CHAPTER 326 Animal Models of Traumatic Brain Injury
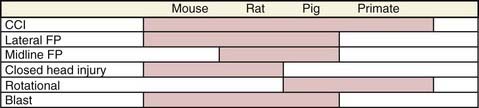
Traumatic brain injury (TBI) is a complex condition that is well recognized to consist of a spectrum of insults involving a number of mechanisms of injury, degrees of severity, and types of pathology. This is readily apparent when comparing cranial computed tomographic scans of patients with severe TBI, in whom contusion, diffuse axonal injury (DAI), and subdural, subarachnoid, or parenchymal hemorrhages, as well as other pathologic findings, can be seen in various combinations.1 A number of additional factors can affect the clinical outcome of patients with TBI, such as secondary insults, gender, age, genetic polymorphisms, drug use, and others.2 It is thus logical that a menu of experimental models is needed both to study these pathologies and to successfully translate new therapies to clinical care. Several well-characterized experimental animal models of TBI have been developed and are the focus of this chapter (Fig. 326-1). We discuss these models, the relevant outcomes in experimental TBI, the covariates that have been incorporated into models to mimic the clinical condition, and confounding factors that affect the animal models, such as anesthetics and strain differences.
Experimental Models
Controlled Cortical Impact
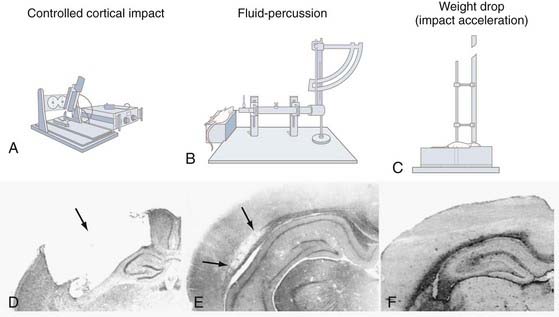
Experimental TBI induced with a pneumatic impactor was first introduced for use in laboratory ferrets by Lighthall and colleagues3,4 and subsequently adapted for rats by Dixon and associates5 in an attempt to better control the biomechanical parameters of brain injury. Unlike the fluid percussion (FP) injury device, which disperses a stream of solution intracranially that cannot be readily quantified, the controlled cortical impact (CCI) model of experimental TBI takes advantage of biomechanical events contributing to injury (Fig. 326-2A and D). These events can be analyzed by establishing a quantifiable relationship between measurable engineering parameters, such as force, velocity, and tissue deformation, and the magnitude of tissue damage or functional impairment (or both). These controlled mechanical variables enable accurate, reliable, and independent control of the deformation parameters over a wide range of contact velocities.
The CCI injury device typically consists of a small-bore (1.975 cm), double-acting stroke-constrained pneumatic cylinder with a 5.0-cm stroke. The cylinder is rigidly mounted on a crossbar in either an angled (perpendicular to the dura surface) or vertical position. The lower rod end has an impact tip attached that varies in geometry (rounded or flat edge) and diameter (5 to 6 mm for rat CCI), and the upper rod end is attached to a velocity-measuring sensor system. The impactor tip is pneumatically driven at a predetermined velocity, depth, and duration of tissue deformation. In rats, a depth of penetration of this device of 2.6 to 2.8 mm with a velocity of 4.0 m/sec and a dwell time of 50 to 150 msec consistently produces an injury of moderate severity. However, the exact injury parameters depend on the particular laboratory. The velocity of the impacting shaft is controlled by varying the gas pressure. Other CCI devices have recently been developed that use electromagnetic actuators to drive the impactor tip in an effort to provide greater control of the impact parameters.6–8
Rat Controlled Cortical Impact Model
The rat CCI model also produces morphologic and cerebrovascular injury responses that resemble certain aspects of human TBI. Commonly observed are graded histologic and axonal derangements,3–5,9,10 as well as disruption of the blood-brain barrier (BBB),11,12 subdural and intraparenchymal hematoma, edema, inflammation, and alterations in cerebral blood flow (CBF).12 Similarly, the CCI model also produces neurobehavioral and cognitive impairments similar to those observed in human patients. In contrast to other TBI models, the CCI device induces a significantly pronounced cortical contusion.
Rat CCI produces graded morphologic and functional responses that can be used to monitor injury severity and evaluate therapies. Lesion volumes produced by CCI are calculated by measuring tissue loss in serial coronal sections stained with hematoxylin and eosin. Hippocampal neuronal loss/survival is measured semiquantitatively by counting healthy-appearing neurons or by using unbiased stereologic methods.13 Recent studies of CCI with the de Olmos amino-cupric-silver staining method have indicated that CCI can induce neurodegeneration in regions distant from the site of impact in rats.14 A variety of tests are used to evaluate neurological and cognitive function after CCI. Gross vestibulomotor function is assessed in the rat with a beam-balancing task. Finer components of vestibulomotor function and coordination are assessed with a beam-walking task.15–18 Cognitive function in rodents after CCI has primarily been assessed with the Morris water maze test.19–24 Conditioned fear response can also be used to assess cognitive function after TBI.25
Mouse Controlled Cortical Impact Model
With the development of mutant strains of mice, including both gene knockout and transgenic lines, a version of the CCI model in mice has logically followed. Smith and colleagues characterized this model in the C57BL6 mouse—the background commonly used to produce relevant mutant strains.26 The depth of deformation was scaled down to 1.0 to 1.2 mm based on the cortical thickness of the mouse versus rat, and for a given impact velocity, an insult of similar severity to that commonly seen in rats was produced. Outcome testing is similar to that used in rats except that neurological status can also be measured in mice with a “grip test” adapted from Hall.27 CCI is unquestionably the most common model used to produce experimental TBI in mice, and because mice are steadily becoming the most popular species, the mouse CCI model is taking on greater importance in the field.
