Chapter 13 Anesthesia for Myocardial Revascularization
Cardiac anesthesiologists who have been in practice for the past several decades have seen a variety of anesthetic and surgical practices come into vogue and fall out of favor based on new research and economic pressures. Perhaps the most striking example is the rise and fall of high-dose opioid anesthesia, which was initially driven by concern about excessive cardiovascular depression by volatile anesthetics in the 1970s and further accelerated in the mid-1980s by concerns about potential coronary steal with isoflurane. The prolonged postoperative mechanical ventilation resulting from the shift to high-dose opioids was also thought important to reduce stress on the recently revascularized myocardium. However, during the following decade, this approach was completely reversed by new basic and clinical research, such as lack of evidence for adverse effects of volatile agents, particularly as related to potential effects of coronary vasodilation on coronary steal, and by strong evidence of their benefits via rapid preconditioning; by social and economic factors (i.e., safety and efficacy of fast-tracking for most patients and recognition that time on the ventilator for many patients is an uncomfortable experience); and by the rapid rise in off-pump coronary artery bypass grafting (OPCAB), which by avoiding adverse physiologic effects of cardiopulmonary bypass (CPB) facilitates more rapid emergence and recovery in many patients.1,2 Given the increasing emphasis on pain control in all surgical patients and its reported association with enhanced postoperative outcome in a variety of surgical subgroups, there has been a resurgence in the use of neuraxial techniques in cardiac surgery, particularly in European and Asian countries.3 Although not commonly used in the United States because of logistical issues and liability concerns, the rapidly growing literature base mandates that clinicians familiarize themselves with their potential benefits and risks.
EPIDEMIOLOGY AND RISK ASSESSMENT
The Society of Thoracic Surgeons (STS) instituted a voluntary clinical database system with this approach in the early 1990s that has continued to grow rapidly as cardiac surgical groups are increasingly interested in benchmarking their practices against others.4 Many tertiary centers (e.g., Cleveland Clinic) and regional consortiums of hospitals (e.g., Northern New England Cardiovascular Disease Study Group) maintain databases, and some publish statistical models. Many states have established and maintain risk-adjusted mandatory reporting systems for hospital and individual surgeon performance (with New York State being an early and influential pioneer). A new scoring system (EuroSCORE) based on outcomes in 128 centers in eight European countries has received increasing attention. It appears to compare favorably with the STS model in North American patients.5 It is freely accessible by means of an interactive web-based calculator (www.euroscore.org) and is decidedly simpler and faster to use than the STS’s scoring system, which is now also freely accessible to the public at http://www.sts.org/sections/stsnationaldatabase/riskcalculator/index.html.
PATHOPHYSIOLOGY OF CORONARY ARTERY DISEASE
Anatomy
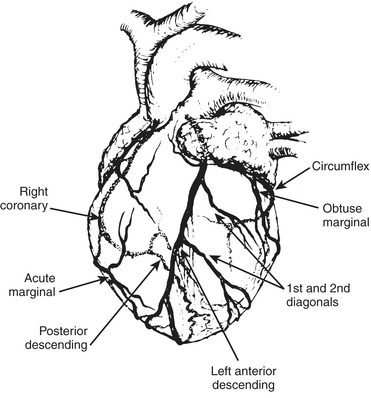
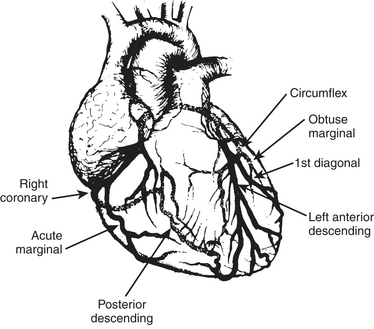
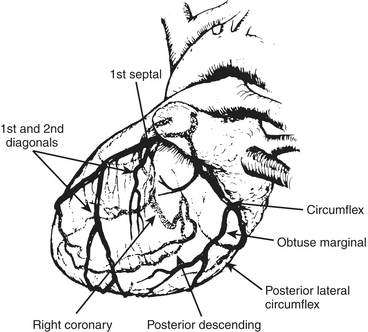
The anesthesiologist should be familiar with coronary anatomy if only to interpret the significance of angiographic findings. The coronary circulation and common sites for placement of distal anastomoses during CABG are shown in Figures 13-1 to 13-3.
