CHAPTER 24 Anesthesia for Pediatric Otorhinolaryngologic Surgery
The art and science of anesthesia must be well integrated in caring for the child undergoing ENT anesthesia. Many procedures, including adenoidectomy, rigid bronchoscopy, and laser removal of papillomas, require short intervals of deep planes of anesthesia and limited movement of the patient. To effectively manage operating room services, the patient must awake in a timely manner without airway irritability. One of the major changes in pediatric anesthesia over the past decade has been the abandonment of halothane in favor of sevoflurane. Although sevoflurane appears to be a safer anesthetic agent than halothane, sevoflurane-induced postoperative delirium continues to challenge the pediatric anesthesiologist (Vlajkovic and Sindjelic, 2007; Kuratani and Oi, 2008).
Propofol has many properties that make it a useful anesthetic agent for ENT surgery. It causes less airway irritability than thiopental. Propofol may be protective against and therapeutic for laryngospasm (Brown et al., 1991; Scanlon et al., 1993; Allsop et al., 1995; Afshan et al., 2002). Propofol also has useful antiemetic properties and a low incidence of emergence delirium (Uezono et al., 2000; Moore et al., 2003; Voepel-Lewis et al., 2003), making this anesthetic a good choice for ENT anesthesia.
Anesthesia for otologic procedures
Otitis media is the most frequently diagnosed childhood malady, with the highest incidence occurring among children between the ages of 6 and 18 months. Its frequency decreases after the first year of life. By age 7 years, otitis media is a less frequent pediatric diagnosis. Otitis media is inflammation of the middle ear, without reference to pathogenesis or cause. The other areas of the temporal bone that are contiguous with the middle ear, including the mastoid, petrous apex, and perilabyrinthine air cells, also may be involved. In those who have had a single episode of acute otitis media, residual middle ear fluid remains in up to 40% of patients at 1 month, 20% at 2 months, and 10% at 3 months (Pelton et al., 1977; Teele et al., 1980; Gluckman, 1990).
Many patients with congenital anomalies are candidates for myringotomy tubes. Patients with cleft palate, craniofacial malformations, Down syndrome, Turner’s syndrome, and human immunodeficiency virus (HIV) infection have a higher incidence of otitis media and therefore have a greater need for myringotomy and insertion of tympanostomy tubes than the general population (Bluestone and Klein, 2003). These children have other airway and medical issues that complicate the usual approach to the healthy child requiring myringotomy tubes. Critically ill patients with a history of prolonged nasotracheal intubation have a high incidence of persistent middle ear effusions requiring middle ear ventilation.
Adenoidectomy for chronic otitis media with effusion may benefit some children. The effectiveness of adenoidectomy for chronic otitis media with effusion is not directly related to adenoid size. For children who have recurrent or chronic otitis media with effusion and who had one or more myringotomy and tympanostomy tube insertion procedure in the past, adenoidectomy may be a reasonable option (Paradise et al., 2003). Upper airway obstruction, recurrent acute or chronic adenoiditis, and sinusitis are additional indications for adenoidectomy in children who have chronic otitis media with effusion.
Myringotomy and Insertion of Tympanostomy Tubes
Candidates for myringotomy and tube insertion present with subacute or chronic upper respiratory tract infection with or without fever. In these children, surgical intervention appears to improve the symptoms of upper respiratory infection postoperatively (Tait and Knight, 1987). However, the finding by these investigators is applicable only to myringotomy for children with middle ear effusion; it should not be generalized or applied to children with upper respiratory infection (especially in its acute phase with fever) who are scheduled for other surgical procedures (Hinkle, 1989).
Preoperative sedation should be administered based on the child’s anxiety level. Mask induction of anesthesia with nitrous oxide, oxygen, and sevoflurane provides effective and smooth induction and maintenance anesthesia with prompt emergence. Preoperative or intraoperative analgesia is advocated for early postoperative pain relief. Because these children are usually anesthetized without intravascular access, preoperative administration of oral acetaminophen or nonsteroidal antiinflammatory drugs (NSAIDs) (Watcha et al., 1992) and intraoperative intranasal fentanyl or butorphanol (Bennie et al., 1998; Galinkin et al., 2000; Finkel et al., 2001) or intramuscular ketorolac (Pappas et al., 2003) has been studied and recommended. It appears that intraoperative ketorolac (0.5 mg/kg, up to 30 mg) administered by the intramuscular route provides better analgesia than oral or rectal acetaminophen. Ketorolac is associated with less vomiting and minimal sedation compared with intranasal butorphanol and perhaps with fentanyl. However, ketorolac, along with other NSAIDs, should be used with caution because ketorolac affects platelet function, and increased postoperative bleeding during tonsillectomy or adenoidectomy has been reported (Dahl and Kehlet, 1994; Nikanne et al., 1997).
Because children undergoing myringotomy commonly have adenoidal hypertrophy, they may exhibit signs of nasal and upper airway obstruction (Bluestone and Klein, 2001). These children often require continuous positive airway pressure (CPAP) during inhalational induction and insertion of an oropharyngeal airway after reaching a surgical plane of anesthesia to maintain a patent airway (see Chapter 3, Respiratory Physiology, and Chapter 13, Induction, Maintenance, and Recovery). Although myringotomy and tube insertion may last less than 10 minutes, it should be performed with standard monitoring (i.e., using a precordial stethoscope, pulse oximeter, electrocardiogram, thermometer, and automated blood pressure cuff). Myringotomy is one of a few exceptions in modern pediatric anesthesia practice in the United States for which intravenous infusion is not routinely administered.
Postoperative hypoxemia occurs frequently in infants and children during transport and in the recovery room, even after minor surgical procedures such as myringotomy (Motoyama and Glazener, 1986). After myringotomy, patients are prone to upper airway obstruction, and these children should be transferred to the recovery room in the lateral position while receiving supplemental oxygen (see Chapter 13, Induction, Maintenance, and Recovery).
Mastoidectomy and Tympanoplasty
The clinical importance of the mastoid process is related to its contiguous structures, which include the posterior and middle cranial fossa, sigmoid and lateral sinuses, facial nerve, semicircular canals, and petrous tip of the temporal bone. The distal part of the middle ear and mastoid are connected by the aditus ad antrum, making the mastoid susceptible to infection by infectious processes from the middle ear. With improved medical and surgical management of otitis media, mastoidectomy for mastoiditis has declined considerably over the past several decades. However, mastoidectomies may also be performed in association with cholesteatoma removal and tympanoplasty. Tympanoplasty is performed in patients with chronic perforation or atelectasis of the tympanic membrane (Bluestone and Klein, 2003). Deep retraction pockets may lead to squamous epithelium proliferation in the middle ear and mastoid cavities, causing a cholesteatoma that may grow by the enzymatic activity of the skin tissues and accumulation of squamous debris.
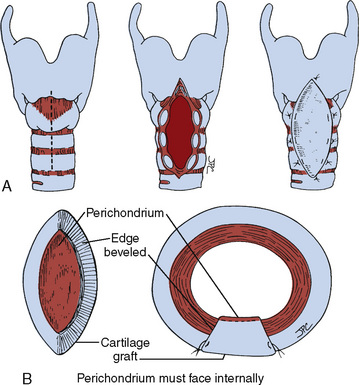
Tympanoplasty is an operation to reconstruct the tympanic membrane with or without grafting. Its primary indications are repair of tympanic perforation; stabilization or improvement of hearing; removal or prevention of congenital, iatrogenic, or primary or secondary acquired cholesteatoma; and removal of atelectatic or diseased areas of the tympanic membrane (Haynes and Harley, 2002). Grafting materials include fat, fascia, perichondrium, periosteum, cartilage, vein, and paper patch.
Mastoidectomy involves the surgical exposure and removal of mastoid air cells. There are several types of mastoidectomy (Bluestone and Klein, 2003). In a complete simple cortical mastoidectomy, the mastoid air-cell system is removed, but the canal wall is left intact. The operation is performed for acute or chronic mastoid osteitis, and it is frequently part of the surgical procedure advocated by some surgeons for cholesteatoma.
Anesthetic Management
For mastoidectomy and tympanoplasty, the patient is positioned supine, with the head laterally rotated away from the affected side. It is important to carefully rotate the neck of a patient under general anesthesia because of the risk for atlantoaxial rotational subluxation (Brisson et al., 2000).
Induction of anesthesia can entail inhalational or intravenous induction. The anesthesiologist should consider endotracheal intubation without muscle relaxation or with a short-acting muscle relaxant so the facial nerve can be monitored during surgery. The use of nitrous oxide may be contraindicated during and after placement of the tympanic graft, because nitrous oxide accumulates in a closed gas space and increases the ambient pressure; some surgeons believe this tends to lift the graft away from its new site (Koivunen et al., 1996; Doyle and Banks, 2003).
The most common postoperative complication requiring unscheduled admission to the hospital for children who have undergone tympanomastoidectomy is postoperative nausea and vomiting (PONV) (Megerian et al., 2000). PONV has been attributed to surgical stimulation of the vestibular labyrinth, anesthetic techniques, or a combination of the two (Jellish et al., 1995; Dornhoffer and Manning, 2000). Propofol has antiemetic properties (Ved et al., 1996; Erb et al., 2002b). It appears that propofol used for induction and maintenance of anesthesia is superior to isoflurane or sevoflurane in reducing PONV after middle ear surgery (Jellish et al., 1995, 1999; Moore et al., 2003). The use of intravenous dexamethasone during surgery appears to decrease PONV in patients after undergoing tympanomastoid surgery (Liu et al., 2001).
Cochlear Implantation
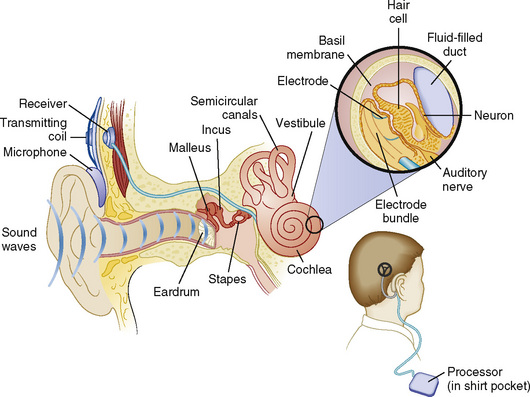
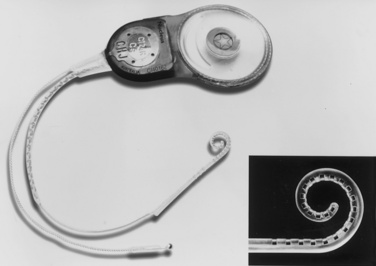
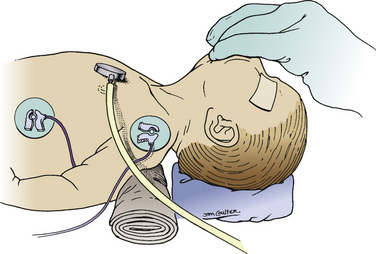
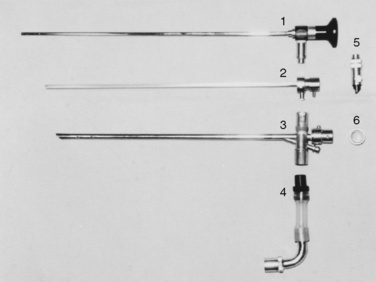
Cochlear implantation has become a feasible choice for profoundly hearing-impaired children (Figs. 24-1 and 24-2). Profoundly deaf children’s auditory nerve fibers remain intact, but the sensory neuroepithelium in the cochlea is absent. This damage occurs because of a genetic defect, infection, cochlear ossification, or aging (Fischetti, 2003). Cochlear implants bypass the damage by receiving and converting sound into signals sent along electrodes to cells adjacent to the auditory nerve. Hearing aids are ineffective because sound cannot be converted into an electrical impulse. Cochlear implants have eight to 22 electrodes that are placed through a cochleostomy. Sounds received from an external microphone are converted to an electrical signal that is received and transmitted by the cochlear nerve (Balkany et al., 2001; Miyamoto and Kirk, 2003). Because the cochlea is full size at birth, there is no anatomic difficulty with electrode insertion in very young children.
Anesthesia for rhinologic procedures
Reduction of Nasal Fracture
A nasal fracture may cause considerable bleeding, and the blood may be swallowed into the stomach. These patients therefore are assumed to have a full stomach, regardless of the time of last food intake. Reduction of a nasal fracture may take only a few moments, but in the acute situation, an oral endotracheal tube is mandatory to protect the airway from pulmonary aspiration. More commonly, surgery is delayed for several days after an isolated nasal fracture to allow swelling to subside. In these cases, a flexible laryngeal mask airway (LMA) can be substituted for an endotracheal tube. The LMA cannot prevent aspiration of stomach contents into the lungs, but it can prevent blood from the nose from passing through the vocal cords (John et al., 1991; Williams and Bailey, 1993). The LMA may be removed either when the patient is deeply anesthetized or when awake.
Nasal Polypectomy
Nasal polypectomy is performed under general anesthesia, often using a preformed Ring, Adair, Ellwyn (RAE) endotracheal tube, which was originally developed by Ring and associates (1975) (see Chapter 10, Equipment). Packing of the pharynx is indicated in most intranasal procedures. One end of the oropharyngeal gauze packing should be tagged with a hemostat and left hanging externally at one corner of the mouth. A note should be prominently placed in the room for all to see, or a labeled tape should be placed across the exit door, reminding all operating room personnel that a throat pack is in place and needs to be removed. Historically, as much as 10 mcg/kg of epinephrine, usually in a 1:200,000 solution (5 mcg/mL), can be used in children for hemostasis under halothane anesthesia without apparent myocardial irritability (Karl et al., 1983; Ueda et al., 1983). More recently, however, our anesthesia practice has decided to use isoflurane, sevoflurane, desflurane, propofol, or remifentanil as maintenance anesthetic, without changing the epinephrine dosage (see Chapter 13, Induction, Maintenance, and Recovery).
Special consideration should be given to the management of children with cystic fibrosis, in whom nasal polyps often occur (Hulka, 2000) (see Chapter 36, Systemic Disorders). The polyps are multiple and recur after removal. These children often have severe obstructive lung disease with recurrent pulmonary infection and thick, tenacious secretions. They are frequently hypoxemic in room air. During general anesthesia, the cystic fibrosis patient may require frequent endotracheal lavage with saline and deep suctioning. Secretions can hamper oxygenation and ventilation; a rise in the peak inspiratory pressure intraoperatively may indicate that the endotracheal tube requires suctioning. An inhaled bronchodilator should be immediately available to treat bronchospasm. Inhaled anesthetics and nitrous oxide or opioids and a relaxant may be used for patients with mild to moderate airway obstruction. Nitrous oxide should be avoided in those with severe lower airway obstruction with air trapping, because of possible hyperinflation of trapped air spaces. These patients with lower airway obstruction should be ventilated with an oxygen-air mixture, and appropriate positive end-expiratory pressure (5 to 7 cm H2O) should be added throughout the procedure to minimize airway closure and atelectasis (see Chapter 3, Respiratory Physiology in Infants and Children). Because of the association of nasal/ethmoidal polyposis with asthma and aspirin sensitivity (Samter’s triad), NSAIDs should be used with caution.
Sinus Surgery
Sinus surgery is indicated for children who have failed maximal medical therapy. Children with allergy or gastroesophageal reflux disease should receive maximal medical therapy and should rarely require surgery (Goldsmith and Rosenfeld, 2003). Children with immune deficiency, immotile cilia syndrome (i.e., Kartagener’s syndrome), or cystic fibrosis (Gysin et al., 2000) are at high risk for chronic sinusitis. Unfortunately, because of their underlying disease, these children have a significant surgical failure rate (Herbert and Bent, 1998).
In children with recurrent sinusitis without chronic disease, adenoidectomy should be the initial procedure if the quantity of adenoid tissue visualized on endoscopy is considered sufficient to serve as a reservoir of bacterial pathogens. Adenoidectomy or adenotonsillectomy is usually the first-line surgical intervention for preschoolers, and it is often appropriate in older children. The expected rate of improvement is 70% to 80% (Goldsmith and Rosenfeld, 2003).
Endoscopic sinus surgery (ESS) should be performed only when children have failed previous therapies. In contrast to older traditional techniques of sinus surgery, ESS focuses on enlarging the natural ostia of the maxillary and ethmoid sinuses, while preserving most or all of the sinus mucosa (Goldsmith and Rosenfeld, 2003). In addition to children with chronic sinusitis who fail medical management, Lusk (2003) lists the accepted indications for ESS as complete nasal obstruction in patients with cystic fibrosis, antrochoanal polyps, intracranial complications, mucoceles and mucopyoceles, orbital abscess, traumatic injury in the optic canal, dacryocystorhinitis resulting from sinusitis, fungal sinusitis, certain neoplasms, and meningoencephalocele. In properly selected children, the results are good, with an expected improvement of 80% (Lusk, 2003). Preoperative computed tomography (CT) is essential in defining the specific diseased sinuses and in looking for anatomic abnormalities that need to be addressed, including septal deviation, concha bullosa cells, obstructing Haller air cells, and abnormal middle turbinates (Goldsmith and Rosenfeld, 2003).
Choanal Atresia
Choanal atresia is a congenital malformation in which no connection exists between the nasal cavity and the aerodigestive tract (Prasad et al., 2002); it has an incidence of 1 in 7000 births. The atresia is bony (30%) or mixed membranous and bony (70%). The existence of purely membranous atresia has come into question (Brown et al., 1996). Choanal atresia is unilateral in 50% to 60% of patients. Syndromes associated with choanal atresia include Apert’s syndrome, DiGeorge syndrome, trisomy 18, Treacher Collins syndrome, camptomelic dysplasia, and CHARGE association (i.e., coloboma, heart defects, atresia choanae, retardation of growth and development, genitourinary problems, and ear anomalies) (Tewfick et al., 1997).
The symptoms caused by choanal atresia depend on whether the obstruction is unilateral or bilateral. Because neonates are obligatory nasal breathers, those with bilateral disease present with acute respiratory distress. Respiratory distress can be attenuated if the mouth is kept open with an oral airway strapped in place or by a large rubber nipple with a large hole cut in it and kept in place with an umbilical tape around the neck (McGovern, 1961; Hengerer and Wein, 2003). For the neonate with bilateral choanal atresia, surgical nasal correction or tracheostomy must be performed within the first few days of life. Other infants may have only unilateral atresia, with minimal symptoms that can go undiagnosed for months or even years. The most common complaint is intractable unilateral anterior nasal discharge.
A number of surgical procedures have been described for the correction of choanal atresia, including endoscopic, transnasal, transseptal, and transpalatal procedures (Holland and McGuirt, 2001). Timing of surgery for bilateral choanal atresia varies and depends on the infant’s ability to adapt to oral breathing and acquire adequate nutrition. Hengerer and Wein (2003) state that some surgeons advocate “a rule of tens” to guide the timing of surgical intervention. The child must reach 10 weeks of age, weigh 10 pounds, and have a hemoglobin level of 10 g/dL. Other surgeons have demonstrated routine success in newborns 48 to 72 hours old and weighing as little as 1900 g (Werkhaven J, 2003, personal communication).
The anesthetic approach depends on the child’s condition. In general, intravenous induction with a muscle relaxant of intermediate duration, endotracheal intubation with an oral RAE tube, and maintenance with an inhaled agent and opioid or propofol and remifentanil infusion suffice. Rarely, algorithms for difficult neonatal intubation must be used for securing the airway in infants with bilateral choanal atresia. After the atresia is surgically corrected, the surgeon is often faced with the problem of restenosis (Pirsig, 1986); however, recent innovations have created optimism that the restenosis rate can be reduced. Newer endoscopic techniques with powered instrumentation have enhanced the safety and efficacy for choanal atresia repair (Prasad et al., 2002). Mitomycin-C, an aminoglycoside and alkylating agent used as an intravenous antineoplastic agent, can be used topically after choanal atresia repair. Topical application inhibits fibroblast growth and migration and granulation tissue formation responsible for restenosis (Holland and McGuirt, 2001). Topical mitomycin has not caused systemic effects and has not contributed to anesthetic complications. Patients with unilateral obstruction usually do well in the postoperative period and require no special monitoring. However, infants undergoing bilateral repair can exhibit partial or intermittent upper airway obstruction that persists for some time. The infant should be observed closely in the ICU with appropriate monitoring until breathing dynamics have normalized.
