CHAPTER 20 Anesthetic and Intensive Care Management of the Patient with a Meningioma
ANESTHETIC CONSIDERATIONS
Preoperative Evaluation and Preparation
When intracranial procedures are planned, the preoperative evaluation must address various physiologic aspects of the patient’s condition. Neurologically there must be documentation of the patient’s level of consciousness, cranial nerve function, neurologic deficits, and the presence or absence of elevated intracranial pressure (ICP). Patients with a history of hypertension, cardiovascular disease, cerebral vascular insufficiency, or previous carotid endarterectomy may have altered levels of cerebral autoregulation, impaired cerebral perfusion, or abnormal baroreceptor function.1–3 Intravascular volume depletion may be a result of diminished oral intake from nausea and vomiting, preoperative osmotic diuresis, and even the use of intravenous contrast administration for diagnostic studies.
Choice of Patient Position
Complications related to maintaining abnormal positions may occur. Extreme care is required regarding prophylactic padding. Hyperflexion–extension of the head is to be avoided. Head flexion can result in oropharyngeal complications. Compression by artificial airways and endotracheal tubes can occur.4 Leg pneumatic venous compression devices are used to relieve the incidence of deep venous thrombosis (DVT) from prolonged immobilization. This is true for all neurosurgical interventions, but is particularly important in patients with meningiomas, as these patients often present with a hypercoagulable state and are prone to the development of DVT.
Prone
A dreaded complication of the prone position is retinal ischemia and blindness. The mechanism is unknown, although orbital compression, low arterial blood pressure, and poor venous drainage have been suggested. Prolonged procedures greater than 7 hours seem to be an important factor.5
Lateral
The lateral position can be an alternative to the sitting or prone position. A vacuum mattress conforms to the patient’s anatomy. The patient must not move after head fixation. This can cause strain in the cervical area.6 The lateral position is used particularly for approaches to the cerebellopontine angle, and for skull base approaches to the foramen magnum, petrous bone, and petroclival junction. It is the most complex positioning and requires great attention to detail to prevent axillary compression, brachial plexus stretch injuries, vascular compromise, and adequacy of monitoring placement.
Sitting
The term sitting is a misnomer. The patient is in a modified recumbent position, as shown in Figure 20-1A and B. The legs are high to promote venous return and to elevate central venous pressure (CVP). This enhances circulatory stability and may reduce the chance for air embolism. Modifications to the sitting position permit lowering of the head without taking the patient out of the head holder. This is important if venous air embolus (VAE) is suspected because it allows rapid lowering of the head in a critical situation.
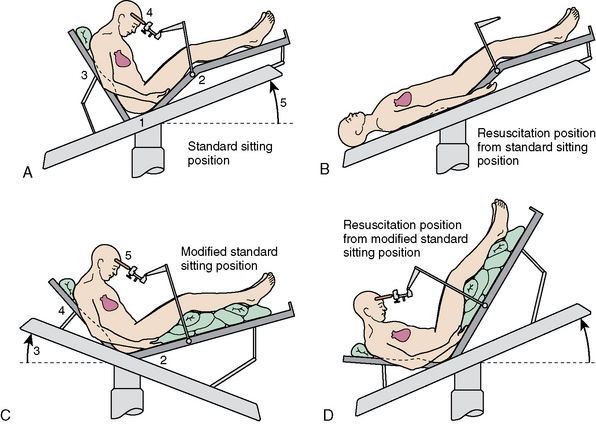
FIGURE 20-1 A–D, Variations of sitting position.
(From Miller RD, ed [2004]. Anesthesia, 4th ed, chapter 56, p. 1900, figure 56-1. Philadelphia: Churchill Livingstone, reproduced with permission.)
Head flexion is required to improve access to posterior structures. A two-fingerbreadth distance between the chin and the chest must be maintained to prevent compression ischemia. The bite block or oral airway must be positioned to prevent pressure on the base of the tongue. Large increases in ICP can occur with extreme head flexion and rotation or the addition of the positive end-expiratory pressure (PEEP).7 Head flexion may also result in downward migration of the endotracheal tube down the right mainstream bronchus. The positions shown in Figure 20-1C and D permit surgery to continue with the head to heart venous pressure gradient dimensions. Only the position shown in Figure 20-1D is compatible with closed-chest cardiac massage.
The sitting position for neurosurgery remains controversial. Its use has been diminishing because of potential serious complications, although many surgeons believe it is highly advantageous. The sitting position may be used because it provides better access to midline lesions, improved cerebral venous decompression, lower ICP, and gravity drainage of blood and CSF. The latter minimizes the need for exhaustive cautery with improved preservation of arachnoid plains. The sitting position also facilitates direct observation of facial musculature as a means of determining irritation of the facial nerve. Intraoperative facial electromyography improves direct observation by providing a continuous and more sensitive monitor of facial nerve functions.1,8
Complications related to the use of the sitting position include venous air embolism (VAE), paradoxical VAE, circulatory instability, pneumocephalus, subdural hematoma, compressive peripheral neuropathy, quadriplegia, and skin compressive lesions.9 The severity of these complications have made most neurosurgeons in the United States abandon the position, while it is still used extensively in other parts of the world, particularly in Europe. Mild transient postural hypotension (–20 to –30 mm Hg) occurs in about one third of anesthetized patients placed in the sitting position. Marked hypotension (–50% of supine values) occurs in 2% to 5% of cases.10,11 Patients with heart failure or severe coronary/or cerebral occlusive vascular disease are a relative contraindication to the sitting position.