Pig Controlled Cortical Impact Model
In general, large-animal models of TBI are justified for complex physiologic and biomechanical studies that require large brain mass. By increasing the size of the impact tip and depth of impact, the CCI model has easily been scaled up to larger animals such as the pig. Using a direct focal impact method, Duhaime and associates demonstrated in piglets of different ages a vulnerability to mechanical trauma that increased progressively during maturation.28 CCI produced in swine with a pneumatic impactor has been shown to result in clinically relevant pathophysiology such as edema, cell death, white matter damage, and cerebrovascular dysregulation.29,30 Pig CCI models may be the most useful TBI model to mimic the neurological intensive care unit environment.
Primate Controlled Cortical Impact Model
Recently, the CCI technique has been applied to monkeys.31 Impact applied to the right frontal cortex produced edema (as assessed by magnetic resonance imaging [MRI]) and histopathologic changes, including necrotic cell death, axonal spheroids, astrocytosis, and accumulation of macrophages. The monkey CCI model may prove valuable for preclinical safety studies to facilitate the translation of experimental neurotherapeutics to humans.
Fluid Percussion
First described by Lindgren and Rinder in a rabbit model of TBI,32 the FP device has since been used in several other animal species, including cats,33,34 rats,35–37 pigs,38–40 and mice.41 The FP device (see Fig. 326-2B and E) consists of a Plexiglas cylinder filled with physiologic saline and enclosed at one end by a male Luer-Lock fitting that is subsequently paired with a female fitting. Injury is produced when a metal pendulum strikes the piston of the injury device from a predetermined height and causes rapid injection of saline into the closed cranium. The resulting pressure pulse induces a brief increase in intracranial pressure (ICP) with associated displacement and deformation of neural tissue. The severity of injury is regulated by varying the height of the pendulum, which corresponds to variations in extracranial pressure pulses expressed in atmospheres. Increased magnitudes of tissue deformation are associated with increased brain injury.
Rat Midline Fluid Percussion
Midline FP in rats is produced by placing the injury screw along the central sagittal suture midway between the bregma and lambda. Midline FP has been used to induce concussive injuries35 and can produce cognitive deficits in the absence of overt hippocampal cell death.42 Although the lateral FP model has been popular for studying neuronal cell death mechanisms, there is a recent resurgence of interest in midline FP because of the increased interest in diffuse brain injury associated with sports concussions and blast-induced TBI.
Rat Lateral Fluid Percussion
Lateral FP in rats is produced by placing the injury screw over the parietal cortex midway between the bregma and lambda. Lateral FP is advantageous for producing hippocampal cell death and cortical contusions.43 Furthermore, lateral FP injury in the rat has been shown to produce vascular and BBB disruption,44 reductions in CBF,45 cerebral edema, tissue shearing,46 and intraparenchymal hemorrhage, all of which contribute to the formation of a focal lesion in the injured cortex.47–53
Pig Fluid Percussion
The immature pig FP model is useful for studying CBF, pial artery diameter, and cerebral oxygenation.54 Moreover, rapid increases in ICP have been observed in piglets after FP-induced TBI.38 Midline FP can result in a reproducible secondary increase in ICP accompanied by patterns of diffuse brain damage in immature and juvenile piglets.55,56 FP injury in pigs has also been used in combination with secondary insults (see later).
Closed Head Injury Models
Closed head injury models are thought to produce injury by transmitting mechanical forces through the skull to the brain. There are three main variations of closed head injury models. The first is a focal impact applied to the intact skull. Hall and coworkers produced concussive injury in mice by dropping a 50-g weight 18 cm onto the intact skull.57 The Shohomi laboratory has characterized and applied a closed head impact model in both rats58 and mice59 that produces edema, functional deficits, and significant hippocampal neuronal cell death. For weight drop models, the height and mass of the falling weight are adjusted according to the desired severity of injury, with further distances and increased weight producing more injury than less distant and lighter weights.
The second type of closed head model is the Marmarou impact acceleration model (see Fig. 326-2C and F). In this model, a weight is attached to a string, and when the predetermined height above the cranium is obtained, the weight is released through a guide tube and subsequently strikes a cemented disk (“helmet”) on the skull to prevent skull fracture.60 During impact the rat’s head is rapidly accelerated downward into a foam pad, which facilitates relatively slower head deceleration. Impact acceleration produces a diffuse injury without noticeable contusions or hippocampal cell loss.60 Brainstem injury or DAI (or both)61 and elevations in ICP have also been observed after closed head impact acceleration injury.62
Finally, the group at the University of Pennsylvania developed and described a closed head rotation model originally characterized in monkeys63 and successfully adapted to swine. DAI and transient posttraumatic unconsciousness have been shown to be produced in miniature swine by rapid acceleration and deceleration of the head in the coronal plane, without impact.64 Recently, this model has been further applied to immature piglets and found to produce a range of clinically relevant functional deficits that correlate with neuropathologic axonal damage.65
Complex Models of Traumatic Brain Injury—Secondary Insults, Polytrauma, and Blast Injury
It is well recognized that TBI is often complicated by secondary insults such as hypoxemia, hemorrhagic shock, or polytrauma.66 The injured brain is highly vulnerable to these insults because autoregulatory mechanisms are often compromised early after the injury and metabolic demands are generally high in the early postinjury period.67 Thus, secondary insults can have devastating consequences. A number of modifications of established models have been developed to mimic the pathophysiology of these various insults.
Traumatic Brain Injury Plus Hypoxemia
Ishige and colleagues incorporated secondary hypoxemia into a lateral FP injury model in rats.68 A period of hypoxemia (PaO2 of 40 mm Hg for 30 minutes, beginning immediately after the insult) that alone had no consequences in rats dramatically exacerbated the pathology as determined by numerous outcome criteria, including electrophysiology, function, blood flow, edema, and histology. Subsequently, Clark and associates characterized an analogous model of combined CCI plus hypoxemia in rats—again using 30 minutes of hypoxemia at a level similar to previous studies.69 Marked exacerbation of DNA damage and neuronal death was seen in the hippocampus underlying the contusion. This model has been used to study the effect of various neuroprotective therapies.70,71 In the work of Clark and colleagues, monitoring of arterial blood pressure revealed that hypotension commonly develops after approximately 20 minutes of hypoxemia69; thus, it is likely that the models using hypoxemia are actually models of combined hypoxemia and hypotension.