Anesthesia for pharyngeal and laryngeal procedures
Tonsillectomy and Adenoidectomy
Tonsillectomy and adenoidectomy (perhaps with the exception of myringotomies) are the most common pediatric surgical procedures performed in the United States. Although tonsillectomy and adenoidectomy are frequently performed procedures, the benefits in relation to cost and risk are still hotly debated (Paradise, 2003). Just about every indication has its advocates and detractors (Bluestone, 2001; Paradise, 2003).
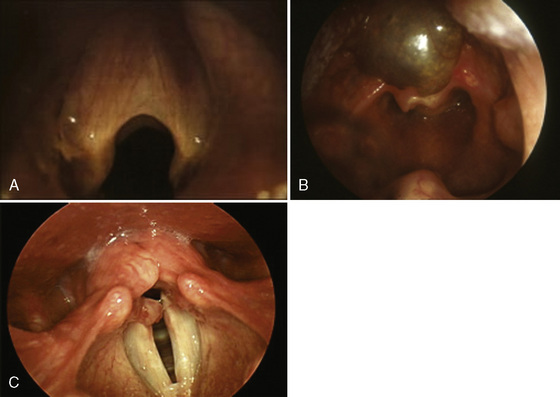
Nocturnal upper airway obstruction, with or without obstructive sleep apnea (OSA), is a common indication for adenotonsillectomy. A mild, partial obstruction in otherwise healthy children is exacerbated by conditions such as achondroplasia, Down syndrome, mucopolysaccharidosis, and obesity. Adenotonsillectomy is considered curative when adenotonsillar hypertrophy is the primary cause of childhood sleep-related breathing disorders (Schechter, 2002). Indications for tonsillectomy include recurrent pharyngotonsillitis, chronic tonsillitis, hemorrhagic tonsillitis, peritonsillar abscess, streptococcal carriage, dysphagia, abnormal dentofacial growth, halitosis, and suspicion of malignant disease (i.e., tonsil asymmetry) (Darrow and Siemans, 2002). Additional indications for adenoidectomy include recurrent or chronic rhinosinusitis or adenoiditis, and recurrent otitis media (Darrow and Siemans, 2002). Adenotonsillar hypertrophy with resultant upper airway obstruction is the most frequent cause of OSA in children (Fig. 24-3).
Obstructive Sleep Apnea Syndrome
Obstructive sleep apnea syndrome (OSAS) is a disorder of breathing during sleep characterized by prolonged partial upper airway obstruction (obstructive hypopnea) or intermittent complete obstruction (OSA) with or without snoring and associated with moderate to severe oxygen desaturation that disrupts normal sleep-time breathing and normal sleep patterns (American Thoracic Society, 1996). The most common cause of OSAS among children is upper airway narrowing with adenotonsillar hypertrophy. OSAS also occurs in infants and children with upper airway narrowing resulting from craniofacial anomalies, and in those with neuromuscular diseases, including cerebral palsy and muscular dystrophy (Marcus, 2001; Schwengel et al., 2009) (Box 24-1). See related video online at www.expertconsult.com.![]()
Box 24-1 Some Congenital and Medical Conditions Associated with Obstructive Sleep Apnea Syndrome
From Sterni LM, Tunkel DE: Obstructive sleep apnea in children: an update, Pediatr Clin North Am 50:427, 2003.
In recent years, the epidemic increase in the prevalence of obesity during childhood seems to be contributing to substantial changes in the cross-sectional demographic and anthropometric characteristics of the children being referred for evaluation of OSAS. Although less than 15% of all symptomatic habitually snoring children were obese (i.e., >95th percentile for age and gender) in the early 1990s, more than 50% fulfilled the criteria for obesity among all referrals to a Kentucky sleep center by the mid 2000s (Gozal et al., 2006; Schwengel et al., 2009).
Adenotonsillar hypertrophy is the most common cause of OSAS in children. Many published papers, primarily case reports and case series, support the idea that tonsillectomy with or without adenoidectomy is often the cure for OSAS (Schechter et al., 2002; Garetz, 2008).
The peak prevalence of OSAS in children occurs between 2 and 8 years, which is the age when the tonsils and adenoids are large in relation to the child’s upper airways. The site of collapse is most commonly at the level of the adenoids or velopharynx (Isono et al., 1998; Isono, 2006). Although OSAS is associated with adenotonsillar hypertrophy, there must be other neuromuscular factors involved. These patients usually do not obstruct while awake, implying that sleep induces another dimension to OSAS. Some otherwise normal children with OSAS but with smaller tonsils and adenoids are cured by adenotonsillectomy, whereas others with larger tonsils and adenoids are not (Marcus, 2001). The patency of pharyngeal airway is maintained by tonic and phasic contractions of the upper airway dilator muscles, such as the genioglossus, geniohyoid, and velopalatine muscles (Isono, 2006). Compared with other inspiratory muscles (i.e., diaphragm and intercostal muscles), these upper airway muscles are preferentially depressed with sleep, sedatives, and general anesthetics (Ochiai et al., 1989, 1992) (see Chapter 3, Respiratory Physiology in Infants and Children). It is hypothesized that children with OSAS may have abnormal centrally mediated activation of their airway muscles, leading to a more collapsible upper airway (Marcus et al., 1994).
Fatty deposits of the upper airway structures, and subcutaneous fat deposits in the anterior neck region are the direct causes of airway obstruction in obese children (mostly adolescents), as well as in obese adults, with OSAS. In addition, increased adipose deposits in the chest wall and abdomen can reduce chest wall compliance as well as cephalad displacement of the diaphragm, causing inefficient ventilation by mass loading and decreased functional residual capacity of the lung (Dayyat et al., 2007; Schwengel et al., 2009). Obese patients have symptoms that differ from those in children with OSAS caused by adenotonsillar hypertrophy. Obese patients tend to have a significantly greater incidence of daytime sleepiness, insulin resistance, and systemic as well as pulmonary hypertension than children with adenotonsillar hypertrophy (Tauman et al., 2004). Furthermore, Tauman and coworkers recently observed a high failure rate for obese children with OSAS undergoing adentotonsillectomy.
Dayyat and colleagues (2007) differentiated between children with OSAS into two types. The type 1 child with OSAS is not obese but has disordered breathing secondary to marked lymphadenoid hypertrophy. The type 2 patient is obese with minimal lymphadenoid hyperplasia. Table 24-1 compares the common symptoms experienced by the two types of patients (Dayyat et al., 2007).
TABLE 24-1 Clinical Presentation of Pediatric Obstructive Sleep Apnea Types I and II
| Symptoms and Findings Seen with Similar Frequencies in Types I and II |
| Symptoms and Findings that Differ Between Types | Type I | Type II |
| Excessive daytime sleepiness | + | ++++ |
| Weight gain | − | ++ |
| Hyperactive behavior | ++++ | − or + |
| Truncal obesity | − or + | +++ |
| Enlarged neck circumference | − or + | +++ |
| Enlarged tonsils or adenoids | ++++ | ++ |
| Depression and low self-esteem | + | +++ |
| Shyness and social withdrawal | + | +++ |
| Left ventricular hypertrophy | ++ | ++++ |
| Systemic hypertension | + | ++++ |
| Recurrent ear infections | +++ | − or + |
| Insulin resistance | − | ++++ |
| Dyslipidemia | + | ++++ |
| Elevated C-reactive protein | ++ | ++++ |
| Elevated liver enzymes | − | ++ |
−, Absent; + infrequent to ++++ very frequent.
From Dayyat E, Kheirandish-Gozal L, Gozal D: Childhood obstructive sleep apnea: one or two distinct disease entities? Sleep Med Clin 2:433, 2007.
One of the most interesting areas of childhood OSAS research has been on the effects that OSAS has on systemic inflammation. Children with OSAS are at risk for developing inflammatory responses that may lead to endothelial dysfunction and atherogenesis (Gozal et al., 2008). School-aged children with OSAS have a higher serum C-reactive protein level than controls, and the elevation correlates with the severity of OSAS, and levels decrease after adenotonsillectomy (Gozal et al., 2007). Serum proteomic patterns in children with OSAS differ significantly from those in children who have primary snoring but do not have OSAS (Shah et al., 2006). In the Shah group’s study, proteomic profiling of serum samples in children with OSAS revealed differential expression of circulating proteins. Proteomics may play a future role in diagnosing OSAS in the snoring child.
Symptoms of OSAS include nocturnal snoring, breathing pauses, gasping, use of accessory muscles of respiration, enuresis, and excessive sweating (Messner, 2003). In addition, children with OSAS have a host of sequelae, which are usually reversible after adenotonsillectomy but can lead to perioperative complications during and after surgery. Children with OSAS may present with failure to thrive, although the cause of this growth failure is unclear (Sterni and Tunkel, 2003). Many of these children experience a growth spurt after surgery. Children with OSAS may have neurocognitive deficits such as poor learning, behavioral problems, and lower grades in school than non-OSAS children. Adenotonsillectomy improves functioning in these children (Marcus, 2001). Recent studies demonstrate that despite negative polysomnographic findings, the benefits of adenotonsillectomy are still observed in children with symptomatic airway obstruction, including improvements in behavior and quality of life (Leong and Davis 2007).
The diagnosis of OSAS is based on a thorough history and physical examination along with appropriate sleep studies, including polysomnography (Table 24-2 and Boxes 24-2 and 24-3). Snoring, increased respiratory efforts, periodic obstructive apnea, and oxygen desaturation while sleeping are the universal features of OSAS, which must be differentiated from the benign condition referred to as primary snoring.
TABLE 24-2 Polysomnographic Characteristics of Obstructive Sleep Apnea in Children and Adults
| Characteristic | Adults | Children |
| Apnea duration | 10 sec | 2 breaths |
| Hypopnea desaturation | ≥ 4% | ≥ 3% |
| Hypopnea duration | 10 sec | 2 breaths |
| Hypopnea nasal pressure drop | > 30% | ≥ 50% |
| Cortical arousals | Common | Uncommon |
| Normal apnea-hypopnea index | <5 | <1 |
From Karlson KH Jr: What’s new in pediatric obstructive sleep apnea? Clin Pulm Med 15:226, 2008.
Polysomnography
Pediatric polysomnography includes measurement of end-tidal CO2, electroencephalogram, chin EMG, chest wall movement, airflow through the nose and mouth, leg movement, and oxygen saturation via pulse oximetry (Box 24-4). After all events are reviewed and scored, several indices can be calculated. These include the apnea index and the apnea-hypopnea index, which are calculated for the entire sleep period, for both rapid eye movement sleep and non–rapid eye movement sleep. The term index refers to the number of events divided by the number of hours of sleep (Table 24-3). This calculation allows the comparison of polysomnograms of varying time lengths. The apnea index is determined using only apneas, whereas the apnea-hypopnea index includes both apneas and hypopneas (Wagner and Torrez, 2007).
Box 24-4 Components of Polysomnography*
From American Thoracic Society: Standards and indications for cardiopulmonary sleep studies in children, Am J Respir Crit Care Med 153:866, 1996.
TABLE 24-3 Respiratory Events that Can Be Seen During Polysomnography
| Event | Definition |
| Central apnea | Pause in airflow with absent respiratory effort, scored when >20 sec or 2 missed breaths and a >3% drop in oxygen saturation |
| Obstructive apnea | >90% reduction of airflow despite continuing respiratory effort, scored when event lasts at least 2 missed breaths in children |
| Obstructive hypopnea | >50% reduction of airflow with associated respiratory effort, scored when at least 2 missed breaths and >3% drop in oxygen saturation or arousal |
| Mixed apneas | ≥ 90% reduction in airflow, lasting at least 2 missed breaths, and containing absent respiratory effort initially (a central apneic pause), followed by resumption of respiratory effort without a resumption of airflow (an obstructive apnea) |
| Obstructive hypoventilation | End-tidal CO2 > 50 mm Hg for >25% of the total sleep time, with paradoxical respirations, snoring, and no baseline lung disease |
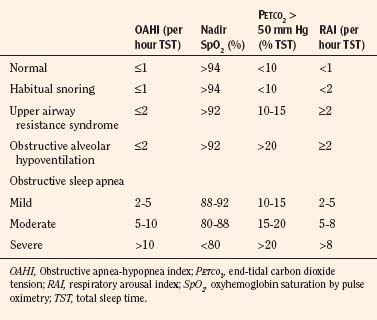
It is helpful to divide the scores into severity-based categories (Table 24-4). OSAS severity can be used to determine the appropriate surgical center where the surgery should be performed and whether the child requires overnight observation (Box 24-5).
Data from McGowan et al., 1992; Gerber et al., 1996; Blum et al., 2004; Fricke et al., 2006; Guilleminault et al., 2007.
Although definitive diagnosis of OSAS is made by a positive polysomnogram, many children are not tested because the polysomnography laboratories specializing in children are few, and the tests are expensive and require an overnight stay (Sterni and Tunkel, 2003). Most children are diagnosed by recommendations set forth by the section of Pediatric Pulmonology Subcommittee on Obstructive Sleep Apnea, sans Polysomnography. This committee’s recommendations for otherwise healthy children older than 1 year are as follows (Schechter, 2002):
Severe, untreated OSAS may lead to pulmonary hypertension and cor pulmonale caused by nocturnal hypoxia and hypercarbia, resulting in compensatory changes in the pulmonary vasculature. Pulmonary vascular resistance increases, causing increased right ventricular strain. Severe cases may progress to pulmonary hypertension, arrhythmias, and cor pulmonale, which are reversible by performing early adenotonsillectomy (Miman et al., 2000). Children with OSAS also tend to have higher diastolic blood pressures. The cardiovascular changes appear to be the result of an increase in sympathetic tone that results from obstructive respiratory events (Marcus et al., 1998). Fortunately, few children develop clinically significant heart failure. It is prudent to pursue an aggressive cardiac evaluation if the child has a loud second heart sound, exercise intolerance, or diastolic hypertension. Because children are diagnosed earlier today, significant heart disease is rarely an issue (Marcus, 2001).
Preoperative Preparation
A careful review of the history, laboratory data, and physical examination results are essential for the optimal outcome of adenotonsillectomy. Careful evaluation of coagulation status is important before performing this procedure. If there is a history of easy bruising, frequent epistaxis, or positive family history, the prothrombin time, partial thromboplastin time, and platelet count should be obtained to rule out coagulopathies. In many pediatric institutions, a coagulation profile is obtained routinely for patients scheduled for adenotonsillectomy. However, in a prospective study of hemostatic assessment of patients before tonsillectomy, routine measurements of a coagulation profile were not useful predictors of postoperative bleeding (Close et al., 1994).
Although relatively mild, von Willebrand’s disease (i.e., reduced factor VIII, decreased platelet adhesiveness because of deficient von Willebrand factor) is the most common coagulopathy seen among patients scheduled for adenotonsillectomy. Children with von Willebrand’s disease or mild hemophilia A are treated preoperatively with desmopressin (1-desamino-8- d-arginine vasopressin [DDAVP]) (Prinsley et al., 1993). DDAVP is a synthetic analogue of vasopressin, which, in addition to its antidiuretic effect, stimulates endothelial cells and releases stored factor VIII and von Willebrand factor (Mannucci, 1988). The intravenous dosage of DDAVP is 0.3 mcg/kg, given over 20 minutes before anesthetic induction.
The examiner should elicit a history of drug ingestion, especially acetylsalicylic acid. If the patient was given such drugs recently, surgery should be postponed, because these drugs cause platelet dysfunction for as long as 10 days and may cause excessive bleeding intraoperatively and postoperatively (Davies and Steward, 1977; Paradise, 2003).
Anesthetic Management
Children who are anxious preoperatively may receive sedation with oral midazolam. In older children scheduled for intravenous induction, EMLA cream (i.e., eutectic mixture of local anesthetics, 2.5% lidocaine and 2.5% prilocaine) is applied to the dorsum of both hands and sealed with plastic adhesives at least 1 hour before intravenous catheter insertion (see Chapter 9, Preoperative Preparation, and Chapter 13, Induction, Maintenance, and Recovery).
Adenoidectomy is a relatively short procedure (15 to 45 minutes), and many centers perform anesthesia without neuromuscular blockade and with less opioid than suggested in the following descriptions. Anesthesia is induced most commonly with oxygen, nitrous oxide, and sevoflurane. As with those undergoing myringotomies, children with adenotonsillar hypertrophy often have partial or complete nasal airway obstruction. The mouth must be kept open during the induction of anesthesia until the gag reflex is abolished and the oral airway is inserted. If necessary, moderate CPAP (10 to 15 cm H2O), with jaw thrust during induction (before the patient is deep enough for oral airway insertion) helps to prevent the pharyngeal airway collapse that results from the relaxation of upper airway muscle tone (Reber et al., 2001; Bruppacher et al., 2003).
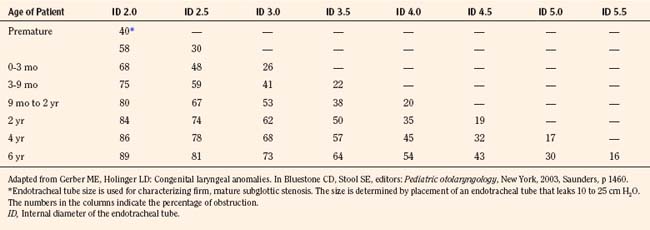
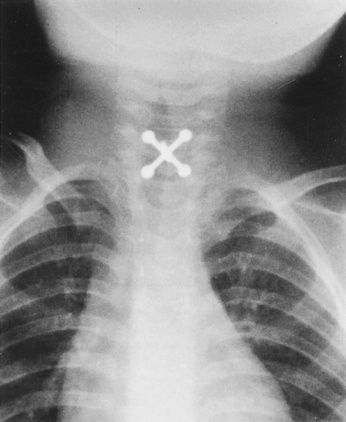
After the intravenous route is established, atropine or glycopyrrolate may be given for its anticholinergic effects. The patient’s trachea is intubated with a preformed oral (RAE) tube under deep inhalational anesthesia supplemented with a topical lidocaine spray to the vocal cords under a direct vision, an intravenous bolus of propofol, or an intermediate-acting, nondepolarizing muscle relaxant (e.g., cisatracurium, vecuronium). In older children, anesthesia may be induced intravenously with propofol (2 to 3 mg/kg) mixed with lidocaine (1 to 2 mg/mL of propofol) to reduce pain at the injection site, with or without anticholinergic agent or a muscle relaxant. Inhalation anesthetic or propofol infusion is continued thereafter with spontaneous or controlled ventilation. A cuffed endotracheal tube is recommended to reduce the chance of aspiration of blood and secretions and to reduce gas leaks around the tube. A cuffed endotracheal tube, 0.5 to 1.0 mm (inner diameter) smaller than the age-appropriate size, should be chosen to accommodate the passage of the cuff through the subglottis (Khine et al., 1997; James, 2001; Fine and Borland, 2004). The endotracheal tube is immobilized with adhesive tape over the middle of the lower lip (Fig. 24-4). The breath sounds on both sides of the chest should be auscultated carefully to avoid endobronchial intubation. The endotracheal tube is then held in place by the groove-bladed tongue depressor that is a part of the Ring adaptation of the Brown-Davis mouth gag (Fig. 24-5).
Anesthesia is maintained with supplemental opioids to reduce the requirement of the anesthesia maintenance agent and to provide postoperative analgesia. The physician may start with a loading dose of 1 to 2 mcg/kg of fentanyl, 50 to 100 mcg/kg of morphine, or 10 to 20 mcg/kg of hydromorphone given intravenously, with additional doses administered as needed. Children with moderate and severe OSAS should receive lower dosages of opioids to minimize the risk for prolonged apnea during emergence and postoperative upper airway obstruction. Brown and coworkers (2004) have shown that young age and low preoperative oxygen saturation were associated with lower postoperative morphine requirement. It appears that children with a minimal oxygen saturation of 85% or less during preoperative sleep required half the postoperative analgesic dosage required by children whose saturations were 85% or greater (Brown et al., 2006).
