Chapter 21 Anomalous Origin of the Left Coronary Artery from the Pulmonary Trunk
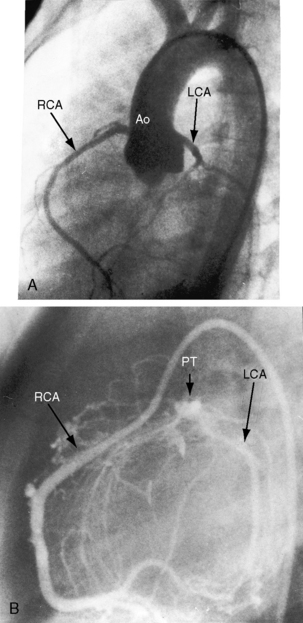
In 1886, St. John Brooks1 described two cases of “an abnormal coronary artery arising from the pulmonary artery.” The diagnosis was subsequently called into question when the abnormal communication was attributed to a coronary arterial fistula. The seminal report of Bland, White, and Garland2 in 1933 referred to Maude Abbott’s case of a 60-year-old woman with anomalous origin of the left coronary artery from the pulmonary trunk (Figure 21-1B). Bland, White, and Garland remain with us as eponyms.3 In 1962, Fontana and Edwards4 reported a series of 58 postmortem specimens.
Anomalous origin of the left coronary artery from the pulmonary trunk is the most common major congenital malformation of the coronary circulation, with an incidence rate estimated at 1 in 300,000 live births.5–8 More rarely, the right coronary artery (Figure 21-2), the left anterior descending coronary artery,9 the circumflex coronary artery,10–12 both coronary arteries,13–15 or a single coronary artery16 arises from the pulmonary trunk or its right or left branch.11,17 Stenosis of the ostium of the anomalous left coronary artery has been reported,18 and rarely, the anomalous artery courses within the aortic wall (intramural).19
The anomalous left coronary artery is a thin-walled vessel that resembles a venous channel (Figures 21-1, 21-3, and 21-4).20,21 The right coronary artery originates from its normal aortic sinus, is dilated and tortuous (see Figures 21-1 and 21-4), and, on rare occasions, is aneurysmal.22 The portion of left ventricle perfused by the anomalous left coronary artery is thin, scarred, and dilated21 and occasionally forms a ventricular aneurysm. Conversely, the hypoperfused but viable portion of left ventricle increases its mass, often appreciably,20 because immature cardiomyocytes replicate in response to the hypoxemic stimulus.23 The left ventricular endocardium exhibits fibroelastosis and rarely is focally calcified. Interestingly, in adults with typical ischemic heart disease, newly formed elastic fibers appear within 3 or 4 weeks after a myocardial infarction and culminate in endocardial fibroelastosis in the vicinity of the infarct.24
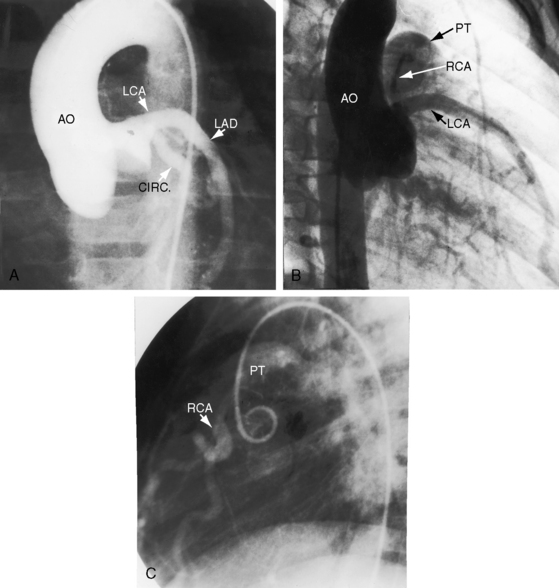
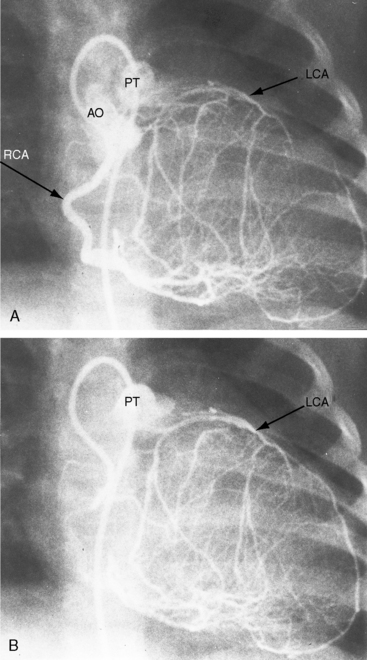
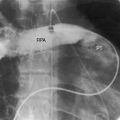
Figure 21-3 Aortogram (anteroposterior projection) from a 4-year-old boy with anomalous origin of the right coronary artery from the pulmonary trunk. A, The left coronary artery (LCA) originates from its aortic (AO) sinus and divides into the left anterior descending (LAD) and circumflex (CIRC) arteries. B, Intercoronary anastomoses visualize the left coronary artery (LCA) that originates from the pulmonary trunk as illustrated in Figure 21-5. C, Lateral projection shows the right coronary artery (RCA) entering the pulmonary trunk (PT).
