Chapter 1 Anatomy and Biomechanics of the Anterior Cruciate Ligament
Introduction
Anterior cruciate ligament (ACL) reconstruction is the sixth most common procedure performed in orthopaedics, and it is estimated that between 75,000 and 100,000 ACL repair procedures are performed annually in the United States alone.1,2 The ACL has therefore been intensively studied, and outcomes of ACL surgery have received considerable attention. This has included research on surgical technique factors such as tunnel position, graft choices, and fixation methods, as well as postoperative rehabilitation protocols.
Traditional single-bundle ACL reconstruction has focused on reconstruction of one portion of the ACL, the anteromedial (AM) bundle, and although outcomes are generally good, with success rates between 69% and 95%, there remains room for improvement.3,4 A prospective study of a cohort of ACL reconstructed patients 7 years after surgery revealed degenerative radiographic changes in 95% of patients, and only 47% were able to return to their previous activity level following ACL reconstruction.5 However, it should be noted that some studies of long-term follow-up have more encouraging results. Jarvela et al demonstrated tibiofemoral degenerative changes in only 18% of patients at 7 years follow-up post ACL reconstruction with bone–patella–bone grafts.6 In addition, Roe et al reported on a cohort of patients reconstructed with bone–patellar tendon–bone grafts and found an incidence of 45% with degenerative radiographic changes at 7 years follow-up, as well as an incidence of 14% with degenerative changes in a group with hamstring grafts.7
Anterior Cruciate Ligament Anatomy
Historical Descriptions
Two bundles of the ACL were described for the first time in 1938 by Palmer et al, followed by Abbott et al in 1944 and Girgis et al in 1975.8–10 Each author described an AM bundle and a posterolateral (PL) bundle, named for the relative location of the tibial insertion sites of each bundle. More recently, in 1979 and again in 1991, Norwood et al and Amis et al, respectively, described a third bundle of the ACL anatomy, the intermediate (IM) bundle.11,12 Although it may be said that a two-bundle description of the ACL anatomy is an oversimplification of the complete anatomy, many studies have been based on this functional division, and it has been accepted as a reasonable way to understand the anatomy and biomechanics of the ligament. The IM bundle is most similar to the AM bundle in both anatomical and biomechanical considerations, and for the purposes of this chapter it is therefore considered as part of the AM bundle.
Anatomy of the Anteromedial and Posterolateral Bundles
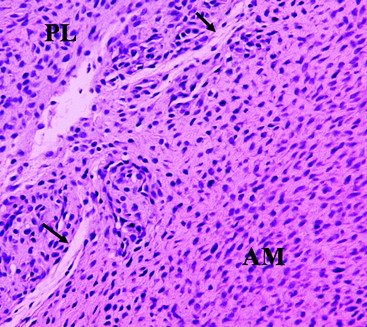
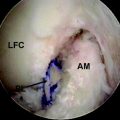