The vertical and superior orientation of the RCA ostium allows easy passage of air bubbles during aortic cannulation, CPB, or open valve surgery. In sufficient concentration (e.g., coronary air embolus), myocardial ischemia involving the inferior LV wall segments and the right ventricle may occur (Fig. 13-4). In contrast, the nearperpendicular orientation of the left main coronary ostium makes air embolization much less common.
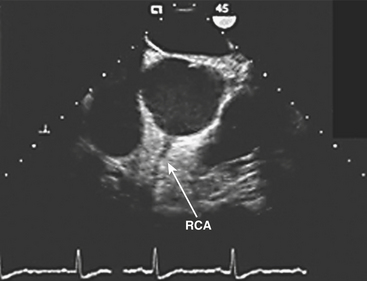
(Image courtesy of Martin J. London, MD, University of California, San Francisco, CA [www.ucsf.edu/teeecho].)
Venous drainage of the myocardium is primarily to the coronary sinus, which drains 96% of the LV free wall and septum, and the remainder of the venous return goes directly into the right atrium.6 A small fraction may enter other cardiac chambers directly through the anterior-sinusoidal, anterior-luminal, and thebesian veins.
Myocardial Ischemia and Infarction
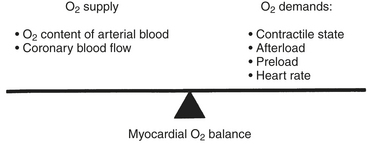
In patients with CAD, myocardial ischemia usually results from increases in myocardial oxygen demand (Fig. 13-5) that exceed the capacity of the stenosed coronary arteries to increase their oxygen supply. However, the determinants of myocardial oxygen balance are complex, and alterations may have several effects. For example, an increase in blood pressure (i.e., increased afterload) increases wall tension and oxygen demand while also increasing coronary blood flow (CBF). It is now appreciated that myocardial ischemia may occur without changes in systemic hemodynamics and in awake patients may occur in the absence of chest pain (i.e., silent ischemia), particularly in diabetic patients.
In atherosclerotic heart disease, the fundamental lesion is an intimal lipid plaque that causes chronic stenosis and episodic thrombosis, occurring most often in an epicardial coronary artery, thereby reducing myocardial blood supply. Characteristics of the vulnerable plaque include high lipid content, a thin fibrous cap, a reduced number of smooth muscle cells, and increased macrophage activity. The lipid core is the most thrombogenic component of the plaque. Fuster described five phases in the progression of CAD by plaque morphology. Phase 1 is a small plaque present in many people younger than 30 years and usually progresses very slowly depending on the presence of risk factors associated with CAD (i.e., elevated low-density lipoprotein cholesterol). Phase 2 is a plaque with a high lipid content that has the potential to rupture. If it ruptures, it will lead to thrombosis and increased stenosis (phase 5), possibly producing unstable angina or an acute coronary syndrome. The phase 2 plaque usually does not rupture; it instead progresses onto phases 3 and 4, with enlargement and fibrous tissue organization, which may ultimately produce an occlusive plaque at phase 5.7
ANESTHESIA FOR CORONARY ARTERY BYPASS GRAFTING
Conventional CABG with CPB is still the most commonly performed cardiac surgical procedure (Box 13-1). Fast-track management with early extubation (4 to 8 hours postoperatively) has become the standard of care in nearly all centers. OPCAB is increasing steadily, although its use tends to be very frequent in some centers or infrequent in others as various surgeons have become “early adopters” or are waiting for firm evidence-based recommendations from future randomized, controlled trials. However, anesthetic management of the sickest patients undergoing multivessel operations combined with valve repair or replacement, repeat operations, and other complex procedures (e.g., ventricular septal defect repairs along with CABG after acute myocardial infarction) has changed relatively little over the past decade as the long duration of surgery usually mandates greater cumulative doses of fixed anesthetic agents with overnight or even prolonged postoperative mechanical ventilation. However, many clinicians have adopted use of infusions of shorter-acting agents (e.g., sufentanil, propofol, remifentanil), avoided large cumulative dosing of fixed agents with potentially long half-lives (e.g., midazolam), and now rely on a volatile anesthesia “base,” taking a “wait and see” attitude toward early extubation if surgery is “smooth” and physiologic parameters remain within acceptable limits (e.g., good urine output, normothermic, adequate hematocrit).