Three theories have been proposed to explain the origin of a coronary artery from the pulmonary trunk.15 The first two theories are related to division of the embryologic truncus arteriosus.25 In the early embryo, two opposing truncal cushions enlarge and fuse to form the truncal septum, which divides the truncus arteriosus into aortic and pulmonary channels.25 Assuming that the coronary arteries originate as two endothelial buds (see Chapter 32), displacement of the origin of one or both of these buds could assign either or both coronary arteries to the portion of the truncus arteriosus destined to become the pulmonary artery. Alternatively, faulty division of the truncus could incorporate one or both coronary artery buds into the pulmonary artery. The higher incidence of anomalous origin of the left rather than the right coronary artery from the pulmonary trunk has been attributed to the proximity of the left aortic sinus to the truncal septum, so a relatively small displacement of the left coronary artery anlage suffices to cause the left coronary artery ostium to lie within the pulmonary artery.15 These theories presuppose that human coronary arteries originate as two endothelial buds that develop before or simultaneously with the division of the truncus arteriosus (see Chapter 32).15 Nor do these theories explain the presence of a third (accessory) coronary artery or anomalous origin of one branch of the left coronary artery from the pulmonary trunk or explain why the relative sizes of the pulmonary artery, the aorta, and their valves are not altered by the proposed displacement of the truncal septum.15 The involution and persistence theory postulates that there are originally six coronary artery anlagen: three from the aorta, and three from the pulmonary artery (see Chapter 32).15 The coronary arteries that are destined to originate normally are believed to arise from two persistent anlagen in two separate aortic sinuses, whereas the anlage in the third aortic sinus, in addition to all three anlagen in the pulmonary sinuses, undergo involution.15 According to this theory, anomalous origin of one or both coronary arteries could result from persistence of pulmonary artery coronary anlagen together with involution of the normally persistent aortic coronary anlagen.15 In the presence of a bicuspid aortic valve, anomalous origin of the left coronary artery from the pulmonary trunk is believed to be the expression of a single morphogenic defect.26,27 In the Syrian hamster, a relationship has been proposed between anomalous origin of the left coronary artery and the developmental morphology of the semilunar valves.26,27
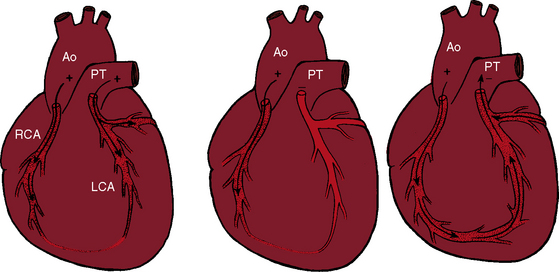
Myocardial ischemia is a serious sequela of anomalous origin of the left coronary artery from the pulmonary trunk.28 Ischemia does not stem from the fact that only one coronary artery originates from the aorta because the functional consequences of a congenitally single coronary artery are benign,29–31 with few exceptions (see Chapter 32).32,33 Nor is perfusion of the anomalous coronary artery by unoxygenated blood from the pulmonary trunk a satisfactory explanation because in cyanotic congenital heart disease the oxygen content of coronary arterial blood can be exceedingly low without producing myocardial ischemia. Why then does cardiac muscle become ischemic when the left coronary artery arises from the pulmonary trunk? The answer lies in the direction of blood flow through the coronary bed,34,35 as illustrated in the circulatory patterns of Figure 21-5. In fetal and early neonatal life, relatively high pulmonary arterial pressure results in antegrade blood flow into the anomalous left coronary artery (see Figure 21-5, first panel). The subsequent fall in pulmonary arterial pressure is accompanied by a parallel fall in blood flow into the anomalous left coronary (see Figure 21-5, middle panel). During this crucial transition, myocardial perfusion depends almost entirely on perfusion from the right coronary artery (see Figure 21-3).35,36 Myocardial ischemia is unavoidable unless adequate circulation from right to left coronary artery is established via intercoronary anastomoses, on which survival largely depends (see Figure 21-3B).21,35,37 Intercoronary anastomoses represent low-resistance pathways between the aorta and pulmonary trunk, in essence, arteriovenous fistulae,34 that have two opposing effects: (1) a desirable effect of reestablishing left coronary arterial perfusion; and (2) the undesirable effect of a coronary steal that bypasses the capillary bed and deprives the myocardium of oxygen.34,35,38 The idea of retrograde flow through an anomalous left coronary artery was originally proposed by 1886 by St. John Brooks:
“Here are two arteries belonging to the different circulations—the pulmonary and the systemic—anastomosing with each other. In these circulations, as is well known, the arterial pressure is very much greater in the systemic than in the pulmonary; how then did the blood flow in the anomalous coronary artery? There cannot be a doubt that it acted very much after the manner of a vein, and that blood flowed through it towards the pulmonary artery, and from thence into the lungs.”1
Physiologic, pathologic, and clinical derangements arise from the ischemic consequences of the transition from decreased antegrade perfusion of the anomalous left coronary artery, to flow from the right coronary artery through the low-resistance intercoronary anastomoses into the left coronary artery, followed by retrograde flow into the pulmonary trunk (see Figure 21-5). Ischemia causes the left ventricle to labor under three handicaps: first, viable myocardium is compromised, so contractility is depressed39; second, mitral regurgitation occurs as a consequence of ischemic papillary muscle dysfunction40 that adds to the hemodynamic burden (Figure 21-6); and third, flow via the intercoronary anastomoses constitutes a left-to-right shunt that is occasionally large enough to impose volume overload on the left ventricle.41 Although regional abnormalities of wall motion characterize anomalous origin of the left coronary artery from the pulmonary trunk,42,43 infants tend to exhibit global hypokinesis.42,43
History
The outlook is most grave when both coronary arteries originate from the pulmonary trunk13–15 and is most favorable when the right coronary artery alone, or a left anterior descending or circumflex coronary artery alone, originates anomalously.10–12 The clinical course is a continuum that ranges from death in infancy to asymptomatic adult survival, with all gradations in between.35,44–47 Nevertheless, there are three general patterns: (1) serious symptoms in early infancy with death before 1 year of age; (2) early symptoms followed by gradual attenuation or disappearance; and (3) absence or virtual absence of early symptoms with asymptomatic survival to adulthood. Adult survival, even to age 90 years,48 has been reported and is much more likely with anomalous origin of the right coronary artery from the pulmonary trunk, which, however, is not necessarily benign.