Retrospective reviews of surgeons who performed procedures in the sitting and various horizontal positions concluded that each position has its own benefits and risks. They found no support of increased morbidity or mortality with either position.12,13 In one series, although VAE occurred more than three times as often in seated patients as in the horizontally positioned patients, no increased clinical complication rate was found. Seated patients lost less blood and required fewer blood transfusions when compared with supine, prone, and lateral patients.13
Venous Air Embolism
Venous air entrainment (VAE) results from both an open vein and negative intravenous pressure relative to atmospheric pressure. It occurs when the head is positioned above the heart to encourage cerebral venous drainage. One study found VAE incidence rates of 25%, 18%, 15%, and 10% associated with sitting, lateral, supine, and prone positions, respectively. Low CVP and poor surgical techniques increase VAE incidence. With the use of a precordial Doppler monitor, the VAE incidence ranges form 25% to 50% during suboccipital craniotomy.14–16
The highest risk portion of the operation for VAE is during skin–muscle incisions and when bone venous sinusoids are exposed during the dissection.9 Highly vascular lesions also predispose to VAE. VAE is most severe when a dural sinus is open, and therefore skull-base approaches, with their exposure of multiple venous sinuses, are particularly prone to VAE. It may also occur from headholder pins, burr holder, and connections in venous catheter systems.9
The clinical significance of VAE is influenced by several factors, including the volume of intravascular gas, its rate of entrainment, the presence of a patent foramen ovale, elevated right heart pressure, the presence of nitrous oxide, anesthetic depression of cardiovascular function, and the patient’s cardiopulmonary compensatory capacity. Small bubbles of air entrained slowly are of little physiologic significance. The venous gas bubbles are removed by the lungs at a rate that depends mainly on a compensatory rise in pulmonary artery pressure (PAP).17,18 The PAP plateaus as the rate of venous entrainment of the gas equals the rate of its pulmonary excretion. At this point equilibrium occurs. If this excretion capacity overloads it leads to further PAP increases, pulmonary shunting, and reduced cardiac output and circulatory collapse. VAE-reduced cardiac output and/or increased dead space leads to a decrease in end-expired carbon dioxide, making end-tidal carbon dioxide monitoring extremely useful in this setting. A fall in end-tidal CO2 is the first sign of a VAE. Mild carbon dioxide retention occurs as dead space increases in embolized regions. The diagnosis of VAE may be confirmed by a blood gas measurement. A large end-tidal carbon dioxide to PaCO2 gradient in the absence of a recent change in controlled ventilation results. A late occurrence during an air embolism is hypoxemia, which occurs as a result of the shunting of pulmonary blood flow.18
Chronic postoperative perfusion deficits associated with increased pulmonary vascular permeability have been seen with small amounts of air over a prolonged period.19,20 Acute respiratory distress syndrome may result.
Paradoxical air embolism is another serious complication of VAE. With a patent foramen ovale, intravenous gas may pose to the left side of the heart and lodge in the brain, heart, or other vital organs. A patent foramen ovale and elevated right heart pressure occurs in about 5% to 10% of adult neurosurgical patients.21 A transesophageal echocardiogram (TEE) can be used to detect the presence of left-sided aerated saline.22 The operative TEE, however, is of limited value and can only provide guidance of a positive test result. In the presence of a known patent foramen ovale, a position other than seated should be used. PAP monitoring can detect a right-to-left arterial pressure gradient. It may reveal a paradoxical VAE.23 Volume loading to elevate left atrial pressure or lowering the table can be helpful. PEEP elevates cerebral venous pressure and may reduce the potential for VAE, but its use remains controversial. Its ability to reverse the normal PAP to left atrial pressure gradient remains unclear.24
When nitrous oxide is used as part of the anesthetic technique, the volume of entrained intravascular gas is increased.22 Nitrous oxide is 34 times more soluble in blood than nitrogen. It rapidly diffuses into an intravascular air bubble. It has been suggested that reducing the inspired concentration of nitrous oxide enhances safety.25 A clinical study comparing 50% nitrous oxide with nitrogen showed that the use of 50% nitrous oxide has no effect on clinical outcome in the presence of minor episodes of VAE.16
The surgeon should be informed and the prevention of additional air entry should be prevented. The wound should be packed, and cerebral venous pressure should be increased by applying jugular venous compression or by lowering the patient’s head. Pharmacologic cardiovascular support should be used if deterioration occurs and the patient should be placed supine and resuscitation should proceed. The left lateral decubitus position may remove air from the pulmonary artery back into the right ventricle.5,26
The sitting position promotes CSF drainage form the intracranial space with resultant air entry and consequent pneumocephalus. Some degrees of pneumocephalus can occur in any craniotomy, but is accentuated in procedures performed in the sitting position.27 Air entry is further facilitated by surgical decompression, diuretics, and hyperventilation. Air can remain trapped inside the skull when the dura is closed and can produce a mass effect. This mass effect may be asymptomatic or may be associated with headache, confusion, impaired memory, and lethargy. The symptoms usually resolve over 4 days.9 Breathing 100% oxygen will hasten reabsorption of the pneumocephalus.
Cervical spinal cord ischemia due to neck flexion can result in quadriplegia.28 Hypotension in the sitting position could potentiate this injury. Patient should be questioned perioperatively about upper extremity paresthesia related to neck position. Vigilance should he exerted to avoid extreme neck flexion, which in addition to cord ischemia can obstruct venous return resulting in marked swellings of the head and tongue. Small oral airway and late blocks should be used to ensure jugular venous patency.9 One should consider evoked potential monitoring to detect intraoperative cervical spinal cord ischemia.
Padding should be used in dependent areas and anatomically correct positioning should be maintained. Stretching the sciatic nerve and compression ischemia of the nerves and skin should be avoided.9
Maintenance of Anesthesia
Pinaud and colleagues29 found only minor and clinically insignificant differences among propofol-fentanyl, isoflurane nitrous oxide, and fentanyl-nitrous oxide anesthetics. They concluded that overemphasis on minor ICP effects of anesthetic drugs do not warrant such caution.