BOX 13-1 Management Strategies for Anesthesia for Myocardial Revascularization
Preoperative Evaluation and Management
Assessment of Cardiac Characteristics
Assessment of Major Comorbidities
Preoperative Medication Management
Intraoperative Management
Management before Revascularization
Revascularization Management
Management after Revascularization
Premedication
Management of Antianginal and Antihypertensive Medications
β-adrenergic receptor antagonists
For the newly trained anesthesiologist who is told to provide “perioperative β-blockade” to all patients with known CAD, or those with multiple risk factors undergoing major noncardiac (particularly vascular) surgery, it may come as a surprise that in 1972, a case series from the prestigious Cleveland Clinic admonished clinicians to withdraw such medication at least 2 weeks before CABG surgery reporting that 5 such patients died within 24 hours of surgery. Several years later, Kaplan and coworkers found that it was safe to continue β-blockade and that operative mortality was similar in patients in whom propranolol had been continued within 24 to 48 hours of surgery. Randomized trials evaluating the safety of administration of propranolol within 12 hours of surgery showed a significantly greater increase in the incidence of pre-CPB ischemia in patients withdrawn from propranolol (within 24 to 72 hours); they also recommended continuation of therapy up until the time of surgery. Further work in the 1980s documented the efficacy of continuation of β-blockers through surgery with regard to reducing pre-CPB ischemia. These studies were instrumental in laying the groundwork for the subsequent noncardiac surgery studies in the late 1980s. These led to the contemporary randomized perioperative β-blocker trials, which despite ongoing controversy regarding the exact efficacy of this therapy resulted in its becoming a clinical routine.8 Several contemporary observational studies have documented associations of β-blocker therapy with reduction in perioperative mortality in CABG patients. The largest of these by Ferguson and colleagues9 considered 629,877 patients in the STS database (1996 to 1999) in which a modest but statistically significant reduction in 30-day risk-adjusted mortality was reported (OR = 0.94, 95% CI = 0.91 to 0.97). This treatment effect was observed in many high-risk subgroups, although a trend toward increased mortality was seen in patients with an ejection fraction (EF) less than 30% (OR = 1.13; 95% CI, 0.96 to 1.33). Considerable efforts are being expended by major organizations (STS, ACC) in increasing compliance with existing guidelines for use of β-blockers at the time of hospital discharge (along with use of aspirin, statins, and angiotensin-converting enzyme [ACE] inhibitors).
other medications
Aspirin (and other platelet inhibitors such as dipyridamole) have long been recognized to have strong efficacy in the prevention of early graft thrombosis after CABG, and are a well-recognized component of primary and secondary prevention strategies for all patients with ischemic heart disease. A large observational analysis reported substantial reduction in overall mortality (1.3% vs. 4.0%) and ischemic complications of the heart, brain, kidneys, and gastrointestinal tract when aspirin was administered within 48 hours after surgery. However, patients receiving aspirin immediately before surgery have more mediastinal bleeding and may receive more blood products. A consensus conference of the American College of Chest Physicians on antithrombotic and thrombolytic therapies recommended institution of aspirin within 6 hours after CABG surgery over continuation of preoperative therapy (level of evidence Ia).10
ACE inhibitors and statins are receiving attention as agents given a variety of important “pleiotropic” effects (e.g., effects independent of their primary actions of antihypertensive effects or lipid-lowering effects, respectively).11 Potent anti-inflammatory effects and beneficial effects on endothelial function have been reported for both agents, as well as less clear effects on angiogenesis. Both agents are commonly administered acutely during PCI for their purported benefits. ACE inhibitors are widely considered to be vasculoprotective, particularly with regard to ventricular remodeling after acute myocardial infarction, and they appear to reduce damage after ischemic reperfusion (likely related to reduction in ischemic-induced vasoconstriction and reduction in leukocyte adhesion). Statins have been reported to reduce circulating levels of adhesion molecules, which have been implicated in endothelial dysfunction after CPB. Both agents appear to have direct effects on platelet aggregation and plasminogen activator inhibitors. Of the two classes of drugs, the greatest interest appears to be on the statins because of the greater number of publications and intense concurrent interest with regard to cardioprotection for noncardiac (particularly vascular) surgery. Several investigators have published similar reports of efficacy in observational cohorts of CABG patients.
