Chapter 17 Anesthetic Monitoring
1. What is the purpose of intraoperative patient monitoring?
2. What are some monitors that have been mandated for use by the American Society of Anesthesiologists? How frequently is it mandated that intraoperative blood pressure be measured?
Electrocardiographic Monitoring
3. What are some potential intraoperative problems during anesthesia that can be detected by an anesthesiologist through the use of an electrocardiogram?
4. Which lead is selected on the electrocardiogram for continuous tracing on the monitor to best detect cardiac dysrhythmias? Why?
5. Which lead is selected on the electrocardiogram for continuous tracing on the monitor to best detect inferior wall myocardial ischemia? Which lead is selected for continuous tracing on the monitor to best detect anterior or lateral wall myocardial ischemia?
Systemic Blood Pressure Monitoring
6. How does an automated oscillometric blood pressure measuring device, such as the Dinamap, work?
7. What is the appropriate-sized cuff for use with an automated oscillometric blood pressure measuring device?
8. When using an automated oscillometric blood pressure measuring device, will the blood pressure be falsely high or low with a cuff that is too small? When using an automated oscillometric blood pressure measuring device, will the blood pressure be falsely high or low with a cuff that is too loose?
9. What is a potential problem that can result from too frequent cycling of an automated oscillometric blood pressure measuring device?
10. What are some possible indications for intraarterial blood pressure monitoring?
11. What are some arteries that may be used for intraarterial blood pressure monitoring? Which of these is most commonly selected?
12. How does the intraarterial blood pressure waveform change with increasing distance from the heart?
Central Venous Pressure Monitoring
13. What are some indications for the placement of a central venous catheter?
14. What veins are used for central venous access? What are some potential complications of the cannulation of veins for central access?
15. Which is the preferred jugular vein for cannulation? Why?
16. What are some advantages and disadvantages of cannulation of the internal jugular vein over other central veins?
17. What are some advantages and disadvantages of cannulation of the subclavian vein over other central veins?
18. What does the central venous waveform look like? What do each of the peaks and descents represent relative to the cardiac cycle?
19. Why is the central venous pressure able to be used to estimate a patient’s intravascular fluid volume status?
20. Under which circumstances does central venous pressure not estimate a patient’s intravascular fluid volume status? What invasive monitor can be used instead of a central venous catheter under these conditions?
Pulmonary Artery Catheter Monitoring
21. Name six possible indications for the placement of a pulmonary artery catheter. What information can be obtained regarding the patient’s status with the use of a pulmonary artery catheter?
22. Of what is the pulmonary capillary wedge pressure a reflection? What other measurement derived by the pulmonary artery catheter can also be used in lieu of the pulmonary capillary wedge pressure?
23. How is estimation of the cardiac output accomplished through the use of a pulmonary artery catheter?
24. What are some potential complications of pulmonary artery catheterization?
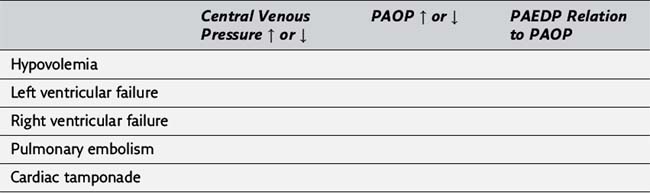
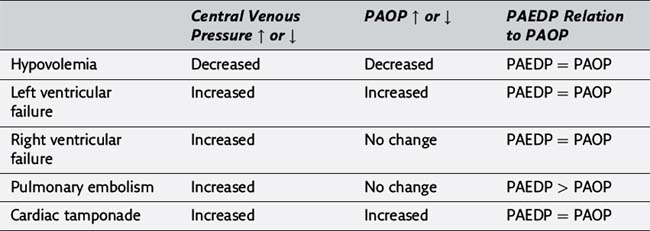
25. Please complete the following table illustrating the usefulness of central venous catheters and pulmonary artery catheters in the evaluation of various hemodynamic disorders (PAEDP, pulmonary artery end-diastolic pressure; PAOP, pulmonary artery occlusion pressure).