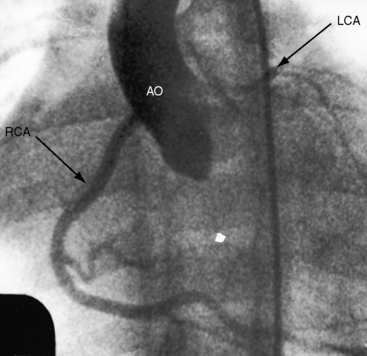
Eighty percent to 90% of patients with anomalous origin of the left coronary artery from the pulmonary trunk die in their first year.21,47 Presentation in the neonate is unusual because elevated pulmonary arterial pressure results in forward flow into the anomalous left coronary artery.49 Accordingly, infants appear healthy at birth and often remain so for about 2 months, after which symptoms usually begin.47 Irritability, dyspnea, wheezing, cough, diaphoresis, and ashen gray pallor are precipitated or aggravated by feeding, crying, or a bowel movement.47,50 Occasionally, the initial symptom is hoarseness thought to be caused by impingement of a dilated pulmonary artery on the recurrent laryngeal nerve.51 Chronic congestive heart failure is responsible for poor growth and development and is usually the cause of death. More than a third of patients presents with sudden cardiac death,52 in which case the diagnosis is necessarily post mortem. Symptomatic infants occasionally have improvement53 only to die suddenly during a relatively asymptomatic childhood or adolescence. One of the first known patients with the anomaly was the 60-year-old woman described by Maude Abbott54 in 1908. An elderly asymptomatic man played football in his youth and presented at age 67 years with sustained ventricular fibrillation.55 Angina may be delayed until the teens, or the anomaly may be discovered in asymptomatic children56 or adults because of mitral regurgitation (Figures 21-6 and 21-7). A 39-year-old mother of three presented with exertional fatigue.57 About 15% of individuals with anomalous origin of the left coronary artery survive to adulthood,58 some reaching the seventh or eighth decade.22,59 The malformation reveals itself in adults because of detection of the murmur of mitral regurgitation,22,59,60 because of a continuous murmur mistaken for a patent ductus (see Figure 21-7), or because of angina pectoris, myocardial infarction, congestive heart failure, atrial fibrillation, ventricular tachyarrhythmias, cardiac arrest, or sudden cardiac death.34,45,46,61–64
Arterial pulse, jugular venous pulse, precordial movement, and palpation
Pulsus alternans results from left ventricular failure.21 Diastolic pressure is occasionally low because of an aortic runoff through the fistulous communication.41 The jugular venous pulse is elevated in the presence of congestive heart failure but cannot be clinically assessed in symptomatic infants. A left parasternal impulse is the result of an enlarged left atrium that expands in systole in response to mitral regurgitation.
Auscultation
Anomalous origin of the left coronary artery from the pulmonary trunk is accompanied by systolic, diastolic, or continuous murmurs or by no murmur at all. The holosystolic murmur of mitral regurgitation (see Figure 21-7) is the consequence of ischemic papillary muscle dysfunction (see previous).20,65,66 Short apical mid-diastolic murmurs are analogous to mid-diastolic murmurs in other forms of mitral regurgitation.66
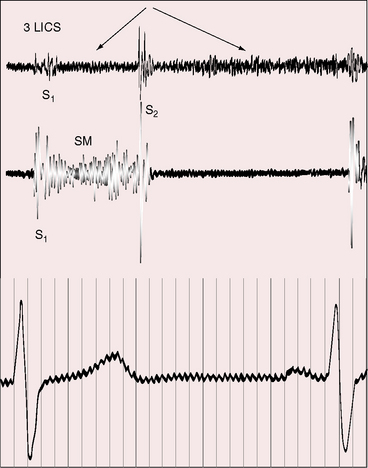
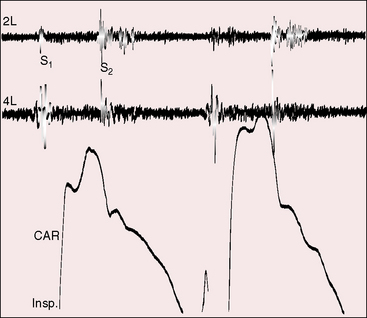
Continuous murmurs are generated by flow through intercoronary anastomoses (Figures 21-7 and 21-8).41 Their configuration and location are only occasionally similar to patent ductus arteriosus,67 which only rarely coexists.68 The continuous murmur of the intercoronary anastomoses does not peak around the second heart sound but is softer in systole and louder in diastole (see Figures 21-7 and 21-8) because transmural systolic pressure generated by ventricular contraction reduces intercoronary systolic flow. The occasional isolated diastolic murmur has been attributed to a reduction in systolic intercoronary flow caused by elevated pulmonary arterial pressure provoked by left ventricular failure or mitral regurgitation.41
The continuous murmurs are located at the base of the heart or somewhat lower either to the left or right of the sternum, but usually along the left sternal border (see Figures 21-7 and 21-8). An isolated diastolic murmur along the sternal edge is occasionally mistaken for semilunar valve regurgitation. Third heart sounds coincide with left ventricular failure and mitral regurgitation.
