CHAPTER 46 Anomalous Pulmonary Venous Connections and Drainage
Controversy exists about the origin of the primordial pulmonary vein.1 Regardless, it is generally accepted that a common pulmonary vein forms in the dorsal mesocardium and is progressively incorporated into the posterior wall of the left atrium. As the atrium expands and the common vein is absorbed, the four major branches (two left and two right) achieve their discrete insertions.
PARTIAL ANOMALOUS PULMONARY VENOUS CONNECTION
Prevalence and Epidemiology
The incidence of PAPVC at autopsy has been reported to range between 0.6% and 0.7%.2,3 The clinical frequency is less, indicating that many cases remain asymptomatic. Ethnic and gender predilections are unknown, probably because of the relative infrequency of this process.
Etiology and Pathophysiology
The anatomic manifestations of PAPVC are varied. Anomalous connections can occur both above and below the diaphragm, generally to an ipsilateral systemic vein. The right-sided pulmonary veins most often drain into the embryologic derivatives of the right cardinal vein, usually the inferior vena cava (IVC) or superior vena cava (SVC). Anomalous left-sided veins typically empty into derivatives of the left cardinal vein, most often the left innominate vein or the coronary sinus. Anomalous veins may also connect through remnants of the primitive splanchnic plexus to contralateral systemic vessels, although this is less common. Connection of a pulmonary vein (usually the right upper lobe branch) to the posterior SVC at the junction of the right atrium due to a defect of the common wall between the SVC and the right upper lobe pulmonary vein represents a unique process known as a sinus venosus defect (see following section). Drainage of the right pulmonary veins to the IVC may be associated with systemic arterial supply and hypoplasia of the ipsilateral lung with secondary dextroposition of the heart. This condition, variably referred to as congenital venolobar syndrome or scimitar syndrome (Fig. 46-1), is often placed within the continuum of bronchopulmonary dysplasias.4,5 A horseshoe lung refers to the fusion of the lower lobes across the midline without an intervening fissure. This anomaly is highly associated with scimitar syndrome, with up to 80% of patients with horseshoe lung also affected with PAPVC in some series.5
Manifestations of Disease
Imaging Indications and Algorithm
The suggestion of anomalous venous return on chest radiography or echocardiography warrants further evaluation with cardiac CT angiography (CTA) or MRI. The choice of which cross-sectional modality to employ varies with institution. Patient factors (such as contraindications to MRI or contrast media) must also be considered. In addition, because of the increased frequency of PAPVC reported in patients with Turner syndrome, routine screening in this population may be warranted.6
Imaging Techniques and Findings
Radiography
Signs of right ventricular overload, including a lateralized or upturned cardiac apex on the frontal radiograph and filling in of the retrosternal clear space on the lateral view, may be evident. Increased pulmonary blood flow is often present; however, it is nonspecific. The abnormal vein itself may be visualized, as in anomalous pulmonary venous drainage of the right lung to the IVC, when the so-called scimitar vein (named for the crescent shape of the vessel likened to a Turko-Mongol saber; Fig. 46-2) can be visualized. As described previously, patients with scimitar syndrome display hypoplasia of the right lung and often some degree of cardiac dextroposition. Aberrant drainage into the SVC or azygos vein may result in dilation of these structures that is radiographically apparent. Anomalous left pulmonary veins emptying into the left innominate vein can create a bulbous appearance of the superior mediastinum (Fig. 46-3).
Ultrasonography
Echocardiography is often the first imaging modality employed to evaluate a child with suspected structural heart disease. Defining the pulmonary venous connections is an integral step in every echocardiographic examination. The pulmonary venous connections are often best demonstrated through a subxiphoid approach in infants. Suprasternal, parasternal, apical, and subcostal windows are often more revealing in older children. When not all of the pulmonary venous connections can be accounted for, a more detailed search for systemic connections is required. The presence of dilated systemic veins can be a clue to an unsuspected anomalous venous connection. Right ventricular volume overload resulting from PAPVC is well evaluated with echocardiography. Because of limitations in field of view of the pulmonary veins and left atrium from a transthoracic technique, transesophageal scanning may be needed for more detailed anatomic study of the pulmonary vein insertion.7
Computed Tomography
CTA timed to maximize left atrial and pulmonary venous opacification provides high spatial resolution images of the course and connections of anomalous pulmonary veins.8 The isotropic acquisition of current multidetector CT scanners allows multiplanar reformation, maximum intensity projection, and volume rendered reconstruction. These techniques provide a degree of anatomic visualization previously available only with angiography. In addition, surrounding noncardiac structures, such as the lung parenchyma, are well demonstrated. This can aid in the characterization of an associated hypoplastic or horseshoe lung (see Fig. 46-1).
Magnetic Resonance
Cardiac MRI is another useful tool for the evaluation of PAPVC. MRI has been shown to be accurate in the evaluation of pulmonary vein anomalies and is often considered the method of choice for preoperative characterization of PAPVC (see Fig. 46-3).9–11 Black blood images, like CTA, provide high spatial resolution for the evaluation of anatomic connections. Phase contrast cine images allow accurate quantification of pulmonary and systemic blood flow for shunt fraction calculation. Whereas the evaluation of lung parenchyma is less optimal than with CT, the lack of ionizing radiation and the functional information about the quantification of right ventricular volume overload and shunt fraction make MR superior for guiding clinical management.