The ACL is a structure composed of numerous fascicles of dense connective tissue that connect the distal femur and the proximal tibia. Histological studies have demonstrated that a septum of vascularized connective tissue is present that separates the AM and PL bundles (Fig. 1-1). In addition, it has been shown that the histological properties of the ligament are variable at different stages in ACL development. At the time of fetal ACL development, the ACL is observed to be hypercellular with circular, oval, and fusiform-shaped cells. Later, in the adult ACL, the histology reveals a relatively hypocellular pattern with predominantly fibroblast cells with spindle-shaped nuclei.13,14
The ligament finds its origin on the medial surface of the lateral femoral condyle (LFC), runs an oblique course within the knee joint from lateral and posterior to medial and anterior, and inserts into a broad area of the central tibial plateau. The cross-sectional area of the ligament varies significantly throughout its course from approximately 44 mm2 at the midsubstance to more than three times as much at both its origin and insertion.10,15,16 The total length of the ligament is approximately 31 to 38 mm and varies by as much as 10% throughout a normal range of motion.17
Anterior Cruciate Ligament Development
ACL formation has been observed in fetal development as early as 8 weeks, corresponding to O’Rahilly stages 20 and 21.18,19 A leading hypothesis holds that the ACL originates as a ventral condensation of the fetal blastoma and gradually migrates posteriorly with the formation of the intercondylar space.20 The menisci are derived from the same blastoma condensation as the ACL, a finding that is consistent with the hypothesis that these structures function in concert.21 Another proposed mechanism of fetal ACL formation is from a confluence between ligamentous collagen fibers and fibers of the periosteum.22 Following the initial formation of the ligament, no major organizational or compositional changes are observed throughout the remainder of fetal development.19
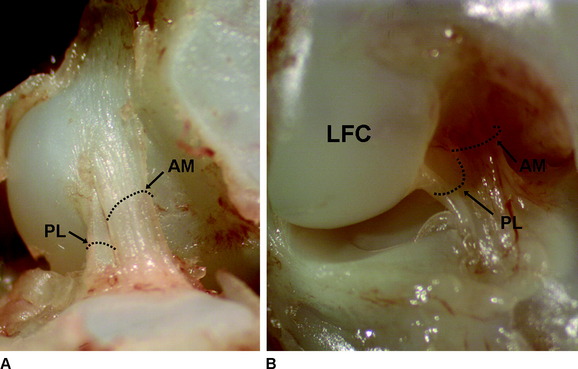
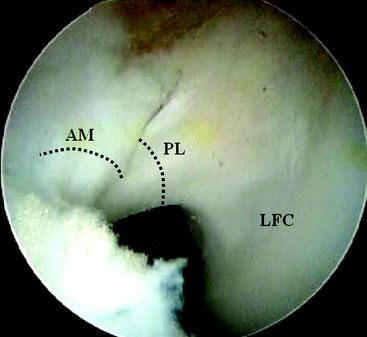
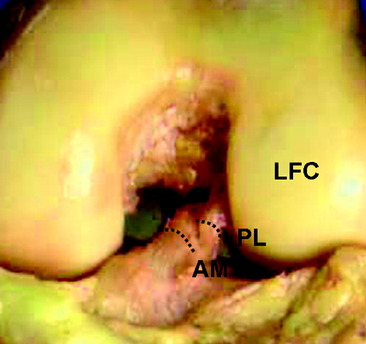
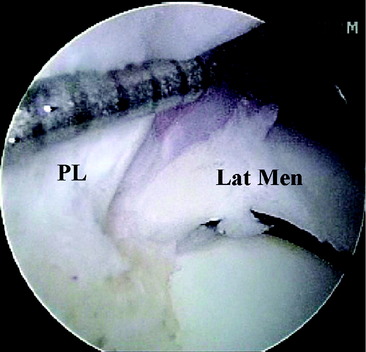
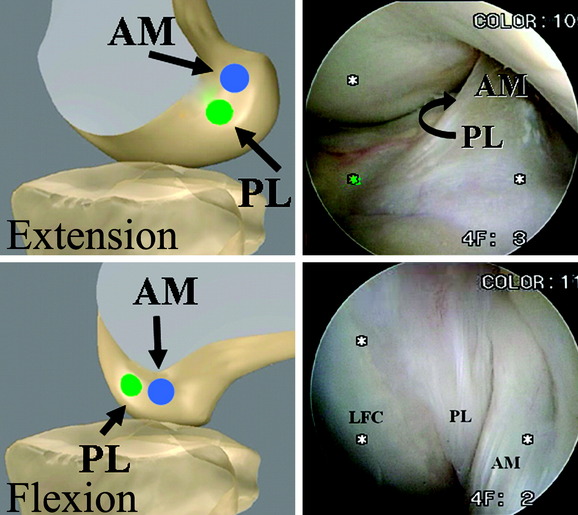
Two distinct bundles of the ACL are present at 16 weeks of gestation (Fig. 1-2). In arthroscopy, the AM and PL bundles can also be appreciated, particularly with the knee held in 90 to 120 degrees of flexion (Fig. 1-3). Finally, cadaveric dissection also reveals two anatomical bundles of the ACL (Fig. 1-4). In summary, there is a considerable amount of interindividual variability with respect to the relative sizes of the AM and PL bundles, as seen in fetal, arthroscopic, and cadaveric studies; however, all individuals with an intact ACL have both bundles of ligament.
