Chapter 12 Anesthesia Delivery Systems
Anesthesia workstation
1. What are some components of an anesthesia workstation?
2. What is the purpose of the fail-safe valve? What triggers the fail-safe valve on the anesthesia machine?
3. Can a hypoxic mixture be delivered from the anesthesia machine with an intact fail-safe valve? Explain.
4. How are oxygen, nitrous oxide, and air gases that are used in anesthesia typically delivered to the anesthesia machine? At what pressure must these gases be delivered for proper function of the anesthesia machine?
5. How is the delivery of erroneous gases to the anesthesia machine minimized?
6. What is the purpose of the cylinders of oxygen and nitrous oxide that are found on the back of the anesthesia machine?
7. How is an erroneous hookup of a gas cylinder to the anesthesia machine minimized?
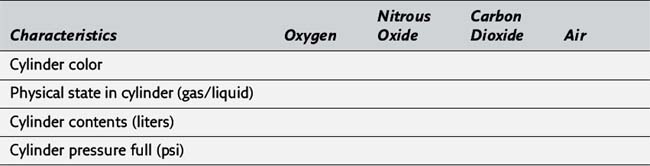
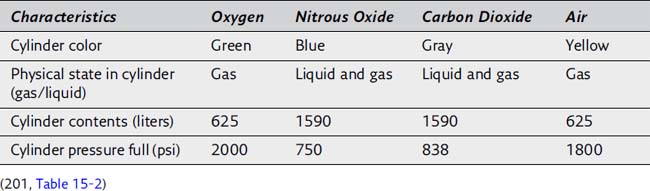
8. Please complete the following table illustrating the characteristics of compressed gases stored in E-sized cylinders:
9. How is the pressure of oxygen related to the volume of oxygen in an oxygen gas cylinder? What does this mean with regard to calculating the volume of oxygen remaining in a used oxygen cylinder?
10. How is the pressure of nitrous oxide related to the volume of nitrous oxide in a nitrous oxide gas cylinder?
11. Why does atmospheric water vapor accumulate as frost on the outside surface of oxygen tanks and nitrous oxide tanks in use?
12. What is the purpose of flowmeters on an anesthesia machine?
13. How do flowmeters on an anesthesia machine work?
14. Are flowmeters for various gases interchangeable?
15. Why is the oxygen flowmeter the last flowmeter in a series on the anesthesia machine with respect to the direction in which the gas flows?
16. What is the purpose of the oxygen flush valve?
17. What is the flow of oxygen delivered to the patient when the oxygen flush valve is depressed?
18. What is the risk of activating the oxygen flush valve during a mechanically delivered inspiration?
Vaporizers
19. Why do volatile anesthetics require placement in a vaporizer for their inhaled delivery to patients via the anesthesia machine?
20. What is the heat of vaporization?
21. What is vapor pressure? What influence does temperature have on vapor pressure?
22. Why are contemporary vaporizers unsuitable for use with desflurane?
23. Describe how contemporary vaporizers for volatile anesthetics are classified.
24. What does the term agent-specific refer to?
25. What do the terms variable-bypass and flow-over refer to?
26. What does the term temperature-compensated refer to? Between what temperatures is vaporizer output reliably constant?
27. What does the term out of circuit refer to?
28. How does tipping of a vaporizer affect vaporizer output?
29. How is the delivery of two different volatile anesthetics to the same patient via the same anesthesia machine prevented?
30. How is the potential risk of filling the agent-specific vaporizer with the erroneous volatile anesthetic minimized?
Anesthetic breathing systems
31. What is the function of anesthetic breathing systems?
32. How do anesthetic breathing systems impart resistance to the spontaneously ventilating patient?
33. What are some features of an anesthetic breathing system that enable them to be classified as either open, semiopen, closed, or semiclosed?
34. What are the most commonly used anesthetic breathing systems?
35. What characterizes the Mapleson systems?
36. Describe the Mapleson F anesthetic breathing system. What is another name for this anesthetic breathing system?
37. When is the Mapleson F system commonly used?
38. What are some advantages of the Mapleson F anesthetic breathing system?
39. What are some disadvantages of the Mapleson F anesthetic breathing system?
40. Describe the Bain circuit anesthetic breathing system.
41. What are some advantages of the Bain circuit anesthetic breathing system?
42. What are some disadvantages of the Bain circuit anesthetic breathing system?
43. How does the circle anesthetic breathing system get its name?
44. How does the circle system prevent rebreathing of carbon dioxide?
45. What are the classifications of a circle system and on what does this depend?
46. What is the most commonly used circle breathing system used in the United States?
47. What are some advantages of the semiclosed and closed circle systems?
48. What are some disadvantages of the circle anesthetic breathing system?
49. What is the impact of the rebreathing of anesthetic gases in a semiclosed circle system?
50. What are the components of a circle system?
51. What is the purpose of unidirectional valves in the circle system? What would occur if one of the unidirectional valves should become incompetent?
52. Where is the dead space in the circle system?
53. What is advantageous about the corrugated tubing in the circle system?
54. What is disadvantageous about the corrugated tubing in the circle system?
55. Describe the Y-piece connector in the circle system circuit.
56. What are other names for the adjustable pressure-limiting (APL) valve?
57. Describe the function of the APL valve when the “bag/vent” selector switch is set to “bag.”
58. What are the advantages of the reservoir bag on the circle system?
59. Describe a closed anesthetic breathing system. What is the inflow volume of fresh gases in a closed anesthetic breathing system?
60. What are some advantages to the closed circle anesthetic breathing system?
61. What is a disadvantage to the closed circle anesthetic breathing system?
62. What are the dangers of the closed circle anesthetic breathing system?
63. Are inspired concentrations of oxygen more or less predictable when nitrous oxide is also being delivered in a closed circle anesthetic breathing system? Why?
64. How can the potential problem of the inadequate delivery of oxygen using a closed circle anesthetic breathing system be minimized?
65. In a closed circle anesthetic breathing system, to what extent is the inhaled concentration of anesthetic dependent on the exhaled concentration of anesthetic? What is the potential problem with this? How can this problem be partially offset?
