Obstetrics and gynecology
A Assisted reproductive technologies
Assisted reproductive technologies (ARTs) refers to all techniques used to retrieve and fertilize human oocytes. In vitro fertilization (IVF) is the most common technique used to artificially fertilize human oocytes.
The procedure is performed by initially stimulating maturation of the follicle with a gonadotropin-releasing hormone agonist that induces pituitary gland suppression and creates quiescent ovaries to prevent the production of a single dominant follicle. Follicle-stimulating hormone (FSH) and human menopausal gonadotropin are then administered, which induces 10 to 15 ovarian follicles. The patient is then given human chorionic gonadotropin (hCG), which induces the follicle to then mature and move into the follicular fluid. The oocyte is retrieved transvaginally, transabdominally, or via laparoscopy with an ultrasonically guided probe 34 to 36 hours after hCG administration. All visible follicles are collected, washed, incubated for 4 to 6 hours in a culture medium, and examined microscopically. Most follicles contain only one oocyte. Fertilization occurs in the IVF laboratory. The oocyte is identified and has minimal exposure to ambient room temperature, room air, and especially any chemical odors. Sperm are washed and centrifuged. Fresh media is added next to the centrifuged sperm, and those sperm that swim to the media, which can number 50,000, are placed with the oocyte. Timing must be coordinated with proper maturation of the uterine endometrium. ART is found to increase the risk of multiple gestations. Also, it has been reported that atypical implantations of the fertilized ovum or zygote, such as abdominal, cervical, ovarian, or tubal pregnancy, occur more frequently with ART. Common ART techniques are listed in the table on pg. 413.
Common Assisted Reproductive Technology Techniques
| In vitro fertilization (IVF) | Oocytes are removed, fertilization occurs in the laboratory, and the embryo is placed transcervically into the uterus or into the distal portion of the fallopian tube(s). |
| Gamete intrafallopian transfer (GIFT) | Oocytes and sperm are transferred into one or both fallopian tubes for fertilization. |
| Advantage: oocyte retrieval and gamete transfer occur with a single procedure. | |
| Disadvantages: Requires at least one patent fallopian tube and laparoscopic surgery. Fertilization cannot be confirmed. | |
| Zygote intrafallopian transfer (ZIFT) | Fertilized embryos are placed into the fallopian tube. |
| Advantages: Fertilization is confirmed. Laparoscopic surgery can be avoided if fertilization has not occurred. The embryos can be transferred at an appropriate developmental stage. | |
| Disadvantage: Requires a two-stage procedure, with added risks and costs. Requires at least one patent fallopian tube. | |
| Tubal embryo transfer (TET) | Cleaving embryos are placed into the fallopian tube. |
| Peritoneal oocyte and sperm transfer (POST) | Oocytes and sperm are placed into the pelvic cavity. |
Modified from Speroff L. Clinical gynecologic endocrinology and infertility. 6th ed. Baltimore: Lippincott Williams & Wilkins; 1999: 1133-1148; Tsen LC. Anesthesia for assisted reproductive technologies. Int Anesthesiol Clin 2007;45:99-113.
Patients are assessed for antibodies to human immunodeficiency virus types 1 and 2 (HIV-1, HIV-2) and human T-cell lymphotropic virus type 1 (HTLV-1), hepatitis B antigen, and antibodies to hepatitis B and C. Patients are also tested for Chlamydia, syphilis, gonorrhea, and cytomegalovirus. Smokers require twice as many attempts at successful IVF than nonsmokers, so smoking is extremely discouraged.
a) IVF is generally performed on patients who are American Society of Anesthesiologists (ASA) class 1 or 2 in their third or fourth decade of life.
b) Although IVF is a relatively simple procedure for the reproductive endocrinologist to perform, especially outside the operating room, IVF is an uncomfortable procedure and requires that patients do not move for the probe to be guided for retrieval and later reimplantation.
c) The vaginal wall must be pierced for the desired ovary to be accessed. Also, major blood vessels are present in the proximity of the ovaries, and their injury could lead to complications.
d) Anesthesia requirements vary with the individual needs of the patient and the reproductive endocrinologist. Multiple ART procedures may need to be performed until one of them is successful, so safe yet inexpensive anesthetic techniques are desirable.
e) Minimal sedation, moderate sedation and analgesia, regional intrathecal anesthesia, paracervical block, or general anesthesia can be administered to assist in making the procedure as comfortable and successful as possible.
f) Moderate sedation with analgesia is usually sufficient for most patients. None of the anesthetic procedures caused differences in reproductive outcome.
g) Anesthetic medications generally considered safe for use in anesthesia for ART are listed in the box on pg. 414. Anesthesia providers should consider using safe anesthetic techniques with quick onset and a short duration.
h) It should be noted that propofol, lidocaine, thiopental, thiamylal, and alfentanil have been shown to accumulate in the follicular fluid.
i) Midazolam, when titrated in small doses to provide mild to moderated sedation and anxiolysis, has been shown to be safe, with no accumulation in follicular fluid or teratogenicity.
j) Ketamine (0.75 mg/kg) with midazolam (0.06 mg/kg) moderate sedation and analgesia has been safely used as an alternative to general anesthesia with isoflurane.
k) The literature has suggested the use of caution when using a potent inhaled agent (especially sevoflurane and desflurane) because of possible negative effects to ART outcomes.
l) Nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen, indomethacin, ketoprofen, ketorolac, meloxicam, naproxen, or oxaprozin are avoided because of inhibition of prostaglandin synthesis and possible effects on embryo implantation.
m) Droperidol and metoclopramide have both been shown to induce rapid hyperprolactinemia and should be avoided. Low plasma prolactin levels are associated with higher incidences of pregnancy.
n) The common anesthetic agents that could cause potential problems with ART are listed in the box below. The necessity for any medication given to the patient should be carefully considered, and the anesthetic technique should be kept simple and basic.
B Cesarean section
A cesarean section (C-section) is the surgical removal of a fetus through an abdominal and uterine incision. A low transverse incision is the most common; in an emergency, a rapid vertical midline incision may be used. Indications for a C-section are failure of labor to progress, previous C-section, fetal distress, malpresentation of the fetus or umbilical cord, placenta previa, and genital herpes or other local infections.
a) A focused assessment should include obstetric and anesthetic history, maternal health, airway, allergies, and baseline vital signs. In emergency cases, the time for assessment will be brief. Special attention should be paid to airway assessment because failed intubation is a major cause of maternal morbidity and mortality.
b) Key points regarding physiological changes in pregnancy
(1) Cardiac output increases mostly because of the increase in stroke volume but also because of increase in heart rate.
(2) The greatest demand on the heart is immediately after delivery when cardiac output increases 180%.
(3) Blood volume is markedly increased and prepares the parturient for the blood loss associated with delivery.
(4) Plasma volume is increased to a greater extent than red blood cell volume, resulting in a dilutional anemia.
(5) Minute ventilation increases 45%, and this change is mostly caused by the large increase in tidal volume.
(6) Oxygen consumption is markedly increased. Carbon dioxide production is similarly increased.
(7) Pregnant women have an increased sensitivity to local anesthetics and a decreased minimum alveolar concentration (MAC) for all general anesthetics.
(8) Platelets, factor VII, and fibrinogen are increased.
(9) Intragastric pressure is increased in the last trimester, which, in combination with increased acid volume, often results in heartburn.
(10) All pregnant women are at increased risk of aspiration because of the physiologic changes to the gastrointestinal system.
a) A nonparticulate antacid (e.g., sodium citrate, 30 mL) and metoclopramide 10 mg IV are routinely administered at most institutions regardless of the anesthetic technique chosen. Sedation is best avoided. Benzodiazepines have been implicated as possible teratogens, and it is best to avoid maternal amnesia during childbirth.
b) Laboratory tests should include a type and screen, complete blood count (CBC), electrolytes, blood urea nitrogen, creatinine, glucose, prothrombin time, and partial thromboplastin time. In emergency C-sections, there may not be time to complete these tests.
c) The parturient should have at least one large-bore intravenous (IV) lines in place before induction of anesthesia.
d) An IV antibiotic should be administered either before abdominal incision or immediately after clamping of umbilical cord.
(2) If there is a history of pregnancy-induced hypertension, an arterial line is recommended.
(3) If there is severe preeclampsia, a central line is also recommended, with a pulmonary catheter in cases of hemodynamic instability.
b) Positioning: Supine with left lateral uterine displacement. This is accomplished by placing a wedge under the right hip. Failure to use left lateral uterine displacement can result in aortocaval compression.
(1) The tabletop should be set up for a general anesthetic.
(2) Set out a smaller endotracheal tube (6-6.5) as well (because of airway edema).
(3) Have ephedrine, phenylephrine, and oxytocin drawn up. Ensure immediate availability of Methergine and Hemabate.
(4) Have difficult airway equipment available.
(5) Unless there is maternal hypoglycemia, avoid giving IV solutions with glucose because they may lead to neonatal hypoglycemia.