Synopsis of Treatment Options
Surgical/Interventional
Indications for surgical treatment are controversial. Generally speaking, all patients who are symptomatic and do not have a contraindication should be treated surgically. Specifically, the current consensus holds that if the Qp:Qs (shunt fraction) is greater than 1.5 : 1, surgical closure is performed. PAPVC with shunt ratios below 1.5 : 1 are usually well tolerated and can be clinically observed. The particular repair will vary according to the site of anomalous drainage and the coexistence of any other form of heart disease. Anomalous left pulmonary veins may be reanastomosed to the left atrial appendage. Right-sided anomalous veins are often anastomosed to the right atrium and connected to the left atrium with a patch or baffle through a preexisting or surgically created ASD. In general, surgical repair of PAPVC is associated with very good outcomes.12 Patients with scimitar syndrome, however, often do poorly and suffer from high degrees of postoperative pulmonary venous stenosis related to baffle obstruction (Fig. 46-4).12
Reporting: Information for the Referring Physician
KEY POINTS
 PAPVC results from failure of at least one of the pulmonary veins to become incorporated into the left atrium.
PAPVC results from failure of at least one of the pulmonary veins to become incorporated into the left atrium. The anomalous pulmonary vein can connect to a variety of systemic veins, including the IVC, SVC, innominate and azygos veins, and coronary sinus.
The anomalous pulmonary vein can connect to a variety of systemic veins, including the IVC, SVC, innominate and azygos veins, and coronary sinus. Patients with PAPVC can present with dyspnea on exertion or murmur related to right-sided heart enlargement and volume overload.
Patients with PAPVC can present with dyspnea on exertion or murmur related to right-sided heart enlargement and volume overload.SINUS VENOSUS DEFECT
Prevalence and Epidemiology
The SVD has been estimated to account for up to 10% of ASDs.13 The exact prevalence is difficult to measure because of the often subclinical nature of the condition and the difficulty in detection with standard first-line cardiac imaging techniques (plain radiography and echocardiography). Most studies have found a female-to-male preponderance of roughly 2 : 1. No racial or ethnic predilection has been established.
Etiology and Pathophysiology
The lack of an intact intervening wall between a pulmonary vein and the SVC or right atrium results in “unroofing” of the pulmonary vein, thereby creating anomalous pulmonary venous drainage to the right atrium.14 An interatrial connection posterior and superior to the fossa ovalis is commonly present. This does not represent an ASD, but rather it is a connection between the atria formed by the unroofed insertion of the pulmonary vein. Because of higher left-sided pressures, flow of blood can pass retrograde from the left atrium through the orifice of the pulmonary vein and then enter the SVC through the defect and continue to the right atrium, resulting in a left-to-right shunt. Additional anomalous connecting pulmonary veins to the SVC superior to the defect as well as other systemic veins may be present.
Manifestations of Disease
Clinical Presentation
The often large interatrial communication of an SVD results in a significant left-to-right shunt with increased pulmonary blood flow; some patients can be relatively asymptomatic while others may present with congestive heart failure.13,14 Symptoms include dyspnea on exertion, palpitations, and angina.13,15 As with conventional ASDs, SVD may be the cause of an otherwise unexplained stroke due to paradoxic embolization.15
Imaging Techniques and Findings
Radiography
Plain film findings in SVD are nonspecific but include cardiomegaly with right-sided heart enlargement and increased pulmonary blood flow (Fig. 46-5).
Ultrasonography
Echocardiography demonstrates abnormal continuity between the right upper lobe pulmonary vein and the SVC in the superior type of SVD (Fig. 46-6). Right atrial and ventricular enlargement is present, and the atrial septum proper is intact.
Computed Tomography
Although there have been case reports,16 no large case series has been published on the accuracy of CT in the evaluation of SVDs. That being said, the high spatial detail of CTA allows definition of the anomalous drainage.
Magnetic Resonance
Cardiac MR has been shown to be well suited for the evaluation of SVD.17 In addition to defining the defect (Fig. 46-7), right ventricular volume and function and pulmonary-to-systemic blood flow ratio (shunt fraction) can be quantified. MR angiography (MRA) can also depict additional pulmonary veins that may be connecting anomalously to the SVC or other systemic veins.
Differential Diagnosis
Synopsis of Treatment Options
Surgical/Interventional
The traditional means of SVD repair consists of a single patch closure of the defect that baffles the anomalous right upper lobe pulmonary vein back to the left atrium. This technique can be complicated by narrowing and obstruction of the SVC postoperatively. A second technique involves placement of a second patch to widen the SVC–right atrial junction to reduce the incidence of postoperative SVC narrowing,20 but the sutures placed at the SVC–right atrial junction can lead to sinoatrial node dysfunction.21 The Warden procedure involves transection of the cranial portion of the SVC above the anomalous venous connection and anastomosis of the SVC to the right atrial appendage. The connection of the right upper lobe pulmonary vein and the caudal end of the SVC is then patch closed. Because of the decrease in reported complications of obstruction and nodal dysfunction, the Warden technique has gained in popularity.21,22
Reporting: Information for the Referring Physician
KEY POINTS
 The superior type of SVD is due to absence of the common wall between the right upper lobe pulmonary vein and the SVC.
