Chapter 20 Anesthesia for Heart, Lung, and Heart-Lung Transplantation
HEART TRANSPLANTATION
The history of heart transplantation spans almost a century. Canine heterotopic cardiac transplantation was first reported in 1905, but such efforts were doomed by ignorance of the workings of the immune system (Box 20-1). Further research in the late 1950s and early 1960s set the stage for the first human cardiac transplant by Barnard in 1966. However, there were few long-term survivors in this era, owing to continued deficiency in understanding and in modulating the human immune system, and the procedure fell into general disfavor. Continued research at selected centers (e.g., Stanford University) and lessons learned from renal transplantation led to greater understanding of the technical issues and immunology required, and by the early 1980s cardiac transplantation gained widespread acceptance as a realistic option for patients with end-stage cardiomyopathy.
Heart transplantation experienced explosive growth in the mid to late 1980s, but the annual number of heart transplants worldwide plateaued by the early 1990s at approximately 3500 per year. The factor limiting continued growth has been a shortage of suitable donors. In 2004, there were approximately 3500 patients on the United Network for Organ Sharing cardiac transplant waiting list (includes all U.S. candidates), whereas only 2055 heart transplantations were performed in the United States during the 2003 calendar year. The median waiting time for a cardiac graft varies widely according to blood type (approximately 39 days for type AB recipients but up to 303 days for type O recipients). In aggregate, more than 48% of patients on the heart transplant list had spent more than 2 years waiting for a transplant.1 The most frequent recipient indications for adult heart transplantation remain either idiopathic or ischemic cardiomyopathy. Other less common diagnoses include viral cardiomyopathy, systemic diseases such as amyloidosis, and complex congenital heart disease.
Donor Selection and Graft Harvest
Donors can exhibit major hemodynamic and metabolic derangements that can adversely affect organ retrieval. The vast majority of brain-dead donors will be hemodynamically unstable.2 Reasons for such instability include hypovolemia (secondary to diuretics or diabetes insipidus), myocardial injury (possibly a result of “catecholamine storm” during periods of increased intracranial pressure), and inadequate sympathetic tone due to brainstem infarction. Donors often also have abnormalities of neuroendocrine function such as low T3 and T4 levels. Donor volume status should be assiduously monitored, and inotropic and vasopressor therapy should be guided by data from invasive monitors.
SURGICAL PROCEDURES
Orthotopic Heart Transplantation
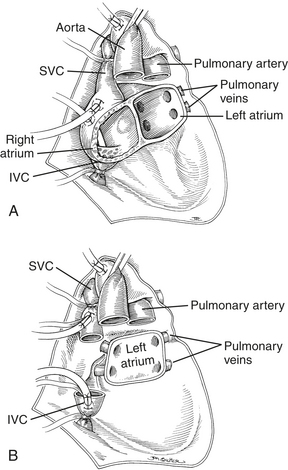
Orthotopic heart transplantation is carried out via a median sternotomy, and the general approach is similar to that used for coronary revascularization or valve replacement. Patients will frequently have undergone a prior median sternotomy; repeat sternotomy is cautiously performed using an oscillating saw. The groin should be prepped and draped to provide a rapid route for cannulation for cardiopulmonary bypass (CPB) if necessary. After the pericardium is opened, the aorta is cannulated as distally as possible, and the IVC and SVC are individually cannulated via the high right atrium (RA). Manipulation of the heart before institution of CPB is limited if thrombus is detected in the heart with transesophageal echocardiography (TEE). After initiation of CPB and cross-clamping of the aorta, the heart is arrested and excised (Fig. 20-1). The aorta and PA are separated and divided just above the level of their respective valves, and the atria are transected at their grooves. A variant of this classic approach totally excises both atria, mandating bicaval anastomoses. This technique may reduce the incidence of atrial arrhythmias, better preserve atrial function by avoiding tricuspid regurgitation, and enhance cardiac output (CO) after transplantation.
