CHAPTER 19 Anesthesia for Fetal Surgery
Fetal interventions present a unique therapeutic opportunity. With advances in medical imaging technology, fetuses can be screened for anatomic congenital defects early in the course of a pregnancy. Although postnatal therapy is adequate for many fetuses, some congenital defects will not allow a successful transition to extrauterine life. Other birth defects may result in death in utero or serious disability after birth (Harrison, 1996). The logistics of fetal interventions can be quite challenging, but the ideas behind many of the treatments are conceptually quite simple. Some examples of interventions include bypass of fetal airway obstruction that would be fatal at birth, resection of a lung lesion causing hydrops fetalis, and repair of a myelomeningocele to minimize long-term disability (Table 19-1). Prenatal therapy opens new territory by making the fetus an active recipient of a given intervention. While the fetus is actively treated, the mother, by necessity, also receives medical care. Because of the high risk of these procedures, patient selection is an important consideration. The mothers must be at a sufficiently low anesthesia risk, and they must be highly motivated to comply with frequent follow-up and activity restrictions. Providing anesthesia for these patients requires a thorough understanding of maternal, fetal, and placental physiology. Familiarity with minimally invasive and open procedures and an understanding of the diseases amenable to fetal therapy are important. A list of key terms and acronyms is given in Box 19-1.
Box 19-1 Acronyms and Glossary
Amnioreduction: Percutaneous needle drainage of excess amniotic fluid
CDH: Congenital diaphragmatic hernia
CHAOS: Congenital high airway obstruction syndrome
EXIT: Ex utero intrapartum therapy
HLHS: Hypoplastic left heart syndrome
Polyhydramnios: Excess amniotic fluid, which may be a sign of fetal pathology
Diseases
Nonimmune hydrops threatens the lives of both the fetus and mother (van Selm et al., 1991). The aptly named maternal mirror syndrome is a state of maternal edema that mirrors that of the fetus. The pathophysiology of this process is unclear, but it may involve a maternal inflammatory response to shedding of debris from a hydropic placenta (Redman and Sargent, 2000). In contrast to preeclampsia, the mother is hemodiluted rather than hemoconcentrated, and the fetus, by definition, must show signs of hydrops (Vidaeff et al., 2002). Pulmonary edema secondary to the mirror syndrome may be life threatening, and treatment of maternal mirror syndrome involves either delivery of the fetus or treatment of the cause of the hydrops (Heyborne and Chism, 2000; Pirhonen and Hartgill, 2004; Livingston et al., 2007).
Complicated Multiple Gestations
Monozygotic twin gestations are at increased risk for complications. If a monozygotic cell mass splits within 3 days of fertilization, a dichorionic diamniotic twin gestation should result. If the mass splits later than 13 days after fertilization, conjoined twins will result. If the cell mass splits between days 3 and 13, the twins will be “conjoined” to varying degrees at the level of the placenta and placental vessels (Lewi et al., 2003). These conjoined placental vessels may result in a net flow of blood from one twin (donor) to the other twin (recipient). The donor will suffer from hypovolemia, oligohydramnios, and growth restriction, whereas the recipient will suffer from hypervolemia, polyhydramnios, hydrops, and heart failure (Luks et al., 2005). Ultrasound and fetal echocardiographic changes may be used to describe the severity of disease (Quintero et al., 1999; Rychik et al., 2007). This process, twin-twin transfusion syndrome, places both twins at risk for preterm delivery, death, and neurologic disability.
Traditional treatment of twin-twin transfusion syndrome involves serial amnioreduction to relieve the polyhydramnios. This may decrease the risk of preterm labor and may allow better perfusion of the donor twin; however, this therapy does not target the physiologic problem, which arises from the connected placental vessels (Lewi et al., 2005). Newer therapies target the placental vessels, employing percutaneous placement of endoscopes into the amniotic fluid to allow visualization and ablation of the connected placental vessels (Fig. 19-1). Minimally invasive fetoscopic laser ablation of the vessels has been shown to be superior to amnioreduction in a randomized clinical trial (Senat et al., 2004). Another trial showed no difference between amnioreduction and laser ablation, but there were differences in the studies that do not allow direct comparison (Crombleholme et al., 2007). More research is needed to fully define the role of minimally invasive laser therapy and amnioreduction in the treatment of twin-twin transfusion syndrome.
Twin reversed arterial perfusion sequence (TRAPS) is a similar clinical entity in which one of the twin cell masses may be acardiac, acephalic, or both. The normal or “pump” twin is at risk for death from high-output heart failure because it must supply cardiac output for itself and the acardiac cell mass (Tan and Sepulveda, 2003). In these cases, selective feticide of the acardiac cell mass will allow the normal pump twin to survive. This is accomplished by occlusion of blood flow to the umbilical cord of the acardiac mass either by bipolar cautery or radiofrequency ablation (Tsao et al., 2002). If the twins are monochorionic and monoamniotic, the umbilical cord of the acardiac cell mass must also be divided to prevent cord entanglement and death of the normal twin. These umbilical cord occlusions are accomplished with minimally invasive techniques, using ultrasound or fetoscopic guidance.
Neurologic
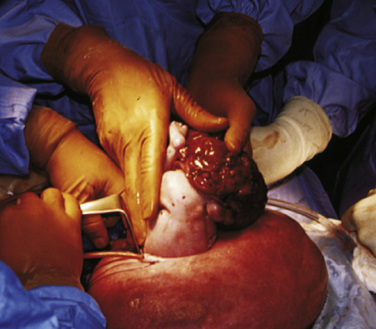
Fetal therapies for prenatally diagnosed hydrocephalus and myelomeningocele (MMC) have been described. Results for in utero treatment of hydrocephalus with ventriculoamniotic shunts have not been encouraging, and this therapy is no longer actively studied or offered (Manning et al., 1986; Bruner et al., 2006). However, in utero closure of MMC defects is more promising. This closure involves accessing the midgestation fetus via maternal laparotomy and hysterotomy (Fig. 19-2). The MMC is repaired, and uterine and abdominal incisions are closed. Pregnancy is continued with the goal of delivering as close to term as possible.
The rationale for in utero closure of MMC is based on animal models of fetuses with these lesions, where it appears that prolonged bathing of neurologic elements in the amniotic fluid worsens the neurologic outcome (Meuli et al., 1995; Meuli et al., 1996). In human studies, closure of fetal MMC decreases the need for postnatal ventriculoperitoneal shunting and the incidence of hindbrain herniation and Chiari malformation. Motor function may be somewhat improved, and cognitive behavioral testing does not appear to be adversely affected (Bruner et al., 1999; Tulipan et al., 1999; Johnson et al., 2003; Johnson et al., 2006). A randomized trial sponsored by the National Institutes of Health (NIH) is currently enrolling patients who are randomized either to standard postnatal closure of MMC or to in utero closure of MMC. Enrollment in the trial is, at this time, the only avenue for this surgery.
Airway
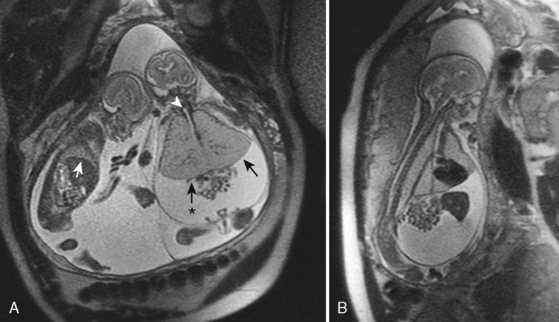
Airway obstruction is life threatening in the postnatal period, especially when airway patency cannot be established immediately on delivery by intubation. Extrinsic causes of airway obstruction include cystic hygroma and oral teratoma. Cystic hygroma is a type of lymphatic malformation that may develop in the neck, resulting in large, fluid-filled cystic masses (Fig. 19-3). Teratomas are germ cell tumors composed of tissues foreign to their normal location (Fig. 19-4). Polyhydramnios may result from an inability of the fetus to swallow amniotic fluid. Intrinsic causes of airway obstruction include laryngeal cysts, webs, and atresia. The spectrum of disease due to intrinsic compression has been called congenital high airway obstruction syndrome (CHAOS) (Hedrick et al., 1994; Lim et al., 2003).
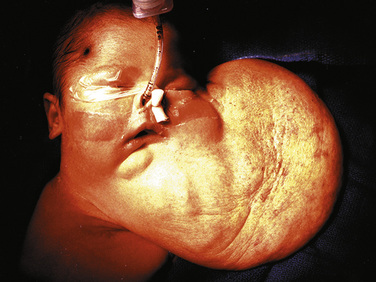
FIGURE 19-3 A newborn who underwent aspiration of a cystic hygroma and subsequent intubation during an EXIT procedure.
(Photo courtesy of Alan W. Flake, MD, Children’s Hospital of Philadelphia.)
Airway obstruction may be fatal in utero. This is not immediately intuitive, because the placenta serves as the organ of respiration, but complete airway obstruction will not allow egress of lung fluid into the amniotic space. This fluid will collect, and massive pulmonary hyperplasia may result. The fetal diaphragm may be flattened or even project into the abdominal space (Fig. 19-5). The intrathoracic pressure is presumably increased and will impede cardiovascular filling and function, resulting in nonimmune hydrops and fetal demise (Mong et al., 2008). If hydrops is not present, fetal airway obstruction is well served by the ex utero intrapartum therapy (EXIT) procedure. The EXIT procedure for a near-term fetus involves a maternal laparotomy and hysterotomy, exteriorization of the fetus, and securing of the fetal airway before clamping and division of the umbilical cord. After the cord is divided, the fetus is delivered and given to a neonatology team for further stabilization. If hydrops is present and the lungs are immature, midgestation tracheal decompression may be a treatment option (Kohl et al., 2006; Kohl et al., 2009).
Lung Lesions
Lung lesions that may be diagnosed in utero include congenital diaphragmatic hernia (CDH), congenital cystic adenomatoid malformation (CCAM), and bronchopulmonary sequestration (BPS). CDH has been a target for in utero therapy for decades. Results with both open and minimally invasive techniques have thus far been equivalent to those with optimal postnatal therapy. Thus, the risk-benefit analysis would support postnatal therapy. However, efforts at refining minimally invasive therapy continue and may prove to be of benefit in the future (Harrison et al., 1997; Harrison et al., 2003; Deprest et al., 2005; Doné et al., 2008; Peralta et al., 2008).
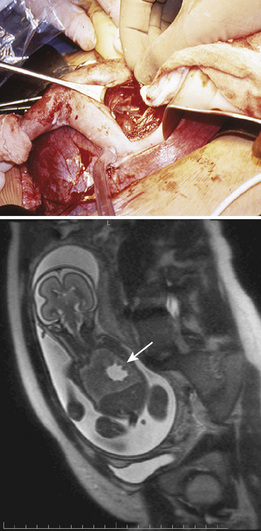
The management of prenatally diagnosed CCAM is instructive because its natural history is variable (Fig. 19-6). CCAM results from an overgrowth of terminal bronchioles. These masses do not participate in gas exchange, may predispose to infection and malignancy, may cause mediastinal shift and cardiac compression, and may result in hypoplasia of the remaining lung tissue. Some CCAMs will grow and some will shrink—each at unpredictable, variable rates. Regular prenatal assessment is required. Lesion composition (microcystic or macrocystic) and size have varying physiologic effects. Taking pulmonary maturity into consideration, the treatment of CCAM may occur in early, mid, or late gestation. Therapies may be minimally invasive or require open fetal surgery. Compromise results mostly from mass effect or fluid collection, resulting in a tamponade physiology. Solitary lesions with a large single cyst may be treated minimally invasively with one-time or serial needle aspiration. Ultrasound-guided minimally invasive placement of a thoracoamniotic shunt may negate the need for serial procedures in large, cystic lesions.
