTRIDIUM DIFFICILE PROCTITIS
A, Typical-appearing thick yellow plaques in the rectum. B, CT shows marked thickening of the rectum both on standard and coronal views (C).
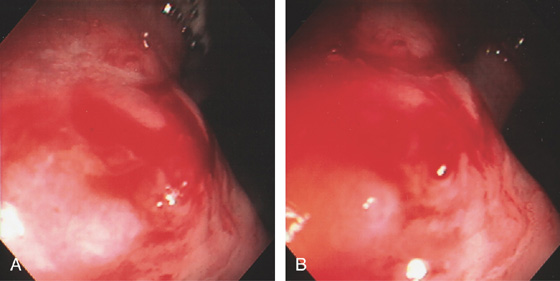
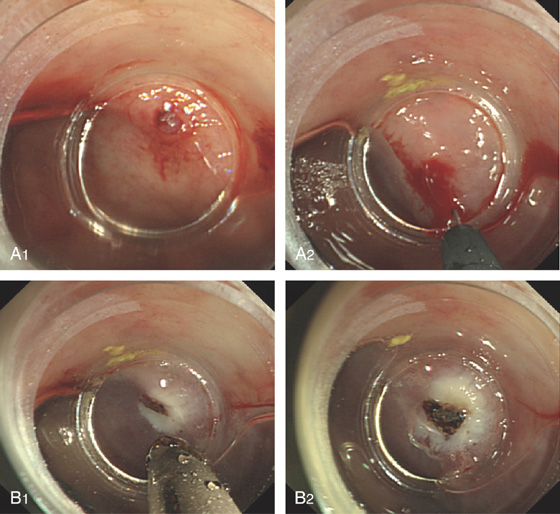
Figure 6.58 DIEULAFOY LESION
A, Active bleeding in the distal rectum. B, Close-up shows a pinpoint arterial bleeding source.
Figure 6.59 DIEULAFOY LESION
A, Visible vessel in rectum as shown with a cap device (A1). Epinephrine is injected (A2). B, After injection, thermal therapy is applied.
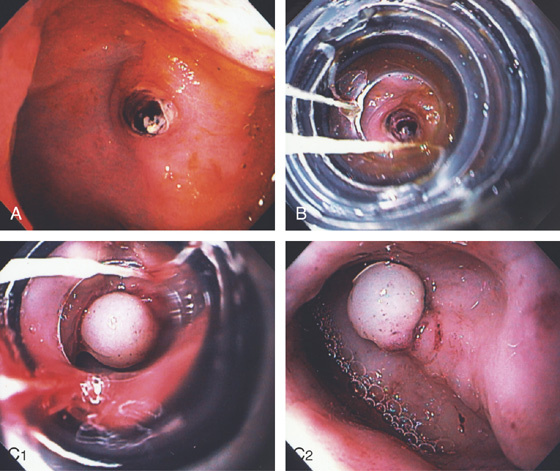
Figure 6.60 DIEULAFOY LESION
A, Pinpoint clot/visible vessel in the rectum without associated ulceration. B, The banding device has been placed over the lesion. C1, The band has been successfully deployed. C2, The banded tissue has a whitish appearance caused by ischemia.
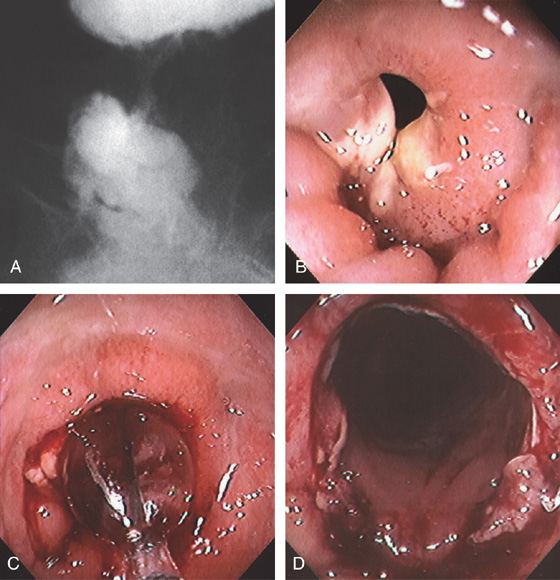
Figure 6.61 ANASTOMOTIC STRICTURE
A, Barium enema demonstrating a stricture of the distal rectum. B, Tight stricture of the distal rectum after low anterior colonic resection. C, Balloon dilatation performed. D, Widely patent anastomosis after dilatation.