Insertion Site Anatomy
Anatomical studies have characterized the individual contributions of both the AM and PL bundles to the overall ACL architecture. Odensten and Gillquist described the femoral origin of the ACL as an ovoid area measuring 18 mm in length and 11 mm in width.23 Within this area, the AM bundle occupies a position located on the proximal portion of the medial wall of the LFC, and the PL bundle occupies a more distal position near the anterior articular cartilage surface of the LFC (Fig. 1-5, A). Harner et al studied the digitized origin and insertion of the AM and PL bundles in five cadavers and concluded that each bundle occupies approximately 50% of the total femoral origin, with cross-sectional areas of 47 ± 13 mm2 and 49 ± 13 mm2 for AM and PL, respectively. 16
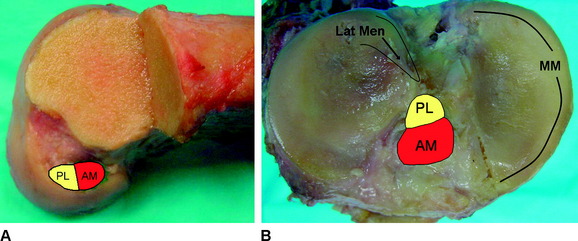
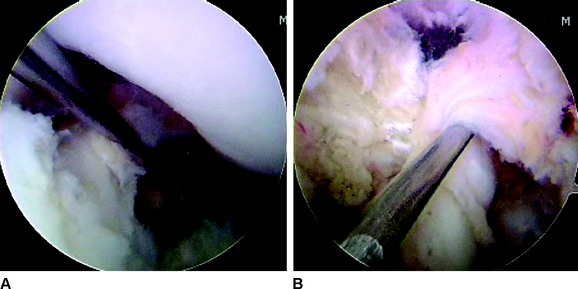
On the tibia, the insertions of the AM and PL bundles are located between the medial and lateral tibial spine over a broad area stretching as far posterior as the posterior root of the lateral meniscus. The full ACL insertion has been described as an oval area measuring 11 mm in diameter in the coronal plane and 17 mm in the sagittal plane.10,15,24 Within this area the AM bundle insertion can be found in an anterior and medial position, whereas the PL bundle insertion is located more posteriorly and laterally (Fig. 1-5, B). Posteriorly, fibers of the PL bundle are in close approximation to the posterior root of the lateral meniscus and, in some individuals, may attach to the meniscus itself (Fig. 1-6). The overall size of the tibial insertion is approximately 120% of the femoral origin; however, as is the case with the femoral origin, the two bundles share approximately equal tibial insertion site areas: the AM bundle occupies 56 ± 21 mm2, and the PL bundle occupies 53 ± 21 mm2.16
The size and length of each bundle is also unique. The AM bundle is approximately 38 mm in length.10,15,17 The PL bundle has been less well studied. Kummer and Yamamoto measured the PL bundle in 50 cadavers and determined an average length of 17.8 mm.25 However, the AM and PL bundles have a similar diameter.
Crossing Pattern
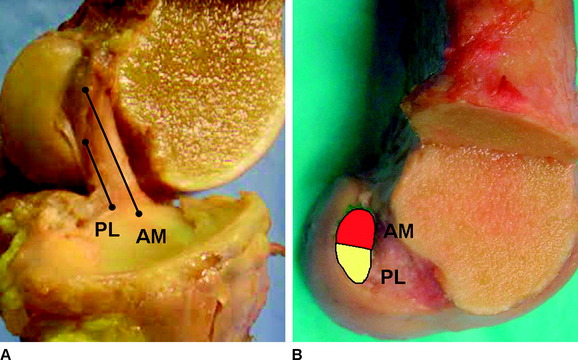
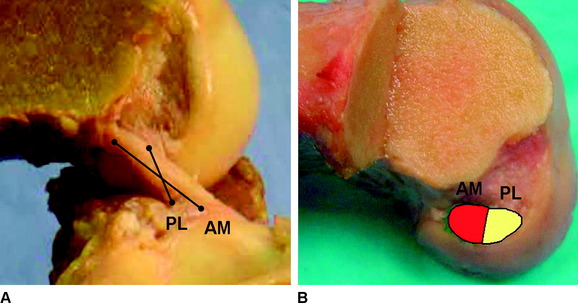
Based on their anatomical positions, the AM and PL bundles change alignment as the knee moves from extension to flexion. The femoral insertion sites are oriented vertically when the knee is in zero degrees, and the two bundles of the ACL are oriented in parallel (Fig. 1-7). As the knee moves into 90 degrees of flexion, the AM bundle insertion site on the femur rotates posteriorly and inferiorly, in contrast to the femoral insertion of the PL bundle, which rotates anteriorly and superiorly. This change in alignment of the insertion sites leads to a horizontal plane of insertions for the AM and PL bundle with the knee in 90 degrees of flexion (Fig. 1-8). The change in insertion site alignment causes the two bundles to twist around each other and become crossed. As the knee is flexed, the PL bundle can be seen anterior to the AM bundle at its femoral insertion (Fig. 1-9).