In contrast to large lesions with a single cyst, microcystic lesions are not amenable to aspiration or shunting. If a midgestation fetus with immature lungs has significant physiologic compromise, an in utero fetal pulmonary lobectomy may be pursued. After the lobectomy is completed, the fetus is returned to the uterus for further growth and development (Adzick et al., 2003). If the fetus is late in gestation and the lesion is causing distress or may interfere with neonatal resuscitation, a delivery via the EXIT procedure can be undertaken, with fetal thoracotomy and pulmonary lobectomy before umbilical cord clamp (Hedrick et al., 2005). If the mass is asymptomatic, the child can be delivered normally, and a postnatal pulmonary lobectomy can be electively scheduled.
Cardiac Disease
Most of the therapies for congenital cardiac malformations are minimally invasive and catheter based, with most of the reported literature describing fetal aortic valvuloplasty for treatment of severe aortic stenosis—a possible precursor to hypoplastic left heart syndrome (Tworetzky et al., 2004; Marshall et al., 2005; Wilkins-Haug et al., 2006). In utero atrial septoplasty (Fig. 19-7) has also been described for patients with hypoplastic left heart with an intact or highly restrictive atrial septum (Marshall et al., 2008). Pulmonary atresia with an intact ventricular septum may also be amenable to fetal cardiac intervention, as this is the “right-sided” equivalent of hypoplastic left heart syndrome (Tworetzky and Marshall, 2004). Earlier surgical techniques involved a maternal laparotomy and exteriorization of the uterus. The necessary needles and catheters were then inserted through an otherwise intact uterus, and subsequently guided through the fetal chest wall and myocardium to the chambers or vessels of interest. Currently, an even less invasive approach to the fetal heart is performed. The approach is entirely percutaneous: through the maternal abdomen, uterus, and fetal chest wall. Great care and skill come into play as the trajectory of the needles, catheters, and wires is planned. Outcomes from fetal cardiac interventions have been encouraging but are not definitive yet.
Bladder Outlet Obstruction
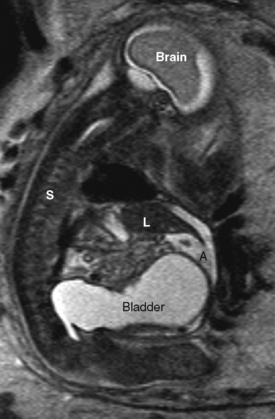
Fetal urinary tract obstruction (Fig. 19-8) may result in a host of complications, such as pulmonary hypoplasia, renal dysplasia, bladder dysfunction, renal failure, skeletal abnormalities, and abdominal wall muscular abnormalities (Harrison et al., 1981; Harrison et al., 1982). Patient selection for therapy is challenging, because the disease process must be severe enough to warrant a prenatal surgical intervention, but not so far advanced that the renal damage is irreversible (Cendron et al., 1994). Fetal evaluation is quite involved and includes ultrasound examination of the urinary tract, measurement of amniotic fluid volumes, and analysis of fetal urine electrolytes and proteins (Crombleholme et al., 1990). Minimally invasive therapy may involve needle decompression of the bladder, vesicoamniotic shunting, or ablation of posterior urethral valves. Open surgery for bladder marsupialization or ureterostomies has also been reported (Crombleholme et al., 1988). Decompression may reduce the severity of oligohydramnios and the resulting pulmonary hypoplasia, but it may not allow recovery of renal function. Placement of a vesicoamniotic shunt may be technically successful, but the shunt may become dislodged. Bladder function may still not be optimal, as the fetal bladder is not exposed to cyclic filling and emptying. Open fetal surgery has been performed, but the risks and benefits must be carefully considered (Crombleholme et al., 1988).
Sacrococcygeal Teratoma
Sacrococcygeal teratoma is a germ cell tumor arising from the coccyx (Fig. 19-9). Prenatal diagnosis is associated with much higher mortality than postnatal diagnosis (Flake, 1993). In utero, the teratoma places the fetus at risk of hydrops and death from high-output heart failure (Bond et al., 1990). Serial echocardiography is used to monitor the cardiac status of the fetus, and ultrasound is used to monitor tumor growth (Tran et al., 2008). The tumor may cause a vascular steal phenomenon, where much of the fetal cardiac output goes to supplying the tumor instead of the fetus. This is evidenced by increased flow in the descending aorta and increased diameter of the fetal inferior vena cava (Hedrick et al., 2004). The tumor may also rupture in utero, resulting in fetal anemia. Treatment may be minimally invasive in select cases of cystic tumors and involves aspiration of the cystic components. Therapy with radiofrequency ablation has not yielded consistent results (Paek et al., 2001; Ibrahim et al., 2003). In certain cases, midgestation debulking of the tumor may be attempted. An EXIT procedure for resection of a massive sacrococcygeal teratoma has also been performed. For large tumors, at the very least, a planned cesarean delivery is necessary, with provisions for immediate resection if necessary.
Physiology
Maternal
Sensitivity to anesthetic agents increases during pregnancy. Early in gestation, the minimum alveolar concentrations (MACs) for isoflurane and for halothane are approximately 30% lower in pregnant women than in nonpregnant women (Gin and Chan, 1994; Chan et al., 1996). CSF volume decreases, and the epidural venous plexus becomes distended (Kerr et al., 1964). Pregnancy enhances the spread of local anesthetics in the spinal fluid (Hirabayashi et al., 1995b); in the epidural space, the spread of small dosages of local anesthesia is increased, but the spread of large dosages is about the same in pregnant and nonpregnant women (Kalas et al., 1966; Grundy et al., 1978). Increased dermatomal spread of epidural anesthetics is likely to result from increased nerve sensitivity, hormonal changes, reduced protein levels, and pH changes in the CSF (Flanagan et al., 1987; Hirabayashi et al., 1995a; Hirabayashi et al., 1996; Popitz-Bergez et al., 1997).
Plasma cholinesterase activity decreases during pregnancy, but this decrease does not clinically affect the dosing of succinylcholine. Pregnancy also increases sensitivity to nondepolarizing muscle relaxants. Onset time is faster for vecuronium, and recovery time is longer for rocuronium (Baraka et al., 1992; Puhringer et al., 1997).
Airway management in a pregnant woman is potentially more difficult than in a nonpregnant woman. Capillary engorgement of the airway mucosal tissues has multiple implications. Resultant airway narrowing forces downsizing of endotracheal tubes, and nasal intubation is likely to cause epistaxis. The potential for difficult intubation is increased and airway complications are a significant factor in anesthesia-related morbidity and mortality (Cormack and Lehane, 1984; Samsoon and Young, 1987; Pilkington et al., 1995; Ezri et al., 2001; Ross, 2003; Munnur et al., 2005; Rudra et al., 2006; Goldszmidt, 2008). Respiratory and metabolic changes place the mother at increased risk of hypoxia. Oxygen consumption increases by as much as 60% (Spatling et al., 1992) and functional residual capacity (FRC) begins to decrease in the second trimester, reaching 80% of the nonpregnant volume at term (Alaily and Carrol, 1978). FRC decreases further in the supine position. Tidal volume and minute ventilation increase by 45% at term, and Paco2 decreases to 30 mm Hg by the second trimester (Kelman and Templeton, 1975; Templeton and Kelman, 1976; Alaily and Carrol, 1978). The risk of pulmonary edema increases after open fetal surgery (DiFederico et al., 1998). This increased risk may be secondary to decreased plasma protein concentration, decreased colloid oncotic pressure, or medications used to terminate premature labor, or a combination of these (Mendenhall, 1970; Wu et al., 1983). In addition, surgical manipulation of the uterus and hysterotomy may release factors that increase pulmonary vascular permeability (DiFederico et al., 1998).
Pregnancy is a high cardiac output state. Heart rate and stroke volume increase as early as the first trimester, and at term cardiac output is increased approximately 50% from nonpregnant values (Laird-Meeter et al., 1979; Hunter and Robson, 1992). Cardiac output increases even more during labor and immediately postpartum (Robson et al., 1987). Systemic vascular resistance decreases by about 20% secondary to vasodilation and the addition of the placenta, which is a low-resistance vascular circuit (Clark et al., 1989). Supine hypotension may occur because of compression of the inferior vena cava by the gravid uterus. This compression results in a decrease of right atrial pressure in the supine position (Kerr, 1965). Compression of the aorta is most pronounced in the supine position and becomes negligible in the full lateral position (Eckstein and Marx, 1974). Human studies suggest a reduced response to arterial vasoconstriction by alpha agonists but an increased sensitivity to venous constriction (Nisell et al., 1985). Thus, any pressor response may be more likely to result from increased venous return and increased stroke volume. Plasma volume increases relatively more than red blood cell volume increases, and hemoglobin concentrations decrease in pregnancy.
The pregnant patient is at increased risk for pulmonary aspiration of gastric contents. Hormonal changes and displacement of the stomach with resultant reduction of tone in the lower esophageal high-pressure zone may allow reflux of gastric contents (Ulmsten and Sundstrom, 1978). Intragastric pressure is highest in the third trimester. Gastric emptying of solids and liquids is not slowed during pregnancy, but gastric emptying does slow down during labor (Davison et al., 1970; O’Sullivan et al., 1987; Macfie et al., 1991; Sandhar et al., 1992; Whitehead et al., 1993; Chiloiro et al., 2001; Wong et al., 2002). Although gastric acid production has not definitively been shown to be greater in pregnancy, acid aspiration is still a major concern.
Fetal
Fetal physiology is as complex as maternal physiology. Logistical considerations necessitate some extrapolation from animal studies. Neurologic pathways for cortical transmission of noxious stimuli in humans are still developing into the third trimester (Lee et al., 2005). Fetal sensitivity to volatile anesthetic agents is increased. With both isoflurane and halothane, the anesthesia requirement of fetal lambs is lower than that of a pregnant ewe (Gregory et al., 1983; Bachman et al., 1986). Perception and processing of pain are controversial, but noxious stimuli will elicit a physiologic response in the human fetus, as evidenced by increases in cortisol and β-endorphin, and decreases in the pulsatility index of the fetal middle cerebral artery (Fisk et al., 2001). These physiologic responses are attenuated by administration of fentanyl directly to the fetus. The pharmacokinetics and pharmacodynamics of fentanyl in the fetus remain to be studied.
The cardiopulmonary physiology of the fetus is not dependent on the lungs, because the placenta acts as the organ of respiration. A major role of the lungs in utero is the production of amniotic fluid. The lung epithelium actively secretes fluid, which fills the lungs. Excess fluid passes out the trachea and into the oropharynx. This fluid is then either swallowed or expelled into the amniotic space and becomes a component of amniotic fluid. Fluid in the lungs causes distention and allows lung development (Olver and Strang, 1974; Perks and Cassin, 1985; Harding and Hooper, 1996). Restriction of egress of this fluid from the lungs results in pulmonary hyperplasia, whereas continuous drainage results in pulmonary hypoplasia (Alcorn et al., 1977).
