CHAPTER 154 Anatomy and Physiology of Pain
Primary Afferent Nociceptors
Nociceptive information is conveyed from the periphery to the spinal cord by small myelinated and unmyelinated primary afferents that travel through the dorsal root to synapse in the dorsal horn. As described in detail in Chapter 158, recent years have seen an explosion of information about the mechanisms of nociceptive signal transduction and the properties of primary afferents. Individual afferents manifest a high degree of specificity, with nociceptive neurons differentiated from low-threshold afferents in terms of physiology, morphology, and neurochemistry. The primary afferent fibers also exhibit significant plasticity in response to tissue conditions, with alterations in neuronal phenotype and enhanced responsiveness during inflammation or in response to damage to the nerve itself. This “primary afferent sensitization” is now recognized to be an important contributor to hyperalgesia and abnormal pain states.
Dorsal Horn and Ascending Pathways
Nociceptive afferents enter the dorsal horn and terminate both superficially (in laminae I and II) and more deeply (in laminae V, VI, and VII), as well as around the central canal. By contrast, low-threshold tactile afferents spare the superficial laminae and send their terminals primarily to laminae III and IV (sometimes called the nucleus proprius).1 The different terminations of nociceptive and low-threshold input to the dorsal horn highlight the link between anatomic and functional organization of nociceptive and non-nociceptive somatosensory pathways and extend the specificity seen at the level of primary afferents to the central nervous system. Second-order neurons in the nociceptive pathways are found primarily in the superficial layers and more deeply in laminae V and VI of the dorsal horn and are usually divided into two classes: wide dynamic range (WDR) and nociceptive specific (NS).2,3 WDR neurons receive convergent input from both nociceptive and non-nociceptive primary afferents. They consequently exhibit low thresholds within the innocuous range but, unlike low-threshold tactile dorsal horn neurons, code stimulus intensity through the noxious range. WDR neurons are distributed somatotopically within the dorsal horn, and although the receptive fields are relatively large, they are not so large that a contribution to stimulus localization is precluded. WDR neurons are more common in the deeper dorsal horn but are also found more superficially in laminae I and II. NS neurons do not receive non-nociceptive input and respond exclusively to noxious stimuli carried either by Aδ mechanoreceptors or by both Aδ and C nociceptors. Their receptive fields are small, which points to an important role in stimulus localization. They are concentrated in the more superficial layers.
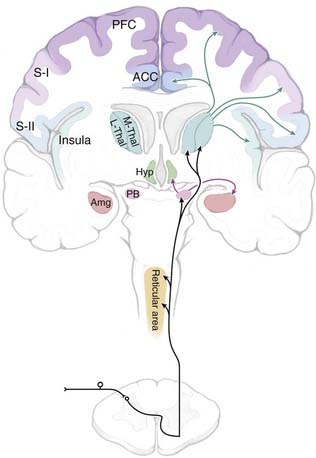
Nociceptive information is conveyed to the brain primarily via the spinoreticular, spinomesencephalic, spinoparabrachial, and spinothalamic tracts, all of which ascend through the anterolateral quadrant (Fig. 154-1). The importance of the anterolateral systems in pain (and temperature) sensibility is confirmed by the ability of anterolateral chordotomy to relieve pain, at least in the short term, in both patients and experimental studies.1 The spinothalamic tract also carries non-nociceptive information (innocuous warming and cooling, innocuous tactile information). In addition, tactile information is conveyed by the spinocervical tract and through the dorsal columns (which include ascending branches of large-diameter low-threshold primary afferents, as well as the postsynaptic dorsal column system, which consists of second-order projections of low-threshold dorsal horn neurons).
Direct spinothalamic projections terminate in both the medial and lateral thalamus, and these two targets can be considered more important in the affective-motivational and sensory-discriminative aspects of pain, respectively.4,5 Parallel spinoreticular and spinomesencephalic pathways may contribute to conscious sensation, but these pathways may be more important for arousal, autonomic and motor responses to noxious input, and recruitment of descending control systems (see the later section “Descending Modulatory Systems”). The spinoparabrachial pathway consists of projections of neurons primarily in lamina I and relays information to the amygdala and hypothalamus, as well as the midbrain periaqueductal gray (PAG) matter and caudal ventrolateral medulla. The spinoparabrachial pathway is considered particularly important in the emotional and autonomic aspects of pain.6
Role of the Dorsal Column Pathway in Visceral Pain
The classic view that ascending pathways through the dorsal columns are involved exclusively in the transmission of non-noxious information has recently been called into question by evidence that a postsynaptic dorsal column component contributes to visceral pain sensibility.7 These studies were motivated by the clinical observation that midline myelotomy relieves pain in patients with cancer involving pelvic visceral structures. Experimental studies subsequently documented a significant projection ascending ipsilaterally through the dorsal columns and transmitting information to the ventroposterolateral nucleus of the thalamus. Importantly, this pathway may not contribute to pain sensation under normal conditions but could become sensitized by visceral inflammation.8,9
Supraspinal Nociceptive Targets
Thalamus
The thalamus has been proposed to be involved in pain since as far back as 1911. Indeed, at that time pain was considered uniquely primitive among sensory systems and as being perceived at the level of the thalamus, without important cortical involvement.10 It is now recognized that reentrant interconnections between different thalamic nuclei and cortical areas set the stage for conscious perception of pain.11–14
When considering nociception, the thalamus is generally separated into medial and lateral aspects. Although with recent discoveries this division is no longer as clear-cut as was once thought, the lateral system is most strongly linked to the processing of sensory-discriminative information related to pain, whereas the medial system is more closely associated with emotional aspects of pain. The lateral system encompasses the ventral and posterior nuclei of the thalamus and their linkages with the lateral somatosensory cortices. The lateral thalamic nuclei receive direct spinothalamic input from both superficial and deep layers of the dorsal horn.15,16 Thalamic nuclei assigned to the lateral system include the ventral posterior medial (VPM) and ventral posterior lateral (VPL) nuclei, as well as the ventral posterior inferior (VPI) nucleus. Together, the VPM and VPL nuclei in humans are referred to as the ventrocaudal (Vc) nucleus. Medial and intralaminar nuclei along with their targets in the anterior cingulate cortex (ACC) and the medial prefrontal cortex (PFC) have been thought to constitute the medial pathway. In a recent and still controversial remapping of these systems, Dostrovsky and Craig have argued that a novel, primate-specific nucleus, the posterior portion of the ventromedial (VMpo) nucleus, represents the main thalamic relay for sensory-discriminative information, with the insula as the principal cortical target.13 The validity of this framework remains a topic of debate,15–19 and the controversy probably reflects the somewhat artificial nature of the medial/lateral pain system concept. Certainly, it should be recognized that these divisions are not as well demarcated as was once thought, and overlap and interconnections must exist in function and structure.
Lateral Thalamic Nuclei
Ventral Caudal Nucleus
The structures of the lateral thalamus primarily associated with nociception are the three nuclei that make up the Vc nucleus, also known as the principal sensory nucleus of the thalamus in humans or the ventral posterior (VP) complex in primates. In both humans and primates, the constituent nuclei are the VPM and VPL, which are functionally matched. Spinothalamic projections to the VPL nucleus arise from the spinal dorsal horn, whereas input to the VPM nucleus comes from the spinal trigeminal nucleus (i.e., the “medullary dorsal horn”).20–22 Spinothalamic/trigeminothalamic terminations are interdigitated with terminals of the medial lemniscal pathway, which carries somatotopically organized tactile information through the dorsal columns and to the dorsal column nuclei.23,24 The spinothalamic terminations in the VPL nucleus arrive in clusters and are roughly somatotopically organized in concordance with the same medial-lateral somatotopic organization as the lemniscal pathway.25–27
The output of the Vc nucleus is primarily to sensory cortical areas, most notably to the primary somatosensory (SI) cortex. Via retrograde tracing in primates, it was found that the Vc nucleus provides the majority of thalamic input to the SI cortex.28 Importantly, as many as 90% of identified nociceptive neurons of the VPL nucleus could be activated antidromically from the SI cortex.29 Other identified targets of Vc projections include the secondary somatosensory (SII) cortex, insula, and posterior parietal cortex.30–33
In addition to anatomic approaches, recording and stimulation of Vc neurons provides evidence for involvement of the region in sensory and discriminative aspects of pain. The majority of neurons in this region respond to innocuous or low-threshold mechanical stimuli, but as many as 10% are activated by noxious stimuli or changes in temperature.34–37 Clusters of these nociceptive-responding neurons can be identified most consistently near the posterior and inferior aspect.23,26,29,38,39 Neurons analogous to the NS and WDR classes first described in the dorsal horn can be identified.40 The Vc nucleus receives visceral as well as cutaneous input, and neurons in this nucleus have been shown to respond to noxious visceral stimuli, although the organization of responsive neurons is not apparently viscerotopic.41,42
Stimulation of the Vc nucleus in humans most often produces contralateral, nonpainful paresthesias even at higher intensities, at least in patients without a chronic pain complaint, although temperature and pain sensations can be evoked from some sites at the lowest stimulus intensity.43,44 Stimulation sites near the posterior and inferior border were significantly more likely to evoke painful or thermal sensations than were those in the core region.43 Lenz and colleagues45,46 have been able to distinguish stimulation sites at which a binary (pain versus no pain) sensation can be elicited and others at which an analog sensation, in which the sensory magnitude is related to stimulus intensity, is produced. They suggest that the information conveyed at sites associated with binary signaling are related to an “alarm” aspect of pain processing whereas processing at analog signaling sites is more important for coding stimulus intensity.