5. Perioperative management and anesthetic techniques
The guidelines for general, spinal, and epidural anesthesia are listed below.
a) General anesthesia for C-section
(1) Histamine2-receptor antagonist or proton pump inhibitor or metoclopramide intravenously
(b) Denitrogenation (administration of 100% oxygen)
(c) Traditional 3 to 5 minutes versus 4 vital capacity breaths
(5) Rapid-sequence IV induction
(a) Propofol, ketamine, or etomidate
(b) Succinylcholine (rocuronium or vecuronium if succinylcholine is contraindicated)
(6) Intubation with a 6.0- to 7.0-mm cuffed endotracheal tube
(7) Administration of a low concentration (e.g., MAC) of an inhalation agent with nitrous oxide
b) Epidural anesthesia for C-section
(3) Intravascular volume replacement with Ringer’s lactate or normal saline (10-20 mL/kg)
(5) Supplemental oxygen by face mask or nasal prongs
(6) Epidural catheter at L2 to L3 or L3 to L4
(9) Therapeutic dose: 5-mL boluses of 2% lidocaine + 1:400,000 epinephrine. Alternatively, 5-mL boluses of 0.5% bupivacaine, 0.5% ropivacaine, or 3% 2-chloroprocaine (boluses of lidocaine or 2-chloroprocaine every 1 to 2 minutes; boluses of bupivacaine or ropivacaine every 2 to 5 minutes)
(10) Aggressive treatment of hypotension: Exaggerated left uterine displacement; IV fluids; ephedrine or low-dose phenylephrine (or both)
c) Spinal anesthesia for C-section
(4) Replacement with Ringer’s lactate or normal saline (15-20 mL/kg)
(6) Supplemental oxygen by face mask or nasal prongs
(7) Prophylactic intramuscular (IM) ephedrine (25-50 mg) in patients with a baseline systolic blood pressure of less than 105 mmHg
d) Tasks at delivery and postdelivery maintenance
(1) The length of time from uterine incision to delivery has been shown to correlate with the degree of neonatal acidosis. The interval should be recorded. An interval of 3 minutes seems to be the critical value; neonates delivered later than 3 minutes after uterine incision are more likely to be depressed.
(2) After the umbilical cord has been clamped, the anesthesia provider’s options for anesthetic maintenance increase because the administered drugs will no longer reach the baby. If adequate uterine contraction is achieved with oxytocin, there is no reason why the use of an inhalation anesthetic cannot be continued.
(3) A low-dose inhalation agent (up to 1 MAC), with or without nitrous oxide, and either fentanyl or sufentanil may be used. If uterine tone does not allow the use of an inhalation agent, an opioid and nitrous oxide technique is useful.
(4) When opioids are used, nitrous oxide is usually needed. If nitrous oxide is not used, the anesthesia provider may consider giving a small dose of midazolam for amnesia. Opioid administration should be customized to the circumstances. One option is giving up to 5 mcg of fentanyl per kilogram, adjusting the dose according to the expected duration of the case and the patient’s response. Another opioid choice is morphine 10 mg IV and 10 mg IM.
(5) After the placenta has been delivered, oxytocin should be given immediately unless the obstetrician’s plan for uterine contraction calls for the use of another agent.
(a) The half-life of oxytocin varies from 4 to 17 minutes. It is metabolized by the liver, kidney, and plasma enzyme pathways in the parturient.
(b) Commercially available preparations of oxytocin contain a preservative that causes systolic and especially diastolic hypotension, flushing, and tachycardia when infused at high doses.
(c) The amount of oxytocin added to the IV solution should be tailored to the volume of solution remaining in the bag, the flow rate of the IV, and the patient’s condition. If the IV bag is nearly empty or if IV solution is being administered rapidly, less oxytocin should be added to the bag.
(d) In general, the obstetrician is likely to desire the administration of 20 to 40 units of oxytocin over the first hour postpartum.
(e) If an unusually large blood loss results in hypotension and if fluid resuscitation is needed, it may be helpful to infuse the oxytocin at an appropriate rate and to start a second IV line for administering fluid volume at a rapid rate.
(f) If the solution with the added oxytocin is infused fast enough to replace volume, then it is likely that the high dose of oxytocin may cause further hypotension.
(6) If oxytocin does not adequately stimulate uterine contraction, the next drug used is usually an ergot alkaloid (Methergine, Ergotrate).
(a) Because of their potent vascular effects, ergot alkaloids are not administered intravenously. Ergot alkaloids normally cause an increase in blood pressure, central venous pressure, and pulmonary capillary wedge pressure.
(b) IV administration may result in arterial and venous constriction, coronary artery constriction, severe hypertension, cerebral bleeding, headache, nausea, and vomiting.
(c) An IM dose of 0.2 mg is commonly administered for stimulating uterine contractions.
(d) In some cases, the obstetrician may choose to administer oxytocin or ergotamine directly into the uterine muscle to maximize effect.
(e) Ergot alkaloids are metabolized and eliminated chiefly by the liver. The plasma half-life is approximately 2 hours, but uterine effects last much longer.
(f) Ergot alkaloids potentiate sympathomimetics, especially α-agonists (including ephedrine). Severe hypertension, cerebrovascular accidents, and retinal detachment have occurred when the two drugs were used simultaneously. These effects may persist even when the vasopressor is given well after the last dose of methylergonovine maleate.
(7) When the uterus does not contract well despite the use of oxytocin and ergot alkaloids, prostaglandin F2a (Hemabate, 250 mcg) is administered either IM or directly into the uterine muscle.
(a) Prostaglandins are potent stimulators of uterine contractions. The contractions induced by prostaglandins are strong and painful.
(b) Nausea, vomiting, and diarrhea are frequent side effects. In addition to causing uterine contractions, prostaglandins may cause hypotension by relaxing vascular smooth muscle; however, cases of severe hypertension after prostaglandin administration have also been reported.
(c) Prostaglandins may cause a recalcitrant uterus to contract and stop bleeding. If they do not, the surgeon is likely to extend the procedure and include hysterectomy, for which the anesthesia provider must be prepared.
(1) Even with the administration of metoclopramide, the parturient often has a large volume of gastric contents. Suctioning of the stomach with an orogastric tube while the patient is anesthetized decreases the incidence of vomiting after awake extubation.
(2) Before extubation, the anesthesia provider should verify full recovery of neuromuscular function. Because C-section is usually a brief procedure involving fairly limited exposure to anesthetic, emergence is often quick. Advance preparation limits patient discomfort before extubation.
C Dilatation and curettage
Dilatation and curettage (D & C) involves dilation of the cervix and scraping of the endometrial lining of the uterus. The procedure is done to diagnose and treat uterine bleeding, cervical lesions, or stenosis. D & Cs are also used to complete an incomplete or missed abortion and are then referred to as suction D & Cs with the gestational week.
2. Preoperative assessment and patient preparation
a) History and physical examination: Assess for any cardiac, respiratory, neurologic, or renal abnormalities. Assess for a history of hiatal hernia or reflux; if a suction D & C, assess the gestational week; if greater than 16 weeks, consider the patient to have a full stomach.
4. Perioperative management and anesthetic technique
a) Drugs: Anxiolytic agents (midazolam [Versed], 0.01-0.02 mg/kg), narcotics (fentanyl, 1-2 mcg/kg), oxytocin (Pitocin) for suction D & Cs, and induction agents (propofol) can be used.
b) This procedure can be done either with a short-acting spinal or saddle block as a general anesthetic by mask, laryngeal mask airway, or with an endotracheal tube with inhalation agent or nitrous oxide or with heavy sedation (midazolam [Versed], fentanyl, and propofol).
c) Postoperatively, assess for bleeding, nausea, and cramping. Treat with narcotics, NSAIDs, and antiemetics.
d) Volume depletion and anemia can occur rapidly or slowly over time. IV rehydration and assessment of hemoglobin and hematocrit values are imperative.
D Gynecologic laparoscopy
Laparoscopy is a common endoscopic technique in gynecologic procedures. It is frequently used to diagnose or treat pelvic conditions that may include sterilization, adhesions, pain, endometriosis, ectopic pregnancies, ovarian cysts and tumors, infertility, and vaginal hysterectomy. A pneumoperitoneum is achieved by insertion of a trocar and insufflation of carbon dioxide.
2. Preoperative assessment and patient preparation
a) Monitoring equipment: Standard
b) Additional equipment: Fluid warmer and Bair Hugger; other equipment as needed
4. Perioperative management and anesthetic technique
E Hysterectomy: Vaginal or total abdominal
Hysterectomy is commonly performed to treat uncontrolled uterine bleeding, dysmenorrhea, uterine myoma, gynecologic cancer, adhesions, endometriosis, and pelvic relaxation syndrome. Frequently, laparoscopy is used; for ovarian cancer prophylaxis, a bilateral salpingo-oophorectomy may be performed as well.
a) History and physical examination: As indicated by the patient’s history and medical condition
(1) Laboratory tests: As indicated by the patient’s history and medical condition
(2) Diagnostic tests: As indicated by the patient’s history and medical condition
(3) Preoperative medications: Anxiolytics are given as indicated; consider prophylaxis for postoperative nausea and vomiting.
(4) IV therapy: Two 16- to 18-gauge IV lines are used; consider a central line or an arterial line (or both) if the procedure is radical.
(5) An epidural catheter may be placed for intraoperative or postoperative pain relief.
a) Monitoring equipment is standard.
b) Consider an arterial line and central venous pressure catheter if large blood loss is expected.
c) Additional equipment includes a fluid warmer and Bair Hugger.
(1) Anesthetic and adjunct agents and antibiotics are used.
(2) IV fluids: For vaginal hysterectomy, calculate for moderate blood loss; crystalloids at 4 to 6 mL/kg/hr. Estimated blood loss is 750 to 1000 mL. For abdominal hysterectomy, calculate for a moderate to large blood loss; crystalloids at 6 to 10 mL/kg/hr. Estimated blood loss is 1000 to 1500 mL.
(3) Blood: Type and crossmatch for 2 to 4 units of packed red blood cells if significant blood loss is expected.
4. Perioperative management and anesthetic technique
General or regional anesthesia is used, with a subarachnoid block or epidural with a sensory level of anesthesia of T4 to T6.
a) Complications: Nausea, vomiting, anemia
b) Pain management: Patient-controlled analgesia; epidural opiates or an epidural local anesthetic such as 0.125% or 0.25% bupivacaine with fentanyl, 1 mcg/mL at an infusion of 8 to 10 mL/hr. Ketorolac 15 to 30 mg IV or IM every 6 hours may also be used for pain relief.