Echocardiographic Monitoring
26. What is some information that can be derived intraoperatively through the use of a transesophageal echocardiogram?
27. What is the difference between M-mode and B-mode echocardiography?
28. When would one use continuous-wave Doppler as opposed to using pulsed-wave Doppler? What is the Nyquist limit?
Pulse Oximetry Monitoring
29. How does a pulse oximeter work?
30. Enumerate five factors that influence the accuracy of pulse oximetry.
31. Is the SpO2 read by the pulse oximeter falsely high or falsely low in the presence of carboxyhemoglobin?
32. What is the SpO2 read by the pulse oximeter in the presence of methemoglobinemia?
33. What is the SpO2 read by the pulse oximeter in the presence of intravenous dyes?
Electrophysiologic Monitoring
34. What is an evoked potential? What are some evoked potentials that can be monitored?
35. What are some intraoperative uses of evoked potentials? What is the most common procedure for which evoked potentials are monitored intraoperatively?
36. How do evoked potentials appear when the patient is under general anesthesia? What is the potential problem with this?
37. What are some factors that may limit the usefulness of evoked potentials in the intraoperative period?
38. The integrity of which spinal cord neurologic tissue is monitored by somatosensory evoked potentials and motor evoked potentials?
Capnography Monitoring
40. Please refer to Figure 20-10. What portion of the ventilatory cycle is represented by each letter in the figure?
41. What does the absence of carbon dioxide in a patient’s exhaled gases indicate during endotracheal intubation? What does the absence of carbon dioxide in a person’s exhaled gases indicate after proper and confirmed endotracheal intubation?
42. What are some possible causes of a decrease in the concentration of carbon dioxide in a patient’s exhaled gases?
43. What are some possible causes of an increase in the concentration of carbon dioxide in a patient’s exhaled gases?
44. How does the end-tidal carbon dioxide concentration measured on a capnogram compare with the arterial carbon dioxide concentration? Why?
Electroencephalographic Monitoring
45. What are some intraoperative uses of an electroencephalogram?
46. What factors may influence the tracings obtained by an intraoperative electroencephalogram?
47. What is the bispectral index monitor?
48. What are some potential clinical uses of a bispectral index monitor? What are its limitations?
Inhaled Gas Monitoring
52. What are some methods by which the exhaled concentrations of multiple gases, including respiratory and anesthetic gases, may be measured?
53. What are some advantages and disadvantages of mass spectrometry techniques for measuring a patient’s exhaled gases?
54. What are some advantages and disadvantages of Raman spectrometry techniques for measuring a patient’s exhaled gases?
Answers*
1. The primary purpose of intraoperative patient monitoring is to gather data regarding the physiologic status of the patient. Monitoring provides the anesthesiologist with the information to respond appropriately to any salutary or adverse physiologic changes. In addition, the patient’s response to the therapeutic interventions can be assessed. (320)
2. The American Society of Anesthesiologists has mandated that qualified anesthesia personnel shall be present in the room to administer anesthesia and monitor the patient throughout the conduct of all general anesthetics, regional anesthetics, and monitored anesthesia care. The standard adopted by the American Society of Anesthesiologists is that during all anesthetics the patient’s oxygenation, ventilation, circulation, and temperature shall be continually evaluated. The full description of these standards (Appendix B) also provides an explanation of each of these objectives and specific methods by which they can be achieved. In brief, the use of pulse oximetry, capnography, an oxygen analyzer, a disconnect alarm, and a visual display of the electrocardiogram are all addressed. In addition, the blood pressure and heart rate are to be evaluated at least every 5 minutes during the course of anesthesia. (320)
Electrocardiographic monitoring
3. Potential intraoperative problems such as cardiac dysrhythmias, myocardial ischemia, and electrolyte abnormalities may all be detected through the use of an electrocardiogram. (321)
4. Lead II provides for the best visualization of the P wave on the electrocardiogram, making it the best lead for the detection of cardiac dysrhythmias on a continuous tracing. (321)
