Chapter 87 Ankylosing Spondylitis and Related Disorders
Seronegative spondyloarthritis is a group of inflammatory diseases that affect the axial spine, causing progressive pain, deformity, and dysfunction. The common features of seronegative spondyloarthropathies include negative tests for rheumatoid factors, absence of subcutaneous (rheumatoid) nodules, radiologic sacroiliitis with or without spondylitis (often asymmetrical), peripheral inflammatory arthritis, evidence of clinical overlap between group members, and tendency to familial aggregation.1 Ankylosing spondylitis (AS) is a disabling form of seronegative spondyloarthritis that affects the entheses, where ligaments, tendons, and capsules are attached to the bone. Three processes are observed at the entheses: inflammation, bone erosion, and syndesmophyte (spur) formation. Clinically, this disease is characterized by arthritis of the spine and pelvis, initially manifesting with pain and stiffness and progressing to joint fusion and deformity.2 Other members of this group of diseases are psoriatic arthritis, reactive arthritis, enteropathic spondylitis, and other undifferentiated forms that do not meet the criteria for a definitive category. Extraskeletal involvement is seen in the iris, myocardium and aorta, lungs, and kidneys.3–5 Fibrosis of the septum and the atrioventricular bundle may cause conduction defects. Focal necrosis at the root of the aorta often leads to dilation of the aortic ring, causing aortic incompetence. Detailed classification criteria for the entire group of the seronegative spondyloarthropathies have been described by Amor et al.6 and by the European Spondyloarthropathy Study Group.7
Prevalence of seronegative spondyloarthropathies, including AS, are directly correlated with prevalence of HLA-B27 in the population.8 Over 90% of patients with AS are HLA-B27 positive. The risk of an antigen-positive individual developing AS is under 2%.9 However, if a patient is antigen-positive and has a family history of AS, the likelihood of developing the clinical syndrome is 20%.10,11
The highest prevalence of the disease (4.5%) is found in Canadian Haida Indians, 50% of whose population is HLA-B27 positive. Among Europeans, the prevalence of B27 antigen in the general population ranges between 3% and 13%, and the prevalence of AS is estimated at 0.1% to 0.23%.1 In the Chinese population, approximately 5% carry the HLA-B27 gene.2 AS is rare in people of African descent and Australian Aboriginals. Some African populations, such as those in Gambia and Senegal, have a prevalence of HLA-B27 of 3% to 6%, but AS is still rare. Most occurrences of AS in Africans are in HLA-B27-negative individuals.12 Over 50 subtypes of HLA-B27 have been reported, and each subtype has a different strength of association with AS. Other major histocompatibility complex genes are associated as well with AS and other seronegative spondyloarthritides.2
Pathogenesis
The mechanism by which HLA-B27 leads to AS is unknown, but the association of HLA-B27 with AS is one of the strongest genetic associations with any common disease. Other genetic factors are certainly involved, since familial studies have shown that the overall genetic risk of HLA-B27 is less than 50%. Furthermore, first-degree relatives of AS patients are 5 to 16 times more likely to develop AS than are HLA-B27 individuals in the general population.2
Population and peptide-specificity analysis of HLA-B27 suggest that it has a pathogenic function related to antigen presentation.13 Several theories have been proposed to explain these associations, but only one, molecular mimicry, has provided a specific etiologic agent for these diseases. It has been suggested that AS may be triggered by the presentation of certain antigens by enterobacteria. Klebsiella pneumoniae shares a sequence of six consecutive amino acids with certain subtypes of the HLA-B27 antigen. Elevated immune responses to Klebsiella microbes have been demonstrated in AS patients from 10 different countries. This wide geographic distribution suggests that the same etiologic agent is probably related in producing this condition.14 Furthermore, HLA-B27 transgenic rats do not appear to develop AS if they are maintained in a germ-free environment.15 The HLA-B27 antigen may distinguish a group of people whose immune response to such an infectious agent predisposes them to develop spondyloarthropathy through the phenomenon of molecular mimicry.
Pathology
Inflammation occurs initially at the sacroiliac (SI) joint but may also affect entheses, vertebral bodies that are adjacent to intervertebral discs and the peripheral joint synovium.16 The lumbar spine is usually involved progressively upward. Skip lesions may occur, especially in women.17 Hips and shoulders are affected frequently, but peripheral joints are rarely involved.
Although the synovium is the primary site of joint disease in rheumatoid arthritis, the primary site in the spondyloarthritides is less well-defined.18 It is also thought that enthesitis (inflammation at sites where ligaments, tendons, or joint capsules are attached to bone) is the hallmark of AS and other spondyloarthropathies.19 Likely, the course of events is more complex than was earlier claimed and may be a combination of enthesitis, synovitis, and subchondral marrow changes, followed by fibrosis, cartilage metaplasia, and ossification; none can thus far be distinguished as a unique hallmark.20
In the early stages, chronic inflammatory cells localize to the subchondral bone at the sites of ligament attachment in the SI joints and discovertebral joints, resulting in periarticular osteopenia. Enthesitis at the insertion of the anulus fibrosus on the vertebral bodies in the erosive phase result in square appearance of the vertebrae on lateral radiographs. As the disease progresses, articular cartilage is destroyed by osteoclasts and replaced by granulation tissue, which in radiographs show extensive erosion and destruction of the joint space. In the late stage, granulation tissues are replaced with fibrous tissue that undergoes ossification and completely obliterates the joint, leading to “bamboo” or “poker” spine.21 Surprisingly, the anterior longitudinal ligament remains free from ossification.