Tensioning Pattern
The change in alignment of the AM and PL femoral insertion sites allows the ACL to twist around itself as it is moved through a complete range of motion. Clearly, this crossing pattern, along with the differences in the length of each bundle, has implications for the tensioning pattern of the overall ligament and each individual bundle. In a study by Gabriel et al, forces were measured in each bundle during an anterior load of 134N over several flexion angles, as well as for a combined rotatory load of 10 Nm valgus and 5 Nm internal tibial torque.26 The results showed that the PL bundle is tightest in extension (in situ force of 67 ± 30N) and becomes relaxed as the knee is flexed, whereas the AM bundle is more relaxed in extension, and reaches a maximum tightness as the knee approaches 60 degrees of flexion (in situ forces of 90 ± 17N).12,26 This tensioning pattern also can be observed grossly in cadaveric and arthroscopic views of the bundles (Fig. 1-10). The PL bundle is also observed to tighten during internal and external rotation.
Biomechanics
Anterior-Posterior Translation Control
The dynamic nature of the two bundles of the ACL during knee flexion demonstrates the complex role of the ACL in stabilization of the knee joint. However, initial biomechanical studies of the ACL focused mainly on its function of resisting anterior tibial translation.27,28 From this work we know that the in situ forces of the ACL vary considerably during a normal range of motion of the knee joint. With a 110N anterior tibial load applied, the ACL demonstrates high in situ forces between 0 and 30 degrees flexion, with a maximum occurring at 15 degrees. In situ forces are at their lowest point between 60 and 90 degrees, with a minimum occurring at 90 degrees.
As mentioned earlier, recent studies have also been completed to evaluate the individual roles of each bundle of the ACL in anterior-posterior translation. These studies have shown that the AM bundle has relatively constant levels of in situ forces during knee flexion, whereas the PL bundle is more variable, with high in situ forces at 0, 15, and 30 degrees of flexion but rapidly decreasing in situ forces beyond this angle.28
Rotational Stability
Clinical experience has suggested that biomechanical considerations of anterior-posterior translation alone do not correlate with subjective evaluations of knee stability and that a more complete evaluation of the role of rotational stability is relevant.29 Therefore, in recent years closer attention has been given to the rotational stabilizing function of the ACL.26,30,31 Included in the study by Gabriel et al was an analysis of a combined rotatory load of 10 Nm valgus and 5 Nm internal tibial torque at 15 and 30 degrees flexion. For the PL bundle, in situ forces of 21N were recorded at 15 degrees and 14N at 30 degrees. For the AM bundle, in situ forces were 30N and 35N, respectively. This demonstrates that the both the AM and PL bundles contribute to rotational stability of the knee at these angles.
In addition to biomechanical studies, recent studies using in vivo kinematics analysis have assessed rotational stability in the ACL during various functional activities such as walking, running, and cutting.32–34 Andriacchi et al studied the in vivo kinematics of normal and ACL-deficient subjects during four phases of walking and determined that an ACL-deficient knee is positioned differently than a normal knee. During walking, the intact ACL maintains a balance of rotation during the interval of swing phase to heel strike. However, in the ACL-deficient knee, an increased internal rotation occurs between these phases of walking, which is maintained through the stance phase.30 A study of running and cutting in ACL-deficient patients demonstrated normal anterior-posterior stability during running but abnormal rotational movements compared with subjects with an intact ACL.34
Finally, a magnetic resonance imaging (MRI)-based study of the in vivo kinematics of the normal ACL during weight-bearing knee flexion has demonstrated that several components of ACL kinematics change during weight-bearing knee flexion. First, as the flexion angle increases, axial rotation (or twist) of the ACL increases as well. At full extension the ACL is internally twisted by approximately 10 degrees; however, this increases to approximately 20 degrees when the knee is moved to 30 degrees flexion, and it increases to approximately 40 degrees with the knee at 60 to 90 degrees flexion. Second, the orientation of the ligament within the joint space changes with the flexion angle. As the knee flexion angle increases, so does the lateral angulation of the ligament. Therefore, the ligament may possess a lateral force component, functioning to constrain internal tibial rotation.32,33
Biomechanics Considerations in Anterior Cruciate Ligament Surgery
Based on the aforementioned research into the role of rotational stability, work has been completed to assess the ability of different surgical techniques in restoring both anterior-posterior translation of the knee and rotational stability. Yagi et al performed a study comparing a single-bundle reconstruction with the femoral tunnel placed at the 11- or 1-o’clock position with anatomical double-bundle ACL reconstruction and the femoral tunnels placed based on the insertion site anatomy of the transected ACL.35 In this study, the double-bundle ACL reconstruction was better able to resist anterior tibial translation at full extension and 30 degrees flexion, compared with the single-bundle technique. Additionally, when a combined internal tibial and valgus torque was applied at 15 and 30 degrees flexion, the double-bundle ACL reconstruction had a response closer to the intact ACL compared with the single-bundle technique.
