Anaesthesia-related techniques
TECHNIQUES TO ASSESS PERIOPERATIVE RISK
Aims of preoperative assessment in respect of the high-risk patient
 To quantify known disease, and to identify subclinical disease, aiming to intervene and optimize where possible
To quantify known disease, and to identify subclinical disease, aiming to intervene and optimize where possible
 To facilitate informed patient consent: a better appreciation of risk allows patients and clinicians to discuss the risk–benefit ratios of alternative procedures and/or conservative treatment
To facilitate informed patient consent: a better appreciation of risk allows patients and clinicians to discuss the risk–benefit ratios of alternative procedures and/or conservative treatment
 To assist in appropriate allocation of critical care or high-dependency beds
To assist in appropriate allocation of critical care or high-dependency beds
 To assist decision-making in respect of both anaesthesia and surgery: for example, in deciding between open or laparoscopic surgery, or whether to use regional techniques as an adjunct or alternative to general anaesthesia.
To assist decision-making in respect of both anaesthesia and surgery: for example, in deciding between open or laparoscopic surgery, or whether to use regional techniques as an adjunct or alternative to general anaesthesia.
Assess
Cardiac risk indices
The first widely used cardiac risk index was that proposed by Goldman et al in 1977.1 Nine independent criteria were identified as indicators of increased risk (Box 2.1). The Goldman Index has been revised by subsequent workers, notably Detsky2 and Lee.3
In 2007, the American College of Cardiology (ACC) and American Heart Association (AHA)4 sought to stratify apparent cardiac risk factors into three categories – those that require further investigation, and others that may or may not actually impose increased risk (Box 2.2).
A step-by-step approach to risk assessment
Subsequent guidelines propose a stepwise approach to the evaluation of a potential high-risk surgical patient. The aim is to assist in creating an individualized cardiac risk assessment, and to suggest appropriate interventions before surgery in terms of optimization. The process is summarized in Box 2.3 and expanded upon in the sections that follow.
Assessing the risk of the surgical procedure
The risk of serious cardiac complications following surgery depends not only on the presence of risk factors, such as those described above, but also varies according to the type of surgery performed. Surgery induces a physiological stress response, with sympatho-humoral activation, increased myocardial oxygen demands and hyper-coagulability. With regard to cardiac risk, surgical interventions fall into one of three categories: low, intermediate or high-risk, according to the risk of myocardial infarction (MI) and cardiac death within 30 days of surgery (Table 2.1).
Table 2.1
Risk of MI/cardiac death within 30 days of surgery
| Low risk (<1%) | Intermediate risk (1–5%) | High risk (>5%) |
| Breast | Abdominal | Aortic and major vascular surgery |
| Dental | Carotid | Peripheral vascular surgery |
| Endocrine | Endovascular aneurysm repair | |
| Eye | Head and neck | |
| Gynaecology | Neurosurgery | |
| Plastic/reconstructive | Major orthopaedic | |
| Minor orthopaedic | Renal transplant | |
| Minor urology | Major urology |
Action
Tests of functional capacity including cardiopulmonary exercise testing
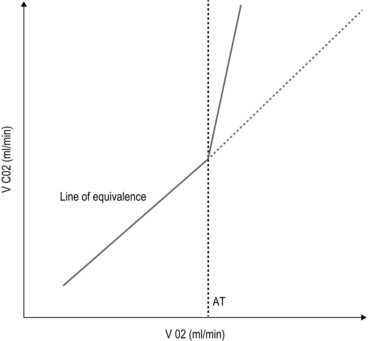
Cardiopulmonary exercise (CPEX) testing is increasingly regarded as a gold-standard for preoperative exercise testing, yielding considerable data on oxygen uptake and utilization. CPEX testing is cheap and relatively non-invasive, and aims to determine the patient’s anaerobic threshold. Since it evaluates both the cardiovascular and respiratory systems, it is ideal for investigation of the patient with exertional breathlessness. The patient exercises on a bicycle ergometer, with measurement of gas exchange at the mouth together with ECG monitoring. CPEX detects the change from aerobic to partial anaerobic metabolism (Fig. 2.1): at the anaerobic threshold (AT), production of CO2 relative to consumption of O2 increases. An AT of less than 11 ml/min/kg has been associated with a higher perioperative cardiovascular mortality.