The use of NSAIDs during or after tonsillectomy is controversial. A meta-analysis suggested that there is an increased risk for bleeding and increased return to the operating room when NSAIDs such as ketorolac are used (Marret, 2003). Criticism of this meta-analysis focuses on the small number of patients evaluated, the varying types and dosages of NSAID, and the varying surgical techniques used for the tonsillectomy. A Cochrane database review evaluated the role of NSAIDs in posttonsillectomy bleeding and found no significant correlation between NSAID use and increased risk for posttonsillectomy bleeding (Cardwell et al., 2005). Practice among pediatric anesthesiologists varies. Some centers do not use NSAIDs during adenoidectomy or tonsillectomy, whereas others use them routinely (Allford, 2009).
There is evidence that high dosages of dexamethasone (up to 1 mg/kg, 25 mg maximum) reduce postoperative swelling and pain and decrease the incidence of PONV without apparent adverse effects attributable to dexamethasone (Pappas et al., 1998). Children receiving dexamethasone are more likely to advance to a soft-solid diet on the first postoperative day (Steward et al., 2003).
Bleeding during tonsil and adenoid surgery is seldom excessive, but massive hemorrhage has occurred with tearing of the carotid vessels (Smith, 1972, 1980). Homeostasis is usually obtained using electrocautery. There have been several reports of fires due to electrocautery-induced ignition of the endotracheal tube or packing during tonsillectomy. Fires are caused by combustible material in an oxygen-rich environment (Mattucci and Militana, 2003); management of airway fires is discussed later.
Children with OSAS tend to emerge from anesthesia more slowly than children without OSAS. This may be explained by their deficit in sleep arousal mechanisms. They seem to have elevated sleep arousal mechanisms in response to hypercarbia and increased upper airway obstruction (Marcus et al., 1998, 1999). Other subtle disturbances of sleep architecture may also be present (Bandla et al., 1999).
Techniques of Tonsillectomy and Adenoidectomy
The adenoidectomy is performed either with a mechanical or a thermal technique (Hesham, 2009). Mechanical removal of the adenoids may be performed using curettes, adenotomes, or a micro-debrider. These mechanical techniques require nasopharyngeal packing to prevent bleeding, and the surgeon must remember to remove the packing at the end of the procedure. Thermal removal of adenoid tissue may employ electrosurgical cautery, radiofrequency ablation, or lasers, and it usually results in little bleeding.
Mechanical removal of the tonsil has many technical variations. In general, an incision is made in the anterior pillar mucosa to expose the tonsil capsule, and using sharp or blunt dissection, the tonsil and capsule are removed together. Alternatively, a guillotine, or snare, is occasionally used to remove the tonsil without making an incision. This results in exposure of the underlying pharyngeal musculature with the perforating vascular supply to the tonsil, and hemostasis must be established. At the turn of the previous century, snare tonsillectomy with ether anesthesia was the standard, and hemostasis was achieved after prolonged observation of the surgical field to ensure passive wound clotting. This was less than ideal, and the mortality rate was unacceptably high. Today, active hemorrhage control is performed after removal of the tonsils. Some surgeons use dissolvable suture to ligate the bleeding points, but this is becoming less common. More often, electrosurgical cautery is used for hemostasis. This technique completes an electrical arc between the patient and the surgical instrument and has the potential to ignite flammable gases and combustible materials (such as the sponges in the nasopharynx used for tamponade of the adenoid bleeding). Case reports of airway fires have been reported with the use of electrosurgical cautery in tonsillectomy (Mattucci and Militana, 2003). It is suggested that the inspired oxygen levels be maintained as they are for the use of lasers in the airway (see Laser Surgery of the Larynx, later).
Thermal removal of the tonsil uses the electrosurgical unit or a radiofrequency unit for tonsil dissection. The most common radiofrequency unit in use is Coblation (ArthroCare, Austin, TX). Thermal removal of the tonsils and adenoids generally results in less blood loss, but it may be accompanied by slightly more postoperative pain than other techniques. A cuffed endotracheal tube, using moist throat packing and inspired oxygen levels of less than 40%, will minimize the risk for an airway fire (Johnson et al., 2002).
Intracapsular tonsillectomy is a newer technique that uses either the micro-debrider or the radiofrequency ablator to remove the tonsil tissue while preserving the tonsil capsule (Koltai et al., 2002). In effect, this may be considered a selective removal of the tonsillar mass, which is believed to be the most common cause of OSAS. Preservation of the capsule protects the underlying pharyngeal musculature, resulting in less postoperative pain and hemorrhage. Because the vascular supply is interrupted at a more terminal point, the muscular bed can contract around the vessels, providing better hemostasis. Initially, this technique was recommended only for those patients with OSAS. Recurrent infection is considered a contraindication, because this technique leaves behind residual tonsil tissue that may become reinfected. This technique is especially appropriate in those OSAS children 3 years of age and younger because of the lower postoperative pain scores and thus less opioid need (Solares et al., 2005; Colen et al., 2008). In general, the only risk of an intracapsular tonsillectomy is the rare (<1 in 1500) need to return for a complete tonsillectomy because of tissue regrowth leading to recurrent infectious tonsillitis.
At the conclusion of surgery, the surgeon usually suctions the pharynx and larynx under direct vision to prevent bleeding caused by agitation of raw mucosal surfaces by a suction catheter. Suctioning of the stomach contents may also remove swallowed secretions, blood, or residual preoperative sedative medications. Removal of these preoperative medications may decrease excess sedation in the postoperative period. Some surgeons elect to infiltrate 0.25% bupivacaine or other local anesthetic medications into the tonsillar fossa to aid in postoperative pain relief (Naja et al., 2005), whereas others feel that this analgesic technique may lead to premature discharge of patients who will then experience rebound pain when the local anesthetic wears off. This practice has occasionally been associated with airway obstruction after extubation.
Laryngospasm may occur when the patient is extubated after tonsillectomy. Methods for avoiding this problem include extubating the trachea while the child is deeply anesthetized (not recommended for children with OSAS) or almost completely awake (see Chapter 13, Induction, Maintenance, and Recovery). Although intravenous lidocaine has not consistently proved helpful in preventing laryngospasm, application of local anesthetic before intubation or manipulation of the airway can reduce the incidence of laryngospasm during intubation and after extubation (Leicht et al., 1985; McCulloch et al., 1992; Landsman, 1997). It appears that lidocaine deposited locally in the laryngeal area suppresses laryngeal mucosa neuroreceptor transmission. However, lidocaine’s duration of action at laryngeal receptor sites is only 30 minutes, and its administration before intubation may not protect against laryngospasm at the time of extubation (Warner, 1996).
Before extubation, the lungs should be well expanded with an oxygen-air mixture at sustained high positive pressure (35 to 40 cm H2O) several times (the vital capacity maneuver), to reopen intrathoracic airways and reverse atelectasis, which frequently develops during anesthesia and surgery; the patient should then be extubated under positive pressure (Benoit et al., 2002; Tusman et al., 2003) to prevent postoperative oxygen desaturation. Positive airway pressure at the moment of extubation causes a coughing motion, which helps to expel secretions around the vocal cords. Positive extending pressure on the upper airway walls also attenuates the excitation of the superior laryngeal nerve and may diminish the risk for laryngospasm (Suzuki and Sasaki, 1977; Sasaki, 1979) (see Chapter 3, Respiratory Physiology in Infants and Children, and Chapter 13, Induction, Maintenance, and Recovery).
Postoperative Management
Children with hypertrophic tonsils and adenoids tend to have increased airway obstruction in the immediate postoperative period. The presence of blood and secretions in the pharynx and larynx may provoke upper airway reflexes, leading to laryngospasm. These patients tend to become hypoxemic more often and perhaps more severely during the first several hours after surgery than patients undergoing procedures not involving the upper airways (Motoyama and Glazener, 1986). However, they seem to maintain their preoperative levels of oxygen saturation in room air thereafter, as determined with pulse oximetry (Motoyama and Borland, unpublished observations). Fortunately, serious complications after adenotonsillectomy are infrequent. Postoperative hemorrhage, however, does occur and can become a life-threatening catastrophe (discussed later). Postoperative emesis is relatively common because of pharyngeal mucosal irritation from surgery, which may stimulate the glossopharyngeal nerves, or from bloody secretions that are swallowed. It is therefore prudent for the anesthesiologist to administer antiemetics (e.g., ondansetron or metoclopramide) prophylactically, in addition to dexamethasone before the end of surgery (Pappas et al., 1998). Pain after tonsillectomy is mild to moderate and may be controlled by opioids given intraoperatively as part of anesthetic management or by supplementation (morphine, 50 mcg/kg; fentanyl, 0.5 to 1.0 mcg/kg; hydromorphone, 10 mcg/kg) in the postanesthetic care unit. Postoperative pain after adenoidectomy is relatively mild. Acetaminophen is a good adjunct to pain control in these patients.
It has been reported that children with OSAS have reduced opioid requirements after adenotonsillectomy. In addition, children with OSAS spontaneously breathing during halothane anesthesia are more likely to become apneic than non-OSAS children when treated with the same dosage of fentanyl (Waters et al., 2002). However, others have found no morbidity from opioid or benzodiazepine treatment in OSAS pediatric patients undergoing adenotonsillectomy (Helfaer et al., 1996; Sanders et al., 2006; Leong and Davis, 2007).
Preoperative sedation with benzodiazepines and postoperative treatment with opioids should be tailored to meet the needs of the patient. It would be prudent to titrate opioids, anticipating that the more severe the OSAS, the higher is the risk of opioid and benzodiazepine sensitivity. For patients with moderate or severe OSAS, many anesthesiologists prefer not to administer opioids until the patient has fully emerged from the anesthetic, has regained spontaneous ventilation, has been extubated, and is able to maintain an open airway with spontaneous ventilation. The patient’s opioid therapy may then be titrated carefully to achieve analgesia without respiratory compromise (Leong and Davis, 2007). During the immediate postoperative period, monitoring these patients in a postanesthetic care unit staffed with pediatric recovery room nurses allows a safe postoperative recovery with pain control.
Children with OSAS have a higher incidence of postoperative respiratory complications, including prolonged oxygen requirements, airway obstruction requiring nasal airway, and major respiratory compromise requiring airway instrumentation, than children without OSAS (Biavati et al., 1997; Wilson and Robertson 2002). Because OSAS is a disorder of anatomic and dynamic factors of upper airway function, it is not surprising that a procedure that is curative in many of these children would cause significant postoperative respiratory complications. Because these children are presumed to have impaired neuromuscular control of upper airway patency, residual anesthetic effects combined with blood, edema, and residual lymphoid tissue obstructing the postsurgical airway can lead to postoperative respiratory events (Wilson et al., 2002). McColley and colleagues (1992) reported a 58% incidence of severe respiratory compromise in children younger than 3 years that was associated with severe oxygen desaturation (SpO2, ≤ 70%) or hypoventilation from upper airway obstruction. Among children with OSAS undergoing adenotonsillectomy (mean age, 5 years), intraoperative and postoperative complications are particularly high among those who were born prematurely (85% versus 25% to 34% in full-term infants).
Nixon and coworkers (2005) demonstrated that, not infrequently, children with OSAS continue to have polysomnogram- documented airway obstruction the night after adenotonsillectomy. Obstructive apneas and hypopneas were four times as frequent and associated with greater oxygen desaturation in the severe OSAS group as opposed to the mild OSAS group.
The subpopulation of children with OSAS who must be monitored in the hospital is still unknown. Children who are most likely to experience postoperative respiratory complications and have a higher postoperative respiratory disturbance index on their postoperative polysomnogram include children 3 years of age and younger, children with severe OSAS diagnosed by preoperative polysomnography, and those with associated medical conditions such as hypotonia, morbid obesity, failure to thrive, or severe structural airway abnormalities (Statham et al., 2006; Karlson 2008). These children are not candidates for outpatient surgery facilities and should receive medical care in centers with pediatric inpatient facilities and pediatric intensive care support. High-risk patients should be monitored overnight with continuous pulse oximetry, because standard apnea monitoring is unable to detect obstructive apnea and hypopnea. Patients can be discharged when significant oxygen desaturation during sleep has resolved.
A rare complication of adenotonsillectomy in children with severe OSAS is pulmonary edema resulting from relief of upper airway obstruction (Mehta et al., 2006). The exact cause is unknown. Galvis and colleagues (1980) hypothesized that during obstructed breathing, extreme negative pressures occur in the intrapleural and intrathoracic compartments. Intubation results in sudden equalization of pressure. The pulmonary venous pressure is suddenly much higher than intrathoracic pressure, resulting in pulmonary hyperemia and edema. Furuhashi-Yanaha and colleagues (2000) hypothesized that the abrupt switch from nasal to oral breathing during emergence from anesthesia might create acute obstruction, which creates an increase in negative intrapleural pressure, leading to pulmonary edema. Regardless of the cause, these children require oxygen and possible reintubation to provide CPAP or positive end-expiratory pressure. This condition usually resolves in 24 to 48 hours (Carcillo, 2003), and the use of diuretics is controversial.
Bleeding after Tonsillectomy
Occasionally, bleeding continues or recurs after tonsillectomy, and the child must be anesthetized to suture or pack the bleeding area. Posttonsillectomy bleeding is classified as primary or secondary bleeding. Primary bleeding occurs within the first 24 hours after surgery and is usually more brisk and profuse than secondary bleeding. Most fatal hemorrhages occur within the first 24 hours after surgery (Randall and Hoffer, 1998). Most posttonsillectomy bleeding, however, occurs within the first 6 hours after surgery (Crysdale and Russel, 1986; Carithers et al., 1987; Guida and Mattucci, 1990).
Secondary bleeding usually occurs 24 hours to 5 to 10 days after surgery, when the eschar covering the tonsillar bed sloughs (Verghese and Hannallah, 2001). Allen and coworkers (1973) reported a 0.1% incidence of posttonsillectomy bleeding necessitating repeat exploration in the operating room. Crysdale and Russel (1986) reported a 2.15% incidence of bleeding during the overnight stay in the hospital for 9400 children undergoing tonsillectomy with or without adenoidectomy. Of these, 3% (0.06% of all patients) required reexploration under general anesthesia.
The child with tonsillar bleeding may be brought back to the anesthesia service several hours or even days or weeks after the surgery. Because primary bleeding tends to be more rigorous, bleeding may obstruct the view of the larynx, and emergent tracheostomy may be needed. The otolaryngologist should be present before anesthetic induction. Whether the bleeding is primary or secondary, several issues must be addressed before induction of anesthesia; the child usually is hypovolemic, anemic, agitated, or in shock, with a stomach full of blood clots and often without an intravenous catheter. This is one of the most dangerous and challenging situations in pediatric anesthesia practice, and the failure to initiate prompt action has been one of the main causes of death associated with tonsillectomy (Alexander et al., 1965).
The anesthesiologist must be aware that the child and parents are extremely frightened. If the condition permits, the patient may be sedated with intravenous midazolam to relieve anxiety. Before the induction of anesthesia, precautions for a patient with a full stomach should be taken. These include at least one, but preferably two, well-functioning suction apparatuses with large-bore suction tubes, extra laryngoscope handles and blades, and several cuffed endotracheal tubes with lubricated stylets in place. An experienced assistant should always be available to help the anesthesiologist and to apply cricoid pressure during the rapid-sequence induction (see Chapter 13, Induction, Maintenance, and Recovery).
The death of a child as a consequence of hemorrhage after tonsil and adenoid surgery is particularly tragic because the operation in most cases is elective and the child is in relatively good health. Mortality rates reported in the literature range from 1 in 1000 to 1 in 27,000 (10 to 0.37 per 10,000) in old reports (Bluestone et al., 1975; Avery and Harris, 1976). The anesthesia-related mortality unadjusted for age is reported to be 1 in 14,000 (Avery and Harris, 1976). A more recent survey in Europe shows the posttonsillectomy mortality rates of 0.62 to 0.63 per 10,000 (Windfuhr et al., 2009). Windfuhr and colleagues (2008a, 2008b) also reported life-threatening posttonsillectomy hemorrhage in 79 patients between 1980 and 2006. There were 36 children involved in the study. Out of 29 deaths, 18 were children. Nearly 90% of the life-threatening crises were related to secondary hemorrhage (>24 hours), and all deaths were directly or indirectly attributable to posttonsillectomy hemorrhage.
Peritonsillar Abscess
Peritonsillar cellulitis and abscesses occur more frequently in older children and adults than in young children. Most infections appear to originate in the tonsils and spread to the peritonsillar space between the tonsillar capsule and the superior constrictor muscle. The infection may spread upward into the palate, but it usually does not invade the posterior tonsillar pillar or the posterior pharyngeal wall (Teele, 1983). The patient presents with complaints of severe sore throat, difficulty in swallowing, and high fever. Progressive difficulty in opening the mouth may develop because of spasm of the pterygoid muscles. It is therefore imperative to determine the extent of limitation of mouth opening preoperatively. The affected tonsillar area is markedly inflamed and swollen, and the uvula is displaced to the opposite side (Cook, 1982). In older children and adolescents, it is possible to perform incision and drainage of a peritonsillar abscess with intravenous sedation using an opioid and topical or local anesthesia, with the patient in a head-down position and the head turned to the side of the abscess. If this is not possible, general endotracheal anesthesia is necessary.
Use of the Laryngeal Mask Airway During Adenotonsillectomy
Since the early 1990s, several reports (but no scientific studies) have described the use of the armored LMA rather than an endotracheal tube for airway support during tonsil or adenoid surgery. The armored LMA can be held within the Boyle-Davis gag and remain patent (Alexander, 1990) (Fig. 24-6). A reported benefit of using the LMA is avoiding tracheal intubation and its associated complications (trauma, cardiovascular stimulation, endobronchial intubation, coughing, laryngospasm, and subglottic edema) (Hatcher and Stack, 1999). The disadvantages of the armored LMA include the risk for aspiration of stomach contents and the risk of inadequate positioning (Hatcher and Stack, 1999).
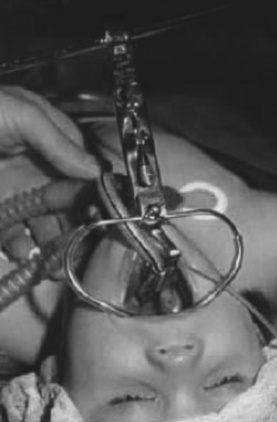
FIGURE 24-6 Armored laryngeal mask airway used for adenotonsillectomy in a child.
(From Kretz FJ, Reimann B, Stelzner J, et al: The laryngeal mask in pediatric adenotonsillectomy: a meta-analysis of medical studies [in German]. Anaesthesist 49:706, 2000. With permission of Springer Science and Business Media.)
Hern and coworkers (1999) reported the surgeon’s perspective on the use of LMA for tonsillectomy. In their study, 44 children were anesthetized using an LMA, and 47 patients were anesthetized using an endotracheal tube before tonsillectomy. There was an 11.4% failure rate for the LMA, requiring change to an endotracheal tube before the end of the procedure. The LMA was reported to hamper the surgeon’s visualization of the field and might have led to removal of less tonsillar tissue in the LMA group. It is difficult to suggest that the LMA is a superior choice for airway management during tonsillectomy. Additional objective data must be accumulated before recommending this technique.
Laser Surgery of the Larynx
The laser (light amplification by stimulated emission of radiation) has been used increasingly in otolaryngology since the introduction of the CO2 laser for laryngeal surgery (Strong et al., 1973). The laser is a beam of coherent electromagnetic radiation that can be focused to a very small spot with precision, resulting in controlled coagulation, incision, or vaporization of the target tissue without affecting the neighboring tissues (Geffin et al., 1986; Keon, 1988). Clinical uses of the laser for upper and lower airways and its anesthetic implications have been reviewed elsewhere (Beamis et al., 1991; Rampil, 1992; Pashayan, 1994).