The superior type of SVD is due to absence of the common wall between the right upper lobe pulmonary vein and the SVC. The SVD results in a left-to-right shunt between the atria and presents with symptoms related to right ventricular volume overload similar to ASD and PAPVC.
The SVD results in a left-to-right shunt between the atria and presents with symptoms related to right ventricular volume overload similar to ASD and PAPVC.COR TRIATRIATUM
Prevalence and Epidemiology
The reported incidence of cor triatriatum ranges between 0.1% and 0.4%.23–25 No racial or gender predilections have been described.
Etiology and Pathophysiology
Like the previously described pulmonary vein anomalies, there is ample controversy surrounding the embryogenesis of cor triatriatum. The most commonly held theory is based on incomplete incorporation of the common primordial pulmonary vein into the left atrium. The common vein becomes a “third atrium” separated from the left atrium by a fibromuscular membrane. This accessory chamber typically receives all the pulmonary veins and connects to the left atrium through a stenotic orifice (classic cor triatriatum or cor triatriatum sinister). The common vein may also communicate with the right atrium (cor triatriatum dexter) or the systemic veins (cor triatriatum with PAPVC). Yet another variation is subtotal cor triatriatum, in which some of the pulmonary veins drain normally into the left atrium and others drain into an accessory chamber that then empties in any of the patterns previously described.24
Manifestations of Disease
Imaging Indications and Algorithm
Imaging indications are the same as those described in the previous sections.
Imaging Technique and Findings
Radiography
In addition to otherwise unexplained pulmonary edema, pulmonary artery enlargement may be seen secondary to pulmonary hypertension. The heart is often small; however, enlargement of the left atrium due to a dilated accessory chamber may be visualized (Fig. 46-8).
Ultrasonography
Echocardiography is often the only modality needed in the evaluation of cor triatriatum (Fig. 46-9). Visualization of the thin echogenic membrane is often best appreciated through a parasternal, apical, or subcostal approach. Supravalvular mitral stenosis due to a supramitral ring has an appearance similar to cor triatriatum and also results in venous obstruction. The two entities can be differentiated by the location and appearance of the membrane. The left atrial appendage and foramen ovale are located distal to the relatively thin curvilinear membrane of cor triatriatum, whereas the appendage and foramen are proximal to the stiff membrane of the supramitral ring. Right atrial dilation and ventricular dilation secondary to venous obstruction are nonspecific and are seen in both diseases.
Computed Tomography
CTA can demonstrate the accessory chamber26 and, depending on the size of the membranous ostia, may allow differentiation of drainage to the left or right atrium. If the cor triatriatum is associated with partial anomalous venous connection, these aberrant pathways can be well demonstrated. As stated before, the requisite ionizing radiation and the relative lack of functional information pertaining to right-sided heart overload are drawbacks compared with MRI.
Magnetic Resonance
Like CTA, cardiac MRI allows excellent anatomic depiction of the accessory atrial chamber in cor triatriatum27–30 (Fig. 46-10). Functional information about right-sided heart strain or overload may also be obtained. Flow from the high-pressure accessory chamber through the obstructed membrane into the low-pressure left atrium can produce visible turbulent jets or dephasing on cine images. Additional anomalies, such as PAPVC and the presence of an ASD, can be evaluated.
Angiography
Conventional angiography may demonstrate filling of the accessory chamber with a discrete membrane separating it from the true atrium. Contrast material may pool in this chamber for a prolonged period if the outflow obstruction is severe. Because the walls of the accessory chamber most likely form from the common pulmonary vein, they are noncontractile. Angiography, though, is often unsuccessful in discriminating between cor triatriatum, total anomalous pulmonary venous connection, and intrinsic pulmonary vein obstruction.25,31,32
Reporting: Information for the Referring Physician
TOTAL ANOMALOUS PULMONARY VENOUS CONNECTION
Prevalence and Epidemiology
TAPVC is generally estimated to represent not more than 2.0% of congenital heart disease.36 For the most part, no strong gender prevalence has been noted.37 The curious exception to this is total anomalous connection to the portal system, which affects males 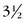 times more often than females.38 There is a common association of TAPVC with heterotaxy syndrome. More important, patients with heterotaxy and TAPVC (particularly those complicated by post-repair stenosis) are more likely to have poor outcomes.39
times more often than females.38 There is a common association of TAPVC with heterotaxy syndrome. More important, patients with heterotaxy and TAPVC (particularly those complicated by post-repair stenosis) are more likely to have poor outcomes.39
Etiology and Pathophysiology
As described before, failure of the normal incorporation of the common pulmonary vein into the dorsal wall of the left atrium with persistent connections of all pulmonary veins to systemic vessels, right atrium, or coronary sinus results in total anomalous venous connection. Various classification schemes for anomalous drainage patterns have been described. One commonly used classification put forth by Craig and Darling includes supracardiac drainage (type I), drainage at the cardiac level (type II), infracardiac drainage (type III), and mixed patterns (type IV).40 The most frequently described connection is to the left innominate vein.37 Other frequently encountered sites of drainage include the coronary sinus, the right atrium (secondary to malposition of the septum primum), the SVC, and the portal system. Patients with TAPVC often have a concurrent ASD, VSD, or patent ductus arteriosus (PDA). Postnatal survival in patients with TAPVC is dependent on the presence of a shunt. In addition, many cases of TAPVC demonstrate some degree of pulmonary venous obstruction, especially the infradiaphragmatic type. Causes of venous obstruction include intrinsic abnormality of the vessel wall with medial hypertrophy, compression by adjacent structures, and narrowing at the level of the diaphragm or ductus venosus. The presence of venous obstruction portends a worse prognosis.37
Manifestations of Disease
Imaging Techniques and Findings
Radiography
When the pulmonary venous connection to the systemic circulation is unobstructed, cardiomegaly with right atrial and ventricular enlargement and increased pulmonary blood flow are present on the chest radiograph. Patients with total drainage to the left innominate vein may demonstrate the snowman sign, in which the upper portion of the snowman is derived from the vertical vein on the left, the enlarged innominate vein superiorly, and the enlarged SVC on the right. The cardiac silhouette constitutes the lower portion of the snowman (Fig. 46-11).