The effects of lesions and inactivation of the Vc nucleus further support the idea that this nucleus is a functional relay for nociceptive information. Focal application of lidocaine in this region in nonhuman primate results in reduced detection of small changes in skin temperature in the noxious range.47 In humans, lesioning of the Vc nucleus and the lateral thalamus more broadly has been attempted in the treatment of neuropathic pain, with resulting decreases in contralateral detection of thermal and mechanical pain, as well as touch and proprioception.48–50 However, such surgeries are necessarily approached with caution because of the risk of triggering iatrogenic central pain.51,52
Ventralis Caudalis Parvocellularis Nucleus
The human ventralis caudalis parvocellularis (Vcpc) nucleus is thought to be analogous to the region referred to as the VPI nucleus in primates.53 Although not as well studied as the Vc nucleus, this region is strongly associated with nociception, and one of its functions is as a relay nucleus for nociceptive signals. Primate studies show that input to the VPI nucleus travels via the spinothalamic tract and arises from neurons in laminae I, IV, and V.25,54,55 The VPI nucleus importantly differs from the neighboring VP nucleus in that the former projects primarily to the SII and insular cortices, both of which are involved in the perception of pain.56 Although the VPI nucleus is anatomically contiguous with the VPL/VPM regions, it is functionally distinct and has been tied to both the sensory and affective components of pain.57 Recording studies in both humans and primates have found numerous neurons in this region that respond specifically to the application of noxious stimuli.36,58,59 In humans, stimulation of the Vcpc nucleus apparently elicits painful sensations more reliably than does stimulation of the Vc nucleus itself.43,60
Posterior Part of the Ventral Medial Nucleus
The VMpo nucleus is the most recently identified of the pain-related nuclei of the lateral thalamus and remains a topic of significant debate.16–19 First identified by Craig and colleagues in primates on the basis of direct spinothalamic input from lamina I,55,61,62 the VMpo nucleus is found in the caudal aspect of the thalamus and is in a location that was formerly included within the posterior complex. A similar region posteromedial to the Vc nucleus has since been identified in humans.63 No analogue has been identified in nonprimate species. The VMpo nucleus projects primarily to the insula, with additional projections to the SI cortex.55
This area is of particular interest because from current evidence it appears to be a specifically nociceptive region of the thalamus. In initial recordings of neurons from primate VMpo nuclei, 97% of the neurons characterized were found to respond to either thermal or noxious stimuli. Further studies have confirmed the presence of WDR, NS, and thermoreceptive-specific neurons and the lack of low-threshold neurons. The recorded neurons have an anterior-posterior topographic organization and small receptive fields.55 Subsequent electrophysiologic recordings in awake human patients found that neurons in a region tentatively identified as the VMpo nucleus respond to innocuous or noxious cooling of the skin and that stimulation of the region elicits perceptions of cold or cooling.64 These authors suggest that the VMpo nucleus could be important not specifically as a nociceptive relay but rather for cooling/cold information.
Notably, however, debate has arisen regarding the location, identification, and characterization of the primate and human VMpo nucleus. Arguments regarding the projection of lamina I neurons in the VMpo nucleus are based on questions of identifying terminals versus fibers of passage. Using a different antibody to the same antigen, another group failed to replicate the staining results that first identified the VMpo nucleus in primates, and the human VMpo nucleus was identified primarily on the basis of antibody staining against the same antigen.16,17,19,53 In a study of poststroke lesions in humans, the authors found that poststroke pain and changes in temperature perception still occurred in the absence of VMpo damage, although lesions not including a significant part of the Vc nucleus did not cause these symptoms.52 Further research is needed to confirm previous studies on the boundaries and connectivity of the VMpo nucleus in primates and to characterize the physiology and responsiveness of VMpo cells in humans and primates.
Medial Thalamic Nuclei
Intralaminar Nuclei
The intralaminar nuclei have long been considered part of a “nonspecific” medial complex that sends diffuse projections to the entirety of the cerebral cortex.65 Intralaminar nuclei postulated to be involved in nociception include the central lateral (CL), center median (CM), and parafascicular (Pf) nuclei, with the Pf and CM nuclei sometimes considered together as the CM-Pf complex. Nuclear boundaries appear to be well matched among species, with only few significant differences. One of the primary interspecies differences in intralaminar nuclei is in their input. In humans, the CL is the only one of the three nuclei that receives spinothalamic projections, whereas in primates there are additional spinothalamic connections to the CM and Pf nuclei.21,22,66–68 Input to the CL nucleus arises from deeper aspects of the spinal gray matter, laminae V and VII.20,69,70 Output from the intralaminar nuclei goes diffusely to the cortex, including the posterior parietal and motor cortex. However, organized connections with striatal structures suggest that these nuclei convey important motor responses to noxious input.71–75
Recordings made in intralaminar nuclei support the idea that they are involved in processing nociceptive information, although their specific role still remains unclear. Neurons responding only to stimuli of noxious intensity have been identified in humans and primates in all three nuclei and in general have large, bilateral receptive fields.76,77 Recordings from the CM-Pf complex have shown that a large proportion of neurons respond to pinprick or noxious heat but none to non-noxious stimuli.78,79
Lesioning of the medial thalamus has been performed for intractable or neuropathic pain and has generally had positive results. In one study of 69 patients with neurogenic pain, medial thalamotomy was found to relieve the pain in 67%.79,80 Stimulation of the intralaminar nuclei includes pain and thermal sensation, along with unpleasant sensations such as dyspnea and dizziness.50,81,82
Ventral Caudal Part of the Medial Dorsal Nucleus
This area of the medial thalamus is often grouped with the intralaminar nuclei because of similarities in afferents and physiology, although the ventral caudal portion of the medial dorsal (MDvc) nucleus has received additional attention as a nociceptive relay after it was shown to receive a spinothalamic projection from lamina I of the spinal cord.16,55 Output from the MDvc nucleus is argued to be primarily to the ACC and frontal lobe. Based on these projections and on identification of NS neurons localized to the MDvc nucleus, this region is proposed to be important in the motivational aspects of pain.
Brainstem
The spinoreticular pathway arises in deeper laminae of the dorsal horn and sends projections to the medial and lateral brainstem core areas, including the lateral reticular nucleus, nucleus reticularis dorsalis, nucleus gigantocellularis, and “rostral ventromedial medulla” (RVM), and to the internal parabrachial nucleus (see Gauriau and Bernard6 for an up-to-date review). These connections are probably important in somatomotor integration of nociceptive responses, recruitment of descending modulatory systems, and engagement of arousal mechanisms.
The spinoparabrachial pathway, which consists of projections from lamina I to the lateral parabrachial region, is receiving increasing attention for its role in pain and especially chronic pain.6 The majority of neurons in the lateral/external parabrachial area are strongly activated by noxious stimulation over wide areas of the body. The nociceptive portion of the parabrachial complex projects heavily to the central nucleus of the amygdala and to the ventromedial hypothalamus. The connection through the amygdala to the extended amygdala has been implicated in emotional reactions to painful stimuli, and this input through the spinoparabrachial system is probably reinforced by direct projections to the amygdala from deeper spinal laminae, which have been demonstrated in both rodents and primates.83,84 The connection from the nociceptive parabrachial complex to the hypothalamus seems more likely to be related to motivated behavior triggered by pain, including defensive behavior, flight, and aggression.
Spinomesencephalic pathways terminate primarily in the lateral and ventrolateral PAG and are thought to be important in active versus passive coping strategies for dealing with escapable and inescapable pain, respectively.85,86 Such input presumably also triggers descending control mechanisms (see “Descending Modulatory Systems” later).
Cortical Processing
The inability to identify a specific cortical “center” that when lesioned eliminates pain gave rise to an idea that pain was uniquely primitive among sensory systems in being processed entirely at subcortical levels.10 However, the advent of functional imaging approaches reawakened interest in the cortical representation of pain, and the notion that the cerebral cortex is uninvolved in detection of noxious stimuli has since been discounted. It is now appreciated that there is not a single cortical “pain center” that mediates all aspects of the complex sensation that is pain but that a reasonably well-defined cortical network is recruited by acute noxious stimulation. Notably, although no lesion at a single site can eliminate the perception of pain, stimulation of any one of many sites can elicit painful perceptions, in conjunction with or independent of other somatosensory sensations. Such stimulation experiments along with recent advances in both noninvasive and invasive techniques has shed light on the contributions that the numerous cortical regions make to the “pain matrix.” Importantly, the cortical representation of long-standing chronic pain adds new layers of complexity to this picture.
