Headache and vertigo of cervical origin
Headache
Headache is one of the most prevalent pain disorders, affecting 66% of the global population, with a daily prevalence of 4–5%.1 This major health problem, disturbing both quality of life and work, is comparable to back pain in that it affects mostly the active population and thus has socio-economic consequences, generating high costs for therapy as well as for absence from work. It was reported in 1999 that in the US alone, migraine headache cost American employers about $US13 billion per year because of missed workdays and impaired work function.2 Headache is not an easy issue. It occurs in many forms and the causal mechanisms are still not well understood.3 Furthermore, the patient’s description may be so vague that distinction between the common benign forms and more serious syndromes is sometimes difficult. Sensitive structures within the skull can become stimulated by tension, stretching, compression or displacement, all of which can be the consequence of inflammation (e.g. meningitis), increase of pressure (e.g. tumour, haematoma) or decrease in pressure (e.g. lumbar puncture). The same happens when vascular structures dilate or constrict.
Very often the problem lies outside the skull, as in tension-type headache of muscular contraction, trigeminal neuralgia, temporal arteritis and conditions affecting the teeth, the temporomandibular joint, the eyes, the sinuses or other structures in this region. Headache may also be psychogenic.4
The differential diagnosis of headache is challenging and should proceed in an orderly fashion. Crucial elements include a thorough history, supplemented by general medical and neurological examinations, as well as laboratory testing and neuroimaging in selected patients. By using a logical and clinical classification system, the clinician will be able to identify those patients with headache suited to orthopaedic therapy.
The first step is to distinguish primary from secondary headaches. The International Headache Society (IHS) has classified headaches as primary, where there is no other causative factor, or secondary, where the headache occurs in close relationship to another disorder to which it is attributed.5 The former tends to decline with age, while the prevalence of the latter increases.6
Primary headache is usually classified into three main groups. The most common form is tension-type headache, a mild to moderate dull pain, often brought on by stress which has a global prevalence of 83%.7 Migraine has a prevalence of 10%, and cluster headache constitutes for about 3% (Box 1). Except migraine, which can sometimes be treated with techniques described in this book, these primary headache disorders fall outwith the scope of this work and will, therefore, only be discussed briefly.
Secondary headaches are related to other disorders. This can be a local lesion in the head or neck or a generalised lesion (Box 2). Orthopaedic medicine is interested in the secondary headaches in which the cause must be sought in the cervical spine.8
Primary headache
Tension-type headache
Tension-type headaches (TTH) are the most common chronic headaches. Rasmussen and colleagues surveyed 1000 adults from the general population and reported a lifetime prevalence of TTH of 69% in men and 88% in women.9 Jensen reported a lifetime prevalence of 78% in a general adult population.10 Some 30% were affected more than 2 weeks a year and 3% were labelled as chronic. The term describes headaches that have previously been grouped under various ill-defined headings such as ‘tension headache’, ‘stress headache’ and ‘muscle contraction headache’.
TTH is described as a dull, non-pulsatile pain, affecting the entire head, of oppressive and progressive character, moderate or severe intensity, variable duration (up to several days) and lacking the typical features of migraine. In 90% of cases the pain is bilateral, the typical location being in the occipital, parietal, temporal and frontal areas.11,12 Though the duration and intensity of the pain is variable, this headache is not as debilitating as migraine, and sufferers are usually able to continue their daily activities.
Box 3 cites the IHS operational criteria for tension-type headache.13
Although TTH is the most frequent type of primary headache (two-thirds of the population have suffered an episodic TTH), its physiopathology is still the cause of controversy. For many years it has been thought that TTH was directly related to muscular tension. However, more recently it has been postulated that, although muscular tension is usually present in most cases, it is possible that the origin is more central, due to the hyperexcitability of the trigeminal caudal nucleus and of other structures of the central nervous system that register, modulate and interpret head pain. Precipitating factors for tension-type headache are emotional stress, anxiety, depression and myofascial pain.14,15 This pathology type may also be induced, intensified or made chronic by analgesic abuse.16
Treatment
For acute episodes, the most common treatment involves the use of simple analgesics and anti-inflammatory medications. For chronic tension-type headache and for prevention, amitriptyline17,18 is considered the treatment of choice, but also nortriptyline, mirtazapine19 and tizanidine can be used. Amitriptyline (AMT) is a tricyclic antidepressant, possessing an analgesic effect that is independent of its antidepressive effect. The analgesic mechanism is not precisely known. Probably, serotonin (5-HT) and noradrenaline reuptake inhibition of the CNS plays a fundamental role in the control of the pain.20
Migraine headache
Migraine is a neurovascular disorder characterized by neuronal aura symptoms and vascular headache. Since 1988 migraine is defined by the criteria set by the Headache Classification Committee of the International Headache Society (IHS).13 It consists of two subgroups: migraine with aura and migraine without aura. In 20% of cases, the migraine headache is preceded by a visual hallucination/illusion known as an aura. Typically, the aura is a serrated arc of scintillating, shining, crenulated shapes, beginning adjacent to central vision and expanding peripherally over 5–20 min, within one visual field, usually followed by headache. The scintillations are followed temporarily by a blind region, after the same retinotopic progression from central to peripheral visual fields. Sometimes the visual hallucinations are accompanied by unilateral paraesthesia or numbness, hemiparesis and dysphasia. The typical characteristics of migraine headache are unilateral location, pulsating quality, moderate or severe intensity and aggravation by routine physical activity. The pain peaks and then subsides, and usually lasts between 4 and 72 hours in adults and 1 and 48 hours in children. The frequency of attacks is extremely variable, from a few in a lifetime to several times a week, and the average migraineur experiences from one to three headaches a month. The pain is accompanied by other features. Nausea occurs in almost 90% of patients, while vomiting occurs in about one-third of patients. Many patients experience sensory hyperexcitability manifested by photophobia, phonophobia, osmophobia and seek a dark and quiet room. Blurred vision, nasal stuffiness, diarrhoea, polyuria, pallor or sweating may be noted during the headache phase. There may be localized oedema of the scalp or face, scalp tenderness, prominence of a vein or artery in the temple, or stiffness and tenderness of the neck. Impairment of concentration and mood are common. Box 4 presents the diagnostic criteria.21
Many theories have been formulated in the last six decades about the pathogenesis of migraine, but the problem is still not fully clarified.
The first theory, conceived by Wolff in 1948, is build up on a vascular basis, considering three observations: (a) during the migraine attack extra-cranial vessels dilate and are throbbing in many patients; (b) the stimulation of intra-cranial vessels provokes an ipsilateral headache; (c) vasoconstrictor drugs, like ergot derivatives, show a curative effect. On these observations, he hypothesized that an intra-cranial vasoconstriction could be responsible of the migraine aura and of the following hyperaemic reaction with local vasodilatation and activation of the perivascular nociceptive endings, resulting in acute unilateral headache.22
Another possible interpretation of the migraine pain is proposed on the basis of a depression of the cortical electrical activity, the so-called ‘cortical spreading depression theory’. Shortly before migraine headache begins, a spreading depression develops in the cortex of one hemisphere. This cortical spreading depression (CSD) is a relatively short-lasting wave of depolarization that spreads across the surface of the brain, moving from the back (occipital region) of the cerebral cortex toward the front at about 3–5 mm/minute. This phenomenon is frequently referred to in the literature as the ‘spreading depression of Leao’.23 CSD begins with a brief wave of excitation, followed by a prolonged period of neuronal depression, which is associated with disturbances in nerve cell metabolism and regional reductions in blood flow.24 Experimental evidence supports a relationship between CSD as a cause of migraine aura.25 Also during an aura, cerebral blood flow abnormalities are often seen. Support for the CSD theory comes from observations that, in patients who have migraine with aura, a gradual spread of reduced blood flow that mimics the rate of progression of CSD can be measured during the aura phase.26
Current theories however explain migraine as a neurovascular disorder27 in which the trigeminocerebrovascular system plays an unique pivotal role. It is hypothesized that the primary dysfunction in migraine occurs within the central nervous system and that this evokes changes in blood vessels within pain-producing intracranial meningeal structures that give rise to headache pain.28 The brain itself is not provided with pain sensitive endings, while meninges are rich of nociceptors.29 Tracing studies have identified the trigeminal nerve as the major afferent pathway for pain from the vessels and dura mater.30 This trigeminovascular system consists of the neurons that innervate the cerebral vessels and the dura and whose cell bodies are located in the trigeminal ganglion. This ganglion contains bipolar cells. Peripheral fibres innervate blood vessels in the meninges, the extracranial arteries, and those in the circle of Willis. These nerve fibres contain nociceptors that are capable of generating pain impulses, and the endings of these nerve fibres contain peptide neurotransmitters.31 The centrally projecting fibre synapses in the caudal brain stem or high cervical cord. The trigeminal innervation is predominantly to the forebrain but extends posteriorly to the rostral basilar artery, whereas the more caudal vessels are innervated by the C2 and C3 dorsal roots, which also synapse with the central trigeminal neurons.