Yamamoto et al compared the double-bundle ACL reconstruction with a lateral single-bundle reconstruction, with the femoral tunnel placed approximately at a 10-o’clock position for the right knee.36 The double-bundle anatomical reconstruction better restored the anterior tibial translation at 60 degrees and 90 degrees flexion when compared with the single-bundle technique.36
Finally, Tashman et al performed an in vivo kinematics analysis of normal and single-bundle reconstructed knees.31 Subjects with a normal ACL were compared with a group of single-bundle ACL reconstructed patients to evaluate anterior-posterior translation and knee rotation during downhill jogging. Single-bundle ACL reconstructed patients had fully restored anterior-posterior translation as compared with subjects with a normal ACL but were found to lack normal rotational kinematics.31 Because the single-bundle reconstruction is an approximation of the position of the AM bundle, it can be concluded that part of the rotational stability is derived from the actions of the PL bundle.
In summary, Yagi et al and Yamamoto et al have demonstrated that normal anterior-posterior translation may be restored using traditional single-bundle reconstruction techniques. However, it is not possible to restore rotational stability using this approach.35,36 In addition, Tashman et al have shown that single-bundle reconstruction is not capable of restoring normal rotational kinematics.36 Anatomical double-bundle reconstruction, in contrast, offers an opportunity to restore both components of normal knee stability as demonstrated in cadaveric biomechanics studies, and it is possible that this will soon be demonstrated in an in vivo kinematics model as well.35,36
1 ABOS. Diplomat. www.abos.org, 2004.
2 Griffin LY, Agel J, Albohm MJ, et al. Non-contact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8:141-150.
3 Yunes M, Richmond JC, Engels EA, et al. Patellar versus hamstring tendons in anterior cruciate ligament reconstruction: a meta-analysis. Arthroscopy. 2001;17:248-257.
4 Freedman KB, D’Amato MJ, Nedeff DD, et al. Arthroscopic anterior cruciate ligament reconstruction: a metaanalysis comparing patellar tendon and hamstring tendon autografts. Am J Sports Med. 2003;31:2-11.
5 Fithian DC, Paxton EW, Stone ML, et al. Prospective trial of a treatment algorithm for the management of the anterior cruciate ligament-injured knee. Am J Sports Med. 2005;33:335-346.
6 Jarvela T, Paakkala T, Kannus P, et al. The incidence of patellofemoral osteoarthritis and associated findings seven years after anterior cruciate ligament reconstruction with a bone-patellar tendon-bone autograft. Am J Sports Med. 2001;29:18-24.
7 Roe J, Pinczewski LA, Russell VJ, et al. A seven year follow-up of patellar tendon and hamstring tendon grafts for arthroscopic anterior cruciate ligament reconstruction. Am J Sports Med. 2005;33:1337-1345.
8 Palmer I. On the injuries to the ligaments of the knee joint. Acta Chir Scand. 1938;91:282.
9 Abbott LC, Saunders JB, Bost FC, et al. Injuries to the ligaments of the knee joint. J Bone Joint Surg Am. 1944;26A:503-521.
10 Girgis FG, Marshall JL, Al Monajem ARS. The cruciate ligaments of the knee joint. Clinic Orthop. 1975;106:216-231.
11 Norwood LA, Cross MJ. Anterior cruciate ligament: functional anatomy of its bundles in rotary instabilities. Am J Sports Med. 1979;7:23.
