CHAPTER 31 Anesthesia for Burn Injuries
Epidemiology
The most recent edition of the American Burn Association’s National Burn Repository includes a comprehensive analysis of data from acute burn admissions within the United States that were collected between 1998 and 2007. Pediatric burn patients account for almost one third of the total projected volume. Burn-related trauma is the second leading cause of accidental death in children between 1 and 4 years of age and remains the third leading cause of accidental death in individuals younger than age 18 years, exceeded only by motor vehicle accidents and drowning. Approximately 70% of pediatric burns up to the age of 4 years old are the result of scald injuries, whereas flame burns are the most common pattern among children 5 years of age and older (American Burn Association, 2007). In general, younger children are at higher risk for sustaining burn injuries, and abuse or neglect may account for as much as 15% to 20% of these cases (Tucker, 1986; Sheridan et al., 1997a).
The mortality previously associated with severe burn trauma has been significantly reduced since the 1980s. Current survival expectations associated with management of burn wounds may be attributed to improved access to emergency medical care and targeted resuscitation, advanced ventilation modalities and a more comprehensive understanding of the pathophysiology of inhalation injury, rigorous infection control practices, enhanced nutritional support, early burn wound excision and grafting, and the attenuation of the hypermetabolic response (Herndon and Spies, 2001). The development of evidence-based practice guidelines and multidisciplinary care models available at regional burn care centers have aided these efforts. Long-term outcomes have subsequently improved, and pediatric burn survivors are able to report a satisfying quality of life as measured by several psychometric tools (Sheridan et al., 2000; Baker et al., 2007, 2008).
Burn-wound assessment
Injury depth, size, and location are the three components that contribute to the overall severity of burn wounds and are the direct results of exposure to thermal, chemical, electrical, ultraviolet, and radiologic sources. Regardless of the medium, the protective functions of the skin are impaired or destroyed. Burn wounds are dynamic, in that dermal changes evolve over time with damage extending to adjacent or deeper tissues (Palmieri and Greenhalgh, 2002).
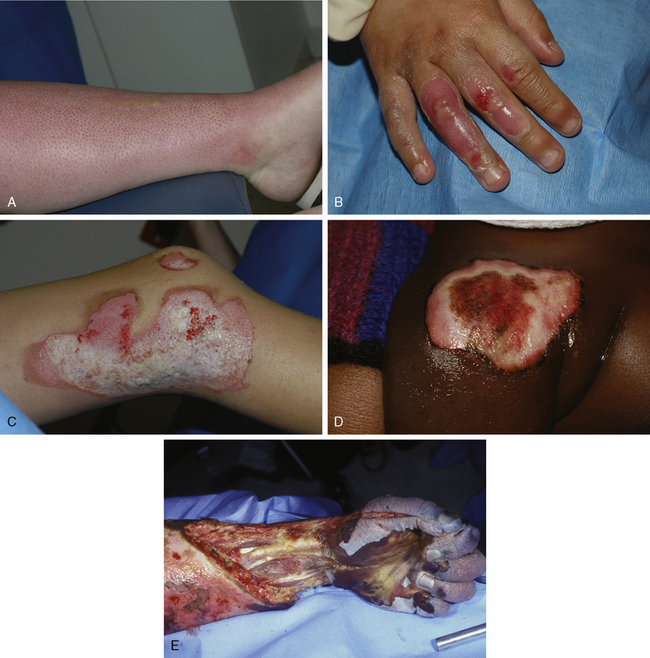
The traditional classification of burn depth (first through fourth degrees) has been supplanted by the use of more comprehensive terminology to include the superficial, partial-thickness, and full-thickness categories, although there remains considerable overlap between these designations (Table 31-1). First-degree (superficial) burns are restricted to the epidermis and generally heal quickly without scarring, pigmentation changes, or contractures. Sunburn is the most common example and is associated with erythema, mild pain, and possible minor blistering (Fig. 31-1, A). Second-degree (partial-thickness) burns involve the epidermis and variable portions of underlying dermal structures and are further categorized as superficial or deep partial-thickness injuries, with different implications for the progression of tissue damage and the level of anticipated care. A superficial partial-thickness burn poses minimal risk of scar formation, because the dermal structures (e.g., nail beds, hair follicles, sebaceous glands, and nerves) are largely unaffected, allowing for injuries to heal within 2 weeks (Fig. 31-1, B). In contrast, deep partial-thickness injuries disrupt portions of the dermal matrix, and epithelial regeneration is commonly associated with scar formation. Pain may not be severe, despite extensive injury, which probably reflects variable degrees of nerve dysfunction or loss. Many of these wounds require excision and grafting in order to heal properly (Fig. 31-1, C). Full-thickness burns (formerly third-degree burns) are characterized by deep-tissue destruction where the necrotic debris adheres tightly to the dermal-matrix remnant as a thick, waxy layer of eschar (Fig. 31-1, D). In the absence of normal regenerative capabilities, wound healing can only occur by prolonged peripheral granulation and contraction, with a significantly increased incidence of infection and debilitating scar formation. Surgical intervention is required to reestablish normal barrier functions and prevent infectious sequelae (Sheridan, 2002). Even with early excision and grafting, hypertophic scarring persists years after the original injury. Fourth-degree burns are full-thickness burns with extensions beyond the fascia and include the destruction of gross muscle mass or the disruption of major joint-capsule integrity (Fig. 31-1, E). Injuries of this type are often a precursor to surgical amputation.
| First degree | Superficial | Epidermal layer only Mild painNo scarring Barrier functions preserved(Sunburn) |
| Second degree | Superficial/partial thickness Deep/partial thickness |
Epidermal layer with varying degree of dermal extension Wet appearance Hyperemic Edematous Blistering PainfulHeals in 7 to 10 days Scar formation is uncommon(Scald) Dry May appear red or pale Moderate-severe blistering Less pain (nerve damage) May advance to full-thickness injury Heals in 2 to 8 weeks Probable scar formation without surgical therapy (Flame/chemical exposure) |
| Third degree | Full thickness | Epidermal and complete dermal layer involvement Dry Waxy white or leathery appearance Painless (nerve destruction) Evolving wound siteExcision and grafting required (Prolonged flame contact) |
| Fourth degree | Full thickness | Epidermal/dermal loss Fascia violation down to tendon or bone Muscle necrosis (Electrical injury) |
Modified from de Campo T, Aldrete JA: The anesthetic management of the severely burned patient, Int Care Med 7:55, 1981.
Reassessments of burn-wound size and depth are necessary, because injuries have the potential to evolve from their initial presentation (dermal dysfunction worsens and affects a broader and deeper area than originally observed). Therapeutic fluid administration, nutritional requirements, and prognostic determinations are dependent on the physician’s ability to perform consistent serial evaluations and can be influenced to varying degrees by a clinician’s experience and subjectivity. Several methods have been developed to minimize potential inaccuracies and include laser Doppler, thermography, vital dye fluorescence, and video angiography (Mandal, 2006; McGill et al., 2007; Monstrey et al., 2008). These techniques are used to identify viable tissue by documenting the presence of adequate vascular flow to the site of injury.
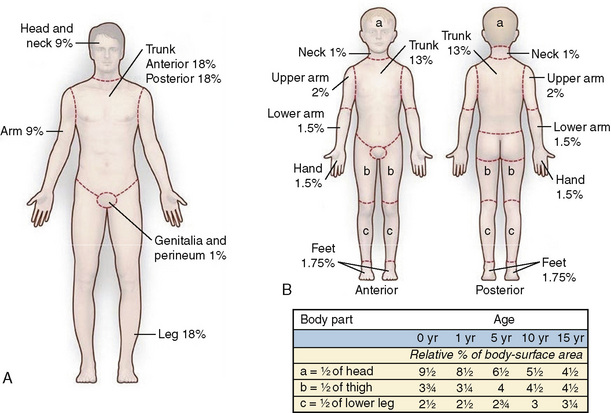
Estimates of dermal involvement are recorded as a percentage of total body surface area (TBSA), and several clinometric instruments are available (Wachtel et al., 2000; Jose et al., 2004). Each method seeks to balance ease of use with consistent assessments among different care providers. The Lund-Browder diagram remains a commonly used tool, because it addresses the differences observed with patient size and body proportions in relation to growth (Fig. 31-2) (Lund and Browder, 1994). It has been adapted for the evaluation of pediatric patients. The “rule of nines” is well-suited for a rapid field-estimate of burn injuries to facilitate prehospital management and identify criteria for transfer to a regional burn center. This technique has also been modified to appropriately account for the alterations in body proportions observed with infants, small children, and the morbidly obese (Livingston and Lee, 2000; Smith et al., 2005). Pediatric burn patients have also been evaluated using the palmar method, in which the size of the patient’s palm represents the equivalent of 1% TBSA (Sheridan et al., 1995b; Rossiter et al., 1996). Complex burn wounds often have highly irregular distributions across multiple body segments, introducing additional inaccuracies to surface-area assessments. Newer techniques have been pioneered to improve the precision and reproducibility of clinician assessments. They include a flexible single-use nomogram that can be applied directly to the patient’s wound sites and an advanced graphics software platform that creates a three-dimensional virtual display (Neuwalder et al., 2002; Dirnberger et al., 2003; Malic et al., 2007).
Burns are further classified by their overall severity (minor, moderate, or major) as defined by the American Burn Association and the American College of Surgeons Committee on Trauma (Table 31-2). These parameters may serve as useful indicators of the anticipated degree of physiologic derangement, but their prognostic value has not been validated.
TABLE 31-2 Burn Wound Severity
| Minor | Superficial burns <15% TBSA |
| Moderate | Superficial burns = 15%-25% TBSA Superficial burns = 10%-20% TBSA in children Full-thickness burns <10% TBSA and burns not involving eyes, ears, face, hands, feet, or perineum |
| Major | Partial-thickness burns >25% TBSA Full-thickness burns >10% TBSA Concomitant inhalation injury Electrical burns Any complicated burn injury, i.e., patients with co-morbid conditions, patients with burns to the eyes, ears, face, hands, feet, or perineumBurns involving the face, eyes, ears, hands, feet, or perineum that may result in functional or cosmetic impairment |
TBSA, Total body surface area (%).
Modified from the American Burn Association: Guidelines for service standards and severity classifications in the treatment of burn injury, Am Coll Surg Bull 69:24, 1984.
Pathophysiology
An evidence-based, multidisciplinary approach directed by a pediatric specialist is the most effective way to minimize mortality and improve long-term, functional outcomes among pediatric burn-trauma victims (Thombs et al., 2006; Gore et al., 2007). The spectrum of burn-related physiologic abnormalities includes, but is not limited to, loss of thermal insulation and antimicrobial barriers, distortions of airway anatomy and pulmonary derangements, fluctuating intravascular volumes and the need for individualized fluid replacement, hypermetabolism accompanied by grossly elevated caloric needs, septicemia, altered responses to common anesthetic agents, and a prolonged inflammatory response to systemic trauma. Most patients benefit from the capabilities of a regional burn unit to deal with these complex pathophysiologic changes, and the American Burn Association has forwarded selection criteria to facilitate referrals (Box 31-1).
Box 31-1 Burn-Center Transfer Criteria
Modified from the American Burn Association: Hospital and Prehospital Resources for Optimal Care of Patients with Burn Injury: Guidelines for development and operation of burn centers, Burn Care Rehab 11:980, 1990; American College of Surgeons: Resources for optimal care of the injured patient, 1993, American College of Surgeons, p 64.
Dermal Barrier Disruption
The epidermis is an effective barrier to heat loss, evaporation, and infection; the dermis and its supporting neurovascular structures provide elasticity, flexibility, and the mechanism for epithelial regeneration. Burn trauma induces localized tissue coagulation and microvascular reactions in the underlying dermis that can lead to injury extension (Aggarwal et al., 1994a, 1994b). Protective functions are immediately lost with dermal disruption and result in significantly increased risk of infection and hypothermia. The risk of hypothermia is particularly high in infants and young children because of their disproportionate surface area/body mass ratio; burn patients benefit from aggressive control of ambient temperature and humidity to restrict heat loss and limit the caloric expense of shivering.
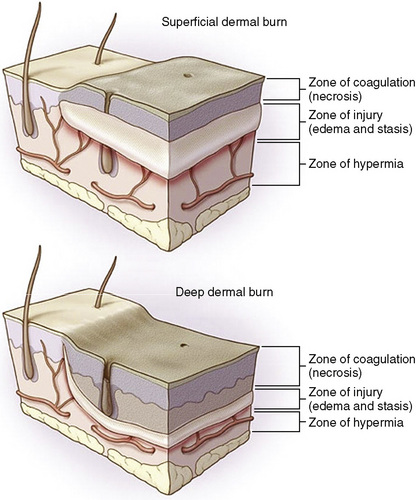
The severity of skin damage is a function of temperature and its duration of contact. Burn wounds have three zones of heterogeneous tissue damage, radiating from the epicenter of maximal tissue destruction (Jackson, 1953). The zone of edema and stasis is of particular importance, because it represents affected tissues that are potentially salvageable with supportive care. Successful management helps reduce the final TBSA measurement (Fig. 31-3) (Hettiaratchy and Dziewulski, 2004). During the first 24 to 48 hours, systemic hypotension, acidosis, and developing sepsis contribute to injury extension. These conditions are likely to exacerbate tissue edema, impaired microcirculation, and perfusion deficits. Serial examination is the most prudent method to address the variability of burn-wound progression. Early detection of nonviable areas with subsequent surgical excision and grafting has been consistently demonstrated to decrease morbidity and mortality in this patient population (Ong et al., 2006).
Respiratory Abnormalities
It is often difficult to definitively distinguish respiratory insufficiency caused by the toxic products of combustion from the pulmonary derangements that are commonly observed with systemic responses to severe burn trauma. Inhalational injury is a term that encompasses both the direct thermal and subsequent inflammatory damage to the upper and lower airways. Several studies have demonstrated that inhalation injury associated with burns increases mortality, although no specific indicators are reliably predictive of the overall degree of pulmonary dysfunction (Shirani et al., 1987; Hollingsed et al., 1993; Ryan et al., 1998; Edelman et al., 2006; Endorf and Gamelli, 2007).