Monitoring
Pulmonary Artery Catheterization
Based on the existing literature it is not possible to give precise criteria for use of a PA catheter in CABG.12 The higher the patient risk (based primarily on established preoperative clinical predictors), the more favorable is the risk-benefit ratio. Risk factors include the following:
Transesophageal Echocardiography
It is appreciated that the earliest signs of myocardial ischemia include diastolic dysfunction followed by systolic segmental wall motion abnormalities that occur within seconds of acute coronary occlusion. Comparison of TEE with continuous (Holter) ECGs have shown a greater incidence of segmental wall motion abnormalities than ECG changes in patients with CAD. However, it is thought that new segmental wall motion abnormalities detected in the intraoperative period may frequently occur due to nonischemic causes, particularly changes in loading conditions, and alteration in electrical conduction in the heart. The use of inotropic agents or elevations of catecholamine levels can aggravate (by increased oxygen demand) or improve wall motion. Changes in preload and afterload are likely the most important factors in the CABG setting; transient reversible segmental wall motion abnormalities are commonly related to acute myocardial stunning due to ischemia before or during weaning from CPB. TEE is highly sensitive but not specific for myocardial ischemia. The short-axis midpapillary muscle view, commonly used because of its inclusion of myocardium supplied by the three major coronary arteries, may entirely miss segmental wall motion abnormalities occurring in the basal or apical portions of the heart. These changes necessitate interrogation of additional components of the comprehensive TEE examination recommended by the ASE/SCA Task Force before and after CPB or after completion of revascularization in OPCAB. The ASA Practice Guidelines for TEE (which have not been revised since their initial publication in 1996) list the perioperative uses in patients with increased risk of myocardial ischemia or infarction as a category II indication (supported by weaker evidence and expert consensus). The indication is strengthened when ECG monitoring cannot be used to diagnose ischemia (e.g., in patients with left bundle-branch block, extensive Q waves, or ST-T abnormalities on the baseline ECG), and it is weakened when baseline segmental wall motion abnormalities are present (particularly akinesis or dyskinesis due to fibrotic, calcified, or aneurysmal myocardium). Use of perioperative TEE to evaluate myocardial perfusion, coronary anatomy, or graft patency is listed as a category III indication, but newer technology will upgrade this indication. Evaluation of ischemic mitral regurgitation by TEE may even influence the surgical management during CABG.13
Induction and Maintenance
Primary Induction Agents
The clinical effects of propofol are in general similar to those of thiopental. However, it has numerous advantages over thiopental based on its predictable pharmacokinetics and dynamics. Based on these, it has been widely adopted in the operating room for anesthesia delivery by computer-controlled devices and for postoperative ICU sedation. It is often used for sedation after CABG surgery.14
Opioids
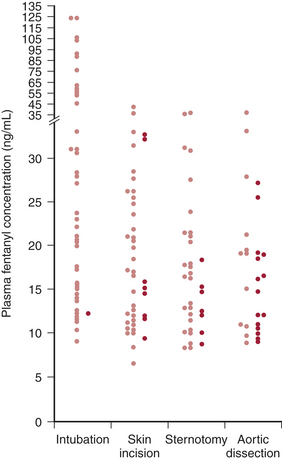
These drugs are pure opioid agonists and none provides complete anesthesia as defined by predictable dose-response relations for suppression of the stress response and release of endogenous catecholamines even with high serum concentrations. Hypertension and tachycardia have been commonly reported in response to induction/intubation and surgical stimuli (particularly with sternotomy) in older studies of high-dose opioid anesthesia with fentanyl or sufentanil. Figure 13-6 demonstrates this lack of association of serum levels with hemodynamic responses.
Inhalation Anesthetics and Myocardial Protection
myocardial protection and preconditioning
Inhalation anesthetics are thought to protect the myocardium against ischemia by their ability to mimic ischemic preconditioning. They have been shown to reduce myocardial infarction size after periods of ischemia, protect the heart against postischemic LV dysfunction, and reduce the incidence of arrhythmias after cardiac surgery. Intravenous anesthetics such as propofol do not appear to have the same cardioprotective properties. There is increasing evidence from prospective randomized clinical studies in patients undergoing CABG surgery that volatile anesthetic agents should be part of the anesthetic regimen, particularly in patients at high risk for ischemic events.15
sevoflurane
Sevoflurane has been increasingly used during cardiac surgery owing to its favorable hemodynamic effects and cardioprotective properties. It is a potent trigger of the preconditioning cascade.16 It has beneficial effects on intraoperative myocardial function after CPB, and it may also favorably influence long-term morbidity and mortality after CABG.
desflurane
Desflurane has been shown to have preconditioning-like cardioprotective effects in vitro and in vivo.17 It preconditions human myocardium by activation of KATP channels, stimulation of adenosine A1 receptors, and nitric oxide release.