F Loop electrosurgical excision procedure
The loop electrosurgical excision procedure is performed for the diagnosis and treatment of cervical intraepithelial neoplasia. This form of electrosurgery uses a loop electrode for excision and fulguration to prevent cervical bleeding. Other types of therapies that may be used to ablate cervical lesions are cryosurgery and carbon dioxide laser surgery.
2. Preoperative assessment and patient preparation
a) Monitoring equipment: Standard. If a pregnancy is more than 16 weeks’ gestation, fetal monitoring may be used.
b) Drugs: Standard tabletop agents are used.
c) IV fluids: Calculate for minimal blood loss, 2 to 4 mL/kg/hr. Estimated blood loss is 50 to 200 mL.
4. Perioperative management and anesthetic technique
a) Local, monitored anesthesia care, or regional or general anesthesia is used.
b) Induction: Standard induction is indicated.
c) In pregnant patients, rapid-sequence induction is used.
d) In nonpregnant patients, mask ventilation may be appropriate.
e) Maintenance: Standard, inhalational agent, oxygen, and opioid. Muscle relaxation is not required.
G Pelvic exenteration
Pelvic exenteration is performed for the treatment of advanced, recurrent, radioresistant cervical carcinoma. It is considered a radical surgical approach because all pelvic tissues, including the cervix, bladder, lymph nodes, rectum, uterus, and vagina, are resected. Vaginal reconstruction and appropriate colon and urinary diversions are also performed.
2. Preoperative assessment and patient preparation
a) History and physical examination: As indicated by the patient’s history and medical condition
(1) Laboratory tests: CBC, electrolytes, blood urea nitrogen, creatinine, calcium, magnesium, phosphate, prothrombin time, partial thromboplastin time, urinalysis, and renal function tests
(2) Diagnostic tests: As indicated by the patient’s history and physical examination
(3) Premedication: Anxiolytics as indicated
(4) IV therapy: Two 14- to 16-gauge IV catheters
(5) Central and arterial line; pulmonary arterial catheter considered if the patient has a significant cardiac history
(6) An epidural catheter possible for postoperative pain relief
a) Monitoring equipment: Standard; arterial line and central venous pressure useful because of major fluid shift, potential for major bleeding, obtaining laboratory values for a long duration, and possible need for vasoactive infusion. Foley catheter to monitor fluid status.
4. Perioperative management and anesthetic technique
a) General anesthesia with an epidural block is used.
b) Induction is standard, as indicated. Keep in mind that the surgery may be brief if metastatic tumor is found to be inoperable during initial exploration
c) Maintenance is with inhalational agent, oxygen, and opioid anesthesia.
d) Consider a local anesthetic through an epidural catheter.
e) Use intermediate- or long-acting nondepolarizing muscle relaxants.
f) Maintain normocarbia, mean arterial pressure of 60 to 88 mmHg, and urinary output at 0.5 to 1 mL/kg/hr; transfuse as indicated.
g) Position: Both lithotomy and supine positions are used throughout the procedure.
h) Emergence: The patient generally is transported to the intensive care unit for 2 to 3 days; postoperative ventilation may be necessary. If the patient is hemodynamically stable, extubation may be considered.
H Vaginal delivery
See the discussion of C-section earlier in this section.
a) All patients should have an IV catheter 18 gauge or larger placed and should receive a 500- to 1000-mL bolus before an epidural placement.
b) Lumbar epidural analgesia provides segmental levels of analgesia that block pain impulses from the uterus but maintain sensation in the perineum and avoid motor blockade.
(1) Generally, this is only administered when labor is well established, and the cervix is dilated 5 to 6 cm in primiparas and 3 to 4 cm in multiparas, with regular contractions.
(2) Bupivacaine is frequently used because it has little effect on the fetus and a longer duration of action. Solutions commonly used are 1/8% or 1/16% with fentanyl, 1 to 2 mcg/mL, with an infusion at 8 to 12 mL/hr and a bolus of 5 to 10 mL.
(3) Monitor blood pressure frequently for the first half hour and treat any blood pressure less than 100 mmHg with ephedrine, fluids, and left uterine displacement.
(4) Epidural topoffs may be used for forceps delivery or an extensive episiotomy repair with 5 to 10 mL of the infusion or 3% chloroprocaine (Nesacaine).
(5) Fetal heart tone needs to be assessed before and after placement of lumbar epidural.
(6) Monitor for signs of high level neuraxial block.
(7) Imperative to have emergency airway equipment available in situations where airway management is required.
c) A saddle block may be used for forceps delivery to block the perineum and inner thigh; 7.5 to 10 mg of lidocaine may be given while the patient is in the sitting position.
d) Pudendal nerve block: This blocks the pudendal nerves of S2 to S4 during the second stage of labor and results in low forceps delivery and episiotomy. It is administered transvaginally, with the local anesthetic injected posterior to the ischial spines beneath the sacrospinous ligaments. There is risk of puncture of the fetal scalp.
e) Paracervical block: This is injected into the fornix of the vagina lateral to the cervix. Nerve fibers from the uterus, cervix, and upper vagina are anesthetized; fibers from the perineum are not blocked. There is a high frequency of fetal bradycardia, so it is generally avoided.
f) Normal blood loss for a vaginal delivery is 400 to 600 mL.
I Obstetric complications
From 1990 to 2005, the maternal mortality rate in the United States was 15 per 100,000 live births. The most common causes of maternal death include hemorrhage, embolic disorders, preeclampsia, infection, and cardiomyopathy.
1. Abnormal placental implantation
(1) The placenta normally implants into the endometrium. A placenta implanted on or in the myometrium, the underlying muscular layer of the uterus, is termed placenta accreta (on the myometrium), placenta increta (into the myometrium), or placenta percreta (completely through the myometrium).
(2) Any of these abnormal placental implantations means that separation of the placenta from the uterine wall will be difficult and may be accompanied by severe bleeding.
(3) Placenta accreta, placenta increta, and placenta percreta are commonly associated with placenta previa and are more common in women who have had a previous C-section than in those who have not.
(4) The anesthetic implications are the same as those for other causes of increased blood loss.
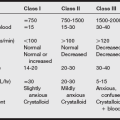
(1) Blood loss is difficult to estimate in obstetric patients. Often, lost blood is hidden inside the patient’s body, soaked in laparotomy sponges, absorbed by drapes, or spilled onto the floor.
(2) In general, approximately 500 mL is lost during a spontaneous vaginal delivery and approximately 700 mL during a C-section with general anesthesia; 1500 mL or more is lost if a hysterectomy is performed during C-section.
(3) Because the term parturient has a 50% increase in blood volume, a great amount of blood can often be lost before the vital signs begin to change in response to the loss; 15% of the total blood volume may be lost without the occurrence of any compensatory tachycardia or vasoconstriction.
(4) Hypotension may not occur until 30% of the total blood volume has been lost.
(5) Approximately 4% of all parturients who deliver vaginally experience excessive postpartum bleeding.
(1) Many obstetricians now choose to deliver fetuses in breech presentations by C-section. In this case, C-section usually is elective, and either a regional or a general anesthetic can be used.
(2) If the baby is to be delivered vaginally, an epidural anesthetic may be requested and is considered strongly indicated at some centers.
(3) The muscle relaxation that it provides is helpful, and analgesia is required, at least for the forceps delivery of the fetal head.
(4) Breech deliveries often result in laceration of the birth canal and therefore cause more bleeding than head-first deliveries.
(1) After delivery, when hemostasis is unobtainable despite the use of some combination of oxytocin, ergot alkaloids, and prostaglandin, the surgeon performs a hysterectomy to stop uterine bleeding.
(2) An atonic uterus, especially an incised uterus, can lose several liters of blood within a few minutes, outpacing the ability of even the most prepared anesthesia providers to replace intravascular volume.
(3) Anesthesia at this point becomes trauma anesthesia, the primary purpose of which is the maintenance of vital signs, vital organ perfusion, and oxygenation; maternal analgesia and amnesia are important but secondary concerns. Etomidate, ketamine, benzodiazepines, and opioids are useful because they cause minimal hemodynamic depression.
(4) If rapid blood loss begins during C-section with regional anesthesia, the anesthesia provider should consider the rapid induction of general anesthesia. It is difficult to manage volume resuscitation and to keep an awake patient both mentally and physically comfortable.
5. Disseminated intravascular coagulation
(1) Disseminated intravascular coagulation (DIC) is frequently associated with three obstetric problems: retention of a dead fetus, placental abruption, and amniotic fluid embolism.
(2) Circulatory shock, which often accompanies DIC, worsens the problem by decreasing peripheral and hepatic blood flow and causing further cell damage. Renal failure may result from the deposit of fibrin and cellular debris in the filtration system.
(3) Clinically, patients with DIC have uncontrolled bleeding because of the consumption of clotting factors. Laboratory studies show decreased levels of fibrinogen and platelets, increased prothrombin and partial thromboplastin times, and excessive amounts of fibrin degradation products.
(1) Patients with DIC need fluid resuscitation, and they almost always are hemorrhaging. Increasing intravascular volume dilutes activated clotting factors and slows the clotting process. Increased peripheral and hepatic perfusion limits cellular damage and improves clearance of activated clotting factors.
(2) Because the patient is bleeding and many clotting factors are depleted, it appears as if repletion of clotting factors is necessary; however, administration of clotting factors fuels an already out-of-control coagulation process.
(3) Definitive treatment of DIC first requires elimination of the cause. Replacement of clotting factors in obstetric patients should probably be postponed until the DIC has subsided.
(a) Thrombotic pulmonary embolism occurs in pregnant individuals fivefold more often than it does in nonpregnant individuals and is more likely to occur postpartum than antepartum.
(b) It is associated with prolonged inactivity, cesarean delivery, obesity, and increasing age and parity.