Heterotopic Heart Transplantation
Donor harvesting for heterotopic placement is performed in the previously described manner, except that the azygos vein is ligated and divided to increase the length of the donor SVC; the PA is extensively dissected to provide the longest possible main and right PA; and the donor IVC and right pulmonary veins are oversewn, with the left pulmonary veins incised to create a single large orifice. The operation is performed via a median sternotomy in the recipient, but the right pleura is entered and excised. The recipient SVC is cannulated via the RA appendage, and the IVC is cannulated via the lower RA. After arrest of the recipient heart, the LA anastomosis is constructed by incising the recipient LA near the right superior pulmonary vein and extending this incision inferiorly and then anastomosing the respective LA. The recipient RA-SVC is then incised and anastomosed to the donor RA-SVC, following which the donor aorta is joined to the recipient aorta in an end-to-side manner. Finally, the donor PA is anastomosed to the recipient main PA in an end-to-side manner if it is sufficiently long; otherwise, they are joined via an interposed vascular graft (Fig. 20-2).
Special Situations
Mechanical ventricular assist devices have been successfully used to “bridge” patients who would otherwise die of acute heart failure awaiting transplantation.3 The technique of transplantation is virtually identical in such patients to that for ordinary orthotopic transplantation. However, repeat sternotomy is obligatory, and patients will often have been exposed to aprotinin during the assist device placement, increasing the probability of an anaphylactic response to the second aprotinin exposure. Although the incidence of anaphylaxis seems to be low, the team should be in a position to expeditiously initiate CPB before administering aprotinin in this setting. Placement of large-bore intravenous access is prudent because excessive hemorrhage can occur during the transplant procedure.
Pathophysiology after Transplantation
Denervation has important implications in the choice of pharmacologic agents used after cardiac transplantation. Drugs that act indirectly on the heart via either the sympathetic (ephedrine) or parasympathetic (atropine, pancuronium, edrophonium) nervous systems will generally be ineffective. Drugs with a mixture of direct and indirect effects will exhibit only their direct effects (leading to the absence of the normal increase in refractory period of the atrioventricular node with digoxin, tachycardia with norepinephrine infusion, and bradycardia with neostigmine). Thus, agents with direct cardiac effects (e.g., epinephrine or isoproterenol) are the drugs of choice for altering cardiac physiology after transplantation. However, the chronically high catecholamine levels found in cardiac transplant recipients may blunt the effect of α-adrenergic agents, as opposed to normal responses to β-adrenergic agents.4
Allograft coronary vasculopathy remains the greatest threat to long-term survival after heart transplantation. Allografts are prone to the accelerated development of an unusual form of coronary atherosclerosis that is characterized by circumferential, diffuse involvement of entire coronary arterial segments, as opposed to the conventional form of coronary atherosclerosis with focal plaques often found in eccentric positions in proximal coronary arteries. The pathophysiologic basis of this process remains elusive, but it is likely due to an immune cell-mediated activation of vascular endothelial cells to upregulate the production of smooth muscle cell growth factors. More than half of all heart transplant recipients have evidence of concentric atherosclerosis 3 years after transplant, and more than 80% have this condition at 5 years.5 Because afferent cardiac reinnervation is rare, a substantial portion of recipients with accelerated vasculopathy have silent ischemia. Noninvasive methods of detecting coronary atherosclerosis are insensitive for detecting allograft vasculopathy. Furthermore, coronary angiography often underestimates the severity of allograft atherosclerosis; other diagnostic regimens such as intravascular ultrasound and dobutamine stress echocardiography may detect morphologic abnormalities or functional ischemia, respectively, in the absence of angiographically significant lesions. Therefore, the anesthesiologist should assume that there is a substantial risk of coronary vasculopathy in any heart transplant recipient beyond the first 2 years, regardless of symptoms, the results of noninvasive testing, and even angiography.