Primary Somatosensory Cortex
The SI cortex is part of the anterior parietal lobe and forms the postcentral gyrus. The role of the SI cortex in somatosensory and mechanosensory function is well documented elsewhere.87,88 This region is thus well suited for the proposed role in nociception of discriminating location and intensity, although its specific role has been debated.31,89,90
The primary thalamic input to the SI cortex is via the VP nuclei of the thalamus, which as noted earlier receives both spinothalamic and lemniscal projections. In monkeys, nociceptive input to the SI cortex has been demonstrated,28 and studies in rats have also shown clusters of nociceptive neurons in this region of cortex.91 Nociceptive neurons in the SI cortex are much rarer in primates, however, and although some reports mention their existence, mechanosensory neurons are far more common.92 Nonetheless, subdural and other recording methods in humans have shown evidence of neurons specifically activated by noxious stimuli and specific activation of the SI cortex in pain-related tasks.93–95
A majority of recent imaging studies demonstrate activation of the SI cortex with experimental noxious stimuli, and SI activation has been linked specifically to pain intensity.96–98 Interestingly, a sustained noxious stimulation (immersion of the hand in hot water for 3 minutes under laboratory conditions) has been reported to give rise to decreased signal (blood flow) in this region.99
Despite the findings of nociceptive neurons and activation of the SI cortex in imaging studies, SI stimulation predominantly produces contralateral sensations of temperature, paresthesias, and other innocuous sensations; few, if any studies have reported sensations of pain evoked by stimulation of the human SI cortex.100–102 Studies of surgical and ischemic lesions in the SI cortex and surrounding areas have also shed light on its role in pain processing. Early reports of SI damage from head trauma pointed to hypoalgesia and deficits in spatial and temporal discrimination of noxious stimuli.103–105 A more recent case report of stroke-related damage to the right SI cortex described a patient’s difficulty in localizing laser-evoked noxious stimuli on the left side of the body; this patient was unable to discriminate the location of the stimuli further than to a single limb.106 However, surgical interventions in the SI cortex for pain control have been reported to generally be ineffective.107
Secondary Somatosensory Cortex
The SII cortex is located on the parietal operculum at the superior bank of the sylvian fissure, and although the functional specifics of pain processing are still being described, the SII cortex holds an unambiguous role in cortically mediated nociception. The SII cortex is proposed to be involved in recognition of painful and thermal stimuli, pain-related learning, and integration of tactile and nociceptive information. Thalamic input to the SII cortex comes largely from the VPI nucleus.108 Individual neurons in the SII cortex may be NS and tend to have large receptive fields with contralateral or bilateral activation.109
The SII cortex is consistently activated in imaging studies involving nociception. Its proximity to the temporal lobe has made it amenable to clinical research involving implantable, intracortical electrodes in patients with temporal lobe epilepsy, and a wealth of knowledge about function has been gained in recent years from such studies. With laser stimuli and implantable electrodes, SII responses were shown to be graded according to stimulation intensity, from the lowest sensory threshold to detection of stimuli as painful. Interestingly, however, the level of SII activation did not increase with increasing stimulation intensity above the threshold for detection of pain.110 In another study using depth electrodes, Frot and coworkers showed increased latency in SII activation from tactile input as compared with noxious stimuli, again supporting the idea of SII involvement in integration and learning.111 In imaging studies, the SII cortex is activated concurrently with or even before the SI cortex, thus indicating a parallel rather than a serial relationship between these two regions.112
Stimulation and lesion studies are few as a result of interpretative difficulties related to location and because ischemic lesions often extend into other areas. Only a few stimulation protocols have focused specifically on the SII cortex. One group reported that SII stimulation in humans did not invoke any painful sensations, although this work was focused primarily on the insula.113,114 In a systematic exploration of stimulation of the SII cortex, another group found the responses to be a mix of somatosensory, temperature, and painful sensations, with a similar percentage of evoked painful sensations as seen in the insula.102 Difficulties with location have also inhibited studies based on SII lesions, although in general, deficits in the SII cortex after lesions support the idea of its direct involvement in pain processing. In a case involving a pure sensory stroke involving only the SII cortex, the patient showed contralateral restricted deficits in light touch, pain, and temperature sensation.115 Other studies involving the SII cortex report central pain syndrome, hyperalgesia, and thermal and mechanical deficits but, notably, with parietal lesions that do not involve the SII cortex, no such changes are observed.116,117 Although some impediments to interpreting data related to the SII cortex exist, several lines of research unequivocally indicate the involvement of this region in pain processing and integration of tactile and nociceptive signals.
Insula
When viewed sagittally, the insula is a triangular region of the cortex located fully inside the sylvian sulcus. Before recent advances in imaging technology, the role of the insula in nociception was unclear, although it is now considered to be a central structure in the pain matrix and is the most commonly activated site in cortical imaging studies on pain.11 Many structural subdivisions of the insula have been proposed, but the most commonly used divisions are along the anterior-posterior axis. The anterior or mid/anterior insula is often implicated in processing of nociceptive information, whereas the posterior insula is more involved in tactile processing.113,114,118 Functional magnetic resonance imaging points to a somatotopic organization.119,120 Baliki and colleagues recently provided evidence that the insula includes circuits both specific to pain per se and more generally linked to encoding the intensity or magnitude of sensory stimuli, including painful stimuli.121
Recording and stimulation studies confirm the role of the insula in nociception and shed light on differences in function and processing between it and neighboring or functionally similar areas such as the SII cortex. Depth electrodes implanted in humans show activation of insular activity from painful stimuli with slightly higher latency (40 to 60 msec) than similarly activated suprasylvian areas, which indicates serial rather than parallel processing.122 Stimulation of the insula results in a range of somatosensory responses (temperature, pain, and paresthesias), as well as some nonsomatosensory responses, including pharyngolaryngeal constriction and interruption of speech. Stimulation of the insula shows a crude somatosensory map with much larger areas of sensation, including the whole body, than is seen with stimulation of the SI or SII cortex.102 Previous work found a somatotopic pain map that overlapped with a nonpain somatotopic map, thus supporting the idea of integration as a key role of the insula.114 In patients with implanted electrodes, a laser was used as the noxious stimulus to evoke responses within the insula. In contrast to activation in the SII cortex, cells in the insula did not show activation until the stimulus reached the threshold for pain. Increasing intensity above threshold correlated with increased activity of cells, thus indicating that one function of the insula is to code for the intensity of painful stimuli.110 With lesions localized to the insular cortex, some patients show an increase in pain tolerance or loss of affective quality of pain while retaining their ability to detect intensity and heat-pain thresholds.117
Anterior Cingulate Cortex
The cingulate cortex is commonly divided functionally into anterior and posterior segments, and the ACC, which encompasses Brodmann’s areas 24 and 32, is further divided into the midcingulate cortex (MCC) and the ACC proper. The entire ACC has been specifically implicated in the affective and motivational aspects of pain, although the region has many other functions, including overall affect, response selection, and autonomic control. Activation of the MCC in response to noxious stimuli has been show to occur with the same latency as that of the SII cortex, which suggests that processing of affective and sensory-discriminative processing occurs in parallel rather than serially. The ACC has few afferents, and most input to the ACC comes from medial thalamic nuclei, the mediodorsal and Pf nuclei.123 In contrast, the posterior cingulate cortex receives afferents from anterior thalamic nuclei and numerous other cortical regions and notably also has strong interconnections with the ACC.124
Recordings from neurons in the ACC in rabbits and rats reveal NS neurons with whole-body receptive fields and no apparent somatotopic order or mapping.125,126 In psychiatric patients awaiting cingulotomy, recordings made from the ACC clearly show pain-specific neurons that respond to contralateral thermal, noxious thermal, and noxious mechanical stimuli. Interestingly, with excitatory stimulation, even with high-current stimulation at the site of an NS neuron, painful sensations were not evoked.127 Earlier work involving stimulation of the ACC in humans was consistent with these results in that no painful responses were elicited with stimulation, although such autonomic sensations as changes in blood pressure and heart rate occurred.128 One explanation for the discrepancy between stimulation and recording results is that the ACC requires coactivation of other pain-related areas to interpret the ACC response as painful, but an experiment verifying this idea would be very difficult to conduct.
Lesions and selective modulation of the affective aspect of pain additionally shed light on the role of the ACC in pain responses. An imaging study in which the unpleasantness of a painful stimulus was manipulated by using hypnosis revealed consistent correlates of perceived unpleasantness with activation of only the ACC, thus highlighting its role in the emotional and motivation aspects of pain.129 In addition, in a study of 12 patients who underwent therapeutic cingulotomy for intractable pain, pain ratings were improved only modestly, but the pain was considered less bothersome or distressing. On a long-term basis, however, these same patients also reported significant deficits in executive functioning and intention.130
Imaging studies of activation of the cingulate cortex in response to noxious stimuli indicate that the primary site of activation is the rostral ACC or MCC, although different patterns of activation can occur through modulation of higher cognitive function.131–133 With anticipation of pain or the early onset of a painful stimulus, the rostral segment of the ACC is primarily activated, whereas if the stimulus is maintained, activation of the caudal ACC can be noted.134 These data taken together indicate that the ACC is a primary cortical site of the pain matrix, but its role is in the emotional distress of pain and selection of responses to painful stimuli.