Traumatic Brain Injury Plus Hemorrhage
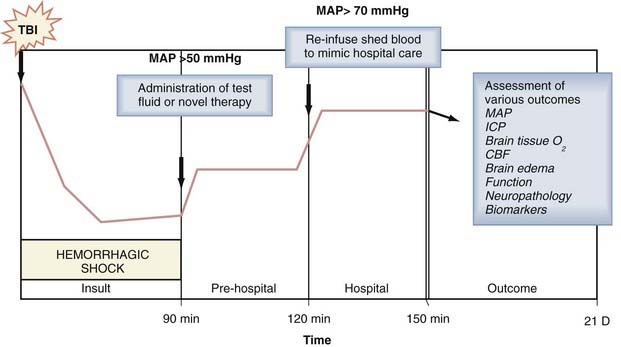
Hemorrhagic hypotension and hemorrhagic shock after TBI are common insults in the setting of polytrauma and have taken on greater significance in combat casualty care with the recent surge in blast-induced TBI from the improvised explosive devices (IEDs) used in terrorist attacks.72 TBI greatly enhances vulnerability of the organism to the hypotensive effects of hemorrhage, and blood loss that would otherwise be well tolerated can lead to shock.73,74 In addition, hemorrhage produces anemia and may result in extracerebral organ injury, thus further increasing secondary brain damage, mortality, or both. Much of the work incorporating this combined insult has focused on evaluation of the effect of various resuscitation strategies on survival and intracranial dynamics in large-animal models of FP injury such cats75 and pigs.76 In these studies, volume-controlled hemorrhage was generally induced and followed after various durations of shock by resuscitation with fluids such as crystalloids, hypertonic saline, colloids, or pressors, and resuscitation of hemorrhagic shock with standard crystalloid solutions after TBI was found to markedly exacerbate brain swelling and ICP. This earlier work has been followed by numerous studies, such as studies involving CCI plus hemorrhagic shock in pigs to evaluate the effect of resuscitation with hemoglobin solutions77 on cerebral hemodynamics, MRI outcomes, and brain tissue PO2. Studies of TBI plus hemorrhagic shock are less common in rodent models. Matsushita and coworkers examined the effect of moderate hypotension (mean arterial blood pressure [MAP] of 60 mm Hg for 30 minutes) induced by hemorrhage in rats after lateral FP and reported reduced CBF and an increase in the contusion area,78 whereas Schütz and coauthors reported that hemorrhagic hypotension (MAP of 50 to 60 mm Hg for 30 minutes) failed to worsen the histologic damage but increased the functional deficits.79 More recently, Dennis and colleagues reported the first model of combined TBI and hemorrhagic shock in mice and demonstrated that 90 minutes but not 60 minutes of hemorrhagic shock to a MAP of 30 to 40 mm Hg immediately after CCI exacerbated hippocampal neuronal death assessed 7 days after the combined insult.80 In that model, as in many large-animal models of TBI plus hemorrhage, a protocol that included insult, “prehospital,” and “hospital” phases was incorporated to maximize its clinical relevance (Fig. 326-3). Rodent models of combined TBI and hemorrhagic shock have only begun to be used to explore the effects of novel therapies.
Traumatic Brain Injury Plus Polytrauma
Given the recent interest in blast injury and polytrauma, a number of new models of experimental TBI accompanied by extracerebral trauma have been developed, including models such as combined blunt trauma to the head, chest, and femur in pigs, among others.81 Most of the studies in these polytrauma models have focused on the hemodynamic effects of resuscitation fluids rather than on neuroprotection. In this regard, a key fact is that extracerebral trauma leads to cytokine production, which can further exacerbate the brain injury. This hypothesis was recently directly supported by Utagawa and associates, who reported that systemic administration of interleukin-1β at doses that did not produce hypotension exacerbated the histologic damage and functional deficits in rats after lateral FP injury.82
Blast-Induced Traumatic Brain Injury
Because blast-induced injuries have been the leading cause of TBI in Operation Iraqi Freedom, this form of TBI has taken on tremendous importance in experimental TBI research.83,84 Blast injuries result from blast overpressure waves and a variety of other forces as a consequence of the detonation of explosive materials—most commonly IEDs. The recent use of body armor in the U.S. military has protected the highly vulnerable lung and gut from blast forces and shifted the site of primary damage in exposed soldiers to the limbs and brain. Recent studies have begun to model blast-induced TBI in rats, mice, and pigs. There are a number of potentially unique facets of blast-induced TBI, including pronounced acute cerebral swelling, vasospasm, and hemorrhage—particularly with severe insults.85 Penetrating injury from shrapnel and burns can also accompany a severe blast.86 Similarly, a retrograde pulse of venous or cerebrospinal fluid pressure into the brain from a blast has also been suggested to mediate vascular injury.87 Cernak and coauthors provided some of the earliest reports on the effect of blast in rodent models with the use of blast overpressure waves produced by a shock tube and highlighted an important role for oxidative stress.88 More recently, seminal work by Long and associates reported that body armor could markedly reduce mortality from blast overpressure injury in rats, again in a shock tube, and that rats protected by body armor and exposed to blast overpressure injury exhibited marked axonal injury as an important neuropathologic feature.89 Work in large-animal models of explosive blast and studies of therapies have only begun to be explored.
