Anesthesia for hypophysectomy
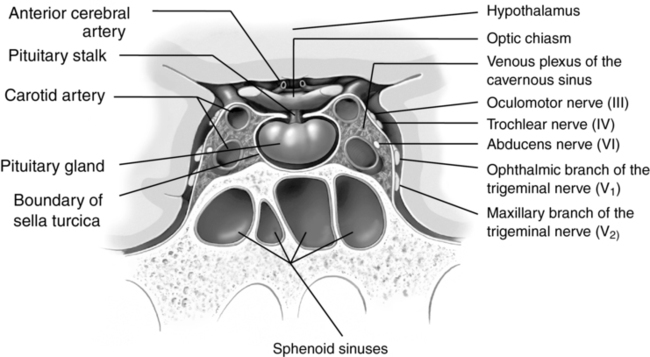
In adults, the pituitary gland has a volume of 0.5 to 1.5 mm3 and is located inferior to the hypothalamus within the sella turcica (Figure 135-1). Despite its small size, the pituitary gland plays a crucial role in human physiology. It consists of two functionally separate regions: (1) the anterior pituitary or adenohypophysis and (2) the posterior pituitary or neurohypophysis. The pituitary gland secretes a variety of hormones that either directly affect other tissues or control the regulation of other endocrine substances. Pituitary tumors are a common cause of primary pituitary dysfunction and can manifest by hypersecretion or hyposecretion of hormones or by invasion of the structures surrounding the sella turcica.
Adenohypophysis
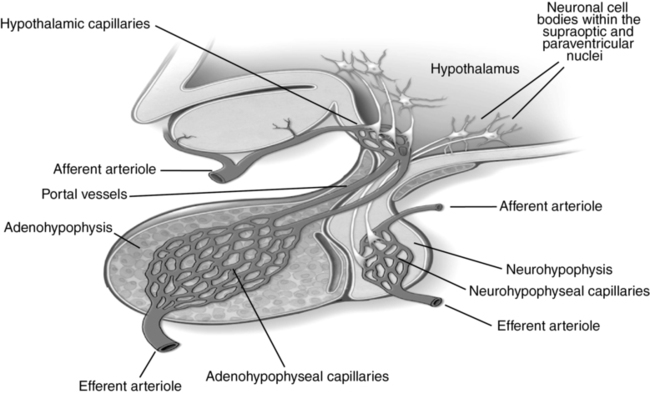
The adenohypophysis secretes an array of hormones under the regulation of the hypothalamus. Releasing and inhibiting factors are secreted into a capillary network within the hypothalamus (Table 135-1). Via portal vessels, these compounds then enter a second capillary network within the adenohypophysis, where they either enhance or inhibit secretion of adenohypophyseal hormones (Figure 135-2). Further secretion of hormones by the anterior pituitary is then regulated via feedback control of hypothalamic and adenohypophyseal secretion in response to concentrations of hormones secreted by target glands. Given the complex interactions among the hypothalamus, anterior pituitary, target endocrine glands, and end organs, disease or dysregulation at any point within these pathways can cause dysfunction of one or more hormone axes.
Table 135-1
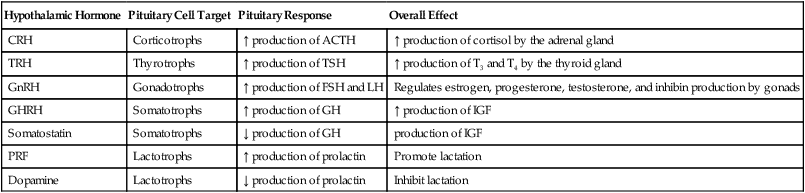
Hypothalamic Hormones and Adenohypophyseal Responses
| Hypothalamic Hormone | Pituitary Cell Target | Pituitary Response | Overall Effect |
| CRH | Corticotrophs | ↑ production of ACTH | ↑ production of cortisol by the adrenal gland |
| TRH | Thyrotrophs | ↑ production of TSH | ↑ production of T3 and T4 by the thyroid gland |
| GnRH | Gonadotrophs | ↑ production of FSH and LH | Regulates estrogen, progesterone, testosterone, and inhibin production by gonads |
| GHRH | Somatotrophs | ↑ production of GH | ↑ production of IGF |
| Somatostatin | Somatotrophs | ↓ production of GH | production of IGF |
| PRF | Lactotrophs | ↑ production of prolactin | Promote lactation |
| Dopamine | Lactotrophs | ↓ production of prolactin | Inhibit lactation |
Neurohypophysis
Unlike the adenohypophysis, which contains hormone-secreting cells, the neurohypophysis contains distal axons of peptidergic neurons with cell bodies located in the supraoptic and paraventricular nuclei of the hypothalamus. These neurons synthesize and secrete either oxytocin or vasopressin (i.e., antidiuretic hormone), which are released into the systemic circulation via capillaries located in the neurohypophysis (see Figure 135-2).