Anesthesia machine ventilators
66. What parts of a circle system are eliminated in anesthesia machine ventilators when the “bag/vent” selector switch is set to “vent”?
67. What are two different ways in which anesthesia machine ventilators are powered?
68. Describe the mechanics of a conventional anesthesia machine ventilator during inspiration.
69. Why is oxygen preferred over air as the ventilator driving gas?
70. Describe the mechanics of a conventional anesthesia machine ventilator during exhalation.
71. Describe the mechanically driven piston type of ventilators found on some newer anesthesia machines.
72. Why are standing or ascending bellows preferred over hanging or descending bellows?
73. How are inhaled gases normally humidified in awake patients breathing through their native airway?
74. What effect does tracheal intubation or the use of a laryngeal mask airway have on airway humidification? What are the negative consequences of this?
75. Describe anesthetic breathing system humidification. What effect does chemical neutralization of carbon dioxide have on this process?
76. What are three types of humidifiers used for anesthesia and in the intensive care unit?
77. Describe heat and moisture exchanger (HME) humidifiers. What is the difference between an HME and an HMEF?
78. What are the advantages of HME humidifiers over other types of humidifiers?
79. What are the disadvantages of HME humidifiers?
80. What is the advantage of heated water vaporizers and humidifiers over HME humidifiers? When are they used most frequently?
81. What are the risks of heated water vaporizers and humidifiers?
82. Describe nebulizer humidifiers used for anesthesia and in the intensive care unit.
Pollution of the atmosphere with anesthetic gases
83. In the operating room, what are the Occupational Safety and Health Administration (OSHA) recommendations for the maximum concentrations of nitrous oxide and volatile anesthetics in parts per million?
84. What is required to control pollution of the atmosphere with anesthetic gases?
85. Describe operating room scavenging.
86. Describe the two types of scavenging systems used in the operating room.
87. What are the advantages of active scavenging with a waste gas receiver mounted on the side of the anesthesia machine?
88. What are the potential hazards of scavenging systems?
89. What two features do scavenging systems have to minimize their potential hazards?
90. Where might be the source of a high-pressure leak of nitrous oxide?
91. Where might be the source of a low-pressure leak of nitrous oxide?
92. What anesthetic techniques can lead to operating room pollution?
93. How often should the air in the operating room be exchanged?
Elimination of Carbon Dioxide
94. How is carbon dioxide eliminated in open and semiopen breathing systems?
95. How is carbon dioxide eliminated in a semiclosed or closed anesthetic breathing system?
96. What are two types of chemicals that are used to neutralize carbon dioxide? What products are formed? Are the neutralization reactions endothermic or exothermic?
97. What does soda lime consist of?
98. Why is silica added to soda lime?
99. Why is the water in the soda lime carbon dioxide absorbent canister hazardous?
100. What does Amsorb Plus consist of?
101. Why are calcium sulfate and polyvinylpyrrolidine added to Amsorb Plus?
102. Why is the water formed by the neutralization of carbon dioxide useful? What if the carbon dioxide absorbent canister fails to become warm during use?
103. What two factors influence the efficiency of carbon dioxide neutralization?
104. How does the size of the carbon dioxide absorbent granules affect the efficiency of carbon dioxide neutralization?
105. What is the optimal carbon dioxide absorbent granule size? How is this sizing system defined?
106. What does channeling in the carbon dioxide absorbent granule-containing canister refer to? How does channeling in the canister affect the efficiency of carbon dioxide neutralization?
107. What is the most frequent cause of channeling in the carbon dioxide absorbent granule-containing canister? How can it be minimized?
108. Define carbon dioxide absorbent absorptive capacity. What can cause a decrease in absorptive capacity?
109. Why do the carbon dioxide absorbent granules change color?
110. Contrast the color change of soda lime granules with those of Amsorb Plus.
111. Describe the degradation of inhaled anesthetics by soda lime to carbon monoxide.
112. Describe the degradation of inhaled anesthetics by soda lime to compound A.
113. Does Amsorb Plus degrade inhaled anesthetics?
114. What factor contributes to the degradation of inhaled anesthetics by soda lime?
115. Why do most instances of increased blood concentrations of carboxyhemoglobin occur in anesthetized patients on a Monday?
116. What causes the development of fire and extreme heat in the breathing system? How can this be avoided?
117. Complete the following table:
| Feature | Soda Lime | Amsorb Plus |
|---|---|---|
| Mesh size | ||
| Generation of compound A with sevoflurane | ||
| Generation of carbon monoxide with inhaled anesthetics | ||
| Risk of exothermic reactions and fire in the presence of sevoflurane |
Checking the anesthesia machine and circle system function
118. What are the current recommendations for preanesthesia checkout procedures? How do these apply to newer machines with automated checkout procedures?
119. How often should these checkout procedures be performed?
120. What are the most important preoperative checks?
121. Does the presence of a Jackson-Rees circuit along with a full oxygen E-cylinder mounted on the back of the anesthesia machine comply with the current checkout recommendations?