Anesthetic Management
Induction
Most patients presenting for heart transplantation will not be in a fasting state and should be considered to have a “full stomach.” Therefore, the induction technique should aim to rapidly achieve control of the airway to prevent aspiration while avoiding myocardial depression. A regimen combining a short-acting hypnotic with minimal myocardial depression (etomidate, 0.3 mg/kg), a moderate dose of narcoticto blunt the tachycardia response to laryngoscopy and intubation (fentanyl, 10 μg/kg), and succinylcholine (1.5 mg/kg) is popular; high-dose narcotic techniques with or without benzodiazepines have also been advocated. Vasodilation should be countered with an α-agonist. Anesthesia can be maintained with additional narcotic and sedatives (benzodiazepines or scopolamine).6
Postoperative Management and Complications
Early complications after heart transplantation include acute and hyperacute rejection, cardiac failure, systemic and pulmonary hypertension, cardiac arrhythmias, renal failure, and infection. Hyperacute rejection is an extremely rare but devastating syndrome mediated by preformed recipient cytotoxic antibodies against donor heart antigens. The donor heart immediately becomes cyanotic from microvascular thrombosis and ultimately ceases to contract. This syndrome is lethal unless the patient can be supported mechanically until a suitable heart is found. Acute rejection is a constant threat in the early postoperative period and may present in many forms (e.g., low CO, arrhythmias). Acute rejection occurs most frequently during the initial 6 months after transplantation, so its presence is monitored by serial endomyocardial biopsies, with additional biopsies to evaluate any acute changes in clinical status. Detection of rejection mandates an aggressive increase in the level of immunosuppression, usually including pulses of glucocorticoid or a change from cyclosporine to tacrolimus. Low CO after transplantation may reflect a number of causes: hypovolemia, inadequate adrenergic stimulation, myocardial injury during harvesting, acute rejection, tamponade, or sepsis. Therapy should be guided by invasive monitoring, TEE, and endomyocardial biopsy. Systemic hypertension may be due to pain, so adequate analgesia should be obtained before treating blood pressure with a vasodilator. Because fixed pulmonary hypertension will have been excluded during the recipient evaluation, pulmonary hypertension after heart transplantation is usually transient and responsive to vasodilators such as prostaglandin E1, nitrates, or hydralazine after either orthotopic or heterotopic placement.7 Atrial and ventricular tachyarrhythmias are common after heart transplantation; once rejection has been ruled out as a cause, antiarrhythmics are used for conversion or control (except those acting via indirect mechanisms such as digoxin, or those with negative inotropic properties such as β-blockers and calcium channel blockers). Almost all recipients will require either β-adrenergic agonists or pacing to increase heart rate in the immediate perioperative period, but 10% to 25% of recipients will also require permanent pacing.8 Renal function often improves immediately after transplantation, but immunosuppressives such as cyclosporine and tacrolimus may impair renal function. Finally, infection is a constant threat to immunosuppressed recipients. Bacterial pneumonia is frequent early in the postoperative period, with opportunistic viral and fungal infections becoming more common after the first several weeks.
LUNG TRANSPLANTATION
History and Epidemiology
Although the first human lung transplant was performed in 1963, surgical technical problems and inadequate preservation and immunosuppression regimens prevented widespread acceptance of this procedure until the mid 1980s (Box 20-2). Advances in these areas have since made lung transplantation a viable option for many patients with end-stage lung disease. The frequency of both single- and double-lung transplants increased exponentially during the period up until 1993, with the sharpest growth in unilateral transplants. According to data collected by UNOS between 2000 and 2002, the annual frequency of lung transplantation remained stagnant, with the total number averaging in the vicinity of 1000. This is unchanged from the time between 1993 and 1995 when the numbers first leveled off. Further growth in lung transplantation is constrained by a shortage of donor organs, with demand for organs still vastly exceeding supply. It is estimated that in excess of a million individuals with end-stage lung disease are potential recipients of lung transplants.9 Some had hoped that non−heart-beating donors would provide an alternative source of organs, but this has not been the case. The Organ Procurement and Transplantation Network currently registers approximately 4000 patients for lung transplantation. This number does not accurately reflect the number of organs required as some patients will require bilateral lung transplantation. Average time to transplant increased to as much as 451 days in 1999, but now about one fourth of patients receive a transplant within 251 days. Most of this improvement has been seen with recipients who are 50 years of age and older. One explanation for this may be increasing leniency in organ selection criteria. This seems to have not been associated with increasing mortality rates. Mortality for patients on the waiting list has also continued to decline, from a 1993 high of close to 250 per 1000 patient-years to approximately 140 in 2002. Although some of this improvement may be ascribed to better medical management of patients on the waiting list, it is also likely due to broadened criteria for acceptance for transplantation and subsequent inclusion of patients with less severe illness.