When the pulmonary and systemic circulations are in a series, as in a normal adult heart, cardiac output can be quantitatively described by either the left or the right ventricular output. Because the fetus has a parallel circulatory system, fetal cardiac output is measured and reported as the sum of both the left and right ventricular output, or combined cardiac output (CCO). Echocardiographic studies have found that a normal human fetus has a CCO of 425 to 550 mL/kg per minute, with the right ventricle contributing 60% to 70% of the total output (De Smedt et al., 1987; Mielke and Benda, 2001; Rychik, 2004). The fetal myocardium has a greater proportion of noncontractile elements and is also stiffer with impaired relaxation compared with adult myocardium (Friedman, 1972; Rychik, 2004). Functioning close to the upper limit of the Starling curve, increases in preload provide only minimal, if any, incremental increases of stroke volume and cardiac output (Gilbert, 1980). Variation in heart rate provides a relatively greater contribution to variation in cardiac output. This lack of response to preload has been attributed to poor compliance of the myocardium, but it may also result from extrinsic compression of the fetal heart that is relieved with aeration of the lungs and clearance of lung fluid (Grant, 1999; Grant et al., 2001).
The blood volume of a fetus varies over gestation. At 16 to 22 weeks, blood volume of the fetoplacental unit has been estimated to be 120 to 162 mL/kg of fetal weight (Morris et al., 1974; Nicolaides et al., 1987). At 31 weeks, blood volume has been reported as 93 mL/kg of fetal weight (Nicolaides et al., 1987). It is important to note that about two thirds of the blood volume is contained on the placental side of the fetoplacental unit (Barcroft and Kennedy, 1939; Yao et al., 1969). A 16-week fetus weighs approximately 100 g, at 22 weeks 430 g, and at 31 weeks 1500 g.
Studying hemostasis is challenging because of the rapid evolution of the coagulation system in the fetal and neonatal period. The fetus produces coagulation factors independently of the mother, and these factors do not cross the placenta (Cade et al., 1969). The plasma concentrations of these proteins increase with increasing gestational age (Reverdiau-Moalic et al., 1996). A fetus at 19 to 23 weeks of gestation has a mean prothrombin time (PT) of 32.5 seconds with an International Normalized Ratio (INR) of 6.4 and a mean activated partial thromboplastin time (aPTT) of 168.8 seconds. At 30 to 38 weeks of gestation, mean PT is 22.6 seconds with an INR of 3.0 and an aPTT of 104.8 seconds (Reverdiau-Moalic et al., 1996).
While in utero, fetal temperature is closely linked to maternal variations in temperature. Fetal tissue is more metabolically active than adult tissue, and fetal heat production results in a fetal-to-maternal gradient of 0.5 °C. Excess heat flows from the fetus and is dissipated by the mother. After birth, an increase in heat production is required for homeostasis with the loss of the maternal “heat clamp,” and with new evaporative and convective heat losses. The sympathetic nervous and endocrine systems generally activate and regulate this heat production. Shivering and nonshivering thermogenesis take a primary role in the generation of heat for the newborn (Power et al., 2004). A fetus removed from the uterus during open surgery has a similar need for increased heat production as a newborn, but it cannot increase its production adequately. Both fetal shivering and nonshivering thermogenesis responses are largely absent in utero if the umbilical cord is patent (Gunn et al., 1986). Maintenance of normothermia in a fetus exposed during open fetal surgery can be challenging, with a lack of shivering and nonshivering thermogenesis, immature skin barriers, and increased evaporative losses.
Uteroplacental Blood Flow
The fetus depends on intact uteroplacental blood flow and patent umbilical vessels for respiration and nutrition. Uterine blood flow, a surrogate for fetal oxygen delivery, correlates with fetal umbilical venous Po2 (Skillman et al., 1985; Bilardo et al., 1990). Human uterine blood flow at term is extrapolated to be 700 mL/min (12% of cardiac output), compared with 200 mL/min in the nonpregnant uterus (Thaler et al., 1990).
Uterine blood flow is directly related to uterine perfusion pressure (the difference between uterine arterial pressure and uterine venous pressure) and inversely related to uterine vascular resistance (Box 19-2). Of note for fetal surgical procedures, maternal hypotension, aortocaval compression, and uterine contractions will decrease uterine blood flow. The effect of vasopressors, vasodilators, and anesthetic agents on uterine blood flow is variable because these agents affect uterine arterial pressure and uterine vascular resistance at the same time. Ephedrine has been suggested as the vasopressor of choice in obstetrics, as it may not cause as much uterine arterial vasoconstriction as phenylephrine. Several studies have shown no dramatic clinical differences in neonatal outcome and lend slightly more support to phenylephrine to support maternal blood pressure. When phenylephrine is compared with ephedrine to support blood pressure during cesarean section, newborn pH measurements are statistically but not clinically different (Thomas et al., 1996; Lee et al., 2002; Ngan Kee et al., 2008). Apgar scores are similar. In clinical practice, ephedrine would be a logical choice if the maternal heart rate were low, whereas phenylephrine could be used if the maternal heart rate were high. Local factors such as nitric oxide are released by the uterine vascular endothelium and modulate the effect of systemically administered vasoactive medications (Weiner et al., 1991).
Neuraxial and general anesthetics have variable effects on uterine blood flow. As long as maternal systemic pressure is maintained, epidural anesthesia does not seem to alter uterine blood flow in elective cesarean sections (Alahuhta et al., 1991). If a neuraxial technique causes significant hypotension, uterine blood flow will be decreased. In sheep models, pain and stress decrease uterine blood flow (Shnider et al., 1979). Relief of pain with an epidural may attenuate this reduction of flow. Barring resultant hypotensive decreases in blood flow, intravenous (IV) induction agents, thiopental, propofol, etomidate, and ketamine do not affect uterine blood flow dramatically. Volatile anesthetics decrease uterine tone and increase risk of bleeding (Cullen et al., 1970). Light and moderate levels of volatile anesthesia slightly depress blood pressure, but uterine vasodilation maintains blood flow. In a sheep model of fetal surgery, with deeper levels of volatile anesthesia, uterine vasodilation cannot compensate for the reductions in maternal blood pressure and cardiac output, and fetal acidosis occurs (Palahniuk and Shnider, 1974). It is important to note, however, that no medications were given to the pregnant ewes to support their blood pressure while undergoing general anesthesia with high dosages of volatile agent.
Although the studies are not entirely in agreement, maternal hypocapnia, with or without the mechanical effect of hyperventilation with positive pressure, is likely to decrease uterine blood flow, placental–umbilical blood flow, and fetal oxygenation in sheep, monkey, and human studies. In contrast, maternal hypercapnia is associated with an increase in fetal oxygenation and may be beneficial during labor and delivery (Motoyama et al., 1966, 1967; James, 1967; Rivard et al., 1967; Parer et al., 1970; Peng et al., 1972; Levinson et al., 1974).
Placental Anatomy and Transport
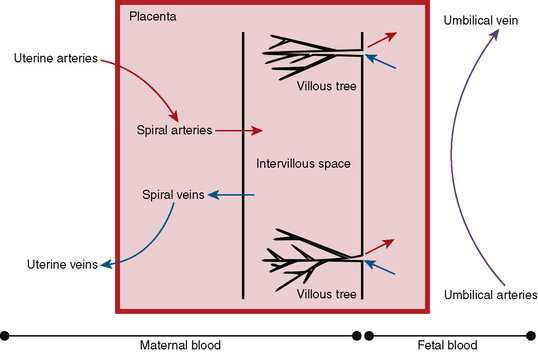
Maternal spiral arteries arise from the uterus and deposit oxygenated blood and nutrients into the intervillus space. The space is a cavernous expanse, into which the villous trees from the fetal circulation extend. The intervillus space contains maternal blood and is bounded on the maternal side by the basal plate, and on the fetal side by the chorionic plate. The villous trees and terminal villi contain the terminal umbilical arterioles and capillaries that arise from the umbilical arteries and transport the deoxygenated blood and waste products from the fetus for exchange in the intervillus space. These vessels return blood to the fetus via the umbilical veins (Fig. 19-10).
In maternal and fetal blood, the difference in partial pressures of oxygen drives oxygen across the placenta and into the fetal circulation. Fetal hemoglobin has a higher affinity for oxygen than maternal hemoglobin, and this affinity may also help with oxygen transport. Normal newborn umbilical arterial Po2 is 17 mm Hg, and umbilical venous Po2 is 29 mm Hg (Helwig et al., 1996). At term, fetal oxygen consumption is 6.8 ± 1.4 mL/kg per minute (Bonds et al., 1986). Fetal oxygen saturation has been shown to be 60% to 70% in term human fetuses (measured by scalp oximetry) and in fetal sheep (Johnson et al., 1991; Luks et al., 1998). Glucose is transported to the fetus by facilitated diffusion (Rice et al., 1979; Challier et al., 1985).
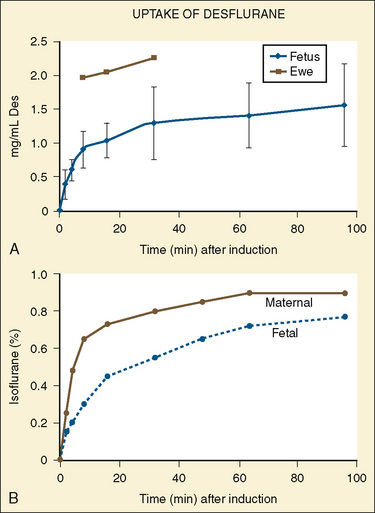
Although the newer volatile anesthetics have not been studied as thoroughly as halothane and isoflurane, the low molecular weight and lipid insolubility of these medications should allow rapid transfer with relatively high fetal-to-maternal (F/M) ratios. A higher F/M ratio indicates that more of the agent in question is found in the fetal blood than in the maternal blood. Most human in vivo studies have been performed in patients undergoing elective termination of pregnancy or elective cesarean section. Isoflurane has an F/M ratio of 0.7 (Biehl et al., 1983b; Kangas et al., 1976; Dwyer et al., 1995), whereas the F/M ratio of desflurane is reported to be 0.5 (Schwarz et al., 2003). Cautious extension of the findings with halothane and isoflurane to desflurane and sevoflurane is reasonable (Fig. 19-11). After 3 minutes, nitrous oxide has an F/M ratio of 0.83 (Polvi et al., 1996).