Clinical Factors Incorporated Into Experimental Models of Traumatic Brain Injury
Many factors besides the mechanism of injury influence outcome after TBI, and several of these factors have been incorporated into experimental TBI models. Age at injury has a complex effect on TBI, with outcomes being poorest in infants and the elderly. Most TBI models in rodents and large animals have used young adults—which mimics the most common clinical condition. The field of TBI is fortunate in this regard because the same cannot be said for stroke or cardiac arrest. There have been a number of experimental TBI studies that have examined the issue of age at injury. Experimental TBI in developing animals has been studied, with the most common model being CCI in 17-day-old rats90,91 or 21-day-old mice.92 CCI has also been used to model TBI in geriatric mice—with injury as late as at 24 months of age.93 Critical differences in a number of secondary injury mechanisms such as blood flow, metabolism, and neuroinflammation have been reported.93,94
Gender also has a complex effect on secondary injury mechanisms and outcome after clinical TBI. Surprisingly, the influence of gender in TBI has only recently been the focus of investigation in experimental models. The effects of gender related to the circulating sex hormones estrogen and progesterone have significant impact, notably in attenuating oxidative damage in TBI,95,96 but innate gender differences have also been reported.97 Gender effects have been examined in most of the major models of TBI, although there has been little study in models of TBI plus secondary insults.
A large number of genetic polymorphisms influence the evolution of secondary damage, plasticity, and recovery after brain injury, and among these, polymorphisms related to dopamine, serotonin, poly(adenosine diphosphate ribose) polymerase (PARP), tumor necrosis factor-α (TNF-α), adenosine, and apolipoprotein E play a role in TBI. The mouse model of CCI has been useful in facilitating the study of a number of these factors, and the major effects of these factors on the pathologic mechanisms and outcomes of TBI have been shown. For example, TNF-α knockout mice show early neuroprotection but delayed functional impairment after CCI.98 In adenosine A1 receptor knockout mice, seizures and lethal status epilepticus develop after CCI,99 and PARP knockout mice show neuroprotection after CCI.100
Several commonly used substances such as ethanol and caffeine101,102 influence the outcomes of TBI. These and other drugs have been incorporated into various experimental TBI models and been shown to affect neuropathology or functional outcome (or both).103–107 The ubiquitous substances ethanol and caffeine can be neuroprotective or deleterious, depending on the dose and timing.103–105
Confounding Factors Inherent In Experimental Models of Traumatic Brain Injury
Anesthesia is necessary for the humane care of animals subjected to experimental TBI. This obviously differs from the clinical condition. Most anesthetic agents are neuroprotective, particularly when administered before the injury. Isoflurane is the most commonly used anesthetic in experimental TBI, probably because of its ease of administration, its ability to facilitate rapid recovery, and its limited street value. Its impact on neuropathology and outcome has been studied extensively in the CCI model, where it is neuroprotective specifically when given at or near the time of impact.108,109 In a comparison of isoflurane and fentanyl anesthesia after CCI in rats, protection against both neuropathology and adverse functional outcomes was observed with isoflurane,109 and it was additionally shown to blunt stress more effectively than fentanyl did in rats.110 Other anesthetics such as pentobarbital have also been used,26 particularly in rodent models, where neuroprotection is also well recognized111; however, in CCI, pentobarbital and other commonly used anesthetic agents do not appear to confer as much neuroprotection as isoflurane does and may be less reliable.112 Whatever anesthetic is used, its effects must be recognized and appreciated, and appropriate anesthesia sham controls should be included in the experimental design.
Once a species and model have been selected, strain differences can still impart important effects on outcome in experimental TBI. This has been most extensively characterized in mice. Fox and coauthors reported marked differences in the behavioral responses of C57BL/6, FVB/N, and 129/SvEMS mouse strains to experimental TBI produced by CCI.113 Poor baseline performance in both FVB/N and 129/SvEMS mice—relative to the C57BL/6 strain—was observed on the Morris water maze task, which is fortunate given the importance of C57BL/6 mice in experimental TBI. Murine strain differences with regard to global cerebral ischemia114 and neuronal death after lateral FP have also been reported.115 Although less well characterized in rats with TBI, strain differences are well known in rat models of stroke,116 where they have been shown to influence the effects of therapies such as hypothermia.117
Dennis AM, Haselkorn ML, Vagni VA, et al. Hemorrhagic shock after experimental traumatic brain injury in mice: effect on neuronal death. J Neurotrauma. 2009;26:889-899.
DeWitt DS, Prough DS, Taylor CL, et al. Reduced cerebral blood flow, oxygen delivery, and electroencephalographic activity after traumatic brain injury and mild hemorrhage in cats. J Neurosurg. 1992;76:812-821.
Dixon CE, Clifton GL, Lighthall JW, et al. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253-262.
Dixon CE, Lighthall JW, Anderson TE. A physiologic, histopathologic, and cineradiographic characterization of a new fluid percussion model of experimental brain injury in the rat. J Neurotrauma. 1988;5:91-104.
Dixon CE, Lyeth BG, Povlishock JT, et al. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1987;67:110-119.
Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855-857.
Gennarelli TA, Thibault LE, Adams JH, et al. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol. 1982;12:564-574.
Hall ED, Sullivan PG, Gibson TR, et al. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22:252-265.
Hall ED, Yonkers PA, McCall JM, et al. Effects of the 21-aminosteroid U74006F on experimental head injury in mice. J Neurosurg. 1988;68:456-461.
Ishige N, Pitts LH, Hashimoto T, et al. Effect of hypoxia on traumatic brain injury in rats: part 1. Changes in neurological function, electroencephalograms, and histopathology. Neurosurgery. 1987;20:848-853.
Lewelt W, Jenkins LW, Miller JD. Autoregulation of cerebral blood flow after experimental fluid percussion injury of the brain. J Neurosurg. 1980;53:500-511.
Long JB, Bentley TL, Wessner KA, et al. Blast over pressure in rats: recreating a battlefield injury in the laboratory. J Neurotrauma. 2009;26:827-840.
Lyeth BG, Jenkins LW, Hamm RJ, et al. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249-258.
Manley GT, Rosenthal G, Lam M, et al. Controlled cortical impact in swine: pathophysiology and biomechanics. J Neurotrauma. 2006;23:128-139.