The neurovascular hypothesis proposes that either migraine triggers or CSD (cortical spreading depression)32 can activate trigeminal nerve axons, which then release neuropeptides (such as substance P, neurokinin A, and CGRP) from axon terminals near the meningeal and other blood vessels.33 Substance P and neurokinin A cause vasodilation and promote the extravasation of plasma proteins and fluid from nearby meningeal blood vessels, where they produce an inflammatory response. This response is termed sterile neurogenic perivascular inflammation. The neuropeptides may also sensitize nerve endings, providing a mechanism for sustaining the headache. When activated, the trigeminal nerve also transmits pain impulses to the trigeminal nucleus caudalis, which relays pain impulses to higher centres of the brain. According to the neurovascular theory, vasodilation is not the cause of migraine headaches but is an accompanying phenomenon attributable to trigeminal nerve activation. Although the cause of this activation is not known, it may be due to ionic and metabolic disturbances in brain function, such as those associated with CSD. It has also been proposed that abnormal activity in brain stem sensory nuclei may cause antidromic activation of trigeminal sensory pathways.34
The integrated hypothesis of migraine pathogenesis is an attempt to consolidate various theories and explain several observations related to migraine pain. According to this theory, triggers such as stress, glare, noise, the patient’s internal clock, the dilation of the internal or external carotid arteries, or other factors may activate specific centres in the brain stem. One such centre, the locus ceruleus, causes changes in epinephrine levels. Another centre, the dorsal raphe nucleus, affects serotonin levels in the brain.35 They cause constriction of cerebral blood vessels and a localized deficiency in blood flow, provoking CSD. This, in turn, stimulates trigeminovascular fibres, eliciting neurogenic inflammation and headache pain. Nerve fibres from the locus ceruleus, the dorsal raphe nucleus, and the trigeminal nerve cause a stimulation of cranial nerves that dilate both cerebral and extracranial blood vessels. The dilation of meningeal vessels contributes to pain generation.36 The locus ceruleus also sends fibres to higher centres of the cerebral cortex, where it influences a person’s state of arousal and awareness, and descending projections interact with the body’s pain control mechanisms. Likewise, the dorsal raphe nucleus sends multiple fibres to blood vessels and upward toward the cerebral cortex. These serotonin-secreting fibres help regulate sleep and neuro-endocrine functions. Other connections are made with lower brain stem areas and with the hypothalamus. A disruption in the normal function of the hypothalamus may be responsible for prodromal signs and symptoms of migraine such as mood changes, food cravings, drowsiness, thirst and yawning.37 These signs and symptoms may occur several hours, or even as long as 1 day, before headache pain begins.
Treatment
The classic therapeutic approach has three major pillars: avoidance of migraine triggers, treatment of the acute attack by medication and regular use of preventive medications.
Acute attacks are treated with simple analgesics and NSAIDS, and more migraine-specific drugs, such as ergot-derivates38 and triptans, which are active at 5-HT1 receptors. However, triptans (sumatriptan and its six licensed successors), because of their better tolerability, have replaced ergotamine in most cases.39 They reduce neuronal activity via these receptors at the trigeminocortical complex and thalamic level. There are still situations where tolerability and contraindications to use are a problem. The main issue for triptans relates to their vasoconstrictor properties and related cardiovascular and cerebrovascular safety concerns. This necessitates that triptans are not used in patients with cerebrovascular or cardiovascular contraindications.40
Prevention
Migraine prevention is an important component of therapy aimed at reducing the attack frequency and severity. Unfortunately, the mechanisms of action of current preventives are not well understood. A potential mechanism is the inhibition of cortical spreading depression but, as noted above, the efficacy against cortical spreading depression does not necessarily predict the efficacy in treating migraine without aura. Substances that have proven beneficial in migraine, with and without aura, broadly comprise compounds from the following classes: beta-blockers (propranolol), antidepressants (amitriptyline), anticonvulsants (valproate and topiramate), calcium channel blockers (flunarizine) and serotonin antagonists (methysergide). According to the pathophysiological concepts discussed above, these drugs most probably target the activity of modulatory circuits as well as the neuronal activity in afferent sensory pathways such as the trigeminal system.41 Although many patients can be effectively managed using the available substances, side effects and contraindications because of co-morbidities can complicate treatment. A particular problem is the prediction of which patients will respond to which substance as treatment is still largely conducted by trial and error.
Migraine and orthopaedic medicine
On empirical grounds Cyriax42 found that manipulation of the cervical spine may have a therapeutic effect. He discovered that the onset of an acute attack could sometimes be aborted by 30 seconds of strong neck traction. Other observations have also demonstrated some relationship between mechanical stimulation of the occipital nerve and induction or abortion of migraine attacks.43 Also nerve blocks of the greater occipital nerves proved to be curative in a high percentage of migraine sufferers.44,45 These results reinforce previous evidence of convergence of cervical afferents on the trigeminal sensory circuit and suggest that, by modifying the central processing of pain signals in migraine in the thalamus, greater occipital nerve blocks shut down several symptom generators.46,47 Therefore, manipulation of the cervical spine can have a preventive effect in some patients, especially the middle-aged or elderly and it is always worthwhile manipulating the cervical spine in migraineurs of over 40 years of age. When the neck movements are painless, one session usually suffices. Manipulation is performed in four directions: both rotations and both lateral flexions. Painful neck movements may require two or three sessions. The techniques are described in Chapter 11.
Cluster headache
Also called ‘Horton’s neuralgia’, this headache is characterized by recurrent short-lasting attacks (15 to 180 minutes) of excruciating unilateral periorbital pain accompanied by ipsilateral autonomic signs (lacrimation, nasal congestion, ptosis, miosis, lid oedema, redness of the eye).48,49 It affects young adults, predominantly males. Prevalence is estimated at 0.5–1.0/1000. Cluster headache (CH) has a circannual and circadian periodicity, attacks being clustered (hence the name) in bouts that can occur during specific months of the year. An attack can be triggered by alcohol, strong odours and napping. During bouts, attacks may happen at precise hours, especially during the night. Cluster headache seems to be associated with trigeminovascular activation and neuroendocrine and vegetative disturbances, though the precise causative mechanisms remain unknown. Involvement of the hypothalamus has been confirmed, explaining, at least in part, the cyclic aspects of CH.50 The disease is familial in about 10% of cases. Genetic factors play a role in the susceptibility, and a causative role has been suggested for the hypocretin receptor gene. Diagnosis is clinical. Differential diagnoses include other primary headache diseases such as migraine. At present, there is no curative treatment. There are efficient treatments to shorten the painful attacks and to reduce the number of daily attacks. Acute treatment is based on subcutaneous or intra-nasal administration of sumatriptan.51 Verapamil, lithium, methysergide, prednisone, greater occipital nerve blocks and topiramate may be used for prophylaxis. In refractory cases, deep-brain stimulation of the hypothalamus and greater occipital nerve stimulators have been tried in experimental settings.52 The disease course over a lifetime is unpredictable. Some patients have only one period of attacks, while in others the disease evolves from episodic to chronic form.
Cervicogenic headache
Cervicogenic headache (CEH) is a syndrome characterized by chronic hemi-cranial pain that is referred to the head from either bony structures or soft tissues of the neck. Sjaastad and colleagues53,54 have, in consecutive publications since 1990, established criteria for the diagnosis of headache of cervical origin (Box 5), since 1983 known as ‘cervicogenic headache’.