Thiopental crosses rapidly into the fetal circulation, but F/M ratios range widely, between 0.4 and 1.1 (Levy and Owen, 1964; Finster et al., 1966). Propofol has been studied at term after bolus administration, with and without infusions. F/M ratios range between 0.5 and 0.85 (Dailland et al., 1989; Valtonen et al., 1989; Gin et al., 1990; Gregory et al., 1990). If used only for induction of general anesthesia for open fetal surgery, differences between propofol and thiopental are not likely to be clinically significant. Propofol infusions may be used for maternal sedation in early pregnancy for minimally invasive cases. In women between 12 and 18 weeks of gestation, the F/M ratio was 0.5 and was independent of time between 5 and 20 minutes of infusion (Jauniaux et al., 1998). Diazepam is a commonly used drug for maternal and fetal sedation; within minutes of injection, the F/M ratio approaches unity and ratios approach 2.0 after an hour (Erkkola et al., 1973; Mandelli et al., 1975). Although midazolam has a lower F/M ratio of 0.76, at term it is still frequently used as a sedative in noninvasive surgery (Wilson et al., 1987). No studies have shown any teratogenic relationship between oral clefts and benzodiazepines given in pregnancy (Koren et al., 1998). Morphine is hydrophilic, and also quite commonly used for maternal and fetal analgesia and sedation. Intramuscular maternal administration of morphine (0.12 to 0.19 mg/kg) decreases fetal breathing movement; however, fetal tone and gross movement are not as affected by morphine, and the F/M ratio is 0.61 (Kopecky et al., 2000). In early gestation, fentanyl is detectable in fetal brain tissue between 10 and 30 minutes after maternal administration (Cooper et al., 1999). The F/M ratio of fentanyl varied from 0.16 to 1.2 in a small study of maternal IV administration (Shannon et al., 1998). Remifentanil, a short-acting potent opioid, is finding some use in both obstetric anesthesia and anesthesia for fetal surgery. With remifentanil infusions at term for cesarean section, umbilical vein to maternal artery ratio was 0.88, and umbilical artery to umbilical vein ratio was 0.29 (Kan et al., 1998). Succinylcholine in large (300 mg) or repeated doses crosses the placenta and affects the fetus. Nondepolarizing muscle relaxants and anticholinesterase agents are large, ionized molecules that do not easily cross the placenta. Vecuronium F/M ratios are 0.06 to 0.11 (Dailey et al., 1984; Iwama et al., 1999). Rocuronium has been used in mothers undergoing cesarean section with no adverse effects (Abouleish et al., 1994; Baraka et al., 1997). Atropine readily crosses the placenta, as opposed to glycopyrrolate, which has a mean F/M ratio of 0.22. A case of fetal bradycardia has been attributed to placental passage of neostigmine (Clark et al., 1996). Ephedrine crosses the placenta readily, with an F/M ratio of 0.7 (Hughes et al., 1985) (Table 19-2).
TABLE 19-2 Documented Fetal-to-Maternal Ratios of Medications Administered to the Mother
| Drug | Fetal-to-Maternal Ratio |
| Halothane | 0.7-0.9 |
| Isoflurane | 0.7 |
| Nitrous oxide | 0.83 |
| Thiopental | 0.4-1.1 |
| Propofol | 0.5-0.85 |
| Diazepam | 1-2 |
| Midazolam | 0.76 |
| Morphine | 0.61 |
| Fentanyl | 0.16-1.2 |
| Remifentanil | 0.29-0.88 |
| Vecuronium | 0.06-0.11 |
| Glycopyrrolate | 0.22 |
| Ephedrine | 0.7 |
Surgical issues
Minimally Invasive Interventions
Minimally invasive interventions are the most frequently performed fetal surgical procedures. The uterine cavity is accessed percutaneously with needles and small sheaths. Visualization of structures is provided by ultrasound guidance or fetoscopes inserted through the sheaths. Because minimally invasive techniques allow a wide range of therapeutic options via a wide range of operative techniques, a clear understanding of the surgical plan helps to shape the anesthesia plan. Placental vessels allowing unequal blood flow between monochorionic twins (twin-twin transfusion syndrome) may be ablated with lasers (Lewi et al., 2005; Habli et al., 2008). The umbilical cord of an acephalic acardiac twin mass (twin reversed arterial perfusion sequence) can be coagulated with radiofrequency to prevent harm to the normal twin (Tan and Sepulveda, 2003). With ultrasound guidance, fetal thoracoamniotic shunts can be placed to allow drainage of fluid from cystic lung lesions into the amniotic fluid (Adzick and Kitano, 1993). Tracheal occlusion can be performed to prevent egress of lung fluid, and this may attenuate the lung hypoplasia that results from congenital diaphragmatic hernia (Peralta et al., 2008). The efficacy of tracheal occlusion continues to be studied. Fetal cardiac catheterization and intervention may allow fetuses with evolving hypoplastic left heart syndrome to develop two ventricle circulations (Tworetzky and Marshall, 2003). Vesicoamniotic shunts have been placed in the bladders of fetuses with lower urinary tract obstruction to relieve hydroureter and hydronephrosis and perhaps allow improved bladder function (Crombleholme et al., 1990). Attempted treatment of fetal sacrococcygeal teratomas with radiofrequency ablation has been associated with too much morbidity at this time (Paek et al., 2001; Ibrahim et al., 2003). Techniques for endoscopic closure of fetal MMC also continue to be refined (Fichter et al., 2008).
Because of the wide range of therapeutic options, the requirements for surgical access to the fetus are also quite varied. The access may be as minimal as one small-gauge radiofrequency probe or may be as involved as multiple trocars for a robot-assisted MMC repair. Endoscopes range from 1.0 to 3.8 mm in external diameter (Klaritsch et al., 2009). The timing of these procedures is typically in early or middle gestation. However, given the varied pathophysiology of the disease processes to be treated and the varied nature of these interventions, it is possible that minimally invasive interventions may be done at any time in gestation. Tracheal balloons placed at around 26 weeks gestation to obstruct the egress of lung fluid are removed in late gestation before delivery (Deprest et al., 2005).
Open Midgestation Surgery
Open fetal surgery has been performed to relieve bladder outlet obstruction, repair congenital diaphragmatic hernia, resect lung lesions causing significant mass effect and hydrops, debulk sacrococcygeal teratomas, and close MMCs (Crombleholme et al., 1988; Harrison et al., 1997; Adzick et al., 2003; Hedrick et al., 2004; Fichter et al., 2008). Presently, open midgestation surgery is largely reserved for closure of MMC and resection of lung lesions causing hydrops. Open treatment for bladder outlet obstruction is also being performed at some institutions. Treatment of MMC in utero is currently restricted to patients enrolled in the NIH-sponsored Management of Myelomeningocele Study (MOMS trial).
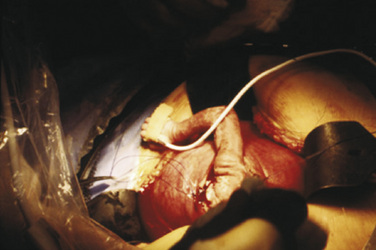
After induction of anesthesia, a maternal laparotomy is performed. The location of this incision is transverse, but more cephalad than that performed for a low-segment transverse cesarean section, to allow easier access to the uterus and fetus. Once the uterus is exposed, the placental edges are mapped carefully with sterile ultrasound to plan for an appropriate hysterotomy. Implantation of the placenta on the anterior or posterior aspect of the uterus will influence the surgical approach, and an anterior placenta may necessitate exteriorization of the uterus for a posterior hysterotomy. Special uterine stapling devices are used to minimize blood loss (Bond et al., 1989). The fetus is exposed, but only the necessary anatomy is delivered via the hysterotomy. For example, in an MMC closure, the lesion is exposed while the rest of the fetus remains bathed in amniotic fluid in the uterus. If a fetal thoracotomy is planned, an arm is delivered, and the shoulder and chest are exposed while the rest of the fetus remains in the uterus (see Fig. 19-6). After surgery and skin closure, the fetus is placed back into the uterus, warmed crystalloid is infused to restore amniotic volume, and intrauterine antibiotics are given. The uterus is closed and a patch of omentum is sewn over a two-layer uterine closure to further prevent amniotic fluid leakage.
EXIT Procedure
The EXIT procedure is similar in approach to an open midgestation procedure, but with several key differences. Because the fetus will be delivered at the end of the case, these procedures are performed at or near term to optimize fetal lung maturity. Before the umbilical cord is clamped, surgical intervention is performed that will allow successful transition to extrauterine life (Mychaliska et al., 1997). This intervention may involve laryngoscopy, rigid bronchoscopy, and intubation, or it may involve resection of airway tumors or tracheostomy (Liechty et al., 1997; Bouchard et al., 2002; Rahbar et al., 2005). Pulmonary pathology necessitating an EXIT procedure includes large lung lesions that would make neonatal resuscitation difficult because of mediastinal shift, air trapping, or compression of the normal lung (Hedrick et al., 2005). After completion of the procedure, the umbilical cord is clamped and cut. The newborn is taken to a team headed by a neonatologist for further neonatal resuscitation and management in an intensive care unit.
Anesthetic plan
Minimally Invasive Procedures
Because these cases are the most variable in terms of degree of invasiveness, need for maternal analgesia and anesthesia, and need for fetal analgesia or immobility, communication and understanding of needs of the case are vital. An anesthesia plan can range from local anesthetic infiltration to sedation to neuraxial technique to general anesthesia. Medications can be given directly to the mother by the anesthesia team and thus indirectly to the fetus by placental transfer (Van de Velde et al., 2005). Medications can also be given directly to the fetus by the surgical team. Route of direct administration can be variable, and intramuscular, IV, and intracardiac routes have been described (Fisk et al., 2001; Deprest et al., 2005; Mizrahi-Arnaud et al., 2007). Sheep models of intraamniotic opioid instillation have also been reported (Strumper et al., 2003). Maternal analgesia can often be accomplished with local anesthetic infiltration, whereas in other cases a neuraxial technique or general anesthesia may be necessary.
As instrumentation for treatment of twin-twin transfusion syndrome has shrunk and invasiveness has decreased, anesthesia techniques at some institutions have similarly changed. Initially, procedures were performed with general or neuraxial anesthesia techniques, but these procedures are now done with sedation. Routine use of magnesium sulfate tocolysis and severe IV fluid restriction are no longer needed. Pulmonary edema has been reported after fetoscopic surgery, but this was more likely the result of absorption of irrigation fluids through venous channels in the myometrium than a capillary leak phenomenon (Robinson et al., 2008). As surgical techniques vary, fluid restriction may be necessary, as well as close observation of irrigation fluids used during these cases.
At some institutions, the current practice for laser ablation of placental vessels includes maternal fasting, placement of one IV catheter, aspiration prophylaxis, and tocolysis with preoperative indomethacin. Light sedation and analgesia are administered to the mother to provide maternal comfort and decreased fetal movement to make the surgical procedure easier. Multiple regimens for sedation have been used successfully, including combinations of opioids and other sedatives such as benzodiazepines or propofol. In a randomized double-blind trial comparing diazepam and remifentanil for fetal immobilization in minimally invasive surgery, the remifentanil group (0.1 mcg/kg per minute) had significantly less fetal movement and surgeons reported better operating conditions (Van de Velde et al., 2005). The degree of maternal sedation needed often competes with the need to avoid paradoxical chest and abdominal breathing motions that make the surgical procedure impossible.
In contrast to the anesthesia for laser ablation of placental vessels, providing anesthesia for balloon dilation of fetal aortic stenosis involves maternal general endotracheal anesthesia and intramuscular administration of fentanyl, vecuronium, and atropine to the fetus (Tworetzky and Marshall, 2003). The potential risks of administration of general anesthesia in a pregnant woman are outweighed by the need for a completely immobile mother and fetus, along with the potential need for fetal analgesia as the catheters and needles are advanced through the fetal chest wall and heart. These different techniques for minimally invasive surgery illustrate the need for collaboration between the teams to prioritize needs and balance risks and benefits to arrive at an optimal anesthesia plan.