Upper-Airway Effects
Direct-heat injury caused by superheated gas inhalation and toxic smoke exposure may manifest as rapidly progressive edema of the tongue, epiglottis, and aryepiglottic folds (Pruitt et al., 1970). Macroglossia, micrognathia, and tonsillar hypertrophy are common findings in previously healthy children, and the normal presence of these features may further restrict airway patency (Benjamin and Herndon, 2002). Reduced cross-sectional area and increased flow resistance are poorly tolerated by infants and young children because of their elevated baseline minute ventilation, oxygen consumption, and limited endurance of the major respiratory muscle groups (Keens et al., 1978). Large-volume intravenous fluid administration is likely to accelerate oropharyngeal edema and convert a marginal airway into a severely compromised one, with subsequent anatomic distortions that make successful direct laryngoscopy challenging or impossible. Avoiding the “lost airway scenario” in the pediatric burn patient is a critical step during initial management, and the clinician should be guided by serial examinations to avoid airway catastrophe. Early, definitive control of the airway using context-appropriate methods is preferable for patients with evidence of progressive respiratory distress. This principle also applies to other clinical circumstances where the oropharyngeal architecture has been altered (e.g., ingestion of caustic agents such as lye).
Lower-Airway Effects (Smoke-Inhalation Injury)
Lower-airway injury is a clinical diagnosis supported by the presenting history and serial examination. Chest radiographs and bedside pulmonary function tests usually remain normal until infectious complications arise, and then their results often show an underestimation of the severity of lung damage (Lee and O’Connell, 1988; Wittram and Kenny, 1994). Fiberoptic bronchoscopy and bronchoalveolar lavage can be used to document the presence of carbonaceous debris and mucosal sloughing, although the absence of these findings should not preclude the diagnosis (Hunt et al., 1975). Technetium scanning is an assessment of pulmonary vascular endothelium damage represented as abnormal lung/liver uptake ratios of the isotope. In the future, this method may be accepted as a more objective diagnostic tool to detect lung injury in those patients with pulmonary signs and symptoms (but negative chest radiographs or pulmonary function test [PFT] findings), although it will require additional study (Shiau et al., 2003).
Toxicologic analysis of the victims with smoke-inhalation injury has demonstrated exposure to many poisonous aerosols, including carbon monoxide (CO), hydrogen cyanide, various aldehydes, hydrogen chloride, and other aromatic hydrocarbons (e.g., benzene). Even a brief exposure has significant adverse effects on the ciliated respiratory epithelial cells; toxic gases induce separation of cells from basement membranes, leukocyte migration, and exudate formation with severe impairment of the mucociliary-clearance mechanism (Fein et al., 1980). Severe ciliary dysfunction permits the accumulation of cellular debris, secretions, and bacteria. Endobronchial sloughing and edema contribute to small airway narrowing. Diminished flow capacity promotes widespread atelectasis that is magnified by surfactant loss (Herndon et al., 1985). Leukocytes aggregate in the affected lung tissue and release potent inflammatory mediators and other chemotaxins that increase endothelial permeability (Wright and Murphy, 2005). Dyspnea, tachypnea, diffuse rhonchi, and bronchospasm are common clinical signs observed with evolving pulmonary insufficiency and impaired gas exchange in the presence of persistent aerosolized irritants (Table 31-3).
| Early resuscitation phase (0-48 hours) | Upper-airway compromise Persistent bronchospasm Conducting-airway obstruction Impaired ciliary clearance Decreased lung and chest-wall compliance |
| Late resuscitation phase (48+ hours) | Surfactant loss Increased dead space Increased closing volume Decreased functional residual capacity Tracheobronchitis ARDS/pulmonary edema/pneumonia |
ARDS, Acute respiratory distress syndrome.
The cascade of endobronchial cell destruction and protracted inflammatory changes predispose the patient to barotrauma (lung injury secondary to elevated ventilatory pressures and air trapping in the smaller airways), significant ventilation-perfusion distortions, and the potential for bacterial infection that leads to bronchopneumonia after several days (Pruitt et al., 1975; Rue et al., 1993). Lower-airway injuries also substantially increase systemic fluid requirements. Paradoxically, efforts to minimize pulmonary edema with fluid restriction increase mortality in these patients because inadequate intravascular volume invariably results in systemic hypoperfusion and multiorgan dysfunction (Ansermino and Hemsley, 2004).
During this period, clinical efforts must focus on the maintenance of effective gas exchange to provide time for the resolution of pulmonary tissue injury. Vigorous pulmonary toilet is needed to clear accumulated endobronchial debris, purulent exudates, mucous plugs, and residual particulates (Demling, 2008). A number of studies have reported that the administration of aerosolized heparin and mucolytics may reduce cast formation and attenuate respiratory failure (Desai et al., 1998; Holt et al., 2008). Ventilation modalities should be selected to minimize barotrauma and reperfusion injury, because elevated peak inspiratory pressures and hyperoxygenation worsen pulmonary insufficiency (Shapiro et al., 1980; Slutsky, 1993). Low-volume, pressure-limited ventilation with permissive hypercapnia (allowing for the deliberate elevation of measured arterial carbon dioxide tension [Paco2] while maintaining pH greater than 7.25 and an arterial oxygen saturation of hemoglobin [SpO2] of greater than 90%) is one method that may preserve tissue oxygenation in the presence of severe pulmonary dysfunction and is associated with a reduction in short-term mortality (Hickling et al., 1994; Sheridan et al., 1995a). Other methods (e.g., high-frequency percussive ventilation) have also been used, but outcomes data remain unclear (Rodeberg et al., 1994; Micak et al., 1997; Sheridan et al., 1997b; Cortiella et al., 1999; Silver et al., 2004).
Infection is the most common complication after the accumulation of denuded endobronchial tissue, small-airway obstruction, persistent edema, and extended intubation intervals (Rue et al., 1995). The incidence has been reported as high as 30% in pediatric burn patients, with infection occurring after the loss of antimicrobial barriers and the onset of generalized immunosuppression that follows severe burn trauma (Fitzpatrick et al., 1994). Fever, mucopurulent secretions, and lobar consolidation documented by chest radiographs support the diagnosis of pneumonia (or tracheobronchitis if no x-ray changes are noted). Bronchoalveolar lavage and brushings may help support the diagnosis (Ramzy et al., 2003; Wood et al., 2003; Goldberg et al., 2008; Malhotra et al., 2008). Culture-guided and Gram-stain–guided antibiotic therapies should be reevaluated often and adjusted to optimize bacteriocidal effects and limit the development of antibiotic resistance.
Tracheostomy for pediatric patients has been advocated as a useful adjunct for airway and ventilatory management, but its application is not without risk (Sellers et al., 1997; Coin et al., 1998; Palmieri et al., 2002). The typical challenges encountered during pediatric direct laryngoscopy are made considerably more difficult in the presence of facial burns and progressive edema. Tracheostomy provides a stable, definitive airway and should be considered when the treatment plan requires multiple transports to and from the operating room or frequent repositioning of the patient for wound care. Improved secretion clearance, dead-space reduction, decreased airway resistance, and facilitation of the weaning process are also perceived benefits of a tracheostomy. However, surgical airways in small children may be associated with a greater incidence of structural abnormalities (e.g., subglottic stenosis and tracheoesophageal fistula), and subsequent life-long restricted tolerance of exercise. The routine use of this technique is not recommended (Calhoun et al., 1988; Desai et al., 1993; Barret et al., 2000).
Carbon-Monoxide Exposure
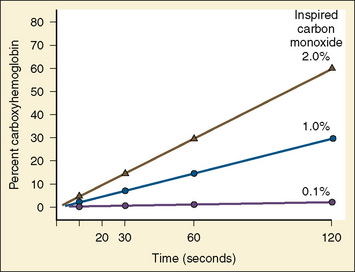
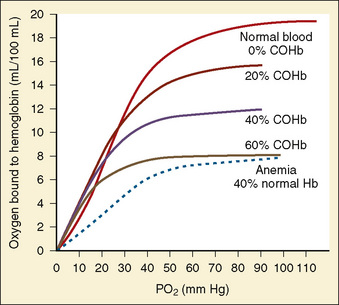
CO is a by-product of incomplete organic combustion and a persistent toxin of heme-containing species because of its strong binding affinity. Although CO reversibly binds hemoglobin, it does so at approximately 200 times the strength of oxygen, producing a functional anemia. Only a brief exposure is necessary to produce clinical symptoms (Fig. 31-4). A person exposed to 1% CO vapor attains measured carboxyhemoglobin (COHb) levels of 30% within 2 minutes. Severe CO poisoning may occur even without evidence of overt burn trauma, and delayed diagnosis contributes to significant morbidity and mortality after exposure (Cone et al., 2008; Stefanidou et al., 2008). The half-life of CO in a patient breathing room air approaches 200 minutes, but by applying 100% oxygen therapy this time can be reduced to approximately 40 minutes (Weaver et al., 2000). The clinician should always maintain a high index of suspicion for occult CO intoxication.
CO binding of hemoglobin and cytochrome P450 produces a leftward shift of the oxygen-hemoglobin dissociation curve and subsequently interferes with erythrocyte transport and oxygen use within the mitochondria (Fig. 31-5). CO-induced alterations of oxygen delivery and cellular usage have the greatest impact on those organ systems with the highest baseline oxygen requirements (i.e., the brain and heart). Ambient levels of 100 ppm are sufficient to produce acute neurologic symptoms (e.g., agitation, dizziness, lethargy, and seizures) in addition to chest pain, dyspnea, and noncardiogenic pulmonary edema. In animal models, CO has been observed to have direct myocardial depressant properties that are independent from the effects of global hypoxemia (Suner and Jay, 2008). The classically-described cherry-red appearance of patients with CO toxicity is rare and should actually be considered a late sign associated with high mortality (“when you’re cherry red, you’re dead”). Most patients have pallor that reflects the functional anemia previously described.
Standard peripheral oximetry overestimates oxygen saturation in the patient with acute CO intoxication and masks profound hypoxemia (Kao and Nañangas, 2004). Arterial sampling of Pao2 is also misleading, because this technique quantifies plasma levels of dissolved oxygen rather than actual hemoglobin saturation. COHb may be assayed with blood samples or measured directly with a noninvasive cooximeter (Masimo Rad-57, Masimo Corporation; Irvine, Calif) that measures and compares multiple light wavelengths to identify COHb and methemoglobin levels (Suner and McMurdy, 2009).
Long-term sequelae may manifest as chronic headaches, memory disturbances, learning disabilities, and neuromotor dysfunction in as many as 10% of those patients who experience severe exposure (Ginsberg, 1985). Some studies suggest that CO induces brain-lipid peroxidation and leukocyte-mediated inflammation, which culminate in white matter demyelination or focal necrosis (Wang et al., 2009). Neuropsychiatric effects may appear days to weeks after the initial exposure, and although most patients eventually achieve complete clinical recovery, radiographic evidence of CO-induced changes persist for far longer.
Currently, it is difficult to determine the precise risk of long-term neurologic effects, although some clinicians have advocated the use of hyperbaric oxygen therapy (HBOT) to limit neuropsychiatric changes. There is conflicting evidence pertaining to the efficacy of HBOT, in part because the neurocognitive symptoms may be attributable to several factors other than CO exposure (Chou et al., 2000; Gilmer et al., 2002). The benefits of this treatment remain controversial, and HBOT is often impractical for those patients with severe comorbid injuries.
Cyanide Toxicity
Cyanide inactivates cytochrome oxidase and prevents mitochondrial oxidative phosphorylation, even in the presence of adequate oxygen; therefore, normal cellular aerobic metabolism is altered to an anaerobic state (Geller et al., 2006). Toxicity manifests as supranormal venous oxygen levels with a concurrent metabolic lactic acidosis that does not resolve with oxygen therapy. The patient displays many similar neurologic and cardiovascular symptoms as observed with CO poisoning, and in fact may require treatment for the toxic effects from both vapors. Treatment involves the administration of agents that scavenge intracellular cyanide and promote its conversion to nontoxic metabolites. Sodium thiosulfate donates a sulfur group to cyanide and increases its conversion rate to thiocyanate, which then undergoes renal elimination (Gracia and Shepard, 2004). Nitrites (e.g., amyl nitrite and sodium nitrite) induce methemoglobin, which then interacts with cyanide to release it from cytochrome-oxidase binding sites. Nitrites should be used with caution in children because concurrent methemoglobin and carboxyhemoglobinemias significantly reduce oxygen-carrying capacity (Fidkowski et al., 2009). Hydroxocobalamin combines with cyanide to form nontoxic cyanocobalamin (vitamin B12), and this compound is excreted by the kidneys (Borron et al., 2007).
Additional Injury Mechanisms
Acute dermal necrosis from burn trauma is characterized by protein denaturation, loss of elasticity, and tense contractures of the dermal remnant. The rigid layer of eschar that accompanies circumferential full-thickness burns of the abdomen and thorax may severely restrict diaphragmatic movement and chest-wall expansion, quickly progressing to respiratory failure within the first hours after trauma (Quinby, 1972). Markedly elevated intraabdominal and intrathoracic pressures impair venous return, with subsequent reduction of cardiac output (Demling, 1986). Compressive effects of circumferential burns involving the upper or lower extremities lead to neurovascular compromise and render the limbs nonviable. Abdominothoracic and extremity escharotomies are performed to relieve compartment syndromes and restore effective chest wall compliance and limb perfusion.