Prefrontal Cortex
The PFC encompasses a large part of the frontal cortex just anterior to the motor cortex. Although no evidence exists for direct nociceptive connections, the area is worth mentioning here because of its role in higher cognitive function and endogenous modulation of pain. The PFC has been implicated in a majority of pain-related imaging studies and is even more frequently involved during chronic pain.11 The medial PFC and dorsolateral PFC are the subdivisions more commonly activated during pain, and both areas are involved in executive function, attention, and execution of high-order tasks. The dorsolateral PFC is specifically involved in the placebo response, in which pain sensations are modulated by expectation.135
Sensitization of Ascending Pain Transmission Pathways
Stimulation of injured or inflamed tissue is well known to give rise to the exaggerated pain responses of hyperalgesia (enhanced pain in response to a normally painful stimulus) and allodynia (pain produced by a normally innocuous stimulus). This can be attributed at least in part to dynamic regulation of the pain transmission elements in accord with stimulus history, the state of the tissue (inflamed versus normal), and immune signals. Plasticity in nociceptive processing has now been documented at every level of processing, such that an increased signal carried by sensitized primary afferents is further amplified at the dorsal horn and supraspinally. Changes at the dorsal horn are probably the best studied, and the net effect at this level is often referred to as “central sensitization” because molecular, cellular, and circuit-level changes in the dorsal horn give rise to an increase in the sensitivity of nociceptive neurons, with lowered thresholds, increased responsiveness, and enlarged receptive fields.136–141 More recently, this concept has been extended to include the supraspinal systems, and there is mounting evidence of reorganization of thalamocortical and subcortical forebrain circuits in chronic pain states, not all of which may be reversible.142,143
Descending Modulatory Systems
The variability in pain experience has long been recognized and frequently underpins a characterization of pain reports as “subjective” and therefore not to be trusted. One of the first systematic scientific discussions of the variability of pain and the importance of cognitive and emotional factors in pain sensation was advanced by Beecher,144 who quantified the pain experienced by wounded soldiers according to the amount of narcotics that they required. He noted that the pain experienced by many of these soldiers was much less than would have been predicted based on their injuries and argued that the apparent absence of pain reflected a positive cognitive appraisal of the injury, which would remove that soldier from the war, at least temporarily.
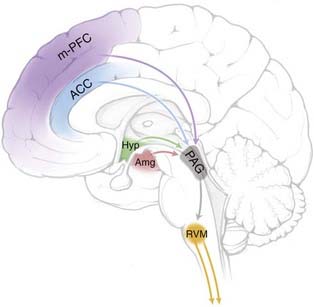
We now appreciate that cognitive and emotional factors, including attention, learning, and mood, can all influence pain perception in situations nowhere nearly as extreme as the battlefield.145,146 The brain is not a passive receiver of information about noxious stimuli but actively regulates its own input. Indeed, we now recognize that the coding of afferent information is shaped dynamically by descending modulatory systems that influence nociceptive processing at the first central relay, the dorsal horn. These descending control systems are themselves regulated by afferent sensory input but are also recruited by higher centers to modulate spinal nociceptive processing in accord with behavioral priorities. In this section we consider the organization of descending modulatory systems in the central core of the brainstem, with a particular focus on the PAG/RVM system.
Descending Modulation and the Periaqueductal Gray Matter/Rostral Ventromedial Medulla System
It has been known since the work of Sherrington at the turn of the last century that spinal nocifensive reflexes are altered in the absence of descending influences.147 However, the idea that pain modulation is a specific function of the nervous system is usually traced to a report in the late 1960s in which electrical stimulation in the midbrain PAG was found to produce potent antinociception in rats.148 These original observations were relatively crude, but subsequent work confirmed the antinociceptive potency of PAG stimulation149 and extended it to other species, including humans.150 This phenomenon of “stimulation-produced analgesia” inspired experimental studies in a number of laboratories and ultimately led to definition of a brainstem pain-modulating circuit (Fig. 154-2) with critical links in the PAG and RVM. Electrical stimulation at either site resulted in antinociception, an effect mediated by inhibition of nociceptive activity in the dorsal horn. The anatomic substrate for this descending control is a significant projection from the PAG to the RVM, which in turn projects via the dorsolateral funiculus to the dorsal horn to influence sensory processing. The PAG itself sends only minor projections to the spinal cord and receives substantial input from limbic forebrain structures, including the ACC, PFC, insula, amygdala, and hypothalamus. This information is integrated and transmitted to the RVM. Both the RVM and especially the PAG also receive significant nociceptive input from the dorsal horn via the spinoreticular and spinomesencephalic tracts. Based on the connectivity of this system alone, it can be seen that the PAG/RVM system is well situated to integrate higher order influences important in cognition and emotion with afferent input from the dorsal horn. Other brainstem systems, such as the nucleus reticularis dorsalis in the caudal medulla (which mediates the effects of counterirritation) and the noradrenergic systems in the dorsolateral pontine tegmentum, are also known to modulate nociceptive transmission, but the PAG/RVM system is the best studied and probably has the greatest impact on pain.
Interest in the PAG/RVM system was heightened when it became clear that it was an important substrate for opioid analgesic drugs.151–153 It had long been recognized that morphine and other opioid agents act primarily in the brain. Using microinjection mapping to delineate central sites that were directly sensitive to opioids, it became clear that both the PAG and RVM could support opioid analgesia and that these regions are in fact required for the analgesic actions of systemically administered opioids.152 This system also uses endogenous opioids as neurotransmitters, and there is evidence that endogenous opioids in the PAG and RVM contribute to the pain-modulating function of this system.
Given that the direct cellular effects of opioids are inhibitory,154 it may be surprising that focal opioid application and electrical stimulation in these regions have the same net behavioral effect, analgesia. However, the answer to this apparent paradox lies in the circuitry within the PAG and RVM, where opioids act directly on inhibitory neurons that normally inhibit the pain-inhibiting output neurons. Opioids thus activate descending inhibition via disinhibition.
Bidirectional Control
Along with the evidence that this system mediates the analgesic actions of exogenous and endogenous opioids, the initial discovery of the PAG/RVM circuit as the basis for stimulation-produced analgesia led to an early view of this circuit as an “analgesia system.” We now recognize that this view was incomplete and that the brainstem exerts bidirectional control in which pain is inhibited or facilitated under different conditions. The bidirectional nature of descending modulation is best studied in the RVM. A number of early behavioral and electrophysiologic studies suggested that electrical stimulation of the RVM could facilitate pain, depending on the stimulating current and exact location of the electrode.155 The implications of these observations were unclear until evidence developed that the RVM contributes to increased pain in a number of paradigms. Thus, for example, blocking activity in the RVM interferes with secondary hyperalgesia after inflammation, with nerve injury pain, and with hyperalgesia induced by chronic opioid administration (“opioid-induced hyperalgesia”).156,157 Current thinking therefore recognizes a modulatory system rather than an “analgesia” system, with the potential for bidirectional control.145
Neural Basis for Bidirectional Control
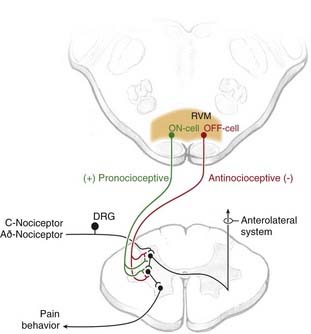
Functional studies thus clearly demonstrate that as a region the RVM exerts bidirectional control of nociceptive processing, with a net pronociceptive action under some conditions and a net antinociceptive role in others. The neural basis for the pain-inhibiting and pain-facilitating influences from the RVM is now recognized to be two classes of RVM neurons, termed OFF-cells and ON-cells, respectively (Fig. 154-3).146,158 OFF-cells mediate the analgesic actions of mu-opioid agents and are probably recruited in behavioral states associated with decreased pain, such as stress-induced analgesia. ON-cells are activated as part of a positive feedback loop that amplifies pain after tissue or nerve injury and during inflammation. These neurons are also activated by higher structures (e.g., the hypothalamus) in the absence of noxious input and could thus account for increased pain sensitivity stimulated by cognitive and emotional processes, such as during mild stress.159
Recruitment of the Periaqueductal Gray Matter/Rostral Ventromedial Medulla Modulatory System
The discussion of descending modulatory systems has to this point focused on anatomic and physiologic delineation of the PAG/RVM circuitry and the effects of experimental manipulations on dorsal horn processing and nociceptive behavior. This understanding prompts the question of how these systems come to be activated in behaving animals. Although our information is in many ways still incomplete, we now have strong evidence that the balance between pain-facilitating and pain-inhibiting outflow from the RVM is dynamic and allows the organism to amplify or suppress nociceptive afferent transmission in accord with behavioral priorities.145,146
Activation by Noxious Input: A Positive Feedback Loop
An early view of descending modulatory systems focused on the phenomenon of counterirritation in which pain at one site on the body suppressed input from other regions. A reasonable assumption was that this remote inhibition was mediated by the PAG/RVM system as part of a negative feedback loop. We now know that negative feedback processes triggered by noxious input are mediated not by the PAG/RVM system but by the dorsal reticular nucleus in the caudal medulla.160,161 It can be argued that this process is important as a contrast mechanism and for integration of multiple sensory inputs with motor output. This negative feedback process is in contrast to the PAG/RVM system, which mediates an acute positive feedback process. Discrete noxious stimuli or more prolonged inflammatory events activate ON-cells, thereby amplifying responses to subsequent input.162,163
Stress
A very different approach to the function of the RVM system is to consider descending modulation within the larger context of integrating and organizing defensive responses to noxious or threatening aspects of the environment.146,164,165 The phenomenon of “stress-induced analgesia,” as seen, for example, on encountering biologically relevant threat stimuli such as a predator or during major trauma, is well documented. Stress-induced analgesia presumably functions to suppress nociception in situations in which distress or overt behavior that would otherwise be evoked by a noxious stimulus might interfere with effective coping.149,164,166,167 A role for the RVM in stress-induced analgesia is well documented, and RVM OFF-cells are recruited in a model of fear-induced analgesia, in which these neurons are activated via the amygdala.168,169
Stress can also give rise to stress-induced hyperalgesia, an effect probably more relevant than stress-induced analgesia to everyday experience and to the clinical environment. In humans, anxiety or anticipation of pain can be shown to enhance pain sensitivity,170–174 and stress is often asserted to exacerbate chronic clinical pain.175–177 Hyperalgesia mediated by activation of RVM ON-cells has recently been demonstrated in an animal model of mild or emotional stress.159
Contribution to Chronic Pain States
Descending facilitation from the PAG/RVM system has recently been shown to contribute to chronic pain states. Inactivation of the RVM attenuates or blocks allodynia and hyperalgesia in animal models of inflammation and nerve injury pain, thus indicating that this region functions as part of a positive feedback loop triggered by injury and inflammation.156,178–181 There is further evidence pointing specifically to recruitment and sensitization of RVM ON-cells as being important in this positive feedback process.156,163,182 Importantly, the recent observations that RVM ON-cells can be engaged by the hypothalamus and preoptic areas in models of stress and illness show that pain processing can be facilitated by top-down influences in the absence of an initial noxious trigger. Such top-down modulation could be relevant to functional pain disorders such as fibromyalgia or irritable bowel syndrome.