Aftercare
Pharmacological strategies to reduce risk
β-blockers
Part of the physiological stress response to surgery is a catecholamine surge with increased heart rate and myocardial oxygen consumption. In surgical patients with known ischaemic heart disease, Mangano et al5 reported a reduced 2 year mortality after 7 days’ perioperative β-blockade.1 These findings were swiftly incorporated into new guidelines recommending use of β-blockade in patients with overt ischaemic heart disease or with risk factors. Subsequent studies produced more equivocal results and a more cautious approach followed, recommending use of β-blockers in high-risk patients rather than in all patients at risk.
Then came the POISE (PeriOperative Ischaemia Study Evaluation) study,6 which measured 30-day mortality and morbidity after oral metoprolol. There was a significant reduction in the number of cardiac events, but the overall mortality rate actually increased, with a significant excess of strokes – possibly because of the excess of patients suffering from hypotension and bradycardia amongst those treated.
Close monitoring of blood pressure and heart rate intra- and postoperatively is, however, essential.
Management of antiplatelet therapy
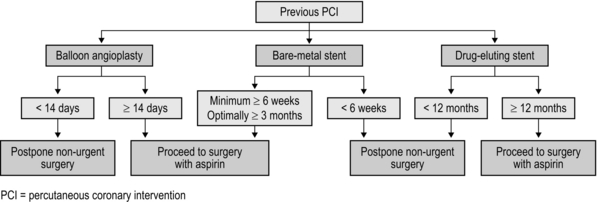
Two sorts of stent are commonly employed: bare-metal stents have generally been superseded by drug-eluting stents which carry a reduced risk of re-stenosis but a higher risk of stent thrombosis. Drug eluting stents require continuous dual antiplatelet therapy (aspirin + clopidogrel) for at least 12 months after implantation. It is now generally accepted that elective surgery should not take place within 12 months of drug-eluting stent implantation. After 12 months, surgery can proceed, but with at least continuation of aspirin therapy. It is no longer acceptable simply to discontinue all antiplatelet therapy in all patients, and discussion between surgeon, anaesthetist and cardiologist is to be recommended. The recommendations in respect of the timing of non-cardiac surgery after PCI are summarized in Figure 2.2.
REFERENCES
1. Goldman L, Caldera DL, Nussbaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med 1977;297(16):845–50.
2. Detsky AS. Cardiac assessment for patients undergoing non cardiac surgery: a multifactorial clinical risk index. Arch Intern Med 1996;146(11):2131–4.
3. Lee TH. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043–9.
4. Fleisher LA. ACC / AHA 2007 Guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol 2007;50(17):e159–242.
5. Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischaemia Research Group. N Engl J Med 1996;335(23):1713–20.
6. POISE Study Group. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008;371:1839–47.
FURTHER READING
Atkinson D, Carter A. Pre-operative assessment for aortic surgery. Current Anaesthesia and Critical Care 2008;19:115–27.
Foex P, Sear JW. Challenges of β-blockade in surgical patients. Anesthesiology 2010;113:767–71.
Poldermans D, Bax JJ, Boersma E, et al. Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009;30(22):2769–812.
OXYGEN THERAPY
Appraise
Rationale for oxygen therapy
Mild-to-moderate hypoxaemia during the postoperative period is extremely common and may contribute to poor outcome in a variety of areas (Box 2.4).
Factors contributing to postoperative hypoxaemia
From first principles, adequate tissue oxygenation depends on:
 diffusion of oxygen across alveolus into the pulmonary capillaries
diffusion of oxygen across alveolus into the pulmonary capillaries
 delivery of arterial blood to the tissues and uptake of oxygen.
delivery of arterial blood to the tissues and uptake of oxygen.
Anaesthesia and surgery may disrupt each of these processes. The main factors that contribute to postoperative hypoxaemia are conveniently classified anatomically from respiratory drive onwards, and are summarized in Box 2.5.
Assess
Assessment and detection of hypoxaemia
 altered mental state (disorientation, confusion, etc.)
altered mental state (disorientation, confusion, etc.)
 dyspnoea or tachypnoea (difficulty completing sentences, use of accessory muscles, etc.)
dyspnoea or tachypnoea (difficulty completing sentences, use of accessory muscles, etc.)
 cyanosis (often difficult to detect clinically)
cyanosis (often difficult to detect clinically)
 cardiovascular: tachycardia/hypertension/arrhythmias
cardiovascular: tachycardia/hypertension/arrhythmias
 vasodilatation (headache/bounding pulses) if accompanying hypercarbia.
vasodilatation (headache/bounding pulses) if accompanying hypercarbia.