The chest radiograph appearance in patients with infradiaphragmatic obstructed TAPVC is very different. The heart size is normal or mildly enlarged, and there is normal pulmonary blood flow with a variable degree of pulmonary edema, depending on the degree of obstruction (Fig. 46-12).
Ultrasonography
The lack of pulmonary veins entering the left atrium together with signs of right ventricular volume overload establishes the diagnosis of TAPVC on echocardiography. At this point, a detailed survey must be performed to account for all of the anomalous veins. The individual veins should be carefully examined with two-dimensional and color Doppler techniques. The size of the pulmonary veins at initial diagnosis has prognostic implications.40 The orientation of aberrant vessels as well as presence or absence of obstruction should be clearly defined to aid surgical management. Because of the association with heterotaxy syndrome, the cardiac segmental anatomy and abdominal visceral morphology as well as intracardiac anomalies are also evaluated.
Computed Tomography
There are limited published data concerning the use of CTA for the evaluation of TAPVC. The small studies that have been performed indicate that the modality is capable of demonstrating the anatomy of the pulmonary veins in detail.8,41–43 The course and size of the pulmonary veins can be determined with CTA, and the presence of venous obstruction is well demonstrated with isotropic multidetector-row CT acquisition with multiplanar reformats, maximum intensity projections, and volume rendered techniques (Fig. 46-13). Functional assessment (particularly in the neonatal period) is well evaluated by echocardiography. The pulmonary and abdominal visceral morphology is well demonstrated with CT, aiding in the evaluation of patients with concomitant heterotaxy.
Magnetic Resonance
In patients with TAPVC who have incomplete evaluation of all of the anomalous pulmonary venous connections by echocardiography, MRA can provide highly detailed comprehensive information.9,44 The number of pulmonary veins and their sizes, courses, and drainage patterns are well depicted. As described before, the size of the pulmonary veins is clinically relevant in that those patients with smaller and obstructed anomalous veins tend to have a worse outcome40 (Fig. 46-14). Improved MRA techniques now allow excellent spatial resolution to detect the presence or absence of venous obstruction. The anomalously draining veins should be carefully assessed for any intrinsic narrowing or extrinsic compression (i.e., from an adjacent pulmonary artery or bronchus). Functional evaluation by MRI is often unnecessary in the neonate and is usually performed by echocardiography. The cardiac segmental anatomy, intracardiac and great vessel connections, and abdominal visceral morphology in patients with heterotaxy can also be evaluated on MRI (Fig. 46-15).
Angiography
The increasing accuracy of two-dimensional echocardiography with Doppler examination, cardiac MRI, and CTA have eliminated the need for diagnostic angiography in the work-up of TAPVC. If it is performed, selective pulmonary angiography with attention to the levophase will demonstrate the anomalous venous channels (Fig. 46-16).
Synopsis of Treatment Options
Surgical/Interventional
The definitive treatment of TAPVC is surgical. In the case of supracardiac TAPVC draining to the left innominate vein, the common vein is anastomosed side-to-side with the left atrium, and the communication to the systemic veins as well as additional shunts, such as ASD or VSD, are closed. Other types of TAPVC are treated in a similar manner. Patients with preexisting venous obstruction and coexistent complex heart disease, especially single ventricle, have worse outcomes.45,46 Other features that have been correlated with poor outcome include younger age and type II connection.46 Pulmonary vein stenosis after repair is a common complication, particularly in patients with heterotaxy.
Reporting: Information for the Referring Physician
KEY POINTS
 The distinction between the obstructed and nonobstructed forms of TAPVC is important because the surgical repair of the obstructed veins is more difficult and patients tend to have a worse outcome with obstruction.
The distinction between the obstructed and nonobstructed forms of TAPVC is important because the surgical repair of the obstructed veins is more difficult and patients tend to have a worse outcome with obstruction.Alphonso N, Norgaard MA, Newcomb A, et al. Cor triatriatum: presentation, diagnosis and long-term surgical results. Ann Thorac Surg. 2005;80:1666-1671.
Darling RC, Rothney WB, Craig JM. Total pulmonary venous drainage into the right side of the heart: report of 17 autopsied cases not associated with other major cardiovascular anomalies. Lab Invest. 1957;6:44-64.