5. Lead II on the electrocardiogram provides for the best detection of inferior wall myocardial ischemia on a continuous tracing. The V5 precordial lead on the electrocardiogram provides for the best detection of anterior or lateral wall myocardial ischemia. (321)
Systemic blood pressure monitoring
6. Automated oscillometric blood pressure monitoring devices work by inflating a pneumatic cuff encircling a limb until arterial blood flow through the limb is occluded. The cuff is then deflated until pressure oscillations are detected. The pressure at which oscillations are initially detected is considered to be the systolic blood pressure. The cuff continues to deflate and the oscillations increase for a time and then begin to decrease. The diastolic pressure is defined as the point at which further deflation of the cuff provides no further evidence of pressure oscillations. The most reliable blood pressure parameter measured by this noninvasive blood pressure monitoring device is the mean arterial blood pressure. (321-322, Figure 20-3)
7. The appropriate cuff size for use with a noninvasive blood pressure measuring device is one whose width is about 40% of the circumference of the patient’s limb. (322)
8. When using an automated oscillometric blood pressure measuring device, the blood pressure will be falsely high when the blood pressure cuff is too small. Conversely, the blood pressure will be falsely low when the blood pressure cuff is too large. (322)
9. Cycling an automated oscillometric blood pressure measuring device too frequently can result in limited perfusion to the extremity distal to the cuff. Complications such as edema, nerve paresthesia, superficial thrombophlebitis, and compartment syndrome have all been reported as a result of noninvasive blood pressure devices that have been repeatedly cycled. These complications are rare. (321)
10. Possible indications for intraarterial blood pressure monitoring include the need for continuous blood pressure monitoring, access for frequent arterial blood gas samplings, need for monitoring intentional pharmacologic cardiovascular manipulation, and failure of indirect blood pressure measurement. (322)
11. Arteries that may be used for intraarterial blood pressure monitoring include the radial, ulnar, brachial, axillary, femoral, dorsalis pedis, and the superficial temporal arteries. Of these, the radial artery is the most frequently used artery for cannulation. (323, Table 20-3)
12. The waveform from an intraarterial catheter changes progressively with increasing distance from the heart. The waveform peak is higher and the trough lower at more distal arterial sites. The mean arterial pressure, however, remains approximately the same. (323, Figure 20-4)
Central venous pressure monitoring
13. Indications for the placement of a central venous catheter include the measurement of central venous pressures, access through which to provide long-term intravenous feedings, access for the administration of large volumes of fluids, intravascular access when no peripheral access is available, the administration of vasoactive or caustic drugs, to initiate transvenous cardiac pacing, for temporary hemodialysis, and for the aspiration of air emboli. (324)
14. Veins that are cannulated for central venous access include the internal jugular, subclavian, femoral, and antecubital veins. Potential complications of cannulation of the central veins include arterial puncture, hematoma, hemothorax, pneumothorax, nerve injury, emboli, cardiac dysrhythmias, thrombosis, and infection. Accidental arterial puncture while attempting cannulation of the jugular vein can result in the need to surgically explore and repair the artery. A pneumothorax occurs more frequently after placement of a subclavian catheter. This is the basis for the recommendation that a chest radiograph be done after failed subclavian catheterization and before attempting catheterization on the other side. (323, Table 20-4)
15. The right internal jugular vein is preferred over the left jugular vein for cannulation because of its short, straight, valveless route to the superior vena cava. (323)
16. Advantages of cannulation of the internal jugular vein include its predictable anatomic location with palpable landmarks, its location at the head of the patient’s bed allowing the anesthesiologist easy access to the catheter intraoperatively, and the relatively decreased complications associated with cannulation of this central vein. Disadvantages of cannulation of the internal jugular vein include the potential for puncture of the carotid artery and pleural cavity and trauma to the brachial plexus. (323)