Cardiovascular Abnormalities
Pediatric burn patients experience profound changes in intravascular fluid dynamics and impairments of systemic organ perfusion. Hypotension is the result of volume deficits and low peripheral vascular resistance that lead to diminished measures of cardiac output, central venous pressure, and pulmonary artery occlusion pressure. Additionally, pediatric patients often experience varying degrees of the systemic inflammatory response syndrome (SIRS), a complex milieu of cytokines, chemokines, and proinflammatory mediators that alter capillary permeability, depress myocardial contractility, and impair the normal function of many other organ systems (Table 31-4).
| System | Early | Late |
| Cardiovascular | ↓ CO caused by decreased circulating blood volume, myocardial depression (TNFα) | ↑ CO caused by sepsis or hypermetabolism Hypertension |
| Pulmonary | Upper- and lower-airway obstruction ↓ FRC ↓ Pulmonary compliance ↓ Chest-wall compliance |
Bronchopneumonia Tracheal stenosis Restricted chest-wall expansion |
| Renal | ↓ GFR caused by:
Tubular dysfunction |
↑ Synthetic function caused by
Dysfunction caused by sepsis or drug interaction
Thrombocytopenia
↑ Fibrin split products
Coagulopathies
Coagulopathies
Transfusion reactions
Transfusion-related infection
Seizures
↑ ICP
Seizures
ICU disorientation
↑ CO2 production
↓ Ionized calcium
Altered protein binding
Altered pharmacokinetics
Altered pharmacodynamics
Enzyme induction
Altered receptor function
Drug interactions
TNFα, Tumor necrosis factor-α; GFR, glomerular filtration rate; ICU, intensive care unit.
Modified from Szyfelbein SK, et al.: Burn injuries. In Coté CJ, et al., editors: A practice of anesthesia for infants and children, ed 2, Philadelphia, 1993, Saunders.
The release of tumor necrosis factor-α (TNFα) from cardiac myocytes has been demonstrated to contribute to progressive cardiac contractile dysfunction in several models of trauma, thermal injury, and sepsis (Horton et al., 2004; Niederbichler et al., 2006). TNFα is a well-recognized inflammatory mediator that normally modulates the antimicrobial and metabolic response to tissue injury. In the context of burn trauma, this mediator recruits additional neurohumoral agents that exacerbate organ injury and initiate cellular apoptosis. Interleukins (IL-1β, IL-6, and others) also possess negative inotropic effects and may act synergistically with TNFα to perpetuate postburn myocardial cardiac depression (Maass et al., 2002a, 2002b). Nuclear factor κβ (NFκβ) is a transcription agent involved in the regulation of many of the cytokines and chemokines that contribute to the progression of SIRS. Burn trauma appears to activate myocardial NFκß, which promotes the secretion of TNFα (Maass et al., 2002a). Prolonged hypotension that is resolved with volume replacement may result in reperfusion injury and the formation of oxidized free radicals and lipid peroxidation by-products. These agents exacerbate the tissue damage incurred during the low-flow state (Parihar et al., 2008). Free radical generation is accompanied by impaired antioxidant mechanisms and the up-regulation of inducible nitric oxide synthetase (iNOS). This cascade promotes peripheral vasodilation, enhanced NFκβ release, and production of additional reactive tissue injury mediators such as peroxynitrite (Horton, 2003). Leukotrienes, platelet activation factor, thromboxane A2, and complement are other factors that potentially modulate burn-induced inflammatory responses. Cellular oxidative stress is an important component of burn-mediated injury with a complex and extensive impact on organ function. Further studies are needed to fully describe the interactions of many neurohumoral mediators detected during the systemic burn response, and their appropriate manipulation may result in therapeutic benefits.
Burn shock is the clinical summation of intravascular fluid deficits and cellular ischemia that is initially characterized by decreased cardiac output, impaired contractility, and hypoperfusion of organ systems. Thermal injury also creates significant alterations in endothelial permeability, resulting in pronounced fluid translocation and generalized edema formation (Warden, 2002). Transcellular electrolyte shifts and the loss of circulating plasma proteins further impair tissue perfusion. Pediatric burn patients possess limited compensatory responses to systemic hypotension and vasoactive infusions (e.g., dopamine or norepinephrine) may be needed to supplement intravenous fluid administration to achieve adequate blood pressure and cardiac output. Resuscitation guidelines have evolved to become goal-oriented rather than formula-driven; current recommendations advocate periodic reassessment and adjustment of individual fluid requirements to restore organ perfusion and tissue oxygenation.
Survivors of the acute injury phase often develop a hyperdynamic circulation that is characterized by marked elevation of cardiac output and lowered peripheral vascular resistance. Hypertension has been observed with increased incidence in patients who have sustained more than 20% TBSA thermal injuries (Falkner et al., 1978). Elevations of plasma renin, aldosterone, and catecholamines have been noted. Untreated hypertension may manifest as seizures, encephalopathy, and ultimately, end-organ dysfunction in up to 7% of pediatric patients with severe burns (Popp et al., 1980). Although desirable, antihypertensive therapy may have adverse side effects in this setting and should be guided by clinically relevant measures of systemic perfusion.
Renal Abnormalities
Acute renal dysfunction occurs in the context of hypoperfusion, intravascular depletion, myoglobin-induced tubular damage, and mechanical obstruction secondary to cast formation by the products of hemolysis (Aikawa et al., 1990). Hyperglycemia is a function of the stress response and may add to intravascular volume depletion by promoting an osmotic diuresis. Efforts to maintain urine output within a recommended range of 1 to 1.5 mL/kg per hour should be confirmed with serial urimeter measurements that demonstrate ongoing evidence of adequate fluid resuscitation. Tubular dysfunction in the form of altered responses to plasma renin and aldosterone may also contribute to renal derangements (Mariano et al., 2008).
Significant interpatient differences concerning the pharmacodynamics and pharmacokinetics of commonly used antibiotics have been reported (Weinbrem, 1999; Kiser et al., 2006; Conil et al., 2007). Alterations of creatinine clearance and glomerular filtration rates impact drug-dosing schedules and effective plasma concentrations, necessitating customized dosing regimens guided by serum plasma levels (Boucher et al., 1992). Antibiotics with nephrotoxic properties are likely to exacerbate renal dysfunction.
Perturbations of extracellular water and macromolecules affect electrolyte composition, and rapid changes of serum sodium, potassium, and calcium concentrations are associated with increased morbidity; serial monitoring and correction of these alterations is an essential component of acute burn management. Elevated levels of stress hormones (e.g., aldosterone, angiotensin, catecholamines, and plasma renin) also contribute to renal dysfunction observed in the early postburn period. Patients with thermal injuries release elevated levels of atrial natriuretic peptide (ANP), which may mitigate the adverse effects of low-flow states by improving renal blood flow and urine output (Onuoha et al., 2000). Animal studies have shown intrarenal redistribution of blood flow with the preservation of inner cortical perfusion. Reductions in urinary chloride and sodium concentration reflect the increased perfusion of the juxtaglomerular nephrons that possess significant salt-retention capacity (Carter and Well, 1975).
Hepatic Abnormalities
Burn-related hepatic dysfunction is a significant contributor to morbidity and mortality. Hepatic blood flow is compromised in the early burn phase secondary to blood loss, large-volume fluid shifts, and compensatory vasoconstriction. The extent of hepatocellular derangement is also influenced by the onset of sepsis, toxic drug interactions, and the potential infectious risks of multiple blood product transfusions. Liver dysfunction is likely modulated by cytokines IL-1β and TNFα, both of which are potent inflammatory mediators (Jeschke et al., 1999). Elevations of serum transaminases, reduced synthesis of constitutive proteins (e.g., albumin, transferrin, and retinol-binding protein), and focal hypertrophy are all clinical markers of evolving hepatic dysfunction (Jeschke et al., 2007a).
Restoration of hepatic blood flow precedes an extended phase of hypermetabolism and initiates a catabolic cascade with adverse systemic effects. Acute phase protein production occurs at the expense of albumin synthesis, and subsequent reductions of the protein binding capacity result in unpredictable drug plasma levels. The acute-phase process may continue for months, prolonging systemic organ dysfunction and delaying efforts to restore lean body mass (Jeschke et al., 2004). The duration and severity of hepatic dysfunction are variable and ultimately dependent on the ability to reconvert the patient’s hepatic function to an anabolic state; this process requires successful wound closure, infection control, and adequate nutritional support.
Metabolic and Gastrointestinal Abnormalities
The severity of burn trauma correlates directly with the anticipated degree of inflammatory and hypermetabolic responses, and these physiologic derangements are associated with increased mortality for those patients who have experienced more than 60% TBSA burn (Jeschke et al., 2007b). Endogenous catecholamines and other stress hormones (e.g., glucagon and glucocorticoids) act synergistically to produce metabolic changes that include a hyperdynamic circulation, sharply elevated basal energy expenditure, and the catabolism of skeletal muscle proteins. Additional clinical findings include refractory tachycardia, increased cardiac output and oxygen consumption, hyperpyrexia, and increased carbon dioxide production (Tredget and Yu, 1992).
The prolonged catabolic phase of thermal injury is also marked by accelerated gluconeogenesis, glucose intolerance, lipolysis, glycogen mobilization, and insulin resistance. Increased amino-acid oxidation causes urea formation and nitrogen loss. These processes manifest as delayed wound healing, muscle wasting, and long-term rehabilitative failure if they are not adequately treated. Recent experimental efforts have examined the pharmacologic attenuation of burn-related metabolic disturbances with the administration of exogenous β-adrenergic blockade, human growth hormone, insulin, synthetic testosterone analogues, and nutrient-specific enteral formulae to restore systemic anabolic balance (Herndon and Tompkins, 2004; Pereira and Herndon, 2005; Mayes et al., 2008; Jeschke et al., 2008).
Gastroduodenal ulceration is a common complication of extensive burns that can be prevented with the early administration of proton-pump inhibitors, H2-receptor antagonists, and neutralizing agents such as sulcrafate. Acid prophylaxis is an important adjunctive therapy, because thermal injury is strongly associated with disruptions of the mucosal intestinal barrier. Severe burns induce enlargement of the mucosal gap junctions and are followed by bacterial translocation and systemic absorption of endotoxins; these two factors contribute to an increased risk of sepsis (Ziegler et al., 1988). Enteral feeding is superior to intravenous caloric administration as a means of preserving gastrointestinal motility and mucosal barrier integrity and reducing the incidence of enterogenic infection (Chen et al., 2007).
Hematologic Abnormalities
The immediate postburn period features a significant contraction of red blood cell mass secondary to destruction by heat-induced denaturation, lysis by circulating free radicals, and sequestration in the extravascular space. Hemoglobinuria (particularly after electrical injury) and the accumulation of cellular debris promote cast formation and impair renal function. Normal erythrocyte deformability may also be affected with a subsequent decrease in functional half-life and an increased risk of thrombosis (Schachar et al., 1974). During the acute phase, normal or supranormal hemoglobin measurements may represent hemoconcentration—an indirect indicator of inadequate resuscitation. After restoration of intravascular volume, anemia can persist despite elevated levels of erythropoietin; persistent bone marrow suppression is likely mediated by circulating inflammatory factors (Jelkmann et al., 1990). Repetitive excisional procedures, gastroduodenal ulceration, and phlebotomy are other important factors to consider during assessment of ongoing blood loss. Because of the limited circulating blood volumes of infants and small children, these may become disproportionately significant sources of anemia.
Platelet abnormalities initially manifest as thrombocytopenia secondary to platelet aggregation, microemboli consumption, and dilution secondary to large-volume fluid administration. A biphasic pattern is seen when thrombocytosis occurs 10 to 14 days postburn and continues for several months (Heideman, 1979). Qualitative platelet function may be altered after thermal injury, and clinical evidence of coagulopathy should be treated with platelet transfusions. Persistent thrombocytopenia in the immediate postburn period is associated with sepsis and is considered to be a poor prognostic indicator.
Elevations of factors V, VII, VIII, fibrinogen, and fibrinogen split products normally occur with burn trauma and indicate thrombotic and fibrinolytic pathway activity. Despite increased factor levels that may continue for months, there does not seem to be a higher incidence of hypercoagulable states (Simon et al., 1977). The response to extensive burn trauma may include disseminated intravascular coagulation (DIC); the clinical diagnosis is supported by thrombocytopenia, decreased fibrinogen, prothrombin time (PT)/partial thromboblastin time (PTT) prolongation, and elevated levels of fibrin split products. Underlying etiologies (e.g., sepsis, hypoxemia, and burn shock) need to be identified and aggressively treated using blood product transfusions as supportive therapy.
Neurologic Injury
Varying degrees of neurologic dysfunction may occur through multiple mechanisms, and the evaluation of these deficits remains particularly challenging in infants and young children. Direct nerve trauma from crush or electrical injuries and compartment syndromes often appear as peripheral neuropathies and profound sensorimotor loss. Neurologic dysfunction may also arise from the acute or delayed toxic effects of hypoxia, metabolic derangements, and potential drug interactions. Burn encephalopathy is a complex syndrome characterized by a variable course of delirium, hallucinations, seizure activity, coma, and behavioral changes that afflict as many as 1 in 7 pediatric burn patients (Antoon et al., 1972). One third of all pediatric burn patients are predisposed to acute stress disorders, and the incidence increases with burn severity (Stoddard et al., 2006). Additional neuropsychiatric disturbances may arise from the complex interactions of stress, anxiety, anticipated procedural pain, and generalized sleep deprivation that often occurs in the intensive care environment but remains underappreciated.
Children with severe burn injuries often become obtunded in the first hours after trauma as a result of rapid fluid shifts, side effects of analgesia, and stress-related exhaustion. CO intoxication must always be suspected and promptly treated; diagnostic delays may manifest as persistent neurologic deficits in the survivors (Meert et al., 1998). Occult brain trauma should be investigated with computerized axial tomography when the elicited patient history supports a mechanism of injury.