Most intravenous anesthetic drugs either reduce ICP or have little effect on it, provided ventilation is controlled to prevent PaCO2 elevation. These drugs elicit a coupled reduction in cerebral metabolism and cerebral blood flow (CBF) and thereby ICP. Use of barbiturates, propofol, and etomidate are followed by a relative fall in ICP. Benzodiazepines create a moderate fall and narcotics have little to no direct ICP reducing action.29–33 Under clinical conditions, all volatile anesthetic agents have the potential to elevate CBF, CBV, and ICP. Relative potency differences with regard to ICP elevation exist: halothane >> enflurane > sevoflurane, isoflurane, desflurane.20,34
The addition of nitrous oxide to an established volatile anesthetic under normocapnic conditions result in a dose-dependent elevation in CBV and CBF. With hypocapnia, the CBF-elevating action of the addition of nitrous oxide to an established volatile anesthetic is blocked with isoflurane.35,36
A small dose of furosemide (5–10 mg) will promote diuresis of excess fluids reabsorbed from the extravascular space. Glucose-containing solutions are avoided owing to the possible detrimental effect of hyperglycemia on areas of the brain at risk for cerebral ischemia.37 Normal saline is the preferred intravenous fluid. Lactated Ringer’s may be used if the patient does not have elevated ICP and its use is limited. Approximately 180 mL of free water is produced with each liter of lactated Ringer’s. It should be remembered that the osmolarity of normal plasma is about 285 mOsmol. Lactated Ringer’s is 275 mOsmol and saline is 305 mOsmol. The use of osmotic and loop diuretics may predispose patients to electrolyte disturbance and hypotension. Intravenous colloid can be used to maintain cerebral perfusion pressure. It has minimal effect on the cerebral dehydrating effect of the diuretic.
CRITICAL CARE CONSIDERATIONS
Intracranial Venous Congestion
One of the most difficult intraoperative aspects of meningioma removal is the network of cerebral veins and venous sinuses with which the tumor is often intimately related, especially in the parasagittal location. The neurosurgical goal of complete tumor resection, especially in benign lesions such as meningiomas, often is compromised by the association of these tumors with venous channels. On occasion, either by design or through unexpected thrombosis, venous channels are compromised. If collateral drainage exists, this may transpire without consequence. If this is not the case, however, venous congestion will result with consequent parenchymal swelling,40 which at is extremes can be joined by intraparenchymal hemorrhage typical of venous infarction. Edematous cerebral parenchyma can lose function, generate seizures,41 and, if a large enough territory is involved, lead to intracranial hypertension or herniation.
Cerebral edema can also be caused directly by the meningioma itself, especially if the tumor is high grade with penetration through the pial membrane into the parenchyma. One study found a 2.9% incidence of postoperative edema caused directly by the tumor among patients who underwent surgery for a supratentorial meningioma.42
Proximity to or direct involvement of a venous sinus is an especially challenging problem, as venous congestion caused by sinus compromise is frequently severe and can have fatal consequences, especially in the region of the superior sagittal sinus. Whereas complete ligation of the anterior third of the superior sagittal sinus is acceptable, compromise of the posterior third or even the middle third is not. There is less risk if the sinus is reconstructed, but it is often best not to resect the portion of the meningioma adherent to or invading the sinus.43–46 Recent advances in radiosurgery have made it possible to treat tumor remnants in the sinus effectively. In the posterior fossa, unilateral transverse or sigmoid sinus ligation is possible if the contralateral sinus is present and test occlusion is tolerated (even if the contralateral sinus is nondominant).47
Surgery to resect a nearby meningioma can also lead to venous sinus thrombosis. The possible prothrombotic combination of flow alteration, endothelial injury, and postoperative state (i.e., Virchow’s triad) places the patient at risk for the same venous congestion complications as direct surgical compromise of a sinus or vein. Unlike direct surgical compromise, however, sinus thrombosis is potentially treatable albeit not without risk. The treatment is full anticoagulation, which is obviously relatively contraindicated in the postoperative setting. If the benefits of anticoagulation in a particular case outweigh the risks, then it should be achieved with unfractionated heparin without bolus dose. Contrary to anticoagulation with low molecular weight heparin, unfractionated heparin can be easily titrated and, more importantly, rapidly reversed with protamine. Mechanical endovenous thrombectomy has been described but these patients also received full anticoagulation.48
Seizure
Another frequent complication of meningioma surgery is seizure. Among patients undergoing resection of a supratentorial meningioma, studies have found that 36.5% to 37.3% who had preoperative seizures and 8.4% to 20.0% of those who did not, suffered postoperative seizures.49,50 Risk of postoperative seizure has been shown to be higher in patients with preoperative seizures,50,51 parietal tumor location,50 subtotal tumor resection,50 and peritumoral edema.51 As described earlier, one cause of peritumoral edema is venous compromise. Seizures are a classic symptom of a venous infarct. Overall, intraoperative manipulation and irritation of an adherent cortex is also thought to contribute to the generation of seizures after the resection of some meningiomas. Seizure prophylaxis with antiepileptic medication(s) is a common practice for high-risk or, frequently, all postoperative meningioma patients.
Systemic Venous Thromboembolism
Among complications of meningioma surgery, deep venous thrombosis (DVT) and pulmonary embolism (PE) are not uncommon. A meta-analysis found an incidence of 4.3% for DVT and 1.4% for PE without prophylaxis.52 Risk factors include older age (one study defined this as greater than 65 years old),42,53 male gender,53 and postoperative nonambulatory status.53 As the mortality of a postcraniotomy PE has been shown to be 51%,54 prophylaxis against PE and its precursor DVT is now standard of care in postoperative patients. Multiple methods of prophylaxis exist in singular or in combination and can be grouped into mechanical, low-dose unfractionated heparin, and low molecular weight heparin.