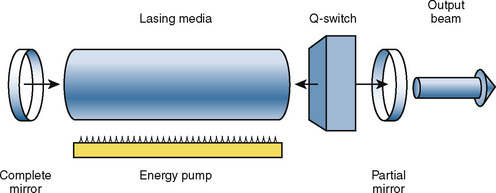
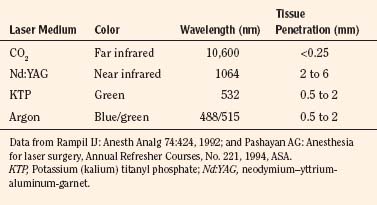
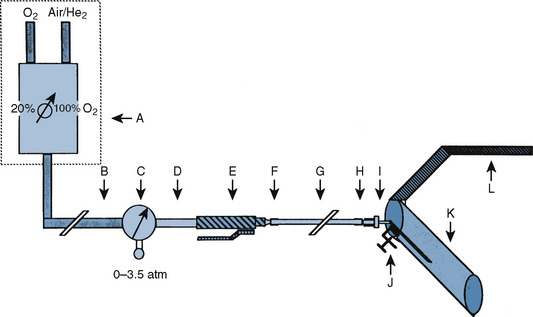
The principal components of a laser system include a lasing medium (gaseous or solid) that holds the molecules whose electrons create the laser light, resonating mirrors to boost lasing efficiency, and an energy source that pumps the lasing molecules into producing laser light (Rampil, 1992) (Fig. 24-7). Some lasers use a gaseous lasing media, such as CO2, argon, or helium-neon, whereas others use solid rods of laser-passive material that contain small quantities of ionic impurities, such as chromium (for ruby laser) and neodymium (Nd). A synthetic gem crystal, yttrium-aluminum-garnet (YAG), is commonly used as a passive host matrix (Rampil, 1992). Fiberoptic bundles are used for beam delivery of lasers of visible and near-infrared wavelengths. For the CO2 laser with invisible far-infrared wavelength, the most common delivery system is an articulated arm containing front-surface mirrors at each junction, which is used together with a low-powered visible helium-neon gas laser beam through the same optical path as a beam guide (Rampil, 1992; Pashayan, 1994). Recently, a flexible, hollow waveguide for delivery of the CO2 has become available. Physical characteristics of commonly used lasers are described in Table 24-5.
The most commonly used laser for laryngeal surgery is the CO2 laser. This has a long, far-infrared wavelength and is completely absorbed by the bending frequency of the water molecules; it is absorbed completely in the first few layers of cells and produces very little scatter of energy. The powerful, focused CO2 laser beam therefore produces explosive vaporization of the target tissue surface with minimal damage to the underlying tissues (Beamis et al., 1991; Rampil, 1992). The thermal effect (i.e., coagulation lateral to the zone of vaporization) can be varied between 80 and 400 mcm and is diminished with increasing water content or vascularity of the tissue or by the use of less power. Thermal coagulation also decreases with shorter pulse duration. The CO2 laser beam is delivered to the target tissue by means of an articulated arm and a micromanipulator attached to the microscope or a bronchoscope coupler for tracheobronchial applications (Werkhaven, 1995). The micromanipulator allows surgical precision on the order of 250 mcm.
The potassium (kalium) titanyl phosphate (KTP) laser and argon laser operate in the green and blue-green wavelength range of the spectrum. The KTP and argon laser beams are transmitted through clear substances and are well absorbed by pigments in the tissues, especially melanin and hemoglobin. They therefore have widespread application for certain lesions, such as hemangiomas and granulation tissue (Werkhaven, 1995). The energy scatter is intermediate between the CO2 and Nd:YAG laser beams. Because the depth of thermal damage produced by a laser beam beyond the immediate vaporization site partially depends on scatter, it produces vaporization (but less than that of the CO2 laser) in addition to coagulation and cutting (Beamis et al., 1991). The depth of tissue penetration is 0.5 to 2 mm (Pashayan, 1994). KTP and argon laser beams, as well as the Nd:YAG beam, can be transmitted by means of fiberoptic bundles through the rigid or flexible bronchoscope to vaporize or coagulate lesions in the tracheobronchial lumen (Geffin et al., 1986; Ward, 1992).
The Nd:YAG laser has a near-infrared wavelength, is transmitted through clear fluids, and is readily absorbed by proteins. It is absorbed much less by water, and the beam is transmitted and scattered through much larger volumes of tissue. The energy of the Nd:YAG laser beam is therefore widely disseminated and produces less vaporization and more thermal coagulation. The depth of tissue penetration may range from 2 to 6 mm (Pashayan, 1994). The Nd:YAG laser is transmitted by means of flexible optic fibers that can be directed through the side suction port of ventilating bronchoscope. The ablation of granulation tissue, mixed capillary-cavernous hemangioma, or obstructive vascular tumor in the tracheobronchial wall may be accomplished successfully with the Nd:YAG laser (Werkhaven, 1995).
Risks Associated with Laser Surgery
Because of the potential danger with the use of laser beams in the operating room, the users and other operating room personnel should follow the safety guidelines established by the American National Standard for the Safe Use of Laser in Health Care Facilities and published by the American National Standard Institute (the ANSI Z136 Standard) (ANSI, New York). Doors to operating rooms where laser surgery is in progress should be clearly marked to prevent unprotected personnel from accidentally straying into the path of laser beams. Warning signs should specify the class and type of the laser being used as well as appropriate protective measures specific for the wavelength (Rampil, 1992; Pashayan, 1994).
All laser beams for medical use are transmitted through air and are well reflected by smooth metal surfaces. Patients and health care personnel involved in laser surgery are at risk for laser injury, particularly of the eyes. The nature and extent of eye injury depend on the wavelength of the laser beam and its energy. Because the laser beam of far-infrared wavelength is absorbed by the surface it first encounters, errant CO2 laser beams can cause serious corneal ulcerations and scar formation (Liebowitz and Peacock, 1980). Visible and near-infrared lasers (e.g., KTP, argon, Nd:YAG) penetrate through the transparent cornea and anterior chamber of the eye, are absorbed by the retina, and cause serious damage. The anesthetized patient’s eyes should be taped closed and covered with saline-soaked eye pads or metal shields, or both (Keon, 1988). Operating room personnel must wear safety goggles that are specific for the laser wavelength in use. Safety goggles should provide wraparound protection from reflected beams (Rampil, 1992). For CO2 lasers, any plastic or regular eyeglasses (not contact lenses or thin plastic splash protection shields) will suffice as protection (provided that lateral aspects of the eye are also covered), because far-infrared beams are completely absorbed. Visible and near-infrared lasers require specific filters for particular laser wavelengths.
Incandescent debris resulting from the explosive vaporization of the tissue in contact with the laser beam produces a plume of smoke with fine particulates (0.1 to 0.8 mcm), which may contain infectious or mutagenic viral particles (Nezhat et al., 1987; Kokosa and Eugene, 1989). Fragments of DNA have been found in the smoke plume, but only one study has found viral transmission from the laser plume (Garden et al., 2002). The same risk exists for the smoke plume from electrocautery. Ordinary surgical masks do not effectively filter particles smaller than 3.0 mcm; special high-efficiency masks are therefore needed to filter laser plume particles (Rampil, 1992).
A laser beam can ignite inflammable materials used for general anesthesia, such as endotracheal tubes, anesthesia circuits, oil-based lubricants, ointments, sponges, and drapes (Snow et al., 1976; Cozine et al., 1981; Hermens et al., 1983). All materials used for endotracheal tubes, with the exception of special metallic tubes, are quite vulnerable to laser impact (Geffin et al., 1986; Sosis, 1989a, 1989b). Polyvinyl chloride (PVC) tubes appear to be more easily punctured and ignited by CO2 laser than red-rubber tubes, and to produce more toxic combustion fumes (Ossof et al., 1983), although black smoke from burning rubber is probably just as damaging to the airway mucosa. Wolf and Simpson (1987) found that once PVC is ignited, it is less flammable than silicone or red rubber, with a flammability index (i.e., the fraction of inspired oxygen [Fio2] at which the material ignites) of 0.26, compared with silicone (0.19) and red rubber (0.18); this means that silicone and rubber tubes continue to burn in room air, whereas PVC tubes do not. Red-rubber tubes, however, are more resistant to puncture than PVC tubes (41 versus 0.8 seconds with PVC, at the same power density) and intramural fires are less likely to occur (Ossof, 1989). For these reasons, metal tubes are preferred overall.
During laser surgery, when a nonmetallic endotracheal tube is used, the Fio2 should be reduced to 30% or less, as long as the patient is adequately oxygenated, to reduce combustibility of endotracheal tubes (Hermens et al., 1983). Oxygen may be diluted by nitrogen or helium to reduce the flammability of endotracheal tubes (Pashayan and Gravenstein, 1985; Simpson et al., 1990). Oxygen concentrations up to 60% may be used if helium is the diluent gas. Nitrous oxide supports combustion above 450° C and should therefore be avoided (Wolf and Simpson, 1987; Keon, 1988). It is imperative that the patient be immobilized during laser surgery. The choice of anesthetics depends largely on the technique of ventilation during the laser surgery.
Airway Fire
In the unfortunate event of an airway fire, a mnemonic of the 4 Es—extract, eliminate, extinguish, and evaluate (Box 24-6)—may help guide management. In the event of an airway fire, immediately extract all combustible materials from the airway such as pledgets or endotracheal tubes, even if they are still burning. Second, eliminate the source of oxygen being delivered to the endotracheal tube. The continued delivery of oxygen to a combustible source produces a blowtorch effect that can further ignite material in the vicinity. Third, extinguish any other fires in the vicinity. If a combustible material such as a pledget is still in the airway and cannot be removed, saline flush should be used to extinguish the fire. Fourth, evaluate any damage that may have been caused by the fire or the combustion byproducts. The operative field needs to be examined along with the lower tracheobronchial tree. Standards and guidelines for the management of surgical fires, including airway fires, have been published (A Clinician’s Guide to Surgical Fires, 2003).
Ventilatory Management During Laser Surgery
Numerous anesthetic approaches have been reported for airway management during laryngeal laser surgery. General transtracheal anesthesia, through a preexisting tracheostomy, is the easiest and probably the safest method. It is performed by replacing the preexisting tracheostomy cannula with a metal tracheostomy tube. Manually operated intermittent jet ventilation (Rontal et al., 1980; Scamman and McCabe, 1986; Ravussin et al., 1987) or high-frequency jet ventilation (Smith et al., 1975) by means of a needle or a catheter through the cricothyroid membrane has been used. These techniques, however, have a potential risk for barotrauma to the upper airways and overdistention of lungs (e.g., pneumothorax, pneumomediastinum), particularly when the laryngeal airway is obstructed. Subcutaneous emphysema and pneumomediastinum also may occur because of needle misplacement or a direct leak at the tracheal puncture site (Borland and Reilly, 1987). Without tracheostomy or transtracheal puncture, the maintenance of airways and pulmonary ventilation during laser surgery can be accomplished by means of manual jet ventilation (i.e., Saunder’s jet) without an endotracheal tube. A combination of intravenous propofol, topical lidocaine, and small dosages of opioids (i.e., fentanyl or remifentanil infusion) has been used successfully with the patient breathing spontaneously (Borland LM, unpublished data).
Nonflammable Endotracheal Tubes
Red-rubber tubes wrapped with reflective aluminum or copper adhesive-backed tape, originally described by Snow and colleagues (1974), have been used effectively (Norton et al., 1976), but the use of these homemade, laser-resistive tubes has decreased considerably over the past decade with the advent of commercially available nonflammable endotracheal tubes. Before wrapping a red-rubber tube with a metal tape, the tube should be wiped with alcohol to remove greasy or oily residues that interfere with adhesion. It should also be wiped with tincture of benzoin or an equivalent before spiral wrapping with a metal tape (Rampil, 1992). There should be no windows of exposed tube; care should be taken to prevent wrinkles, which may cause abrasion of the laryngotracheal mucosa.
Certain metallic tapes do not sufficiently prevent the CO2, Nd:YAG, or KTP laser beams from penetrating the endotracheal tube and igniting a blowtorch fire. The CO2 laser can ignite the adhesive backing of all these tapes and perforate the aluminum tapes within 0.1 second (Sosis, 1989a). Other aluminum tapes (3M No. 425 and 433; 3M Corp., St. Paul, MN) and a copper foil tape (Venture Tape Corp., Rockland, MA; 3M Corp.) are effective in shielding at least 60 seconds of direct exposure (Sosis and Dillon, 1990). A metallic foil wrap for endotracheal tubes, specifically manufactured for the laser, is also available (Laser Guard, Merocel Corp., Mystic, CT) and is approved by the U.S. Food and Drug Administration. This metal-based surgical sponge provides protection against CO2, argon, and KTP lasers but not against Nd:YAG lasers, according to the study by the Emergency Care Research Institute (ECRI, 1990). It also provides a smoother surface than metal tapes on the endotracheal tube for better airway mucosal protection, but it is bulky, adding about 2 mm to the diameter of the endotracheal tube.
A number of laser-resistant endotracheal tubes with metal exteriors have become available for clinical use. The Laser Flex tube (Mallinckrodt, St. Louis, MO; Bivona, Gary, IN) is a stainless steel spiral with a PVC tip, with or without two distal saline-inflatable cuffs (Fig. 24-8). This tube is resistant to CO2 and the Nd:YAG laser. The Fome Cuff tube (Bivona) is an aluminum spiral tube with an outer coating of silicone that is approved by the manufacturer for the CO2 laser only. This tube has a self-inflating foam sponge–filled cuff. The foam in the cuff prevents deflation of the cuff after puncture, but damage to the filling tube may cause difficulty in deflating the cuff (Rampil, 1992). The Laser Shield II (Medtronic/Xomed, Jacksonville, FL) is silicon-based endotracheal tube wrapped with metal foil and then wrapped in polytetrafluoroethylene tape to smooth the tube. These tubes are available only in two sizes, and the smaller size (5.0) has an outside diameter with a cuff roughly equivalent to a size 5.5 endotracheal tube.
Anesthesia with Manual Jet Ventilation
A more commonly accepted and practiced method for ventilation during laryngeal laser surgery is the use of a Venturi apparatus (i.e., Saunders’ jet injector), originally described by Saunders (1967) and Spoerel and coworkers (Donlon and Benumof (1996) and applied in pediatric patients by Miyasaka and colleagues (1980) as well as others (Johans and Reichert, 1984; Scamman and McCabe, 1986; Borland and Reilly, 1987). The apparatus consists of an injector and tubing, a toggle switch, a reducing valve or regulator, and tubing with a connector to a high-pressure wall oxygen supply (50 psi) (Figs. 24-9 and 24-10). After a precordial stethoscope, a pulse oximeter sensor, electrocardiogram leads, and a blood pressure cuff are positioned on the child, and anesthesia is induced, usually by mask with nitrous oxide, sevoflurane, and oxygen. An intravenous catheter is inserted, an axillary or rectal temperature probe is placed, and a nerve stimulator is positioned. Cisatracurium (or other intermediate-acting muscle relaxant), fentanyl (2 to 3 mcg/kg), and lidocaine (1 mg/kg) are injected intravenously. Midazolam may be given to ensure amnesia during the laser surgery. Alternatively, anesthesia may be switched to intravenous propofol with or without a small dosage of fentanyl. Nitrous oxide is discontinued, and the patient is ventilated with 100% oxygen for several minutes before the mask is removed. The surgeon positions the child’s head and inserts a Jako suspension laryngoscope to secure a patent laryngeal airway.

FIGURE 24-10 A Saunders-type, hand-held jet ventilation apparatus.
(Courtesy Manujet III, VBM Medizintechnik, Sulz am Neckar, Germany.)
For supraglottic ventilation, a 10-French metal injector is inserted into the side channel of the suspension laryngoscope and secured with the distal end 1 to 2 cm above the glottic opening; the metal injector is readjusted as needed to obtain the optimal distance and direction. Oxygen concentration of the jet injector is adjusted with an oxygen blender to reduce the inspired oxygen concentration with room air entrainment to 30% or less, provided that oxygen saturation on the pulse oximeter is 98% or higher. The anesthesiologist begins jet ventilation by intermittently squeezing the toggle switch. Short bursts of inspiration with air entrainment (1 to 2 seconds) with a driving pressure of 15 psi or less (the pressure may vary with different setups), followed by enough expiration time (2 to 4 seconds) to complete exhalation, provides adequate pulmonary ventilation. Close observation of the child’s chest motion during each breath is crucial to prevent air trapping, hyperinflation, and volutrauma to the lungs (Borland and Reilly, 1987). Inspired oxygen concentration is decreased as low as possible by adjusting the oxygen-blender to maintain SpO2 between 98% and 100% throughout the procedure. If passive exhalation is unusually prolonged, measures to improve airway patency must be taken immediately. This can usually be accomplished by readjusting or redirecting the injector. Occasionally, when papillomatous growth about the glottis is extensive, the child may require intubation with a small tube and manual ventilation while the surgeon improves the glottic aperture by debulking papillomas on and around the vocal cords.
The manual jet ventilation technique offers several advantages in laryngeal laser surgery, including adequate ventilation, excellent surgical access to the larynx (especially to the anterior and posterior commissures where an endotracheal tube obliterates the exposure), and the absence of ignitable material in the surgical field (Norton et al., 1976; Goforth et al., 1983). Potential risks of this technique include pneumothorax, pneumomediastinum, distention of the stomach and regurgitation, aspiration of resected material, and dehydration of mucosal surface (Lines, 1973; Norton et al., 1976; Rontal et al., 1980; Ruder et al., 1981; Keon, 1988).
Tracheostomy
Tracheostomy is indicated in numerous situations before development of respiratory distress (Stool and Eavey, 1990). The three primary indications for the creation of an artificial airway are airway obstruction, chronic assisted ventilation, and pulmonary toilet (Wetmore et al., 1999; Carron et al., 2000). Table 24-6 lists the conditions for which tracheotomy has been advocated in pediatric patients (Wetmore, 2003).
The ideal tracheotomy tube conforms to the trachea and neck without causing undue pressure or injury to the skin or tracheal mucosa. Plastic tracheotomy tubes are commonly used because they cause minimal tissue reaction. Tracheotomy tubes should be manufactured in metric sizes that correspond to endotracheal tube sizes. However, metal tracheotomy tubes are still measured in French sizes (Wetmore, 2003). Table 24-7 compares the sizes of the endotracheal tubes and commonly used tracheotomy tubes in infants and children (Wetmore, 2003).
Anesthetic Approach for Tracheostomy
Urgent tracheotomy may be required in neonates for congenital airway defects and for older children because of upper airway trauma, corrosive ingestion, infections, and tumors (see Table 24-6). Although these children may initially maintain oxygenation and ventilation, they are at risk for acute respiratory failure. They may have airway anomalies that contribute to difficult or impossible tracheal intubation. The location of the obstruction dictates the surgical and anesthetic approach. The anesthesiologist must establish whether the child can maintain a patent airway under anesthesia and whether they can be intubated by standard laryngoscopy or fiberoptic bronchoscopy. Sedation with ketamine may also be an option. Otherwise, anesthesia is induced with an inhaled anesthetic agent after preoxygenation in the position in which the child is most comfortable, often the sitting position. If no intravenous catheter is in place, one is inserted, and atropine (0.01 to 0.02 mg/kg) or glycopyrrolate (0.01 mg/kg) may be injected intravenously as indicated after induction of anesthesia. Unless a normal, unobstructed airway can be maintained, muscle relaxants should not be used because total upper airway obstruction may result. After the child is anesthetized but spontaneously breathing, topical lidocaine (up to 5 mg/kg) may be sprayed on the larynx to ablate laryngeal reflexes before laryngoscopy. If intubation is impossible, an LMA may be used to maintain an airway until the tracheotomy is performed. Tracheostomy is best performed in infants with an endotracheal tube or a rigid bronchoscope in place to ensure adequate pulmonary gas exchange. The endotracheal tube also helps the surgeon to identify the trachea. Many surgeons prefer to use a rigid bronchoscope held in place rather than an endotracheal tube. The rigid bronchoscope allows a firmer anatomic reference for the trachea, but it cannot be taped in place and must be held by hand. Elective tracheotomy is usually performed in children already intubated for chronic ventilation or pulmonary toilet or performed in combination with tumor removal or repair of tracheal abnormalities.