Kanter KR. Surgical repair of total anomalous pulmonary venous connection. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2006:40-44.
Kim TH, Kim YM, Suh CH, et al. Helical CT angiography and three-dimensional reconstruction of total anomalous pulmonary venous connections in neonates and infants. AJR Am J Roentgenol. 2000;175:1381-1386.
Uçar T, Fitoz S, Tutar E, et al. Diagnostic tools in the preoperative evaluation of children with anomalous pulmonary venous connections. Int J Cardiovasc Imaging. 2008;24:229-235.
Valsangiacomo ER, Levasseur S, McCrindle BW, et al. Contrast-enhanced MR angiography of pulmonary venous abnormalities in children. Pediatr Radiol. 2003;33:92-98.
Wang ZJ, Reddy GP, Gotway MB, et al. Cardiovascular shunts: MR imaging evaluation. Radiographics. 2003;23:S181-S194.
Zylak CJ, Eyler WR, Spizarny DL, Stone CH. Developmental lung anomalies in the adult: radiologic-pathologic correlation. Radiographics. 2002;22:S25-S43.
1 Webb S, Kanani M, Anderson RH, et al. Development of the human pulmonary vein and its incorporation in the morphologically left atrium. Cardiol Young. 2001;11:632-642.
2 Healy JEJr. An anatomic survey of anomalous pulmonary veins: their significance. J Thorac Cardiovasc Surg. 1952;23:433-444.
3 Hughes C, Rumore P. Anomalous pulmonary veins. Arch Pathol. 1944;37:364-366.
4 Panicek DM, Heitzman ER, Randall PA, et al. The continuum of pulmonary developmental anomalies. Radiographics. 1987;7:747-772.
5 Freedom RM, Yoo SH, Goo HW, et al. The bronchopulmonary foregut malformation complex. Cardiol Young. 2006;16:229-251.
6 Moore JW, Kirby WC, Rogers WM, Poth MA. Partial anomalous pulmonary venous drainage associated with 45,X Turner’s syndrome. Pediatrics. 1990;86:273-276.
7 Stümper O, Vargas-Barron J, Rijlaarsdam M, et al. Assessment of anomalous systemic and pulmonary venous connections by transoesophageal echocardiography in infants and children. Br Heart J. 1991;66:411-418.
8 Uçar T, Fitoz S, Tutar E, et al. Diagnostic tools in the preoperative evaluation of children with anomalous pulmonary venous connections. Int J Cardiovasc Imaging. 2008;24:229-235.
9 Masui T, Seelos KC, Kersting-Sommerhoff BA, Higgins CB. Abnormalities of the pulmonary veins: evaluation with MR imaging and comparison with cardiac angiography and echocardiography. Radiology. 1991;181:645-649.
10 Festa P, Ait-Ali L, Cerillo AG, et al. Magnetic resonance imaging is the diagnostic tool of choice in the preoperative evaluation of patients with partial anomalous pulmonary venous return. Int J Cardiovasc Imaging. 2006;22:685-693.
11 Greil GF, Powell AJ, Gildein HP, Geva T. Gadolinium-enhanced three-dimensional magnetic resonance angiography of pulmonary and systemic venous anomalies. J Am Coll Cardiol. 2002;39:335-341.
12 Alsoufi B, Cai S, Van Arsdell GS, et al. Outcomes after surgical treatment of children with partial anomalous pulmonary venous connection. Ann Thorac Surg. 2007;84:2020-2026.
13 Davia JE, Cheitlin MD, Bedynek JL. Sinus venosus atrial septal defect: analysis of fifty cases. Am Heart J. 1973;85:177-185.
14 Van Praagh S, Carrera ME, Sanders SP, et al. Sinus venosus defects—anatomic and echocardiographic findings and surgical treatment. Am Heart J. 1994;128:365-379.
15 Attenhofer Jost CH, Connolly HM, Danielson GK, et al. Sinus venosus atrial septal defect: long term postoperative outcome for 115 patients. Circulation. 2005;112:1953-1958.
16 Otsuka M, Itoh A, Haze K. Sinus venosus type of atrial septal defect with partial anomalous pulmonary venous return evaluated by multislice CT. Heart. 2004;90:901.
17 Valente AM, Sena L, Powell AJ, et al. Cardiac magnetic resonance imaging evaluation of sinus venosus defects: comparison to surgical findings. Pediatr Cardiol. 2007;28:51-56.
18 Kharouf R, Luxenberg DM, Khalid O, Abdulla R. Atrial septal defect: spectrum of care. Pediatr Cardiol. 2008;29:271-280.
19 Oliver JM, Gallego P, Gonzalez A, et al. Sinus venosus syndrome: atrial septal defect or anomalous venous connection? A multiplane transoesophageal approach. Heart. 2002;88:634-638.
20 Iyer AP, Somanrema K, Pathak S, et al. Comparative study of single- and double-patch techniques for sinus venosus atrial septal defect with partial anomalous pulmonary venous connection. J Thorac Cardiovasc Surg. 2007;133:656-659.
21 Stewart RD, Bailliard F, Kelle AM, et al. Evolving surgical strategy for sinus venosus atrial septal defect: effect on sinus node function and late venous obstruction. Ann Thorac Surg. 2007;84:1651-1655.