Electrocardiogram
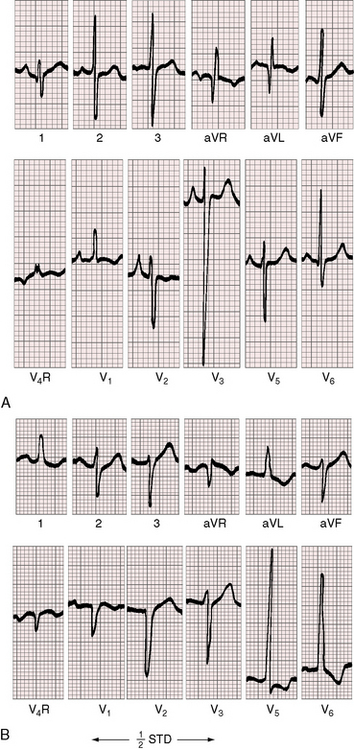
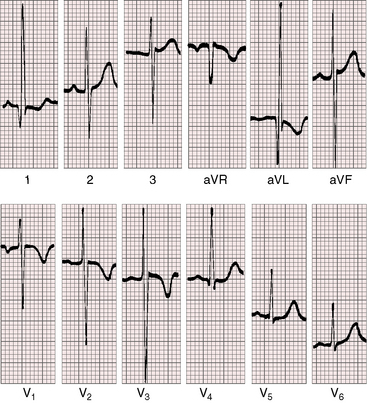
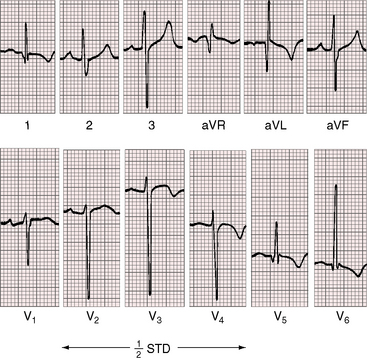
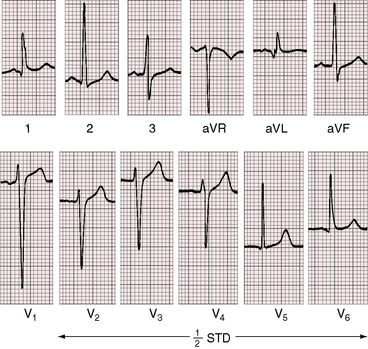
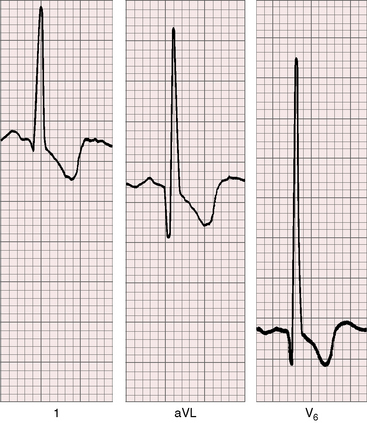
Three features characterize the scalar electrocardiogram of anomalous origin of the left coronary artery from the pulmonary trunk: (1) deep narrow q waves; (2) left ventricular hypertrophy; and (3) left axis deviation. In healthy infants and children, q waves are consistently absent in leads 1 and aVL. In anomalous origin of the left coronary artery from the pulmonary trunk, deep narrow q waves are present in these leads (Figures 21-9A and 21-10)8 and are in striking contrast to the shallow broad q waves of adult ischemic heart disease. The depth of the q wave in lead aVL can equal or exceed the height of the R wave (Figures 21-9A and 21-11). In infants, these q waves can be small or absent but become progressively more prominent. An abnormal tracing may improve with age69 or may become more abnormal (see Figure 21-9A, B). Q waves are rarely present in right precordial leads (see Figure 21-9B).
The second electrocardiographic characteristic, left ventricular hypertrophy,7,20,21 was in the title of the original Bland, White, and Garland2 publication, “Report of an Unusual Case Associated with Cardiac Hypertrophy.” Although heart weights are increased, it is the posterobasal region of the left ventricle that is selectively increased in mass (see previous).20,23 From the gross morphologic point of view, this regional increase in mass is hypertrophy, but from the cell biologic point of view, the posterobasal increase in mass is hyperplasia (i.e., replication of cardiomyocytes).23 The basis for this cellular pattern is the capacity of immature cardiomyocytes to replicate in response to hypoxemia that is a feature of the hypoperfused but viable posterobasal left ventricular wall (see previous).23
In the electrocardiogram, left ventricular hypertrophy is represented by typical voltage and repolarization criteria (Figures 21-11 and 21-12).69 However, in leads aVL and V6, the deep narrow q waves may reflect the initial force deformity of the congenital coronary artery anomaly rather than left ventricular hypertrophy (Figure 21-13). Electrocardiograms recorded during angina pectoris or exercise stress testing disclose ischemic ST segment depressions and T wave inversions.
Left axis deviation is the third electrocardiographic characteristic of anomalous origin of the left coronary artery from the pulmonary trunk (see Figure 21-9B).70 The mechanism has been attributed to a disproportionate increase in posterobasal left ventricular muscle mass that results a left superior direction of the major depolarization vector.20,70
Left atrial P wave abnormalities occur because of mitral regurgitation (see Figure 21-9B), which is responsible for occasional atrial fibrillation.