Increased experience with lung transplantation has been accompanied by a decrease in both operative and long-term mortality. For example, 30-day mortality for double-lung transplantation decreased from 44% in 1988 to 13.6% in 1991, whereas that for single-lung transplantation decreased from 22.7% to 12.6%.10 As of the end of 1995, 3-year actuarial survival for recipients of both single- and double-lung transplants performed in the era 1992 to 1995 was approximately 60%, 10% better than for recipients transplanted in the previous 3-year interval. Even better survival data have been reported from centers with extensive experience with these procedures (1-year survival rates of 82% for double-lung recipients and 90% for single-lung recipients). Infection is the most frequent cause of death in the first year after transplant, but this is superceded in later years by bronchiolitis obliterans.
Some of the most challenging patients are those with cystic fibrosis. The 1-year survival of 79% and 5-year survival of 57% after lung transplantation have shown that despite the high incidence of poor nutrition and the almost ubiquitous colonization by multidrug-resistant organisms, these patients can still successfully undergo lung transplantation with acceptable outcome data.11
It is a sign of the maturity of lung transplantation procedures that survival data for repeat lung transplantation are also becoming available. A late 1991 survey of centers reported that actuarial survival after repeat transplantation was significantly worse than that of first-time recipients (e.g., 35% vs. greater than 75% at 1 year),12 and subsequent data have confirmed this observation. Infection and multiorgan failure before repeat transplant are associated with an almost uniformly fatal outcome.
Donor Selection and Graft Harvest
The ongoing shortage of suitable donor organs has led to a liberalization of selection criteria. Prospective lung donors who were cigarette smokers are no longer rejected simply based on a pack-year history. CT has been used to assess the structural integrity of the lung, particularly in donors who have sustained traumatic chest injury. Lungs that have contusion limited to less than 30% of a single lobe can be considered adequate.13 Greater use has also been made of organs from older but otherwise healthy donors (55 to 60 years old) especially when the ischemic period will be short. A clear chest radiograph, normal blood gas results, unremarkable findings on bronchoscopy and sputum stain, and direct intraoperative evaluation confirm satisfactory lung function. The lungs are matched to the recipient for ABO blood type and size (oversized lungs can result in severe atelectasis and compromise of venous return in the recipient, especially after double-lung transplantation). Donor serology and tracheal cultures guide subsequent antibacterial and antiviral therapy in the recipient.
Single-Lung Transplant
The choice of which lung to transplant is usually based on multiple factors, including avoidance of a prior operative site, preference for removing the native lung with the worst ventilation-perfusion ratio, and donor lung availability. The recipient is positioned for a posterolateral thoracotomy, with the ipsilateral groin prepped and exposed in case CPB becomes necessary. With the lung deflated, a pneumonectomy is performed, with special care to preserve as long a PA segment as possible. After removal of the diseased native lung, the allograft is positioned in the chest with precautions to maintain its cold tissue temperature. The bronchial anastomosis is performed first. A “telescoping” anastomosis is used if there is significant discrepancy in size between the donor and the recipient. The object of the technique is to minimize the chance of dehiscence. Although it was once common to wrap bronchial anastomoses with omentum, wrapping produces no added benefit when a telescoping anastomosis is performed. The PA is anastomosed next, and finally the pericardium is opened and the allograft LA cuff containing the pulmonary venous orifices is anastomosed to the native LA. The pulmonary circuit is then flushed with blood and de-aired. The initial flush solution is usually cold (4°C) but is followed by a warm (37°C) flush. The warm flush is usually performed during final completion of the vascular anastomoses. The goal of the flushing is to achieve a controlled reperfusion. The contents of this solution are listed in Box 20-3. After glucocorticoid administration, the vascular clamps are removed, reperfusion is begun, and the lung reinflated with a series of ventilations to full functional residual capacity. After achieving adequate hemostasis and satisfactory blood gases, chest tubes are placed, the wound is closed, and the patient is transported to the ICU.