The sequence of events in the synovial joints between the facets, the caudal part of the SI joints, and the peripheral joints resembles rheumatoid arthritis, although the exact target of inflammatory changes and the nature of cellular exudates may differ.20 Proliferation of synovial tissue and accumulation of plasma cells and lymphocytes at the joint margin lead to the formation of pannus, which infiltrates and destroys the articular cartilage and the subchondral bone. This destruction is followed by fibrosis and, later, by bony ankylosis in the reparative phase.
Clinical Manifestations
AS onset is often in the third decade, with 80% of cases diagnosed prior to age 30. Fewer then 5% are diagnosed after age 45. There is also a gender predominance, with a male-to-female ratio of 2:1.22
Juvenile-onset AS, with the onset of symptoms earlier than 16 years, has a higher incidence of hip disease. Patients with hip joint involvement show a faster progression of the spine disease. In the absence of hip disease, there is little difference between juvenile-onset and adult-onset AS in severity of spinal and extraskeletal manifestations.23
Inflammatory back pain distinguishes itself from mechanical back pain by insidious onset before age 40, pain persisting for at least 3 months, morning stiffness, and improvement with exercise. Buttock pain typically alternates from side to side. Pain and stiffness in the cervical spine generally start at a later stage.9 With the progress of the disease, the spine is gradually ankylosed with a generalized kyphotic deformity. To maintain upright posture, the patient hyperextends the hips and flexes the knees. Involvement of the hip joints seriously compromises the compensatory postures. Hyperextension of the cervical spine helps in maintaining the forward gaze. In advanced cases, when the cervical spine becomes ankylosed in flexion, forward gaze and jaw opening are affected. As the disease process immobilizes the spine, the majority of painful symptoms resolve. The inflammatory process tends to become inactive as bony ankylosis sets in, in the late fourth or fifth decade.24 Spondylodiscitis has a prevalence of 5% to 8% in patients with AS, and most lesions are asymptomatic.25 In half the cases, there may be multiple lesions. The most common location is in the lower thoracic and upper lumbar region, but the cervicothoracic junction may also be involved. Neither trauma nor infection has been found to be involved in most cases.25,26 However, owing to a distribution similar to that of thoracolumbar fracture, many researchers believe that these lesions represent pseudarthrosis resulting from the instability of a chronic nonunion, and histologic studies appear to confirm this.27,28
The incidence of peripheral joint involvement is 20% to 40%. Peripheral arthritis in AS and related conditions is oligoarticular and asymmetrical and often affects the hips or shoulders. Temporomandibular joint pain and stiffness occur in about 10% of patients. Less frequently involved joints include the knees, ankles, elbows, and wrists. Involvement of the hip joint is more important from the clinical viewpoint, since hip disease is far more disabling than is spinal rigidity.1 Painful peripheral enthesitis often involves heel insertion of the Achilles tendon and plantar fascia. Other sites of enthesitis include the superior and inferior poles of the patella, tibial tubercle, pubic attachment of the adductor longus, femoral trochanters, humeral epicondyles, and nuchal crests.
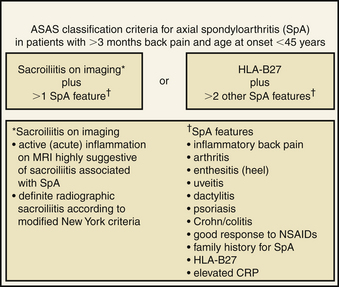
The classic diagnostic criteria for the classification of AS are the modified New York criteria. This scheme requires radiographic presence of sacroiliitis and one of the following: back pain and stiffness for more than 3 months that is relieved with exercise and not relieved by rest, limitation of motion of the lumbar spine in the sagittal and coronal planes, or limitation of chest expansion relative to normal values.29 The limitation of these criteria is that they may exclude patients without clear radiographic abnormality and may delay diagnosis and treatment.30 Recently, the Assessment of Spondyloarthritis International Society (ASAS) published classification criteria that have been validated and take into account the clinical, laboratory, and radiologic findings31 (Fig. 87-1). Other classification systems include the European Spondyloarthropathy Study Group and the Amor criteria.6,7
Laboratory and Radiologic Features
The HLA-B27 status is important in the early diagnosis of AS and associated conditions, but this test is not helpful in screening chronic low back pain. Erythrocyte sedimentation rate and serum C-reactive protein values may indicate systemic inflammation, but these tests have sensitivities of less than 50%.32
On radiographs, a common radiologic feature is squaring of the ventral corners of the thoracic and lumbar vertebrae due to osteopenia at the attachment of the ventral anulus. Vertebral osteopenia also accompanies the loss of normal concavity of the end plates.33 Symmetrical bilateral patchy areas of osteoporosis along the ill-defined SI joint are often suggestive of early disease. Later, subchondral erosions followed by patchy areas of ossifications develop that eventually lead to obliteration of the SI joint. Ossification extends within the substance of the anulus forming syndesmophytes, which bridges the adjacent vertebral bodies and develops into the bamboo spine in late stages34 (Fig. 87-2). Dorsal vertebral structures are also ossified. These include the capsule of the facet joints, supraspinous and interspinous ligaments, and ligamentum flavum. In the subaxial cervical spine, extensive ankylosis with varying degrees of kyphosis is seen in the advanced stages. In contrast, the upper cervical spine may demonstrate hypermobility resulting from atlantoaxial instability.35
Early diagnosis of AS has been helped, in large part, by the development of MRI, which can detect early signs of inflammation and structural damage to the SI joints and the spine. In particular, increased signal on short-tau inversion recovery and T1 imaging with gadolinium correlate with inflammatory infiltrate.36 Identification of inflammation at the SI joints is paramount in the early diagnosis of this disease. Various radiographic grading schemes exist for severity of SI and/or spine involvement.37