Action
Oxygen therapy devices
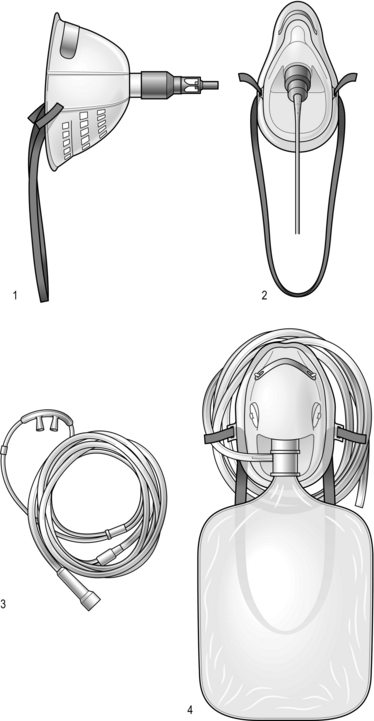
Increasing the inspired concentration of oxygen provides a higher gradient for diffusion of oxygen from the alveolar gas into the pulmonary capillary blood. Two sorts of device are available – variable and fixed performance (Fig. 2.3).
Variable performance devices
The addition of a reservoir bag increases the amount of inspired oxygen, up to about 80%.
Recognition and management of respiratory failure
The common causes in surgical patients are as listed in Box 2.5 and the clinical manifestations are as described above. In terms of investigations, these should include arterial blood gas analysis and an urgent chest X-ray (CXR). It is important to note that arterial gases do not require to be taken on air for a diagnosis to be made – this is dangerous, and may provoke severe desaturation.
 Continuous positive airways pressure (CPAP): delivered via a close-fitting mask, maintaining a positive expiratory pressure of 5-15 cm H2O. It increases FRC and reduces the work of breathing. The increase in intrathoracic pressure reduces cardiac preload and afterload and hence CPAP may be of great benefit in acute heart failure. Gastric distension may be a problem.
Continuous positive airways pressure (CPAP): delivered via a close-fitting mask, maintaining a positive expiratory pressure of 5-15 cm H2O. It increases FRC and reduces the work of breathing. The increase in intrathoracic pressure reduces cardiac preload and afterload and hence CPAP may be of great benefit in acute heart failure. Gastric distension may be a problem.
 Non-invasive ventilation (NIV): this applies a positive inspiratory, as well as expiratory, pressure (e.g. BiPAP), and is particularly useful in the presence of raised PaCO2.
Non-invasive ventilation (NIV): this applies a positive inspiratory, as well as expiratory, pressure (e.g. BiPAP), and is particularly useful in the presence of raised PaCO2.
 Invasive ventilation: requires sedation and endotracheal intubation and may supervene from BiPAP in a patient whose gases are deteriorating or who is becoming exhausted.
Invasive ventilation: requires sedation and endotracheal intubation and may supervene from BiPAP in a patient whose gases are deteriorating or who is becoming exhausted.
PERIPHERAL VENOUS ACCESS
Appraise
Peripheral venous access is used for fluid and intravenous drug administration. When selecting an appropriate cannula (Fig. 2.4), it is important to remember that flow rates increase in proportion to the fourth power of the radius (Poiseuille’s law). Hence volume resuscitation requires a large-bore (14 G or 16 G), short cannula. Smaller diameter devices are suitable for maintenance fluids and/or drug administration.
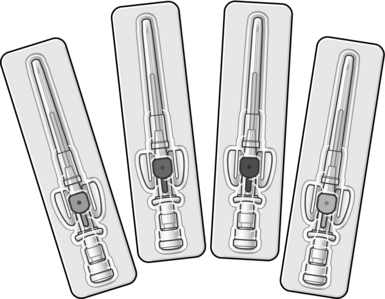
Fig. 2.4 Peripheral intravenous cannulae.
Action
Venous access in the lower limb is generally avoided and carries a greater risk of thrombosis.
A few tips for successful peripheral venous cannulation are given in Box 2.6.
CENTRAL VENOUS ACCESS
Appraise
Central venous lines are usually multichannel devices comprising three to five lumens (ranging from 20 G to 14 G size). Indications for central venous access are summarized in Box 2.7.
INSERTION TECHNIQUE
Ultrasound guidance (Fig. 2.5) is strongly recommended for internal jugular lines, and has been demonstrated to reduce the incidence of complications. The surface landmark for the vein lies over a triangle formed from the two heads of sternomastoid (medial and lateral) and the clavicle (inferior). In the absence of ultrasound, the needle should be advanced at an angle of about 300 towards the ipsilateral nipple. A high approach reduces the risk of pneumothorax but increases the risk of arterial puncture – the converse is true of a low approach.