Descending Modulation in Humans: Evidence from Imaging Studies
Existence of the brainstem modulatory system in humans was first demonstrated by the analgesic action of deep brain stimulation in the periventricular gray/PAG area in some forms of clinical pain.150 With the advent of functional imaging approaches, we now have increasing understanding of when brainstem pain-modulating systems are recruited in humans.98,183 Placebo analgesia has probably been the most intensively studied model, with coactivation of the ACC and PAG during both opioid- and placebo-induced analgesia.184,185 PAG activation has also been inversely correlated with pain rating in paradigms associated with increased pain.186–188 Although correlational, these studies confirm the recruitment of brainstem core systems in humans by cognitive and emotional variables.
Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol. 2008;18:464-468.
Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81-97.
Apkarian AV, Bushnell MC, Treede RD, et al. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463-484.
Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiology. 2008;23:371-380.
Blomqvist A, Zhang ET, Craig AD. Cytoarchitectonic and immunohistochemical characterization of a specific pain and temperature relay, the posterior portion of the ventral medial nucleus, in the human thalamus. Brain. 2000;123:601-619.
Coderre TJ, Katz J, Vaccarino AL, et al. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259-285.
Craig AD. Pain mechanisms: labeled lines versus convergence in central processing. Annu Rev Neurosci. 2003;26:1-30.
DeLeo JA, Sorkin LS, Watkins LR, editors. Immune and Glial Regulation of Pain. Seattle: International Association for Study of Pain Press. 2007:443.
Dostrovsky JO, Craig AD. The thalamus and nociceptive processing. In: Basbaum AI, Bushnell MC, editors. The Senses: A Comprehensive Reference, Vol 5. Pain. San Diego, CA: Academic Press; 2008:635-654.
Fields H. State-dependent opioid control of pain. Nat Rev Neurosci. 2004;5:565-575.
Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. 5th ed. London: Elsevier; 2006:125-142.
Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251-258.
Heinricher MM, Ingram SL. The brainstem and nociceptive modulation. In: Bushnell MC, Basbaum AI, editors. The Senses, a Comprehensive Reference, Vol 5. Pain. San Diego, CA: Academic Press; 2008:593-626.
Heinricher MM, Tavares I, Leith JL, et al. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214-225.
Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169-203.
McMahon SB, Lewin GR, Wall PD. Central hyperexcitability triggered by noxious inputs. Curr Opin Neurobiol. 1993;3:602-610.
Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319-325.
Rainville P, Carrier B, Hofbauer RK, et al. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain. 1999;82:159-171.
Ren K, Dubner R. Central nervous system plasticity and persistent pain. J Orofac Pain. 1999;13:155-163.
Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377-391.
Treede RD, Apkarian AV. Nociceptive processing in the cerebral cortex. In: Basbaum AI, Bushnell MC, editors. The Senses: A Comprehensive Reference, Vol 5. Pain. San Diego: Academic Press; 2008:669-698.
Willis WD, editor. Hyperalgesia and Allodynia. New York: Raven Press. 1992:1.
Willis WDJr, Westlund KN. Dorsal columns and visceral pain. In: Basbaum AI, Bushnell MC, editors. The Senses: A Comprehensive Reference, Vol 5. Pain. San Diego, CA: Academic Press; 2008:527-542.
1 Willis WD, Coggleshall RE. Sensory Mechanisms of the Spinal Cord. New York: Plenum Press; 1991.
2 Dubner R, Bennett GJ. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci. 1983;6:381-418.
3 Price DD, Dubner R. Neurons that subserve the sensory-discriminative aspects of pain. Pain. 1977;3:307-338.
4 Bushnell MC. Thalamic processing of sensory-discriminative and affective-motivational dimensions of pain. In: Besson JM, Guilbaud G, Ollat H, editors. Forebrain Areas Involved in Pain Processing. Paris: John Libbey Eurotext, 1985.
5 Melzack R, Casey KL. Sensory, motivational, and central control determinants of pain. In: Kenshalo DR, editor. The Skin Senses. Springfield, IL: Charles C. Thomas; 1968:423-443.
6 Gauriau C, Bernard JF. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251-258.
7 Willis WD, Westlund KN. The role of the dorsal column pathway in visceral nociception. Curr Pain Headache Rep. 2001;5:20-26.
8 Palecek J, Paleckova V, Willis WD. Postsynaptic dorsal column neurons express NK1 receptors following colon inflammation. Neuroscience. 2003;116:565-572.
9 Palecek J. The role of dorsal columns pathway in visceral pain. Physiol Res. 2004;53(suppl 1):S125-S130.
10 Head H, Holmes G. Sensory disturbances from cerebral lesions. Brain. 1911;34:102-254.
11 Apkarian AV, Bushnell MC, Treede RD, et al. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463-484.
12 Ralston HJ3rd. Pain and the primate thalamus. Prog Brain Res. 2005;149:1-10.
13 Dostrovsky JO, Craig AD. The thalamus and nociceptive processing. In: Basbaum AI, Bushnell MC, editors. The Senses: A Comprehensive Reference, Vol 5. Pain. San Diego, CA: Academic Press; 2008:635-654.
14 Willis WDJr. Pain pathways in the primate. Prog Clin Biol Res. 1985;176:117-133.
15 Willis WD, Zhang X, Honda CN, et al. Projections from the marginal zone and deep dorsal horn to the ventrobasal nuclei of the primate thalamus. Pain. 2001;92:267-276.
16 Willis WDJr, Zhang X, Honda CN, et al. A critical review of the role of the proposed vmpo nucleus in pain. J Pain. 2002;3:79-94.
17 Jones EG, Lensky KM, Chan VH. Delineation of thalamic nuclei immunoreactive for calcium-binding proteins in and around the posterior pole of the ventral posterior complex. Thalamus Relat Syst. 2001;1:213-224.
18 Jones EG. A pain in the thalamus. J Pain. 2002;3:102-104.
19 Graziano A, Jones EG. Widespread thalamic terminations of fibers arising in the superficial medullary dorsal horn of monkeys and their relation to calbindin immunoreactivity. J Neurosci. 2004;24:248-256.
20 Applebaum AE, Leonard RB, Kenshalo DRJr, et al. Nuclei in which functionally identified spinothalamic tract neurons terminate. J Comp Neurol. 1979;188:575-585.
21 Apkarian AV, Hodge CJ. Primate spinothalamic pathways: III. Thalamic terminations of the dorsolateral and ventral spinothalamic pathways. J Comp Neurol. 1989;288:493-511.
22 Mehler WR. The anatomy of the so-called “pain tract” In man: an analysis of the course and distribution of the ascending fibers of the fasciculus anterolateralis. In: Basic Research in Paraplegia. Springfield, IL: Thomas; 1962.