Nonsurgical Management
Treatment of AS should be tailored to the manifestations of the disease. The best combination of treatments includes both pharmacologic and nonpharmacologic treatments. Treatment recommendations have been proposed by a task force made up of the assessment of the ASAS working group and the European League against Rheumatism (EULAR) group.38
With early diagnosis, nonsurgical treatment, including pharmacologic and physical therapy, can benefit all patients with AS or related conditions. Physical therapy can provide short-term improvement in functionality.39 Cognitive and behavioral modification may also improve symptoms.40
Nonsteroidal anti-inflammatory drugs (NSAIDs) are the first-line agents in the treatment of AS.41 These medications are symptom-modifying as well as disease modifying.42 Sulfasalazine has shown clinical benefit in the treatment of peripheral arthritis and spinal stiffness, but there were no statistical improvements in spinal pain, function, or global assessment.43
More recently, anti-TNF (tumor necrosis factor) agents have been developed for use in AS patients who failed standard treatments. These include golimumab, infliximab, etanercept, and rituximab. The proinflammatory mediator zTNF is produced by macrophages and activated lymphocytes, and TNF results in increased cytokine expression that results in bone resorption and proteoglycan breakdown. Furthermore, TNF has been shown to be overexpressed in the SI joints in AS patients.44
Surgical Management of Ankylosing Spondylitis
Patients with AS often have increased rigidity throughout the spine in addition to poor bone density and osteoporosis, which result in an increased incidence in spine deformity and instability. Therefore, spine surgery in AS patients is often focused on either deformity correction or stabilization of an unstable fracture.
Spine Fractures
As the spine undergoes autofusion through ligamentous ossification and syndesmophytosis, a rigid kyphotic deformity develops that is unable to dissipate energies from a traumatic event.45–49 Hyperextension is the most often observed mechanism of spine fracture in AS.50 Spine fractures in AS are pathologic fractures and differ from fractures in the normal spine in many aspects. These fractures (1) often result after trivial trauma, (2) are usually highly unstable and displace more frequently, (3) almost always involve all three columns, (4) are more often associated with neurologic complications, and (5) are associated with epidural bleeding.51 Because of the syndesmophyte formation, fracture often extends through the disc space.49,52 Associated conditions, such as peripheral arthritis, may further impair the patient’s mobility and increase the incidence of falls.
Neurologic deficit is frequent with these fractures and may be as high as 75%.46,47 The incidence of spinal cord injury in patients with AS is 11.4 times higher than that in the general population.50 In a review of 31 consecutive patients with fractures in AS, Olerud et al.48 reported immediate neurologic impairment in one third of patients, and a further one third subsequently developed neurologic impairment. The secondary neurologic deterioration could be a result of fracture displacement or development of an epidural hematoma. Displacement in hyperextension is considered to be most dangerous for neurologic impairment.51,53 Because of the kyphotic deformity, the spine tends to displace in hyperextension when the patient lies in a supine position.48
The cumulative incidence of spine fractures in patients with AS is approximately 17%, and the incidence of these injuries peaks around the sixth decade of life.45,54 The midcervical spine is the most frequent site of fracture, followed by the thoracolumbar junction.48,55 Radiologic demonstration of the fractures may be difficult because of poor bone density and may be missed on plain radiographs.49 Patients with a history of back or neck pain after trivial trauma or sudden progression of deformity should undergo a thorough radiologic evaluation using CT, and the clinician should have a very high index of suspicion for occult fracture. Multiple fractures are also a strong consideration in patients with AS.
In comparison to the general population, patients with AS and spinal cord injury have poor outcomes. Morbidity is as high as 85%,52 and mortality rates range from 35% to 50%13,56,57; pulmonary complications play a major role relating to decreased chest wall expansion.49 Vascular injuries such as aortic rupture have been reported in fractures resulting from higher-energy trauma.58–60
Because of the frequent incidence of neurologic deterioration, failure of union, or inadequate immobilization at the fracture site with conservative treatment, most surgeons support surgical stabilization as the treatment of choice. A recent study of 122 spine fractures in patients with AS demonstrated that the most common level of fracture was C6-7. Most of these patients (67%) were treated surgically, and the remainder were treated with bracing, secondary to a poor medical condition.52 If bracing is used, serial imaging should be utilized to recognize persistent movement or progressive subluxation, which may require surgical stabilization. For the most part, nonoperative immobilization alone is difficult and frequently inadequate because of marked instability. Neurologic deterioration may result from fracture movement with simple maneuvers such as transfer to a stretcher61 or during halo-vest application.54
For cervical fractures, the surgical treatment may consist of dorsal, ventral, or combined approaches to stabilize the fracture. Generally, a long construct is recommended spanning multiple levels above and below the fracture.52 Taggard and Traynelis62 described a technique of three-point internal fixation combining lateral mass plate and interspinous wiring, spanning at least two levels above and below the fracture. Lateral mass screw placement is difficult in this patient population because the usual landmarks of the facet joints are lost as a result of bony ankylosis. Extrapolation from a recognizable landmark may be necessary to find the point of screw entry. As an additional measure of safety, these authors suggested use of screws that are 14 mm or shorter to avoid nerve root damage.