INTRAOSSEOUS ACCESS
In cases where immediate resuscitation is required in the absence of intravenous access, intraosseous access may be life-saving, most especially in children (Fig 2.6).
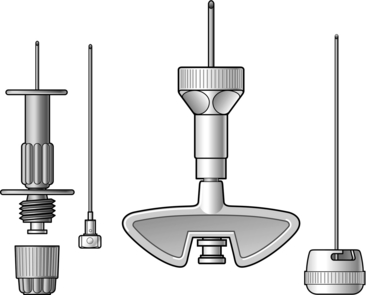
Fig. 2.6 Intraosseous devices.
GENERAL ANAESTHESIA TECHNIQUES
Action
Induction of anaesthesia
General anaesthesia may be induced by either an intravenous or inhalational technique.
Airway management
 Holding a facemask (with or without airway adjuncts such as an oropharyngeal airway)
Holding a facemask (with or without airway adjuncts such as an oropharyngeal airway)
Endotracheal intubation
A key aspect of the preoperative assessment is an evaluation of the likely difficulty or otherwise of endotracheal intubation (and, even more importantly, of mask ventilation). Certain clinical features predict possible difficulty (Box 2.9).
Failed intubation
 Reattempt intubation under optimal conditions: improved head and neck position; different laryngoscope blade; gum-elastic bougie
Reattempt intubation under optimal conditions: improved head and neck position; different laryngoscope blade; gum-elastic bougie
 Insert an LMA (which will usually re-establish the airway and may itself provide a conduit for intubation)
Insert an LMA (which will usually re-establish the airway and may itself provide a conduit for intubation)
 If now able to ventilate but unable to intubate, consider waking the patient and postponing surgery
If now able to ventilate but unable to intubate, consider waking the patient and postponing surgery
 In a ‘can’t intubate, can’t ventilate’ scenario, proceed without delay to cricothyroidotomy.
In a ‘can’t intubate, can’t ventilate’ scenario, proceed without delay to cricothyroidotomy.
Principles of monitoring
Essential requirements
The Association of Anaesthetists of Great Britain and Ireland (AAGBI) have published clear guidelines regarding minimum standards of monitoring (Box 2.10).1
Indications for invasive monitoring
In complex cases, an enhanced level of monitoring may be indicated. An arterial line allows continuous, beat-to-beat recording of the arterial blood pressure (Box 2.11). Further advanced cardiovascular monitoring may include measurement of cardiac filling pressures (most commonly the central venous pressure), cardiac output and mixed venous oxygen saturation.
Aftercare
Levels of postoperative care
An ever-increasing number of patients undergo day case surgery or are discharged from hospital within 24 hours. For those who require inpatient management postoperatively, several levels of care are defined (Box 2.12).
Analgesic techniques
A robust strategy for managing postoperative pain is essential since untreated pain has a variety of adverse consequences (Box 2.13).
The pharmacological management of acute pain includes:
 Classical analgesics: simple analgesics, NSAIDs, opioids
Classical analgesics: simple analgesics, NSAIDs, opioids
 Local anaesthetics: neuraxial, regional and local blocks
Local anaesthetics: neuraxial, regional and local blocks
 Adjuvant drugs: clonidine, ketamine, pregabalin,amitriptyline, etc.
Adjuvant drugs: clonidine, ketamine, pregabalin,amitriptyline, etc.
Pre-emptive analgesia (i.e. before the skin incision) may have an impact on postoperative pain.
LOCAL ANAESTHESIA TECHNIQUES
Appraise
LA agents may be administered in a variety of ways according to the required area of analgesia:
 Topical anaesthesia: application of LA to the skin, and to the mucous membranes of the conjunctival sac, mouth, nose, tracheo-bronchial tree and urethra
Topical anaesthesia: application of LA to the skin, and to the mucous membranes of the conjunctival sac, mouth, nose, tracheo-bronchial tree and urethra
 Local infiltration: direct injection of LA into the operative site
Local infiltration: direct injection of LA into the operative site
 Field block: injection of LA around the operative site, so as to create an analgesic zone
Field block: injection of LA around the operative site, so as to create an analgesic zone
 Individual peripheral nerve blocks: e.g. median, ulnar, femoral or pudendal nerves
Individual peripheral nerve blocks: e.g. median, ulnar, femoral or pudendal nerves
 Regional block: injection of LA around nerve trunks supplying the region to be operated upon, e.g. brachial plexus block
Regional block: injection of LA around nerve trunks supplying the region to be operated upon, e.g. brachial plexus block
 Neuroaxial blocks: spinal and epidural anaesthesia
Neuroaxial blocks: spinal and epidural anaesthesia
 Intravenous regional anaesthesia: injection of a large, dilute LA volume into the veins of a previously exsanguinated limb.