Open Midgestation Procedures
Open midgestation surgery requires significant uterine relaxation. General endotracheal anesthesia with deep inhalational anesthesia (twice the minimum alveolar concentration) is most often used to achieve the necessary uterine relaxation for open surgery. Desflurane is the agent chosen at some institutions because its low solubility allows for timely emergence from deep levels of anesthesia, but isoflurane has also been used successfully. Nitroglycerin boluses can be used to further relax the uterus if sufficient relaxation cannot be achieved (Clark et al., 2004; George et al., 2007). Uterine relaxation allows easier fetal manipulation and decreases the likelihood of initiation of labor from uterine surgical manipulation. Uterine relaxation may allow increased uterine blood flow as long as maternal blood pressure is maintained, and it results in fetal exposure to some volatile anesthetic agents. The mother is at risk for hypotension both from the anesthetic agents and from aortocaval compression. With careful patient positioning and judicious use of vasopressors, a goal of systemic blood pressure within 10% of normal blood pressure should be achievable.
After fasting, placement of a peripheral IV catheter, and aspiration prophylaxis, a high lumbar epidural catheter is placed for postoperative analgesia. A test dose of local anesthetic is given, but a standard epidural dose is not given if volatile anesthesia is to be used. Oral tocolysis with indomethacin is also given. Standard monitors are placed, the mother is placed supine with left uterine displacement, and she is preoxygenated. After rapid-sequence induction of general anesthesia, she is intubated, and an orogastric tube and a Foley catheter are placed. Ventilation should maintain normocapnia. Hypocapnia should be avoided to prevent umbilical vasoconstriction and fetal hypoxia, but hypercapnia may be beneficial (Motoyama et al., 1966; Peng et al., 1972). Because of the risk for rapid bleeding and hemodynamic instability, a second large-bore peripheral IV line is placed, as well as an arterial catheter. Small changes in maternal blood pressure may have dramatic effects on the fetal oxygen saturation and heart rate and function. Swings in blood pressure are likely to be exacerbated by the restrictive administration of fluids and frequent use of vasopressors. Intravenous crystalloid administration is kept to a minimum because of the risk of maternal pulmonary edema after fetal surgery (DiFederico et al., 1998). Administration of 500 mL of crystalloid is typical. Restricting fluid administration may be more of an issue when nitroglycerin is used more liberally. Clinically, the maternal blood pressure improves with exteriorization of the uterus and with surgical manipulation. Phenylephrine and ephedrine should be prepared for use. Infusions of phenylephrine allow smoother blood pressure control and may allow more rigorous control of crystalloid administration. The anesthesia literature is still exploring the relative advantages and disadvantages of phenylephrine and ephedrine in the obstetric population, but no specific data exist to guide the choices in fetal surgery. Central venous pressure measurement has guided fluid therapy in the past but has not been used recently because it is very restrictive (500 cc), and hypotension is treated with either vasopressors or transfusion if bleeding is noted. Low central venous pressure would probably not trigger much change in fluid management.
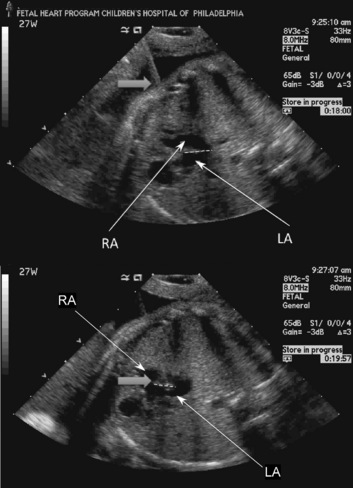
When the uterus is exposed, some time is taken for mapping of the placenta to optimize the surgical approach. After hysterotomy and exposure of the fetus, an intramuscular injection of fentanyl (20 mcg/kg), atropine (20 mcg/kg), and vecuronium (0.2 mg/kg) is given by the surgical team. Amniotic fluid is lost through the hysterotomy, but it is replaced with a continuous pressurized infusion of warmed lactated Ringer’s solution. If fetal IV access is deemed necessary, it is obtained at this time, and sterile IV tubing is handed over the surgical drapes to the anesthesia team. Monitoring of the fetus in these cases may include fetal heart rate by ultrasound, fetal echocardiography, and pulse oximetry (Fig. 19-12) (Luks et al., 1998; Keswani et al., 2005) in addition to direct fetal observation. The sterile pulse oximeter is placed by the surgical team, the hand is covered with sterile foil to prevent artifact from the operating room lights, and a sterile cable is passed to the anesthesia team over the surgical drapes. Values for fetal oxygen saturation range from 40% to 70% (Johnson et al., 1991; Helwig et al., 1996). Fetal echocardiography may be intermittent or continuous depending on the nature of the surgery. Cardiac filling, contractility, and rate, along with patency of the ductus arteriosus are helpful in intraoperative management of the fetus. Umbilical blood gas measurement may be used in select cases, although cord injury is a risk. Drugs may be given to the fetus intravenously or intramuscularly.
The anesthesia team must watch closely for fetal bradycardia, maternal or fetal bleeding, and maternal blood pressure changes. Careful observation and understanding of the events occurring in the surgical field are important. Maternal bleeding when the uterus is opened is often difficult to appreciate. A decrease in fetal oxygen saturation seems to be the most sensitive indicator of fetal distress (Luks et al., 1998). In the absence of a decrease in fetal oxygen saturation, the most common sign of fetal distress is bradycardia. Blood products should be readily available during this time. Prior to the resection of large chest lesions, a 10- to 20-mg/kg fluid bolus (usually packed red blood cells) to the fetus maintains fetal hemodynamic stability by preserving cardiac output when large shifts in mediastinal anatomy occur during the resection. With closure of the uterus, tocolysis is begun with a bolus of IV magnesium sulfate, the epidural block is initiated, and the volatile anesthetic is reduced. The maternal abdomen is closed, and the mother is extubated awake. Postoperative tocolysis continues with a magnesium infusion that is later transitioned to oral nifedipine.
EXIT Procedure
Several key differences for the EXIT procedure result from the fact that the fetus is to be delivered at the conclusion of the case. Because magnesium tocolysis is no longer needed, pulmonary edema is less of a problem, so fluid management can be more generous. Another difference is the need for two operating rooms and a resuscitation area for the neonatal team. Although general endotracheal anesthesia is often used to provide high-dosage volatile anesthetics and is the technique of choice at some institutions, adequate uterine relaxation with nitroglycerin infusion is possible. Several reports have described neuraxial techniques for the EXIT procedure in conjunction with infusions of nitroglycerin (Clark et al., 2004; George et al., 2007).
Pain and the Fetus
Controversy surrounds our understanding of the experience of pain by the fetus (Wise, 1997; Glover and Fisk, 1999; Lee et al., 2005; Lowery et al., 2007). Impeded by our inability to critically study human fetal physiology and neural development, a variety of contradictions drawn from available animal and human sources obfuscate our knowledge of this area. At what point, if ever, a fetus “feels” or experiences pain is unknown. The functional neurodevelopment of pain pathways in several experimental animal models is well described, although the exact correlation to human development and experience is unclear (Fitzgerald and Walker, 2009). Even less understood is the experience of pain. Some animal data imply that the fetus is unaware until birth; other data question the need for cortical function in the experience of pain (Mellor et al., 2005). Just as confusing is the relationship between pain and stress. A fetus in the second trimester can mount a stress response to a stimulus that is blocked by the administration of analgesics to the fetus; yet how this phenomenon relates to pain is unclear, especially because a similar stress response can be noted in an adult under deep anesthesia (Giannakoulopoulos et al., 1994; Teixeira et al., 1999; Fisk et al., 2001; Gruber et al., 2001; Baldini et al., 2008). The late effects of this stress response are worrisome, although these effects are as ambiguous and unsettled. Also ambiguous are the possible deleterious effects on the developing nervous system from the medications used to block this response and provide analgesia and anesthesia to the fetus (Jevtovic-Todorovic et al., 2003). In animal models, opioids have also been associated with adverse hemodynamic changes in fetuses (Sedgwick et al., 2005; Mizrahi-Arnaud et al., 2007). Finally, the significant risks to the mother when using her as a conduit to deliver medications to the fetus have to be considered (Golombeck et al., 2006). With the lack of clarity in this area, continued observation, research, and questioning are of paramount importance for the anesthesiologist caring for both mother and fetus.
Currently, transplacental administration of medications for fetal surgery is the norm. Procedures are performed either with maternal general anesthesia or with local anesthesia with maternal systemic anxiolysis and analgesia. Halothane and probably the other volatile anesthetics are transferred to the fetus through the placenta (Dwyer et al., 1995). Opioids and sedatives similarly pass through the placenta to the fetus (Bakke and Haram, 1982; Kan et al., 1998). Open procedures are often supplemented with IV or intramuscular analgesics, neuromuscular blockers, and anticholinergics. Until microsampling and microanalysis techniques are improved and long-term outcome studies are executed, the anesthesiologist will need to strive to provide the best possible conditions for the surgeon while subjecting the mother and fetus to as little risk or harm as possible.
Summary
More outcome studies are needed, as well as further studies to refine anesthesia techniques and methods. Some institutions are exploring the use of IV anesthesia to minimize fetal exposure to high-dosage volatile agents during portions of the case that do not require intense uterine relaxation (Boat, 2008). Research in this field is limited by practical, medicolegal, and ethical considerations, and the number of these cases is small in comparison with other fields. Protection of vulnerable populations such as pregnant mothers and fetuses is important, but these protections will limit flexibility in study design. Most anesthesia studies in this area are, however, still in the descriptive, hypothesis-generating stages, as compared with rigorous hypothesis testing. Many questions remain to be answered, and much progress remains to be made.
For questions and answers on topics in this chapter, go to “Chapter Questions” at www.expertconsult.com.
Abouleish E., Abboud T., Lechevalier T., et al. Rocuronium (Org 9426) for caesarean section. Br J Anaesth. 1994;73:336.
Adzick N.S., Flake A.W., Crombleholme T.M. Management of congenital lung lesions. Semin Pediatr Surg. 2003;12:10.
Adzick N.S., Kitano Y. Fetal surgery for cystic adenomatoid malformation of the lung. J Pediatr Surg. 1993;28:806.
Alahuhta S., Räsänen J., Jouppila R., et al. Uteroplacental and fetal haemodynamics during extradural anaesthesia for caesarean section. Br J Anaesth. 1991;66:319.
Alaily A.B., Carrol K.B. Pulmonary ventilation in pregnancy. Br J Obstet Gynaecol. 1978;85:518.
Alcorn D., Adamson T.M., Lambert T.F., et al. Morphological effects of chronic tracheal ligation and drainage in the fetal lamb lung. J Anat. 1977;123:649.
Bachman C.R., Biehl D.R., Sitar D., et al. Isoflurane potency and cardiovascular effects during short exposures in the foetal lamb. Can Anaesth Soc J. 1986;33:41.
Bakke O.M., Haram K. Time-course of transplacental passage of diazepam: influence of injection-delivery interval on neonatal drug concentrations. Clin Pharmacokinet. 1982;7:353.