(c) Presentation varies from a few minor complaints to massive cardiovascular collapse. Pleuritic chest pain, dyspnea, hyperventilation, hypocapnia, coughing, hemoptysis, and distention of neck veins are associated with the disorder.
(d) Thromboembolism is a major cause of maternal mortality, but while the parturient is in the delivery area, it is less likely to occur than either amniotic fluid or air embolism.
(a) Venous air embolism can occur during labor, spontaneous vaginal delivery, and operative delivery and is frequently associated with placenta previa.
(b) The overall incidence of subclinical venous air embolism in parturients has been reported to be as high as 29% during general anesthesia; the incidence in the parturient may be as high as 97%.
(c) Most venous air emboli are detected between delivery and uterine repair. The signs and symptoms of venous air embolism are as follows:
(a) Although rare, amniotic fluid embolism is almost uniformly fatal. It may occur during labor, vaginal delivery, or operative delivery and is associated with placental abruption.
(b) The pathogenesis is almost identical to that of venous air embolism except that patients who develop amniotic fluid embolism are prone to develop DIC if they survive the initial embolism.
(c) Signs and symptoms of amniotic fluid embolism include a chill, shivering, anxiety, cough, dyspnea, cyanosis, tachypnea, pulmonary edema, and cardiovascular collapse. O2 saturation has been reported to decrease quickly.
(1) The incidence of postpartum thromboembolism can be affected by anesthetic interventions. C-sections performed with general anesthesia are associated with accelerated maternal coagulation compared with those performed with regional anesthesia, so the use of regional anesthesia may help reduce the incidence of postoperative thromboemboli.
(2) The anesthesia provider can help prevent prolonged inactivity in those who have had a C-section by providing analgesia sufficient to allow comfortable ambulation. Use of epidural opioid analgesia is often an appropriate solution to this problem. It may be specifically indicated in those at risk for thromboembolism even if it must be administered after a general anesthetic has been given.
(3) If embolism is suspected during spontaneous or operative delivery, the obstetrician should be informed immediately. The obstetrician can take steps to stop the entrainment of air or amniotic fluid, which include flooding the surgical field with saline, returning the uterus to within the abdomen, and stimulating uterine contractions.
(4) One hundred percent O2 should be administered by positive-pressure ventilation through a cuffed endotracheal tube.
(a) Nitrous oxide administration should be discontinued because it rapidly expands the volume of an air embolus and prevents the delivery of 100% O2.
(5) An arterial line may be needed for monitoring of oxygenation and blood pressure. IV fluids are administered as needed to bolster central venous pressure.
(6) A generous preload is necessary to enable the right side of the heart to pump volume forward against increased pulmonary vascular resistance.
(7) If the fetus has not been delivered, left uterine displacement improves uterine blood flow and facilitates venous return to the heart. Pharmacologic support of the cardiovascular system is likely to be needed.
(8) Patient position has been suggested to hinder the movement of the foreign substance into the pulmonary arteries. A slight anti-Trendelenburg (head-up) position with left lateral tilt of at least 15 degrees is designed to trap air in the right atrium, from which it can be aspirated via a central venous catheter. Unfortunately, it often is difficult to place the patient in this position and insert a central line in time to prevent pulmonary artery embolization.
(9) In the case of amniotic fluid embolism, prompt recognition and action are necessary to prevent maternal mortality. Immediate support of maternal circulation are necessary. Inotropic support should not be delayed (epinephrine, dopamine). Treatment for coagulopathy must also begin immediately and ideally with the consultation of a hematologist. Large-volume infusion devices may also be helpful for the resuscitation effort.
(1) Multiple-gestation pregnancies carry higher risk for the both mother and fetuses than singleton pregnancies. Many of the risk factors affect anesthetic management.
(2) Multiple-gestation pregnancies, especially rare monoamniotic pregnancies, are associated with complications requiring emergent surgical intervention more often than singleton pregnancies.
(3) The anesthesia provider should constantly be prepared to provide anesthesia for an emergency C-section. The multiple fetuses are often small and premature.
(4) The large uterus compounds the problems of aortocaval compression; therefore left uterine displacement should be maintained at all times when the parturient is not lying on her side.
(5) If the fetuses are to be delivered vaginally, an epidural is valuable for maternal analgesia and neonatal safety. Because the neonate often is small and premature, a slow, controlled delivery through a well-relaxed birth canal makes birth trauma less likely.
(6) The epidural provides pelvic relaxation and reduces maternal discomfort, decreasing the likelihood that pain will induce a forceful reflexive expulsion of the fetus.
(7) Either regional or general anesthesia is appropriate for a C-section. After the babies have been delivered, the uterus may not contract well because it has been overstretched for many weeks.
(8) Larger-than-usual doses of oxytocin may be needed to induce the uterus to contract well and to stop bleeding. However, it is imperative that oxytocin administration not be started until after all the neonates have been delivered. Strong uterine contractions before the delivery of all neonates deprive any remaining fetuses of blood supply and oxygenation.
(1) Abruption occurs when the placenta begins to separate from the uterus before delivery; this allows bleeding behind the placenta and jeopardizes the fetal blood supply.
(2) Placental abruption results in bleeding (often hidden), uterine irritability (often hypertonic), abdominal pain, and fetal distress or death.
(3) Open venous sinuses in the uterine wall may allow products of hemostasis and amniotic fluid to enter the maternal circulation; this results in an incidence of DIC of up to 50%.
(4) The reported incidence of abruption in the general population varies widely but is much higher in women with hypertension (up to 23% among women with preeclampsia). When fetal death occurs, maternal mortality can exceed 10%.
(1) In cases of placental abruption without fetal distress, vaginal delivery may still be possible. Because fetal distress can occur without warning, the anesthesia provider should be prepared to administer anesthesia for an emergency C-section.
(2) Taking an anesthetic history as soon as the diagnosis of placental abruption becomes known and checking for adequate IV access is recommended. If the mother is unstable or if fetal distress is present, operative delivery is necessary.
(3) Regional anesthesia usually is not indicated because of the potential for coagulopathy and because of the uncertainty of uteroplacental blood flow and therefore of fetal oxygenation.
(4) Generous venous access should be established as soon as possible. Although placental abruption does not usually result in sudden blood loss, a large volume of blood may be lost.
(5) When abruption results in fetal death, the volume of lost maternal blood can be as great as 5 L, all of which may be concealed. Volume resuscitation should begin as soon as IV access has been secured. Large volumes of crystalloid and colloid solutions and of red blood cells may be needed.
(6) General anesthesia can be induced with ketamine, up to 1 mg/kg. If the uterus is hypertonic, another drug should be chosen because the use of ketamine may further increase uterine tone, decreasing fetal O2 supply.
(7) An alternate choice is etomidate, 0.3 mg/kg. If uterine tone is excessive, a volatile inhalation agent may be useful for maintenance of anesthesia and uterine relaxation.
(8) After the baby has been delivered, the uterus often becomes atonic; therefore, the use of inhalation agents should normally be discontinued. IV or intramyometrial oxytocin and intramyometrial ergotamine may be used with uterine massage to facilitate uterine contraction and to halt bleeding.
(1) When the placenta has implanted on the lower uterine segment and either partially or completely covers the opening of the cervix, placenta previa is present.
(2) Placenta previa has an incidence of up to 1%, and the mortality rate for those with it approaches 1%.
(3) Placenta previa is more common in women who have had it during a prior pregnancy.
(4) It most often results in painless vaginal bleeding before the onset of labor that may stop without intervention or hemodynamically significant blood loss. The potential exists, however, for sudden loss of large amounts of blood.
(5) The risk of bleeding increases if the placenta is disturbed by manual examination or cervical dilation. Postpartum bleeding is often increased as well because the lower uterine segment, where the placenta previa was implanted, does not contract as well as the rest of the uterus.
(1) The diagnosis of placenta previa normally indicates an operative delivery. The anesthesia provider should prepare for heavy blood loss.
(2) The anesthesia provider may choose either a general or regional anesthetic technique, taking into consideration the parturient’s current volume status and the potential for blood loss.
(3) Regional techniques should be performed only by an anesthesia provider who is very experienced with regional anesthesia and only after careful assessment and preparation.
(1) Postpartum bleeding in moderate amounts is a normal event. Excessive bleeding may occur because of uterine atony (which accounts for 80% of all postpartum bleeding), placental retention, abnormalities of the uterus, lacerations of the delivery channel, uterine inversion, and abnormalities of coagulation.
(2) Uterine atony is associated with multiparity, prolonged infusions of oxytocin before delivery, polyhydramnios, and multiple gestation.
(3) A retained placenta or retained placental fragments must be removed manually to stop the bleeding. In the past, this has often required the administration of an inhalation agent for uterine relaxation.
(4) Nitroglycerin, a potent uterine relaxant with a relatively short duration of action, has been used successfully to provide uterine relaxation adequate for placental extraction. A dose of approximately 1 mcg/kg IV appears to be adequate.
(5) Sublingual nitroglycerin spray has also been used effectively and offers the added benefits of long shelf life and a ready-to-use preparation. Because nitroglycerin is a potent venodilator when given at low doses and is an arteriolar dilator when administered intravenously at a rate of 1 mcg/kg/min or higher, care should be taken to ensure that intravascular volume is adequate before this drug is administered.
(6) Analgesia for the procedure can be accomplished with a variety of methods, including the use of an already established epidural catheter or the administration of small IV doses of ketamine.