Double-Lung Transplant
Early attempts at double-lung transplantation using an en bloc technique via a median sternotomy were plagued by frequent postoperative airway dehiscence due to poor vascular supply of the tracheal anastomosis; by hemorrhage due to extensive mediastinal dissection (which also resulted in cardiac denervation); by the requirement for complete CPB and cardioplegic arrest (to facilitate pulmonary arterial and venous anastomoses); and by poor access to the posterior mediastinum. The subsequent development of the bilateral sequential lung transplant technique via a “clamshell” thoracosternotomy (essentially two single-lung transplants performed in sequence) has avoided many of the problems inherent in the en bloc technique.14 An alternative to using a clamshell incision in slender patients is an approach through two individual anterolateral thoracotomies. This results in a particularly pleasing cosmetic result in female patients because the scar falls in the breast crease. Use of CPB is optional, exposure of the posterior mediastinum is enhanced (improving hemostasis), and cardiac denervation can usually be avoided. Pleural scarring is usually extensive in patients with cystic fibrosis, and postoperative hemorrhage and coagulopathy are the rule if CPB is required.
Pathophysiology before Transplantation
Patients with highly compliant lungs and obstruction of expiratory airflow cannot completely exhale the delivered tidal volume, resulting in positive intrapleural pressure throughout the respiratory cycle (“auto-PEEP” [positive end-expiratory pressure] or “intrinsic PEEP”), which decreases venous return and causes hypotension. The presence of auto-PEEP is highly negatively correlated with FEV1 (percent predicted) and highly positively correlated with pulmonary flow resistance and resting hypercarbia. Hyperinflation is a frequent complication of single-lung ventilation during lung transplantation in patients with obstructive lung disease. Hyperinflation-induced hemodynamic instability can be diagnosed by turning off the ventilator for 30 seconds and opening the breathing circuit to the atmosphere. If the blood pressure returns to its baseline value, hyperinflation is the underlying cause. Hyperinflation can be ameliorated with deliberate hypoventilation (decreasing both the tidal volume and/or rate).15 Although this may result in profound hypercarbia, high carbon dioxide tensions are well tolerated in the absence of hypoxemia. PEEP may also decrease air trapping because it decreases expiratory resistance during controlled mechanical ventilation. However, the application of PEEP requires close monitoring, because if the level of extrinsic PEEP applied exceeds the level of auto-PEEP, further air trapping may result.
RV failure is frequently encountered in lung transplant recipients with pulmonary hypertension due to chronically elevated RV afterload. The response of the RV to a chronic increase in afterload is to hypertrophy, but eventually this adaptive response is insufficient. As a result, RV volume decreases and chamber dilation results. The following should be kept in mind when caring for patients with severe RV dysfunction (Box 20-4). First, increases in intrathoracic pressure may markedly increase PVR, leading to frank RV failure in patients with chronic RV dysfunction. Changes in RV function may occur immediately after adding PEEP, increasing tidal volume or decreasing expiratory time, and can have devastating consequences. In addition, although intravascular volume expansion in the presence of normal PVR increases CO, overzealous infusion in patients with elevated PVR increases RV end-diastolic pressure and RV wall stress, decreasing CO. Inotropes with vasodilating properties (such as dobutamine or milrinone) are often a better choice than volume for augmenting CO in the setting of elevated PVR. Furthermore, the RV has a higher metabolic demand yet a lower coronary perfusion pressure than normal. RV performance can be augmented by improving RV coronary perfusion pressure with α-adrenergic agents, provided these vasoconstrictors do not disproportionately elevate PVR. This can sometimes be a better choice than augmenting the perfusion pressure with β-adrenergic agents because the oxygen supply is increased without a large increase in oxygen demand. Finally, vasodilators such as nitroprusside or prostaglandin E1 may be effective in decreasing PVR and improving RV dysfunction early in the disease process, when only mild to moderate pulmonary hypertension is present. However, they are of notably limited value in the presence of severe, end-stage pulmonary hypertension. Systemic vasodilation and exacerbation of shunting often limit their use. Inhaled nitric oxide has shown promise as a means of acutely decreasing PVR without altering systemic hemodynamics both during the explanation phase and after lung transplantation.16 Nitric oxide decreases both PA pressure and intrapulmonary shunting. Further, the combination of inhaled nitric oxide and aerosolized prostacyclin had a synergistic effect, without causing deleterious effects on the systemic perfusion pressure. The use of nitric oxide with or without inhaled prostacyclin may be helpful in avoiding CPB in patients having lung transplantation.