Mechanical methods of DVT/PE prophylaxis include graded compression stockings, intermittent pneumatic compression devices, early ambulation, and early physical and occupational therapy. These methods are usually well tolerated, and unlike other methods, do not carry a risk of harm to the patient. A meta-analysis of postcraniotomy patients found an incidence of 1.42% for DVT and 0.68% for PE with mechanical prophylaxis alone, rates lower than in patients without any prophylaxis.52
Low-dose unfractionated heparin (LDUH) is usually given subcutaneously at a dose of 5000 units twice daily. Most studies have shown it to effectively reduce the incidence of DVT/PE when combined with mechanical prophylaxis versus mechanical prophylaxis alone in craniotomy for tumor patients (to a symptomatic DVT/PE incidence of 0% in one randomized controlled trial [RCT]).55 Although a recent meta-analysis did not agree,54 most believe that it is effective. The use of postoperative anticoagulation in any form has in the past provoked trepidation in neurosurgeons owing to the fear of intracranial hemorrhage (ICH). The mortality of postcraniotomy ICH has been estimated to be 27.5% with a severe morbidity, defined as a permanent, serious neurologic deficit, of 36.7%.54 Numerous studies including at least one RCT, however, have found no increased risk of ICH with LDUH, leading most to accept it as a safe means of DVT/PE prophylaxis in neurosurgery.56,57
Low molecular weight heparin (LMWH), including enoxaparin and dalteparin, are given subcutaneously once daily. LMWH is favored over LDUH (both combined with mechanical prophylaxis) in other surgical subspecialties owing to its greater efficacy in preventing DVT and lower risk of causing heparin-induced thrombocytopenia.58,59 Patients appreciate the once daily dosing, but the monetary cost is higher than for LDUH. The use of LMWH versus LDUH (both combined with mechanical prophylaxis) in neurosurgery remains controversial, however, because of the results of conflicting studies regarding its safety and efficacy. One RCT of mechanical and LMWH versus mechanical alone in postoperative neurosurgical patients found a statistically significant decrease in rates of DVT with the addition of LMWH and no difference in rates of ICH.60 Two RCTs of LMWH versus LDUH (both combined with mechanical prophylaxis) showed no statistically significant difference in DVT/PE rates between the two groups.55,61 One of these two RCTs also examined ICH rates and found no statistically significant difference.61 A large meta-analysis of LMWH versus LDUH (both combined with mechanical prophylaxis), however, showed a decrease in DVT rates from 1.83% to 0.50% and a decrease in PE rates from 0.34% to 0.15% but an increase in ICH rates from 1.87% to 3.16% in the LMWH group.54 To complicate matters further, a decision analysis model taking into account the deleterious effects of potential anticoagulant side effects (i.e., ICH) concluded that overall outcomes were best with mechanical prophylaxis alone. Differences between the treatment groups, however, were modest and reached statistical significance only when comparing the LMWH group to the other groups. In addition, assumptions used in this type of analysis limit its power and generalizability.54
Hydrocephalus
The incidence of hydrocephalus after meningioma surgery is estimated to be 3.4% to 8.2%.42,62 It is more common after resections of skull base or intraventricular meningiomas and, as such, some advocate the use of a preemptive ventriculostomy for these procedures. Intraoperatively, this strategy would allow CSF drainage to create more working room during skull base meningioma resections63 and would allow the drainage of any intraventricular hemorrhage or debris after resection of an intraventricular meningioma.64 Postoperatively, this group of patients can potentially demonstrate a prolonged emergence from anesthesia commensurate with an often prolonged operative duration. A ventriculostomy in these patients would allow intracranial pressure measurement during a prolonged emergence period to rule out hydrocephalus as the cause. It would also allow rapid treatment of hydrocephalus should it occur in this group of patients who are prone to its development.
Hydrocephalus can also lead to wound complications. In a patient who develops a pseudomeningocele, one must consider the possibility of underlying hydrocephalus. Moreover, the incidence of pseudomeningocele has been shown to be increased among patients with preoperative hydrocephalus.63 Postoperative hydrocephalus can also increase the risk for CSF leak.
CSF Leak
CSF leak after meningioma surgery is, not surprisingly, most common after resection of a skull-base lesion. The reported incidence varies, but one study found that 17% of 257 skull-base tumor resections (mostly meningiomas) were complicated by a CSF leak.62 The diagnosis is often obvious but in cases of uncertainty one can assay for beta-2-transferrin in the leaking fluid, as this protein is found only in CSF.65
CSF leak is an emergency due the risk of ascending meningitis66,67 and tension pneumocephalus.68 If the leak is from an incision, that incision should be oversewn but that alone is not sufficient. All patients with a postoperative CSF leak should undergo a computed tomography (CT) scan to assess for the source. Fine image cuts and/or the instillation of radiopaque dye into the subarachnoid space can aid in its location. Reports of leak sources distant from the operative site must be kept in mind.69
If the CT scan reveals a repairable source or if the surgeon is not completely confident of meticulous air sinus obliteration and wound closure, the patient should return to the operating room expeditiously for an exploration and repair of potential leak sources. If appropriate to the leak source or original surgery, an endonasal endoscopic repair may be considered.70 If the CT scan does not reveal a leak source and the surgeon is completely confident of meticulous air sinus obliteration and wound closure, a trial of external lumbar drainage may be attempted to divert CSF flow away from the leak source and allow it to heal. During the trial, a 10 mL/hr drainage rate is advised as higher rates risk overdrainage (normal CSF production is approximately 20 mL/hr). We strongly recommend that the hourly 10 mL be drained using an “open/close” method rather than a continuous drip. The latter is prone to accidental catastrophic overdrainage, which can lead to devastating complications such as pneumocephalus with or without tension,71,72 extra-axial hemorrhage from sagging parenchyma and stretched blood vessels, herniation,72 and reports of temporary blindness.72 We also recommend that the duration of drainage be approximately 5 days to give the leak source an adequate chance to heal. Frequent, premature “clamping trials” or “leak challenges” should be avoided as they may cause fluid to break through sites of weak preliminary healing. It should be noted that external lumbar drainage is unlikely to heal a leak in cases where the leak was unexpected and no primary repair was attempted. In these cases, the leak will be through bony openings with no chance of secondary scarring and it is often wise to return immediately to the operating room. In cases where a leak was considered a potential problem and a primary skull base repair was attempted, then the likelihood of success with lumbar spinal drainage trail is reasonable.