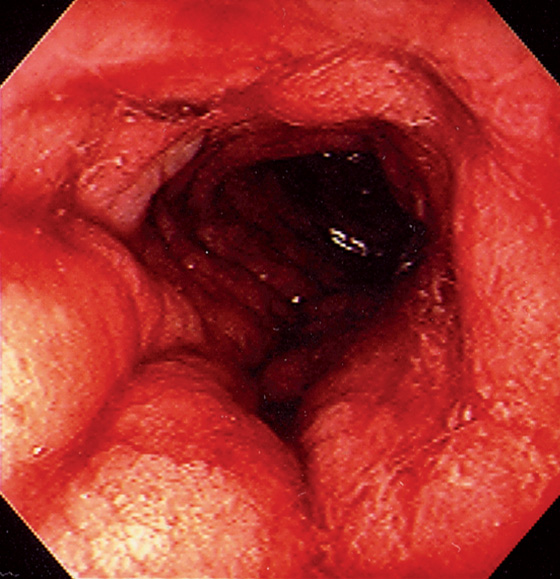
Figure 6.62 ACUTE RADIATION INJURY
Marked edema and fresh hemorrhage in the distal rectum.
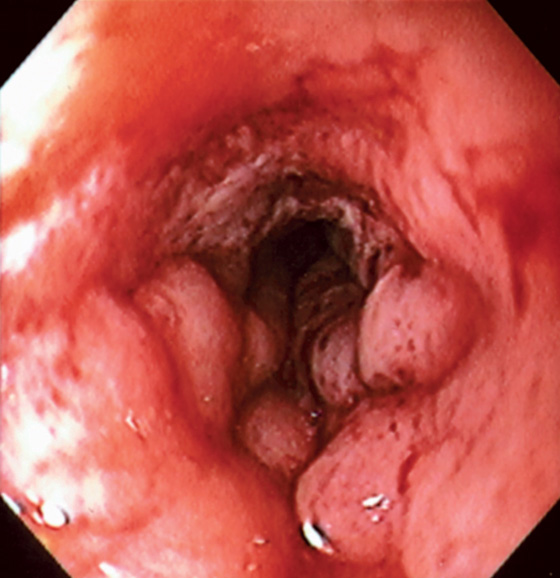
Figure 6.63 RADIATION-INDUCED STRICTURE
Tight stricture with ulceration and edema related to prior radiation therapy.
Figure 6.64 FOCAL RADIATION–INDUCED ECTASIA
A, Focal area of ectasias anteriorly in the distal rectum related to prior radiation seeds placed for prostate cancer. B, Appearance after argon plasma coagulation.
Figure 6.65 RADIATION-INDUCED ECTASIAS
MILD INJURY
A1, Multiple vascular ectasias in the rectum as shown from the anal verge. A2, Note the ectasias begin just inside the anal verge.
SEVERE INJURY
B1, Numerous large ectasias with active bleeding. The argon laser is being used to treat the lesions (B2, B3). Multiple areas of coagulation are visible after thermal therapy. Hemostasis is achieved (B4).
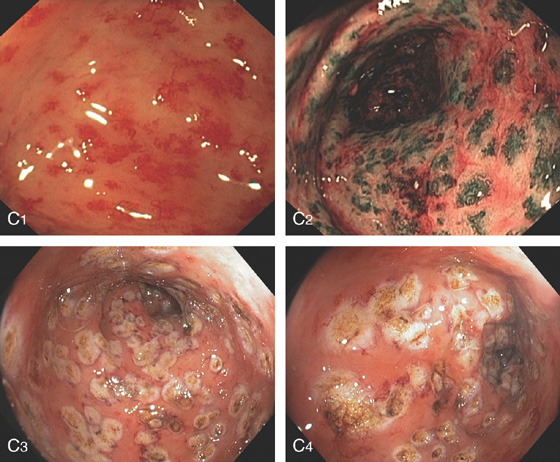
Figure 6.65 RADIATION-INDUCED ECTASIAS
C1, C2, Standard and narrow band imaging. C3, C4, Appearance of the lesions after ablation.