Marmarou A, Foda MA, van den Brink W, et al. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J Neurosurg. 1994;80:291-300.
Matsushita Y, Bramlett HM, Kuluz JW, et al. Delayed hemorrhagic hypotension exacerbates the hemodynamic and histopathologic consequences of traumatic brain injury in rats. J Cereb Blood Flow Metab. 2001;21:847-856.
McIntosh TK, Nobel L, Andrews B, et al. Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent Nerv Syst Trauma. 1987;4:119-134.
McIntosh TK, Vink R, Yamakami I, et al. Traumatic brain injury in the rat: characterization of a midline fluid percussion model. J Neuroscience. 1989;28:233-244.
Morris RG, Garrud P, Rawlins JN, et al. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681-683.
Nemoto EM, Rao G, Robinson T, et al. Effect of local cooling (15 degrees C for 24 hours) with the Chillerpad after traumatic brain injury in the nonhuman primate. Adv Exp Med Biol. 2006;578:311-315.
Smith DH, Okiyama K, Thomas MJ, et al. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J Neurotrauma. 1991;8:259-269.
Smith DH, Soares HD, Pierce JS, et al. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169-178.
Statler KD, Jenkins LW, Dixon CE, et al. The simple model versus the super model: translating experimental traumatic brain injury research to the bedside. J Neurotrauma. 2001;18:1195-1206.
Tanno H, Nockels RP, Pitts LH, et al. Breakdown of the blood-brain barrier after fluid percussive brain injury in the rat. Part 1: distribution and time course of protein extravasation. J Neurotrauma. 1992;9:21-32.
Yamakami I, McIntosh TK. Alterations in regional cerebral blood flow following brain injury in the rat. J Cereb Blood Flow Metab. 1991;11:655-660.
1 Saatman KE, Duhaime AC, Bullock R, et al. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719-738.
2 Statler KD, Jenkins LW, Dixon CE, et al. The simple model versus the super model: translating experimental traumatic brain injury research to the bedside. J Neurotrauma. 2001;18:1195-1206.
3 Lighthall JW. Controlled cortical impact: a new experimental brain injury model. J Neurotrauma. 1988;5:1-15.
4 Lighthall JW, Goshgarian HG, Pinderski CR. Characterization of axonal injury produced by controlled cortical impact. J Neurotrauma. 1990;7:65-76.
5 Dixon CE, Clifton GL, Lighthall JW, et al. A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253-262.
6 Bilgen M. A new device for experimental modeling of central nervous system injuries. Neurorehabil Neural Repair. 2005;19:219-226.
7 Bayly PV, Dikranian KT, Black EE, et al. Spatiotemporal evolution of apoptotic neurodegeneration following traumatic injury to the developing rat brain. Brain Res. 2006;1107:70-81.
8 Brody DL, Mac Donald C, Kessens CC, et al. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J Neurotrauma. 2007;24:657-673.
9 Goodman JC, Cherian L, Bryan RMJr, et al. Lateral cortical impact injury in rats: pathologic effects of varying cortical compression and impact velocity. J Neurotrauma. 1994;11:587-597.
10 Meaney DF, Ross DT, Winkelstein BA, et al. Modification of the cortical impact model to produce axonal injury in the rat cerebral cortex. J Neurotrauma. 1994;11:599-612.
11 Dhillon HS, Donaldson D, Dempsey RJ, et al. Regional levels of free fatty acids and Evans blue extravasation after experimental brain injury. J Neurotrauma. 1994;11:405-415.
12 Kochanek PM, Marion DW, Zhang W, et al. Severe controlled cortical impact in rats: assessment of cerebral edema, blood flow, and contusion volume. J Neurotrauma. 1995;12:1015-1025.
13 Bladwin SA, Gibson T, Callihan CT, et al. Neuronal cell loss in the CA3 subfield of the hippocampus following cortical contusion utilizing the optical dissector method for cell counting. J Neurotrauma. 1997;14:385-398.
14 Hall ED, Sullivan PG, Gibson TR, et al. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J Neurotrauma. 2005;22:252-265.
15 Feeney DM, Gonzalez A, Law WA. Amphetamine, haloperidol, and experience interact to affect rate of recovery after motor cortex injury. Science. 1982;217:855-857.
16 Kline AE, Massucci JL, Dixon CE, et al. The therapeutic efficacy conferred by the 5-HT(1A) receptor agonist 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) after experimental traumatic brain injury is not mediated by concomitant hypothermia. J Neurotrauma. 2004;21:175-185.
17 Kline AE, Wagner AK, Westergom BP, et al. Acute treatment with the 5-HT(1A) receptor agonist 8-OH-DPAT and chronic environmental enrichment confer neurobehavioral benefit after experimental brain trauma. Behav Brain Res. 2007;177:186-194.
18 Dixon CE, Kline AE, Ma X, et al. Acute etomidate treatment reduces cognitive deficits and histopathology in rats with traumatic brain injury. Crit Care Med. 2003;31:2222-2227.
19 Morris RG, Garrud P, Rawlins JN, et al. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681-683.
20 Smith DH, Okiyama K, Thomas MJ, et al. Evaluation of memory dysfunction following experimental brain injury using the Morris water maze. J Neurotrauma. 1991;8:259-269.
21 Hamm RJ, Dixon CE, Gbadebo DM, et al. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J Neurotrauma. 1992;9:11-20.
22 Kline AE, Bolinger BD, Kochanek PM, et al. Acute systemic administration of interleukin-10 suppresses the beneficial effects of moderate hypothermia following traumatic brain injury in rats. Brain Res. 2002;937:22-31.
23 Kline AE, Massucci J, Ma X, et al. Bromocriptine reduces lipid peroxidation and enhances spatial learning and hippocampal neuron survival in a rodent model of focal brain trauma. J Neurotrauma. 2004;21:1712-1722.
24 Kline AE, Massucci JL, Zafonte RD, et al. Differential effects of single versus multiple administrations of haloperidol and risperidone on functional outcome after experimental brain trauma. Crit Care Med. 2007;35:919-924.