Baldini G., Bagry H., Carli F. Depth of anesthesia with desflurane does not influence the endocrine-metabolic response to pelvic surgery. Acta Anaesthesiol Scand. 2008;52:99.
Baraka A., Jabbour S., Tabboush Z., et al. Onset of vecuronium neuromuscular block is more rapid in patients undergoing caesarean section. Can J Anaesth. 1992;39:135.
Baraka A.S., Sayyid S.S., Assaf B.A., et al. Thiopental-rocuronium versus ketamine-rocuronium for rapid-sequence intubation in parturients undergoing cesarean section. Anesth Analg. 1997;84:1104.
Barcroft J., Kennedy J.A. The distribution of blood between the foetus and the placenta in sheep. J Physiol. 1939;95:173.
Biehl D.R., Yarnell R., Wade J.G., et al. The uptake of isoflurane by the foetal lamb in utero: effect on regional blood flow. Can Anaesth Soc J. 1983;30:581.
Bilardo C.M., Nicolaides K.H., Campbell S. Doppler measurements of fetal and uteroplacental circulations: relationship with umbilical venous blood gases measured at cordocentesis. Am J Obstet Gynecol. 1990;162:115.
Boat A.. Anesthetic challenges during EXIT procedures: Presented at the Annual Meeting of the International Fetal Medical and Surgical Society. 2008.
Bond S.J., Harrison M.R., Schmidt K.G., et al. Death due to high-output cardiac failure in fetal sacrococcygeal teratoma. J Pediatr Surg. 1990;25:1287.
Bond S.J., Harrison M.R., Slotnick R.N., et al. Cesarean delivery and hysterotomy using an absorbable stapling device. Obstet Gynecol. 1989;74:25.
Bonds D.R., Crosby L.O., Cheek T.G., et al. Estimation of human fetal-placental unit metabolic rate by application of the Bohr principle. J Dev Physiol. 1986;8:49.
Bouchard S., Johnson M.P., Flake A.W., et al. The EXIT procedure: experience and outcome in 31 cases. J Pediatr Surg. 2002;37:418.
Bruner J.P., Davis G., Tulipan N. Intrauterine shunt for obstructive hydrocephalus: still not ready. Fetal Diagn Ther. 2006;21:532.
Bruner J.P., Tulipan N., Paschall R.L., et al. Fetal surgery for myelomeningocele and the incidence of shunt-dependent hydrocephalus. JAMA. 1999;282:1819.
Cade J.F., Hirsh J., Martin M. Placental barrier to coagulation factors: its relevance to the coagulation defect at birth and to haemorrhage in the newborn. Br Med J. 1969;2:281.
Cendron M., D’Alton M.E., Crombleholme T.M. Prenatal diagnosis and management of the fetus with hydronephrosis. Semin Perinatol. 1994;18:163.
Challier J.C., Nandakumaran M., Mondon F. Placental transport of hexoses: a comparative study with antipyrine and amino acids. Placenta. 1985;6:497.
Chan M.T., Mainland P., Gin T. Minimum alveolar concentration of halothane and enflurane are decreased in early pregnancy. Anesthesiology. 1996;85:782.
Chiloiro M., Darconza G., Piccioli E. Gastric emptying and orocecal transit time in pregnancy. J Gastroenterol. 2001;36:538.
Clark K.D., Viscomi C.M., Lowell J., et al. Nitroglycerin for relaxation to establish a fetal airway (EXIT procedure). Obstet Gynecol. 2004;103:1113.
Clark R.B., Brown M.A., Lattin D.L. Neostigmine, atropine, and glycopyrrolate: does neostigmine cross the placenta? Anesthesiology. 1996;84:450.
Clark S.L., Cotton D.B., Lee W., et al. Central hemodynamic assessment of normal term pregnancy. Am J Obstet Gynecol. 1989;161:1439.
Cooper J., Jauniaux E., Gulbis B., et al. Placental transfer of fentanyl in early human pregnancy and its detection in fetal brain. Br J Anaesth. 1999;82:929.
Cormack R.S., Lehane J. Difficult tracheal intubation in obstetrics. Anaesthesia. 1984;39:1105.
Crombleholme T.M., Harrison M.R., Golbus M.S., et al. Fetal intervention in obstructive uropathy: prognostic indicators and efficacy of intervention. Am J Obstet Gynecol. 1990;162:1239.
Crombleholme T.M., Harrison M.R., Langer J.C., et al. Early experience with open fetal surgery for congenital hydronephrosis. J Pediatr Surg. 1988;23:1114.
Crombleholme T.M., Shera D., Lee H., et al. A prospective, randomized, multicenter trial of amnioreduction vs selective fetoscopic laser photocoagulation for the treatment of severe twin-twin transfusion syndrome. Am J Obstet Gynecol. 2007;197:396 e1.
Cullen B.F., Margolis A.J., Eger E.I. The effects of anesthesia and pulmonary ventilation on blood loss during elective therapeutic abortion. Anesthesiology. 1970;32:108.
Dailey P.A., Fisher D.M., Shnider S.M., et al. Pharmacokinetics, placental transfer, and neonatal effects of vecuronium and pancuronium administered during cesarean section. Anesthesiology. 1984;60:569.
Dailland P., Cockshott I.D., Lirzin J.D., et al. Intravenous propofol during cesarean section: placental transfer, concentrations in breast milk, and neonatal effects. A preliminary study. Anesthesiology. 1989;71:827.
Davison J.S., Davison M.C., Hay D.M. Gastric emptying time in late pregnancy and labour. J Obstet Gynaecol Br Commonw. 1970;77:37.
De Smedt M.C., Visser G.H., Meijboom E.J. Fetal cardiac output estimated by Doppler echocardiography during mid- and late gestation. Am J Cardiol. 1987;60:338.
Deprest J., Jani J., Gratacos E., Vandecruys H., et al. Fetal intervention for congenital diaphragmatic hernia: the European experience. Semin Perinatol. 2005;29:94.
DiFederico E.M., Burlingame J.M., Kilpatrick S.J., et al. Pulmonary edema in obstetric patients is rapidly resolved except in the presence of infection or of nitroglycerin tocolysis after open fetal surgery. Am J Obstet Gynecol. 1998;179:925.
Doné E., Gucciardo L., Van Mieghem T. Prenatal diagnosis, prediction of outcome and in utero therapy of isolated congenital diaphragmatic hernia. Prenat Diagn. 2008;28:581.
Dwyer R., Fee J.P., Moore J. Uptake of halothane and isoflurane by mother and baby during caesarean section. Br J Anaesth. 1995;74:379.
Eckstein K.L., Marx G.F. Aortocaval compression and uterine displacement. Anesthesiology. 1974;40:92.
Erkkola R., Kangas L., Pekkarinen A. The transfer of diazepam across the placenta during labour. Acta Obstet Gynecol Scand. 1973;52:167.
Ezri T., Szmuk P., Evron S., et al. Difficult airway in obstetric anesthesia: a review. Obstet Gynecol Surv. 2001;56:631.
Fichter M.A., Dornseifer U., Henke J., et al. Fetal spina bifida repair: current trends and prospects of intrauterine neurosurgery. Fetal Diagn Ther. 2008;23:271.
Finster M., Mark L.C., Morishima H.O., et al. Plasma thiopental concentrations in the newborn following delivery under thiopental: nitrous oxide anesthesia. Am J Obstet Gynecol. 1966;95:621.
Fisk N.M., Gitau R., Teixeira J.M., et al. Effect of direct fetal opioid analgesia on fetal hormonal and hemodynamic stress response to intrauterine needling. Anesthesiology. 2001;95:828.
Fitzgerald M., Walker S.M. Infant pain management: a developmental neurobiological approach. Nat Clin Pract Neuro. 2009;5:35.
Flake A.W. Fetal sacrococcygeal teratoma. Semin Pediatr Surg. 1993;2:113.
Flanagan H.L., Datta S., Lambert D.H., et al. Effect of pregnancy on bupivacaine-induced conduction blockade in the isolated rabbit vagus nerve. Anesth Analg. 1987;66:123.
Friedman W.F. The intrinsic physiologic properties of the developing heart. Prog Cardiovasc Dis. 1972;15:87.
George R.B., Melnick A.H., Rose E.C., et al. Case series: combined spinal epidural anesthesia for cesarean delivery and ex utero intrapartum treatment procedure. Can J Anaesth. 2007;54:218.
Giannakoulopoulos X., Sepulveda W., Kourtis P., et al. Fetal plasma cortisol and beta-endorphin response to intrauterine needling [see comment]. Lancet. 1994;344:77.
Gilbert R.D. Control of fetal cardiac output during changes in blood volume. Am J Physiol. 1980;238:H80.
Gin T., Chan M.T. Decreased minimum alveolar concentration of isoflurane in pregnant humans. Anesthesiology. 1994;81:829.
Gin T., Gregory M.A., Chan K., et al. Maternal and fetal levels of propofol at caesarean section. Anaesth Intensive Care. 1990;18:180.
Glover V., Fisk N.M. Fetal pain: implications for research and practice. Br J Obstet Gynaecol. 1999;106:881.
Goldszmidt E. Principles and practices of obstetric airway management. Anesthesiol Clin. 2008;26:109. vii
Golombeck K., Ball R.H., Lee H., et al. Maternal morbidity after maternal-fetal surgery. Am J Obstet Gynecol. 2006;194:834.
Grant D.A. Ventricular constraint in the fetus and newborn. Can J Cardiol. 1999;15:95.
Grant D.A., Fauchère J.C., Eede K.J., et al. Left ventricular stroke volume in the fetal sheep is limited by extracardiac constraint and arterial pressure. J Physiol. 2001;535:231.
Gregory G.A., Wade J.G., Beihl D.R., et al. Fetal anesthetic requirement (MAC) for halothane. Anesth Analg. 1983;62:9.
Gregory M.A., Gin T., Yau G., et al. Propofol infusion anaesthesia for caesarean section. Can J Anaesth. 1990;37:514.
Gruber E.M., Laussen P.C., Casta A., et al. Stress response in infants undergoing cardiac surgery: a randomized study of fentanyl bolus, fentanyl infusion, and fentanyl-midazolam infusion. Anesth Analg. 2001;92:882.
Grundy E.M., Zamora A.M., Winnie A.P. Comparison of spread of epidural anesthesia in pregnant and nonpregnant women. Anesth Analg. 1978;57:544.
Gunn T.R., Butler J., Gluckman P. Metabolic and hormonal responses to cooling the fetal sheep in utero. J Dev Physiol. 1986;8:55.
Habli M., Michelfelder E., Livingston J., et al. Acute effects of selective fetoscopic laser photocoagulation on recipient cardiac function in twin-twin transfusion syndrome. Am J Obstet Gynecol. 2008;199:412 e1.
Harding R., Hooper S.B. Regulation of lung expansion and lung growth before birth. J Appl Physiol. 1996;81:209.
Harrison M.R. Fetal surgery. Am J Obstet Gynecol. 1996;174:1255.
Harrison M.R., Adzick N.S., Bullard K.M., et al. Correction of congenital diaphragmatic hernia in utero VII: a prospective trial. J Pediatr Surg. 1997;32:1637.