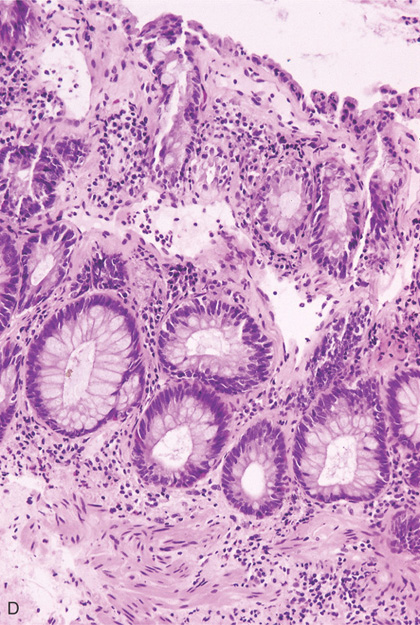
D, Blood vessels in the subepithelium are shown as empty spaces.
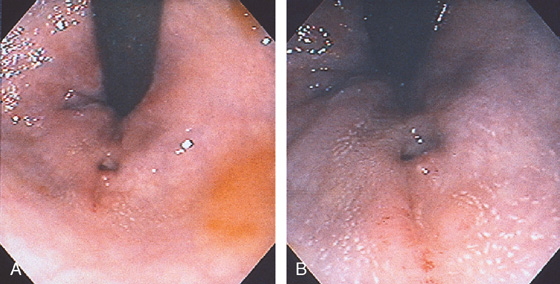
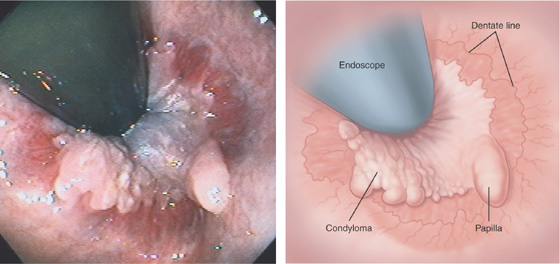
Figure 6.66 CONDYLOMA
Retroflexion demonstrates the dentate line and a hypertrophied papilla. In addition, a verrucous-appearing sessile lesion overlies the dentate line, typical of condyloma.
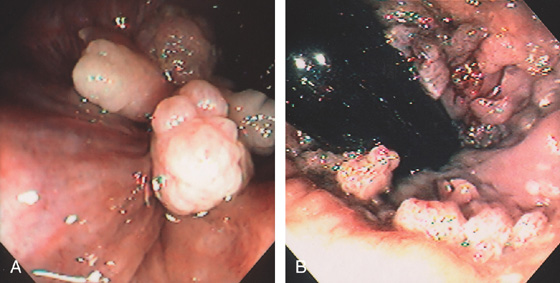
Figure 6.67 CONDYLOMA
A, Polypoid lesions at the anal verge shown on retroflexion. These may mimic anal papillae. B, Multiple small verrucous lesions at the anal verge.
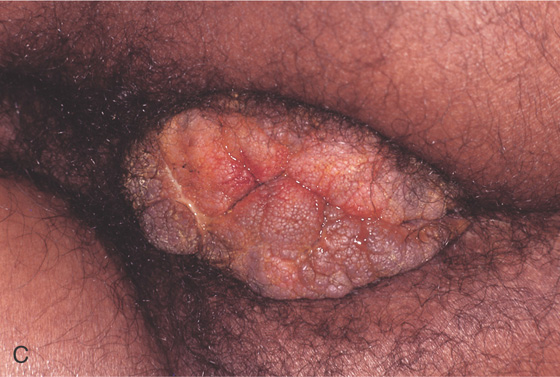
C, Large condylomatous mass at the anal verge in a transplant patient.
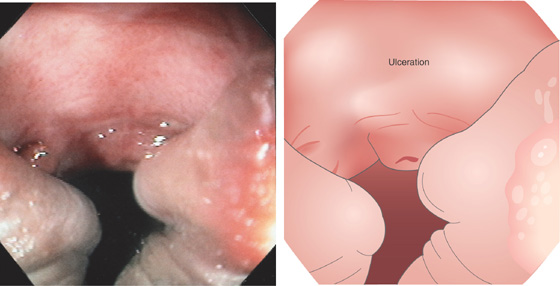
Figure 6.68 CYTOMEGALOVIRUS ULCER
Large hemicircumferential ulcer of the anal canal in a patient with AIDS. Squamous mucosa is shown posteriorly, documenting the lesion’s location. This lesion was associated with severe anorectal pain. Other lesions causing anorectal pain, especially with defecation, include ulcerations, fistulae, fissures, and hemorrhoidal disease.