Although the subaxial cervical spine is the most common site of fracture in AS, the odontoid process is vulnerable. Bony ankylosis of the atlanto-occipital and/or atlantoaxial joint or the lower cervical spine may make the odontoid susceptible to fracture after minor trauma.63,64 Spontaneous fracture of the odontoid without history of trauma has also been reported.65 These injuries may be treated by halo-jacket immobilization alone66 or in combination with dorsal occipitocervical fusion.
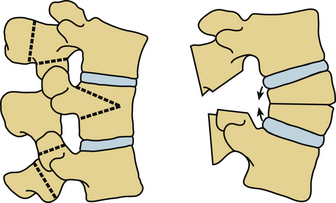
Fractures in the thoracic and lumbar spine involve all three columns and are inherently unstable with a high possibility of secondary neurologic deterioration. Fractures often occur across an ankylosed disc space (Fig. 87-3). Displacements are common, particularly toward hyperextension when the patient is positioned supine.48,60 Surgical stabilization is therefore mandatory. Stabilization is often attained through long dorsal constructs, three or more segments included above and below the fracture. Anterior column reconstruction is often not needed.52
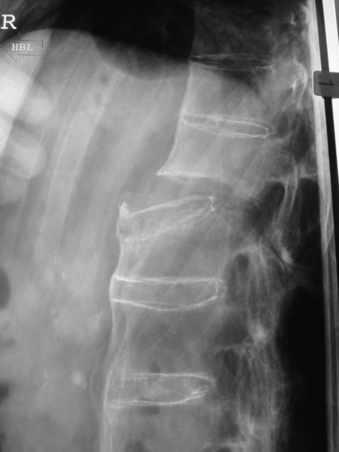
FIGURE 87-3 Plain radiograph of a transdiscal fracture of L1 in a patient with ankylosing spondylitis.
Patients with AS with spine fracture present significant perioperative challenges. First, intubation may be difficult, owing to decreased cervical mobility, instability, or ventral osteophytes.67 Second, proper positioning can be extremely difficult and requires significant planning. During head positioning, one must take into account the degree of cervical kyphosis and plan accordingly. Cases of spinal cord injury and/or death have been reported with improper head position in patients with AS.47 With unstable cervical fracture, preoperative halo placement can provide stability for positioning. Neurophysiologic monitoring can provide important information during positioning as well. Increased blood loss should be expected during spine surgery in patients with AS.68
Spine Deformity
A progressive generalized kyphotic deformity, focused at both the cervicothoracic and thoracolumbar junctions, is typical in AS.69 This kyphotic deformity leads to a shift in the center of gravity both forward and downward, resulting in loss of sagittal balance and difficulty in forward gaze. In the early phase of the disease, patients try to compensate their sagittal balance by hyperextension of the hips and flexion of the knees. Hyperextension of the cervical spine helps to maintain forward gaze. When hips are involved with the disease, fixed flexion deformity develops and further interferes with the sagittal balance. In advanced stages of the disease, the cervical spine is ankylosed, resulting in a global kyphotic deformity, interfering severely with compensation of the gaze angle. Severe kyphotic deformities may produce compression of the abdominal viscera and limitation of the pulmonary function by restriction of the diaphragmatic excursion.
Indications for Deformity Correction
Surgical intervention is indicated when kyphosis is decompensated. This means that the patient cannot maintain a horizontal gaze when the hips and knees are extended and the eyeballs are in the neutral position.70 In practice, however, the general condition of the patient, the feasibility of correction, and, perhaps above all else, the morale and earnest desire of the patient to accept the risks and rehabilitative measures required for correction become more important decisive factors before surgery is considered. As was mentioned earlier in the chapter, many perioperative challenges exist in these patients.
Preoperative Planning
The level of osteotomy to correct a global kyphotic deformity has a disparate effect on gaze angle and sagittal balance. Sagittal balance is restored by redirecting the spine dorsally at the level of osteotomy so that the head lies vertically above the pelvis. The angle by which the spine is redirected at the osteotomy site (the osteotomy angle) for full restoration of the sagittal balance depends on the level of osteotomy. When the osteotomy is performed at a higher level, the osteotomy angle must be greater than that required for osteotomy at a lower level in the spine to achieve the same degree of correction in the sagittal balance.71,72 In contrast, the visual angle (by which the gaze is redirected forward) will always be the same as the osteotomy angle, irrespective of the level of the osteotomy.
As was mentioned earlier, in the presence of global kyphosis in AS, patients try to extend their hips and flex their knees as much as possible to correct the sagittal balance and to achieve a forward gaze. An estimate of gaze angle and sagittal balance correction from the lateral radiograph taken in this posture will underestimate both these parameters. The simple way to resolve this problem is to take the lateral radiograph while the patient stands with relaxed hips, without making an effort to correct the spine balance or forward gaze. The degree of gaze angle correction needed is determined by the chin-brow to vertical angle (CBVA).73 The CBVA is an angle between the line drawn from the chin to the brow and a vertical line and is used as a clinical measurement of sagittal balance and its effect on horizontal gaze.69 The sagittal balance correction is estimated from the degree of dorsal shift of the plumb line required to bring it back to the sacrum. Presence of hip flexion deformity severely affects both the sagittal balance and the gaze angle, and this must be corrected by total hip replacement before spine osteotomy is undertaken.