Intraoperative Awareness and Recall
Patients undergoing cardiac surgery have always been considered to be at increased risk due to anesthetic regimens intentionally devoid of cardiodepressant inhalation anesthetics and due to frequent periods of light anesthesia in the presence of hemodynamic instability resulting from surgical manipulation of the heart and great vessels, depressed contractility after CPB, or bleeding. The published incidence of awareness in patients undergoing cardiac surgery is significantly higher than that reported for general surgery with older reports of up to 23%. However, the introduction of fast-track anesthesia techniques and the recognition that inhalation agents are useful in intraoperative preconditioning before CPB have changed anesthetic regimens for cardiac surgery. It is now recognized that use of inhalation agents during cardiac surgery (including during CPB) reduces the risk of awareness.18
Choice of Technique
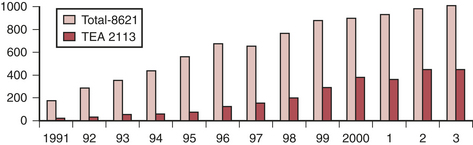
Interest in the use of thoracic epidural anesthesia (TEA) for cardiac surgery has steadily increased over the past 15 years. It has been long appreciated that thoracic sympathectomy has favorable effects on the heart and coronary circulation. Its coronary vasodilating effects have been well documented and it has been used to treat unstable angina for many years. There has been a resurgence in interest, and it is frequently used as a supplement to general anesthesia for cardiac surgery, particularly in Europe and Asia (Fig. 13-7). However, in the United States, medicolegal concerns about the rare but present danger of a devastating neurologic injury and the substantial logistical issues regarding placement the night before surgery, increased time to place relative to inducing general anesthesia, and the potential for cancellation of a case in event of bloody return are major limiting factors. The advent of fast-tracking could be considered a potential driving force (e.g., ability to extubate faster and have a more comfortable patient with TEA), although most evidence suggests that a wide variety of techniques can be effectively used to facilitate early extubation and that the cardioprotective effects of volatile agents may be as effective as the beneficial effects of thoracic sympathectomy.
Safety concerns are a major consideration in use of TEA in this setting given chronic use of antiplatelet agents, use of systemic anticoagulation and platelet inhibition for acute therapy of unstable angina, and systemic anticoagulation and potential coagulopathy induced by CPB. The true incidence of serious complications (particularly epidural hematoma) is unknown. A widely quoted estimate is 1 in 1528 for TEA with 95% confidence. Intrathecal risks are quoted as 1 in 3610. These estimates were based on consideration of more than 4000 reported cases (cardiac surgery) in which no complications were reported. Chakravarthy and coworkers presented an audit of 2113 cardiac surgery TEA cases over a 13-year period with no permanent neurologic deficits, a 0.9% dural puncture rate, and 0.2% transient neurologic deficits.19
FAST-TRACK MANAGEMENT FOR CORONARY ARTERY BYPASS GRAFTING
The fast-track clinical pathway encompasses a variety of perioperative (and after hospital discharge) management strategies, but early extubation is the one that has received the greatest attention (Box 13-2). Because it is a simple, continuous variable (e.g., hours to extubation), it is one that many observational reports and a smaller number of randomized, controlled trials have reported. It also appears to be the only one that has resulted in meta-analyses showing the practice is safe and effective.20 Early extubation is acknowledged as a key component of the fast-track clinical pathway and one that was considered perhaps the most radical change in practice during the peak of scrutiny of the fast-track pathway (Box 13-3).
BOX 13-2 Perioperative Goals of Fast-Track Management
BOX 13-3 Suggested Criteria for Early Extubation
Cardiovascular
Respiratory
CORONARY ARTERY BYPASS GRAFTING WITHOUT CARDIOPULMONARY BYPASS
Off-pump coronary artery bypass grafting is the single greatest change in CABG techniques in the past 2 decades.21,22 Although this term encompasses a range of surgical approaches (based on the degree of invasiveness encompassing full, limited, or no sternotomy), the technique that is most commonly performed is OPCAB with a full sternotomy. The precise number of such procedures performed remains largely speculative; however, it is likely in the 20–30% range at the present time. Surgeons appear to adopt it enthusiastically or not at all, an unsurprising finding given the unsettled state of the literature. Although the literature base is increasing rapidly, the final word is still years away (given several large ongoing randomized, controlled trials). However, it appears that OPCAB is as effective as routine CABG, is safer for certain patient subsets, and may be associated with improvement in several outcomes at slightly lower resource use. Its use is likely to increase, especially for high-risk patients with serious comorbidities associated with higher morbidity/mortality from CPB (e.g., severe cerebrovascular and renal disease). The clinician will immediately notice that the pace and tempo of anesthetic management differs substantially from that of conventional CABG. The focused involvement of the anesthesiologist is perhaps more important in OPCAB than during on-pump CABG (including use of TEE and PA catheterization).