Increased intracranial pressure from cerebral edema may occur within 72 hours postinjury (Gueugniaud et al., 1997). Patients requiring massive volume resuscitation may be subject to electrolyte shifts that potentiate the risk of cerebral edema and subsequent seizure activity. Aggressive correction of iatrogenic hyponatremia results in central pontine demyelination (Cohen et al., 1991). Serial monitoring of electrolyte concentrations and correcting imbalances help avert undesirable consequences.
In addition to direct nerve trauma, metabolic derangements, and tissue hypoxia, peripheral neuropathies may evolve secondary to constrictive dressings complicated by progressive edema (Dagum et al., 1993). Careful attention to patient positioning and periodic documentation of peripheral pulses by Doppler ultrasound helps detect vascular compromise.
The pediatric burn patient is exposed to varying degrees of polypharmacy during efforts to achieve clinically adequate sedation and analgesia, often through the use of supranormal doses of benzodiazepines, narcotics, and other drugs for extended periods. Burn-related derangements of hepatic and renal function alter the normal pharmacodynamics and kinetics of these agents and contribute to unpredictable plasma concentrations. The clinician should be aware that these agents may be associated with transient or sustained neurocognitive impairments observed in some pediatric burn patients. Several animal studies have identified the potential for premature apoptotic neurodegeneration with the use of drugs that interact with N-methyl-D-aspartate (NMDA) and γ-aminobutyric acid (GABA) receptors (Jevtovic-Todorovic et al., 2003; Young et al., 2005). Many of the anesthetic agents expected to be used throughout the conduct of pediatric burn procedures (e.g., midazolam and ketamine) act at one or both receptor sites and are implicated as potential sources for neuroapoptosis; there are data suggesting that some combinations of these agents may possess greater neurotoxic potency. No information is currently available to reliably identify the least harmful pharmacologic choice (Mellon et al., 2007) (see Chapter 7, Pharmacology).
Immunosuppression
The child with major burn trauma suffers from epithelial barrier loss and a dynamic proinflammatory cascade that creates a grossly immunocompromised state. Absence of the normal dermal functions is further complicated by the infectious potential of invasive monitoring devices and passive exposure to drug-resistant organisms (Weber et al., 1997). Bacterial translocation secondary to gastrointestinal-permeability changes increases the incidence of sepsis. Common inflammatory mediators have been demonstrated to impair neutrophil chemotaxis and cytotoxic activity (Church et al., 2006). Strict observance of sterile technique, early burn wound excision, restoration of dermal barriers, and targeted antibiotic administration are all important components to limit infectious complications.
Pharmacologic alterations
Pediatric burn patients experience a spectrum of metabolic and organ-system derangements, with implications for the pharmacodynamics and kinetics of commonly-used anesthetic drugs. Significant interpatient variability observed with drug uptake and elimination necessitates the titration of all prescribed medications toward a desirable clinical endpoint, and each individual should be frequently reassessed for developing side effects. The more extensive the burn injury, the more pronounced this phenomenon (Martyn, 1986).
Intravascular depletion, transcompartmental fluid shifts, and blood-flow deficits in organs are factors that alter the distribution volume and clearance mechanisms of the drugs used throughout the acute burn admission. Major organ perfusion is preserved at the expense of blood flow to the gastrointestinal tract and extremity muscle groups and thereby renders oral and intramuscular administration routes of uptake unreliable. Organ blood flow improves with the onset of the hypermetabolic phase, but it is not clear if there is a concurrent increase in the rate of drug metabolism (Wilmore et al., 1980; Aulick et al., 1981). Hepatic protein synthesis shifts from albumin to α1-acid glycoprotein during the hypermetabolic phase. Albumin binds acidic drugs (e.g., benzodiazepines), and a reduction of circulating protein levels increases the pharmacologically active fraction. Conversely, α1-acid glycoprotein provides competitive binding sites for basic drugs (e.g., muscle relaxants) and reduces muscle bioavailability (Martyn et al., 1984).
Neuromuscular Relaxants
Succinylcholine is normally an appropriate drug selection to facilitate rapid sequence inductions and establish a definitive airway; however, in the context of the acutely burned patient, the risk of a severe hyperkalemic response must be carefully considered (Tolmie et al., 1967; Gronert and Theye, 1975). Excessive succinylcholine-induced potassium efflux may be observed as early as 12 hours postinjury and can persist for up to 2 years (Gronert, 1999). It has been posited that extensive proliferation of acetylcholine receptors and multiple isoforms occur throughout the muscle membrane, even at sites distant from the original injury (Martyn et al., 2006). As large masses of extrajunctional tissues are depolarized, serum potassium levels rapidly increase to induce lethal cardiac arrhythmias. Some controversy remains concerning the onset and duration of receptor proliferation with the subsequent risk of hyperkalemic responses, but the availability of rapid-acting nondepolarizing alternatives (e.g., rocuronium) obviate the need for succinylcholine in acute burn management.
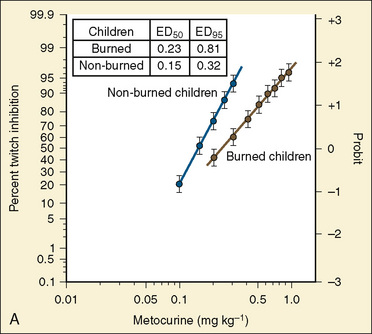
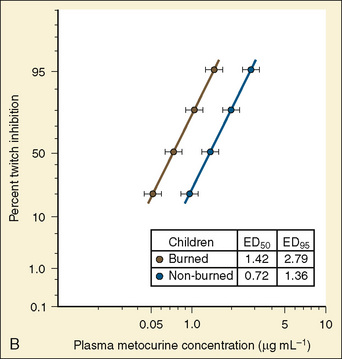
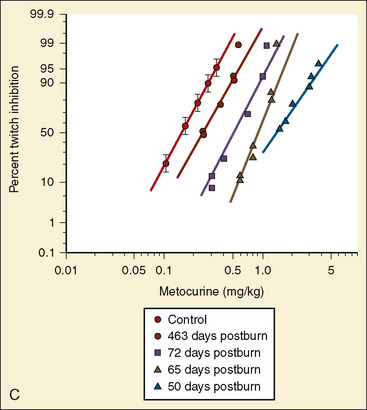
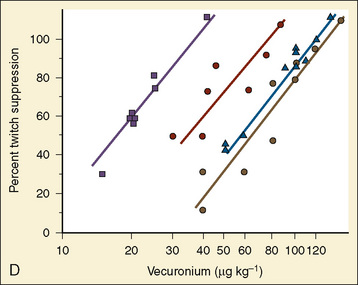
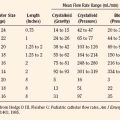
Resistance to a variety of nondepolarizing muscle relaxants (NDMRs) has been previously reported (Martyn et al., 1983a, 1983b; Mills and Martyn, 1989). Although there are notable distinctions concerning the pharmacokinetics and protein-binding capacities between patient cohorts with and without burns, these factors probably do not contribute significantly to observed clinical differences. Increases in receptor density are believed to be the predominant mechanism attributable to developing NDMR tolerance, and this effect correlates with burn severity (Martyn et al., 1982). Studies of vecuronium and rocuronium in adults with thermal injuries demonstrated the clinical effects of this resistance phenomenon; longer onset times and shorter recovery profiles were observed and attributed to decreased drug saturation of an enlarged receptor population (Fig. 31-6) (Mills and Martyn, 1989; Han et al., 2004). Burn patients generally require larger doses of NDMRs to produce clinically relevant responses for airway manipulation and surgical procedures (Table 31-5). The serum half-lives of the short- and intermediate-acting relaxants do not appear to be prolonged, and appropriate reversal of neuromuscular blockade is successful using the normal dosing ranges of neostigmine and glycopyrrolate (Martyn et al., 2006).
TABLE 31-5 Emergency-Department Values of Vecuronium in Burn Patients and Control Patients
| Study Group | ||
|---|---|---|
| (% TBSA Burn) | ED50 (mcg/kg) | ED95 (mcg/kg) |
| Controls | 17.6 (15.4-19.9)† | 35.3 (30.6-42.3) |
| <40 | 34.0* (29.3-38.7)‡ | 68.2* (59.8-79.9) |
| 40-60 | 55.4* (48.1-62.1) | 111.1* (99.8-126.2) |
| >60 | 64.5* (57.4-71.9) | 129.4* (113.0-154.0) |
TBSA, Total body surface area; ED50 and ED95, effective doses for 50% and 95% twitch suppression.
* Significantly different from controls, p < 0.01.
† Numbers in parentheses represent lower and upper 95% confidence limits.
‡ ED50 and ED95 for vecuronium are significantly increased in burned children compared with controls; the shift in the dose-response curve is related to the magnitude of burn.
From Mills AK, Martyn JA: Neuromuscular blockade with vecuronium in paediatric patients with burn injury, Br J Clin Pharm 28:155, 1989.
Analgesics
Effective pain management for pediatric burn patients is highly desirable but difficult to achieve consistently because of the varying intensities of pain associated with recurrent débridement, dressing changes, and physical therapy occurring throughout treatment. Previously, insufficient analgesic administration was often because of unsupported but lingering reservations concerning the risk of addiction to potent narcotics (Perry and Heidrich, 1982). Additional confounders included patient- or family-held misconceptions concerning dependence and associated social stigma, clinicians’ fear of potential litigation, and individual variations of nociception attributable to cultural differences. Frequent assessment with age-appropriate rating systems, education, and candid discussions about reasonable expectations for pain relief have largely eliminated opiate underuse.
Morphine sulfate is widely used, and although its metabolism to active glucuronidated metabolites suggests a prolonged duration of action, this has not been substantiated (Furman et al., 1990; Perreault et al., 2001). Fentanyl is another common choice, and kinetics studies in adults have demonstrated increased distribution volumes and clearance; these changes have partially explained observed increases in opiate requirements (Han et al., 2007; Kaneda and Han, 2009). Agonist-antagonist drugs (e.g., nalbuphine) theoretically offer the advantage of analgesia without significant respiratory depression; when administered to adult patients for limited debridement procedures, results have compared favorably with morphine (Lee et al., 1989). Methadone has been used for patients who were unresponsive to other analgesics, but its duration of action may be variable in the context of burn-related drug kinetics (Concilus et al., 1989). Methadone also possesses weak NMDA antagonism and as such may be an appropriate drug selection to minimize opioid tolerance and pathologic hyperalgesia (the paradoxical sensitization to painful stimuli) (Callahan et al., 2004). With its ultrashort half-life (7 to 10 minutes), remifentanil has no practical utility in the burn unit.
Tolerance develops in pediatric burn patients, but this fact should not preclude opioid administration. Addiction and the subsequent abuse of narcotics are rare when opiates are prescribed to achieve clinically appropriate endpoints (Porter and Jick, 1980; Goodman and McGrath, 1991). The supranormal dosing ranges commonly needed to provide effective analgesia in burn patients are the consequence of thermal-injury–induced quantitative and qualitative changes of receptor populations. After skin wound closure, opioid requirements rapidly decline. Undesirable withdrawal symptoms can be avoided by prescribing a gradual taper that parallels the decreasing frequency of procedures conducted during the acute injury phase (see Chapter 15, Pain Management).
Although opioid addiction has not been observed with regularity among the pediatric burn population, some animal studies cite potential adverse effects from exposure to high-dose narcotic regimens. Hyperalgesia has been observed in burn patients who have been the recipients of opiate-dose escalation, and this condition is thought to be secondary to neuroplastic changes in nociception pathways (Chu et al., 2008). Modulations of spinal receptors may produce hyperalgesic conditions secondary to inhibitory neuron apoptosis (Angst and Clark, 2006). Tolerance and opioid-induced hyperalgesia are clinically similar (both show evidence of decreased analgesic efficacy) but the molecular mechanisms are distinct. Other studies have examined opioids and their contribution to postburn immunosuppression, possibly through cytokine modulation (Alexander et al., 2005; Schwacha et al., 2006).
Anxiolytics
Adequate sedation improves acceptance of prolonged mechanical ventilation, decreases narcotic requirements, and reduces anxiety associated with dressing changes or other bedside procedures. Benzodiazepine infusions are administered concurrently with opioids, and patient requirements are elevated in the presence of developing tolerance and altered pharmacokinetics. Daily background infusions up to 0.1 mg/kg per hour are typical for severely burned patients and may be titrated to desired clinical effect throughout the weaning period (Sheridan et al., 2001). Clinicians should be aware of potential side effects; in one pediatric study, neurocognitive and behavioral changes attributed to midazolam fully resolved with drug cessation (Sheridan et al., 1994).
Dexmedetomidine is an α2-agonist with analgesic, anxiolytic, and sedative properties that has been used successfully as a supplement for mechanically ventilated children with opiate and benzodiazepine resistance (Walker et al., 2006). It is insufficient for primary analgesia but offers minimal respiratory depression and reasonable hemodynamic stability. In contrast to other sedative agents, treatment with dexmedetomidine mimics a physiologic sleep state that may attenuate sleep-related disorders observed in the intensive-care environment. Hypotension and bradycardia are potential side-effects of dexmedetomidine, presumably mediated through unopposed vagal stimulation (Carroll et al., 2008).
Patients treated with protracted opioid and benzodiazepine infusions are required to metabolize several half-lives worth of drug. In this setting, it is often difficult to balance the need for clinically effective sedation with the desire for the prompt return of protective airway reflexes. Short-term infusions of propofol have been used in the hours preceding extubation, so that opiate and benzodiazepine administration may be adequately weaned (Sheridan et al., 2003). Extended infusions of propofol are not suitable for pediatric burn patients, because there is an ill-defined risk of refractory acidosis and myocardial depression specific to the lipid-based emulsion (Parke et al., 1992; Vasile et al., 2003). Additionally, bacterial contamination of a centrally-infused sedative would be poorly tolerated in the setting of postburn immunosuppression (see Chapter 7, Pharmacology).