X-ray
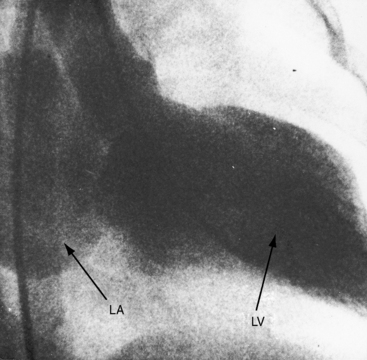
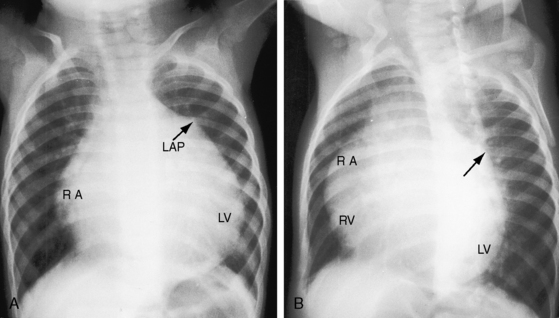
The cardiac silhouette varies from massive cardiomegaly in symptomatic infants (Figure 12–14) to normal or nearly so in an occasional older child or adult (Figure 21-15). The most consistent radiologic features are enlargement of the left ventricle (see Figure 21-14)37,71 and left atrium (see Figures 21-6, 21-14, and 21-15).37,71 Increased vascularity represents the pulmonary venous congestion of left ventricular failure (see Figure 21-14). Dystrophic calcification of the left ventricle has been described but is not visible in the x-ray.
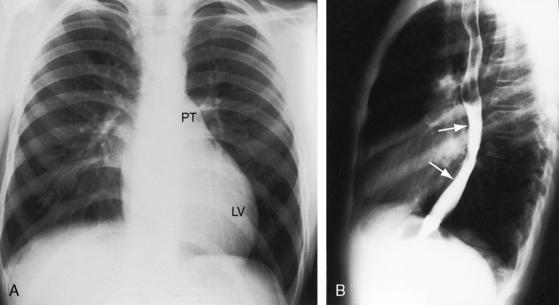
Figure 21-15 X-rays from the 13-year-old boy whose phonocardiogram is shown in Figure 21-7. A convex left ventricle (LV) occupies the apex, and there is mild prominence of the pulmonary trunk (PT). The lateral view shows displacement of the barium-filled esophagus (arrows) by a moderately enlarged left atrium.
Echocardiogram
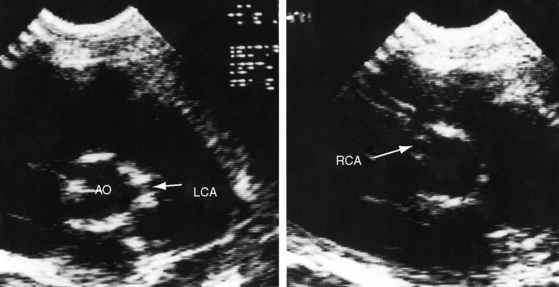
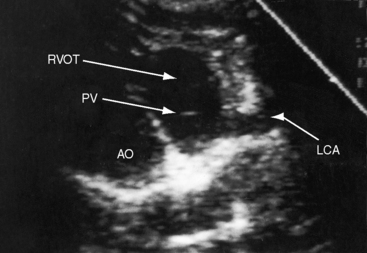
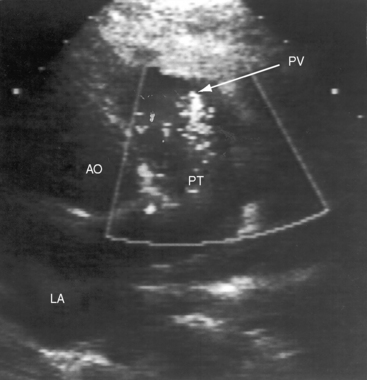
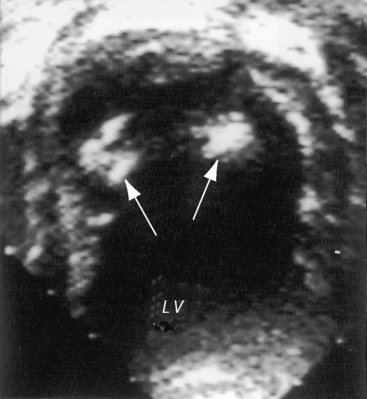
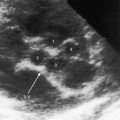
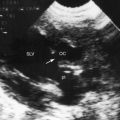
Echocardiography establishes the aortic origins of the left and right coronary arteries (Figure 21-16), identifies the origin of an anomalous left coronary artery from the pulmonary trunk (Figure 21-17 and video 21-1), and defines the flow patterns. Color flow imaging establishes the presence of diastolic or continuous flow entering the pulmonary trunk just distal to the pulmonary valve72 and adhering to the medial wall of the pulmonary trunk (Figure 21-18).73–75 Although a coronary arterial fistula into the pulmonary artery can exhibit similar flow patterns (see Chapter 22), two coronary arteries are identified originating in their normal respective aortic sinuses.75,76 Patent ductus arteriosus is characterized by color flow that enters near the left pulmonary artery, adheres to the lateral wall of the pulmonary trunk, and is directed toward the pulmonary valve (see Chapter 20). In a 72-year-old woman, the diagnosis was made with both echocardiographic myocardial blush and coronary arteriography.77 Echocardiography also determines left ventricular size, wall motion, and ejection fraction, and color flow imaging quantifies mitral regurgitation. Echogenic left ventricular papillary muscles indicate ischemic scarring (Figure 21-19).