Outcomes in Off-Pump Coronary Artery Bypass Grafting
Several investigators and working groups have comprehensively reviewed outcomes in patients undergoing OPCAB. Investigators have analyzed 37 randomized trials of 3369 patients with comparable treatment groups with the exception of a marginal difference in number of grafts performed (2.6 OPCAB versus 2.8 CABG).21 All but one of the studies specifically excluded “high-risk” patients. Although various definitions were used, most excluded patients with low ejection fractions, repeat procedures, and renal failure, and several studies excluded patients with diseased circumflex vessels. As expected, not all studies reported on all outcomes. The investigators found no significant differences in 30-day or 1- to 2-year mortality, myocardial infarction, stroke (30 day and 1 to 2 years), renal dysfunction, need for IABP, wound infection, or reoperation for bleeding or reintervention (for ischemia). OPCAB was associated with a significant reduction in atrial fibrillation (OR = 0.58), numbers of patients transfused (OR = 0.43), respiratory infections (OR = 0.41), need for inotropes (OR = 0.48), duration of ventilation (weighted mean difference [WMD] of 3.4 hours), ICU length of stay (WMD of 0.3 day), and hospital length of stay (WMD of 1.0 days). Changes in neurocognitive dysfunction were not different in the immediate postoperative period; they were significantly improved at 2 to 6 months (OR = 0.57), but there were no differences seen at 12 months. The critical issue of graft patency was addressed in only four studies, and these varied substantially with regard to when this was assessed (3 months in two and 12 months in two). Only one study reported a difference (reduction in circumflex patency with OPCAB). Because of the small numbers of patients, the overall data for this category were considered inadequate for meta-analysis. A large-scale, randomized, controlled trial is being conducted by the Department of Veterans Affairs with evaluation of graft patency at 1 year postoperatively. Four randomized, controlled trials have analyzed quality of life. Various methods precluded inclusion in the meta-analysis, but it generally appears there is little difference between operations. Of the 20 trials reporting conversion rates, 8% of OPCAB patients required conversion to CABG, whereas only 1.7% were converted from CABG to OPCAB. The conversion rate for OPCAB in these low- to medium-risk patients is substantial and would be expected to be even higher in higher-risk patients with greater disease burdens, more complex lesions, or impaired ventricular function in whom tolerance of stabilization and verticalization may be less. The anesthesiologist must be prepared for rapid institution of CPB at all times.
MYOCARDIAL ISCHEMIA DURING REVASCULARIZATION
Incidence of Myocardial Ischemia in Patients Undergoing Revascularization Surgery
In addition to providing anesthesia, a major concern of the anesthesiologist is the prevention and treatment of myocardial ischemia. Numerous laboratory and clinical studies have examined the incidence of myocardial ischemia in the perioperative period, as it relates to the administration of anesthetic drugs. Although the incidence of pre-bypass ischemia in patients appears to be between 10% and 50%, it is not at all clear that the anesthetic drug combination per se is a determinant of this incidence. Several studies have addressed whether the anesthetic technique (e.g., fast-track or early extubation, high thoracic epidural) is related to perioperative morbidity and myocardial ischemic events. An extensive meta-analysis of more than 30 randomized, controlled trials of patients undergoing cardiac surgery showed no difference in the relative risk of postoperative myocardial ischemia when early extubation protocols were compared with conventional extubation management.22
Hemodynamic Changes Related to Myocardial Ischemia
Besides ECG abnormalities, some hemodynamic changes should alert the anesthesiologist to the possibility of intraoperative myocardial ischemia. The association of tachycardia with hypotension or increased LV filling pressure (both of which reduce coronary perfusion pressure [CPP] is a particularly undesirable combination jeopardizing the oxygen supply-demand relationship. Figure 13-8 demonstrates how hypertension in the absence of tachycardia, in response to surgical stress (skin incision), can be associated with pulmonary hypertension, elevated pulmonary capillary wedge pressure, and prominent A and V waves on the PCWP waveform. Although ECG changes occurred later, the early hemodynamic abnormalities almost certainly were the result of ischemic LV dysfunction. Treatment included deepening anesthesia and administering a nitroglycerin infusion.