The pain is mild, usually has an undulating course and may eventually become chronic. There is a marked female preponderance. The neck seems to be involved, either because there has been a trauma, for example whiplash, or because there is limitation of neck movement. There may be accompanying shoulder and/or arm pain. An attack can be precipitated either as the result of neck movement or of direct pressure, for example on examination.55 In their early publications Sjaastad et al mention the headache as being strictly unilateral. Later they restate this and define unilaterality as follows: the headache dominates on one side; when weak, the pain may be only on that side; when severe, it may also be felt on the contralateral side, but to a lesser extent. It never dominates on the contralateral side.56
The fact that neck structures can give rise to headache is now generally accepted. There seems to be a consensus on the pathophysiology. The neuroanatomical basis for CEH is the ‘trigemino-cervical nucleus’. This is a region of the upper cervical spinal cord where sensory nerve fibres in the descending tract of the trigeminal nerve (trigeminal nucleus caudalis) are believed to interact with sensory fibres from the upper C1–C4 cervical roots. This functional convergence of upper cervical and trigeminal sensory pathways allows the bi-directional referral of painful sensations between the neck and trigeminal sensory receptive fields of the face and head. C1 spinal nerve has some ectopic sensory ganglia and it innervates the short muscles of the suboccipital triangle.57 The C2 spinal nerve gives sensory supply to the median and lateral atlantoaxial joints; to several neck muscles (prevertebral, sternocleidomastoid, trapezius, semispinalis and splenius muscles); to the dura of the posterior cranial fossa and the upper spinal canal. Both the C2 and C3 spinal nerves supply the zygapophyseal joints of the adjacent segments. The atlantoaxial ligaments and the dura mater of the spinal canal are innervated by the sinuvertebral nerves stemming from the C1–C3 spinal nerves. The origin is sympathetic; the nerves contain nociceptive, proprioceptive, vasomotor and vaso-sensory fibres.58
Cervicogenic headache and orthopaedic medicine
There are a number of orthopaedic medical conditions that can give rise to headache and that often can be approached very successfully by the use of local treatment (see p. 122). Lesions of the extracranial soft tissues of the locomotor system, especially the capsulo-ligamentous structures of the occipito-atlantoaxial complex, may give rise to segmental headache. The cervical dura mater is often responsible for the vague, multisegmental occipito-frontal, temporal or retro-orbital pain (see p. 16).
Headache, referred from the cervical dura mater
A disco-dural interaction at any cervical level or any space-occupying lesion in the spinal canal may give rise to pain felt in the head. The pain usually radiates from the mid-neck up to the temple, the forehead and behind one or both eyes but rarely to the bridge of the nose. This ‘multi-segmentally referred pain’ is dural in origin and will disappear when treatment to the neck is accomplished. For further details see p. 16.
Matutinal headache in the elderly
As the result of arthrosis at the upper cervical joints, ligamentous contracture may develop and can result in ‘segmental’ pain felt in the upper cervical dermatomes which cover the head. The patient typically complains of occipito-frontal headache felt especially in the morning. Treatment includes capsulo-ligamentous stretching. For a detailed description see p. 163.
Postconcussional headache
The upper cervical capsules and ligaments can also become sprained during trauma, for example an accident that causes concussion of the brain after which a period of immobilization follows. The subsequent build-up of ligamentous adhesions finally results in ‘segmental’ headache. Several authors recognize the possibility of upper cervical capsuloligamentous conditions causing pain in the head. The adhesions can be broken manipulatively. A full description can be found on p. 168.
Vertigo
Vertigo or dizziness is not an uncommon complaint of patients who suffer from disorders that affect the cervical spine. Most often these symptoms are not related to the conditions but just occur at the same time as the cervical disorder. If the dizziness is caused by the functional disturbance in the neck, it may be expected to be as ‘treatable’ as any other symptom originating in that area. It would therefore be very helpful to be able to differentiate ‘cervical vertigo’ from other possible causes.
Vertigo originating as the result of movements of the neck initially suggests a cervical origin. These movements, however, influence not only the proprioceptive system of the cervical spine but also the blood flow through the vertebrobasilar arteries. At the same time, the vestibular apparatus may also be disturbed. All these factors in control of balance are interrelated.
Anatomy
Man is aware of his position in space. The cortex therefore receives information from three different systems: optical, vestibular and proprioceptive (superficial and deep). Disturbances in one of these areas often result in vertigo.
Optical system
Visual stimuli pass via the optical nerve, the optic chiasma and the optic tracts, mainly to the occipital cortex, though some traverse the mesencephalon and interact with eye movement, vestibular activity and the muscles of the cervical spine.
Vestibular system
The vestibular system consists of a peripheral part (organ of balance and vestibulocochlear nerve) and a central part (vestibular nuclei and vestibulospinal tract).
Peripheral part
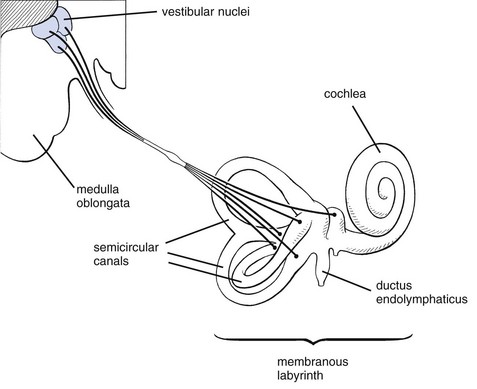
The osseous labyrinth lies in the temporal bone and has three membranous semicircular canals (membranous labyrinth) (Fig. 1). Each lies in contact with an ampulla that contains sensory receptors – the cupulae – a gelatinous substance that moves in the endolymph and in which the otoliths float. It also contains the sensory hair cells. Impulses generated by movement of otoliths within the endolymph pass via the vestibulocochlear nerve (vestibular part) to one of the four vestibular nuclei.
Central part
This is formed by the four vestibular nuclei in the white substance of the pons cerebri from which several tracts originate (e.g. the vestibulospinal tract). These connections facilitate orientation of the position of the body.
There are also many cerebellovestibular connections, responsible for good judgement and adaptation of the direction of movements and for control of anti-gravity muscles.
Much information comes from the vestibular nuclei to modulate the activity of the oculomotor nuclei and thus the ocular muscles. These stimuli cause nystagmus, the main sign of a vestibular disorder.
Proprioceptive system
Superficial, fine tactile sensations are conveyed through the anterior spinothalamic tract. Deep tactile sensations (conscious proprioception is a combination of deep sensation, discrimination sense and vibratory sensation) are transmitted along the fasciculus gracilis and the fasciculus cuneatus. Reflex proprioception (unconscious proprioception) is conveyed centrally via the ventral and dorsal spinocerebellar tracts and the olivospinal tract.
Blood supply
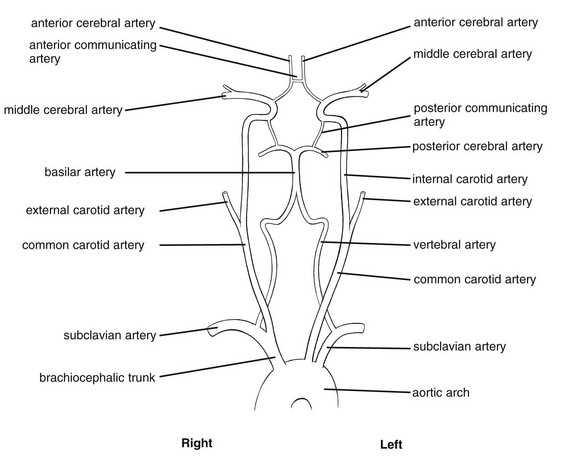
The vertebrobasilar system is a ‘closed’ circuit, starting below in the subclavian arteries and ending above in the arterial circle of Willis (Fig. 2).
The left subclavian artery originates directly from the aortic arch as does the left common carotid artery. The right subclavian artery and the right common carotid artery are branches of the brachiocephalic trunk. The common carotid artery divides (usually at the level of the fourth cervical vertebra) into the external and internal carotid arteries.
The external carotid artery is of less importance in this context and supplies the outer parts of the head – the face, the temporal area, the occipital region, the skin of the head and the mouth.
The internal carotid artery gives off very few branches during its extracranial course but supplies a considerable part of the brain via the anterior cerebral (medial aspect of the hemispheres) and the middle cerebral artery (a continuation of the internal carotid artery, which supplies the entire outer and lateral aspects of the brain), before it anastomoses again with the vertebrobasilar system.
The vertebral arteries originate bilaterally from the subclavian arteries, of which they usually form the first and biggest branches. They run parallel on both sides of the spinal column and form the main blood supply for the brainstem, the cervical spinal cord and the cervical spine. They are closely related to this part of the spinal column and the vertebrae are adapted to the presence of the artery in that the transverse processes C1–C6 contain a transverse foramen through which the artery runs (see online chapter Applied anatomy of the cervical spine). A groove for the artery lies above the posterior arch of the atlas, dorsal to the lateral masses. Occasionally, this groove is closed to form an arterial canal.