25 Dash PK, Mach SA, Blum S, et al. Intrahippocampal wortmannin infusion enhances long-term spatial and contextual memories. Learn Mem. 2002;9:167-177.
26 Smith DH, Soares HD, Pierce JS, et al. A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169-178.
27 Hall E. Glucocorticoid treatment of head-injured mice. J Neurosurg. 1985;62:882-887.
28 Duhaime AC, Margulies SS, Durham SR, et al. Maturation-dependent response of the piglet brain to scaled cortical impact. J Neurosurg. 2000;93:455-462.
29 Alessandri B, Heimann A, Filippi R, et al. Moderate controlled cortical contusion in pigs: effects on multi-parametric neuromonitoring and clinical relevance. J Neurotrauma. 2003;20:1293-1305.
30 Manley GT, Rosenthal G, Lam M, et al. Controlled cortical impact in swine: pathophysiology and biomechanics. J Neurotrauma. 2006;23:128-139.
31 Nemoto EM, Rao G, Robinson T, et al. Effect of local cooling (15 degrees C for 24 hours) with the Chillerpad after traumatic brain injury in the nonhuman primate. Adv Exp Med Biol. 2006;578:311-315.
32 Lindgren S, Rinder L. Experimental studies in head injury. I. Pressure propagation in “percussion concussion.”. Biophysik. 1966;3:174-180.
33 Sullivan HG, Martinez J, Becker DP, et al. Fluid percussion model of mechanical brain injury in the cat. J Neurosurg. 1976;45:520-534.
34 Thibault LE, Meaney DF, Anderson BJ, et al. Biomechanical aspects of a fluid percussion model of brain injury. J Neurotrauma. 1992;9:311-322.
35 Dixon CE, Lyeth BG, Povlishock JT, et al. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1987;67:110-119.
36 McIntosh TK, Nobel L, Andrews B, et al. Traumatic brain injury in the rat: characterization of a midline fluid-percussion model. Cent Nerv Syst Trauma. 1987;4:119-134.
37 Dixon CE, Lighthall JW, Anderson TE. A physiologic, histopathologic, and cineradiographic characterization of a new fluid percussion model of experimental brain injury in the rat. J Neurotrauma. 1988;5:91-104.
38 Pfenninger EG, Reith A, Breitig D, et al. Early changes of intracranial pressure, perfusion pressure, and blood flow after acute head injury. Part 1: an experimental study of the underlying pathophysiology. J Neurosurg. 1989;70:774-779.
39 Zink BJ, Walsh RF, Feustel PJ. Effects of ethanol in traumatic brain injury. J Neurotrauma. 1993;10:275-286.
40 Armstead WM, Kurth CD. Different cerebral hemodynamic responses following fluid percussion brain injury in the newborn and juvenile pig. J Neurotrauma. 1994;11:487-497.
41 Carbonell WS, Maris DO, McCall T, et al. Adaptation of the fluid percussion injury model to the mouse. J Neurotrauma. 1998;15:217-229.
42 Lyeth BG, Jenkins LW, Hamm RJ, et al. Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249-258.
43 McIntosh TK, Vink R, Yamakami I, et al. Traumatic brain injury in the rat: characterization of a midline fluid percussion model. J Neurosci. 1989;28:233-244.
44 Cortez SC, McIntosh TK, Noble LJ. Experimental fluid percussion brain injury: vascular disruption and neuronal and glial alterations. Brain Res. 1989;482:271-282.
45 Yamakami I, McIntosh TK. Alterations in regional cerebral blood flow following brain injury in the rat. J Cereb Blood Flow Metab. 1991;11:655-660.
46 Graham DI, Raghupathi R, Saatman KE, et al. Tissue tears in the white matter after lateral fluid percussion brain injury in the rat: relevance to human brain injury. Acta Neuropathol. 2000;99:117-124.
47 Tanno H, Nockels RP, Pitts LH, et al. Breakdown of the blood-brain barrier after fluid percussive brain injury in the rat. Part 1: distribution and time course of protein extravasation. J Neurotrauma. 1992;9:21-32.
48 Tanno H, Nockels RP, Pitts LH, et al. Breakdown of the blood-brain barrier after fluid percussion brain injury in the rat. Part 2: effect of hypoxia on permeability to plasma proteins. J Neurotrauma. 1992;9:335-347.
49 Schmidt RH, Grady MS. Regional patterns of blood-brain barrier breakdown following central and lateral fluid percussion injury in rodents. J Neurotrauma. 1993;10:415-430.
50 Soares HD, Hicks RR, Smith D, et al. Inflammatory leukocytic recruitment and diffuse neuronal degeneration are separate pathological processes resulting from traumatic brain injury. J Neurosci. 1995;15:8223-8233.
51 Qian L, Ohno K, Maehara T, et al. Changes in lCBF, morphology and related parameters by fluid percussion injury. Acta Neurochir (Wien). 1996;138:90-98.
52 Iwamoto Y, Yamaki T, Murakami N, et al. Investigation of morphological change of lateral and midline fluid percussion injury in rats, using magnetic resonance imaging. Neurosurgery. 1997;40:163-167.
53 Dietrich WD, Alonso O, Busto R, et al. Posttraumatic cerebral ischemia after fluid percussion brain injury: an autoradiographic and histopathological study in rats. Neurosurgery. 1998;43:585-594.
54 Armstead WM. Cerebral hemodynamics after traumatic brain injury of immature brain. Exp Toxicol Pathol. 1999;51:137-142.
55 Bauer R, Walter B, Torossian A, et al. A piglet model for evaluation of cerebral blood flow and brain oxidative metabolism during gradual cerebral perfusion pressure decrease. Pediatr Neurosurg. 1999;30:62-69.
56 Fritz HG, Walter B, Holzmayr M, et al. A pig model with secondary increase of intracranial pressure after severe traumatic brain injury and temporary blood loss. J Neurotrauma. 2005;22:807-821.