If a CSF leak is accompanied by persistent hydrocephalus, a shunt should be considered. One should keep in mind the possibility of pneumocephalus (with a risk of tension) from flow reversal through an unhealed leak source after shunting. Among postoperative skull-base tumor (mostly meningioma) patients with a CSF leak, 23% ultimately required shunting in one retrospective study.62
The use of prophylactic antibiotics to prevent meningitis when a CSF leak is present is debated; however, the literature regarding posttraumatic CSF leaks seems to suggest that their use does decrease meningitis rates, especially if the leak is present for greater than 7 days.73,74
In an attempt to decrease rates of CSF leak after skull-base tumor resection, some neurosurgeons begin CSF diversion preoperatively. One retrospective review of skull-base tumor resections found a statistically significant decrease in postoperative CSF leak rate when a lumbar drain was placed preoperatively.75 A review of cerebellopontine angle tumors revealed a trend toward increased rates of CSF otorrhea or rhinorrhea if hydrocephalus was present preoperatively. The authors suggested that the preoperative placement of a ventriculostomy may aid in leak prevention in these patients.63
Pneumocephalus
Pneumocephalus following craniotomy for meningioma can be troublesome to the patient, as it can cause headache, nausea, and vomiting, as we mentioned earlier in this chapter. It can also be troublesome to the surgeon because there is the risk of progressive mass effect from tension development (the accumulation of intracranial air under pressure through an entry site acting as a one-way valve). Most postoperative pneumocephalus is caused by ambient air entering the craniotomy at the time of surgery and is usually not under tension. In an attempt to reduce the volume of postoperative pneumocephalus, the subarachnoid space is routinely filled with saline just before final dural closure. One group has attempted to augment this technique using carbon dioxide, which is heavier and more readily absorbed than air. Their technique consisted of a sterile cannula that delivered carbon dioxide gas to the surgical field at a rate of 2 L/min during the intradural portion of the operation. The subarachnoid space was filled with saline just before final dural closure per routine. In their randomized population of 40 patients with intraventricular or paraventricular tumors (20 experimental, 20 controls) they found the technique to be safe. They also concluded that the technique was associated with statistically significant decreases in postoperative intraventricular gas volume, time to complete resolution of gas, incidence of postoperative emesis, and duration of postoperative emesis.76 This type of routine postoperative pneumocephalus can be treated with normobaric 100% oxygen via a non-rebreather facemask (or endotracheal tube if already intubated) to increase the nitrogen gradient that facilitates air absorption.77
More worrisome causes of postoperative pneumocephalus are a CSF leak or overdrainage of an external lumbar drain because they can both lead to tension development. This is especially true for perinasal CSF leak sources that are susceptible to maneuvers that increase airway pressure (e.g., coughing, sneezing, nose blowing, Valsalva).78
Hematoma
In a population operated on for a supratentorial meningioma, the incidence of postoperative hematoma has been estimated to be 2.5% among 19- to 64-year-old patients and 7.4% among 65- to 84-year-old patients.42 Another study reported 21 postoperative hematomas requiring surgical evacuation in a population of 296 intracranial meningioma patients (7.1%). There was an increased risk among older patients in this study as well.79 Intra-axial hematomas are likely caused by intraoperative brain manipulation, inadequate hemostasis, delayed vessel rupture, and of great significance in the meningioma population, venous infarction with secondary hemorrhage. In the case of intra-axial hematomas associated with venous infarction, all attempts should be made to treat the problem medically, as the tissue involved with the hematoma has significant capacity for recovery once the swelling and hematoma subside.
Rare Conditions
A few conditions at the case report level merit mentioning because they can be diagnostically challenging. There has been a case report of adrenal apoplexy following craniotomy for meningioma resection.80 This is extremely rare, but can cause significant complications and be both easily treated and easily misdiagnosed. Risk factors include sepsis, hypotension, use of anticoagulants, administration of adrenocorticotropic hormone (ACTH), and long-term corticosteroid use. Treatment is with steroid replacement, but some patients will require adrenalectomy. There has also been a case series reporting that psychogenic pseudoseizure may develop after craniotomy for a nonepilepsy indication.81 This may be considered as a diagnosis of exclusion in a patient whose extensive seizure workup is negative.
Specific Skull Base Sites
Certain skull-base operative sites are associated with a specific but nonexclusive cluster of potential complications. A primary risk of anterior fossa meningioma resection (e.g., anterior falcine, olfactory groove, tuberculum sella) is involvement of the nearby air sinuses without adequate postoperative obliteration and seal. This can lead to CSF rhinorrhea, ascending meningitis, or tension pneumocephalus. As maneuvers that increase airway pressure (e.g., coughing, sneezing, nose blowing, Valsalva) can contribute to tension development, some recommend prophylactic tracheostomy for those with significant bony defects between the sinonasal cavity and anterior fossa.78 Others, however, believe that prophylactic tracheostomy is unnecessary.82
Cavernous sinus compromise can cause orbital venous stasis with cavernous sinus syndromes and ophthalmoplegia. In the case of a meningioma with cavernous sinus involvement, most now recommend not resecting the intracavernous portion because of the high risk of complications and little benefit to tumor control.83 This can be followed by radiosurgery to the region of residual tumor. In our opinion, a strategic approach to these lesions should be carried out preoperatively where a clear surgical plan for complete resection of all tumor outside the cavernous sinus, and specifically in the superior portion of the sinus should be performed. This surgical resection should clear the inferior portion of the optic apparatus from tumor by at least 1 cm so that postoperative radiation can be given with a high enough dose for tumor control, while still allowing the optic system to receive minimal radiation, ideally less than 9 Gy.