122. What does a leak check of the machine’s low-pressure system evaluate? Why is this so important?
123. Why is calibration of the oxygen monitor so important?
124. Does a manual positive-pressure leak test check the integrity of the unidirectional valves?
Answers*
Anesthesia workstation
1. Components of an anesthesia workstation include what was previously recognized as the anesthesia machine, (the pressure-regulating and gas-mixing components), as well as the vaporizers, anesthesia breathing circuit, ventilator, scavenging system, and respiratory and physiologic monitoring systems (electrocardiogram, arterial blood pressure, temperature, pulse oximeter, and inhaled and exhaled concentrations of oxygen, carbon dioxide, anesthetic gases, and vapors). (198, Table 15-1)
2. The purpose of the fail-safe valve is to prevent the delivery of hypoxic gas mixtures from the anesthesia machine in the event of failure of the oxygen supply. The fail-safe valve is triggered when the pressure in the oxygen delivery line decreases to less than 30 psi. When the fail-safe valve is triggered, it either shuts off or proportionally decreases the flow of all gases. Note that it is only the pressure of oxygen that triggers the fail-safe valve. (199)
3. An intact fail-safe valve is actually only a pressure-sensor valve. A hypoxic mixture may still be delivered to the patient if the fail-safe valve is sensing an adequate gas pressure in the circuit of the anesthesia machine when the oxygen flow is zero. This confirms the importance of the oxygen analyzer on the anesthesia machine. Far superior to the fail-safe valve or an oxygen analyzer is the continuous presence of a vigilant anesthesiologist. (199)
4. The oxygen, nitrous oxide, and air gases that are used in anesthesia are most often delivered to the anesthesia machine as compressed gases from a central supply source located in the hospital. These hospital supplied gases enter the operating room from a central source through pipelines to operating room wall outlets. Pressure hoses then connect the wall outlets to the anesthesia machine. These gases must be delivered at a pressure of about 50 psi for the anesthesia machine to function properly. (199-200).
5. The delivery of erroneous gases from the central supply source to the pipeline inlet connections on the anesthesia machine is minimized in two ways. First, the wall outlets and pressure hoses are color-coded. Second, and more importantly, the pressure hoses are connected to the wall outlet and anesthesia machine by noninterchangeable gas-specific diameter fittings. This diameter index safety system (DISS) is designed to prevent misconnections of pipeline gases. (199-200)
6. The purpose of the cylinders of oxygen and nitrous oxide that are found on the back of the anesthesia machine is for the delivery of those gases should the central gas supply fail. (200)
7. An erroneous hookup of a gas cylinder to the anesthesia machine is minimized in two ways. First, the cylinders are color-coded. Second, and more importantly, the color-coded cylinders are attached to the anesthesia machine by a hanger yoke assembly, which consists of two metal pins that correspond to holes in the valve casing of the gas cylinder. This pin index safety system (PISS) makes it impossible to attach an oxygen cylinder to any yoke on the anesthesia machine other than that designed for oxygen. Otherwise, a cylinder containing nitrous oxide could be attached to the oxygen yoke, which would result in the delivery of nitrous oxide when the oxygen flowmeter was activated. (200)
9. The pressure in an oxygen cylinder is directly proportional to the volume of oxygen in the cylinder. For example, a full oxygen cylinder is evidenced by a pressure of approximately 2000 psi. If the pressure gauge on an oxygen cylinder were to read 500 psi, one fourth of the initial pressure, it can be estimated that only one fourth of the volume remains in the oxygen cylinder. The volume in the cylinder could be estimated to be 625/4, or about 155 L. (201)
10. In contrast to oxygen, the pressure gauge for nitrous oxide does not indicate the amount of gas remaining in the cylinder because the pressure in the gas cylinder remains at 750 psi as long as any liquid nitrous oxide is present. When nitrous oxide leaves the cylinder as a vapor, additional liquid is vaporized to maintain an unchanging pressure in the cylinder. After all the liquid nitrous oxide is vaporized, the pressure begins to decrease, and it can be assumed that about 75% of the contents of the gas cylinder have been exhausted. Because a full nitrous oxide cylinder (E-size) contains about 1590 L, approximately 400 L of nitrous oxide remains when the pressure gauge begins to decrease from its previously constant value of 750 psi. (201)
11. Vaporization of a liquefied gas (nitrous oxide), as well as expansion of a compressed gas (oxygen), absorbs heat, which is extracted from the metal cylinder and the surrounding atmosphere. For this reason, atmospheric water vapor often accumulates as frost on gas cylinders and in valves, particularly during high gas flow from these tanks. Internal icing does not occur because compressed gases are free of water vapor. (201)
12. Flowmeters on the anesthesia machine precisely control and measure gas flow to the common gas inlet. (201)
13. Measurement of the flow of gases is based on the principle that flow past a resistance is proportional to pressure. Typically, gas flow enters the bottom of a vertically positioned and tapered (the cross-sectional area increases upward from site of gas entry) glass flow tube. Gas flow into the flowmeter tube raises a bobbin or ball-shaped float. The float comes to rest when gravity is balanced by the decrease in pressure caused by the float. The upper end of the bobbin or the equator of the ball indicates the gas flow in milliliters or liters per minute. (201)
14. Proportionality between pressure and flow is determined by the shape of the tube (resistance) and the physical properties (density and viscosity) of the gas. The flowmeters are initially calibrated for the indicated gas at the factory. Because few gases have the same density and viscosity, flowmeters are not interchangeable with other gases. (201)
15. The oxygen flowmeter should be the last in the sequence of flowmeters, and thus oxygen should be the last gas added to the manifold. This arrangement reduces the possibility that leaks in the apparatus proximal to oxygen inflow can diminish the delivered oxygen concentration, whereas leaks distal to that point result in loss of volume without a qualitative change in the mixture. Nevertheless, an oxygen flowmeter tube leak can produce a hypoxic mixture regardless of the flowmeter tube arrangement. (201, Figure 15-4)
16. The purpose of the oxygen flush valve is to provide a large volume of oxygen to the patient quickly. Oxygen delivered to the patient when the oxygen flush valve is depressed bypasses the flowmeters and manifold. (201-202)
17. The flow of oxygen that is delivered to the patient via the oxygen flush valve is 35 to 75 L/min. (201-202, Figure 15-3)