23 Kaas JH, Nelson RJ, Sur M, et al. The somatotopic organization of the ventroposterior thalamus of the squirrel monkey, Saimiri sciureus. J Comp Neurol. 1984;226:111-140.
24 Jones EG. Correlation and revised nomenclature of ventral nuclei in the thalamus of human and monkey. Stereotact Funct Neurosurg. 1990;54-55:1-20.
25 Mehler WR. The posterior thalamic region in man. Confin Neurol. 1966;27:18-29.
26 Lenz FA, Dostrovsky JO, Tasker RR, et al. Single-unit analysis of the human ventral thalamic nuclear group: somatosensory responses. J Neurophysiol. 1988;59:299-316.
27 Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14:2-31.
28 Gingold SI, Greenspan JD, Apkarian AV. Anatomic evidence of nociceptive inputs to primary somatosensory cortex: relationship between spinothalamic terminals and thalamocortical cells in squirrel monkeys. J Comp Neurol. 1991;308:467-490.
29 Kenshalo DRJr, Giesler GJJr, Leonard RB, et al. Responses of neurons in primate ventral posterior lateral nucleus to noxious stimuli. J Neurophysiol. 1980;43:1594-1614.
30 Burton H, Carlson M. Second somatic sensory cortical area (SII) in a prosimian primate, Galago crassicaudatus. J Comp Neurol. 1986;247:200-220.
31 Kenshalo DRJr, Willis WD. The Role of the Cerebral Cortex in Pain Sensation. New York: Plenum Press; 1991.
32 Burton H, Jones EG. The posterior thalamic region and its cortical projection in New World and Old World monkeys. J Comp Neurol. 1976;168:249-301.
33 Jones EG, Burton H. Areal differences in the laminar distribution of thalamic afferents in cortical fields of the insular, parietal and temporal regions of primates. J Comp Neurol. 1976;168:197-247.
34 Lenz FA, Dougherty PM. Neurons in the human thalamic somatosensory nucleus (ventralis caudalis) respond to innocuous cool and mechanical stimuli. J Neurophysiol. 1998;79:2227-2230.
35 Willis WD. Nociceptive pathways: anatomy and physiology of nociceptive ascending pathways. Philos Trans R Soc Lond B Biol Sci. 1985;308:253-270.
36 Apkarian AV, Shi T. Squirrel monkey lateral thalamus. I. Somatic nociresponsive neurons and their relation to spinothalamic terminals. J Neurosci. 1994;14:6779-6795.
37 Bushnell MC, Duncan GH, Tremblay N. Thalamic VPM nucleus in the behaving monkey. I. Multimodal and discriminative properties of thermosensitive neurons. J Neurophysiol. 1993;69:739-752.
38 Lee J, Dougherty PM, Antezana D, et al. Responses of neurons in the region of human thalamic principal somatic sensory nucleus to mechanical and thermal stimuli graded into the painful range. J Comp Neurol. 1999;410:541-555.
39 Lenz FA, Garonzik IM, Zirh TA, et al. Neuronal activity in the region of the thalamic principal sensory nucleus (ventralis caudalis) in patients with pain following amputations. Neuroscience. 1998;86:1065-1081.
40 Lenz FA, Gracely RH, Rowland LH, et al. A population of cells in the human thalamic principal sensory nucleus respond to painful mechanical stimuli. Neurosci Lett. 1994;180:46-50.
41 Bruggemann J, Shi T, Apkarian AV. Squirrel monkey lateral thalamus. II. Viscerosomatic convergent representation of urinary bladder, colon, and esophagus. J Neurosci. 1994;14:6796-6814.
42 Chandler MJ, Hobbs SF, Fu QG, et al. Responses of neurons in ventroposterolateral nucleus of primate thalamus to urinary bladder distension. Brain Res. 1992;571:26-34.
43 Lenz FA, Seike M, Richardson RT, et al. Thermal and pain sensations evoked by microstimulation in the area of human ventrocaudal nucleus. J Neurophysiol. 1993;70:200-212.
44 Davis KD, Kiss ZH, Tasker RR, et al. Thalamic stimulation–evoked sensations in chronic pain patients and in nonpain (movement disorder) patients. J Neurophysiol. 1996;75:1026-1037.
45 Bagley CA, Ohara S, Lawson HC, et al. Psychophysics of CNS pain–related activity: binary and analog channels and memory encoding. Neuroscientist. 2006;12:29-42.
46 Lenz FA, Ohara S, Gracely RH, et al. Pain encoding in the human forebrain: binary and analog exteroceptive channels. J Neurosci. 2004;24:6540-6544.
47 Duncan GH, Bushnell MC, Oliveras JL, et al. Thalamic VPM nucleus in the behaving monkey. III. Effects of reversible inactivation by lidocaine on thermal and mechanical discrimination. J Neurophysiol. 1993;70:2086-2096.
48 Albe-Fessard D, Dondey M, Nicolaidis S, et al. Remarks concerning the effect of diencephalic lesions on pain and sensitivity with special reference to lemniscally mediated control of noxious afferences. Confin Neurol. 1970;32:174-184.
49 Mark VH, Ervin FR, Yakovlev PI. Correlation of pain relief, sensory loss, and anatomical lesion sites in pain patients treated with stereotactic thalamotomy. Trans Am Neurol Assoc. 1961;86:86-90.
50 Richardson DE. Thalamotomy for intractable pain. Confin Neurol. 1967;29:139-145.
51 Tasker RR, Kiss ZH. The role of the thalamus in functional neurosurgery. Neurosurg Clin N Am. 1995;6:73-104.
52 Kim JH, Greenspan JD, Coghill RC, et al. Lesions limited to the human thalamic principal somatosensory nucleus (ventral caudal) are associated with loss of cold sensations and central pain. J Neurosci. 2007;27:4995-5004.
53 Treede RD. Spinothalamic and thalamocortical nociceptive pathways. J Pain. 2002;3:109-112.
54 Ralston HJ3rd, Ralston DD. The primate dorsal spinothalamic tract: Evidence for a specific termination in the posterior nuclei (po/sg) of the thalamus. Pain. 1992;48:107-118.
55 Craig AD, Bushnell MC, Zhang ET, et al. A thalamic nucleus specific for pain and temperature sensation. Nature. 1994;372:770-773.
56 Friedman DP, Murray EA. Thalamic connectivity of the second somatosensory area and neighboring somatosensory fields of the lateral sulcus of the macaque. J Comp Neurol. 1986;252:348-373.
57 Millan MJ. The induction of pain: an integrative review. Prog Neurobiol. 1999;57:1-164.
58 Pollin B, Albe-Fessard D. Organization of somatic thalamus in monkeys with and without section of dorsal spinal tracts. Brain Res. 1979;173:431-449.
59 Casey KL, Morrow TJ. Nociceptive neurons in the ventral posterior thalamus of the awake squirrel monkey: observations on identification, modulation, and drug effects. In: Besson JM, Guilbaud G, Peschanski M, editors. Thalamus and Pain. Amsterdam: Elsevier, 1987.
60 Halliday AM, Logue V. Painful sensations evoked by electrical stimulation in the thalamus. In: Somjen GG, editor. Neurophysiology Studied in Man. Amsterdam: Excerpta Medica; 1972:221-230.
61 Craig AD. Distribution of brainstem projections from spinal lamina I neurons in the cat and the monkey. J Comp Neurol. 1995;361:225-248.
62 Han ZS, Zhang ET, Craig AD. Nociceptive and thermoreceptive lamina I neurons are anatomically distinct. Nat Neurosci. 1998;1:218-225.
63 Blomqvist A, Zhang ET, Craig AD. Cytoarchitectonic and immunohistochemical characterization of a specific pain and temperature relay, the posterior portion of the ventral medial nucleus, in the human thalamus. Brain. 2000;123:601-619.
64 Davis KD, Lozano RM, Manduch M, et al. Thalamic relay site for cold perception in humans. J Neurophysiol. 1999;81:1970-1973.
65 Jones EG. A new view of specific and nonspecific thalamocortical connections. Adv Neurol. 1998;77:49-71.
66 Apkarian AV, Hodge CJ. Primate spinothalamic pathways: I. A quantitative study of the cells of origin of the spinothalamic pathway. J Comp Neurol. 1989;288:447-473.
67 Bowsher D. Some afferent and efferent connections of the parafascicular-center median complex. In: The Thalamus. New York: Columbia University Press; 1966.
68 Kerr FW. The ventral spinothalamic tract and other ascending systems of the ventral funiculus of the spinal cord. J Comp Neurol. 1975;159:335-356.
69 Craig ADJr, Linington AJ, Kniffki KD. Cells of origin of spinothalamic tract projections to the medial and lateral thalamus in the cat. J Comp Neurol. 1989;289:568-585.
70 Giesler GJJr, Yezierski RP, Gerhart KD, et al. Spinothalamic tract neurons that project to medial and/or lateral thalamic nuclei: evidence for a physiologically novel population of spinal cord neurons. J Neurophysiol. 1981;46:1285-1308.
71 Sadikot AF, Parent A, Francois C. The centre median and parafascicular thalamic nuclei project respectively to the sensorimotor and associative-limbic striatal territories in the squirrel monkey. Brain Res. 1990;510:161-165.