22 Warden HE, Gustafson RA, Tarnay TJ, Neal WA. An alternative method for repair of partial anomalous pulmonary venous connection to the superior vena cava. Ann Thorac Surg. 1984;38:601-605.
23 Van Praagh R, Corsini I. Cor triatriatum: pathologic anatomy and a consideration of morphogenesis based on 13 postmortem cases and a study of normal development of the pulmonary vein and atrial septum in 83 human embryos. Am Heart J. 1969;78:379-405.
24 Buchholz S, Jenni R. Doppler echocardiographic findings in 2 identical variants of a rare cardiac anomaly, “subtotal” cor triatriatum: a critical review of the literature. J Am Soc Echocardiogr. 2001;14:846-849.
25 Jegier W, Gibbons JE, Wiglesworth FW. Cor triatriatum: clinical, hemodynamic and pathological studies: surgical correction in early life. Pediatrics. 1963;31:255-267.
26 Chen K, Thng CH. Multislice computed tomography and two-dimensional echocardiographic images of cor triatriatum in a 46-year-old man. Circulation. 2001;104:2117.
27 Rumancik WM, Hernanz-Schulman M, Rutkowski MM, et al. Magnetic resonance imaging of cor triatriatum. Pediatr Cardiol. 1988;9:149-151.
28 Sakamoto I, Matsunaga N, Hayashi K, et al. Cine-magnetic resonance imaging of cor triatriatum. Chest. 1994;106:1586-1589.
29 Ibrahim T, Schreiber K, Dennig K, et al. Assessment of cor triatriatum sinistrum by magnetic resonance imaging. Circulation. 2003;108:e107.
30 Steen H, Merten C, Lehrke S, et al. Two rare cases of left and right atrial congenital heart disease: cor triatriatum dexter and sinister. Clin Res Cardiol. 2007;96:122-124.
31 Gheissari A, Malm JR, Bowman FOJr, Bierman FZ. Cor triatriatum sinistrum: one institution’s 28-year experience. Pediatr Cardiol. 1992;13:85-88.
32 Richardson JV, Doty DB, Siewers RD, Zuberbuhler JR. Cor triatriatum (subdivided left atrium). J Thorac Cardiovasc Surg. 1981;81:232-238.
33 Alphonso N, Norgaard MA, Newcomb A, et al. Cor triatriatum: presentation, diagnosis and long-term surgical results. Ann Thorac Surg. 2005;80:1666-1671.
34 Huang YK, Chu JJ, Chang JP, et al. Cor triatriatum sinistrum: surgical experience in Taiwan. Surg Today. 2007;37:449-454.
35 Oglietti J, Cooley DA, Izquierdo JP, et al. Cor triatriatum: operative results in 25 patients. Ann Thorac Surg. 1983;35:415-420.
36 Mehrizi A, Hirsch MS, Taussig HB. Congenital heart disease in the neonatal period: autopsy study of 170 cases. J Pediatr. 1964;65:721-726.
37 Delisle G, Ando M, Calder AL, et al. Total anomalous pulmonary venous connection: report of 93 autopsied cases with emphasis on diagnostic and surgical considerations. Am Heart J. 1976;91:99-122.
38 Lucas RVJr, Adams PJr, Anderson RC. Total anomalous pulmonary venous connection to the portal system: a cause of pulmonary venous obstruction. AJR Am J Roentgenol. 1961;86:561-575.
39 Foerster SR, Gauvreau K, McElhinney DB, Geva T. Importance of total anomalous pulmonary venous connection and postoperative pulmonary vein stenosis in outcomes of heterotaxy syndrome. Pediatr Cardiol. 2008;29:536-544.
40 Jenkins KJ, Sanders SP, Orav EJ, et al. Individual pulmonary vein size and survival in infants with totally anomalous pulmonary venous connection. J Am Coll Cardiol. 1993;22:201-206.
41 Kim TH, Kim YM, Suh CH, et al. Helical CT angiography and three-dimensional reconstruction of total anomalous pulmonary venous connections in neonates and infants. AJR Am J Roentgenol. 2000;175:1381-1386.
42 Shiraishi I, Yamagishi M, Iwasaki N, et al. Helical computed tomographic angiography in obstructed total anomalous pulmonary venous drainage. Ann Thorac Surg. 2001;71:1690-1692.
43 Sridhar PG, Kalyanpur A, Suresh PV, et al. Total anomalous pulmonary venous connection: helical computed tomography as an alternative to angiography. Indian Heart J. 2003;55:624-627.
44 Valsangiacomo ER, Levasseur S, McCrindle BW, et al. Contrast-enhanced MR angiography of pulmonary venous abnormalities in children. Pediatr Radiol. 2003;33:92-98.
45 Hancock Friesen CL, Zurakowski D, Thiagarajan RR, et al. Total anomalous pulmonary venous connection: an analysis of current management strategies in a single institution. Ann Thorac Surg. 2005;79:596-606.
46 Karamlou T, Gurofsky R, Sukhni EA, et al. Factors associated with mortality and reoperation in 377 children with total anomalous pulmonary venous connection. Circulation. 2007;115:1591-1598.