18. Activation of the oxygen flush valve during a mechanically delivered inspiration from the anesthesia machine ventilator permits the transmission of high airway pressure to the patient’s lungs, with the possibility of barotrauma. (202)
Vaporizers
19. Volatile anesthetics are liquids at room temperature and atmospheric pressure. Vaporization, which is the conversion of a liquid to a vapor, takes place in a closed container, referred to as a vaporizer. The inhaled delivery of volatile anesthetics requires that they be vaporized. The vapor concentration resulting from vaporization of a volatile liquid anesthetic must be delivered to the patient with the same accuracy and predictability as other gases (oxygen, nitrous oxide). (202)
20. The heat of vaporization of a liquid is the number of calories required at a specific temperature to convert 1 g of a liquid into a vapor. (202)
21. Vaporization in the closed confines of a vaporizer ceases when equilibrium is reached between the liquid and vapor phases such that the number of molecules leaving the liquid phase is the same as the number reentering. The molecules in the vapor phase collide with each other and the walls of the container, thereby creating pressure. This pressure is termed vapor pressure and is unique for each volatile anesthetic. Vapor pressure is temperature dependent such that a decrease in the temperature of the liquid is associated with a lower vapor pressure and fewer molecules in the vapor phase. Cooling of the liquid anesthetic reflects a loss of heat (heat of vaporization) necessary to provide energy for vaporization. This cooling is undesirable because it lowers the vapor pressure and limits the attainable vapor concentration. (202)
22. Desflurane has a vapor pressure near 1 atm (664 mm Hg) at 20° C. For this reason, a desflurane vaporizer is electrically heated to 23° C and 25° C and pressurized with a backpressure regulator to 1500 mm Hg to create an environment in which the anesthetic has a relatively lower, but predictable volatility. (202)
23. Contemporary vaporizers are classified as agent-specific, variable-bypass, flow-over, temperature-compensated, and out of circuit. (202, Figure 15-5)
24. Vaporizers are calibrated to accommodate a single volatile anesthetic. (203)
25. Variable-bypass describes dividing (splitting) the total fresh gas flow through the vaporizer into two portions. The first portion of the fresh gas flow (20% or less) passes into the vaporizing chamber of the vaporizer, where it becomes saturated (flow-over) with the vapor of the liquid anesthetic. The second portion of the fresh gas flow passes through the bypass chamber of the vaporizer. Both portions of the fresh gas flow mix at the patient outlet side of the anesthesia machine. The proportion of fresh gas flow diverted through the vaporizing chamber, and thus the concentration of volatile anesthetic delivered to the patient, is determined by the concentration control dial. (202, Figure 15-5)
26. As the vaporizer temperature changes, a temperature-sensitive bimetallic strip or an expansion element inside the vaporizer influences proportioning of total gas flow between the vaporizing and bypass chambers. For example, as the temperature of the liquid anesthetic in the vaporizer chamber decreases, the temperature-sensing elements allow increased gas inflow into this chamber to offset the effect of decreased anesthetic liquid vapor pressure. Vaporizers are often constructed of metals with high thermal conductivity (copper, bronze) to further minimize heat loss. As a result, vaporizer output is nearly linear between 20° C and 35° C. (203, Figure 15-5)
27. Out of circuit describes the fact that vaporizers are isolated from the anesthetic breathing system. (203)
28. Tipping of vaporizers can cause liquid anesthetic to spill from the vaporizing chamber into the bypass chamber with a resultant increased vapor concentration exiting from the vaporizer. (203)
29. A safety interlock mechanism ensures that only one vaporizer at a time can be turned on. (203)
30. Use of an anesthetic-specific keyed filler device prevents placement of a liquid anesthetic into the vaporizing chamber that is different from the anesthetic for which the vaporizer was calibrated. This is uniquely important for desflurane because its vapor pressure is near 1 atm and accidental placement of desflurane in a contemporary vaporizer could result in an anesthetic overdose. (203)
Anesthetic breathing systems
31. The function of anesthetic breathing systems is to deliver oxygen and anesthetic gases to the patient and to eliminate carbon dioxide. (204)
32. Anesthetic breathing systems can add considerable resistance to inhalation because peak flows as high as 60 L/min are reached during spontaneous inspiration. This resistance is influenced by unidirectional valves and connectors. The components of the breathing system, particularly the tracheal tube connector, should have the largest possible lumen to minimize this resistance to breathing. Right-angle connectors should be replaced with curved connectors to minimize resistance. Substituting controlled ventilation of the patient’s lungs for spontaneous breathing can offset the increased resistance to inhalation imparted by anesthetic breathing systems. (204)
33. Anesthetic breathing systems are classified as open, semiopen, semiclosed, and closed according to the presence or absence of (1) a gas reservoir bag in the circuit, (2) rebreathing of exhaled gases, (3) means to chemically neutralize exhaled carbon dioxide, and (4) unidirectional valves. (204, Table 15-3)
34. The most commonly used anesthetic breathing systems are the (1) Mapleson F (Jackson-Rees) system, (2) Bain circuit, and (3) circle system. (204)
35. The Mapleson systems are characterized by the absence of valves to direct gases to or from the patient and the absence of chemical carbon dioxide neutralization. (204, Figures 15-6 and 15-8)
36. The Mapleson F system is a T-piece arrangement with a reservoir bag and an adjustable pressure-limiting overflow valve on the distal end of the gas reservoir bag. Another name for this anesthetic breathing system is the Jackson-Rees circuit. (204, Figure 15-6)
37. The Mapleson F system is commonly used for controlled ventilation during transport of endotracheally intubated patients. (206)
38. Advantages of the Mapleson F anesthetic breathing system include its minimal dead space and resistance. This makes this system ideal for pediatric anesthesia. (206)
39. Disadvantages of the Mapleson F system include (1) the need for high fresh gas inflow to prevent rebreathing, (2) the possibility of high airway pressure and barotrauma should the overflow valve become occluded, and (3) the lack of humidification. Lack of humidification can be offset by allowing the fresh gas to pass through an in-line heated humidifier. (206)
40. The Bain circuit is a coaxial version of the Mapleson D system in which the fresh gas supply tube runs coaxially inside the corrugated expiratory tubing. The fresh gas tube enters the circuit near the reservoir bag, but the fresh gas is actually delivered at the patient end of the circuit. The exhaled gases are vented through the overflow valve near the reservoir bag. (206, Figure 15-7)