72 Casey KL, Minoshima S, Morrow TJ, et al. Comparison of human cerebral activation pattern during cutaneous warmth, heat pain, and deep cold pain. J Neurophysiol. 1996;76:571-581.
73 Sadikot AF, Parent A, Francois C. Efferent connections of the centromedian and parafascicular thalamic nuclei in the squirrel monkey: a PHA-L study of subcortical projections. J Comp Neurol. 1992;315:137-159.
74 Powell TP, Cowan WM. The interpretation of the degenerative changes in the intralaminar nuclei of the thalamus. J Neurol Neurosurg Psychiatry. 1967;30:140-153.
75 Groenewegen HJ, Berendse HW. The specificity of the ‘nonspecific’ midline and intralaminar thalamic nuclei. Trends Neurosci. 1994;17:52-57.
76 Albe-Fessard D, Berkley KJ, Kruger L, et al. Diencephalic mechanisms of pain sensation. Brain Res. 1985;356:217-296.
77 Bushnell MC, Duncan GH. Sensory and affective aspects of pain perception: is medial thalamus restricted to emotional issues? Exp Brain Res. 1989;78:415-418.
78 Ishijima B, Yoshimasu N, Fukushima T, et al. Nociceptive neurons in the human thalamus. Confin Neurol. 1975;37:99-106.
79 Jeanmonod D, Magnin M, Morel A. Thalamus and neurogenic pain: physiological, anatomical and clinical data. Neuroreport. 1993;4:475-478.
80 Tsubokawa T, Moriyasu N. Follow-up results of centre median thalamotomy for relief of intractable pain. A method of evaluating the effectiveness during operation. Confin Neurol. 1975;37:280-284.
81 Richardson DE. Thalamotomy for control of chronic pain. Acta Neurochir (Wien) Suppl. 1974;21:77-88.
82 Sano K. Intralaminar thalamotomy (thalamolaminotomy) and postero-medial hypothalamotomy in the treatment of intractable pain. Prog Neurol Surg. 1977;8:50-103.
83 Newman HM, Stevens RT, Apkarian AV. Direct spinal projections to limbic and striatal areas: anterograde transport studies from the upper cervical spinal cord and the cervical enlargement in squirrel monkey and rat. J Comp Neurol. 1996;365:640-658.
84 Burstein R, Potrebic S. Retrograde labeling of neurons in the spinal cord that project directly to the amygdala or the orbital cortex in the rat. J Comp Neurol. 1993;335:469-485.
85 Keay KA, Feil K, Gordon BD, et al. Spinal afferents to functionally distinct periaqueductal gray columns in the rat: an anterograde and retrograde tracing study. J Comp Neurol. 1997;385:207-229.
86 Lumb BM. Inescapable and escapable pain is represented in distinct hypothalamic-midbrain circuits: specific roles for Adelta- and c-nociceptors. Exp Physiol. 2002;87:281-286.
87 Kaas JH, Nelson RJ, Sur M, et al. Multiple representations of the body within the primary somatosensory cortex of primates. Science. 1979;204:521-523.
88 Ploner M, Schmitz F, Freund HJ, et al. Differential organization of touch and pain in human primary somatosensory cortex. J Neurophysiol. 2000;83:1770-1776.
89 Bushnell MC, Duncan GH, Hofbauer RK, et al. Pain perception: is there a role for primary somatosensory cortex? Proc Natl Acad Sci U S A. 1999;96:7705-7709.
90 Treede RD, Kenshalo DR, Gracely RH, et al. The cortical representation of pain. Pain. 1999;79:105-111.
91 Lamour Y, Willer JC, Guilbaud G. Rat somatosensory (smi) cortex: I. Characteristics of neuronal responses to noxious stimulation and comparison with responses to non-noxious stimulation. Exp Brain Res. 1983;49:35-45.
92 Kenshalo DRJr, Isensee O. Responses of primate SI cortical neurons to noxious stimuli. J Neurophysiol. 1983;50:1479-1496.
93 Kanda M, Nagamine T, Ikeda A, et al. Primary somatosensory cortex is actively involved in pain processing in human. Brain Res. 2000;853:282-289.
94 Timmermann L, Ploner M, Haucke K, et al. Differential coding of pain intensity in the human primary and secondary somatosensory cortex. J Neurophysiol. 2001;86:1499-1503.
95 Ohara S, Crone NE, Weiss N, et al. Amplitudes of laser evoked potential recorded from primary somatosensory, parasylvian and medial frontal cortex are graded with stimulus intensity. Pain. 2004;110:318-328.
96 Rainville P, Carrier B, Hofbauer RK, et al. Dissociation of sensory and affective dimensions of pain using hypnotic modulation. Pain. 1999;82:159-171.
97 Tracey I. Nociceptive processing in the human brain. Curr Opin Neurobiol. 2005;15:478-487.
98 Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377-391.
99 Apkarian AV, Stea RA, Manglos SH, et al. Persistent pain inhibits contralateral somatosensory cortical activity in humans. Neurosci Lett. 1992;140:141-147.
100 Penfield WB, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389-443.
101 Coghill RC, Talbot JD, Evans AC, et al. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14:4095-4108.
102 Mazzola L, Isnard J, Mauguiere F. Somatosensory and pain responses to stimulation of the second somatosensory area (SII) in humans. A comparison with SI and insular responses. Cereb Cortex. 2006;16:960-968.
103 Russell W. Transient disturbances following gunshot wounds of the head. Brain. 1945;68:79-97.
104 Marshall J. Sensory disturbances in cortical wounds with special reference to pain. J Neurol Neurosurg Psychiatry. 1951;14:187-204.
105 Bassetti C, Bogousslavsky J, Regli F. Sensory syndromes in parietal stroke. Neurology. 1993;43:1942-1949.
106 Ploner M, Freund HJ, Schnitzler A. Pain affect without pain sensation in a patient with a postcentral lesion. Pain. 1999;81:211-214.
107 Sweet W. Cerebral localization of pain. In: Thompson RGJr, editor. New Perspectives in Cerebral Localization. New York: Raven Press; 1982:205-242.
108 Krubitzer L, Clarey J, Tweedale R, et al. A redefinition of somatosensory areas in the lateral sulcus of macaque monkeys. J Neurosci. 1995;15:3821-3839.
109 Disbrow E, Roberts T, Krubitzer L. Somatotopic organization of cortical fields in the lateral sulcus of Homo sapiens: evidence for SII and PV. J Comp Neurol. 2000;418:1-21.
110 Frot M, Magnin M, Mauguiere F, et al. Human SII and posterior insula differently encode thermal laser stimuli. Cereb Cortex. 2007;17:610-620.
111 Frot M, Garcia-Larrea L, Guenot M, et al. Responses of the supra-sylvian (SII) cortex in humans to painful and innocuous stimuli. A study using intra-cerebral recordings. Pain. 2001;94:65-73.
112 Ploner M, Schmitz F, Freund HJ, et al. Parallel activation of primary and secondary somatosensory cortices in human pain processing. J Neurophysiol. 1999;81:3100-3104.
113 Ostrowsky K, Isnard J, Ryvlin P, et al. Functional mapping of the insular cortex: clinical implication in temporal lobe epilepsy. Epilepsia. 2000;41:681-686.
114 Ostrowsky K, Magnin M, Ryvlin P, et al. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb Cortex. 2002;12:376-385.
115 Horiuchi T, Unoki T, Yokoh A, et al. Pure sensory stroke caused by cortical infarction associated with the secondary somatosensory area. J Neurol Neurosurg Psychiatry. 1996;60:588-589.
116 Schmahmann JD, Leifer D. Parietal pseudothalamic pain syndrome. Clinical features and anatomic correlates. Arch Neurol. 1992;49:1032-1037.
117 Greenspan JD, Lee RR, Lenz FA. Pain sensitivity alterations as a function of lesion location in the parasylvian cortex. Pain. 1999;81:273-282.
118 Treede RD, Apkarian AV, Bromm B, et al. Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus. Pain. 2000;87:113-119.
119 Brooks JC, Zambreanu L, Godinez A, et al. Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage. 2005;27:201-209.
120 Henderson LA, Gandevia SC, Macefield VG. Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: a single-trial fMRI study. Pain. 2007;128:20-30.
121 Baliki MN, Geha PY, Apkarian AV, et al. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28:1398-1403.
122 Frot M, Mauguiere F. Dual representation of pain in the operculo-insular cortex in humans. Brain. 2003;126:438-450.
123 Vogt BA, Nimchinsky EA, Vogt LJ, et al. Human cingulate cortex: surface features, flat maps, and cytoarchitecture. J Comp Neurol. 1995;359:490-506.
124 Vogt BA, Rosene DL, Pandya DN. Thalamic and cortical afferents differentiate anterior from posterior cingulate cortex in the monkey. Science. 1979;204:205-207.
125 Sikes RW, Vogt BA. Nociceptive neurons in area 24 of rabbit cingulate cortex. J Neurophysiol. 1992;68:1720-1732.
126 Yamamura H, Iwata K, Tsuboi Y, et al. Morphological and electrophysiological properties of accx nociceptive neurons in rats. Brain Res. 1996;735:83-92.