Pathophysiology after Lung Transplantation
Lung transplantation also profoundly alters the vascular system. The ischemia and reperfusion that are an obligatory part of the transplantation process damage endothelia. Cold ischemia alone decreases β-adrenergic cyclic adenosine monophosphate (cAMP)-mediated vascular relaxation by approximately 40%, and subsequent reperfusion produces even greater decreases in both cyclic guanosine monophosphate (cGMP)-mediated and β-adrenergic cAMP-mediated pulmonary vascular smooth muscle relaxation. Endothelial damage in the pulmonary allograft also results in “leaky” alveolar capillaries and the development of pulmonary edema. Pulmonary endothelial permeability is approximately three times greater in donor lungs than in healthy volunteers. Regulation of pulmonary vasomotor tone solely by circulating humoral factors is another side effect of denervation. Changes in either the levels of circulating mediators or in the responsiveness of the pulmonary vasculature to such mediators may result in dramatic effects on the pulmonary vasculature. An example of the former is the finding that the potent vasoconstrictor endothelin is present at markedly elevated levels (two to three times normal) immediately after transplantation and remains elevated for up to 1 week thereafter. Alterations in the response of denervated pulmonary vasculature to α1-adrenergic agents and prostaglandin E1, as well as a reduction in nitric oxide activity, have also been demonstrated in acutely denervated lung. Dysfunctional responses to mediators may be exaggerated if CPB is required. Pulmonary vascular resistance can be substantially decreased with the administration of inhaled nitric oxide after reperfusion. It remains unclear whether nitric oxide also ameliorates reperfusion injury. Several studies suggest that nitric oxide prevents or modulates reperfusion injury as measured by decreased lung water, lipid peroxidase activity, and neutrophil aggregation in the graft.17 However, there are a number of studies that suggest that although nitric oxide has an effect on pulmonary hemodynamics, it does not ameliorate reperfusion injury.
Anesthetic Management
Induction of Anesthesia
Three main principles should guide the formulation of a plan for induction: (1) protection of the airway; (2) avoidance of myocardial depression and increases in RV afterload in patients with RV dysfunction; and (3) avoidance and recognition of lung hyperinflation in patients with increased lung compliance and expiratory airflow obstruction (Box 20-5). All lung transplants are done on an emergency basis, and the majority of patients will have recently had oral intake and must be considered to have “full stomachs.” Since aspiration during induction would be catastrophic, every measure must be taken to protect the airway. Patients with known or suspected abnormalities of airway anatomy should be intubated awake after topical anesthesia is applied to the airway. Although a conventional rapid-sequence intravenous induction with a short-acting hypnotic (e.g., etomidate 0.2 to 0.3 mg/kg), a small amount of narcotic (e.g., up to 10 μg/kg of fentanyl), and succinylcholine will usually be tolerated, patients with severe RV dysfunction may exhibit profound hemodynamic instability in response to this induction regimen. For such patients, a more gradual induction is recommended, with greater reliance on high doses of narcotics and ventilation with continuous application of cricoid pressure. Patients with bullous disease or fibrotic lungs requiring high inflation pressures may develop a pneumothorax during initiation of positive-pressure ventilation. Acute reductions in SaO2 accompanied by difficulty in ventilating the lungs and refractory hypotension should generate strong suspicions that a tension pneumothorax has developed. RV function can be impaired during induction by drug-induced myocardial depression, by increases in afterload, or by ischemia secondary to acute RV dilation. Agents that act as myocardial depressants (e.g., thiopental) should be avoided in such patients. Increases in RV afterload can result from inadequate anesthesia, exacerbation of chronic hypoxemia and hypercarbia, and metabolic acidosis, as well as increases in intrathoracic pressure due to positive-pressure ventilation. Systemic hypotension is poorly tolerated because increased RV end-diastolic pressure will diminish net RV coronary perfusion pressure. In addition, chronic elevation of RV afterload increases the metabolic requirements of RV myocardium. Once the trachea is intubated and positive-pressure ventilation initiated, the avoidance of hyperinflation in patients with increased pulmonary compliance or bullous disease is crucial. Small tidal volumes and low respiratory rates and inspiratory/expiratory (I/E) ratios should be used (“permissive hypercapnia”). If hemodynamic instability does occur with positive-pressure ventilation, the ventilator should be disconnected from the patient. If hyperinflation is the cause of hypotension, blood pressure will increase within 10 to 30 seconds of the onset of apnea. Ventilation can then be resumed at a tidal volume and/or rate compatible with hemodynamic stability.