41. Advantages of the Bain circuit include (1) warming of the fresh gas inflow by the surrounding exhaled gases in the corrugated expiratory tube, (2) conservation of moisture as a result of partial rebreathing, and (3) ease of scavenging waste anesthetic gases from the overflow valve. It is lightweight, easily sterilized, reusable, and useful when access to the patient is limited, such as during head and neck surgery. (207)
42. Hazards of the Bain circuit include unrecognized disconnection or kinking of the inner fresh gas tube. The outer expiratory tube should be transparent to allow inspection of the inner tube. (207)
43. The essential components of a circle anesthetic breathing system are arranged in a circular manner. (207, Figure 15-9)
44. The circle system prevents rebreathing of carbon dioxide by chemical neutralization of carbon dioxide with carbon dioxide absorbents. (207)
45. A circle system can be classified as semiopen, semiclosed, or closed, depending on the amount of fresh gas inflow. (207)
46. A semiclosed system is associated with rebreathing of gases and is the most commonly used breathing system in the United States. (207)
47. The semiclosed and closed circle system are both advantageous in that they allow for the rebreathing of exhaled gases. The rebreathing of exhaled gases results in (1) some conservation of airway moisture and body heat and (2) decreased pollution of the surrounding atmosphere with anesthetic gases when the fresh gas inflow rate is set at less than the patient’s minute ventilation. (207)
48. Disadvantages of the circle system include (1) increased resistance to breathing because of the presence of unidirectional valves and carbon dioxide absorbent, (2) bulkiness with loss of portability, and (3) enhanced opportunity for malfunction because of the complexity of the apparatus. (207)
49. The rebreathing of exhaled gases in a semiclosed circle system influences the inhaled anesthetic concentrations of these gases. For example, when uptake of the anesthetic gas is high, as during induction of anesthesia, rebreathing of exhaled gases depleted of anesthetic greatly dilutes the concentration of anesthetic in the fresh gas inflow. This dilutional effect of uptake is offset clinically by increasing the delivered concentration of anesthetic. As uptake of anesthetic diminishes, the impact of dilution on the inspired concentration produced by rebreathing of exhaled gases is lessened. (207)
50. The circle system consists of (1) a fresh gas inlet, (2) inspiratory and expiratory unidirectional check valves, (3) inspiratory and expiratory corrugated tubing, (4) a Y-piece connector, (5) an adjustable pressure-limiting (APL) valve, also referred to as an overflow or “pop-off” valve, (6) a reservoir bag, (7) a canister containing carbon dioxide absorbent, (8) a bag/vent selector switch, and (9) a mechanical anesthesia ventilator. (207-208)
51. Two unidirectional valves are situated in different limbs of the corrugated tubing in a circle system such that one functions for inhalation and the other for exhalation. These valves (1) permit positive-pressure breathing and (2) prevent the rebreathing of exhaled gases until they have passed through the carbon dioxide absorbent canister and have had their oxygen content replenished. Rebreathing and hypercapnia can occur if the unidirectional valves stick in the open position, and total occlusion of the circuit can occur if they are stuck in the closed position. If the expiratory valve is stuck in the closed position, breath stacking and barotrauma can occur. (208)
52. Dead space in the circle system is between the Y-piece and the patient. (208)
53. The inspiratory and expiratory corrugated tubes serve as conduits for delivery of gases to and from the patient. Their large bore provides minimal resistance, and the corrugations provide flexibility, resist kinking, and promote turbulent instead of laminar flow. (208)
54. During positive-pressure ventilation, some of the delivered gas distends the corrugated tubing and some is compressed within the circuit, which leads to a smaller delivered tidal volume. (208)
55. The Y-piece connector at the patient end of the circuit has (1) a curved elbow, (2) an outer diameter of 22 mm to fit inside a facemask, and (3) an inner diameter of 15 mm to fit onto an endotracheal tube connector. (208)
56. The APL valve is also known as the overflow or “pop-off” valve. (208)
57. When the “bag/vent” selector switch is set to “bag,” the APL (overflow or “pop-off”) valve (1) allows venting of excess gas from the breathing system into the waste gas scavenging system and (2) can be adjusted to allow the anesthesiologist to provide assisted or controlled ventilation of the patient’s lungs by manual compression of the gas reservoir bag. The APL valve should be fully open during spontaneous ventilation so that circuit pressure remains negligible throughout inspiration and expiration. (208)
58. When the “bag/vent” selector switch is set to “bag,” the gas reservoir bag maintains an available reserve volume of gas to satisfy the patient’s spontaneous inspiratory flow rate (up to 60 L/min), which greatly exceeds conventional fresh gas flows (commonly 3 to 5 L/min) from the anesthesia machine. The bag also serves as a safety device because its distensibility limits pressure in the breathing circuit to less than 60 cm H2O, even when the APL valve is closed. (208)
59. In a closed anesthetic breathing system, there is total rebreathing of exhaled gases after absorption of carbon dioxide, and the APL valve or relief valve of the ventilator is closed. A closed system is present when the fresh gas inflow into the circle system (150 to 500 mL/min) satisfies the patient’s metabolic oxygen requirements (150 to 250 mL/min during anesthesia) and replaces anesthetic gases lost by virtue of tissue uptake. If sidestream gas analyzers are used, the analyzed gas exiting the analyzer must be returned to the breathing system to maintain a closed system. (208)
60. Advantages of a closed circle anesthetic breathing system over a semiclosed circle anesthetic breathing system include (1) maximal humidification and warming of inhaled gases, (2) less pollution of the surrounding atmosphere with anesthetic gases, and (3) economy in the use of anesthetics. (208)
61. A disadvantage of a closed circle anesthetic breathing system is an inability to rapidly change the delivered concentration of anesthetic gases and oxygen because of the low fresh gas inflow. (208)
62. The principal dangers of a closed anesthetic breathing system are delivery of (1) unpredictable and possibly insufficient concentrations of oxygen and (2) unknown and possibly excessive concentrations of potent anesthetic gases. (208)
63. Unpredictable and possibly insufficient delivered concentrations of oxygen when using a closed anesthetic breathing system are more likely if nitrous oxide is included in the fresh gas inflow. For example, decreased tissue uptake of nitrous oxide with time in the presence of unchanged uptake of oxygen can result in a decreased concentration of oxygen in the alveoli. (208, Table 15-4)