Intravenous regional anaesthesia: injection of a large, dilute LA volume into the veins of a previously exsanguinated limb.
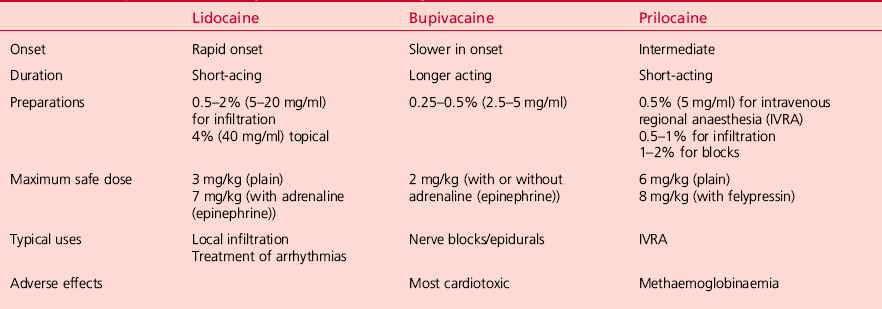
LOCAL ANAESTHETIC AGENTS
Addition of a vasoconstrictor (e.g. adrenaline (epinephrine)) prolongs the duration of action of LAs. Epinephrine is added to LA in concentrations ranging from 1:80 000 to 1:300 000. The commonest strength is a 1:200 000 (5 μg per ml) concentration of adrenaline (epinephrine) (Box 2.14).
Important features of the different LA agents are summarized in Table 2.2.
LOCAL ANAESTHETIC TOXICITY
Strategies to reduce the risk and/or impact of LA toxicity include:
 Ensure patent IV access, availability of resuscitation equipment and presence of a trained assistant before LA administration
Ensure patent IV access, availability of resuscitation equipment and presence of a trained assistant before LA administration
 Use the least toxic drug, in the lowest dose, and reduce doses in the elderly and frail
Use the least toxic drug, in the lowest dose, and reduce doses in the elderly and frail
 Calculate the dose carefully (this point cannot be overstated!)
Calculate the dose carefully (this point cannot be overstated!)
 Inject slowly, aspirating during injection in case of inadvertent vascular puncture.
Inject slowly, aspirating during injection in case of inadvertent vascular puncture.
 CNS: lightheadedness, dizziness, taste disturbance, tinnitus, circumoral paraesthesiae; progressing to agitation, convulsions, coma and respiratory arrest
CNS: lightheadedness, dizziness, taste disturbance, tinnitus, circumoral paraesthesiae; progressing to agitation, convulsions, coma and respiratory arrest
 CVS: hypotension, myocardial depression, arrhythmias and cardiac arrest.
CVS: hypotension, myocardial depression, arrhythmias and cardiac arrest.
Initial management
 Stop injecting the LA and call for help
Stop injecting the LA and call for help
 Assess patient according to ABC principles
Assess patient according to ABC principles
 Maintain the airway: if necessary, secure it by endotracheal intubation
Maintain the airway: if necessary, secure it by endotracheal intubation
 Give 100% oxygen and ensure adequate ventilation
Give 100% oxygen and ensure adequate ventilation
 Confirm or establish IV access: administer fluids ± vasopressors
Confirm or establish IV access: administer fluids ± vasopressors
 Control seizures with thiopentone or benzodiazepines
Control seizures with thiopentone or benzodiazepines
 If the patient is in cardiac arrest, perform cardiopulmonary resuscitation (CPR) according to ALS protocol.
If the patient is in cardiac arrest, perform cardiopulmonary resuscitation (CPR) according to ALS protocol.