Intraoperative Treatment of Myocardial Ischemia
Close attention to hemodynamic control and rapid treatment of abnormalities are fundamental principles of the intraoperative management of the patient with CAD. If there is a hemodynamic abnormality that is temporally related to the onset of ischemia, it should be treated accordingly. Hemodynamic treatment to ensure an adequate CPP (diastolic BP − LVEDP) should be a priority, as should control of heart rate, the single most important treatable determinant of myocardial oxygen consumption. Table 13-1 summarizes the treatment of acute perioperative myocardial ischemia.23,24
Table 13-1 Acute Treatments for Suspected Intraoperative Myocardial Ischemia*
| Associated Hemodynamic Finding | Therapy | Dosage |
|---|---|---|
| Hypertension, tachycardia† | Deepen anesthesia | |
| Intravenous (IV) β-blockade |
* Ensure adequacy of oxygenation, ventilation, and intravascular volume status and consider surgical factors, such as manipulation of heart of coronary grafts.
† Tachyarrhythmias (e.g., paroxysmal atrial tachycardia, atrial fibrillation) should be treated directly with synchronized cardioversion or specific pharmacologic agents.
‡ Bolus doses (25-50 μg) and high infusion rate may be required initially.
Intravenous Nitroglycerin
Preoperatively, it is often used to treat patients with unstable angina or ischemic mitral regurgitation and to limit the size of an evolving myocardial infarction, reduce associated complications, and reverse segmental wall motion abnormalities. In the pre-CPB period and during OPCAB, nitroglycerin is used to treat signs of ischemia such as ST-segment depression, hypertension uncontrolled by the anesthetic technique, ventricular dysfunction, or coronary artery spasm (Box 13-4). During CPB, nitroglycerin can be used to control the mean arterial pressure; however, nitroglycerin is not always effective in controlling mean arterial pressure during CPB (approximately 60% of patients respond) because of alterations of the pharmacokinetics and pharmacodynamics of the drug with CPB. Factors contributing to the reduction of its effectiveness include adsorption to the plastic in the CPB system, alterations in regional blood flow, hemodilution, and hypothermia. Different oxygenators and filters sequester up to 90% of circulating nitroglycerin during CPB. After revascularization, nitroglycerin is used to treat residual ischemia or coronary artery spasm and reduce preload and afterload, and it may be combined with vasopressors (e.g., phenylephrine) to increase the CPP when treating coronary air embolism (Box 13-5).
Calcium Channel Antagonists
Calcium antagonists have been found to be cardioprotective against reperfusion injury. This is due to their energy-saving actions of negative inotropy and chronotropy. These antagonists have also been shown to reduce reperfusion arrhythmias and attenuate myocardial stunning. In a review of methods to reduce ischemia during OPCAB, Kwak included the calcium antagonists along with newer drugs such as nitric oxide–releasing agents, free radical scavengers, and Na+/H+ exchange inhibitors in the management of ischemia-reperfusion injury.23
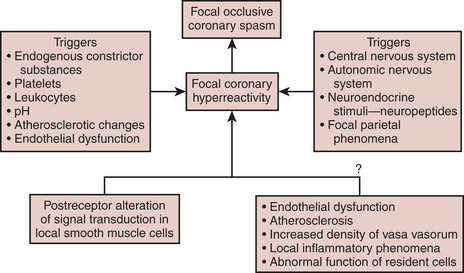
Coronary Artery and Arterial Conduit Spasm
Spasm has usually been associated with profound ST-segment elevation on the ECG, hypotension, severe dysfunction of the ventricles, and myocardial irritability. Many hypotheses have been put forward to explain the origin of coronary artery spasm; some of the mechanisms that may play a role are demonstrated in Figure 13-9. The mechanism of postoperative spasm may or may not be the same as that underlying Prinzmetal’s variant angina, but the same stimuli seem to be present and therapy is usually effective with a wide range of vasodilators such as nitroglycerin, calcium channel blockers, milrinone, or combinations of nitroglycerin and calcium channel blockers in both situations. Arterial grafts such as the internal mammary artery, and particularly radial artery grafts, are prone to spasm after revascularization and its prevention and recognition are crucial to prevent serious complications.
SUMMARY
1. Myles P.S., Daly D.J., Djaiani G., et al. A systematic review of the safety and effectiveness of fast-track cardiac anesthesia. Anesthesiology. 2003;99:982.
2. Cheng D.C., Bainbridge D., Martin J.E., et al. Does off-pump coronary artery bypass reduce mortality, morbidity, and resource utilization when compared with conventional coronary artery bypass? A meta-analysis of randomized trials. Anesthesiology. 2005;102:188.