57 Hall ED, Yonkers PA, McCall JM, et al. Effects of the 21-aminosteroid U74006F on experimental head injury in mice. J Neurosurg. 1988;68:456-461.
58 Shapira Y, Shohami E, Sidi A, et al. Experimental closed head injury in rats: mechanical, pathophysiologic, and neurologic properties. Crit Care Med. 1988;16:258-265.
59 Chen Y, Constantini S, Trembovler V, et al. An experimental model of closed head injury in mice: pathophysiology, histopathology, and cognitive deficits. J Neurotrauma. 1996;13:557-568.
60 Marmarou A, Foda MA, van den Brink W, et al. A new model of diffuse brain injury in rats. Part I: pathophysiology and biomechanics. J Neurosurg. 1994;80:291-300.
61 Okonkwo DO, Povlishock JT. An intrathecal bolus of cyclosporin A before injury preserves mitochondrial integrity and attenuates axonal disruption in traumatic brain injury. J Cereb Blood Flow Metab. 1999;19:443-451.
62 Engelborghs K, Verlooy J, Van Reempts J, et al. Temporal changes in intracranial pressure in a modified experimental model of closed head injury. J Neurosurg. 1998;89:796-806.
63 Gennarelli TA, Thibault LE, Adams JH, et al. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol. 1982;12:564-574.
64 Ross DT, Meaney DF, Sabol MK, et al. Distribution of forebrain diffuse axonal injury following inertial closed head injury in miniature swine. Exp Neurol. 1994;126:291-299.
65 Friess SH, Ichord RN, Owens K, et al. Neurobehavioral functional deficits following closed head injury in the neonatal pig. Exp Neurol. 2007;204:234-243.
66 Chesnut RM, Marshall LF, Klauber MR, et al. The role of secondary brain injury in determining outcome from severe head injury. J Trauma. 1993;34:216-222.
67 Lewelt W, Jenkins LW, Miller JD. Autoregulation of cerebral blood flow after experimental fluid percussion injury of the brain. J Neurosurg. 1980;53:500-511.
68 Ishige N, Pitts LH, Hashimoto T, et al. Effect of hypoxia on traumatic brain injury in rats: part 1. Changes in neurological function, electroencephalograms, and histopathology. Neurosurgery. 1987;20:848-853.
69 Clark RS, Kochanek PM, Dixon CE, et al. Early neuropathologic effects of mild or moderate hypoxemia after controlled cortical impact injury in rats. J Neurotrauma. 2004;14:179-189.
70 Clark RS, Nathaniel PD, Zhang X, et al. boc-Aspartyl(OMe)-fluoromethylketone attenuates mitochondrial release of cytochrome c and delays brain tissue loss after traumatic brain injury in rats. J Cereb Blood Flow Metab. 2007;27:316-326.
71 Clark RS, Kochanek PM, Watkins SC, et al. Caspase-3 mediated neuronal death after traumatic brain injury in rats. J Neurochem. 2000;74:740-753.
72 Gawande A. Casualties of war—military care for the wounded from Iraq and Afghanistan. N Engl J Med. 2004;351:2471-2475.
73 Law MM, Hovda DA, Cryer HG. Fluid percussion brain injury adversely affects control of vascular tone during hemorrhagic shock. Shock. 1996;6:213-217.
74 Yuan XQ, Wade CE. Traumatic brain injury attenuates the effectiveness of lactated Ringer’s solution resuscitation of hemorrhagic shock in rats. Surg Gynecol Obstet. 1992;174:305-312.
75 DeWitt DS, Prough DS, Taylor CL, et al. Reduced cerebral blood flow, oxygen delivery, and electroencephalographic activity after traumatic brain injury and mild hemorrhage in cats. J Neurosurg. 1992;76:812-821.
76 Glass TF, Fabian MJ, Schweitzer JB, et al. Secondary neurologic injury resulting from nonhypotensive hemorrhage combined with mild traumatic brain injury. J Neurotrauma. 1999;16:771-782.
77 Rosenthal G, Morabito D, Cohen M, et al. Use of hemoglobin-based oxygen-carrying solution-201 to improve resuscitation parameters and prevent secondary brain injury in a swine model of traumatic brain injury and hemorrhage: laboratory investigation. J Neurosurg. 2008;108:575-587.
78 Matsushita Y, Bramlett HM, Kuluz JW, et al. Delayed hemorrhagic hypotension exacerbates the hemodynamic and histopathologic consequences of traumatic brain injury in rats. J Cereb Blood Flow Metab. 2001;21:847-856.
79 Schütz C, Stover JF, Thompson HJ, et al. Acute, transient hemorrhagic hypotension does not aggravate structural damage or neurologic motor deficits but delays the long-term cognitive recovery following mild to moderate traumatic brain injury. Crit Care Med. 2006;34:492-501.
80 Dennis AM, Haselkorn ML, Vagni VA, et al. Hemorrhagic shock after experimental traumatic brain injury in mice: effect on neuronal death. J Neurotrauma. 2009;26:889-899.
81 Earle SA, de Moya MA, Zuccarelli JE, et al. Cerebrovascular resuscitation after polytrauma and fluid restriction. J Am Coll Surg. 2007;204:261-275.
82 Utagawa A, Truettner JS, Dietrich WD, et al. Systemic inflammation exacerbates behavioral and histopathological consequences of isolated traumatic brain injury in rats. Exp Neurol. 2008;211:283-291.
83 Shanker T. Iraqi bombers thwart efforts to shield GIs. New York: Times; June 2, 2007.
84 Hanley CJ. US Military struggles to defeat IEDs. Washington Post. August 20. 2007.
85 Armonda RA, Bell RS, Vo AH, et al. Wartime traumatic cerebral vasospasm: recent review of combat casualties. Neurosurgery. 2006;59:1215-1225.