Use of Intralipid®
The use of Intralipid® may reverse LA toxicity. CPR should be continued throughout treatment with lipid emulsion. Recovery may take more than an hour. The Association of Anaesthetists of Great Britain and Ireland has produced comprehensive guidelines (2010)1 detailing the management of severe local anaesthetic toxicity and the use of lipid emulsion:
CERVICAL PLEXUS BLOCK
 Deep: identify the lateral border of the sternomastoid at the level of the thyroid cartilage (C4) and feel for the interscalene groove. Aim the needle in a caudal and medial direction 10–20 mm towards the contralateral elbow, until paraesthesiae are felt or contact made with the C4 transverse process. After aspiration, inject 8–10 ml of LA solution. Complications include blockade of the phrenic nerve, recurrent laryngeal nerve and stellate ganglion
Deep: identify the lateral border of the sternomastoid at the level of the thyroid cartilage (C4) and feel for the interscalene groove. Aim the needle in a caudal and medial direction 10–20 mm towards the contralateral elbow, until paraesthesiae are felt or contact made with the C4 transverse process. After aspiration, inject 8–10 ml of LA solution. Complications include blockade of the phrenic nerve, recurrent laryngeal nerve and stellate ganglion
 Superficial: the superficial plexus is blocked by a 10 ml ‘sausage-shaped’ injection along the posterior border of sternomastoid.
Superficial: the superficial plexus is blocked by a 10 ml ‘sausage-shaped’ injection along the posterior border of sternomastoid.
CENTRAL NEURO-AXIAL BLOCKS
Spinal or subarachnoid block and epidural blocks are the major neuro-axial techniques.
INDICATIONS FOR EPIDURAL ANAESTHESIA/ANALGESIA
1. Hip and knee surgery: Internal fixation of a fractured hip is associated with less blood loss when central neuro-axial blocks are used. The incidence of deep vein thrombosis is reduced in patients undergoing total hip and knee replacement under an epidural technique.
2. Vascular reconstruction of the lower limbs and endovascular arterial reconstructions: Epidural anaesthesia improves distal blood flow and can be used as the sole anaesthetic technique. Patients undergoing lower limb amputation may have a reduced incidence of phantom limb pain if neuro-axial blockade is established before surgery.
3. Postoperative pain relief following abdominal and thoracic surgery: Low concentration bupivacaine (0.125%), often in combination with an opioid such as fentanyl or preservative-free morphine provides effective pain relief. It also minimizes the effects of surgery on cardiopulmonary reserve, such as diaphragmatic splinting and the inability to cough effectively. This is especially important in patients with compromised respiratory function, e.g. chronic obstructive airways disease, morbid obesity and the elderly. Adequate analgesia allows better cooperation with chest physiotherapy. Epidural analgesia also facilitates earlier mobilization and reduces deep vein thrombosis.
EFFECTS ON ORGAN SYSTEMS
Cardiovascular: Sympathetic blockade (sympathetic outflow T1–L2) results in vasodilatation of resistance and capacitance vessels, causing relative hypovolaemia and tachycardia, with a resulting fall in blood pressure. This is managed with fluid loading and/or a vasoconstrictor. If the block is as high as T2 the sympathetic supply to the heart (T2–T5) is also interrupted, leading to bradycardia.
Respiratory: Usually unaffected, unless the blockade is high enough to affect the intercostal muscle nerve supply (thoracic nerve roots) leading to reliance on diaphragmatic breathing alone.
Gastrointestinal: Blockade of the sympathetic outflow to the GI tract leads to a predominance of parasympathetic (vagus and sacral parasympathetic) tone, with active peristalsis and relaxed sphincters and a small contracted gut which can enhance surgical access. Urinary retention is a common problem with epidural anaesthesia.
CONTRAINDICATIONS
Absolute
1. Patient refusal: A primary absolute contraindication
2. Coagulopathy: Clotting abnormalities may lead to the development of a large haematoma and spinal cord compression. In warfarinized patients the international normalized ratio (INR) should be below 1.4 prior to catheter insertion. A platelet count below 100 000 is a relative contraindication
3. Skin infection at proposed injection site: Insertion of an epidural needle through an area of skin infection may introduce pathogenic bacteria into the epidural space, leading to abscess formation or even meningitis
4. Raised intracranial pressure: Accidental dural puncture in patients with raised ICP may lead to brainstem herniation (coning).
Relative
1. Bacteraemia: Some may consider this an absolute contraindication. Epidural abscesses have been described occurring de novo, even when no epidural has been inserted
2. Fixed cardiac output states: E.g. severe aortic stenosis, hypertrophic cardiomyopathy, complete heart block. These patients are unable to increase their cardiac output to compensate for the peripheral vasodilatation that occurs and can develop profound circulatory collapse. Hypovolaemia is also a relative contraindication
3. Neurological disorders: E.g. multiple sclerosis – since any new neurological symptoms may be ascribed to the epidural.