12 Amis AA, Dawkins GPC. Functional anatomy of the anterior cruciate ligament. Fibre bundle actions related to ligament replacement and injuries. J Bone Joint Surg Br. 1991;73:260-267.
13 Shino K, Inoue M, Horibe S, et al. Maturation of allografts tendons transplanted into the knee. An arthroscopic and histological study. J Bone Joint Surg Br. 1988;70:556-560.
14 Falconiero RP, DiStefano VJ, Cook TM. Revascularization and ligamentization of autogenous anterior cruciate ligament grafts in humans. Arthroscopy. 1998;14:197-205.
15 Arnoczsky SP. Anatomy of the anterior cruciate ligament. Clin Orthop. 1983;172:19-25.
16 Harner CD, Baek GH, Vogrin TM, et al. Quantitative analysis of human cruciate ligament insertions. Arthroscopy. 1999;15:741-749.
17 Fu FH, Bennett CH, Lattermann C, et al. Current trends in anterior cruciate ligament reconstruction. Part 1: biology and biomechanics of reconstruction. Am J Sports Med. 1999;27:821-830.
18 O’Rahilly R. The early prenatal development of the human knee joint. J Anat. 1951;85:166-170.
19 Gardner E, O’Rahilly R. The early development of the knee joint in staged human embryos. J Anat. 1968;102:289-299.
20 Ellison AE, Berg EE. Embryology, anatomy, and function of the anterior cruciate ligament. Orthop Clin North Am. 1985;16:3-14.
21 Galleazzi R. Clinical and experimental study of the semilunar cartilage of the knee joint. J Bone Joint Surg. 1929;9:515.
22 Behr CT, Potter HG, Paletta GAJr. The relationship of the femoral origin of the anterior cruciate ligament and the distal femoral physeal plate in the skeletally immature knee. An anatomic study. Am J Sports Med. 2001;29:781-787.
23 Odensten M, Gillquist J. Functional anatomy of the anterior cruciate ligament and a rationale for reconstruction. J Bone Joint Surg Am. 1985;67:257-262.
24 Petersen W, Tillmann B. Anatomy and function of the anterior cruciate ligament. Orthopade. 2002;31:710-718.
25 Kummer B, Yamamoto Y. [Funktionelle Anatomie der Kreuzbaender]. Arthroskopie. 1988;1:2-10.
26 Gabriel MT, Wong EK, Woo SL, et al. Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res. 2004;22:85-89.
27 Takai S, Woo SL-Y, Livesay GA, et al. Determination of the in situ loads on the human anterior cruciate ligament. J Orthop Res. 1993;11:686-695.
28 Sakane M, Fox RJ, Woo SL-Y, et al. In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J Orthop Res. 1997;15:285-293.
29 Kocher MS, Steadman JR, Briggs KK, et al. Relationships between objective assessment of ligament stability and subjective assessment of symptoms and function after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:629-634.
30 Andriacchi TP, Dyrby CO. Interactions between kinematics and loading during walking for the normal and ACL deficient knee. J Biomech. 2005;38:293-298.
31 Tashman S, Collon D, Anderson K, et al. Abnormal rotational knee motion during running after anterior cruciate ligament reconstruction. Am J Sports Med. 2004;32:975-983.
32 Li G, DeFrate LE, Rubash HE, et al. In vivo kinematics of the ACL during weight-bearing knee flexion. J Orthop Res. 2005;23:340-344.
33 Li G, DeFrate LE, Sun H, et al. In vivo elongation of the anterior cruciate ligament and posterior cruciate ligament during knee flexion. Am J Sports Med. 2004;32:1415-1420.
34 Waite JC, Beard DJ, Dodd CA, et al. In vivo kinematics of the ACL-deficient limb during running and cutting. Knee Surg Sports Traumatol Arthrosc. 2005;13:377-384.
35 Yagi M, Wong EK, Kanamori A, et al. Biomechanical analysis of an anatomic anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30:660-666.
36 Yamamoto Y, Hsu Y, Woo SL, et al. Knee stability and graft function after anterior cruciate ligament reconstruction: a comparison of a lateral and an anatomical femoral tunnel placement. Am J Sports Med. 2004;32:1825-1832.