64. The potential problem of the inadequate delivery of oxygen using a closed circle anesthetic breathing system can be minimized by the use of an oxygen analyzer placed on the inspiratory or expiratory limb of the closed circle system. (208)
65. Exhaled gases, devoid of carbon dioxide, form a major part of the inhaled gases when a closed anesthetic breathing system is used. This means that the composition of the inhaled gases is influenced by the concentration present in the exhaled gases. The concentration of anesthetic in exhaled gases reflects tissue uptake of the anesthetic. Initially, tissue uptake is maximal, and the concentration of anesthetic in the exhaled gases is minimal. Subsequent rebreathing of these exhaled gases dilutes the inhaled concentration of anesthetic delivered to the patient. Therefore, high inflow concentrations of anesthetic are necessary to offset maximal tissue uptake. Conversely, only small amounts of anesthetic need to be added to the inflow gases when tissue uptake has decreased. The unknown impact of tissue uptake on the concentration of anesthetic in exhaled gases makes it difficult to estimate the inhaled concentration delivered to the patient through a closed anesthetic breathing system. This disadvantage can be partially offset by administering higher fresh gas inflow (3 L/min) for about 15 minutes before instituting the use of a closed anesthetic breathing system. This approach permits elimination of nitrogen from the lungs and corresponds to the time of greatest tissue uptake of anesthetic. (208-209, Table 15-4)
Anesthesia machine ventilators
66. When the anesthesia machine ventilator “bag/vent” selector switch is set to “vent,” the gas reservoir bag and APL valve are eliminated from the circle anesthetic system and the patient’s ventilation is delivered from the mechanical anesthesia ventilator. (209, Figure 15-10)
67. Anesthesia ventilators are powered by compressed gas, electricity, or both. (209)
68. Most conventional anesthesia machine ventilators are pneumatically driven by oxygen or air that is pressurized and, during the inspiratory phase, routed to the space inside the ventilator casing between the compressible bellows and the rigid casing. Pressurized air or oxygen entering this space forces the bellows to empty its contents into the patient’s lungs through the inspiratory limb of the breathing circuit. This pressurized air or oxygen also causes the ventilator relief valve to close, thereby preventing inspiratory anesthetic gas from escaping into the scavenging system. (209)
69. Oxygen is preferable to air as the ventilator driving gas because if there is a leak in the bellows, the fraction of inspired oxygen will be increased. If there is a leak in the bellows in a ventilator driven by 50 psi oxygen or air, the peak inspiratory pressure will rise. (209)
70. During exhalation, the driving gas is either vented into the room or directed to the scavenging system, and the bellows refills as the patient exhales. (209)
71. Some newer anesthesia machines have mechanically driven piston type of ventilators. The piston operates much like the plunger of a syringe to deliver the desired tidal volume or airway pressure to the patient. (209)
72. Ventilators with bellows that rises during exhalation (standing or ascending bellows) are preferred because the bellows will not rise (fill) if there is a leak in the anesthesia breathing system or the system becomes accidentally disconnected. Ventilators with a bellows that descends during exhalation (hanging or descending bellows) are potentially dangerous because the bellows will continue to rise and fall during a disconnection. Whenever a ventilator is used, a disconnect alarm must be activated and audible. (209)
73. The upper respiratory tract (especially the nose) functions as the principal heat and moisture exchanger (HME) to bring inspired gas to body temperature and 100% relative humidity in its passage to the alveoli. (209)
74. Water is removed from medical gases (cylinders or piped) to prevent corrosion and condensation. Tracheal intubation or the use of a laryngeal mask airway bypasses the upper airway and thus leaves the tracheobronchial mucosa the burden of heating and humidifying inspired gases. Humidification of inspired gases by the lower respiratory tract in intubated patients can lead to dehydration of the mucosa, impaired ciliary function, impaired surfactant function, inspissation of secretions, atelectasis, and a rise in the alveolar-to-arterial gradient. Breathing of dry and room temperature gases in intubated patients is associated with water and heat loss from the patient. Heat loss is more important than water loss, and the most important reason to provide heated humidification for intubated patients is to decrease heat loss and associated decreases in body temperature. This is especially true in infants and children, who are rendered poikilothermic by general anesthesia. (209)
75. Humidification is a form of vaporization in which water vapor (moisture) is added to the gases delivered by the anesthetic breathing system to minimize water and heat loss. The water formed and the heat generated by chemical neutralization of carbon dioxide help humidify and heat the gases in the breathing circuit. (210)
76. Humidifiers used for anesthesia and in the intensive care unit include (1) heat and moisture exchanger (HME) humidifiers, (2) heated water vaporizers and humidifiers, and (3) nebulizers. (210)
77. HME humidifiers are devices that, when placed between the endotracheal tube and Y-piece of the circle system, conserve some of the exhaled water and heat and return it to the inspired gases. They contain a porous hydrophobic or hygroscopic membrane that traps exhaled humidified gases and returns them to the patient on inspiration. Bacterial and viral filters can be incorporated in HME humidifiers to convert them into heat and moisture exchanging filters (HMEFs). (211)
78. The advantages of HME humidifiers over other types of humidifiers are that they are (1) simple and easy to use, (2) lightweight, (3) not dependent on an external power source, (4) disposable, and (5) low cost. (211)
79. The disadvantages of HME humidifiers are that they (1) are not as effective as heated water vaporizers and humidifiers in maintaining patient temperature, (2) add resistance and increase the work of breathing and therefore should be used with caution in spontaneously ventilating patients, (3) can become clogged with patient secretions or blood, and (4) can increase dead space, which can cause significant rebreathing in pediatric patients. Special low-volume HME humidifiers are available for pediatric patients. (211)
80. Heated water vaporizers and humidifiers are used to deliver a relative humidity higher than that delivered by HME humidifiers. Heated water vaporizers are more frequently used in pediatric anesthesia and intensive care unit patients. (211)
81. Risks from heated water vaporizers and humidifiers include (1) thermal injury, (2) nosocomial infection, (3) increased work of breathing, and (4) increased risk of malfunction because of the complexity of these systems. (211)