Ketamine
Some animal models that studied thermal injury have demonstrated an increase in NMDA receptors, which suggests that ketamine (a noncompetitive antagonist) may be a more effective analgesic than opioids for burn patients. Ketamine may also prove useful in reducing the incidence of opioid-induced hyperalgesia. Its analgesic potency is enhanced by several metabolites, the most active of which is norketamine (which possesses one third of the potency of ketamine). Used in combination with benzodiazepines, its half-life increases 20% to 30% (Reich and Silvay, 1980).
Ketamine produces a dose-dependent shift of the carbon dioxide response curve but unlike other hypnotic agents, does not alter the slope; hypercarbic respiratory drive remains intact at clinically relevant doses (Bourke et al., 1987). Preservation of spontaneous respiratory drive is complemented with a degree of hemodynamic stability secondary to centrally mediated sympathetic stimulation. However, in the hypovolemic burn patient who has exhausted the compensatory endogenous catecholamines, ketamine acts as a direct myocardial depressant (Owens et al., 2006). Dosing should be adjusted to avoid hemodynamic collapse in these patients or a vasoactive infusion (e.g., norepinephrine) can be used to provide a background level of sympathomimetic support.
Because concurrent benzodiazepine administration is so prevalent in burn patients, the unpleasant psychomimetic residua attributed to ketamine have not been clinically significant. Ketamine has been used as an adjunct for other drugs, and multiple delivery routes make it particularly suitable for those burn patients with behavioral difficulties or developmental delay (Allison and Smith, 1998; Heinrich et al., 2004; Tosun et al., 2008).
Volatile Agents
All of the common volatile agents have been successfully used for anesthetic maintenance in pediatric burn patients. Halothane is inexpensive and possesses some residual anesthetic effect, whereas sevoflurane promotes more rapid awakening and return of protective airway reflexes. Sevoflurane is an appropriate choice for inhalation induction, but its rapid taper may be associated with an increased incidence of emergence delirium (Kain et al., 2005). Patients with acute injuries and decompensated burn shock generally do not tolerate the vasodilation associated with potent volatile agents; concurrent vasoactive support may be needed. Pediatric burn patients with residual pulmonary injury and elevated metabolism demonstrate variable degrees of dead-space ventilation and elevated cardiac output that may alter the speed of inhalation inductions.
Considerations for initial management
Airway
Supraglottic edema has the potential to evolve rapidly and severely reduce airway patency. Aspiration of scalding liquids or caustic agents may have a presentation similar to that of epiglottitis (Sheridan, 1996). A focused history should identify factors supporting possible inhalational injury; prolonged extrication from a confined space is consistent with increased risk (Box 31-2). Preexisting pulmonary disease and prior intubation attempts in the field by paramedic personnel should also be reviewed.
Fiberoptic bronchoscopy has been used to support the diagnosis of inhalation injury but should not supersede clinical judgment (Hunt et al., 1975). Arterial blood gas values and chest radiographs are often normal with initial presentation and may therefore provide false reassurance of patient stability. The absence of overt thermal injury does not preclude significant CO poisoning and 100% fraction of inspired oxygen (Fio2) should be empirically applied. Although it is not always possible to avoid the “lost airway scenario,” the clinician should be aware that the American Society of Anesthesiologists’ (ASA) difficult-airway algorithm has practical limitations when applied to pediatric burn patients. Fiberoptic intubation performed when the patient is awake is rarely feasible for injured children who are not profoundly sedated, and the presence of oropharyngeal debris or edema reduces the likelihood of success. See related video online at www.expertconsult.com. Small ketamine boluses titrated to maintain spontaneous ventilation provide a reasonable margin of safety over the protocolized administration of rapid-sequence hypnotics and muscle relaxants. Ketamine is thought to leave protective airway reflexes intact; however, this may not be the case depending on the level of sedation (Drummand, 1996; Peña and Krauss, 1999).
Small ketamine boluses titrated to maintain spontaneous ventilation provide a reasonable margin of safety over the protocolized administration of rapid-sequence hypnotics and muscle relaxants. Ketamine is thought to leave protective airway reflexes intact; however, this may not be the case depending on the level of sedation (Drummand, 1996; Peña and Krauss, 1999).
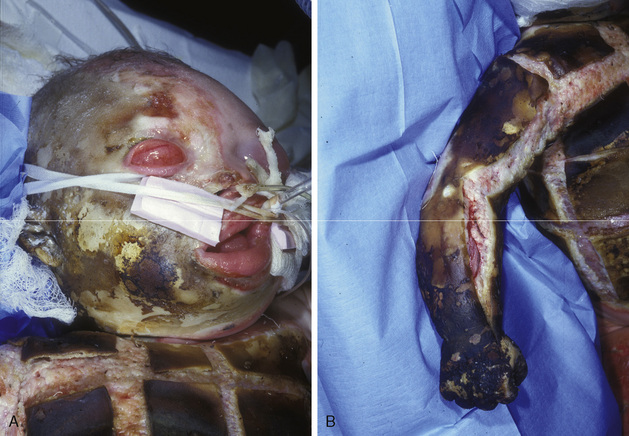
A semielective surgical airway created early may be considered for facial burns, because ensuing edema alters anatomy and renders endotracheal tube stabilization difficult at best (Fig. 31-7) (Palmieri et al., 2002). Perceived advantages of early tracheostomy in the setting of prolonged mechanical ventilation remain controversial (Saffle et al., 2002). Laryngeal mask airways have been used as a bridging technique to definitive airway control, but direct injuries to the oral cavity and unpredictable swelling may prevent an adequate seal and preclude delivery of sufficient tidal volumes. Progressive edema distorts facial and neck circumference, leading to inadvertent malposition or extubation; the endotracheal tube must be thoroughly stabilized (often using nonconventional methods such as dental anchoring with sutures or wire). Potentially catastrophic dislodgement may be minimized with frequent reassessments and secondary confirmation by chest radiographs.
Volume Resuscitation
Adequate intravenous access is problematic with extensive tissue destruction, and patients often require central venous catheters (femoral or subclavian) for extended periods. Intraosseous routes offer a safe and reliable alternative in the presence of severe injury and are suitable for large-volume infusions (Horton and Beamer, 2008) (see Chapter 30, Anesthesia for the Pediatric Trauma Patient). Bladder catheterization is performed to accurately quantitate urine output and continuously evaluate the efficacy of ongoing resuscitation.
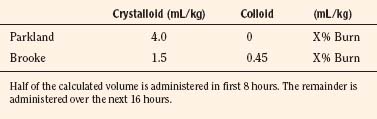
Treatment of burn shock initially depends on weight-based calculations for crystalloid administration. There are several formulas in use, but none is ideal or suitable for every patient and should only provide guidelines; actual fluid volumes need to be based on the patient’s clinical response (Warden, 2002). The Parkland formula recommends 4mL/kg per percentage of burn over 24 hours, with half of the calculated volume delivered during the first 8 hours of treatment, followed by the projected balance over the remaining 16 hours. The Brooke formula uses a combination of crystalloid and colloid delivered over the same time period (Table 31-6). However, a recent adult study demonstrated that volume requirements based on accepted values of minimal hourly urine output were higher than those predicted by the either formula (Blumetti et al., 2008). The formulas are inadequate when calculating resuscitation requirements for children who weigh less than 10 kg. A suggested guideline for these patients is to administer the sum of normal hourly maintenance fluids and the volumes derived from the Parkland or Brooke formula over a 24-hour period (Graves et al., 1988).
Capillary permeability increases in patients with burns more than 20% TBSA, and the degree of change correlates with burn severity. Some centers advocate the use of colloids as the primary fluid choice during early resuscitation, because colloids offer improved intravascular retention and may decrease the total volume requirements while minimizing tissue edema (Sheridan, 2002). There has been no definitive demonstration of improved outcomes when isotonic crystalloid- and colloid-resuscitation regimens have been compared (Bowser-Wallace and Caldwell, 1986). Hypertonic saline has been proposed as an alternative choice for resuscitation fluids, but the outcomes data are unclear and there is a potential risk of iatrogenic hypernatremia (Huang et al., 1995; Junger et al., 1997). Normal vascular integrity is usually restored within 24 to 36 hours if resuscitation efforts are adequate.
Achieving clinometric targets consistent with normal organ perfusion (goal-oriented resuscitation) has prove superior to the fulfillment of rote volume algorithms, and this principle has been an important advance of modern burn care (Box 31-3). Urine output (1 to 1.5 mL/kg per hour) has been cited as an indicator of systemic normoperfusion, and the maintenance of target outputs often requires intravenous volumes that exceed the initial formulaic predictions (Yowler and Fratianne, 2000; Hoskins et al., 2006). Closed-loop and decision-assist algorithms have been introduced to manage infusion rates directed by desired levels of urinary flow and have the potential to reduce total volume requirements and minimize edema (Salinas et al., 2008). Despite these advances, there is growing controversy concerning the suitability of accepted invasive monitors and clinical endpoints as valid indicators of adequate cellular perfusion (Ahrns, 2004). Further research is needed to refine monitoring strategies and identify physiologic markers that accurately reflect oxygen derangements and perfusion deficits.
Nutritional Supplementation
Early enteral feeding via a nasoduodenal tube provides caloric support and helps maintain the integrity of the intestinal mucosal barrier that restricts bacterial translocation (McDonald et al., 1991). These devices offer the opportunity for continuous feeding throughout surgical procedures without the risk of aspiration. Patients with extensive burns or inadequate volume replacement may not tolerate these feedings because of splanchnic hypoperfusion (Jenkins et al., 1994). Poor caloric replacement results in delayed wound healing, an increased risk of sepsis, and resistance to ventilator weaning from catabolic loss of lean body mass. Modulation of hormonal, adrenergic, and inflammatory mediators continues to be the focus of therapeutic research (Ipaktchi and Arbabi, 2006).
Surgical Excision
The goal of current surgical techniques is to remove necrotic debris and achieve biological wound closure. The early reestablishment of a physical barrier, which provides protection from bacteria, mechanical trauma, and insensible water loss, improves long-term outcomes even for those patients who have experienced significant thermal injury. Variable mesh sizes may expand harvested autograft coverage, but staged procedures are to be expected in patients with more than 50% TBSA as suitable donor sites are rapidly depleted. Split-thickness cadaveric allograft provides physiologic protection similar to native skin (Sheridan and Tompkins, 1999). A number of dermal analogs have been manufactured and used clinically. Integra (Integra Life Sciences; Plainboro, New Jersey) is a bovine-derived acellular dressing that promotes fibroblast migration and growth. Alloderm (LifeCell Corporation; The Woodlands, Texas) is an acellular collagen matrix procured from screened cadaveric donors. These dermal substitutes restore protective barriers and provide a structural framework for the rapid revascularization and fibroblast repopulation of the recipient sites (Sheridan, 2002). As wound healing progresses, temporary biological dressings are resurfaced with autograft. Physiologic closure is most advantageous when performed within the first 24 hours of injury to circumvent wound colonization and reduce the systemic inflammatory response.
Excisional procedures have been refined so that blood loss is minimized through the use of extremity tourniquets, subcutaneous deposition of diluted epinephrine, and controlled operative times. These operations are still associated with hemorrhage in the range of 3% to 5% of estimated blood volume for each 1% TBSA excised (Budny et al., 1993; Housinger et al., 1993). Intraoperative fluid selection and replacement should be guided by oxygen delivery needs and serial lab analysis.
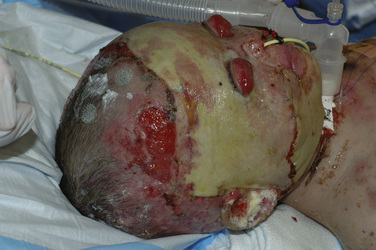
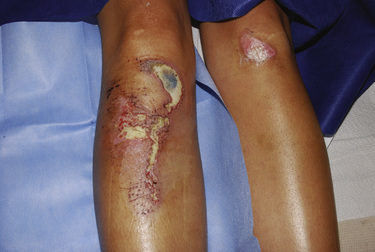
Circumferential burns are notable for the inelastic eschar that can quickly induce neurovascular compromise, possibly leading to limb loss. Patients with thoracic-wall involvement develop a restrictive form of respiratory insufficiency. Extensive burns involving the trunk may be complicated by abdominal compartment syndrome, which has symptoms of diminished venous return, cardiac output, and urinary flow (Jensen et al., 2006). Escharotomies decompress these insults to restore perfusion (Fig. 31-8).
Perioperative considerations
Induction and Airway Management
Mechanical trauma and edema may sufficiently alter facial features to impair mask fit and assisted ventilation. Tight eschar restricts mandibular mobility and mouth opening despite effective muscle relaxation. Mask-seal maintenance may require the participation of a second anesthesia provider. In general, difficult airways should be anticipated for the first 24 to 48 hours, with alternative techniques and necessary equipment immediately available. The surgeon should be physically present to perform an emergent tracheostomy in the event of an unrecoverable airway. In rare circumstances, the use of extracorporeal membrane oxygenation has been used as the initial technique to maintain oxygenation in a patient who had surgery for delayed closure with gross neck deformity that was judged sufficient to preclude tracheostomy rescue (Sheridan et al., 2008). Primary tracheostomy might be considered in the presence of gross deformities of the face, neck or head, but there are risks of long-term sequelae (Palmieri et al., 2002).
Fiberoptic-assisted endotracheal intubation is the preferred method for securing a potentially difficult pediatric airway. Appropriate sedation and maintenance of spontaneous respirations may be accomplished by the judicious use of low-dose ketamine or dexmedetomidine infusion. Manual distraction of the tongue, jaw thrust, and antisialogue therapy improve conditions. The success of additional methods such as retrograde wire, light wand, and GlideScope video laryngoscopy (Verathon; Bothell, Washington) have been reported (Hsu et al., 2007, 2008). Some practitioners have advocated the use of laryngeal mask airways to avoid the potential trauma of repetitive intubations, but this method is impractical if an adequate seal cannot be maintained secondary to anatomic distortion (McCall et al., 1999). Laryngeal mask airways may also be used as an assist-device to facilitate fiberoptic intubation as previously described (see Chapter 12, Airway Management). All airway devices possess positive qualities and disadvantages, and the specific use of any technique should be guided by the anticipated needs of the patient and the clinician’s experience.