Intraoperative Management
Institution of one-lung ventilation (OLV) occurs before hilar dissection and may compromise hemodynamics and/or gas exchange (Box 20-6). Patients with diminished lung compliance can often tolerate OLV with normal tidal volumes and little change in hemodynamics. In contrast, patients with increased lung compliance and airway obstruction will often exhibit marked hemodynamic instability, unless the tidal volume is decreased and the expiratory time is increased. The magnitude of hypoxemia generally peaks about 20 minutes after beginning OLV. Hypoxemia during OLV may be treated with continuous positive airway pressure (CPAP) applied to the nonventilated lung,18 PEEP to the ventilated lung, or both. CPAP attempts to oxygenate the shunt fraction but may interfere with surgical exposure. PEEP attempts to minimize atelectasis in the ventilated lung but may concomitantly increase shunt through the nonventilated lung. Definitive treatment of shunt in the nonventilated lung is provided by rapid isolation and clamping of the PA of the nonventilated lung. Pneumothorax on the nonoperative side may result during OLV if a large tidal volume is used.
BOX 20-6 Management Principles for One-Lung Ventilation during Lung Transplantation
PA clamping is usually well tolerated, except in the face of pulmonary hypertension with diminished RV reserve. If the degree of RV compromise is uncertain, a 5- to 10-minute trial of PA clamping is attempted, then the RV is evaluated by serial CO, RVEF measurements, and TEE. A significant decrease in CO may predict patients who will require extracorporeal support. Other indications for CPB in lung transplantation are listed in Box 20-7.
BOX 20-7 Indications for Cardiopulmonary Bypass during Lung Transplantation
| Cardiac index | <2 L/min/m2 |
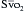 |
<60% |
| Mean arterial pressure | <50 to 60 mm Hg |
| SaO2 | <85% to 90% |
| pH | <7.00 |
Patients with severe pulmonary hypertension (greater than two thirds of systemic pressure) will generally be placed on CPB before PA clamping. The intraoperative use of nitric oxide (20 to 40 ppm) may allow some procedures to proceed without the use of CPB.19
Extracorporeal membrane oxygenation (ECMO) has also been suggested as an alternative method of CPB during lung transplantation. It has been suggested that the use of ECMO with heparin-bonded circuits might improve the outcome of both single- and double-lung transplants by lessening the amount of pulmonary edema especially in those patients who need CPB due to hemodynamic instability or with primary pulmonary hypertension. An added benefit of this technique is also that it clears the operative field of bypass cannulas making left-sided transplant as unimpeded as right-sided transplant. There is no apparent increase in transfusion requirement. Another added benefit of using ECMO in situ is that reperfusion of the lungs can be more easily controlled since the CO transiting the newly transplanted lung can be precisely controlled. This is especially the case for patients with advanced pulmonary hypertension.20
Living-Related Lung Transplantation
The scarcity of suitable donor lungs has resulted in waiting times on transplant lists in excess of 2 years, during which as many as 30% of candidates succumb to their illness. Living-related lung transplantation programs have developed to address the needs of lung transplant candidates with acute deterioration expected to preclude survival. Successful grafting of a single lobe for children with bronchopulmonary dysplasia or Eisenmenger’s syndrome, or two lobes for children and young adults with cystic fibrosis, has encouraged several centers to consider such procedures. The anesthetic management issues related to such undertakings have been reviewed.21 Donor candidates will have undergone a rigorous evaluation to ensure that there are no contraindications to lobe donation and that the donation is not coerced. Donor lobectomy is performed via a standard posterolateral thoracotomy. Of special note to the anesthesiologist during such procedures is the requirement for OLV to optimize surgical exposure, the continuous infusion of prostaglandin E1 to promote pulmonary vasodilation, and the administration of heparin and steroids just before lobe harvest. Anesthetic management of the recipient is identical to that for a standard lung transplant, except that the use of CPB is mandatory for bilateral lobar transplant.