17. Advantages of cannulation of the subclavian vein include its landmarks, its capacity to remain patent despite hypovolemia, easier nursing care, and the relative increase in patient comfort associated with cannulation of this central vein. Disadvantages of cannulation of the subclavian vein include the potential for puncture of the subclavian artery and pleural cavity and for thoracic duct damage on the left. (323)
18. The central venous pressure waveform has a typical trace in a normally functioning heart. The a wave correlates with atrial contraction, the c wave correlates with closure of the tricuspid valve and its bulging into the right atrium, and the v wave correlates with blood accumulation in the vena cava and right atrium against a closed tricuspid valve. The x descent correlates with atrial relaxation, and the y descent correlates with opening of the tricuspid valve and right ventricular filling. (324, Figure 20-5)
19. The central venous pressure parallels right atrial pressure in a patient with normal cardiovascular physiology. In these patients, the central venous pressure can be used to estimate the patient’s intravascular fluid volume status. (324)
20. The central venous pressure does not estimate the patient’s intravascular fluid volume status in the face of right-sided heart dysfunction, left ventricular dysfunction, or pulmonary hypertension. Under these conditions, a pulmonary artery catheter may be used for cardiovascular monitoring. (324)
Pulmonary artery catheter monitoring
21. Possible indications for the placement of a pulmonary artery catheter perioperatively include poor left ventricular function, valvular heart disease, recent myocardial infarction, adult respiratory distress syndrome or any pulmonary vascular disease process, massive trauma, and major vascular surgery. In general, the pulmonary artery catheter allows for more accurate assessment of cardiac filling pressure than a central venous monitor in the presence of pulmonary vascular disease, left-sided heart dysfunction, or potential left-sided heart dysfunction due to myocardial ischemia. The pulmonary artery catheter also measures cardiac output and calculates systemic and pulmonary vascular resistance. (324, Table 20-5)
22. The pulmonary capillary wedge pressure is a reflection of left atrial pressure. The pulmonary artery diastolic pressure may be used as an approximation of left atrial pressure in lieu of the pulmonary artery wedge pressure. This allows for continuous monitoring. The pulmonary artery diastolic pressure does not accurately reflect left atrial pressure in conditions in which pulmonary vascular resistance is increased, as with hypoxia, hypercarbia, hypothermia, and various forms of pulmonary disease. (324, Figure 20-6)
23. Cardiac output can be estimated through the use of a pulmonary artery catheter via the thermodilution method. To do this, cold saline is rapidly injected through the proximal central venous port. A thermistor located at the distal end of the pulmonary artery catheter senses the change in temperature. Because blood flow is the source of dilution of temperature, the flow, or cardiac output, can be calculated. It is the right ventricular cardiac output that is actually measured by this technique, whereas left ventricular cardiac output can only be estimated based on the results. (325)
24. Potential complications of pulmonary artery catheterization include pulmonary ischemia or infarction from prolonged wedging of the catheter, cardiac dysrhythmias, infection, catheter knotting, and, rarely, pulmonary artery rupture. (324)
Echocardiographic monitoring
26. Intraoperative cardiac imaging with a transesophageal echocardiogram is now widely accepted as a monitor for cardiac function during surgery, especially cardiac surgery. Information that can be derived from an intraoperative echocardiogram includes regional ventricular and atrial wall motion, ejection fraction, cardiac valve function, the presence of intracardiac air, and the effects of surgery and anesthesia on cardiac function. The use of a transesophageal echocardiogram requires advanced technical training. Complications associated with the use of transesophageal echocardiography include pharyngeal and esophageal injury and bleeding, but these occurrences are rare. (326, Table 20-7)