For tracheotomy, the patient is positioned with the shoulders elevated by placing a roll under the neck so that the neck is hyperextended. Some surgeons request that the anesthesiologist hold the chin with the left hand to keep the neck stretched so that soft tissue over the trachea is stabilized (Fig. 24-11) (Stool and Eavey, 1990). The surgeon palpates the neck to identify the thyroid and cricoid cartilages, sometimes with difficulty. The trachea is palpated down to the sternal notch, guided by the presence of an endotracheal tube. A skin incision is made about one fingerbreadth above the sternal notch, either horizontally or vertically. When the fascial layer is incised under the subcutaneous fat and the trachea is identified, a pair of stay sutures, one on each side of the tracheal incision, is placed for traction during the cannulation of the tracheotomy tube. The stay sutures also serve to prevent asphyxiation in the event that the tracheotomy tube becomes displaced in the immediate postoperative period before the tract has formed (Myers et al., 1985). Before the tracheotomy cannula is inserted in the tracheal incision, the endotracheal tube or bronchoscope is slowly pulled back to accommodate the insertion. The endotracheal tube or bronchoscope should not be removed completely until successful ventilation is demonstrated after placement of the tracheotomy tube. The anesthesia delivery tube is then disconnected from the endotracheal tube under the drapes and reconnected to the proximal universal adapter end of the tracheotomy tube over the operative field.
An esophageal stethoscope or a nasogastric tube has occasionally been misidentified as an endotracheal tube in the trachea by the surgeon, and inadvertent esophageal incision has been made (Schwartz and Downes, 1977); esophageal stethoscopes therefore should be avoided during tracheotomy. The child is monitored with a precordial stethoscope, pulse oximeter, capnography, electrocardiography, automated blood pressure cuff, and rectal or skin thermometer.
Laryngeal Stenosis
Subglottic Stenosis
Neonatal subglottic stenosis can be categorized as congenital or acquired. The incidence of congenital subglottic stenosis is 5%; the remaining cases are acquired. Subglottic stenosis in the full-term infant is defined as a subglottic airway diameter of less than 4 mm at the level of the cricoid cartilage, and less than 3 mm in the premature infant (Walner et al., 2001). This narrowing can be compared with the reference point of a neonatal endotracheal tube. The outer diameter of a 2.5 endotracheal tube is 3.6 mm, and the outer diameter of a 3.0 endotracheal tube is 4.2 mm (Rutter et al., 2003). The diagnosis of subglottic stenosis is made by rigid endoscopy while the patient is anesthetized. The extent of the subglottic stenosis is determined by placement of an endotracheal tube that allows an airway leak between 10 and 25 cm H2O (Table 24-8). This leak allows accurate measure of the airway size. The airway stenosis is then graded as follows: grade I, less than 50% obstruction; grade II, 51% to 70% obstruction; grade III, 71% to 91% obstruction; and grade IV, no detectable lumen (Gerber and Holinger, 2003).
In the absence of trauma, an abnormality of the cartilage or subglottic tissues is usually considered congenital. The cause of congenital subglottic stenosis is thought to be a failure of the laryngeal lumen to recanalize after completion of normal epithelial fusion at the end of the third month of gestation (Walander, 1955). Congenital subglottic stenosis lies on a continuum of embryologic failure that includes laryngeal atresia, stenosis, and webs. In its mildest form, congenital subglottic stenosis merely represents a normal-appearing cricoid with a smaller-than-average diameter, usually with an elliptical shape. Infants and children with mild subglottic stenosis may present with a history of recurrent upper respiratory infections, often diagnosed as croup, in which minimal glottic swelling precipitates airway obstruction. The location of the stenosis is usually 2 to 3 mm below the true vocal cords (Rutter et al., 2003).
Severe congenital subglottic stenosis can be a life-threatening airway emergency manifesting immediately after the infant is delivered. See related video online at www.expertconsult.com.![]() If endotracheal intubation is successful, the patient may require intervention before extubation. In more severe cases, tracheotomy can be life saving at the time of delivery. Infants with congenital subglottic stenosis may have surprisingly few symptoms and may not present for treatment for weeks or months after birth (Rutter et al., 2003). After the initial management of congenital subglottic stenosis, the larynx will grow with the patient and may not require further surgical intervention.
If endotracheal intubation is successful, the patient may require intervention before extubation. In more severe cases, tracheotomy can be life saving at the time of delivery. Infants with congenital subglottic stenosis may have surprisingly few symptoms and may not present for treatment for weeks or months after birth (Rutter et al., 2003). After the initial management of congenital subglottic stenosis, the larynx will grow with the patient and may not require further surgical intervention.
Laryngomalacia is a congenital condition of excessive flaccidity of the laryngeal structures, especially the epiglottis and arytenoids, most likely caused by the lack of neural control of laryngeal muscles. See related video online at www.expertconsult.com.![]() Laryngomalacia accounts for more than 70% of persistent stridor in neonates and young infants (Fig. 24-12); the remaining 10% of neonatal stridor is caused by vocal cord paralysis (Yellon et al., 2002) (Fig. 24-13).
Laryngomalacia accounts for more than 70% of persistent stridor in neonates and young infants (Fig. 24-12); the remaining 10% of neonatal stridor is caused by vocal cord paralysis (Yellon et al., 2002) (Fig. 24-13).
In the mid-1960s, long-term intubation was introduced as the preferred artificial airway in neonates (McDonald and Stocks, 1965). As neonatal survival rates increased with the advent of neonatology and improved neonatal intensive care, subglottic stenosis occurred as a frequent morbidity caused by long-term tracheal intubation. Acquired subglottic stenosis results from intubation trauma and accompanying inflammatory responses. Factors suspected of contributing to this problem include prematurity, the size and amount of movement of the endotracheal tube, duration of intubation, laryngeal or tracheal injury during intubation, and presence of infection during the course of tracheal intubation. The incidence of acquired subglottic stenosis has been decreased over the past decade by minimizing the issues known to exacerbate subglottic injury (Walner et al., 2001). Patients presenting to the otolaryngologist for diagnosis or treatment of subglottic stenosis are former preterm infants who were intubated in the neonatal ICU for extended periods and have a tracheotomy in place with an established diagnosis, or are examined on an outpatient basis for symptoms suggesting subglottic stenosis (Cotton, 2000).
Surgical Management of Acquired Subglottic Stenosis in the Neonate
Neonatal subglottic stenosis unresponsive to nonoperative therapy may require tracheotomy or an anterior cricoid split procedure. Tracheotomy is performed if, in addition to severe subglottic injury, there is substantial glottic or tracheal involvement. After tracheotomy and without the endotracheal tube to act as a stent, the stenosis becomes more severe. Over the next 2 years, the airway may heal, allowing for decannulation, but more often, surgical reconstruction of the trachea will be necessary (Rutter et al., 2003).
The anterior cricothyroidotomy, or cricoid split, is an effective treatment for some cases of acquired neonatal subglottic stenosis in the absence of substantial glottic or tracheal or pulmonary pathology (Rutter et al., 2003). The neonate must meet the following criteria (Cotton, 2000):
The procedure is performed under general anesthesia with muscle relaxation. The shoulders are elevated, and the neck is extended as in the tracheotomy position (see Fig. 24-11). A horizontal incision is made in the skin over the cricoid cartilage. The cricoid cartilage, upper trachea, and lower edge of the thyroid cartilage are exposed. An incision is made through the cricoid ring and underlying mucosa through the first two tracheal rings and the lower one third of the thyroid cartilage. Stay sutures are placed on both severed edges of the cricoid cartilage, as in a tracheotomy (Healy, 1995b). The endotracheal tube that was used for anesthesia and airway management is then removed. It is replaced, while the larynx is still split, with a nasotracheal tube that is one size (0.5 mm) larger than that predicted for the patient’s age and body size. The surgeon helps guide the nasotracheal tube into position through the open incision, and the wound is closed loosely, with a drain. The nasotracheal tube is left in place for 7 days to stent the subglottic aperture. The patient is kept well sedated with or without mechanical ventilation in the pediatric ICU. A modification of the anterior cricoid split is to place a cartilage graft over the split in an effort to allow the airway to seal more rapidly, leading to earlier extubation. Thyroid alar cartilage and occasionally auricular cartilage are used (Rutter et al., 2003).
Laryngotracheal Reconstruction in Children
Chronically ventilated infants who have undergone tracheotomy because of prolonged intubation and subglottic stenosis will most likely require laryngeal reconstructive surgery. Children with greater than 70% obstruction of their laryngeal lumen usually require this surgery to allow decannulation (Rutter et al., 2003).
Laryngotracheal reconstruction has become the standard of care for symptomatic subglottic stenosis in the pediatric age group. There are five stages: characterization of the stenosis, expansion of the tracheal lumen, stabilization of the framework, healing of the airway, and decannulation (Rothschild et al., 1995). The procedure has evolved to include a variety of techniques for expanding the laryngotracheal complex to provide a stable airway of sufficient size. These include, but are not limited to, anterior cartilage graft with the tracheotomy left in place without stent, long-term (several months) stenting with or without cartilage grafts, and short-term (4 to 6 weeks) stenting with anterior or posterior cartilage grafts (Cotton, 2000).
Anterior cartilage graft with a tracheotomy left in place without a stent is indicated primarily for isolated anterior subglottic stenosis with no or relatively mild posterior subglottic components. A variation of this procedure is to remove the tracheotomy at the time of surgery and perform a single-stage laryngotracheoplasty. Posterior division of the cricoid plate and the introduction of a cartilage graft between the cut ends are indicated particularly for children with persistent posterior glottic pathology or primarily posterior subglottic pathology (Cotton, 2000).
Single-stage laryngotracheal reconstruction (LTR) uses cartilage grafts to obtain stability of the reconstructed airway. Single-stage LTR may include an anterior cartilage graft, a posterior cartilage graft, or both, and reconstruction often includes a cartilage graft at the former stoma site. The grafts are supported temporarily by a full-length endotracheal tube fixed in position through the nasal route (Cotton, 2000). The optimal time for extubation has not been established definitively. In general, children remain intubated for 7 to 10 days for anterior cartilage grafts alone, and 12 to 14 days if a posterior and anterior graft is required (Cotton, 2000).
Through a horizontal skin incision over the cricoid cartilage, laryngeal structures are exposed. A vertical incision is made through the lower thyroid cartilage, the cricoid cartilage, and the upper tracheal rings (Fig. 24-14, A). The rib cartilage graft, which had been taken from the anterior chest wall with the external perichondrium intact, is used for the anterior or posterior grafts (see Fig. 24-14, B). Auricular cartilage and septal cartilage have also been used as graft materials. Toward the conclusion of surgery, the armored tube is removed from the tracheotomy stoma and replaced with a cuffed nasotracheal tube, one size larger than is appropriate for the patient’s age and size, to stent the larynx. This intraoperative transition needs to be carefully coordinated between the anesthesiologist and the surgeon.
One requirement of single-stage LTR is meticulous postoperative management of the patient’s condition in the ICU. The nasotracheal airway must be maintained securely during the time of extended stenting without accidental extubation. The postoperative management of patients with single-stage LTR is not standardized (Cotton, 2000). Some centers use sedation to prevent agitation and accidental self-extubation and to avoid pharmacologic paralysis (Rothschild et al., 1995). However, many children do not tolerate nasotracheal intubation and require prolonged sedation and neuromuscular blockade to ensure maintenance of an airway with minimal trauma during healing (Brandom, 1997; Yellon et al., 1997). If neuromuscular blockade is used, daily recovery of neuromuscular function and the avoidance of prolonged use of corticosteroids will be associated with less muscle weakness after extubation (Yellon et al., 1997). Prolonged sedation with benzodiazepines or opioids can lead to withdrawal syndrome, and close observation after weaning from these agents is paramount. The role of dexmedetomidine in pediatric patients for prolonged sedation in the ICU setting continues to be investigated (Chrysostomou et al., 2006, 2009; Bejian et al., 2009).
Acute Supraglottitis or Epiglottitis
Acute infectious epiglottitis or supraglottitis is a relatively uncommon but truly life-threatening disease of childhood. See related video online at www.expertconsult.com.![]() Acute epiglottitis is an acute bacterial infection principally involving supraglottic structures, including the lingular surface of the epiglottis, the aryepiglottic folds, and the arytenoids, with little or no involvement of subglottic structures, including the laryngeal surface of the epiglottis. It is therefore more appropriate to call it supraglottitis. Until the late 1980s, before the vaccination became available, Haemophilus influenzae type b (Hib) was the causative organism in more than 75% of cases. Group A β-hemolytic streptococci account for most of the rare cases of epiglottitis (Gorelick and Baker, 1994). With the advent of effective Hib conjugate vaccines in 1988, the incidence of Hib disease declined dramatically in the United States (Adams et al., 1993; Hoekelman, 1994). The age-specific incidence of Hib disease among children younger than 5 years old decreased by 71%, and that of Hib meningitis by 82%, between 1985 and 1991 (Adams et al., 1993). Similarly, the incidence of acute supraglottitis, one of the most dreaded childhood emergencies, has declined by 84% (Gorelick and Baker, 1994).
Acute epiglottitis is an acute bacterial infection principally involving supraglottic structures, including the lingular surface of the epiglottis, the aryepiglottic folds, and the arytenoids, with little or no involvement of subglottic structures, including the laryngeal surface of the epiglottis. It is therefore more appropriate to call it supraglottitis. Until the late 1980s, before the vaccination became available, Haemophilus influenzae type b (Hib) was the causative organism in more than 75% of cases. Group A β-hemolytic streptococci account for most of the rare cases of epiglottitis (Gorelick and Baker, 1994). With the advent of effective Hib conjugate vaccines in 1988, the incidence of Hib disease declined dramatically in the United States (Adams et al., 1993; Hoekelman, 1994). The age-specific incidence of Hib disease among children younger than 5 years old decreased by 71%, and that of Hib meningitis by 82%, between 1985 and 1991 (Adams et al., 1993). Similarly, the incidence of acute supraglottitis, one of the most dreaded childhood emergencies, has declined by 84% (Gorelick and Baker, 1994).
Until the late 1980s, before the era of Hib conjugate vaccination, preschool children between the ages of 2 and 6 years had the highest incidence of supraglottitis, although it occurred in infants, older children, and occasionally adults (Schloss et al., 1979; Blackstock et al., 1987; Crysdale and Sendi, 1988). The highest number of cases is reported in the spring and fall, but sporadic cases are reported in other seasons as well. The condition usually begins with complaints of severe sore throat associated with dysphagia and a thick, muffled voice without a preceding history of runny nose. Rarely is there a croupy or barking cough, except in atypical cases in infants (Blackstock et al., 1987). The symptoms rapidly progress, and typical pathognomonic signs of dysphagia, dysphonia, dyspnea, and drooling (the 4 Ds) appear (Diaz, 1985).
In most children presenting with stridor in the emergency room, the most common symptoms found in those with acute supraglottitis are drooling, agitation, and absence of spontaneous cough (Mauro et al., 1988). The child looks toxic and usually has a high fever and tachycardia. The disease progresses very rapidly and may be fatal, with severe airway obstruction within 6 to 12 hours, unless immediate steps are taken to restore the patient’s upper airway patency. Classically, the child is sitting up, is dyspneic with the mouth open and drooling, and resists attempts to get him or her to lie down. There is forward chin thrust, slight cervical flexion, and forward flexion at the waist, together with tripod placement of the arms to support this posture. The inspiratory phase is slow, with stridor and retraction, whereas the expiratory phase is unobstructed. The inspiratory sound has been compared with that of a quacking duck.
A laryngoscope with several blades, endotracheal tubes with stylets, a bronchoscope, and a self-inflating bag with face mask and oxygen must accompany the patient for emergency intubation (Oh and Motoyama, 1977). Throat examination should not be attempted in the emergency room, because the risk for complete airway obstruction is great. If the child’s condition permits or when the diagnosis is questionable, the patient may be transferred to the pediatric ICU, where a radiograph of the lateral neck is taken with a portable x-ray machine (Butt et al., 1988). In the ICU, all the help and expertise needed for an emergency should be readily available. In most patients, a swollen epiglottis obstructing the air space (shadow) is seen on the lateral neck radiograph (Fig. 24-15, A). Negative radiographs, however, do not necessarily rule out supraglottitis.
A lateral neck radiograph typically shows a narrowing of the airway shadow below the vocal cords (see Fig. 24-15, B). An anteroposterior radiograph of the neck may show a long area of airway narrowing resembling the steeple of a church (“steeple sign”) (Fig. 24-16).
Anesthetic Management of Acute Supraglottitis
Endotracheal intubation is performed orally with a styletted tube one or two sizes smaller than usual. Visualization of the classic cherry-red epiglottis under direct laryngoscopy confirms the diagnosis (Fig. 24-17). However, the picture may be atypical or only part of the epiglottis may be inflamed or swollen. Inflammatory swelling of other supraglottic structures, including the uvula, arytenoids, aryepiglottic folds, and false vocal cords, also causes severe obstruction. To visualize the vocal cords for intubation, the epiglottis is lifted by the curved tip of a straight laryngoscope blade (e.g., Phillips 1). In children with severe obstruction and mucosal swelling, identification of supraglottic structures and glottic opening with a laryngoscope blade may be extremely difficult. Under these circumstances, forcible manual chest compression by the assistant may open up the expiratory passages momentarily (or produce a few bubbles of expired air). By aiming the tip of the endotracheal tube at that spot and advancing the tube with a gentle twisting motion, the physician may be successful in inserting the tube in the glottis (Smith, 1980). In case intubation proves to be impossible, a surgeon who can perform an emergency tracheotomy in the operating room with the surgical instruments and various tracheotomy tubes should be immediately available.
After the endotracheal tube is in place, breath sounds are checked on both sides of the chest, and the tube is fixed with adhesive tape. The breath sounds are monitored carefully with the precordial stethoscope while continuous positive pressure (10 to 15 cm H2O) is maintained, because pulmonary edema may occur after the relief of severe upper airway obstruction (Travis et al., 1977; Galvis et al., 1980), with an incidence of about 7% (Davis et al., 1981). Throat and blood cultures are taken during this period of stabilization but not before the intubation and restoration of adequate pulmonary gas exchange.
After the child’s condition is stabilized and adequate alveolar ventilation is reestablished, some anesthesiologists replace the orotracheal tube with a nasotracheal tube that has a leak, at or below 30 cm H2O, for postoperative management in the ICU with minimal sedation of the patient (Oh and Motoyama, 1977). This is not a necessary step, and the physician should be confident that the switch could be made without losing the airway. Because acute epiglottitis has become an uncommon phenomenon, many anesthesiologists are happy with the oral intubation and leave the tube in place. The child can be deeply sedated for 24 hours to avoid premature extubation.
Since the mid 1970s, the management of epiglottitis with tracheotomy has been replaced by the less invasive long-term nasotracheal or orotracheal intubation as the standard treatment of choice. This has significantly reduced the duration of hospital stay for children with acute supraglottitis (Oh and Motoyama, 1977; Schloss et al., 1983). Accidental or self-extubation is a potentially disastrous complication. Careful taping of the tube and proper restraint of elbows and hands can prevent untimely extubation. Because most children are bacteremic, antimicrobial therapy, consisting of ampicillin with sulbactam, cefotaxime, or ceftriaxone, is started after blood and throat cultures are obtained. Any fluid deficits should be corrected parenterally. After emergence from anesthesia, the child often falls back to sleep for some time because of sleep deprivation and exhaustion. After this period, most patients require minimal or no sedation (Oh and Motoyama, 1977), although deep sedation has been used in some centers. Usually, it is possible to extubate these patients in 24 to 48 hours. A return of normal body temperature and increased leaks around the nasotracheal tube are major signs of recovery. The epiglottis may be examined under direct vision, with intravenous propofol to determine the time for extubation. Alternatively, a flexible fiberoptic bronchoscope may be used transnasally with intravenous sedation.