The vertebral artery is divided into four segments59,60 (Fig. 3):
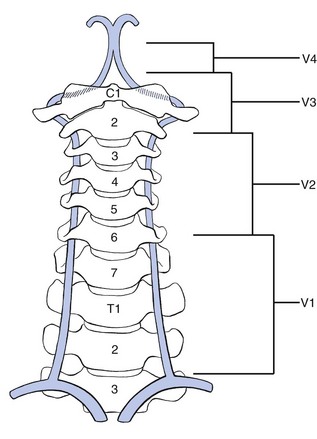
Fig 3 The course of the vertebral artery: V1, extravertebral segment; V2, intervertebral segment; V3, atlantoaxial segment; V4, subforaminal and intracranial segment.
Extravertebral segment
The artery runs upwards from the subclavian artery to the transverse foramen of the sixth cervical vertebra. It is surrounded anteriorly by the anterior scalenus and longus colli muscles; posteriorly and distally it is adjacent to the first rib and the transverse processes of the first thoracic and the seventh cervical vertebrae.
Intervertebral segment
From the sixth to the second vertebrae the vertebral artery passes through a canal formed by the transverse foramina and, between the vertebrae, by ligamentous and muscular structures. The anteromedial border is formed by the uncovertebral joints (von Luschka’s joints).
The vertebral artery runs anterior to the nerve roots and spinal nerves, the latter lying in the sulcus for the spinal nerve. An uncoarterioradicular junction is thus formed. This is of clinical importance because arthrotic changes at these levels may have important consequences for the blood flow in these arteries.
The contents of the transverse foramina include, in addition to the vertebral artery, two vertebral veins, the periarterial venous plexus of the vertebral artery and the vertebral nerve.
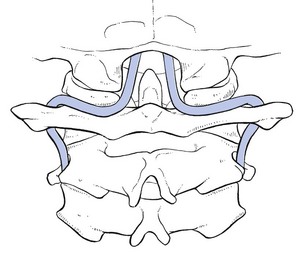
Atlantoaxial segment
Between the axis and atlas the artery curves backwards and outwards (Fig. 4) because the transverse foramina of the atlas lie more laterally than those of the other vertebrae. The artery runs dorsally around the lateral mass of the atlas and loops over the posterior arch in the arterial groove. It is surrounded anteriorly by the joint capsules of the atlanto-occipital joints and posteriorly by the obliquus capitis superior and rectus capitis posterior major muscles.
Subforaminal and intracranial segment
The artery curves upwards again and runs cranially from posterolateral to anteromedial to pierce the posterior atlanto-occipital membrane, the dura mater and the arachnoid mater, and to enter the skull via the foramen magnum.
Just below the base of the brain and at the level of the pons, the left and right arteries join to form the basilar artery. The latter trunk then splits into a left and right posterior cerebral artery. From the vertebral and basilar arteries, branches originate to supply parts of the brain, especially the cerebellum.
A characteristic of the blood supply of the brain is the close connection of the different arteries by means of communicating arteries. Together they form the arterial circle of Willis. From posterior to anterior the circle of Willis contains two posterior cerebral arteries, both originating from the basilar artery. They are both connected via posterior communicating arteries with their respective internal carotid artery. From here depart both anterior cerebral arteries, connected by one anterior communicating artery. The circle is thus completed.
Blood supply by the vertebral arteries
At the cervical level the vertebral arteries give off cervical branches and cranial branches, the latter anastomosing with the spinal rami.
There are extensive anastomoses with the deep cervical artery (originating via the costocervical trunk from the subclavian artery), the thyrocervical trunk and its branch (the ascending cervical artery) and the occipital artery (branch of the external carotid artery). Spinal rami supply the anterior and posterior roots, the epidural blood vessels and the vertebral canal, especially the upper two vertebrae. Articular rami supply the joint capsules of the intervertebral and uncovertebral joints. Muscular rami supply the intrinsic muscles of the cervical spinal column and cutaneous branches supply the skin.
Just before both vertebral arteries join to form the basilar artery, they give off, unilaterally or bilaterally, a branch that forms (together with its fellow) the anterior spinal artery, which supplies the anterior aspect of the spinal cord.
The posterior spinal arteries also originate from the vertebral arteries and supply the posterior aspect of the spinal cord, where they anastomose extensively with the spinal rami.
Intracranially, the branches that form the posterior inferior cerebellar arteries supply the posterolateral part of the medulla oblongata, part of the posterior lobe of the cerebellum, the vermis and the cerebellar nuclei.
The basilar artery and its branches form the blood supply for the medulla oblongata, the reticular formation, the pons, the mesencephalon, parts of the cerebellum (via the anterior inferior cerebellar artery and the superior cerebellar artery), the vestibular system and its nuclear complex (via the labyrinthine artery).
The posterior cerebral arteries are part of the circle of Willis via anastomoses with the internal carotid artery. They supply parts of the thalamus and hypothalamus, the occipital lobe, large parts of the temporal lobe, the red nucleus, the substantia nigra, the nuclei of the oculomotor nerve (III) and the trochlear nerve (IV).
Blood flow
Together the vertebral arteries form a functional unit. In normal circumstances, disturbance of the function of one artery is immediately compensated for by the other, provided it is healthy. Failure adequately to compensate leads to complaints and symptoms, especially vertigo and tinnitus. Provocation tests, directed at the function of the vertebrobasilar system, are designed to test this compensation mechanism (see later).
Severely diminished flow in one vertebral artery may well lead to occlusion of the posterior inferior cerebellar artery on that side, resulting in a lateral medullary infarction – Wallenberg’s syndrome (Box 6).
Blood flow in the vertebral arteries may be influenced by movement, compression, trauma or vascular factors.61 However, the functional adaptability of structures within the spinal canal, and intervertebral and transverse foramina is so high that anatomical changes such as narrowing do not necessarily cause symptoms.
Movement
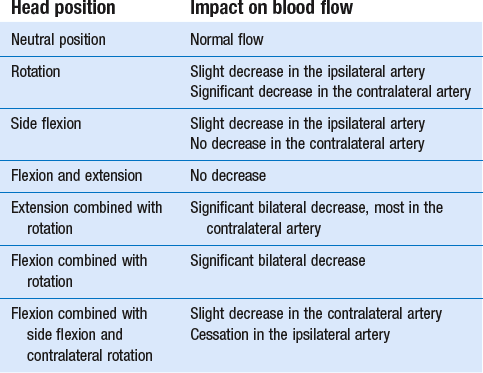
Different authors agree that maximal rotation of the cervical spine diminishes blood flow in the contralateral vertebral artery. This effect is greatly increased when rotation is combined with flexion, extension and/or side flexion.
Chrost, cited by Gutmann and Biedermann,62 has made a survey in terms of percentage of the blood flow in the vertebral arteries in different positions of the head (Table 1). Flexion and extension movements have very little influence on the blood flow in the vertebral arteries because the axis of flexion and extension of each segment lies in the same course.
Side flexion of the local spine gives rise to moderate diminution of blood flow in the ipsilateral artery.
Rotations have greatest influence on the blood flow. ‘Redundancy’ of length may also help to accommodate movements. For example, the distance between the vertebral artery and the axis of rotation is greatest in the atlantoaxial segment and redundancy is therefore required at this level in the atlantoaxial loop of the artery so that, even if it is extended during full rotation of C1 on C2, neither the lumen nor flow is reduced. During 30° of rotation there is an influence on the contralateral vertebral artery and during 45° of rotation the artery is almost completely occluded. On the other hand, there is an atlanto-occipital loop between C1 and the occiput. This curve does not change during movement and may be considered as a buffer mechanism against strong arterial pulsations.
Compressing factors
There are a number of compressing factors.
In the extravertebral segment
Exceptionally, compression may be related to anatomical anomalies (an unusual origin of the artery) or to shortening in the prevertebral musculature.
In the intervertebral segment
Osseous growth at the edges of the vertebral bodies (spondylosis) and degenerative changes in one or more intervertebral joints (spondylarthrosis) may cause compression.63 The intervertebral disc is of lesser importance here, except in large disc protrusions where the vertebral artery may rarely become threatened.
In the atlantoaxial segment
Circulation in the vertebral artery is impaired, on the one hand, by congenital arterial anomalies (hypoplasia of one or both vertebral arteries, aplasia of one artery, anomalies in the circle of Willis) and, on the other hand, anomalies and positional changes in the upper cervical vertebrae, such as basilar impression, an extraoccipital condyle, assimilation of the atlas (fusion of the atlas with a part of the base of the skull), osseous canal for the vertebral artery at the upper part of the posterior arch of the atlas, odontoid anomalies (aplasia, asymmetry), os odontoideum (articulation between odontoid process and the body of axis) and fusion of C1–C2.
These anomalies are rare and are not detected on routine examination.