86 Ling G, Bandak F, Armonda R, et al. Explosive blast neurotrauma. J Neurotrauma. 2009;26:815-825.
87 Knudsen SK, Øen EO. Blast-induced neurotrauma in whales. Neurosci Res. 2003;46:377-386.
88 Cernak I, Wang Z, Jiang J, et al. Ultrasound and functional characteristics of blast injury–induced neurotrauma. J Trauma. 2001;50:695-706.
89 Long JB, Bentley TL, Wessner KA, et al. Blast over pressure in rats: recreating a battlefield injury in the laboratory. J Neurotrauma. 2009;26:827-840.
90 Kochanek AR, Kline AE, Gao WM, et al. Gel-based hippocampal proteomic analysis 2 weeks following traumatic brain injury to immature rats using controlled cortical impact. Dev Neurosci. 2006;28:410-419.
91 Prins ML, Fujima LS, Hovda DA. Age-dependent reduction of cortical contusion volume by ketones after traumatic brain injury. J Neurosci Res. 2005;82:413-420.
92 Tong W, Igarashi T, Ferriero DM, et al. Traumatic brain injury in the immature mouse brain: characterization of regional vulnerability. Exp Neurol. 2002;176:105-116.
93 Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol. 2008;213:372-380.
94 Biagas KV, Grundl PD, Kochanek PM, et al. Posttraumatic hyperemia in immature, mature, and aged rats: autoradiographic determination of cerebral blood flow. J Neurotrauma. 1996;13:189-200.
95 Roof RL, Duvdevani R, Stein DG. Gender influences outcome of brain injury: progesterone plays a protective role. Brain Res. 1993;607:333-336.
96 Hurn PD, Macrae IM. Estrogen as a neuroprotectant in stroke. J Cereb Blood Flow Metab. 2000;20:631-652.
97 Du L, Bayır H, Lai Y, et al. Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. J Biol Chem. 2004;279:38563-38570.
98 Scherbel U, Raghupathi R, Nakamura M, et al. Differential acute and chronic responses of tumor necrosis factor–deficient mice to experimental brain injury. Proc Natl Acad Sci U S A. 1999;96:8721-8726.
99 Kochanek PM, Vagni VA, Janesko KL, et al. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:565-575.
100 Whalen MJ, Clark RS, Dixon CE, et al. Reduction of cognitive and motor deficits after traumatic brain injury in mice deficient in poly(ADP-ribose) polymerase. J Cereb Blood Flow Metab. 1999;19:835-842.
101 Kelly DF. Alcohol and head injury: an issue revisited. J Neurotrauma. 1995;12:883-890.
102 Sachse KT, Jackson EK, Wisniewski SR, et al. Increases in cerebrospinal fluid caffeine concentration are associated with favorable outcome after severe traumatic brain injury in humans. J Cereb Blood Flow Metab. 2008;28:395-401.
103 Gottesfeld Z, Moore AN, Dash PK. Acute ethanol intake attenuates inflammatory cytokines after brain injury in rats: a possible role for corticosterone. J Neurotrauma. 2002;19:317-326.
104 Zhang L, Maki A, Dhillon HS, et al. Effects of six weeks of chronic ethanol administration on the behavioral outcome of rats after lateral fluid percussion brain injury. J Neurotrauma. 1999;16:243-254.
105 Kelly DF, Lee SM, Pinanong PA, et al. Paradoxical effects of acute ethanolism in experimental brain injury. J Neurosurg. 1997;86:876-882.
106 Al Moutaery K, Al Deeb S, Ahmad Khan H, et al. Caffeine impairs short-term neurological outcome after concussive head injury in rats. Neurosurgery. 2003;53:704-711.
107 Chan F, Lanctôt KL, Feinstein A, et al. The serotonin transporter polymorphisms and major depression following traumatic brain injury. Brain Inj. 2008;22:471-479.
108 Statler KD, Alexander H, Vagni V, et al. Isoflurane exerts neuroprotective actions at or near the time of severe traumatic brain injury. Brain Res. 2006;1076:216-224.
109 Statler KD, Kochanek PM, Dixon CE, et al. Isoflurane improves long-term neurologic outcomes versus fentanyl after traumatic brain injury in rats. J Neurotrauma. 2000;17:1179-1189.
110 Statler KD, Alexander HL, Vagni VA, et al. Moderate hypothermia may be detrimental after traumatic brain injury in fentanyl-anesthetized rats. Crit Care Med. 2003;31:1134-1139.
111 Popovic R, Liniger R, Bickler PE. Anesthetics and mild hypothermia similarly prevent hippocampal neuron death in an in vitro model of cerebral ischemia. Anesthesiology. 2000;92:1343-1349.
112 Statler KD, Alexander H, Vagni V, et al. Comparison of seven anesthetic agents on outcome after experimental traumatic brain injury in adult, male rats. J Neurotrauma. 2006;23:97-108.
113 Fox GB, LeVasseur RA, Faden AI. Behavioral responses of C57BL/6, FVB/N, and 129/SvEMS mouse strains to traumatic brain injury: Implications for gene targeting approaches to neurotrauma. J Neurotrauma. 1999;16:377-389.
114 Wellons JC3rd, Sheng H, Laskowitz Dt, et al. A comparison of strain-related susceptibility in two murine recovery models of global cerebral ischemia. Brain Res. 2000;868:14-21.
115 Witgen BM, Lifshitz J, Grady MS. Inbred mouse strains as a tool to analyze hippocampal neuronal loss after brain injury: a stereological study. J Neurotrauma. 2006;23:1320-1329.
116 Walberer M, Stolz E, Müller C, et al. Experimental stroke: ischemic lesion volume and oedema formation differ among rat strains (a comparison between Wistar and Sprague-Dawley rats using MRI). Lab Anim. 2006;40:1-8.
117 Ren Y, Hashimoto M, Pulsinelli WA, et al. Hypothermic protection in rat focal ischemia models: strain differences and relevance to “reperfusion injury.”. J Cereb Blood Flow Metab. 2004;24:42-53.