Hemostasis
Tangential excisions are invariably associated with hemorrhage, although the amount of blood loss during the procedure is difficult to quantitate, because blood is distributed among the drapes, table, and floor. Excisions of the face, neck, and head involve greater losses attributable to the increased vascularity of these areas. One study offered guidelines suggesting that 3% to 5% of estimated blood volume (EBV) will be lost for every 1% TBSA excised; increasing the precision of anticipated blood-volume loss may improve the efficiency of product ordering (Housinger et al., 1993). Where significant volume depletion is expected, transfusion therapy should begin preoperatively to maintain adequate oxygen delivery. Despite the known inaccuracies involved with blood-loss estimates, some practical intraoperative recommendations have been forwarded; the surgical time should be limited to no more than 2 hours, with the planned excision restricted to 15% TBSA. The procedure should be aborted if blood loss exceeds 50% EBV (Engrav et al., 1983).
Multiple blood conservation techniques have been described and include the injection of a saline solution mixed with diluted epinephrine, epinephrine-soaked compressive wraps, topical sealants, extremity tourniquets, and preoperative acute normovolemic hemodilution (Gomez et al., 2001; Losee et al., 2005). The tumescent technique uses a solution (consisting of isotonic crystalloid with 1:100,000 epinephrine) that is subdermally injected to facilitate eschar débridement and donor-site harvest by creating a smooth, tense skin surface (Robertson et al., 2001). Blood loss from freshly debrided areas can be further reduced by the liberal application of fibrin sealants or thrombin sprays that promote hemostasis and graft adherence (Nervi et al., 2001; Foster, 2007). Few adverse affects have been noted.
Postoperative Pain Management
Significant pain is to be expected, and opiate and benzodiazepine infusions should be continued in the immediate postoperative period, with additional bolus doses of narcotics titrated to clinical response. Developing tolerance and elevated baseline drug requirements often confound pain assessments but need not delay monitored dose escalation (Abdi and Zhou, 2002). Pain tends to be most intense from freshly harvested donor sites. The tumescent solution may be supplemented with bupivicaine (2 to 2.5 mg/kg) or lidocaine (5 to 7 mg/kg) for locoregional analgesia (Jellish et al., 1999; Bussolin et al., 2003). Serum measurements of local anesthetic absorption have demonstrated that this technique is safe and effective. Intravenous lidocaine infusion has also been used to improve analgesia and may possibly modulate nociceptive pathways in burn patients (Cassuto and Tarnow, 2003). However, the experience with local anesthetic infusion is limited to case reports and has yet to be validated in well-designed clinical trials (Wasiak and Cleland, 2007).
Considerations for other burn injuries
Electrical Injury
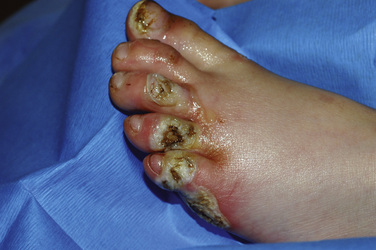
Low voltage events (those less than or equal to 500 volts) typically occur in the residential setting and are associated with full-thickness burns at the point of contact (most commonly the hands and oral cavity of young children). The voltage is insufficient to traverse large body segments, so that deep tissue damage is generally limited to the area demarcated by the contact burn (Fig. 31-9). These injuries can still present significant trauma and airway management challenges, such as when an unfortunate young child suffers bilateral oral commissure burns from biting into an electrical cord. New-onset cardiac conduction abnormalities have also been documented (Garcia et al., 1995). Low-voltage burns are treated using the same principles as previously described for the care of thermal injuries.
High-voltage events (of 1000 volts or more) are generally encountered in commercial or industrial environments and involve catastrophic neurovascular and skeletal muscle damage that follows the conductive path from its source to the ground. Several distinct forms of trauma occur with these events: plasma arcing and its associated flash create localized heat in excess of 2000° F that often ignites clothing; high-energy electrical transfer produces acute cardiac and neurologic injury; and loss of coordination and profound muscle spasm is associated with falls, potential fractures, or organ rupture. Energy that passes through the axis of the trunk and limbs affects muscle, nerves, and vasculature. Unlike the entrance and exit wounds that demarcate the path of current flow, these devastating internal injuries are not immediately apparent. Victims of high-voltage electrical exposure should therefore be evaluated as polytrauma patients because there can be such a variety of resulting systemic manifestations (Edlich et al., 2005).
Initial treatment is focused on airway support and the identification of cardiac conduction abnormalities. The risk of delayed and unpredictable cardiac arrhythmias increases when the presenting history supports transthoracic current propagation, tetany, loss of consciousness, and abnormal electrocardiography results from admission (Bailey et al., 2007). Early fasciotomy is often necessary to preserve the extremities, as myonecrosis causes severe edema with subsequent compartment syndrome. Myoglobin and hemoglobin are released in large quantities, and their precipitation as glomerular casts impairs renal function. Urine output should be maintained with fluids, sodium bicarbonate-induced alkalinization, and osmotic diuretics. Global and focal neurologic dysfunction resulting from electrical exposure and its associated trauma is variable and may continue to affect the patient throughout the rehabilitation course (Cherington, 2005).
Chemical Exposure
Caustic liquids continue to damage adjacent tissues until the offending agent is neutralized, and clinician safety may be compromised if these active residuals are not properly handled (Fig. 31-10). Before definitive treatment begins, decontamination requires the removal of the patient’s clothing and copious irrigation of affected surfaces with warm water until the efficacy of rinsing is confirmed with litmus paper applied to the wound (Sheridan, 2002). Consultation with a poison-control center is warranted if there is a potential risk of systemic toxicity from chemical absorption. Many caustic agents are potent respiratory irritants, and concurrent inhalation injury should be considered and treated appropriately.
Acknowledgments
For questions and answers on topics in this chapter, go to “Chapter Questions” at www.expertconsult.com.
Abdi S., Zhou Y. Management of pain after burn injury. Curr Opin Anaesthesiol. 2002;15:563-567.
Aggarwal S.J., Diller K.R., Blake G.K., et al. Burn-induced alterations in vasoactive function of the peripheral cutaneous microcirculation. J Burn Care Rehabil. 1994;15:1-12.
Aggarwal S.J., Diller K.R., Yeung H.K., et al. Local macromolecular extravasation in thermal burns quantified by fluorescent video microscopy and computer vision. J Burn Care Rehabil. 1994;15:104-120.
Ahrns K.S. Trends in burn resuscitation: shifting the focus from fluids to adequate endpoint monitoring, edema control and adjuvant therapies. Crit Care Nurs Clin North Am. 2004;16:75-98.
Aikawa N., Wakabayashi G., Ueda M., et al. Regulation of renal function in thermal injury. J Trauma. 1990;30:S174-S178.
Alexander M., Daniel T., Chaudry I.H., et al. Opiate analgesics contribute to the development of post-injury immunosuppression. J Surg Res. 2005;129:161-168.
Allison K.P., Smith G. Burn management in a patient with autism. Burns. 1998;24:484-486.
American Burn Association. National burn repository 2007 report dataset version 4.0. Available at http://www.ameriburn.org/resources_factsheet.php Accessed November 15, 2008
Angst M.S., Clark J.D. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570-587.
Ansermino M., Hemsley C. ABC of burns: intensive care management and control of infection. BMJ. 2004;329:220-223.
Antoon A.Y., Volpe J.J., Crawford J.D. Burn encephalopathy in children. Pediatrics. 1972;50:609-616.
Aulick L.H., Goodwin C.W., Becker R.A., et al. Visceral blood flow following thermal injury. Ann Surg. 1981;193:112-116.
Bailey B., Gaudreault P., Thivierge R.L. Cardiac monitoring of high-risk patients after an electrical injury: a prospective multicentre study. Emerg Med J. 2007;24:348-352.
Baker C.P., Russell W.J., Meyer W.3(rd), et al. Physical and psychologic rehabilitation outcomes for young adults burned as children. Arch Phys Med Rehabil. 2007;88:S57-S64.
Baker C.P., Rosenberg M., Mossberg K.A., et al. Relationships between the Quality of Life Questionnaire (QLQ and the SF-36): among young adults burned as children. Burns. 2008;34:1163-1168.
Barret J.P., Desai M.H., Herndon D.N. Effects of tracheostomies on infection and airway complications in pediatric burn patients. Burns. 2000;26:190-193.
Benjamin D., Herndon D.N. Special considerations of age: the pediatric burned patient. In: Herndon D.N., editor. Total Burn Care. ed 2. London: Saunders; 2002:427-438.
Blumetti J., Hunt J.L., Arnoldo B.D., et al. The Parkland formula under fire: is the criticism justified? J Burn Care Res. 2008;29:180-186.
Borron S.W., Baud F.J., Barriot P., et al. Prospective study of hydroxocobalamin for acute cyanide poisoning in smoke inhalation. Ann Emerg Med. 2007;49:794-801.
Boucher B.A., Kuhl D.A., Hickerson W.L. Pharmacokinetics of systemically administered antibiotics in patients with thermal injury. Clin Infect Dis. 1992;14:458-463.
Bourke D.L., Malit L.A., Smith T.C. Respiratory interactions of ketamine and morphine. Anesthesiology. 1987;66:153-156.
Bowser-Wallace B.H., Caldwell F.T. A prospective analysis of hypertonic lactated saline v. Ringer’s lactate-colloid for the resuscitation of severely burned children. Burns Incl Therm Inj. 1986;12:402-409.
Budny P.G., Regan P.J., Roberts A.H. The estimation of blood loss during burns surgery. Burns. 1993;19:134-137.
Bussolin L., Busoni P., Giorgi L., et al. Tumescent local anesthesia for surgical treatment of burns and postburn sequelae in pediatric patients. Anesthesiology. 2003;99:1371-1375.
Calhoun K.H., Deskin R.W., Garza C., et al. Long-term airway sequelae in a pediatric burn population. Laryngoscope. 1988;98:721-725.
Callahan R.J., Au J.D., Paul M., et al. Functional inhibition by methadone of N– methyl D-aspartate receptors expressed in Xenopus oocytes: stereospecific and subunit effects. Anesth Analg. 2004;98:653-659.
Carroll C.L., Kreiger D., Campbell M., et al. Use of dexmedetomidine for sedation of children hospitalized in the intensive care unit. J Hosp Med. 2008;3:142-147.
Carter J.G., Well C.H.2nd. Intrarenal redistribution of blood flow in the early postburn period. J Trauma. 1975;15:877-886.
Cassuto J., Tarnow P. Potent inhibition of burn pain without use of opiates. Burns. 2003;29:163-166.
Chen Z., Wang S., Yu B., et al. A comparison study between early enteral nutrition and parenteral nutrition in severe burn patients. Burns. 2007;33:708-712.
Cherington M. Spectrum of neurologic complications of lightning injuries. Neurorehabilitation. 2005;20:3-8.
Chou K.J., Fisher J.L., Silver E.J. Characteristics and outcome of children with carbon monoxide poisoning with and without smoke exposure referred for hyperbaric oxygen therapy. Pediatr Emerg Care. 2000;16:151-155.
Chu L.F., Angst M.S., Clark D. Opioid-induced hyperalgesia in humans: molecular mechanisms and clinical considerations. Clin J Pain. 2008;24:479-496.
Church D., Sameer E., Reid O., et al. Burn wound infections. Clin Micro Rev. 2006;19:403-434.
Cohen B.J., Jordan M.H., Chapin S.D., et al. Central pontine myelinolysis after correction of hyponatremia during burn resuscitation. J Burn Care Rehabil. 1991;12:153-156.
Coin C.E., Purdue G.F., Hunt J.L. Tracheostomy in the young pediatric burn patient. Arch Surg. 1998;133:539-540.
Concilus R., Denson D.D., Knarr D., et al. Continuous intravenous infusion of methadone for control of burn pain. J Burn Care Rehabil. 1989;10:406-409.
Cone D.C., MacMillan D., Parwani V., et al. Threats to life in residential structure fires. Prehosp Emerg Care. 2008;12:297-301.
Conil J.M., Georges B., Lavit M., et al. Pharmacokinetics of ceftazadime and cefepime in burn patients: the importance of age and creatinine clearance. Int J Clin Pharmaco Ther. 2007;45:529-538.
Cortiella J., Micak R., Herndon D. High frequency percussive ventilation in pediatric patients with inhalation injury. J Burn Care Rehabil. 1999;20:232-235.
Dagum A.B., Peters W.J., Neligan P.C., et al. Severe multiple mononeuropathy in patients with major thermal burns. J Burn Care Rehabil. 1993;14:440-445.
Demling R.H. Postgraduate course: respiratory injury: III. Pulmonary dysfunction in the burn patient. J Burn Care Rehabil. 1986;7:277-284.
Demling R.H. Smoke inhalation injury: an update. Eplasty. 2008;8:254-282.
Desai M.H., Micak R., Richardson J., et al. Reduction in mortality in pediatric patients with inhalation injury with aerosolized heparin/N-acetylcystine therapy. J Burn Care Rehabil. 1998;19:210-212.
Desai M.H., Mlcak R.P., Robinson E., et al. Does inhalation injury limit exercise endurance in children convalescing from thermal injury? J Burn Care Rehabil. 1993;14:12-16.
Dirnberger J., Giretzlehner M., Ruhmer M., et al. Modelling human burn injuries in a three-dimensional virtual environment. Stud Health Technol Inform. 2003;94:52-58.
Drummand G.B. Comparison of sedation with midazolam and ketamine: effects on airway muscle activity. Br J Anaesth. 1996;76:663-667.