Nystagmus
The main sign that characterizes vertigo is the presence of nystagmus, an involuntary repetitive movement of the eyes – the cardinal sign of a vestibular disorder.59,64
Classification
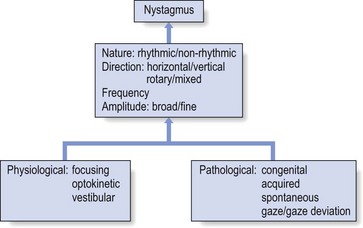
Nystagmus can be classified according to its nature (rhythmic or not), its direction (horizontal, vertical, rotary or mixed), and its frequency or its amplitude (broad or fine) (Fig. 5).
Types65
Nystagmus can be physiological or pathological.
Physiological nystagmus
Focusing nystagmus occurs when a fixed point is looked at.
Optokinetic nystagmus occurs when objects pass by the onlooker with a certain regularity or when a moving onlooker passes by a number of stationary objects (e.g. looking out of a window when travelling in a train).
Vestibular nystagmus is seen when the head is quickly turned in one direction. Movement of endolymph within the canals lies in relation to that of the head and continues momentarily after the head has stopped. The cupula organs in the ampullae of the semicircular canals are moved in the direction of lymph flow and are thus stimulated. Muscles, especially the extraocular muscles, are influenced by this and the position of the eyes changes as the head is turned, in a direction opposite to the rotation. At first the eyes remain behind but are then brought back to their normal position. This eye movement, slow first and opposite to the turning direction, followed by a quick movement in the direction of the rotation is called ‘nodding eye movements’ or ‘nystagmus’. The slow phase is caused by impulses from the labyrinth; the quick phase is the result of corrective mechanisms in the ocular muscles.
Pathological nystagmus
Nystagmus can be either congenital or acquired. In the latter, it occurs spontaneously or as the result of movement (positioning or provoking nystagmus).
Spontaneous nystagmus can have a peripheral cause. In this case, a one-sided viewing-direction nystagmus is found: a nystagmus that is latently present becomes worse when the patient is asked to look in one direction. When the problem is central, nystagmus may present in different situations:
• Fixation nystagmus occurs when the patient tries to fix a certain point.
• Two-sided viewing-direction nystagmus (symmetrical nystagmus) is seen when a person looks in one direction and then in the opposite direction.
• Exclusive one-sided viewing-direction nystagmus is not latently present but occurs only when looking in one direction.
• Flagging movements or dysconjugation of movements of the eyes is seen when the examiner makes a pendulum movement with the arm.
• Gaze nystagmus or gaze deviation nystagmus always has a central cause. It may be present for as long as the patient gazes and the eye movement may not be horizontal.
Aetiology and classification of vertigo
It is usual to classify vertigo into two groups, depending on the cause: vestibular and non-vestibular vertigo. Vestibular vertigo is divided into peripheral and central types.
Vestibular vertigo
Peripheral
Vertigo is peripheral when the cause of the symptoms lies in the peripheral vestibular system, containing the labyrinth and the vestibular part of the vestibulocochlear nerve.
Peripheral vestibular vertigo is characterized by short-lived heavy turning sensations with a sudden onset. It may be accompanied by hearing disturbances66 (tinnitus, deafness) and sometimes important autonomic nervous system symptoms, such as palpitations, anxiety, nausea, vomiting, sweating and fluctuations in blood pressure. There are some disorders that selectively involve the peripheral vestibular system to cause dizziness without hearing loss, for example benign paroxysmal positional vertigo, vestibular neuritis and bilateral idiopathic vestibulopathy.67 Transitory spontaneous nystagmus, rotating or horizontal, and in the same direction may also be present. It is regular and with a latency period.
Most disorders in the peripheral vestibular complex lead to vertigo: injuries, infections, neoplasms, labyrinthine vascular accident, Ménière’s syndrome and vestibular neuronitis.
Central
The lesion lies in the vestibular nuclei or their tracts. Central vertigo may result from injury, multiple sclerosis, tumours, cerebral arteriosclerosis and vertebrobasilar insufficiency leading to brain stem ischaemia or cerebellar stroke.68
Disturbances of equilibrium accompany the vertigo. Symptoms are persistent, sometimes with acute exacerbations. Auditory disturbances are rare and neurovegetative symptoms are also less pronounced.
There is persistent spontaneous nystagmus, which sometimes varies and changes direction. It is irregular and has no latency period.
Non-vestibular vertigo
This type of vertigo may have different causes: ophthalmological, psychiatric, orthostatic hypotension, visual and auditory disorders, and possibly alteration in the proprioception from the cervical joints. Hyperventilation, circulatory disorders, arterio-sclerosis, brain sclerosis, skull injury and anaemia are other possibilities.
A special form of non-vestibular vertigo occurs in disorders which affect the cervical spine. In this form, a distinction can be made between either a vascular or a proprioceptive cause. The former may follow circulatory disorders in the area supplied by the vertebral arteries; the latter results from disturbances in the proprioception of the joints of the upper cervical segments, mainly as the result of arthrosis or a disc protrusion.
Important features in the functional examination of a patient complaining of vertigo
In view of the treatment of the neck patient within orthopaedic medicine it is of extreme importance to differentiate between the following types of patients:
• The patient who shows a contraindication for any type of cervical treatment, when the problem lies within the vertebrobasilar system. The examination is then meant to recognize the possible risk factors.
• The patient whose vertigo has nothing to do with the neck, when the vestibular system is affected and in whom a normal treatment can be given to the cervical spine.
• The patient in whom the vertigo is cervicogenic and can be treated as such. This is so when the proprioceptive system is disturbed.
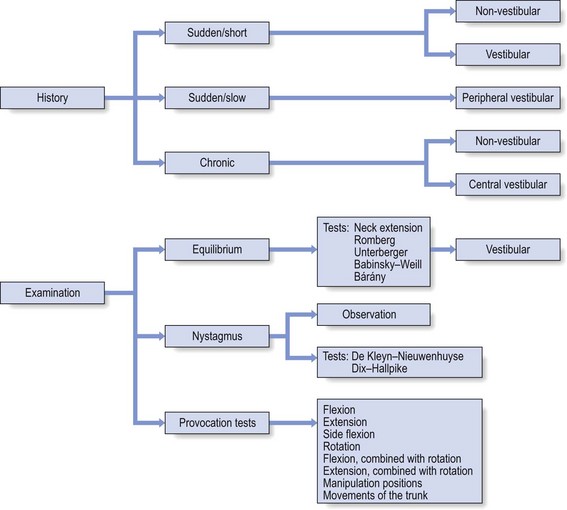
Requests came from several sources for the recognition of risk factors in patients who may need to receive manual and/or manipulative treatment to the neck. This has led to the development of ‘Clinical Standards for Pre-manipulative Testing of the Cervical Spine’. The first and so far most important and internationally recognized standard is the Australian one,69 from which other standards have been derived.70–74 There are mutual differences but from these standards emerges a consensus on some important features: a patient with complaints of vertigo should be put through meticulous history-taking, an orientating otoneurological examination and a number of dizziness provocation tests.
History
The history is extremely important. The moment the patient mentions symptoms that could relate to vertigo (dizziness, tinnitus, anxiety, palpitations, excessive perspiration), the examiner should determine whether or not vertigo is truly present. This is achieved by the following sequence of questions:
• Are there real turning sensations or just a dizzy feeling? Turning sensations point towards a vestibular disorder, usually in the peripheral part. This also applies to motion sickness, for example car sickness, for which the cause is usually vestibular.
• How did it start – suddenly or gradually? This may indicate the type of vertigo present (see below).
• How severe are the complaints?
• Is the vertigo influenced by certain positions?
Questions should also be asked about other symptoms, such as deafness, tinnitus, headache, nausea/vomiting, neurological symptoms (disturbances of vision or speech, paraesthesia, diminution of consciousness) and infection/fever.
Clinical examination is not wholly reliable and much attention should be paid to history. The diagnosis is made on the recognition of certain patterns of symptoms. The importance for the orthopaedic physician is that a correct provisional and general diagnosis is made and the patient correctly referred for specialist attention.
Based on the history, three types of vertigo can be distinguished:
• Sudden, severe and short: a sudden attack, coming on rapidly, lasts only a short time (from a few seconds to some hours) and disappears quite quickly. The causes can be non-vestibular (benign paroxysmal positioning vertigo, orthostatic vertigo, hyperventilation, cervical vertigo) or vestibular as the result of insufficiency of vertebral and/or basilar arteries. Differential diagnosis should be made from Ménière’s syndrome, hypoglycaemia or syncopation.