(1) When postpartum bleeding is excessive, the anesthesia provider performs fluid resuscitation while simultaneously working with the obstetrician to eliminate the cause of the bleeding. Fundal massage, IV oxytocin, IM methylergonovine maleate, or IM prostaglandin often is all that is needed. In some cases, anesthesia may be necessary for an additional procedure.
a) Introduction: Preeclampsia is a vasospastic disease of pregnancy that affects 2.6% to 6% of parturients. The incidence of preeclampsia is highest in primigravidas younger than 20 years or older than 35 years of age and in women who have had preeclampsia during a previous pregnancy. The exact cause of preeclampsia is unknown but probably involves an abnormality in the ratio of thromboxanes to prostacyclins. Whereas thromboxanes are potent vasoconstrictors and platelet aggregators, prostacyclins have the opposite effect. Thromboxane A2 and prostacyclin levels normally increase during pregnancy. An imbalance of prostacyclins and thromboxanes, both of which are produced by the placenta, has been demonstrated in preeclampsia.
(1) Preeclampsia results in hypertension, 1+ to 2+ proteinuria, and edema after the 20th week of gestation. Generally, the diagnosis of preeclampsia is made when two of the three signs are present.
(2) Hypertension is defined as a blood pressure greater than 140/90 mmHg or more than 30 mmHg above systolic baseline and more than 15 mmHg above diastolic baseline.
(3) Severe preeclampsia is said to exist when the following conditions are present: maternal blood pressure greater than 160/110, 3+ or 4+ proteinuria, urine output less than 20 mL/hr, central nervous system (CNS) signs (blurred vision or changes in mentation), pulmonary edema, and epigastric pain. Blood pressure monitoring is a key indicator because it is technically easy to perform, and the severity of the hypertension frequently parallels the severity of the disease.
(4) Preeclampsia results in maternal, fetal, and neonatal morbidity and mortality.
(5) The chief cause of maternal mortality is cerebral hemorrhage caused by hypertension.
(6) Pulmonary edema, renal failure, hepatic rupture, cerebral edema, and DIC also may cause maternal death.
(7) Brain edema results in CNS irritability, seizures (a significant percentage of which occur postpartum), and an increase in sensitivity to depressant drugs. Fetal death results primarily from placental abruption or infarct. Delivery of the fetus is curative.
(8) HELLP syndrome consists of hemolysis, elevated liver enzymes, and a low platelet count. From 5% to 10% of the sickest women with preeclampsia develop HELLP syndrome. Clinical signs of HELLP syndrome include epigastric pain, upper abdominal tenderness, proteinuria, hypertension, jaundice, nausea, and vomiting. Rarely, HELLP syndrome may result in liver rupture. Some experts believe that a degree of compensated DIC is present in all patients with HELLP syndrome.
No uniform agreement exists with regard to the pathophysiology of preeclampsia. One view is that preeclampsia is a hyperdynamic state involving an early increase in cardiac output and elevated systemic vascular resistance (SVR). Another view is that preeclampsia is characterized by an increase in SVR and variable decrease in cardiac output. Thromboxane A2 is found in increased levels during preeclampsia and has been correlated with disease severity.
(1) Increased vascular permeability results in extravasation of fluid and protein (proteinuria in the kidneys).
(2) Hypertension results in compensatory decreases in circulating blood volume and a loss of intravascular water and electrolytes via the kidney.
(3) Capillary injury stimulates platelet aggregation and fibrin deposition and may result in thrombocytopenia and, occasionally, DIC. It also results in multiple organ system dysfunction. Often, total body water is increased.
(4) Intravascular volume may decrease by as much as 40%.
(5) Marked peripheral and end-organ vasoconstriction is common, and it either causes or occurs in response to the decrease in vascular volume. An increased vascular sensitivity to vasopressin, angiotensin, and catecholamines has been demonstrated. Catecholamine levels often increase, and this results in decreased perfusion to the uterus, placenta, and fetus. Arteriolar constriction increases left ventricular work. When preeclampsia becomes severe, intravascular volume is either contracted or shifted centrally.
(6) Central venous pressure may be low because of a contracted blood volume, or it may be relatively normal because of a redistribution of vascular volume into the central circulation. Pulmonary capillary occlusion pressure may be normal or high because of left-sided heart failure and often does not correlate well with the central venous pressure.
(7) Uteroplacental insufficiency can result from a combination of decreased intravascular volume, vascular intimal deterioration, and increased vascular resistance. Placental perfusion in the preeclampsia patient may decrease by 70% compared with that in a healthy parturient. Decreased placental perfusion leads to intrauterine growth retardation and can cause fetal hypoxia and placental infarction.
(8) Platelet aggregation and fibrin deposition increase in preeclampsia. Platelet counts may drop as the platelets are consumed; however, even in the presence of a normal platelet count, platelet function may be below normal.
(1) Magnesium sulfate is almost always administered to women with preeclampsia in the United States. Although it is not curative, it has been shown to reduce the likelihood of eclampsia by 58% and the risk of maternal death by 45%.
(2) Delivery presently is the only definitive way of ending the disease process of preeclampsia. When a fetus is at a gestational age of more than 37 weeks, obstetricians generally proceed with delivery. If the fetus is immature, delivery is delayed to allow the fetus time to mature.
(3) If preeclampsia is severe or fetal distress occurs, delivery is usually accomplished expeditiously. In any case, obstetric treatment is aimed at preventing eclampsia (seizures), avoiding decreases in uteroplacental blood flow, and maximizing organ perfusion.
(4) Magnesium sulfate, which is also used as a tocolytic, causes venodilation, mild CNS depression, a decrease in the rate of fibrin deposition, and a reduction in uterine activity, if present.
(5) Decreasing fibrin deposition prevents further decay in organ perfusion and often greatly decreases liver pain in parturients with hemolysis, elevated liver enzymes, and a low platelet count (HELLP syndrome).
(6) Magnesium therapy is continued after delivery for the suppression of seizures.
(7) Regional anesthesia and preeclampsia
(a) Epidural analgesia and anesthesia generally are preferred for both spontaneous vaginal delivery and C-section in the preeclampsia patient when they are not contraindicated.
(b) A carefully initiated epidural infusion helps control maternal hypertension and may improve organ blood flow. Careful initiation of the block is necessary in women with preeclampsia because their mean blood pressure tends to decrease more than that of healthy parturients.
(c) During a C-section, epidural anesthesia avoids stimulation of the airway, which can aggravate hypertension and possibly cause cerebral bleeding.
(d) During a vaginal delivery, epidural analgesia allows a slower, more controlled expulsion of the premature infant and decreases the likelihood of trauma to the fetal head. Even these advantages, however, must be weighed against the risks of regional anesthesia, primarily hypotension and bleeding.
(e) Because thrombocytopenia and other coagulation problems are associated with preeclampsia, careful consideration should be given to the patient’s coagulation status before regional anesthesia is begun.
(f) A careful history of bleeding should be taken and a platelet count evaluated before insertion of an epidural catheter in a patient with a diagnosis of preeclampsia. Coagulation problems are found almost exclusively in women with platelet counts below 100,000/mm3.
(g) Some evidence indicates that epidural analgesia can improve uteroplacental perfusion, and therefore fetal oxygenation, by decreasing plasma catecholamines. Epidural analgesia causes vascular dilation; this decreases blood pressure while maintaining—and, in some cases, improving—perfusion of the uterus and other organs, as long as hypotension is not allowed. Uterine artery systolic-to-diastolic ratios in women with preeclampsia decrease after the initiation of epidural analgesia, suggesting that resistance to blood flow is lowered.
(h) Diastolic blood pressure should not be reduced to less than 90 mmHg in women with severe preeclampsia because such a reduction will probably result in inadequate uteroplacental blood flow.
(i) Hypotension is a significant concern in parturients with severe preeclampsia because of the sometimes constricted intravascular volume and the likelihood that the patient has already received an antihypertensive agent such as hydralazine or labetalol. When hypotension does occur, ephedrine and other vasopressors should be used cautiously because they may produce an exaggerated response in the preeclampsia patient.
(j) Epidural anesthesia can be accomplished safely in these patients if careful attention is devoted to volume status. Most but not all preeclampsia patients have a contracted blood volume.
(k) IV preloading may be necessary because of the constricted intravascular volume; however, some patients have a normal central venous pressure or left ventricular dysfunction. Preloading in these patients can result in pulmonary edema. Placement of a central venous pressure line or pulmonary catheter may be indicated in severe preeclampsia when the patient is oliguric or hypoxic.
(l) Bupivacaine is usually the local anesthetic of choice because of its long history of safe use in preeclampsia patients and because it has a slower onset than lidocaine and chloroprocaine. The slower onset allows time for the anesthesia provider to react to hemodynamic changes. Often, it is necessary to begin administering epidural anesthesia slowly with bupivacaine in these patients without any intravascular prehydration. Small incremental doses of bupivacaine can be administered and fluid given intravenously as needed when and if changes in blood pressure occur.
(m) After epidural analgesia or anesthesia has been instituted, the sympathectomy should not be allowed to wear off abruptly, because the increased intravascular volume could precipitate a hypertensive crisis or pulmonary edema. Instead, the block should be allowed to recede slowly, when the body is able to eliminate the intravascular fluid load and adequate monitoring is available.
(8) General anesthesia and preeclampsia
(a) Coagulopathy or decay in maternal or fetal condition is the most common indication for general anesthesia in the patient with preeclampsia. The maternal brain is edematous and more sensitive to CNS-depressant drugs.
(b) Induction of general anesthesia is hazardous in patients with preeclampsia. Their exaggerated hypertensive response to laryngoscopy and endotracheal intubation is potentially lethal. Compounding the problem, upper airway swelling may make identification of landmarks and intubation more difficult. Swelling may preclude the insertion of an endotracheal tube of normal size. Difficulty in intubation increases the duration of airway stimulation and worsens hypertension.
(c) The challenge during induction of general anesthesia is prevention of a further increase in blood pressure, which may result in intracranial hemorrhage. Control of blood pressure during induction of general anesthesia in these patients demands careful planning and skill in implementation.