MANAGEMENT OF SURGICAL PATIENTS RECEIVING LONG-TERM ANTICOAGULANT OR ANTIPLATELET THERAPY
ANTICOAGULANT THERAPY
Patients on oral anticoagulants undergoing elective surgery
 Identifying patients who can safely undergo an invasive procedure whilst continuing their VKA
Identifying patients who can safely undergo an invasive procedure whilst continuing their VKA
 Identifying patients who are at high risk of thromboembolism and who require bridging therapy with UFH or LMWH when the VKA is stopped
Identifying patients who are at high risk of thromboembolism and who require bridging therapy with UFH or LMWH when the VKA is stopped
 Determining the optimal dose and timing of parenteral anticoagulants during the perioperative period.
Determining the optimal dose and timing of parenteral anticoagulants during the perioperative period.
Procedures which do not require warfarin interruption: Patients on warfarin may undergo minor procedures such as dental extraction without discontinuing their treatment, provided their INR is in the therapeutic range and they receive tranexamic acid mouthwashes.1
For minor dermatological and ophthalmological (e.g. cataract extraction) procedures, it is also recommended that patients do not stop their VKA therapy.
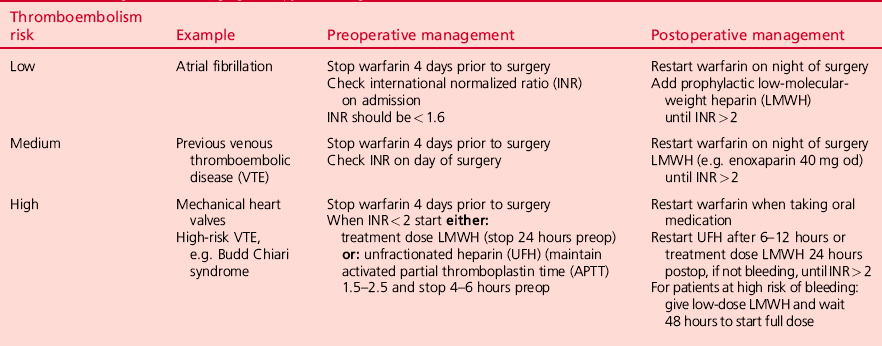
Stratification of thromboembolism risk
 Atrial fibrillation: approximately 50% of all patients receiving warfarin therapy have atrial fibrillation (AF) which is, therefore, the most common clinical condition requiring a decision about bridging therapy. The average risk of perioperative stroke in patients with AF who do not receive antithrombotic therapy is 4.5%. The risk can be further stratified based on a ‘CHADS’ score (1 point each for congestive cardiac failure, hypertension, age > 75 years and diabetes, and 2 points for history of stroke or transient ischaemic attack). The American College of Physicians recommends low dose LMWH or no bridging for a score of 0–2, and bridging with therapeutic LMWH or UFH for CHADS scores of 4 and above. Intermediate levels of risk can be managed with higher prophylactic doses of LMWH.
Atrial fibrillation: approximately 50% of all patients receiving warfarin therapy have atrial fibrillation (AF) which is, therefore, the most common clinical condition requiring a decision about bridging therapy. The average risk of perioperative stroke in patients with AF who do not receive antithrombotic therapy is 4.5%. The risk can be further stratified based on a ‘CHADS’ score (1 point each for congestive cardiac failure, hypertension, age > 75 years and diabetes, and 2 points for history of stroke or transient ischaemic attack). The American College of Physicians recommends low dose LMWH or no bridging for a score of 0–2, and bridging with therapeutic LMWH or UFH for CHADS scores of 4 and above. Intermediate levels of risk can be managed with higher prophylactic doses of LMWH.
 Mechanical heart valves: the risk of thromboembolism is such that bridging therapy is essential. The risk varies according to the type of valve and also its position (mitral > aortic). If the patient’s target INR is 3, then bridging therapy with therapeutic/full-dose LMWH is required. If the target INR is 2.5, then low-dose LMWH bridging is sufficient. Whenever surgery is planned, the risk of procedure-related bleeding must be balanced against the possible risk of thromboembolic events.
Mechanical heart valves: the risk of thromboembolism is such that bridging therapy is essential. The risk varies according to the type of valve and also its position (mitral > aortic). If the patient’s target INR is 3, then bridging therapy with therapeutic/full-dose LMWH is required. If the target INR is 2.5, then low-dose LMWH bridging is sufficient. Whenever surgery is planned, the risk of procedure-related bleeding must be balanced against the possible risk of thromboembolic events.