Thoracolumbar Kyphotic Correction
Three methods are used to correct kyphosis in the thoracolumbar spine in the setting of AS: opening wedge osteotomy, also known as Smith-Peterson osteotomy (SPO); polysegmental wedge osteotomy; and pedicle subtraction osteotomy (PSO). Because these procedures involve resection of stabilizing elements of the spine, they are always supplemented with dorsal instrumentation.
SPO was first introduced by Smith-Petersen et al.74 and involves two- or three-level osteotomies through the facet joints at the L1-3 levels and closing the gap by osteoclasis of the vertebral body. The wedge is then closed by hyperextension of the spine with manual pressure. This manipulation can cause disruption of the anterior longitudinal ligament and creates a large gap in the ventral aspect of the vertebral column. The main disadvantage of this technique was serious vascular and neurologic complications resulting from sudden elongation of the anterior column during closed osteoclasis maneuver and a large ventral opening of a monosegmental procedure.5,69,75,76 Mortality rates up to 12% and complication rates up to 50% are not unusual.70 In general, every 1 mm of facet that is resected results in 1 degree of correction, so each SPO can result in 10 degrees of correction.77
The polysegmental wedge osteotomy was developed to reduce disruption at the anterior longitudinal ligament. In this technique, multiple SPOs are performed in the thoracolumbar spine, creating a more gradual correction and small bony defects ventrally. Wilson and Turkell78 first reported polysegmental osteotomy in one patient in 1949. Mortality rates as low as 4% and complication rates of about 27% have been reported.70 The total degree of correction that is achieved may be the same as that with open wedge osteotomy, which corresponds to about 10 degrees per level of osteotomy.
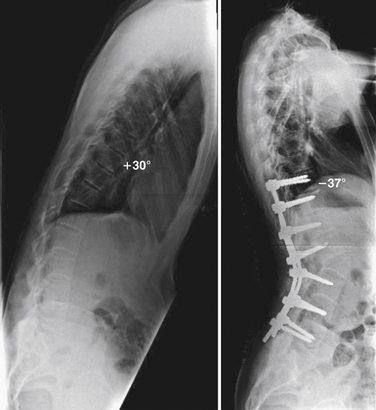
PSO, or lumbar closing wedge osteotomy, is a technique in which a transpedicular vertebral wedge resection is performed extending from the dorsal elements through the pedicles into the vertebral body77 (Fig. 87-4). The wedge is closed by hyperextension of the lumbar spine, hinging on the ventral cortex of the vertebral body. Since there is no elongation of the anterior column, vascular complications are rare. Care must be taken to avoid disrupting the ventral cortical surface, as this may destabilize the segment. The consolidation at the osteotomy site is rapid. This technique was initially described by Scudese and Calabro79 but was later popularized by Thomasen80 in 1985. The PSO is typically performed at L2 or L3, as one of these is the typical apex of normal lumbar lordosis (Fig. 87-5). Approximately 30 to 40 degrees of correction may be achieved from a single-level PSO.81 Kim et al.82 have reported outcomes on 45 patients with AS and a kyphotic deformity. The average correction was 34 degrees, with improvement of sagittal balance from 94 mm to 8 mm. Complications included ileus in five patients, neurologic deficit in five patients, and visual disturbance in two patients.
Ideally, kyphotic deformity is best corrected when the osteotomy is placed at the site of maximum deformity. It is possible to perform a PSO in both the thoracic and lumbar regions, but thoracic PSOs are technically more difficult and carry a higher risk of neurologic sequelae.69 In practice, a combination of polysegmental wedge osteotomies and a PSO can provide sufficient sagittal deformity correction.
Cervical Osteotomy
Urist83 first described a case of cervical osteotomy under local anesthesia with the patient in the sitting position in 1953. Simmons et al.84 recently published a large retrospective series of patients with AS who underwent cervical extension osteotomy. Over a 36-year period, 131 cases were performed in a sitting position under local anesthesia. CBVA angles were improved on average 37 degrees. The 3-month mortality rate was 3%. Complications included transient C8 radiculopathy (12%), pseudarthrosis (5%), spinal cord injury (2%), and stroke (<1%).
In brief, the spinous processes of C6 and C7 are removed, and a complete laminectomy is performed at C7. The caudal portion of the C6 lamina and the rostral portion of T1 are also removed. The fused C7-T1 facet is removed bilaterally, and the C8 nerve roots are exposed. A portion of the C7 and T1 pedicles is also removed. The head is then extended, breaking the ankylosed anterior column.69,85 Dorsal instrumentation is placed for stabilization. Preoperative halo application can help to control head position on the table after osteotomy.
Berens D.L. Roentgen features of ankylosing spondylitis. Clin Orthop Relat Res. 1971;74:20-33.
Kabasakal Y., Garrett S.L., Calin A. The epidemiology of spondylodiscitis in ankylosing spondylitis: a controlled study. Br J Rheumatol. 1996;35(7):660-663.
Olerud C., Frost A., Bring J. Spinal fractures in patients with ankylosing spondylitis. Eur Spine J. 1996;5(1):51-55.