82. Nebulizers produce a mist of microdroplets of water suspended in a gaseous medium. The quantity of water droplets delivered is not limited by the temperature of the carrier gas. In addition to water, nebulizers can deliver medications to peripheral airways. (211)
Pollution of the atmosphere with anesthetic gases
83. In the operating room, OSHA recommends that the concentration of nitrous oxide not exceed 25 ppm and exposure concentrations of volatile anesthetics not exceed 2 ppm. (211)
84. Control of pollution of the atmosphere with anesthetic gases requires (1) scavenging of waste anesthetic gases, (2) periodic preventive maintenance of anesthesia equipment, (3) attention to the anesthetic technique, and (4) adequate ventilation of the operating rooms. (211)
85. Scavenging is the collection and subsequent removal of vented gases from the operating room. The excess gas comes from either the APL valve if the bag/vent selector switch is set to “bag,” or from the ventilator relief valve if the bag/vent selector switch is set to “vent.” All excess gas from the patient exits the breathing system through these valves. The amount of delivered gas used to anesthetize a patient commonly far exceeds the patient’s needs. In addition, when the bag/vent selector switch is set to vent, some anesthetic breathing systems direct the drive gas inside the bellows canister to the scavenging system. If sidestream gas analyzers are used, the analyzed gas exiting the analyzer must be directed to the scavenging system or returned to the breathing system. (211)
86. Scavenging systems may be characterized as active or passive. An active system is connected to the hospital’s vacuum system and gases are drawn from the machine by a vacuum. A passive system is connected to the hospital’s ventilation duct and waste gases flow out of the machine on their own. (212)
87. Many anesthesia machines provide active scavenging with a waste gas receiver mounted on the side of the anesthesia machine. Advantages of this system include (1) a needle valve that allows the clinician to manually adjust the amount of vacuum flow through the scavenging system, (2) a needle valve that can be adjusted such that the 3-L reservoir bag will be slightly inflated and appear to “breathe” with the patient, and (3) unlike other active scavenging systems, a waste gas receiver that does not require a strong vacuum to operate. (212)
88. Hazards of scavenging systems include (1) obstruction of the scavenging pathways, which can result in excessive positive pressure in the breathing circuit and possible barotrauma, and (2) excessive vacuum applied to the scavenging system, which can cause negative pressures in the breathing system. (212)
89. Scavenging systems contain two relief valves to minimize their potential hazards. If gas accumulates in the scavenging system and cannot leave the anesthesia machine properly, the positive-pressure scavenge relief valve opens when the pressure reaches 10 cm H2O to allow the gas to escape into the room. If negative pressure is applied to the scavenging system, the negative-pressure scavenge relief valve opens and allows room air to be drawn in (instead of drawing gas from the patient). Additionally, if the amount of fresh gas flow exceeds the capacity of the scavenging system, the excess waste anesthetic gas exits the scavenging system through the positive-pressure relief valve and pollutes the operating room. (212)
90. High-pressure leakage of nitrous oxide can occur as a result of faulty yokes attaching the nitrous oxide tank to the anesthesia machine or faulty connections from the central nitrous oxide gas supply to the anesthesia machine. (212)
91. Low-pressure leakage of anesthetic gases can occur because of leaks inside the anesthesia machine and leaks between the machine and patient. (212)
92. Anesthetic techniques that can lead to operating room pollution include (1) poorly fitting facemasks, (2) flushing the anesthetic delivery circuit, (3) filling anesthetic vaporizers, (4) the use of uncuffed endotracheal tubes, (5) failure to turn off the nitrous oxide flow or vaporizers at the end of the anesthesia, and (6) the use of semiopen breathing circuits such as the Jackson-Rees, which are difficult to scavenge. (212)
93. The air in the operating room should be exchanged at least 15 times per hour by the operating room ventilation system. This rate should be checked periodically by the hospital’s clinical engineering department. (212)
Elimination of carbon dioxide
94. Open and semiopen breathing systems eliminate carbon dioxide by venting all exhaled gases to the atmosphere. (212)
95. Semiclosed and closed breathing systems eliminate carbon dioxide by chemical neutralization. (212)
96. Soda lime and Amsorb Plus absorbents are used to neutralize carbon dioxide. The products formed by their reactions with carbon dioxide are carbonates, water, and heat, making them exothermic reactions. (212-213)
97. Soda lime granules consist of water, calcium hydroxide, and small amounts of sodium and potassium hydroxide that serve as activators. (212, Table 15-6)
98. Soda lime granules fragment easily and produce alkaline dust, which can lead to bronchospasm if inhaled. Silica is added to the granules to provide hardness and minimize alkaline dust formation. (213)
99. The water formed from the neutralization of carbon dioxide, the water present in the soda lime granules, and the water condensed from the patient’s exhaled gases leach the alkaline bases from the soda lime granules and produce a slurry containing NaOH and KOH in the bottom of the canister. These monovalent bases can be corrosive to the skin. (213)
100. Amsorb Plus granules consist of water, calcium hydroxide, and calcium chloride. (213, Table 15-6)
101. Calcium sulfate and polyvinylpyrrolidine are added to Amsorb Plus to increase hardness. (213)
102. The water formed by the neutralization of carbon dioxide with soda lime and Amsorb Plus is useful for humidifying the gases and for dissipating some of the heat generated in these exothermic reactions. The heat generated during the neutralization of carbon dioxide can be detected by the warmness of the canister. Failure of the canister to become warm should alert the anesthesia provider to the possibility that chemical neutralization of carbon dioxide is not taking place. (213)
103. The efficiency of carbon dioxide neutralization is influenced by the size of the carbon dioxide granules and the presence or absence of channeling in the carbon dioxide canister. (213)
104. The optimal absorbent granule size represents a compromise between absorptive efficiency and resistance to airflow through the carbon dioxide absorbent canister. Absorbent efficiency increases as absorbent granule size decreases because the total surface area coming in contact with carbon dioxide increases. The smaller the absorbent granules, however, the smaller the interstices through which gas must flow and the greater the resistance to flow. (213)