HEART-LUNG TRANSPLANTATION
SUMMARY
1. Hunt S.A. Taking heart: cardiac transplantation past, present, and future. N Engl J Med. 2006;355:231.
2. Wood K.E., Becker B.N., McCartney J.G., et al. Care of the potential organ donor. N Engl J Med. 2004;351:2730.
3. Mehta S.M., Aufiero T.X., Pae W.E. Combined Registry for the Clinical Use of Mechanical Ventricular Assist Pumps and the Total Artificial Heart in Conjunction with Heart Transplantation: Sixth official report. J Heart Lung Transplant. 1994;14:585.
4. Borow K.M., Neumann A., Arensman F.W., et al. Cardiac and peripheral vascular responses to adrenoceptor stimulation and blockade after cardiac transplantation. J Am Coll Cardiol. 1989;14:1229.
5. Gao S.Z., Hunt S.A., Schroeder J.S., et al. Early development of accelerated graft coronary artery disease: Risk factors and course. J Am Coll Cardiol. 1996;28:673.
6. Hensley F.A., Martin D.E., Larach D.R., et al. Anesthetic management for cardiac transplantation in North America. J Cardiothorac Anesth. 1987;1:429.
7. Villanueva F.S., Murali S., Uretsky B.F., et al. Resolution of severe pulmonary hypertension after heterotopic cardiac transplantation. J Am Coll Cardiol. 1989;14:1239.
8. Jacquet L., Ziady G., Stein K., et al. Cardiac rhythm disturbances early after orthotopic heart transplantation: Prevalence and clinical importance of the observed abnormalities. J Am Coll Cardiol. 1990;16:832.
9. Olson CM (ed): Diagnostic and therapeutic technology assessment: Lung transplantation. JAMA 269:931, 1993
10. Kaye M.P. The Registry of the International Society for Heart and Lung Transplantation: Ninth official report. J Heart Lung Transplant. 1992;11:599.
11. Quinlan J.J., Gasior T.A., Firestone S., et al. Anesthesia for living-related (lobar) lung transplantation. J Cardiothorac Vasc Anesth. 1996;1996:391.
12. Novick R.J., Kaye M.P., Patterson G.A., et al. Redo lung transplantation: A North American-European experience. J Heart Lung Transplant. 1993;12:5.
13. Gilbert S., Dauber J., Hattler B., et al. Lung and heart-lung transplantation at the University of Pittsburgh 1982-2002. Clin Transplants. 253, 2002.
14. Kaiser L.R., Pasque M.K., Trulock E.P., et al. Bilateral sequential lung transplantation: The procedure of choice for double-lung replacement. Ann Thorac Surg. 1991;52:438.
15. Quinlan J.J., Buffington C.W. Deliberate hypoventilation in a patient with air trapping during lung transplantation. Anesthesiology. 1993;78:1177.
16. Adatia I., Lillehei C., Arnold J.H., et al. Inhaled nitric oxide in the treatment of postoperative graft dysfunction after lung transplantation. Ann Thorac Surg. 1994;57:1311.
17. Lang J.D.Jr., Leill W. Pro: Inhaled nitric oxide should be used routinely in patients undergoing lung transplantation. J Cardiothorac Vasc Anesth. 2001;15:7859.
18. Triantafillow A.N., Benumof J.L., Lecamwasam H.S. Physiology of the lateral decubitus position, the open chest, and one lung ventilation. In: Kaplan J.A., Slinger P.D., editors. Thoracic Anesthesia. 3rd ed. Philadelphia: Churchill Livingstone; 2003:71-94.
19. Chetham P. Anesthesia for heart or single or double lung transplantation. J Card Surg. 2000;15:167.
20. Pereszlenyi A., Lang G., Steltzer H., et al. Bilateral lung transplantation with intra- and postoperatively prolonged ECMO support in patients with pulmonary hypertension. Eur J Cardiothorac Surg. 2002;21:858.
21. Veeken C., Palmer S.M., Davis R.D., Gricknik K.P. Living-related lobar lung transplantation. J Cardiothorac Vasc Anesth. 2004;18:506.


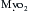 . This can be achieved with a warming blanket on the bed, on the patient’s head and arms, and on the legs below the knees. A fluid warmer is also useful in this regard.
. This can be achieved with a warming blanket on the bed, on the patient’s head and arms, and on the legs below the knees. A fluid warmer is also useful in this regard.