Smith-Petersen M.N., Larson C.B., Aufranc O.E. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. Clin Orthop Relat Res. 1969;66:6-9.
van der Linden S., Valkenburg H., Cats A. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals: a family and population study. Br J Rheumatol. 1983;22(4 Suppl 2):18-19.
Wanders A., Heijde D., Landewe R., et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005;52(6):1756-1765.
Weinstein P.R., Karpman R.R., Gall E.P., Pitt M. Spinal cord injury, spinal fracture, and spinal stenosis in ankylosing spondylitis. J Neurosurg. 1982;57(5):609-616.
1. Olivieri I., Barozzi L., Padula A., et al. Clinical manifestations of seronegative spondylarthropathies. Eur J Radiol. 1998;27(Suppl 1):S3-S6.
2. Thomas G.P., Brown M.A. Genetics and genomics of ankylosing spondylitis. Immunol Rev. 2010;233(1):162-180.
3. Gran J.T., Husby G. Clinical, epidemiologic, and therapeutic aspects of ankylosing spondylitis. Curr Opin Rheumatol. 1998;10(4):292-298.
4. Rumancik W.M., Firooznia H., Davis M.S.Jr., et al. Fibrobullous disease of the upper lobes: an extraskeletal manifestation of ankylosing spondylitis. J Comput Tomogr. 1984;8(3):225-229.
5. Camargo F.P., Cordeiro E.N., Napoli M.M. Corrective osteotomy of the spine in ankylosing spondylitis: Experience with 66 cases. Clin Orthop Relat Res. 1986;208:157-167.
6. Amor B., Dougados M., Mijiyawa M. Criteria of the classification of spondylarthropathies. Rev Rhum Mal Osteoartic. 1990;57(2):85-89.
7. Dougados M., van der Linden S., Juhlin R., et al. The European Spondylarthropathy Study Group preliminary criteria for the classification of spondylarthropathy. Arthritis Rheum. 1991;34(10):1218-1227.
8. Khan M.A., van der Linden S.M. Ankylosing spondylitis and other spondyloarthropathies. Rheum Dis Clin North Am. 1990;16(3):551-579.
9. Calin A., Porta J., Fries J.F., Schurman D.J. Clinical history as a screening test for ankylosing spondylitis. JAMA. 1977;237(24):2613-2614.
10. Lawrence J.S. The prevalence of arthritis. Br J Clin Pract. 1963;17:699-705.
11. van der Linden S., Valkenburg H., Cats A. The risk of developing ankylosing spondylitis in HLA-B27 positive individuals: a family and population study. Br J Rheumatol. 1983;22(4 Suppl 2):18-19.
12. Belachew D.A., Sandu N., Schaller B., Guta Z. Ankylosing spondylitis in sub-Saharan Africa. Postgrad Med J. 2009;85(1005):353-357.
13. Tico N., Ramon S., Garcia-Ortun F., et al. Traumatic spinal cord injury complicating ankylosing spondylitis. Spinal Cord. 1998;36(5):349-352.
14. Ebringer A., Ahmadi K., Fielder M., et al. Molecular mimicry: the geographical distribution of immune responses to Klebsiella in ankylosing spondylitis and its relevance to therapy. Clin Rheumatol. 1996;15(Suppl 1):57-61.
15. Taurog J.D., Richardson J.A., Croft J.T., et al. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180(6):2359-2364.
16. Shamji M.F., Bafaquh M., Tsai E. The pathogenesis of ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E3.
17. McGonagle D., Khan M.A., Marzo-Ortega H., et al. Enthesitis in spondyloarthropathy. Curr Opin Rheumatol. 1999;11(4):244-250.
18. Benjamin M., McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001;199(Pt 5):503-526.
19. Ball J. Enthesopathy of rheumatoid and ankylosing spondylitis. Ann Rheum Dis. 1971;30(3):213-223.
20. Francois R.J., Braun J., Khan M.A. Entheses and enthesitis: a histopathologic review and relevance to spondyloarthritides. Curr Opin Rheumatol. 2001;13(4):255-264.
21. Cruickshank B. Pathology of ankylosing spondylitis. Clin Orthop Relat Res. 1971;74:43-58.
22. Feldtkeller E., Khan M.A., van der Heijde D., et al. Age at disease onset and diagnosis delay in HLA-B27 negative vs. positive patients with ankylosing spondylitis. Rheumatol Int. 2003;23(2):61-66.
23. Brophy S., Calin A. Ankylosing spondylitis: interaction between genes, joints, age at onset, and disease expression. J Rheumatol. 2001;28(10):2283-2288.
24. Carette S., Graham D., Little H., et al. The natural disease course of ankylosing spondylitis. Arthritis Rheum. 1983;26(2):186-190.
25. Kabasakal Y., Garrett S.L., Calin A. The epidemiology of spondylodiscitis in ankylosing spondylitis: a controlled study. Br J Rheumatol. 1996;35(7):660-663.
26. Rasker J.J., Prevo R.L., Lanting P.J. Spondylodiscitis in ankylosing spondylitis, inflammation or trauma? A description of six cases. Scand J Rheumatol. 1996;25(1):52-57.
27. Fang D., Leong J.C., Ho E.K., et al. Spinal pseudarthrosis in ankylosing spondylitis: clinicopathological correlation and the results of anterior spinal fusion. J Bone Joint Surg [Br]. 1988;70(3):443-447.