27. M-mode echocardiography provides a unidimensional view of the myocardium, while B-mode echocardiography provides a two-dimensional image of the myocardium. The M-mode is most useful for determining velocities, while the B-mode is most useful for evaluating changes in myocardium function. (326)
28. One would use continuous–wave Doppler when measuring velocities with high Doppler shifts. Pulsed-wave Doppler can only be used when the velocities measured are relatively slow. However an advantage is that the location of the moving object is also measurable. In pulsed-wave Doppler echocardiography, the maximal Doppler shift measurable by echocardiography is limited to half the pulse repetition frequency, also called the Nyquist limit. (326)
Pulse oximetry monitoring
29. A pulse oximeter works by emitting a light through a diode and sensing the light, usually on the opposite side of a digit. The wavelength of light that is absorbed by oxyhemoglobin relative to reduced hemoglobin in the pulsatile (and therefore arterial) vessel allows the device to calculate the saturation of oxygen in the peripheral artery. (327, Figure 20-8)
30. Factors that influence the accuracy of pulse oximetry include low flow conditions, motion artifact, nail polish, ambient light interference, dysfunctional hemoglobins, methylene blue, and a shift in the oxyhemoglobin dissociation curve. (327, Table 20-8)
31. The SpO2 read by the pulse oximeter in the presence of carboxyhemoglobin is falsely high. This occurs because carboxyhemoglobin has an absorbance of light that is markedly similar to oxyhemoglobin. (327)
32. The SpO2 read by the pulse oximeter in the presence of methemoglobinemia approaches 85% regardless of the true arterial hemoglobin oxygen saturation. (327)
33. The SpO2 read by the pulse oximeter in the presence of intravenous dyes (methylene blue, indigo carmine) will be artificially low regardless of the true arterial hemoglobin oxygen saturation. (326)
Electrophysiologic monitoring
34. An evoked potential is a measured low amplitude signal from the central nervous system that occurs in response to sensory or motor nerve stimulation. Evoked potentials that can be monitored include visual, auditory, sensory, and motor. (328)
35. Evoked potentials can be used intraoperatively to assess the integrity of the neural pathways during anesthesia. The most common evoked potentials monitored intraoperatively are somatosensory evoked potentials from the spinal cord during surgery on the spinal cord or vertebral column. (328)
36. Evoked potentials may undergo changes in the latency period and amplitude while patients are under general anesthesia. These changes are similar to the changes that are seen with neural ischemia, which can complicate interpretation of the evoked potential values. Limiting the minimum alveolar concentration (MAC) of volatile anesthetics to 0.5 to 0.75 facilitates monitoring of evoked potentials. Opioids and propofol have less of an effect on evoked potentials, and muscle relaxants do not affect somatosensory evoked potentials at all. (328)
37. Factors that may limit the intraoperative usefulness of evoked potentials because of their influence on the results include age and gender of the patient, arterial blood gas tensions, and body temperature. In addition, the cost and complexity of performing evoked potentials may limit their use. (328)
38. Somatosensory evoked potentials of the lower extremities monitor the integrity of the dorsal column of the spinal cord. Motor evoked potentials monitor the corticospinal tract. Unlike somatosensory evoked potentials, motor evoked potentials are sensitive to muscle relaxants. (328)
Capnography monitoring
39. A capnograph is a waveform display that illustrates the patient’s inhaled and exhaled concentrations of carbon dioxide. (328-329)
40. In the capnogram, the point A designates the exhalation of anatomic dead space gas just before the exhalation of alveolar gas. Point B designates the beginning of exhalation of alveolar gas that contains carbon dioxide. Phase C-D designates the exhalation of alveolar gas, while point D designates the end-tidal carbon dioxide concentration. Phase D-E designates the beginning of inspiration and the entrainment of inspired gases. (329, Figure 20-10)
41. The absence of carbon dioxide in a patient’s exhaled gases just after attempted endotracheal intubation with properly functioning equipment provides evidence that the patient’s lungs are not being ventilated. That is, the endotracheal tube may not be in the trachea. The absence of carbon dioxide in a patient’s exhaled gases after intubation of the trachea has been confirmed may indicate that there is either a malfunction of equipment, a malfunction in the interface between the patient and the equipment (as in disconnection from the anesthesia circuit), movement or dislodgment of the endotracheal tube from its previously proper position, or a physiologic patient problem such as a cardiac arrest. (328)