• Sudden, severe and gradually disappearing: a sudden and severe attack is much slower to disappear (over several days to several weeks). It is caused by a peripheral vestibular condition, such as vestibular neuritis, labyrinthine injury, labyrinthine vascular accident, (peri)labyrinthitis or herpes zoster oticus. Differential diagnosis is required from multiple sclerosis.
• Chronic, permanent/long-standing and not severe: not very severe, but continuously present, chronic vertigo with slight exacerbations may go on for months without change. It can be caused by non-vestibular or central vestibular lesions (cerebral atherosclerosis, hypertension, tumour between the pons and cerebellum and skull injury).
Clinical examination
The information presented here is a summary of some of the clinical tests which enable distinction of a non-vestibular vertigo from a vestibular one, the latter having either a central or peripheral cause.
Orientating examination of equilibrium
The following tests are appropriate for the vestibular system:
Neck extension
The patient is asked to look at the ceiling for about 20 seconds or less if he is not able to maintain that position.
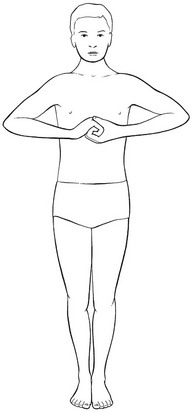
Romberg’s test
Assesses static equilibrium. The patient stands with both feet together, if possible, and performs Jendrassik’s grip (reinforcement) (Fig. 6). If this seems impossible, he holds his arms outstretched in front of him. When it is possible to hold the position with the eyes open, but not with the eyes closed, the test is considered positive. The patient then usually tends to fall to one side. Further neurological examination is then required.
Unterberger’s test
The patient stands with the arms forwards and must try to ‘step’ on the spot, lifting the knees. Rotation of 45° per 50 steps is considered normal, more than this is pathological. The examiner should note the direction in which the patient moves (Fig. 7).
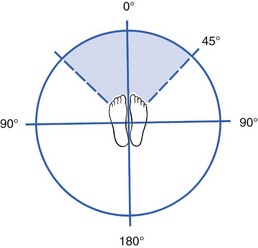
Fig 7 Unterberger’s test: the patient walks on the spot. Rotation of more than 45° per 50 steps is pathological.
There seems to be disagreement about the value of this test. However, it was demonstrated in a prospective study of 100 patients with unilateral, subsequently histologically proven acoustic neuroma, that Unterberger’s test achieved a higher sensitivity than the Romberg test.75
In a recent prospective, controlled study Kuipers and Oosterhuis concluded that this test does not appear to be useful for detection of abnormalities in the vestibular system or for distinguishing normal individuals from patients.76 A few years earlier Hickey et al came to the same conclusion.77
Examination of nystagmus
The examiner observes and analyses the involuntary eye movements. The following should be borne in mind:
• Nystagmus has a slow (pathological) phase and a quick (recovery) phase, and its direction is described by the latter. However, the side of the lesion is better indicated by the former (e.g. a quick recovery phase to the right implies a problem to the left).
• The patient cannot influence the examination, nor can compensation be achieved.
Nystagmus may occur spontaneously (Fig. 8) and a combination of opening and closing the eyes may point towards the type of nystagmus present. The examiner next undertakes tests to recognize positioning or provoking nystagmus (see types of nystagmus above). In orthopaedic medicine two tests are important: the De Kleyn–Nieuwenhuyse and the Dix–Hallpike tests.
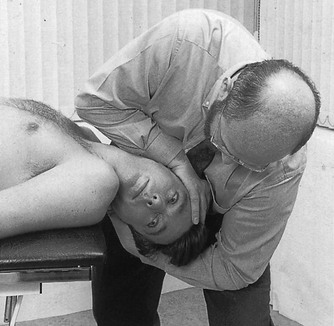
De Kleyn–Nieuwenhuyse test
The patient is in a supine lying position with his head maximally rotated in more-or-less maximum extension (Fig. 9). This provokes diminution or abolition of the blood flow in the contralateral vertebral artery, thereby testing the compensating mechanism in the ipsilateral vertebral artery and in the basilar artery and its branches. Disturbance of blood flow in the ipsilateral artery may result in the appearance of nystagmus or vertigo.
Dix–Hallpike test
The patient sits on the couch with his head turned away. The examiner then brings the patient quickly backwards under guidance. In pathological circumstances nystagmus results, but if it disappears within 30 seconds it is a benign paroxysmal positioning vertigo. Positional nystagmus together with the precipitating mechanism and the Dix–Hallpike test seem to be the best predictors of benign paroxysmal positioning vertigo.78
Provocation tests for dizziness
The patient, either sitting or lying, is subjected to different neck movements: they are performed rapidly and repeatedly at first and then sustained in the end range for at least 10 seconds. The neck movements are purely physiological but may be combined:
• Flexion, combined with rotation
• Extension, combined with rotation
• If manipulation is considered, the patient’s head is also put in the different manipulation positions
• Movements of the trunk while the patient’s head is held motionless.
Technical investigations
The most valuable otological examination methods are electronystagmography and audiometry. Electronystagmography seems to be useful when the cause is thought to be central or is uncertain. It is not significantly helpful in the diagnosis of peripheral lesions except as confirmation.79
Of the clinical neurophysiological methods, brainstem auditory evoked potentials are more useful than electroencephalography. Computed tomography and magnetic resonance imaging should be used when a central nervous system disorder is suspected.80
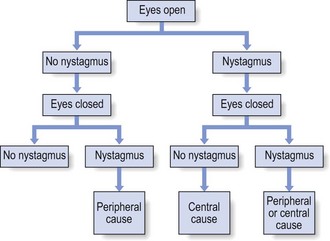
Interpretation of the examination
Careful interpretation of the data from the history and of the findings from the clinical examination (see Fig. 10) must enable the examiner to get an idea of the area where the problem lies: in the vestibular system, the vertebrobasilar system or the cervical spinal joints.
Disturbance in the vestibular system
Disturbance of the vestibular system is movement induced. The rapid execution of different neck movements may cause dizziness and probably also nausea (‘motion sickness’). The examiner must keep in mind that vestibular vertigo may also be the result of acute ischaemia of the vestibular tissues, because of their blood supply via the basilar artery.
Disturbance of the vertebrobasilar system
The most important symptoms that are experienced by patients suffering from vertebrobasilar insufficiency are described by Coman64 as the 5 Ds: dizziness, dysarthria, dysphagia, diplopia, drop attacks. Other symptoms, such as weakness, numbness, gait disturbance and visual abnormalities are also possible.81
The sustained neck positions provoke dizziness and/or nystagmus.
Disturbance of proprioception of the cervical spine
Elements that point towards a vestibular disorder (i.e. turning sensations, motion sickness) are absent. The patient does not mention one of the 5 D symptoms. There is no nystagmus. The neck movements are positive in that they indicate a cervical disorder: pain, stiffness, possibly diminished range of motion, articular pattern. The movements may or may not provoke dizziness. There may be balance disturbance.82
Benign positional paroxysmal vertigo is common in the elderly and even more common in patients with migraine.83 Multivariate analysis demonstrated that the presence of a turning sensation and the absence of a lightheaded sensation predicted its presence.84
Some authors describe upper cervical joint dysfunction as a possible cause of vertigo.85 Others doubt the existence of cervical vertigo as an independent entity and strongly emphasize the need to exclude neurological, vestibular and psychosomatic disorders first.86,87 Posturographic assessment of human posture dynamics could be a possible future tool for use in diagnosing cervical vertigo.88
Oostendorp et al describe a turning/stop turning test (without head and neck movements) that could act as a differentiating element between vestibulogenous dizziness and cervicogenic dizziness.
Turning/stop turning test
The patient sits on a revolving stool with closed eyes. The examiner turns the patient ten times with constant speed in one direction. He then stops the movement and waits until an eventual dizziness has disappeared. The same is then performed in the other direction. The test is positive when the patient recognizes the symptoms, i.e. turning sensations.
When this test is positive the problem is rather vestibulogenous. If the cervical provocation tests are also positive there is clearly a cervicogenous component as well. When the test is negative and the provocation tests positive, the dizziness is of cervical origin.