Anesthesia for endoscopy
Anesthesia for rigid and flexible bronchoscopy in infants and children requires meaningful cooperation and communication between the endoscopist and anesthesiologist (Donlon and Benumof, 1996). The surgeon and the anesthesiologist are both working in the same anatomic field. The anesthesiologist is concerned about maintaining a patent airway, oxygenation, adequate ventilation, preventing aspiration, minimizing laryngeal motion, and preventing cardiac dysrhythmias, and the surgeon needs a clear view of a motionless field for a reasonable time. Sometimes, these goals conflict. Donlon and Benumof (1996) described the anesthetic goals during bronchoscopy:
Rigid bronchoscopy has broad applications in the diagnostic and therapeutic arenas. Common indications for pediatric patients include investigation of stridor, upper and central (tracheobronchial) airway obstruction, gastroesophageal reflux disease, and removal of airway foreign bodies (Hoeve and Rombout, 1992; Cohen et al., 2001). Flexible bronchoscopy is frequently used for examination of the nasal cavity, nasopharynx, larynx, trachea, and bronchi in motion. Technological advances, such as smaller instruments and improved forceps, will continue to expand the diagnostic and therapeutic role of the flexible bronchoscopy (Cohen et al., 2001). Flexible and rigid bronchoscopies are used complementarily, and these procedures are often performed during the same examination. Box 24-7 lists the common indications for fiberoptic and rigid bronchoscopy. In many scenarios, either instrument can be used for the same indication.
Box 24-7 Indications for Flexible or Rigid Bronchoscopy
From Hartnick CJ, Cotton RT: Stridor and airway obstruction. In Bluestone CD, Stool SE, Alper CM, et al., editors: Pediatric otolaryngology, ed 4, Philadelphia, 2003, Saunders, p 1437.
The Storz-Hopkins rigid bronchoscope, with a ventilating sidearm, provides excellent visibility for pediatric bronchoscopy (Szekely and Farkas, 1978; Johnson et al., 1986). This bronchoscope consists of several components: Storz bronchoscope (sizes 3.0 to 6.0); Hopkins glass rod telescope (2.8 and 4.0 mm outer diameter); antifog sheath for the telescope; adapter for anesthesia circuit connecting to the sidearm, which shares the same narrow channel for aspiration and instrumentation; glass obturator cap; and the light source attached to the telescope and prism (Fig. 24-18). Pediatric Storz rigid bronchoscopes are available in three lengths and various diameters (Fig. 24-19). A variety of forceps is available for biopsy and foreign body removal.
FIGURE 24-19 Different bronchoscope sizes (3.5 to 6.0) (left) and lengths (20, 26, and 30 cm) (right).
(From Casselbrant ML, Alper CM: Methods of examination. In Bluestone CD, Stool SE, editors: Pediatric otolaryngology, ed 4, New York, 2003, Saunders, p 1379.)
It is important to use the smallest optical telescope through the rigid bronchoscope to not completely occlude or unduly increase airflow resistance. For example, use of the standard optical telescope (4-mm outer diameter), as recommended in the literature (Szekely and Farkas, 1978), almost completely occludes the lumen of small bronchoscopes (sizes 3.5 and smaller). The total airway resistance increases sixfold to sevenfold (Widlund, et al., 1982) unless a smaller telescope (2.8-mm outer diameter) is used (Table 24-9). When airway resistance is too high, neither spontaneous nor controlled ventilation is sufficient for adequate gas exchange during rigid bronchoscopy. High airway resistance would be expected to cause hyperinflation of the lungs, and hypercapnia or respiratory acidosis (Rah et al., 1979). Spontaneous breathing during rigid bronchoscopy, especially in neonates and infants, should be limited primarily to evaluations of the larynx and documentation of tracheobronchomalacia in the patient with stridor. The high resistance caused by the telescope in the bronchoscope, together with central respiratory depression caused by general anesthesia, makes spontaneous breathing nearly impossible in these young children (Motoyama, 1992).
TABLE 24-9 Flow Resistance of Storz Bronchoscope in Relation to Estimated Airway Resistance in Infants and Children
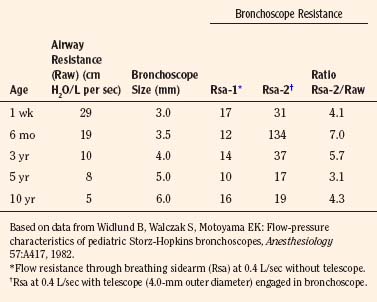
With the advances in fiberoptic technology, diagnostic bronchoscopy can be performed with a flexible fiberoptic bronchoscope during spontaneous breathing with topical anesthesia and minimal sedation even in the sick premature infant. Bronchoscopy also may be performed under general anesthesia through an endotracheal tube or LMA by means of a right-angle adapter with a diaphragm (Maekawa et al., 1981; Wood and Postma, 1988). The use of the flexible fiberoptic bronchoscope for difficult intubation is described in Chapter 13, Induction, Maintenance, and Recovery, and Chapter 12, Airway Management.
Laryngoscopy and Bronchoscopy for Stridor
Stridor in pediatric patients may be congenital or acquired (Fig. 24-20). Most cases are of extrathoracic origin, but some originate in the large intrathoracic airways (i.e., trachea or major bronchi) (Maze and Bloch, 1979) (Box 24-8). See related video online at www.expertconsult.com.![]() Stridor may be caused by fixed obstruction (i.e., airway cross-section does not change with transmural pressure) or varying degrees of obstruction (i.e., airway caliber responds to changes in transmural pressure). These children must be examined while they are actively breathing to document underlying pathophysiology.
Stridor may be caused by fixed obstruction (i.e., airway cross-section does not change with transmural pressure) or varying degrees of obstruction (i.e., airway caliber responds to changes in transmural pressure). These children must be examined while they are actively breathing to document underlying pathophysiology.
Box 24-8 Causes of Stridor in the Pediatric Patient
Modified from Maze A, Bloch E: Stridor in pediatric patients, Anesthesiology 50:132, 1979.
In the newborn infant, flexible and rigid forms of bronchoscopy are used to determine the cause of stridor. Flexible bronchoscopic examination of the infant’s larynx by the nasal approach is possible when the child is awake. The infant should be swaddled for restraint. Information about cord mobility and laryngeal dynamics can be made without much stress to the infant. Atropine or glycopyrrolate can be used as an anti-sialagogue and to prevent vagally mediated bradycardia. The nasal mucosa can be anesthetized with topical lidocaine to prevent discomfort during the transnasal approach, but the vocal cords should not be sprayed, because topical anesthesia affects their motion. Midazolam, dexmedetomidine, or low-dosage propofol may be helpful to reduce the infant’s excessive movement while still maintaining breathing dynamics. Because the upper airway muscles, including the vocal cords (i.e., cricoarytenoid muscles), are extremely sensitive to the depressant effect of sedatives and anesthetics (Ochiai et al., 1989, 1992), anesthesia should be maintained at a minimal level; otherwise, the vocal cord motions will cease.
Only by rigid bronchoscopy under the controlled conditions of general anesthesia can a magnified clear view of the larynx and lower airways be achieved (Hartnick and Cotton, 2003). The use of the rigid bronchoscope allows lower airway inspection while maintaining complete and secure control over oxygenation and ventilation. After airway dynamics are inspected by fiberoptic or rigid bronchoscopy, the child may be paralyzed with an intermediate muscle relaxant. Anesthesia can be maintained with an inhaled anesthetic agent or total intravenous anesthesia. Communication must continue with the bronchoscopist to maintain adequate oxygenation and ventilation. When the lower airways are explored, the endoscopist may need to be reminded to intermittently to return to above the carina for the anesthesiologist to ventilate and oxygenate both lungs. In older infants and children, spontaneous breathing may be maintained during the entire procedure.
This technique for laryngoscopy and bronchoscopy with spontaneous breathing is useful for the evaluation of stridor, laryngomalacia, and tracheobronchomalacia. It is also a technique some anesthesiologists advocate for foreign body removal from the airway. Maintenance of spontaneous breathing can be achieved with an inhalational or an intravenous anesthetic technique. For example, after inhalational induction, the concentration of sevoflurane in oxygen is maintained at a minimum alveolar concentration (MAC) of 2 or 3 for about 5 minutes to establish a sufficient tissue level of anesthesia to allow endotracheal instrumentation (Smith, 1980). An alternative is to discontinue the inhaled anesthetic, slowly provide a bolus (2 to 3 mg/kg) of propofol, and begin a propofol infusion between 200 and 400 mcg/kg per minute while maintaining spontaneous breathing. Supplementation with fentanyl or morphine can further ablate laryngeal responses. Before endoscopy, the glottis and trachea are topically anesthetized with 2% to 4% lidocaine (up to 5 mg/kg) under direct vision.
Bronchoscopy for Foreign Body Aspiration
Foreign body aspiration occurs most frequently in children between 1 and 3 years of age. See related video online at www. expertconsult.com.![]() Commonly aspirated objects include peanuts, seeds, and other food particles and less frequently plastic and metal particles (Baraka, 1974; Blazer et al., 1980). Foreign bodies are embedded more commonly in the right main bronchus than in the left, and less frequently in the larynx and trachea (Blazer et al., 1980; Cohen et al., 2001). Symptoms and signs associated with bronchial aspiration include coughing, wheezing, dyspnea, and decreased air entry in the affected side, whereas dyspnea, stridor, coughing, and cyanosis are more common with laryngeal or tracheal foreign bodies (Blazer et al., 1980).
Commonly aspirated objects include peanuts, seeds, and other food particles and less frequently plastic and metal particles (Baraka, 1974; Blazer et al., 1980). Foreign bodies are embedded more commonly in the right main bronchus than in the left, and less frequently in the larynx and trachea (Blazer et al., 1980; Cohen et al., 2001). Symptoms and signs associated with bronchial aspiration include coughing, wheezing, dyspnea, and decreased air entry in the affected side, whereas dyspnea, stridor, coughing, and cyanosis are more common with laryngeal or tracheal foreign bodies (Blazer et al., 1980).
In addition to the usual preoperative assessment, physical examination should focus on the location, degree of airway obstruction, and gas exchange. A review of the latest chest radiographs is helpful in determining the location of the foreign body and for evidence of secondary pathologic changes such as atelectasis, air trapping, or pneumonia (Fig. 24-21). If significant hyperinflation of one lung or lobe exists, nitrous oxide should be withheld because of the potential danger of further increase in gas volume and possible rupture of the affected lung. Although it is desirable to keep the child NPO (waiting 6 or more hours for solids and 2 hours for clear liquids), the patient’s condition determines the timing of the bronchoscopic examination. If foreign body aspiration causes life-threatening respiratory distress, its removal takes precedence over NPO guidelines.
FIGURE 24-21 Foreign body in the left bronchus with hyperinflation of the ipsilateral portion of the lung.
(Courtesy Dr. K. S. Oh, Department of Radiology, Children’s Hospital of Pittsburgh.)
A major controversy in the anesthetic management of patients undergoing bronchoscopy for foreign body removal is whether to control ventilation or to maintain spontaneous ventilation (Verghese and Hannallah, 2001; Chen et al., 2009). There are few data to justify one technique over the other. Woods (1990) prefers spontaneous ventilation, and Kosloske (1982) advocates neuromuscular blockade and controlled ventilation. The risk of controlled ventilation is to force the foreign body deeper into the small airways, and the risk for the spontaneously breathing patient is unexpected movement or cough (Donlon and Benumof, 1996). In a report of four patients in whom the bronchoscopist had the foreign body slip from the forceps back into the airway, neither controlled ventilation with muscle paralysis nor spontaneous breathing under a deep plane of anesthesia played a role in the mishaps. It was thought that the experience of the endoscopist and the availability of proper equipment were more important factors than the method of ventilation (Pawar, 2000).
Muscle relaxation is particularly useful for bronchoscopy involving the removal of a foreign body distal to the carina, especially because the duration of these procedures can extend to more than an hour. If the spontaneous ventilation technique is employed, meticulous topical anesthesia of the vocal cords with lidocaine can decrease the risks for coughing and laryngospasm. Propofol-based total intravenous anesthesia would be a better choice than inhalational anesthesia because propofol provides a steady level of anesthesia regardless of ventilation and perfusion mismatches (Verghese and Hannallah, 2001).
Foreign bodies in the larynx are more likely to cause total airway obstruction than foreign bodies below the glottis. Foreign bodies located in the bronchi may dislodge from cough or change in position and cause total obstruction (Woods, 1990). In case of acute respiratory distress and hypoxemia with a laryngeal foreign body, anesthesia is induced with the patient in a sitting position with an inhaled anesthetic and oxygen while the patient is monitored with a precordial stethoscope, pulse oximeter, and electrocardiogram. Spontaneous breathing is preferable; positive pressure ventilation may cause the foreign body to be displaced and further obstruct the airway (Darrow and Holinger, 2003). After inhalational induction, intravenous access is established (if not already available), and a vagolytic dose of atropine (0.02 to 0.03 mg/kg) is administered. After intravascular access is established, inhalational anesthesia may be switched to total intravenous anesthesia with propofol with or without opioids (e.g., fentanyl, remifentanil). For children in stable condition with a foreign body presumed to be in the bronchus, an intravenous catheter is inserted before the induction, and the child is monitored in the usual manner. If a full stomach is suspected, the physician must weigh the risk for aspiration against loss of a patent airway before rapid-sequence induction with succinylcholine and cricoid pressure is considered.
During the procedure, the anesthesiologist’s attention should be focused on the breath sounds detected by the precordial stethoscope, the symmetry of respiratory excursion, and oxygen saturation measured by a pulse oximeter. Close communication and cooperation between the bronchoscopist and the anesthesiologist are essential throughout bronchoscopy. It is important to remember that the lumen of the bronchoscope is narrowed by the telescope and other instruments, especially when a suction catheter is inserted through the side port, the same narrow channel through which the patient must be ventilated (Cotton and Reilly, 1990). The rate of rise in end-tidal Pco2 in apneic infants and young children is extremely high—approximately 9 mm Hg/min (Motoyama et al., 2001). The period of apnea or severe hypoventilation therefore should be closely observed and communicated to the endoscopist. The physician must make sure that after each period of hypoventilation or apnea the telescope, forceps, and endobronchial suction catheter through the bronchoscope’s side arm are removed. The distal end of the bronchoscope should be pulled above the carina, and the proximal open end of the bronchoscope is occluded with the endoscopist’s thumb or a glass obturator cap (see Fig. 24-18), so that the child can be hyperventilated before instrumentation is resumed. During the crucial moment of foreign body retrieval, ventilation must sometimes be held until the oxygen saturation begins to fall.
After the completion of bronchoscopy for foreign body retrieval, the child is usually intubated with an endotracheal tube. Tracheal intubation allows tracheobronchial suction, lung expansion, and oxygenation and ventilation until adequate reversal of muscle relaxation and return to spontaneous breathing. Dexamethasone (0.4 to 1.0 mg/kg, up to a total of 20 mg/kg) is given prophylactically to prevent laryngeal edema (Tunnessen and Feinstein, 1980; Postma et al., 1987). Postoperative croup is treated with inhalation of racemic epinephrine (0.5 mL of 2.25% solution diluted in 3 mL of saline solution) (Adair et al., 1971).
Endoscopy for Foreign Body Ingestion
According to the National Safety Council, suffocation from foreign body ingestion and aspiration is the third leading cause of accidental death in children younger than 1 year and the fourth leading cause in children between 1 and 6 years old. Ingestions are often asymptomatic, unrecognized, and self-resolving. Because retained esophageal foreign bodies are so much more common than aspirations, procedures for their removal outnumber those for aspirated foreign bodies (Manning and Stool, 2003). The most commonly ingested foreign body is a coin, followed by food or bones. Other foreign bodies include buttons, batteries, pins, safety pins, thumbtacks, and small toys (McGahren, 1999). Once in the stomach or bowel, coins usually pass through the remainder of the gastrointestinal tract. Many coins, however, become lodged in the esophagus. The most frequent symptoms of upper esophageal foreign body include dysphagia, drooling, gagging, retching, and vomiting. The child may also experience coughing, choking, and significant airway compromise. Serious complications, such as esophageal erosion, caused by retained esophageal coins are rare and occur only after a coin has been lodged for more than 24 hours. Soprano and coworkers (1999) recommend that children with a single coin in any part of the esophagus who have no history of esophageal disease, or respiratory compromise on presentation to the emergency department, be observed for 12 to 24 hours. These authors found that within 24 hours there is a 28% chance of spontaneous passage of the coin into the stomach, regardless of esophageal location.
A chest radiograph should be obtained in all cases of suspected foreign body ingestion. Ingested foreign bodies are predisposed to lodge in three areas of anatomic constriction in the esophagus: the proximal esophagus at the level of the cricopharyngeal muscle and thoracic inlet (the foreign body is seen at the level of the clavicles on chest radiograph), the middle esophagus at the level of the carina and the aortic arch, and the distal esophagus just proximal to the esophageal gastric junction (the foreign body is seen two to four vertebral bodies above the stomach bubble on chest radiography). The most common site for foreign bodies to lodge is at the upper esophagus at the level of the thoracic inlet. If the foreign body is a coin, it will be oriented in a transverse position because the opening of the esophagus is widest in a transverse position (McGahren, 1999).
Removal of a foreign body from the esophagus is not usually a complicated procedure. As long as the child is not dyspneic, the physician should wait 4 to 6 hours after the last meal, depending on the child’s age, until the stomach is empty. The child should be well sedated, and anesthesia is induced with inhaled or intravenous anesthetics. During esophagoscopy, the mucosa over the cricoid cartilage may be traumatized by compression between the endotracheal tube anteriorly and the rigid esophagoscope posteriorly. A prospective review involving more than 50,000 general pediatric anesthetic cases revealed that the incidence of postintubation croup after esophagoscopy was 20 times higher than that of the general pediatric surgical population during the same time period (Moro, Borland, and Motoyama, unpublished observations). A reduced-size endotracheal tube may be helpful to minimize subglottic swelling (Smith, 1980). Dexamethasone (0.4 to 1 mg/kg, up to 20 mg) may also reduce the incidence of postoperative stridor.
The only potential major hazard with foreign body ingestion is when the foreign body, usually a coin, is held in the hypopharynx, and on gagging and coughing, it dislodges and slips into the larynx, completely occluding the airway. When a radiograph of the neck demonstrates a foreign body high in the esophagus (Fig. 24-22), the child is sedated heavily with an opioid and a sedative to avoid excitement, gagging, and coughing. After intravenous access is established and atropine is given, the trachea is intubated under deep sevoflurane anesthesia or propofol, with or without muscle relaxation. The use of neuromuscular blockade is determined by the ability to ventilate the child with positive pressure. Cricoid pressure is avoided under these circumstances because it may irritate the upper airway or dislodge the foreign body.
Summary
For questions and answers on topics in this chapter, go to “Chapter Questions” at www.expertconsult.com.
A clinician’s guide to surgical fires: how they occur, how to prevent them, how to put them out. Health Devices. 2003;32:5.
Adair J.C., Ring W.H., Jordan W.S., et al. Ten-year experience with IPPB in the treatment of acute laryngotracheobronchitis. Anesth Analg. 1971;50:649.
Adams W.G., Deaver K.A., Cochi S.L., et al. Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA. 1993;269:221.
Afshan G., Chohan U., Qamar U.L., et al. Is there a role of a small dose of propofol in the treatment of laryngeal spasm? Paediatr Anaesth. 2002;12:625.
Alexander C.A. A modified Intravent laryngeal mask for ENT and dental anaesthesia. Anaesthesia. 1990;45:892.
Alexander D.W., Graff T.W., Kelley E. Factors in tonsillectomy mortality. Arch Otolaryngol. 1965;82:409.
Allen T.H., Steven I.M., Sweeney D.B. The bleeding tonsil: anesthesia for control of haemorrhage after tonsillectomy. Anaesth Intensive Care. 1973;1:517.
Allford M. A national survey of anesthetic management of tonsillectomy surgery in children. Paediatr Anaesth. 2009;19(2):1.
Allsop E., Innes P., Jackson M., et al. Dose of propofol required to insert the laryngeal mask airway in children. Paediatr Anaesth. 1995;5:47.
American Thoracic Society. Standards and indications for cardiopulmonary sleep studies in children. Am J Respir Crit Care Med. 1996;153:866.