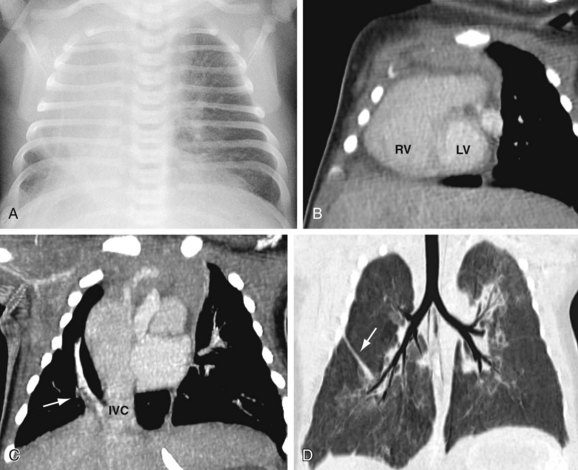
 FIGURE 46-1
FIGURE 46-1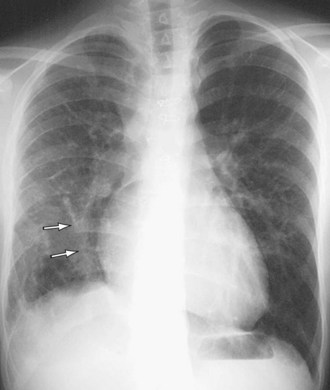
 FIGURE 46-2
FIGURE 46-2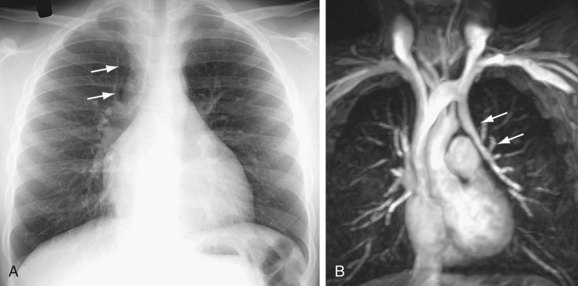
 FIGURE 46-3
FIGURE 46-3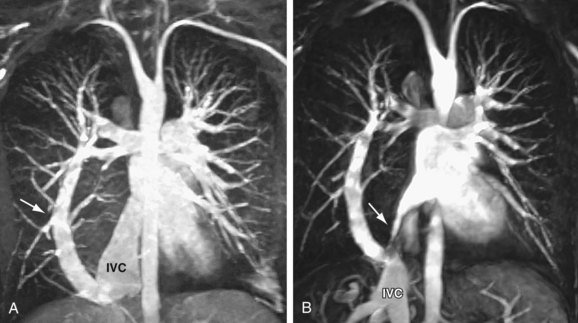
 FIGURE 46-4
FIGURE 46-4

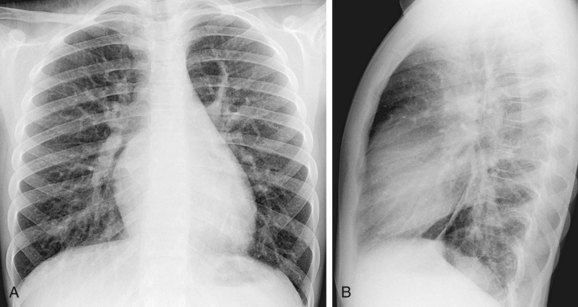
 FIGURE 46-5
FIGURE 46-5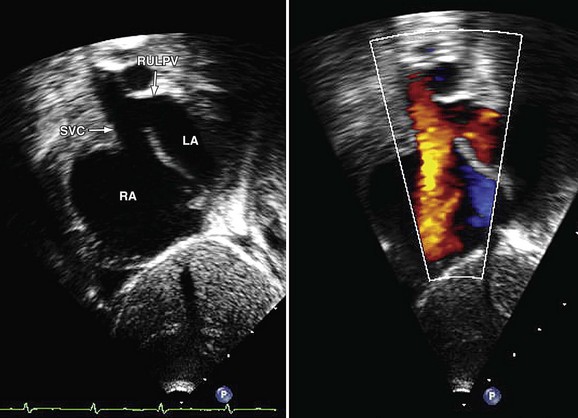
 FIGURE 46-6
FIGURE 46-6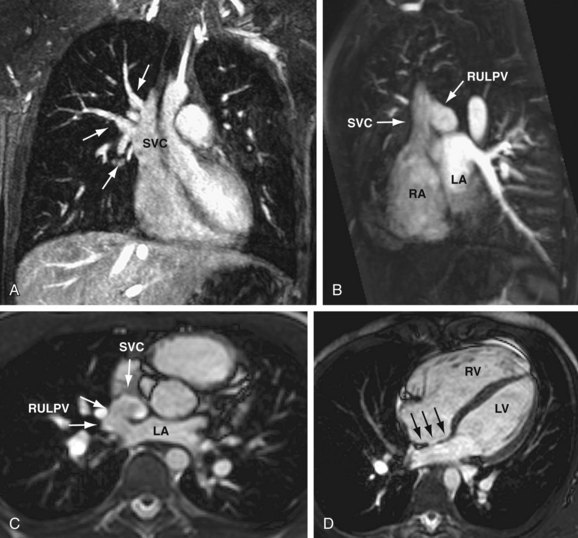
 FIGURE 46-7
FIGURE 46-7


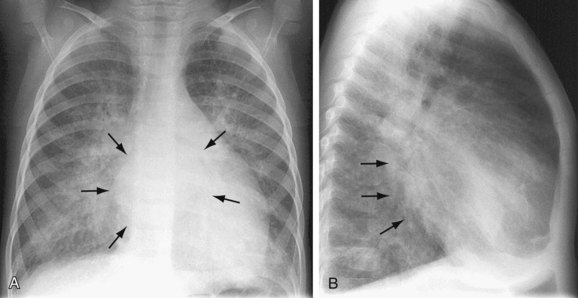
 FIGURE 46-8