Edelman D.A., White M.T., Tyburski J.G., et al. Factors affecting prognosis of inhalation injury. J Burn Care Res. 2006;27:848-853.
Edlich R.F., Farinholt H.M., Winters K.L., et al. Modern concepts of treatment and prevention of electrical burns. J Long Term Eff Med Implants. 2005;15:511-532.
Endorf F.W., Gamelli R.L. Inhalation injury, pulmonary perturbations and fluid resuscitation. J Burn Care Res. 2007;28:80-83.
Engrav L.H., Heimbach D.M., Reus J.L., et al. Early excision and grafting vs. non-operative treatment of burns of indeterminate depth: a randomized prospective study. J Trauma. 1983;23:1001-1004.
Falkner B., Roven S., DeClement F.A., et al. Hypertension in children with burns. J Trauma. 1978;18:213-217.
Fein A., Leff A., Hopewell P.C. Pathophysiology and management of the complications resulting from fire and the inhaled products of combustion: review of the literature. Crit Care Med. 1980;8:94-98.
Fidkowski C.W., Fuzaylov G., Sheridan R.L., et al. Inhalation burn injury in children. Paediatr Anaesth. 2009;19:202-211.
Fitzpatrick J.C., Cioffi W.G.Jr, Cheu H.W., et al. Predicting ventilation failure in children with inhalation injury. J Ped Surg. 1994;29:1122-1126.
Foster K. The use of fibrin sealant in burn operations. Surgery. 2007;142:S50-S54.
Furman W.R., Munster A.M., Cone E.J. Morphine pharmacokinetics during anesthesia and surgery in patients with burns. J Burn Care Rehabil. 1990;11:391-394.
Garcia C.T., Smith G.A., Cohen D.M., Fernandez K. Electrical injuries in a pediatric emergency department. Ann Emerg Med. 1995;26:604-608.
Geller R.J., Barthold C., Saiers J.A., et al. Pediatric cyanide poisoning: causes, manifestations, management, and unmet needs. Pediatrics. 2006;118:2146-2158.
Gilmer B., Kilkenny J., Tomaszewski C., et al. Hyperbaric oxygen does not prevent neurologic sequelae after carbon monoxide poisoning. Acad Emerg Med. 2002;9:1-8.
Ginsberg M.D. Carbon monoxide intoxication: clinical features, neuropathology and mechanisms of injury. J Toxicol Clin Toxicol. 1985;23:281-288.
Goldberg A.E., Malhotra A.K., Riaz O.J., et al. Predictive value of broncho-alveolar lavage fluid Gram’s stain in the diagnosis of ventilator-associated pneumonia: a prospective study. J Trauma. 2008;65:871-876.
Gomez M., Logsetty S., Fish J.S. Reduced blood loss during burn surgery. J Burn Care Rehabil. 2001;22:111-117.
Goodman J.E., McGrath P.J. The epidemiology of pain in children and adolescents: a review. Pain. 1991;46:247-264.
Gore D.C., Hawkins H.K., Chinkes D.L., et al. Assessment of adverse events in the demise of pediatric burn patients. J Trauma. 2007;63:814-818.
Gracia R., Shepard G. Cyanide poisoning and its treatment. Pharmacotherapy. 2004;24:1358-1365.
Graves T.A., Cioffi W.G., McManus W.F., et al. Fluid resuscitation of infants and children with massive thermal injury. J Trauma. 1988;28:1656-1659.
Gronert G.A., Theye R.A. Pathophysiology of hyperkalemia induced by succinylcholine. Anesthesiology. 1975;43:89-99.
Gronert G.A. Succinylcholine hyperkalemia after burns. Anesthesiology. 1999;91:320-322.
Gueugniaud P.Y., Jauffray M., Bertin-Maghit M., et al. Cerebral oedema after extensive thermal injury: prognostic significance of early intracranial and cerebral perfusion pressures. Ann Burns Fire Disast. 1997;10:72.
Han T., Harmatz J.S., Greenblatt D.J., et al. Fentanyl clearance and volume of distribution are increased in patients with major burns. J Clin Pharmacol. 2007;47:674-680.
Han T., Kim H., Bae J., et al. Neuromuscular pharmacodynamics of rocuronium in patients with major burns. Anesth Analg. 2004;99:386-392.
Heideman M. The effect of thermal injury on hemodynamic, respiratory and hematologic variables in relationship to complement activation. J Trauma. 1979;19:239-247.
Heinrich M., Wetzstein V., Muensterer O.J., et al. Conscious sedation: off-label use of rectal s+ketamine and midazolam for wound dressing changes in paediatric heat injuries. Eur J Pediatr Surg. 2004;14:235-239.
Herndon D.N., Spies M. Modern burn care. Semin Pediatr Surg. 2001;10:28-31.
Herndon D.N., Thompson P.B., Traber D.L. Pulmonary injury in burned patients. Crit Care Clin. 1985;1:79-96.
Herndon D.N., Tompkins R.G. Support of the metabolic response to burn injury. Lancet. 2004;363:1895-1902.
Hettiaratchy S., Dziewulski P. ABC of burns: pathophysiology and types of burns. BMJ. 2004;328:1427-1429.
Hickling K.G., Walsh J., Henderson S., et al. Low mortality rate in adult respiratory distress syndrome using low-volume, pressure-limited ventilation with permissive hypercapnia: a prospective study. Crit Care Med. 1994;22:1568-1578.
Hollingsed T.C., Saffle J.R., Barton R.G., et al. Etiology and consequences of respiratory failure in thermally injured patients. Am J Surg. 1993;166:592-597.
Holt J., Saffle J.R., Morris S.E., et al. Use of inhaled heparin/N-acetylcystine in inhalation injury: does it help? J Burn Care Res. 2008;29:192-195.
Horton J.W. Free radicals and lipid peroxidation mediated injury in burn trauma: the role of antioxidant therapy. Toxicology. 2003;189:75-88.
Horton J.W., Maass D.L., White D.J., et al. Effects of burn serum on myocardial inflammation and function. Shock. 2004;22:438-445.
Horton M.A., Beamer C. Powered intraosseous insertion provides safe and effective vascular access for pediatric emergency patients. Pediatr Emerg Care. 2008;24:347-350.
Hoskins S.L., Elgjo G.I., Liu J., et al. Closed-loop resuscitation of burn shock. J Burn Care Res. 2006;27:377-385.
Housinger T.A., Lang D., Warden G. A prospective study of blood loss with excisional therapy in pediatric burn patients. J Trauma. 1993;34:262-263.
Hsu W.T., Hsu S.C., Lee Y., et al. Penetrating injury of the soft palate during GlideScope intubation. Anesth Analg. 2007;104:1609-1610.
Hsu W.T., Tsao S.L., Chen K.Y., et al. Penetrating injury of the palatoglossal arch associated with the use of GlideScope videolaryngoscope in a flame burn patient. Acta Anaesthesiol Taiwan. 2008;46:39-41.
Huang P.P., Stucky F.S., Dimick A.R., et al. Hypertonic sodium resuscitation is associated with renal failure and death. Ann Surg. 1995;221:543-554.
Hunt J.L., Agee R.N., Pruitt B.A.Jr. Fiberoptic bronchoscopy in acute inhalation injury. J Trauma. 1975;15:641-649.
Ipaktchi K., Arbabi S. Advances in burn critical care. Crit Care Med. 2006;34:S239-S244.
Jackson D.M. The diagnosis of the depth of burning. Br J Surg. 1953;40:588-596.
Jelkmann W., Wolff M., Fandrey J. Modulation of the production of erythropoietin by cytokines: in vitro studies and their clinical implications. Contrib Nephrol. 1990;87:68-77.
Jellish W.S., Gamelli R.L., Furry P.A., et al. Effect of topical local anesthetic application to skin harvest sites for pain management in burn patients undergoing skin-grafting procedures. Ann Surg. 1999;229:115-120.
Jenkins M.E., Gottschlich M.M., Warden G.D. Enteral feeding during operative procedures in thermal injuries. J Burn Care Rehabil. 1994;15:199-205.
Jensen A.R., Hughes W.B., Grewal H. Secondary abdominal compartment syndrome in children with burns and trauma: a potentially lethal combination. J Burn Care Res. 2006;27:242-246.
Jeschke M.G., Barrow R.E., Herndon D.N. Extended hypermetabolic response of the liver in severely burned pediatric patients. Arch Surg. 2004;139:641-647.
Jeschke M.G., Finnerty C.C., Kulp G.A., et al. Combination of recombinant human growth hormone and propranolol decreases hypermetabolism and inflammation in severely burned children. Pediatr Crit Care Med. 2008;9:209-216.
Jeschke M.G., Herndon D.N., Wol S.E., et al. Recombinant human growth hormone alters acute phase reactant proteins, cytokine expression and liver morphology in burned rats. J Surg Res. 1999;83:122-129.
Jeschke M.G., Micak R.P., Finnerty C.C., et al. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11:R90.
Jeschke M.G., Micak R.P., Finnerty C.C., et al. Changes in liver function and size after a severe thermal injury. Shock. 2007;28:172-177.
Jevtovic-Todorovic V., Hartman R.E., Izumi Y., et al. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876-882.
Jose R.M., Roy D.K., Vidyadharan R., et al. Burns area estimation: an error perpetuated. Burns. 2004;30:481-482.
Junger W.G., Coimbra R., Liu F.C., et al. Hypertonic saline resuscitation: a tool to modulate immune function in trauma patients? Shock. 1997;8:235-241.
Kain Z.N., Caldwell-Andrews A.A., Weinberg M.E., et al. Sevoflurane versus halothane: postoperative maladaptive behavioral changes: a randomized, controlled trial. Anesthesiology. 2005;102:720-726.
Kaneda K., Han T.H. Comparative population pharmacokinetics of fentanyl using nonlinear mixed effect modeling: burns vs. non-burns. Burns. 2009;35(6):790-797.
Kao L.W., Nañangas K.A. Carbon monoxide poisoning. Emerg Med Clin North Am. 2004;22:985-1018.
Keens T.G., Bryan A.C., Levison H., et al. Developmental pattern of muscle fiber types in human ventilatory muscles. J Appl Physiol. 1978;44:909-913.
Kiser T.H., Hoody D.W., Obritsch M.D., et al. Levofloxacin pharmacokinetics and pharmacodynamics in patients with severe burn injury. Antimicrob Agents Chemother. 2006;50:1937-1945.
Lee J.J., Marvin J.A., Heimbach D.M. Effectiveness of nalbuphine for relief of burn debridement pain. J Burn Care Rehabil. 1989;10:241-246.
Lee M.J., O’Connell D.J. The plain chest radiograph after acute smoke inhalation. Clin Radiol. 1988;39:33-37.
Livingston E.H., Lee S. Percentage of burned body surface area determination in obese and non-obese patients. J Surg Res. 2000;91:106-110.
Losee J.E., Fox I., Hua L.B., et al. Transfusion-free pediatric burn surgery: techniques and strategies. Ann Plast Surg. 2005;54:165-171.
Lund C.C., Browder N.C. The estimation of areas of burns. Surg Gynecol Obstet. 1994;79:352-357. now J Am Coll Surg
Maass D.L., Hybki D.P., White J., et al. The time course of cardiac NF-kappaß activation and TNF-alpha secretion by cardiac myocytes after burn injury: contribution to burn-related cardiac contractile dysfunction. Shock. 2002;17:293-299.
Maass D.L., White J., Horton J.W. IL-1 beta and IL-6 act synergistically with TNF-alpha to alter cardiac contractile function after burn trauma. Shock. 2002;18:360-366.
Malhotra A.K., Riaz O.J., Duane T.M., et al. Subthreshold quantitative bronchoalveolar lavage: clinical and therapeutic implications. J Trauma. 2008;65:580-588.
Malic C.C., Karoo R.O., Austin O., et al. Resuscitation burn card: a useful tool for burn injury assessment. Burns. 2007;33:195-199.
Mandal A. Burn wound depth assessment: is laser Doppler imaging the best measurement tool available? Int Wound J. 2006;3:38-143.
Mariano F., Cantaluppi V., Stella M., et al. Circulating plasma factors induce tubular and glomerular alterations in septic burn patients. Crit Care. 2008;12:R42.
Martyn J.A. Clinical pharmacology and drug therapy in the burned patient. Anesthesiology. 1986;65:67-75.
Martyn J.A., Abernethy D.R., Greenblatt D.J. Plasma protein binding of drugs after severe burn injury. Clin Pharmacol Ther. 1984;35:535-539.
Martyn J.A., Fukushima Y., Chon J.Y., et al. Muscle relaxants in burns, trauma and critical illness. Int Anesthesiol Clin. 2006;44:123-143.
Martyn J.A.J., Matteo R.S., Szyfelbein S.K., Kaplan R.F. Unprecendented resistance to neuromuscular locking effects of metocurine with persistence after complete recovery in a burned patient. Anesth Analg. 1982;61:614-617.
Martyn J.A.J., Goudsouzian N.G., Matteo R.S., et al. Metocurine requirements and plasma concentrations in burned paediatric patients. Br J Anaesth. 1983;55:263.
Martyn J.A., Liu L.M., Szyfelbein S.K., et al. The neuromuscular effects of pancuronium in burned children. Anesthesiology. 1983;59:561-564.
Mayes T., Gottschlich M.M., Kagan R.J. An evaluation of the safety and efficacy of an anti-inflammatory, pulmonary enteral formula in the treatment of pediatric burn patients with respiratory failure. J Burn Care Res. 2008;29:82-88.
McCall J.E., Fischer C.G., Shomaker E., et al. Laryngeal mask airway use in children with acute burns: intraoperative airway management. Paediatr Anaesth. 1999;9:515-520.
McDonald W.S., Sharp C.W.Jr, Deitch E.A. Immediate enteral feeding in burn patients is safe and effective. Ann Surg. 1991;213:177-183.