42. Possible causes of a decrease in the patient’s exhaled concentration of carbon dioxide include hyperventilation, hypothermia, low cardiac output, pulmonary embolism, accidental disconnection, tracheal extubation, or cardiac arrest. (328, Table 20-9)
43. Possible causes of an increase in the patient’s exhaled concentration of carbon dioxide include hypoventilation, hyperthermia, sepsis, rebreathing, the administration of bicarbonate, and the insufflation of carbon dioxide during laparoscopy. (328, Table 20-9)
44. The end-tidal carbon dioxide concentration measured on a capnogram is less than the true arterial concentration of carbon dioxide, typically by a 2- to 5-mm Hg gradient. This occurs as a result of the alveolar-to-arterial difference for carbon dioxide concentrations secondary to dead space ventilation. (328)
Electroencephalographic monitoring
45. Intraoperative uses of an electroencephalogram include monitoring for cerebral ischemia and monitoring the depth of anesthesia. (329)
46. Among the factors that influence the tracings obtained by an electroencephalogram and limit its usefulness intraoperatively are anesthetics, changes in body temperature, and alterations in the arterial carbon dioxide concentration. (329)
47. The bispectral index monitor performs a bispectral analysis of the electroencephalogram and provides the clinician with a processed evaluation of its analysis through its display of a number between 0 to 100. The analysis is done through superficial scalp electrodes typically on the forehead of the patient. The number provided by the bispectral index monitor reflects the state of wakefulness of the central nervous system. (329, Figure 20-11)
48. The bispectral index monitor may be used clinically to predict loss of consciousness and lack of recall during anesthesia. A bispectral index numerical value of 0 is consistent with an isoelectric encephalogram. A numerical value of 60 or less corresponds to a low probability of recall or awareness. Thus the use of the bispectral index monitor for the titration of medicines to achieve adequate but not excessive loss of consciousness may result in more rapid awakening at the end of the procedure. The bispectral index has not been shown to be well correlated with the hemodynamic or movement responses to noxious stimuli. In addition, a recent study has shown that the use of a bispectral index monitor showed no decrease in the incidence of awareness when using volatile anesthetics. (329)
Temperature monitoring
49. Patients will typically have a passive decrease in body temperature by 1° C to 4° C during anesthesia. Intraoperative passive cooling occurs because of anesthesia-induced vasodilation, environmental temperature, and surgical exposure. The mechanism for core heat loss is through redistribution, and for peripheral heat loss is through radiation, convection, conduction, and evaporation. (330)
50. Sites for body temperature monitoring include the esophagus, nasopharynx, rectum, bladder, and tympanic membrane. Axillary and skin temperature monitors are less reliable. (330)
51. Maintaining intraoperative normothermia may decrease risks associated with hypothermia, namely coagulopathies, impaired wound healing, and potentially increased myocardial oxygen requirements as from shivering. (330)
Inhaled gas monitoring
52. Multiple gas analysis can be achieved by infrared absorption, mass spectrometry, and Raman spectroscopy. (330)
53. Advantages of mass spectrometry techniques for measuring a patient’s exhaled gases are that it can measure the gases continuously, it can measure all gases including inhaled anesthetics, oxygen, and nitrogen, and it can measure the inspired gas concentrations as well as the exhaled concentrations. A disadvantage of the mass spectrometry technique is that it has traditionally used large and expensive monitors. (330)
54. Advantages of Raman spectrometry techniques for measuring a patient’s exhaled gases are that they can measure all gases including inhaled anesthetics, oxygen, and nitrogen and they do not alter the gas molecule so that it can be returned to the anesthetic delivery system. A disadvantage of Raman spectrometry techniques is that they require a very high intensity light source to work, such as a laser. (330)