[1] Niparko J.K., Kileny P.R., Kemink J.L., Lee H.M., Graham M.D. Neurophysiologic intraoperative monitoring: II. Facial nerve function. Am J Otol. 1989;10:55-61.
[2] Bristow J.D., Gribbin B., Honour A.J., Pickering G.W., Sleight P. Diminished baroreflex sensitivity in high blood pressure and ageing man. J Physiol. 1969;202:45P-46P.
[3] Wade J.G., Larson C.P., Hickey R.F., Ehrenfeld W.K., Severinghaus J.W. Effect of carotid endarterectomy on carotid chemoreceptor and baroreceptor function in man. N Engl J Med. 1970;282:823-829.
[4] Teeple E., Maroon J., Rueger R. Hemimacroglossia and unilateral ischemic necrosis of the tongue in a long-duration neurosurgical procedure. Anesthesiology. 1986;64:845-846.
[5] ASA Task Force on Perioperative Blindness. Practice Advisory for Perioperative Visual Loss Associated with Spine Surgery. Anesthesiology. 2009;104(6):1319-1328.
[6] Anderson J.M., Keen R.I., Neave R. Positioning the Surgical Patient. Boston: Butterworths, 1988.
[7] Lodrini S., Montolivo M., Pluchino F., Borroni V. Positive end-expiratory pressure in supine and sitting positions: its effects on intrathoracic and intracranial pressures. Neurosurgery. 1989;24:873-877.
[8] Grimaldi M., Dall’Olio D. Electrophysiologic monitoring of facial nerve during otoneurosurgery. Acta Otorhinolaryngol Ital. 1990;10:593-606.
[9] Standefer M., Bay J.W., Trusso R. The sitting position in neurosurgery: a retrospective analysis of 488 cases. Neurosurgery. 1984;14:649-658.
[10] Albin M.S., Babinski M., Wolf S. Cardiovascular responses to the sitting position. Br J Anaesth. 1980;52:961-962.
[11] Marshall W.K., Bedford R.F., Miller E.D. Cardiovascular responses in the seated position – impact of four anesthetic techniques. Anesth Analg. 1983;62:648-653.
[12] Matjasko J., Petrozza P., Cohen M., Steinberg P. Anesthesia and surgery in the seated position: analysis of 554 cases. Neurosurgery. 1985;17:695-702.
[13] Black S., Ockert D.B., Oliver W.C., Cucchiara R.F. Outcome following posterior fossa craniectomy in patients in the sitting or horizontal positions. Anesthesiology. 1988;69:49-56.
[14] Michenfelder J.D., Miller R.H., Gronert G.A. Evaluation of an ultrasonic device (Doppler) for the diagnosis of venous air embolism. Anesthesiology. 1972;36:164-167.
[15] Buckland R.W., Manners J.M. Venous air embolism during neurosurgery: a comparison of various methods of detection in man. Anaesthesia. 1976;31:633-643.
[16] Losasso T.J., Muzzi D.A., Dietz N.M., Cucchiara R.F. Fifty percent nitrous oxide does not increase the risk of venous air embolism in neurosurgical patients operated upon in the sitting position. Anesthesiology. 1992;77:21-30.
[17] Verstappen F.T., Bernards J.A., Kreuzer F. Effects of pulmonary gas embolism on circulation and respiration in the dog III. Excretion of venous gas bubbles by the lung. Pflugers Arch. 1977;370:67-70.
[18] Verstappen F.T., Bernards J.A., Kreuzer F. Effects of pulmonary gas embolism on circulation and respiration in the dog IV. Origin of arterial hypoxemia during pulmonary gas embolism. Pfleugers Arch. 1977;370:71-75.
[19] Flick M.R., Hoeffel I.M., Staub N.C. Superoxide dismutase with heparin prevents increased lung vascular permeability during air emboli in sheep. J Appl Physiol. 1981;55:1284-1291.
[20] Albin M.S., Bunegin L., Garcia C., McKay W. The transcranial Doppler can image microaggregates of intracranial air and particulate matter. J Neurosurg Anesth. 1989;1:134-135.
[21] Gronert G.A., Messick J.M., Cucchiara R.F., Michenfelder J.D. Paradoxical air embolism from a patent foramen ovale. Anesthesiology. 1979;50:548-549.
[22] Black S., Muzzi D.A., Nishimura R.A., Cucchiara R.F. Preoperative and intraoperative echocardiography to detect right-to-left shunt in patients undergoing neurosurgical procedures in the sitting position. Anesthesiology. 1990;72:436-438.
[23] Perkins N.A., Bedford R.F. Hemodynamic consequences of PEEP in the seated neurological patients – implications for paradoxical air embolism. Anesth Analg. 1984;63:429-432.
[24] Zasslow M.A., Pearl R.P., Larson C.P., Silverberg G., Shuer L.F. PEEP does not affect left atrial-right atrial pressure difference in neurosurgical patients. Anesthesiology. 1988;68:760-763.
[25] Munson E.S., Merrick H.C. Effect of nitrous oxide on venous air embolism. Anesthesiology. 1966;27:783-787.
[26] Alvaran S.B., Toung J.K., Graff T.E., Benson D.W. Venous air embolism: comparative merits of external cardiac massage, intracardiac aspiration, and left lateral decubitus position. Anesth Analg. 1978;57:166-170.
[27] Toung T.J., McPherson R.W., Ahn H., Donham R.T., Alano J., Long D. Pneumocephalus: effects of patient position on the incidence and location of aerocele after posterior fossa and upper cervical cord surgery. Anesth Analg. 1986;65:65-70.
[28] Wilder B.L. Hypothesis: the etiology of midcervical quadriplegia after operation with the patient in the sitting position. Neurosurgery. 1982;11:530-531.
[29] Pinaud M., Lelausque J.N., Chetanneau A., Fauchoux N., Ménégalli D., Souron R. Effects of propofol on cerebral hemodynamics and metabolism in patients with brain trauma. Anesthesiology. 1990;73:404-409.