(a) Nondepolarizing relaxants are markedly potentiated in women with preeclampsia and therapeutic levels of magnesium. In these patients, half of an effective dose in 95% of the population dose produced 100% block for 35 minutes.
(b) In healthy parturients not receiving magnesium sulfate, the same dose on average produced 42% blockade for approximately 9 minutes. Reduced doses of nondepolarizers can be used, if desired, but these drugs yield a longer-than-usual block.
(1) Premature delivery is estimated to occur in 12% to 13% of all pregnancies in the United States and is implicated in both maternal and neonatal death and considerable morbidity.
(2) Premature labor is defined as regular uterine contractions that occur between 20 and 37 weeks of gestation and that result in dilation or effacement of the cervix. When labor begins prematurely, the ability to halt it can allow the fetus additional time to mature. Stopping labor is termed tocolysis (from the Greek tokos, meaning “childbirth,” and lysis, meaning “breaking up”).
(3) The cause of preterm labor is not well understood; however, four pathways are supported by a considerable body of clinical and experimental evidence: excessive myometrial and fetal membrane overdistention, decidual hemorrhage, precocious fetal endocrine activation, and intrauterine infection or inflammation. The processes leading to preterm parturition may originate from one or more of these pathways.
(4) Factors associated with preterm labor are listed in the box below.
(a) A variety of agents are used as tocolytics. These include β-adrenergic receptor agonists, nitric oxide donors, magnesium sulfate, calcium channel blockers, prostaglandin-synthesis inhibitors, and oxytocin antagonists. Labor-inhibiting drugs are only marginally effective.
(b) Tocolytics act by two primary mechanisms: through generation or alteration of intracellular messengers or by inhibiting the synthesis or blocking the action of a known myometrial stimulant.
(c) Although more than 80% of women with preterm labor who are treated with tocolytics have their pregnancies maintained for 24 to 48 hours, few data suggest that tocolysis maintains pregnancy for a longer period.
(d) A critical goal of tocolysis is to delay delivery long enough to allow for the administration of corticosteroids, which reduces the risks of neonatal respiratory distress syndrome, intraventricular hemorrhage, necrotizing enterocolitis, and overall perinatal death. The initial benefit of corticosteroid therapy occurs approximately 18 hours after administration of the first dose with maximal effort at about 48 hours.
(e) Thus, treatment of acute preterm labor may allow time for the onset of the therapeutic effect of corticosteroids.
(1) Magnesium sulfate has been used for decades as a tocolytic; however, recently it has been noted that given its lack of benefit, possible harm, and expense, magnesium sulfate should not be used for tocolysis. Others believe it is still of some benefit when used properly.
(2) Magnesium causes relaxation of vascular, bronchial, and uterine smooth muscle by altering calcium transport and availability. Motor end-plate sensitivity and muscle membrane excitability also are depressed. Magnesium hyperpolarizes the plasma membrane and inhibits myosin light-chain kinase activity by competing with intracellular calcium, which in turn reduces myometrial contractility.
(3) The normal serum magnesium level during pregnancy is 1.8 to 3 mg/dL. A serum magnesium level of 4 to 8 mg/dL is therapeutic as a tocolytic, but even toxic levels do not eliminate uterine contractility. At 10 to 12 mg/dL, the patellar reflex is eliminated. Levels above 12 mg/dL cause respiratory depression; at approximately 18 mg/dL, respiratory depression progresses to apnea. The presence of higher levels (25 mg/dL) can cause cardiac arrest.
(a) The side effects of magnesium sulfate administration are dose dependent. As magnesium levels increase, skeletal muscle weakness increases, and CNS depression and vascular dilation occur. Magnesium sulfate infusion commonly results in a slight decrease in blood pressure during epidural anesthesia.
(b) Magnesium antagonizes the vasoconstrictive effect of α-agonists, so ephedrine and phenylephrine are less effective at increasing maternal blood pressure when administered concomitantly with magnesium.
(c) Cardiac muscle is not affected to a clinically evident degree when magnesium is administered at therapeutic levels, although magnesium can have profound myocardial effects during a gross overdose.
(d) Magnesium is eliminated unchanged by the kidneys. In a patient who is receiving a maintenance infusion of magnesium and who has decreasing urine output, blood levels of magnesium quickly increase, as do related side effects.
(e) Other side effects of magnesium sulfate include the following:
(f) Patients on magnesium sulfate therapy have partial, if subclinical, neuromuscular blockade. Both depolarizing and nondepolarizing neuromuscular blocking drugs are potentiated by magnesium. Administration of priming or defasciculating doses of neuromuscular blocking drugs may cause significant paralysis when combined with magnesium therapy. The neuromuscular blocking effects of magnesium can be at least partially antagonized by calcium.
(g) Neonatal side effects after maternal magnesium administration are rare.
(h) Magnesium is also used in the treatment of preeclampsia, a vasospastic disease of pregnancy that can result in severe hypertension, coagulopathy, and seizure.
(i) Magnesium sulfate causes relaxation of vascular smooth muscle, a decrease in SVR, and a decrease in blood pressure. At serum levels of 7 to 9.5 mg/dL, it is an anticonvulsant. It also decreases fibrin deposition, improving circulation to visceral organs that are vulnerable to vasospasm and failure.
(1) Stimulation of the β2-receptor system causes smooth muscle relaxation, including relaxation of the uterus. The myometrium has β2-receptors in cell membranes. Stimulation of these receptors triggers a cascade of biochemical effects, resulting in inhibition of myometrial contractility at the cellular level.
(2) β2 stimulation also causes an increase in progesterone production. Progesterone, in turn, causes histologic changes in myometrial cells that limit the spread of contractile impulses. β-Adrenergic receptor agonists cause myometrial relaxation by binding to β2-adrenergic receptors and subsequently increasing the levels of intracellular cyclic AMP, which activates protein kinase, inactivating myosin light-chain kinase, thus diminishing myometrial contractility.
(3) The administration of β-agonists results in downregulation of β-receptors over time. This results in a decreased tocolytic effect during long-term β-agonist therapy that has been demonstrated in animals after as few as 24 hours of ritodrine administration.
(a) Available β-agonists have both β1 and β2 effects, although some agents are fairly selective for one receptor subset over the other.
(b) The side effects of β-agonist therapy can be predicted on the basis of a knowledge of systemic β effects. Cardiovascular effects are generally the most clinically important and troublesome. β1 stimulation causes an increase in heart rate, myocardial contractility, and myocardial O2 demand. Palpitations and premature ventricular contractions are common. β2 stimulation causes vascular dilation, bronchial dilation, an increase in secretions, and various metabolic effects.
(c) Maternal side effects of β-agonists include the following:
(d) β-Agonist therapy further increases the demand on the cardiovascular system. Complaints of palpitations and chest pain are common. ECG changes are sometimes seen, although myocardial ischemia is not always documented.
(i) β stimulation increases blood glucose and insulin levels. When a β-agonist infusion is started, the blood glucose level increases within a few hours and returns to baseline within 72 hours without treatment.
(ii) Potassium is redistributed from the extracellular to intracellular compartments. This results in a decrease in serum potassium level, sometimes to less than 3 mEq/L. As with glucose levels, serum potassium levels return to normal within 72 hours after initiation of β-agonist therapy.
(a) There is a small but notable incidence of pulmonary edema among healthy parturients receiving β-agonists. The mechanism for the development of pulmonary edema in these patients is unclear.
(b) Fluid overload resulting from a physiologic increase in intravascular volume, antidiuresis, and IV fluid administration may have a role.
(c) Myocardial fatigue caused by tachycardia also has been suggested as a possible cause. Pulmonary artery pressures are not uniformly elevated, however, and sometimes they are low; this finding can be used to argue against both of these hypotheses. However, it is clear that the danger of pulmonary edema increases when parturients receiving β-agonists are preloaded for regional anesthesia.
(d) Risk factors associated with pulmonary edema during β-agonist tocolysis include anemia, fluid overload, magnesium, multiple gestation, and prolonged maternal tachycardia.
(6) Fetal and neonatal side effects
(a) Clinically used β-agonists cross the placenta and have fetal and neonatal effects. Fetal tachycardia (fetal heart rate [FHR] >160 beats/min) is common.
(b) Ritodrine (Yutopar) is a selective β2-agonist. Ritodrine therapy increases maternal heart rate by an average of 40 beats/min. Systolic blood pressure commonly increases, and diastolic pressure decreases. The manufacturer’s literature recommends that patients on ritodrine therapy receive no more than 2 L of IV fluid over 24 hours.
(c) Before spinal or epidural anesthesia for C-section is initiated, it is common for a 2-L IV preload to be given in less than 30 minutes. However, even smaller IV preloads are not recommended in patients receiving ritodrine until use of the drug has been discontinued for at least 1 hour. Ritodrine is eliminated by the kidneys and has an elimination half-life of approximately 30 minutes.
(d) Terbutaline (Brethine, Bricanyl) is a synthetic, relatively β2-receptor–selective, noncatecholamine sympathomimetic amine. When administered parenterally, terbutaline is less β2-receptor–selective than ritodrine. Arrhythmias are more likely to occur with terbutaline use than during ritodrine administration, and tachycardia can be a problem. Terbutaline is approximately 50% eliminated by the kidneys and has a half-life of up to 16 hours. Similar to ritodrine, terbutaline has been associated with pulmonary edema when it is used for tocolysis.
(1) Nifedipine is the most commonly used agent because it can be administered orally. The calcium channel blockers inhibit the influx of calcium ions through the cell membrane and the release of intracellular calcium from the sarcoplasmic reticulum.
(2) This decreases intracellular free calcium leading to inhibition of calcium-dependent myosin light-chain kinase–mediated phosphorylation, resulting in myometrial relaxation.