28. Chan F.L., Ho E.K., Fang D., et al. Spinal pseudarthrosis in ankylosing spondylitis. Acta Radiol. 1987;28(4):383-388.
29. van der Linden S., Valkenburg H.A., Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984;27(4):361-368.
30. Mease P.J. Spondyloarthritis update: new insights regarding classifcation, pathophysiology, and management. Bull NYU Hosp Jt Dis. 2008;66(3):203-209.
31. Sieper J., Rudwaleit M., van der Heijde D., et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part II): validation and final selection. Ann Rheum Dis. 2009;68(6):777-783.
32. Rudwaleit M., van der Heijde D., Khan M.A., et al. How to diagnose axial spondyloarthritis early. Ann Rheum Dis. 2004;63(5):535-543.
33. Hanson C.A., Shagrin J.W., Duncan H. Vertebral osteoporosis in ankylosing spondylitis. Clin Orthop Relat Res. 1971;74:59-64.
34. Barozzi L., Olivieri I., De Matteis M. Seronegative spondylarthropathies: imaging of spondylitis, enthesitis and dactylitis. Eur J Radiol. 1998;27(Suppl 1):S12-S17.
35. Berens D.L. Roentgen features of ankylosing spondylitis. Clin Orthop Relat Res. 1971;74:20-33.
36. Bollow M., Fischer T., Reisshauer H., et al. Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis-cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis. 2000;59(2):135-140.
37. Rostom S., Dougados M., Gossec L. New tools for diagnosing spondyloarthropathy. Joint Bone Spine. 2010;77(2):108-114.
38. Zochling J., van der Heijde D., Burgos-Vargas R., et al. ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis. 2006;65(4):442-452.
39. Dagfinrud H., Kvien T.K., Hagen K.B. Physiotherapy interventions for ankylosing spondylitis. Cochrane Database Syst Rev. 2004;4:CD002822.
40. Basler H.D., Rehfisch H.P. Cognitive-behavioral therapy in patients with ankylosing spondylitis in a German self-help organization. J Psychosom Res. 1991;35(2-3):345-354.
41. Zochling J., van der Heijde D., Dougados M., Braun J. Current evidence for the management of ankylosing spondylitis: a systematic literature review for the ASAS/EULAR management recommendations in ankylosing spondylitis. Ann Rheum Dis. 2006;65(4):423-432.
42. Wanders A., Heijde D., Landewe R., et al. Nonsteroidal antiinflammatory drugs reduce radiographic progression in patients with ankylosing spondylitis: a randomized clinical trial. Arthritis Rheum. 2005;52(6):1756-1765.
43. Chen J., Liu C. Sulfasalazine for ankylosing spondylitis. Cochrane Database Syst Rev. 2005;2:CD004800.
44. Braun J., Sieper J. Ankylosing spondylitis. Lancet. 2007;369(9570):1379-1390.
45. Cooper C., Carbone L., Michet C.J., et al. Fracture risk in patients with ankylosing spondylitis: a population based study. J Rheumatol. 1994;21(10):1877-1882.
46. Graham B., Van Peteghem P.K. Fractures of the spine in ankylosing spondylitis: diagnosis, treatment, and complications. Spine (Phila Pa 1976). 1989;14(8):803-807.
47. Hunter T., Dubo H. Spinal fractures complicating ankylosing spondylitis. Ann Intern Med. 1978;88(4):546-549.
48. Olerud C., Frost A., Bring J. Spinal fractures in patients with ankylosing spondylitis. Eur Spine J. 1996;5(1):51-55.
49. Jacobs W.B., Fehlings M.G. Ankylosing spondylitis and spinal cord injury: origin, incidence, management, and avoidance. Neurosurg Focus. 2008;24(1):E12.
50. Alaranta H., Luoto S., Konttinen Y.T. Traumatic spinal cord injury as a complication to ankylosing spondylitis: an extended report. Clin Exp Rheumatol. 2002;20(1):66-68.
51. Bohlman H.H. Acute fractures and dislocations of the cervical spine: an analysis of three hundred hospitalized patients and review of the literature. J Bone Joint Surg [Am]. 1979;61(8):1119-1142.
52. Caron T., Bransford R., Nguyen Q. Spine fractures in patients with ankylosing spinal disorders. Spine (Phila Pa 1976). 2010;35(11):E458-E464.
53. Surin V.V. Fractures of the cervical spine in patients with ankylosing spondylitis. Acta Orthop Scand. 1980;51(1):79-84.
54. Fox M.W., Onofrio B.M., Kilgore J.E. Neurological complications of ankylosing spondylitis. J Neurosurg. 1993;78(6):871-878.
55. Wade W., Saltzstein R., Maiman D. Spinal fractures complicating ankylosing spondylitis. Arch Phys Med Rehabil. 1989;70(5):398-401.
56. Foo D., Sarkarati M., Marcelino V. Cervical spinal cord injury complicating ankylosing spondylitis. Paraplegia. 1985;23(6):358-363.
57. Murray G.C., Persellin R.H. Cervical fracture complicating ankylosing spondylitis: a report of eight cases and review of the literature. Am J Med. 1981;70(5):1033-1041.
58. Savolaine E.R., Ebraheim N.A., Stitgen S., Jackson W.T. Aortic rupture complicating a fracture of an ankylosed thoracic spine: a case report. Clin Orthop Relat Res. 1991;272:136-140.