Echocardiography coupled with scalar electrocardiography is a particularly useful diagnostic combination.7 More recent imaging techniques include multislice computed tomography (CT),78,79 magnetic resonance imaging,80 and CT coronary angiography.81
1 Brooks H.S.J. Two cases of an abnormal coronary artery aising from the pulmonary artery. J Anat Physiol. 1886;20:26.
2 Bland E.F., White P.D., Garland J. Congenital anomalies of the coronary arteries: report of an unusual case associated with cardiac hypertrophy. Am Heart J. 1933;8:787-801.
3 Gasul B.M., Loeffler F. Anomalous origin of the left coronary artery from the pulmonary artery (Bland-White-Garland syndrome) report of four cases. Pediatrics. 1949;4:498-507.
4 Fontana R.S., Edwards J.E. Congenital cardiac disease: a review of 357 Case studies pathologically. Philadelphia: WB Saunders; 1962.
5 Lee A.C., Foster E., Yeghiazarians Y. Anomalous origin of the left coronary artery from the pulmonary artery: a case series and brief review. Congenit Heart Dis. 2006;1:111-115.
6 Lin C-P., Chen Y-P., Chen T-H., et al. Anomalous origin of the left main coronary artery from the main pulmonary artery in a young adult. Circulation. 2001;104:1575-1576.
7 Angelini P., Velasco J.A., Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002;105:2449-2454.
8 Teixeira A.M., Pereira J., Anjos R. Anomalous origin of the left coronary artery from the pulmonary trunk. Cardiol Young. 2004;14:439-440.
9 Subban V., Jeyaram B., Sankardas M.A. Anomalous origin of the left anterior descending artery from the pulmonary artery. Heart. 2010;96:170.
10 Evans J.J., Phillips J.F. Origin of the left anterior descending coronary artery from the pulmonary artery. 3 year angiographic follow-up after saphenous vein bypass graft and proximal ligation. J Am Coll Cardiol. 1984;3:219-224.
11 Garcia C.M., Chandler J., Russell R. Anomalous left circumflex coronary artery from the right pulmonary artery: first adult case report. Am Heart J. 1992;123:526-528.
12 Tamer D.F., Mallon S.M., Garcia O.L., Wolff G.S. Anomalous origin of the left anterior descending coronary artery from the pulmonary artery. Am Heart J. 1984;108:341-345.
13 Bharati S., Szarnicki R.J., Popper R., Fryer A., Lev M. Origin of both coronary arteries from the pulmonary trunk associated with hypoplasia of the aortic tract complex: a new entity. J Am Coll Cardiol. 1984;3:437-441.
14 Colmers R.A., Siderides C.I. Anomalous origin of both coronary arteries from pulmonary trunk. Myocardial infarction in otherwise normal heart. Am J Cardiol. 1963;12:263-269.
15 Heifetz S.A., Robinowitz M., Mueller K.H., Virmani R. Total anomalous origin of the coronary arteries from the pulmonary artery. Pediatr Cardiol. 1986;7:11-18.
16 Ogden J.A. Origin of a single coronary artery from the pulmonary artery. Am Heart J. 1969;78:251-253.
17 Bharati S., Chandra N., Stephenson L.W., Wagner H.R., Weinberg P.M., Lev M. Origin of the left coronary artery from the right pulmonary artery. J Am Coll Cardiol. 1984;3:1565-1569.
18 Legendre A., Ou P., Bonnet D. Ostial stenosis of an anomalous left coronary artery from the pulmonary artery in a teenager. Pediatr Cardiol. 2009;30:1194-1195.
19 Goldberg S.P., Mitchell M.B., Campbell D.N., Tissot C., Lacour-Gayet F. Anomalous left coronary artery from the pulmonary artery with an intramural course within the aortic wall: report of 3 surgical cases. J Thorac Cardiovasc Surg. 2008;135:696-698.
20 Burch G.E., Depasquale N.P. The anomalous left coronary artery: an experiment of nature. Am J Med. 1964;37:159-161.
21 Case R.B., Morrow A.G., Stainsby W., Nestor J.O. Anomalous origin of the left coronary artery; the physiologic defect and suggested surgical treatment. Circulation. 1958;17:1062-1068.
22 Arsan S., Naseri E., Keser N. An adult case of Bland White Garland syndrome with huge right coronary aneurysm. Ann Thorac Surg. 1999;68:1832-1833.
23 Perloff J.K. Normal myocardial growth and the development and regression of increased ventricular mass. In Perloff J.K., Child J.S., Aboulhosn J., editors: Congenital heart disease in adults, 3rd ed, Philadelphia: Saunders/Elsevier, 2009.
24 Hutchins G.M., Bannayan G.A. Development of endocardial fibroelastosis following myocardial infarction. Arch Pathol. 1971;91:113-118.
25 Kutsche L.M., Van Mierop L.H. Anatomy and pathogenesis of aorticopulmonary septal defect. Am J Cardiol. 1987;59:443-447.
26 Cardo M., Fernandez B., Duran A.C., Fernandez M.C., Arque J.M., Sans-Coma V. Anomalous origin of the left coronary artery from the dorsal aortic sinus and its relationship with aortic valve morphology in Syrian hamsters. J Comp Pathol. 1995;112:373-380.