Therapy
A patient with vestibular vertigo should be referred for specialist examination and treatment. Acute vertigo as the result of dysfunction of the labyrinth or of a serious central nervous system process demands prompt intervention to avoid long-term disability. Chronic vertigo may need surgery or rehabilitative measures, for example physiotherapeutic adaptation exercises, for lasting relief.89
Non-vestibular proprioceptive vertiginous complaints can have a discal origin. In these cases, manipulation with maximum traction and no articular movement (straight pull, traction with leverage) usually gives good results, especially in the elderly. This should of course only be performed by an experienced operator. For the technique, see Chapter 11). If there is any doubt, other conditions must first be excluded by specialized examination. The same techniques have the surprising result of also curing tinnitus. Kessinger mentions good results on cervical manipulation for vertigo, tinnitus and diminished hearing.90
Physical therapy also seems to be an effective treatment for benign positional paroxysmal vertigo.91,92
References
1. Goadsby, PJ, Bench to bedside: what have we learnt recently about headache? Curr Opin Neurol 1997; 10:215–220. ![]()
2. Hu, H, Markson, L, Lipton, R, Stewart, W, Berger, M, Burden of migraine in the United States: Disability and economic costs. Arch Intern Med 1999; 1:813–818. ![]()
3. Schreiber, CP, The pathophysiology of primary headache. Prim Care 2004; 31:261–276. ![]()
4. Silberstein, SD, Lipton, RB, Chronic daily headache. Curr Opin Neurol 2000; 13:277–283. ![]()
5. International Headache Society. The International Classification of Headache Disorders. 2nd ed. Cephalalgia. 2004; 24(Suppl 1):9–160.
6. Lipton, RB, Pfeffer, D, Newman, LC, Solomon, S, Headaches in the elderly. J Pain Symptom Management 1993; 8:87–97. ![]()
7. Stovner, L, Hagen, K, Jensen, R, et al, The global burden of headache: A documentation of headache prevalence and disability worldwide. Cephalalgia 2007; 27:193–210. ![]()
8. Haldeman, S, Dagenais, S, Cervicogenic headaches: a critical review. Spine J 2001; 1:31–46. ![]()
9. Rasmussen, BK, Jensen, R, Schrook, M, Olsen, J, Epidemiology of headache in a general population: a prevalence study. J Clin Epidemiol 1991; 44:1147–1157. ![]()
10. Jensen, R, Pathophysiological mechanisms of tension-type headache: a review of epidemiological and experimental studies. Cephalalgia 1999; 19:602–621. ![]()
11. Sacco, S, Diagnostic issues in tension-type headache. Curr Pain Headache Rep 2008; 12:437–441. ![]()
12. Ailani, J, Chronic tension-type headache. Curr Pain Headache Rep 2009; 13:479–483. ![]()
13. International Headache Society. Operational criteria for tension-type headache. Cephalgia. 24(Supp 1), 2004.
14. Chen, Y, Advances in the pathophysiology of tension-type headache: from stress to central sensitization. Curr Pain Headache Rep 2009; 13:484–494. ![]()
15. Jensen, R, Mechanisms of tension-type headache. Cephalalgia 2001; 21:786–789. ![]()
16. Monteith, TS, Oshinsky, ML, Tension-type headache with medication overuse: pathophysiology and clinical implications. Curr Pain Headache Rep 2009; 13:463–469. ![]()
17. Bigal, ME, Rapoport, AM, Hargreaves, R, Advances in the pharmacologic treatment of tension–type headache. Curr Pain Headache Rep 2008; 12:442–446. ![]()
18. Bendtsen, L, Jensen, R, Olesen, J, A non-selective (amitriptyline), but not a selective (citalopram), serotonin reuptake inhibitor is effective in the prophylactic treatment of chronic tension-type headache. J Neurol Neurosurg Psychiatry 1996; 61:285–290. ![]()
19. Martín-Araguz, A, Bustamante-Martínez, C, de Pedro-Pijoán, JM, Treatment of chronic tension type headache with mirtazapine and amitriptyline. Rev Neuro 2003; 37:101–105. ![]()
20. Magni, G, The use of antidepressants in the treatment of chronic pain. A review of the current evidence. Drugs 1991; 42:730–748. ![]()
21. Salomone, S, Caraci, F, Capasso, A, Migraine: an overview. Open Neurol J 2009; 3:64–71. ![]()
22. Wolff, HG. Headache and other head pain. New York: Oxford University Press; 1993.
23. Leao, AA, Spreading depression. Funct Neurol 1986; 1:363–366. ![]()
24. Lauritzen, M, Cerebral blood flow in migraine and cortical spreading depression. Acta Neurol Scand Suppl 1987; 76:1–40. ![]()
25. Lauritzen, M, Pathophysiology of the migraine aura: the spreading depression theory. Brain 1994; 117:199–210. ![]()
26. Cutrer, FM, Sorensen, AG, Weisskoff, RM, Ostegaard, L, Sanchez del Rio, M, Lee, EJ, Perfusion-weighted imaging defects during spontaneous migrainous aura. Ann Neurol 1998; 43:25–31. ![]()
27. Silberstein, SD, Migraine pathophysiology and its clinical implications. Cephalalgia. 2004;24(Suppl 2):2–7. ![]()
28. Hargreaves, RJ, Shepheard, ST. Pathophysiology of migraine – new insights. Can J Neurol Sci. 1999; 26(Supp I3):S12–S19.
29. Moskowitz, MA, Basic mechanisms in vascular headache. Neurol Clin 1990; 8:801–815. ![]()
30. Mayberg, M, Langer, RS, Zervas, NT, Moskowitz, MA, Perivascular meningeal projections from cat trigeminal ganglia: possible pathway for vascular headaches in man. Science 1981; 213:228–230. ![]()
31. Goadsby, PJ, Hoskin, KL, The distribution of trigeminovascular afferents in the non-human primate brain Macaca nemestrina: a c-fos immunocytochemical study. J Anat 1997; 190:367–375. ![]()
32. Friberg, L, Olesen, J, Nicolic, I, et al. Interictal “patchy” regional cerebral blood flow patterns in migraine patients: a single photon emission computerized tomographic study. Eur J Neurol. 1994; 1:35–43.
33. Mason, RT, Peterfreund, RA, Sawchenko, PE, Corrigan, AZ, Rivier, JE, Vale, WW, Release of the predicted calcitonin gene-related peptide from cultured rat trigeminal ganglion cells. Nature 1984; 308:653–655. ![]()
34. Dalkara, T, Zervas, NT, Moskowitz, MA, From spreading depression to the trigeminovascular system. Neurol Sci. 2006;27(Suppl 2):S86–S90. ![]()
35. Aston-Jones, G, Cohen, JD, An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 2005; 28:403–450. ![]()
36. Olesen, J, Burstein, R, Ashina, M, Tfelt-Hansen, P, Origin of pain in migraine: evidence for peripheral sensitisation. Lancet Neurol 2009; 8:679–690. ![]()
37. Alstadhaug, KB, Migraine and the hypothalamus. Cephalalgia 2009; 29:809–817. ![]()
38. Tfelt-Hansen, P, Saxena, PR, Dahlof, C, et al. Ergotamine in the acute treatment of migraine: a review and European consensus. Brain. 2000; 123(Pt 1):9–18.
39. Sprenger, T, Goadsby, PJ, Migraine pathogenesis and state of pharmacological treatment options. BMC Med 2009; 7:71. ![]()
40. Dodick, D, Lipton, RB, Martin, V, et al, Consensus statement: cardiovascular safety profile of triptans (5–HT1B/1D agonists) in the acute treatment of migraine. Headache 2004; 44:414–425. ![]()
41. Lance, JW, Goadsby, PJ. Mechanism and management of headache, 7th ed. Philadelphia: Elsevier Butterworth Heinemann; 2005.
42. Cyriax, JH. Textbook of orthopaedic medicine, vol 1. Diagnosis of soft tissue lesions, 8th ed. London: Baillière Tindall; 1982.