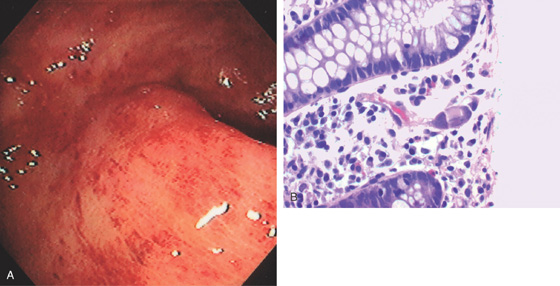
Figure 6.69 CYTOMEGALOVIRUS PROCTITIS
A, Diffuse subepithelial hemorrhage of the rectum with loss of mucosal vascular pattern. B, Characteristic large eosinophilic intranuclear inclusion of CMV. Note the colonic edema.
Figure 6.70 PNEUMATOSIS CYSTOIDES
A, Multiple submucosal cystic projections. B, Ultrasound confirms the lesions to be cystic in nature.
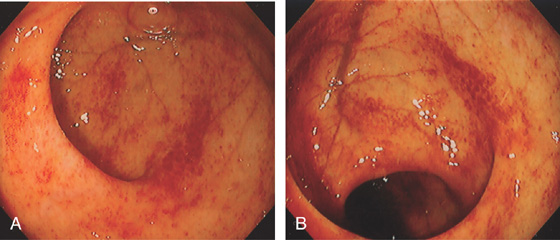
Figure 6.71 GRAFT-VERSUS-HOST DISEASE
Patchy subepithelial hemorrhage is present in the rectum (A) and sigmoid (B).
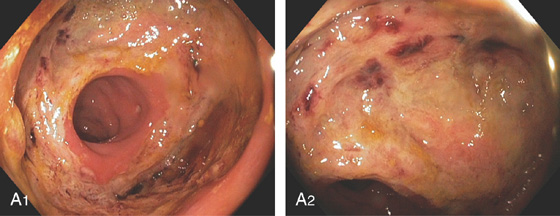
Figure 6.72 RECTOCOLONIC ANASTOMOTIC DEHISCENCE
A1, A2, Large ulceration at the surgical anastomosis.
Figure 6.73 EXTRINSIC LESION
Extrinsic lesion in the anterior rectum in a patient with ulcerative proctitis, who was 22 weeks pregnant.
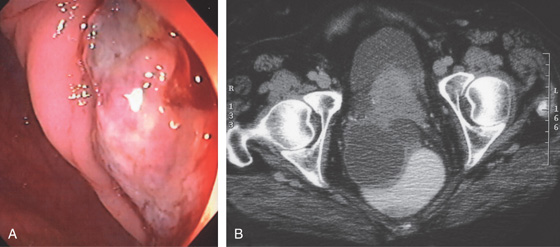
Figure 6.74 EXTRINSIC LESION: OVARIAN CANCER
A, Ulcerated lesion in rectum. B, Extrinsic mass lesion.
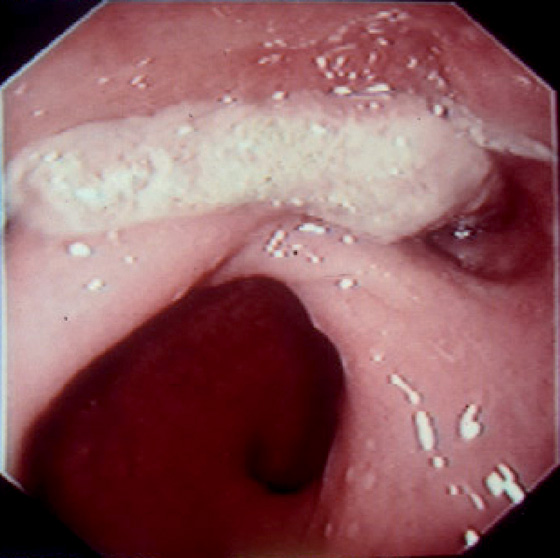
Figure 6.75 DESMOID TUMOR
Whitish lesion representing infiltrating tumor from the gluteus.
Figure 6.76 RECTAL PROLAPSE
A, Prolapsing rectal tissue visible at the anal verge. B, Retroflexion shows the reddish tissue is now reduced.
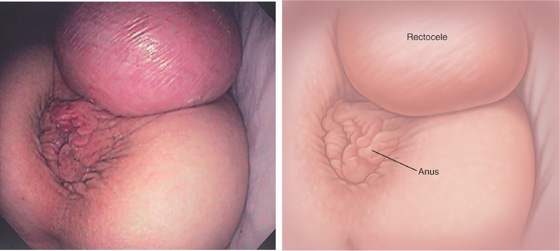
Figure 6.77 RECTOCELE
Prolapse of the rectum through the vagina. The anus is inferior to the rectocele.