59. Schaberg F.J.Jr. Aortic injury occurring after minor trauma in ankylosing spondylitis. J Vasc Surg. 1986;4(4):410-411.
60. Trent G., Armstrong G.W., O’Neil J. Thoracolumbar fractures in ankylosing spondylitis. High-risk injuries. Clin Orthop Relat Res. 1988;227:61-66.
61. Weinstein P.R., Karpman R.R., Gall E.P., Pitt M. Spinal cord injury, spinal fracture, and spinal stenosis in ankylosing spondylitis. J Neurosurg. 1982;57(5):609-616.
62. Taggard D.A., Traynelis V.C. Management of cervical spinal fractures in ankylosing spondylitis with posterior fixation. Spine (Phila Pa 1976). 2000;25(16):2035-2039.
63. Miller F.H., Rogers L.F. Fractures of the dens complicating ankylosing spondylitis with tlantooccipital fusion. J Rheumatol. 1991;18(5):771-774.
64. Peh W.C., Ho E.K. Fracture of the odontoid peg in ankylosing spondylitis: case report. J Trauma. 1995;38(3):361-363.
65. Kremer P., Despaux J., Benmansour A., Wendling D. Spontaneous fracture of the odontoid process in a patient with ankylosing spondylitis: nonunion responsible for compression of the upper cervical cord. Rev Rhum Engl Ed. 1995;62(6):455-458.
66. Kaplan S.L., Tun C.G., Sarkarati M. Odontoid fracture complicating ankylosing spondylitis: a case report and review of the literature. Spine (Phila Pa 1976). 1990;15(6):607-610.
67. Sciubba D.M., Nelson C., Hsieh P., et al. Perioperative challenges in the surgical management of ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E10.
68. Tetzlaff J.E., Yoon H.J., Bell G. Massive bleeding during spine surgery in a patient with ankylosing spondylitis. Can J Anaesth. 1998;45(9):903-906.
69. Sansur C.A., Fu K.M., Oskouian R.J.Jr., et al. Surgical management of global sagittal deformity in ankylosing spondylitis. Neurosurg Focus. 2008;24(1):E8.
70. Giehl J.P., Hehne H-J., Zielke K. Kyphosis correction in ankylosing spondylitis using transpedicular instrumentation. In: Bridwell K.H., DeWald R.L., editors. The textbook of spinal surgery. Philadelphia: Lippincott-Raven; 1997:1159-1167.
71. Lazennec J.Y., Saillant G., Saidi K., et al. Surgery of the deformities in ankylosing spondylitis: our experience of lumbar osteotomies in 31 patients. Eur Spine J. 1997;6(4):222-232.
72. Sengupta D.K., Khazim R., Grevitt M.P., Webb J.K. Flexion osteotomy of the cervical spine: a new technique for correction of iatrogenic extension deformity in ankylosing spondylitis. Spine (Phila Pa 1976). 2001;26(9):1068-1072.
73. Simmons E. Ankylosing spondylitis: surgical considerations. In: Rothman R.H., Simeone F.A., editors. The spine. Philadelphia: WB Saunders; 1992:1447-1511.
74. Smith-Petersen M.N., Larson C.B., Aufranc O.E. Osteotomy of the spine for correction of flexion deformity in rheumatoid arthritis. Clin Orthop Relat Res. 1969;66:6-9.
75. Law W.A. Osteotomy of the spine. Clin Orthop Relat Res. 1969;66:70-76.
76. Weatherley C., Jaffray D., Terry A. Vascular complications associated with osteotomy in ankylosing spondylitis: a report of two cases. Spine (Phila Pa 1976). 1988;13(1):43-46.
77. Gill J.B., Levin A., Burd T., Longley M. Corrective osteotomies in spine surgery. J Bone Joint Surg [Am]. 2008;90(11):2509-2520.
78. Wilson M.J., Turkell J.H. Multiple spinal wedge osteotomy: its use in a case of Marie-Strumpell spondylitis. Am J Surg. 1949;77(6):777-782.
79. Scudese V.A., Calabro J.J. Vertebral wedge osteotomy: correction of rheumatoid (ankylosing) spondylitis. JAMA. 1963;186:627-631.
80. Thomasen E. Vertebral osteotomy for correction of kyphosis in ankylosing spondylitis. Clin Orthop Relat Res. 1985;194:142-152.
81. Yang B.P., Ondra S.L., Chen L.A., et al. Clinical and radiographic outcomes of thoracic and lumbar pedicle subtraction osteotomy for fixed sagittal imbalance. J Neurosurg Spine. 2006;5(1):9-17.
82. Kim K.T., Suk K.S., Cho Y.J., et al. Clinical outcome results of pedicle subtraction osteotomy in ankylosing spondylitis with kyphotic deformity. Spine (Phila Pa 1976). 2002;27(6):612-618.
83. Urist M.R. Osteotomy of the cervical spine: report of a case of ankylosing rheumatoid pondylitis. J Bone Joint Surg [Am]. 1958;40(4):833-843.
84. Simmons E.D., DiStefano R.J., Zheng Y., Simmons E.H. Thirty-six years experience of cervical extension osteotomy in ankylosing spondylitis: techniques and outcomes. Spine (Phila Pa 1976). 2006;31(26):3006-3012.
85. McMaster M.J. Osteotomy of the cervical spine in ankylosing spondylitis. J Bone Joint Surg [Br]. 1997;79(2):197-203.