127 Hutchison WD, Davis KD, Lozano AM, et al. Pain-related neurons in the human cingulate cortex. Nat Neurosci. 1999;2:403-405.
128 Talairach J, Bancaud J, Geier S, et al. The cingulate gyrus and human behaviour. Electroencephalogr Clin Neurophysiol. 1973;34:45-52.
129 Rainville P, Duncan GH, Price DD, et al. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968-971.
130 Cohen RA, Kaplan RF, Moser DJ, et al. Impairments of attention after cingulotomy. Neurology. 1999;53:819-824.
131 Casey KL. Forebrain mechanisms of nociception and pain: analysis through imaging. Proc Natl Acad Sci U S A. 1999;96:7668-7674.
132 Derbyshire SW. Exploring the pain “Neuromatrix.”. Curr Rev Pain. 2000;4:467-477.
133 Derbyshire SW. A systematic review of neuroimaging data during visceral stimulation. Am J Gastroenterol. 2003;98:12-20.
134 Casey KL, Morrow TJ, Lorenz J, et al. Temporal and spatial dynamics of human forebrain activity during heat pain: analysis by positron emission tomography. J Neurophysiol. 2001;85:951-959.
135 Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162-1167.
136 McMahon SB, Lewin GR, Wall PD. Central hyperexcitability triggered by noxious inputs. Curr Opin Neurobiol. 1993;3:602-610.
137 Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169-203.
138 Coderre TJ, Katz J, Vaccarino AL, et al. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259-285.
139 DeLeo JA, Sorkin LS, Watkins LR, editors. Immune and Glial Regulation of Pain. Seattle: International Association for Study of Pain Press. 2007:443.
140 Ren K, Dubner R. Central nervous system plasticity and persistent pain. J Orofac Pain. 1999;13:155-163.
141 Willis WD, editor. Hyperalgesia and Allodynia. New York: Raven Press, 1992.
142 Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol. 2009;87:81-97.
143 Apkarian AV. Pain perception in relation to emotional learning. Curr Opin Neurobiol. 2008;18:464-468.
144 Beecher HK. Relationship of significance of wound to pain experienced. J Am Med Assoc. 1959;161:1609-1613.
145 Fields HL, Basbaum AI, Heinricher MM. Central nervous system mechanisms of pain modulation. In: McMahon S, Koltzenburg M, editors. Wall and Melzack’s Textbook of Pain. 5th ed. London: Elsevier; 2006:125-142.
146 Heinricher MM, Ingram SL. The brainstem and nociceptive modulation. In: Bushnell MC, Basbaum AI, editors. The Senses, a Comprehensive Reference, Vol 5. Pain. San Diego, CA: Academic Press; 2008:593-626.
147 Sherrington CS. The Integrative Action of the Nervous System. New Haven, CT: Yale University Press; 1906.
148 Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;154:444-445.
149 Watkins LR, Mayer DJ. Organization of endogenous opiate and nonopiate pain control systems. Science. 1982;216:1185-1192.
150 Barbaro NM. Studies of PAG/PVG stimulation for pain relief in humans. Prog Brain Res. 1988;77:165-173.
151 Yaksh TL, Rudy TA. Narcotic analgesics: CNS sites and mechanisms of action as revealed by intracerebral injection techniques. Pain. 1978;4:299-359.
152 Yaksh TL, Al-Rodhan NRF, Jensen TS. Sites of action of opiates in production of analgesia. Prog Brain Res. 1988;77:371-394.
153 Heinricher MM, Morgan MM. Supraspinal mechanisms of opioid analgesia. In: Stein C, editor. Opioids and Pain Control. Cambridge: Cambridge University Press; 1999:46-69.
154 Duggan AW, North RA. Electrophysiology of opioids. Pharmacol Rev. 1983;35:219-281.
155 Gebhart GF. Descending modulation of pain. Neurosci Biobehav Rev. 2004;27:729-737.
156 Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319-325.
157 Ossipov MH, Lai J, King T, et al. Antinociceptive and nociceptive actions of opioids. J Neurobiol. 2004;61:126-148.
158 Fields HL, Heinricher MM. Anatomy and physiology of a nociceptive modulatory system. Philos Trans of the R Soc Lond B Biol Sci. 1985;308:361-374.
159 Martenson ME, Cetas JS, Heinricher MM. A possible neural basis for stress-induced hyperalgesia. Pain. 2009;142:236-244.
160 Le Bars D. The whole body receptive field of dorsal horn multireceptive neurones. Brain Res Brain Res Rev. 2002;40:29-44.
161 Villanueva L, Bouhassira D, Le Bars D. The medullary subnucleus reticularis dorsalis (srd) as a key link in both the transmission and modulation of pain signals. Pain. 1996;67:231-240.
162 Ramirez F, Vanegas H. Tooth pulp stimulation advances both medullary off-cell pause and tail flick. Neurosci Lett. 1989;100:153-156.
163 Kincaid W, Neubert MJ, Xu M, et al. Role for medullary pain facilitating neurons in secondary thermal hyperalgesia. J Neurophysiol. 2006;95:33-41.
164 Fanselow MS. The midbrain periaqueductal gray as a coordinator of action in response to fear and anxiety. In: DePaulis A, Bandler R, editors. The Midbrain Periaqueductal Gray Matter. New York: Plenum; 1991:151-173.
165 Bandler R, DePaulis A. Midbrain periaqueductal gray control of defensive behavior in cat and rat. In: DePaulis A, Bandler R, editors. Midbrain Periaqueductal Gray Matter. New York: Plenum; 1991:175-198.
166 Rodgers RJ, Randall JI. Environmentally induced analgesia: Situational factors, mechanisms and significance. In: Rodgers RJ, Cooper SJ, editors. Endorphins, Opiates and Behavioral Processes. New York: Wiley; 1988:107-144.
167 Lewis JW, Cannon JT, Liebeskind JC. Opioid and nonopioid mechanisms of stress analgesia. Science. 1980;208:623-625.
168 Helmstetter FJ, Tershner SA, Poore LH, et al. Antinociception following opioid stimulation of the basolateral amygdala is expressed through the periaqueductal gray and rostral ventromedial medulla. Brain Res. 1998;779:104-118.
169 McGaraughty S, Heinricher MM. Microinjection of morphine into various amygdaloid nuclei differentially affects nociceptive responsiveness and RVM neuronal activity. Pain. 2002;96:153-162.
170 al Absi M, Rokke PD. Can anxiety help us tolerate pain? Pain. 1991;46:43-51.
171 Rhudy JL, Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84:65-75.
172 Benedetti F, Amanzio M, Vighetti S, et al. The biochemical and neuroendocrine bases of the hyperalgesic nocebo effect. J Neurosci. 2006;26:12014-12022.
173 Rhudy JL, Dubbert PM, Parker JD, et al. Affective modulation of pain in substance-dependent veterans. Pain Med. 2006;7:483-500.
174 Thompson T, Keogh E, French CC, et al. Anxiety sensitivity and pain: generalisability across noxious stimuli. Pain. 2008;134:187-196.
175 Vierck CJJr. Mechanisms underlying development of spatially distributed chronic pain (fibromyalgia). Pain. 2006;124:242-263.
176 Leistad RB, Nilsen KB, Stovner LJ, et al. Similarities in stress physiology among patients with chronic pain and headache disorders: evidence for a common pathophysiological mechanism? J Headache Pain. 2008;9:165-175.
177 Chapman CR, Tuckett RP, Song CW. Pain and stress in a systems perspective: reciprocal neural, endocrine, and immune interactions. J Pain. 2008;9:122-145.
178 Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci U S A. 1999;96:7687-7692.
179 Suzuki R, Dickenson A. Spinal and supraspinal contributions to central sensitization in peripheral neuropathy. Neurosignals. 2005;14:175-181.
180 Ren K, Dubner R. Descending modulation in persistent pain: an update. Pain. 2002;100:1-6.
181 Heinricher MM, Tavares I, Leith JL, et al. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev. 2009;60:214-225.
182 Carlson JD, Maire JJ, Martenson ME, et al. Sensitization of pain-modulating neurons in the rostral ventromedial medulla after peripheral nerve injury. J Neurosci. 2007;27:13222-13231.
183 Bingel U, Tracey I. Imaging CNS modulation of pain in humans. Physiology. 2008;23:371-380.
184 Petrovic P, Kalso E, Petersson KM, et al. Placebo and opioid analgesia—imaging a shared neuronal network. Science. 2002;295:1737-1740.
185 Bingel U, Lorenz J, Schoell E, et al. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8.
186 Mainero C, Zhang WT, Kumar A, et al. Mapping the spinal and supraspinal pathways of dynamic mechanical allodynia in the human trigeminal system using cardiac-gated fMRI. Neuroimage. 2007;35:1201-1210.
187 Zambreanu L, Wise RG, Brooks JC, et al. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain. 2005;114:397-407.
188 Tracey I, Ploghaus A, Gati JS, et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. J Neurosci. 2002;22:2748-2752.