(1) Cyclooxygenase converts arachidonic acid to prostaglandin H2. Prostaglandin H2 serves as a substrate for tissue-specific enzymes, which is critical in parturition.
(2) Prostaglandins enhance the formation of myometrial gap junctions and increase available intracellular calcium by raising transmembrane influx and sarcolemmal release of calcium. Indomethacin is the most commonly used tocolytic agent in this class.
(1) Nifedipine appears to be a reasonable choice for initial tocolysis, given the oral route of administration, low frequency of side effects, and efficacy in reducing neonatal complications. Nifedipine can be used at any gestational age when labor-inhibition therapy is being considered.
(2) For pregnancies of less than 32 weeks’ gestation, an alternative to nifedipine is indomethacin. These agents have been shown to be more effective than the β-adrenergic receptor agonists in comparative studies.
(3) Indomethacin should be avoided in women with a platelet dysfunction or bleeding disorder, hepatic or renal dysfunction, gastrointestinal ulcerative disease, or asthma (in women with hypersensitivity to aspirin).
(4) The use of β-adrenergic receptor agonists is an alternative to therapy with nifedipine and indomethacin. The side effect profile of this class of drugs is less favorable than that of nifedipine, but their effectiveness in stopping contractions appears to be similar.
g) Anesthetic technique: When an anesthetic intervention is planned for a patient who is receiving a tocolytic agent, knowledge of maternal and fetal physiology and of the pharmacology of the tocolytic agent must be integrated.
(a) When tocolysis fails, preterm deliveries are often accomplished by C-section. In this situation, 1- and 5-minute Apgar scores have been shown to be higher in neonates delivered with epidural anesthesia than in those delivered with general anesthesia.
(b) Patients on magnesium therapy are often candidates for subarachnoid or epidural blocks as long as careful attention is devoted to volume status. Magnesium causes vasodilation, and maternal hemorrhage is tolerated poorly by both parturients on magnesium and their fetuses.
(c) Subarachnoid block has the advantage of involving very small amounts of local anesthetic, and this reduces the chance for fetal local anesthetic toxicity.
(d) Epidural anesthesia can be used throughout labor for analgesia and can be induced slowly; this minimizes the risk of sudden hypotension caused by sympathetic block.
(e) Even when volume status is accurately assessed, IV preloads before subarachnoid or epidural anesthesia are associated with an increased risk of pulmonary edema in parturients receiving β-agonist drugs.
(f) Use of ritodrine (and perhaps terbutaline) should almost always be discontinued, and enough time for the drug to be largely eliminated should be allowed to pass before regional anesthesia is induced.
(g) If time constraints do not permit the needed delay, induction of general anesthesia for an urgent or emergent procedure is almost always preferable.
(h) If the patient is already in pulmonary edema or has marginal to poor uterine artery blood flow because of vascular constriction, slowly induced epidural anesthesia may provide a beneficial vasodilation.
(i) Anesthesia-induced hypotension must be carefully avoided, however, because almost all therapies directed at restoration of blood pressure would be detrimental. Ephedrine could increase an already rapid heart rate, and IV fluid administration could precipitate or worsen pulmonary edema. A low dose of an α-agonist (e.g., 50-100 mcg of phenylephrine given intravenously) may be the least detrimental choice.
(a) Succinylcholine is the muscle relaxant of choice during the rapid-sequence induction of an obstetric patient. In patients on magnesium therapy, defasciculation with a small dose of a nondepolarizing neuromuscular blocking agent is not recommended because significant paralysis may result, increasing the risk of aspiration of gastric contents.
(b) Magnesium potentiates depolarizing and, especially, nondepolarizing relaxants. The amount of potentiation is variable, and a peripheral nerve stimulator is invaluable. The duration of paralysis after administration of a standard dose of succinylcholine may give a clue as to how much longer than normal the effect of a nondepolarizer will last.
(c) Induction of general anesthesia in a patient receiving a β-agonist tocolytic can present a challenge. As with regional anesthesia, there are advantages to delaying induction of general anesthesia whenever possible until ritodrine has been largely eliminated (at least 1 hour).
(d) Thiopental has a long, safe history of use in obstetric anesthesia, and its cardiovascular depression may offset some of the cardiac stimulation caused by the β-agonist. Propofol may also be used. The use of a vagolytic, such as atropine, glycopyrrolate, or pancuronium bromide, is counterproductive.
(e) Induction of general anesthesia should usually be delayed until the patient has been prepared and the operating surgeon and assistants are ready for incision. Preterm neonates have a significantly higher incidence of low Apgar scores at 1 minute. Reducing the interval from the induction of anesthesia to the delivery of the infant minimizes the depressant effects of the anesthetic that the neonate must overcome.
(f) In pregnant patients, effective doses of fentanyl (5-8 mcg/kg) cross the placenta and result in significant neonatal depression. Fentanyl (1 mcg/kg) administered before the induction of general anesthesia for C-section does not affect the Apgar score or neurobehavioral test results of neonates significantly.
(g) β-Blockers may be used before the induction of anesthesia and instrumentation of the airway. Labetalol has been used successfully to decrease maternal blood pressure while uteroplacental blood flow is maintained. Neonatal side effects (hypotension, bradycardia) are apparently minimal. In women with preeclampsia, labetalol has been administered before the induction of general anesthesia to decrease mean blood pressure at induction and during the first 10 minutes of anesthesia.
(h) Anesthetic depth has important implications for fetal oxygenation. Light anesthesia results in maternal catecholamine outflow in response to surgical stimulation, which in turn results in uterine artery constriction and a decrease in uterine artery blood flow. Anything that decreases uterine artery blood flow decreases uteroplacental blood flow and therefore results in fetal hypoxia.
A prolapsed umbilical cord is present when the cord protrudes through the cervix ahead of the fetus. Danger arises when compression of the cord against the wall of the cervix by the presenting part cuts off blood flow and oxygenation to the fetus.
The obstetrician attempts either to restore blood flow in the umbilical cord by pushing the presenting part back into the uterus or to deliver the fetus abdominally before asphyxia causes permanent injury. In the first situation, anesthesia is likely to be needed for uterine relaxation; in the second, it is necessary for emergent C-section.
(1) Uterine rupture is most commonly associated with labor in the presence of a previous uterine incision (vaginal birth after C-section [VBAC]) but may occur in an unscarred uterus.
(2) The incidence of uterine rupture during attempted VBAC is approximately 0.6%. Uterine rupture has also been associated with cocaine abuse during pregnancy.
(3) The classic description of complete uterine rupture includes sudden, severe, tearing abdominal pain in a multiparous woman in hard labor. The pain may break through labor epidural anesthesia. Next, labor stops, and shock and fetal distress rapidly develop.
(4) Unfortunately, uterine rupture often does not present classically. For example, some ruptures occur during periods of mild labor. The clinical finding most commonly associated with uterine rupture is an abnormal FHR tracing.
(5) Whatever the presentation, bleeding is often severe. The uterus receives approximately 800 mL of blood per minute (≈10% of the cardiac output); therefore, a tear in this organ holds the potential for rapid exsanguination.
(6) Mortality from uterine rupture accounts for half of the maternal deaths attributed to blood loss each year. Fetal mortality rate after uterine rupture is nearly 80%.
b) Anesthetic technique: Uterine rupture requires surgery for hemostasis and often for delivery. Anesthesia providers should be prepared for heavy bleeding commensurate with any severe abdominal trauma. In the operating room, as much as 3500 mL of blood has been found in the abdomen at incision.
15. Postdural puncture headache
Postdural puncture headache (PDPH) results from a loss of cerebrospinal fluid (CSF) from the subarachnoid space. The total volume of CSF present within this sac in an adult is approximately 150 mL. Approximately 500 mL of CSF is produced and reabsorbed each day.
PDPH occurs when CSF leaks out through a hole in the dura made during the performance of a subarachnoid block or accidentally during the attempted performance of an epidural block. Because the Tuohy needle used to place an epidural catheter has a large diameter, puncture of the dura with the needle results in the loss of a significant volume of CSF. Loss of CSF is not the only cause of headache after regional anesthesia in obstetric patients. Meningitis, although rare, can develop despite the adherence to aseptic technique, and this disease shares many of the signs and symptoms of PDPH (headache, nausea, and photophobia) in the initial stages.
b) Incidence: The incidence and severity of PDPH vary with factors thought to be related to the volume and rate of CSF leakage out of the subarachnoid space.
(1) PDPH is infrequent in elderly adults and most frequent in young adults. Women may be slightly more susceptible than men.
(2) Generally, the use of large needles is more likely to be associated with PDPH than use of small ones.
(3) The configuration of the tip of the needle is also important. Other factors being equal, the use of beveled needles (e.g., the Quincke needle) results in headache more frequently than the use of pencil-point or bullet-tip needles (e.g., the Whitacre or Sprotte needle).
(4) The orientation of the needle as it punctures the dural fibers also may be important. Spreading the fibers along their cephalad-to-caudad axis may result in less CSF leakage than cutting the fibers by inserting the bevel perpendicular to the axis of the dural fibers.
(5) The angle at which the needle approaches the dura may also modify the amount of CSF leakage and therefore the incidence of PDPH; however, the angle of approach most often is dictated by anatomy and therefore is difficult for the anesthesia provider to modify effectively.
(1) The hallmark of a PDPH is its postural nature. The headache is relieved by lying down and returns when sitting or standing up.
(2) It is commonly fronto-occipital and sometimes is associated with neck and shoulder stiffness.
(3) Photophobia may be present in patients with severe headaches; double vision occurs less frequently. Temporary deafness has occurred rarely. Nausea and vomiting may also occur.
(4) The onset of the headache is usually not immediate, but it may take 1 to 2 days to become bothersome. It may be mild or severe, and it often becomes worse if the patient feels sick and does not consume liquids.