27 Cardo M., Fernandez B., Duran A.C., Arque J.M., Franco D., Sans-Coma V. Anomalous origin of the left coronary artery from the pulmonary trunk and its relationship with the morphology of the cardiac semilunar valves in Syrian hamsters. Basic Res Cardiol. 1994;89:94-99.
28 Browne L.P., Kearney D., Taylor M.D., et al. ALCAPA: the role of myocardial viability studies in determining prognosis. Pediatr Radiol. 2010;40:163-167.
29 Dent E.D.Jr, Fisher R.S. Single coronary artery: report of two cases. Ann Intern Med. 1956;44:1024-1030.
30 Murphy M.L. Single coronary artery. Am Heart J. 1967;74:557-561.
31 Smith J.C. Review of single coronary artery with report of 2 cases. Circulation. 1950;1:1168-1175.
32 Newton M.C.Jr, Burwell L.R. Single coronary artery with myocardial infarction and mitral regurgitation. Am Heart J. 1978;95:126-127.
33 Pachinger O., Vandenhoven P., Judkins M. Single coronary artery-a cause of angina pectoris. Eur J Cardiol. 1974;2:161-165.
34 Baue A.E., Baum S., Blakemore W.S., Zinsser H.F. A later stage of anomalous coronary circulation with origin of the left coronary artery from the pulmonary artery. Coronary artery steal. Circulation. 1967;36:878-885.
35 Edwards J.E. The direction of blood flow in coronary arteries arising from the pulmonary trunk. Circulation. 1964;29:163-166.
36 Armer R.M., Shumacker H.B., Lurie P.R., Fisch C. Origin of the left coronary artery from the pulmonary artery without collateral circulation. Report of a case with a suggested surgical correction Pediatrics. 1963;32:588-593.
37 Sabiston D.C.Jr, Neill C.A., Taussig H.B. The direction of blood flow in anomalous left coronary artery arising from the pulmonary artery. Circulation. 1960;22:591-597.
38 Rudolph A.M. The effects of postnatal circulatory adjustments in congenital heart disease. Pediatrics. 1965;36:763-772.
39 Menke J.A., Shaher R.M., Wolff G.S. Ejection fraction in anomalous origin of the left coronary artery from the pulmonary artery. Am Heart J. 1972;84:325-329.
40 Hofmeyr L., Moolman J., Brice E., Weich H. An unusual presentation of an anomalous left coronary artery arising from the pulmonary artery (ALCAPA) in an adult: anterior papillary muscle rupture causing severe mitral regurgitation. Echocardiography. 2009;26:474-477.
41 Rudolph A.M., Gootman N.L., Kaplan N., Rohman M. Anomalous left coronary artery arising from the pulmonary artery with large left-to-right shunt in infancy. J Pediatr. 1963;63:543-549.
42 Carvalho J.S., Redington A.N., Oldershaw P.J., Shinebourne E.A., Lincoln C.R., Gibson D.G. Analysis of left ventricular wall movement before and after reimplantation of anomalous left coronary artery in infancy. Br Heart J. 1991;65:218-222.
43 Rein A.J., Colan S.D., Parness I.A., Sanders S.P. Regional and global left ventricular function in infants with anomalous origin of the left coronary artery from the pulmonary trunk: preoperative and postoperative assessment. Circulation. 1987;75:115-123.
44 Jameson A.G., Ellis K., Levine O.R. Anomalous left coronary artery arising from pulmonary artery. Br Heart J. 1963;25:251-256.
45 Letcher J.R., Mccormick D., Tendler S., Ross J.Jr, Chandrasekaran K., Brockman S. Left main coronary artery arising from the pulmonary trunk in a 56-year-old patient presenting with acute myocardial infarction. Am J Cardiol. 1991;68:1257-1258.
46 Suzuki Y., Murakami T., Kawai C. Detection of anomalous origin of left coronary artery from pulmonary artery by real-time Doppler color flow mapping in a 53-year-old asymptomatic female. Int J Cardiol. 1992;34:339-342.
47 Talner N.S., Halloran K.H., Mahdavy M., Gardner T.H., Hipona F. Anomalous origin of the left coronary artery from the pulmonary artery: a clinical spectrum. Am J Cardiol. 1965;15:689-695.
48 Cronk E.S., Sinclair J.G., Rigdon R.H. An anomalous coronary artery arising from the pulmonary artery. Am Heart J. 1951;42:906-911.
49 Garty Y., Guri A., Shinwell E.S., Matitiau A. An unusual neonatal presentation of anomalous origin of the left coronary artery arising from the pulmonary artery. Neonatology. 2008;93:248-250.
50 Castaneda A.R., Indeglia R.A., Varco R.L. Anomalous origin of the left coronary artery from the pulmonary artery. Certain therapeutic considerations. Circulation. 1966;33:I52-56.
51 Allen D.R., Schieken R.M., Donofrio M.T. Hoarseness as the initial clinical presentation of anomalous left coronary artery from the pulmonary artery. Pediatr Cardiol. 2005;26:668-671.
52 Moustafa S.E., Zehr K., Mookadam M., Lorenz E.C., Mookadam F. Anomalous interarterial left coronary artery: an evidence based systematic overview. Int J Cardiol. 2008;126:13-20.
53 Ihenacho H.N., Singh S.P., Astley R., Parsons C.G. Anomalous left coronary artery. Report of an unusual case with spontaneous remission of symptoms. Br Heart J. 1973;35:562-565.
54 Abbott M. Congenital cardiac disease. In: Osler W., McCrae T., editors. Modern medicine, its theory and practice. Philadelphia: Lea & Febiger; 1908:323-425.