105. Absorbent granule size is designated as mesh size, which refers to the number of openings per linear inch in a sieve through which the granular particles can pass. The granular size of carbon dioxide absorbents in anesthesia practice is between 4 and 8 mesh, a size at which absorbent efficiency is maximal with minimal resistance. A 4-mesh screen means that there are four quarter-inch openings per linear inch. An 8-mesh screen has eight eighth-inch openings per linear inch. (213)
106. Channeling is the preferential passage of exhaled gases through the carbon dioxide absorber canister via pathways of low resistance such that the bulk of the carbon dioxide absorbent granules are bypassed. (213)
107. Channeling results from loose packing of absorbent granules and can be minimized by gently shaking the canister before use to ensure firm packing of the absorbent granules. Carbon dioxide absorbent canisters are designed to facilitate uniform dispersion of exhaled gas flow through the absorbent granules. (213)
108. Absorptive capacity is determined by the maximum amount of carbon dioxide that can be absorbed by 100 g of carbon dioxide absorbent. Channeling of exhaled gases through the absorbent granules can substantially decrease their efficiency. Carbon dioxide absorber canister design also influences the absorptive capacity of the carbon dioxide absorbent. (213)
109. Carbon dioxide absorbents contain a pH-sensitive indicator dye that changes color when the carbon dioxide absorbent granules are exhausted. When the absorptive components of the granules are exhausted, carbonic acid accumulates and produces a change in the pH and thus in the indicator dye color. (213-214)
110. Soda lime contains the indicator dye ethyl violet, which changes granule color from white to purple when exhausted. Over time, exhausted granules may revert to their original white color even though absorptive capacity does not recover with time. On reuse, the dye quickly produces the purple color change again. Amsorb Plus contains an indicator dye that changes granule color from white to purple when exhausted and, once changed, does not revert to its original color. (214)
111. Desiccated soda lime may degrade sevoflurane, isoflurane, enflurane, and desflurane to carbon monoxide. (214)
112. Soda lime, whether moist or dry, degrades sevoflurane and halothane to unsaturated nephrotoxic compounds (compound A). (214)
113. In contrast to soda lime, Amsorb Plus, whether desiccated or moist, does not degrade inhaled anesthetics. (214)
114. Desiccation of soda lime increases the degradation of inhaled anesthetics. Without a patient attached to the conventional circle system, desiccation of soda lime is enhanced by retrograde gas flow, which is facilitated by fresh gas flows greater than 5 L/min, an open APL valve, and removing the breathing bag. (214)
115. Desiccation of soda lime requires a prolonged period (usually 48 hours) of retrograde gas flow. Accordingly, most instances of increased blood concentrations of carboxyhemoglobin occur in patients anesthetized on a Monday after continuous flow of oxygen (flowmeter accidentally left on) through the carbon dioxide absorbent over the weekend. (214)
116. Desiccation of the carbon dioxide absorbent Baralyme (no longer clinically available) can lead to fire within the circle system with sevoflurane use. A poorly characterized chemical reaction between sevoflurane and Baralyme can produce sufficient heat and combustible degradation products to lead to the spontaneous generation of fires within the carbon dioxide absorber canister and breathing circuit. Cases of extreme heat without fire associated with desiccated soda lime have been reported in Europe. To avoid this problem, anesthesia providers should make every effort to not use desiccated carbon dioxide absorbents. (214, Table 15-8)
| Feature | Soda Lime | Amsorb Plus |
|---|---|---|
| Mesh size | 4 – 8 | 4 – 8 |
| Generation of compound A with sevoflurane | Yes | No |
| Generation of carbon monoxide with inhaled anesthetics | Yes | No |
| Risk of exothermic reactions and fire in the presence of sevoflurane | No | No |
(212-214, Table 15-6)
Checking the anesthesia machine and circle system function
118. In 2008 the American Society of Anesthesiologists developed new recommendations for preanesthesia checkout (PAC) procedures to provide guidelines applicable to all anesthesia delivery systems. This allows individual departments to develop a PAC specific to the anesthesia delivery systems currently used at their facilities that can be performed consistently and expeditiously. Specifically, for newer anesthesia delivery systems that incorporate automated checkout features, items that are not evaluated by the automated checkout need to be identified, and supplemental manual checkout procedures included as needed. (215, Table 15-9)
119. A complete anesthesia machine and circle system function checkout procedure should be performed each day before the first case. An abbreviated checkout should be performed before each subsequent use that day. (215, Table 15-9)
120. The most important preoperative checks are (1) verification that an auxiliary oxygen cylinder and self-inflating manual ventilation device (Ambu bag) are available and functioning, (2) a leak check of the machine’s low-pressure system, (3) calibration of the oxygen monitor, and (4) a positive-pressure leak check of the breathing system. (215-216)
121. No. Failure to ventilate is a major cause of morbidity and death related to anesthesia care. Because equipment failure with resulting inability to ventilate the patient can occur at any time, a self-inflating manual ventilation device (e.g., Ambu bag) should be present at every anesthetizing location for every case and should be checked for proper function. In addition, a source of oxygen separate from the anesthesia machine and pipeline supply, specifically an oxygen cylinder with a regulator and a means to open the cylinder valve, should be immediately available and checked. (215)
122. A leak check of the machine’s low-pressure system is performed to confirm the integrity of the anesthesia machine from the flowmeters to the common gas outlet. It evaluates the portion of the anesthesia machine that is downstream from all safety devices, except the oxygen monitor. The low-pressure circuit is the most vulnerable part of the anesthesia machine because the components located within this area are the ones most subject to breakage and leaks. The machine’s low-pressure system must be checked because leaks in this circuit can lead to hypoxia or patient awareness, or both. (215)
123. The oxygen monitor is the only machine safety device that detects problems downstream from the flowmeters. The other machine safety devices (the fail-safe valve, the oxygen supply failure alarm, and the proportioning system) are all upstream from the flowmeters. (216)
124. No. A positive-pressure leak check of the breathing system must be performed before every procedure. This test does not check the integrity of the unidirectional valves inasmuch as the breathing system will pass the leak check even if the unidirectional valves are incompetent or stuck shut. (216, Table 15-9, items 12-13)