(1) Patients should be given a choice of treatments ranging from the most conservative to the most aggressive. PDPH may be a mild irritation for a few days, or it may be debilitating.
(2) Patients with a mild PDPH may elect to rest in bed because the horizontal position often provides complete relief from the headache or to take over-the-counter analgesics if they are up and about. Adequate hydration should be encouraged. In hospitalized patients who are not taking fluids by mouth, IV hydration is warranted. Liberal hydration does not increase the production of CSF; however, it is important that the patient not be allowed to become dehydrated because dehydration decreases CSF production.
(3) Caffeine is a cerebral vasoconstrictor and is effective in preventing or treating PDPH in some patients.
(4) The most effective treatment available for PDPH is an epidural blood patch. This treatment entails some risk because it is invasive; also, the headache is likely to become worse if another dural puncture is made in the course of placing the epidural (with a 17- or 18-gauge Tuohy needle). The usual contraindications to an epidural procedure also apply to this treatment.
(5) A blood patch is performed by placing a Tuohy needle in the epidural space, preferably at the same interspace as the dural puncture or one interspace below. After the Tuohy needle is in place, an assistant performs a peripheral venipuncture and draws 20 mL of the patient’s blood using strict aseptic technique. The blood is slowly injected into the epidural space. The ideal volume for injection appears to be between 15 and 20 mL; the use of smaller volumes is associated with a significantly lower success rate, especially in the placement of prophylactic blood patches. If discomfort develops in the back or neck, the injection is temporarily stopped. After the discomfort has passed, injection of the target volume of 15 to 20 mL may continue (unless discomfort returns). After the desired volume is injected, the Tuohy needle is withdrawn, and the patient should lie quietly for at least 1 hour.
(6) For the next several hours, the patient should rest and not be overly active. This may need to be specifically discussed with the patient because she may feel quite well for the first time in days and therefore may want to be more active. Excessive activity before complete clot consolidation may result in the clot being dislodged from the dural puncture site, allowing CSF leakage to resume.
(7) The epidural blood patch is thought to plug the dural rent with a fibrin clot; the injected volume of blood applies pressure to the dura, in effect “autotransfusing” the cerebrum with CSF from around the spinal cord. An epidural blood patch is effective in more than 90% of patients when it is performed 24 hours after the dural puncture. A second patch is effective in approximately half of those who do not obtain relief from the first; this brings the total success rate to approximately 95%.
J Anesthesia for the pregnant patient undergoing a nonobstetric procedure
a) Surgery for nonobstetric procedures occurs in up to 2% of women.
b) Approximately 42% of procedures occur in the first trimester, 35% during the second, and 23% during the third.
c) Acute abdominal problems are most common, with appendectomy ranking first followed by cholecystectomy.
d) Other common problems include adnexal disease (e.g., ovarian cysts, which may rupture or become torted) and trauma.
a) Aortocaval compression becomes clinically relevant from approximately 20 weeks of gestation. It can be relieved by a left lateral tilt of 15 degrees, which is therefore essential in all pregnant patients in the supine position after 20 weeks.
b) There may be delay in the onset of the classical symptoms and signs of hypovolemia because of the increase in blood volume along with a resting tachycardia.
c) Pregnancy is a hypercoagulable state with an increase in most clotting factors. The platelet count may fall, but there is actually an increase in production and consumption.
d) Pregnancy is a significant risk factor for thromboembolism; therefore, thromboprophylaxis is essential in the postoperative period when the risk is further increased by immobility and the hypercatabolic state.
e) Airway management may be challenging during pregnancy. Bag-mask ventilation may be more difficult because of increased soft tissue in the neck. Laryngoscopy can be hindered by weight gain and breast engorgement. Increased edema of the vocal cords because of increased capillary permeability can hinder intubation and increase the risk of bleeding.
f) Increased maternal oxygen consumption and reduced functional residual capacity results in rapid oxygen desaturation during attempts at intubation.
g) Smaller sized endotracheal tubes may be needed.
h) Careful preoxygenation in a slightly head-up position is essential before induction.
i) It is recommended that from 16 weeks’ gestation, patients undergoing general anesthesia should be given prophylaxis against aspiration pneumonitis. This usually includes a nonparticulate antacid such as sodium citrate 0.3 M 30 mL and an H2 receptor antagonist (e.g., ranitidine 150 mg orally or 50 mg intravenously).
j) Induction of anesthesia should be by a rapid-sequence technique with cricoid pressure.
k) At the end of the procedure, the patient should be extubated fully awake in the lateral position.
l) Pharmacokinetic and pharmacodynamic profiles are altered in pregnancy, and drugs should be titrated accordingly.
(1) Prevention of fetal asphyxia
(a) One of the most serious risks to the fetus during maternal surgery is intrauterine asphyxia. This must be avoided by maintaining maternal oxygenation and hemodynamic stability.
(b) It is extremely important to avoid hypoxia, extreme hyper- and hypocarbia, hypotension, and uterine hypertonus.
(c) Maternal hypoxemia causes uteroplacental vasoconstriction and decreased perfusion, causing fetal hypoxia, acidosis, and ultimately death.
(d) Uteroplacental circulation is not autoregulated, and hence perfusion is entirely dependent on the maintenance of an adequate maternal blood pressure and cardiac output.
(e) Hypotension can be caused by anesthetic drugs, central neuraxial blockade, hypovolemia or aortocaval compression.
(f) Maternal hypotension needs to be treated aggressively by ensuring left lateral tilt and boluses of IV fluids. Additional vasopressors may be required, and currently it is believed that α-agonists such as phenylephrine and metaraminol produce a better fetal acid balance than indirect sympathomimetic agents such as ephedrine.
(a) Teratogenicity is defined as the observation of any significant change in the function or form of a child secondary to prenatal treatment.
(b) During the first 2 weeks of human gestation, the teratogens have an all-or-none phenomenon; the fetus is lost or is preserved fully intact.
(c) The period from the third to the eighth weeks of gestation represents the most important time for organogenesis during which drugs can exert their most serious teratogenic effects.
(d) After this, drug exposure should not cause organ abnormalities, but fetal growth retardation may occur.
(e) Studies looking at the outcomes of women who underwent surgery during pregnancy suggest no increase in congenital anomalies in their offspring but an increase in fetal loss, growth restriction, and low birth weight attributed to the requirement for surgery (not anesthetic administration).
(f) Nitrous oxide inhibits methionine synthetase; therefore, there is concern that it could affect DNA synthesis in the developing fetus and should be avoided.
(g) Ketamine causes increased uterine tone and fetal asphyxia and should not be used in the first two trimesters. The effect is not seen in the third trimester.
(3) Prevention of preterm labor and fetal monitoring
(a) Surgery during pregnancy increases the risk of spontaneous abortion, preterm labor, and preterm delivery. This risk is increased with intra abdominal procedures.
(b) Uterine manipulation should be kept to a minimum, and drugs that increase uterine tone (e.g., ketamine) should be avoided.
(c) Prophylactic tocolytic therapy is controversial because there are associated maternal side effects, and efficacy during nonobstetric surgery has not been proven.
(d) Perioperative fetal monitoring is also an area of controversy. From 18 to 22 weeks, FHR monitoring is feasible, and from 25 weeks FHR variability can be observed.
(e) Continuous monitoring may be technically difficult during abdominal operations or in cases of maternal obesity.
(f) Anesthetic agents reduce both baseline FHR and FHR variability; therefore, interpretation is difficult and may lead to unnecessary interventions.
(g) Anesthetic agents do not cause decelerations or persistent fetal bradycardia, and these changes may indicate fetal distress.
(h) Monitoring may enable swift action to be taken such as the optimization of maternal hemodynamics, oxygenation, and ventilation.
a) Attention to thromboprophylaxis is essential. This should include early mobilization, maintaining adequate hydration, thromboembolic deterrent (TED) stockings and other calf compression devices and consideration of pharmacological prophylaxis (e.g., subcutaneous low-molecular-weight heparin).
b) Adequate analgesia is important because pain causes increased circulating catecholamines, impairing uteroplacental perfusion. Analgesia may mask the signs of early preterm labor, so tocometry is useful to detect contractions. This enables tocolysis to be administered without delay.
c) If a pregnancy continues beyond the first postoperative week, the incidence of premature labor is no higher than in nonsurgical pregnant patients.
a) Covers the 6-week period after childbirth during which time the various changes that occurred during pregnancy revert to the nonpregnant state.
b) The cardiovascular system and blood volume return to normal by the end of 2 weeks.
c) After delivery of the placenta, the uterus is the size of a 20-week pregnancy and decreases by 1 finger breadth each day, so that by day 12, it is no longer palpable.
d) Avoid elective surgery in the initial 6-week postpartum period to allow the body to return to its normal physiological function.
e) If anesthesia is undertaken, explanation of the effects of the drugs on breastfeeding should occur. Administration of drugs to a breastfeeding mother can inhibit lactation or cause direct harmful effects to the infant because of excretion in breast milk. For many medications, there is insufficient evidence available to provide accurate guidance on drug safety during breastfeeding.
f) Breast milk production is dependent on adequate maternal hydration and regular stimulation (either by the baby feeding or by the mother expressing). If scheduled for anesthesia or surgery, encourage the mother to breastfeed as near as possible to the procedure.
(1) Propofol and thiopental are found in breast milk in insignificant amounts, as are levels of volatile agents. Because neuromuscular blocking agents are large, ionized, and water soluble, they are not excreted into breast milk.
(2) After general anesthesia, women can be advised to express and discard the first sample of milk and to resume infant feeding after this.
(3) All of the commonly used antiemetics are advised to be used with caution or only if essential by manufacturers.
(1) Local anesthetics are not excreted into breast milk in amounts sufficient to be harmful.
(2) Breastfeeding can continue as normal after regional anesthesia.