55 Facciorusso A., Lanna P., Vigna C., et al. Anomalous origin of the left coronary artery from the pulmonary artery in an elderly patient, football player in youth. J Cardiovasc Med (Hagerstown). 2008;9:1066-1069.
56 Irving C., Wren C. Asymptomatic anomalous origin of the left coronary artery from the pulmonary artery. Pediatr Cardiol. 2009;30:385-386.
57 Maeder M., Vogt P.R., Ammann P., Rickli H. Bland-White-Garland syndrome in a 39-year-old mother. Ann Thorac Surg. 2004;78:1451-1453.
58 Lampe C.F., Verheugt A.P. Anomalous left coronary artery. Adult type. Am Heart J. 1960;59:769-776.
59 Fierens C., Budts W., Denef B., Van De Werf F. A 72 year old woman with ALCAPA. Heart. 2000;83:E2.
60 Kerwin R.W., Westaby S., Davies G.J., Blackwood R.A. Anomalous left coronary artery from the pulmonary artery presenting with infective endocarditis in an adult. Eur Heart J. 1985;6:545-547.
61 Agustsson M.H., Gasul B.M., Lundquist R. Anomalous origin of the left coronary artery from the pulmonary artery (adult type). A case report. Pediatrics. 1962;29:274-282.
62 Alexi-Meskishvili V., Berger F., Weng Y., Lange P.E., Hetzer R. Anomalous origin of the left coronary artery from the pulmonary artery in adults. J Card Surg. 1995;10:309-315.
63 Frapier J.M., Leclercq F., Bodino M., Chaptal P.A. Malignant ventricular arrhythmias revealing anomalous origin of the left coronary artery from the pulmonary artery in two adults. Eur J Cardiothorac Surg. 1999;15:539-541.
64 Harthorne J.W., Scannell J.G., Dinsmore R.E. Anomalous origin of the left coronary artery. Remediable cause of sudden death in adults. N Engl J Med. 1966;275:660-663.
65 Cayler G.G., Smeloff E.A., Miller G.E.Jr. A new clinical sign of anomalous coronary artery. Dis Chest. 1969;55:163-166.
66 Perloff J.K., Roberts W.C. The mitral apparatus. Functional anatomy of mitral regurgitation. Circulation. 1972;46:227-239.
67 Davis C.Jr, Dillon R.F., Fell E.H., Gasul B.M. Anomalous coronary artery simulating patent ductus arteriosus. J Am Med Assoc. 1956;160:1047-1050.
68 Jurishica A.J. Anomalous left coronary artery; adult type. Am Heart J. 1957;54:429-436.
69 Puri P.S., Rowe R.D., Neill C.A. Varying vectorcardiographic patterns in anomalous left coronary artery arising from pulmonary artery. Am Heart J. 1966;71:616-626.
70 Perloff J.K., Roberts N.K., Cabeen W.R.Jr. Left axis deviation: a reassessment. Circulation. 1979;60:12-21.
71 Sabiston D.C.Jr, Pelargonio S., Taussig H.B. Myocardial infarction in infancy. The surgical management of a complication of congenital origin of the left coronary artery from the pulmonary artery. J Thorac Cardiovasc Surg. 1960;40:321-336.
72 Kudo Y., Suda K., Koteda Y. Pitfalls of echocardiographic evaluation of anomalous origin of the left coronary artery from the pulmonary trunk. Cardiol Young. 2008;18:537-538.
73 Houston A.B., Pollock J.C., Doig W.B., et al. Anomalous origin of the left coronary artery from the pulmonary trunk: elucidation with colour Doppler flow mapping. Br Heart J. 1990;63:50-54.
74 Schmidt K.G., Cooper M.J., Silverman N.H., Stanger P. Pulmonary artery origin of the left coronary artery: diagnosis by two-dimensional echocardiography, pulsed Doppler ultrasound and color flow mapping. J Am Coll Cardiol. 1988;11:396-402.
75 Swensson R.E., Murillo-Olivas A., Elias W., Bender R., Daily P.O., Sahn D.J. Noninvasive Doppler color flow mapping for detection of anomalous origin of the left coronary artery from the pulmonary artery and for evaluation of surgical repair. J Am Coll Cardiol. 1988;11:659-661.
76 Hsu S.Y., Lin F.C., Chang H.J., Yeh S.J., Wu D. Multiplane transesophageal echocardiography in diagnosis of anomalous origin of the left coronary artery from the pulmonary artery: a case report. J Am Soc Echocardiogr. 1998;11:668-672.
77 Kandzari D.E., Harrison J.K., Behar V.S. An anomalous left coronary artery originating from the pulmonary artery in a 72-year-old woman: diagnosis by color flow myocardial blush and coronary arteriography. J Invasive Cardiol. 2002;14:96-99.
78 Ichikawa M., Lim Y.-J., Komatsu S., et al. Detection of Bland-White-Garland Syndrome by multislice computed tomography in an elderly patient. Int J Cardiol. 2007;114:288-290.
79 Sato Y., Inoue F., Matsumoto N., et al. Detection of anomalous origins of the coronary artery by means of multislice computed tomography. Circ J. 2005;69:320-324.
80 Komocsi A., Simor T., Toth L., et al. Magnetic resonance studies in management of adult cases with Bland-White-Garland syndrome. Int J Cardiol. 2007;123:e8-e11.
81 Rha S.W., Yong H.S., Park C.G. Anomalous origin of the left coronary artery from the pulmonary artery in an elderly patient visualised by three dimensional multidetector CT coronary angiography. Heart. 2005;91:947.