[30] Shapiro H.M. Intracranial hypertension: therapeutic and anesthetic considerations. Anesthesiology. 1975;43:445-471.
[31] Hoffman W.E., Miletich D.J., Albrecht R.F. The effects of midazolam on cerebral blood flow and oxygen consumption and its interaction with nitrous oxide. Anesth Analg. 1986;65:729-733.
[32] Frizzell R.T., Meyer Y.J., Borchers D.J., Weprin B.E., Allen E.C., Pogue W.R., et al. The effects of etomidate on cerebral metabolism and blood flow in a canine model for hypoperfusion. J Neruosurg. 1991;74:263-269.
[33] Bristow A., Shalev D., Rice B., Lipton J.M., Giesecke AH Jr. Low-dose synthetic narcotic infusions for cerebral relaxation during craniotomies. Anesth Analg. 1987;66:413-416.
[34] Kotani J., Sugioka S., Momota Y., Ueda Y. Effect of sevoflurane on intracranial pressure, saggital sinus pressure and the intracranial volume-pressure relation in cats. J Neurosurg Anesthesiol. 1992;4:194-198.
[35] Archer D.P., Labrecque P., Tyler J.L., Meyer E., Trop D. Cerebral blood volume is increased in dogs during administration of nitrous oxide or isoflurane. Anesthesiology. 1987;67:642-648.
[36] Drummond J.C., Scheller M.S., Todd M.M. The effect of nitruos oxide on cortical cerebral blood flow during anesthesia with halothane and isoflurane, with and without morphine, in the rabbit. Anesth analg. 1987;66:1083-1094.
[37] Sieber F.E., Smith D.S., Traystman R.J., Wollman H. Glucose: a reevaluation of its intraoperative use. Anesthesiology. 1987;67:72-81.
[38] Artu A.A., Cucchiara R.F., Mesick J.M. Cardiorespiratory and cranial nerve sequelae of surgical procedures involving the posterior fossa. Anesthesiology. 1980;52:83-686.
[39] Howard R., Mahoney A., Thurlow A.C. Respiratory obstruction after posterior fossa surgery. Anaesthesia. 1990;45:222-224.
[40] Kiya K., Satoh H., Mizoue T., Kinoshita Y. Postoperative cortical venous infarction in tumours firmly adherent to the cortex. J Clin Neurosci. 2001;8(Suppl. 1):109-113.
[41] Lieu A.S., Howng S.L. Intracranial meningiomas and epilepsy: incidence, prognosis and influencing factors. Epilepsy Res. 2000;38(1):45-52.
[42] Boviatsis E.J., Bouras T.I., Kouyialis A.T., Themistocleous M.S., Sakas D.E. Impact of age on complications and outcome in meningioma surgery. Surg Neurol. 2007;68(4):407-411.
[43] Sindou M.P., Alvernia J.E. Results of attempted radical tumor removal and venous repair in 100 consecutive meningiomas involving the major dural sinuses. J Neurosurg. 2006;105(4):514-525.
[44] Caroli E., Orlando E.R., Mastronardi L., Ferrante L. Meningiomas infiltrating the superior sagittal sinus: surgical considerations of 328 cases. Neurosurg Rev. 2006;29(3):236-241.
[45] DiMeco F., Li K.W., Casali C., Ciceri E., Giombini S., Filippini G., et al. Meningiomas invading the superior sagittal sinus: surgical experience in 108 cases. Neurosurgery. 2004;55(6):1263-1272.
[46] Sindou M., Hallacq P. Venous reconstruction in surgery of meningiomas invading the sagittal and transverse sinuses. Skull Base Surg. 1998;8(2):57-64.
[47] Hwang S.K., Gwak H.S., Paek S.H., Kim D.G., Jung H.W. The experience of ligation of transverse or sigmoid sinus in surgery of large petroclival meningiomas. J Korean Med Sci. 2002;17(4):544-548.
[48] Soleau S.W., Schmidt R., Stevens S., Osborn A., MacDonald J.D. Extensive experience with dural sinus thrombosis. Neurosurgery. 2003;52(3):534-544.
[49] Lieu A.S., Howng S.L. Intracranial meningiomas and epilepsy: incidence, prognosis and influencing factors. Epilepsy Res. 2000;38(1):45-52.
[50] Chozick B.S., Reinert S.E., Greenblatt S.H. Incidence of seizures after surgery for supratentorial meningiomas: a modern analysis. J Neurosurg. 1996;84(3):382-386.
[51] Rothoerl R.D., Bernreuther D., Woertgen C., Brawanski A. The value of routine electroencephalographic recordings in predicting postoperative seizures associated with meningioma surgery. Neurosurg Rev. 2003;26(2):108-112. Epub 2002 Oct 10
[52] Danish S.F., Burnett M.G., Stein S.C. Prophylaxis for deep venous thrombosis in patients with craniotomies: a review. Neurosurg Focus. 2004;17(4):E2.
[53] Gerber D.E., Segal J.B., Salhotra A., Olivi A., Grossman S.A., Streiff M.B. Venous thromboembolism occurs infrequently in meningioma patients receiving combined modality prophylaxis. Cancer. 2007;109(2):300-305.
[54] Danish S.F., Burnett M.G., Ong J.G., Sonnad S.S., Maloney-Wilensky E., Stein S.C. Prophylaxis for deep venous thrombosis in craniotomy patients: a decision analysis. Neurosurgery. 2005;56(6):1286-1292.
[55] Goldhaber S.Z., Dunn K., Gerhard-Herman M., Park J.K., Black P.M. Low rate of venous thromboembolism after craniotomy for brain tumor using multimodality prophylaxis. Chest. 2002;122(6):1933-1937.
[56] Constantini S., Kanner A., Friedman A., Shoshan Y., Israel Z., Ashkenazi E., et al. Safety of perioperative minidose heparin in patients undergoing brain tumor surgery: a prospective, randomized, double-blind study. J Neurosurg. 2001;94(6):918-921.