Avery A.D., Harris L.J. Tonsillectomy, adenoidectomy, and tonsillectomy with adenoidectomy: Assessing the quality of care using short-term outcome measures. Quality of medical care assessment using outcome measures: Eight disease- specific applications. Santa Monica, CA: Rand Corp, 1976.
Balkany T.J., Hodges A., Miyamoto R.T., et al. Cochlear implants in children. Otolaryngol Clin North Am. 2001;34:455.
Bandla H.P., Hopkins R.L., Beckerman R.C., et al. Pulmonary risk factors compromising postoperative recovery after surgical repair for congenital heart disease. Chest. 1999;116:740.
Baraka A. Bronchoscopic removal of inhaled foreign bodies in children. Br J Anaesth. 1974;46:124.
Beamis J.E., Vergos K., Rebeiz E.E., et al. Endoscopic laser therapy for obstructive tracheobronchial lesions. Ann Otol Rhinol Laryngol. 1991;100:413.
Bejian S., Valasek C., Nigro J.J., et al. Prolonged use of dexmedetomidine in the paediatric cardiothoracic intensive care unit. Cardiol Young. 2009;19:98-104.
Bennie R.E., Boehringer L.A., Dierdorf S.F., et al. Transnasal butorphanol is effective for postoperative pain relief in children undergoing myringotomy. Anesthesiology. 1998;89:385.
Benoit Z., Wicky S., Fisher J.-F., et al. The effect of increased FIO2 before tracheal extubation on postoperative atelectasis. Anesth Analg. 2002;954:1777-1781.
Biavati M.J., Manning S.C., Phillips D.L. Predictive factors for respiratory complications after tonsillectomy and adenoidectomy in children. Arch Otolaryngol Head Neck Surg. 1997;123:517.
Blackstock D., Adderley R.J., Steward D.J. Epiglottitis in young infants. Anesthesiology. 1987;67:97.
Blazer S., Naveh Y., Friedman A. Foreign body in the airway: a review of 200 cases. Am J Dis Child. 1980;134:68.
Bluestone C.D. Controversies in tonsillectomy, adenoidectomy and tympanostomy tubes. In: Bailey B., editor. Head and neck surgery—otolaryngology. Philadelphia: Lippincott Williams & Wilkins; 2001:993-1006.
Bluestone C.D., Klein J.O. Intracranial complications of otitis media. In: Bluestone C.D., Stool S.E., et al, editors. Pediatric otolaryngology. Philadelphia: WB Saunders; 2003:474-686.
Bluestone C.D., Paradise J.L., Kass E.H., et al. The workshop on tonsillectomy and adenoidectomy. Ann Orol Rhinol Laryngol suppl. 1975;19:84.
Blum R.H., McGowan F.X.Jr. Chronic upper airway obstruction and cardiac dysfunction: anatomy, pathophysiology and anesthetic implications. Paediatr Anaesth. 2004;14:75.
Borland L.M., Reilly I.S. Jet ventilation for laser laryngeal surgery in children: modification of the Saunders jet ventilation technique. Int J Pediatr Otorhinolaryngol. 1987;14:65.
Brandom B.W. Postoperative management of laryngotracheal reconstruction. Int Anesthesiol Clin. 1997;35:127.
Brisson P., Patel H., Scorpio R., et al. Rotary atlanto-axial subluxation with torticollis following central-venous catheter insertion. Pediatr Surg Int. 2000;16:421.
Brown G.W., Patel N., Ellis F.R. Comparison of propofol and thiopentone for laryngeal mask insertion. Anaesthesia. 1991;46:771.
Brown K.A., Laferrière A., Lakheeram I., et al. Recurrent hypoxemia in children is associated with increased analgesic sensitivity to opiates. Anesthesiology. 2006;15:665.
Brown K.A., Laferrière A., Moss I.R. Recurrent hypoxemia in young children with obstructive sleep apnea is associated with reduced opioid requirement for analgesia. Anesthesiology. 2004;100:806-810.
Brown O.E., Pownell P., Manning S.C. Choanal atresia: a new anatomic classification and clinical management applications. Laryngoscope. 1996;106:97.
Bruppacher H., Reber A., Keller J.P., et al. The effects of common airway maneuvers on airway pressure and flow in children undergoing adenotonsillectomies. Anesth Analg. 2003;97:29.
Butt W., Shann F., Walker C., et al. Acute epiglottitis: a different approach to management. Crit Care Med. 1988;16:43.
Carcillo J. Intensive care management of infection-related acute upper airway obstruction in children. In: Bluestone C.D., Stool S.E., editors. Pediatric otolaryngology. Philadelphia: WB Saunders; 2003:1599.
Cardwell M., Siviter G., Smith A. Non-steroidal anti-inflammatory drugs and perioperative bleeding during pediatric tonsillectomy. Cochrane Database Syst Rev. (2):2005. CD003591
Carithers J.S., Gebhart D.E., Williams J.A. Postoperative risks of pediatric tonsilloadenoidectomy. Laryngoscope. 1987;97:422.
Carron J.D., Derkay C.S., Strope G.L., et al. Pediatric tracheotomies: changing indications and outcomes. Laryngoscope. 2000;110:1099.
Chen L., Zhang X., Li S., et al. Risk factors for hypoxemia in children less than 5 years of age undergoing rigid bronchoscopy for foreign body removal. Anesth Analg. 2009;109(4):1079.
Chrysostomou C., Di Filippo S., Manrique A.M., et al. Use of dexmedetomidine in children after cardiac and thoracic surgery. Pediatr Crit Care Med. 2006;7:186-187.
Chrysostomou C., Sanchez De Toledo J., Avolio T., et al. Dexmedetomidine use in a pediatic cardiac intensive care unit: can we use it in infants after cardiac surgery? Pediatr Crit Care Med. 2009;10(6):654.
Close H.L., Kryzer T.C., Nowlin J.R., et al. Hemostatic assessment of patients before tonsillectomy: a prospective study. Otolaryngol Head Neck Surg. 1994;111:733.
Cohen S., Pine H., Drake A. Use of rigid and flexible bronchoscopy among pediatric otolaryngologists. Arch Otolaryngol Head Neck Surg. 2001;127:505.
Colen T.Y., Seidman C., Weedon J., Goldstein N.A. Effect of intracapsular tonsillectomy on quality of life for children with obstructive sleep-disordered breathing. Arch Otolaryngol Head Neck Surg. 2008;134:124.
Coté C.J., Roll N., Liu L.M.P., Gousouzian N.G. A single-blind study of pulse oximetry and capnography in children. Anesthesiology. 1991;74:984.
Cotton R.T. Management of subglottic stenosis. Otolaryngol Clin North Am. 2000;33:111.
Cotton R.T., Reilly J.S. Congenital malformations of the larynx. In: Bluestone C.D., Stool S.E., editors. Pediatric otolaryngology. ed 2,. Philadelphia: WB Saunders; 1990:1121-1128.
Cozine K., Rosenbaum L.M., Askanazi J., et al. Laser-induced endotracheal tube fire. Anesthesiology. 1981;55:583.
Crysdale W.S., Russel D. Complications of tonsillectomy and adenoidectomy in 9,409 children observed overnight. Can Med Assoc J. 1986;135:1139.
Crysdale W.S., Sendi K. Evolution in the management of acute epiglottitis: a 10-years experience with 242 children. Int Anesthesiol Clin. 1988;26:32-38.
Dahl J.B., Kehlet H. Non-steroidal anti-inflammatory drugs: rationale for use in severe postoperative pain. Br J Anaesth. 1994;72:375.
Darrow D.H., Holinger L.D. Foreign bodies of the larynx, trachea, and bronchi. In: Bluestone C.D., Stool S.E., editors. Pediatric otolaryngology. Philadelphia: WB Saunders; 2003:1543-1557.
Darrow D.H., Siemens C. Indications for tonsillectomy and adenoidectomy. Laryngoscope. 2002;112:6.
Davies D.W., Steward D.J. Unexpected excessive bleeding during operation: role of acetylsalicylic acid. Can Anaesth Soc J. 1977;24:452.
Davis H.W., Gartner J.C., Galvis A.G., et al. Acute upper airway obstruction: croup and epiglottitis. Rev Pediatr Clin North Am. 1981;28:859.
Dayyat E., Kheirandish-Gozal L., Gozal D. Childhood obstructive sleep apnea: one or two distinct disease entities? Clin Sleep Med. 2007;2:433.
Diaz J.H. Croup and epiglottitis in children: the anesthesiologist as diagnostician. Anesth Analg. 1985;64:621.
Donlon J.V., Benumof J.L. Anesthetic and airway management of laryngoscopy and broncoscopy. St Louis: Mosby, 1996.
Dornhoffer J., Manning L. Unplanned admissions following outpatient otologic surgery: the University of Arkansas experience. Ear Nose Throat J. 2000;79:710.
Doyle W.J., Banks J.M. Middle ear pressure change during controlled breathing with gas mixtures containing nitrous oxide. J Appl Physiol. 2003;94:199.
Erb T.O., Schulman S.R., Sugarman J. Permission and assent for clinical research in pediatric anesthesia. Anesth Analg. 2002;94:1155.
Fine G.F., Borland L.M. The future of the cuffed endotracheal tube. Paediatr Anaesth. 2004;14:38-42.
Finkel J.C., Cohen I.T., Hannallah R.S., et al. The effect of intranasal fentanyl on the emergence characteristics after sevoflurane anesthesia in children undergoing surgery for bilateral myringotomy tube placement. Anesth Analg. 2001;92:1164.
Fischetti M. Cochlear implants: to hear again. Sci Am. 2003;288:82.
Fricke B.L., Donnelly L.F., Shott S.R., et al. Comparison of lingual tonsil size as depicted on MR imaging between children with obstructive sleep apnea despite previous tonsillectomy and adenoidectomy and normal controls. Pediatr Radiol. 2006;36:518.
Furuhashi-Yanaha A., Dohi S., Oshima T., et al. Acute pulmonary edema caused by impaired switching from nasal to oral breathing in the emergence from anesthesia. Anesthesiology. 2000;92:1209.
Galinkin J.L., Fazi L.M., Cuy R.M., et al. Use of intranasal fentanyl in children undergoing myringotomy and tube placement during halothane and sevoflurane anesthesia. Anesthesiology. 2000;93:1378.
Galvis A.G., Stool S.E., Bluestone C.D. Pulmonary edema following relief of acute upper airway obstruction. Ann Otol Rhinol Laryngol. 1980;89:124.
Garden J.M., O’Banion M.K., Bakus A.D., et al. Viral disease transmitted by laser-generated plume (aerosol). Arch Dermatol. 2002;138:1303.
Garetz S.L. Behavior, cognition, and quality of life after adenotonsillectomy for pediatric sleep-disordered breathing: summary of the literature. Otolaryngol Head Neck Surg. 2008;138(1 Suppl):S19.
Geffin B., Shapshay S.M., Bellack G.S., et al. Flammability of endotracheal tubes during Nd-YAG laser application in the airway. Anesthesiology. 1986;65:5-11.
Gerber M.E., Holinger L.D. Congenital laryngeal anomalies. In: Bluestone C.D., Stool S.E., editors. Pediatric otolaryngology. Philadelphia: WB Saunders; 2003:1460-1472.
Gerber M.E., O’Connor D.M., Adler E., et al. Selected risk factors in pediatric adenotonsillectomy. Arch Otolaryngol Head Neck Surg. 1996;122:811.
Gluckman I.L. Inflammatory diseases of the mouth and pharynx. In Bluestone C.D., Stool S.E., editors: Pediatric otolaryngology, ed 2., Philadelphia: WB Saunders, 1990.
Goforth A.J., Cooke J.E., Putney F.J. An anesthesia technique for laser surgery of the larynx. Laryngoscope. 1983;93:822.
Goldsmith A.J., Rosenfeld R.M. Treatment of pediatric sinusitis. Pediatr Clin North Am. 2003;50:2.
Gorelick M.H., Baker M.D. Epiglottitis in children, 1979 through 1992: effects of Haemophilus influenzae type b immunization. Arch Pediatr Adolesc Med. 1994;148:47.
Gozal D., Capdevila O.S., Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008;177:1142.
Gozal D., Crabtree V.M., Sans Cadevila O., et al. C-reactive protein, obstructive sleep apnea and cognitive dysfunction in school-aged children. Am J Respir Crit Care Med. 2007;176:188.
Gozal D., Simakajornboon N., Holbrook C.R., Crabtree V.M. Secular trends in obesity and parentally reported daytime sleepiness among children referred to a pediatric sleep center for snoring and suspected sleep-disordered breathing (SDB). (Abstract) Sleep. 2006;29:A74.
Guida R.A., Mattucci K.F. Tonsillectomy and adenoidectomy: an inpatient or outpatient? Laryngoscope. 1990;100:491.
Guilleminault C., Huang Y.S., Glamann C., et al. Adenotonsillectomy and obstructive sleep apnea in children: a prospective survey. Otolaryngol Head Neck Surg. 2007;136:169.
Gysin C., Alothman G.A., Papsin B.C. Sinonasal disease in cystic fibrosis: clinical characteristics, diagnosis, and management. Pediatr Pulmonol. 2000;30:481.
Hartnick C.J., Cotton R.T. Stridor and airway obstruction. In: Bluestone C.D., Stool S.E., editors. Pediatric otolaryngology. Philadelphia: WB Saunders; 2003:1437.
Hatcher I.S., Stack C.G. Postal survey of the anaesthetic techniques used for paediatric tonsillectomy surgery. Paediatr Anaesth. 1999;9:311.
Haynes D.S., Harley D.H. Surgical management of chronic otitis media: beyond tympanotomy tubes. Otolaryngol Clin North Am. 2002;35:827.
Helfaer M.A., McColley S.A., Pyzik P.L.R.P.T., et al. Polysomnography after adenotonsillectomy in mild pediatric obstructive sleep apnea. Crit Care Med. 1996;24:1323.
Hengerer A.S., Wein R.O. Congenital malformations of the nose and paranasal sinuses. In: Bluestone C.D., Stool S.E., editors. Pediatric otolaryngology. Philadelphia: WB Saunders; 2003:979-994.
Herbert R.L., Bent J.P. Meta-analysis of outcomes of pediatric functional endoscopic sinus surgery. Laryngoscope. 1998;108:796.
Hermens J.M., Bennett M.J., Hirshman C.A. Anesthesia for laser surgery. Anesth Analg (Paris). 1983;62:218.
Hern J.D., Jayaraj S.M., Sidhu V.S., et al. The laryngeal mask airway in tonsillectomy: the surgeon’s perspective. Clin Otolaryngol Allied Sci. 1999;24:122.
Hesham A. Bipolar diathermy versus cold dissection in paediatric tonsillectomy. Int J Pediatr Otorhinolaryngol. 2009;73:793.
Hinkle A.J. What wisdom is there in administering elective general anesthesia to children with active upper respiratory tract infection? Anesth Analg. 1989;68:413.
Hoekelman R.A. Epiglottitis: another dying disease? Pediatr Ann. 1994;23:229.
Hoeve L.J., Rombout J. Pediatric laryngobronchoscopy: 1332 procedures stored in a database. Int J Pediatr Otorhinolaryngol. 1992;24:73.
Holland B.W., McGuirt W.F.Jr. Surgical management of choanal atresia: Improved outcome using mitomycin. Arch Otolaryngol Head Neck Surg. 2001;127:1375.
Hulka G.F. Head and neck manifestations of cystic fibrosis and ciliary dyskinesia. Otolaryngol Clin North Am. 2000;33:1333.
Isono S. Developmental changes of pharyngeal airway patency: implications for pediatric anesthesia. Pediatr Anes. 2006;16:109-122.
Isono S., Shimada A., Utsugi M., et al. Comparison of static mechanical properties of the passive pharynx between normal children and children with sleep-disordered breathing. Am J Respir Crit Care Med. 1998;157:1204.
James I. Cuffed tubes in children. Paediatr Anaesth. 2001;11:259.
Jellish W.S., Jensen R.L., Anderson D.E., et al. Intraoperative electromyographic assessment of recurrent laryngeal nerve stress and pharyngeal injury during anterior cervical spine surgery with Caspar instrumentation. J Neurosurg. 1999;91:170.
Jellish W.S., Leonetti J.P., Murdoch J.R., et al. Propofol-based anesthesia as compared with standard anesthetic techniques for middle ear surgery. J Clin Anesth. 1995;7:292.
Johans T.G., Reichert T.I. An insufflation device for anesthesia during subglottic carbon dioxide laser microsurgery in children. Anesth Analg. 1984;63:368.
John R.E., Hill S., Hughes T.J. Airway protection by the laryngeal mask: a barrier to dye placed in the pharynx. Anaesthesia. 1991;46:366.
Johnson J.T., Schuller D.E., Silver F., et al. Antibiotic prophylaxis in high-risk head and neck surgery: one-day vs. five-day therapy. Otolaryngol Head Neck Surg. 1986;95:554.
Johnson L.B., Elluru R.G., Myer C.M. Complications of adenotonsillectomy. Larygoscope. 2002;112:35.
Karl H.W., Swedlow D.B., Lee K.W., Downes J.J. Epinephrine interactions in children. Anesthesiology. 1983;58:142.
Karlson K.H. What’s new in pediatric obstructive sleep apnea? Clin Pulm Med. 2008;15:226.
Keon T.P. Anesthetic considerations for laser surgery. Int Anesthesiol Clin. 1988;26:50.
Khine H.H., Corddry D.H., Kettrick R.G., et al. Comparison of cuffed and uncuffed endotracheal tubes in young children during general anesthesia. Anesthesiology. 1997;86:627.
Koivunen P., Alho O.P., Uhari M., et al. General anesthesia with and without nitrous oxide (N2O) and the weight of middle ear effusion in children undergoing adenoidectomy and tympanostomy. Laryngoscope. 1996;106:724.
Kokosa I., Eugene I. Chemical composition of laser-tissue interaction smoke plume. J Laser Appl. 1989;2:59.
Koltai P.J., Solares C.A., Mascha E.J., et al. Intracapsular partial tonsillectomy for tonsillar hypertrophy in children. Laryngoscope. 2002;112:17.
Kosloske A.M. Bronchoscopic extraction of aspirated foreign bodies in children. Am J Dis Child. 1982;136:924.
Kuratani N., Oi Y. Greater incidence of emergence agitation in children after sevoflurane anesthesia as compared with halothane: a meta-analysis of randomized controlled trials. Anesthesiology. 2008;109:225-232.
Landsman I.S. Mechanisms and treatment of laryngospasm. Int Anesthesiol Clin. 1997;35:67.
Leicht P., Wisborg T., Chraeinrner-Jorgensen B. Does intravenous lidocaine prevent laryngospasm after extubation in children? Anesth Analg. 1985;64:1193.
Leong A.C., Davis J.P. Morbidity after adenotonsillectomy for paediatric obstructive sleep apnoea syndrome: waking up to a pragmatic approach. J Laryngol Otol. 2007;121:809.
Licameli G.R., Healy G.B. Surgery of the larynx and trachea. In: Bluestone C.D., Stool S.E., editors. Atlas of pediatric otolaryngology. Philadelphia: WB Saunders, 1995.
Liebowitz H.M., Peacock G.R. Cortical injury produced by carbon dioxide laser radiation. Arch Ophthalmol. 1980;52:993.
Lines V. Anaesthesia for laryngoscopy and microlaryngeal surgery in children. Anaesth Intensive Care. 1973;1:507.
Liu Y.H., Li M.J., Wang P.C., et al. Use of dexamethasone on the prophylaxis of nausea and vomiting after tympanomastoid surgery. Laryngoscope. 2001;111:1271.
Lusk R. Surgical management of chronic rhinosinusitis. In: Bluestone C.D., Stool S.E., editors. Pediatric otolaryngology. Philadelphia: WB Saunders; 2003:1013-1020.
Maekawa S., Fukuda K., Yamauchi T., et al. Follow-up study of pharyngeal carriers of beta-hemolytic streptococci among school children in Sapporo City during a period of 2 years and 5 months. J Clin Microbiol. 1981;13:1017.