43. Piovesan, EJ, Di Stani, F, Kowacs, PA, et al, Massaging over the greater occipital nerve reduces the intensity of migraine attacks: evidence for inhibitory trigemino-cervical convergence mechanisms. Arq Neuropsiquiatr 2007; 65:599–604. ![]()
44. Young, W, Cook, B, Malik, S, Shaw, J, Oshinsky, M, The first 5 minutes after greater occipital nerve block. Headache 2008; 48:1126–1128. ![]()
45. Rozen, T, Cessation of hemiplegic migraine auras with greater occipital nerve blockade. Headache 2007; 47:917–919. ![]()
46. Piovesan, EJ, Kowacs, PA, Tatsui, CE, Lange, MC, Referred pain after painful stimulation of the greater occipital nerve in humans: evidence of convergence of cervical afferences on trigeminal nuclei. Cephalalgia 2001; 21:107–109. ![]()
47. Matharu, MS, Bartsch, T, Ward, N, Frackowiak, RS, Weiner, R, Goadsby, PJ, Central neuromodulation in chronic migraine patients with suboccipital stimulators: a PET study. Brain. 2004;127(Pt 1):220–230. ![]()
48. Ekbom, K, Evaluation of clinical criteria for cluster headache with special reference to the classification of the International Headache Society. Cephalalgia 1990; 10:195–197. ![]()
49. Torelli, P, Manzoni, GC, Pain and behaviour in cluster headache. A prospective study and review of the literature. Funct Neurol 2003; 18:205–210. ![]()
50. Leone, M, Franzini, A, Bussone, G, Stereotactic stimulation of posterior hypothalamic gray matter in a patient with intractable cluster headache. N Engl J Med 2001; 345:1428–1429. ![]()
51. Rapoport, AM, Mathew, NT, Silberstein, SD, et al, Zolmitriptan nasal spray in the acute treatment of cluster headache: a double–blind study. Neurology 2007; 69:821–826. ![]()
52. Afridi, SK, Shields, KG, Bhola, R, Goadsby, PJ, Greater occipital nerve injection in primary headache syndromes – prolonged effects from a single injection. Pain 2006; 122:126–129. ![]()
53. Sjaastad, O, Bovin, G, Cervicogenic headache. The differentiation from common migraine. An overview. Functional Neurol 1991; 6:93–100. ![]()
54. Sjaastad, O, Cervicogenic headache: the controversial headache. Clin Neurol Neurosurg. 1992;94(Suppl):S147–S149. ![]()
55. Bovim, G, Cervicogenic headache, migraine, and tension-type headache. Pressure-pain threshold easurements. Pain 1992; 51:169–173. ![]()
56. Sjaastad, 0, Frederiksen, TA, Cervicogenic headache: criteria, classification and epidemiology. Clin E’.I;p Rheumatol. 2000;18(2 Suppl 19):S3–S6. ![]()
57. Bogduk, N, The clinical anatomy of the cervical dorsal rami. Spine 1982; 7:319–330. ![]()
58. Mendel, T, Wink, CS, Zimny, ML, Neural elements in human cervical intervertebral discs. Spine 1992; 17:130–135. ![]()
59. Sjaastad, O, Fredriksen, TA, Pfaffenrath, V, Cervicogenic headache: diagnostic criteria. The Cervicogenic Headache International Study Group. Headache 1998; 38:442–445. ![]()
60. Argenson, C, Francke, JP, Sylla, S, et al. The vertebral arteries (segments V1 and V2). Anat Clin. 1980; 2:24–41.
61. Francke, JP, Di Marino, V, Pannier, M, Argenson, C, Libersa, C. The vertebral arteries: the V3 atlanto–axoidal and V4 intracranial segments – collaterals. Anat Clin. 1981; 2:229–242.
62. Oostendorp, RAB, Bernards, ATM, Querido, C, Hagenaars, LHA, Meldrum, HA. Neurologie en manuele therapie. De vertebrobasilaire insuffici’ntie. Ned Tijdschr Manuele Ther. 1985; 85/4(2):33.
63. Gutmann, G, Biedermann, H. Funktionelle Pathologie und Klinik der Wirbelsäule, Band I, Die Halswirbelsäule (Teil 2). Stuttgart: Fischer; 1984.
64. Strek, P, Reron, E, Maga, P, Modrzejewski, M, Szybist, N, A possible correlation between vertebral artery insufficiency and degenerative changes in the cervical spine. Eur Arch Otorhinolaryngol. 1998;255(9):437–440. ![]()
65. Coman, WB. Dizziness related to ENT conditions. In: Grieve GP, ed. Modern Manual Therapy of the Vertebral Column. New York: Churchill Livingstone; 1986:304.
66. Guerrier, Y, Basseres, F. Le Vertige et le Vertigineux. Brussels: Duphar & Cie; 1984.
67. Ruckenstein, MJ, Vertigo and dysequilibrium with associated hearing loss. Otolaryngol Clin North Am. 2000;33(3):535–562. ![]()
68. El–Kashlan, HK, Telian, SA, Diagnosis and initiating treatment for peripheral system disorders: imbalance and dizziness with normal hearing. Otolaryngol Clin North Am. 2000;33(3):563–578. ![]()
69. Solomon, D, Distinguishing and treating causes of central vertigo. Otolaryngol Clin North Am. 2000;33(3):579–602. ![]()
70. Anon, Protocol for pre–manipulative testing of the cervical spine. Aust J Physiother 1988; 34:97–100. ![]()
71. Anon. Protocol for pre–manipulative testing of the cervical spine. Physiotherapy. 1991; 41:15–17.
72. Aspinall, W, Clinical testing for cervical mechanical disorders which produce ischemic vertigo. J Orthop Sports Phys Ther 1989; 11:1176–1182. ![]()
73. Michaeli, A. Dizziness testing of the cervical spine: can complications of manipulations be prevented? Physiother Theory Pract. 1991; 7:243–250.
74. Hutchison, MS. An investigation of pre–manipulative dizziness testing. In: Jones HM, et al, eds. Proceedings of the 6th Biennial Conference. Adelaide: Manipulative Therapists Association of Australia, 1989.
75. Oostendorp, RAB, Hagenaars, LHA, Fischer, AJEM, et al. Dutch standard ‘cervicogenic dizziness’. In: Paris SV, ed. Proceedings of the 5th International Conference, International Federation of Orthopaedic Manipulative Therapists, June 1–5. Colorado: Vail, 1992.
76. Moffat, DA, Harries, ML, Baguley, DM, Hardy, DG, Unterberger’s stepping test in acoustic neuroma. J Laryngol Otol. 1989;103(9):839–841. ![]()
77. Kuipers–Upmeijer, J, Oosterhuis, HJ, Unterberger’s test not useful in testing of vestibular function. Ned Tijdschr Genees. 1994;138(3):136–139. ![]()
78. Hickey, SA, Ford, GR, Buckley, JG, Fitzgerald O’Connor, AF, Unterberger stepping test: a useful indicator of peripheral vestibular dysfunction? J Laryngol Otol. 1990;104(8):599–602. ![]()
79. Lopez–Escamez, J, Lopez–Nevot, A, Gamiz, M, et al, Diagnosis of common causes of vertigo using a structured clinical history. Acta Otorhinolaringol Espanica. 2000;51(1):25–30. ![]()
80. Bakr, MS, Saleh, EM, Electronystagmography: how helpful is it? J Laryngol Otol. 2000;114(3):178–183. ![]()
81. Ojala, M, Palo, J, The aetiology of dizziness and how to examine a dizzy patient. Ann Med. 1991;23(3):225–230. ![]()
82. Bose, B, Northrup, BE, Osterholm, JL, Delayed vertebro–basilar insufficiency following cervical spine injury. Spine. 1985;10(1):108–110. ![]()
83. Karlberg, M, Persson, L, Magnusson, M, Impaired postural control in patients with cervico–brachial pain. Acta Oto–Laryngologica. 1995;Suppl 520(Pt 2):440–442. ![]()
84. Ishiyama, A, Jacobson, KM, Baloh, RW, Migraine and benign positional vertigo. Ann Otol Rhinol Laryngol. 2000;109(4):377–380. ![]()
85. Oghalai, JS, Manolidis, S, Barth, JL, Stewart, MG, Jenkins, HA, Unrecognized benign paroxysmal positional vertigo in elderly patients. Otolaryngol Head Neck Surg. 2000;122(5):630–634. ![]()
86. Galm, R, Rittmeister, M, Schmitt, E, Vertigo in patients with cervical spine dysfunction. Eur Spine J. 1998;7(1):55–58. ![]()
87. Norre, ME, Cervical vertigo. Diagnostic and semiological problem with special emphasis upon ‘cervical nystagmus ’. Acta Otorhinolaryngol Belg. 1987;41(3):436–452. ![]()
88. Brandt, T, Cervical vertigo – reality or fiction? Audiol Neuro–Otol. 1996;1(4):187–196. ![]()
89. Karlberg, M, Johansson, R, Magnusson, M, Fransson, PA, Dizziness of suspected cervical origin distinguished by posturographic assessment of human postural dynamics. J Vestib Res. 1996;6(1):37–47. ![]()
90. Goebel, JA, Management options for acute versus chronic vertigo. Otolaryngol Clin North Am. 2000;33(3):483–494. ![]()
91. Kessinger, RC, Boneva, DV, Vertigo, tinnitus, and hearing loss in the geriatric patient. J Manip Physiol Ther. 2000;23(5):352–362. ![]()
92. Garrido, MC, Rodrigo, FJ, Garcia, AJ, et al, Benign positional paroxysmal vertigo in the general ear, nose, and throat clinic. Acta Otorhinolaryngol Espanica. 2000;51(1):14–18. ![]()