Harrison M.R., Golbus M.S., Filly R.A. Management of the fetus with a urinary tract malformation. JAMA. 1981;246:635.
Harrison M.R., Golbus M.S., Filly R.A., et al. Fetal surgery for congenital hydronephrosis. N Engl J Med. 1982;306:591.
Harrison M.R., Keller R.L., Hawgood S.B., et al. A randomized trial of fetal endoscopic tracheal occlusion for severe fetal congenital diaphragmatic hernia. N Engl J Med. 2003;349:1916.
Hedrick H.L., Flake A.W., Crombleholme T.M., et al. Sacrococcygeal teratoma: prenatal assessment, fetal intervention, and outcome. J Pediatr Surg. 2004;39:430. discussion 438
Hedrick H.L., Flake A.W., Crombleholme T.M., et al. The ex utero intrapartum therapy procedure for high-risk fetal lung lesions. J Pediatr Surg. 2005;40:1038. discussion 44
Hedrick M.H., Ferro M.M., Filly R.A., et al. Congenital high airway obstruction syndrome (CHAOS): a potential for perinatal intervention. J Pediatr Surg. 1994;29:271.
Helwig J.T., Parer J.T., Kilpatrick S.J., et al. Umbilical cord blood acid-base state: what is normal? Am J Obstet Gynecol. 1996;174:1807. discussion 12
Heyborne K.D., Chism D.M. Reversal of Ballantyne syndrome by selective second-trimester fetal termination: a case report. J Reprod Med. 2000;45:360.
Hirabayashi Y., Shimizu R., Saitoh K., et al. Cerebrospinal fluid progesterone in pregnant women. Br J Anaesth. 1995;75:683.
Hirabayashi Y., Shimizu R., Saitoh K., et al. Spread of subarachnoid hyperbaric amethocaine in pregnant women. Br J Anaesth. 1995;74:384.
Hirabayashi Y., Shimizu R., Saitoh K., et al. Acid-base state of cerebrospinal fluid during pregnancy and its effect on spread of spinal anaesthesia. Br J Anaesth. 1996;77:352.
Hughes S.C., Ward M.G., Levinson G., et al. Placental transfer of ephedrine does not affect neonatal outcome. Anesthesiology. 1985;63:217.
Hunter S., Robson S.C. Adaptation of the maternal heart in pregnancy. Br Heart J. 1992;68:540.
Ibrahim D., Ho E., Scherl S.A., et al. Newborn with an open posterior hip dislocation and sciatic nerve injury after intrauterine radiofrequency ablation of a sacrococcygeal teratoma. J Pediatr Surg. 2003;38:248.
Iwama H., Kaneko T., Tobishima S., et al. Time dependency of the ratio of umbilical vein/maternal artery concentrations of vecuronium in caesarean section. Acta Anaesthesiol Scand. 1999;43:9.
James J.T. Maternal hyperventilation during labor. Anesthesiology. 1967;28:804-805.
Jauniaux E., Gulbis B., Shannon C., et al. Placental propofol transfer and fetal sedation during maternal general anaesthesia in early pregnancy. Lancet. 1998;352:290.
Jevtovic-Todorovic V., Hartman R.E., Izumi Y., et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876.
Johnson M.P., Gerdes M., Rintoul N., et al. Maternal-fetal surgery for myelomeningocele: neurodevelopmental outcomes at 2 years of age. Am J Obstet Gynecol. 2006;194:1145. discussion 50
Johnson M.P., Sutton L.N., Rintoul N., et al. Fetal myelomeningocele repair: short-term clinical outcomes. Am J Obstet Gynecol. 2003;189:482.
Johnson N., Johnson V.A., Fisher J., et al. Fetal monitoring with pulse oximetry. Br J Obstet Gynaecol. 1991;98:36.
Kalas D.B., Senfield R.M., Hehre F.W. Continuous lumbar peridural anesthesia in obstetrics. IV. Comparison of the number of segments blocked in pregnant and nonpregnant subjects. Anesth Analg. 1966;45:848.
Kan R.E., Hughes S.C., Rosen M.A., et al. Intravenous remifentanil: placental transfer, maternal and neonatal effects. Anesthesiology. 1998;88:1467.
Kangas L., Erkkola R., Kanto J., et al. Halothane anaesthesia in caesarean section. Acta Anaesthesiol Scand. 1976;20:189.
Kelman G.R., Templeton A. Maternal blood gases during human pregnancy. J Physiol. 1975;244:66P.
Kerr M.G. The mechanical effects of the gravid uterus in late pregnancy. J Obstet Gynaecol Br Commonw. 1965;72:513.
Kerr M.G., Scott D.B., Samuel E. Studies of the inferior vena cava in late pregnancy. Br Med J. 1964;1:532.
Keswani S.G., Crombleholme T.M., Rychik J., et al. Impact of continuous intraoperative monitoring on outcomes in open fetal surgery. Fetal Diagn Ther. 2005;20:316.
Klaritsch P., Albert K., Van Mieghem T., et al. Instrumental requirements for minimal invasive fetal surgery. BJOG. 2009;116:188.
Kohl T., Hering R., Bauriedel G., et al. Fetoscopic and ultrasound-guided decompression of the fetal trachea in a human fetus with Fraser syndrome and congenital high airway obstruction syndrome (CHAOS) from laryngeal atresia. Ultrasound Obstet Gynecol. 2006;27:84. discussion 8
Kohl T., Tchatcheva K., Merz W., et al. Percutaneous fetoscopic laser decompression of congenital high airway obstruction syndrome (CHAOS) from laryngeal atresia via a single trocar: current technical constraints and potential solutions for future interventions. Fetal Diagn Ther. 2009;25:67.
Kopecky E.A., Ryan M.L., Barrett J.F., et al. Fetal response to maternally administered morphine. Am J Obstet Gynecol. 2000;183:424.
Koren G., Pastuszak A., Ito S. Drugs in pregnancy. N Engl J Med. 1998;338:1128.
Laird-Meeter K., van de Ley G., Bom T.H., et al. Cardiocirculatory adjustments during pregnancy: an echocardiographic study. Clin Cardiol. 1979;2:328.
Lee A., Ngan Kee W.D., Gin T. A quantitative, systematic review of randomized controlled trials of ephedrine versus phenylephrine for the management of hypotension during spinal anesthesia for cesarean delivery. Anesth Analg. 2002;94:920.
Lee S.J., Ralston H.J., Drey E.A., et al. Fetal pain: a systematic multidisciplinary review of the evidence. JAMA. 2005;294:947.
Levinson G., Shnider S.M., DeLorimier A.A., et al. Effects of maternal hyperventilation on uterine blood flow and fetal oxygenation and acid-base status. Anesthesiology. 1974;40:340.
Levy C.J., Owen G. Thiopentone transmission through the placenta. Anaesthesia. 1964;19:511.
Lewi L., Jani J., Deprest J. Invasive antenatal interventions in complicated multiple pregnancies. Obstet Gynecol Clin North Am. 2005;32:105. x
Lewi L., Van Schoubroeck D., Gratacós E., et al. Monochorionic diamniotic twins: complications and management options. Curr Opin Obstet Gynecol. 2003;15:177.
Liechty K.W., Crombleholme T.M., Flake A.W., et al. Intrapartum airway management for giant fetal neck masses: the EXIT (ex utero intrapartum treatment) procedure. Am J Obstet Gynecol. 1997;177:870.
Lim F.Y., Crombleholme T.M., Hedrick H.L., et al. Congenital high airway obstruction syndrome: natural history and management. J Pediatr Surg. 2003;38:940.
Livingston J.C., Malik K.M., Crombleholme T.M., et al. Mirror syndrome: a novel approach to therapy with fetal peritoneal-amniotic shunt. Obstet Gynecol. 2007;110:540.
Lowery C.L., Hardman M.P., Manning N., et al. Neurodevelopmental changes of fetal pain. Semin Perinatol. 2007;31:275.
Luks F.I., Carr S.R., De Paepe M.E., et al. What and why the pediatric surgeon should know about twin-to-twin transfusion syndrome. J Pediatr Surg. 2005;40:1063.
Luks F.I., Johnson B.D., Papadakis K., et al. Predictive value of monitoring parameters in fetal surgery. J Pediatr Surg. 1998;33:1297.
Macfie A.G., Magides A.D., Richmond M.N., et al. Gastric emptying in pregnancy. Br J Anaesth. 1991;67:54.
Mandelli M., Morselli P.L., Nordio S., et al. Placental transfer to diazepam and its disposition in the newborn. Clin Pharmacol Ther. 1975;17:564.
Manning F.A., Harrison M.R., Rodeck C. Catheter shunts for fetal hydronephrosis and hydrocephalus. Report of the International Fetal Surgery Registry. N Engl J Med. 1986;315:336.
Marshall A.C., Levine J., Morash D., et al. Results of in utero atrial septoplasty in fetuses with hypoplastic left heart syndrome. Prenat Diagn. 2008;28:1023.
Marshall A.C., Tworetzky W., Bergersen L., et al. Aortic valvuloplasty in the fetus: technical characteristics of successful balloon dilation. J Pediatr. 2005;147:535.
Mellor D.J., Diesch T.J., Gunn A.J., et al. The importance of “awareness” for understanding fetal pain. Brain Research Reviews. 2005;49:455.
Mendenhall H.W. Serum protein concentrations in pregnancy. I. Concentrations in maternal serum. Am J Obstet Gynecol. 1970;106:388.
Meuli M., Meuli-Simmen C., Yingling C.D., et al. Creation of myelomeningocele in utero: a model of functional damage from spinal cord exposure in fetal sheep. J Pediatr Surg. 1995;30:1028. discussion 32
Meuli M., Meuli-Simmen C., Yingling C.D., et al. In utero repair of experimental myelomeningocele saves neurological function at birth. J Pediatr Surg. 1996;31:397.
Mielke G., Benda N. Cardiac output and central distribution of blood flow in the human fetus. Circulation. 2001;103:1662.
Mizrahi-Arnaud A., Tworetzky W., Bulich L.A., et al. Pathophysiology, management, and outcomes of fetal hemodynamic instability during prenatal cardiac intervention. Pediatr Res. 2007;62:325.
Mong A., Johnson A.M., Kramer S.S., et al. Congenital high airway obstruction syndrome: MR/US findings, effect on management, and outcome. Pediatr Radiol. 2008;38:1171.
Morris J.A., Hustead R.F., Robinson R.G., et al. Measurement of fetoplacental blood volume in the human previable fetus. Am J Obstet Gynecol. 1974;118:927.
Motoyama E.K., Rivard G., Acheson F., et al. Adverse effect of maternal hyperventilation on the foetus. Lancet. 1966;1:286.
Motoyama E.K., Rivard G., Acheson F., et al. The effect of changes in maternal pH and P-CO2 on the P-O2 of fetal lambs. Anesthesiology. 1967;28:891.
Munnur U., de Boisblanc B., Suresh M.S. Airway problems in pregnancy. Crit Care Med. 2005;33:S259.
Mychaliska G.B., Bealer J.F., Graf J.L., et al. Operating on placental support: the ex utero intrapartum treatment procedure. J Pediatr Surg. 1997;32:227. discussion 30
Myers L.B., Cohen D., Galinkin J., et al. Anaesthesia for fetal surgery. Paediatr Anaesth. 2002;12:569.