Manning S.C., Stool S.E. Foreign bodies of the pharynx and esophagus. In: Bluestone C.D., Stool S.E., editors. Pediatric otolaryngology. Philadelphia: WB Saunders; 2003:1324-1337.
Marcus C.L. Sleep-disordered breathing in children. Am J Respir Crit Care Med. 2001;164:16.
Marcus C.L., Greene M.G., Carroll J.L. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157:1098.
Marcus C.L., Lutz J., Hamer A., et al. Developmental changes in response to sub atmospheric pressure loading of the upper airway. J Appl Physiol. 1999;87:626.
Marcus C.L., McColley S.A., Carroll J.L., et al. Upper airway collapsibility in children with obstructive sleep apnea syndrome. J Appl Physiol. 1994;77:918.
Marrett E., Flahault A., Samama C.M., et al. Effects of postoperative, nonsteroidal, antiinflammatory drugs on bleeding risk after tonsillectomy. Meta-analysis of randomized, controlled trials. Anesthesiology. 2003;98:1497.
Mattucci K.F., Militana C.J. The prevention of fire during oropharyngeal electrosurgery. Ear Nose Throat J. 2003;82:107-109.
Mauro R.D., Poole S.R., Lockhart C.H. Differentiation of epiglottitis from laryngotracheitis in the child with stridor. Am J Dis Child. 1988;142:679.
Maze A., Bloch E. Stridor in pediatric patients. Anesthesiology. 1979;50:132.
McColley S.A., April M.M., Carroll J.L., et al. Respiratory compromise after adenotonsillectomy in children with obstructive sleep apnea. Arch Otolaryngol Head Neck Surg. 1992;118:940.
McCulloch T.M., Richardson M.A., Flint P.W., et al. Lidocaine effects on the laryngeal chemoreflex, mechanoreflex, and afferent electrical stimulation reflex. Ann Otol Rhinol Laryngol. 1992;101:583.
McDonald I.H., Stocks J.G. Prolonged nasotracheal intubation: a review of its development in a pediatric hospital. Br J Anaesth. 1965;37:161.
McGahren E.D. Esophageal foreign bodies. Pediatr Rev. 1999;20:129.
McGovern P.H. Bilateral choanal atresia in newborns: a new method of medical management. Laryngoscope. 1961;71:480.
McGowan F.X., Kenna M.A., Fleming J.A., et al. Adenotonsillectomy for upper airway obstruction carries increased risk in children with a history of prematurity. Pediatr Pulmonol. 1992;13:222.
Megerian C.A., Reily J., O’Connell F.M., et al. Outpatient tympanomastoidectomy: factors affecting hospital admission. Arch Otolaryngol Head Neck Surg. 2000;126:1345.
Mehta V., Har-El G., Goldstein N. Postobstructive pulmonary edema after laryngospasm in the otolaryngology patient. Laryngoscope. 2006;116:1693.
Messner A.H. Treating pediatric patients with obstructive sleep disorders: an update. Otolaryngol Clin North Am. 2003;36:519.
Miyamoto R.T., Kirk K.I. Cochlear implants in children. In: Bluestone C.D., Stool S.E., editors. Pediatric otolaryngology. Philadelphia: WB Saunders; 2003:808-816.
Miyasaka K., Sloan I.A., Froese A.B. An evaluation of the jet injector (Saunders) technique for bronchoscopy in paediatric patients. Can Anaesth Soc J. 1980;27:117.
Moore J.K., Moore E.W., Elliott R.A., et al. Propofol and halothane versus sevoflurane in paediatric day-case surgery: Induction and recovery characteristics. Br J Anaesth. 2003;90:461.
Motoyama E.K. Anesthesia and the upper airway in infants and children. Int Anesthesiol Clin. 1992;30:17.
Motoyama E.K., Fine G.F., Jacobson K.H., et al. Accelerated increases in end-tidal CO2 (Petco2) in anesthetized infants and children during rebreathing. Anesthesiology. 2001;94:A1276.
Motoyama E.K., Glazener C.I. Hypoxemia after general anesthesia in children. Anesth Analg. 1986;65:267.
Naja M.Z., El-Rajab M., Sidani H., et al. Modified infiltration technique in tonsillectomy: expanded case report of 25 children. Int J Pediatr Otorhinolaryngol. 2005;69:35.
Nezhat C., Winen W.K., Nezhat C., et al. Smoke from laser surgery: is there a health hazard? Laser Surg Med. 1987;7:376.
Nikanne E., Kokki H., Tuovinen K. Perioperative ketoprofen in small children during adenoidectomy. Br J Anaesth. 1997;78:24.
Nixon G.M., Kermack A.S., McGregor C.D., et al. Sleep and breathing on the first night after adenotonsillectomy for obstructive sleep apnea. Pediatr Pulmonol. 2005;39:332.
Norton M.L., Strong M.S., Vaughan C.W., et al. Endotracheal intubation and Venturi (jet) ventilation for laser microsurgery of the larynx. Ann Otol Rhinol Laryngol. 1976;85:656.
Ochiai R., Guthrie R.D., Motoyama E.K. Differential sensitivity to halothane anesthesia of the genioglossus, intercostals and diaphragm in kittens. Anesth Analg. 1992;74:338.
Ochiai R., Guthrie R.D., Motoyama E.K. Effects of varying concentrations of halothane on the activity of the genioglossus, intercostals, and the diaphragm in cats: An electromyographic study. Anesthesiology. 1989;70:812.
Oh T.H., Motoyama E.K. Comparison of nasotracheal intubation and tracheostomy in management of acute epiglottitis. Anesthesiology. 1977;46:214.
Ossof R.H. Laser safety in otolaryngology—head and neck surgery: Anesthetic and educational considerations for laryngeal surgery. Laryngoscope. 1989;99:1.
Ossof R.H., Duncavage I.A., Eisenman T.S., et al. Comparison of tracheal damage from laser-ignited endotracheal tube fires. Ann Otol Rhinol Laryngol. 1983;92:333.
Pappas A.L., Fluder E.M., Creech S., et al. Postoperative analgesia in children undergoing myringotomy and placement equalization tubes in ambulatory surgery. Anesth Analg. 2003;96:1621.
Pappas A.L., Sukhani R., Hotaling A.J., et al. The effect of preoperative dexamethasone on the immediate and delayed postoperative morbidity in children undergoing adenotonsillectomy. Anesth Analg. 1998;87:57.
Paradise J.L. Tonsillectomy and adenoidectomy. In: Bluestone C.D., Stool S.E., editors. Pediatric otolaryngology. Philadelphia: WB Saunders; 2003:1210-1222.
Paradise J.L., Feldman H.M., Campbell T.F., et al. Early versus delayed insertion of tympanostomy tubes for persistent otitis media: developmental outcomes at the age of three years in relation to prerandomization illness patterns and hearing levels. Pediatr Infect Dis J. 2003;22:309.
Pashayan A.G. Anesthesia for laser surgery. Park Ridge, IL: American Society of Anesthesiologists, 1994. ASA Refresher Course, No. 221
Pashayan A.G., Gravenstein J.S. Helium retards endotracheal tube fires from carbon dioxide lasers. Anesthesiology. 1985;62:274.
Pawar D.K. Dislodgement of bronchial foreign body during retrieval in children. Paediatr Anaesth. 2000;10:333.
Pelton S.I., Shurin P.A., Klein J.O. Persistence of middle ear effusion after otitis media. Pediatr Res. 1977;11:504.
Pirsig W. Surgery of choanal atresia in infants and children: historical notes and updated review. Int J Pediatr Otorhinolaryngol. 1986;11:153.
Postma D.S., Prazma J., Woods C.I., et al. Use of steroids and a long-acting vasoconstrictor in the treatment of postintubation croup: a ferret model. Arch Otolaryngol Head Neck Surg. 1987;113:844.
Prasad M., Ward R.F., April M.M., et al. Topical mitomycin as an adjunct to choanal atresia repair. Arch Otolaryngol Head Neck Surg. 2002;128:398.
Prinsley P., Wood M., Lee C.A. Adenotonsillectomy in patients with inherited bleeding disorders. Clin Otolaryngol. 1993;18:206.
Rah K.H., Salzberg A.M., Boyan C.P., et al. Respiratory acidosis with the small Storz-Hopkins bronchoscopes: occurrence and management. Ann Thorac Surg. 1979;27:197.
Rampil I.J. Anesthetic considerations for laser surgery. Anesth Analg. 1992;74:424.
Randall D.A., Hoffer M.E. Complications of tonsillectomy and adenoidectomy. Otolarygol Head Neck Surg. 1998;118:61.
Ravussin P., Bayer-Berger M., Monnier P., et al. Percutaneous transtracheal ventilation for laser endoscopic procedures in infants and small children with laryngeal obstruction: report of two cases. Can J Anaesth. 1987;34:83.
Reber A., Pagonini R., Frei F.J. Effect of common airway manoeuvres on upper airway dimensions and clinical signs in anaesthetized, spontaneously breathing children. Br J Anaesth. 2001;86:217.
Ring W.H., Adair J.C., Elwyn R.A. The new pediatric endotracheal tube. Anesth Analg. 1975;54:237.
Rontal M., Rontal E., Wenokur M. Jet insufflation anesthesia for endolaryngeal surgery. Laryngoscope. 1980;90:1162.
Rothschild M.A., Cotcamp D., Cotton R.T. Postoperative medical management in single-stage laryngotracheoplasty. Arch Otolaryngol Head Neck Surg. 1995;121:1175.
Ruder C.B., Raphael N.L., Abramson A.L., et al. Anesthesia for carbon dioxide laser microsurgery of the larynx. Otolaryngol Head Neck Surg. 1981;89:732.
Rutter M.J., Yellon R.F., Cotton R.T. Management and prevention of subglottic stenosis in infants and children. In: Bluestone C.D., Stool S.E., editors. Pediatric otolaryngology. Philadelphia: WB Saunders; 2003:1519-1542.
Sanders J.C., King M.A., Mitchell R.B., Kelly J.P. Perioperative complications of adenotonsillectomy in children with obstructive sleep apnea syndrome. Anesth Analg. 2006;103:1115.
Sanders R.D. Two ventilating attachments for bronchoscopes. Del Med J. 1967;39:170.
Sasaki C.T. Development of laryngeal function: etiologic significance in the sudden infant death syndrome. Laryngoscope. 1979;89:1964.
Scamman F.L., McCabe B.F. Supraglottic jet ventilation for laser surgery of the larynx in children. Ann Otol Rhinol Laryngol. 1986;95:142.
Scanlon P., Carey M., Power M., et al. Patient response to laryngeal mask insertion after induction of anaesthesia with propofol or thiopentone. Can J Anaesth. 1993;40:816.
Schechter M.S. Section on pediatric pulmonology, Subcommittee on Obstructive Sleep Apnea Syndrome. Technical report: diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2002;109:e69.
Schloss M.D., Hannallah R., Baxter J.D. Acute epiglottitis: 26 years’ experience at the Montreal Children’s Hospital. J Otolaryngol. 1979;8:259.
Schloss M.D., Pham-Dang H., Rosales J.K. Foreign bodies in the tracheobronchial tree: a retrospective study of 217 cases. J Otolaryngol. 1983;12:212.
Schwartz A.J., Downes J.J. Hazards of a simple monitoring device, the esophageal stethoscope. Anesthesiology. 1977;47:64.
Schwengel D.A., Sterni L.M., Tunkel D.E., et al. Perioperative management of children with obstructive sleep apnea. Anesth Analg. 2009;109:60-75.
Shah Z., Jortani S., Tauman R., et al. Serum proteomic patterns associated with sleep-disordered breathing in children. Pediatr Res. 2006;59:466.
Simpson J.I., Schiff G.A., Wolf G.L. The effect of helium on endotracheal to be flammability. Anesthesiology. 1990;73:538.
Smith R.B., Schaer W.B., Pfaeffle H. Percutaneous transtracheal ventilation for anesthesia and resuscitation: a review and report of complications. Can Anaesth Soc J. 1975;22:607-612.
Smith R.M. Anesthesia for infants and children, ed 4. St Louis: CV Mosby, 1980.
Smith R.M. Anesthesia for pediatric otolaryngology. In: Ferguson C.E., Kendig E.L.Jr, editors. Pediatric otolaryngology. Philadelphia: WB Saunders, 1972.
Snow J.C., Kripke B.J., Strong M.S., et al. Anesthesia for carbon dioxide laser microsurgery on the larynx and trachea. Anesth Analg (C/eve). 1974;53:507.
Snow J.C., Norton M.L., Suluja I.S., et al. Fire hazard during CO2, laser microsurgery on the larynx and trachea. Anesth Analg. 1976;55:146T.
Solares C.A., Koempel J.A., Hirose K., et al. Safety and efficacy of powered intracapsular tonsillectomy in children: a multi-center retrospective case series. Int J Pediatr Otorhinolaryngol. 2005;69:21.
Soprano J.V., Fleisher G.R., Mandl K.D. The spontaneous passage of esophageal coins in children. Arch Pediatr Adolesc Med. 1999;153:1073.
Sosis M., Dillon F. What is the safest foil tape for endotracheal tube protection during Nd-YAG laser surgery? A comparative study. Anesthesiology. 1990;72:553.
Sosis M.B. Evaluation of five metallic tapes for protection of endotracheal tubes during CO laser surgery. Anesth Analg. 1989;68:392.
Sosis M.B. What is the safest endotracheal tube for Nd-YAG laser surgery? A comparative study. Anesth Analg. 1989;69:802.
Statham M.M., Elluru R.G., Buncher R., et al. Adenotonsillectomy for obstructive sleep apnea syndrome in young children prevalence of pulmonary complications. Arch Otolaryngol Head Neck Surg. 2006;132:476.
Sterni L.M., Tunkel D.E. Obstructive sleep apnea in children: an update. Pediatr Clin North Am. 2003;50:427.
Steward D.L., Welge J.A., Myer C.M. Steroid for improving recovery following tonsillectomy in children. Cochrane Database Syst Rev. 1, 2003. CD003997
Stool S.E., Eavey R. Tracheotomy. In Bluestone C.D., Stool S.E., editors: Pediatric otolaryngology, ed 2., Philadelphia: WB Saunders, 1990.
Strong M.S., Jako E.J., Polyani T., et al. Laser surgery in the aerodigestive tract. Am J Surg. 1973;126:529.
Suzuki M., Sasaki C.T. Laryngeal spasm: a neurophysiologic redefinition. Ann Otol Rhinol Laryngol. 1977;86:150.
Tait A.R., Knight P.R. The effects of general anesthesia on upper respiratory tract infections in children. Anesthesiology. 1987;67:930.
Tauman R., O’Brien L.M., Holbrook C.R., et al. Sleep pressure score: a new index of sleep disruption in snoring children. Sleep. 2004;27:274.
Teele D.W. Inflammatory diseases of the mouth and pharynx. In: Bluestone C.D., Stool S.E., editors. Pediatric otolaryngology. Philadelphia: WB Saunders, 1983.
Teele D.W., Healy G.B., Tally F.P. Persistent effusions of the middle ear: cultures for anaerobic bacteria. Ann Otol Rhinol Laryngol. 1980;89:102.
Tewfik T.L., Kaloustian V.M. Congenital anomalies of the ear, nose and throat. New York: Oxford University Press, 1997.
Travis K.W., Todres I.D., Shannon D.C. Pulmonary edema associated with croup and epiglottitis. Pediatrics. 1977;59:695.
Tunnessen W.W.Jr, Feinstein A.R. The steroid-croup controversy: an analytic review of methodologic problems. J Pediatr. 1980;96:751.
Tusman G., Bohn S.H., Tempra A., et al. Effect of recruitment maneuver on atelectasis in anethetized children. Anethesiology. 2003;98:14.
Ueda W., Hirakawa M., Mae O. Appraisal of epinephrine administration to patients under halothane anesthesia for closure of cleft palate. Anesthesiology. 1983;58:574.
Uezono S., Goto T., Terui K., et al. Emergence agitation after sevoflurane versus propofol in pediatric patients. Anesth Analg. 2000;91:563.
Ved S.A., Walden T.L., Montana J., et al. Vomiting and recovery after outpatient tonsillectomy and adenoidectomy in children: comparison of four anesthetic techniques using nitrous oxide with halothane or propofol. Anesthesiology. 1996;85:4.
Verghese S.T., Hannallah R.S. Pediatric otolaryngologic emergencies. Anesthesiol Clin North Am. 2001;19:237.
Vlajkovic G.P., Sindjelic R.P. Emergence delirium in children: many questions, few answers. Anesth Analg. 2007;204:84-91.
Voepel-Lewis T., Malviya S., Tait A.R. A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit. Anesth Analg. 2003;96:1625.
Wagner M.H., Torrez D.M. Interpretation of the polysomnogram in children. Otolaryngol Clin North Am. 2007;40:745.
Walander A. The mechanism of origin of congenital malformation of the larynx. Acta Otolaryngol. 1955;45:426.
Walner D.L., Loewen M.S., Kimura R.E. Neonatal subglottic stenosis: incidence and trends. Laryngoscope. 2001;111:48.
Ward R.F. Treatment of tracheal and endobronchial lesions with the potassium titanyl phosphate laser. Ann Otol Rhinol Laryngol. 1992;10:205.
Warner D.O. Airway pharmacology. In: Benumof J.L., editor. Airway management. Boston: Mosby; 1996:74.
Watcha M.F., Ramirez-Ruiz M., White P.F., et al. Perioperative effects of oral ketorolac and acetaminophen in children undergoing bilateral myringotomy. Can J Anaesth. 1992;39:649.
Waters K.A., McBrien F., Stewart P., et al. Effects of OSA, inhalational anesthesia, and fentanyl on the airway and ventilation of children. J Appl Physiol. 2002;92:1987.
Werkhaven J.A. Laryngotracheal laser surgery. In: Bluestone C.D., Stool S.E., editors. Atlas of pediatric otolaryngology. Philadelphia: WB Saunders, 1995.
Wetmore R.F. Tracheotomy. In: Bluestone C.D., Stool S.E., editors. Pediatric otolaryngology. Philadelphia: WB Saunders; 2003:1583-1598.
Wetmore R.F., Marsh R.R., Thompson M.E., et al. Pediatric tracheostomy: a changing procedure? Ann Otol Rhinol Laryngol. 1999;108:695.
Widlund B., Walczak S., Motoyama E. Flow-pressure characteristics of pediatric Storz-Hopkins bronchoscopes. Anesthesiology. 1982;57:A417.
Williams P.J., Bailey P.M. Comparison of the reinforced laryngeal mask airway and tracheal intubation for adenotonsillectomy. Br J Anaesth. 1993;70:30.
Windfuhr J.P., Schloendorff G., Baburi D., et al. Lethal outcome of post-tonsillectomy hemorrhage. Eur Arch Otorhinolaryngol. 2008;265:1527-1534.
Windfuhr J.P., Schloendorff G., Sesterhenn A.M., et al. A devastating outcome after adenoidectomy and tonsillectomy: ideas for improved prevention and management. Otolaryngol Head Neck Surg. 2009;140:191-196.
Windfuhr J.P., Schloendorff G., Baburi D. Kremer: Life-threatening posttonsillectomy hemorrhage. Laryngoscope. 2008;118:1389-1394.
Wolf G.L., Simpson J.I. Flammability of endotracheal tubes in oxygen and nitrous oxide-enriched atmosphere. Anesthesiology. 1987;67:236.
Wood R.E., Postma D. Endoscopy of the airway in infants and children. J Pediatr. 1988;112:1.
Woods A.M. Pediatric endoscopy. In: Berry F.A., editor. Anesthetic management of difficult and routine pediatric patients. ed 2. New York: Churchill Livingstone; 1990:199.
Yellon R.F., McBride T.P., Davis H.W. Otolaryngology. In: Zitelli B.J., Davis H.W., editors. Atlas of pediatric physical diagnosis. ed 4,. St Louis: Mosby; 2002:818-865.
Yellon R.F., Parameswaran M., Brandom B.W. Decreasing morbidity following laryngotracheal reconstruction in children. Int Anesthesiol Clin. 1997;35:145.