McGill D.J., Sørensen K., MacKay I.R., et al. Assessment of burn depth: a prospective, blinded comparison of laser Doppler imaging and videomicroscopy. Burns. 2007;33:833-842.
Meert K.L., Heidemann S.M., Saranaik A.P. Outcome of children with carbon monoxide poisoning treated with normobaric oxygen. J Trauma. 1998;44:149-154.
Mellon R.D., Simone A.F., Rappaport B.A. Use of Anesthetic agents in neonates and young children. Anesth Analg. 2007;104:509-520.
Micak R., Cortiella J., Desai M., et al. Lung compliance, airway resistance and work of breathing in children after inhalation injury. J Burn Care Rehabil. 1997;18:531-534.
Mills A.K., Martyn J.A. Neuromuscular blockade with vecuronium in paediatric patients with burn injury. Br J Clin Pharmacol. 1989;28:155-159.
Monstrey S., Hoeksema H., Verbelen J., et al. Assessment of burn wound healing potential. Burns. 2008;34:761-769.
Nervi C., Gamelli R.L., Greenhalgh D.G., et al. A multicenter clinical trial to evaluate the topical hemostatic efficacy of fibrin sealant in burn patients. J Burn Care Rehabil. 2001;22:99-103.
Neuwalder J.M., Sampson C., Breuing K.H., et al. A review of computer-aided body surface area determination: SAGE II and EPRI’s 3D Burn Vision. J Burn Care Rehabil. 2002;23:55-59.
Niederbichler A.D., Westfall M.V., Su G.L., et al. Cardiomyocyte function after burn injury and lipopolysaccharide exposure: single-cell contraction analysis and cytokine secretion profile. Shock. 2006;25:176-183.
Ong Y.S., Samiel M., Song C. Meta-analysis of early excision of burns. Burns. 2006;32:145-150.
Onuoha G.N., Alpar E.K., Gowar J. Plasma levels of atrial natriuretic peptide in severe burn injury. Burns. 2000;26:449-453.
Owens V.F., Palmieri T.L., Comroe C.M., et al. Ketamine: a safe and effective agent for painful procedures in the pediatric burn patient. J Burn Care Res. 2006;27:211-216.
Palmieri T.L., Greenhalgh D.G. Topical treatment of pediatric patients with burns: a practical guide. Am J Clin Dermatol. 2002;3:529-534.
Palmieri T.L., Jackson W., Greenhalgh D.G. Benefits of early tracheostomy in severely burned children. Crit Care Med. 2002;30:922-924.
Parihar A., Parihar M.S., Milner S., et al. Oxidative stress and anti-oxidative mobilization in burn injury. Burns. 2008;34:6-17.
Parke T.J., Stevens J.E., Rice A.S., et al. Metabolic acidosis and fatal myocardial failure after propofol infusion in children: five case reports. BMJ. 1992;305:613-616.
Peña B.M., Krauss B. Adverse effects of procedural sedation and analgesia in a pediatric emergency department. Ann Emerg Med. 1999;34:483-491.
Pereira C.T., Herndon D.N. The pharmacologic modulation of the hypermetabolic response to burns. Adv Surg. 2005;39:245-261.
Perreault S., Choinière M., du Souich P.B., et al. Pharmacokinetics of morphine and its glucuronidated metabolites in burn injuries. Ann Pharmacother. 2001;35:1588-1592.
Perry S., Heidrich G. Management of pain during debridement: a survey of U. S. burn units. Pain. 1982;13:267-280.
Popp M.B., Friedberg D.L., MacMillan B.G. Clinical characteristics of hypertension in burned children. Ann Surg. 1980;191:473-478.
Porter J., Jick H. Addiction rare in patients treated with narcotics. N Eng J Med. 1980;302:123.
Pruitt B.A.Jr, Erickson D.R., Morris A. Progressive pulmonary insufficiency and other pulmonary complications of thermal injury. J Trauma. 1975;15:369-379.
Pruitt B.A.Jr, Flemma R.J., Divincenti F.C., et al. Pulmonary complications in burn patients: a comparative study of 697 patients. J Thorac Cardiovasc Surg. 1970;59:7-20.
Quinby W.C. Restrictive effects of thoracic burns in children. J Trauma. 1972;12:646-655.
Ramzy P.I., Jeschke M.G., Wolf S.E., et al. Correlation of bronchoalveolar lavage with radiographic evidence of pneumonia in thermally injured children. J Burn Care Rehabil. 2003;24:382-385.
Reich D.L., Silvay G. Ketamine: an update on the first twenty-five years of clinical experience. Can J Anaesth. 1980;36:186-197.
Robertson R.D., Bond P., Wallace B., et al. The tumescent technique to significantly reduce blood loss during burn surgery. Burns. 2001;27:835-838.
Rodeberg D.A., Housinger T.A., Greenhalgh D.G., et al. Improved ventilatory function in burn patients using volumetric diffusive respiration. J Am Coll Surg. 1994;179:518-522.
Rossiter N.D., Chapman P., Haywood I.A. How big is a hand? Burns. 1996;22:230-231.
Rue L.W.3rd, Cioffi W.G., Mason A.D., et al. Improved survival of burned patients with inhalation injury. Arch Surg. 1993;128:772-778.
Rue L.W.3rd, Cioffi W.G., Mason A.D.Jr, et al. The risk of pneumonia in thermally injured patients requiring ventilatory support. J Burn Care Rehabil. 1995;16:262-268.
Ryan C.M., Schoenfeld D.A., Thorpe W.P., et al. Objective estimates of the probability of death from burn injuries. N Engl J Med. 1998;338:362-366.
Saffle J.R., Morris S.E., Edelman L. Early tracheostomy does not improve outcome in burn patients. J Burn Care Rehabil. 2002;23:431-438.
Salinas J., Drew G., Gallagher J., et al. Closed-loop and decision-assist resuscitation of burn patients. J Trauma. 2008;64:S321-S332.
Schachar N.S., Jay A.W., Rowlands S., et al. Decreased red cell deformability in burn patients. Can J Surg. 1974;17:239-243.
Schwacha M.G., McGwin G.Jr, Hutchison C.B., et al. The contribution of opiate analgesics to the development of infectious complications in burn patients. Am J Surg. 2006;192:82-86.
Sellers B.J., Davis B.L., Larkin P.W., et al. Early prediction of prolonged ventilator dependence in thermally injured patients. J Trauma. 1997;43:899-903.
Shapiro B.A., Cane R.D., Harrison R.A., et al. Changes in intrapulmonary shunting with administration of 100 percent oxygen. Chest. 1980;77:138-141.
Sheridan R.L. Recognition and management of hot liquid aspiration in children. Ann Emerg Med. 1996;27:89-91.
Sheridan R.L. Burns. Crit Care Med. 2002;30:S500-S514.
Sheridan R.L., Hinson M.I., Liang M.H., et al. Long-term outcome of children surviving massive burns. JAMA. 2000;283:69-73.
Sheridan R.L., Kacmarek R.M., McEttrick M.M., et al. Permissive hypercapnia as a ventilatory strategy in burned children: effect on barotrauma, pneumonia and mortality. J Trauma. 1995;39:854-859.
Sheridan R.L., Petras L., Basha G., et al. Planimetry study of the percent of body surface represented by the hand and palm: sizing irregular burns is more accurately done with the palm. J Burn Care Rehabil. 1995;16:602-604.
Sheridan R.L., Keaney T., Stoddard F., et al. Short-term propofol infusions as an adjunct to extubation in burned children. J Burn Care Rehabil. 2003;24:356-360.
Sheridan R.L., McEttrick M., Bacha G., et al. Midazolam infusion in pediatric patients with burns who are undergoing mechanical ventilation. J Burn Care Rehabil. 1994;15:515-518.
Sheridan R.L., Ryan C.M., Petras L.M., et al. Burns in children younger than two years of age: an experience with 200 consecutive admissions. Pediatrics. 1997;100:721-723.
Sheridan R.L., Hurford W.E., Kacmarek R.M., et al. Inhaled nitric oxide in burn patients with respiratory failure. J Trauma. 1997;42:629-634.
Sheridan R.L., Ryan D.P., Fuzaylov G., et al. Case records of the Massachusetts General Hospital Case 2–2008. Case 5–2008: an 18-month-old girl with an advanced neck contracture after a burn. N Engl J Med. 2008;358:729-735.
Sheridan R.L., Stoddard F., Querzoli E. Management of background pain and anxiety in critically burned children requiring protracted mechanical ventilation. J Burn Care Rehabil. 2001;22:150-153.
Sheridan R.L., Tompkins R.G. Skin substitutes in burns. Burns. 1999;25:97-103.
Shiau Y.C., Liu F.Y., Tsai J.J., et al. Usefulness of technetium-99m hexamethylpropylene amine oxime lung scan to detect inhalation injury of patients with pulmonary symptoms/signs but negative chest radiograph and pulmonary function test findings after a fire accident-a preliminary report. Ann Nucl Med. 2003;17:435-438.
Shirani K.Z., Pruitt B.A., Mason A.D.Jr. The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205:82-87.
Silver G.M., Freiburg C., Halerz M., et al. A survey of airway and ventilator management strategies in North American pediatric burn units. J Burn Care Rehabil. 2004;25:435-440.
Simon T.L., Curreri P.W., Harder L.A. Kinetic characterization of hemostasis in thermal injury. J Lab Clin Med. 1977;89:702-711.
Slutsky A.S. Mechanical ventilation: American College of Chest Physicians’ Consensus Conference. Chest. 1993;104:1833-1859.
Smith J.J., Malyon A.D., Scerri G.V., et al. A comparison of serial halving and the rule of nines as a pre-hospital assessment tool in burns. Br J Plast Surg. 2005;58:957-967.
Stefanidou M., Athanaselies S., Spiliopoulou C. Health impacts of fire smoke inhalation. Inhal Toxicol. 2008;20:761-766.
Stoddard F.J., Saxe G., Ronfeldt H., et al. Acute stress symptoms in young children with burns. J Am Acad Child Adolesc Psychiatry. 2006;45:87-93.
Suner S., Jay G. Carbon monoxide has direct toxicity on the myocardium distinct from effects of hypoxia in an ex vivo rat heart model. Acad Emerg Med. 2008;15:59-65.
Suner S., McMurdy J. Masimo Rad-57 Pulse Co-Oximeter for noninvasive carboxyhemoglobin measurement. Expert Rev Med Devices. 2009;6:125-130.
Thombs B.D., Singh V.A., Milner S.M. Children under 4 years are at greater risk of mortality following acute burn injury: evidence from a national sample of 12,902 pediatric admissions. Shock. 2006;26:348-352.
Tolmie J.D., Joyce T.H., Mitchell G.D. Succinylcholine danger in the burned patient. Anesthesiology. 1967;28:467-470.
Tosun Z., Esmaoglu A., Coruh A. Propofol-ketamine vs propofol-fentanyl combinations for deep sedation and analgesia in pediatric patients undergoing burn dressing changes. Paediatr Anaesth. 2008;18:43-47.
Tredget E.E., Yu Y.M. The metabolic effects of thermal injury. World J Surg. 1992;16:68-79.
Tucker P. The burn victim: a review of psychosocial issues. Aust N Z J Psychiatry. 1986;20:413-420.
Vasile B., Rasulo F., Candiani A., et al. The pathophysiology of propofol infusion syndrome: a simple name for a complex syndrome. Intensive Care Med. 2003;29:1417-1425.
Wachtel T.L., Berry C.C., Wachtel E.E., et al. The inter-rater reliability of estimating the size of burns from various burn area chart drawings. Burns. 2000;26:156-170.
Walker J., MacCallum M., Fischer C., et al. Sedation using dexmedetomidine in pediatric burn patients. J Burn Care Res. 2006;27:206-210.
Wang P., Zeng T., Zhang C.L., et al. Lipid peroxidation was involved in the memory impairment of carbon-monoxide induced delayed neuron damage. Neurochem Res. 2009;34:1293-1298.
Warden G. Fluid resuscitation and early management. In: Herndon D., editor. Total burn care. ed 2,. London: WB Saunders; 2002:88-90.
Wasiak J., Cleland H. Lidocaine for pain relief in burn injured patients. Cochrane Database Syst Rev. 18, 2007. CD005622
Weaver L.K., Howe S., Hopkins R., et al. Carboxyhemoglobin half-life in carbon monoxide-poisoned patients treated with 100% oxygen at atmospheric pressure. Chest. 2000;119:661-663.
Weber J.M., Sheridan R.L., Pasternack M.S., et al. Nosocomial infections in pediatric patients with burns. Am J Infect Control. 1997;25:195-201.
Weinbrem M.J. Pharmacokinetics of antibiotics in burn patients. J Antimicrob Chemother. 1999;44:319-327.
Wilmore D.W., Goodwin C.W., Aulick L.H., et al. Effect of injury and infection on visceral metabolism and circulation. Ann Surg. 1980;192:491-504.
Wittram C., Kenny J.B. The admission chest radiograph after acute inhalation injury and burns. Br J Radiol. 1994;67:751-754.
Wood A.Y., Davit A.J.2nd, Ciraulo D.L., et al. A prospective assessment of diagnostic efficacy of blind protective bronchial brushings compared to bronchoscope-assisted lavage, bronchoscope-directed brushings and blind endotracheal aspirates in ventilator-associated pneumonia. J Trauma. 2003;55:825-834.
Wright M.J., Murphy J.T. Smoke inhalation enhances early leukocyte responsiveness to endotoxin. J Trauma. 2005;59:64-70.
Young C., Jevtovic-Todorovic V., Qin Y.Q., et al. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. Bt J Pharmacol. 2005;146:189-197.
Yowler C.J., Fratianne R.B. Current status of burn resuscitation. Clin Plast Surg. 2000;27:1-10.
Ziegler T.R., Smith R.J., O’Dwyer S.T., et al. Increased intestinal permeability associated with infection in burn patients. Arch Surg. 1988;123:1313-1319.