Ngan Kee W.D., Lee A., Khaw K.S., et al. A randomized double-blinded comparison of phenylephrine and ephedrine infusion combinations to maintain blood pressure during spinal anesthesia for cesarean delivery: the effects on fetal acid-base status and hemodynamic control. Anesth Analg. 2008;107:1295.
Nicolaides K.H., Clewell W.H., Rodeck C.H., et al. Measurement of human fetoplacental blood volume in erythroblastosis fetalis. Am J Obstet Gynecol. 1987;157:50.
Nisell H., Hjemdahl P., Linde B., et al. Cardiovascular responses to circulating catecholamines in normal pregnancy and in pregnancy-induced hypertension. Clin Physiol. 1985;5:479.
O’Sullivan G.M., Sutton A.J., Thompson S.A., et al. Noninvasive measurement of gastric emptying in obstetric patients. Anesth Analg. 1987;66:505.
Olver R.E., Strang L.B. Ion fluxes across the pulmonary epithelium and the secretion of lung liquid in the foetal lamb. J Physiol. 1974;241:327.
Paek B.W., Jennings R.W., Harrison M.R., et al. Radiofrequency ablation of human fetal sacrococcygeal teratoma. Am J Obstet Gynecol. 2001;184:503.
Palahniuk R.J., Shnider S.M. Maternal and fetal cardiovascular and acid-base changes during halothane and isoflurane anesthesia in the pregnant ewe. Anesthesiology. 1974;41:462.
Parer J.T., Eng M., Aoba H., et al. Uterine blood flow and oxygen uptake during maternal hyperventilation in monkeys at cesarean section. Anesthesiology. 1970;32:130.
Peng A.T., Blancato L.S., Motoyama E.K. Effect of maternal hypocapnia v. eucapnia on the foetus during caesarean section. Br J Anaesth. 1972;44:1173.
Peralta C.F., Jani J.C., Van Schoubroeck D., et al. Fetal lung volume after endoscopic tracheal occlusion in the prediction of postnatal outcome. Am J Obstet Gynecol. 2008;198:60 e1.
Perks A.M., Cassin S. The rate of production of lung liquid in fetal goats, and the effect of expansion of the lungs. J Dev Physiol. 1985;7:149.
Pilkington S., Carli F., Dakin M.J., et al. Increase in Mallampati score during pregnancy. Br J Anaesth. 1995;74:638.
Pirhonen J.P., Hartgill T.W. Spontaneous reversal of mirror syndrome in a twin pregnancy after a single fetal death. Eur J Obstet Gynecol Reprod Biol. 2004;116:106.
Polvi H.J., Pirhonen J.P., Erkkola R.U. Nitrous oxide inhalation: effects on maternal and fetal circulations at term. Obstet Gynecol. 1996;87:1045.
Popitz-Bergez F.A., Leeson S., Thalhammer J.G., et al. Intraneural lidocaine uptake compared with analgesic differences between pregnant and nonpregnant rats. Reg Anesth. 1997;22:363.
Power G.G., Blood A.B., Hunter C.J. Perinatal thermal physiology. In: Polin R.A., Fox W.W., Abman S.H., editors. Fetal and neonatal physiology. ed 3. Philadelphia: Saunders; 2004:541.
Puhringer F.K., Sparr H.J., Mitterschiffthaler G., et al. Extended duration of action of rocuronium in postpartum patients. Anesth Analg. 1997;84:352.
Quintero R.A., Morales W.J., Allen M.H., et al. Staging of twin-twin transfusion syndrome. J Perinatol. 1999;19:550.
Rahbar R., Vogel A., Myers L.B., et al. Fetal surgery in otolaryngology: a new era in the diagnosis and management of fetal airway obstruction because of advances in prenatal imaging. Arch Otolaryngol Head Neck Surg. 2005;131:393.
Redman C.W., Sargent I.L. Placental debris, oxidative stress and pre-eclampsia. Placenta. 2000;21:597.
Reverdiau-Moalic P., Delahousse B., Body G., et al. Evolution of blood coagulation activators and inhibitors in the healthy human fetus. Blood. 1996;88:900.
Rice P.A., Rourke J.E., Nesbitt R.E.Jr. In vitro perfusion studies of the human placenta. VI. Evidence against active glucose transport. Am J Obstet Gynecol. 1979;133:649.
Rivard G., Motoyama E.K., Acheson F.M., et al. The relation between maternal and fetal oxygen tensions in sheep. Am J Obstet Gynecol. 1967;97:925.
Robinson M.B., Crombleholme T.M., Kurth C.D. Maternal pulmonary edema during fetoscopic surgery. Anesth Analg. 2008;107:1978.
Robson S.C., Dunlop W., Boys R.J., et al. Cardiac output during labour. Br Med J (Clin Res Ed). 1987;295:1169.
Ross B.K. ASA closed claims in obstetrics: lessons learned. Anesthesiol Clin North America. 2003;21:183.
Rudra A., Mondal M., Acharya A., et al. Anaesthesia-related maternal mortality. J Indian Med Assoc. 2006;104:312.
Rychik J. Fetal cardiovascular physiology. Pediatr Cardiol. 2004;25:201.
Rychik J., Tian Z., Bebbington M., et al. The twin-twin transfusion syndrome: spectrum of cardiovascular abnormality and development of a cardiovascular score to assess severity of disease. Am J Obstet Gynecol. 2007;197:392 e1.
Samsoon G.L., Young J.R. Difficult tracheal intubation: a retrospective study. Anaesthesia. 1987;42:487.
Sandhar B.K., Elliott R.H., Windram I., et al. Peripartum changes in gastric emptying. Anaesthesia. 1992;47:196.
Schwarz U., Galinkin J.L., Biard J.M., et al. The uptake of desflurane in fetal sheep: preliminary results: Presented at the Annual Meeting of the Society for Pediatric Anesthesiology, Fort Myers, FL, Feb 20-23. 2003.
Sedgwick J.A., Schenbeck J.L., Eghtesady P. Harmful effects of fentanyl on the fetus and placenta? Am J Obstet Gynecol. 2005;193:303.
Senat M.V., Deprest J., Boulvain M., et al. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med. 2004;351:136.
Shannon C., Jauniaux E., Gulbis B., et al. Placental transfer of fentanyl in early human pregnancy. Hum Reprod. 1998;13:2317.
Shnider S.M., Wright R.G., Levinson G., et al. Uterine blood flow and plasma norepinephrine changes during maternal stress in the pregnant ewe. Anesthesiology. 1979;50:524.
Skillman C.A., Plessinger M.A., Woods J.R., et al. Effect of graded reductions in uteroplacental blood flow on the fetal lamb. Am J Physiol. 1985;249:H1098.
Spatling L., Fallenstein F., Huch A., et al. The variability of cardiopulmonary adaptation to pregnancy at rest and during exercise. Br J Obstet Gynaecol. 1992;99(Suppl 8):1.
Strumper D., Durieux M.E., Gogarten W., et al. Fetal plasma concentrations after intraamniotic sufentanil in chronically instrumented pregnant sheep. Anesthesiology. 2003;98:1400. discussion 5A
Tan T.Y., Sepulveda W. A cardiac twin: a systematic review of minimally invasive treatment modalities. Ultrasound Obstet Gynecol. 2003;22:409.
Teixeira J.MA., Glover V., Fisk N.M. Acute cerebral redistribution in response to invasive procedures in the human fetus. Am J Obstet Gynecol. 1999;181:1018.
Templeton A., Kelman G.R. Maternal blood-gases, PAo2-Pao2), physiological shunt and VD/VT in normal pregnancy. Br J Anaesth. 1976;48:1001.
Thaler I., Manor D., Itskovitz J., et al. Changes in uterine blood flow during human pregnancy. Am J Obstet Gynecol. 1990;162:121.
Thomas D.G., Robson S.C., Redfern N., et al. Randomized trial of bolus phenylephrine or ephedrine for maintenance of arterial pressure during spinal anaesthesia for caesarean section. Br J Anaesth. 1996;76:61.
Tran K.M., Flake A.W., Kalawadia N.V., et al. Emergent excision of a prenatally diagnosed sacrococcygeal teratoma. Paediatr Anaesth. 2008.
Tsao K., Feldstein V.A., Albanese C.T., et al. Selective reduction of a cardiac twin by radiofrequency ablation. Am J Obstet Gynecol. 2002;187:635.
Tulipan N., Bruner J.P., Hernanz-Schulman M., et al. Effect of intrauterine myelomeningocele repair on central nervous system structure and function. Pediatr Neurosurg. 1999;31:183.
Tworetzky W., Marshall A.C. Balloon valvuloplasty for congenital heart disease in the fetus. Clin Perinatol. 2003;30:541.
Tworetzky W., Marshall A.C. Fetal interventions for cardiac defects. Pediatr Clin North Am. 2004;51:1503. vii
Tworetzky W., Wilkins-Haug L., Jennings R.W., et al. Balloon dilation of severe aortic stenosis in the fetus: potential for prevention of hypoplastic left heart syndrome: candidate selection, technique, and results of successful intervention. Circulation. 2004;110:2125.
Ulmsten U., Sundstrom G. Esophageal manometry in pregnant and nonpregnant women. Am J Obstet Gynecol. 1978;132:260.
Valtonen M., Kanto J., Rosenberg P. Comparison of propofol and thiopentone for induction of anaesthesia for elective caesarean section. Anaesthesia. 1989;44:758.
Van de Velde M., Van Schoubroeck D., Lewi L.E., et al. Remifentanil for fetal immobilization and maternal sedation during fetoscopic surgery: a randomized, double-blind comparison with diazepam. Anesth Analg. 2005;101:251.
van Selm M., Kanhai H.H., Gravenhorst J.B. Maternal hydrops syndrome: a review. Obstet Gynecol Surv. 1991;46:785.
Vidaeff A.C., Pschirrer E.R., Mastrobattista J.M., et al. Mirror syndrome: a case report. J Reprod Med. 2002;47:770.
Weiner C., Liu K.Z., Thompson L., et al. Effect of pregnancy on endothelium and smooth muscle: their role in reduced adrenergic sensitivity. Am J Physiol. 1991;261:H1275.
Whitehead E.M., Smith M., Dean Y., et al. An evaluation of gastric emptying times in pregnancy and the puerperium. Anaesthesia. 1993;48:53.
Wilkins-Haug L.E., Tworetzky W., Benson C.B., et al. Factors affecting technical success of fetal aortic valve dilation. Ultrasound Obstet Gynecol. 2006;28:47.
Wilson C.M., Dundee J.W., Moore J., et al. A comparison of the early pharmacokinetics of midazolam in pregnant and nonpregnant women. Anaesthesia. 1987;42:1057.
Wise J. Fetuses cannot feel pain before 26 weeks. BMJ. 1997;315:1112.
Wong C.A., Loffredi M., Ganchiff J.N., et al. Gastric emptying of water in term pregnancy. Anesthesiology. 2002;96:1395.
Wu P.Y., Udani V., Chan L., et al. Colloid osmotic pressure: variations in normal pregnancy. J Perinat Med. 1983;11:193.
Yao A.C., Moinian M., Lind J. Distribution of blood between infant and placenta after birth. Lancet. 1969;2:871.