3. Chakravarthy M., Thimmangowda P., Krishnamurthy J., et al. Thoracic epidural anesthesia in cardiac surgical patients: A prospective audit of 2,113 cases. J Cardiothorac Vasc Anesth. 2005;19:44.
4. Shroyer A.L., Coombs L.P., Peterson E.D., et al. The Society of Thoracic Surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg. 2003;75:1856.
5. Nashef S.A., Roques F., Hammill B.G., et al. Validation of European System for Cardiac Operative Risk Evaluation (EuroSCORE) in North American cardiac surgery. Eur J Cardiothorac Surg. 2002;22:101.
6. Wei K., Kaul S. The coronary microcirculation in health and disease. Cardiol Clin. 2004;22:221.
7. Fuster V., Moreno P.R., Fayad Z.A., et al. Atherothrombosis and high-risk plaque. J Am Coll Cardiol. 2005;46:937.
8. London M.J., Zaugg M., Schaub M.C., et al. Perioperative beta-adrenergic receptor blockade: Physiologic foundations and clinical controversies. Anesthesiology. 2004;100:170.
9. Ferguson T.B.Jr., Coombs L.P., Peterson E.D. Preoperative beta-blocker use and mortality and morbidity following CABG surgery in North America. JAMA. 2002;287:2221.
10. Stein P.D., Schunemann H.J., Dalen J.E., et al. Antithrombotic therapy in patients with saphenous vein and internal mammary artery bypass grafts: The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:600S.
11. O’Neil-Callahan K., Katsimaglis G., Tepper M.R., et al. Statins decrease perioperative cardiac complications in patients undergoing noncardiac vascular surgery: The Statins for Risk Reduction in Surgery (StaRRS) study. J Am Coll Cardiol. 2005;45:336.
12. Murphy G.S., Vender J.S. Is the pulmonary artery catheter dead? con position. J Cardiothorac Vasc Anesth. 2007;21:147.
13. Minhaj M., Patel K., Muzic D., et al. The effect of routine intraoperative transesophageal echocardiography on surgical management. J Cardiothorac Vasc Anesth. 2007;21:800.
14. Herr D.L., Sum-Ping S.T., England M. ICU sedation after coronary artery bypass graft surgery: Dexmedetomidine based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17:576.
15. Zaugg M., Lucchinetti E., Garcia C., et al. Anaesthetics and cardiac preconditioning: II. Clinical implications. Br J Anaesth. 2003;91:566.
16. De Hert S.G., Van der Linden P.J., Cromheecke S., et al. Cardioprotective properties of sevoflurane in patients undergoing coronary surgery with cardiopulmonary bypass are related to the modalities of its administration. Anesthesiology. 2004;101:299.
17. Piriou V., Chiari P., Gateau-Roesch O., et al. Desflurane-induced preconditioning alters calcium-induced mitochondrial permeability transition. Anesthesiology. 2004;100:581.
18. Ranta S.O., Herranen P., Hynynen M. Patients’ conscious recollections from cardiac anesthesia. J Cardiothorac Vasc Anesth. 2002;16:426.
19. Chakravarthy M., Thimmangowda P., Krishnamurthy J., et al. Thoracic epidural anesthesia in cardiac surgical patients: A prospective audit of 2,113 cases. J Cardiothorac Vasc Anesth. 2005;19:44.
20. Hawkes C.A., Dhileepan S., Foxcroft D. Early extubation for adult cardiac surgical patients. Cochrane Database Syst Rev. 2003;4:CD003587.
21. Raja S.G., Dreyfus G.D. Off-pump coronary artery bypass surgery: To do or not to do? Current best available evidence. J Cardiothorac Vasc Anesth. 2004;18:486.
22. Sellke F.W., DiMaio J.M., Caplan L.R., et al. Comparing on-pump and off-pump coronary artery bypass grafting: Numerous studies but few conclusions: A scientific statement from the American Heart Association council on cardiovascular surgery and anesthesia in collaboration with the interdisciplinary working group on quality of care and outcomes research. Circulation. 2005;111:2858.
23. Kwak Y.L. Reduction of ischemia during off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth. 2005;19:667.
24. Hannan E.L., Wu C., Walford G., et al. Drug-eluting stents versus coronary-artery bypass grafting in multivessel coronary disease. N Engl J Med. 2008;358:331.

 , minimal base deficit
, minimal base deficit