FIGURE 46-8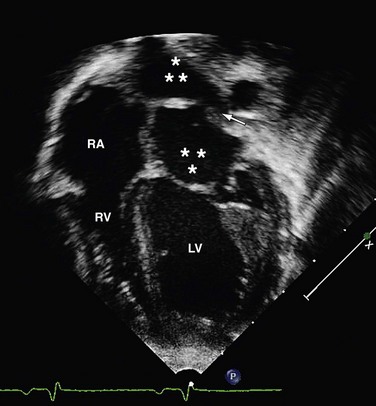
 FIGURE 46-9
FIGURE 46-9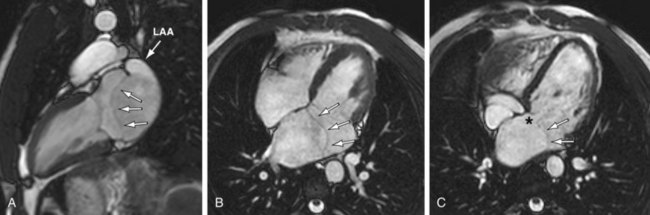
 FIGURE 46-10
FIGURE 46-10




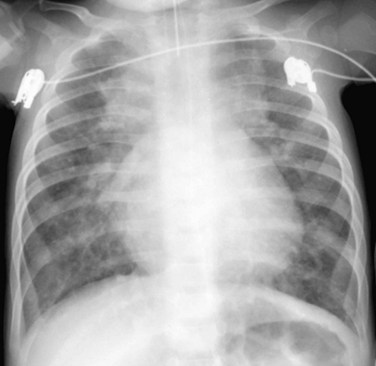
 FIGURE 46-11
FIGURE 46-11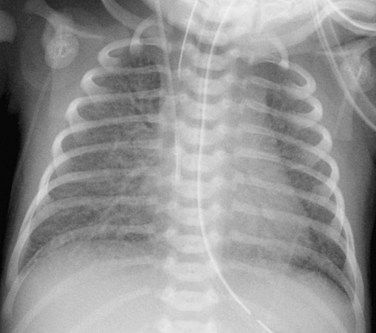
 FIGURE 46-12
FIGURE 46-12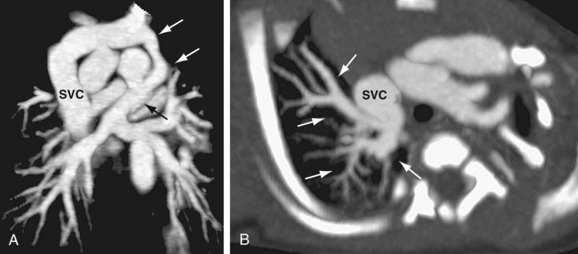
 FIGURE 46-13
FIGURE 46-13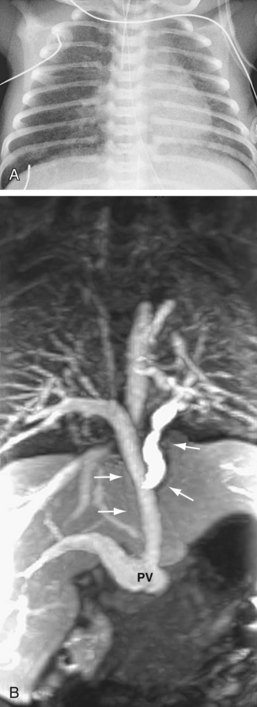
 FIGURE 46-14
FIGURE 46-14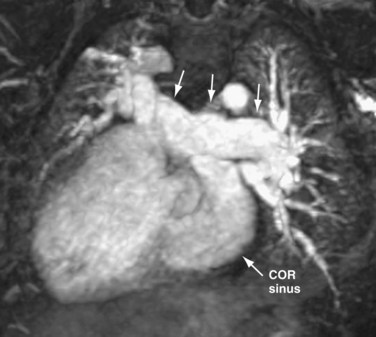
 FIGURE 46-15
FIGURE 46-15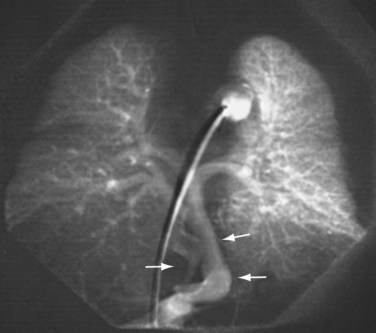
 FIGURE 46-16
FIGURE 46-16