[57] Macdonald R.L., Amidei C., Lin G., Munshi I., Baron J., Weir B.K., et al. Safety of perioperative subcutaneous heparin for prophylaxis of venous thromboembolism in patients undergoing craniotomy. Neurosurgery. 1999;45(2):245-251.
[58] Browd S.R., Ragel B.T., Davis G.E., Scott A.M., Skalabrin E.J., Couldwell W.T. Prophylaxis for deep venous thrombosis in neurosurgery: a review of the literature. Neurosurg Focus. 2004;17(4):E1.
[59] Geerts W.H., Pineo G.F., Heit J.A., Bergqvist D., Lassen M.R., Colwell C.W., et al. Prevention of venous thromboembolism: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(Suppl. 3):338S-400s.
[60] Agnelli G., Piovella F., Buoncristiani P., Severi P., Pini M., D’Angelo A., et al. Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. NEJM. 1998;339:1639-1640.
[61] Macdonald R.L., Amidei C., Baron J., Weir B., Brown F., Erickson R.K., et al. Randomized, pilot study of intermittent pneumatic compression devices plus dalteparin versus intermittent pneumatic compression devices plus heparin for prevention of venous thromboembolism in patients undergoing craniotomy. Surg Neurol. 2003;59(5):363-372.
[62] Duong D.H., O’Malley S., Sekhar L.N., Wright D.G. Postoperative hydrocephalus in cranial base surgery. Skull Base Surg. 2000;10(4):197-200.
[63] Pirouzmand F., Tator C.H., Rutka J. Management of hydrocephalus associated with vestibular schwannoma and other cerebellopontine angle tumors. Neurosurgery. 2001;48(6):1246-1253.
[64] Lyngdoh B.T., Giri P.J., Behari S., Banerji D., Chhabra D.K., Jain V.K. Intraventricular meningiomas: a surgical challenge. J Clin Neurosci. 2007;14(5):442-448.
[65] Oberascher G. Cerebrospinal fluid otorrhea–new trends in diagnosis. Am J Otol. 1988;9(2):102-108.
[66] Bernal-Sprekelsen M., Alobid I., Mullol J., Trobat F., Tomás-Barberán M. Closure of cerebrospinal fluid leaks prevents ascending bacterial meningitis. Rhinology. 2005;43(4):277-281.
[67] Leonetti J.P., Anderson D., Marzo S., Moynihan G. Prevention and management of cerebrospinal fluid fistula after transtemporal skull base surgery. Skull Base. 2001;11(2):87-92.
[68] Sprague A., Poulgrain P. Tension pneumocephalus: a case report and literature review. J Clin Neurosci. 1999;6(5):418-424.
[69] Nadkarni T.D., Menon R.K., Desai K.I., Goel A. Spontaneous cerebrospinal fluid rhinorrhea following excision of a massive torcular meningioma. J Clin Neurosci. 2006;13(1):118-121.
[70] Bernal-Sprekelsen M., Alobid I., Mullol J., Trobat F., Tomás-Barberán M. Closure of cerebrospinal fluid leaks prevents ascending bacterial meningitis. Rhinology. 2005;43(4):277-281.
[71] Mirza S., Saeed S.R., Ramsden R.T. Extensive tension pneumocephalus complicating continuous lumbar CSF drainage for the management of CSF rhinorrhoea. ORL J Otorhinolaryngol Relat Spec. 2003;65(4):215-218.
[72] Açikbas S.C., Akyüz M., Kazan S., Tuncer R. Complications of closed continuous lumbar drainage of cerebrospinal fluid. Acta Neurochir (Wien). 2002;144(5):475-480.
[73] Brodie H.A., Thompson T.C. Management of complications from 820 temporal bone fractures. Am J Otol. 1997;18(2):188-197.
[74] Brodie H.A. Prophylactic antibiotics for posttraumatic cerebrospinal fluid fistulae. A meta-analysis. Arch Otolaryngol Head Neck Surg. 1997;123(7):749-752.
[75] Bien A.G., Bowdino B., Moore G., Leibrock L. Utilization of preoperative cerebrospinal fluid drain in skull base surgery. Skull Base. 2007;17(2):133-139.
[76] Beppu T., Ogasawara K., Ogawa A. Alleviation of intracranial air using carbon dioxide gas during intraventricular tumor resection. Clin Neurol Neurosurg. 2006;108(7):655-660.
[77] Dexter F., Reasoner D.K. Theoretical assessment of normobaric oxygen therapy to treat pneumocephalus. Anesthesiology. 1996;84(2):442-447.
[78] Ducic Y., Zuzukin V. A Rational Approach to the Use of Tracheotomy in Surgery of the Anterior Skull Base. Laryngoscope. 2007.
[79] Gerlach R., Raabe A., Scharrer I., Meixensberger J., Seifert V. Post-operative hematoma after surgery for intracranial meningiomas: causes, avoidable risk factors and clinical outcome. Neurol Res. 2004;26(1):61-66.
[80] Gutenberg A., Lange B., Gunawan B., Larsen J., Brück W., Rohde V., et al. Spontaneous adrenal hemorrhage: a little-known complication of intracranial tumor surgery. J Neurosurg. 2007;106(6):1086-1088.
[81] Reuber M., Kral T., Kurthen M., Elger C.E. New-onset psychogenic seizures after intracranial neurosurgery. Acta Neurochir (Wien). 2002;144(9):901-907.
[82] Gil Z., Cohen J.T., Spektor S., Shlomi B., Fliss D.M. Anterior skull base surgery without prophylactic airway diversion procedures. Otolaryngol Head Neck Surg. 2003;128(5):681-685.
[83] Sindou M., Wydh E., Jouanneau E., Nebbal M., Lieutaud T. Long-term follow-up of meningiomas of the cavernous sinus after surgical treatment alone. J Neurosurg. 2007;107(5):937-944.