 Venous thromboembolic disease (VTE): therapeutic dose bridging is recommended for high-risk patients. These include patients who have suffered an episode of VTE within the previous 3 months, or those with known thrombophilia (such as deficiency of Protein S, Protein C or antithrombin III, or the presence of antiphospholipid antibodies). Moderate-risk patients (e.g. those with VTE within 3–12 months or with Factor V Leiden mutation) also require full-dose bridging therapy. Low-risk patients require either no bridging or prophylactic dose LMWH only.
Venous thromboembolic disease (VTE): therapeutic dose bridging is recommended for high-risk patients. These include patients who have suffered an episode of VTE within the previous 3 months, or those with known thrombophilia (such as deficiency of Protein S, Protein C or antithrombin III, or the presence of antiphospholipid antibodies). Moderate-risk patients (e.g. those with VTE within 3–12 months or with Factor V Leiden mutation) also require full-dose bridging therapy. Low-risk patients require either no bridging or prophylactic dose LMWH only.
Bleeding risk with bridging therapy: The risk of surgery when a patient is on full-dose bridging therapy varies markedly with the type of surgery. The risk of major bleeding is low for minor surgery such as inguinal hernia repair, but for major surgery, including knee and hip replacement, the risk of major bleeding is significantly greater. LMWH bridging therapy should be stopped 24 hours before surgery and therapeutic doses resumed 24–48 hours postoperatively. Low-dose LMWH may be considered as an alternative option during resumption of anticoagulant bridging after major surgery. LMWHs are very dependent on adequate renal function for their elimination, and reduced doses may be required in the presence of renal impairment or in the very elderly. In general, monitoring of LMWH activity is not required, but factor Xa levels can be measured where necessary.
A scheme for management of perioperative bridging therapy according to the risk of thromboembolic events is presented in Table 2.3.
New oral anticoagulant drugs
Rivaroxaban: Is a direct inhibitor of factor Xa. It has been licensed for the prevention of VTE following major joint replacement surgery. There is no specific reversal agent for this drug. It is recommended that this drug be discontinued 24 hours prior to surgery. If it is used for postoperative VTE prophylaxis 24 hours should elapse before epidural catheter removal.
Dabigatran: Is a direct thrombin inhibitor. Both the INR and aPTT are prolonged by the drug, but not in a dose-dependent manner. The thrombin clotting time (TT) is highly sensitive for quantifying its anticoagulant effects. It is almost entirely dependent on renal excretion for its elimination. There is no reversal agent for this drug.
ANTIPLATELET THERAPY
Stratifying the bleeding risk: Because there is considerable inter individual variability in response to both aspirin and, especially, clopidogrel therapy, some patients may be at greater risk than others for adverse bleeding outcomes. It is now becoming apparent that the degree of platelet inhibition in patients treated with the same antiplatelet regime is highly variable and up to 30% of patients may show no demonstrable platelet inhibition on standard therapy. This has implications not only for the risks of recurrent ischaemic events in ‘hypo-responders’, but at the other end of the spectrum for bleeding risks in ‘hyper-responders’. In terms of antiplatelet therapy there is undoubtedly an optimal therapeutic window, but there are many challenges left to define the best method for monitoring platelet function and to identify ‘cut-off’ values where the risk of ischaemic events or bleeding becomes a significant risk. There is accumulating evidence that bleeding risk increases as the degree of irreversible platelet inhibition increases. Prasugrel is a third generation thienopyridine that achieves 4–5 times more potent ADP P2Y12 receptor blockade than clopidogrel. It significantly reduced ischaemic events in the TRITON – TIMI trial but the occurrence of major bleeding was also significantly increased. Prasugrel is increasingly used in patients with coronary stents who have had a poor response to conventional therapy. Point of care platelet function monitoring as a means of assessing the efficacy of these drugs is still under evaluation, but the most promising techniques in terms of assessing bleeding risk are platelet mapping™, which is a modification of the thromboealstographic technique and the Multiplate® analyser.
Perioperative management: It is currently recommended that patients on aspirin as primary prevention should continue therapy up until the day of surgery and those on clopidogrel should discontinue treatment at least 5 days prior to surgery. However, there are serious thrombotic risks associated with the discontinuation of these agents when they are used for secondary prevention of vascular disease or after coronary revascularization. It is generally agreed that aspirin should never be discontinued before surgery unless the risk of bleeding is thought unacceptable, e.g. intracranial surgery. Clopidogrel alone appears to increase the bleeding risk more than for aspirin alone. Dual therapy increases the relative risk of bleeding by 50% and the absolute risk by 1%. This risk remains increased in patients who stopped clopidogrel less than 5 days before surgery.